Abstract
Sensory features are common and impairing in autism spectrum disorder (ASD), but there are few observational sensory assessments that are valid across ages. We used the Sensory Processing 3-Dimensional (SP3-D) observed Assessment and parent-reported Inventory to examine sensory responsivity in 41 ASD and 33 typically-developing (TD) youth across 7–17 years. ASD youth had higher and more variable observed and reported sensory responsivity symptoms compared to TD, but the two measures were not correlated. Observed sensory over-responsivity (SOR) and sensory craving (SC) decreased with age in ASD, though SOR remained higher in ASD versus TD through adolescence. Results suggest that in ASD, the SP3-D Assessment can identify SOR through adolescence, and that there is value in integrating multiple sensory measures.
Keywords: Autism spectrum disorder, Sensory modulation, Sensory reactivity, Sensory responsivity, Sensory assessment, Sensory over-responsivity, Adolescence
Autistic individuals often exhibit differences in how they modulate, or react to, sensory stimuli. These modulation differences include sensory features such as sensory over-responsivity, sensory seeking, and/or sensory under-responsivity. Sensory over-responsivity (SOR), or hyper-responsivity, is an exaggerated, negative response to or avoidance of sensory experiences. Sensory seeking (SS), also referred to as Sensory craving (SC) or Sensory interests, repetitions, and seeking behaviors (SIRS) can include a fascination with or intensely craving sensory stimulation, wanting increased sensory input, having trouble disengaging from stimuli, or having unusual or repetitive sensory interests. SUR, or hyporesponsivity, is a slow response or lack of awareness to aversive sensory input (Liss et al., 2006; Miller et al., 2007). The study of sensory processing in individuals with ASD is crucial, because sensory features are both extremely prevalent (with estimates ranging from 70 to 95% of children with ASD; Baranek et al., 2006; Leekam et al., 2007; Baker et al., 2008; Tomchek et al., 2014; Perez-Repetto, Jasmin, Fombonne, Gisel, & Couture, 2017; Kirby et al., 2022), and often have profound impacts on daily life: SOR symptoms in particular have been associated with increased impairment in a wide range of areas including reduced daily living and adaptive functioning skills (Hitoglou et al., 2010; Dellapiazza et al., 2018; Neufeld et al., 2021), social skill deficits (Hilton et al., 2007; Glod et al., 2015) anxiety (Hofmann & Bitran, 2007; Uljarević et al., 2016) and externalizing behaviors (O’Donnell et al., 2012).
While existing literature has included a multitude of measures to examine sensory processing, including questionnaires, direct assessment, physiology, and neural reactivity (Cascio et al., 2016; DuBois et al., 2017; Burns et al., 2017), parent reports are used the vast majority of the time (Rogers et al., 2003; Dickie et al., 2009). A fairly recent systematic review of 93 studies assessing sensory features in autistic individuals found that only 6.5% of the studies used direct assessment data, whereas 10.8% used self-reported data and 80.7% used parent-reported or caregiver-reported data (Burns et al., 2017). Commonly used questionnaires include the Sensory Experience Questionnaire (SEQ; Baranek et al., 2006), the Sensory Profile (McIntosh et al., 1999), the Sensory Processing Measure (Kuhaneck et al., 2007), and the Sensory Processing 3-Dimensional Inventory (Schoen et al., 2017; Miller et al., 2017). Using parent-reported data can be advantageous, as it provides cross-contextual information from someone very familiar with the child, and further can often be collected more rapidly and easily when compared to direct assessments (Cascio et al., 2016; Jorquera-Cabrera et al., 2017). However, reports completed by caregivers may also be subjective and influenced by expectations that may differ from children’s actual behavior (Tomchek et al., 2014; Tavassoli et al., 2019), and there is often limited consistency between observational assessments and sensory questionnaires (Schoen et al., 2008; Baranek et al., 2008; Tavassoli, Hoekstra, & Baron-Cohen, 2014; Cascio et al., 2016; Tavassoli et al., 2016; Tavassoli et al., 2019). A small amount of prior research has also suggested that, compared to parent report, direct observational assessments may be more highly correlated with biological correlates of sensory processing (Chang et al., 2016). There are fewer observational measures compared to questionnaires that assess sensory modulation behaviors, and these are generally aimed at use with very young children through late childhood/early adolescence (e.g., the Sensory Processing Assessment (Baranek, 1999), the Sensory Processing 3-Dimensional Assessment (SP3-D:A; Mulligan et al., 2019), the Sensory Integration and Praxis Test (SIPT; Ayres 1996), and the Sensory Assessment for Neurodevelopmental Disorders (SAND; Siper & Tavassoli, 2021). Parent-reported data and direct assessment data can provide different but potentially complementary information; parents can observe a wide range of behaviors across multiple settings and developmental periods. Conversely, direct assessments provide insight into participants’ sensory processing at a single timepoint in a controlled environment, which is more limited than parent report but can also be more objective and thus more easily comparable across participants. Additionally, observed assessments administered by trained professionals may be able to better identify and categorize sensory impairments when compared to parent reports.
Furthermore, despite evidence showing that sensory features may persist into older adolescence and adulthood, there are few standardized observational assessments that are tailored specifically for adolescents and adults (DuBois et al., 2017). Of the few existing assessments for children, most are not validated for adolescents (DuBois et al., 2017), which limits the ability to understand how sensory processing may change across development. As children grow older, they may exhibit fewer sensory features, as they learn to self-regulate in response to aversive sensory stimuli (Baranek et al., 2007, 2019; Ben-Sasson et al., 2010; Perez-Repetto, Jasmin, Fombonne, Gisel, & Couture, 2017), which is consistent with findings that prevalence of observed sensory symptoms is greater in toddlers than in young school-aged children (Baranek et al., 2007). However, some cross-sectional parent report studies have found either no significant change (McCormick et al., 2016; Baranek et al., 2019) or an increase (Talay-Ongan & Wood, 2000) in sensory over-responsivity from childhood into early adolescence, and evidence from a meta-analysis even indicates a non-linear change, in which sensory features increase until 6 to 9 years old and later decrease (Ben-Sasson et al., 2009). These inconsistent findings could reflect the wide variability in individual trajectories and highlights the need for further study of sensory responsivity across development. Of the few studies examining the relationship between age and sensory processing symptoms in ASD, none to our knowledge have examined specifically how observed sensory behaviors relate to age across later childhood and adolescence. Thus, there is a strong need to develop standardized observational assessments of sensory features that can be used across a wide range of ages, including adolescents.
The Sensory Processing 3-Dimensional (SP3-D) Assessment is an observational behavioral assessment that can be used to examine a number of sensory features including modulation, and has been shown to distinguish sensory modulation between TD youth and youth with sensory processing atypicalities, which in some cases included an ASD diagnosis (Mulligan et al., 2019; Schoen et al., 2014; Tavassoli et al., 2019). However, the measure has primarily been validated in preschool- to school-aged children, and in particular ASD versus TD groups have only been directly compared on this assessment in school-aged children (Tavassoli et al., 2016). An initial pilot study of the SOR items of the SP3-D Assessment indicated that the measure could differentiate individuals with high and low SOR across young children through adults (Schoen et al., 2008), which suggests that the measure may be valid in older children and adolescents, but this measure has not been extensively studied in this age range.
Therefore, a primary goal of this study was to characterize how the SP3-D Assessment is able to differentiate ASD and typically-developing (TD) school-aged children and adolescents on measures of sensory modulation, including SOR, SC, and SUR, across visual, auditory, and tactile stimulation. Additionally, we aimed to examine the distribution of assessment scores within our ASD compared to TD groups as to introduce potential score thresholds for determining atypically high sensory modulation behavior. Given the varied strengths and weaknesses of observed and parent-reported data, we also sought to examine the relationship between the SP3-D Assessment and parent-reported data provided in the Sensory Processing 3-Dimensional (SP3-D) Inventory, and the unique ability of each to predict diagnosis, to inform the utility of collecting both observed and reported data. Finally, we examined relationships between age, diagnostic group, and sensory processing behaviors, first, to determine whether the SP3-D Assessment can differentiate sensory processing behaviors specifically in adolescents with ASD compared to those with typical development, and second, to understand how observed versus parent-reported measures can contribute to understanding of how sensory processing behaviors change with age.
Methods
Participants
Participants included 74 children and adolescents between the ages of 7 and 17 years (mean age, 13.7 years) who participated in the Sensory Processing 3-Dimensional Assessment (SP3-D; Mulligan et al., 2019). Forty-one of the participants had an ASD diagnosis, and 33 participants were typically developing (TD) youth. ASD and TD groups did not differ significantly in sex, race/ethnicity, age, or performance IQ [Table 1]. All participants had a full-scale IQ within the typical range based on the Weschler Abbreviated Scales of Intelligence- Second Edition (WASI-II; Wechsler 2011). Participants in the ASD group had a formal diagnosis according to the Autism Diagnostic Interview – Revised (ADI-R; Lord et al., 1994) and/or the Autism Diagnostic Observation Schedule – Second Edition (ADOS-2; Lord et al., 2000), and clinical judgment. To ensure that our sample was generalizable to the general autistic population, we did not exclude for prevalent comorbidities with autism (e.g., attention-deficit/hyperactivity disorder (ADHD), anxiety, and depression). The study was approved by the University of California Los Angeles (UCLA) Institutional Review Board, and informed consent and assent were obtained from all participants.
Table 1.
Participants’ demographics, including diagnostic group, assigned sex at birth, age, and Performance IQ
| ASD (n = 41) | TD (n = 33) | t or χ2 | |||||
|---|---|---|---|---|---|---|---|
| Assigned Sex at Birth | 0.72 | ||||||
| Female | 10 | 11 | |||||
| Male | 31 | 22 | |||||
| Race/Ethnicity | |||||||
| White, not Hispanic or Latino/a | 13 | 12 | 0.62 | ||||
| Asian, not Hispanic or Latino/a | 5 | 5 | |||||
| Black or African American, not Hispanic or Latino/a | 2 | 2 | |||||
| Hispanic or Latino/a | 9 | 6 | |||||
| Multiracial, not Hispanic or Latino/a | 7 | 5 | |||||
| Multiracial, Hispanic or Latino/a | 5 | 3 | |||||
| Mean | SD | Range | Mean | SD | Range | ||
| Age (years) | 14.19 | 3.11 | 7.64–17.98 | 13.09 | 3.01 | 8.62–17.67 | −1.54 |
| Performance IQ | 108.9 | 16.99 | 112.67 | 11.49 | 1.09 | ||
Study Procedure
Participants were recruited as part of a larger, cross-sectional neuroimaging study and came in at one timepoint.
Measures
Sensory Processing 3-Dimensional (SP3-D) Assessment
The Sensory Processing 3-Dimensional (SP3-D) Assessment examines multiple domains of sensory processing, including sensory modulation, discrimination, and sensorimotor abilities across multiple sensory modalities including visual, auditory, tactile, proprioceptive, and vestibular domains (Mulligan et al., 2019). The assessment has been shown to have good inter-rater reliability and internal consistency (Mulligan et al., 2019), as well as construct validity in identifying sensory modulation features (Schoen et al., 2014). For the purposes of this study, only the auditory, tactile, and visual modulation items on the SP3-D Assessment were administered, which consisted of a series of sensory “games” that lasted about 30 minutes in total. The assessment was adapted to allow for standardized collection of physiological data (i.e., heart rate and galvanic skin response) during administration of the sensory modulation items. This adaptation included standardizing the amount of time for which each item was administered as well as adding two experimenter-administered components to complement two groups of items that were self-administered in the original assessment. Visual stimuli included a spinning disk, strobe lights, and a sparkle wheel. The tactile domain included removing plastic animals from goo, as well as two different experimenter-administered brushes on the participants’ forearm and one brush on the participants’ lips. Participants were then asked to self-administer the three brushes, for a total of seven tactile stimuli. The auditory domain included six tasks, in which participants listened to an experimenter play three instruments (cymbals, a cymbal and a stick, and a whistle) along with music and then were asked to play the same three instruments as loudly as they could. After each task, the stimuli were left in front of the participants for 10–15 seconds to examine if participants exhibited any additional atypical sensory responses outside of the structure of the task.
Examiners were trained by a licensed clinical psychologist, who also acted as the master coder, based on the SP3-D administration manual (as yet unpublished; see Mulligan et al., 2019), as well as consultation with one of the developers of the assessment. SP3-D sensory behaviors were scored using standardized scoring guidelines in consensus with a master coder and with developers of the assessment; inter-rater reliability was not established because every assessment was reviewed by the master coder. Potentially atypical modulation behaviors were categorized into sensory-under responsivity (SUR), sensory seeking/craving (SC), and sensory over-responsivity (SOR). SUR is defined as “decreased awareness” or a lack of an expected response to a strong or aversive stimulus (e.g., pressing very hard on arm with a scratchy brush; no reaction to loud cymbal noise). SC is defined as “wanting increased input” or difficulty disengaging with the stimulus (e.g., peering at the sparkle wheel from the side; continuing to play with goo after the task). SOR is defined as an “adverse” response to or avoidance of the stimulus (e.g., grimace in response to tactile brush; putting hands over ears during musical instruments). Consistent with scoring established by Tavassoli et al., (2016), for each item or task in the assessment, participants’ responses were scored as typical (0) or atypical (1) for each category of SUR, SC, and SOR. Additionally, each task received a score of typical or atypical both during the task and during the 10–15 seconds after the task. Total SUR, SC, and SOR domain scores were calculated by summing the number of atypical responses throughout all tasks, across both during and after time periods, and all three domains (visual, tactile, and auditory). Scores were summed across the during and after periods of 16 tasks (3 visual, 7 tactile, 6 auditory), so that the highest possible score for total SUR, SC, and SOR was 32.
Sensory Processing 3-Dimensional (SP3-D) Inventory (Miller et al., 2017)
Parents of participants completed the SP3-D Inventory, which asks respondents to indicate which of a list of visual, tactile, and auditory stimuli participants are over-responsive to, under-responsive to, or seek out. SUR, SC, and SOR total counts were calculated by summing the number of items for which parents indicated atypical responses. The maximum total SUR, SC, and SOR counts on the parent-reported SP3-D Inventory were 20, 20, and 44, respectively.
Data Analytic Plan
Diagnostic group differences
To understand how sensitive the reported and observed measures are in differentiating sensory processing behaviors in ASD compared to TD groups, independent samples t-tests were run for SUR, SS, and SOR domains on the observed SP3-D Assessment and in the parent-reported SP3-D Inventory.
Relationship between reported and observed data
Pearson correlations were used to examine the relationship between reported and observed data. Regression “uniqueness” analyses were then run including both reported and observed measures as a predictor of diagnosis to identify the extent to which the observed SP3-D assessment uniquely predicted diagnostic group over and above the parent-reported SP3-D Inventory. Cohen’s D was also calculated for each instrument testing the separability of test scores between the ASD and TD groups. Cohen’s D, a measure of effect size, calculates how much overlap exists between the distributions of test scores for the two groups. Finally, we ran “tipping point” analyses to identify the cutoff scores that best differentiated sensory processing behaviors in the ASD versus TD groups.
Here, our focus was on diagnostic group differences in the SP3-D observed Assessment, as group differences in parent-reported sensory modulation has already been published on extensively in this measure and others (Baranek et al., 2006; Tomchek & Dunn, 2007; Tavassoli et al., 2018). Thus, we did not correct for multiple comparisons in this study given that there were only 3 main comparisons of interest in the paper.
We defined the tipping point as the score in each measure and behavior at which a child had > = 50% chance of an ASD diagnosis. We then also calculated scores for which the chances were > = 75% and > = 90% for having ASD. Mathematically, the tipping point was identified using a logistic regression model (Rice, 2007).
The logit equation states that:
This implies that
Substituting p(x) = 0.5, the tipping point score cutoff
For each of the six measures (SP3-D Assessment SOR, parent-reported SOR, SP3-D Assessment SC, parent-reported SC, SP3-D Assessment SUR, parent-reported SUR), the logistic regression parameters from the models were used to calculate the subscale’s tipping point, x, when the subtest was significantly associated with ASD diagnoses. If the subtest was not significantly associated with ASD diagnosis, then the tipping point was not computed.
Age-related changes in sensory processing behaviors
Hierarchical regression analyses were used to examine the main effect of age on each sensory domain as well as the interaction between age and diagnostic group. Diagnostic group status (ASD or TD) and age main effects were entered in a first step, with the interaction term (group*age) entered in a second step. The interaction term was removed if not significant so that the main effects could be interpreted.
Results
Diagnostic group differences
Mean scores
An independent-samples t-test showed that the ASD group showed higher SOR and SC behaviors on the SP3-D Assessment, but there were no group differences in observed SUR. As expected, the ASD group was also rated higher in all three categories on the parent-reported SP3-D Inventory [Table 2]. Due to the very low frequency in SUR demonstrated on SP3-D Assessment, these SUR scores were not examined in subsequent analyses. Table 2 also shows descriptive statistics for the SP3-D parent report for reference and comparison with the observed assessment.
Table 2.
Mean scores and standard deviations across all sensory modulation behaviors in the observed SP3-D Assessment and parent-reported SP3-D Inventory in ASD and TD groups
| ASD (n = 41) | TD (n = 33) | Mean Differences | SD Differences | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | P-value | F | P-value | |
| Observed SOR | 2.76 | 2.78 | 1.27 | 1.89 | 2.61 | 0.01 | 1.35 | 0.25 |
| Parent SOR | 9.1 | 8.38 | 1.45 | 2.28 | 5.08 | < 0.001 | 29.82 | < 0.001 |
| Observed SC | 2.39 | 2.97 | 1.00 | 2.14 | 2.62 | 0.03 | 5.07 | 0.03 |
| Parent SC | 4.02 | 4.05 | 0.85 | 1.92 | 4.14 | < 0.001 | 15.92 | < 0.001 |
| Observed SUR | 0.37 | 0.66 | 0.18 | 0.39 | 1.41 | 0.16 | 9.62 | 0.003 |
| Parent SUR | 6.73 | 5.44 | 1.97 | 3.49 | 4.36 | < 0.001 | 11.35 | 0.001 |
Distribution
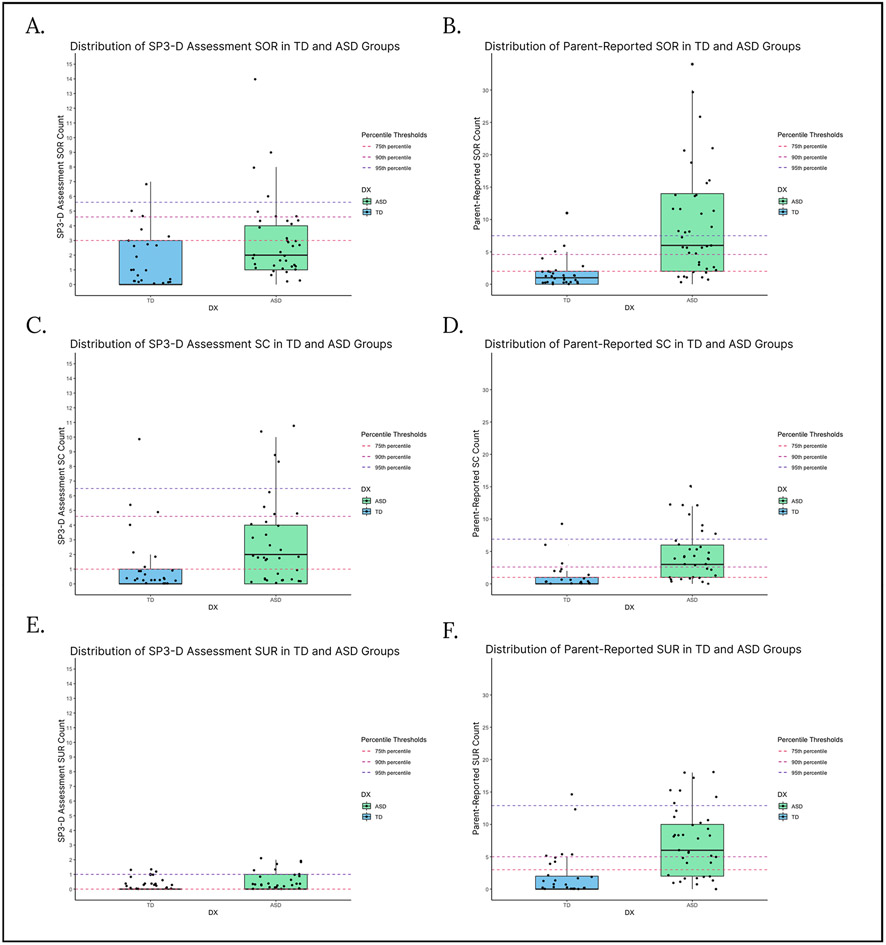
Distributions of scores in each group are shown in Fig. 1. Dotted lines show the 75th, 90th, and 95th percentiles of the TD group to illustrate a possible “typical” range of scores and how the ASD scores differed from this range. The 75th, 90th, and 95th percentile scores were calculated using the distribution of the scores within the TD group (i.e., the 75th percentile cutoff indicated that 75% of the TD group fell below that score). Percentiles were used as potential score thresholds instead of using standard deviations, as the TD and ASD groups’ scores were not normally distributed. Additionally, Table 2 shows that, compared to the TD group, the ASD group had significantly higher standard deviations across every domain other than observed SOR, indicating significantly greater variance in scores in the ASD group.
Fig. 1.
Box plots for parent-reported and observed SOR, SC, and SUR in ASD and TD groups, with thresholds indicating the 75th, 90th, and 95th percentile scores for the TD group (Fig. 1a: observed SOR, Fig. 1b: reported SOR, Fig. 1c: observed SC, Fig. 1d: reported SC, Fig. 1e: observed SUR, Fig. 1f: reported SUR)
Relationship between reported and observed data
There were no significant correlations between SUR, SC, and SOR behaviors observed in the SP3-D Assessment and the corresponding sensory modulation behaviors as reported by the participants’ parents in the SP3-D Inventory [Table 3]. However, there were significant correlations between sensory subscales within each measure, with observed SOR and SC showing a correlation of r = 0.50 (p < 0.001), and parent-reported SOR, SUR, and SC correlated with an r = 0.50–0.80 (p < 0.001).
Table 3.
Correlations between observed SP3-D Assessment and parent-reported SP3-D Inventory
| Reported SC |
Reported SOR |
Observed SUR |
Observed SC |
Observed SOR |
|
|---|---|---|---|---|---|
| Observed SC | 0.46*** | ||||
| Observed SUR | 0.16 | 0.15 | |||
| Reported SOR | 0.18 | 0.22† | 0.18 | ||
| Reported SC | 0.51*** | 0.20† | 0.21† | 0.20† | |
| Reported SUR | 0.80*** | 0.56*** | 0.09 | 0.25* | 0.15 |
p < 0.1
p < 0.05
p < 0.01
p < 0.001
Uniqueness analyses
Three regression analyses (one each for SOR, SC, and SUR) examined whether parent-reported and observed sensory modulation behaviors uniquely predicted diagnosis [Table 4]. Both SOR measures showed unique ability to predict ASD diagnosis (i.e., variability in observed SOR predicted diagnosis over and above variability in parent report). However, observed SC and SUR did not add additional predictive value over and above parent-reported SC and SUR, respectively.
Table 4.
Uniqueness analysis: regression analyses across observed and reported SOR, SC, and SUR behaviors
| SOR | SC | SUR | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B Final Model | Std. Error | Pseudo-R2* | B Final Model | Std. Error | Pseudo-R2* | B Final Model | Std. Error | Pseudo-R2* | |
| (Intercept) | −1.87*** | 0.54 | 0.37 | −0.80* | 0.35 | 0.20 | −0.90* | 0.37 | 0.19 |
| Observed | 0.33* | 0.15 | 0.16 | 0.12 | 0.62 | 0.55 | |||
| Reported | 0.43*** | 0.13 | 0.40** | 0.14 | 0.25*** | 0.07 | |||
p < 0.05
p < 0.01
p < 0.001, B final model indicates the unstandardized beta-values for each variable included in the final model
Pseudo-R2 values provided are McFadden Pseudo R2 values used for logistic regression
Tipping point analyses
Logistic regression “tipping point” analyses were used to determine the score for each measure subscale at which the likelihood was greater than 50%, 75%, and 90% for having ASD. Consistent with the independent-samples t-tests conducted above, for all but observed SUR, each sensory subscale was significant in predicting ASD diagnoses. The Cohen’s D for these tests ranged from 0.30 to 1.18 [Table 5].
Table 5.
Tipping point analysis for all sensory modulation behaviors in reported and observed measures
| Subtest | 50% Threshold |
75% Threshold |
90% Threshold |
Cohen’s D |
|---|---|---|---|---|
| SP3-D SOR * | 1.20 | 4.61 | 8.02 | 0.61 |
| Parent-reported SOR *** | 3.01 | 5.69 | 8.37 | 1.19 |
| SP3-D SC * | 0.66 | 4.83 | 9.31 | 0.53 |
| Parent-reported SC ** | 1.42 | 3.98 | 6.54 | 0.97 |
| SP3-D SUR | NA | NA | NA | 0.33 |
| Parent-reported SUR *** | 2.99 | 7.35 | 11.72 | 1.02 |
Significance of subtest in predicting ASD diagnoses in logistic regression model, as follows:
p < 0.05
p < 0.01
p < 0.001
Tipping point values and Cohen’s D scores indicated that the most separable measures (largest Cohen’s D) also had the largest tipping point score cutoff. This occurs because the TD groups have lower reported values on these measures.
Age-related changes in sensory processing
Age by diagnostic group interaction
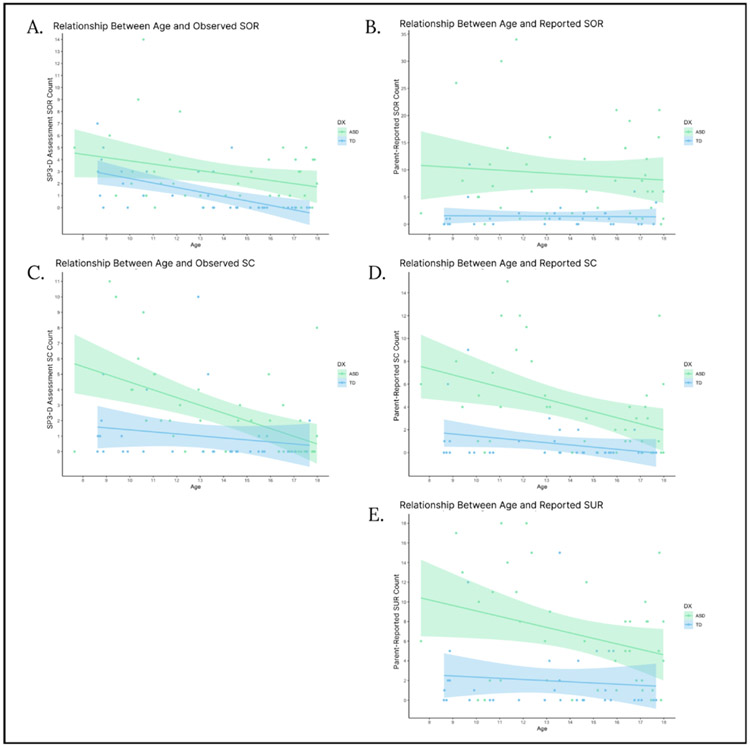
Regression analyses were run to examine the relationship between age and each sensory subscale as well as to determine whether these relationships differed by diagnostic group. There was a significant age by diagnostic group interaction in the regression predicting observed SC behaviors, indicating that sensory craving decreased with age for the ASD group only [Table 6; Fig. 2c]. There were no other age by diagnostic group interactions, so the interaction term was removed from all of the other final regression models [Table 6]. There was a main effect of age on observed SOR, parent-reported SC, and parent-reported SUR, indicating that these scores decreased with age across both diagnostic groups. These main effects remained significant even after removing outliers (2 ASD participants in observed SOR, 1 ASD and 2 TD participants in reported SOR, 3 TD and 1 ASD participant in observed SC, 3 TD and 1 ASD participant in reported SC, and 2 TD participants in reported SUR). Age was not significantly associated with parent-reported SOR.
Table 6.
Regression analysis analyzing effects of diagnostic group, age, and interaction between diagnostic group and age on SP3-D Assessment and parent-reported SP3-D Inventory scores
| SP3-D SOR | Parent-Reported SOR | SP3-D SC | Parent-Reported SC |
Parent-Reported SUR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B Final Model |
Std. Error | ΔR2 | B Final Model | Std. Error | ΔR2 | B Final Model |
Std. Error | ΔR2 | B Final Model |
Std. Error | B Final Model | Std. Error | ΔR2 | |
| Step 1: | 0.23*** | 0.27*** | 0.21*** | 0.26*** | ||||||||||
| DX Group | 1.83** | 0.53 | 7.82*** | 1.54 | 1.77** | 0.58 | 3.61*** | 0.73 | 5.17*** | 1.09 | ||||
| Age | 0.09 | −0.16 | 0.25 | 0.09 | −0.39** | 0.12 | −0.37* | 0.18 | ||||||
| Step 2: | 0.26* | |||||||||||||
| DX x Age | - | - | - | - | −0.37 | 0.19 | - | - | - | - | ||||
p < 0.1
p < 0.05
p < 0.01
p < 0.001, B final model indicates the unstandardized beta-values for each variable included in the final model
Fig. 2.
Correlations between age and reported and observed sensory modulation behaviors in ASD and TD groups (Fig. 2a: observed SOR, Fig. 2b: reported SOR, Fig. 2c: observed SC, Fig. 2d: reported SC, Fig. 2e: reported SUR). Across both diagnostic groups, age was significantly negatively correlated with observed SOR, reported SC, and reported SUR, and age by diagnostic group interactions were not significant. There was a significant age by diagnostic group interaction in observed SC, in which observed SC was negatively related to age in the ASD group but not in the TD group
Discussion
This study examined how performance on an observed assessment of sensory responsivity differs across ages and across autistic youth compared to those with typical development. Broadly, goals included determining the utility of the SP3-D sensory Assessment in differentiating sensory responsivity in ASD compared to TD youth, creating a baseline for normative performance on the SP3-D, and assessing how observed versus parent-reported sensory responsivity related to age.
Results demonstrated that the SP3-D Assessment identified higher SOR and SC behaviors in the ASD compared to TD youth, across children and adolescents, which is consistent with existing literature using parent-reported data in this age range (e.g., Kientz & Dunn 1997; Baranek et al., 2006; Tomchek & Dunn, 2007; Miller et al., 2017; Tavassoli et al., 2018). While observed measures have shown higher rates of sensory modulation behaviors in younger children (e.g., Baranek et al., 2007; Tavassoli et al., 2016; Siper et al., 2017), there are no studies to our knowledge that compare observed behaviors in older ASD versus TD youth. Our results suggest that the SP3-D is sensitive to higher rates of sensory features even in older children and adolescents with cognitive abilities in the average range or higher, who may be better than young children at regulating their responses. However, the SP3-D did not show diagnostic group differences in SUR, which could be due to a floor effect, as there were very few SUR behaviors observed in either group. The SP3-D may be less effective at identifying SUR in this age range compared to SOR and SS, especially given that parents did report higher levels of all three types of sensory features in the ASD group. So, although there was a low frequency of observed SUR behaviors, this does not necessarily imply a low prevalence of SUR, considering that there were significant diagnostic group differences in SUR endorsed by parents. Rather, the SP3-D Assessment stimuli may not elicit SUR behaviors in this population of older children and adolescents with relatively high cognitive abilities. Further, parents may be more aware of whether or not sensory differences are associated with daily life impairment, which is an important aspect of determining a clinical diagnosis, and which is hard to assess in a lab setting.
Our analyses also found that observed sensory behaviors did not significantly correlate with behaviors reported by parents, which is consistent with previous literature (Schoen et al., 2017; Tavassoli et al., 2019). This lack of correlation suggests that the observed assessment is measuring a different aspect of sensory responsivity than that reported by parents and emphasizes the importance of including both in clinical evaluation and research. Further analysis showed that both reported and observed SOR contributed unique predictive value to ASD diagnosis, indicating that the observed assessment captured variability in ASD-related SOR behaviors beyond those measured by parents. In contrast, only parent-reported SC uniquely predicted diagnosis; interestingly, the observed and reported data were not correlated, but each measure independently showed diagnostic group differences. To the extent that observed sensory craving on the SP3-D is measuring a unique aspect of individual variability beyond that measured by the parent report, the observed data may capture something other than ASD-related SC, such as impulsivity or age (see further discussion below on age and sensory seeking and craving behaviors). Overall, diagnostic group differences in sensory behaviors appeared larger in the parent reports compared to the observed measure in this sample, which could suggest that lab assessments are either less sensitive to existing sensory symptoms or more stringent in measuring clinical levels of sensory features. For example, an observed assessment may identify fewer sensory symptoms but could be more specific in identifying the most severe symptoms. Notably, there is wide variability in sensory responsivity within ASD (Uljarević et al., 2017; Tillmann et al., 2020) and it is important for a behavioral assessment to capture this variability as well as to determine which behaviors differ from a typical range. We thus explored two different methods of determining potential score thresholds for differentiating typical versus atypical sensory modulation behaviors. First, we provided threshold scores at the TD group’s 75th, 90th, and 95th percentiles so that scores can be compared to these typical distributions. For example, 25% of TD youth demonstrated three or more SOR behaviors on the observed assessment, indicating that some degree of over-responsivity may be typical but that more than three of these behaviors may suggest a significant level of over-responsivity. A second method used diagnostic group differences to identify “tipping point” scores at which the likelihood of having ASD was greater than 50%, 75%, or 90% for each sensory domain.
Age-related analyses indicated that observed SOR behaviors decreased with age in both ASD and TD participants, though these remained higher in the ASD compared to the TD group into adolescence. These findings are consistent with prior findings that even as sensory features change with age, there is stability in how children rank on severity of symptoms (Baranek et al., 2019). Notably, whereas observed SOR was significantly associated with age, parent-reported SOR was not. This could potentially indicate that older participants in the sample are better able to regulate their behaviors during the lab assessment or not show outward behaviors while their bodies continue to respond physiologically. Alternatively, as children get older, parents may be less attuned to improvements in their children’s SOR symptoms or still notice SOR symptoms across settings despite their children’s regulation in certain contexts.
In contrast to SOR, observed sensory craving behaviors decreased with age more so for the ASD than for the TD group such that the diagnostic group differences declined with age. While parent-reported SC showed a similar trend, the diagnostic group by age interaction did not reach significance, likely because the TD group also showed some age-related declines. A reduction in sensory craving across adolescence could reflect the extent to which these behaviors are associated with impulsivity. In particular, observed sensory craving behaviors were most commonly scored when participants continued to engage with stimuli after the task was completed. In this case, a decline in actively seeking out more sensory input could relate more to improvements in response inhibition or ability to resist the temptation to play with the stimulus, which is associated with age (Steinberg et al., 2008), rather than internal changes in the extent to which an individual wants to engage with the sensory stimulus. In other words, our scoring of sensory craving in the SP3-D Assessment reflects outward behavior and cannot necessarily determine the underlying cause of the behavior. This could further explain the discrepancy between the observed assessment and parent report: potentially, the “sensory craving” score on the SP3-D Assessment might be identifying an impulsivity-driven enjoyment of exploring the stimuli rather than a clinical level of sensory seeking or craving that interferes in everyday life. It can be difficult to determine a strong need for sensory exploration in adolescents during an observed assessment as they improve their ability to inhibit such responses. Parents may have more opportunities to observe such behaviors across different environments where their children are either less able or less interested in response inhibition.
Overall, findings suggest that the SP3-D Assessment and parent report can be used to measure sensory processing differences across older children and adolescents, and that the observed SP3-D Assessment is particularly sensitive in identifying SOR in ASD across ages, which is consistent with the initial development of the measure, in which it was piloted across a wide age range specifically to identify SOR (Schoen et al., 2008). In contrast, for sensory craving in ASD youth with high cognitive and verbal skills, the observed assessment may be identifying impulsivity rather than ASD-related sensory modulation differences. Given that some sensory features have a strong impact on quality of life, including associations with reduced daily living and adaptive functioning skills (Hitoglou et al., 2010; Dellapiazza et al., 2018; Neufeld et al., 2021), social skill deficits (Hilton et al., 2007; Glod et al., 2015), anxiety (Hofmann & Bitran, 2007; Uljarević et al., 2016), and externalizing symptoms (O’Donnell et al., 2012), understanding the development of these symptoms can help inform early interventions for autistic populations.
Taken together, results further inform the value of collecting both direct observational assessments and parent-reported data, and potentially combining these measures for clinical and/or research purposes as suggested in prior research (Risi et al., 2006; Kim & Lord, 2011; Tavassoli et al., 2016), to gain a greater understanding of individuals’ sensory modulation behaviors. Parents have more opportunities to recognize sensory processing across varied intensities and environments whereas direct lab assessments can use standardized stimuli (in length of exposure and aversiveness of stimuli) to objectively compare participants’ responses across diagnostic groups and age ranges (Tavassoli et al., 2019). For example, parents of autistic youth may be more likely to rate their child high on a sensory assessment because they are more familiar with sensory responsivity as a symptom of ASD and thus have a greater likelihood of both noticing these behaviors and considering them as atypical.
Observed data and parent reports can also provide complimentary information about the development of sensory responsivity, as observed assessments may be more sensitive to age-related changes in ability to regulate sensory responses, whereas parents may be more aware the sensory stimuli continue to bother their children despite their reduced overt responsivity to the stimuli.
Limitations and future directions
As mentioned, this study used a modified version of the SP3-D Assessment visual, tactile, and auditory modulation domains only, in part due to the need to standardize physiological data collection as well as because the assessment was a part of a larger study focusing on visual, tactile, and auditory sensory responses. Given the limitations of our sample size and inclusionary criteria, the thresholds provided here are exploratory, and meant as reference for future study as a larger standardization sample should be used to further establish cutoff scores for clinical and research use. Further research with a larger sample is also needed to understand the distribution of behaviors within separate sensory subdomains. Additionally, our sample is representative of the autistic population in the sense that it includes many participants who exhibit comorbid symptoms, such as ADHD and anxiety, so future research is needed to determine how these symptoms may affect observed sensory behaviors.
While this study demonstrated clear strengths of the SP3-D Assessment, particularly in identifying SOR in autism across ages, there are also some limitations to using an observed behavioral assessment. In particular, such assessments are designed to measure outward behavior as objectively as possible, but similar behaviors can arise from different underlying causes. For example, we cannot solely use behavior to know why participants are showing increased engagement with the stimuli (e.g., whether they truly are craving more of the input or if they are fidgeting with a stimulus due to impulsivity). Especially for adolescents and adults who are able to report on their internal experiences, future studies can explore integrating self-reported data with behavioral observations to determine what participants are experiencing while displaying particular behaviors. Additionally, to address the discrepancies between observed and reported data, combining observed behavior with physiology may provide additional insight into sensory responsivity (e.g., Jung et al., 2021), particularly in older adolescents who may find sensory stimulation unpleasant but are more likely to control their behavioral responses. In fact, there is some evidence from prior studies that autistic youth who show reduced SOR behaviors have reduced heart rate acceleration (Jung et al., 2021) and greater prefrontal down-regulation of the amygdala (Green et al., 2015, 2019) during aversive sensory stimulation. Future research should also continue to explore how observed versus reported sensory responsivity relate to biological mechanisms, as there is some preliminary data suggesting that observed behavior may be more highly correlated with biology (Chang et al., 2016).
Conclusions
In conclusion, the SP3-D Assessment differentiated both sensory over-responsivity and sensory craving in ASD compared to TD children and adolescents, and it was particularly sensitive to identifying higher SOR across ages. Assessment scores were not correlated with parent report, though both observed and reported SOR uniquely contributed to predicting ASD diagnosis. Some low-level sensory responsivity appeared normative within the TD sample, particularly at younger ages, though the ASD group had more variability in sensory responsivity in addition to overall higher scores. Results highlight the importance of integrating observational and questionnaire data and of using consistent assessments across childhood and adolescence to track developmental changes in sensory processing.
References
- Ayres AJ (1996). Sensory integration and praxis tests (SIPT). Los Angeles, CA: Western Psychological Services (WPS) [Google Scholar]
- Baker AEZ, Lane A, Angley MT, & Young RL (2008). The relationship between sensory processing patterns and behavioural responsiveness in autistic disorder: A pilot study. Journal of Autism and Developmental Disorders, 38(5), 867–875. 10.1007/s10803-007-0459-0 [DOI] [PubMed] [Google Scholar]
- Baranek GT (1999). Sensory processing assessment for young children (SPA). Unpublished manuscript, University of North Carolina at Chapel Hill [Google Scholar]
- Baranek GT, Boyd BA, Poe MD, David FJ, & Watson LR (2007). Hyperresponsive sensory patterns in young children with autism, developmental delay, and typical development. American Journal of Mental Retardation: AJMR, 112(4), 233–245. 10.1352/0895-8017(2007)112[233:HSPIYC[2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Baranek GT, David FJ, Poe MD, Stone WL, & Watson LR (2006). Sensory Experiences Questionnaire: Discriminating sensory features in young children with autism, developmental delays, and typical development. Journal of Child Psychology and Psychiatry and Allied Disciplines, 47(6), 591–601. 10.1111/j.1469-7610.2005.01546.x [DOI] [PubMed] [Google Scholar]
- Baranek GT, Roberts JE, David FJ, Sideris J, Mirrett PL, Hatton DD, & Bailey DB (2008). Developmental trajectories and correlates of sensory processing in young boys with fragile X syndrome. Physical & Occupational Therapy in Pediatrics, 28(1), 79–98. 10.1300/j006v28n01_06 [DOI] [PubMed] [Google Scholar]
- Baranek GT, Carlson M, Sideris J, Kirby AV, Watson LR, Williams KL, & Bulluck J (2019). Longitudinal assessment of stability of sensory features in children with autism spectrum disorder or other developmental disabilities. Autism Research, 12(1), 100–111. 10.1002/aur.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sasson A, Hen L, Fluss R, Cermak SA, Engel-Yeger B, & Gal E (2009). A Meta-Analysis of Sensory Modulation Symptoms in Individuals with Autism Spectrum Disorders. Journal of Autism and Developmental Disorders, 39(1), 1–11. 10.1007/s10803-008-0593-3 [DOI] [PubMed] [Google Scholar]
- Ben-Sasson A, Carter AS, & Briggs-Gowan MJ (2010). The Development of Sensory Over-responsivity From Infancy to Elementary School. Journal of Abnormal Child Psychology, 38( 8), 1193–1202. 10.1007/s10802-010-9435-9“ [DOI] [PubMed] [Google Scholar]
- Burns CO, Dixon DR, Novack M, & Granpeesheh D (2017). A Systematic Review of Assessments for Sensory Processing Abnormalities in Autism Spectrum Disorder. Review Journal of Autism and Developmental Disorders, 3(4), 209–224. 10.1007/s40489-017-0109-1 [DOI] [Google Scholar]
- Cascio CJ, Woynaroski T, Baranek GT, & Wallace MT (2016). Toward an interdisciplinary approach to understanding sensory function in autism spectrum disorder. Autism Research: Official Journal of the International Society for Autism Research, 9(9), 920–925. 10.1002/aur.1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YS, Gratiot M, Owen JP, Brandes-Aitken A, Desai SS, Hill SS … Mukherjee P. (2016). White Matter Microstructure is Associated with Auditory and Tactile Processing in Children with and without Sensory Processing Disorder. Frontiers in Neuroanatomy, 9. 10.3389/fnana.2015.00169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellapiazza F, Vernhet C, Blanc N, Miot S, Schmidt R, & Baghdadli A (2018). Links between sensory processing, adaptive behaviours, and attention in children with autism spectrum disorder: A systematic review. Psychiatry Research, 270, 78–88. 10.1016/j.psychres.2018.09.023 [DOI] [PubMed] [Google Scholar]
- Dickie VA, Baranek GT, Schultz B, Watson LR, & McComish CS (2009). Parent Reports of Sensory Experiences of Preschool Children With and Without Autism: A Qualitative Study. The American Journal of Occupational Therapy : Official Publication of the American Occupational Therapy Association, 63(2), 172–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois D, Lymer E, Gibson BE, Desarkar P, & Nalder E (2017). Assessing Sensory Processing Dysfunction in Adults and Adolescents with Autism Spectrum Disorder: A Scoping Review. Brain Sciences, 7(8), E108. 10.3390/brainsci7080108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glod M, Riby DM, Honey E, & Rodgers J (2015). Psychological Correlates of Sensory Processing Patterns in Individuals with Autism Spectrum Disorder: A Systematic Review. Review Journal of Autism and Developmental Disorders, 2(2), 199–221. 10.1007/s40489-015-0047-8 [DOI] [Google Scholar]
- Green SA, Hernandez L, Lawrence KE, Liu J, Tsang T, Yeargin J … Bookheimer SY (2019). Distinct Patterns of Neural Habituation and Generalization in Children and Adolescents With Autism With Low and High Sensory Overresponsivity. The American Journal of Psychiatry, 176(12), 1010–1020. 10.1176/appi.ajp.2019.18121333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Hernandez L, Tottenham N, Krasileva K, Bookheimer SY, & Dapretto M (2015). Neurobiology of Sensory Overresponsivity in Youth With Autism Spectrum Disorders. JAMA Psychiatry, 72(8), 778–786. 10.1001/jamapsychiatry.2015.0737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton C, Graver K, & Lavesser P (2007). Relationship between social competence and sensory processing in children with high functioning autism spectrum disorders. Research in Autism Spectrum Disorders, 1, 164–173. 10.1016/j.rasd.2006.10.002 [DOI] [Google Scholar]
- Hitoglou M, Ververi A, Antoniadis A, & Zafeiriou DI (2010). Childhood autism and auditory system abnormalities. Pediatric Neurology, 42(5), 309–314. 10.1016/j.pediatrneurol.2009.10.009 [DOI] [PubMed] [Google Scholar]
- Hofmann SG, & Bitran S (2007). Sensory-processing sensitivity in social anxiety disorder: Relationship to harm avoidance and diagnostic subtypes. Journal of Anxiety Disorders, 21(7), 944–954. 10.1016/j.janxdis.2006.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorquera-Cabrera S, Romero-Ayuso D, Rodriguez-Gil G, & Triviño-Juárez JM (2017). Assessment of Sensory Processing Characteristics in Children between 3 and 11 Years Old: A Systematic Review. Frontiers in Pediatrics, 5, 57. 10.3389/fped.2017.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Zbozinek TD, Cummings KK, Wilhelm FH, Dapretto M, Craske MG … Green SA (2021). Associations between physiological and neural measures of sensory reactivity in youth with autism. Journal of Child Psychology and Psychiatry, 62(10), 1183–1194. 10.1111/jcpp.13387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kientz MA, & Dunn W (1997). A comparison of the performance of children with and without autism on the Sensory Profile. The American Journal of Occupational Therapy: Official Publication of the American Occupational Therapy Association, 51(7), 530–537. 10.5014/ajot.51.7.530 [DOI] [PubMed] [Google Scholar]
- Kim SHS, & Lord C (2011). New Autism Diagnostic Interview-Revised Algorithms for Toddlers and Young Preschoolers from 12 to 47 Months of Age. Journal of Autism and Developmental Disorders, 42, 82–93. 10.1007/s10803-011-1213-1 [DOI] [PubMed] [Google Scholar]
- Kirby AV, Bilder DA, Wiggins LD, Hughes MM, Davis J, Hall-Lande JA … Bakian AV (2022). Sensory features in autism: Findings from a large population-based surveillance system. Autism Research, 15(4), 751–760. 10.1002/aur.2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhaneck HM, Henry D, & Glennon TJ (2007). Sensory Processing Measure: SPM. Western Psychological Services (WPS) [Google Scholar]
- Leekam SR, Nieto C, Libby SJ, Wing L, & Gould J, (2007). Describing the sensory abnormalities of children and adults with autism. Journal of Autism and Developmental Disorders, 37(5), 894–910. 10.1007/s10803-006-0218-7 [DOI] [PubMed] [Google Scholar]
- Liss M, Saulnier C, Fein D, & Kinsbourne M (2006). Sensory and attention abnormalities in autistic spectrum disorders. Autism: The International Journal of Research and Practice, 10(2), 155–172. 10.1177/1362361306062021 [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC … Rutter M (2000). The Autism Diagnostic Observation Schedule–Generic: A Standard Measure of Social and Communication Deficits Associated with the Spectrum of Autism. 19. [PubMed] [Google Scholar]
- Lord C, Rutter M, & Le Couteur A (1994). Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685. 10.1007/BF02172145 [DOI] [PubMed] [Google Scholar]
- McCormick C, Hepburn S, Young GS, & Rogers SJ (2016). Sensory symptoms in children with autism spectrum disorder other developmental disorders and typical development: A longitudinal study. Autism, 20(5), 572–579. 10.1177/1362361315599755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh DN, Miller LJ, Shyu V, & Dunn W (1999). Development and validation of the short sensory profile. Sensory profile manual, 61, 59–73 [Google Scholar]
- Miller LJ, Anzalone ME, Lane SJ, Cermak SA, & Osten ET (2007). Concept Evolution in Sensory Integration: A Proposed Nosology for Diagnosis. The American Journal of Occupational Therapy, 61(2), 135–140. 10.5014/ajot.61.2.135 [DOI] [PubMed] [Google Scholar]
- Miller LJ, Schoen SA, Mulligan S, & Sullivan J (2017). Identification of Sensory Processing and Integration Symptom Clusters: A Preliminary Study. Occupational Therapy International, 2017. 10.1155/2017/2876080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan S, Schoen SA, Miller LJ, Valdez A, & Magalhaes D (2019). The Sensory Processing 3-Dimensions Scale: Initial Studies of Reliability and Item Analyses. The Open Journal of Occupational Therapy, 7(1), 10.15453/2168-6408.1505 [DOI] [PubMed] [Google Scholar]
- Neufeld J, Hederos Eriksson L, Hammarsten R, Lundin Remnélius K, Tillmarm J, Isaksson J, & Bölte S (2021). The impact of atypical sensory processing on adaptive functioning within and beyond autism: The role of familial factors. Autism, 25(8), 2341–2355. 10.1177/13623613211019852 [DOI] [PubMed] [Google Scholar]
- O’Donnell S, Deitz J, Kartin D, Nalty T, & Dawson G (2012). Sensory processing, problem behavior, adaptive behavior, and cognition in preschool children with autism spectrum disorders. The American Journal of Occupational Therapy: Official Publication of the American Occupational Therapy Association, 66(5), 586–594. 10.5014/ajot.2012.004168 [DOI] [PubMed] [Google Scholar]
- Perez Repetto L, Jasmin E, Fombonne E, Gisel E, & Couture M, (2017). Longitudinal Study of Sensory Features in Children with Autism Spectrum Disorder. Autism Research and Treatment, 2017, 1934701. 10.1155/2017/1934701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JA (2007). Mathematical statistics and data analysis (3rd ed.). Thomson/Brooks/Cole [Google Scholar]
- Risi S, Lord C, Gotham K, Corsello C, Chrysler C, Szatmari P … Pickles, A. (2006). Combining Information From Multiple Sources in the Diagnosis of Autism Spectrum Disorders. Journal of the American Academy of Child & Adolescent P sychiatry, 45(9), 1094–1103. 10.1097/01.chi.0000227880.42780.0e [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Hepburn S, & Wehner E (2003). Parent Reports of Sensory Symptoms in Toddlers with Autism and Those with Other Developmental Disorders. Journal of Autism and Developmental Disorders, 33(6), 631–642. 10.1023/B:JADD.0000006000.38991.a7 [DOI] [PubMed] [Google Scholar]
- Schoen SA, Miller LJ, & Green KE (2008). Pilot study of the Sensory Over-Responsivity Scales: Assessment and inventory. The American Journal of Occupational Therapy: Official Publication of the American Occupational Therapy Association, 62(4), 393–406. 10.5014/ajot.62.4.393 [DOI] [PubMed] [Google Scholar]
- Schoen SA, Miller LJ, & Sullivan J (2017). The development and psychometric properties of the Sensory Processing Scale Inventory: A report measure of sensory modulation. Journal of Intellectual & Developmental Disability, 42(1), 12–21. 10.3109/13668250.2016.1195490 [DOI] [Google Scholar]
- Schoen SA, Miller LJ, & Sullivan JC (2014). Measurement in Sensory Modulation: The Sensory Processing Scale Assessment. The American Journal of Occupational Therapy, 68(5), 522–530. 10.5014/ajot.2014.012377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siper PM, Kolevzon A, Wang AT, Buxbaum JD, & Tavassoli T (2017). A clinician-administered observation and corresponding caregiver interview capturing DSM-5 sensory reactivity symptoms in children with ASD. Autism Research: Official Journal of the International Society for Autism Research, 10(6), 1133–1140. 10.1002/aur.1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siper PM, & Tavassoli T (2021). Sensory assessment for neurodevelopmental disorders.Wood Dale: Stoelting Co [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, & Woolard J (2008). Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: Evidence for a dual systems model. Developmental Psychology, 44(6), 1764–1778. 10.1037/a0012955 [DOI] [PubMed] [Google Scholar]
- Talay-Ongan A, & Wood K (2000). Unusual Sensory Sensitivities in Autism: A possible crossroads. International Journal of Disability Development and Education, 47(2), 201–212. 10.1080/713671112 [DOI] [Google Scholar]
- Tavassoli T, Bellesheim K, Siper PM, Wang AT, Halpern D, Gorenstein M … Buxbaum, J. D. (2016). Measuring Sensory Reactivity in Autism Spectrum Disorder: Application and Simplification of a Clinician-Administered Sensory Observation Scale. Journal of Autism and Developmental Disorders, 46(1), 287–293. 10.1007/s10803-015-2578-3 [DOI] [PubMed] [Google Scholar]
- Tavassoli T, Brandes-Aitken A, Chu R, Porter L, Schoen S, Miller LJ … Marco EJ (2019). Sensory over-responsivity: Parent report, direct assessment measures, and neural architecture. Molecular Autism, 10(1), 4. 10.1186/s13229-019-0255-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavassoli T, Miller LJ, Schoen SA, Nielsen DM, & Baron-Cohen S (2014). Sensory over-responsivity in adults with autism spectrum conditions. Autism: The International Journal of Research and Practice, 18(4), 428–432. 10.1177/1362361313477246 [DOI] [PubMed] [Google Scholar]
- Tavassoli T, Miller LJ, Schoen SA, Jo Brout J, Sullivan J, Baron-Cohen S (2018). Sensory reactivity empathizing and systemizing in autism spectrum conditions and sensory processing disorder. Developmental Cognitive Neuroscience 2972–2977. 10.1016/j.dcn.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillmann J, Uljarevic M, Crawley D, Dumas G, Loth E, Murphy D … de Bruijn Y, … the AIMS-2-TRIALS LEAP group. (2020). Dissecting the phenotypic heterogeneity in sensory features in autism spectrum disorder: A factor mixture modelling approach.Molecular Autism, 11( 1),67. 10.1186/s13229-020-00367-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchek SD, Huebner RA, & Dunn W (2014). Patterns of sensory processing in children with an autism spectrum disorder. Research in Autism Spectrum Disorders, 8(9), 1214–1224. 10.1016/j.rasd.2014.06.006 [DOI] [Google Scholar]
- Tomchek SD, & Dunn W (2007). Sensory processing in children with and without autism: A comparative study using the short sensory profile. The American Journal of Occupational Therapy, 61(2) 190–200. 10.5014/ajot.61.2.190 [DOI] [PubMed] [Google Scholar]
- Uljarević M, Baranek G, Vivanti G, Hedley D, Hudry K, & Lane A (2017). Heterogeneity of sensory features in autism spectrum disorder: Challenges and perspectives for future research. Autism Research: Official Journal of the International Society for Autism Research, 10(5), 703–710. 10.1002/aur.1747 [DOI] [PubMed] [Google Scholar]
- Uljarević M, Lane A, Kelly A, & Leekam S (2016). Sensory subtypes and anxiety in older children and adolescents with autism spectrum disorder. Autism Research, 9(10), 1073–1078. 10.1002/aur.1602 [DOI] [PubMed] [Google Scholar]
- Wechsler D (2011). Wechsler Abbreviated Scale of Intelligence—Second Edition. 10.1037/t15171-000 [DOI] [Google Scholar]