Abstract
The objective of this study was to investigate whether dietary supplementation with benzoic acid, Enterococcus faecium, and essential oil complex (BEC) could help laying hens recover from coccidia and Clostridium perfringens type A challenge. A total of 60 (35-wk-old) Lohmann-laying hens were randomly assigned to 3 experimental groups (10 replicates with 2 hens per replicate): I) control group (CON), II) challenge group (CC), and III) BEC group (2,000 mg/kg BEC). The total experimental period was 8 wk. The results shown that the challenge layers had lower egg-laying rate and average daily feed intake (ADFI) (P < 0.05), and addition of BEC after challenge increased egg-laying rate (P < 0.05). The content of propionic acid (PA) and butyric acid (BA) in short-chain fatty acid (SCFA) was significantly decreased by challenge (P < 0.05). CC and BEC groups had lower villus height to crypt depth ratio (V/C) and higher pathological scores in duodenum (P < 0.05), whereas the BEC group had lower pathological scores in jejunum when compared with the CC group (P < 0.05). The challenge increased the concentration of proinflammatory cytokines (IL-1β and IL-6) (P < 0.05). An increase in the abundance of Bacteroidoes (genus), Bacteroidaceae (family), Bacteroidoes sp. Marseille P3166 (species), Bacteroidoes caecicola (species) was observed in the CC group, whereas the BEC group had higher abundance of Bacteroides caecigallinarum (species). The genera Faecalibacterium and Asterolplasma were positively correlated with egg-laying rate (r = 0.57, 0.60; P < 0.01); and the genera Bacteroides and Romboutsia were negatively correlated with egg-laying rate (r = −0.58, −0.74; P < 0.01). The genera Bacteroides, Lactobacillus, and Rombutzia were positively correlated with jejunal mucosa proinflammatory factor IL-1β level (r = 0.61, 0.60, 0.59; P < 0.01), which were negatively correlated with genera Rikenbacteriaceae RC9, Faecalibacterium, and Olsenlla (r = −0.56, −0.57, −0.61; P < 0.01). There genera UCG.005 was positively correlated with proinflammatory factor IL-6 level in jejunal mucosa (r = 0.58; P < 0.01), which was negatively correlated with Rikenbacteriaceae RC9 (r = −0.62; P < 0.01). The experiment results revealed that the addition of BEC to the diet restored the production performance of the laying hens. In addition, supplementation of 2,000 mg/kg BEC modulated gut health by reducing gut damage scores and modulating microbial composition, thereby promoting recovery of laying hens after coccidia and Clostridium perfringens challenge.
Key words: benzoic acid, intestinal health, proinflammatory factor, modulating microbial composition
INTRODUCTION
For decades, the necrotic enteritis (NE) induced by Clostridium (C.) perfringens and coccidia had caused great economic loss in the global poultry industry (Wade and Keyburn, 2015). NE is one of the most important enteric diseases in poultry as it impaired performance and food safety, in which coccidia and C. perfringens are 2 important factors of this disease (Biggs, 1985). In general, C. perfringens is a commensal bacterium in the intestine, which does not develop disease under normal circumstances. The pathogenic process of the bacteria requires inducers, such as special dietary composition, immune suppression, intestinal machinery damage, and intestinal flora disturbance, etc. Coccidia can parasitize in the intestinal epithelium, thereby causing intestinal mucosa destruction and immune response, creating conditions for the colonization of C. perfringens, and continuously promoting the development of NE (Immerseel et al., 2008). Adding antibiotics to the diet is an effective method to control NE in poultry. However, some studies have shown that with the prohibition of the use of antibiotics, the incidence of NE caused by C. perfringens of broilers has increased, which could lead to the production performance have a sharp decline. And the studies have shown that the diarrhea and mortality of piglets due to Escherichia coli infection has increased (Mark et al., 2003; Dahiya et al., 2006). Thereby, the development of new antibiotic alternatives is essential for the treatment of NE. In recent years, organic acids, essential oils (EOs), probiotics, and other feed additives have been widely used in promoting livestock production performance and protecting intestinal health. Kaya et al. (2014) shown that the addition of organic acids has a positive effect on the performance and egg quality of laying hens. Further research shown that an appropriate amount of benzoic acid improved egg quality and intestinal morphology, and promoted the gastrointestinal health of laying hens (Gong et al., 2021). Several studies have demonstrated that EOs may improve laying performance and health status by its anti-inflammatory, anthelmintic, antimicrobial, and antioxidant properties as well as stimulation of digestive secretions and immune modulation. And it reported that Eos, such as thymol and carvacrol, can destroy the structure of coccidia oocysts and inhibit the growth of both gram-positive and gram-negative bacteria (Remmal et al., 2011; Achahbar et al., 2012; Du et al., 2015). In addition, previous studies have shown that Enterococcus faecium (E. faecium) significantly improved production performance and nutrient digestibility of laying hens and breeders (Park et al., 2016; Zhao et al., 2019; Wang et al., 2021a). And Placha et al. (2010) believed that E. faecium as a probiotic could neutralize the negative effects of EOs on the intestinal integrity of laying hens. Our previous study found that the dietary supplementation of BEC could effectively alleviate the stress of laying hens challenged by coccidia and C. perfringens (Zhang et al., 2022). Then whether higher level of BEC can recover its stress damage, further research is needed. Therefore, the purpose of this study was to investigate whether the dietary supplementation with the high level of BEC help laying hens recover more quickly from coccidia and C. perfringens challenged.
MATERIALS AND METHODS
Chemicals, Pathogen, and Bacterial Strain
The commercial BEC product used contained 70% of benzoic acid (99.5% of purity), 5% of EO (thymol:carvacrol = 1:1), 5% of Enterococcus faecium (EF, 2 × 108 CFU/kg diet), and 20% of its own carrier (50% silica and 50% dextrin) from DSM (DSM Nutritional Products Inc., Shanghai, China).
The avian coccidiosis quadrivalent live vaccine (provided by the Foshan Standard Biotech Co., Ltd., Guangdong, China), containing Eimeria tenella, Eimeria poisonous, Eimeria acerola, and Eimeria giant. The chicken C. perfringens was purchased from the China Veterinary Drug Administration (CVCC2030). After activation, it was inoculated into a sterile thioglycolate liquid medium at a volume ratio of 2% and cultured in a sterile incubator at 37°C for 24 h.
Experimental Birds, Management, and Diets
At 35 wk of ages, a total of 60 Lohmann gray hens were randomly assigned to 3 experimental groups including 10 replicates with 2 hens per replicate. I) Control group, basal diet; II) challenge group, from the sixth week (d 42–48), the hens form the challenge group and BEC group were treated with 80-fold anticoccidia vaccine (55,000 coccidia sporangia/mL/hen) via oral gavage and 40 mL of C. perfringens (2.5 × 1010 CFU/mL) via mix into feed individually; III) BEC group (dietary supplementation of 2,000 mg/kg BEC complex after challenge). The CON group sterile phosphate-buffered saline was administered instead. The challenge was performed at every day and lasted a week. All hens were housed individually in an environmentally controlled room where temperature was maintained at approximately 20°C to 22°C and artificial light by a daily lighting schedule of 16 h light and 8 h dark (challenged and unchallenged were kept in 2 separate room with the same facilities and equipment). Hens were given free access to water and a complete feeding mixture in mash form, the experimental diets meet the National Research Council (Kim, 1994) requirements, as shown in Supplementary Table 1.
Productive Performance and Sample Collection
After the challenge, egg numbers in each replicate were recorded from d 49 to d 62. Egg production was calculated as the average production per day.
On d 63, 8 hens/treatment were randomly selected to slaughter and collect samples. The hens were sacrificed by cervical dislocation. The middle of the duodenum and jejunum segments (about 3 cm) was fixed in 4% paraformaldehyde for mucosal morphology. Then, the intestine tissues (duodenum, jejunal) and cecum chyme were taken and then stored at −80°C till gene expression analysis.
Intestinal Morphology Analysis
Duodenum and jejunal mucosa morphology were analyzed as described previously (Yang et al., 2020; Gong et al., 2021). Briefly, following fixing in 10% paraformaldehyde, the intestinal segments were embedded in paraffin and sectioned (the section thickness was 3 μm), then stained with hematoxylin and eosin. The middle of duodenum, jejunum, and ileum (1 × 2 cm) under an optical microscope to collect images (Microscope: NIKON Eclipse ci, imaging system: NIKON digital sight DS-FI2, MADE in Japan). Observed the villus height and crypt depth, calculated the V/C.
Macroscopic Lesion Scoring of Small Intestines
Lesions in the small intestine (duodenum, jejunum) were scored as described by Keyburn et al. (2006) as follows: 0 = no gross lesions; 1 = congested intestinal mucosa; 2 = small focal necrosis or ulceration (1–5 foci); 3 = focal necrosis or ulceration (6–15 foci); 4 = focal necrosis or ulceration (16 or more foci); 5 = patches of necrosis 2- to 3-cm long; 6 = diffuse necrosis typical of field cases. Lesion scores of 2 or more were classified as necrotic enteritis positive.
Real-Time PCR for Jejunal Barrier-Related mRNA Expression
Total RNA of jejunum mucosa was extracted with TRIzol reagent (TaKaRa, Dalian, China) on basis of the manufacturer's instructions. The concentration of RNA was measured by using DU 640 UV spectrophotometer detection (Beckman Coulter Inc., Fullerton, CA). cDNA was synthesized by using primeScript RT reagent kit (Takara).
The primers of genes (Claudin-1, Claudin-2, zonula occudens (ZO)-1, ZO-2, Occludin, Mucin-1, Mucin-2), listed in Supplementary Table 2, were purchased from TaKaRa Biotechnology (Dalian Co., Ltd., Dalian, China), and the real-time PCR was performed using the SYBR Premix Ex Tap (Takara). The PCRs were run on an Applied Biosystems 7900HT Real-Time PCR system (Applied Biosystems, CA). The house keeping gene (β-actin) was chosen to correct for variance in the amount of RNA input in the reaction. The relative mRNA expression compared to the house keeping gene was obtained with previous methods (Wang et al., 2021a,b).
The Levels of Intestinal Inflammatory Factor
Enzyme-linked immunosorbent assay (ELISA) test kits (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China) were utilized to analyze the cytokine levels in jejunum, including interleukin (IL)-1β (IL-1β), IL-4, IL-6, and tumor necrosis factor alpha (TNF-α) following the previous method (Wang et al., 2021b).
Gut Microbiota Analysis and Short-Chain Fatty Acids Quantification in Cecum
Microbial profile in the cecum digesta (n = 8) was evaluated by the sequencing and clustering of 16S rRNA gene with high-throughput pyrosequencing, the sequencing and bioinformatics analysis were performed by Novogene Bioinformatics Technology Co. (Tianjin, China), and the method were used as recently described by Yang et al. (2020). SCFA (n = 8) including acetate, propionate, valerate, and butyrate in the cecum content were also analyzed using Agilent 6890 gas chromatograph (Agilent Technologies, Santa Clara, CA) following previous protocols (Wang et al., 2021b).
Statistical Analysis
All data were analyzed by one-way ANOVA using GLM procedure of SAS 9.0 software (SAS Institute, Cary, NC) and GraphPad Prism (GraphPad Inc., La Jolla, CA). For the microbiota, data were analyzed by Wilcox rank sum test, differences among treatments were considered significant at P < 0.05 or extremely significant at P < 0.01. Beta diversity based on the weighted UniFrac distance matrices were calculated with QIIME (Version 1.7.0) and Cluster analysis was preceded by principal coordinate analysis (PCoA). Differentially represented bacterial taxa between different samples were analyzed using the linear discriminant analysis effect size (LEfSe). The results were expressed as the mean and SEM.
RESULTS
Production Performance and Cecum SCFA
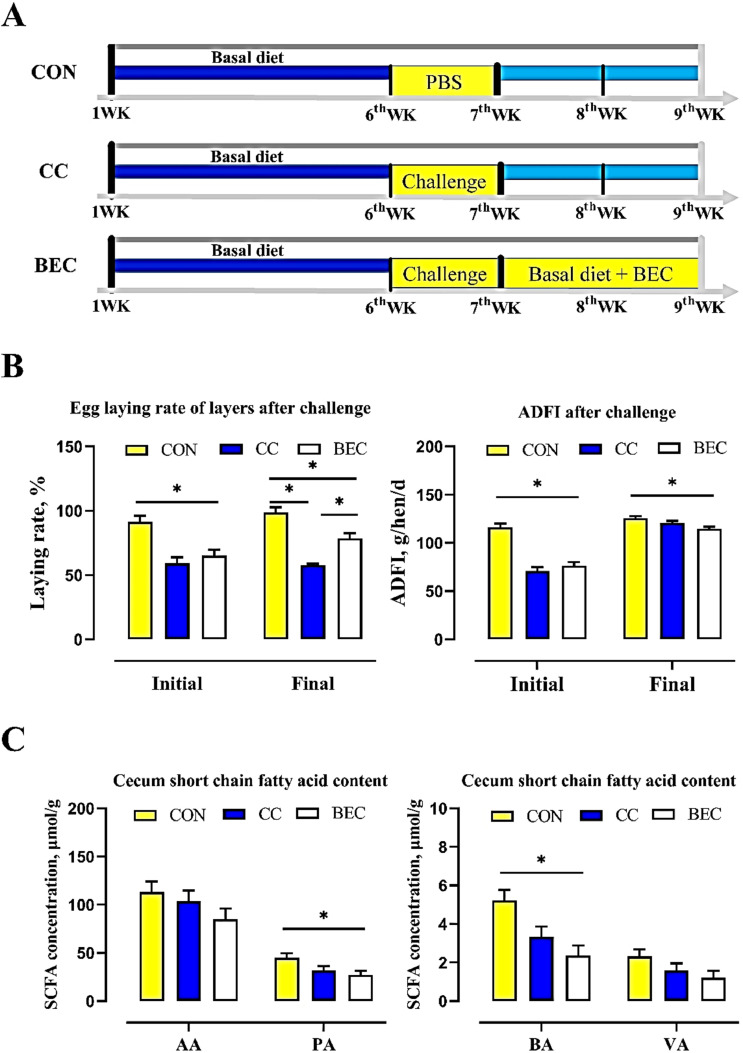
As shown in Figure 1B, after challenged the layers had lower egg-laying rate (ELR), ADFI compared with the CON group (P < 0.05). Dietary supplementation with BEC after challenge was shown to alleviate ELR than challenged ones (P < 0.05). The CC and BEC group had lower cecum content concentration of the main SCFA (propionate, butyrate) than those observed in the CON group (Figure 1C; P < 0.05).
Figure 1.
The effect of dietary BEC supplementation on the egg-laying rate, ADFI and SCFA. (A) Experiment design. (B) Egg-laying rate and average daily feed intake after challenge. (C) Short-chain fatty acid concentration in cecal digestion. Abbreviations: AA, acetate; ADFI, average daily feed intake; BA, butyrate; BEC, 2,000 mg/kg BEC (1,400 mg/kg benzoic acid, 2 × 108 CFU/kg Enterococcus faecium, and 100 mg/kg essential oil complex) after challenge; CC, challenge group; CON, control group; PA, propionate; SCFA, short-chain fatty acid; VA, valerate. Data are means ± SEM, *P < 0.05.
Intestinal Lesions Score and Intestinal Morphology
The CC and BEC groups had higher pathological scores in duodenum, whereas the BEC group had lower pathological scores in jejunum when compared with the CC group (Figure 2A; P < 0.05).
Figure 2.
The effect of dietary BEC supplementation on the intestinal lesion score, morphology, and inflammatory cytokine levels of laying hens after challenged. (A) Intestinal lesion score. (B) Intestinal morphology. (C) Jejunum inflammatory cytokine levels. Abbreviations: BEC, 2,000 mg/kg BEC (1,400 mg/kg benzoic acid, 2 × 108 CFU/kg Enterococcus faecium, and 100 mg/kg essential oil complex) after challenge; CC, challenge group; CON, control group; IL-4, interleukin-4; IL-1β, interleukin-1β; IL-6, interleukin-6; TNF-α, tumor necrosis factor alpha; V/C, villus height/crypt depth. Data are means ± SEM, *P < 0.05.
The layers in CC group had lower V/C ratio, and the layers fed BEC diet had higher crypt depth in jejunum than CON group; but had no effect on crypt depth and V/C ratio when compared with the challenged ones (Figure 2B; P < 0.05). No effect of BEC supplementation after challenge was observed on villus height in duodenum and jejunum, and crypt depth in jejunum (P > 0.05).
Jejunal Inflammatory Cytokine Levels and Intestinal Barrier Function
The challenge upregulated the concentration of proinflammatory cytokines (IL-1β and IL-6) (Figure 2C; P < 0.05), whereas the BEC addition after challenge didn't influence the proinflammatory cytokines levels and intestinal barrier function-related gene expression (Mucin-1, Mucin-2, ZO-1, ZO-2, Claudin-1, Claudin-2, and occludin) in jejunum of layers (Figure 3A–D; P > 0.05).
Figure 3.
The effect of dietary BEC supplementation on the expression of genes related to intestinal barrier function of laying hens after challenged. (A–D) Intestinal barrier function-related genes. Abbreviations: BEC, 2,000 mg/kg BEC (1,400 mg/kg benzoic acid, 2 × 108 CFU/kg Enterococcus faecium, and 100 mg/kg essential oil complex) after challenge; CC, challenge group; CON, control group; ZO-1, zonula occluden-1; ZO-2, zonula occluden-2. Data are means ± SEM, *P < 0.05.
Cecum Microbiota Composition
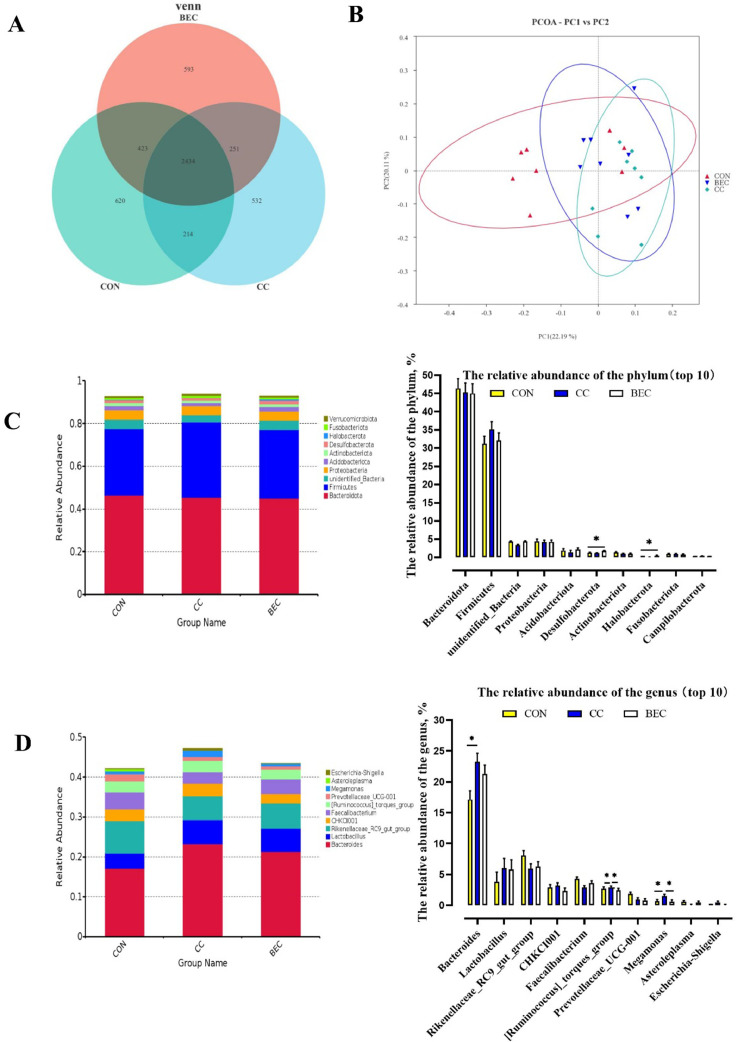
The shared OUT among 3 groups were presented in Figure 4A. Relative microbial abundances of the cecum at phylum level indicated that Firmicutes and Bacteroidota were the dominant phylum in all dietary treatments (CON, 77.56%; CC, 80.42%; BEC, 77.10%). The BEC group had higher abundance of Desulfobacterota and Halobacterota ratio value at the phylum level (Figure 4B; P < 0.01). At the genus level, we observed that enrichment of Lactobacillus and Bacteroides on 3 groups. The Bacteroides and Megamonas were increased in CC group, the Faecalibacterium was decreased compare with CON and BEC groups (Figure 4C; P < 0.05). These data showed that while the CC and BEC led to microbial variation but did not change the dominant species at phylum and genus level in the layer cecum.
Figure 4.
The effect of dietary BEC supplementation in microbiota diversity. (A) Venn diagram. (B) The principal coordinate analysis (PCoA) of the cecum microbiota based on unweighted UniFrac metric. (C, D) The relative abundance of the top 10 phylum (C) and genus (D) from groups. Abbreviations: BEC, 2,000 mg/kg BEC (1,400 mg/kg benzoic acid, 2 × 108 CFU/kg Enterococcus faecium, and 100 mg/kg essential oil complex) after challenge; CC, challenge group; CON, control group. Data are means ± SEM, * P < 0.05.
Beta Diversity of Cecum Microbiota
PCA of related bacterial communities indicate that the separation in the CON, CC and BEC groups could be hardly detected (Figure 4B; PC1 vs. PC2). As shown in Figure 5A and B (LEfSe), an increase in the abundance of Bacteroidoes (genus), Bacteroidaceae (family), Bacteroidoes sp. Marseille P3166 (species), Bacteroidoes caecicola (species) was observed in the CC group, whereas the BEC group had higher abundance of Bacteroides caecigallinarum (species).
Figure 5.
The effect of dietary BEC supplementation in microbiota diversity. (A) Taxonomic cladogram obtained from LEfSe analysis of 16S rRNA sequencing. Biomarker taxa are heighted by colored circles and shaded areas. Each circle's diameter is relative to abundance of taxa in the community. (B) Only taxa meeting an LDA significant threshold >3.5 are shown. (Red) BEC enriched taxa; (Green) CC enriched taxa; (Blue) CON enriched taxa. Abbreviations: CC, challenge group; CON, control group; BEC, 2,000 mg/kg BEC (1,400 mg/kg benzoic acid, 2 × 108 CFU/kg Enterococcus faecium, and 100 mg/kg essential oil complex) after challenge.
Correlations Between Cecum Microbiota and Laying Rate or Inflammatory Cytokines of Laying Hens
A Spearman correlation analysis was performed to evaluate the potential link between alterations in gut microbiota composition and the ELR or inflammatory cytokines in laying hens (Figure 6A and B). The ELR positively correlated with genus Faecalibacterium, Asteroleplasma, Rikenellaceae RC9 gut, Olsenella, and Alloprevotella (r = 0.57, 0.60, 0.48, 0.48, 0.42; P < 0.05), but negatively correlated with Bacteroides, Romboutsia, and Megamonas (r = −0.58, −0.73, −0.48; P < 0.05). The genera Bacteroides, Lactobacillus, Romboutasi, and Megamonas were positively correlated with IL-1β (r = 0.61, 0.60, 0.59, 0.53; P < 0.05), but genera Rikenellaceae RC9 gut, Faecalibacterium, Olsenella, Prevotellaceae UGG. 001, and Escherichia. Shigella (r = −0.56, −0.57, −0.61, −0.47; P < 0.05) were negatively correlated with IL-1β. The genera UCG. 005, Bacteroides, Lactobacillus, and UCG.008 positively correlated with IL-6 (r = 0.58, 0.48, 0.47, 0.47; P < 0.05), but genera Rikenellaceae RC9 gut, Faecalibacterium, Asteroleplasma, and Olsenella (r = −0.62, −0.46, −0.47, −0.46; P < 0.05) were negatively correlated with IL-6.
Figure 6.
Heatmap of spearman r correlations between the gut microbiota significantly modified by different (A) egg-laying rate (B) inflammatory factors at genus level (Top 35). Red indicates positive correlation, and blue indicates negative correlation; while the color is darker, the correlation is higher. *P < 0.05 and **P < 0.01. Abbreviations: BEC, 2,000 mg/kg BEC (1,400 mg/kg benzoic acid, 2 × 108 CFU/kg Enterococcus faecium, and 100 mg/kg essential oil complex) after challenge; CC, challenge group; CON, control group; ELR, egg-laying rate; IL1B, IL-1β (interleukin-1 β); IL-6, interleukin-1.
DISCUSSION
NE generally present as acute clinical and subclinical, the acute clinical NE was characterized by livestock mortality, while the subclinical NE caused intestinal mucosa damage, disrupted the villous crypt microarchitecture, and prevented digestion and absorption of nutrients, thereby impaired the growth performance of the chicken (Immerseel et al., 2004). In our study, we used a cochallenge of C. perfringens (CVCC2030) with coccidia, similar to our previous study using the same challenge model to create subclinical NE (Zhang et al., 2022). Different from the previous study, the BEC were supplemented after rather than before challenge, and the objective of current study is to evaluate whether the effect of BEC could help the laying recover from coccidia and C. perfringens challenge.
Usually, NE could lead to the decrease in feed intake, body weight gain and feed conversion of broilers (Du et al., 2015; Awawdeh, 2021). Similar to our findings, both egg production and ADFI decreased significantly after challenge. And the dietary supplementation BEC after challenge had an increase trendy in laying rate. Soltan (2008) showed that organic acids had no significant effect on egg production. But there was a study had shown that adding 150 mg/kg of organic acid and essential oil mixture could increase egg production and reduce ADFI of laying hens (Wang et al., 2019a). Therefore, the effects of organic acids and EOs on production performance may be influenced by different type and concentration, different dietary compositions, feeding, and management conditions (Gheisar et al., 2014; Habibi et al., 2014; Zeng et al., 2015).
The indigestible dietary fiber produced SCFA in the cecum of poultry under the action of anaerobic microbial fermentation, which could promote intestinal mucus secretion and protect the intestinal barrier by regulating the tight junction protein (Walugembe et al., 2015; Molnár et al., 2020). In this experiment, the concentration of cecal butyrate and propionate decreased when the laying hens were challenged with coccidia and C. perfringens, which indicated that challenge-induced stress lead to a depression in nutrients fermentation of cecum. Our observation was in agreement with that of Lin et al. (2022), who found that the content of acetate, butyrate and total SCFA in the cecum of broiler been lowered after challenged with the Eimeria. The short-chain fatty acids played a critical role in maintaining the physical health of animals. Among them, butyrate was an important energy source for promoting intestinal development and intestinal epithelial cells growth, while propionate was closely related to liver metabolism (Liao et al., 2020). There were some studies have shown that adding plant essential oil, organic acids or beneficial bacteria to the diet increased the concentration of SFCA (Yang et al., 2019; Xu et al., 2022). However, we suggested that the dietary supplementation with high level of BEC could not increase the content of cecal butyrate and propionate of laying hens which had been challenged with it was speculated that the NE caused by challenging might be more severe, or the individual beneficial substance in the BEC was lower coccidia and C. perfringens.
The intestine is an important digestive organ of the body, and the morphology of intestine is one of the most critical indicators that reflect the intestine health. The intestinal surface area including villus height, crypt depth, and V/C, which could determine the rate of intestinal epithelial turnover and the nutrient digestion and absorption capacity (Yang et al., 2016). If the crypt depth deepened, it means that the body may rebuild the villi by increasing the rate of cell turnover to fight the damage caused by pathogenic bacteria or toxin infection (Paiva et al., 2014). In our study, the CC and BEC groups significantly increased crypt depth and decreased V/C of duodenum compared with the control group. In our previous study, C. perfringens and coccidia challenged could make the crypt depth was higher and V/C was lower in duodenum, jejunum, and ileum (Zhang et al., 2022). It is proved that the challenge had a certain damaged to the integrity of intestinal morphology. Gong et al. (2021) shown that dietary supplemented with 2,000 mg/kg benzoic acid had increased crypt depth in duodenum. In addition, Placha et al. (2010) shown that 0.4% EO may had effects on intestine integrity, and the probiotic strain E. faecium was able to eliminate negative effects and can strengthen nonspecific immunity, which is similar to our findings but not identical. Coccidia and C. perfringens challenge lesions in different parts of the poultry gut that depend entirely on the extent of the infection, thus leading to differences in pathological conditions (Tyzzer et al., 1932). Keyburn et al. (2006) defined the intestinal lesions scores include thin or friable walls, focal necrosis or ulceration and diffuse necrosis typical of field cases. Our results shown that the lesions scores of duodenum and jejunum in challenge group were higher than, the BEC group had higher lesions scores of duodenum, and the BEC group had lower pathological scores of jejunum than control group. Our findings were similar to many studies, which also determined that coccidia and C. perfringens challenged significantly increased duodenum and jejunum lesions scores (Attia et al., 2012; Rodrigues et al., 2017; Gharib-Naseri et al., 2019). Mustafa et al. (2021) indicated that the dietary supplementation with organic acids in coccidial challenged group could improve intestinal health, and reduce intestinal lesions scores of broiler chickens. Another study showed that adding 120 mg/kg of essential oils (thymol and carvacrol) after C. perfringes challenged significantly reduced lesions scores in chickens (Yin et al., 2017). Recently, there were many studies about the effects of organic acids, essential oils or probiotics on the intestinal of challenged poultry, the results were inconsistent and further research was needed.
NE is understood to be a stepwise process that begins with depletion of the mucosal layer of the intestine, it appears that under conditions where birds are already subject to infection, the activity of mucolytic bacteria becomes more than the host mucogenic activity, with the result that pathogenesis of NE proceeds in the presence of increased host mucogenic activity (Collier et al., 2008). Forder et al. (2012) considered that challenged with Eimeria, and C. perfringens could affect the intestinal mucin synthesis genes, and they reported that the expression of Mucin2 and Mucin13 were depressed by challenge. However, we observed that the CC and BEC groups had no significant effects on the expression of genes-related intestinal barrier. Although our result was different from previous studies, the experiment had shown that the intestinal barrier-related genes was only expressed when it yield an acute challenge response (Kitessa et al., 2014). Further study is required to elucidate the pattern of change in mucin synthesis genes as NE progresses clinically or subclinically.
The upregulation of intestinal inflammatory factors can induce inflammatory responses in the intestinal tract of broilers. The occurrence of inflammatory diseases in animals is often accompanied by an increase in the secretion of inflammatory factors such as IL-Iβ and IL-6. In our study, compared with the control group, the CC and BEC groups upregulated the levels of IL-1β and IL-6. Studies have shown that the challenge activates the intracellular signaling cascade, and finally activates the nuclear transcription factor NF-κB, which promotes the synthesis and secretion of proinflammatory cytokines such as IL-1β and IL-6, and activates the inflammatory response (Paul et al., 2011). Al-Sadi et al. (2008) found that the inflammatory factors increased the permeability of the mucosal barrier of intestinal epithelial cells, allowing foreign antibodies to enter, leading to the occurrence of intestinal diseases.
To further elucidate the effect of BEC on the gut health of laying hens challenged with coccidia and C. perfringens, we focused on the analysis of gut microbial community was closely related to NE (Alizadeh et al., 2021). The present study showed that coccidia and C. perfringens challenged and the dietary was supplemented with BEC all had no significantly effect on richness and diversity of cecum microbial community. There was a previous study demonstrated that C. perfringens was used to challenge broiler did not affect α-diversity index of cecal microbial community (Latorre et al., 2018), while another study had reported that C. perfringens infection of broiler reduced the diversity of ileum microbial community (Bortoluzzi et al., 2019). This phenomenon correlated with the time of infection of coccidia and C. perfringens, the site of sample collection and the growth stage of the animal. According to the PCA analysis of β diversity, we found that the bacterial community structure of each treatment group was relatively similar, and Bacteroidota were the dominant bacteria at the phylum level. It was reported that Firmicutes and Bacteroidetes constituted vast majority of intestinal microbiota in laying hens, and dynamic changing occurred at different physiological stages (Dai et al.,2022). The Firmicutes to Bacteroidetes ratio was considered a critical biomarker of healthy intestinal function and could be indicative of microecological balance conditions in the gastrointestinal tract (Pereira et al., 2016). Similar to previous reports (Wang et al., 2019b; Gong et al., 2021), the current study revealed that the coccidia and C. perfringens challenge and the dietary supplementation with BEC induced a conversion in gut microenvironment, increased the abundance of Bacreroides and Lactobacillus at the genus level. Simultaneously, we observed that the genus Romboutsia, Lactobacillus and Bacreroides were positively correlated with proinflammatory factor IL-1β. There was a study have suggested that the presence of Bacreroides and Lactobacillus linked to intestinal inflammation and barrier dysfunction (Las-Heras et al., 2019), which might be involved in regulating intestinal anti-inflammatory response. In addition, we also found that the genus Asteroleplasma and Faecalibactenium were positively correlated with ELR. According to experimental researches (Min et al., 2015; Maioli et al., 2021), an increase in the abundance of Asteroleplasma resulted in intestinal inflammation and other intestinal disease and Faecalibactenium was a highly abundant butyrate-producing bacterium that could product anti-inflammatory metabolites. We thought that the alternation in microbiota associated with inflammation such as Bacreroides, Lactobacillus, Asteroleplasma, Faecalibactenium, and so on might affect intestinal health, metabolic function, and production performance of laying hens. Therefore, understanding how NE and BEC induced variations on the intestinal microbiota could help reduce the negative impact of the disease in animal health.
CONCLUSIONS
In conclusion, the C. perfringens and coccidia challenge resulted in reduced performance, sustained damage to the intestine and changed cecal microbiomes. Dietary supplementation of BEC complex right after challenge (1,400 mg/kg benzoic acid, 2 × 108 CFU/kg Enterococcus faecium, and 100 mg/kg essential oil) could help the layer recover from challenge by alleviating production performance, enriching microbial compositions, and promoting intestinal health of laying hens (Figure 7).
Figure 7.
Graphical summary of the effect of benzoic acid, Enterococcus faecium and essential oil complex on intestinal microbiota of laying hens under coccidia and Clostridium perfringens challenge.
ACKNOWLEDGMENTS
We would like to address great gratitude to the National Natural Science Foundation of China (31872792), and Sichuan Science and Technology Program (2022YFH0070, 2018NZ20009) and also to 720 lab and 111 project.
Author contributions: H. Z. and J. W. conceived and designed the experiments; J. W., and H. Z. performed the experiments; H. Z. and J. W. analyzed the data; H. Z. wrote the paper; X. D., S. B., Z. Q., K. Z., X. M., C. L., and J. W. helped revise this manuscript. All authors read and approved the final manuscript.
DISCLOSURES
The authors declare no conflict of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2023.102490.
Appendix. Supplementary materials
REFERENCES
- Achahbar S., Chami F., Chami N., Remmal A. In vitro destructive effect of essential oil components against chicken, eimeria oocysts. Trends Biotechnol. 2012;5:266–271. [Google Scholar]
- Alizadeh M., Shojadoost B., Boodhoo N., Astill J., Taha-Abdelaziz K., Hodgins D.C., Kulkarni R.R., Sharif S. Necrotic enteritis in chickens: a review of pathogenesis, immune responses and prevention, focusing on probiotics and vaccination. Anim. Health Res. Rev. 2021;22:147–162. doi: 10.1017/S146625232100013X. [DOI] [PubMed] [Google Scholar]
- Al-Sadi R., Ye D., Dokladny K., Ma T.Y. Mechanism of IL-1beta-induced increase in intestinal epithelial tight junction permeability. J. Immunol. 2008;15:5653–5661. doi: 10.4049/jimmunol.180.8.5653. 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia Y., Ellakany H., El-Hamid A., Bovera F., Ghazaly S. Control of Salmonella Enteritidis infection in male layer chickens by acetic acid and/or prebiotics, probiotics and antibiotics. Arch. Geflügelk. 2012;76:239–245. [Google Scholar]
- Awawdeh M.S. Assessment of immune response and efficacy of essential oils application on controlling necrotic enteritis induced by Clostridium perfringens in broiler chickens. Molecules. 2021;26:15. doi: 10.3390/molecules26154527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs P.M. Infectious animal disease and its control. Philos. T. R. Soc. B. 1985;310:259–274. doi: 10.1098/rstb.1985.0115. [DOI] [PubMed] [Google Scholar]
- Bortoluzzi C., Vieira B.S., Hofacre C., Applegate T.J. Effect of different challenge models to induce necrotic enteritis on the growth performance and intestinal microbiota of broiler chickens. Poult Sci. 2019;98:2800–2812. doi: 10.3382/ps/pez084. [DOI] [PubMed] [Google Scholar]
- Collier C.T., Hofacre C.L., Payne A.M., Anderson D.B., Kaiser P., Mackie R.I., Gaskins H.R. Coccidia induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet. Immunol. 2008;122:104–115. doi: 10.1016/j.vetimm.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Dahiya J.P., Wilkie D.C., Kessel A.G.V., Drew M.D. Potential strategies for controlling necrotic enteritis in broiler chickens in post-antibiotic era. Anim. Feed. Sci. Tech. 2006;129:60–88. [Google Scholar]
- Dai D., Qi G.H., Wang J., Zhang H.J., Qiu K., Wu S.G. Intestinal microbiota of layer hens and its association with egg quality and safety. Poult Sci. 2022;101 doi: 10.1016/j.psj.2022.102008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du E., Gan L., Li Z., Wang W., Liu D., Guo Y. In vitro antibacterial activity of thymol and carvacrol and their effects on broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2015;6:58. doi: 10.1186/s40104-015-0055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forder R.E.A., Nattrass G.S., Hughes M.S.R., Geier J., Hynd P.I. Quantitative analyses of genes associated with mucin synthesis of broiler chickens with induced necrotic enteritis. Poult. Sci. 2012;91:1335–1341. doi: 10.3382/ps.2011-02062. [DOI] [PubMed] [Google Scholar]
- Gharib-Naseri K., Kheravii S.K., Keerqin C., Morgan N., Swick R.A., Choct M., Wu S.B. Two different Clostridium perfringens strains produce different levels of necrotic enteritis in broiler chickens. Poult. Sci. 2019;98:6422–6432. doi: 10.3382/ps/pez480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheisar M.M., Hosseindoust A., Kim I.H. Evaluating the effect of microencapsulated blends of organic acids and essential oils in broiler chicken diet. J. Appl. Poult. Res. 2014;24:511–519. [Google Scholar]
- Gong H., Yang Z., Celi P., L.Yan X.Ding, Bai S., Zeng Q.F., Xu S.Y., Su Z.W., Zhuo Y. Effect of benzoic acid on production performance, egg quality, intestinal morphology, and cecal microbial community of laying hens. Poult. Sci. 2021;100:196–205. doi: 10.1016/j.psj.2020.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibi R., Sadeghi G., Karimi A. Effect of different concentrations of ginger root powder and its essential oil on growth performance, serum metabolites and antioxidant status in broiler chicks under heat stress. Br. Poult. Sci. 2014;55:228–237. doi: 10.1080/00071668.2014.887830. [DOI] [PubMed] [Google Scholar]
- Immerseel F.V., Buck J.D., Pasmans F., Huyghebaert G., Haesebrouck F., Ducatelle R. Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathol. 2004;33:537–549. doi: 10.1080/03079450400013162. [DOI] [PubMed] [Google Scholar]
- Immerseel F.V., Rood J.I., Moore R.J., Titball R.W. Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens. Trends. Microbiol. 2008;17:32–36. doi: 10.1016/j.tim.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Kaya H., Kaya A., Gul M., Celebi S., Timurkaan S., Apaydin B. Effects of supplementation of different levels of organic acids mixture to the diet on performance, egg quality parameters, serum traits and histological criteria of laying hens. Eur. Poult. Sci. 2014;78:12. [Google Scholar]
- Keyburn A.L., Sheedy S.A., Ford M.E., Williamson M.M., Awad M.M., Rood J.I., Rood M.J. Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infect. Immun. 2006;74:6496–6500. doi: 10.1128/IAI.00806-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D S., US National Research Council. 1994.
- Kitessa S.M., Nattrass G.S., Forder R.E., McGrice H.A., Wu S.B., Hughes R.J. Mucin gene mRNA levels in broilers challenged with eimeria and/or Clostridium perfringens. Avian Dis. 2014;58:408–414. doi: 10.1637/10757-122313-Reg.1. [DOI] [PubMed] [Google Scholar]
- Las-Heras V., Clooney A.G., Ryan F.J., Cabrera-Rubio R., Casey P.G., Hueston C.M., Pinheiro J., Rudkin J.K., Melgar S., Cotter P.D. Short-term consumption of a high-fat diet increases host susceptibility to Listeria monocytogenes infection. Microbiome. 2019;7:7. doi: 10.1186/s40168-019-0621-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre J.D., Adhikari B., Park S.H., Teague K.D., Graham L.E., Mahaffey B.D., Baxter M.F.A., Hernandez-Velasco X., Kwon Y.M., Ricke S.C. Evaluation of the epithelial barrier function and ileal microbiome in an established mecrotic enteritis challenge model in broiler chickens. Front. Vet. Sci. 2018;21:199. doi: 10.3389/fvets.2018.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X., Shao Y., Sun G., Yang Y., Zhang L., Guo Y., Luo X., Lu L. The relationship among gut microbiota, short-chain fatty acids, and intestinal morphology of growing and healthy broilers. Poult. Sci. 2020;99:5883–5895. doi: 10.1016/j.psj.2020.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Teng P.Y., Olukosi O.A. The effects of xylo-oligosaccharides on regulating growth performance, nutrient utilization, gene expression of tight junctions, nutrient transporters, and cecal short chain fatty acids profile in Eimeria-challenged broiler chickens. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maioli T.U., Borras-Nogues E.L., Chatel J.M. Possible benefits of Faecalibacterium prausnitzii for obesity-associated gut disorders. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.740636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark C., Christian F., Enric M., Paul M.M., Ian P. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 2003;52:159–161. doi: 10.1093/jac/dkg313. [DOI] [PubMed] [Google Scholar]
- Min B.R., Perkins D., Wright C., Dawod A., Min B.J., Terrill T.H. Effects of feeding two different tannin containing diets on ruminal fermentation profiles and microbial community changes in meat goats. Agricult. Food Anal. Bacteriol. 2015;5:153–165. [Google Scholar]
- Molnár A., Such N., Farkas V., Pál L., Menyhárt L., Wágner L., Husveth F., Dublecz K. Effects of wheat bran and clostridium butyricum supplementation on cecal microbiota, short-chain fatty acid concentration, pH and histomorphometry in broiler chickens. Animals (Basel) 2020;10:2230. doi: 10.3390/ani10122230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa A., Bai S., Zeng Q., Ding X., Wang J., Xuan Y., Su Z.W., Zhang K.Y. Effect of organic acids on growth performance, intestinal morphology, and immunity of broiler chickens with and without coccidial challenge. AMB Express. 2021;11:140. doi: 10.1186/s13568-021-01299-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva D., Walk C., Mcelroy A. Dietary calcium, phosphorus, and phytase effects on bird performance, intestinal morphology, mineral digestibility, and bone ash during a natural necrotic enteritis episode. Poult. Sci. 2014;93:2752–2762. doi: 10.3382/ps.2014-04148. [DOI] [PubMed] [Google Scholar]
- Park J.W., Jeong J.S., Lee S.I., Kim I.H. Effect of dietary supplementation with a probiotic (Enterococcus faecium) on production performance, excreta microflora, ammonia emission, and nutrient utilization in ISA brown laying hens. Poult. Sci. 2016;95:2829–2835. doi: 10.3382/ps/pew241. [DOI] [PubMed] [Google Scholar]
- Paul M.S., Mallick A.I., Haq K., Orouji S., Abdul-Careem M.F., Sharif S. In vivo administration of ligands for chicken toll-like receptors 4 and 21 induces the expression of immune system genes in the spleen. Vet. Immunol. Immunopathol. 2011;144:228–237. doi: 10.1016/j.vetimm.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Placha I., Simonova M.P., Cobanova K., Laukova A., Faix S. Effect of Enterococcus faecium AL41 and thymus vulgaris essential oil on small intestine integrity and antioxidative status of laying hens. Res. Vet. Sci. 2010;89:257–261. doi: 10.1016/j.rvsc.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Pereira T.M.C., Pimenta F.S., Porto M.L., Baldo M.P., Campagnaro B.P., Gava A.L., Meyrelles S.S., Vasquez E.C. Coadjuvants in the diabetic complications: nutraceuticals and drugs with pleiotropic effects. Int. J. Mol. Sci. 2016;17:1273. doi: 10.3390/ijms17081273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmal A., Achahbar S., Bouddine L., Chami N., Chami F. In vitro destruction of eimeria oocysts by essential oils. Vet. Parasitol. 2011;182:121–126. doi: 10.1016/j.vetpar.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Rodrigues I., Svihus B., Bedford M., Gous R., Choct M. Intermittent lighting improves resilience of broilers during the peak phase of sub-clinical necrotic enteritis infection. Poult. Sci. 2017;97:438–446. doi: 10.3382/ps/pex315. [DOI] [PubMed] [Google Scholar]
- Soltan M. A.Effect of dietary organic acid supplementation on egg production, egg quality and some blood serum parameters in laying hens. Int. J. Poult. Sci. 2008;7:613–621. [Google Scholar]
- Tyzzer E.E., Theiler H., Jones E.E. Coccidiosis in gallinaceous birds: II. A comparative study of species of eimeria of the chicken. Am. J. Epidemiol. 1932;15:319–393. [Google Scholar]
- Wade B., Keyburn A.L. The true cost of necrotic enteritis. World Poult. 2015;31:16–17. [Google Scholar]
- Walugembe M., Hsieh J.C., Koszewski N.J., Lamont S.J., Persia M.E., Rothschild M.F. Effects of dietary fiber on cecal short-chain fatty acid and cecal microbiota of broiler and laying-hen chicks. Poult. Sci. 2015;94:2351–2359. doi: 10.3382/ps/pev242. [DOI] [PubMed] [Google Scholar]
- Wang J., Jia R., Gong H., Celi P., Zhuo Y., Ding X., Bai S., Zeng Q.F., Yin H.D., Xu S.Y. The effect of oxidative stress on the chicken ovary: involvement of microbiota and melatonin interventions. Antioxidants. 2021;10:1422. doi: 10.3390/antiox10091422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.W., Jia H.J., Zhang H.J., Wang J., Lv H.Y., Wu S.G., Qi G.H. Supplemental plant extracts from flos lonicerae in combination with baikal skullcap attenuate intestinal disruption and modulate gut microbiota in laying hens challenged by salmonella pullorum. Front. Microbiol. 2019;10:1681. doi: 10.3389/fmicb.2019.01681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Liang S., Li X., Yang X., Long F., Yang X. Effects of encapsulated essential oils and organic acids on laying performance, egg quality, intestinal morphology, barrier function, and microflora count of hens during the early laying period. Poult. Sci. 2019;98:6751–6760. doi: 10.3382/ps/pez391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wan C., Zhao S., Yang Z., Celi P., Ding X., Bai S., Zeng Q.F., Mao X.B., Xu S.Y. Differential analysis of gut microbiota and the effect of dietary Enterococcus faecium supplementation in broiler breeders with high or low laying performance. Poult. Sci. 2021;100:1109–1119. doi: 10.1016/j.psj.2020.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Zhou Y., Zhan Z., Zhang W., Fu D., Zhao R., Chen X.D. Research note: effects of Bacillus coagulans X26 on the production performance, intestinal structure, short-chain fatty acids and flora composition of laying hens during the peak laying period. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Wang X., Xia X., Yin Y. Energy metabolism in intestinal epithelial cells during maturation along the crypt-villus axis. Sci. Rep. 2016;6:31917–31930. doi: 10.1038/srep31917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Liu Y., Yan F., Yang C., Yang X. Effects of encapsulated organic acids and essential oils on intestinal barrier, microbial count, and bacterial metabolites in broiler chickens. Poult. Sci. 2019;98:2858–2865. doi: 10.3382/ps/pez031. [DOI] [PubMed] [Google Scholar]
- Yang Z., Zhang C., Wang J., Celi P., Ding X., Bai S. Characteristics of the intestinal microbiota of broiler breeders with difference egg laying rate. Front. Vet. 2020;7 doi: 10.3389/fvets.2020.599337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin D., Du E., Yuan J., Gao J., Wang Y., Aggrey S.E., Guo Y.M. Supplemental thymol and carvacrol increases ileum Lactobacillus population and reduces effect of necrotic enteritis caused by Clostridium perfringes in chickens. Sci. Rep. 2017;7:7334. doi: 10.1038/s41598-017-07420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z.K., Zhang S.H., Wang L., Piao X.S. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: a review. J. Anim. Sci. Technol. 2015;6:7–16. doi: 10.1186/s40104-015-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Ding X., Bai S., Zeng Q., Zhang K., Mao X., Chu L.C., Hou D.X., Xuan Y., Wang J.P. Alleviating effect of dietary supplementation of benzoic acid, Enterococcus faecium and essential oil complex on coccidia and Clostridium perfringens challenge in laying hens. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Zhang K., Ding X., Pietro C., Yan L., Bai S., Zeng Q.F., Mao X.B., Xu S.Y., Wang J.P. The impact of dietary supplementation of different feed additives on performances of broiler breeders characterized by different egg-laying rate. Poult. Sci. 2019;98:6091–9099. doi: 10.3382/ps/pez316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.