Abstract
Background:
Failures have been reported across the cancer care continuum in patients with hepatocellular carcinoma (HCC); however, the impact of treatment delays on outcomes has not been well characterized. We described the prevalence of treatment delays in a racially and ethnically diverse cohort of patients and its association with overall survival.
Methods:
Using the Surveillance, Epidemiology, and End Results (SEER) – Medicare database, we identified patients diagnosed with HCC between 2001 and 2015. We performed multivariable logistic regression analysis to identify factors associated with treatment delay, i.e., receipt of HCC-directed therapy > three months after diagnosis. Cox proportional hazards regression analysis with a 5-month landmark was used to characterize the association between treatment delay and overall survival, accounting for immortal time bias.
Results:
Of 8450 patients with treatment within 12 months of HCC diagnosis, 1205 (14.3%) experienced treatment delays. The proportion with treatment delays ranged from 6.8% of patients undergoing surgical resection to 21.6% of those undergoing liver transplantation. In multivariable analysis, Black patients (OR 1.96, 95%CI 1.21 – 3.15) and those living in high poverty neighborhoods (OR 1.55, 95%CI 1.25 – 1.92) were more likely to experience treatment delays than White patients and those living in low poverty neighborhoods, respectively. Treatment delay was independently associated with worse survival (HR 1.15 95%CI 1.05 – 1.25).
Conclusion:
Nearly one in seven patients with HCC experience treatment delays, with higher odds in Black patients and those living in high poverty neighborhoods. Treatment delays are associated with worse survival, highlighting a need for interventions to improve time-to-treatment.
Keywords: Treatment, prognosis, survival, inequity, liver cancer
INTRODUCTION
Hepatocellular carcinoma (HCC) accounts for 75% to 85% of cases of primary liver cancer and is the third leading cause of cancer-related death worldwide.1 With increased hepatitis B vaccination and hepatitis C treatment uptake, viral-related HCC is decreasing. However, in parallel with the high prevalence of metabolic syndrome, metabolic-associated fatty liver disease (MALFD)-related HCC is rapidly increasing in most countries, including the United States.2
Despite advances in treatment options, the 5-year survival for HCC remains poor at less than 20%.3 This poor prognosis is partly related to failures across the cancer care continuum, with demonstrated underuse of HCC screening and treatment.4–7 In addition, HCC disproportionately affects racial, ethnic, and low socioeconomic status (SES) populations, with both higher incidence and mortality, especially in Black and Hispanic patients.8–10 However, few studies have characterized the prevalence of treatment delays and the potential association with survival in large, racially, and socioeconomically diverse populations.11,12 These data are important as studies in breast and colorectal cancers have demonstrated that treatment delays are common and associated with worse survival.13–15 Understanding the implications of timely treatment for patients with HCC is particularly important in light of the COVID-19 pandemic, during which failures and delays in cancer treatment were common.16
The aims of our study were to (1) describe the prevalence and disparities in HCC treatment delay and (2) evaluate the association between treatment delay and overall survival in a large population-based sample of patients with HCC in the United States.
METHODS
Data source and Study population
The Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database is a population-based dataset providing information on diagnosis, survival, demographics, and health services utilization of cancer patients from Medicare eligibility until death.17 We included Medicare beneficiaries aged ≥65 years who had diagnostically confirmed HCC (International Classification of Diseases for Oncology, Third Edition, [ICD]- [O] histology code 8170 and site code C22.0 for the liver with positive histology, cytology, laboratory test, positive radiology tests) between the years 2001 and 2015.18 Patients were excluded from the final sample if they: (1) were not continuously enrolled in Medicare Part A and B during the study period; (2) enrolled in health maintenance organizations17,19; (3) diagnosed with other cancers within one year prior to HCC diagnosis; (4) died within 30 days post HCC diagnosis; (5) had missing sociodemographic characteristics that could not be imputed or (6) did not receive HCC treatment (Supplemental Figure).19 This study protocol was deemed exempt by the IRB at Texas A&M University.
Sociodemographic and clinical predictors
We obtained patient sociodemographic information from the SEER Patient Entitlement and Diagnosis Summary File (PEDSF), including age, sex, race, ethnicity, census tract poverty level, geographic region, metropolitan status (using rural-urban continuum codes), and the year of HCC diagnosis. Based on prior literature, neighborhood SES was categorized based on census tract poverty level (0% to <10% poverty as low-poverty neighborhoods, 10% to <20% poverty as moderate-poverty neighborhoods, and ≥20% poverty as high-poverty neighborhoods).20–22 Race and ethnicity were categorized as non-Hispanic White (White), non-Hispanic Black (Black), Hispanic, Asian/Pacific Islander (Asian), and “other/unknown.”
Early-stage HCC was defined as a unifocal lesion ≤5 cm with no evidence of vascular invasion or distant metastases, as previously described.23 We conducted a sensitivity analysis using SEER stage, classified as localized, regional, or distant. In addition, we abstracted information on liver disease etiology, ascites, and hepatic encephalopathy. Liver disease etiology was classified hierarchically as hepatitis C virus (HCV), hepatitis B virus (HBV), alcohol-associated liver disease, other liver diseases, MAFLD, and no identifiable liver disease. MAFLD was defined by the presence of metabolic syndrome in the absence of other liver disease etiologies. NCI comorbidity index was used as a measure of non-cancer comorbidity.24,25
Outcomes and Statistical analysis
Our primary outcome was the presence of treatment delay, evaluated as a dichotomous variable, with delayed treatment defined as the time from diagnosis to first treatment exceeding three months, based on tumor doubling time and prior literature.6,12,26,27 HCC-specific treatments were abstracted from Medicare claims data using the ICD-9, ICD-10-Procedure Coding System, HCPCS, and CPT codes within 12 months post HCC diagnosis. HCC treatments were categorized into the most definitive treatment, defined hierarchically as liver transplantation, surgical resection, local ablation, transarterial embolization, external radiation, and systemic therapy. For patients who underwent liver transplantation, those who received bridging therapy within 3 months while awaiting transplant were considered to have received timely treatment. Chi-square tests were used to compare patient characteristics between those who received timely treatment (i.e., ≤3 months) versus delayed treatment (i.e., >3 months). Variables associated with delays were (p<0.10) were included in multivariable analysis. Factors of known clinical significance, e.g., liver dysfunction and sex, were selected a priori. We performed multivariable logistic regression, with an interaction between race, ethnicity, and SES with time fixed effects, to examine the association between race and ethnicity with treatment delay across socioeconomic strata. We adjusted standard errors for clustering at the census tract level.
We conducted landmark analysis to examine our secondary outcome of overall survival, accounting for immortal time bias.28,29 We used landmark analysis instead of time-dependent Cox regression analyses given our aim was to evaluate if timely treatment, instead of simply receipt of treatment, was associated with survival.30,31 Survival was defined from the time of the landmark to death from any cause. A landmark of 5 months was selected for the primary analysis based on prior literature and tumor doubling times.26,27 Patients whose HCC was treated within 3 months were classified as timely treatment, therapy between 4–5 months as delayed treatment, and those with therapy beyond 5 months were excluded. Patients who died prior to the 5-month landmark were also excluded. Patients who remained alive on December 31, 2017, were censored at that date. We performed univariable and multivariable Cox proportional hazards analyses to examine the association between treatment delay and overall survival. We conducted post-hoc sensitivity analyses using 6-, 7-, 8- and 9-month landmarks. We also conducted a post-hoc sensitivity analysis excluding patients who underwent liver transplantation as first therapy given anticipated wait times before being awarded MELD exception points.
All p-values were two-sided with a statistical significance of 5%. All statistical analyses were performed using Stata version 16.1 (Stata Corp, College Station, TX).
RESULTS
Patient characteristics
Of 13,874 patients with HCC, 8,450 (60.9%) were treated within 12 months of diagnosis (Supplemental Figure 1). Median age was 73 years, and more than two-thirds (67.2%) were male. The racial and ethnic composition of the cohort was 68.1% White, 7.4% Black, 13.4% Asian, and 4.0% Hispanic patients. Most patients resided in low-poverty neighborhoods (48.2%) and in metropolitan areas with more than 1 million people (62.7%). The most common liver disease etiology was MAFLD (36.4%), followed by HCV (31.0%). More than half of patients (60.4%) were identified as having localized SEER stage; however, only 23.1% had a unifocal lesion ≤5 cm without vascular invasion or distant metastases.
Prevalence and Correlates of Treatment delay
Median time from HCC diagnosis to first treatment was 1 (IQR 1 to 3) month, with treatment delays observed in 1205 (14.3%) patients. The proportion of patients with delayed treatment remained stable over the study period (Figure 1). Characteristics of patients receiving timely versus delayed treatment are shown in Table 1. Patients receiving delayed treatment were more likely to be Black, reside in poorer neighborhoods, have a higher comorbidity burden, and have underlying hepatitis C infection.
Figure 1.
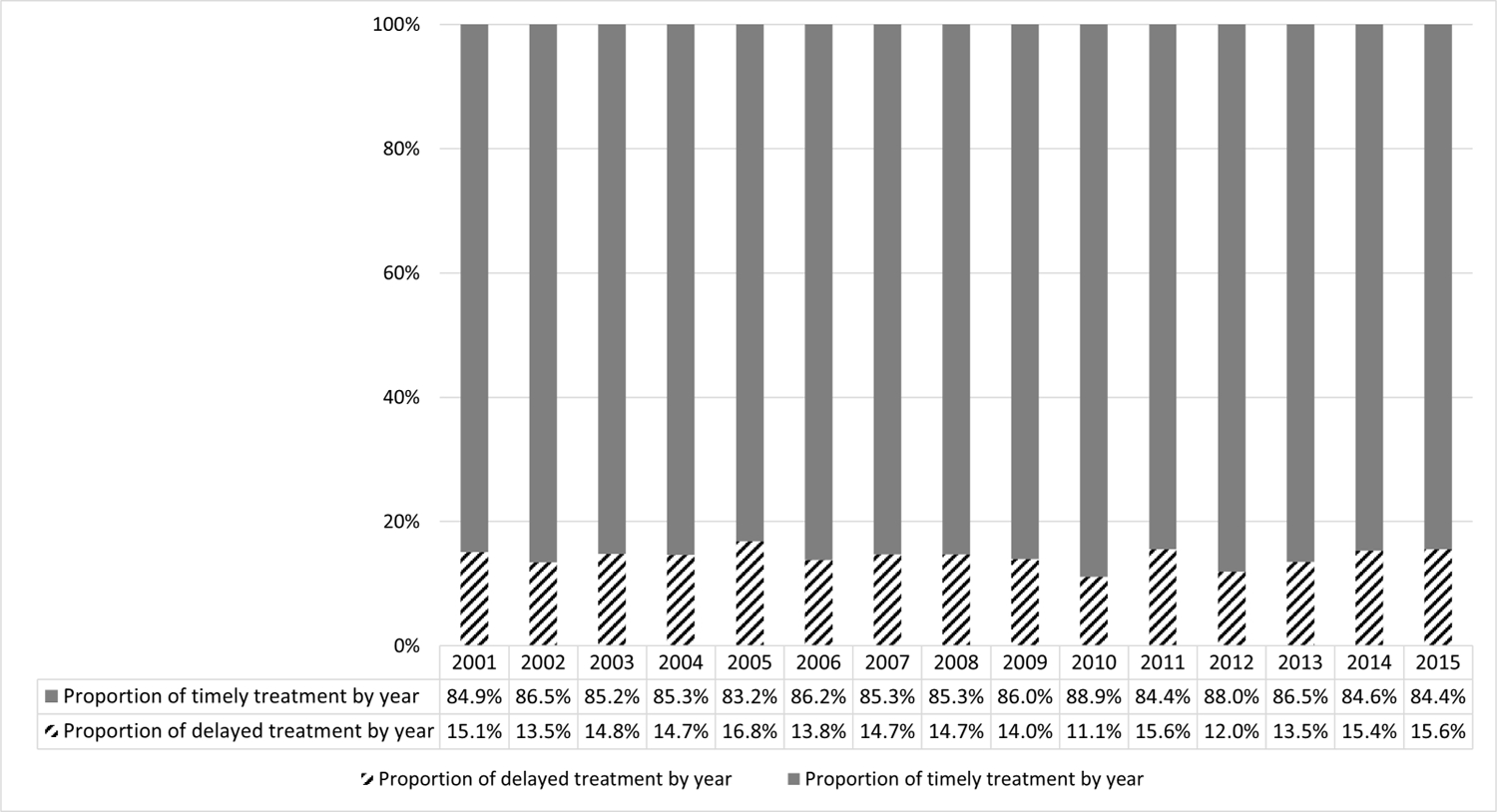
Proportion of patients with delayed vs. timely HCC treatment over time
Table 1.
Patient characteristics by presence or absence of treatment delay
| Patients receiving timely treatment n=7245 | Patients receiving delayed treatment n=1205 | P-value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Total | 7245 | 100.0% | 1205 | 100.0% | |
| Age at diagnosis | |||||
| 65 – 69 years | 1940 | 26.8% | 390 | 32.4% | |
| 70 – 74 years | 2065 | 28.5% | 369 | 30.6% | <0.001 |
| 75 – 79 years | 1727 | 23.8% | 232 | 19.3% | |
| 80 years and older | 1513 | 20.9% | 214 | 17.8% | |
| Gender | |||||
| Female | 2382 | 32.9% | 393 | 32.6% | 0.86 |
| Male | 4863 | 67.1% | 812 | 67.4% | |
| Race and ethnicity | |||||
| White | 4986 | 68.8% | 771 | 64.0% | |
| Black | 498 | 6.9% | 124 | 10.3% | <0.001 |
| Asian | 967 | 13.3% | 165 | 13.7% | |
| Hispanic | 276 | 3.8% | 61 | 5.1% | |
| Other/Unknown | 518 | 7.1% | 84 | 7.0% | |
| Neighborhood-level SES | |||||
| Low poverty neighborhoods | 3558 | 49.1% | 518 | 43.0% | <0.001 |
| Moderate poverty neighborhoods | 2093 | 28.9% | 381 | 31.6% | |
| High poverty neighborhoods | 1594 | 22.0% | 306 | 25.4% | |
| Geographic region | |||||
| Northeast | 1338 | 18.5% | 228 | 18.9% | |
| West | 3792 | 52.3% | 696 | 57.8% | <0.001 |
| Midwest | 691 | 9.5% | 101 | 8.4% | |
| South | 1424 | 19.7% | 180 | 14.9% | |
| Metropolitan status | |||||
| Metro > 1 million | 4549 | 62.8% | 750 | 62.2% | |
| Metro 250,000 – 1 million | 1415 | 19.5% | 255 | 21.2% | 0.02 |
| Metro <250,000 | 522 | 7.2% | 103 | 8.5% | |
| Non-Metro | 759 | 10.5% | 97 | 8.0% | |
| Tumor Staging | |||||
| Unifocal <=5 cm without vascular invasion and metastasis | 1642 | 22.7% | 310 | 25.7% | |
| Beyond unifocal without vascular invasion and metastasis | 3605 | 49.8% | 641 | 53.2% | |
| Vascular invasion or metastasis | 313 | 4.3% | 50 | 4.1% | <0.001 |
| Non-determinable | 1685 | 23.3% | 204 | 16.9% | |
| SEER stage | |||||
| Localized | 4531 | 62.5% | 751 | 62.3% | |
| Regional | 1753 | 24.2% | 298 | 24.7% | 0.01 |
| Distant | 766 | 10.6% | 90 | 7.5% | |
| Unknown | 375 | 5.2% | 66 | 5.5% | |
| NCI comorbidity index | |||||
| 0 | 1566 | 21.6% | 237 | 19.7% | |
| 1 | 1601 | 22.1% | 218 | 18.1% | |
| 2 | 1422 | 19.6% | 245 | 20.3% | 0.001 |
| 3 | 1315 | 18.2% | 259 | 21.5% | |
| 4 | 405 | 5.6% | 85 | 7.1% | |
| >=5 | 936 | 12.9% | 161 | 13.4% | |
| Liver disease etiology | |||||
| HCV | 2175 | 30.0% | 448 | 37.2% | |
| HBV | 402 | 5.5% | 59 | 4.9% | |
| Alcohol related liver disease | 737 | 10.2% | 137 | 11.4% | <0.001 |
| Other liver diseases | 148 | 2.0% | 25 | 2.1% | |
| MAFLD | 2705 | 37.3% | 372 | 30.9% | |
| No identifiable liver disease | 1078 | 14.9% | 164 | 13.6% | |
| Liver dysfunction | |||||
| Presence of hepatic encephalopathy | 476 | 6.6% | 80 | 6.6% | 0.93 |
| Presence of ascites | 900 | 12.4% | 149 | 12.4% | 0.96 |
The proportion of patients experiencing treatment delays differed by HCC therapy, with the highest delays observed in patients who underwent liver transplantation and lowest in those treated with surgical resection (Figure 2 and Supplemental Table 1). Among 480 patients who underwent transplantation over a time horizon of 56 months, 327 (68.1%) had prior bridging therapy, with 285 (87.1%) doing so within 3 months. Of 153 patients who underwent transplantation as initial treatment, 120 (78.3%) did so within 3 months of HCC diagnosis.
Figure 2.
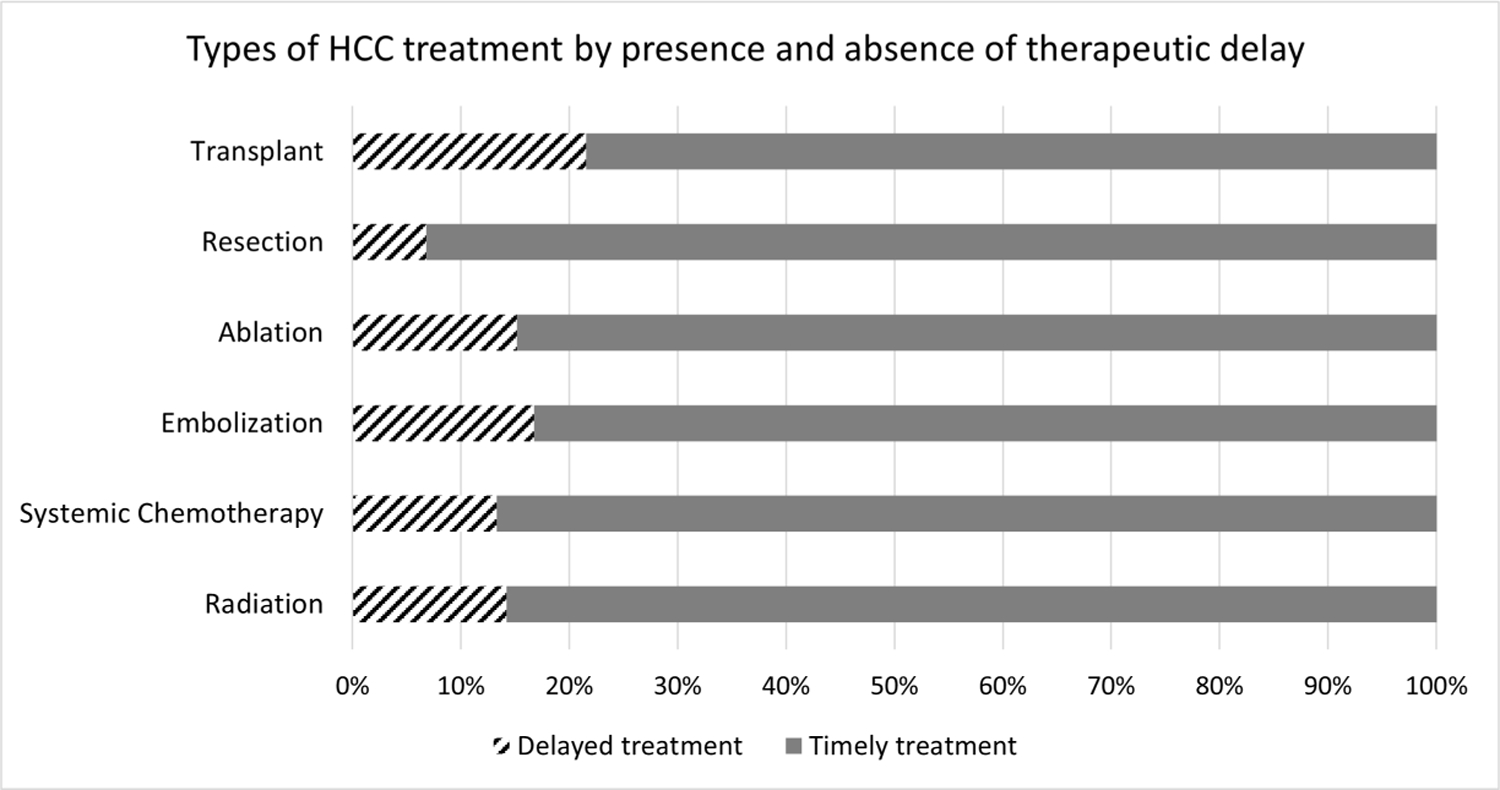
Type of HCC treatment, by presence and absence of treatment delay
We also noted sociodemographic disparities in time-to-treatment. Treatment delays were observed in 19.9% of Black and 18.1% of Hispanic patients, compared to 13.4% and 14.6% of White and Asian patients, respectively. Similarly, treatment delays were observed in 12.7%, 15.4%, and 16.1% of those living in low, moderate, and high poverty neighborhoods, respectively.
In multivariable analysis (Table 2), we continued to observe sociodemographic disparities in treatment delays. Specifically, Black patients (OR 1.91 95%CI 1.20 – 3.05) and patients in moderate-high poverty neighborhoods (moderate poverty: OR 1.30 95%CI 1.08 – 1.57; high poverty: OR 1.53 95%CI 1.24 – 1.89) were more likely to experience treatment delays compared to White patients and those living in low poverty neighborhoods, respectively. The interaction between the Black race and neighborhood poverty was not statistically significant.
Table 2.
Correlates of delayed treatment (with and without type of first HCC treatment)
| Delayed treatment (without first HCC treatment) n=8450 OR (95% CI)1 | Delayed treatment (with first HCC treatment) n=8450 OR (95% CI)2 | |
|---|---|---|
| Age at diagnosis | ||
| 65 – 69 years | Ref | Ref |
| 70 – 74 years | 0.94 (0.80,1.10) | 0.95 (0.81,1.12) |
| 75 – 79 years | 0.71 (0.59,0.85) | 0.72 (0.60,0.86) |
| 80 years and older | 0.78 (0.65,0.95) | 0.77 (0.63,0.93) |
| Male sex | 1.03 (0.90,1.17) | 1.02 (0.89,1.16) |
| Race and ethnicity | ||
| White | Ref | Ref |
| Black | 1.91 (1.20,3.05) | 1.96 (1.21,3.15) |
| Asian | 1.27 (0.96,1.68) | 1.30 (0.98,1.72) |
| Hispanic | 1.02 (0.53,1.97) | 1.02 (0.53,1.96) |
| Other/Unknown | 1.01 (0.70,1.45) | 1.02 (0.71,1.45) |
| Neighborhood-level SES | ||
| Affluent neighborhoods | Ref | Ref |
| Moderate poverty neighborhoods | 1.30 (1.08,1.57) | 1.29 (1.07,1.55) |
| Poor neighborhoods | 1.53 (1.24,1.89) | 1.55 (1.25,1.92) |
| Interaction of race, ethnicity, and poverty | ||
| Black#Moderate poverty neighborhoods | 0.72 (0.40,1.32) | 0.71 (0.39,1.32) |
| Black#High poverty neighborhoods | 0.61 (0.34,1.08) | 0.59 (0.33,1.06) |
| Asian#Moderate poverty neighborhoods | 0.82 (0.54,1.25) | 0.82 (0.54,1.26) |
| Asian#High poverty neighborhoods | 0.44 (0.26,0.74) | 0.44 (0.26,0.73) |
| Hispanic#Moderate poverty neighborhoods | 1.63 (0.72,3.69) | 1.58 (0.70,3.58) |
| Hispanic#High poverty neighborhoods | 0.83 (0.37,1.85) | 0.82 (0.37,1.83) |
| Geographic region | ||
| West | Ref | Ref |
| Northeast | 1.05 (0.88,1.26) | 1.07 (0.89,1.28) |
| Midwest | 0.83 (0.66,1.05) | 0.82 (0.65,1.04) |
| South | 0.65 (0.54,0.79) | 0.65 (0.54,0.79) |
| Metropolitan status | ||
| Metro > 1 million | Ref | Ref |
| Metro 250,000 – 1 million | 1.10 (0.94,1.28) | 1.10 (0.94,1.29) |
| Metro <250,000 | 1.29 (1.01,1.65) | 1.29 (1.00,1.66) |
| Non-Metro | 0.87 (0.69,1.09) | 0.88 (0.69,1.11) |
| Tumor Staging | ||
| Unifocal <=5 cm without vascular invasion and metastasis | Ref | Ref |
| Beyond unifocal without vascular invasion and metastasis | 1.00 (0.86,1.16) | 0.95 (0.81,1.11) |
| Vascular invasion or metastasis | 0.89 (0.60,1.32) | 0.83 (0.55,1.26) |
| NCI comorbidity index | ||
| 0 | Ref | Ref |
| 1 | 0.93 (0.76,1.15) | 0.92 (0.75,1.14) |
| 2 | 1.05 (0.86,1.29) | 1.01 (0.82,1.24) |
| 3 | 1.18 (0.96,1.45) | 1.12 (0.91,1.38) |
| 4 | 1.35 (1.02,1.78) | 1.31 (0.99,1.73) |
| >=5 | 1.06 (0.84,1.34) | 1.01 (0.80,1.28) |
| Liver disease etiology | ||
| HCV | Ref | Ref |
| HBV | 0.74 (0.55,1.01) | 0.79 (0.58,1.07) |
| Alcohol related liver disease | 1.02 (0.81,1.27) | 1.00 (0.80,1.24) |
| Other liver diseases | 0.99 (0.64,1.54) | 1.02 (0.66,1.57) |
| MAFLD | 0.81 (0.68,0.95) | 0.85 (0.72,1.00) |
| No identifiable liver disease | 0.87 (0.69,1.09) | 0.92 (0.73,1.15) |
| Liver dysfunction | ||
| Presence of hepatic encephalopathy | 0.88 (0.68,1.15) | 0.82 (0.63,1.07) |
| Presence of ascites | 0.89 (0.72,1.10) | 0.86 (0.70,1.07) |
| First HCC treatment type | ||
| Liver transplantation | 1.24 (0.82,1.87) | |
| Surgical resection | 0.38 (0.29,0.49) | |
| Local ablation | 0.81 (0.68,0.98) | |
| Embolization | Ref | |
| Systemic chemotherapy | 0.81 (0.69,0.96) | |
| Radiation | 0.90 (0.68,1.20) |
Model included year fixed effects (not reported)
Model included year fixed effects (not reported)
Overall survival
In the 5-month landmark analysis (n=6644), 5954 patients (89.6%) received timely treatment while 690 patients (10.4%) received delayed treatment. Median overall survival of the cohort was 25 (IQR 11 to 61) months – 25 months for patients with timely treatment versus 23 months for those with treatment delay. Treatment delay was associated with worse survival in univariable (HR 1.13 95% CI 1.04 – 1.23) (Figure 3) and multivariable (HR 1.15, 95%CI 1.05 – 1.25) analyses. In multivariable analysis, compared to White patients, Hispanic patients had worse survival (HR 1.40 95%CI 1.08 – 1.82), whereas Asian patients had better survival (HR 0.82 95% CI 0.73 – 0.93). There was no significant difference in survival between Black patients and White patients. Median overall survival was 35 months for Asian patients compared to 23, 22, and 20 months for White, Black, and Hispanic patients, respectively. Other factors associated with worse survival included male sex, older age ≥70 years, higher comorbidity, presence of ascites, more advanced tumor burden, living in the Midwest, and living in non-metropolitan areas. When type of HCC treatment was added to the model, treatment delay was not associated with worse survival (Table 3).
Figure 3.
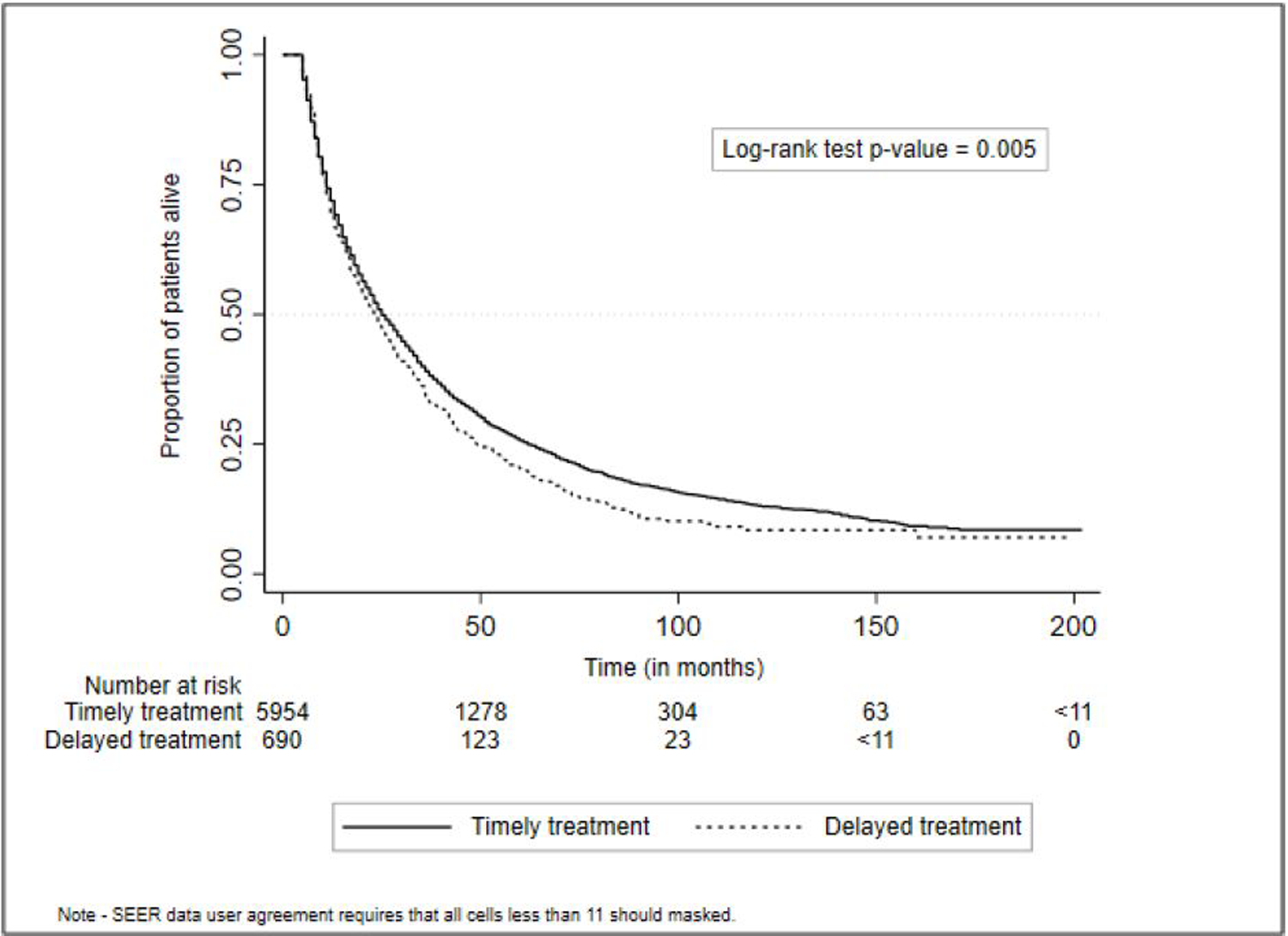
Delayed treatment is associated with worse survival
Table 3.
Correlates of overall survival – 5-month landmark (without and with type of first HCC treatment)
| Overall survival (Without first HCC treatment) n=6644 HR (95% CI) | Overall survival (with first HCC treatment) n=6644 HR (95% CI) | |
|---|---|---|
| Delayed treatment | 1.15 (1.05,1.25) | 1.07 (0.98,1.17) |
| Age at diagnosis | ||
| 65 – 69 years | Ref | Ref |
| 70 – 74 years | 1.15 (1.06,1.24) | 1.16 (1.07,1.25) |
| 75 – 79 years | 1.31 (1.21,1.41) | 1.25 (1.16,1.36) |
| 80 years and older | 1.44 (1.33,1.57) | 1.36 (1.25,1.48) |
| Male | 1.13 (1.06,1.20) | 1.10 (1.04,1.18) |
| Race and ethnicity | ||
| White | Ref | Ref |
| Black | 0.84 (0.63,1.11) | 0.79 (0.58,1.07) |
| Asian | 0.83 (0.73,0.93) | 0.79 (0.69,0.90) |
| Hispanic | 1.40 (1.08,1.82) | 1.32 (1.03,1.69) |
| Other/Unknown | 0.75 (0.64,0.89) | 0.78 (0.66,0.92) |
| Neighborhood-level SES | ||
| Low poverty neighborhoods | Ref | Ref |
| Moderate poverty neighborhoods | 1.04 (0.96,1.12) | 1.02 (0.94,1.10) |
| High poverty neighborhoods | 1.07 (0.97,1.18) | 1.02 (0.92,1.13) |
| Interaction of race, ethnicity, and poverty | ||
| Black#Moderate poverty neighborhoods | 1.17 (0.83,1.64) | 1.19 (0.83,1.70) |
| Black#High poverty neighborhoods | 1.33 (0.96,1.84) | 1.31 (0.93,1.86) |
| Asian#Moderate poverty neighborhoods | 0.99 (0.83,1.19) | 1.05 (0.87,1.27) |
| Asian#High poverty neighborhoods | 0.93 (0.75,1.16) | 0.97 (0.77,1.23) |
| Hispanic#Moderate poverty neighborhoods | 0.94 (0.67,1.32) | 0.96 (0.69,1.33) |
| Hispanic#High poverty neighborhoods | 0.72 (0.51,1.01) | 0.72 (0.52,1.01) |
| Geographic region | ||
| West | Ref | Ref |
| Northeast | 0.94 (0.87,1.01) | 0.97 (0.90,1.05) |
| Midwest | 1.10 (0.99,1.22) | 1.12 (1.01,1.25) |
| South | 1.08 (0.99,1.18) | 1.16 (1.06,1.27) |
| Metropolitan status | ||
| Metro > 1 million | Ref | Ref |
| Metro 250,000 – 1 million | 1.03 (0.96,1.11) | 1.03 (0.96,1.10) |
| Metro <250,000 | 1.02 (0.91,1.15) | 0.99 (0.87,1.11) |
| Non-Metro | 0.92 (0.83,1.03) | 0.93 (0.83,1.04) |
| Tumor Staging | ||
| Unifocal <=5 cm without vascular invasion and metastasis | Ref | Ref |
| Beyond unifocal without vascular invasion and metastasis | 1.58 (1.47,1.69) | 1.46 (1.36,1.57) |
| Vascular invasion or metastasis | 2.16 (1.87,2.49) | 2.15 (1.84,2.52) |
| Non-determinable | 1.96 (1.80,2.14) | 1.83 (1.68,2.00) |
| NCI comorbidity index | ||
| 0 | Ref | Ref |
| 1 | 1.04 (0.95,1.13) | 1.04 (0.95,1.14) |
| 2 | 1.02 (0.93,1.12) | 1.03 (0.93,1.13) |
| 3 | 1.07 (0.97,1.17) | 1.08 (0.98,1.19) |
| 4 | 1.20 (1.06,1.36) | 1.14 (0.99,1.31) |
| >=5 | 1.27 (1.14,1.42) | 1.22 (1.09,1.37) |
| Liver disease etiology | ||
| HCV | Ref | Ref |
| HBV | 0.68 (0.60,0.78) | 0.72 (0.62,0.82) |
| Alcohol related liver disease | 1.06 (0.96,1.18) | 1.05 (0.95,1.16) |
| Other liver diseases | 0.90 (0.74,1.10) | 0.96 (0.79,1.17) |
| MAFLD | 1.01 (0.93,1.08) | 1.04 (0.97,1.12) |
| No identifiable liver disease | 1.06 (0.96,1.17) | 1.12 (1.01,1.24) |
| Liver dysfunction | ||
| Presence of hepatic encephalopathy | 1.06 (0.93,1.21) | 1.08 (0.95,1.23) |
| Presence of ascites | 1.15 (1.04,1.27) | 1.07 (0.96,1.18) |
| First HCC treatment type | ||
| Liver transplantation | 0.33 (0.26,0.43) | |
| Surgical resection | 0.49 (0.45,0.53) | |
| Local ablation | 0.82 (0.76,0.89) | |
| Embolization | Ref | |
| Systemic chemotherapy | 1.56 (1.44,1.69) | |
| Radiation | 1.56 (1.34,1.82) |
In subgroup analyses by tumor stage (Supplemental Table 2 and 3), treatment delay was associated with worse survival for patients with early-stage HCC (HR 1.21 95%CI 1.02 – 1.44), although this was no longer significant when type of HCC treatment was added to the model (HR 1.11 95%CI 0.92 – 1.33). In non-early-stage patients, treatment delay was associated with higher mortality in both models (Supplemental Table 3). We also conducted subgroup analyses by curative (i.e., transplantation, resection, local ablation) vs. non-curative treatment (i.e., transarterial embolization, radiation, and systemic therapy). Delayed treatment was associated with worse survival among patients who received curative treatment, although this association was mitigated when type of HCC treatment was added to the model (Supplemental Table 4). For non-curative treatments, delayed treatment was not associated with overall survival in either model (Supplemental Table 5). Sensitivity analyses using SEER staging (i.e., local, regional, distant) yielded similar results. In sensitivity analyses using 6-, 7-, 8- and 9-month landmarks, delayed treatment continued to be associated with worse overall survival (Supplemental Table 6). Results were unchanged when excluding patients who underwent liver transplantation as their first therapy (Supplemental Table 7).
DISCUSSION
In this population-based sample, we found nearly one in seven patients with HCC experience treatment delays exceeding 3 months. Several sociodemographic factors were associated with treatment delay; Black patients and those living in moderate and high poverty neighborhoods were more likely to experience treatment delays than White patients and those living in low poverty neighborhoods, respectively. These findings are notable given the association between treatment delay and worse survival, highlighting a need for interventions to improve time-to-treatment for patients with HCC.
Prior studies have described racial and socioeconomic disparities in HCC treatment utilization and overall survival.4,32,33 In a study using the SEER-Medicare database, we found Black patients were less likely to receive curative treatment and had higher mortality, particularly those in high poverty neighborhoods compared to White patients living in similar neighborhoods.23 The current study extends this work by demonstrating racial and ethnic disparities in treatment delays and survival even in a selected population of Medicare beneficiaries. Further, our findings are consistent with a study in the VA system; taken together these data indicate that insurance status alone cannot account for observed disparities in HCC outcomes.34 This is consistent with prior studies that demonstrated significant racial and ethnic disparities among Medicare enrollees in adverse health indicators, timely cancer screening, and in the patient experience of care coordination.35–37 This persistent disparity may be in part related to socioeconomic factors; for example, racial and ethnic minority patients are less likely than Whites to have supplemental coverage to cover gaps in Medicare coverage.38
Racial, ethnic, and socioeconomic disparities in care delivery are well documented in other cancers and can be due to a combination of patient, provider, and system-level factors. Although we found patient-related factors associated with treatment delays, we could not evaluate other important factors, including patient knowledge, attitudes (e.g., level of concern and health locus of control), and barriers to care such as medical mistrust, transportation, and financial barriers.39,40 There are also provider-level factors that can impact cancer care delivery, including cultural barriers, implicit biases against minority populations, and resource constraints faced by providers caring for a greater proportion of disadvantaged patients.41,42 Finally, system-level factors such as resource constraint, scheduling issues, and lack of care coordination may lead to longer wait times and exacerbate disparities in treatment delays even among those with Medicare coverage43. On a broad scale, some inequities observed in this population can be attributed to structural socioeconomic and environmental factors rooted in discrimination and systemic racism.44 For example, in a survey study of over 230,000 Medicare beneficiaries, Black and Hispanic patients reported more difficulty receiving timely follow-up on test results and less help managing their care than White patients.37 Prior studies have also demonstrated wide variability in racial and ethnic disparities in the Medicare population across regions and for different procedures.45 Future studies are needed to assess how these factors impact time to HCC treatment in different practice settings. While expanding Medicare coverage to all would positively impact improving accessibility to cancer care, other issues impacting cancer care disparities must also be addressed, including access to telemedicine, neighborhood conditions, food insecurity, and financial opportunities.46
In our 5-month landmark analysis accounting for immortal time bias, we found HCC treatment delay was associated with worse survival although the difference was no longer statistically significant after type of first treatment was added to the model. Longer delayed treatments of 7 months or greater continued to be associated with worse survival in models with and without type of first treatment. Our findings are consistent with prior studies examining the impact of treatment delay on survival in other cancers, including breast and colorectal cancer.13–15,48,49 Prior studies in HCC have reported discordant findings regarding the association between treatment delays and survival.6,12,47 This discordance may be partly related to specific reasons for treatment delay and type of HCC treatment delivered. For example, providers may be more likely to closely monitor patients and delay treatment in patients with small or slow-growing indolent tumors. Similarly, providers may defer treatment in patients with significant liver dysfunction, including those who are listed for liver transplantation.
Strengths of our study include using a large population-based dataset with linkage to Medicare claims to provide treatment information, liver dysfunction parameters, and liver disease etiology, as well as our use of a landmark analysis to mitigate potential immortal time bias.50 However, we acknowledge limitations of the study. We excluded patients younger than 65 years and did not have access to all the states through the SEER registry, limiting generalizability of the findings.17 Additionally, SEER-Medicare does not have sufficiently granular data to assess robust parameters of liver dysfunction like Child-Pugh score, or validated predictors of survival in patients with cirrhosis like MELD score, or tumor characteristics to determine Milan Criteria or BCLC staging. Furthermore, although use of landmark analysis can best estimate the association of treatment receipt at a certain time (i.e., timely vs delayed treatment), it cannot fully address all inherent biases of a nonrandomized comparison.29 However, our conclusions are strengthened by consistency across sensitivity analyses. Finally, our findings describing racial and ethnic disparities should be interpreted cautiously, as race and ethnicity are self-reported in SEER and do not account for multiracial and/or multiethnic patients.50
In conclusion, our study highlights that treatment delays are experienced by 10–20% of patients, with observed racial, ethnic, and socioeconomic disparities. Given an association between treatment delays and overall survival, interventions to reduce these disparities remain critical.
Supplementary Material
What You Need to Know.
Background
Treatment delays are common and associated with worse survival in several cancers; however, the prevalence and clinical significance of treatment delays in patients with hepatocellular carcinoma (HCC) have not been well characterized in large, diverse patient populations.
Findings
Using the SEER-Medicare database, we found nearly one in seven patients with HCC experience treatment delays exceeding 3 months. Black patients and those living in moderate or high poverty neighborhoods were more likely to experience treatment delays than White patients and those living in low poverty neighborhoods, respectively. Treatment delay was significantly associated with worse overall survival.
Implications
Racial and socioeconomic disparities in timely treatment may partly explain observed disparities in HCC clinical outcomes. Our findings highlight an urgent need for interventions to improve time-to-treatment for patients with HCC.
Grant support
This work was supported by the Population Informatics Lab, the Texas Virtual Data Library (ViDaL) at Texas A&M University, and the National Institutes of Health R01 MD012565 and R01CA256977.
Abbreviations
- CPL
Census tract poverty level
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- HR
hazard ratio
- HCC
Hepatocellular carcinoma
- ICD-9
International Classification of Diseases, 9th revision
- ICD-10
International Classification of Diseases, 10th revision
- MAFLD
Metabolic Associated Liver Disease
- NCI
National Cancer Institute
- OR
odds ratio
- SEER
Surveillance, Epidemiology and End Results
- SES
Socioeconomic status
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Amit Singal has served as a consultant or on advisory boards for Bayer, Eisai, BMS, Exelixis, Genentech, AstraZeneca, FuijFilm Medical Sciences, Exact Sciences, Roche, Glycotest, GRAIL, and TARGET RWE.
None of the other authors have relevant conflicts of interest.
Data sharing statement
“The data underlying this article cannot be shared publicly because the National Cancer Institute does not permit others to use the data except for collaborators at our institution involved with this research as described in our research proposal. However, this data can be obtained through https://healthcaredelivery.cancer.gov/seermedicare/obtain/ by paying the cost mentioned.”
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. [DOI] [PubMed] [Google Scholar]
- 2.Huang D, Singal AG, Kono Y, Tan D, El-Serag H, Loomba R. Changing global epidemiology of liver cancer from 2010–2019: NASH if the fastest growing cause of liver cancer. Cell Metabolism 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin Gastroenterol Hepatol. 2020;18(12):2650–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan D, Yopp A, Beg MS, Gopal P, Singal AG. Meta-analysis: underutilisation and disparities of treatment among patients with hepatocellular carcinoma in the United States. Aliment Pharmacol Ther. 2013;38(7):703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf E, Rich NE, Marrero JA, Parikh ND, Singal AG. Use of Hepatocellular Carcinoma Surveillance in Patients With Cirrhosis: A Systematic Review and Meta-Analysis. Hepatology. 2021;73(2):713–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao A, Rich NE, Marrero JA, Yopp AC, Singal AG. Diagnostic and Therapeutic Delays in Patients With Hepatocellular Carcinoma. J Natl Compr Cancer Netw 2022; 19(9) 1063–71. [DOI] [PubMed] [Google Scholar]
- 7.Singal AG, Lok AS, Feng Z, Kanwal F, Parikh ND. Conceptual Model for the Hepatocellular Carcinoma Screening Continuum: Current Status and Research Agenda. Clin Gastroenterol Hepatol. 2022; 20(1): 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol. 2014;109(4):542–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shebl FM, Capo-Ramos DE, Graubard BI, McGlynn KA, Altekruse SF. Socioeconomic Status and Hepatocellular Carcinoma in the United States. Cancer Epidemiol Prev Biomark. 2012;21(8):1330–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rich NE, Carr C, Yopp AC, Marrero JA, Singal AG. Racial and Ethnic Disparities in Survival Among Patients with Hepatocellular Carcinoma in the United States: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol. 2022; 20(2): e267–e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi DT, Kum HC, Park S, et al. Hepatocellular Carcinoma Screening Is Associated With Increased Survival of Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2019;17(5):976–987.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singal AG, Waljee AK, Patel N, et al. Therapeutic Delays Lead to Worse Survival Among Patients With Hepatocellular Carcinoma. J Natl Compr Cancer Netw 2013;11(9):1101–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bleicher RJ, Ruth K, Sigurdson ER, et al. Time to Surgery and Breast Cancer Survival in the United States. JAMA Oncol. 2016;2(3):330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eaglehouse YL, Georg MW, Shriver CD, Zhu K. Time-to-surgery and overall survival after breast cancer diagnosis in a universal health system. Breast Cancer Res Treat. 2019;178(2):441–450. [DOI] [PubMed] [Google Scholar]
- 15.Corley DA, Jensen CD, Quinn VP, et al. Association Between Time to Colonoscopy After a Positive Fecal Test and Risk of Colorectal Cancer Stage at Diagnosis. JAMA. 2017;317(16):1631–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.COVID-19 Guidance for Practices Translated to Seven Languages. ASCO. Published July 2, 2020. Accessed August 24, 2021. https://www.asco.org/practice-policy/policy-issues-statements/asco-in-action/covid-19-guidance-seven-translations [Google Scholar]
- 17.Brief Description of SEER-Medicare Database. Accessed March 18, 2020. https://healthcaredelivery.cancer.gov/seermedicare/overview/
- 18.Number of Cancer Cases for Selected Cancers in the SEER-Medicare Data. Accessed March 14, 2020. https://healthcaredelivery.cancer.gov/seermedicare/aboutdata/cases.html
- 19.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV–3–18. [DOI] [PubMed] [Google Scholar]
- 20.Pruitt SL, Davidson NO, Gupta S, Yan Yan, Schootman M. Missed opportunities: racial and neighborhood socioeconomic disparities in emergency colorectal cancer diagnosis and surgery. BMC Cancer. 2014;14(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ojinnaka CO, Luo W, Ory MG, McMaughan D, Bolin JN. Disparities in Surgical Treatment of Early-Stage Breast Cancer Among Female Residents of Texas: The Role of Racial Residential Segregation. Clin Breast Cancer. 2017;17(2):e43–e52. [DOI] [PubMed] [Google Scholar]
- 22.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Race/Ethnicity, Gender, and Monitoring Socioeconomic Gradients in Health: A Comparison of Area-Based Socioeconomic Measures-The Public Health Disparities Geocoding Project. Am J Public Health. 2003;93(10):1655–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagle NS, Park S, Washburn D, et al. Racial, Ethnic, and Socioeconomic Disparities in Curative Treatment Receipt and Survival in Hepatocellular Carcinoma. Hepatol Commun. 2022; 6(5): 1186–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. [DOI] [PubMed] [Google Scholar]
- 25.NCI Comorbidity Index Overview. Accessed March 8, 2021. https://healthcaredelivery.cancer.gov/seermedicare/considerations/comorbidity.html
- 26.Nathani P, Gopal P, Rich N, et al. Hepatocellular carcinoma tumour volume doubling time: a systematic review and meta-analysis. Gut. 2021; 70(20): 401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rich NE, John BV, Parikh ND, et al. Hepatocellular Carcinoma Demonstrates Heterogeneous Growth Patterns in a Multicenter Cohort of Patients With Cirrhosis. Hepatology. 2020;72(5):1654–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson JR C K|Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1(11):710–719. [DOI] [PubMed] [Google Scholar]
- 29.Dafni U. Landmark Analysis at the 25-Year Landmark Point. Circ Cardiovasc Qual Outcomes. 2011;4(3):363–371. [DOI] [PubMed] [Google Scholar]
- 30.Agarwal P, Moshier E, Ru M, et al. Immortal Time Bias in Observational Studies of Time-to-Event Outcomes. Cancer Control J Moffitt Cancer Cent. 2018;25(1):1073274818789355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones M, Fowler R. Immortal time bias in observational studies of time-to-event outcomes. J Crit Care. 2016;36:195–199. [DOI] [PubMed] [Google Scholar]
- 32.Hyder O, Dodson RM, Nathan H, et al. Referral Patterns and Treatment Choices for Patients with Hepatocellular Carcinoma: A United States Population-Based Study. J Am Coll Surg. 2013;217(5):896–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nathan H, Hyder O, Mayo SC, et al. Surgical Therapy for Early Hepatocellular Carcinoma in the Modern Era: A 10-Year SEER-Medicare Analysis. Ann Surg. 2013;258(6):1022–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi DT, Davila JA, Sansgiry S, et al. Factors Associated With Delay of Diagnosis of Hepatocellular Carcinoma in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2021;19(8):1679–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ng JH, Bierman AS, Elliott MN, Wilson RL, Xia C, Scholle SH. Beyond Black and White: Race/Ethnicity and Health Status Among Older Adults. Am J Manag Care. 2014;20(3):239–248. [PMC free article] [PubMed] [Google Scholar]
- 36.O’Malley AS, Forrest CB, Feng S, Mandelblatt J. Disparities Despite Coverage: Gaps in Colorectal Cancer Screening Among Medicare Beneficiaries. Arch Intern Med. 2005;165(18):2129–2135. [DOI] [PubMed] [Google Scholar]
- 37.Martino SC, Elliott MN, Hambarsoomian K, et al. Racial/Ethnic Disparities in Medicare Beneficiaries’ Care Coordination Experiences. Med Care. 2016;54(8):765–771. [DOI] [PubMed] [Google Scholar]
- 38.Brunt CS. Supplemental Insurance and Racial Health Disparities under Medicare Part B. Health Serv Res. 2017;52(6):2197–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar S, Al-Balushi M, Dsouza PC, Al-Baimani K, Burney IA, Al-Moundhri M. Beliefs and Perceptions About Cancer Diagnosis and Treatment-Seeking and Decision-Making Behaviors Among Omani Patients with Cancer: A Single-Center Study. J Relig Health. 2022; 61(2): 1351–1365. [DOI] [PubMed] [Google Scholar]
- 40.Schoenberger H, Rich N, Jones P, Yekkaluri S, Yopp A, et al. Racial and Ethnic Disparities in Barriers to Care in Patients with Hepatocellular Carcinoma. Clinical Gastroenterology and Hepatology 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pratt-Chapman ML. Does cultural competency training matter? A national study of anti-gay bias among cancer care professionals. Patient Educ Couns. 2021;104(7):1823–1825. [DOI] [PubMed] [Google Scholar]
- 42.Greene N, Malone J, Adams MA, Dean LT, Poteat T. “This is some mess right here”: Exploring interactions between Black sexual minority women and health care providers for breast cancer screening and care. Cancer. 2021;127(1):74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taplin SH, Anhang Price R, Edwards HM, et al. Introduction: Understanding and Influencing Multilevel Factors Across the Cancer Care Continuum. JNCI Monogr. 2012;2012(44):2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Academies of Sciences E, Division H and M, Practice B on PH and PH, et al. The Root Causes of Health Inequity. National Academies Press (US); 2017. Accessed November 29, 2021. https://www.ncbi.nlm.nih.gov/books/NBK425845/ [Google Scholar]
- 45.Baicker K, Chandra A, Skinner JS, Wennberg JE. Who You Are And Where You Live: How Race And Geography Affect The Treatment Of Medicare Beneficiaries. Health Aff (Millwood). 2004;23:33–44. [DOI] [PubMed] [Google Scholar]
- 46.Roberts ET, Mehrotra A. Assessment of Disparities in Digital Access Among Medicare Beneficiaries and Implications for Telemedicine. JAMA Intern Med. 2020;180(10):1386–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huo TI, Huang YH, Chiang JH, et al. Survival impact of delayed treatment in patients with hepatocellular carcinoma undergoing locoregional therapy: Is there a lead-time bias? Scand J Gastroenterol. 2007;42(4):485–492. [DOI] [PubMed] [Google Scholar]
- 48.Smith EC, Ziogas A, Anton-Culver H. Delay in Surgical Treatment and Survival After Breast Cancer Diagnosis in Young Women by Race/Ethnicity. JAMA Surg. 2013;148(6):516. [DOI] [PubMed] [Google Scholar]
- 49.Tørring ML, Frydenberg M, Hamilton W, Hansen RP, Lautrup MD, Vedsted P. Diagnostic interval and mortality in colorectal cancer: U-shaped association demonstrated for three different datasets. J Clin Epidemiol. 2012;65(6):669–678. [DOI] [PubMed] [Google Scholar]
- 50.Enewold L, Parsons H, Zhao L, et al. Updated Overview of the SEER-Medicare Data: Enhanced Content and Applications. JNCI Monogr. 2020;2020(55):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
“The data underlying this article cannot be shared publicly because the National Cancer Institute does not permit others to use the data except for collaborators at our institution involved with this research as described in our research proposal. However, this data can be obtained through https://healthcaredelivery.cancer.gov/seermedicare/obtain/ by paying the cost mentioned.”


