Abstract
This study evaluated the effect of probiotics (Bacillus subtilis fermentation extract) and its delivery route (in-feed or in ovo) on hatch and growth performance, blood biochemistry, immune status, gut morphology, and microbiota of broiler chickens. Hatching eggs were incubated for 21 d. On d 12, viable eggs were randomly allotted to 4 groups: the noninjected, in ovo saline (S), in ovo Bacillus subtilis 1 (P1), and in ovo Bacillus subtilis 2 (P2). On d 18, S, P1, and P2 groups received 0.2 mL saline diluent, 10 × 106, and 20 × 106 CFU of the bacterium via the amnion, respectively. At hatch, chicks were re-allotted to 5 new treatment groups: P1, P2, 0.005% in-feed Bacillus subtilis extract (P3), 0.05% in-feed bacitracin methylene disalicylate (BMD,), and corn-wheat-soybean diet negative control (NC) in 9 replicate pens (22 birds/pen) and raised for 35 d. Hatch parameters were assessed on d 0, and growth performance indices measured weekly. On d 25, 1 bird/cage was euthanized, and samples collected for further analysis. Data were analyzed by generalized linear model. Treatments S and P2 recorded higher (P = 0.01) chick BW/ Egg Weight values compared to the non-injected eggs. P3 and P2 reduced (P = 0.02) FI at week 5 compared to the NC treatment. However, no change in average body weight gain (ABG) and feed conversion ratio (FCR) were observed during the same period. At d 35, while BMD treatment showed a tendency (P = 0.09) to increase FI compared to the NC treatment, ABG and FCR were similar for all treatments. Blood sodium and chloride levels were increased (P < 0.05) by the BMD treatment compared to the NC treatment. Compared to other treatments, BMD and P3 treatments increased (P < 0.001) jejunal and ileal villus height to crypt depth ratios, respectively. However, P1 and P2 increased (P < 0.001) villus height to crypt depth ratio in the duodenum compared to NC treatment. Treatments did not affect gut microbial diversity; however, BMD treatment increased (P < 0.05) the proportion of bacteria in the genus Enterococcus in the ileum and reduced (P < 0.05) the proportion of bacteria in the genus Streptococcus in the ceca. All probiotics treatments (irrespective of route and dose) reduced (P < 0.001) the levels of serum IgG compared to the NC treatment. However, P1 and P2 had the lowest numerical decrease in serum IgG concentrations, suggesting that Bacillus subtilis (especially in ovo delivered) might provide broiler chickens with better immunological protection by neutralizing pathogenic organisms that could result in the production of natural antibodies.
Key words: Bacillus subtilis, in ovo, performance, immune status, broiler
INTRODUCTION
In a bid to meet the increasing food demands of the growing global population, agriculture continues to be intensified. One such intensification effort led to the adoption of antimicrobial compounds to promote growth in the livestock industry. Interestingly, the livestock industry currently represents the largest user of antimicrobials produced globally (Van Boeckel et al., 2019). The use of antibiotic growth promoters (AGP) subtherapeutically for growth promotion and disease prevention remains a critical part of intensive poultry production (Castanon, 2007; Hedman et al., 2020). In spite of the benefits that AGP use poses to the poultry industry, there is also the risk of the development of antimicrobial resistance, which has undesirable consequences for human and animal health (Van Den Bogaard and Stobberingh, 2000; Diarra and Malouin, 2014; Lekshmi et al., 2017). Hence, it is unsurprising that several country-specific regulatory measures against AGP use in poultry production, as well as increased consumer demands for AGP-free poultry products now exist (Muaz et al., 2018; Oladokun et al., 2021b). As the poultry industry recedes from using AGP, the challenge going forward is finding suitable alternatives and the delivery routes that maximize their effectiveness.
Several bioactive substances, including phytobiotics, prebiotics, essential oils, and probiotics, are thus currently being researched as potential alternatives to AGP in the poultry industry (reviewed by Gadde et al., 2017a). Probiotics, defined as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host” (FAO/WHO, 2001) continue to receive growing interest as an alternative to AGP in poultry production as a result of its immunomodulating properties (Pender et al., 2016; Abd El-Ghany et al., 2022). Evidence abounds in the literature of the potential of probiotics to improve the growth performance of poultry (Torres-Rodriguez et al., 2007; Sen et al., 2012; Bai et al., 2013), improve nutrient digestibility (Mountzouris et al., 2010; Nawaz et al., 2016; Opoola et al., 2021), improve gut health (Oladokun et al., 2021a; Zeng et al., 2021; Gyawali et al., 2022), stimulate immunity (Nawaz et al., 2016; Abd El-Ghany et al., 2022; Hedayati et al., 2022) and positively modulate gut microbiota profile (Mountzouris et al., 2007a, 2010; Hedayati et al., 2022). Popular probiotic strains utilized in poultry include Lactobacillus, Bifidobacterium, Enterococcus, and Bacillus (Bajagai et al., 2016). The use of Bacillus species continues to gain interest in animal production, especially from a commercial standpoint (Kim et al., 2018). This is because spore-based probiotic strains like Bacillus are highly resilient to environmental stressors (Cartman et al., 2008). The use of several Bacillus strains to promote gut health, immunity, and growth of poultry is well documented (Gadde et al., 2017a; Grant et al., 2018; Oladokun et al., 2021a). Despite these reported results, probiotics (including Bacillus strains) have also been reported to not affect growth performance indices like feed intake, weight gain, and feed conversion ratio in broiler chicken studies (Cavazzoni et al., 1998; Li et al., 2019). Other reports have also documented a reduced feed conversion ratio in broiler chickens supplemented with dietary Bacillus subtilis (Knap et al., 2011; Lee et al., 2014). Although popular theories on probiotics mode of action will include bacterial antagonism, immunostimulation, and competitive exclusion (Ohimain and Ofongo, 2012), it is possible that a complete delineation of probiotics mode of actions is yet to be elucidated. Several other factors, including strain-specific mode of action, the health state of the host, housing and environmental conditions, supplemented dose, time of supplementation, and delivery routes, may contribute to the inconsistencies in probiotics results observed in the literature (Yang et al., 2009; Cox and Dalloul, 2015; Untoo et al., 2018).
As a solution to the challenges that characterize the conventional delivery routes in poultry (i.e., in-feed and in-water; summarized in Oladokun et al., 2021a), the in ovo delivery routes continue to gain considerable interest. Asides from other benefits that the in ovo technology affords (documented in Oladokun and Adewole, 2020 and Oladokun et al., 2021a), it also offers the opportunity to colonize the embryonic gut with beneficial microbiota very early on, considering that contact between chick and hen which use to be status quo mode of gut colonization has been eliminated in the present-day poultry industry. Oladokun et al. (2021a) have previously reported that the in ovo delivery of 10 × 106 CFU of Bacillus subtilis improved broiler chicken gut morphology and microbiota profile but with no significant effect on growth performance. As a follow-up to this study, it was hypothesized that modifying the supplemented dose (i.e., 10 × 106 CFU vs. 20 × 106 CFU), rearing period (28 d vs. 35 d), and housing conditions (battery cages vs. floor pens) might influence observed results. Consequently, the objective of this study was to evaluate the effect of the supplementation of two doses of Bacillus subtilis fermentation extract (i.e., 10 × 106 CFU and 20 × 106 CFU), and its delivery routes (in ovo vs. in-feed) on hatch and growth performance, blood biochemistry, immune status, gut morphology, and gut microbiota profile of broiler chickens, compared to in-feed antibiotics.
MATERIALS AND METHODS
Ethics Declarations
The experiment was carried out at the hatchery facility of the Agricultural Campus of Dalhousie University and the broiler rearing facility of the Atlantic Poultry Research Center, Dalhousie Faculty of Agriculture. The experiment was conducted following guidelines recommended by the Canadian Council on Animal Care (Rowsell, 1990). All methods were approved by the Animal Care and Use Committee of Dalhousie University (Protocol number: 2021-032).
Egg Incubation and In ovo Injection Procedure
Hatching broiler eggs (Cobb 500, 52 wk old breeders, average weight = 63 g ± 1.27, n = 1,860) were obtained from a commercial hatchery (Cox Atlantic Chick hatchery, Nova scotia) and incubated in a ChickMaster single-stage incubator (ChickMaster G09, Cresskill, NJ), under standard conditions (37.5°C, 55% relative humidity) from embryonic days (EDs) 1 to 19, and then to an average of 32°C and 68% from EDs 19 to 21. Eggs were candled on ED12 to determine viability. Viable eggs were subsequently assigned to one of 4 experimental groups: 1) noninjected eggs (control; 166 eggs); 2) in ovo saline group (38 eggs; injected with 0.2 mL of physiological saline, i.e., 0.9% NaCl, Baxter Corporation, ON, Canada); 4) in ovo probiotic group 1 (53 eggs; injected with 0.2 mL of Bacillus subtilis fermentation extract, each egg received 10 × 106 CFU of the bacterium/0.2 mL saline diluent), and 4) in ovo probiotic group 2 (53 eggs; injected with 0.2 mL of Bacillus subtilis fermentation extract, each egg received 20 × 106 CFU of the bacterium/0.2 mL saline diluent). The described treatments were replicated in 6 similar incubators operated under similar conditions. The Bacillus subtilis product (strain- Bacillus subtilis 10SI) injected in this experiment was obtained from a commercial source (Probiotech International, St. Hyacinth, QC, Canada) at a concentration of 10 × 1010 CFU/g. Figure S2 details the probiotics manufacturing process as provided by the manufacturers. The Bacillus subtilis 10SI is grown on a media containing yeast extract and glucose under aerobic conditions. At the end of fermentation, the entire culture is concentrated via centrifugation and then spray dried. The Bacillus subtilis product was injected on ED18. The injection procedure utilized in this study have been previously described by Oladokun et al. (2021a). Briefly, eggs were disinfected by cleaning with of 70% alcohol swabs (BD alcohol swabs-catalog 326910, ON, Canada), followed by careful punching of the air cell (the blunt end of the egg) using an 18-gauge needle. The injected probiotics treatments were then delivered to the amnion using a self-refilling injector (Socorex ultra-1810.2.05005, Ecublens, Switzerland) equipped with a 22-gauge needle (injection needle length—3 cm) at a 45° angle. After in ovo injection, the injection sites were sealed with sterile medical tapes (Nexcare Flexible Clear Tape-7100187758, 3M, MN). The non-injected eggs were also taken out and returned to the incubator simultaneously with other injected treatment groups.
Birds, Housing, and Diets
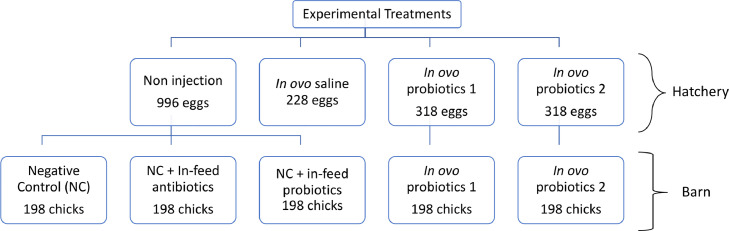
As presented in Figure 1, hatchlings were weighed and randomly assigned to 5 new treatment groups. Chicks from the initial noninjection group were randomly allocated into 3 new treatment groups consisting of (1) chicks fed a basal corn-soybean meal-wheat–based diet (Negative control treatment; NC); (2) chicks fed NC + 0.05% bacitracin methylene disalicylate (in-feed antibiotics); and (3) chicks fed NC + 0.005% Bacillus subtilis containing 1 × 108 CFU/kg of feed. The in ovo probiotics treatments were placed on the control diet to form treatments (4) in ovo probiotics group 1 and (5) in ovo probiotics group 2. Chicks (mixed sex) were weighed and assigned to floor pens (0.93 m × 2.14 m) at a stocking density of 0.076 m2/bird. There were 9 replicate floor pens/treatment containing 22 birds per pen. Two broiler production rooms were utilized. The temperature in the broiler rooms was monitored daily and was gradually reduced from 32 to 22.5°C from d 0 to 35. The lighting program was set to produce 18 h of light and 6 h of darkness throughout the experimental period, and illumination was gradually reduced from 20 lx on d 0 to 5 lx on d 35. Dietary treatments, ingredients, and nutritional composition are presented in Table 1. Birds were provided with feed and water ad libitum; diets were fed as mash in the starter (0–14 d) phase and pellets in the grower (15–25 d) and finisher (26–35 d) phases. Diets were formulated according to Cobb 500 nutrient requirements (Cobb-Vantress, 2018). However, the diets containing probiotics were not further analyzed for probiotics in the diet.
Figure 1.
Schematic presentation of experimental structure in the hatchery and barn. In ovo probiotics group 1- eggs injected with 10 × 106 CFU of Bacillus subtilis fermentation extract /0.2 mL saline diluent; in ovo probiotics group 2- eggs injected with 20 × 106 CFU of Bacillus subtilis fermentation extract /0.2 mL saline diluent; in-feed antibiotics- chicks fed NC + 0.05% bacitracin methylene disalicylate; in-feed probiotics- chicks fed 0.005% Bacillus subtilis containing 1 × 108 CFU/kg of feed; and NC-Negative Control treatment- chicks fed a basal corn-soybean meal-wheat–based diet.
Table 1.
Ingredients, calculated, and analyzed compositions of experimental diets1 (as-fed basis, percentage, unless otherwise stated).
| Ingredients | Phases |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Starter (0–14 d) |
Grower (15–25 d) |
Finisher (26–35 d) |
|||||||
| Negative Control | In-feed Antibiotic | In-feed probiotic | Negative Control | In-feed Antibiotic | In-feed probiotic | Negative Control | In-feed Antibiotic | In-feed probiotic | |
| Ingredient composition | |||||||||
| Corn (ground) | 46.63 | 46.53 | 46.62 | 51.16 | 51.06 | 51.15 | 53.63 | 53.53 | 53.62 |
| Soybean meal-46.5 | 37.12 | 37.14 | 37.13 | 31.87 | 31.89 | 31.88 | 29.2 | 29.22 | 29.21 |
| Wheat | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Soybean Oil (young or mature) | 1.80 | 1.83 | 1.795 | 2.18 | 2.21 | 2.175 | 2.75 | 2.78 | 2.75 |
| Limestone | 1.37 | 1.37 | 1.37 | 1.30 | 1.30 | 1.30 | 1.19 | 1.19 | 1.19 |
| Dicalcium Phosphate 21 P | 1.45 | 1.45 | 1.45 | 1.35 | 1.35 | 1.35 | 1.18 | 1.18 | 1.18 |
| DL Methionine premix2 | 0.58 | 0.58 | 0.58 | 0.57 | 0.57 | 0.57 | 0.52 | 0.52 | 0.52 |
| Vitamin/Mineral Premix or MCB10 3,4 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Salt | 0.38 | 0.38 | 0.38 | 0.36 | 0.36 | 0.36 | 0.36 | 0.36 | 0.36 |
| Lysine HCl | 0.17 | 0.17 | 0.17 | 0.21 | 0.21 | 0.21 | 0.17 | 0.17 | 0.17 |
| Pellet Binding Agent | - | - | - | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| BMD 110G5 | - | 0.05 | - | - | 0.05 | - | - | 0.05 | - |
| Bacillus subtilis | - | - | 0.005 | - | 0.005 | - | - | 0.005 | |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Nutrient | Calculated composition | ||||||||
| Metabolizable energy (kcal/kg) | 2,975 | 2,975 | 2,975 | 3,025 | 3,025 | 3,025 | 3,100 | 3,100 | 3,100 |
| Crude protein | 22.0 | 22.0 | 22.0 | 20.0 | 20 | 20.0 | 19.0 | 19.0 | 19.0 |
| Calcium | 0.90 | 0.90 | 0.90 | 0.84 | 0.84 | 0.84 | 0.76 | 0.76 | 0.76 |
| Available phosphorus | 0.45 | 0.45 | 0.45 | 0.42 | 0.42 | 0.42 | 0.38 | 0.38 | 0.38 |
| Sodium | 0.18 | 0.18 | 0.18 | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 |
| Digestible lysine | 1.22 | 1.22 | 1.22 | 1.12 | 1.12 | 1.12 | 1.02 | 1.02 | 1.02 |
| Digestible methionine + cysteine | 0.91 | 0.91 | 0.91 | 0.85 | 0.85 | 0.85 | 0.80 | 0.80 | 0.80 |
| Digestible Tryptophan | 0.24 | 0.24 | 0.24 | 0.22 | 0.22 | 0.22 | 0.20 | 0.20 | 0.20 |
| Digestible Threonine | 0.84 | 0.84 | 0.84 | 0.76 | 0.76 | 0.76 | 0.72 | 0.72 | 0.72 |
| Analyzed composition | |||||||||
| Dry Matter | 92.2 | 92.2 | 92.2 | 91.5 | 92.1 | 91.4 | 91.7 | 91.8 | 91.8 |
| Crude protein | 24.5 | 24.7 | 23.9 | 21.3 | 21.2 | 21.8 | 19.3 | 20.9 | 21.0 |
| Crude fat | 4.05 | 4.31 | 4.17 | 4.86 | 4.69 | 3.63 | 4.81 | 4.25 | 4.17 |
| Calcium | 0.81 | 0.80 | 1.03 | 0.89 | 0.90 | 0.83 | 0.83 | 0.75 | 0.75 |
| Potassium | 1.05 | 1.00 | 0.98 | 0.94 | 0.91 | 0.95 | 0.84 | 0.92 | 0.89 |
| Phosphorus | 0.62 | 0.65 | 0.72 | 0.66 | 0.65 | 0.65 | 0.57 | 0.60 | 0.59 |
| Sodium | 0.14 | 0.15 | 0.20 | 0.17 | 0.17 | 0.17 | 0.16 | 0.15 | 0.15 |
Basal diet (NC); In-feed antibiotic diet containing NC + 0.05% bacitracin methylene disalicylate (BMD); In-feed probiotics diet containing NC + 0.005% Bacillus subtilis containing- 1 × 108 CFU/kg of feed.
Supplied/kg premix: DL-Methionine, 0.5 kg; wheat middling, 0.5 kg.
Starter vitamin-mineral premix contained the following per kg of diet: 9750 IU vitamin A; 2000 IU vitamin D3; 25 IU vitamin E; 2.97 mg vitamin K; 7.6 mg riboflavin; 13.5 mg Dl Ca-pantothenate; 0.012 mg vitamin B12; 29.7 mg niacin; 1.0 mg folic acid, 801 mg choline; 0.3 mg biotin; 4.9 mg pyridoxine; 2.9 mg thiamine; 70.2 mg manganese; 80.0 mg zinc; 25 mg copper; 0.15 mg selenium; 50 mg ethoxyquin; 1543 mg wheat middling's; 500 mg ground limestone.
Grower and Finisher vitamin-mineral premix contained the following per kg of diet: 9750 IU vitamin A; 2000 IU vitamin D3; 25 IU vitamin E; 2.97 mg vitamin K; 7.6 mg riboflavin; 13.5 mg Dl Ca-pantothenate; 0.012 mg vitamin B12; 29.7 mg niacin; 1.0 mg folic acid, 801 mg choline; 0.3 mg biotin; 4.9 mg pyridoxine; 2.9 mg thiamine; 70.2 mg manganese; 80.0 mg zinc; 25 mg copper; 0.15 mg selenium; 50 mg ethoxyquin; 1543 mg wheat middling's; 500 mg ground limestone.
Bacitracin methylene disalicylate (providing 55 mg/kg mixed feed); Alpharma, Inc., Fort Lee, NJ, USA.
Measurements
Hatch Parameters and Chick Quality
Hatched chicks were counted and weighed individually. Hatchability was calculated as the percentage of hatched chicks to fertile incubated eggs per replicate. The BW/initial egg weight ratio of hatched chicks was also determined and recorded. Chick navel quality was evaluated by adopting Reijrink et al. (2009) scoring method. Chick length was obtained by placing the chick on its ventral side and measuring from the tip of the beak to the middle toe on the right leg.
Growth Performance Parameters
Growth performance parameters, including feed intake and average body weight (BW) were measured on a pen basis weekly. Subsequent calculations, including the average feed intake (AFI), average body weight gain (ABWG), and feed conversion ratio (FCR) were then obtained from the recorded data. Mortality was recorded daily and used to correct for FCR.
Sampling
On d 25, 1 bird per cage (9 replicate birds per treatment group) was randomly selected, weighed, and euthanized by electrical stunning and exsanguination. After euthanasia of the bird, blood samples were collected from each bird into 10 mL blood serum collection tubes (BD Vacutainer Serum Tubes, fisher scientific- BD366430) for further serum assays and into 10 mL heparinized tubes (BD Vacutainer Glass Blood Collection Tubes with Sodium Heparin, fisher scientific- BD366480) for further blood plasma assays. Blood serum and plasma were centrifuged at 1,200 g × 10 min × 18°C. The resulting supernatants were stored in aliquots at −80°C until further analysis. The weights of bursa of Fabricius and spleen were also determined by trained personnel. The small intestinal segments, including the duodenum (region from the gizzard junction to the pancreatic and bile ducts), jejunum (1.5-cm length midway between the point of entry of the bile ducts and Meckel's diverticulum) and ileum (1.5-cm length midway between Meckel's diverticulum and the ileocecal junction), were also excised and fixed in neutral buffered formalin (10%) for further histomorphology processing. Cecal and ileal digesta samples were also collected in RNase and DNase-free tubes, and immediately snap frozen in liquid nitrogen, and later stored at −80°C for subsequent gut microbiota analysis.
Relative Weight of Organs
The weights of the bursa of Fabricius and the spleen were recorded and reported as a percentage of the live BW of the slaughtered chicken (g/Kg BW).
Serum Immunoglobulins
Chicken-specific immunoglobulins enzyme-link immunosorbent assay (ELISA) quantitation kits (Bethyl Laboratories, Montgomery, TX; Catalog No. E33-104-200218 and E33-102-180410, respectively) were used to measure the concentrations of immunoglobulins (IgG and IgM) following manufacturer instructions. Absorbance values were read on a microplate reader (Bio-Tek Instrument Inc., Wonooski, VT) using a software program (KC4, version #3.3, Bio Tek Instruments), and immunoglobulins concentration was extrapolated using the 4-parameter logistic model.
Blood Biochemistry
Samples for blood biochemical analysis were shipped on ice to Atlantic Veterinary College, University of Prince Edward Island Pathology Laboratory, and analyzed using cobas 6000 analyzer series (Roche Diagnostics, Indianapolis, IN).
Gut Morphology
The procedure for intestinal morphometric analysis has previously been reported by Oladokun et al. (2021a). Briefly, fixed intestinal tissues were embedded in paraffin, sectioned (0.5 μm thick), and stained with hematoxylin and eosin for morphological examinations. In each cross-sectioned tissue, ten morphometric measurements including the villus height (from the base of the intestinal mucosa to the tip of the villus excluding the intestinal crypt), villus width (halfway between the base and the tip), crypt depth (from the base upward to the region of transition between the crypt and villi) (Ozdogan et al., 2014) per slide were carried out using Leica 1CC50 W microscope at 4 × Magnification (Leica Microsystems, Wetzlay, Germany) and an image processing and analysis system (Leica Application Suite, Version 3.4.0, Leica Microsystems, Wetzlay, Germany).
DNA Extraction, Quantification, Library Preparation, and Sequencing
Following manufacturer's instructions, DNA was extracted from the ileal and ceca digesta contents using the Qiagen DNeasy PowerSoil Pro Kit (50) (catalog number 47014, Qiagen GmbH, Hilden, Germany). The concentration and purity of extracted DNA were subsequently determined by spectrophotometry (Nanodrop ND1000; Thermo Scientific). Extracted DNA samples (volume-50 µL, concentration-10–200 ng/µL) were then sent to Genome Quebec Innovation Center (Montreal, Canada) for amplicon library preparation and sequencing (primers, V3V4, 341F-CCTACGGGNGGCWGCAG and 805R-GACTACHVGGGTATCTAATCC).
Statistics and Bioinformatic Analysis
Hatch data were analyzed as a randomized complete block design, with the incubator considered as the blocking factor. Datasets from the grow-out trial were also analyzed in a randomized complete block design, with broiler production rooms being the blocking factor. The normality of all data sets was ascertained by testing residuals with the Anderson-Darling test in Minitab statistical package (v.18.1). Data were analyzed using the generalized linear model in the same statistical package. Significant means were separated using Tukey's honest significant difference test in the same statistical package. Analyzed data were presented as means ± SEM and probability values. Values were considered statistically different at P ≤ 0.05 and considered a statistical trend at P < 0.1.
Bioinformatic analysis of the microbiota data was performed by the Canadian Centre for Computational Genomics at McGill University. The GenPipes version 4.0.0 (Bourgey et al., 2019) amplicon-seq pipeline was used to perform analyses. This pipeline is based on the DADA2 package in R environment. First, the trimming was done using Trimmomatic (Bolger et al., 2014), taking off 16 bp from the start of the reads. Then, 8,455,050 paired-end reads passed the quality-filtering parameters applied [truncLen = c(284,176); maxN = 0; maxEE = c(2,2); truncQ = 2] with an average value of 93,945 reads/sample and thus were merged (minimum overlap of 20 bp) and subjected to de novo chimera removal. Taxonomy was assigned to the resulting amplicon sequence variants (ASVs) using Silva database version 123. Visual exploration of the data was then performed in the MicrobiomeAnalyst tool (Dhariwal et al., 2017). Alpha and Beta diversity were calculated based on Shannon and Bray-Curtis indices, respectively, with statistical significance set at P < 0.05.
RESULTS
Hatch Performance and Chick Quality
Results on hatch performance and chick quality are presented in Table 2. The chick BW/ Egg Weight recorded treatment differences. Both the in ovo saline and the in ovo probiotics 2 treatment groups recorded higher (P = 0.01) chick BW/ Egg Weight values compared to the noninjected eggs. The in ovo probiotics 1 treatment group recorded intermediate chick BW/ Egg Weight value compared to other treatment groups. There was no effect of treatment on average navel score, average chick length, average chick weight, and hatchability in this study.
Table 2.
Effect of in ovo delivery of Bacillus subtilis on hatch performance and chick quality.
| Hatch parameters | Noninjected | Treatments1 |
SEM2 | P value3 | ||
|---|---|---|---|---|---|---|
| In ovo saline | In ovo probiotic 1 | In ovo probiotic 2 | ||||
| Hatchability (%) | 96.1 | 95.2 | 96.8 | 96.9 | 0.51 | 0.711 |
| Average chick weight (g) | 43.1 | 43.8 | 43.4 | 43.7 | 0.13 | 0.118 |
| Average chick length (cm) | 18.8 | 18.2 | 19.2 | 18.9 | 0.18 | 0.202 |
| Chick BW/ egg weight (%) | 68.1b | 69.5a | 68.9ab | 69.2a | 0.17 | 0.005 |
| Average navel score | 1.40 | 1.38 | 1.47 | 1.36 | 0.07 | 0.79 |
Treatments include— (1) noninjected eggs; (2) in ovo saline group- injected with 0.2 mL of physiological saline (0.9% NaCl); (3) in ovo probiotics group 1- eggs injected with 10 × 106 CFU of Bacillus subtilis fermentation extract/0.2 mL saline diluent; and (4) in ovo probiotics group 2- eggs injected with 20 × 106 CFU of Bacillus subtilis fermentation extract/0.2 mL saline diluent.
SEM = Standard error of means.
Means within a row with different superscripts.
significantly differ.
Growth Performance
Results on growth performance indexes are presented in Table 3. Compared to other treatments, the in-feed probiotics treatment showed a tendency (P = 0.07) to increase ABG by at least 23.6% in wk 1. However, this tendency soon disappeared in subsequent weeks. Further treatment differences were only recorded in week 5. The AFI of the in-feed antibiotic treatment was higher (P = 0.02) than the in-feed probiotics and the in ovo probiotics 2 treatment groups. Other treatments had statistically similar AFI as the in-feed antibiotic treatment. Both the ABWG and FCR values were similar (P > 0.05) for all treatment groups from wk 2 to wk 5. At the end of the entire trial period (d 0–35), the in-feed antibiotic treatment showed a tendency (P = 0.09) to increase AFI by at least 17.6%, compared to other treatments. However, no corresponding changes in ABWG and FCR were recorded across treatment groups. Furthermore, in order to evaluate if treatment effects on ABWG were sex-linked, ABWG was calculated on a sex basis (males and females separately) at wk 4 and 5, when visual sexual distinction and weighing of birds could be carried out. However, no difference (P > 0.05) in ABWG for males and females was recorded at this time.
Table 3.
Effect of Bacillus subtilis and its delivery routes on the growth performance of broiler chickens raised for 35 days.
| Growth performance parameters | Treatments1 |
P value3 | |||||
|---|---|---|---|---|---|---|---|
| Negative control | In-feed antibiotic | In-feed probiotic | In ovo probiotic 1 | In ovo probiotic 2 | SEM2 | ||
| Week 1 | |||||||
| Average feed intake (g) | 155 | 153 | 159 | 160 | 154 | 1.52 | 0.859 |
| Average body weight gain (g) | 85.7 | 87.4 | 91.6 | 88.7 | 74.1 | 1.00 | 0.071 |
| FCR4 | 1.81 | 1.76 | 1.73 | 1.81 | 2.07 | 0.02 | 0.336 |
| Week 2 | |||||||
| Average feed intake (g) | 184 | 190 | 148 | 163 | 168 | 3.94 | 0.237 |
| Average body weight gain (g) | 213 | 235 | 212 | 200 | 168 | 3.09 | 0.102 |
| FCR4 | 0.87 | 0.81 | 0.69 | 0.81 | 0.99 | 0.02 | 0.193 |
| Week 3 | |||||||
| Average feed intake (g) | 449 | 499 | 416 | 466 | 409 | 13.4 | 0.552 |
| Average body weight gain (g) | 483 | 555 | 480 | 476 | 441 | 9.07 | 0.188 |
| FCR4 | 0.93 | 0.89 | 0.87 | 0.99 | 0.93 | 0.03 | 0.912 |
| Week 4 | |||||||
| Average feed intake (g) | 839 | 917 | 812 | 859 | 853 | 16.6 | 0.222 |
| Average body weight gain (g)-Mixed sex | 714 | 608 | 695 | 773 | 846 | 15.2 | 0.415 |
| Average body weight gain (g)-Males | 761 | 722 | 902 | 954 | 918 | 17.7 | 0.351 |
| Average body weight gain (g)-Females | 635 | 483 | 524 | 677 | 820 | 21.9 | 0.293 |
| FCR4 | 1.31 | 1.24 | 1.43 | 1.38 | 1.39 | 0.09 | 0.516 |
| Week 5 | |||||||
| Average feed intake (g) | 1,378ab | 1,329a | 1,146b | 1,102ab | 970b | 14.8 | 0.024 |
| Average body weight gain (g)-Mixed sex | 1,030 | 1,181 | 906 | 789 | 710 | 143 | 0.324 |
| Average body weight gain (g)-Males | 970 | 1157 | 936 | 926 | 857 | 504 | 0.850 |
| Average body weight gain (g)-Females | 977 | 1,019 | 831 | 679 | 625 | 42.1 | 0.412 |
| FCR4 | 1.37 | 1.14 | 1.27 | 1.43 | 1.48 | 0.04 | 0.655 |
| Total Trial Period (1-35 d) | |||||||
| Average feed intake (g) | 2,974 | 3,051 | 2,656 | 2,753 | 2,595 | 34.4 | 0.087 |
| Average body weight gain (g) | 2,578 | 2,655 | 2,385 | 2,353 | 2,217 | 139 | 0.574 |
| FCR4 | 1.16 | 1.16 | 1.13 | 1.20 | 1.20 | 0.02 | 0.830 |
Treatments include— (1) Negative Control treatment- chicks fed a basal corn-soybean meal-wheat–based diet; (2) In-feed antibiotics- chicks fed NC + 0.05% bacitracin methylene disalicylate; (3) In-feed probiotics- chicks fed NC + 0.005% Bacillus subtilis containing 1 × 108 CFU/kg of feed; (4) In ovo probiotics group 1- eggs injected with 10 × 106 CFU of Bacillus subtilis fermentation extract /0.2 mL saline diluent; and (5) In ovo probiotics group 2- eggs injected with 20 × 106 CFU of Bacillus subtilis fermentation extract /0.2 mL saline diluent.
SEM = Standard error of means.
Means within a row with different superscripts.
Significantly differ.
FCR = Feed Conversion Ratio.
Organ Weight and Serum Immunoglobulin Concentration
According to Table 4, no significant treatment effect on the relative weight of the bursa of Fabricius and spleen was recorded in this study. Conversely, of the two immunoglobulins evaluated, the serum IgG concentration was reduced (P < 0.001) both in the in ovo probiotics 1 and in ovo probiotics 2 treatments, compared to both the NC and the in-feed antibiotics treatment. However, the serum IgG concentration in the in-feed probiotics treatment was statistically similar to that of the in ovo probiotics 1 treatment. Nevertheless, the highest reduction in serum IgG concentration was recorded in the in ovo probiotics 2 treatment, being at least 38% lower than other treatment groups.
Table 4.
Effect of Bacillus subtilis and its delivery routes on relative weight of immune organs and serum immunoglobulin concentrations in broiler chickens.
| Parameters | Treatments1 |
SEM2 | P value3 | ||||
|---|---|---|---|---|---|---|---|
| Negative control | In-feed antibiotic | In-feed probiotic | In ovo probiotic 1 | In ovo probiotic 2 | |||
| Bursa weight (g/Kg BW) | 1.81 | 1.80 | 1.64 | 1.76 | 1.93 | 0.06 | 0.645 |
| Spleen weight (g/Kg BW) | 0.70 | 0.76 | 0.73 | 0.73 | 0.69 | 0.02 | 0.920 |
| Immunoglobulin G (Mg/mL) | 10.3a | 4.75ab | 2.71bc | 0.96cd | 0.06d | 0.31 | <0.001 |
| Immunoglobulin M (Mg/mL) | 2.37 | 0.61 | 0.35 | 0.19 | 0.15 | 0.05 | 0.333 |
Treatments include— (1) Negative Control treatment- chicks fed a basal corn-soybean meal-wheat–based diet; (2) In-feed antibiotics- chicks fed NC + 0.05% bacitracin methylene disalicylate; (3) In-feed probiotics- chicks fed NC + 0.005% Bacillus subtilis containing 1 × 108 CFU/kg of feed; (4) In ovo probiotics group 1- eggs injected with 10 × 106 CFU of Bacillus subtilis fermentation extract/0.2 mL saline diluent; and (5) In ovo probiotics group 2- eggs injected with 20 × 106 CFU of Bacillus subtilis fermentation extract/0.2 mL saline diluent.
SEM = Standard error of means.
Means within a row with different superscripts.
Significantly differ.
Blood Biochemistry
Results on blood biochemistry are presented in Table 5. Only the concentrations of plasma sodium and chloride showed significant treatment effect in this study. Both electrolytes’ minerals recorded similar trend. In both cases, the in-feed antibiotics treatment recorded higher (P < 0.05) concentrations of both minerals compared to the NC treatment. Other treatment groups recorded intermediate statistical values for the concentrations of both minerals (sodium and chloride).
Table 5.
Effect of Bacillus subtilis and its delivery routes on broiler chicken plasma biochemistry indices.
| Parameters | Treatments1 |
||||||
|---|---|---|---|---|---|---|---|
| Negative control | In-feed antibiotic | In-feed probiotic | In ovo probiotic 1 | In ovo probiotic 2 | SEM2 | P value3 | |
| Electrolytes (mmol·L−1) | |||||||
| Sodium | 149.4b | 152.0a | 151.7ab | 151.2ab | 151.6ab | 0.52 | 0.031 |
| Potassium | 6.96 | 6.62 | 6.64 | 6.85 | 6.87 | 0.07 | 0.416 |
| Sodium: Potassium | 21.49 | 23.04 | 23.04 | 22.18 | 22.07 | 0.25 | 0.090 |
| Chloride | 109b | 113a | 111ab | 111ab | 110ab | 0.5 | 0.022 |
| Calcium | 3.16 | 2.80 | 2.98 | 3.03 | 3.11 | 0.06 | 0.148 |
| Phosphorus | 2.47 | 2.18 | 2.29 | 2.38 | 2.34 | 0.05 | 0.317 |
| Magnesium | 0.87 | 0.80 | 0.78 | 0.80 | 0.84 | 0.01 | 0.063 |
| Metabolites (mmol·L−1) | |||||||
| Urea | 0.31 | 0.30 | 0.35 | 0.29 | 0.28 | 0.01 | 0.318 |
| Glucose | 15.5 | 15.3 | 15.6 | 16. | 15.4 | 0.14 | 0.542 |
| Cholesterol | 3.49 | 3.53 | 3.51 | 3.43 | 3.63 | 0.05 | 0.859 |
| Iron | 18.5 | 20.6 | 21.1 | 19.9 | 19.7 | 0.01 | 0.772 |
| Bile acids | 22.5 | 24.4 | 24.4 | 20.7 | 25.2 | 0.94 | 0.584 |
| Uric acid | 364 | 375 | 424 | 384 | 396 | 0.01 | 0.498 |
| Creatinine | 1.99 | 1.69 | 2.00 | 1.55 | 6.42 | 0.06 | 0.083 |
| Enzymes (U·L−1) | |||||||
| Amylase | 606 | 703 | 726 | 795 | 782 | 38.6 | 0.579 |
| Lipase | 22.1 | 23.7 | 24.3 | 20.4 | 20.7 | 0.03 | 0.895 |
| Creatine kinase | 6,496 | 8,291 | 7,562 | 5,111 | 4,598 | 0.04 | 0.411 |
| Alkaline Phosphatase | 10,205 | 7,775 | 11,986 | 13,378 | 9,264 | 1000 | 0.438 |
| Alanine transaminase | 2.62 | 2.25 | 3.56 | 2.00 | 2.38 | 0.04 | 0.287 |
| Aspartate Aminotransferase | 166 | 192 | 184 | 159 | 162 | 0.01 | 0.318 |
| Gamma-Glutamyl Transferase | 9.17 | 10.72 | 9.50 | 9.50 | 10.72 | 0.26 | 0.140 |
| Proteins (g·L−1) | |||||||
| Total Proteins | 28.6 | 27.2 | 28.2 | 29.4 | 29.1 | 0.001 | 0.596 |
| Albumin | 11.8 | 11.9 | 11.7 | 11.9 | 11.6 | 0.14 | 0.973 |
| Globulin | 16.7 | 15.3 | 16.3 | 17.5 | 17.4 | 0.003 | 0.421 |
| Albumin: Globulin | 0.71 | 0.78 | 0.73 | 0.69 | 0.67 | 0.02 | 0.429 |
Treatments include— (1) Negative Control treatment- chicks fed a basal corn-soybean meal-wheat–based diet; (2) In-feed antibiotics- chicks fed NC + 0.05% bacitracin methylene disalicylate; (3) In-feed probiotics- chicks fed NC + 0.005% Bacillus subtilis containing 1 × 108 CFU/kg of feed; (4) In ovo probiotics group 1- eggs injected with 10 × 106 CFU of Bacillus subtilis fermentation extract/0.2 mL saline diluent; and (5) In ovo probiotics group 2- eggs injected with 20 × 106 CFU of Bacillus subtilis fermentation extract/0.2 mL saline diluent.
SEM = Standard error of means.
Means within a row with different superscripts
Significantly differ.
Gut Morphology
Table 6 shows the results on the morphology of the 3 gut sections (duodenum, jejunum, and ileum). Both doses of the in ovo delivered probiotics treatment increased (P < 0.001) duodenal villus height compared to other treatments, with the in ovo probiotics 1 group only comparable to in-feed antibiotics treatment. A similar trend was observed for duodenal villus width, with the in ovo probiotics 1 group being comparable to in-feed probiotics treatment. Interestingly, duodenal crypt depth was observed to be reduced by all treatments compared to the NC treatment, with the exception of the in ovo probiotics 2 treatment which recorded a statistical intermediate crypt depth value. Conversely, jejunal villus height was increased (P = 0.001) by the in ovo probiotics 1 and the in-feed antibiotic treatment compared to the in ovo probiotics 2 treatment. Both the in-feed probiotics and NC treatment recorded statistically intermediate jejunal villus height values. On the contrary, the in ovo probiotics 2 treatment recorded increased (P < 0.001) villus width compared to other treatments (except for the negative control treatment). Jejunal crypt depth was also reduced (P < 0.001) by the in-feed antibiotics and in ovo probiotics 2 treatments compared to other treatments. In terms of jejunal villus height to crypt depth ratio, the in-feed antibiotic treatment was better (P < 0.001) than all other treatments. In the ileum, the in ovo probiotics 2 and in-feed probiotics treatment increased (P = 0.001) villus height compared to the in ovo probiotics 1 treatment group; other treatments had intermediate ileal villus height. The in-feed probiotics treatment also recorded the least ileal crypt depth of all treatments, but this was statistically comparable to the in-feed antibiotics and in ovo probiotics 1 treatment. This was the same trend observed for the ileal villus height: crypt depth, as the in-feed probiotics treatment, recorded the highest ratio of all treatments but was statistically comparable to in-feed antibiotics and in ovo probiotics 2 treatment.
Table 6.
Effect of Bacillus subtilis and its delivery routes on broiler chicken intestinal morphology.
| Parameters | Treatments1 |
SEM2 | P value3 | ||||
|---|---|---|---|---|---|---|---|
| Negative control | In-feed antibiotic | In-feed probiotic | In ovo probiotic 1 | In ovo probiotic2 | |||
| Duodenum | |||||||
| Villus height (mm) | 2.04b | 2.14ab | 2.15ab | 2.28a | 2.21a | 0.02 | <0.001 |
| Villus width (mm) | 0.22b | 0.24ab | 0.22b | 0.22b | 0.25a | 0.00 | 0.003 |
| Crypt depth (mm) | 0.16a | 0.14b | 0.14b | 0.15ab | 0.15ab | 0.00 | 0.004 |
| Villus height: Crypt depth | 12.5b | 15.0a | 14.7a | 15.5a | 14.4a | 0.19 | <0.001 |
| Jejunum | |||||||
| Villus height (mm) | 1.15 | 1.17 | 1.12 | 1.16 | 1.11 | 0.01 | 0.497 |
| Villus width (mm) | 0.25a | 0.20b | 0.23a | 0.24a | 0.26a | 0.00 | <0.001 |
| Crypt depth (mm) | 0.11ab | 0.10c | 0.10bc | 0.12a | 0.11abc | 0.00 | <0.001 |
| Villus height: Crypt depth | 10.2bc | 11.8a | 10.7ab | 9.30bc | 9.88c | 0.18 | <0.001 |
| Ileum | |||||||
| Villus height (mm) | 0.76ab | 0.77ab | 0.82a | 0.70b | 0.74ab | 0.01 | 0.001 |
| Villus width (mm) | 0.18ab | 0.18ab | 0.17b | 0.20a | 0.19ab | 0.00 | 0.007 |
| Crypt depth (mm) | 0.15a | 0.13bc | 0.14ab | 0.13bc | 0.14abc | 0.00 | 0.002 |
| Villus height: Crypt depth | 5.01b | 5.74a | 5.68ab | 5.47ab | 5.21ab | 0.10 | 0.028 |
Treatments include — (1) Negative Control treatment- chicks fed a basal corn-soybean meal-wheat–based diet; (2) In-feed antibiotics- chicks fed NC + 0.05% bacitracin methylene disalicylate; (3) In-feed probiotics- chicks fed NC + 0.005% Bacillus subtilis containing 1 × 108 CFU/kg of feed; (4) In ovo probiotics group 1- eggs injected with 10 × 106 CFU of Bacillus subtilis fermentation extract/0.2 mL saline diluent; and (5) In ovo probiotics group 2- eggs injected with 20 × 106 CFU of Bacillus subtilis fermentation extract/0.2 mL saline diluent.
SEM = Standard error of means.
Means within a row with different superscripts.
Significantly differ.
Gut Microbiota
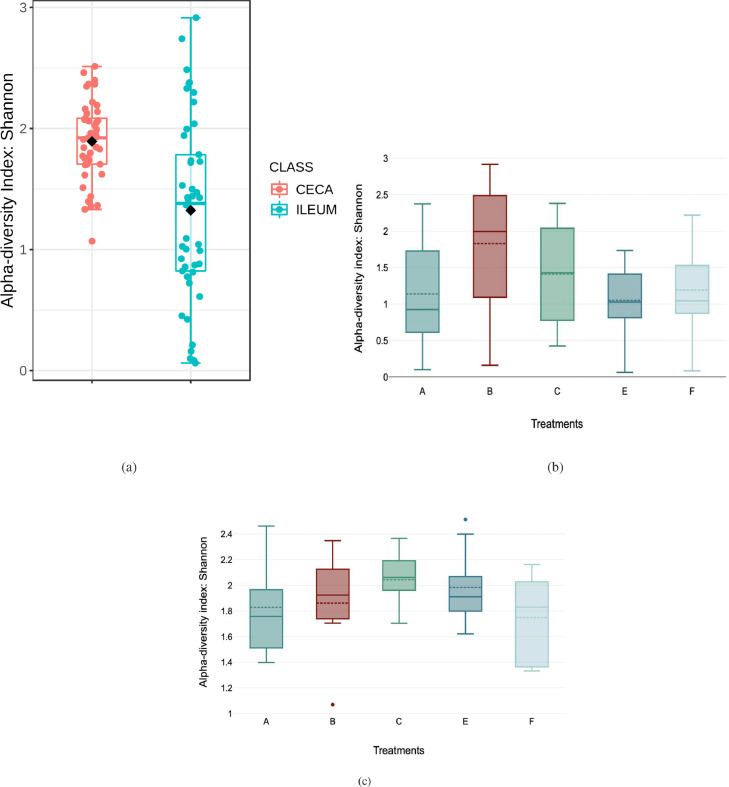
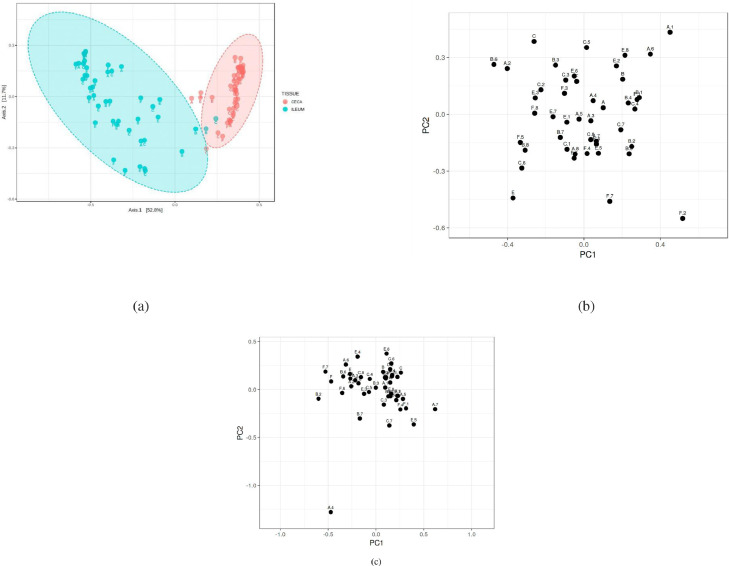
Sequencing analysis yielded a total of 1,712 operational taxonomic units (OTUs) with ≥ 2 counts after quality filtering and demultiplexing. The % of taxon assigned at the Genus level was ∼60%. Rarefaction curve showing specie richness is presented in Supplementary Figure S1. Alpha diversity (Shannon index) showed significant (P < 0.001) diversity between the ileal and cecal samples but not between treatment groups (Figure 2a–c). Similarly, Beta diversity determined by ordination analysis based on Bray-Curtis Index showed unique cluster separation between the ileal and cecal microbiota but not between treatment groups in both gut sections (Figure 3a–c).
Figure 2.
Alpha diversity (Shannon's index) box plots show (a) significant difference between ileal and cecal microbiota (T-test, P > 0.001), (b) no significant effect of treatments on ileal microbiota diversity (ANOVA, P = 0.180), (c) no significant effect of treatment on ceca microbiota (ANOVA, P = 0.320). Treatments include— A) Negative Control treatment- chicks fed a basal corn-soybean meal-wheat–based diet; (B) In-feed antibiotics- chicks fed NC + 0.05% bacitracin methylene disalicylate; (C) In-feed probiotics- chicks fed NC + 0.005% Bacillus subtilis containing 1 × 108 CFU/kg of feed; (E) In ovo probiotics group 1- eggs injected with 10 × 106 CFU of Bacillus subtilis fermentation extract /0.2 mL saline diluent; and (F) In ovo probiotics group 2- eggs injected with 20 × 106 CFU of Bacillus subtilis fermentation extract /0.2 mL saline diluent. Boxes in the boxplots denote interquartile range, solid middle line in the boxes denote the median, and dotted lines denote the means, all symbols outsides the boxes represent outliers.
Figure 3.
Beta diversity (based on analysis based on Bray-Curtis Index) principal coordinate plots show (a) significant difference between ileal and cecal microbiota (PCOA, ANOSIM, P > 0.05), (b) no significant effect of treatments on ileal beta diversity (PCA, ANOSIM, P > 0.05), (c) no significant effect of treatment on ceca beta diversity (PCA, ANOSIM, P > 0.05). Treatments include— A) Negative Control treatment- chicks fed a basal corn-soybean meal-wheat–based diet; (B) In-feed antibiotics- chicks fed NC + 0.05% bacitracin methylene disalicylate; (C) In-feed probiotics- chicks fed NC + 0.005% Bacillus subtilis containing 1 × 108 CFU/kg of feed; (E) In ovo probiotics group 1- eggs injected with 10 × 106 CFU of Bacillus subtilis fermentation extract /0.2 mL saline diluent; and (F) In ovo probiotics group 2- eggs injected with 20 × 106 CFU of Bacillus subtilis fermentation extract /0.2 mL saline diluent. Principal coordinates analysis- PCO-A, Principal component analysis-PCA and ANOSIM- Analysis of Similarities.
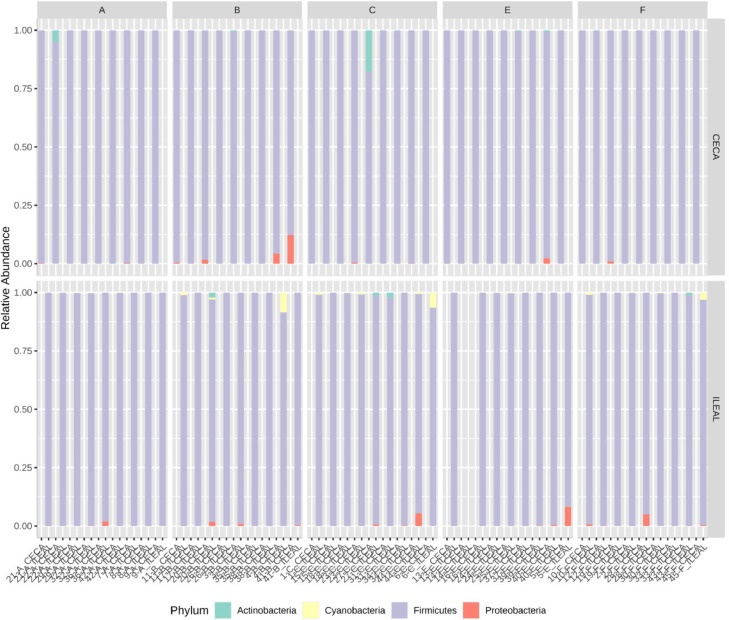
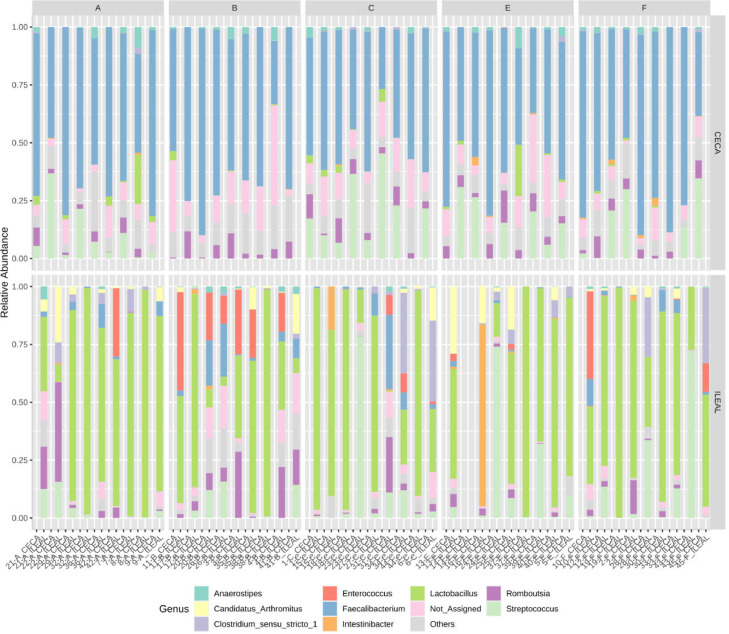
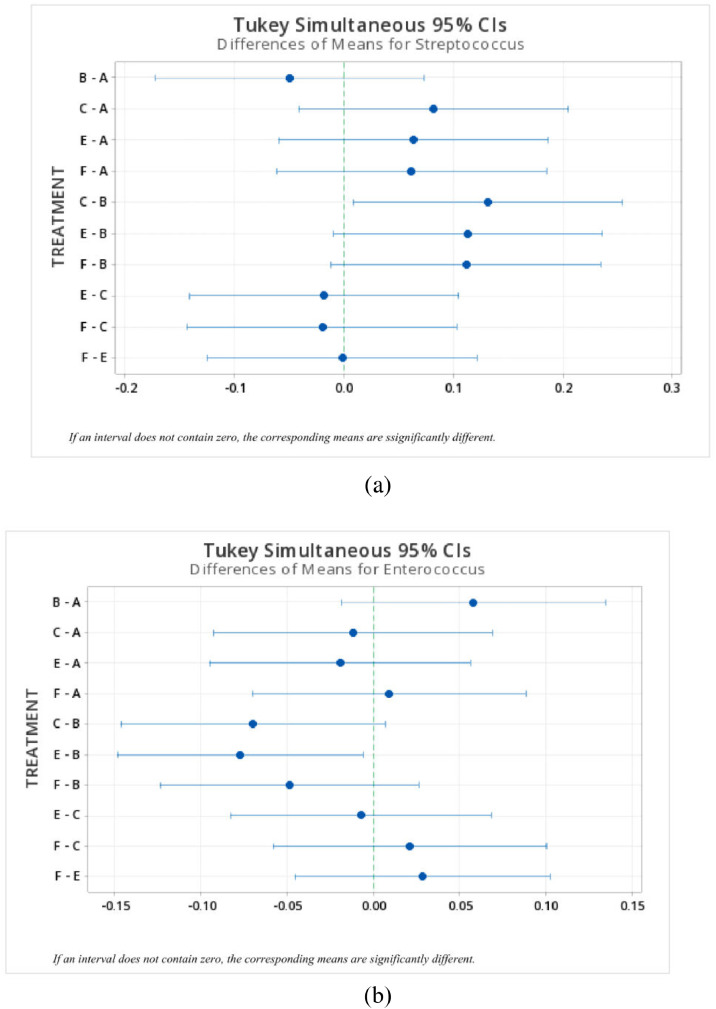
In terms of microbiota composition, the relative abundance of the predominant bacteria phyla and genera in the ileum and ceca are shown in Figure 4, Figure 5. Ileal phyla were dominated by >89% phylum Firmicutes across all treatment groups. The relative abundance of other dominant phyla followed the trend Actinobacteria (range of 0.5–9.8% across treatments) > Cyanobacteria (range of 0.4–2.5%) > Proteobacteria (range of 0.4–1.2%) > Bacteroidetes (range of 0–0.03%). Conversely, ceca phyla were dominated by >96% Firmicutes. Phylum Actinobacteria (range of 0.2–3.4% across treatments) and Proteobacteria (range of 0.1–3.1% across treatments) together accounted for the remainder of the ceca phyla microbiota composition. Phylum Bacteroidetes were not reported in the ceca. At the genus taxa, the ileal microbiota was dominated by ∼54% Lactobacillus, with a 43 to 65% relative abundance across treatment groups. Other predominant genera in the ileum included Streptococcus > Enterococcus > Romboutsia > Clostridium sensu_stricto_1 > Lachnospiraceae Sp. > Candidatus Arthromitus > Faecalibacterium > Peptostreptococcaceae Intestinibacter. Unlike the ileum, the ceca were dominated by ∼48% genus Ruminococcaceae Faecalibacterium, with a 39 to 53% relative abundance across treatment groups. Other predominant genera in the ceca followed the order Lachnospiraceae Sp. > Streptococcus > Romboutsia > Ruminococcaceae Sp. > Lactobacillus > Peptostreptococcaceae Intestinibacter > Clostridium sensu_stricto_1 > Enterococcus. Concurrently, significant differences in the cumulative proportions of bacteria in the genus Enterococcus in the ileum were observed (Figure 6b). While the in-feed antibiotic treatment increased (P = 0.02) the proportion of this bacteria compared to the in-feed probiotics and in ovo probiotic 1 treatment, other treatments recorded intermediate proportions of bacteria in this genus. Similarly, in the ceca, significant differences in the cumulative proportion of bacteria were only detected in the genus Streptococcus (Figure 6a). The in-feed antibiotic treatment reduced (P = 0.03) the proportion of bacteria in this genus compared to the in-feed probiotics treatment. Other treatments recorded statistically intermediate proportions of bacteria in this genus.
Figure 4.
Gut microbiota composition at the phylum taxa for both (a) ileal and (b) cecal digesta in broiler chickens with treatment groups- A) Negative Control treatment- chicks fed a basal corn-soybean meal-wheat–based diet; (B) In-feed antibiotics- chicks fed NC + 0.05% bacitracin methylene disalicylate; (C) In-feed probiotics - chicks fed NC + 0.005% Bacillus subtilis containing 1 × 108 CFU/kg of feed; (E) In ovo probiotics group 1- eggs injected with 10 × 106 CFU of Bacillus subtilis fermentation extract /0.2 mL saline diluent; and (F) In ovo probiotics group 2- eggs injected with 20 × 106 CFU of Bacillus subtilis fermentation extract /0.2 mL saline diluent.
Figure 5.
Gut microbiota composition at the genus taxa for both (a) ileal and (b) ceca digesta in broiler chickens with treatment groups- A) Negative Control treatment- chicks fed a basal corn-soybean meal-wheat–based diet; (B) In-feed antibiotics- chicks fed NC + 0.05% bacitracin methylene disalicylate; (C) In-feed probiotics- chicks fed NC + 0.005% Bacillus subtilis containing 1 × 108 CFU/kg of feed; (E) In ovo probiotics group 1- eggs injected with 10 × 106 CFU of Bacillus subtilis fermentation extract /0.2 mL saline diluent; and (F) In ovo probiotics group 2- eggs injected with 20 × 106 CFU of Bacillus subtilis fermentation extract /0.2 mL saline diluent.
Figure 6.
Significant differences in cumulative proportions of bacteria in the genera (a) Streptococcus in the ceca (ANOVA, P = 0.023) and (b) Enterococcus in the ileum of broiler chickens (ANOVA, P = 0.031) under different treatment groups. Treatment groups include A) Negative Control treatment- chicks fed a basal corn-soybean meal-wheat–based diet; (B) In-feed antibiotics- chicks fed NC + 0.05% bacitracin methylene disalicylate; (C) In-feed probiotics- chicks fed NC + 0.005% Bacillus subtilis containing 1 × 108 CFU/kg of feed; (E) In ovo probiotics group 1- eggs injected with 10 × 106 CFU of Bacillus subtilis fermentation extract /0.2 mL saline diluent; and (F) In ovo probiotics group 2- eggs injected with 20 × 106 CFU of Bacillus subtilis fermentation extract /0.2 mL saline diluent.
DISCUSSION
The use of Bacillus subtilis probiotic strains as prospective alternatives to AGPs due to their spore-forming, immunomodulatory and antibacterial properties continue to gain momentum in the poultry industry (Duc et al., 2004; Griggs and Jacob, 2005). Nonetheless, like other competitive exclusion cultures, strain-specific properties like proteolytic activity, toxin-producing capacity, inoculation dose, and delivery routes are potential factors that could limit their efficacy (Edens et al., 1997; Peebles, 2019). Using selected parameters and direct comparison to an AGP (Bacitracin); this study thus attempts to validate the optimum dose (10 × 106 CFU vs. 20 × 106 CFU) and delivery route (in ovo vs. in-feed) of Bacillus subtilis that qualify it as an effective alternative to AGP.
This study revalidates previous reports from our laboratory (Oladokun et al., 2021a) that showed that amniotic delivery of Bacillus subtilis fermentation extract at embryonic day 18 had no negative effect on embryo viability and hatchability. Both in ovo probiotics treatments in this study recorded ∼96% hatchability, similar to the noninjected eggs. Consistent with the result reported here, other studies (Edens et al., 1997; Pender et al., 2016, 2017; Majidi-Mosleh et al., 2017a,b; Castañeda et al., 2020; Alizadeh et al., 2020) have also affirmed no adverse effect of in ovo delivered probiotics on hatchability. Contrastingly, although dependent on the broiler chicken strain, probiotic strain, injection site, and injection dose (De Oliveira et al., 2014; El-Moneim et al., 2020; Leão et al., 2021), a few studies (Meijerhof and Hulet, 1997; Triplett et al., 2018) have reported reduced hatchability following in ovo delivery of probiotics. Besides, Uni and Ferket (2003) patent has previously recommended that amniotic delivery of enteric modulators between embryonic d 17 and 19 does not impair hatchability, as the developing embryo maximizes in ovo delivered substances at this time-point. Additionally, chicks hatched from the noninjected eggs treatment in this study recorded reduced ratio of chick body weight to egg weight compared to the in ovo saline and in ovo probiotics 2 treatments. Several factors including egg size (Wilson, 1991; Tahir et al., 2011), length of egg storage (Lapão et al., 1999), post-hatch chick-holding time (Pinchasov, 1991; Reis et al., 1997), and age of breeder flock (Leão et al., 2021) are reported to influence the ratio of chick body weight to egg weight. Older breeder flocks are known to lay heavier eggs, and heavier eggs usually undergo less dehydration leading to a high chick body weight to egg weight ratio. Considering that all eggs in this study were sourced from the same source and underwent similar incubation and post-hatch conditions, the observed result might not be attributed to the variabilities associated with egg source, egg storage, or post-hatch handling condition. Additionally, although randomly allotted, the average weight of noninjected eggs in this study was at least 0.2% heavier than other treatments (data not shown), suggesting that egg size could also not have influenced the observed result. Nonetheless, chick body weight to egg weight ratio recorded for all treatments in this study were within the normal range (62–76%) for broiler chickens reported by Kumar et al. (1994). Despite the foregoing, it would be important to limit the use of small-sized eggs in current hatchery practice, as this has practical implications on bird hatch weight and subsequent market weights.
Furthermore, in this study, at the end of the total trial period (d 35) and Wk 5 especially, all probiotics treatments (irrespective of delivery routes) recorded similar feed conversion efficiency (P > 0.05) as the antibiotic treatment, with similar or less feed intake (P < 0.1). Several studies have affirmed the role of AGP (especially BMD) in improved growth performance (especially via increased AFI) in poultry (Gadde et al., 2017a; Karthikeyan et al., 2017; Walters et al., 2019). On the other hand, probiotics (whether in-feed or in ovo) are theorized to improve growth performance in poultry by positively modulating the gut microbiota in favor of host's nutrient utilization and energy uptake (Furuse and Yokota, 1985). Notwithstanding, variable results on the effect of probiotics (especially Bacillus subtilis) supplementation on growth performance are reported in the literature. Consistent with the results presented here, a number of studies (Knap et al., 2011; E Malik et al., 2016; Majidi-Mosleh et al., 2017a,b; ; Duneska and Bustillo, 2020; Castañeda et al., 2021) have reported no significant effect of Bacillus subtilis delivered across several routes (in-feed, in-water, or in ovo) on ABWG in broiler chickens. Conversely, improved ABWG following probiotics supplementation across several routes has also been reported in poultry (Aliakbarpour et al., 2012; Sen et al., 2012; Jeong and Kim, 2014; Gadde et al., 2017b; Hayashi et al., 2018). A plethora of factors, including probiotic viability, diet interaction, bird's genetic potential, and environmental or stress status, could account for the inconsistency in probiotics effect on growth performance recorded across the literature (Patterson and Burkholder, 2003; Mountzouris et al., 2007b; Flint and Garner, 2009). Additionaly, it has been speculated that a single time point delivery of Bacillus subtilis via the in ovo route might only guarantee a transient beneficial effects in the chicken gut (Latorre et al., 2014; Bernardeau et al., 2017). Both Patterson and Burkholder (2003) and Nunes et al. (2012) have submitted that significant improvement in growth performance following probiotics supplementation is mostly feasible in evironmental or imunological challenged birds producing below their genetic potential. Although, the birds utilized in this study have been genetically selected for high growth performance, it is interesting to note that all probiotics treatments recorded at most 3-point less FCR values at d35, compared to the performance objectives metric recommended by the breeders (Cobb, 2018).
Similar to the results on growth performance, all probiotic treatments (regardless of delivery routes) in this study reduced the concentration of serum IgG compared to the control treatment. Serum immunoglobulins are reflective of the humoral immune status of the bird. Despite the considerable number of reports in the literature that have reiterated the immunomodulatory role of probiotics (Haghighi et al., 2006; Bai et al., 2017; Pender et al., 2017; Royan, 2017), a complete mechanistic insight on the specific mode (s) of action is yet to be fully elucidated. A few of the prevailing rationale for the immunomodulatory role of probiotics in the literature will include increased antimicrobial peptide production (Royan, 2017), neutralizing dysbiosis (Cisek and Binek, 2014), mucosal immunostimulation (Nava et al., 2005), and increased antibody production against infectious antigens (Lee et al., 2007). Consistent with the report in the literature, Kabir et al. (2004) and Elkhouly et al. (2016) reported increased antibody production in broiler chickens exposed to sheep red blood cells and pathogenic antigen challenges. On the contrary, considering that birds in this study were raised under experimental conditions and were not subjected to any form of challenge, it is rational to speculate that the reduced levels of serum IgG might be a result of probiotics elimination of pathogenic agents that could have resulted in increased production of natural antibodies. This is corroborated by the report of Munyaka et al. (2012), a similar unchallenged study with broiler chickens. Nothwithstanding, more studies are needed to provide a broader understanding of the immunomodulatory mechanisms of probiotics in poultry.
With regards to blood biochemistry indices, the in-feed antibiotics treatment recorded increased levels of blood plasma sodium and chloride, compared to the NC treatment. The blood is often considered a window to the health status of the bird. This report's findings are consistent with recent data from our laboratory (Oladokun and Adewole, 2022), which also demonstrates that the use of in-feed antibiotics raises the levels of both electrolyte minerals. While all evaluated blood biochemical indices are in the range of published values for healthy broiler chickens (Ilo et al., 2019), both electrolyte minerals were within the upper limit of those ranges (Leeson and Summers, 2001). The effect of antibiotics on the levels of these blood minerals is largely unreported in the literature. However, excessive levels of these minerals in the blood have been linked with the maladies of acidosis, immunosuppression, and poor bone health (Oviedo-Rondón et al., 2001; Pohl et al., 2013). This study may thus offer even another reason to promote the cessation of AGP use in poultry.
In terms of gut morphology, treatment effects were quite variable in this study. Broiler chicken growth rate has been correlated with its gut morphological development (Smith et al., 1990), as the gut is predicted to account for about 1.5% of body weight (Faruq et al., 2019). In this study, both in ovo probiotic treatments and in-feed antibiotics treatment improved duodenal morphology, compared to the NC treatment, as evidenced by increased villus width and villus height to crypt depth ratio. In the jejunum, while the in-feed antibiotic treatment recorded the highest villus height to crypt depth ratio compared to other treatments, all probiotics treatments had wider villus compared to the in-feed antibiotic treatment. In the ileum, the in-feed antibiotics treatment only recorded higher villus height to crypt depth ratio than the NC treatment. Nonetheless, in terms of improved ileal morphology, as evidenced by villus height to crypt depth ratios, both levels of in ovo delivered probiotics displayed statistical similarity. The almost identical growth performance indices observed in this study could be potentially explained by the statistical comparability for evaluated gut morphological indicators demonstrated by most treatments. Although the jejunum is thought to be the primary location of nutrient absorption in the intestine (Zeinali et al., 2017), broiler chickens' duodenum and ileum also play important roles in the digestion and absorption of protein, lipids, fat-soluble vitamins, and starch (Svihus, 2014). Increased villus height and villus height to crypt depth ratio are indicators of higher epithelial cell turnover and a well-differentiated intestinal mucosa, usually suggestive of increased digestive and absorptive ability (Jeurissen et al., 2002). Numerous studies (Viveros et al., 2011; Khodambashi Emami et al., 2012; Adewole and Akinyemi, 2021; Akinyemi and Adewole, 2022) have already documented the beneficial effects of AGP (particularly BMD) on the gut, which are frequently linked to their antibacterial and gut microbiota-modulating capabilities. In agreement with the result presented here, probiotics have also been shown to have a positive effect on broiler chicken gut morphological indices, in numerous studies (Awad et al., 2008, 2010; Aliakbarpour et al., 2012; Deng et al., 2012; Xiang et al., 2019; Castañeda et al., 2020; El-Moneim et al., 2020; Bogusławska-Tryk et al., 2021). Neverthelesss, it is inferable from these studies that this beneficial effect might be dependent on probiotic strains and delivery routes, with lactic acid-based probiotics and in ovo delivery routes affording the most benefits. Probiotics are thought to exert this beneficial effect through competitive exclusion of pathogens (which occurs early enough in the case of in ovo delivery) (Vieco-Saiz et al., 2019; Castañeda et al., 2020).
As highlighted in the introductory section, one of the benefits derivable from in ovo delivery of probiotics is the advantage of colonizing the gut microbiota with beneficial microbes very early on, rather than trying to alter an already established microbiota in later life. In this study, the different evaluated gut sections (i.e., ileum and caecum) revealed distinct microbial diversity (alpha-Shannon index), with the ceca recording higher diversity compared to the ileum. However, treatments had no significant effect on alpha diversity index across both gut sections in this study. This result is in conformation with prevailing knowledge in the literature that microbial diversity is higher in the ceca compared to the ileum as a result of higher fermentation activity (Mohd Shaufi et al., 2015; Qin et al., 2018; Oladokun et al., 2022). Similarly, other studies (Chang et al., 2020; Oladokun et al., 2021a; Zhang et al., 2021; Guo et al., 2021; Deng et al., 2022; Memon et al., 2022) involving probiotics supplementation have also reported no significant effect of probiotics (irrespective of delivery routes) on alpha diversity indices. According to Thibodeau et al. (2015), only extreme events that distort the number of ecological niches across bacterial species can modify alpha diversity indices. Beta diversity analysis also showed no variation in microbial community structure between treatments at the ileum and caecum, but there were clear differences in bacterial community profile across both gut sections. Oladokun et al. (2021a) have previously reported that Bacillus subtilis supplementation across several routes does not cause a shift in beta diversity. Asides from differences in gut sections and nutrition, other potential factors that could cause a shift in beta diversity include broiler chicken age, breed, and environmental/stress condition (Stanley et al., 2014; Oakley et al., 2014; Choi et al., 2015). Regarding microbiota composition, phylum Firmicutes, Actinobacteria and Proteobacteria were the dominant taxa across both ileum and caecum. Similar findings have been reported in ileal and cecal samples from probiotic-supplemented broiler chickens (Wang et al., 2017; Oladokun et al., 2021a; Memon et al., 2022). Furthermore, the in-feed antibiotics treatment increased the abundance of bacteria in the genus Enterococcus in the ileum compared to the in-feed probiotics and in ovo probiotic 1 treatment. The genus Enterococcus potentially consists of harmful and beneficial species. For instance, Tortuero (1973) has previously reported that the implantation of probiotics Lact. acidophilli to leghorn chicks could promote bacterial antagonists that would subsequently inhibit the abundance of bacteria in the genus Enterococci, dubbed to cause a “fat malabsorption syndrome.” Contrarily, Enterococcus faecium is an important lactic acid-producing bacteria famous for its use as probiotics in poultry production (Yu, 2018; Zheng et al., 2020). Beneficial effects associated with Enterococcus faecium includes pathogen exclusion, improved host immunocompetence, improved feed conversion ratio, and weight gain, and enhanced antioxidant status (Capcarova et al., 2011; Kreuzer et al., 2012; Wu et al., 2019). Feed additives, including probiotics, antibiotics, and anticoccidials, have all been reported to enhance the abundance of bacteria in the genus Enterococcus in healthy broiler chickens (Lu et al., 2003). Additionaly, in the cecum, the proportion of bacteria in the genus Streptococcus was reduced by the in-feed antibiotic treatment compared to the in-feed probiotics treatment. Similar to the genus Enterococcus, the activities of bacteria in the genus Streptococcus might also be species-specific. Streptococcus jaecalis has been implicated in the incidence of “fat malabsorption syndrome,” which was counteracted with antibiotics supplementation (Huhtanen and Pensack, 1965). Conversely, a few studies have also reported the capacity of Streptococcus thermophilus to enhance gut integrity (Briskey et al., 2016; Zeng et al., 2017). Consistent with the result observed here, Bauer et al. (2019) have reported that oregano supplementation (1% w/v) on microbial cell cultures obtained from the cecum of broiler chickens significantly reduced bacteria in the genus Streptococcus. Given the healthy state of the flock in this study, it is probable that treatments in this study might have enhanced the abundance of beneficial species of both genera.
CONCLUSIONS
This study has successfully revalidated that amniotic delivery of Bacillus subtilis fermentation extract at embryonic d 18 has no adverse effect on embryo viability and hatchability. In ovo delivered Bacillus subtilis in this study recorded ∼96% hatchability. All Bacillus subtilis treatments (independent of delivery routes and dose) were mostly comparable to the in-feed antibiotics treatment in their ability to ensure gut microbiota homeostasis, enhanced gut morphology, and feed conversion efficiency, even while consuming similar or less feed. Similarly, all Bacillus subtilis treatments reduced serum IgG concentrations compared to the negative control treatment. However, the in ovo delivered Bacillus subtilis treatments showed the lowest numerical decrease in serum IgG concentrations, suggesting that Bacillus subtilis (especially in ovo delivered) might provide broiler chickens with better immunological protection by neutralizing pathogenic organisms that could result in the production of natural antibodies, without adversely affecting hatch and growth performance. As the results obtainable for both in ovo Bacillus subtilis delivered treatments were mostly comparable for most of the evaluated parameters, the in ovo probiotics 1 (10 × 106 CFU) treatment might thus be a more practical option from an economic standpoint. Nonetheless, it would be important for further research to determine if indeed immunological protection is conferred on broiler chickens supplemented with this treatment under some sort of immunological or environmental challenge conditions.
ACKNOWLEDGMENTS
The authors are grateful to Xujie Li, Taiwo Makinde, Nicolas Dionne, Michael McConkey, Sarah Macpherson, and Krista Budgell, who all helped with in ovo procedures, animal care, and bird sampling. Taiwo Erinle and Fisayo Akinyemi are also acknowledged for their help with in ovo procedures, animal care, bird sampling, and gut morphology analysis. Appreciation goes to Probiotech International for supplying the Bacillus subtilis fermentation extract used in this trial. Janice MacIsaac and Jamie Fraser are also appreciated for their help with diet formulation and preparation. The Canadian Center for Computational Genomics (C3G) is acknowledged for support with bioinformatic analysis. The Canadian Center for Computational Genomics (C3G) is a Genomics Technology Platform (GTP) supported by the Canadian Government through Genome Canada.
The support of the following funding agencies is also duly appreciated—Canadian Agricultural Partnership - Pan Atlantic Program (53630), Chicken Farmers of Nova Scotia (53630), and Dalhousie University Start-up grant (34741).
DISCLOSURES
The authors declare no conflict of interest.
Footnotes
Part of this work was presented at the 2022 PSA Annual Meeting, San Antonio, TX, United States.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2022.102473.
Appendix. Supplementary materials
REFERENCES
- Abd El-Ghany W.A., Abdel-Latif M.A., Hosny F., Alatfeehy N.M., Noreldin A.E., Quesnell R.R., Chapman R., Sakai L., Elbestawy A.R. Comparative efficacy of postbiotic, probiotic, and antibiotic against necrotic enteritis in broiler chickens. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adewole D., Akinyemi F. Gut microbiota dynamics, growth performance, and gut morphology in broiler chickens fed diets varying in energy density with or without bacitracin methylene disalicylate (Bmd) Microorganisms. 2021;9:787. doi: 10.3390/microorganisms9040787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinyemi F.T., Adewole D.I. Effect of dietary folic acid and energy density on immune response, gut morphology, and oxidative status in blood and breast muscle of broiler chickens. Can. J. Anim. Sci. 2022;102:243–254. [Google Scholar]
- Aliakbarpour H.R., Chamani M., Rahimi G., Sadeghi A.A., Qujeq D. The Bacillus subtilis and lactic acid bacteria probiotics influences intestinal mucin gene expression, histomorphology and growth performance in broilers. Asian-Australasian J. Anim. Sci. 2012;25:1285–1293. doi: 10.5713/ajas.2012.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh M., Shojadoost B., Astill J., Taha-Abdelaziz K., Karimi S.H., Bavananthasivam J., Kulkarni R.R., Sharif S. Effects of in ovo inoculation of multi-strain lactobacilli on cytokine gene expression and antibody-mediated immune responses in chickens. Front. Vet. Sci. 2020;7:105. doi: 10.3389/fvets.2020.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad W., Ghareeb K., Böhm J. Intestinal structure and function of broiler chickens on diets supplemented with a synbiotic containing Enterococcus faecium and oligosaccharides. Int. J. Mol. Sci. 2008;9:2205–2216. doi: 10.3390/ijms9112205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad W.A., Ghareeb K., Böhm J. Effect of addition of a probiotic micro-organism to broiler diet on intestinal mucosal architecture and electrophysiological parameters. J. Anim. Physiol. Anim. Nutr. (Berl). 2010;94:486–494. doi: 10.1111/j.1439-0396.2009.00933.x. [DOI] [PubMed] [Google Scholar]
- Bai K., Huang Q., Zhang J., He J., Zhang L., Wang T. Supplemental effects of probiotic Bacillus subtilis fmbJ on growth performance, antioxidant capacity, and meat quality of broiler chickens. Poult. Sci. 2017;96:74–82. doi: 10.3382/ps/pew246. [DOI] [PubMed] [Google Scholar]
- Bai S.P., Wu A.M., Ding X.M., Lei Y., Bai J., Zhang K.Y., Chio J.S. Effects of probiotic-supplemented diets on growth performance and intestinal immune characteristics of broiler chickens. Poult. Sci. 2013;92:663–670. doi: 10.3382/ps.2012-02813. [DOI] [PubMed] [Google Scholar]
- Bajagai et al. 2016. Probiotics in animal nutrition - Production, impact and regulation. Food and Agriculture Organization (FAO)
- Bauer B.W., Gangadoo S., Bajagai Y.S., Van T.T.H., Moore R.J., Stanley D. Oregano powder reduces Streptococcus and increases SCFA concentration in a mixed bacterial culture assay. PLoS One. 2019;14 doi: 10.1371/journal.pone.0216853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardeau M., Lehtinen M.J., Forssten S.D., Nurminen P. Importance of the gastrointestinal life cycle of Bacillus for probiotic functionality. J. Food Sci. Technol. 2017;54:2570–2584. doi: 10.1007/s13197-017-2688-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogusławska-Tryk M., Ziółkowska E., Sławińska A., Siwek M., Bogucka J. Modulation of intestinal histology by probiotics, prebiotics and synbiotics delivered in ovo in distinct chicken genotypes. Animals. 2021;11:3293. doi: 10.3390/ani11113293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgey M., Dali R., Eveleigh R., Chen K.C., Letourneau L., Fillon J., Michaud M., Caron M., Sandoval J., Lefebvre F., Leveque G. GenPipes: an open-source framework for distributed and scalable genomic analyses. Gigascience. 2019;8 doi: 10.1093/gigascience/giz037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskey D., Heritage M., Jaskowski L.A., Peake J., Gobe G., Subramaniam V.N., Crawford D., Campbell C., Vitetta L. Probiotics modify tight-junction proteins in an animal model of nonalcoholic fatty liver disease. Therap. Adv. Gastroenterol. 2016;9:463–472. doi: 10.1177/1756283X16645055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capcarova M., Hascik P., Kolesarova A., Kacaniova M., Mihok M., Pal G. The effect of selected microbial strains on internal milieu of broiler chickens after peroral administration. Res. Vet. Sci. 2011;91:132–137. doi: 10.1016/j.rvsc.2010.07.022. [DOI] [PubMed] [Google Scholar]
- Cartman S.T., La Ragione R.M., Woodward M.J. Bacillus subtilis spores germinate in the chicken gastrointestinal tract. Appl. Environ. Microbiol. 2008;74:5254–5258. doi: 10.1128/AEM.00580-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castañeda C.D., Dittoe D.K., Wamsley K.G.S., McDaniel C.D., Blanch A., Sandvang D., Kiess A.S. In ovo inoculation of an Enterococcus faecium–based product to enhance broiler hatchability, live performance, and intestinal morphology. Poult. Sci. 2020;99:6163–6172. doi: 10.1016/j.psj.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castañeda C.D., Gamble J.N., Wamsley K.G.S., McDaniel C.D., Kiess A.S. In ovo administration of Bacillus subtilis serotypes effect hatchability, 21-day performance, and intestinal microflora. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon J.I.R. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 2007;86:2466–2471. doi: 10.3382/ps.2007-00249. [DOI] [PubMed] [Google Scholar]
- Cavazzoni V., Adami A., Castrovilli C. Performance of broiler chickens supplemented with Bacillus coagulons as probiotic. Br. Poult. Sci. 1998;39:526–529. doi: 10.1080/00071669888719. [DOI] [PubMed] [Google Scholar]
- Chang C.H., Teng P.Y., Lee T.T., Yu B. Effects of multi-strain probiotic supplementation on intestinal microbiota, tight junctions, and inflammation in young broiler chickens challenged with Salmonella enterica subsp. enterica. Asian-Australasian J. Anim. Sci. 2020;33:1797–1808. doi: 10.5713/ajas.19.0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K.Y., Lee T.K., Sul W.J. Metagenomic analysis of chicken gut microbiota for improving metabolism and health of chickens - a review. Asian-Australasian J. Anim. Sci. 2015;28:1217–1225. doi: 10.5713/ajas.15.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek A.A., Binek M. Chicken intestinal microbiota function with a special emphasis on the role of probiotic bacteria. Pol. J. Vet. Sci. 2014;17:385–394. doi: 10.2478/pjvs-2014-0057. [DOI] [PubMed] [Google Scholar]
- Cobb. 2018. Cobb500 Broiler Performance & Nutrition Supplement.:1–14 Available at chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.cobb-vantress.com/assets/5a88f2e793/Broiler-Performance-Nutrition-Supplement.pdf (verified 26 September 2022).
- Cox C.M., Dalloul R.A. Immunomodulatory role of probiotics in poultry and potential in ovo application. Benef. Microbes. 2015;6:45–52. doi: 10.3920/BM2014.0062. [DOI] [PubMed] [Google Scholar]
- Deng S., Hu S., Xue J., Yang K., Zhuo R., Xiao Y., Fang R. Productive performance, serum antioxidant status, tissue selenium deposition, and gut health analysis of broiler chickens supplemented with selenium and probiotics—a pilot study. Animals. 2022;12:1086. doi: 10.3390/ani12091086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W., Dong X.F., Tong J.M., Zhang Q. The probiotic Bacillus licheniformis ameliorates heat stress-induced impairment of egg production, gut morphology, and intestinal mucosal immunity in laying hens. Poult. Sci. 2012;91:575–582. doi: 10.3382/ps.2010-01293. [DOI] [PubMed] [Google Scholar]
- De Oliveira J.E., Van Der Hoeven-Hangoor E., Van De Linde I.B., Montijn R.C., M. Van Der Vossen J.M.B. In ovo inoculation of chicken embryos with probiotic bacteria and its effect on posthatch Salmonella susceptibility. Poult. Sci. 2014;93:818–829. doi: 10.3382/ps.2013-03409. [DOI] [PubMed] [Google Scholar]
- Dhariwal A., Chong J., Habib S., King I.L., Agellon L.B., Xia J. MicrobiomeAnalyst: a web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 2017;45:W180–W188. doi: 10.1093/nar/gkx295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diarra M.S., Malouin F. Antibiotics in Canadian poultry productions and anticipated alternatives. Front. Microbiol. 2014;5:282. doi: 10.3389/fmicb.2014.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duc L.H., Hong H.A., Barbosa T.M., Henriques A.O., Cutting S.M. Characterization of bacillus probiotics available for human use. Appl. Environ. Microbiol. 2004;70:2161–2171. doi: 10.1128/AEM.70.4.2161-2171.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duneska C., Bustillo C. PhD Diss. Mississippi State University, Mississippi; 2020. Early administration of probiotics through in ovo inoculation and their impact on gut microflora, immune response, and growth performance of broiler chicks. [Google Scholar]
- Edens F.W., Parkhurst C.R., Casas I.A., Dobrogosz W.J. Principles of ex ovo competitive exclusion and in ovo administration of Lactobacillus reuteri. Poult. Sci. 1997;76:179–196. doi: 10.1093/ps/76.1.179. [DOI] [PubMed] [Google Scholar]
- Elkhouly M., Khairy M., Abd- El Alim A.-E.A., Ali A. Effect of phytobiotics, probiotics and toltrazuril on chicken Coccidiosis. Zagazig Vet. J. 2016;44:214–223. [Google Scholar]
- E.A. El-Moneim A.E.M., El-Wardany I., Abu-Taleb A.M., Wakwak M.M., Ebeid T.A., Saleh A.A. Assessment of In ovo administration of Bifidobacterium bifidum and Bifidobacterium longum on performance, Ileal Histomorphometry, blood hematological, and biochemical parameters of broilers. Probiotics Antimicrob. Proteins. 2020;12:439–450. doi: 10.1007/s12602-019-09549-2. [DOI] [PubMed] [Google Scholar]
- E Malik H.E., Hafzalla R.H.H, H A A.O., M O E.M., Dousa B.M., Ali A.M., Elamin K.M. Effect of probiotics and acidifiers on carcass yield, internal organs, cuts and meat to bone ratio of broiler chicken. J. Agric. Vet. Sci. 2016;9:18–23. [Google Scholar]
- FAO/WHO . Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. Amerian Córdoba Park Hotel; Córdoba, Argentina: 2001. pp. 1–10. [Google Scholar]
- Faruq A.Al, Veterinary C., Rahman M.L., Veterinary C., Islam K.H.N., Veterinary C. Development of small intestinal morphology on the basis of growth and absorption rate in broiler chicken (Cobb 500) of Bangladesh. Bangladesh J. Vet. Anim. Sci. 2019;7:9–14. [Google Scholar]
- Flint J.F., Garner M.R. Feeding beneficial bacteria: a natural solution for increasing efficiency and decreasing pathogens in animal agriculture. J. Appl. Poult. Res. 2009;18:367–378. [Google Scholar]
- Furuse M., Yokota H. Effect of the gut microflora on chick growth and utilisation of protein and energy at different concentrations of dietary protein. Br. Poult. Sci. 1985;26:97–104. doi: 10.1080/00071668508416791. [DOI] [PubMed] [Google Scholar]
- Gadde U., Kim W.H., Oh S.T., Lillehoj H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim. Heal. Res. Rev. 2017;18:26–45. doi: 10.1017/S1466252316000207. [DOI] [PubMed] [Google Scholar]
- Gadde U., Oh S.T., Lee Y.S., Davis E., Zimmerman N., Rehberger T., Lillehoj H.S. The effects of direct-fed microbial supplementation, as an alternative to antibiotics, on growth performance, intestinal immune status, and epithelial barrier gene expression in broiler chickens. Probiotics Antimicrob. Proteins. 2017;9:397–405. doi: 10.1007/s12602-017-9275-9. [DOI] [PubMed] [Google Scholar]
- Grant A., Gay C.G., Lillehoj H.S. Bacillus spp. as direct-fed microbial antibiotic alternatives to enhance growth, immunity, and gut health in poultry. Avian Pathol. 2018;47:339–351. doi: 10.1080/03079457.2018.1464117. [DOI] [PubMed] [Google Scholar]
- Griggs J.P., Jacob J.P. Alternatives to antibiotics for organic poultry production. J. Appl. Poult. Res. 2005;14:750–756. [Google Scholar]
- Guo S., Xi Y., Xia Y., Wu T., Zhao D., Zhang Z., Ding B. Dietary Lactobacillus fermentum and Bacillus coagulans supplementation modulates intestinal immunity and microbiota of broiler chickens challenged by Clostridium perfringens. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.680742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyawali I., Zeng Y., Zhou J., Li J., Wu T., Jiang Q., C. Z.-P. Science, and undefined Effect of novel Lactobacillus paracaesi microcapsule on growth performance, gut health and microbiome community of broiler chickens. Poult. Sci. 2022;8 doi: 10.1016/j.psj.2022.101912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi H.R., Gong J., Gyles C.L., Hayes M.A., Zhou H., Sanei B., Chambers J.R., Sharif S. Probiotics stimulate production of natural antibodies in chickens. Clin. Vaccine Immunol. 2006;13:975–980. doi: 10.1128/CVI.00161-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi R.M., Lourenço M.C., Kraieski A.L., Araujo R.B., Gonzalez-Esquerra R., Leonardecz E., da Cunha A.F., Carazzolle M.F., Monzani P.S., Santin E. Effect of feeding Bacillus subtilis spores to broilers challenged with Salmonella enterica serovar Heidelberg Brazilian strain UFPR1 on performance, immune response, and gut health. Front. Vet. Sci. 2018;5:13. doi: 10.3389/fvets.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedayati M., Khalaji S., Manafi M. Lactobacilli spp. and Zataria multiflora essence as antibiotic substituent on broiler health and performance parameters. Ital. J. Anim. Sci. 2022;21:1–7. [Google Scholar]
- Hedman H.D., Vasco K.A., Zhang L. A review of antimicrobial resistance in poultry farming within low-resource settings. Animals. 2020;10:1–39. doi: 10.3390/ani10081264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtanen C.N., Pensack J.M. The role of Streptococcus Faecalis in the antibiotic growth effect in. Poult. Sci. 1965;44:830–834. doi: 10.3382/ps.0440830. [DOI] [PubMed] [Google Scholar]
- Ilo S.U., Maduneme F.C., Ogbu O.C., Okonkwo M.N. Haematological and biochemical characteristics of broiler finisher fed different feed forms (pelleted and mash) J. Agric. and Sus. 2019;12:2. [Google Scholar]
- Jeong J.S., Kim I.H. Effect of Bacillus subtilis C-3102 spores as a probiotic feed supplement on growth performance, noxious gas emission, and intestinal microflora in broilers. Poult. Sci. 2014;93:3097–3103. doi: 10.3382/ps.2014-04086. [DOI] [PubMed] [Google Scholar]
- Jeurissen S.H.M., Lewis F., van der Klis J.D., Mroz Z., Rebel J.M.J., M. ter Huurne A.A.H. Parameters and techniques to determine intestinal health of poultry as constituted by immunity, integrity, and functionality. Curr. Issues Intest. Microbiol. 2002;3:1–14. [PubMed] [Google Scholar]
- Kabir S.M.L., Rahman M.M., Rahman M.B., Rahman M.M., Ahmed S.U. The dynamics of probiotics on growth performance and immune response in broilers. Int. J. Poult. Sci. 2004;3:361–364. [Google Scholar]
- Karthikeyan N., N.B.P. Veeramani S.N.R., Sivaramakrishnan S. Effect of organic acid salts as an alternative to antibiotic growth promoters on the production performance of commercial broiler chicken. Int. J. Curr. Microbiol. Appl. Sci. 2017;6:3470–3480. [Google Scholar]
- Khodambashi Emami N., Samie A., Rahmani H.R., Ruiz-Feria C.A. The effect of peppermint essential oil and fructooligosaccharides, as alternatives to virginiamycin, on growth performance, digestibility, gut morphology and immune response of male broilers. Anim. Feed Sci. Technol. 2012;175:57–64. [Google Scholar]
- Kim J.A., Bayo J., Cha J., Choi Y.J., Jung M.Y., Kim D.H., Kim Y. Investigating the probiotic characteristics of four microbial strains with potential application in feed industry. PLoS One. 2018;14 doi: 10.1371/journal.pone.0218922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knap I., Kehlet A.B., Bennedsen M., Mathis G.F., Hofacre C.L., Lumpkins B.S., Jensen M.M., Raun M., Lay A. Bacillus subtilis (DSM17299) significantly reduces Salmonella in broilers. Poult. Sci. 2011;90:1690–1694. doi: 10.3382/ps.2010-01056. [DOI] [PubMed] [Google Scholar]
- Kreuzer S., MacHnowska P., Amus J., Sieber M., Pieper R., Schmidt M.F., Brockmann G.A., Scharek-Tedin L., Johne R. Feeding of the probiotic bacterium Enterococcus faecium NCIMB 10415 differentially affects shedding of enteric viruses in pigs. Vet. Res. 2012;43:1–12. doi: 10.1186/1297-9716-43-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B., Bhatt R.P., Joshi M.C. Influence of egg weight on hatching indices and posthatching growth of white rock X red cornish broilers at 1200m altitude. J. Appl. Anim. Res. 1994;6:161–165. [Google Scholar]
- Lapão C., Gama L.T., Soares M.C. Effects of broiler breeder age and length of egg storage on albumen characteristics and hatchability. Poult. Sci. 1999;78:640–645. doi: 10.1093/ps/78.5.640. [DOI] [PubMed] [Google Scholar]
- Latorre J.D., Hernandez-Velasco X., Kallapura G., Menconi A., Pumford N.R., Morgan M.J., Layton S.L., Bielke L.R., Hargis B.M., Téllez G. Evaluation of germination, distribution, and persistence of Bacillus subtilis spores through the gastrointestinal tract of chickens. Poult. Sci. 2014;93:1793–1800. doi: 10.3382/ps.2013-03809. [DOI] [PubMed] [Google Scholar]
- Leão A.P.A., Alvarenga R.R., Zangeronimo M.G. In ovo inoculation of probiotics for broiler chickens: systematic review and meta-analysis. Anim. Feed Sci. Technol. 2021;280 [Google Scholar]
- Lee K.W., Lillehoj H.S., Jang S.I., Lee S.H. Effects of salinomycin and Bacillus subtilis on growth performance and immune responses in broiler chickens. Res. Vet. Sci. 2014;97:305–309. doi: 10.1016/j.rvsc.2014.07.021. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Lillehoj H.S., Dalloul R.A., Park D.W., Hong Y.H., Lin J.J. Influence of Pediococcus-based probiotic on coccidiosis in broiler chickens. Poult. Sci. 2007;86:63–66. doi: 10.1093/ps/86.1.63. [DOI] [PubMed] [Google Scholar]
- Leeson S., Summers J.D. 4th ed. University books; Guelph, Ontario, Canada: 2001. Scott's nutrition of the chicken. Chapter 5. [Google Scholar]
- Lekshmi M., Ammini P., Kumar S., Varela M.F. The food production environment and the development of antimicrobial resistance in human pathogens of animal origin. Microorganisms. 2017;5:11. doi: 10.3390/microorganisms5010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.L., Wang J., Zhang H.J., Wu S.G., Hui Q.R., Yang C.B., Fang R.J., Qi G.H. Erratum: intestinal morphologic and microbiota responses to dietary Bacillusspp. in a broiler chicken model. Front. Physiol. 2019;9:1968. doi: 10.3389/fphys.2018.01968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Idris U., Harmon B., Hofacre C., Maurer J.J., Lee M.D. Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Appl. Environ. Microbiol. 2003;69:6816–6824. doi: 10.1128/AEM.69.11.6816-6824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majidi-Mosleh A., Sadeghi A.A., Mousavi S.N., Chamani M., Zarei A. Ileal MUC2 gene expression and microbial population, but not growth performance and immune response, are influenced by in ovo injection of probiotics in broiler chickens. Br. Poult. Sci. 2017;58:40–45. doi: 10.1080/00071668.2016.1237766. [DOI] [PubMed] [Google Scholar]
- Majidi-Mosleh A., Sadeghi A.A., Mousavi S.N., Chamani M., Zarei A. Effects of in ovo infusion of probiotic strains on performance parameters, jejunal bacterial population and mucin gene expression in broiler chicken. Rev. Bras. Cienc. Avic. 2017;19:97–102. [Google Scholar]
- Meijerhof R., Hulet R.M. In ovo injection of competitive exclusion culture in broiler hatching eggs. J. Appl. Poult. Res. 1997;6:260–266. [Google Scholar]
- Memon F.U., Yang Y., Zhang G., Leghari I.H., Lv F., Wang Y., Laghari F., Khushk F.A., Si H. Chicken gut microbiota responses to dietary Bacillus subtilis Probiotic in the presence and absence of Eimeria infection. Microorganisms. 2022;10:1548. doi: 10.3390/microorganisms10081548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohd Shaufi M.A., Sieo C.C., Chong C.W., Gan H.M., Ho Y.W. Deciphering chicken gut microbial dynamics based on high-throughput 16S rRNA metagenomics analyses. Gut Pathog. 2015;7:1–12. doi: 10.1186/s13099-015-0051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountzouris K.C., Tsirtsikos P., Kalamara E., Nitsch S., Schatzmayr G., Fegeros K. Evaluation of the efficacy of a probiotic containing Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus strains in promoting broiler performance and modulating cecal microflora composition and metabolic activities. Poult. Sci. 2007;86:309–317. doi: 10.1093/ps/86.2.309. [DOI] [PubMed] [Google Scholar]
- Mountzouris K.C., Tsirtsikos P., Kalamara E., Nitsch S., Schatzmayr G., Fegeros K. Evaluation of the efficacy of a probiotic containing Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus strains in promoting broiler performance and modulating cecal microflora composition and metabolic activities. Poult. Sci. 2007;86:309–317. doi: 10.1093/ps/86.2.309. [DOI] [PubMed] [Google Scholar]
- Mountzouris K.C., Tsitrsikos P., Palamidi I., Arvaniti A., Mohnl M., Schatzmayr G., Fegeros K. Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition. Poult. Sci. 2010;89:58–67. doi: 10.3382/ps.2009-00308. [DOI] [PubMed] [Google Scholar]
- Muaz K., Riaz M., Akhtar S., Park S., Ismail A. Antibiotic residues in chicken meat: global prevalence, threats, and decontamination strategies: a review. J. Food Prot. 2018;81:619–627. doi: 10.4315/0362-028X.JFP-17-086. [DOI] [PubMed] [Google Scholar]
- Munyaka P.M., Echeverry H., Yitbarek A., Camelo-Jaimes G., Sharif S., Guenter W., House J.D., Rodriguez-Lecompte J.C. Local and systemic innate immunity in broiler chickens supplemented with yeast-derived carbohydrates. Poult. Sci. 2012;91:2164–2172. doi: 10.3382/ps.2012-02306. [DOI] [PubMed] [Google Scholar]
- Nava G.M., Bielke L.R., Callaway T.R., Castañeda M.P. Probiotic alternatives to reduce gastrointestinal infections: the poultry experience. Anim. Heal. Res. Rev. 2005;6:105–118. doi: 10.1079/ahr2005103. [DOI] [PubMed] [Google Scholar]
- Nawaz H., Irshad M.A., Ali M. Effect of probiotics on growth performance, nutrient digestibility and carcass characteristics in broilers. J. Anim. Plant Sci. 2016;26:599–604. [Google Scholar]
- Nunes J.O., Bertechini A.G., Á. G. De Brito J., Fassani É.J., Mesquita F.R., Makiyama L., Meneghetti C. Evaluation of the use of probiotic (Bacillus subtilis C-3102) as additive to improve performance in broiler chicken diets. Rev. Bras. Zootec. 2012;41:2374–2378. [Google Scholar]
- Oakley B.B., Lillehoj H.S., Kogut M.H., Kim W.K., Maurer J.J., Pedroso A., Lee M.D., Collett S.R., Johnson T.J., Cox N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014;360:100–112. doi: 10.1111/1574-6968.12608. [DOI] [PubMed] [Google Scholar]
- Ohimain E.I., Ofongo R.T.S. The effect of probiotic and prebiotic feed supplementation on chicken health and gut microflora : a review. Int. J. Anim. Vet. Adv. 2012;4:135–143. [Google Scholar]
- Oladokun S., Adewole D. Effect of Bacillus subtilis and its delivery route on hatch and growth performance, blood biochemistry, and immune status of broiler chickens. Poult. Sci Annual Meetings. 2022;115 doi: 10.1016/j.psj.2022.102473. (Abstr.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oladokun S., Adewole D.I. In ovo delivery of bioactive substances: an alternative to the use of antibiotic growth promoters in poultry production—a review. J. Appl. Poult. Res. 2020;29:744–763. [Google Scholar]
- Oladokun S., Clark K.F., Adewole D.I. Microbiota and transcriptomic effects of an essential oil blend and its delivery route compared to an antibiotic growth promoter in broiler chickens. Microorganisms. 2022;10:861. doi: 10.3390/microorganisms10050861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oladokun S., Koehler A., MacIsaac J., Ibeagha-Awemu E.M., Adewole D.I. Bacillus subtilis delivery route: effect on growth performance, intestinal morphology, cecal short-chain fatty acid concentration, and cecal microbiota in broiler chickens. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oladokun S., Macisaac J., Rathgeber B., Adewole D. Essential oil delivery route: effect on broiler chicken's growth performance, blood biochemistry, intestinal morphology, immune, and antioxidant status. Animals. 2021;11:3386. doi: 10.3390/ani11123386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opoola E., Kahuwai C.Z., Olugbemi T.S. Evaluation of the performance characteristics, nutrient digestibility and carcass quality of broiler chickens fed Lacto acidophilus®as a replacement for a commercial antibiotic. Niger. J. Anim. Prod. 2021;48:166–174. [Google Scholar]
- Oviedo-Rondón E.O., Murakami A.E., Furlan A.C., Moreira I., Macari M. Sodium and chloride requirements of young broiler chickens fed corn-soybean diets (one to twenty-one days of age) Poult. Sci. 2001;80:592–598. doi: 10.1093/ps/80.5.592. [DOI] [PubMed] [Google Scholar]
- Ozdogan M., Topal E., Paksuz E.P., Kirkan S. Effect of different levels of crude glycerol on the morphology and some pathogenic bacteria of the small intestine in male broilers. Animal. 2014;8:36–42. doi: 10.1017/S1751731113001833. [DOI] [PubMed] [Google Scholar]
- Patterson J.A., Burkholder K.M. Application of prebiotics and probiotics in poultry production. Poult. Sci. 2003;82:627–631. doi: 10.1093/ps/82.4.627. [DOI] [PubMed] [Google Scholar]
- Peebles, E. D. 2019. In ovo development of the chicken gut microbiome and its impact on later gut function. Accessed October 20, 2022.https://www.taylorfrancis.com/chapters/edit/10.1201/9780429266713-5/ovo-development-chicken-gut-microbiome-impact-later-gut-function-david-peebles:95–120.
- Pender C.M., Kim S., Potter T.D., Ritzi M.M., Young M., Dalloul R.A. Effects of in ovo supplementation of probiotics on performance and immunocompetence of broiler chicks to an Eimeria challenge. Benef. Microbes. 2016;7:699–705. doi: 10.3920/BM2016.0080. [DOI] [PubMed] [Google Scholar]
- Pender C.M., Kim S., Potter T.D., Ritzi M.M., Young M., Dalloul R.A. In ovo supplementation of probiotics and its effects on performance and immune-related gene expression in broiler chicks. Poult. Sci. 2017;96:1052–1062. doi: 10.3382/ps/pew381. [DOI] [PubMed] [Google Scholar]
- Pinchasov Y. Relationship between the weight of hatching eggs and subsequent early performance of broiler chicks. Br. Poult. Sci. 1991;32:109–115. doi: 10.1080/00071669108417332. [DOI] [PubMed] [Google Scholar]
- Pohl H.R., Wheeler J.S., Murray H.E. Sodium and potassium in health and disease. Met. Ions Life Sci. 2013;13:29–47. doi: 10.1007/978-94-007-7500-8_2. [DOI] [PubMed] [Google Scholar]
- Qin C., Gong L., Zhang X., Wang Y., Wang Y., Wang B., Li Y., Li W. Effect of Saccharomyces boulardii and Bacillus subtilis B10 on gut microbiota modulation in broilers. Anim. Nutr. 2018;4:358–366. doi: 10.1016/j.aninu.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijrink I.A.M., Meijerhof R., Kemp B., Graat E.A.M., van den Brand H. Influence of prestorage incubation on embryonic development, hatchability, and chick quality. Poult. Sci. 2009;88:2649–2660. doi: 10.3382/ps.2008-00523. [DOI] [PubMed] [Google Scholar]
- Reis L.H., Gama L.T., Soares M.Chaveiro. Effects of short storage conditions and broiler breeder age on hatchability, hatching time, and chick weights. Poult. Sci. 1997;76:1459–1466. doi: 10.1093/ps/76.11.1459. [DOI] [PubMed] [Google Scholar]
- Rowsell H.C. Canadian council on animal care: its role. ILAR J. 1990;32:39–40. [Google Scholar]
- Royan M. The immune-genes regulation mediated mechanisms of probiotics to control salmonella infection in chicken. Worlds. Poult. Sci. J. 2017;73:603–610. [Google Scholar]
- Sen S., Ingale S.L., Kim Y.W., Kim J.S., Kim K.H., Lohakare J.D., Kim E.K., Kim H.S., Ryu M.H., Kwon I.K., Chae B.J. Effect of supplementation of Bacillus subtilis LS 1-2 to broiler diets on growth performance, nutrient retention, caecal microbiology and small intestinal morphology. Res. Vet. Sci. 2012;93:264–268. doi: 10.1016/j.rvsc.2011.05.021. [DOI] [PubMed] [Google Scholar]
- Smith M.W., Mitchell M.A., Peacock M.A. Effects of genetic selection on growth rate and intestinal structure in the domestic fowl (Callus domesticus) Comp. Biochem. Physiol. – Part A Physiol. 1990;97:57–63. doi: 10.1016/0300-9629(90)90722-5. [DOI] [PubMed] [Google Scholar]
- Stanley D., Hughes R.J., Moore R.J. Microbiota of the chicken gastrointestinal tract: influence on health, productivity and disease. Appl. Microbiol. Biotechnol. 2014;98:4301–4310. doi: 10.1007/s00253-014-5646-2. [DOI] [PubMed] [Google Scholar]
- Svihus B. Function of the digestive system. J. Appl. Poult. Res. 2014;23:306–314. [Google Scholar]
- Tahir M., Cervantes H., Farmer C.W., Shim M.Y., Pesti G.M. Broiler performance, hatching egg, and age relationships of progeny from standard and dwarf broiler dams. Poult. Sci. 2011;90:1364–1370. doi: 10.3382/ps.2010-01165. [DOI] [PubMed] [Google Scholar]
- Thibodeau A., Fravalo P., Yergeau E., Arsenault J., Lahaye L., Letellier A. Chicken Caecal Microbiome modifications induced by campylobacter Jejuni colonization and by a non-antibiotic feed additive. PLoS One. 2015;10 doi: 10.1371/journal.pone.0131978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Rodriguez A., Donoghue A.M., Donoghue D.J., Barton J.T., Tellez G., Hargis B.M. Performance and condemnation rate analysis of commercial turkey flocks treated with a Lactobacillus spp.-based probiotic. Poult. Sci. 2007;86:444–446. doi: 10.1093/ps/86.3.444. [DOI] [PubMed] [Google Scholar]
- Tortuero F. Influence of the implantation of Lactobacillus acidophilus in chicks on the growth, feed conversion, malabsorption of fats syndrome and intestinal flora. Poult. Sci. 1973;52:197–203. doi: 10.3382/ps.0520197. [DOI] [PubMed] [Google Scholar]
- Triplett M.D., Zhai W., Peebles E.D., McDaniel C.D., Kiess A.S. Investigating commercial in ovo technology as a strategy for introducing probiotic bacteria to broiler embryos. Poult. Sci. 2018;97:658–666. doi: 10.3382/ps/pex317. [DOI] [PubMed] [Google Scholar]
- Uni, Z.; Ferket, P. 2003. Enhancement of development of oviparous species by in ovo feeding of enteric modulators. Google Patents. United States Patent US 6:592–878.
- Untoo M., Banday I.M., Khurshid A., Banday M., Afzal I., Adil S., Baba I. Potential of probiotics in poultry production. entomoljournal.com. 2018;6:1293–1300. [Google Scholar]
- Van Boeckel T.P., Pires J., Silvester R., Zhao C., Song J., Criscuolo N.G., Gilbert M., Bonhoeffer S., Laxminarayan R. Global trends in antimicrobial resistance in animals in low- and middle-income countries. Science. 2019;365:eaaw1944. doi: 10.1126/science.aaw1944. [DOI] [PubMed] [Google Scholar]
- Van Den Bogaard A.E., Stobberingh E.E. Epidemiology of resistance to antibiotics: links between animals and humans. Int. J. Antimicrob. Agents. 2000;14:327–335. doi: 10.1016/s0924-8579(00)00145-x. [DOI] [PubMed] [Google Scholar]
- Vieco-Saiz N., Belguesmia Y., Raspoet R., Auclair E., Gancel F., Kempf I., Drider D. Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Front. Microbiol. 2019;10:57. doi: 10.3389/fmicb.2019.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveros A., Chamorro S., Pizarro M., Arija I., Centeno C., Brenes A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult. Sci. 2011;90:566–578. doi: 10.3382/ps.2010-00889. [DOI] [PubMed] [Google Scholar]
- Walters H.G., Jasek A., Campbell J.M., Coufal C., Lee J.T. Evaluation of spray-dried plasma in broiler diets with or without bacitracin methylene disalicylate. J. Appl. Poult. Res. 2019;28:364–373. [Google Scholar]
- Wang Y., Sun J., Zhong H., Li N., Xu H., Zhu Q., Liu Y. Effect of probiotics on the meat flavour and gut microbiota of chicken. Sci. Rep. 2017;7:1–3. doi: 10.1038/s41598-017-06677-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson H.R. Interrelationships of egg size, chick size, posthatching growth and hatchability. Worlds. Poult. Sci. J. 1991;47:5–20. [Google Scholar]
- Wu Y., Zhen W., Geng Y., Wang Z., Guo Y. Effects of dietary Enterococcus faecium NCIMB 11181 supplementation on growth performance and cellular and humoral immune responses in broiler chickens. Poult. Sci. 2019;98:150–163. doi: 10.3382/ps/pey368. [DOI] [PubMed] [Google Scholar]
- Xiang Q., Wang C., Zhang H., Lai W., Wei H., Peng J. Effects of different probiotics on laying performance, egg quality, oxidative status, and gut health in laying hens. Animals. 2019;9:1110. doi: 10.3390/ani9121110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Iji P.A., Choct M. Dietary modulation of gut microflora in broiler chickens: a review of the role of six kinds of alternatives to in-feed antibiotics. Worlds. Poult. Sci. J. 2009;65:97. [Google Scholar]
- Yu L.C.H. Microbiota dysbiosis and barrier dysfunction in inflammatory bowel disease and colorectal cancers: exploring a common ground hypothesis. J. Biomed. Sci. 2018;25:1–14. doi: 10.1186/s12929-018-0483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeinali S., Ghazanfari S., Ebrahimi M.A. MUCIN2 gene expression in the chicken intestinal goblet cells are affected by dietary essential oils. Bulg. J. Agric. Sci. 2017;23:134–141. [Google Scholar]
- Zeng Q., He X., Puthiyakunnon S., Xiao H., Gong Z., Boddu S., Chen L., Tian H., Huang S.H., Cao H. Probiotic mixture golden Bifido prevents neonatal Escherichia coli K1 translocation via enhancing intestinal defense. Front. Microbiol. 2017;8:1798. doi: 10.3389/fmicb.2017.01798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X., Li Q., Yang C., Yu Y., Fu Z., Wang H., Fan X., Yue M., Xu Y. Effects of clostridium butyricum-and bacillus spp.-based potential probiotics on the growth performance, intestinal morphology, immune responses, and caecal microbiota in broilers. Antibiotics. 2021;10:624. doi: 10.3390/antibiotics10060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Wang H., Zhang J., Tang X., Raheem A., Wang M., Lin W., Liang L., Qi Y., Zhu Y., Jia Y., Cui S., Qin T. Modulatory effects of Bacillus subtilis on the performance, morphology, cecal microbiota and gut barrier function of laying hens. Animals. 2021;11:1523. doi: 10.3390/ani11061523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D., Liwinski T., Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.