Abstract
Objective
The purpose of the study is to investigate the performance of radiomics-based analysis in prediction of pure ground-glass nodule (pGGN) lung adenocarcinomas invasiveness using thin-section computed tomography images.
Methods
A total of 382 patients surgically resected single pGGN and pathologically confirmed were enrolled in the retrospective study. The pGGN cases were divided into two groups: the noninvasive group and the invasive adenocarcinoma (IAC) group. 330 patients were randomly assigned to the training and testing cohorts with a ratio of 7:3 (245 noninvasive lesions, 85 IAC lesions), while 52 patients (30 noninvasive lesions, 22 IAC lesions) were assigned to the external validation cohort. A model, radiomics model, and combined clinical-radiographic-radiomic model were built using the LASSO and multivariate backward stepwise regression analysis on the basis of the selected and radiomics features. The area under the curve (AUC) and decision curve analysis (DCA) were used to evaluate and compare the model performance for invasiveness discrimination among the three cohorts.
Results
Three clinical-radiographic features (including age, gender and the mean CT value) and three radiomics features were selected for model building. The combined model and radiomics model performed better than the clinical-radiographic model. The AUCs of the combined model in the training, testing, and validation cohorts were 0.856, 0.859, and 0.765, respectively. The DCA demonstrated the radiomics signatures incorporating clinical-radiographic feature was clinically useful in predicting pGGN invasiveness.
Conclusions
The proposed radiomics-based analysis incorporating the clinical-radiographic feature could accurately predict pGGN invasiveness, providing a noninvasive biomarker for the individualized and precise medical treatment of patients.
Keywords: Radiomics, Lung adenocarcinoma, Ground glass opacity, Tomography (X-Ray Computed), Pulmonary nodules
Key points
Radiomics have potentials to differentiate the invasiveness of pGGNs lung adenocarcinoma.
Clinical-radiographic feature adds discriminative value to radiomics in pGGNs pathological subtype.
Combined clinical-radiographic-radiomic model can successfully stratify patients into noninvasive pGGNs and invasive pGGNs in patients with resectable lung adenocarcinoma.
Introduction
Lung cancer is one of the most common and serious causes of cancer-related deaths worldwide [1]. With the development of high-resolution computed tomography (CT) and the popularization of low-dose CT screening, the incidence of pure ground-glass nodules (pGGNs) is rapidly increasing [2, 3]. A pGGN can be defined as a nodule with a hazy attenuation increase in the lung window, without a solid component when viewed with mediastinal window settings, and without vessel and bronchial structure obscuring [4, 5]. According to the new classification proposed by the International Association for the Study of Lung Cancer, the American Thoracic Society, and the European Respiratory Society, lung adenocarcinomas include atypical adenomatous hyperplasia (AAH), adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), and invasive adenocarcinoma (IAC) [6]. Persistent pGGNs lasting for more than three months have been proven to be associated with early stage lung adenocarcinoma including AAH, AIS, MIA and IAC [7–11]. Most pure GGNs are preinvasive lesions; however, recent histologic studies have shown that approximately 20% to 50% have invasive components [12–15]. In recent study, Ye et al. found that 10.8% of pure ground-glass lung adenocarcinoma nodules were of the IAC subtype among 988 pulmonary nodules [16].
Lobectomy is the standard surgical treatment for IACs; however, AIS and MIA may be candidates for sublobar resection [17]. Previous studies have demonstrated that the 5-year disease-free survival (DFS) of patients with AIS and MIA can reach 100% or almost 100%, while the DFS of patients with IAC is 40–85% [18]. Therefore, distinguishing IACs from preinvasive lesions and MIAs before surgery is crucial for clinical management and prognosis prediction in patients with pGGNs.
Clinical features including age, sex, and smoking history have been found as predictors of nodule growth and pathologic diagnosis [8, 19, 20]. Adenocarcinoma also accounts for a large percentage of lung tumors in female patients [21]. Huang et al. found that non-smoking female patients with lung cancer were more likely to have adenocarcinoma [22]. IAC occurred more often in older patients; Hu et al. found that aged ≥ 60 years was one of independent predictors of pGGNs histologic invasiveness [23].
CT is the most commonly used technique for the detection and differentiation of pGGN invasiveness [24]. Previous studies have shown that IAC differentiation is mainly based on radiographic features, including morphological features (margin, shape, vessel change, bubble sign, and pleural indentation) and quantitative features (lesion size, CT value, and volume) [14, 15, 19, 20, 25, 26]. While morphological features widely depend on the experience of observers and quantitative features are affected by scanning parameters (e.g., consistency of measurement), some features in pGGNs overlap; this is especially true in small nodules with a size of < 6 mm [27].
Radiomics that can extract high-throughput data from medical images and analyses with numerous quantitative descriptors in order to investigate the associations between imaging features and various endpoints have a promising potential for the evaluation of pGGN invasion [28–30]. Most previous studies have only extracted texture features from nonenhanced CT images and have included a small number of lesions. It would be very helpful in deciding on the optimal treatment plan if using clinical, radiographic and radiomics features for the differentiation of IAC from MIA and preinvasion (AAH and AIS) in evaluating pGGNs.
Therefore, the authors of the present study hypothesized that the combination of clinical, radiographic and radiomics features can improve the diagnostic ability to determine the histological invasiveness of adenocarcinomas appearing as pGGNs. The purpose of this study is to develop a combined prediction model in order to help guide an individualized preoperative design of surgical procedures.
Materials and methods
Study population
The present retrospective study was approved by the institutional research board, and the requirement for informed consent was waived.
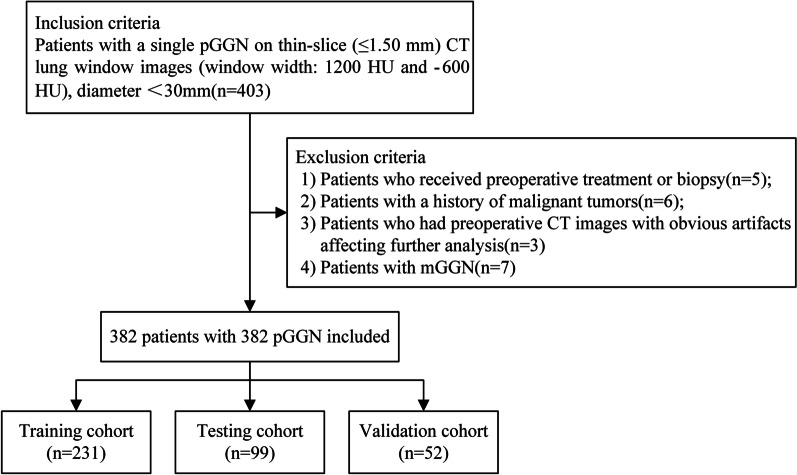
The records and images of patients with pGGNs who underwent a preoperative chest CT were retrospectively reviewed. All nodules were pathologically confirmed as lung adenocarcinomas (AAH, AIS, MIA, and IAC) via surgical resection between January 2017 and December 2020 (Fig. 1 shows the flowchart of study population), lobectomy resection for patients with IAC, whereas limited resections for patients with AAH, AIS or MIA.
Fig. 1.
The flowchart of study population
Inclusion criteria: (1) Patients with a single pGGN on thin-slice (≤ 1.50 mm) CT lung window images (window width: 1200 HU and −600 HU); (2) patients with a nodule diameter of < 30 mm; and (3) patients who underwent surgical resection (including lobectomy resection and limited resections) within one week of receiving a CT scan.
Exclusion criteria: (1) Patients who received preoperative treatment or biopsy which hemorrhage or exudates occurred around pGGNs; (2) patients with a history of malignant tumors; (3) patients who had preoperative CT images with obvious artifacts affecting further analysis, the decision was made by an experienced radiologist before grouping; and (4) patients with multiple GGN (mGGN).
Finally, 382 patients aged 35–73 years (mean age = 56 ± 10 years) with a total of 382 pGGNs were enrolled in the present study. The patients comprised 123 males aged 35–67 years (mean age = 54 ± 11 years) and 259 females aged 38–73 years (mean age = 58 ± 13 years). Of these patients, 231 were assigned to the training cohort and 99 were assigned to the test cohort randomly assigned to the training and testing cohorts with a ratio of 7:3; meanwhile, 52 patients scanned at the third CT were assigned to the external validation cohort. According to the pathological results (AAH, AIS, MIA, and IAC), preinvasive adenocarcinoma and MIA were considered noninvasive lesions. All included pGGNs were divided into two groups: the non-IAC group (n = 275; 72%) and the IAC group (n = 107; 28%).
Acquisition of CT imaging
All patients received a contrast-enhanced chest CT using the Siemens SOMATOM Definition Flash, Siemens SOMATOM Sensation Open CT or GE Revolution. The detailed scanning parameters are listed in Table 1. The contrast-enhanced CT was performed after 25 s of intravenous administration of iodinated contrast material (2 mL/kg) at a rate of 3 mL/sec. The CT scans were acquired from all patients in the supine position at full inspiration. Scan coverage was from the lung base to the thoracic inlet.
Table 1.
CT scanning parameters
| Setting | Siemens SOMATOM definition flash | Siemens SOMATOM sensation open CT | GE revolution |
|---|---|---|---|
| Tube voltage (kV) | 120 | 120 | 120 |
| Tube current (mA) | 35 mAs | 35 mAs | Smart mAs |
| Pitch | 1.2 | 1.2 | 1 |
| Matrix | 512 × 512 | 512 × 512 | 512 × 512 |
| Slice thickness (mm) | 1.0 | 1.0 | 1.25 |
| Reconstruction algorithm | B60 | B60 | STND |
| Window width (HU) | 1200 | 1200 | 1200 |
| Window level (HU) | −600 | −600 | −600 |
Image preprocessing
To minimize noise and processing artifacts, resampling of image data was avoided wherever possible, as previously described in similar investigations [31]. First, linear interpolate was applied to re-sampled the CT images and make voxel isotropic of 1 × 1 × 1 mm. Second, the image was discretized in gray scale and the binwidth was set to 25. Third, a Laplacian of Gaussian convolution kernel filter (σ = 3, 5, and 7) and wavelet transform was employed to decrease noise and enhance features at different spatial scales.
Clinical characteristics and CT image evaluation
According to the research literature related to lung cancer [32], the clinical characteristics, including age, gender, smoking status, and clinical symptoms, were derived from medical records. Two thoracic radiologists with 5 and 10 years of experience, respectively, reviewed the CT images of each patient and identified the radiographic features including morphological and quantitative features without knowledge of the patient’s pathological results. Decisions regarding the CT features were reached by consensus. Morphological features included lobulation, spiculation, pleural indentation, and the vacuole sign. Quantitative features included the lesion maximum diameter and mean CT value. The CT images were read with a lung window setting (width: 1200 HU; level: −600 HU).
Reproducibility analysis
To ensure reader reproducibility, 50 patients were randomly selected for a reproducibility analysis. For the evaluation of the interobserver agreement of the radiomics features, two radiologists with 8 years (Reader 1) and 10 years (Reader 2) of experience in chest CT interpretation, respectively, completed the region of interest (ROI) delineation without any information regarding the patients. Next, Reader 1 repeated the ROI delineation with an interval of one week for the assessment of the interobserver agreement. The interobserver and interobserver agreement of the radiomics feature extraction were evaluated using interclass and intraclass correlation coefficients (ICC). Features with an ICC of > 0.8 were regarded as an acceptable agreement and were included in the subsequent analyses.
Segmentation of pulmonary nodules
Segmentation of pulmonary nodules was performed on the thin-slice enhanced images with a lung window setting (width, 1200 HU; level, −600 HU) using the ITK-SNAP software (version 3.8.0, https://www.itksnap.org). The radiologist delineated the nodule length, and the software automatically drew regions of interest covering the entire range of the tumor on the axial CT images. The radiologist manually adjusted and identified the boundary regions on each section. The involved pleura, blood vessels, and bronchi at the edge of the nodules were excluded from nodule segmentation.
Radiomics feature extraction
Radiomics feature extraction was performed using the PyRadiomics. A total of 1130 radiomics features were extracted from the contrast-enhanced CT image for each patient [33]. The extracted features comprised tumor shape features, first-order statistic features, and texture features, such as the gray-level co-occurrence matrix (GLCM), gray-level run length matrix, gray-level dependence matrix, gray-level zone matrix (GLSZM), and neighborhood gray tone difference matrix, to reflect internal heterogeneity as previously described [34].
clinical-radiographic-feature-based model
Relevant clinical features, including sex, age, smoking history, and clinical symptoms, and radiographic features including morphological and quantitative features were analyzed to build clinical-radiographic-based model.
The clinical feature selection was performed in three steps: (1) The Shapiro–Wilk test was used to test the normality of the data sets; (2) the Student t test or Wilcoxon rank sum test was used for the continuous variables (mean CT value and maximum diameter), and the or Fisher exact test was applied for the categorical variables (sex, smoking history, and clinical symptoms); and (3) the stepwise multivariable logistic regression analysis was applied to obtain the independent clinical risk factors and build a clinical-feature-based model.
Radiomics-feature-based model
The radiomics-feature-based model was built in four steps: (1) The correlation analysis was performed to identify the redundant features (features with a correlation coefficient of > 0.8 were eliminated) [28]; (2) the Mann–Whitney U-test was performed to compare the differences between each radiomics feature in the two groups (radiomics features with a p value of < 0.05 were kept); (3) the least absolute shrinkage and selection operator (LASSO) was performed for feature selection [35–38]; and (4) the stepwise multivariate logistic regression analysis based on the Akaike information criteria was performed to identify the optimal radiomics features for the differentiation of noninvasive lesions and IACs. Then, a combined predictive model was built with the use of selected conventional and radiomics features.
The performance of the three models in the training, testing, and validation cohorts was evaluated by the receiver operating characteristics (ROC) curves; the area under the curve (AUC), accuracy, sensitivity, and specificity were calculated, respectively. A radiomics signature-based score (Rad-score) for each outcome was then obtained from the final model. The Rad-score cut-off values for differentiation of the non-IAC group and the IAC group were chosen according to the Youden index criteria. To estimate model goodness-of-fit, calibration curves were performed, and the Hosmer–Lemeshow test was used to assess model consistency [39, 40]. Decision curve analyses (DCAs) were used to evaluate the potential net benefit based on the clinical diagnosis, radiomics, and the combined model in the different cohorts.
Results
Patient demographic characteristics
A total of 382 patients with pGGNs were enrolled in the present retrospective study according to the inclusion and exclusion criteria. Among them, 275 were diagnosed with noninvasive lesions (AAH, AIS, and MIA) and 107 were diagnosed with IAC. The training cohort comprised 330 patients (330 pGGNs); these patients were divided into the training cohort and the testing cohort according to the ratio of 7:3 with stratified sampling. A total of 52 patients (52 pGGNs) were assigned to the validation cohort. The demographic characteristics of three cohorts of the 382 patients are listed in Table 2.
Table 2.
Demographic characteristics
| Training cohort | Testing cohort | Validation cohort | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NonInvasion | Invasion | p value* | NonInvasion | Invasion | p value | NonInvasion | Invasion | p value* | |
| N = 172 | N = 60 | N = 73 | N = 25 | N = 30 | N = 22 | ||||
| Gender | 0.041 | 0.032 | 0.107 | ||||||
| Female | 124 (72.1%) | 34 (56.7%) | 54 (74.0%) | 12 (48.0%) | 17 (56.7%) | 18 (81.8%) | |||
| Male | 48 (27.9%) | 26 (43.3%) | 19 (26.0%) | 13 (52.0%) | 13 (43.3%) | 4 (18.2%) | |||
| Age | 56.0 [47.0; 62.0] | 59.5 [53.8; 64.0] | 0.016 | 55.0 [48.0; 61.0] | 59.0 [54.0; 64.0] | 0.110 | 57.0 [52.0; 64.0] | 60.5 [53.5; 65.0] | 0.295 |
| Center | 0.302 | 1.000 | 0.317 | ||||||
| Absent | 84 (48.8%) | 24 (40.0%) | 32 (43.8%) | 11 (44.0%) | 19 (63.3%) | 10 (45.5%) | |||
| Present | 88 (51.2%) | 36 (60.0%) | 41 (56.2%) | 14 (56.0%) | 11 (36.7%) | 12 (54.5%) | |||
| AxisMaxLength | 1.02 [0.80; 1.24] | 1.53 [1.19; 1.95] | < 0.001 | 1.01 [0.84; 1.30] | 1.56 [1.17; 1.97] | < 0.001 | 1.29 [0.95; 1.67] | 1.28 [1.18; 1.63] | 0.453 |
| Distance | 0.99 [0.29; 1.82] | 0.74 [0.00; 1.56] | 0.193 | 0.68 [0.29; 1.49] | 0.66 [0.00; 1.20] | 0.427 | 0.50 [0.00; 1.71] | 1.27 [0.59; 1.86] | 0.187 |
| Burr | 0.001 | 0.001 | 1.000 | ||||||
| Absent | 170 (98.8%) | 53 (88.3%) | 71 (97.3%) | 18 (72.0%) | 27 (90.0%) | 20 (90.9%) | |||
| Present | 2 (1.16%) | 7 (11.7%) | 2 (2.74%) | 7 (28.0%) | 3 (10.0%) | 2 (9.09%) | |||
| Lobe | 0.001 | 0.014 | 0.299 | ||||||
| Absent | 170 (98.8%) | 53 (88.3%) | 72 (98.6%) | 21 (84.0%) | 29 (96.7%) | 19 (86.4%) | |||
| Present | 2 (1.16%) | 7 (11.7%) | 1 (1.37%) | 4 (16.0%) | 1 (3.33%) | 3 (13.6%) | |||
| Vacuole | 0.224 | 0.050 | 1.000 | ||||||
| Absent | 163 (94.8%) | 54 (90.0%) | 72 (98.6%) | 22 (88.0%) | 29 (96.7%) | 21 (95.5%) | |||
| Present | 9 (5.23%) | 6 (10.0%) | 1 (1.37%) | 3 (12.0%) | 1 (3.33%) | 1 (4.55%) | |||
| Pleura Involve | 0.017 | 0.255 | 1.000 | ||||||
| Absent | 172 (100%) | 57 (95.0%) | 73 (100%) | 24 (96.0%) | 29 (96.7%) | 22 (100%) | |||
| Present | 0 (0.00%) | 3 (5.00%) | 0 (0.00%) | 1 (4.00%) | 1 (3.33%) | 0 (0.00%) | |||
| MeanCT | −562.50 [−634.72; −480.93] | −501.25 [−545.38; −413.53] | < 0.001 | −508.80 [−588.00; −407.60] | −471.90 [−567.20; −408.10] | 0.544 | −597.55 [−638.65; −551.20] | −493.10 [−566.88; −391.80] | < 0.001 |
| Clinical | 0.497 | 0.855 | 0.475 | ||||||
| Absent | 130 (75.6%) | 42 (70.0%) | 53 (72.6%) | 17 (68.0%) | 23 (76.7%) | 14 (63.6%) | |||
| Present | 42 (24.4%) | 18 (30.0%) | 20 (27.4%) | 8 (32.0%) | 7 (23.3%) | 8 (36.4%) | |||
| Smoke | 0.071 | 0.004 | 0.161 | ||||||
| No | 146 (84.9%) | 44 (73.3%) | 66 (90.4%) | 16 (64.0%) | 22 (73.3%) | 20 (90.9%) | |||
| Yes | 26 (15.1%) | 16 (26.7%) | 7 (9.59%) | 9 (36.0%) | 8 (26.7%) | 2 (9.09%) | |||
*p value is derived from statistical analyses between each of variables and groups. p value < 0.05 indicated statistical significance. Values are presented as no. (%) or mean (95%CI). Chi-square test or Fisher’s exact test was used for the categorical variable. A Student’s t test, Mann–Whitney U-test or Kruskal–Wallis H-test were used for the continuous variable
Feature selection
According to the univariate analysis results, three clinical features (gender, age, and smoking history), six radiographic features including four morphological features (burr, lobe, vacuole sign, and pleural involvement), and two quantitative features (mean CT value and axis max length diameter) were found to be significant in the differentiation between noninvasive lesions and IPAs in the training cohort; only one quantitative feature (mean CT value) was found to be significant between noninvasive lesion and IPAs in the external validation cohort.
According to multivariable logistic regression, age, gender, and the mean CT value were selected to build clinical-radiographic-based model. After LASSO and the stepwise logistic regression analysis were conducted, three radiomics features were ultimately selected to build a radiomics-feature-based model. These features included the Log.5.0_glszm_SmallAreaHighGrayLevelEmphasis, wavelet.LHL_glcm_MCC, and wavelet.LLL_glcm_SumAverage.
Performance of clinical-radiographic, radiomics, and combined models
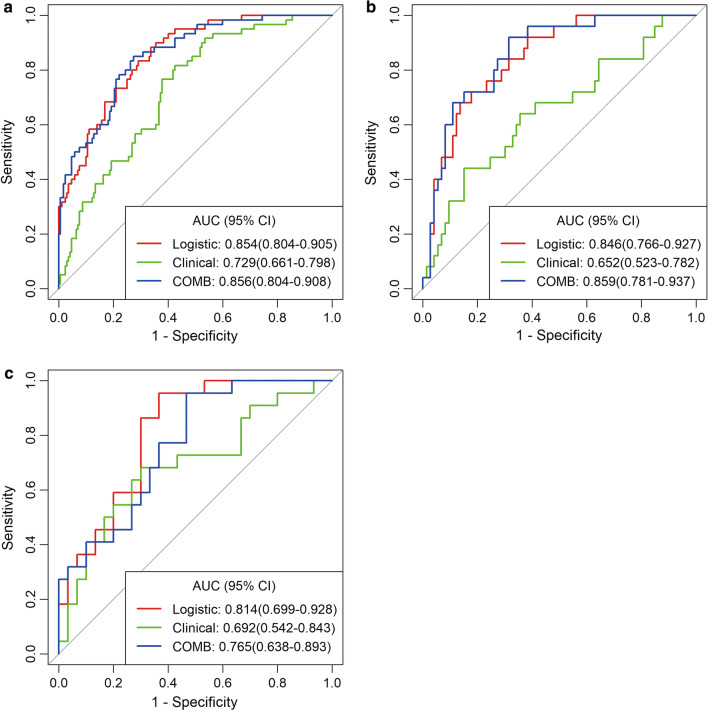
The ROCs and AUCs of the three cohorts are shown in Fig. 2A–C. The best model was the combined model in the training and testing cohorts, with an AUC of 0.856 and 0.859, in the validation cohort, the radiomics model and the combined model performed better than clinical model, with an AUC of 0.814, 0.765 and 0.692, respectively. The radiomics models showed an excellent predictive performance in the discrimination of IACs from noninvasive lesions in the three cohorts. The radiomics signature yielded an AUC of 0.854, 0.846, and 0.814 in the training, testing, and validation cohorts, respectively (Table 3), and the sensitivity was 0.883, 0.840 and 0.955, specificity was 0.663, 0.644 and 0.633 in three cohorts.
Fig. 2.
The ROCs of the three models in the training cohort (a), testing cohort (b), and validation cohort (c). The predictive performance for an invasive pGGN lesion was better in the combined model than in the clinical and radiomics models in the training and testing cohorts. In the validation cohort, the radiomics model and combined model performed better than clinical model
Table 3.
Diagnostic performance of three models for the prediction of pGGN invasiveness
| Cohort | Model | AUC | 95% CI | Sensitivity | Specificity | Accuracy |
|---|---|---|---|---|---|---|
| Training cohort | Clinical-radiographic model | 0.729 | 0.661–0.798 | 0.817 | 0.576 | 0.638 |
| Radiomics model | 0.854 | 0.804–0.905 | 0.883 | 0.663 | 0.720 | |
| Combined model | 0.856 | 0.804–0.908 | 0.850 | 0.727 | 0.759 | |
| Testing cohort | Clinical-radiographic model | 0.652 | 0.523–0.782 | 0.680 | 0.452 | 0.510 |
| Radiomics model | 0.846 | 0.766–0.927 | 0.840 | 0.678 | 0.729 | |
| Combined model | 0.859 | 0.781–0.937 | 0.880 | 0.685 | 0.735 | |
| Validation cohort | Clinical-radiographic model | 0.692 | 0.542–0.843 | 0.682 | 0.600 | 0.635 |
| Radiomics model | 0.814 | 0.699–0.928 | 0.955 | 0.668 | 0.769 | |
| Combined model | 0.765 | 0.638–0.893 | 0.773 | 0.656 | 0.762 |
95% CI means 95% confidence interval
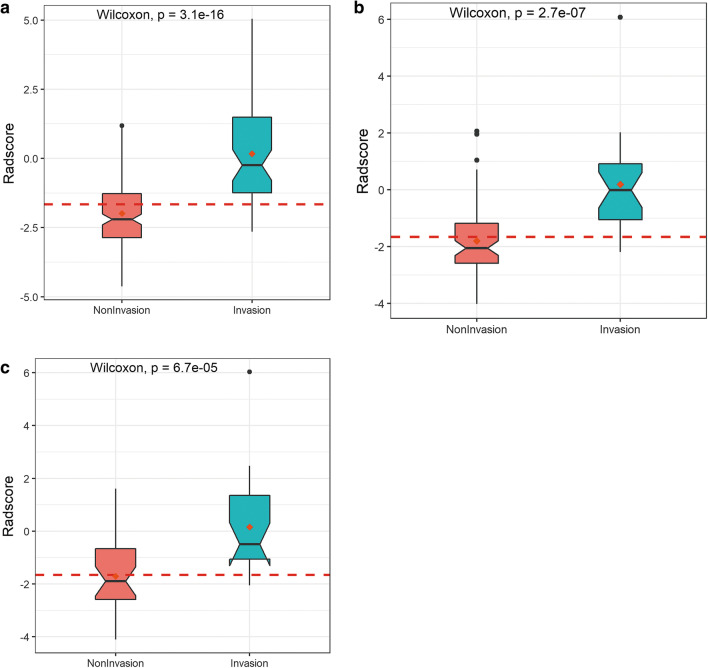
The Rad-score was calculated from the selected radiomics features. The corresponding regression coefficients and distribution in the training, testing, and validation cohorts are presented in Fig. 3. The Rad-score cut-off value was −1.66.
Fig. 3.
The box plots show the distribution between noninvasive and invasive lesions for GGNs in the training cohort (a), testing cohort (b), and validation cohort (c). The Wilcoxon test obtained p values. The Rad-score was higher in the invasive group than in the noninvasive group
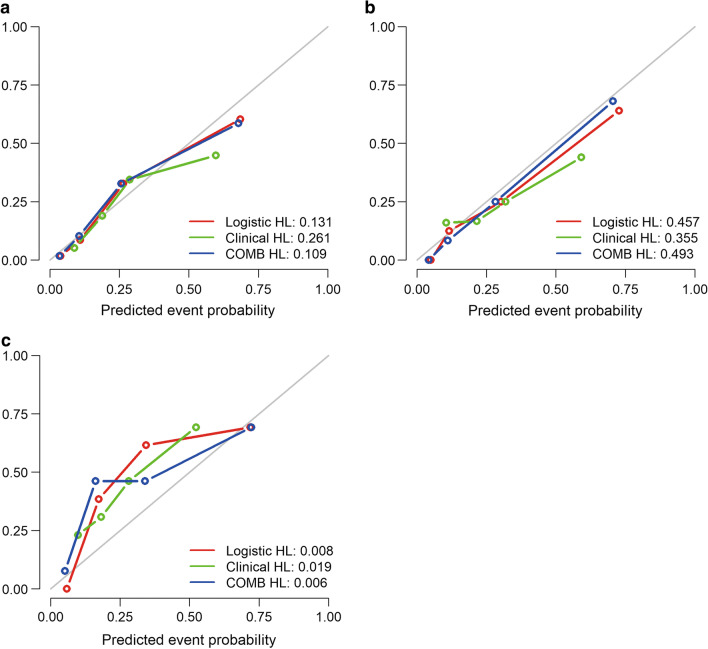
The calibration curves showed good predictability between prediction and observation in the three cohorts (Fig. 4). Compared with the results of the validation cohort, the correspondence between actual and ideal predictions suggested good calibration of the clinical, radiomics, and combined models in the training and testing cohorts.
Fig. 4.
Calibration curves for the prediction of pGGN invasiveness based on the three models in the training cohort (a), testing cohort (b), and validation cohort (c). The x-axis represents the predicted probability of IACs based on the clinical, radiomics, and combined models, and the y-axis represents the actual probability of pGGN invasiveness. The 45° diagonal line represents ideal prediction, and the red, green, and blue lines represent the predictive performance of the nomogram. The closer the line was to the ideal line, the better the predictive nomogram performance
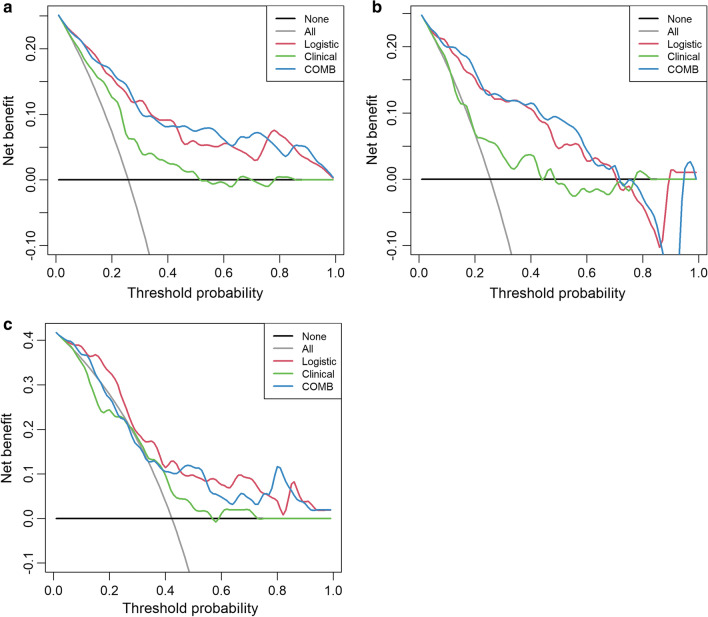
The decision curve analysis for the three models is presented in Fig. 5. The decision curve indicated that the use of a combined model for the prediction of invasive lesions added more net benefit than the use of clinical features or radiomics features alone in the differentiation of IACs from noninvasive lesions; this was especially true in the training and testing cohorts. The DCA in the external validation cohort showed that the combined and radiomics models achieved higher average precision scores than the clinical-radiographic model.
Fig. 5.
Decision curve analysis for the combined model (c) compared with the clinical model (a) and radiomics models (b) alone. The x-axis shows the threshold probability, and the y-axis measures the net benefit. The black line represents the hypothesis that all patients with pGGNs had noninvasive lesions, and the gray line represents the hypothesis that all patients with pGGNs had invasive lesions
Clinical-radiographic features, including gender, age, mean CT value, and radiomics features (Log.5.0_glszm_SmallAreaHighGrayLevelEmphasis, wavelet.LHL_glcm_MCC, and wavelet.LLL_glcm_SumAverage), were significantly associated with an increased risk of IACs. The highest odds ratio (OR) was detected for gender in the clinical model (OR = 2.466), for wavelet.LLL_glcm_SumAverage in the radiomics model (OR = 2.287), and for wavelet.LLL_glcm_SumAverage in the combined model (OR = 2.471) (Table 4).
Table 4.
Associations between features and invasiveness of pGGNs
| OR | 95% CI | p value | |
|---|---|---|---|
| Clinical model | |||
| Gender | 2.466 | 1.266–4.860 | 0.0083 |
| Age | 1.037 | 1.033–1.074 | 0.0356 |
| Mean CT value | 1.006 | 1.003–1.009 | 0.0000 |
| Radiomics moel | |||
| Log.5.0_glszm_SmallAreaHighGrayLevelEmphasis | 2.232 | 1.481–3.441 | 0.0001 |
| wavelet.LHL_glcm_MCC | 0.579 | 0.370–0.884 | 0.0132 |
| wavelet.LLL_glcm_SumAverage | 2.287 | 1.528–3.549 | 0.0001 |
| Combined model | |||
| Log.5.0_glszm_SmallAreaHighGrayLevelEmphasis | 2.201 | 1.446–3.419 | 0.0002 |
| wavelet.LHL_glcm_MCC | 0.603 | 0.382–0.929 | 0.0247 |
| wavelet.LLL_glcm_SumAverage | 2.471 | 1.623–3.928 | 0.0000 |
| Gender | 2.190 | 1.004–4.832 | 0.0491 |
Discussion
Radiomics are a rising field of quantitative imaging that can extract high-throughput data features and describe tumor phenotype characteristics. Radiomics analysis provides a noninvasive and powerful alternative for disease diagnosis, differentiation, clinical treatment, and assessment [34]. Recent studies have succeeded in radiomics analyses in the field of oncology, showing the diagnostic and prognostic values [34, 41, 42].
Therefore, in the present study, the radiomics features and evaluated clinical features were extracted from CT images for the prediction of pGGN invasiveness; they were then investigated, and the performance of the clinical, radiomics, and combined models for classifying noninvasive lesions and IACs was compared.
The results of the present study showed that the combined model performed better than the radiomics and clinical models, with a higher AUC in the training and testing cohorts (0.856, 0.859, respectively), suggesting that it is a noninvasive tool for differentiating IACs from noninvasive lesions.
Some studies have suggested that radiomics features can be used to differentiate pGGN invasiveness. Previous studies reported that in clinical practice, lesion size, CT value, and morphological characteristics were associated with pGGN invasiveness [43–45]. Recent studies have highlighted the combined model, which incorporated clinical features and radiomics features in the diagnosis of lung cancer.
Sun et al. and Liu et al. [46, 47] showed that the predictive model for IAC constructed by integrating the clinical and radiomics features based on the radiomics nomogram exhibited excellent accuracy in the differentiation of noninvasive lesions from IACs (AUC 0.831; 95% CI: 0.765–0.897). Although morphological characteristics, such as lobulation, burr, vacuole sign, and pleural involvement sign, are helpful in the identification of nodule invasiveness, they were not included in the final clinical model construction of this study; this is mainly because the morphological characteristics of early stage lung cancer are usually atypical, especially in IACs with a smaller diameter than 1 cm. Furthermore, identification of the morphological features depended on the radiologist’s diagnostic experience.
Former studies have found that the mean nodule CT value is a significant predictor in the differentiation of pGGN invasiveness. Previous study has shown that pGGNs with a mean CT value higher than −600 HU indicated invasive adenocarcinoma [23].
Wu et al. [48] evaluated CT and histopathologic features of lung adenocarcinoma with pGGNs that were ≤ 10 mm in diameter, and the results showed no statistically significant difference in the CT value between noninvasive lesions and IACs.
In the present study, the multivariate logistic regression analysis revealed that the mean CT value was useful in histopathologic subtype differentiation; IACs reflected a higher mean CT value than noninvasive lesions. The study also showed that the AUC for the clinical model established by using gender, age, and the mean CT value was lower than the AUC for the radiomics model in the three cohorts (AUC = 0.729, 0.652, and 0.692, respectively).
Radiomics show the ability to serve as a bridge between medical imaging and precise medicine [49–51]. Yang et al. [52] utilized 14 radiomics features of lung adenocarcinoma to distinguish IACs and noninvasive lesions; an AUC of 0.77 was achieved. The radiomics signatures performed better than the most commonly used clinical features, such as the mean CT value. Weng et al. [53] identified that the nomogram, which integrated morphology characteristics and radiomics features, showed a high performance in the classification of IACs and MIAs (AUC = 0.888).
In the present study, the radiomics features performed a good differentiation ability in three cohorts (AUC = 0.854, 0,846, and 0.814, respectively); this ability was significantly better at predicting IACs than in the clinical model. The findings of the present study are consistent with the findings of previous studies, which have found various texture and shape features to be significant predictors of IACs.
Furthermore, radiomics features comprising tumor shape features, first-order statistic features, and texture features in a noninvasive, three-dimensional manner may allow for more precise and personalized treatment than traditional modality of these patients with pGGN detected by CT.
The clinical and radiomics models were then combined to improve the diagnosis accuracy; the combined model had a better performance than clinical and radiomics model alone and achieved a satisfactory result in the external validation cohort. The ROC and DCA also indicated that using the combined model to predict invasive lesions added more net benefit than using clinical features or radiomics features alone in differentiating IACs from noninvasive lesions, especially in the training and testing cohorts.
The present study has several limitations. First, it is a single-institutional retrospective study, and the sample size is small; large and multi-institutional cohorts will be recruited in future research. Second, the CT images were acquired from different scanners, and standardization of scanning and reconstruction parameters is required for further study. Third, although an external validation cohort was constructed, the number of patients assigned to it was relatively small; thus, it is necessary to increase the number of cases in this group to identify the performance of the model and usefulness of radiomics signatures.
Conclusion
In conclusion, the radiomics signature from the CT images provided a noninvasive modality for IAC prediction. Radiomics signatures combined with clinical features yielded a better performance than using alone in differentiating IACs from noninvasive lesions appearing as pGGNs on thin-slice CT; this may facilitate clinical diagnosis and treatment in further work.
Abbreviations
- pGGNs
Pure ground glass nodules
- IAC
Invasive adenocarcinoma
- LASSO
Least absolute shrinkage and selection operator
- AUC
Area under the curve
- DCA
Decision curve analysis
- HRCT
High-resolution CT
- AAH
Atypical adenomatous hyperplasia
- AIS
Adenocarcinoma in situ
- MIA
Minimally invasive adenocarcinoma
- DFS
Disease-free survival
- CT
Computed tomography
- ICC
Intra-class correlation coefficients
- GLCM
Gray-level co-occurrence matrix
- GLRLM
Gray-level run length matrix
- GLDM
Gray-level dependence matrix
- GLSZM
Gray-level zone matrix
- NGTDM
Neighborhood grey tone difference matrix
- AIC
Akaike information criterion
Author contributions
H.F. and G.S. were involved in conception and design of the research. H.F. contributed to acquisition of data. J.R. was involved in analysis and interpretation of the data. L.W. and X.C. contributed to statistical analysis. H.F. was involved in writing of the manuscript. Q.X. contributed to critical revision of the manuscript for intellectual content. All authors read and approved the final draft. All authors read and approved the final manuscript.
Funding
Not applicable.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of The Fourth Hospital of Hebei Medical University. A written informed consent was obtained from all participants.
Competing interests
All authors have contributed significantly to the manuscript and declare that the work is original and has not been submitted or published elsewhere. None of the authors have any financial disclosure or conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Tsutsui S, Ashizawa K, Minami K, et al. Multiple focal pure ground-glass opacities on high-resolution CT images: clinical significance in patients with lung cancer. AJR Am J Roentgenol. 2010;195:131–138. doi: 10.2214/AJR.09.3828. [DOI] [PubMed] [Google Scholar]
- 3.Miller A, Markowitz S, Manowitz A, Miller JA. Lung cancer screening using low-dose high-resolution CT scanning in a high-risk workforce:3500 nuclear fuel workers in three US states. Chest. 2004;125(Suppl):152S–153S. [PubMed] [Google Scholar]
- 4.Bak SH, Lee HY, Kim JH, et al. Quantitative CT scanning analysis of pure ground-glass opacity nodules predicts further CT scanning change. Chest. 2016;149(1):180–191. doi: 10.1378/chest.15-0034. [DOI] [PubMed] [Google Scholar]
- 5.Austin JH, Muller NL, Friedman PJ, et al. Glossary of terms for CT of the lungs: recommendations of the Nomenclature Committee of the Fleischner Society. Radiology. 1996;200:327–331. doi: 10.1148/radiology.200.2.8685321. [DOI] [PubMed] [Google Scholar]
- 6.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang B, Hwang JH, Choi YH, et al. Natural history of pure ground-glass opacity lung nodules detected by low-dose CT scan. Chest. 2013;143:172–178. doi: 10.1378/chest.11-2501. [DOI] [PubMed] [Google Scholar]
- 8.Kakinuma R, Muuramatsu Y, Kusumoto M, et al. Solitary pure ground-glass nodules 5 mm or smaller: frequency of growth. Radiology. 2015;276:873–882. doi: 10.1148/radiol.2015141071. [DOI] [PubMed] [Google Scholar]
- 9.MacMahon H, Naidich DP, Goo JM, et al. Guidelines for management of incident pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology. 2017;284:228–243. doi: 10.1148/radiol.2017161659. [DOI] [PubMed] [Google Scholar]
- 10.Lee HY, La Choi Y, Lee KS, et al. Pure ground-glass opacity neoplastic lung nodules: histopathology, imaging, management. AJR Am J Roentgenol. 2014;202:224–233. doi: 10.2214/AJR.13.11819. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen JH, Saghir Z, Wille MM, Thomsen LH, Skov BG, Ashraf H. Ground-glass opacity lung nodules in the era of lung cancer CT screening:radiology, pathology, and clinical management. Oncology (Willston Park) 2016;30:266–274. [PubMed] [Google Scholar]
- 12.Heidinger BH, Anderson KR, Ncmcc U, et al. Lung adenocarcinoma manifesting as pure ground-glass nodules: correlating CT size, volume, density and roundness with histopathologic invasion and size. J Thorac Oncol. 2017;12:1288–1298. doi: 10.1016/j.jtho.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Ichinose J, Kohno T, Fujimori S, Harano T, Suzuki S, Fujii T. Invasiveness and malignant potential of pulmonary lesions presenting as pure ground-glass opacities. Ann Thorac Cardiovasc Surg. 2014;20:347–352. doi: 10.5761/atcs.oa.13-00005. [DOI] [PubMed] [Google Scholar]
- 14.Lim HJ, Ahn S, Lee KS, et al. Persistent pure ground-glass opacity lung nodules ≥10 mm in diameter at CT scan: histopathologic comparisons and prognostic implications. Chest. 2013;144:1291–1299. doi: 10.1378/chest.12-2987. [DOI] [PubMed] [Google Scholar]
- 15.She Y, Zhao L, Dai C, et al. Preoperative nomogram for identifying invasive pulmonary adenocarcinoma in patients with pure ground-glass nodule: a multi-institutional study. Oncotarget. 2017;8:17229–17238. doi: 10.18632/oncotarget.11236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye T, Deng L, Wang S, et al. Lung adenocarcinomas manifesting as radiological part-solid nodules define a special clinical subtype. J Thorac Oncol. 2019;14(4):617–627. doi: 10.1016/j.jtho.2018.12.030. [DOI] [PubMed] [Google Scholar]
- 17.Lee GD, Park CH, Park HS, et al. Lung adenocarcinoma invasiveness risk in pure ground-glass nodules smaller than 2 cm. Thorac Cardiovasc Surg. 2019;67:321–328. doi: 10.1055/s-0037-1612615. [DOI] [PubMed] [Google Scholar]
- 18.Fan L, Fang MJ, Li ZB, et al. Radiomics signature: a biomarker for the preoperative discrimination of lung invasive adenocarcinoma manifesting as a ground-glass nodule. Eur Radiol. 2019;29:889–897. doi: 10.1007/s00330-018-5530-z. [DOI] [PubMed] [Google Scholar]
- 19.Lee SM, Park CM, Goo JM, Lee HJ, Wi JY, Kang CH. Invasive pulmonary adenocarcinomas versus preinvasive lesions appearing as ground-glass nodules: differentiation by using CT features. Radiology. 2013;268:265–273. doi: 10.1148/radiol.13120949. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Sun H, Zhou F, et al. Imaging features of TSCT predict the classification of pulmonary preinvasive lesion, minimally and invasive adenocarcinoma presented as ground glass nodules. Lung Cancer. 2017;108:192–197. doi: 10.1016/j.lungcan.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Minami H, Yoshimura M, Miyamoto Y, Matsuoka H, Tsubota N. Lung cancer in women: sex-associated differences in survival of patients undergoing resection for lung cancer. Chest. 2000;118(6):1603–1609. doi: 10.1378/chest.118.6.1603. [DOI] [PubMed] [Google Scholar]
- 22.Huang C, Wang C, Du J, et al. The prognostic significance of pure ground glass opacities in lung cancer computed tomographic. J Cancer. 2019;10(27):6888–6895. doi: 10.7150/jca.33132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu F, Huang H, Jiang Y, et al. Discriminating invasive adenocarcinoma among lung pure ground-glass nodules: a multi-parameter prediction model [DOI] [PMC free article] [PubMed]
- 24.Han L, Zhang P, Wang Y, et al. CT quantitative parameters to predict the invasiveness of lung pure ground-glass nodules (pGGNs) Clin Radiol. 2018;73(504):e1–504. doi: 10.1016/j.crad.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 25.Jin X, Zhao SH, Gao J, et al. CT characteristics and pathological implications of early stage (T1N0M0) lung adenocarcinoma with pure ground-glass opacity. Eur Radiol. 2015;25:2532–2540. doi: 10.1007/s00330-015-3637-z. [DOI] [PubMed] [Google Scholar]
- 26.Kitami A, Sano F, Hayashi S, et al. Correlation between histological invasiveness and the computed tomography value in pure ground-glass nodules. Surg Today. 2016;46:593–598. doi: 10.1007/s00595-015-1208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Riel SJ, Sánchez CI, Bankier AA, et al. Observer variability for classification of pulmonary nodules on low-dose CT images and its effect on nodule management. Radiology. 2015;277:863–871. doi: 10.1148/radiol.2015142700. [DOI] [PubMed] [Google Scholar]
- 28.Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278(2):563–577. doi: 10.1148/radiol.2015151169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chae HD, Park CM, Park SJ, Lee SM, Kim KG, Goo JM. Computerized texture analysis of persistent part-solid ground-glass nodules: differentiation of preinvasive lesions from invasive pulmonary adenocarcinomas. Radiology. 2014;273(1):285–293. doi: 10.1148/radiol.14132187. [DOI] [PubMed] [Google Scholar]
- 30.Yagi T, Yamazaki M, Ohashi R, et al. HRCT texture analysis for pure or part-solid ground-glass nodules: distinguishability of adenocaricinoma in situ or minimally invasive adenocarcinoma from invasive adenocarcinoma. Jpn J Radiol. 2018;36(2):113–121. doi: 10.1007/s11604-017-0711-2. [DOI] [PubMed] [Google Scholar]
- 31.Scaglia NC, Chatkin JM, Pinto JA, Tsukazan MT, Wagner MB, Saldanha AF (2013) Role of gender in the survival of surgical patients with nonsmall cell lung cancer. Ann Thorac Med, 142–147 [DOI] [PMC free article] [PubMed]
- 32.Rizzo S, Petrellla F, Buscarino V, et al. CT radiogenomic characterization of EGFR, K-RAS, and ALK mutations in non-small cell lung cancer. Eur Radiol. 2016;26:32–42. doi: 10.1007/s00330-015-3814-0. [DOI] [PubMed] [Google Scholar]
- 33.Griethuysen JJM, Fedorov A, Parmar C, et al. Computational radiomics system to decode the radiographic phenotype. Can Res. 2017;77(21):e104–e107. doi: 10.1158/0008-5472.CAN-17-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aerts HJ, Velazquez ER, Leijenaar RT, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006. doi: 10.1038/ncomms5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma C, Huang J. Asymptotic properties of LASSO in high-dimensional partially linear models. Sci China Math. 2016;59:1–20. [Google Scholar]
- 36.Rao SJ. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. J Am Stat Assoc. 2003;98:257–258. [Google Scholar]
- 37.Friedman J, Hastie T, Tibshirani R. Regulariation paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- 38.Lao J, Chen Y, Li ZC, et al. A deep learning-based radiomics model for prediction of survival in glioblastoma multiforme. Sci Rep. 2017;7:10353. doi: 10.1038/s41598-017-10649-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vickers AJ, Cronin AM, Elkin EB, Gonen M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak. 2008;8:53. doi: 10.1186/1472-6947-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kramer AA, Zimmerman JE. Assessing the calibration of mortality benchmarks in critical care: the Hosmer–Lemeshow test revisited. Crit Care Med. 2007;35(9):2052–2056. doi: 10.1097/01.CCM.0000275267.64078.B0. [DOI] [PubMed] [Google Scholar]
- 41.Zhou M, Leung A, Echegaray S, et al. Non-small cell lung cancer radiogenomics map identifies relationships between molecular and imaging phenotypes with prognostic implication. Radiology. 2018;286(1):307–315. doi: 10.1148/radiol.2017161845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang Y, Liu Z, He L, et al. Radiomics signature: a potential biomarker for the prediction of disease-free survival in early-stage (I or II) non-small cell lung cancer. Radiology. 2016;281(3):947–957. doi: 10.1148/radiol.2016152234. [DOI] [PubMed] [Google Scholar]
- 43.Kim H, Goo JM, Park CM. Evaluation of T categories for pure ground-glass nodules with semi-automatic volumetry: is mass a better predictor of invasive part size than other volumetric parameters? Eur Radiol. 2018;28:4288–4295. doi: 10.1007/s00330-018-5440-0. [DOI] [PubMed] [Google Scholar]
- 44.Xiang W, Xing Y, Jiang S, et al. Morphological factors differentiating between early lung adenocarcinomas appearing as pure ground-glass nodules measuring ≤10mm on thin-section computed tomography. Cancer Imaging. 2014;14:33. doi: 10.1186/s40644-014-0033-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao Q, Wang JW, Yang L, Xue LY, Lu WW. CT diagnosis of pleural and stromal invasion in malignant subpleural pure ground-glass nodules: an exploratory study. Eur Radiol. 2019;29:279–286. doi: 10.1007/s00330-018-5558-0. [DOI] [PubMed] [Google Scholar]
- 46.Sun Y, Li C, Jin L, et al. Radiomics for lung adenocarcinoma manifesting as pure ground-glass nodules: invasive prediction. Eur Radiol. 2020;30(7):3650–3659. doi: 10.1007/s00330-020-06776-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu C, Zhang F, Cai Q, Shen Y, Chen S. Establishment of a predictive model for surgical resection of ground-glass nodules. J Am Coll Radiol. 2019;16(4):435–445. doi: 10.1016/j.jacr.2018.09.043. [DOI] [PubMed] [Google Scholar]
- 48.Wu F, Tian SP, Jin X, et al. CT and histopathologic characteristics of lung adenocarcinoma with pure ground-glass nodules 10mm or less in diameter. Eur Radiol. 2017;27(10):4037–4043. doi: 10.1007/s00330-017-4829-5. [DOI] [PubMed] [Google Scholar]
- 49.Lambin P, Rios-Velazquez E, Leijenaar R, et al. Radiomics:extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48:441–446. doi: 10.1016/j.ejca.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar V, Gu Y, Basu S, et al. Radiomics: the process and the challenges. Magn Reson Imaging. 2013;30:1234–1248. doi: 10.1016/j.mri.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lambin P, Leijenaar RTH, Deist TM, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14(12):749–762. doi: 10.1038/nrclinonc.2017.141. [DOI] [PubMed] [Google Scholar]
- 52.Yang B, Guo L, Lu G, Shan W, Duan L, Duan S. Radiomic signature: a non-invasive biomarker for discriminating invasive and non-invasive cases of lung adenocarcinoma. Cancer Manag Res. 2019;11:7825–7834. doi: 10.2147/CMAR.S217887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weng Q, Zhou L, Wang H, et al. A radiomics model for determining the invasiveness of of solitary pulmonary nodules that manifest as part-solid nodules. Clin Radiol. 2019;74(12):933–943. doi: 10.1016/j.crad.2019.07.026. [DOI] [PubMed] [Google Scholar]