Abstract
Background & Aims:
We conducted a meta-analysis to summarize rates of progression to and regression of nonalcoholic fatty liver (NAFL), nonalcoholic steatohepatitis (NASH), and fibrosis in adults with nonalcoholic fatty liver disease (NAFLD).
Methods:
We searched PubMed/Medline and 4 other databases from 1985 through 2020. We included observational studies and randomized controlled trials (RCTs) in any language which used liver biopsy or imaging to diagnose NAFLD in adults with a follow-up period ≥48 weeks. Rates were calculated as incident cases/100 person-years and pooled using the random-effects Poisson distribution model. Heterogeneity was assessed using the I2 statistic.
Results:
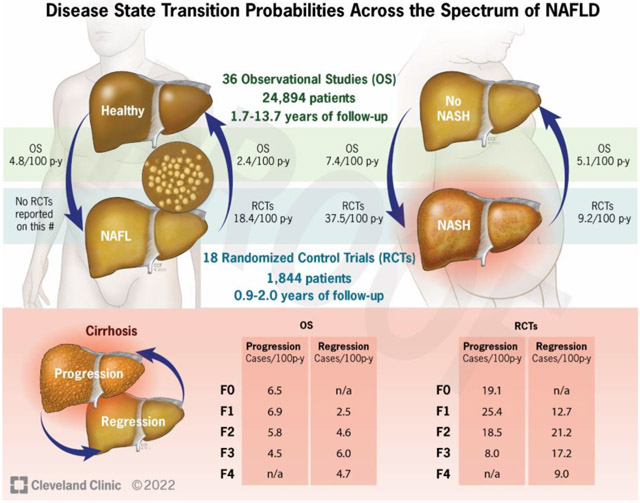
We screened 9,744 articles and included 54 studies involving 26,738 patients. Among observational studies, 20% of healthy adults developed NAFL (incidence rate=4.8/100 person-years) while 21% of people with fatty liver had resolution of NAFL (incidence rate=2.4/100 person-years) after a median of about 4.5 years. In addition, 31% of patients developed NASH after 4.7 years (incidence rate=7.4/100 person-years) whereas in 29% of those with NASH, resolution occurred after a median of 3.5 years (incidence rate=5.1/100 person-years). Time to progress by 1 fibrosis stage was 9.9, 10.3, 13.3 and 22.2 years for F0, F1, F2, and F3, respectively. Time to regress by 1 stage was 21.3, 12.5, 20.4, and 40.0 years for F4, F3, F2, and F1, respectively. Rates estimated from RCTs were higher than those from observational studies.
Conclusions:
In our meta-analysis, progression to NASH was more common than regression from NASH. Rates of fibrosis progression were similar across baseline stage, but patients with advanced fibrosis are more likely to regress than those with mild fibrosis.
Keywords: meta-analysis, simple steatosis, nonalcoholic steatohepatitis, fibrosis
Graphical Abstract
INTRODUCTION
Globally, one in four adults has non-alcoholic fatty liver disease (NAFLD), defined as the presence of ≥5% hepatic fat content without secondary causes such as excessive alcohol use, viral liver diseases, or drug use.1 Most patients have simple steatosis, or non-alcoholic fatty liver (NAFL). About 20% have non-alcoholic steatohepatitis (NASH), a more progressive condition that can lead to cirrhosis and advanced liver-related complications.2 Compared to patients with NAFL, those with NASH have more than 10 times higher incidence of hepatocellular carcinoma and mortality.1 With the rising prevalence of obesity and diabetes—both risk factors for NAFLD—NAFLD/NASH is expected to be the leading cause of liver transplant in the US in the coming decade.3
Understanding the natural process of progression and regression of NAFLD is important for early identification of high-risk patients and appropriate tailoring of disease prevention and control interventions. Longitudinal cohort studies have provided initial insights regarding the evolution of NAFLD over time.4–6 Previous systematic reviews demonstrated that patients with NASH progress more rapidly to advanced fibrosis than do those without.2,7 Also, little is known about the incidence of NAFL in otherwise healthy individuals or the rate of development of NASH from NAFL. Recently, a number of observational studies and randomized controlled trials (RCTs) of NAFLD treatments have been conducted and provide valuable information on the natural history of NAFLD.
Mathematical models including decision modeling are used to simulate the impact of different interventions on disease development and health outcomes to evaluate the comparative long-term benefits and cost-effectiveness of screening and treatment. Estimates of progression and regression rates for each stage must be incorporated to ensure accurate model projections.8,9 However, previous meta-analyses calculated fibrosis progression rates by subtracting the number of stages progressed by the number of stages regressed.2,7 To address these limitations, we conducted a systematic review and meta-analysis to describe the natural history of NAFLD, including both the progression to and regression of NAFL, NASH, and fibrosis. These data will help patient counseling, disease monitoring, and microsimulation modeling.
MATERIALS AND METHODS
We conducted this meta-analysis following the updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.10
Search strategy
A comprehensive search strategy with no language restriction was constructed to capture the relevant literature describing the natural history of NAFLD. The following databases were searched from 1985 through December 9, 2020: Ovid MEDLINE®, Ovid Embase, Scopus, Wiley’s Cochrane Central Register of Controlled Trials, and Web of Science’s Science Citation Index. We also manually searched for additional studies using reference lists from eligible studies and systematic reviews. The complete search strategies are provided in the Supplementary Methods and Appendix Table 1.
Selection criteria
We included studies that: 1) were RCTs or cohort studies; 2) included adult populations (≥18 years); 3) used liver biopsy and/or imaging to diagnose NAFLD at baseline and follow-up; 4) contained data to examine the progression to and/or regression of NAFL, NASH, or fibrosis; and 5) had a follow-up period of at least 48 weeks. We excluded editorials, letters to the editor, conference abstracts, cross-sectional or case-control studies, studies that combined adult and pediatric cohorts, used noninvasive means other than imaging to diagnose NAFLD, had insufficient data to estimate outcomes, only reported mean change of fibrosis stage for the entire cohort, and studies in which patients with other potential causes of fatty liver such as viral hepatitis and alcohol-related liver disease were not excluded. For RCTs, we included only patients in the placebo arm. We also excluded patients if they received intensive lifestyle modifications that could improve hepatic steatosis and other histological findings of NAFLD. When there were multiple studies using the same patient cohort, we included those reporting the most comprehensive data or having the longest follow-up period.
Data extraction
Titles and abstracts of identified studies were reviewed independently by J.Y.P. and L.Z. Full-texts were examined and pre-determined inclusion and exclusion criteria applied. Disagreement was resolved by discussion with a third investigator (P.L.).
Data extraction was also performed independently by J.Y.P and L.Z. using standardized form; P.L. resolved discrepancies. The following was extracted: 1) study characteristics: first author’s name, year of publication, geographic location of the study population, funding source, study design, data collection period, and data source; 2) patient characteristics: sample size, mean age, percent female, mean body mass index, percent with obesity, diabetes, hypertension, metabolic syndrome, and hyperlipidemia; 3) disease progression characteristics: duration of follow-up, diagnostic methods (liver biopsy or imaging), histologic staging criteria (for studies using liver biopsy) or cut-off values (for studies using imaging), attrition rate, proportion of patients having NAFL, NASH, and fibrosis by stage at baseline and follow-up, and proportion of patients whose disease progressed, regressed, or remained unchanged.
Outcomes
Outcomes included rates of progression to or regression of NAFL, NASH, and fibrosis, measured by pooled incidence rates. Progression to or regression of NAFL was based on a priori author-defined criteria (Appendix Table 2). Progression to NASH was defined as developing definite NASH for those with no or borderline NASH at baseline. Regression of NASH was defined as resolution of NASH to borderline or no NASH. Borderline NASH and NASH were based on author-defined criteria (Appendix Table 3). We considered patients to have NASH progression or regression only if development or resolution of NASH was explicitly stated. Fibrosis progression was defined as an increase of ≥1 point and regression as a reduction of ≥1 point on fibrosis score. Among studies using liver biopsies, we used studyspecific fibrosis scores, based on either NASH Clinical Research Network (CRN) or Brunt scoring systems.11 For one study that used METAVIR scoring system12 we converted its 7 stages into 5— equivalent to NASH CRN and Brunt systems for uniform inclusion in the meta-analysis—as follows: METAVIR stage 0 was stage 0, METAVIR stage 1 or 2 was stage 1, METAVIR stage 3 was stage 2, METAVIR stage 4 or 5 was stage 3, and METAVIR stage 6 was stage 4. For imaging studies, progression/regression were based on author-defined cut-offs.
Assessment of risk of bias
Risk of bias of in the studies that were included was identified using the most recent Cochrane tools for non-randomized studies13 and randomized trials.14 Details are described in Supplementary Methods.
Statistical analysis
We reported incidences for RCTs and observational studies separately. Incidences of progression and regression were calculated as number of incident cases divided by person-years. If studies did not report person-years, we calculated them as the total number of patients multiplied by mean follow-up duration (in years), accounting only for patients who had outcome information at follow-up. For fibrosis, we reported incidences based on both cases and stages, stratified by baseline fibrosis stage and NASH versus NAFL. Incident cases equaled number of patients with fibrosis progressing/regressing at follow-up. Incident stages were calculated as number of stages changed at follow-up. Incidences and 95% confidence intervals (CI) were then presented as cases (or stages) per 100 person-years and pooled using the random-effects Poisson distribution model.15 The years required to progress/regress by one stage was calculated as the reciprocal of incidence. Heterogeneity between studies was assessed using the I2 statistic, with <33%, 33–66%, ≥67% representing low, moderate, and high heterogeneity, respectively. We also conducted pre-planned subgroup analyses by geographic location (country- or continent-specific), diagnostic method (liver biopsy vs. imaging), and length of follow-up (<5 years vs. ≥5 years). We constructed funnel plots to assess publication bias. A p-value of <0.05 was considered statistically significant. All analyses were conducted in R v.4.1.2 (Vienna, Austria) using metafor package.
RESULTS
Study identification
A total of 14,387 publications were imported into Covidence™ software (Melbourne, Australia); 4,644 exact duplicates were removed. We identified one additional study through manual search,16 making a total of 9,744 studies for title and abstract screening. Full-texts of 213 studies were retrieved and assessed for eligibility. Of included studies, we contacted the corresponding authors of 5 studies that reported the overall distribution of fibrosis by stage at baseline and/or follow-up without details on the individual patient change (Appendix Table 4). Two authors responded; one provided usable data.17 The remaining four studies were, therefore, excluded. We contacted the corresponding authors of 11 additional studies for further information on the progression and regression of fibrosis but received either no response or no usable data.18–28 However, these 11 studies were eligible due to having information on NAFL and/or NASH. Therefore, full-text review resulted in 60 eligible studies. We further excluded six studies due to repeated use of the same data (Appendix Table 5). Ultimately, 54 studies were included with 18 being RCTs and 36 observational studies. One study was in Spanish;12 the rest were in English. Details on the identification process and exclusions are in Figure 1.
Figure 1.

PRISMA Flow Diagram of study selection
Study characteristics
There were 18 RCTs enrolling 1,844 patients in placebo groups with a median duration of 1.0 year (range: 0.9–2.0 years) and 36 observational studies following 24,894 patients over a median of 4.7 years (range: 1.7–13.7 years) (Table 1). Four-fifths were published after 2010. Seven studies (13%) were conducted in a multinational setting, 11 (20%) in North America (all in USA), 2 (4%) in South America, 11 (20%) in Europe, 22 (41%) in Asia, and 1 (2%) in Israel. All but one RCT and 70% of observational studies used liver biopsy. Sixteen studies (31%) contained data on progression to/regression of NAFL, 28 studies (52%) on NASH, and 31 (57%) studies on fibrosis. Four studies (7%) had data on all 3 conditions. The median age of the patients was 51 years (range: 40–68), 50% (range: 17–100%) were male, and median BMI was 29.5 kg/m2 (range: 21.9–37.7 kg/m2) (Appendix Table 6). Nearly half the patients had hypertension or diabetes mellitus (median: 50% and 48%, respectively).
Table 1.
Characteristics of included studies
| Author, Year | Location | Study Period | Sample Size | Diagnostic Method (scoring system) | Duration of follow-up (mean; total person-years) | Reported data for NAFLD-related condition (Y, N) | ||
|---|---|---|---|---|---|---|---|---|
| Simple Steatosis | NASH | Fibrosis | ||||||
| A. Randomized Controlled Trials | ||||||||
| Armstrong, 201654 | UK | 2010–2014 | 22 | Biopsy (Brunt) | 0.9; 20.3 | N | Y | Y |
| Bril, 201955 | USA | 2010–2016 | 32 | Biopsy (Brunt) | 1.5; 48 | N | Y | N |
| Chan, 201756 | Malaysia | 2012–2015 | 50 | Biopsy (NASH CRN) | 0.9; 46.2 | N | Y | Y |
| Friedman, 201819 | Multinational | 2014–2016 | 126 | Biopsy (NASH CRN) | 1; 126 | N | Y | Y |
| Harrison, 201829 | Multinational | 2012–2016 | 159 | Biopsy (NASH CRN) | 1.8; 293.5 | N | Y | Y |
| Harrison, 201920 | USA | 2016–2019 | 74 | Biopsy (NASH CRN) | 1; 74 | N | Y | N |
| Harrison, 202017 | Multinational | 2016–2019 | 105 | Biopsy (NASH CRN) | 1.4; 145.4 | N | Y | Y |
| Harrison, 202030 | Multinational | 2017–2019 | 331 | Biopsy (NASH CRN) & FibroScan | 0.9; 305.5 | N | Y | Y |
| Loguercio, 201231 | Italy | 2005–2008 | 53 | Biopsy (Brunt) | 1; 53 | N | N | Y |
| Neuschwander-Tetri, 201532 | USA | 2011–2014 | 142 | Biopsy (NASH CRN) | 1.4; 196.6 | Y | Y | Y |
| Newsome, 202057 | UK | 2016–2020 | 80 | Biopsy (Brunt) | 1.4; 110.4 | N | Y | Y |
| Ratziu, 201622 | Multinational | 2012–2015 | 92 | Biopsy (NASH CRN) | 1; 92 | N | Y | N |
| Ratziu, 202023 | Multinational | 2014–2017 | 72 | Biopsy (NASH CRN) | 2; 144 | N | N | Y |
| Sanyal, 201025 | USA | 2005–2009 | 83 | Biopsy (NASH CRN) | 1.8; 132.9 | N | Y | N |
| VanWagner, 201158 | USA | 2005–2009 | 9 | Biopsy (Brunt) | 1; 9 | N | Y | N |
| Wong, 201327 | HK, China | 2009–2012 | 77 | H-MRS | 1; 77 | Y | N | N |
| Younossi, 201928 | Multinational | 2015–2018 | 311 | Biopsy (NASH CRN) | 1.5; 466.5 | N | Y | N |
| Zein, 201159 | USA | 2006–2010 | 26 | Biopsy (Brunt) | 1; 26 | N | Y | N |
| B. Observational Studies | ||||||||
| Ajmera, 201818 | USA | 2009–2017 | 95 | Biopsy (NASH CRN) | 1.7; 161.5 | N | Y | N |
| Akuta, 201652 | Japan | 1980–2016 | 36 | Biopsy (Brunt) | 4.6; 165.6 | N | N | Y |
| Castro, 201933 | Brazil | 2005–2011 | 39 | Biopsy (NASH CRN) & Fibroscan | 10; 390 | N | N | Y |
| Chan, 201460 | Malaysia | 2003–2010 | 35 | Biopsy (NASH CRN) | 6.4; 224 | N | Y | Y |
| Cho, 201961 | Korea | 2000–2010 | 2726 | Ultrasound | 5.2; 14129.8 | Y | N | N |
| Ekstedt, 20065 | Sweden | 1988–2005 | 68 | Biopsy (Brunt) | 13.7; 938.4 | N | N | Y |
| Evans, 200234 | UK | 1985–1999 | 46 | Biopsy (Brunt) | 8.2; 377.2 | N | N | Y |
| Fassio, 200435 | Argentina | 1986–2002 | 22 | Biopsy (Brunt) | 4.3; 94.6 | N | N | Y |
| Hamaguchi, 201062 | Japan | 1997–2008 | 39 | Biopsy (Brunt) | 2.4; 93.6 | Y | Y | Y |
| Harrison, 200321 | USA | 1985–2001 | 22 | Biopsy (Brunt) | 5.7; 125.4 | N | N | Y |
| Hui, 200536 | HK, China | 1996–2004 | 17 | Biopsy (Brunt) | 5.8; 97.8 | N | N | Y |
| Kamarajah, 201844 | Malaysia | 2012–2017 | 113 | Biopsy (NASH CRN) & Fibroscan | 3.1; 348.4 | N | N | Y |
| Kim, 200937 | South Korea | 2000–2005 | 2895 | Ultrasound | 5; 14475 | Y | N | N |
| Kim, 201842 | South Korea | 2007–2013 | 956 | Ultrasound | 4.6; 4397.6 | Y | N | N |
| Kleiner, 201946 | USA | 2004–2013 | 446 | Biopsy (NASH CRN) | 4.9; 2185.4 | Y | Y | Y |
| Lee, 201043 | South Korea | 2004–2007 | 1705 | Ultrasound | 2.2; 3716 | Y | N | N |
| Lin, 201938 | Taiwan | 2001–2010 | 10 | Biopsy (NASH CRN) | 2.4; 23.9 | N | N | Y |
| McPherson, 201563 | UK | 1991–2011 | 108 | Biopsy (NASH CRN) | 6.6; 712.8 | N | Y | Y |
| Moreno Sanchez, 199112 | Spain | 1980–1985 | 10 | Biopsy (METAVIR) | 4.8; 48.3 | N | N | Y |
| Nasr, 201839 | Sweden | 1988–2015 | 32 | Biopsy (NASH CRN) | 9.1; 291.3 | N | N | Y |
| Nogami, 201964 | Japan | 2006–2017 | 34 | Biopsy (NASH CRN) | 10; 340 | N | N | Y |
| Pais, 201140 | France | 1998–2009 | 6 | Biopsy (Kleiner) | 4.8; 28.8 | N | Y | Y |
| Pais, 201365 | France | 1998–2009 | 70 | Biopsy (Brunt) | 3.4; 238 | N | Y | Y |
| Reddy, 202024 | USA | 2006–2016 | 36 | Biopsy (NASH CRN) | 3.8; 136.8 | N | Y | N |
| Seko, 201566 | Japan | 1999–2014 | 52 | Biopsy (NASH CRN) | 2.7; 137.8 | N | Y | Y |
| Seo, 201567 | South Korea | 2006–2009 | 1916 | Ultrasound | 4.2; 8047.2 | Y | N | N |
| Shen, 201226 | HK, China | 2004–2010 | 51 | Biopsy (NASH CRN) | 3; 153 | N | Y | N |
| Teli, 199541 | UK | 1978–1994 | 40 | Biopsy (defined in study) | 8.6; 345.7 | Y | Y | Y |
| Vilar-Gomez, 202068 | USA | 2004–2016 | 90 | Biopsy (NASH CRN) | 5.6; 504 | N | Y | N |
| Wang, 201869 | China | 2006–2014 | 6310 | Ultrasound | 7; 44170 | N | N | N |
| Wang, 202070 | China | 2010–2015 | 4273 | Ultrasound | 4.4; 18801.2 | Y | N | N |
| Wong, 201071 | HK, China | 2006–2009 | 52 | Biopsy (Brunt) | 3; 156 | N | Y | Y |
| Wong, 201516 | HK, China | 2008–2013 | 565 | H-MRS & FibroScan | 3.9; 2212.9 | Y | N | N |
| Zelber-Sagi, 201472 | Israel | 2003–2011 | 147 | Ultrasound | 6.8; 999.6 | Y | N | N |
| Zhang, 201973 | China | 2011–2014 | 1325 | Ultrasound | 3; 3975 | Y | N | N |
| Zhou, 201274 | China | 2005–2009 | 507 | Ultrasound | 4; 2028 | Y | N | N |
Note: HK, Hong Kong; H-MRS, Proton Magnetic Resonance Spectroscopy; NAFLD, Non-alcoholic Fatty Liver Disease; NASH, Non-alcoholic Steatohepatitis; NASH CRN, Non-alcoholic Steatohepatitis Clinical Research Network; N, no; Y, yes
Assessment of study’s risk of bias
Among RCTs, most or all had “low” risk of bias in 4 of 5 domains, the exception being bias due to missing outcomes (Appendix Figure 1). Five studies were at “high” risk of bias because their attrition rate exceeded 20%.28–32
Among observational studies, none had “critical” risk of bias in any domain, but a large percentage of studies (~70%–80%) had “moderate” or “serious” risk for confounding, participant selection, or outcome measurement (Appendix Figure 2). Among 15 studies at “serious” risk of bias in ≥1 domain, twelve were in the confounding domain due to not stratifying their analysis by patient characteristics or not conducting multivariable analysis.12,21,24,33–41 Six were in the participant domain for including <25 patients.12,21,35,36,38,40 One was in the missing data domain because authors did not clearly state the number (and reasons) for loss-to-follow up, had 20% or more of patients lost to follow-up, or missing duration of follow-up.42 Finally, five were in the outcome measurement domain because mean follow-up duration was <2 years or identification of outcomes by pathologists or radiologists were not clearly stated.12,18,33,38,43 All studies had “low” risk of bias in selection of reported results and “low” or “moderate” risk in classification of study population.
Progression to and regression of NAFL
Sixteen studies reported rates of progression to or regression from NAFL. One-fourth used liver biopsy involving 667 patients and the others used imaging (ultrasound, H-MRS) involving 24,727 patients. No RCTs reported progression while two reported regression (pooled incidence=18.4/100 person-years). Among observational studies, 3,871/19,323 (20%) patients with no NAFL at baseline developed NAFL after a median of 4.4 years. In contrast, 892/4,295 patients (21%) with NAFL regressed after a median of 4.6 years. Pooled incidence was 4.8/100 person-years (95%CI: 3.7–6.3) for progression (I2 = 98.43%) and 2.4/100 person-years (95%CI: 1.2–5.0) for regression (I2 = 98.64%) (Figure 2). Heterogeneity was high for observational studies and zero for RCTs.
Figure 2:
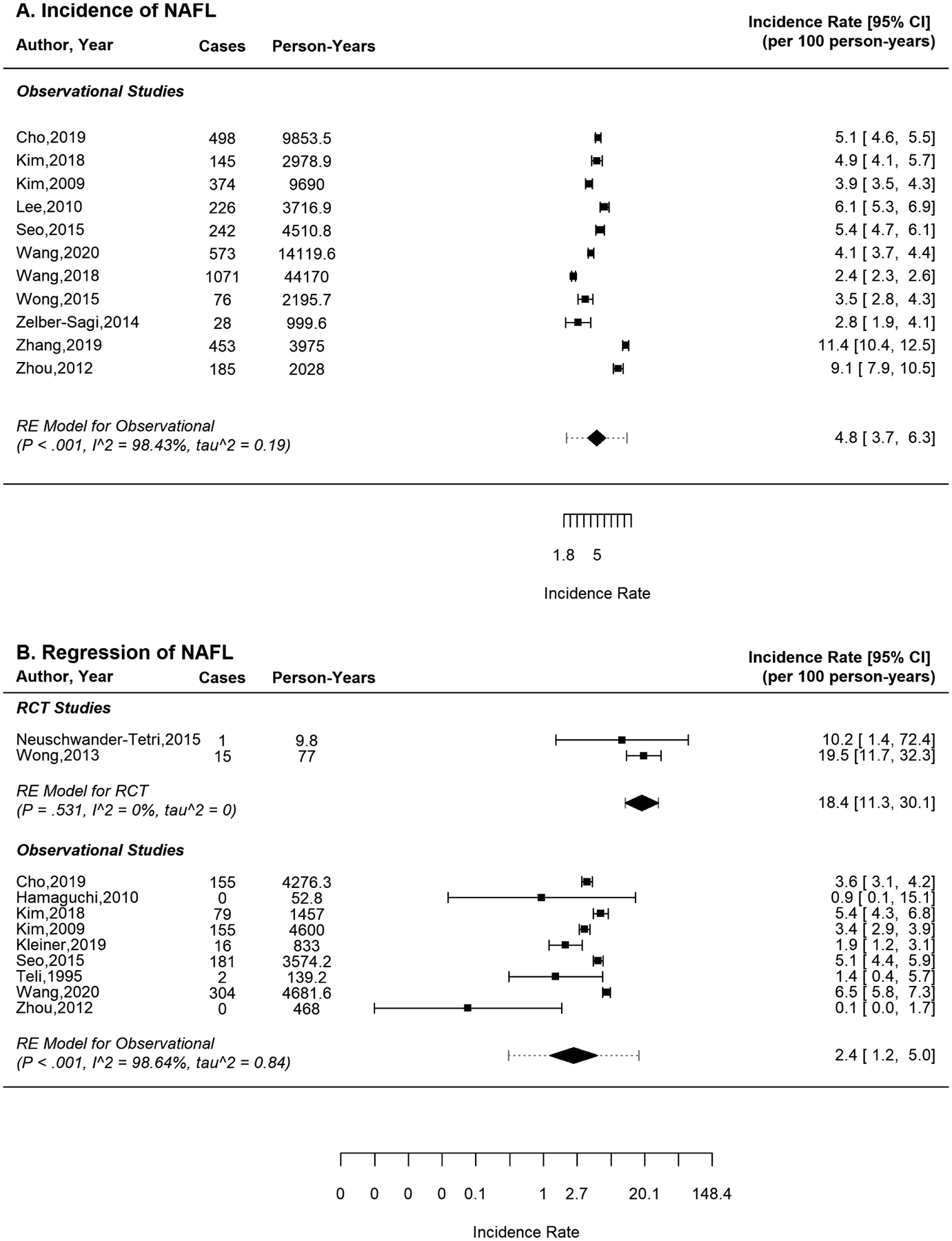
Pooled incidence rate (cases per 100 person-years) of a) progression to and b) regression of nonalcoholic fatty liver (NAFL) or simple steatosis
Progression to and regression of NASH
Among 28 studies in patients with NASH, 12 provided data on progression, and 25 on regression. All studies used liver biopsy. Two RCTs reported progression; pooled incidence was 37.5/100 person-years. In 15 RCTs reporting regression, 165/1,511 (11%) patients resolved NASH after a median of 1.4 years; pooled incidence was 9.2/100 persons-years (95% CI: 6.6–12.9). Among observational studies, 116/372 (31%) patients developed NASH over a median of 4.7 years (range: 2.4–11.6 years). In addition, 189/661 (29%) patients with NASH resolved at follow-up. Pooled incidence was 7.4 and 5.1/100 person-years for progression and regression, respectively (Figure 3). Heterogeneity was high (I2 >67%).
Figure 3:

Pooled incidence rate (cases per 100 person-years) of a) progression to and b) regression of nonalcoholic steatohepatitis (NASH)
Progression to and regression of fibrosis
Twenty-eight studies used liver biopsy alone; 3 used both biopsy and FibroScan® to stage fibrosis. Of the three studies that used both Fibroscan and liver biopsy, two used biopsy and FibroScan® at both baseline and follow-up, but only biopsy data were included.30,44 The third study reported only FibroScan® at follow-up, so we included FibroScan data.33 We excluded one study which used FibroScan® to identify advanced fibrosis or cirrhosis but did not define the fibrosis stage.16
In RCTs, 19/104 (18%) patients with no fibrosis progressed: 84% to F1 and 16% to F2. Among 89 patients with F1, 28 (31%) progressed, 14 (16%) regressed, and 47 (53%) remained stable. From F2, nearly half progressed or regressed, while the others remained stable. From F3, 22% regressed while 13% progressed to cirrhosis. Finally, from F4, 13% of patients regressed to bridging fibrosis. Pooled incidence of progression from F0, F1, F2, and F3 were 19.1, 25.4, 18.5, and 8.0/100 person-years, respectively (Table 2; Appendix Figure 3). Pooled incidence for regression from F1, F2, F3, and F4 were 12.7, 21.2, 17.2, and 9.0/100 person-years, respectively (Appendix Figure 4). Heterogeneity was low or moderate. No RCTs reported data separately for patients with NAFL.
Table 2:
Pooled incidences of fibrosis progression and regression among randomized controlled trials
| Baseline fibrosis stage | No. of studies/patients | Person-years of follow up | Progression | Regression | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of cases | Incidence rate (cases/100 person-years) | No. of stages | Incidence rate (stages/100 person-years) | Time to progress by 1 stage (years) | No. of cases | Incidence rate (cases/100 person-years) | No. of stages | Incidence rate (stages/100 person-years) | Time to regress by 1 stage (years) | |||
| NAFLD | ||||||||||||
| F0 | 4/104 | 108.9 | 19 | 19.1 (9.4–39.1) | 22 | 23.4 (10.9–50.3) | 4.3 (2.0–9.2) | NA | NA | NA | NA | NA |
| F1 | 5/89 | 109.9 | 28 | 25.4 (13.4–48.1) | 37 | 34.0 (19.8–58.4) | 2.9 (1.7–5.1) | 14 | 12.7 (7.5–21.5) | 14 | 12.7 (7.5–21.5) | 7.9 (4.7–13.3) |
| F2 | 5/111 | 146.3 | 27 | 18.5 (12.7–26.9) | 30 | 20.3 (13.4–30.7) | 4.9 (3.3–7.5) | 31 | 21.2 (14.9–30.1) | 36 | 24.6 (17.8–34.1) | 4.1 (2.9–5.6) |
| F3 | 10/423 | 576.8 | 54 | 8.0 (4.5–14.0) | 54 | 8.0 (4.5–14.0) | 12.5 (7.1–22.2) | 94 | 17.2 (12.9–22.9) | 132 | 24.0 (17.4–33.0) | 4.2 (3.0–5.7) |
| F4 | 4/252 | 370.2 | NA | NA | NA | NA | NA | 34 | 9.0 (4.6–17.9) | 34 | 9.0 (4.6–17.9) | 11.1 (5.6–21.7) |
| NASH | ||||||||||||
| F0 | 3/29 | 33.9 | 10 | 29.4 (14.2–60.8) | 13 | 38.4 (22.3–66.1) | 2.6 (1.5–4.5) | NA | NA | NA | NA | NA |
| F1 | 5/89 | 109.9 | 28 | 25.4 (13.4–48.1) | 37 | 34.0 (19.8–58.4) | 2.9 (1.7–5.1) | 14 | 12.7 (7.5–21.5) | 14 | 12.7 (7.5–21.5) | 7.9 (4.7–13.3) |
| F2 | 4/94 | 129.3 | 27 | 20.9 (14.3–30.5) | 30 | 23.2 (16.2–33.2) | 4.3 (3.0–6.2) | 23 | 17.8 (11.8–26.8) | 28 | 21.7 (15.0–31.4) | 4.6 (3.2–6.7) |
| F3 | 9/415 | 568.8 | 54 | 8.4 (4.9–14.3) | 54 | 8.4 (4.9–14.3) | 11.9 (7.0–20.4) | 94 | 17.7 (13.2–23.6) | 132 | 25.0 (18.3–34.3) | 4.0 (2.9–5.5) |
| F4 | 4/252 | 370.2 | NA | NA | NA | NA | NA | 34 | 9.0 (4.6–17.9) | 34 | 9.0 (4.6–17.9) | 11.1 (5.6–21.7) |
Note: No meta-analysis was possible for patients with NAFL because no randomized controlled trials provided data separately for patients with versus without NASH. NAFLD, Non-alcoholic Fatty Liver Disease; NAFL, Non-alcoholic Fatty Liver; NASH, Non-alcoholic Steatohepatitis.
In observational studies, 123/297 (41%) patients progressed from F0 after a median of 5.8 years: 58% to F1, 28% to F2, 12% to F3, and 2% to F4. Among patients with F1, 23% regressed to F0 while 37% progressed to higher stages (59% to F2, 34% to F3, and 7% to F4). In patients with F2, a similar proportion progressed, regressed, or remained stable (32%, 37%, and 31%, respectively) after 5 years. Patients with F3 regressed more often than progressed, 32% vs. 22%, while a larger proportion of patients in the observational studies with stage F4 regressed than noted in RCTs (29% vs. 13%). Pooled incidence of progression from F0, F1, F2, and F3 were 6.5, 6.9, 5.8, and 4.5/100 person-years, respectively (Table 3). Pooled incidence for regression from F1, F2, F3, and F4 were 2.5, 4.6, 6.0, and 4.7/100 person-years, respectively. In addition, patients with NAFL tended to have lower incidence of progression than those with NASH although no tests for subgroup difference were statistically significant. Forest plots of incidence calculated as stages/100 person-years are in Supplementary Results and Appendix Figures 5–6.
Table 3:
Pooled incidences of fibrosis progression and regression among observational studies
| Baseline fibrosis stage | No. of studies/patients | Person-years of follow up | Progression | Regression | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of cases | Incidence rate (cases/100 person-years) | No. of stages | Incidence rate (stages/100 person-years) | Time to progress by 1 stage (years) | No. of cases | Incidence rate (cases/100 person-years) | No. of stages | Incidence rate (stages/100 person-years) | Time to regress by 1 stage (years) | |||
| NAFLD | ||||||||||||
| F0 | 18/297 | 1965.5 | 123 | 6.5 (4.6–9.0) | 196 | 10.0 (7.7–13.2) | 9.9 (7.6–13.0) | NA | NA | NA | NA | NA |
| F1 | 19/385 | 2110.0 | 143 | 6.9 (4.8–10.0) | 212 | 9.7 (6.8–13.7) | 10.3 (7.3–14.7) | 90 | 2.5 (1.4–4.6) | 90 | 2.5 (1.4–4.6) | 40.0 (21.7–71.4) |
| F2 | 19/219 | 1224.4 | 71 | 5.8 (4.6–7.3) | 80 | 7.5 (6.1–9.2) | 13.3 (10.9–16.4) | 92 | 4.6 (2.4–8.8) | 106 | 4.9 (2.3–10.5) | 20.4 (9.5–43.5) |
| F3 | 14/202 | 1009.3 | 45 | 4.5 (3.3–6.0) | 45 | 4.5 (3.3–6.0) | 22.2 (16.7–30.3) | 64 | 6.0 (3.7–9.6) | 101 | 8.0 (4.6–13.8) | 12.5 (7.2–21.7) |
| F4 | 9/41 | 230.7 | NA | NA | NA | NA | NA | 12 | 4.7 (2.1–10.8) | 12 | 4.7 (2.1–10.8) | 21.3 (9.3–47.6) |
| NAFL | ||||||||||||
| F0 | 7/72 | 418.8 | 23 | 6.1 (3.1–11.80 | 34 | 8.6 (4.8–15.5) | 11.6 (6.5–20.8) | NA | NA | NA | NA | NA |
| F1 | 5/58 | 216.2 | 27 | 12.2 (7.6–19.7) | 39 | 17.6 (11.3–27.4) | 5.7 (3.6–8.8) | 6 | 2.6 (0.9–7.3) | 6 | 2.6 (0.9–7.3) | 38.5 (13.7–111.1) |
| F2 | 4/18 | 63.8 | 3 | 4.7 (1.5–14.6) | 4 | 6.3 (2.4–16.7) | 15.9 (6.0–41.7) | 8 | 12.5 (6.0–25.9) | 10 | 14.2 (5.1–39.7) | 7.0 (12.5–19.6) |
| F3* | 1/11 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| F4* | 1/11 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| NASH | ||||||||||||
| F0 | 9/41 | 255.7 | 20 | 8.2 (4.9–13.7) | 30 | 11.9 (8.0–17.8) | 8.4 (5.6–12.5) | NA | NA | NA | NA | NA |
| F1 | 12/120 | 645.3 | 50 | 7.8 (5.7–10.7) | 72 | 10.9 (8.1–14.8) | 9.2 (6.8–12.3) | 19 | 1.5 (0.4–6.2) | 19 | 1.5 (0.4–6.2) | 66.7 (16.1–250.0) |
| F2 | 12/92 | 522.6 | 35 | 6.7 (4.8–9.3) | 44 | 8.4 (6.3–11.3) | 11.9 (8.8–15.9) | 20 | 3.7 (1.0–8.8) | 29 | 4.5 (1.6–12.6) | 22.2 (7.9–62.5) |
| F3 | 8/52 | 256.8 | 13 | 5.1 (2.9–8.7) | 13 | 5.1 (2.9–8.7) | 19.6 (11.5–34.5) | 18 | 5.9 (1.8–19.0) | 23 | 6.3 (1.6–24.6) | 15.9 (4.1–62.5) |
| F4 | 4/11 | 69 | NA | NA | NA | NA | NA | 1 | 1.2 (0.1–24.0) | 1 | 1.2 (0.1–24.0) | 83.3 (4.2–1000.0) |
Note: NAFLD, Non-alcoholic Fatty Liver Disease; NAFL, Non-alcoholic Fatty Liver; NASH, Non-alcoholic Steatohepatitis.
No meta-analysis was possible because there was only one study providing data on patients with NAFL and F3/F4 at baseline.52
Sensitivity analyses
Rates of NASH progression/regression were lower in observational studies with ≥5 years of follow-up or conducted in USA/Europe than studies with <5 years or conducted in Asia, respectively (Appendix Tables 9&10). Fibrosis progression rates in patients with F0/F1 and regression rates in all patients were also lower in studies with longer than shorter follow-up (Appendix Tables 11&12). Detailed subgroup analyses are included in Supplementary Results and Appendix Tables 7–18. Publication bias was not observed (Appendix Figures 7–12).
DISCUSSION
In this systematic review and meta-analysis of 54 studies, we provide a comprehensive assessment of the natural history of NAFLD, ranging from NAFL to NASH and fibrosis. We found that among observational studies, one in every 20 otherwise healthy adults develop NAFL in a year. Interestingly, 10 of 11 studies were conducted in Asia with a pooled rate of 5.1/100 person-years (95%CI: 3.9–6.6) which was similar to that reported in an earlier meta-analysis.1 Our pooled incidence was >10 times higher than that reported by a population-based study of Olmsted County, Minnesota, USA,45 but that study used ICD codes to identify NAFL instead of ultrasound, as was used in the studies we reviewed.
Novel findings from the present study include the meta-analysis based estimate that after developing fatty liver, 2.4% of patients in observational studies have resolution of NAFL each year. These patients were considered to receive no conventional treatment for NAFLD although they might undergo partial lifestyle modifications or change their behaviors during the observational period. Similar estimates for RCTs was not possible due to the limited sample size. Of note, regression rate in observational studies using liver biopsy was nearly half of that in studies using imaging. Patients who had a liver biopsy are likely to have more severe NAFLD-related conditions than those receiving imaging with a lower probability of regression in biopsied patients. Of the three studies using liver biopsy, one was a registry of patients from eight large US medical centers.46 In addition, because ultrasound is only able to reliably detect >33% hepatic steatosis, patients with a reduction between 5–33% steatosis instead of <5% might have been classified as improved, overestimating the regression rate.47,48 At the same time, patients without NAFL who developed 5–33% steatosis might have been missed by ultrasound, underestimating the progression rate. Ultrasound is less sensitive in obese patients, reducing the accuracy of the diagnosis and severity of NAFLD in this population.49 Also, ultrasound provides strata (<5, 5–33, 33–66, >66%) but given the interobserver variability and the challenge to accurately quantify changes, studies using ultrasound alone may have limitations. Therefore, better quality data are needed on development and resolution of NAFL, especially outside of Asia where the majority of published studies were conducted.
Another important finding was that annually, about 7% of NAFL patients developed NASH and 5% of those with NASH had resolution in observational studies. Because the NAFLD activity score used to diagnose NASH does not include fibrosis as a component, and the included studies did not report NASH stratified by presence of fibrosis, we could not determine if baseline fibrosis stage affects NASH progression. Incidence and regression of NASH did not differ much by region but were 4–6 times higher in studies with shorter than longer follow-up. Compared to observational studies, rates of NASH progression and regression were higher in RCTs. One RCT enrolled patients with fibrosis20 and both RCTs required a NAFLD activity score (NAS) ≥4 with a score ≥1 in steatosis, ballooning, and lobular inflammation.20,32 Patients in these studies had NASH and may therefore experience a higher rate of progression than patients in observational studies. A recent meta-analysis estimated ~12% of placebo patients with NASH achieved NASH resolution without worsening of fibrosis, which differ only by older age.50
To estimate fibrosis progression and regression, we included 3 times as many studies as a previous meta-analysis.2 We found that rates of fibrosis progression and regression were much higher in RCTs than in observational studies. In observational studies, 59% of F0 patients remained stable and 6% progressed to bridging fibrosis or cirrhosis at follow-up compared to 82% and 0% in RCTs, respectively. On average, 7% of F0 progressed each year, primarily to stage 1 or 2. Patients with NASH progressed faster than those without, progressing from stage 0 in 8.4 years for NASH versus 11.6 years for NAFL, estimates that are similar to those reported by Singh et al.2 Because NASH patients can develop fibrosis at a more rapid rate than NAFL patients) and advanced fibrosis is associated with increased mortality,51 interventions to prevent patients with NASH from developing fibrosis are important. However, current RCTs have focused mostly on patients with fibrosis and high quality data are needed to determine the prevalence and natural course of NASH patients without fibrosis.
In observational studies, 22% of patients with F1/F2 progressed to F3/F4 with a higher proportion in those with F2 than F1. However, rates in patients with NASH were higher for F2 but lower for F1 than those without NASH. These findings suggest that baseline stage is important in fibrosis development, while the role of active NASH could not be confirmed in patients with F≥F1. Furthermore, 71% of F4 patients remained stable. Since we did not evaluate decompensated cirrhosis or other complications of cirrhosis, we may have overestimated the proportion of stable cirrhotic patients. We also could not estimate incidence among patients with NAFL and F3/F4 because only one study reported data separately for non-NASH patients.52 The overall incidence of fibrosis progression/regression for all NAFLD patients was comparable to that for NASH patients. It is, therefore, possible that most patients with significant fibrosis already had NASH at evaluation. In the NASH CRN registry, ~95% of patients with F3/F4 had definite or borderline NASH.6 Finally, we could not estimate fibrosis progression or regression for patients with NAFL in RCTs because no studies published to date provide these data.
Heterogeneity was moderate to high in most estimates for NAFL and NASH, consistent with previous meta-analyses.1,2,53 Potential reasons include differences in patient characteristics, variability in imaging or biopsy reading, and study design. Subgroup analyses helped minimize the heterogeneity. Importantly, RCTs had little or no heterogeneity compared to observational studies, especially for fibrosis estimates in patients with advanced stages at baseline. RCTs often have shorter duration and smaller sample size than observational studies. In our study, observational studies had a median follow-up that was 5 times longer and sample size that was 13 times larger than those in the RCTs. Also, patients enrolled in RCTs have more severe disease and are evaluated more frequently, with better outcome assessment than in observational studies.
Strengths of our analyses include the large number of studies evaluated that allows us to provide robust estimates of progression and regression of NAFL and NASH without treatment. In addition to examining fibrosis in patients with NAFL vs. NASH and by baseline fibrosis stage separately, we also distinguished rates of progression from those of regression using both cases and stages per 100 person-years. These data provide detailed information on the likelihood of progression/regression and magnitude of the potential change and allows us to estimate progression in people with F3/F4 that could not be identified by previous analyses.2,7
Limitations include our estimates being confounded by the differences among studies in patient characteristics, modality and quality of diagnosis, healthcare settings, or outcome assessment. We mitigated the confounding effect to some extent by including all high quality published data. Second, without access to patient-level data we could not estimate disease progression in high-risk groups such as patients with Hispanic ethnicity, obesity, diabetes, or metabolic syndrome, who might have a faster disease progression. Such analyses will require potentially depositing raw data into a repository to permit investigators to include patient-level data. Third, we used author-defined criteria for determining outcomes, and these varied across studies, leading to potentially inconsistent patient classification. These could also be overcome by the availability of raw data. Finally, because not all studies provided fibrosis data for patients with NAFL vs. NASH, our estimates for subgroups could be biased. However, despite these limitations, the inclusion of a large number of high quality studies allowed for robust analyses.
In conclusion, our meta-analysis provides the most comprehensive, up-to-date data on the natural history of NAFLD in adults. Understanding disease progression is important for stratifying high-risk patients for intervention versus monitoring. Our approach can help identify patients in need of aggressive and early treatment to prevent advanced complications, target them for RCTs, and allow evidence based prognosis with patients. Microsimulation studies will benefit from the detailed estimates provided in our study. Future studies on the natural history of NAFLD in children and other high-risk adults will complete our epidemiological understanding of the disease, which will effectively guide our clinical care efforts and improve patients’ quality-of-life.
Supplementary Material
WHAT YOU NEED TO KNOW.
Background:
Nonalcoholic fatty liver disease affects one-fourth of adults, but its natural history is not well characterized. Our study meta-analyzed rates of progression to and regression of simple steatosis, NASH, and fibrosis.
Findings:
One in 20 adults developed simple steatosis but only 2.4% regressed. Progression to NASH was more common than regression from it, whereas rates of fibrosis progression were similar across baseline stage.
Implications for Patient Care:
Because NASH patients can develop fibrosis more quickly than those with simple steatosis, interventions to prevent fibrosis progression in these patients are in urgent need.
Conflict of interest:
Dr. Alkhouri is a speaker for Echosens (makers of Fibroscan) and received research funding from Gilead, Intercept, Allergan, Cirius, Madrigal, and Genfit which was not related to this study. Dr. Deshpande is a consultant for Merck. All other authors reported no conflict of interest. Dr. Herman serves on a Data Safety Monitoring Board for Merck. Dr. Dasarathy is funded by grants NIH R01 GM119174; R01 DK113196; P50 AA024333; R01 AA021890; 3U01AA026976; U01 AA 026976; R56HL141744; U01 DK061732; 5U01 DK062470-17S2; R21 AR 071046 which are independent of the submitted work.
Financial support:
The study is funded by AHRQ R01HS026937
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. [DOI] [PubMed] [Google Scholar]
- 2.Singh S, Allen AM, Wang Z, et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13(4):643–654 e641–649; quiz e639–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong RJ, Singal AK. Trends in Liver Disease Etiology Among Adults Awaiting Liver Transplantation in the United States, 2014–2019. JAMA Netw Open. 2020;3(2):e1920294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42(1):132–138. [DOI] [PubMed] [Google Scholar]
- 5.Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865–873. [DOI] [PubMed] [Google Scholar]
- 6.Sanyal AJ, Van Natta ML, Clark J, et al. Prospective Study of Outcomes in Adults with Nonalcoholic Fatty Liver Disease. N Engl J Med. 2021;385(17):1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol. 2009;51(2):371–379. [DOI] [PubMed] [Google Scholar]
- 8.Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology (Baltimore, Md). 2016;64(5):1577–1586. [DOI] [PubMed] [Google Scholar]
- 9.Younossi ZM, Tampi R, Priyadarshini M, et al. Burden of Illness and Economic Model for Patients With Nonalcoholic Steatohepatitis in the United States. Hepatology. 2019;69(2):564–572. [DOI] [PubMed] [Google Scholar]
- 10.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. [DOI] [PubMed] [Google Scholar]
- 12.Moreno Sanchez D, Casis Herce B, Martin Algibez A, et al. [Non alcoholic steatohepatitis. Mid-term clinical and histologic course in 10 patients]. Medicina Clinica. 1991;96(19):733–736. [PubMed] [Google Scholar]
- 13.Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JP, Savovic J, Page MJ, Sterne JA, on behalf of the RoB2 Development Group. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2). 2019; https://www.riskofbias.info/welcome/rob-2-0-tool, Accessed on 4/5/2021.
- 15.Spittal MJ, Pirkis J, Gurrin LC. Meta-analysis of incidence rate data in the presence of zero events. BMC Med Res Methodol. 2015;15:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong VW, Wong GL, Yeung DK, et al. Incidence of non-alcoholic fatty liver disease in Hong Kong: a population study with paired proton-magnetic resonance spectroscopy. J Hepatol. 2015;62(1):182–189. [DOI] [PubMed] [Google Scholar]
- 17.Harrison SA, Goodman Z, Jabbar A, et al. A randomized, placebo-controlled trial of emricasan in patients with NASH and F1-F3 fibrosis. Journal of Hepatology. 2020;72(5):816–827. [DOI] [PubMed] [Google Scholar]
- 18.Ajmera V, Park CC, Caussy C, et al. Magnetic Resonance Imaging Proton Density Fat Fraction Associates With Progression of Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2018;155(2):307–310.e302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman SL, Ratziu V, Harrison SA, et al. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology (baltimore, md). 2018;67(5):1754–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison SA, Alkhouri N, Davison BA, et al. Insulin sensitizer MSDC-0602K in non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled phase IIb study. Journal of hepatology. 2019. [DOI] [PubMed] [Google Scholar]
- 21.Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. American Journal of Gastroenterology. 2003;98(9):2042–2047. [DOI] [PubMed] [Google Scholar]
- 22.Ratziu V, Harrison SA, Francque S, et al. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-α and -δ, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology. 2016;150(5):1147–1159.e1145. [DOI] [PubMed] [Google Scholar]
- 23.Ratziu V, Sanyal A, Harrison SA, et al. Cenicriviroc Treatment for Adults with Nonalcoholic Steatohepatitis and Fibrosis: final Analysis of the Phase 2b CENTAUR Study. Hepatology (Baltimore, Md). 2020. [DOI] [PubMed] [Google Scholar]
- 24.Reddy YK, Marella HK, Jiang Y, et al. Natural History of Non-Alcoholic Fatty Liver Disease: A Study With Paired Liver Biopsies. Journal of Clinical and Experimental Hepatology. 2020;10(3):245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. New England Journal of Medicine. 2010;362(18):1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen J, Chan HL, Wong GL, et al. Assessment of non-alcoholic fatty liver disease using serum total cell death and apoptosis markers. Alimentary Pharmacology & Therapeutics. 2012;36(11–12):1057–1066. [DOI] [PubMed] [Google Scholar]
- 27.Wong VW, Chan RS, Wong GL, et al. Community-based lifestyle modification programme for non-alcoholic fatty liver disease: a randomized controlled trial. Journal of Hepatology. 2013;59(3):536–542. [DOI] [PubMed] [Google Scholar]
- 28.Younossi ZM, Ratziu V, Loomba R, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet (london, england). 2019;394(10215):2184–2196. [DOI] [PubMed] [Google Scholar]
- 29.Harrison SA, Abdelmalek MF, Caldwell S, et al. Simtuzumab Is Ineffective for Patients With Bridging Fibrosis or Compensated Cirrhosis Caused by Nonalcoholic Steatohepatitis. Gastroenterology. 2018;155(4):1140–1153. [DOI] [PubMed] [Google Scholar]
- 30.Harrison SA, Wong V-S, Okanoue T, et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: results from randomized phase III STELLAR trials. Journal of hepatology. 2020. [DOI] [PubMed] [Google Scholar]
- 31.Loguercio C, Andreone P, Brisc C, et al. Silybin combined with phosphatidylcholine and vitamin E in patients with nonalcoholic fatty liver disease: a randomized controlled trial. Free radical biology & medicine. 2012;52(9):1658–1665. [DOI] [PubMed] [Google Scholar]
- 32.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet (london, england). 2015;385(9972):956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castro PCS, Alberton HCP, Pedroso MLA, et al. Evaluation of Progression of Hepatic Fibrosis in a Group of Patients with Non-Alcoholic Fatty Liver Disease Accompanied for 10 Years. Arquivos de Gastroenterologia. 2019;56(3):256–260. [DOI] [PubMed] [Google Scholar]
- 34.Evans CD, Oien KA, MacSween RN, Mills PR. Non-alcoholic steatohepatitis: a common cause of progressive chronic liver injury? Journal of Clinical Pathology. 2002;55(9):689–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fassio E, Alvarez E, Dominguez N, et al. Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology. 2004;40(4):820–826. [DOI] [PubMed] [Google Scholar]
- 36.Hui AY, Wong VW, Chan HL, et al. Histological progression of non-alcoholic fatty liver disease in Chinese patients. Alimentary Pharmacology & Therapeutics. 2005;21(4):407–413. [DOI] [PubMed] [Google Scholar]
- 37.Kim HK, Park JY, Lee KU, et al. Effect of body weight and lifestyle changes on long-term course of nonalcoholic fatty liver disease in Koreans. American Journal of the Medical Sciences. 2009;337(2):98–102. [DOI] [PubMed] [Google Scholar]
- 38.Lin TY, Yeh ML, Huang CF, et al. Disease progression of nonalcoholic steatohepatitis in Taiwanese patients: a longitudinal study of paired liver biopsies. European Journal of Gastroenterology & Hepatology. 2019;31(2):224–229. [DOI] [PubMed] [Google Scholar]
- 39.Nasr P, Ignatova S, Kechagias S, Ekstedt M. Natural history of nonalcoholic fatty liver disease: A prospective follow-up study with serial biopsies. Hepatology Communications. 2018;2(2):199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pais R, Pascale A, Fedchuck L, et al. Progression from isolated steatosis to steatohepatitis and fibrosis in nonalcoholic fatty liver disease. Clinics & Research in Hepatology & Gastroenterology. 2011;35(1):23–28. [DOI] [PubMed] [Google Scholar]
- 41.Teli MR, James OF, Burt AD, et al. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995;22(6):1714–1719. [PubMed] [Google Scholar]
- 42.Kim D, Chung GE, Kwak MS, et al. Effect of longitudinal changes of body fat on the incidence and regression of nonalcoholic fatty liver disease. Digestive & Liver Disease. 2018;50(4):389–395. [DOI] [PubMed] [Google Scholar]
- 43.Lee K. Metabolic syndrome predicts the incidence of hepatic steatosis in Koreans. Obesity Research and Clinical Practice. 2010;4(3):e217–e224. [DOI] [PubMed] [Google Scholar]
- 44.Kamarajah SK, Chan WK, Mustapha NRN, Mahadeva S. Repeated liver stiffness measurement compared with paired liver biopsy in patients with non-alcoholic fatty liver disease. Hepatology International. 2018;12(1):44–55. [DOI] [PubMed] [Google Scholar]
- 45.Allen AM, Therneau TM, Larson JJ, et al. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: A 20 year-community study. Hepatology. 2018;67(5):1726–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kleiner DE, Brunt EM, Wilson LA, et al. Association of Histologic Disease Activity with Progression of Nonalcoholic Fatty Liver Disease. JAMA Network Open. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123(3):745–750. [DOI] [PubMed] [Google Scholar]
- 48.Dasarathy S, Dasarathy J, Khiyami A, et al. Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol. 2009;51(6):1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mottin CC, Moretto M, Padoin AV, et al. The role of ultrasound in the diagnosis of hepatic steatosis in morbidly obese patients. Obes Surg. 2004;14(5):635–637. [DOI] [PubMed] [Google Scholar]
- 50.Ng CH, Xiao J, Lim WH, et al. Placebo effect on progression and regression in NASH: Evidence from a meta-analysis. Hepatology. 2022;75(6):1647–1661. [DOI] [PubMed] [Google Scholar]
- 51.Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology. 2017;65(5):1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akuta N, Kawamura Y, Suzuki F, et al. Analysis of association between circulating miR-122 and histopathological features of nonalcoholic fatty liver disease in patients free of hepatocellular carcinoma. BMC Gastroenterology. 2016;16 (1) (no pagination)(141). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Orci LA, Sanduzzi-Zamparelli M, Caballol B, et al. Incidence of Hepatocellular Carcinoma in Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review, Meta-analysis, and Meta-regression. Clin Gastroenterol Hepatol. 2021. [DOI] [PubMed] [Google Scholar]
- 54.Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet (london, england). 2016;387(10019):679–690. [DOI] [PubMed] [Google Scholar]
- 55.Bril F, Biernacki DM, Kalavalapalli S, et al. Role of Vitamin E for Nonalcoholic Steatohepatitis in Patients With Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care. 2019;42(8):1481–1488. [DOI] [PubMed] [Google Scholar]
- 56.Chan WK, Nik Mustapha NR, Mahadeva S. A Randomized Trial of Silymarin for the Treatment of Nonalcoholic Steatohepatitis. Clinical gastroenterology and hepatology. 2017;(no pagination). [DOI] [PubMed] [Google Scholar]
- 57.Newsome PN, Buchholtz K, Cusi K, et al. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. The New England journal of medicine. 2020;13. [DOI] [PubMed] [Google Scholar]
- 58.Van Wagner LB, Koppe SW, Brunt EM, et al. Pentoxifylline for the treatment of non-alcoholic steatohepatitis: a randomized controlled trial. Annals of Hepatology. 2011;10(3):277–286. [PubMed] [Google Scholar]
- 59.Zein CO, Yerian LM, Gogate P, et al. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54(5):1610–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chan WK, Ida NH, Cheah PL, Goh KL. Progression of liver disease in non-alcoholic fatty liver disease: a prospective clinicopathological follow-up study. Journal of Digestive Diseases. 2014;15(10):545–552. [DOI] [PubMed] [Google Scholar]
- 61.Cho HJ, Hwang S, Park JI, et al. Improvement of Nonalcoholic Fatty Liver Disease Reduces the Risk of Type 2 Diabetes Mellitus. Gut & Liver. 2019;13(4):440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hamaguchi E, Takamura T, Sakurai M, et al. Histological course of nonalcoholic fatty liver disease in Japanese patients: tight glycemic control, rather than weight reduction, ameliorates liver fibrosis. Diabetes Care. 2010;33(2):284–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McPherson S, Hardy T, Henderson E, et al. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. Journal of Hepatology. 2015;62(5):1148–1155. [DOI] [PubMed] [Google Scholar]
- 64.Nogami A, Yoneda M, Kobayashi T, et al. Assessment of 10-year changes in liver stiffness using vibration-controlled transient elastography in non-alcoholic fatty liver disease. Hepatology Research. 2019;49(8):872–880. [DOI] [PubMed] [Google Scholar]
- 65.Pais R, Charlotte F, Fedchuk L, et al. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. Journal of Hepatology. 2013;59(3):550–556. [DOI] [PubMed] [Google Scholar]
- 66.Seko Y, Sumida Y, Tanaka S, et al. Serum alanine aminotransferase predicts the histological course of non-alcoholic steatohepatitis in Japanese patients. Hepatology Research. 2015;45(10):E53–61. [DOI] [PubMed] [Google Scholar]
- 67.Seo NK, Koo HS, Haam JH, et al. Prediction of prevalent but not incident non-alcoholic fatty liver disease by levels of serum testosterone. Journal of Gastroenterology & Hepatology. 2015;30(7):1211–1216. [DOI] [PubMed] [Google Scholar]
- 68.Vilar-Gomez E, Vuppalanchi R, Gawrieh S, et al. Vitamin E Improves Transplant-Free Survival and Hepatic Decompensation Among Patients With Nonalcoholic Steatohepatitis and Advanced Fibrosis. Hepatology. 2020;71(2):495–509. [DOI] [PubMed] [Google Scholar]
- 69.Wang J, Ma L, Chen S, et al. Risk for the development of non-alcoholic fatty liver disease: A prospective study. Journal of Gastroenterology and Hepatology (Australia). 2018;33(8):1518–1523. [DOI] [PubMed] [Google Scholar]
- 70.Wang B, Li M, Zhao Z, et al. Glycemic measures and development and resolution of nonalcoholic fatty liver disease in nondiabetic individuals. Journal of Clinical Endocrinology and Metabolism. 2020;105 (5) (no pagination)(dgaa112). [DOI] [PubMed] [Google Scholar]
- 71.Wong VW, Wong GL, Choi PC, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59(7):969–974. [DOI] [PubMed] [Google Scholar]
- 72.Zelber-Sagi S, Salomone F, Yeshua H, et al. Non-high-density lipoprotein cholesterol independently predicts new onset of non-alcoholic fatty liver disease. Liver Int. 2014;34(6):e128–135. [DOI] [PubMed] [Google Scholar]
- 73.Zhang H, Niu Y, Gu H, et al. Low serum adiponectin is a predictor of progressing to nonalcoholic fatty liver disease. Journal of Clinical Laboratory Analysis. 2019;33 (3) (no pagination)(e22709). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou YJ, Li YY, Nie YQ, et al. Natural course of nonalcoholic fatty liver disease in southern China: a prospective cohort study. Journal of Digestive Diseases. 2012;13(3):153–160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


