Highlights
-
•
Xylan is an abundant carbohydrate component of plant cell walls that is vital for proper cell wall structure and vascular tissue development.
-
•
Xylan structure is known to vary between different tissues and species.
-
•
The role of xylan in the plant cell wall is to interact with cellulose, lignin, and hemicelluloses.
-
•
Xylan synthesis is directed by several types of Golgi-localized enzymes.
-
•
Xylan is being explored as an eco-friendly resource for diverse commercial applications.
Abbreviations: GX, glucuronoxylan; GAX, glucuronoarabinoxylan; AGX, arabinoglucuronoxylan; MeGlcpA, 4-O-methylglucuronic acid; GlcpA, glucuronic acid; Araf, L-α-arabinofuranose, TBL, Trichome Birefringence Like; UDP-sugar, uridine diphosphate-linked sugar; XSC, xylan synthase complex; IRX10, Irregular Xylem 10; IRX9, Irregular Xylem 9; IRX14, Irregular Xylem 14; GXMT/GXM, glucuronoxylan methyltransferase; XOATs, xylan O-acetyltransferases; NMR, Nuclear magnetic resonance
Keywords: Xylan, Plant cell wall, glucuronoxylan (GX), glucuronoarabinoxylan (GAX), or arabinoglucuronoxylan (AGX), Glycosyltransferase, Xylan biosynthesis, Hemicellulose
Introduction
The hemicellulose xylan is a plant cell wall polysaccharide that accounts for a considerable mass percentage of many commercially relevant agricultural products, including food, forage and timber. For example, it represents 20–30 % dry weight of the secondary cell wall of dicots, including hardwood trees used for timber, and up to 50 % of the total mass of the walls of grasses, such as forage and cereals (Scheller and Ulvskov, 2010). Thus, xylan is widely considered the second most abundant plant biopolymer on Earth after cellulose (Scheller and Ulvskov, 2010). The emergence of complex β-1,4 xylan in the cell wall was a key evolutionary event that coincided with the colonization of land by plants. Charophyte algae, ancestral to modern land plants, are the most basal plants known to date to synthesize xylans like those found in terrestrial plants (Jensen et al., 2018). The importance of xylan in building a functional and mechanically strong cell wall has been demonstrated by the generation of xylan-deficient mutants that appear stunted and deformed, presumably due to the development of vascular tissue with characteristic misshapen vessels (Brown et al., 2009). The working hypothesis of cell wall structure holds that hemicelluloses such as xylan fill an intermediate role in the cell wall by facilitating interactions between the relatively chemically inert cellulose microfibrils and the pectin/lignin matrix of primary and secondary cell walls, respectively (Kang et al., 2019).
Xylan structure
The xylans of land plants are broadly defined as substituted polymers with a β-1,4 linked xylose backbone, though some algae produce xylan that incorporates β-1,3 linkages (Hsieh and Harris, 2019). Xylan function within the wall is determined by the identity, amount and patterning of glycosyl and non-glycosyl substituents appended to the backbone structure. This Surface Feature is focused on land plant xylans, which are broadly classified as glucuronoxylan (GX), glucuronoarabinoxylan (GAX), or arabinoglucuronoxylan (AGX) depending on the composition of the sugar substituents (Fig. 1). GX is primarily found in the secondary cell walls that make up the vascular tissue of dicots and many monocots (Peña et al., 2016). GX is heavily O-acetylated and features α-1,2 linked glucuronic acid (GlcpA) and 4-O-methylglucuronic acid (MeGlcpA) as the primary sugar substituents. For example, the secondary cell wall xylans from Arabidopsis thaliana are acetylated on more than half of the backbone xyloses, and one of every 8–10 xylosyl residues is substituted with (Me)GlcpA (Smith et al., 2017). The patterning of the (Me)GlcpA and acetyl residues in A. thaliana occurs as distinct domains; one evenly spaced and the other clustered sporadically (Grantham et al., 2017). Additionally, GX often features a unique reducing end sequence Xylp-1,4-β-d-Xylp-1,3-α-l-Rhap-1,2-α-d-GalpA-1,4-d-Xylp, often termed ‘Sequence 1′, for which the function is still uncertain (Pena et al., 2007). In contrast, GAXs are distinguished by the abundance of l-α-arabinofuranose (Araf) substituents on the backbone and can be found in both primary and secondary cell walls of commelinid monocots such as grasses (Scheller and Ulvskov, 2010). GAXs exhibit the greatest diversity of side chains and linkages (Fig. 1), which have been shown to vary significantly between even different tissues of the same plant (Peña et al., 2016). It has been hypothesized that the change in xylan chemical composition that occurred with GAX evolution led to the replacement of the functional role of much of the pectin, classical extensins (Liu et al., 2016), and other hemicelluloses in commelinid primary cell walls with xylan (Carpita, 1996). A notable feature of GAX is the presence of aromatic moieties esterified to Araf residues (Peña et al., 2016). These complex sidechains permit polysaccharide-polysaccharide and polysaccharide-lignin crosslinking and may have had a part in the expansion of the role of GAX within the commelinid cell wall. AGXs are found in the secondary walls of Gymnosperms (Fig. 1). These xylans are mainly substituted with (Me)GlcpA residues at O-2 and to a lesser extent with l-α-Araf at O-3. This broad overview of xylan structure excludes many side chains and substituents for the sake of brevity. In reality, xylans exhibit a large degree of structural heterogeneity depending on plant species, tissue, developmental stage, and cell wall category (Fig. 1). Furthermore, the limited taxonomic diversity of in-depth cell wall analysis suggests that there likely remain undiscovered xylan structural variants.
Fig. 1.
Structural diversity of xylan in the main groups of land plants. The figure shows characteristic structural motifs of xylans from representative members of the plant group based in data described in the literature* (Haghighat et al., 2016, Scheller and Ulvskov, 2010, Smith et al., 2017). Not all xylan side chains identified are represented in the figure. The abbreviation used are FA, Ferulic acid; pCA, p-Coumaric acid; Ac, Acetyl group. *Gymnosperms do not have acetylated xylan with the exception of some Gnetophytes.
Secondary cell wall xylan regulates the interface between cellulose and lignin. Nuclear magnetic resonance (NMR) spectroscopy and molecular dynamics simulations have demonstrated that xylan has two primary configurations in the cell wall: a twofold and a threefold helical screw (Kang et al., 2019). In solution, xylan adopts the threefold screw conformation, a complete rotation of the backbone xylose residues every-three units. Interaction with cellulose promotes the flattening of this threefold screw into a twofold conformation. In A. thaliana, when substituent spacing is accounted for, evenly spaced domains adopting this twofold screw conformation have the substituents facing away from the cellulose microfibril producing a hydrophobic and negatively charged outer layer on the neutral cellulose surface (Grantham et al., 2017). Irregular spacing and position of substituents may prevent or limit proper hydrogen bonding between cellulose and xylan, instead resulting in a threefold xylan that extends away from the cellulose microfibril and deeper into the lignin matrix and into the vicinity of other cellulose microfibrils.
Xylan synthesis
Xylan is synthesized in the Golgi apparatus by the coordinated activity of several enzyme classes (Fig. 2) (Smith et al., 2017). Formation of the glycosidic bonds of the backbone and sugar substituents are catalyzed by glycosyltransferases, acetyl groups are added by xylan O-acetyltransferases (XOATs) from the Trichome Birefringence Like (TBL) family (PFAM 13839), and GlcpA is methyl-etherified by glucuronoxylan methyltransferase (GXMT/GXM) enzymes that contain a Domain of Unknown Function 579 (DUF579; PFAM 04669) (Urbanowicz et al., 2012). Each of these processes is dependent upon a supply of donor substrates and requisite transit into the Golgi lumen from the cytosol (Ebert et al., 2015). In fact, many mutant lines with cell wall abnormalities have been found to be defective in Golgi membrane transporters or enzymes responsible for substrate metabolism, rather than the enzymes directly involved in synthesis. Glycosyltransferases involved in xylan synthesis utilize uridine diphosphate-linked sugars (UDP-sugars) as donor substrates. The relevant substrate pools are maintained by a suite of enzymes that interconvert these UDP-sugars and the membrane transporters that sequester them within the lumen.
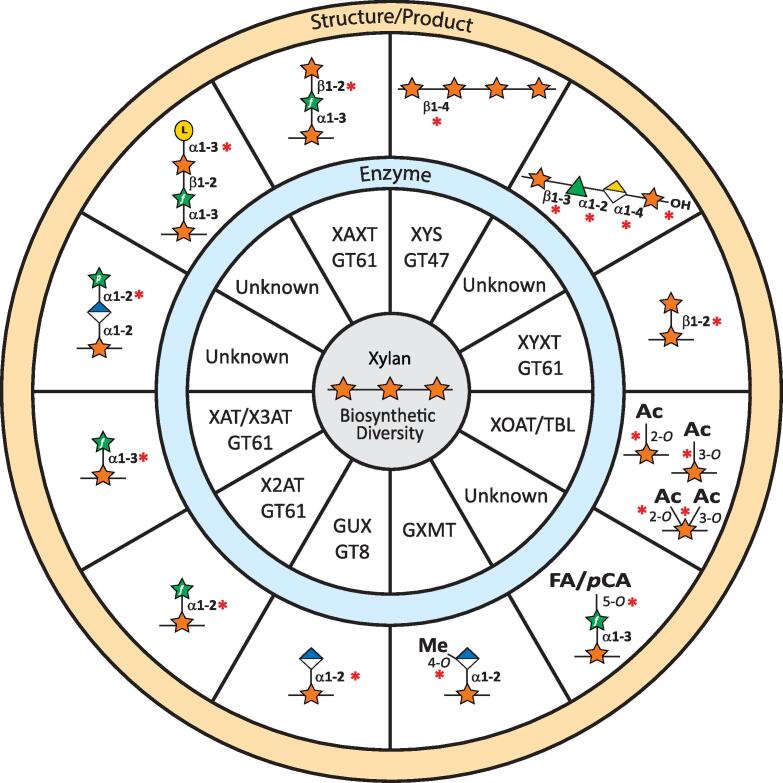
Fig. 2.
The wheel of xylan biosynthetic diversity keeps on turning. Xylan structural features and characterized enzymes involved in xylan synthesis. The enzymes included in this figure have been functionally characterized in vitro and the CAZy family is indicated for glycosyltransferases (GTs). The following references provide supporting data for the proteins listed herein: XYS/IRX10 (Jensen et al., 2014, Urbanowicz et al., 2014); GUX (Lee et al., 2012, Rennie et al., 2012); XAT/XYXT (Anders et al., 2012, Zhong et al., 2022b); XAXT (Zhong et al., 2022a); XYXT (Zhong et al., 2018); GXMT (Urbanowicz et al., 2012); XOAT/TBL (Lunin et al., 2020, Urbanowicz et al., 2014); and X3AT1/X2AT1 (Zhong et al., 2022b). Red asterisks (*) indicate the linkage generated by the action of the respective enzyme. Structures whose biosynthetic enzymes are unknown and/or not functionally characterized are indicated.
Xylan biosynthesis is unique among the hemicelluloses because the backbone is synthesized in the Golgi lumen by glycosyltransferases with a single transmembrane anchor, or no transmembrane region at all, rather than by multi-pass transmembrane proteins. One enzyme, Irregular Xylem 10 (IRX10) or Xylan Synthase 1 (XYS1) has been biochemically confirmed to have β-1,4 xylosyltransferase activity and extends the xylan backbone in vitro (Jensen et al., 2018, Urbanowicz et al., 2014). In vivo, it has been shown to form a xylan synthase complex (XSC) with at least two other glycosyltransferases: IRX9 and IRX14 (Jensen et al., 2014, Zeng et al., 2016). The role of these two proteins remains enigmatic, as neither has a demonstrated enzymatic function, yet mutants are xylan deficient and exhibit stunted growth phenotypes (Wu et al., 2010). Hypotheses for the role of the XSC include assisting in synthesis initiation, acquiring substrates, anchoring the growing xylan backbone, or simply serving as a scaffold for other xylan biosynthetic enzymes to be near the nascent backbone. Initiation of xylan synthesis has been the subject of considerable interest. Recently, XYS1 from rice was demonstrated to have the ability to synthesize xylan de novo from UDP-xylose, albeit with lower catalytic efficiency than its ability to extend existing xylo-oligomers (Wang et al., 2022). It is unknown if this is the dominant xylan chain initiation activity in vivo, an artifact of the in vitro conditions, or if another enzyme conducts initiation with more favorable kinetics. It has been hypothesized that Sequence 1 acts as a primer for xylan backbone extension, but this idea is complicated by the abundance of xylans, such as those from monocots and lower plants, that lack Sequence 1. There remain myriad questions regarding the mechanisms of side chain addition and patterning regulation on xylan that will require new biochemical approaches to decipher.
Xylan biotechnology applications
Xylan has recently gained attention for its potential as an environmentally friendly, renewable feedstock alternative in several industries. Xylan occurs as a significant component of lignocellulosic biomass and agricultural wastes, but due to a combination of complexity and structural diversity depending on the source, it acts as a waste product and/or an inhibitor to bioprocesses. Several approaches to valorize xylan-enriched wastes or engineer biomass feedstocks with more favorable processing potential are underway. Three domains that have attracted particular attention as next-generation, xylan-based biotechnologies are biofuels, biomaterials, and medical applications. Biofuel production requires polysaccharide deconstruction followed by upgrading into usable hydrocarbons. Enzymatic deconstruction of biomass utilizes microorganisms and hydrolytic enzymes to deconstruct biomass under relatively mild conditions while minimizing the energy required and chemical waste produced. Most of the microorganisms commonly utilized for biomass deconstruction readily hydrolyze cellulose and are well suited to utilize the hexose sugars generated. On other hand, xylan presents a problem because these microorganisms often lack the metabolic pathways to deconstruct xylan and utilize pentose sugars. As a result, recalcitrant xylan coating cellulose may act as a physical barrier for hydrolytic enzymes, and xylan oligosaccharides can act as chemical inhibitors (Raud et al., 2019). Therefore, improving the commercial utility of xylan using synthetic biology approaches to produce less recalcitrant energy crops and more efficient microorganisms is an area of interest. Another option is alternative pathways for utilization of xylan-rich wastes from the manufacturing of cellulose products. These wastes are often incinerated, effectively wasting a large portion of fixed carbon. Instead, the xylan poly- and oligosaccharides in these abundant waste streams can be upgraded into valuable chemicals and biomaterials (Vuong and Master, 2021). Materials such as packaging films, bioplastics, hydrogels, and nano- and microparticles have been derived from xylan. Xylan-based films have varying properties based on the substituents present, and along with xylan-based bioplastics, may be biodegradable. As a result, this technology is an intriguing option for green packaging applications (Yilmaz-Turan et al., 2020). Xylan is biodegradable, non-toxic, and has even been shown to have beneficial effects on human and microbiome health (Yan et al., 2022). Accordingly, xylan-based hydrogels and particles are being explored as delivery vehicles for drugs and agricultural chemicals. These characteristics, combined with the crosslinking and self-association properties of xylan polymers, result in materials that can be loaded with active compounds and applied topically, orally, or environmentally, while offering benefits such as protection and delayed release for the payload (Beckers et al., 2020, Elkihel et al., 2021, Fu et al., 2020).
Funding
Funding provided by the Center for Bioenergy Innovation (CBI), from the U.S. Department of Energy Bioenergy Research Centers supported by the Office of Biological and Environmental Research in the DOE Office of Science.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
No data was used for the research described in the article.
References:
- Anders N., Wilkinson M.D., Lovegrove A., Freeman J., Tryfona T., Pellny T.K., Weimar T., Mortimer J.C., Stott K., Baker J.M., Defoin-Platel M., Shewry P.R., Dupree P., Mitchell R.A.C. Glycosyl transferases in family 61 mediate arabinofuranosyl transfer onto xylan in grasses. Proc. Natl. Acad. Sci. U.S.A. 2012;109(3):989–993. doi: 10.1073/pnas.1115858109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers S.J., Wetherbee L., Fischer J., Wurm F.R. Fungicide-loaded and biodegradable xylan-based nanocarriers. Biopolymers. 2020;111:e23413. doi: 10.1002/bip.23413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D.M., Zhang Z., Stephens E., Dupree P., Turner S.R. Characterization of IRX10 and IRX10-like reveals an essential role in glucuronoxylan biosynthesis in Arabidopsis. Plant J. 2009;57:732–746. doi: 10.1111/j.1365-313X.2008.03729.x. [DOI] [PubMed] [Google Scholar]
- Carpita N.C. Structure and Biogenesis of the Cell Walls of Grasses. Annu. Rev. Plant Physiol. 1996;47(1):445–476. doi: 10.1146/annurev.arplant.47.1.445. [DOI] [PubMed] [Google Scholar]
- Ebert B., Rautengarten C., Guo X., Xiong G., Stonebloom S., Smith-Moritz A.M., Herter T., Chan L.J.G., Adams P.D., Petzold C.J., Pauly M., Willats W.G.T., Heazlewood J.L., Scheller H.V. Identification and Characterization of a Golgi-Localized UDP-Xylose Transporter Family from Arabidopsis. Plant Cell. 2015;27(4):1218–1227. doi: 10.1105/tpc.114.133827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkihel A., Christie C., Vernisse C., Ouk T.S., Lucas R., Chaleix V., Sol V. Xylan-Based Cross-Linked Hydrogel for Photodynamic Antimicrobial Chemotherapy. ACS Appl Bio Mater. 2021;4:7204–7212. doi: 10.1021/acsabm.1c00760. [DOI] [PubMed] [Google Scholar]
- Fu G.Q., Zhang S.C., Chen G.G., Hao X., Bian J., Peng F. Xylan-based hydrogels for potential skin care application. Int J Biol Macromol. 2020;158:244–250. doi: 10.1016/j.ijbiomac.2020.04.235. [DOI] [PubMed] [Google Scholar]
- Grantham N.J., Wurman-Rodrich J., Terrett O.M., Lyczakowski J.J., Stott K., Iuga D., Simmons T.J., Durand-Tardif M., Brown S.P., Dupree R., Busse-Wicher M., Dupree P. An even pattern of xylan substitution is critical for interaction with cellulose in plant cell walls. Nat Plants. 2017;3(11):859–865. doi: 10.1038/s41477-017-0030-8. [DOI] [PubMed] [Google Scholar]
- Haghighat M., Teng Q., Zhong R., Ye Z.H. Evolutionary Conservation of Xylan Biosynthetic Genes in Selaginella moellendorffii and Physcomitrella patens. Plant Cell Physiol. 2016;57:1707–1719. doi: 10.1093/pcp/pcw096. [DOI] [PubMed] [Google Scholar]
- Hsieh Y.S.Y., Harris P.J. Xylans of Red and Green Algae: What Is Known about Their Structures and How They Are Synthesised? Polymers (Basel) 2019;11 doi: 10.3390/polym11020354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J.K., Johnson N.R., Wilkerson C.G. Arabidopsis thaliana IRX10 and two related proteins from psyllium and Physcomitrella patens are xylan xylosyltransferases. Plant J. 2014;80:207–215. doi: 10.1111/tpj.12641. [DOI] [PubMed] [Google Scholar]
- Jensen J.K., Busse-Wicher M., Poulsen C.P., Fangel J.U., Smith P.J., Yang J.-Y., Peña M.-J., Dinesen M.H., Martens H.J., Melkonian M., Wong G.-S., Moremen K.W., Wilkerson C.G., Scheller H.V., Dupree P., Ulvskov P., Urbanowicz B.R., Harholt J. Identification of an algal xylan synthase indicates that there is functional orthology between algal and plant cell wall biosynthesis. New Phytol. 2018;218(3):1049–1060. doi: 10.1111/nph.15050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang X., Kirui A., Dickwella Widanage M.C., Mentink-Vigier F., Cosgrove D.J., Wang T. Lignin-polysaccharide interactions in plant secondary cell walls revealed by solid-state NMR. Nat Commun. 2019;10:347. doi: 10.1038/s41467-018-08252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., Teng Q., Zhong R., Ye Z.-H. Arabidopsis GUX proteins are glucuronyltransferases responsible for the addition of glucuronic acid side chains onto xylan. Plant and Cell Physiology. 2012;53(7):1204–1216. doi: 10.1093/pcp/pcs064. [DOI] [PubMed] [Google Scholar]
- Liu X., Wolfe R., Welch L.R., Domozych D.S., Popper Z.A., Showalter A.M., Zabotina O.A. Bioinformatic identification and analysis of extensins in the plant kingdom. PloS one. 2016;11(2):e0150177. doi: 10.1371/journal.pone.0150177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunin V.V., Wang H.-T., Bharadwaj V.S., Alahuhta M., Peña M.J., Yang J.-Y., Archer-Hartmann S.A., Azadi P., Himmel M.E., Moremen K.W., York W.S., Bomble Y.J., Urbanowicz B.R. Molecular mechanism of polysaccharide acetylation by the Arabidopsis xylan O-acetyltransferase XOAT1. The Plant Cell. 2020;32(7):2367–2382. doi: 10.1105/tpc.20.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña M.J., Kulkarni A.R., Backe J., Boyd M., O’Neill M.A., York W.S. Structural diversity of xylans in the cell walls of monocots. Planta. 2016;244:589–606. doi: 10.1007/s00425-016-2527-1. [DOI] [PubMed] [Google Scholar]
- Pena M.J., Zhong R., Zhou G.K., Richardson E.A., O'Neill M.A., Darvill A.G., York W.S., Ye Z.H. Arabidopsis irregular xylem8 and irregular xylem9: implications for the complexity of glucuronoxylan biosynthesis. Plant Cell. 2007;19:549–563. doi: 10.1105/tpc.106.049320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raud M., Kikas T., Sippula O., Shurpali N.J. Potentials and challenges in lignocellulosic biofuel production technology. Renewable and Sustainable Energy Reviews. 2019;111:44–56. doi: 10.1016/j.rser.2019.05.020. [DOI] [Google Scholar]
- Rennie E.A., Hansen S.F., Baidoo E.E., Hadi M.Z., Keasling J.D., Scheller H.V. Three members of the Arabidopsis glycosyltransferase family 8 are xylan glucuronosyltransferases. Plant physiology. 2012;159:1408–1417. doi: 10.1104/pp.112.200964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller H.V., Ulvskov P. Hemicelluloses. Annu Rev Plant Biol. 2010;61:263–289. doi: 10.1146/annurev-arplant-042809-112315. [DOI] [PubMed] [Google Scholar]
- Smith P.J., Wang H.T., York W.S., Pena M.J., Urbanowicz B.R. Designer biomass for next-generation biorefineries: leveraging recent insights into xylan structure and biosynthesis. Biotechnol Biofuels. 2017;10:286. doi: 10.1186/s13068-017-0973-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanowicz B.R., Peña M.J., Ratnaparkhe S., Avci U., Backe J., Steet H.F., Foston M., Li H., O’Neill M.A., Ragauskas A.J., Darvill A.G., Wyman C., Gilbert H.J., York W.S. 4- O -methylation of glucuronic acid in Arabidopsis glucuronoxylan is catalyzed by a domain of unknown function family 579 protein. Proc. Natl. Acad. Sci. U.S.A. 2012;109(35):14253–14258. doi: 10.1073/pnas.1208097109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanowicz B.R., Peña M.J., Moniz H.A., Moremen K.W., York W.S. Two A rabidopsis proteins synthesize acetylated xylan in vitro. The Plant Journal. 2014;80:197–206. doi: 10.1111/tpj.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong T.V., Master E.R. Enzymatic upgrading of heteroxylans for added-value chemicals and polymers. Curr Opin Biotechnol. 2021;73:51–60. doi: 10.1016/j.copbio.2021.07.001. [DOI] [PubMed] [Google Scholar]
- Wang H., Yang H., Wen Z., Gao C., Gao Y., Tian Y., Xu Z., Liu X., Persson S., Zhang B., Zhou Y. Xylan-based nanocompartments orchestrate plant vessel wall patterning. Nat Plants. 2022;8:295–306. doi: 10.1038/s41477-022-01113-1. [DOI] [PubMed] [Google Scholar]
- Wu A.M., Hornblad E., Voxeur A., Gerber L., Rihouey C., Lerouge P., Marchant A. Analysis of the Arabidopsis IRX9/IRX9-L and IRX14/IRX14-L pairs of glycosyltransferase genes reveals critical contributions to biosynthesis of the hemicellulose glucuronoxylan. Plant Physiol. 2010;153:542–554. doi: 10.1104/pp.110.154971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B., Huang C., Lai C., Ling Z., Yong Q. Production of prebiotic xylooligosaccharides from industrial-derived xylan residue by organic acid treatment. Carbohydr Polym. 2022;292 doi: 10.1016/j.carbpol.2022.119641. [DOI] [PubMed] [Google Scholar]
- Yilmaz-Turan S., Jimenez-Quero A., Menzel C., de Carvalho D.M., Lindstrom M.E., Sevastyanova O., Moriana R., Vilaplana F. Bio-based films from wheat bran feruloylated arabinoxylan: Effect of extraction technique, acetylation and feruloylation. Carbohydr Polym. 2020;250 doi: 10.1016/j.carbpol.2020.116916. [DOI] [PubMed] [Google Scholar]
- Zeng W., Lampugnani E.R., Picard K.L., Song L., Wu A.M., Farion I.M., Zhao J., Ford K., Doblin M.S., Bacic A. Asparagus IRX9, IRX10, and IRX14A Are Components of an Active Xylan Backbone Synthase Complex that Forms in the Golgi Apparatus. Plant Physiol. 2016;171:93–109. doi: 10.1104/pp.15.01919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R., Cui D., Phillips D.R., Ye Z.-H. A novel rice xylosyltransferase catalyzes the addition of 2-O-xylosyl side chains onto the xylan backbone. Plant and Cell Physiology. 2018;59:554–565. doi: 10.1093/pcp/pcy003. [DOI] [PubMed] [Google Scholar]
- Zhong R., Lee C., Cui D., Phillips D.R., Adams E.R., Jeong H.Y., Jung K.H., Ye Z.H. Identification of xylan arabinosyl 2-O-xylosyltransferases catalyzing the addition of 2-O-xylosyl residue onto arabinosyl side chains of xylan in grass species. The Plant Journal. 2022;112:193–206. doi: 10.1111/tpj.15939. [DOI] [PubMed] [Google Scholar]
- Zhong R., Phillips D.R., Ye Z.-H. Independent recruitment of glycosyltransferase family 61 members for xylan substitutions in conifers. Planta. 2022;256:70. doi: 10.1007/s00425-022-03989-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.