Abstract
Objective:
Extracellular vesicle (EV) therapy has been shown to mitigate inflammation in animal models of acute myocardial ischemia/reperfusion. This study evaluates the effect of EV therapy on inflammatory signaling in a porcine model of chronic myocardial ischemia and metabolic syndrome.
Methods:
Yorkshire swine were fed a high cholesterol diet for four weeks to induce metabolic syndrome, then underwent placement of an ameroid constrictor to the left circumflex artery to induce chronic myocardial ischemia. Two weeks later, pigs received intramyocardial injection of either saline (control, n=6) or EVs (n=8). Five weeks later, pigs were euthanized and left ventricular myocardial tissue in ischemic and non-ischemic territories were harvested. Protein expression was measured with immunoblotting, and macrophage count was determined by immunofluorescent staining of CD68. Data were statistically analyzed via Wilcoxon rank-sum test.
Results:
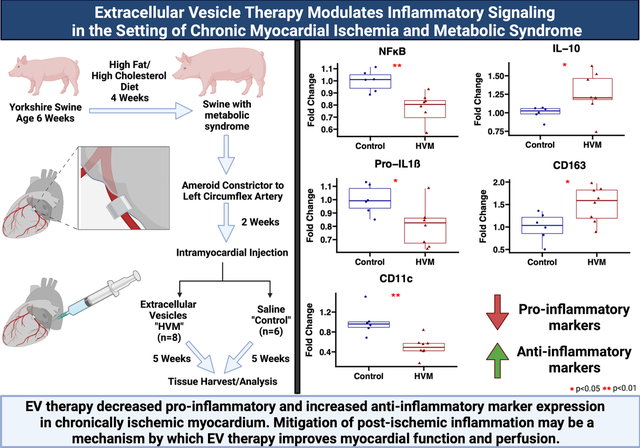
EV treatment was associated with decreased expression of pro-inflammatory markers NFκB (p=0.002), pro-IL1ß (p=0.020), and CD11c (p=0.001) in ischemic myocardium, and decreased expression of NFκB in non-ischemic myocardium (p=0.03) compared to control. EV treatment was associated with increased expression of anti-inflammatory markers IL-10 (p=0.020) and CD163 (p=0.043) in ischemic myocardium compared to control. There were no significant differences in expression of IL-6, TNFα, arginase, HLA-DRA, IKB-α, or p-IKB-α in ischemic myocardium or pro-IL1ß, IL-6, TNFα, IL-10, or IKB-α in non-ischemic myocardium of EV-treated pigs compared to control. There were no differences in macrophage count in ischemic myocardium between EV-treated pigs and control.
Conclusions:
In the setting of metabolic syndrome and chronic myocardial ischemia, intramyocardial EV therapy attenuates pro-inflammatory signaling.
Keywords: Extracellular Vesicles, Ischemia, Inflammation, Metabolic Syndrome, Coronary
Graphical Abstract
INTRODUCTION:
Therapeutic options for diffuse coronary artery disease (CAD) in patients who are poor surgical or catheter-based candidates remain limited. These patients are typically afflicted with chronic myocardial ischemia, which may lead to myocardial necrosis, adverse left ventricular (LV) remodeling, and heart failure.1 Furthermore, metabolic syndrome, which is characterized by obesity, hypertension, insulin resistance, and hypercholesterolemia, affects at least a third of the population in the United States and is often co-morbid with and increases the risk of CAD.2 Therefore there is a growing need to develop adequate therapies for patients with diffuse CAD using a clinically relevant model that reflects common co-morbidities encountered in clinical practice.
A growing body of literature demonstrates that extracellular vesicles (EVs) provide benefit to ischemic myocardium.3 EVs, which may be derived from several sources including human bone marrow mesenchymal stem cells (HBMSC), carry small bioactive molecules such as growth factors, kinase receptors, and mRNAs which can mediate regenerative effects in diseased tissues.4 Our recent studies using a swine model of chronic myocardial ischemia and metabolic syndrome demonstrated that EV therapy improves myocardial function, collateral blood flow formation, perfusion, and remodeling, though the mechanisms of these benefits are incompletely understood and require further investigation5–8.
An important consequence of myocardial ischemia is the inflammatory response that follows the ischemic insult. Though an initial inflammatory response plays an important physiologic role in myocardial recovery9, excessive inflammation adversely affects myocardial remodeling10, increases oxidative stress, promotes endothelial dysfunction,11 and increases risk of plaque rupture.12 Importantly, increased inflammation occurs not only in the setting of an acute ischemic event, but also in the setting of chronic coronary disease.13,14 Further, the conditions associated with metabolic syndrome are known to exacerbate inflammation and oxidative stress.15
EV therapy has been shown in multiple animal models, in the setting of acute myocardial ischemia or ischemia/reperfusion injury, to attenuate excessive inflammatory responses.16 Mechanisms by which EV therapy may mitigate myocardial inflammation in the setting of ischemia include down-regulation of pro-inflammatory signaling, reduction of monocyte infiltration, and modulation of macrophage polarization.16 Though these effects have been extensively demonstrated in animal models of acute ischemia/reperfusion, the effects of EV therapy on myocardial inflammation in the setting of chronic myocardial ischemia have yet to be investigated.
In the present study, we sought to investigate the effects of EV therapy on myocardial inflammation in the setting of metabolic syndrome and chronic myocardial ischemia. Our hypothesis was that EV therapy would attenuate pro-inflammatory signaling and promote anti-inflammatory signaling pathways.
METHODS:
EV Isolation
HBMSC (Lonza, Allendale, NJ) were cultured according to Lonza recommendations in growth media (MSCGM Bulletkit PT-3001; Lonza, Allendale, NJ). Donors were age 18 (female) and 22 (male), with negative viral testing. Cells were grown in normoxia conditions to 80% confluence and passaged up to six times for expansion of the cell line. No extracellular matrix was added. Twenty-four hours before isolation of EVs, growth media was removed, cells were washed with PBS, and media was replaced with serum-free Roswell Park Memorial Institute medium. The serum-free media was collected after 24 hours of incubation and centrifuged at 2,000 rpm at 4 degrees Celsius to remove cellular debris. From this, small EVs, including exosomes and microsomes, were isolated by ultracentrifugation at 100,000g for 70 minutes, washed with PBS at 100,000g for 70 minutes, resuspended in 1% dimethyl sulfoxide in PBS, snap frozen with liquid nitrogen, and stored at −80 degrees Celsius. This ultracentrifugation technique has been previously described as a method to isolate EVs from protein aggregates and lipoproteins.17,18 Protein quantification was performed using a radioimmunoprecipitation assay (Thermo Fisher Scientific) to quantify a standard dose for intramyocardial injection. Characterization was determined by immunoblotting for CD81, CD9, Alix, and Albumin (Supplemental Figure).19 50 μg of EVs, a dose previously effective in chronic myocardial ischemia when delivered intramyocardially,6 were thawed and resuspended in 2mL of 0.9% sterile saline on the day of administration. EVs were not subjected to more than one freeze/thaw cycle. Proteomics data from these EVs have been previously published.6
Animal Model:
Fourteen in-tact male Yorkshire swine at age 6 weeks were started on a high-fat diet, consisting of 500g/d high cholesterol diet comprised of 4% cholesterol, 17.2% coconut oil, 2.3% corn oil, 1.5% sodium cholate, and 75% regular chow (Sinclair Research, Columbia, MO) to induce metabolic syndrome,6 a well-described approach which has been successful in inducing characteristics of metabolic syndrome in our model.20,21 These swine had impaired glucose tolerance and elevated LDL and total cholesterol when compared to swine receiving a normal diet.6,7
Following four weeks of a high fat diet, animals underwent thoracotomy for placement of an ameroid constrictor (Research Instruments SW, Escondido, CA) around the proximal left circumflex artery (LCx) to induce chronic myocardial ischemia. Two weeks later, animals underwent a second thoracotomy procedure for intramyocardial injection of either vehicle (control group, n=6) or EVs (high fat diet with myocardial EV injection group “HVM”, n=8), based on random assignment prior to ameroid procedure. Five weeks after intramyocardial injection, animals were euthanized and their hearts were harvested for analysis (Figure 4). Investigators were not blinded to treatment groups during procedures or analysis. All experiments were approved by the Institutional Animal Care and Use Committee of the Rhode Island Hospital, and animals were cared for in coordination with veterinary technicians at Rhode Island Hospital in compliance with the Principles of Laboratory Animal Care formulated by the National Society of Medical Research and the Guide for the Care and Use of Laboratory Animals.
Figure 4: Graphical abstract.
Swine at age 6 weeks were given four weeks of high fat and high cholesterol diet to induce metabolic syndrome. An ameroid constrictor was placed on the proximal left circumflex artery to induce chronic myocardial ischemia. Two weeks later, animals received intramyocardial injection of either saline vehicle (“control”) or extracellular vesicles (high fat diet with myocardial EV injection group “HVM”). Five weeks later myocardial tissue was harvested for analysis. Ischemic myocardium in the HVM group had decreased expression of pro-inflammatory signaling markers NFκB, pro-IL1ß, and CD11c, and increased expression of anti-inflammatory markers IL-10 and CD163. EV = extracellular vesicle. In boxplots, upper and lower borders of box represent upper and lower quartiles, middle horizontal line represents median, upper and lower whiskers represent maximum and minimum values of non-outliers.
Ameroid Constrictor Placement Procedure
Anesthesia for procedures, perioperative analgesia, and prophylaxis were carried out as previously described.6 After adequate anesthesia and sterile preparation and draping, a left mini-thoracotomy was made, the pericardium was opened to expose the heart, and the left atrium was retracted to expose the LCx. The LCx was isolated near the takeoff of the left main coronary artery. After systemic heparinization, the LCx was occluded for two minutes as confirmed by ST and/or T wave changes on ECG, and 5mL of gold microspheres (BioPal, Worcester, MA) were injected into the left atrium. An ameroid constrictor, consisting of hygroscopic casein material cased in titanium (Research Instruments SW, Escondido, CA), was sized according to the LCx diameter and placed around the artery. Nitroglycerin was administered over the vessel as needed to reverse vasospasm. The incision was closed in multiple layers, and the pigs recovered from anesthesia in a monitored setting. (Video)
Intramyocardial Injection Procedure
After previously described pre-operative preparation, a left mini-thoracotomy incision was made one rib space below the prior thoracotomy incision. The pericardium was opened to expose the at-risk LV myocardium inferior to the previously placed ameroid constrictor. Depending on surgical group, the animals then underwent intramyocardial injection of either EVs (50μg) suspended in 2mL of 0.9% saline (HVM), or 2mL of 0.9% saline (control). Injections with a 27-gauge needle inserted 0.5cm into the myocardium, aspirating to confirm placement, were made into 8 locations of the LV myocardium (0.25cc injection/location) at risk of myocardial ischemia, as determined by tracking branches off the LCx and injecting into myocardium supplied by these branches approximately 2–3cm apart. The wound was closed in layers, and pigs were recovered from anesthesia in a monitored setting. Swine were monitored post-operatively for vital sign abnormalities or other signs of toxicity or immunogenic reactions, of which there were none.
Harvest Procedure
Five weeks after treatment, pigs underwent a harvest procedure. Femoral artery access was obtained with an open incision and Seldinger technique, and a pressure monitor was inserted through a 6F catheter sheath to monitor blood pressure. A median sternotomy was performed, and the heart was exposed. For blood flow analyses, isotope-labeled microspheres were injected into the left atrium while 10mL of blood was withdrawn from the femoral artery catheter. At the end of the procedure, the heart was excised from the pig, and the myocardial tissue was divided into 16 segments based on location with respect to the LAD and LCx. Myocardial tissue segments were snap frozen in liquid nitrogen for Western blot analysis and frozen sectioning, or air dried for microsphere analysis.
Myocardial Perfusion
Myocardial perfusion was determined using isotope-labeled microspheres (BioPal, Worcester, MA) injected at different time points. During the first surgical procedure for ameroid placement, 5mL of gold microspheres were injected into the left atrial appendage to determine the territory of the LV perfused by the LCx. During the harvest procedure, 5mL of Lutetium microspheres were injected into the left atrium while withdrawing 10mL of blood from the femoral artery. This process was repeated during pacing to 150bpm using Samarium microspheres. Blood samples and LV myocardial samples from 10 sections based on proximity of location to the LAD and LCx were weighed, dried, and sent to BioPal for analysis of blood flow to ischemic tissue segments.
Immunoblotting
Total protein (40μg) was fractionated on a 4–12% Bis-Tris gel (ThermoFisher), transferred to a nitrocellulose membrane (ThermoFisher Scientific, Waltham, MA), and membranes were incubated overnight at 4 degrees Celsius with 1:1000 dilutions of individual rabbit polyclonal primary antibodies to nuclear factor kappa B (NFκB), pro-interleukin 1 beta (pro-IL1ß), tumor necrosis factor alpha (TNFα), nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha (IKBα), phosphorylated IKBα (p-IKBα), CD163, CD11c, toll like receptor 2 (TLR2), inducible nitric oxide synthase (iNOS), cluster of differentiation 40 (CD40), HLA class II histocompatibility antigen DR alpha chain (HLA-DRA) (Cell Signaling), arginase, interleukin-6 (IL-6), phosphorylated NFκB (p-NFκB) (Abcam, Cambridge, UK), interleukin-10 (IL-10), and toll like receptor 4 (TLR4) (Novus Biologicals, Centennial, CO). The membranes were then washed and incubated with goat polyclonal anti-rabbit or anti-mouse secondary antibody at 1:3000 dilutions for one hour at room temperature. Membranes were then washed and processed for chemiluminescent detection and captured with a digital camera system (Bio-Rad ChemiDoc MP, Life Science, Hercules, CA). All membranes were probed with GAPDH or α-tubulin (Cell Signaling) to correct for loading error. Densitometric analysis of band intensity was performed using NIH Image J software.
Immunohistochemistry
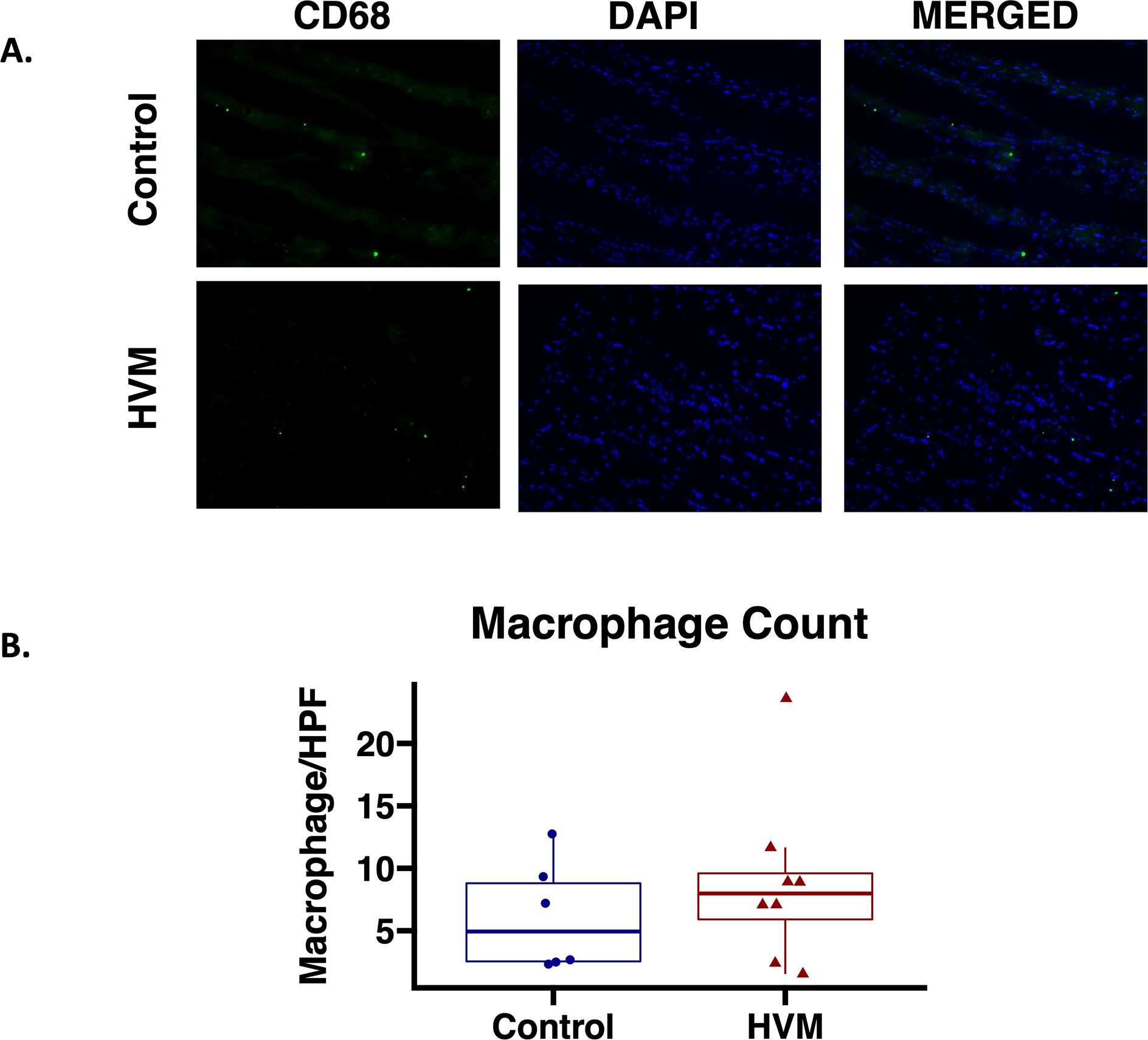
Immunohistochemistry was performed as previously described21. Briefly, frozen section slides were thawed, fixed with 10% paraformaldehyde, blocked, and incubated with antibodies to CD68, CD14, and neutrophil elastase (Cell Signaling) for macrophage, monocyte, and neutrophil staining respectively. Macrophage images were analyzed at 20X magnification with a Nikon E800 Eclipse microscope (Nikon, Tokyo, Japan). Monocyte and neutrophil images were analyzed at 20X magnification with an Olympus VS200 Slide Scanner (Olympus, Tokyo, Japan). Macrophages were counted for each specimen at each high-powered field and averaged for each specimen. Monocytes and neutrophils were counted for each specimen using QuPath software.
Data Analysis:
By analyzing data from prior protocols, using a 2-tailed α-level of 0.01, a ß-error level of 0.20, and the known standard deviation (0.150 mL/mg/min), we obtained a minimum sample size of 6 animals per group. Given that all data were analyzed as non-parametric, western blot results are reported as median fold change values compared to control pigs with interquartile ranges. Fold change was calculated by dividing the individual value with the mean value of all controls. Immunohistochemistry for macrophage staining are reported as median macrophage count/high-powered field with interquartile ranges. Immunohistochemistry for monocytes and neutrophils are reported as median cell count/mm2 with interquartile ranges. Western blot and immunohistochemistry data were statistically analyzed via Wilcoxon rank-sum test. Correlation data were statistically analyzed using Spearman’s rank correlation coefficient. Probability values <0.05 were considered significant.
RESULTS:
Protein Expression
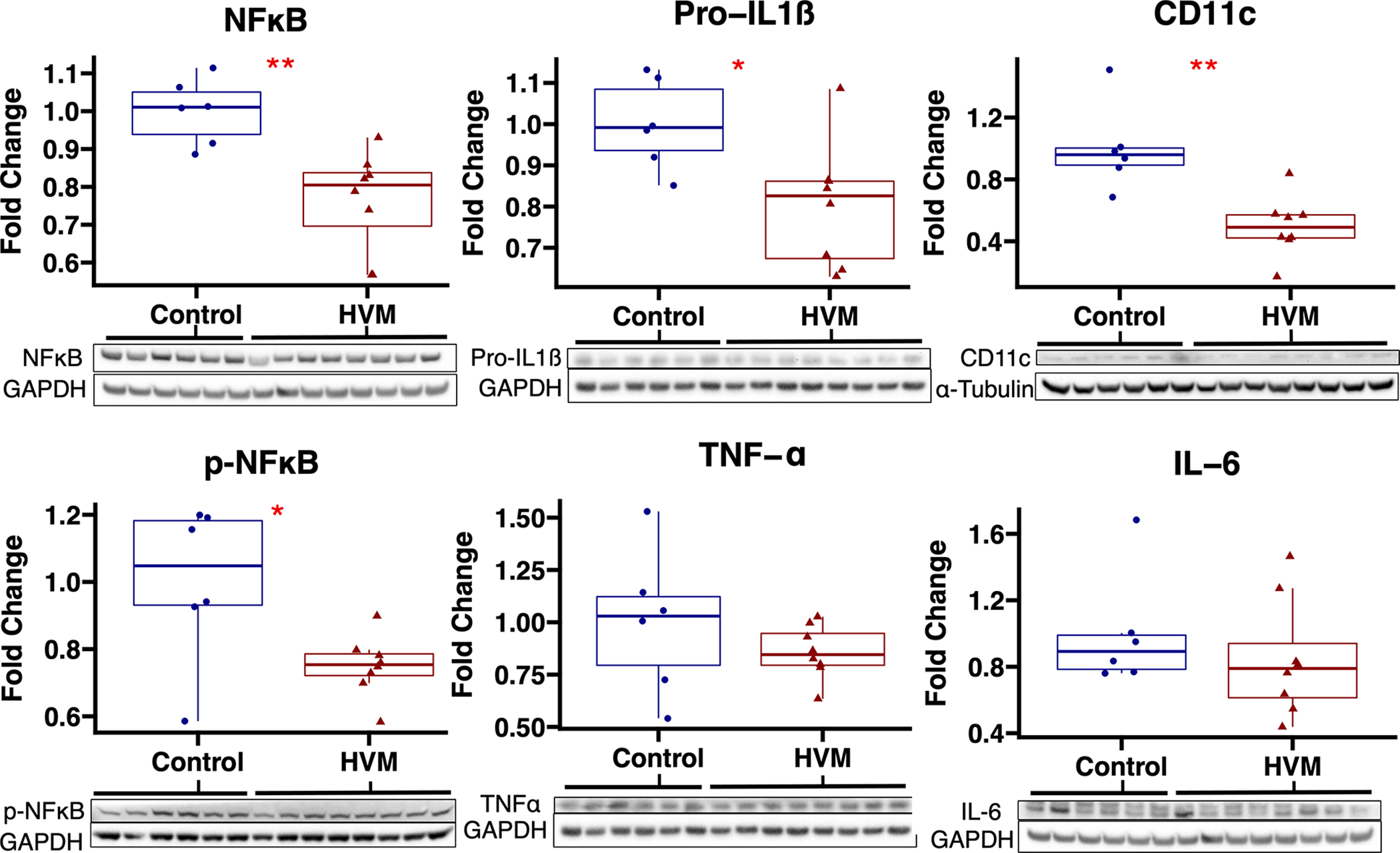
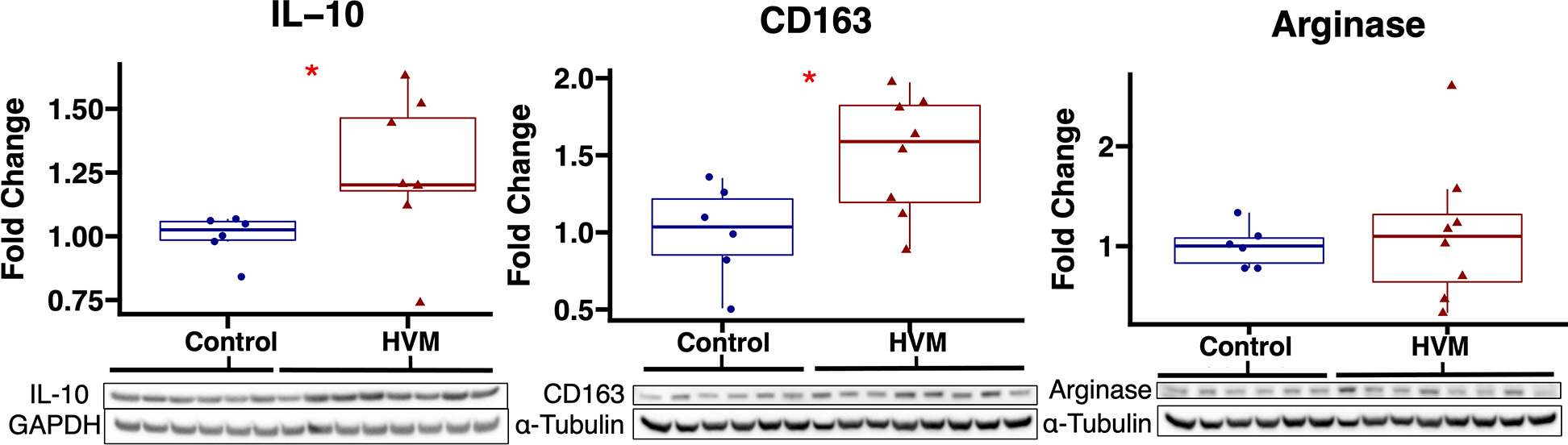
In ischemic myocardium, EV treatment was associated with decreased expression of pro-inflammatory markers NFκß (p=0.002), p-NFκß (p=0.029), pro-IL1ß (p=0.020), and CD11c (p=0.001) and increased expression of anti-inflammatory markers IL-10 (p=0.02) and CD163 (p=0.043) (Figures 1 and 2). There were no significant differences in protein expression of pro-inflammatory markers IL-6, TNF-α, or HLA-DRA, CD40, TLR2, TLR4, iNOS, anti-inflammatory marker arginase, or NFκß regulators IKB-α and p-IKB-α between groups (Table 1).
Figure 1: Pro-inflammatory marker expression in ischemic myocardium of extracellular vesicle (EV) treated vs control pigs.
The high-fat diet with EV injection (HVM) group had significant decreases in expression of pro-inflammatory markers NFκB, p-NFκB, pro-IL1ß, and CD11c compared to the control group. Upper and lower borders of box represent upper and lower quartiles, middle horizontal line represents median, upper and lower whiskers represent maximum and minimum values of non-outliers. *p<0.05 as determined by Wilcoxon rank-sum test.
Figure 2: Anti-inflammatory marker expression in ischemic myocardium of extracellular vesicle (EV) treated vs control pigs.
The high-fat diet with EV injection (HVM) group had significant increases in expression of anti-inflammatory markers IL-10 and CD163 compared to the control group. Upper and lower borders of box represent upper and lower quartiles, middle horizontal line represents median, upper and lower whiskers represent maximum and minimum values of non-outliers. *p<0.05 as determined by Wilcoxon rank-sum test.
Table 1:
Inflammatory protein expression in ischemic myocardium of EV-treated swine.
| Protein | Median (IQR) | P Value |
|---|---|---|
|
| ||
| Pro-Inflammatory | ||
| NFκB | 0.80 (0.70–0.84) | 0.002 |
| P-NFκB | 0.75 (0.72–0.79) | 0.029 |
| Pro-IL1β | 0.83 (0.67–0.86) | 0.020 |
| CD11c | 0.49 (0.42–0.57) | 0.001 |
| HLA-DRA | 0.78 (0.71–1.03) | 0.66 |
| IL-6 | 0.79 (0.61–0.94) | 0.28 |
| TNF-α | 0.85 (0.79–0.95) | 0.35 |
| TLR4 | 1.17 (0.86–1.26) | 0.49 |
| TLR2 | 0.87 (0.67–0.89) | 0.18 |
| iNOS | 1.02 (0.97–1.13) | 0.57 |
| CD40 | 0.79 (0.69–1.0) | 0.18 |
| Anti-Inflammatory | ||
| IL-10 | 1.20 (1.18–1.46) | 0.020 |
| CD163 | 1.59 (1.19–1.83) | 0.043 |
| Arginase | 1.10 (0.64–1.32) | 0.85 |
| NFκβ Regulators | ||
| IKB-α | 0.95 (0.86–1.00) | 0.41 |
| p-IKB-α | 0.92 (0.81–1.02) | 0.85 |
Statistics presented as median fold change (interquartile range) in extracellular vesicle (EV) treated swine (n=8) compared to average control (n=6). P values calculated using Wilcoxon rank-sum test.
In non-ischemic myocardium, EV treatment was associated with decreased expression of pro-inflammatory marker NFκß (p=0.03). There were no significant differences in expression of pro-IL1ß, IL-6, TNF-α, IL-10, or IKB-α between groups in non-ischemic myocardium (Table 2).
Table 2:
Inflammatory protein expression in non-ischemic myocardium of EV-treated swine
| Protein | Median (IQR) | P Value |
|---|---|---|
|
| ||
| Pro-Inflammatory | ||
| NFκB | 0.73 (0.70–0.91) | 0.03 |
| Pro-IL1β | 1.04 (0.82–1.28) | 0.95 |
| IL-6 | 0.85 (0.73–1.15) | 0.85 |
| TNF-α | 0.89 (0.76–0.97) | 0.35 |
| Anti-Inflammatory | ||
| IL-10 | 1.02 (0.88–1.22) | 0.57 |
| NFκβ Regulator | ||
| IKB-α | 0.92 (0.85–0.96) | 0.11 |
Statistics presented as median fold change (interquartile range) in extracellular vesicle (EV) treated swine (n=8) compared to average control (n=6). P values calculated using Wilcoxon rank-sum test.
Inflammatory Cell Counts
In ischemic myocardium, there was no significant difference in total macrophage counts/high powered field in the EV-treated group compared to the control group (Figure 3). There was also no significant difference in monocyte (p=0.63) or neutrophil count (p=0.95) between groups.
Figure 3: Total macrophage count in ischemic myocardium of EV-treated vs control pigs.
A. Representative images in 20x high powered field (HPF). Macrophages identified by staining with CD68 antibody on tissue sections (Green). Nuclei identified with DAPI in tissue sections (Blue). B. There were no differences in total macrophage count in the high-fat diet with EV injection (HVM) group compared to the control group. Upper and lower borders of box represent upper and lower quartiles, middle horizontal line represents median, upper and lower whiskers represent maximum and minimum values of non-outliers. P value calculated using Wilcoxon rank-sum test.
Blood Flow Correlation
Among EV-treated pigs, there was no significant correlation between blood flow to the ischemic territory and protein expression of NFκB, pro-IL1ß, CD11c, CD163, or IL-10 at rest and during pacing. (Table 3)
Table 3:
Correlation of inflammatory marker expression and myocardial perfusion to ischemic territory.
| Rest | Pacing | ||||
|---|---|---|---|---|---|
| Marker | rs | P value | Marker | rs | P value |
| NFκB | −0.45 | 0.27 | NFκB | 0.02 | 0.98 |
| Pro-IL1β | 0.64 | 0.10 | Pro-IL1β | 0.48 | 0.24 |
| CD11c | 0.43 | 0.29 | CD11c | 0.64 | 0.10 |
| CD163 | 0.40 | 0.46 | CD163 | 0.12 | 0.79 |
| IL-10 | −0.24 | 0.58 | IL-10 | 0.45 | 0.27 |
Among pigs treated with extracellular vesicles (HVM group, n=8), there was no significant correlation between inflammatory marker protein expression and blood flow at rest or during pacing in ischemic myocardial tissue. Correlation data were statistically analyzed using Spearman’s rank correlation coefficient, with correlation coefficient expressed as rs.
DISCUSSION:
In the present study, we found that in the setting of metabolic syndrome and chronic myocardial ischemia, EV therapy is associated with a reduction in pro-inflammatory signaling markers NFκß, p-NFκß, pro-IL1ß, and CD11c, and an increase in the anti-inflammatory markers IL-10 and CD163 in ischemic myocardium. We also found EV therapy is associated with reduced expression of NFκß in nonischemic myocardium. These changes were not associated with an absolute reduction in inflammatory cell count and were not correlated with ischemic territory blood flow.
NFκB is a transcription factor that is central in mediating pro-inflammatory signaling in response to stress or injury. NFκB activation increases pro-inflammatory cytokine transcription and is implicated in adverse cardiac remodeling and heart failure in the setting of myocardial infarction.22 We found that EV treatment in ischemic myocardium was associated with decreased expression and activation of NFκB in ischemic territory. Interestingly, there was also reduced NFκB expression in nonischemic myocardium. Myocardial ischemia can stimulate an inflammatory response in myocardium remote from the ischemic insult, an effect that may be mediated by soluble cytokine release.23 Modulation of cytokine production in the ischemic territory by EVs could therefore contribute to decreased NFκB in nonischemic myocardium. Reduced NFκB expression was not secondary to changes in expression of IKB-α, an NFκB regulator. Therefore, decreased NFκB in the setting of EV treatment may be via alternative mechanisms, such as microRNA-driven regulation of genes encoding NFκB, specifically RelA/p65.24
IL1ß is also an important mediator pro-inflammatory signaling in ischemic myocardium, and similar to NFκB has been associated with adverse cardiac remodeling and impaired cardiac function in the setting of ischemic insult.25,26 Included among the pro-inflammatory markers upregulated by NFκB are pro-IL1ß which is subsequently cleaved to the mature form of IL1ß to propagate inflammatory responses.27 IL1ß inhibition is an area of ongoing investigation, with a recent study demonstrating that IL1ß inhibition modestly reduced myocardial infarction and cardiovascular death among patients with chronic CAD.28 Therefore the attenuation of pro-inflammatory signaling with EV therapy could have important clinical consequences.
In addition to our findings of decreased pro-inflammatory signaling in ischemic myocardium of EV-treated pigs, we also found an increase in the expression of the anti-inflammatory cytokine IL-10, which plays an important role in improving structural wound healing and attenuating fibrosis and adverse LV remodeling in the setting of myocardial ischemia.29,30 We have previously demonstrated using the same animal model that EV-therapy reduces perivascular fibrosis and improves diastolic function,8 effects that may be in part due to the modulation of inflammatory signaling demonstrated in the current study.
Interestingly, some inflammatory signaling markers were significantly modulated in EV-treated myocardium while others in the same category were not. This finding could be due to an underpowered study, as several pro-inflammatory markers were downtrending without statistical significance. Furthermore, it is now known that in the setting of myocardial ischemia, cytokines are not regulated uniformly according to strict pro- or anti-inflammatory categories but rather change in clusters, with some groups of cytokines increasing or decreasing more than others.31 Ongoing studies in our lab further characterizing our EV contents, particularly non-coding RNAs, may provide additional mechanistic evidence for how regulation of specific cytokines may occur.
The effects of EV therapy on inflammatory signaling have been investigated in multiple animal models of acute myocardial ischemia. Prior to the current study, these effects had yet to be investigated in a large animal model of chronic myocardial ischemia and metabolic syndrome, more clinically relevant to diabetic patients with CAD. Additionally, the benefit of EV therapy in chronic myocardial inflammation may translate to other clinical contexts such as inflammatory cardiomyopathies. An important mechanism by which EV therapy appears to reduce inflammation is by shifting macrophage polarization from the M1, pro-inflammatory profile, to the M2, anti-inflammatory profile16. Though there is more nuance to M1/M2 classification, generally M1 macrophages secrete pro-inflammatory cytokines, promote inflammation, and are associated with inhibition of angiogenesis and poor myocardial healing.16 M2 macrophages secrete anti-inflammatory cytokines, attenuate inflammation, and promote myocardial wound healing.16 M1 and M2 macrophages can be differentiated by the markers they express and the cytokines they secrete. We found that CD11c, an M1 marker, was decreased, and that CD163, an M2 marker, was increased with EV treatment. The reduction of pro-IL1ß, associated with M1 polarization, and increase in IL-10, secreted by M2 macrophages, could further support a shift in macrophage polarization from the M1 to M2 profile. However, macrophage phenotypes are complex, and many macrophages may co-express M1/M2 markers.32 Furthermore, while there was a nonsignificant trend toward decreased expression of M1 markers TLR2 and CD40 in EV-treated myocardium, there were no differences in M1 markers iNOS and TLR4. These findings taken together preclude definitive conclusions on macrophage polarization phenotypes. Although macrophage count was unchanged, changes in inflammatory marker expression could reflect increased anti-inflammatory macrophage phenotypes among resident macrophages, which often play a more important role in myocardial repair compared to monocyte-derived macrophages. Additionally, inflammatory marker differences could be secondary to modulation of cytokine-related pathways within not only macrophages, but also cardiac fibroblasts and cardiomyocytes, which can also generate pro-inflammatory cytokines.33 However, additional studies are warranted using definitive techniques of macrophage subtype quantification such as flow cytometry, a more quantitative approach than immunohistochemistry,32 to determine whether EV therapy promotes M2 macrophage polarization in the setting of chronic myocardial ischemia.
Several contents within HBMSC-EVs may modulate inflammation. Proteomic analysis done previously on our EVs have shown a high abundance of proteins involved in regulation of immune processes.6 However, perhaps more influential cargo in HBMSC-EVs are microRNAs (miRNA). Multiple miRNAs found in MSC-derived exosomes have been implicated in inhibiting pro-inflammatory cytokine pathways and modifying macrophage polarization.16 Future transcriptomics studies of our EVs will investigate miRNA content that may be involved in proangiogenic, antifibrotic, and anti-inflammatory pathways. Another important consideration is the mechanism by which EVs release cargo for uptake by recipient cells, an area of ongoing investigation that is reviewed in detail elsewhere.34 Importantly, EV content release may be enhanced in acidic environments,34 such as in the setting of myocardial ischemia and inflammation.
Interestingly, we did not find a significant correlation between any single inflammatory marker and perfusion to ischemic myocardium, which could be due to low sample size in subgroup analysis, and limitations of single markers in capturing the summation of pro- and anti-inflammatory signaling in the tissue microenvironment. Finally, it is possible that there is no relationship between inflammatory signaling and perfusion in this model. Decreased inflammatory signaling could still provide mechanistic explanation to other benefits in chronically ischemic myocardium, though correlation analyses may be subject to similar challenges.
Immunogenicity is a consideration with the use of human, rather than porcine, derived MSC-EV in a swine model. We have not noted any signs of rejection or toxicity in pigs after receiving EVs.5,6 One of the important benefits of HBMSC is lower immunogenicity compared to other stem cell therapies, and HBMSC-EVs have even lower immunogenicity due to a lack of major histocompatibility complexes and other co-stimulatory factors.3 Therefore, HBMSC-EVs appear to be safe from an immunogenic standpoint.
There are several limitations in this study to consider. First, only male swine were studied, and we have since modified our protocol to include both male and female swine to encompass any sex-related differences. Another limitation is low sample size, which would especially affect the correlation analyses. An additional limitation is that our molecular data captures only a single time point five weeks after EV treatment, and the early molecular signaling and inflammatory cell changes that occur with EV therapy in our model are still unknown. EVs are short-lived, and therefore the longer-term therapeutic effects that they have are likely secondary to early molecular changes and transcriptional regulation that lead to a sustained response. Prior studies of EV therapy in other tissue beds suggest that epigenetic regulation, mediated by miRNA, may also contribute to long-term effects.35 Therefore, future studies investigating the early molecular signaling pathways affected by EV therapy in chronic myocardial ischemia would be fruitful.
In this study and in previous studies, we have demonstrated beneficial effects with intramyocardial injection of EVs in the setting of chronic myocardial ischemia in pigs with metabolic syndrome. However, intramyocardial injection is limited in clinical practicality. Our lab is currently investigating homing techniques to target myocardial tissue and improve uptake of EVs in myocardium via less invasive routes of delivery. Progress in this area would help translate the benefits of EVs in reducing inflammation, improving blood flow, and promoting myocardial recovery to a more clinically relevant model.
CONCLUSION:
In the setting of metabolic syndrome and chronic myocardial ischemia, EV therapy is associated with decreased expression of the pro-inflammatory and increased expression of the anti-inflammatory markers in ischemic myocardium.
Supplementary Material
Video: Ameroid Constrictor Placement Procedure. The procedure for ameroid constrictor placement involves 1) a left mini-thoracotomy incision, 2) entry into pleural cavity via second intercostal space, 3) spreading the ribs, 4) opening of pericardium, 5) retraction of left atrium to expose left circumflex artery, 6) identification of left circumflex artery, 7) isolation of left circumflex artery near takeoff from left main coronary artery, 8) left circumflex temporary occlusion and injection of gold microspheres into left atrium(not shown), 9) sizing of ameroid constrictor, 10) placement of ameroid constrictor around artery, 11) closure in layers (not shown).
Supplemental Figure: Western Blots Demonstrating Characteristics of Isolated Extracellular Vesicles. Extracellular vesicles (EVs) were characterized based on recommended categories outlined by the International Society for Extracellular Vesicles19. Extracellular vesicles were positive for transmembrane proteins CD81 (Category 1A) and CD9 (Category 1B), cytosolic protein ALIX (Category 2A), and negative for albumin (Category 3). Human bone marrow mesenchymal stem cells (HBMSC) depicted in neighboring lanes.
CENTRAL MESSAGE:
EV therapy reduced pro-inflammatory and increased anti-inflammatory signaling markers in chronically ischemic myocardium.
PERSPECTIVE STATEMENT:
Chronic CAD and metabolic syndrome are often co-morbid and contribute to significant morbidity and mortality globally. Both of these disease processes promote myocardial inflammation, which has adverse consequences on myocardial function, perfusion, and remodeling. In a large animal model of chronic myocardial ischemia and metabolic syndrome, EV therapy attenuated pro-inflammatory signaling.
CENTRAL PICTURE LEGEND:
Intramyocardial EVs reduce inflammatory signaling in chronically ischemic myocardium.
Funding:
This study was supported by the National Heart, Lung, and Blood Institute (NHLBI) 1F32HL160063-01 (S.A.S.); 1R01HL133624 (M.R.A.); R01HL46716 and R01HL128831-01A1 (F.W.S.).
GLOSSARY OF ABBREVIATIONS
- CAD
coronary artery disease
- CD40
cluster of differentiation 40
- EV
extracellular vesicle
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HLA-DRA
HLA class II histocompatibility antigen DR alpha chain
- HVM
high fat diet with myocardial extracellular vesicle injection group
- IKBα
nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha
- IL-6
interleukin-6
- IL-10
interleukin-10
- iNOS
inducible nitric oxide synthase
- LAD
left anterior descending artery
- LCx
left circumflex artery
- NFκB
nuclear factor kappa B
- p-NFκB
phosphorylated nuclear factor kappa B
- p-IKBα
phosphorylated nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha
- pro-IL1ß
pro-interleukin 1 beta
- TLR2
toll like receptor 2
- TLR4
toll like receptor 4
- TNFα
tumor necrosis factor alpha
Biographies



Footnotes
Presentation: 24th Annual C. Walton Lillehei Resident Forum at The American Association for Thoracic Surgery 102nd Annual Meeting, May 14–17, 2022, Boston, MA.
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- 1.Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–477. doi: 10.1093/eurheartj/ehz425 [DOI] [PubMed] [Google Scholar]
- 2.Hirode G, Wong RJ. Trends in the Prevalence of Metabolic Syndrome in the United States, 2011–2016. JAMA. 2020;323(24):2526–2528. doi: 10.1001/jama.2020.4501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karbasiafshar C, Sellke FW, Abid MR. Mesenchymal stem cell-derived extracellular vesicles in the failing heart: past, present, and future. Am J Physiol Heart Circ Physiol. 2021;320(5):H1999–H2010. doi: 10.1152/ajpheart.00951.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.EL Andaloussi S, Mäger I, Breakefield XO, Wood MJA. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12(5):347–357. doi: 10.1038/nrd3978 [DOI] [PubMed] [Google Scholar]
- 5.Potz BA, Scrimgeour LA, Pavlov VI, Sodha NR, Abid MR, Sellke FW. Extracellular Vesicle Injection Improves Myocardial Function and Increases Angiogenesis in a Swine Model of Chronic Ischemia. J Am Heart Assoc. 2018;7(12). doi: 10.1161/JAHA.117.008344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scrimgeour LA, Potz BA, Aboul Gheit A, et al. Extracellular Vesicles Promote Arteriogenesis in Chronically Ischemic Myocardium in the Setting of Metabolic Syndrome. J Am Heart Assoc. 2019;6;8(15:012617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aboulgheit A, Potz BA, Scrimgeour LA, et al. Effects of High Fat Versus Normal Diet on Extracellular Vesicle–Induced Angiogenesis in a Swine Model of Chronic Myocardial Ischemia. J Am Heart Assoc. 2021;10(4):e017437. doi: 10.1161/JAHA.120.017437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aboulgheit A, Karbasiafshar C, Sabra M, et al. Extracellular vesicles improve diastolic function and substructure in normal and high-fat diet models of chronic myocardial ischemia. J Thorac Cardiovasc Surg. Published online October 13, 2021:S0022–5223(21)01420–3. doi: 10.1016/j.jtcvs.2021.07.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonvini RF, Hendiri T, Camenzind E. Inflammatory response post-myocardial infarction and reperfusion: a new therapeutic target? European Heart Journal Supplements. 2005;7(suppl_I):I27–I36. doi: 10.1093/eurheartj/sui077 [DOI] [Google Scholar]
- 10.Westman PC, Lipinski MJ, Luger D, et al. Inflammation as a Driver of Adverse Left Ventricular Remodeling After Acute Myocardial Infarction. J Am Coll Cardiol. 2016;67(17):2050–2060. doi: 10.1016/j.jacc.2016.01.073 [DOI] [PubMed] [Google Scholar]
- 11.Sabe SA, Feng J, Sellke FW, Abid MR. Mechanisms and Clinical Implications of Endothelium-dependent Vasomotor Dysfunction in Coronary Microvasculature. Am J Physiol Heart Circ Physiol. Published online March 25, 2022. doi: 10.1152/ajpheart.00603.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of Plaque Formation and Rupture. Circulation Research. 2014;114(12):1852–1866. doi: 10.1161/CIRCRESAHA.114.302721 [DOI] [PubMed] [Google Scholar]
- 13.Pereira-da-Silva T, Napoleao P, Pinheiro T, et al. Inflammation is associated with the presence and severity of chronic coronary syndrome through soluble CD40 ligand. Am J Cardiovasc Dis. 2020;10(4):329–339. [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta JL, Li DY. Inflammation in ischemic heart disease: Response to tissue injury or a pathogenetic villain? Cardiovascular Research. 1999;43(2):291–299. doi: 10.1016/S0008-6363(99)00132-7 [DOI] [PubMed] [Google Scholar]
- 15.Rana JS, Nieuwdorp M, Jukema JW, Kastelein JJP. Cardiovascular metabolic syndrome - an interplay of, obesity, inflammation, diabetes and coronary heart disease. Diabetes Obes Metab. 2007;9(3):218–232. doi: 10.1111/j.1463-1326.2006.00594.x [DOI] [PubMed] [Google Scholar]
- 16.Viola M, de Jager SCA, Sluijter JPG. Targeting Inflammation after Myocardial Infarction: A Therapeutic Opportunity for Extracellular Vesicles? Int J Mol Sci. 2021;22(15):7831. doi: 10.3390/ijms22157831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3:Unit 3.22. doi: 10.1002/0471143030.cb0322s30 [DOI] [PubMed] [Google Scholar]
- 18.Brennan K, Martin K, FitzGerald SP, et al. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci Rep. 2020;10(1):1039. doi: 10.1038/s41598-020-57497-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. doi: 10.1080/20013078.2018.1535750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Lerman LO. Investigating the metabolic syndrome: Contributions of swine models. Toxicol Pathol. 2016;44(3):358–366. doi: 10.1177/0192623316630835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elmadhun NY, Lassaletta AD, Chu LM, Liu Y, J F, Sellke FW. Atorvastatin increases oxidative stress and modulates angiogenesis in Ossabaw swine with the metabolic syndrome. The Journal of thoracic and cardiovascular surgery. 2012;144:1486–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawano S, Kubota T, Monden Y, et al. Blockade of NF-kappaB improves cardiac function and survival after myocardial infarction. Am J Physiol Heart Circ Physiol. 2006;291(3):H1337–1344. doi: 10.1152/ajpheart.01175.2005 [DOI] [PubMed] [Google Scholar]
- 23.Lee WW, Marinelli B, van der Laan AM, et al. PET/MRI of inflammation in myocardial infarction. J Am Coll Cardiol. 2012;59(2):153–163. doi: 10.1016/j.jacc.2011.08.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma X, Becker Buscaglia LE, Barker JR, Li Y. MicroRNAs in NF-κB signaling. J Mol Cell Biol. 2011;3(3):159–166. doi: 10.1093/jmcb/mjr007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saxena A, Russo I, Frangogiannis NG. Inflammation as a therapeutic target in myocardial infarction: learning from past failures to meet future challenges. Transl Res. 2016;167(1):152–166. doi: 10.1016/j.trsl.2015.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toldo S, Van Tassell BW, Abbate A. Interleukin-1 Blockade in Acute Myocardial Infarction and Heart Failure. JACC Basic Transl Sci. 2017;2(4):431–433. doi: 10.1016/j.jacbts.2017.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Sig Transduct Target Ther. 2017;2(1):1–9. doi: 10.1038/sigtrans.2017.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377(12):1119–1131. doi: 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 29.Krishnamurthy P, Rajasingh J, Lambers E, Qin G, Losordo DW, Kishore R. IL-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of STAT-3 and suppression of HuR. Circ Res. 2009;104(2):e9–18. doi: 10.1161/CIRCRESAHA.108.188243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jung M, Ma Y, Iyer RP, et al. IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic Res Cardiol. 2017;112(3):33. doi: 10.1007/s00395-017-0622-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lebedeva A, Fitzgerald W, Molodtsov I, Shpektor A, Vasilieva E, Margolis L. Differential clusterization of soluble and extracellular vesicle-associated cytokines in myocardial infarction. Sci Rep. 2020;10(1):21114. doi: 10.1038/s41598-020-78004-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jayasingam SD, Citartan M, Thang TH, Mat Zin AA, Ang KC, Ch’ng ES. Evaluating the Polarization of Tumor-Associated Macrophages Into M1 and M2 Phenotypes in Human Cancer Tissue: Technicalities and Challenges in Routine Clinical Practice. Front Oncol. 2019;9:1512. doi: 10.3389/fonc.2019.01512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aoyagi T, Matsui T. The Cardiomyocyte as a Source of Cytokines in Cardiac Injury. J Cell Sci Ther. 2011;2012(0):003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maas SLN, Breakefield XO, Weaver AM. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017;27(3):172–188. doi: 10.1016/j.tcb.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muraca M, Cappariello A. The Role of Extracellular Vesicles (EVs) in the Epigenetic Regulation of Bone Metabolism and Osteoporosis. Int J Mol Sci. 2020;21(22):E8682. doi: 10.3390/ijms21228682 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video: Ameroid Constrictor Placement Procedure. The procedure for ameroid constrictor placement involves 1) a left mini-thoracotomy incision, 2) entry into pleural cavity via second intercostal space, 3) spreading the ribs, 4) opening of pericardium, 5) retraction of left atrium to expose left circumflex artery, 6) identification of left circumflex artery, 7) isolation of left circumflex artery near takeoff from left main coronary artery, 8) left circumflex temporary occlusion and injection of gold microspheres into left atrium(not shown), 9) sizing of ameroid constrictor, 10) placement of ameroid constrictor around artery, 11) closure in layers (not shown).
Supplemental Figure: Western Blots Demonstrating Characteristics of Isolated Extracellular Vesicles. Extracellular vesicles (EVs) were characterized based on recommended categories outlined by the International Society for Extracellular Vesicles19. Extracellular vesicles were positive for transmembrane proteins CD81 (Category 1A) and CD9 (Category 1B), cytosolic protein ALIX (Category 2A), and negative for albumin (Category 3). Human bone marrow mesenchymal stem cells (HBMSC) depicted in neighboring lanes.


