Abstract
Senescence compromises the essential role that the endothelium plays in maintaining vascular homeostasis, so promoting endothelial dysfunction and the development of age-related vascular diseases. Their biological and clinical significance calls for strategies for identifying and therapeutically targeting senescent endothelial cells. While senescence and endothelial dysfunction have been studied extensively, distinguishing what is distinctly endothelial senescence remains a barrier to overcome for an effective approach to addressing it. Here, we review the mechanisms underlying endothelial senescence and the evidence for its clinical importance. Furthermore, we discuss the current state and the limitations in the approaches for the detection and therapeutic intervention of target cells, suggesting potential directions for future research.
Subject terms: Senescence, Ageing, Drug discovery, Therapeutics, Cardiovascular diseases
Vascular disease: Improving vascular health by combatting ‘zombie’ cells
Cells that stop proliferating but remain metabolically active offer a promising therapeutic target for the treatment of age-related vascular diseases. Yeaeun Han and Sung Young Kim from Konkuk University School of Medicine in Seoul, South Korea, review the ways in which cellular senescence, the process by which damaged cells enter irreversible growth arrest, but stay alive and continue to secrete substances that damage surrounding tissues, contributes to blood vessel problems as people age. These so-called ‘zombie’ cells then fuel the development of atherosclerosis, hypertension, and other vascular disorders. Diagnostics that test for indicators of senescence affecting the lining of blood vessels could help reveal the severity of vascular damage. Drugs that remove or modify the function of senescent cells in blood vessels could help treat cardiovascular disease.
Introduction
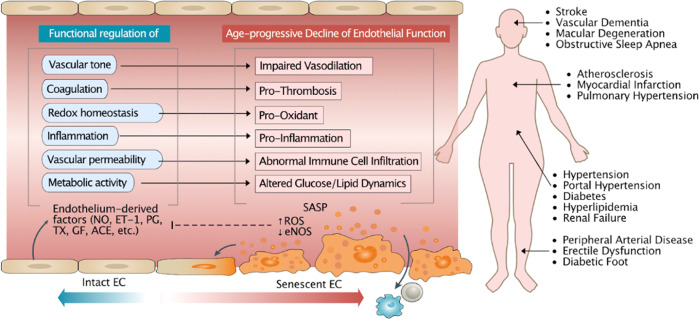
Age-related endothelial dysfunction, regarded as a prominent precursor to the development of cardiovascular diseases (CVDs)1,2, can be characterized by a shift toward a vasoconstrictive, proinflammatory and prothrombotic environment3, resulting in impaired regulation of vascular homeostasis. Endothelial dysfunction can be largely explained by endothelial senescence, which has been implicated in the development of various age-related CVDs, including stroke4, vascular dementia5, macular degeneration6, obstructive sleep apnea7, atherosclerosis8,9, myocardial infarction10, pulmonary hypertension11–13, hypertension14, diabetes15,16, renal failure17, peripheral arterial disease18, erectile dysfunction19 and diabetic foot20 (Fig. 1). The “geroscience hypothesis” aptly summarizes the idea that as aging is the main driver of multiple interrelated diseases, targeting key aging contributors may prevent the onset or mitigate the severity of multimorbidity21. Therefore, there are great clinical motivations and implications for research into endothelial senescence.
Fig. 1. General overview of endothelial senescence and cardiovascular diseases (CVDs).
Endothelial senescence is characterized by an age-associated decline in endothelial function, which includes the loss of control over vasodilation, blood coagulation, oxidative stress, inflammation, immune cell infiltration, and glucose and lipid dynamics mediated by endothelium-derived factors, such as nitric oxide (NO), endothelin-1 (ET-1), prostaglandin (PG), thromboxane (TX), endothelial growth factor (EGF) and angiotensin-converting enzyme (ACE). A reduction in NO, the master regulator of the endothelium, is one of the primary factors driving these changes. Other factors include increased reactive oxygen species (ROS) levels and senescence-associated secretory phenotype (SASP) acquisition. EC endothelial cell, eNOS endothelial nitric oxide synthase.
In this review, we discuss the key alterations found in endothelial senescence and its clinical potential in vascular diseases. The investigation into the clinical applications of endothelial senescence is characterized by two objectives: detecting and targeting senescence. By utilizing endothelial senescence as a molecular biomarker, it may be possible to describe the degree of endothelial dysfunction. Upon detection, senescent cells can be treated to reverse damage and recover endothelial function. Finally, we discuss the latest advances in detection approaches and in senotherapeutics, with a special focus on endothelial cells (ECs) and potential therapeutic applications of these advances to CVDs.
Key pathways involved in endothelial senescence
Senescence serves as a blanket term that encompasses a wide range of states of stable cell cycle arrest states associated with various biological contexts and stimuli. Although such heterogeneity complicates the precise characterization or quantification of senescence, its implication in major age-related diseases makes it critical to investigate for a better understanding of their pathogenesis and the search for therapeutic venues. Rather than a simplistic approach in which various unique types of senescence are understood in the limited confines of generalized senescence, it may be useful to consider the individual features of a particular type of senescence in a defined physiological context.
While endothelial senescence has certain features that can be uniquely attributed to it, it shares several core mechanisms with other types of senescence. Endothelial senescence is triggered by a variety of senescence stressors that include replicative and oxidative stress, oncogenic activation22, telomere attrition23, DNA damage24, and mitochondrial dysfunction25. Mitochondrial dysfunction in senescent ECs can be characterized by decreased mitochondrial mass and alterations in the mitochondrial composition and electron transport chain (ETC)26. It has been demonstrated that the activity of complex IV and cytochrome c oxidase, components of the mitochondrial ETC, declines with age, and the resulting impairment increases mitochondrial oxidative stress26. In turn, oxidative stress can accelerate telomere shortening27 and induce DNA damage24 in ECs. Regardless of the type of stressor, senescent pathways converge on cell cycle arrest, which is primarily mediated by two main tumor suppressive pathways involving p53/p21WAF1 and/or p16Ink4A/RB28. Senescent cells acquire the senescence-associated secretory phenotype (SASP), which includes chemokines, cytokines, growth factors, and insoluble factors. While the SASP is heterogeneous and can vary by the type of cell and stimuli29, a unique feature of endothelial SASP is its characterization by regulators of arterial dysfunction, including increased levels of reactive oxygen species (ROS) and reduced nitric oxide (NO)28.
Impaired redox homeostasis characterized by reduced NO production and increased oxidative stress is widely recognized as a key defining feature of endothelial senescence (Fig. 2). NO is a critical vasodilatory factor that has also been observed in reduction in endothelial dysfunction, owing to age-related impairment of endothelial nitric oxide synthase (eNOS) and decreased levels of the eNOS cofactors tetrahydrobiopterin (BH4) and L-arginine30. It has been reported that hyperglycemia31, hyperuricemia32, and hyperlipidemia33 inhibit eNOS production, while FGF21 has been shown to delay endothelial senescence by enhancing eNOS in mice34 and in a SIRT1-dependent manner in human umbilical vein endothelial cells (HUVECs)34,35. Diminished NO bioavailability may lead to impaired angiogenesis, reduced levels of peroxisome proliferator-activated receptor gamma coactivator 1 (PGC1α)26, and dysregulation in related metabolic processes such as the NAD+ biosynthetic pathway and NAD+-dependent protein deacetylase sirtuin-1 (SIRT1) in the sirtuin family30. Critical cellular sensors, adenosine monophosphate-activated protein kinase (AMPK), which senses the cell energy status, and mammalian target of rapamycin (mTOR), which senses the cell nutritional status, can modulate endothelial function and are altered during endothelial senescence28. In endothelial senescence, protein kinase B (Akt) has been reported to be constitutively activated and to promote cell arrest in a p53/p21-dependent manner through the inhibition of the transcription factor FOXO3a36,37. Inhibition of mTOR has been associated with enhanced endothelium-dependent dilation and higher levels of NO38. Another energy-sensitive metabolic sensor, sirtuin, plays an essential role in facilitating endothelial function through the regulation of the transcription factors FOXO, p53, and NFκB as well as in producing NO through the deacetylation of eNOS28,39,40. SIRT1 can further protect the endothelium by mediating the level of plasminogen activator inhibitor-1 (PAI-1), which can be increased via SIRT1 inhibition and is often increased in endothelial senescence and dysfunction41.
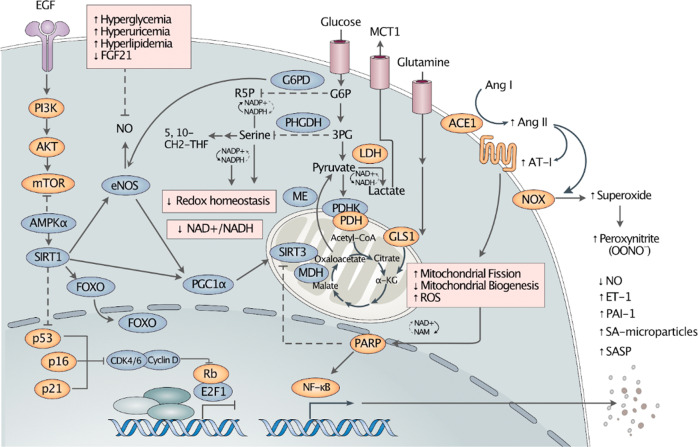
Fig. 2. Altered pathways in endothelial senescence.
We have marked proteins with activities that are believed to be reduced and increased in orange and blue, respectively. A prominent feature of endothelial senescence (ES) is impaired redox homeostasis with reduced antioxidant capacity. Senescent cells exhibit decreased NAD+/NADH levels, which may reduce ROS defense capacity. It has been suggested that potential alterations observed in ES can be summarized by the following: (1) active aerobic glycolysis driven by the activation of LDH and attenuated ME and MDH; (2) decreased PDHK activity leading to activated PDH, causing a shift to the TCA cycle; and 3) decreased PHGDH and G6PD activity leading to disruption of serine synthesis and of pentose phosphate pathway activity, respectively, and resulting in a decreased glutathione level and NADPH synthesis rate. Increased glutamine metabolism, along with an increased GLS1 level, may provide energy for senescent cells. Increased ACE, Ang II, and AT-1 activity can lead to reduced mitochondrial biogenesis and increased mitochondrial fission and ROS production. Impaired mitochondrial function can activate PARP to repair mitochondria. PARP regulates NF-κB, leading to SASP production. In ES, activated NOX may produce superoxide, which forms peroxynitrite with NO, resulting in a positive feedback loop that increases ROS and further decreases NO. EGF epidermal growth factor, PI3K phosphatidylinositol-3-kinase, mTOR mammalian target of rapamycin, AMPK adenosine monophosphate-activated protein kinase, SIRT sirtuin, FOXO forkhead box O, PGC1α peroxisome proliferator-activated receptor γ-coactivator 1α, CDK cyclin-dependent kinase, E2F1 E2F transcription factor 1, NF-κB nuclear factor-kappa B, PARP poly (ADP-ribose) polymerase, NAD nicotinamide adenine dinucleotide, NAM nicotinamide, NADH nicotinamide adenine dinucleotide hydrogen, NADP nicotinamide adenine dinucleotide phosphate, NADPH reduced nicotinamide adenine dinucleotide phosphate, NOX NADPH oxidase, AMP adenosine monophosphate, ADP adenosine diphosphate, ATP adenosine triphosphate, ROS reactive oxygen species, MDH malate dehydrogenase, α-KG α-ketoglutarate, GLS1 glutaminase 1, PDH pyruvate dehydrogenase, PDHK pyruvate dehydrogenase kinase, ME malic enzyme, LDH lactate dehydrogenase, PHGDH D-3-phosphoglycerate dehydrogenase, 5 10-CH2-THF 510-methenyltetrahydrofolate, 3PG 3-phosphoglycerate, G6P glucose 6-phosphate, G6PD glucose 6-phosphate dehydrogenase, R5P ribose 5-phosphate, MCT1 monocarboxylate transporter 1, ACE1 angiotensin-converting enzyme 1, ANG angiotensin, AT-1 angiotensin II type-1 receptor, NO nitric oxide, ET-1 endothelin-1, PAI-1 plasminogen activator inhibitor-1, SA senescence-associated, SASP senescence-associated secretory phenotype, eNOS endothelial nitric oxide synthase, FGF21 fibroblast growth factor 21.
The renin/angiotensin system (RAS) is another key component of vascular tone regulation, which is altered in endothelial senescence. The altered RAS can be identified by increased angiotensin-converting enzyme 1 (ACE1), angiotensin II (Ang II), and angiotensin II type-1 receptor (AT-1), which have been shown to induce fibrosis, inflammation, and oxidative stress in aged mouse thoracic aorta42. In conjunction with increased endothelin-143, a vasoconstrictor peptide produced by ECs44,45, the altered RAS exerts vasoconstrictive pressure on the vasculature46.
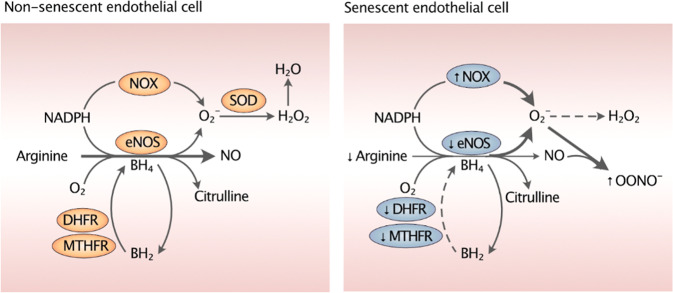
Another cause of vasoconstrictive stress is derived from the uncoupling of eNOS (Fig. 3). The cause of eNOS uncoupling can be attributed to the limited availability of its cofactors such as L-arginine and BH4. Uncoupled eNOS and NADPH oxidase (NOX) can produce superoxide. While superoxide can be scavenged by superoxide dismutases, it has a higher affinity for NO and binds to it to form peroxynitrite (ONOO−), a toxic and reactive nitrogen species with strong oxidizing action47. ONOO−can oxidizes BH4 into 7,8-dihydrobiopterin (BH2) and damage zinc-thiolate clusters of eNOS, further driving eNOS uncoupling, ultimately increasing ROS and reducing NO48. It has been observed that dihydrofolate reductase (DHFR) and methylenetetrahydrofolate reductase (MTHFR), two enzymes responsible for the regeneration of BH4 from BH2, are impaired in senescence, further amplifying NO deficiency in a feed-forward loop. DHFR, a DNA-synthesizing enzyme, is attenuated in senescent cells49. Likewise, deficiency of MTHFR, an enzyme critical for the conversion of homocysteine to methionine, can lead to eNOS uncoupling in a BH4-dependent manner50. Interestingly, it has been reported that inhibition of s-adenosylhomocysteine hydrolase leads to senescence in HUVECs51. As evidenced by the role of the components of folate metabolism in endothelial senescence, it is possible that folate metabolism, which is altered in cancer and targeted for therapy, may also be critically involved in endothelial senescence. Further studies are needed to examine the key factors of folate metabolism, such as methylenetetrahydrofolate dehydrogenase, to elucidate the underlying mechanisms, which remain largely unknown.
Fig. 3. Overview of eNOS uncoupling in endothelial senescence.
eNOS uncoupling occurs due to a reduction in the eNOS cofactors arginine and BH4 and is exacerbated by a decrease in the levels of DHFR and MTHFR, which are enzymes that regenerate BH4 by reducing BH2. eNOS and NOX uncoupling produces superoxide, which shows a higher affinity for NO than for its alternate scavenger, SOD, and ultimately leads to the production of OONO−, a potent oxidant. In addition to taking up NO for its own formation, OONO- oxidizes BH4 into BH2, reducing the eNOS cofactor. These mechanisms collaboratively decrease the NO availability during endothelial senescence. NADPH reduced nicotinamide adenine dinucleotide phosphate, NOX NADPH oxidase, eNOS endothelial nitric oxide synthase, NO nitric oxide, SOD superoxide dismutase, OONO- peroxynitrite, H2O2 hydrogen peroxide, H2O dihydrogen monoxide, BH4 tetrahydrobiopterin, BH2 78-dihydrobiopterin, MTHFR methylenetetrahydrofolate reductase, DHFR dihydrofolate reductase.
Although much remains to be explored regarding the metabolic shifts in endothelial senescence, these shifts appear to play a crucial role in senescence. It is well-established that senescent cells are metabolically active52,53. However, the main source of energy in senescent ECs is still a matter of debate. Different studies of replicative senescence in HUVECs have reported conflicting results, showing that (1) glycolysis decreased with a decline in the expression of nuclear factor E2-related factor 2 (NRF2)54, a regulator of endothelial proliferation and glycolysis55; (2) the rate of glycolysis and the expression of glycolytic enzymes (hexokinase, LDH, aldolase, GAPDH, and pyruvate kinase) did not change significantly56; and (3) glycolysis was increased with increased expression of lactate dehydrogenase A and decreased expression of pyruvate dehydrogenase kinase 1-457. While the latter two studies both noted an increase in glucose consumption and lactate production, the former study did not see a correlation between these two changes and suggested glutaminolysis is the alternative energy source for senescent ECs56. The same study showed that the inhibition of glutaminase (GLS) induced premature senescence in HUVECs. Supporting this finding, it was demonstrated that inhibition of GLS1 reduced the proliferative capacity and increased ROS in HUVECs58. Studies have shown that senescent cells are associated with lower levels of glucose 6-phosphate dehydrogenase (G6PD), which catalyzes the first step of the pentose phosphate pathway59, and of D-3-phosphoglycerate dehydrogenase (PHGDH), which catalyzes the committed step in de novo serine biosynthesis and regulates the critical steps of one-carbon metabolism60. G6PD and PHGDH play essential roles in redox homeostasis, mediating the production of NADPH, and the downregulation of these enzymes may significantly contribute to increased ROS in endothelial senescence61,62.
Endothelial senescence in vascular diseases
Occupying the inner walls of blood vessels, ECs are subject to constant stresses from hemodynamic forces and various substances carried through the flowing blood63. Studies have shown that ECs positioned near arterial geometries such as bifurcations and curvatures in human tissue samples23 or otherwise exposed to disrupted blood flow64,65 undergo higher cell turnover and replicative senescence. Spikes in the partial oxygen pressure and the concentration of hormones and nutrients, which are notably increased during routine activities that cause abrupt metabolic changes in the body, such as exercising and eating, can lead to senescence. It has been demonstrated that high partial oxygen pressure can induce cellular senescence66–68, while culturing ECs in elevated levels of insulin36, glucose69,70 or uric acid71 showed reduced proliferative potential and accelerated onset of senescence. High levels of glucose or other reducing sugars can introduce advanced glycation end-products (AGEs) into the circulation72, and these AGEs can bind to and stimulate ECs, generating ROS73. Similarly, the levels of advanced oxidation protein products (AOPPs), which can be increased by AGEs, tend to be high in uremic patients and have been shown to induce senescence in HUVECs74. Other substances, such as circulating microparticles (MPs), which are shed from the membrane following a variety of stimuli and are circulated through the bloodstream75, can lead to endothelial dysfunction by mediating NO and ROS production76,77. It has been observed that exposure to MPs from acute coronary syndrome patients induces endothelial senescence through Ang 2, ACE1, and AT-1 of the altered RAS system78.
Endothelial senescence has been observed in multiple vascular organs, including the brain and the kidney, undermining critical functions of these organs and has thus been implicated in the development of CVDs. In the brain, cerebrovascular ECs comprise the blood‒brain barrier (BBB) and the neurovascular unit, and senescent phenotypes have been associated with increased BBB permeability and neurovascular uncoupling79. Proinflammatory SASP factors, such as interleukin 6 (IL-6) and interleukin 1β (IL-1β), are upregulated during aging and cerebrovascular diseases. Moreover, altered expression of proteases and protease regulators, such as matrix metalloproteinases (MMPs), can compromise tight junction integrity in the endothelium, all of which may contribute to a leaky BBB and ultimately to cerebrovascular diseases79. A comparison of kidneys in old and young mice showed that SASP factors contributing to glomerulosclerosis in old mice, such as PAI-1, IL-1β, IL-6, or MMPs80, were upregulated, and PAI-1 expressed by glomerular ECs may mediate crosstalk with podocytes to induce podocyte detachment and apoptosis81. The same study showed that endothelial senescence, as represented by high PAI-1 expression, was a negative prognostic marker for kidney transplantation, as recipients with PAI-1-positive kidneys were more likely to develop severe cases of glomerulosclerosis independent of other known clinical covariates81.
Various key features of endothelial senescence enhance susceptibility to the development of CVDs, where reduced capacity for replication and NO production and increased inflammation lead to pathophysiological consequences that can result in endothelial dysfunction82. Several studies have linked reduced replicative capacity and senescence in endothelial cells to reduced angiogenesis, such as impaired neovascularization, as observed in senescent HUVECs83, telomerase-deficient mice84 and SIRT1-silenced mice85. In turn, reduced angiogenic potential can lead to impaired wound healing and neovascularization, which are crucial to recovery from tissue damage, which may predispose patients to subsequent cardiovascular pathologies86. Proinflammatory cytokines contribute to vascular inflammation, which has been associated with atherogenesis and atherosclerosis9. Remodeling of the extracellular matrix (ECM) was shown to be disrupted in senescent ECs82. ECM degradation by MMPs, which are SASP factors, may result in arterial stiffening, exacerbated by reduced eNOS levels87, and an increase in the risk of hypertension88. Vasodilators and related factors, such as NO89, prostacyclin90, and eNOS91, have been observed to be decreased in senescent ECs. The ensuing endothelial dysfunction due to this reduction in NO bioavailability and loss of vascular tone has been implicated in cardiovascular events92.
Several genetic disorders further demonstrate the interconnectedness between senescence and CVD pathology. Hutchinson–Gilford progeria syndrome (HGPS) is a genetic disorder characterized by the accumulation of progerin, a mutant form of lamin A (LMNA)93,94, primarily in the vasculature, including the endothelium95,96. Similarly, Werner syndrome is caused by a mutation in the Werner syndrome helicase (WRN) gene, a DNA helicase gene that has been reported to play a critical role in endothelial homeostasis97. These diseases manifest as accelerated aging and premature death, often due to cardiovascular complications98, predominantly via atherosclerosis99,100. It has been observed that HGPS-derived ECs exhibited senescence traits101,102 and that the expression of progerin in ECs induced inflammation and senescence103, whereas deletion of LMNA in mice resulted in senescence-associated cardiomyopathy with increased SA-β-gal staining intensity and SASP protein levels104. Notably, deficient levels of G6PD, which has been shown to increase ROS and adhesion molecule levels in HUVECs61, has been identified as a risk factor for CVDs in elderly subjects105,106.
Detection of endothelial senescence
Biomarkers frequently used to detect senescent ECs are features that have been broadly established across different types of cellular senescence. A common method to identify senescent cells is by examining their lysosomal content. SA-β-gal staining represents the activity of the lysosomal enzyme beta-galactosidase, which is detectable at the suboptimal cellular pH of 6.0107. Beta-galactosidase has been detected in CVD tissue samples from aged retinal blood vessels108, atherosclerotic plaques in aorta and coronary arteries9,109, and adipose tissue obtained from obese subjects110. Other biomarkers, such as cyclin-dependent kinase (CDK) inhibitors and telomere length111, have been implicated in the pathogenesis of various CVDs. Indeed, it has been reported that telomere length was negatively correlated with atherosclerotic grade in humans112, p16Ink4A limited cell proliferation, and regeneration in response to pancreatic islet injury in mice113, and p53 was elevated in patients with congestive heart failure114 or hypertrophic cardiomyopathy115. Despite its broad use as a biomarker, increased SA-β-gal activity is not unique to senescent cells; it has been observed in cells induced to quiescence through serum starvation or confluence116. Similarly, while a high level of p16Ink4A is a fairly well-established biomarker that is found consistently across aged human tissues117, it has also been observed in cells with inactivated RB, such as cancer cells118, but absent in cells undergoing certain forms of senescence in vitro119,120. To improve the detection of senescent cells, it is common to assess the levels of multiple markers, such as staining for SA-β-gal and γH2AX, which indicates DNA damage response activation121. Nevertheless, the lack of specificity of endothelial senescence biomarkers raises the question of whether the cells identified to date are truly senescent, and the search for a more precise characterization of senescence that caters to different cellular and physiological contexts remains an ongoing challenge.
Moreover, there are challenges to detecting senescent cells outside of a laboratory setting, which limit the clinical exploration of in vivo senescence in patients. The traditional methods of senescence detection rely on immunohistochemical staining of frozen or fixed tissues, which requires physical collection of tissues ex vivo. The need for in situ methods of measuring senescence has led to the development of liquid biopsy, a noninvasive approach that provides an alternative source of senescence identification. Components studied in liquid biopsy include circulating cells, extracellular vesicles, nucleosomes, and various glycoproteins and antigens122, which can serve as viable biomarkers. Circulating ECs, which include endothelial progenitor cells (EPCs) and blood outgrowth endothelial cells (BOECs), hold much promise as potential markers of endothelial senescence measured through liquid biopsy. It is well-established that EPCs actively contribute to cardiovascular homeostasis123 and are used as tools to study endothelial dysfunction124. There have been attempts to use EPCs for their vascular regenerative ability to treat related diseases, such as coronary artery disease125 and liver cirrhosis126. Accumulating evidence supports the idea that dysfunction and a reduction of EPCs are associated with cardiovascular risk factors such as aging, hypertension127–129, coronary artery disease130, and diabetes128. Senescent EPCs have been observed in preeclampsia131, and senescent circulating BOECs in smokers and chronic obstructive pulmonary disease patients132. Moreover, ECs secrete circulating microparticles, as observed in aging porcine coronary artery ECs78 and senescent mouse aortic ECs133. Known as endothelial microparticles (EMPs), these small extracellular vesicles (approximately 100–1000 nm) possess cellular reprogramming potential134 and procoagulant, proinflammatory tendencies135. Moreover, they play a crucial role in the paracrine induction of senescence and propagation of vascular aging134,136. An increase in EMP levels has been associated with various CVDs, such as stroke137, hypertension138,139, heart failure140, and acute coronary syndrome78, demonstrating their potential as a diagnostic biomarker. Other promising biomarkers to be utilized in liquid biopsy include AOPPs, which have been shown to induce senescence in HUVECs74, and AGEs, known to stimulate ECs and promote diabetes through oxidative stress73. While liquid biopsy can offer valuable information that spans various aspects of circulatory biology that can be observed and quantified across space and time, technical challenges remain in actual implementation of liquid biopsy. The low abundance of target components in the bloodstream, the lack of standardized protocols and platforms for analysis and interpretation, and high costs of setup141,142 present significant barriers to the research and technical development of liquid biopsy. Further research is needed to elucidate how potential markers for liquid biopsy can be used to detect senescence and to search for innovative ways to overcome the limitations.
Therapeutic opportunities for endothelial senescence
Potential clinical implications of senescence have galvanized research into therapeutic approaches targeting senescent cells. Considerable progress has been made in two groups of senotherapeutics, senolytics, and senomorphics, in which a few of the compounds have entered clinical trials. Meanwhile, senescence immunotherapy has attracted interest for its potential in senotherapy and its efficacy in boosting the effectiveness of other treatments. We discuss the recent development and limitations of senotherapeutics and introduce several areas in which potential senotherapeutic targets have been identified.
Senolytics eliminate senescent cells by inducing apoptosis by targeting pathways such as BCL-2 family members, p53 and p38 MAPK143 (Table 1). Dasatinib is a tyrosine kinase inhibitor (TKI) that acts on a number of tyrosine kinases, including the Bcr-Abl and Src kinases. It is known for its initial efficacy in treating chronic myelogenous leukemia144,145 and has been repurposed as a senolytic146. It has been demonstrated that quercetin, a Bcl-xL-inhibiting flavonoid, and dasatinib, individually or in combination, are effective at reducing the number of senescent HUVECs146,147 and improving health and cardiac function in vivo148–151 and in idiopathic pulmonary fibrosis patients, where the treatment has led to enhanced physical functions and modest changes in SASP levels152. These promising results have led to a series of clinical trials focused on CVDs, including coronary artery diseases and idiopathic pulmonary fibrosis (Table 1). Fisetin has been reported to selectively induce apoptosis in HUVECs153,154, and three Bcl-xL inhibitors, A1331852, A1155463, and navitoclax, have been shown to induce apoptosis selectively in senescent HUVECs153,154 and in mice, with health improvements155–158. Recently, senolytics inhibiting glutaminolysis159, arginine metabolism160, and angiopoietin-like 2 (ANGPTL2)161, a SASP factor, have shown some potential. Nevertheless, much more evidence is needed to advocate for the clinical usage of senolytics. In 2018, the National Institute on Aging’s Interventions Testing Program162, considered the gold standard for testing longevity drugs, ran a series of studies on fisetin with genetically heterogeneous mice—as opposed to inbred mice commonly used in research—in three independent laboratories. The results showed that fisetin provided no benefits in extending lifespan or clearing senescent cells163. Similarly, despite numerous studies illustrating the in vitro and in vivo efficacy of quercetin, a study showed that quercetin induced cell death in both senescent and nonsenescent primary human coronary artery ECs, showing little specificity for senescence164.
Table 1.
Summary of senotherapeutics and their in vitro and in vivo evidence in endothelial cells or cardiovascular diseases.
| Target mechanism | In vitro anti-senescence evidence | In vivo anti-senescence evidence | Clinical trial stage | Trial | |
|---|---|---|---|---|---|
| Senolytics | |||||
| Quercetin | Inhibits PI3K/AKT, BCL-2 and Serpine activity | Induced apoptosis in IR-induced senescent HUVECs146 | Reversed bleomycin-induced pulmonary fibrosis and reduced SASP marker levels in aged mice149 and Wistar rats150 | Phase 2 in patients with Alzheimer’s disease | NCT04063124 |
| NCT04685590 | |||||
| Phase 2 in patients with coronary artery disease | NCT04907253 | ||||
| Dasatinib | Inhibits tyrosine kinase activity | Induced apoptosis in IR-induced senescent human preadipocytes, and was much less effective on senescent HUVECs146 | Exerted antidiabetic effects on older patients with type 2 diabetes mellitus in a retrospective cohort study151 | Phase 2 with healthy participants | NCT04313634 |
| Quercetin + Dasatinib | Induced apoptosis in IR-induced senescent HUVECs and preadipocytes153 |
1. Improved cardiac function and carotid vascular reactivity in old mice146 2. Reduced hepatic steatosis in old mice148 |
Phase 2 with patients with chronic kidney disease | NCT02848131 | |
| Phase 1 with patients with idiopathic pulmonary fibrosis | NCT02874989 | ||||
| Fisetin | Inhibits BCL-2 and PI3K/AKT | Selectively induced apoptosis in IR-induced senescent HUVEC153 | Reduced senescence marker levels in multiple tissues in progeroid and old mice and restored tissue homeostasis, reduced age-related pathology and extended the lifespan of wild-type mice155 | Phase 2 with patients with knee osteoarthritis | NCT04210986 |
| NCT04815902 | |||||
| Phase 2 with patients with frailty | NCT03675724 | ||||
| NCT03430037 | |||||
| NCT04733534 | |||||
| A1331852, A1155463 | Inhibits BCL-XL | A1331852 and AB1155463 selectively induced apoptosis in IR-induced senescent HUVECs and IMR90 cells153 | Cleared senescent cholangiocytes and reduced SASP factor levels and attenuated liver fibrosis in mice156 | ||
| ABT263 (Navitoclax; UBX0101) | Inhibits BCL-2, BCL-XL and BCL-W | Induced apoptosis in IR-induced senescent HUVEC and IMR90c cells154 |
1.Cleared senescent cells in irradiated mice and normally aged mice157 2. Cleared senescent cells and reduced atherogenesis in murine model of atherosclerosis158 |
||
| SSK1 (gemcitabine with an acetyl group and β-gal-responsive moiety) | Activates the p38 MAPK pathway | Selectively induced apoptosis in HUVECs143 | Eliminated SA-β-gal-positive cells and senescence markers, attenuated lung fibrosis and reduced SASP factors in aged mice143 | ||
| BPTES | Inhibits glutaminase 1 |
Reduced the expression of p16Ink4A, KGA, and IL-6 mRNA and the KGA protein and ameliorated symptoms of diabetes, arteriosclerosis, and NASH in mice159 |
|||
| ABH | Inhibits arginase 1 | Reduced the number of senescent cells in bovine retinal endothelial cells160 | Reduced senescence-associated diabetes-induced alterations (p16Ink4A, p21WAF1, p53, and IGFBP3) in diabetic mice160 | ||
| Senomorphics | |||||
| Rapamycin | Inhibits mTOR | Inhibited SASP acquisition in senescent pulmonary vascular endothelial cells from patients with chronic obstructive pulmonary disease177 |
1. Reduced cardiac inflammation and improved cardiovascular function in mice178 2. Reversed age-related vascular dysfunction and improved arterial function in 30-month-old mice179 |
||
| Metformin | Inhibits IKK/NF-ƙB, activates AMPK | Reduced hyperglycemia-induced senescence and apoptosis in mouse microvascular endothelial cells172 | Restored endothelial function and reduced inflammation in diabetic rats176 | Phase 3 with people at high risk of developing diabetes | NCT00004992 |
| NCT00038727 | |||||
| Resveratrol | Inhibits NF-ƙB (an IĸB-kinase inhibitor), activates AMPK and SIRT1 |
1. Delayed the onset of senescence in human endothelial progenitor cells by increasing telomerase activity170 2. Reduced oxidative reaction and inhibited senescence in endothelial progenitor cells171 |
1. Exerted cardioprotective effects on old senescence-accelerated mice174 2. Reduced aorta media thickness inflammation, fibrosis and oxidative stress and inhibited arterial aging in mice175 |
||
| Ruxolitinib | Inhibits JAK1/2 | Suppressed SASP acquisition in IR-induced senescent human preadipocytes and HUVECs and decreased inflammation in senescent cells173 | Decreased SASP factor levels and enhanced physical activity in aged mice173 | ||
| FGF21 | Activates SIRT1 | Delayed replicative senescence and attenuated senescent phenotypes in HUVECs35 |
1. Protected against immunosenescence in mice180 2. 2. Extended lifespan in transgenic mice with high levels of FGF21181 |
||
IR irradiation, HUVEC human umbilical vein endothelial cell, SASP senescence-associated secretory phenotype, SIRT1 sirtuin-1, AMPK AMP-activated protein kinase, NASH nonalcoholic steatohepatitis.
Moreover, generations of cancer therapeutics have shown that even drugs once believed to be the most innovative, such as TKIs165, are inevitably associated with the risk of acquired resistance to therapy, potentially leading to a more intractable population of surviving tumor cells. Certainly, there is a need for consistent results from independent laboratories as well as longitudinal follow-up studies to observe the long-term effects of these drugs. The use of senolytics can result in far-reaching consequences on the body, as they may not be necessarily cell- or tissue-specific. Targets of senolytics include pathways that are involved in various biological processes in addition to senescence, and inhibition of one pathway may lead to the activation of another, as pathways are not entirely independent of one another and engage in crosstalk. Neglecting the interconnectedness of pathways may create the risk of unexpected adverse events.
Senomorphics are administered with the intention of attenuating the effects of detrimental senescent functions such as SASP, rather than removing senescent cells altogether. Senomorphics target certain components of the SASP factors or pathways that lead to the production of SASP factors, through NF-κB, JAK, SIRT1, and mTOR166. Ruxolitinib is a JAK1/2 inhibitor approved by the FDA to treat myelofibrosis167, and metformin and resveratrol have been shown to attenuate acquisition of the senescent phenotype via NF-ƙB inhibition168,169. The three aforementioned senomorphics showed in vitro and in vivo efficacy in ECs170–172 and aged mice173–176. Rapamycin, which inhibits mTOR, has demonstrated inhibitory effects on SASP acquisition and inflammation in senescent pulmonary vascular ECs177 as well as in mice, which showed cardiac improvement178,179. Furthermore, FGF21, a SIRT1 activator, reduced senescent phenotypes in HUVECs35, protected the immune system against senescence180, and extended the lifespan181 of mice. A caveat with senomorphics is the need for regular administration, as they do not eliminate the source of SASP, highlighting the need to investigate the long-term effects of senomorphics through longitudinal studies, as with senolytics. It is worth noting that while the overall benefits of senomorphics remain to be proven, studies have shown that regular consumption of certain senomorphics, such as metformin and D-glucosamine, has extended the lifespan of participants166.
Senescence immunotherapy mediates senescent clearance by taking advantage of the immune system. While this area of therapeutics needs further research and validation in endothelial senescence, it has been shown in liver ECs that SASP-induced endothelial phenotype plays a critical role in recruiting immunocytes in an endothelium-dependent manner182 and that the immune surveillance of senescent cells is mediated through two distinct EC pathways that depend on the NF-κB component RELA and inducible T-cell costimulator ligand (ICOSLG)183, suggesting possible targets for immunotherapy. Senescence immunotherapy may be an effective complement to existing drugs and treatments. For example, it can be utilized through antibodies targeting senescence-specific antigens to elicit immune responses against senescent cells and thus enhance the antitumor effects of cytotoxic drugs. A recent study reported on D9-HMSN@RSV, which consists of an antibody to CD9, a cell surface protein reported being upregulated in endothelial senescence and atherosclerosis184, conjugated to mesoporous silica nanoparticles (MSNs) to deliver rosuvastatin (RSV), an HMG-CoA reductase inhibitor185. CD9-HMSN@RSV produced positive results both in vitro and in vivo: it attenuated SASP acquisition, cleared senescent endothelial cells, and inhibited both the buildup of senescent plaque and the progression of atherosclerosis in mice. Another example of effective cotreatment involves the use of chimeric antigen receptor (CAR) T cells, where CAR T cells made to recognize urokinase-type plasminogen activator receptor (uPAR)—a cell surface protein widely upregulated in senescent cells—improved outcomes in lung adenocarcinoma and liver fibrosis in mice186. As certain SASP factors can facilitate immune responses, senescence-inducing therapy, followed by senescence immunotherapy, may be viable treatment for various refractory diseases by creating a more immunologically responsive environment. In an experiment with mouse models of pancreatic ductal adenocarcinoma, it was shown that vascular remodeling and endothelial activation mediated by therapy-induced senescence led to the accumulation of T cells in immunologically unresponsive tumors, potentiating PD-1 inhibitor immunotherapy187. Combined, these studies suggest that along with further research into endothelium-specific immunological features, the advent of senescence immunotherapy may be potentially applicable to endothelial senescence and CVDs.
Future perspectives and conclusions
Certain compounds with a hormetic antiaging effect offer new possibilities for senotherapy. Gilbert syndrome (GS) is a genetic disorder characterized by mild hyperbilirubinemia caused by changes in UGT1A1, which encodes the enzyme required for the breakdown of bilirubin. Individuals with GS experience a lower incidence of CVDs, including coronary artery diseases and stroke188,189. While serum bilirubin has been reported to gradually and naturally increase with age190 and can be cytotoxic at high levels191, a moderately elevated level of bilirubin can be effectively antioxidant and anti-inflammatory, leading to improved endothelial function192. Interestingly, knockdown of biliverdin reductase A (BLVRA), an enzyme that converts biliverdin to bilirubin, induced senescence, whereas its overexpression restored certain nonsenescent features in senescent cells193. Although the narrow therapeutic window necessitates further research into its clinical viability, bilirubin is a promising candidate for endothelial senescence therapeutics.
Another promising venue worthy of further research lies in targeting nucleocytoplasmic trafficking (NCT). A study of postmitotic cells in rats revealed the presence of extremely long-lived proteins associated with nuclear pore complex (NPC) over the observational period194, a striking contrast to the majority of proteins, which are usually turned over in a matter of days195. Along with nuclear transport receptors (NTRs) and Ran, NPC comprises the NCT, a specialized machinery involved in the transport of macromolecules, such as signaling proteins and messenger ribonucleoproteins (mRNPs)196. It has been reported that a set of genes associated with NCT—as well as genes associated with the machinery responsible for transport of mRNA, the transcription-export (TREX) complex—is downregulated in various types of senescence, including endothelial senescence196,197. A recent study showed that senescent lens epithelial cells showed concomitant downregulation of a group of NCT genes and failure of the nuclear translocation of BLVRA and NRF2198, suggesting a possible role for NCT in the impaired delivery of these two proteins. A recent study into the disruption of the NCT machinery in fibroblasts revealed a phenotype resembling replicative senescence, indicating that several potential senescence drivers in the NCT may be leveraged for senescence therapy199. Further studies are needed to understand the mechanisms and validate the utility of NCT and TREX factors.
Still, whether or not the elimination of senescent cells is indeed beneficial is yet to be determined. In a study of stress-induced senescence in pulmonary microvascular ECs in mice, senescent cells were removed via the senolytic agent ABT263, leading to a reversal in blood vessel remodeling and pulmonary arterial hypertension12. However, a study of p16Ink4A–Cre knockin mice presented contrasting results. The genetic elimination of cells, mainly liver vascular ECs, with high levels of p16Ink4A with an ablator line (Rosa26-DTA) caused blood tissue barrier disruption, liver fibrosis, and health deterioration in some mice, as the removed senescent cells were not replaced200. Additionally, a study on the effect of navitoclax in atherosclerosis presented contradictory results in which the number of senescent cells and the degree of atherogenesis were reduced in culture but the senescence marker levels were not reduced in vivo201. Moreover, many of the studies of EC senescence tend to be based on HUVECs, at least utilized in the preliminary study. Although a cost-effective and accessible option, HUVECs are collected from the immune-privileged environment of fetal tissues202 and therefore harbor several fundamental differences compared to adult endothelial cells203. These differences present the possibility that results from HUVECs may not necessarily be accurate representations of adult vascular pathophysiology203 and suggest that we may need to find a cell line that better represents in vivo conditions.
Much remains to be explored and further validated in endothelial senescence research. The conflicting results from senescence studies highlight the need to refine the biomarkers to each pathophysiological context, as no single marker can be used to identify senescent cells conclusively. Most likely, collecting a set of markers—those that are universal and those that are more specific—and adjusting them to fulfill different purposes will be necessary. For example, encapsulation of navitoclax, a senolytic associated with thrombocytopenia204, with beads coated with galactooligosaccharides, which respond to β-galactosidase activity, has led to the clearance of senescent cells and showed low toxicity205. This emphasizes the importance of the detection of senescent cells and the delivery of senotherapeutics. Senotherapeutics targeting senescent ECs not only target age-associated deterioration but also lead to improvements in cardiovascular function, which are areas of great interest in view of the burgeoning aging population worldwide. In summary, further exploration into the molecular mechanisms and precise characterization of endothelial senescence and how these mechanisms can be manipulated for therapeutics could have a meaningful impact on developing treatments for vascular diseases.
Acknowledgements
This paper was supported by Konkuk University in 2022.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kinlay S, Ganz P. Role of endothelial dysfunction in coronary artery disease and implications for therapy. Am. J. Cardiol. 1997;80:11I–16I. doi: 10.1016/s0002-9149(97)00793-5. [DOI] [PubMed] [Google Scholar]
- 2.Panza JA, Quyyumi AA, Brush JE, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N. Engl. J. Med. 1990;323:22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 3.Donato AJ, Machin DR, Lesniewski LA. Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ. Res. 2018;123:825–848. doi: 10.1161/CIRCRESAHA.118.312563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cosentino F, et al. Endothelial dysfunction and stroke. J. Cardiovasc. Pharmacol. 2001;38:S75–S78. doi: 10.1097/00005344-200111002-00018. [DOI] [PubMed] [Google Scholar]
- 5.Finger CE, Moreno-Gonzalez I, Gutierrez A, Moruno-Manchon JF, McCullough LD. Age-related immune alterations and cerebrovascular inflammation. Mol. Psychiatry. 2022;27:803–818. doi: 10.1038/s41380-021-01361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shang, X. et al. Associations of ophthalmic and systemic conditions with incident dementia in the UK Biobank. Br. J. Ophthalmol. 10.1136/bjophthalmol-2021-319508 (2021). [DOI] [PubMed]
- 7.Javaheri S, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J. Am. Coll. Cardiol. 2017;69:841–858. doi: 10.1016/j.jacc.2016.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu C-M, Zheng L, Wang Q, Hu Y-W. The emerging role of cell senescence in atherosclerosis. Clin. Chem. Lab. Med. 2020;59:27–38. doi: 10.1515/cclm-2020-0601. [DOI] [PubMed] [Google Scholar]
- 9.Minamino T, et al. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002;105:1541–1544. doi: 10.1161/01.cir.0000013836.85741.17. [DOI] [PubMed] [Google Scholar]
- 10.Jia L, et al. Haplodeficiency of ataxia telangiectasia mutated accelerates heart failure after myocardial infarction. J. Am. Heart Assoc. 2017;6:e006349. doi: 10.1161/JAHA.117.006349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Feen DE, Berger RMF, Bartelds B. Converging paths of pulmonary arterial hypertension and cellular senescence. Am. J. Respir. Cell Mol. Biol. 2019;61:11–20. doi: 10.1165/rcmb.2018-0329TR. [DOI] [PubMed] [Google Scholar]
- 12.van der Feen DE, et al. Cellular senescence impairs the reversibility of pulmonary arterial hypertension. Sci. Transl. Med. 2020;12:eaaw4974. doi: 10.1126/scitranslmed.aaw4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugimoto K, et al. Senescence marker protein 30 deficiency exacerbates pulmonary hypertension in hypoxia-exposed mice. Int. Heart J. 2019;60:1430–1434. doi: 10.1536/ihj.19-190. [DOI] [PubMed] [Google Scholar]
- 14.Jia G, et al. Vascular stiffness in insulin resistance and obesity. Front. Physiol. 2015;6:231. doi: 10.3389/fphys.2015.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmer AK, et al. Cellular senescence in type 2 diabetes: a therapeutic opportunity. Diabetes. 2015;64:2289–2298. doi: 10.2337/db14-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shakeri H, Lemmens K, Gevaert AB, De Meyer GRY, Segers VFM. Cellular senescence links aging and diabetes in cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 2018;315:H448–H462. doi: 10.1152/ajpheart.00287.2018. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell GF, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang M, Wang J, Zhang C, Pintus G. Editorial: arterial aging and age-associated arterial diseases. Front. Genet. 2018;9:444. doi: 10.3389/fgene.2018.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrini MG, Gonzalez-Cadavid NF, Rajfer J. Aging related erectile dysfunction-potential mechanism to halt or delay its onset. Transl. Androl. Urol. 2017;6:20–27. doi: 10.21037/tau.2016.11.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuttolomondo A, et al. Arterial stiffness, endothelial and cognitive function in subjects with type 2 diabetes in accordance with absence or presence of diabetic foot syndrome. Cardiovasc. Diabetol. 2017;16:2. doi: 10.1186/s12933-016-0483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sierra F, Kohanski R. Geroscience and the trans-NIH Geroscience Interest Group, GSIG. Geroscience. 2017;39:1–5. doi: 10.1007/s11357-016-9954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spyridopoulos I, Isner JM, Losordo DW. Oncogenic ras induces premature senescence in endothelial cells: role of p21(Cip1/Waf1) Basic Res. Cardiol. 2002;97:117–124. doi: 10.1007/s003950200001. [DOI] [PubMed] [Google Scholar]
- 23.Chang E, Harley CB. Telomere length and replicative aging in human vascular tissues. Proc. Natl Acad. Sci. USA. 1995;92:11190–11194. doi: 10.1073/pnas.92.24.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhan H, Suzuki T, Aizawa K, Miyagawa K, Nagai R. Ataxia telangiectasia mutated (ATM)-mediated DNA damage response in oxidative stress-induced vascular endothelial cell senescence. J. Biol. Chem. 2010;285:29662–29670. doi: 10.1074/jbc.M110.125138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dai D-F, Rabinovitch PS, Ungvari Z. Mitochondria and cardiovascular aging. Circ. Res. 2012;110:1109–1124. doi: 10.1161/CIRCRESAHA.111.246140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ungvari Z, et al. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H2121–H2128. doi: 10.1152/ajpheart.00012.2008. [DOI] [PubMed] [Google Scholar]
- 27.Kurz DJ, et al. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J. Cell Sci. 2004;117:2417–2426. doi: 10.1242/jcs.01097. [DOI] [PubMed] [Google Scholar]
- 28.Donato AJ, Morgan RG, Walker AE, Lesniewski LA. Cellular and molecular biology of aging endothelial cells. J. Mol. Cell. Cardiol. 2015;89:122–135. doi: 10.1016/j.yjmcc.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faget DV, Ren Q, Stewart SA. Unmasking senescence: context-dependent effects of SASP in cancer. Nat. Rev. Cancer. 2019;19:439–453. doi: 10.1038/s41568-019-0156-2. [DOI] [PubMed] [Google Scholar]
- 30.Ungvari Z, et al. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat. Rev. Cardiol. 2018;15:555–565. doi: 10.1038/s41569-018-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du XL, et al. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J. Clin. Invest. 2001;108:1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia G, Aroor AR, Jia C, Sowers JR. Endothelial cell senescence in aging-related vascular dysfunction. Biochim. Biophys. Acta Mol. Basis Dis. 2019;1865:1802–1809. doi: 10.1016/j.bbadis.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Tian F, et al. κ-opioid receptor stimulation improves endothelial function via Akt-stimulated NO production in hyperlipidemic rats. Sci. Rep. 2016;6:26807. doi: 10.1038/srep26807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ying L, et al. Fibroblast growth factor 21 Ameliorates diabetes-induced endothelial dysfunction in mouse aorta via activation of the CaMKK2/AMPKα signaling pathway. Cell Death Dis. 2019;10:665. doi: 10.1038/s41419-019-1893-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan J, et al. Fibroblast growth factor 21 delayed endothelial replicative senescence and protected cells from H2O2-induced premature senescence through SIRT1. Am. J. Transl. Res. 2017;9:4492–4501. [PMC free article] [PubMed] [Google Scholar]
- 36.Miyauchi H, et al. Akt negatively regulates the in vitro lifespan of human endothelial cells via a p53/p21-dependent pathway. EMBO J. 2004;23:212–220. doi: 10.1038/sj.emboj.7600045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Astle MV, et al. AKT induces senescence in human cells via mTORC1 and p53 in the absence of DNA damage: implications for targeting mTOR during malignancy. Oncogene. 2012;31:1949–1962. doi: 10.1038/onc.2011.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khor E-S, Wong P-F. The roles of MTOR and miRNAs in endothelial cell senescence. Biogerontology. 2020;21:517–530. doi: 10.1007/s10522-020-09876-w. [DOI] [PubMed] [Google Scholar]
- 39.Mattagajasingh I, et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl Acad. Sci. USA. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donato AJ, et al. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J. Physiol. 2011;589:4545–4554. doi: 10.1113/jphysiol.2011.211219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ota H, et al. Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J. Mol. Cell. Cardiol. 2007;43:571–579. doi: 10.1016/j.yjmcc.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Yoon HE, et al. Age-associated changes in the vascular renin-angiotensin system in mice. Oxid. Med. Cell. Longev. 2016;2016:6731093. doi: 10.1155/2016/6731093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uryga AK, Bennett MR. Ageing induced vascular smooth muscle cell senescence in atherosclerosis. J. Physiol. 2016;594:2115–2124. doi: 10.1113/JP270923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freeman BD, Machado FS, Tanowitz HB, Desruisseaux MS. Endothelin-1 and its role in the pathogenesis of infectious diseases. Life Sci. 2014;118:110–119. doi: 10.1016/j.lfs.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeiher AM, Ihling C, Pistorius K, Schächinger V, Schaefer HE. Increased tissue endothelin immunoreactivity in atherosclerotic lesions associated with acute coronary syndromes. Lancet. 1994;344:1405–1406. doi: 10.1016/s0140-6736(94)90571-1. [DOI] [PubMed] [Google Scholar]
- 46.Đambić, V., Pojatić, Đ., Stažić, A. & Kibel, A. Significance of the renin-angiotensin system in clinical conditions. in Selected Chapters from the Renin-Angiotensin System (ed. Kibel, A.) (IntechOpen, 2020).
- 47.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am. J. Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 48.Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 49.Kietadisorn R, Juni RP, Moens AL. Tackling endothelial dysfunction by modulating NOS uncoupling: new insights into its pathogenesis and therapeutic possibilities. Am. J. Physiol. Endocrinol. Metab. 2012;302:E481–E495. doi: 10.1152/ajpendo.00540.2011. [DOI] [PubMed] [Google Scholar]
- 50.Lemarié CA, et al. Mthfr deficiency induces endothelial progenitor cell senescence via uncoupling of eNOS and downregulation of SIRT1. Am. J. Physiol. Heart Circ. Physiol. 2011;300:H745–H753. doi: 10.1152/ajpheart.00321.2010. [DOI] [PubMed] [Google Scholar]
- 51.You Y, et al. Inhibition of S-adenosylhomocysteine hydrolase induces endothelial senescence via hTERT downregulation. Atherosclerosis. 2022;353:1–10. doi: 10.1016/j.atherosclerosis.2022.06.002. [DOI] [PubMed] [Google Scholar]
- 52.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 53.Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nat. Rev. Cancer. 2010;10:51–57. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuosmanen SM, Sihvola V, Kansanen E, Kaikkonen MU, Levonen A-L. MicroRNAs mediate the senescence-associated decline of NRF2 in endothelial cells. Redox Biol. 2018;18:77–83. doi: 10.1016/j.redox.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuosmanen SM, et al. NRF2 regulates endothelial glycolysis and proliferation with miR-93 and mediates the effects of oxidized phospholipids on endothelial activation. Nucleic Acids Res. 2018;46:1124–1138. doi: 10.1093/nar/gkx1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Unterluggauer H, et al. Premature senescence of human endothelial cells induced by inhibition of glutaminase. Biogerontology. 2008;9:247–259. doi: 10.1007/s10522-008-9134-x. [DOI] [PubMed] [Google Scholar]
- 57.Stabenow LK, et al. Oxidative glucose metabolism promotes senescence in vascular endothelial cells. Cells. 2022;11:2213. doi: 10.3390/cells11142213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peyton KJ, et al. Glutaminase-1 stimulates the proliferation, migration, and survival of human endothelial cells. Biochem. Pharmacol. 2018;156:204–214. doi: 10.1016/j.bcp.2018.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leopold JA, Zhang Y-Y, Scribner AW, Stanton RC, Loscalzo J. Glucose-6-phosphate dehydrogenase overexpression decreases endothelial cell oxidant stress and increases bioavailable nitric oxide. Arterioscler. Thromb. Vasc. Biol. 2003;23:411–417. doi: 10.1161/01.ATV.0000056744.26901.BA. [DOI] [PubMed] [Google Scholar]
- 60.Park HS, Kim SY. Endothelial cell senescence: a machine learning-based meta-analysis of transcriptomic studies. Ageing Res. Rev. 2021;65:101213. doi: 10.1016/j.arr.2020.101213. [DOI] [PubMed] [Google Scholar]
- 61.Parsanathan R, Jain SK. Glucose-6-phosphate dehydrogenase deficiency increases cell adhesion molecules and activates human monocyte-endothelial cell adhesion: protective role of l-cysteine. Arch. Biochem. Biophys. 2019;663:11–21. doi: 10.1016/j.abb.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 62.Vandekeere S, et al. Serine synthesis via PHGDH is essential for heme production in endothelial cells. Cell Metab. 2018;28:573–587.e13. doi: 10.1016/j.cmet.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 63.Bloom, S. I., Islam, M. T., Lesniewski, L. A. & Donato, A. J. Mechanisms and consequences of endothelial cell senescence. Nat. Rev. Cardiol. 10.1038/s41569-022-00739-0 (2022). [DOI] [PMC free article] [PubMed]
- 64.Kotla S, et al. Endothelial senescence is induced by phosphorylation and nuclear export of telomeric repeat binding factor 2-interacting protein. JCI Insight. 2019;4:e124867. doi: 10.1172/jci.insight.124867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Warboys CM, et al. Disturbed flow promotes endothelial senescence via a p53-dependent pathway. Arterioscler. Thromb. Vasc. Biol. 2014;34:985–995. doi: 10.1161/ATVBAHA.114.303415. [DOI] [PubMed] [Google Scholar]
- 66.Parrinello S, et al. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat. Cell Biol. 2003;5:741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen Q, Fischer A, Reagan JD, Yan LJ, Ames BN. Oxidative DNA damage and senescence of human diploid fibroblast cells. Proc. Natl Acad. Sci. USA. 1995;92:4337–4341. doi: 10.1073/pnas.92.10.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.von Zglinicki T, Saretzki G, Döcke W, Lotze C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp. Cell Res. 1995;220:186–193. doi: 10.1006/excr.1995.1305. [DOI] [PubMed] [Google Scholar]
- 69.Rogers SC, Zhang X, Azhar G, Luo S, Wei JY. Exposure to high or low glucose levels accelerates the appearance of markers of endothelial cell senescence and induces dysregulation of nitric oxide synthase. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68:1469–1481. doi: 10.1093/gerona/glt033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maeda M, Hayashi T, Mizuno N, Hattori Y, Kuzuya M. Intermittent high glucose implements stress-induced senescence in human vascular endothelial cells: role of superoxide production by NADPH oxidase. PLoS ONE. 2015;10:e0123169. doi: 10.1371/journal.pone.0123169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu M-A, Sánchez-Lozada LG, Johnson RJ, Kang D-H. Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J. Hypertens. 2010;28:1234–1242. [PubMed] [Google Scholar]
- 72.Vlassara H, Uribarri J. Advanced glycation end products (AGE) and diabetes: cause, effect, or both? Curr. Diab. Rep. 2014;14:453. doi: 10.1007/s11892-013-0453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Senatus LM, Schmidt AM. The AGE-RAGE axis: implications for age-associated arterial diseases. Front. Genet. 2017;8:187. doi: 10.3389/fgene.2017.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen Y, et al. p53 SUMOylation mediates AOPP-induced endothelial senescence and apoptosis evasion. Front. Cardiovasc. Med. 2021;8:795747. doi: 10.3389/fcvm.2021.795747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morel O, Jesel L, Freyssinet J-M, Toti F. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler. Thromb. Vasc. Biol. 2011;31:15–26. doi: 10.1161/ATVBAHA.109.200956. [DOI] [PubMed] [Google Scholar]
- 76.Brodsky SV, Zhang F, Nasjletti A, Goligorsky MS. Endothelium-derived microparticles impair endothelial function in vitro. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H1910–H1915. doi: 10.1152/ajpheart.01172.2003. [DOI] [PubMed] [Google Scholar]
- 77.Mostefai HA, et al. Phosphatidylinositol 3-kinase and xanthine oxidase regulate nitric oxide and reactive oxygen species productions by apoptotic lymphocyte microparticles in endothelial cells. J. Immunol. 2008;180:5028–5035. doi: 10.4049/jimmunol.180.7.5028. [DOI] [PubMed] [Google Scholar]
- 78.Abbas M, et al. Endothelial microparticles from acute coronary syndrome patients induce premature coronary artery endothelial cell aging and thrombogenicity: role of the Ang II/AT1 receptor/NADPH oxidase-mediated activation of MAPKs and PI3-kinase pathways. Circulation. 2017;135:280–296. doi: 10.1161/CIRCULATIONAHA.116.017513. [DOI] [PubMed] [Google Scholar]
- 79.Graves SI, Baker DJ. Implicating endothelial cell senescence to dysfunction in the ageing and diseased brain. Basic Clin. Pharmacol. Toxicol. 2020;127:102–110. doi: 10.1111/bcpt.13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang W-J, Cai G-Y, Chen X-M. Cellular senescence, senescence-associated secretory phenotype, and chronic kidney disease. Oncotarget. 2017;5:64520–64533. doi: 10.18632/oncotarget.17327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cohen C, et al. Glomerular endothelial cell senescence drives age-related kidney disease through PAI-1. EMBO Mol. Med. 2021;13:e14146. doi: 10.15252/emmm.202114146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Erusalimsky JD. Vascular endothelial senescence: from mechanisms to pathophysiology. J. Appl. Physiol. 2009;106:326–332. doi: 10.1152/japplphysiol.91353.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang J, et al. Human endothelial cell life extension by telomerase expression. J. Biol. Chem. 1999;274:26141–26148. doi: 10.1074/jbc.274.37.26141. [DOI] [PubMed] [Google Scholar]
- 84.Franco S, Segura I, Riese HH, Blasco MA. Decreased B16F10 melanoma growth and impaired vascularization in telomerase-deficient mice with critically short telomeres. Cancer Res. 2002;62:552–559. [PubMed] [Google Scholar]
- 85.Potente M, et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007;21:2644–2658. doi: 10.1101/gad.435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lähteenvuo J, Rosenzweig A. Effects of aging on angiogenesis. Circ. Res. 2012;110:1252–1264. doi: 10.1161/CIRCRESAHA.111.246116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler. Thromb. Vasc. Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 88.Kaess BM, et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hoffmann J, et al. Aging enhances the sensitivity of endothelial cells toward apoptotic stimuli: important role of nitric oxide. Circ. Res. 2001;89:709–715. doi: 10.1161/hh2001.097796. [DOI] [PubMed] [Google Scholar]
- 90.Nakajima M, et al. Aging decreases the production of PGI2 in rat aortic endothelial cells. Exp. Gerontol. 1997;32:685–693. doi: 10.1016/s0531-5565(97)00089-2. [DOI] [PubMed] [Google Scholar]
- 91.Matsushita H, et al. eNOS activity is reduced in senescent human endothelial cells: Preservation by hTERT immortalization. Circ. Res. 2001;89:793–798. doi: 10.1161/hh2101.098443. [DOI] [PubMed] [Google Scholar]
- 92.Taddei S, Virdis A, Ghiadoni L, Versari D, Salvetti A. Endothelium, aging, and hypertension. Curr. Hypertens. Rep. 2006;8:84–89. doi: 10.1007/s11906-006-0045-4. [DOI] [PubMed] [Google Scholar]
- 93.Eriksson M, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Goldman RD, et al. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc. Natl Acad. Sci. USA. 2004;101:8963–8968. doi: 10.1073/pnas.0402943101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McClintock D, Gordon LB, Djabali K. Hutchinson-Gilford progeria mutant lamin A primarily targets human vascular cells as detected by an anti-Lamin A G608G antibody. Proc. Natl Acad. Sci. USA. 2006;103:2154–2159. doi: 10.1073/pnas.0511133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Olive M, et al. Cardiovascular pathology in Hutchinson-Gilford progeria: correlation with the vascular pathology of aging. Arterioscler. Thromb. Vasc. Biol. 2010;30:2301–2309. doi: 10.1161/ATVBAHA.110.209460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Laarmann K, Kress JM, Kaina B, Fritz G. Werner syndrome (WRN) DNA helicase and base excision repair (BER) factors maintain endothelial homeostasis. DNA Repair. 2019;73:17–27. doi: 10.1016/j.dnarep.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 98.Prakash A, et al. Cardiac abnormalities in patients with Hutchinson-Gilford progeria syndrome. JAMA Cardiol. 2018;3:326–334. doi: 10.1001/jamacardio.2017.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu S, Jin Z-G. Hutchinson-Gilford progeria syndrome: cardiovascular pathologies and potential therapies. Trends Biochem. Sci. 2019;44:561–564. doi: 10.1016/j.tibs.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 100.Kato H, Maezawa Y. Atherosclerosis and cardiovascular diseases in progeroid syndromes. J. Atheroscler. Thromb. 2022;29:439–447. doi: 10.5551/jat.RV17061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Unable to find information for 9014341.
- 102.Mojiri A, et al. Telomerase therapy reverses vascular senescence and extends lifespan in progeria mice. Eur. Heart J. 2021;42:4352–4369. doi: 10.1093/eurheartj/ehab547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bidault G, et al. Progerin expression induces inflammation, oxidative stress and senescence in human coronary endothelial cells. Cells. 2020;9:1201. doi: 10.3390/cells9051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rouhi L, et al. Deletion of the Lmna gene in fibroblasts causes senescence-associated dilated cardiomyopathy by activating the double-stranded DNA damage response and induction of senescence-associated secretory phenotype. J. Cardiovasc. Aging. 2022;2:30. doi: 10.20517/jca.2022.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dore MP, Portoghese M, Pes GM. The elderly with glucose-6-phosphate dehydrogenase deficiency are more susceptible to cardiovascular disease. J. Atheroscler. Thromb. 2021;28:604–610. doi: 10.5551/jat.56531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thomas JE, et al. Glucose-6-phosphate dehydrogenase deficiency is associated with cardiovascular disease in U.S. military centers. Tex. Heart Inst. J. 2018;45:144–150. doi: 10.14503/THIJ-16-6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee BY, et al. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell. 2006;5:187–195. doi: 10.1111/j.1474-9726.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- 108.López-Luppo M, et al. Cellular senescence is associated with human retinal microaneurysm formation during aging. Invest. Ophthalmol. Vis. Sci. 2017;58:2832–2842. doi: 10.1167/iovs.16-20312. [DOI] [PubMed] [Google Scholar]
- 109.Vasile E, Tomita Y, Brown LF, Kocher O, Dvorak HF. Differential expression of thymosin beta-10 by early passage and senescent vascular endothelium is modulated by VPF/VEGF: evidence for senescent endothelial cells in vivo at sites of atherosclerosis. FASEB J. 2001;15:458–466. doi: 10.1096/fj.00-0051com. [DOI] [PubMed] [Google Scholar]
- 110.Villaret A, et al. Adipose tissue endothelial cells from obese human subjects: differences among depots in angiogenic, metabolic, and inflammatory gene expression and cellular senescence. Diabetes. 2010;59:2755–2763. doi: 10.2337/db10-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ogami M, et al. Telomere shortening in human coronary artery diseases. Arterioscler. Thromb. Vasc. Biol. 2004;24:546–550. doi: 10.1161/01.ATV.0000117200.46938.e7. [DOI] [PubMed] [Google Scholar]
- 112.Okuda K, et al. Telomere attrition of the human abdominal aorta: relationships with age and atherosclerosis. Atherosclerosis. 2000;152:391–398. doi: 10.1016/s0021-9150(99)00482-7. [DOI] [PubMed] [Google Scholar]
- 113.Krishnamurthy J, et al. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature. 2006;443:453–457. doi: 10.1038/nature05092. [DOI] [PubMed] [Google Scholar]
- 114.Song H, Conte JV, Foster AH, McLaughlin JS, Wei C. Increased p53 protein expression in human failing myocardium. J. Heart Lung Transplant. 1999;18:744–749. doi: 10.1016/s1053-2498(98)00039-4. [DOI] [PubMed] [Google Scholar]
- 115.Predmore JM, et al. Ubiquitin proteasome dysfunction in human hypertrophic and dilated cardiomyopathies. Circulation. 2010;121:997–1004. doi: 10.1161/CIRCULATIONAHA.109.904557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang N-C, Hu M-L. The limitations and validities of senescence associated-beta-galactosidase activity as an aging marker for human foreskin fibroblast Hs68 cells. Exp. Gerontol. 2005;40:813–819. doi: 10.1016/j.exger.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 117.Tuttle CSL, Luesken SWM, Waaijer MEC, Maier AB. Senescence in tissue samples of humans with age-related diseases: a systematic review. Ageing Res. Rev. 2021;68:101334. doi: 10.1016/j.arr.2021.101334. [DOI] [PubMed] [Google Scholar]
- 118.Shapiro GI, et al. Reciprocal Rb inactivation and p16INK4 expression in primary lung cancers and cell lines. Cancer Res. 1995;55:505–509. [PubMed] [Google Scholar]
- 119.Yamakoshi K, et al. Real-time in vivo imaging of p16Ink4a reveals cross talk with p53. J. Cell Biol. 2009;186:393–407. doi: 10.1083/jcb.200904105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Herbig U, Jobling WA, Chen BPC, Chen DJ, Sedivy JM. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a) Mol. Cell. 2004;14:501–513. doi: 10.1016/s1097-2765(04)00256-4. [DOI] [PubMed] [Google Scholar]
- 121.Biran A, et al. Quantitative identification of senescent cells in aging and disease. Aging Cell. 2017;16:661–671. doi: 10.1111/acel.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kustanovich A, Schwartz R, Peretz T, Grinshpun A. Life and death of circulating cell-free DNA. Cancer Biol. Ther. 2019;20:1057–1067. doi: 10.1080/15384047.2019.1598759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pompilio G, et al. Endothelial progenitor cells and cardiovascular homeostasis: clinical implications. Int. J. Cardiol. 2009;131:156–167. doi: 10.1016/j.ijcard.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 124.Medina RJ, O’Neill CL, O’Doherty TM, Wilson SEJ, Stitt AW. Endothelial progenitors as tools to study vascular disease. Stem Cells Int. 2012;2012:346735. doi: 10.1155/2012/346735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhu J, et al. Safety and efficacy of autologous thymosin β4 pre-treated endothelial progenitor cell transplantation in patients with acute ST segment elevation myocardial infarction: a pilot study. Cytotherapy. 2016;18:1037–1042. doi: 10.1016/j.jcyt.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 126.D’Avola D, et al. Phase 1-2 pilot clinical trial in patients with decompensated liver cirrhosis treated with bone marrow-derived endothelial progenitor cells. Transl. Res. 2017;188:80–91.e2. doi: 10.1016/j.trsl.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 127.Jie KE, Goossens MHJ, van Oostrom O, Lilien MR, Verhaar MC. Circulating endothelial progenitor cell levels are higher during childhood than in adult life. Atherosclerosis. 2009;202:345–347. doi: 10.1016/j.atherosclerosis.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 128.Tepper OM, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 129.Umemura T, et al. Aging and hypertension are independent risk factors for reduced number of circulating endothelial progenitor cells. Am. J. Hypertens. 2008;21:1203–1209. doi: 10.1038/ajh.2008.278. [DOI] [PubMed] [Google Scholar]
- 130.Vasa M, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ. Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 131.Wang Y, Lim R, Nie G. Elevated circulating HtrA4 in preeclampsia may alter endothelial expression of senescence genes. Placenta. 2020;90:71–81. doi: 10.1016/j.placenta.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 132.Paschalaki KE, et al. Dysfunction of endothelial progenitor cells from smokers and chronic obstructive pulmonary disease patients due to increased DNA damage and senescence. Stem Cells. 2013;31:2813–2826. doi: 10.1002/stem.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Burger D, et al. Microparticles induce cell cycle arrest through redox-sensitive processes in endothelial cells: implications in vascular senescence. J. Am. Heart Assoc. 2012;1:e001842. doi: 10.1161/JAHA.112.001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mause SF, Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ. Res. 2010;107:1047–1057. doi: 10.1161/CIRCRESAHA.110.226456. [DOI] [PubMed] [Google Scholar]
- 135.VanWijk MJ, VanBavel E, Sturk A, Nieuwland R. Microparticles in cardiovascular diseases. Cardiovasc. Res. 2003;59:277–287. doi: 10.1016/s0008-6363(03)00367-5. [DOI] [PubMed] [Google Scholar]
- 136.Simoncini S, et al. Biogenesis of pro-senescent microparticles by endothelial colony forming cells from premature neonates is driven by SIRT1-dependent epigenetic regulation of MKK6. Sci. Rep. 2017;7:8277. doi: 10.1038/s41598-017-08883-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Simak J, Gelderman MP, Yu H, Wright V, Baird AE. Circulating endothelial microparticles in acute ischemic stroke: a link to severity, lesion volume and outcome. J. Thromb. Haemost. 2006;4:1296–1302. doi: 10.1111/j.1538-7836.2006.01911.x. [DOI] [PubMed] [Google Scholar]
- 138.Wang JM, et al. Elevated circulating endothelial microparticles and brachial-ankle pulse wave velocity in well-controlled hypertensive patients. J. Hum. Hypertens. 2009;23:307–315. doi: 10.1038/jhh.2008.137. [DOI] [PubMed] [Google Scholar]
- 139.Preston RA, et al. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension. 2003;41:211–217. doi: 10.1161/01.hyp.0000049760.15764.2d. [DOI] [PubMed] [Google Scholar]
- 140.Montoro-García S, et al. Small-size microparticles as indicators of acute decompensated state in ischemic heart failure. Rev. Esp. Cardiol. 2015;68:951–958. doi: 10.1016/j.rec.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 141.Palmirotta R, et al. Liquid biopsy of cancer: a multimodal diagnostic tool in clinical oncology. Ther. Adv. Med. Oncol. 2018;10:1758835918794630. doi: 10.1177/1758835918794630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lone SN, et al. Liquid biopsy: a step closer to transform diagnosis, prognosis and future of cancer treatments. Mol. Cancer. 2022;21:79. doi: 10.1186/s12943-022-01543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cai Y, et al. Elimination of senescent cells by β-galactosidase-targeted prodrug attenuates inflammation and restores physical function in aged mice. Cell Res. 2020;30:574–589. doi: 10.1038/s41422-020-0314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Quintas-Cardama A, et al. Dasatinib (BMS-354825) is active in Philadelphia chromosome-positive chronic myelogenous leukemia after imatinib and nilotinib (AMN107) therapy failure. Blood. 2007;109:497–499. doi: 10.1182/blood-2006-07-035493. [DOI] [PubMed] [Google Scholar]
- 145.Talpaz M, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N. Engl. J. Med. 2006;354:2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 146.Zhu Y, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fan T, Du Y, Zhang M, Zhu AR, Zhang J. Senolytics cocktail dasatinib and quercetin alleviate human umbilical vein endothelial cell senescence via the TRAF6-MAPK-NF-κB Axis in a YTHDF2-dependent manner. Gerontology. 2022;68:920–934. doi: 10.1159/000522656. [DOI] [PubMed] [Google Scholar]
- 148.Ogrodnik M, et al. Cellular senescence drives age-dependent hepatic steatosis. Nat. Commun. 2017;8:15691. doi: 10.1038/ncomms15691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hohmann MS, Habiel DM, Coelho AL, Verri WA, Hogaboam CM. Quercetin enhances ligand-induced apoptosis in senescent idiopathic pulmonary fibrosis fibroblasts and reduces lung fibrosis in vivo. Am. J. Respir. Cell Mol. Biol. 2019;60:28–40. doi: 10.1165/rcmb.2017-0289OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Verma R, et al. Evaluating the ameliorative potential of quercetin against the bleomycin-induced pulmonary fibrosis in wistar rats. Pulm. Med. 2013;2013:921724. doi: 10.1155/2013/921724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Salaami O, et al. Antidiabetic effects of the senolytic agent dasatinib. Mayo Clin. Proc. 2021;96:3021–3029. doi: 10.1016/j.mayocp.2021.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Justice JN, et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–563. doi: 10.1016/j.ebiom.2018.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhu Y, et al. New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging (Albany NY) 2017;9:955–963. doi: 10.18632/aging.101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhu Y, et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell. 2016;15:428–435. doi: 10.1111/acel.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yousefzadeh MJ, et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. 2018;36:18–28. doi: 10.1016/j.ebiom.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Moncsek A, et al. Targeting senescent cholangiocytes and activated fibroblasts with B-cell lymphoma-extra large inhibitors ameliorates fibrosis in multidrug resistance 2 gene knockout (Mdr2-/-) mice. Hepatology. 2018;67:247–259. doi: 10.1002/hep.29464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chang J, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 2016;22:78–83. doi: 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Childs BG, et al. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science. 2016;354:472–477. doi: 10.1126/science.aaf6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Johmura Y, et al. Senolysis by glutaminolysis inhibition ameliorates various age-associated disorders. Science. 2021;371:265–270. doi: 10.1126/science.abb5916. [DOI] [PubMed] [Google Scholar]
- 160.Shosha E, et al. Mechanisms of diabetes-induced endothelial cell senescence: role of arginase 1. Int. J. Mol. Sci. 2018;19:1215. doi: 10.3390/ijms19041215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Caland L, et al. Knockdown of angiopoietin-like 2 induces clearance of vascular endothelial senescent cells by apoptosis, promotes endothelial repair and slows atherogenesis in mice. Aging. 2019;11:3832–3850. doi: 10.18632/aging.102020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Miller RA, et al. An Aging Interventions Testing Program: study design and interim report. Aging Cell. 2007;6:565–575. doi: 10.1111/j.1474-9726.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- 163.Supported Interventions | National Institute on Aging. https://www.nia.nih.gov/research/dab/interventions-testing-program-itp/supported-interventions.
- 164.Hwang HV, Tran DT, Rebuffatti MN, Li C-S, Knowlton AA. Investigation of quercetin and hyperoside as senolytics in adult human endothelial cells. PLoS One. 2018;13:e0190374. doi: 10.1371/journal.pone.0190374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rosenzweig SA. Acquired resistance to drugs targeting tyrosine kinases. Adv. Cancer Res. 2018;138:71–98. doi: 10.1016/bs.acr.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Song S, Tchkonia T, Jiang J, Kirkland JL, Sun Y. Targeting senescent cells for a healthier aging: challenges and opportunities. Adv. Sci. 2020;7:2002611. doi: 10.1002/advs.202002611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Harrison CN, Talpaz M, Mead AJ. Ruxolitinib is effective in patients with intermediate-1 risk myelofibrosis: a summary of recent evidence. Leuk. Lymphoma. 2016;57:2259–2267. doi: 10.1080/10428194.2016.1195501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Moiseeva O, et al. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-κB activation. Aging Cell. 2013;12:489–498. doi: 10.1111/acel.12075. [DOI] [PubMed] [Google Scholar]
- 169.Csiszar A, et al. Resveratrol attenuates TNF-alpha-induced activation of coronary arterial endothelial cells: role of NF-kappaB inhibition. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H1694–H1699. doi: 10.1152/ajpheart.00340.2006. [DOI] [PubMed] [Google Scholar]
- 170.Xia L, et al. Resveratrol reduces endothelial progenitor cells senescence through augmentation of telomerase activity by Akt-dependent mechanisms. Br. J. Pharmacol. 2008;155:387–394. doi: 10.1038/bjp.2008.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shen X, et al. Resveratrol prevents endothelial progenitor cells from senescence and reduces the oxidative reaction via PPAR‑γ/HO‑1 pathways. Mol. Med. Rep. 2016;14:5528–5534. doi: 10.3892/mmr.2016.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Arunachalam G, Samuel SM, Marei I, Ding H, Triggle CR. Metformin modulates hyperglycaemia-induced endothelial senescence and apoptosis through SIRT1. Br. J. Pharmacol. 2014;171:523–535. doi: 10.1111/bph.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xu M, et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc. Natl Acad. Sci. USA. 2015;112:E6301–E6310. doi: 10.1073/pnas.1515386112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sin TK, et al. Resveratrol protects against doxorubicin-induced cardiotoxicity in aged hearts through the SIRT1-USP7 axis. J. Physiol. 2015;593:1887–1899. doi: 10.1113/jphysiol.2014.270101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kim EN, et al. The protective effect of resveratrol on vascular aging by modulation of the renin-angiotensin system. Atherosclerosis. 2018;270:123–131. doi: 10.1016/j.atherosclerosis.2018.01.043. [DOI] [PubMed] [Google Scholar]
- 176.Sena CM, et al. Metformin restores endothelial function in aorta of diabetic rats. Br. J. Pharmacol. 2011;163:424–437. doi: 10.1111/j.1476-5381.2011.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Houssaini A, et al. mTOR pathway activation drives lung cell senescence and emphysema. JCI Insight. 2018;3:e93203. doi: 10.1172/jci.insight.93203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Flynn JM, et al. Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell. 2013;12:851–862. doi: 10.1111/acel.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lesniewski LA, et al. Dietary rapamycin supplementation reverses age-related vascular dysfunction and oxidative stress, while modulating nutrient-sensing, cell cycle, and senescence pathways. Aging Cell. 2017;16:17–26. doi: 10.1111/acel.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Youm Y-H, Horvath TL, Mangelsdorf DJ, Kliewer SA, Dixit VD. Prolongevity hormone FGF21 protects against immune senescence by delaying age-related thymic involution. Proc. Natl Acad. Sci. USA. 2016;113:1026–1031. doi: 10.1073/pnas.1514511113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhang Y, et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. eLife. 2012;1:e00065. doi: 10.7554/eLife.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hoare M, et al. NOTCH1 mediates a switch between two distinct secretomes during senescence. Nat. Cell Biol. 2016;18:979–992. doi: 10.1038/ncb3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yin K, et al. Senescence-induced endothelial phenotypes underpin immune-mediated senescence surveillance. Genes Dev. 2022;36:533–549. doi: 10.1101/gad.349585.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cho JH, et al. CD9 induces cellular senescence and aggravates atherosclerotic plaque formation. Cell Death Differ. 2020;27:2681–2696. doi: 10.1038/s41418-020-0537-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pham LM, et al. Targeting and clearance of senescent foamy macrophages and senescent endothelial cells by antibody-functionalized mesoporous silica nanoparticles for alleviating aorta atherosclerosis. Biomaterials. 2021;269:120677. doi: 10.1016/j.biomaterials.2021.120677. [DOI] [PubMed] [Google Scholar]
- 186.Amor C, et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature. 2020;583:127–132. doi: 10.1038/s41586-020-2403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ruscetti M, et al. Senescence-induced vascular remodeling creates therapeutic vulnerabilities in pancreas cancer. Cell. 2020;181:424–441.e21. doi: 10.1016/j.cell.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rigato I, Ostrow JD, Tiribelli C. Bilirubin and the risk of common non-hepatic diseases. Trends Mol. Med. 2005;11:277–283. doi: 10.1016/j.molmed.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 189.Marconi VC, et al. Bilirubin is inversely associated with cardiovascular disease among HIV-positive and HIV-negative individuals in VACS (Veterans Aging Cohort Study) J. Am. Heart Assoc. 2018;7:e007792. doi: 10.1161/JAHA.117.007792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Boland BS, Dong MH, Bettencourt R, Barrett-Connor E, Loomba R. Association of serum bilirubin with aging and mortality. J. Clin. Exp. Hepatol. 2014;4:1–7. doi: 10.1016/j.jceh.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Vítek L, Ostrow JD. Bilirubin chemistry and metabolism; harmful and protective aspects. Curr. Pharm. Des. 2009;15:2869–2883. doi: 10.2174/138161209789058237. [DOI] [PubMed] [Google Scholar]
- 192.Nitti M, Furfaro AL, Mann GE. Heme oxygenase dependent bilirubin generation in vascular cells: a role in preventing endothelial dysfunction in local tissue microenvironment? Front. Physiol. 2020;11:23. doi: 10.3389/fphys.2020.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kim SY, Kang HT, Choi HR, Park SC. Biliverdin reductase A in the prevention of cellular senescence against oxidative stress. Exp. Mol. Med. 2011;43:15–23. doi: 10.3858/emm.2011.43.1.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Savas JN, Toyama BH, Xu T, Yates JR, Hetzer MW. Extremely long-lived nuclear pore proteins in the rat brain. Science. 2012;335:942. doi: 10.1126/science.1217421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ori A, et al. Integrated transcriptome and proteome analyses reveal organ-specific proteome deterioration in old rats. Cell Syst. 2015;1:224–237. doi: 10.1016/j.cels.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kim SY, et al. Senescence-related functional nuclear barrier by down-regulation of nucleo-cytoplasmic trafficking gene expression. Biochem. Biophys. Res. Commun. 2010;391:28–32. doi: 10.1016/j.bbrc.2009.10.154. [DOI] [PubMed] [Google Scholar]
- 197.Kim SY, et al. Global transcriptional downregulation of TREX and nuclear trafficking machinery as pan-senescence phenomena: evidence from human cells and tissues. Exp. Mol. Med. 2020;52:1351–1359. doi: 10.1038/s12276-020-00490-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Huang Y, et al. Biliverdin reductase A protects lens epithelial cells against oxidative damage and cellular senescence in age-related cataract. Oxid. Med. Cell. Longev. 2022;2022:5628946. doi: 10.1155/2022/5628946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Park J-H, et al. Disruption of nucleocytoplasmic trafficking as a cellular senescence driver. Exp. Mol. Med. 2021;53:1092–1108. doi: 10.1038/s12276-021-00643-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Grosse L, et al. Defined p16 high senescent cell types are indispensable for mouse healthspan. Cell Metab. 2020;32:87–99.e6. doi: 10.1016/j.cmet.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 201.Garrido AM, et al. Efficacy and limitations of senolysis in atherosclerosis. Cardiovasc. Res. 2022;118:1713–1727. doi: 10.1093/cvr/cvab208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.French LE, et al. Fas and Fas ligand in embryos and adult mice: ligand expression in several immune-privileged tissues and coexpression in adult tissues characterized by apoptotic cell turnover. J. Cell Biol. 1996;133:335–343. doi: 10.1083/jcb.133.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tan PH, et al. Phenotypic and functional differences between human saphenous vein (HSVEC) and umbilical vein (HUVEC) endothelial cells. Atherosclerosis. 2004;173:171–183. doi: 10.1016/j.atherosclerosis.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 204.Kile BT. The role of apoptosis in megakaryocytes and platelets. Br. J. Haematol. 2014;165:217–226. doi: 10.1111/bjh.12757. [DOI] [PubMed] [Google Scholar]
- 205.Muñoz-Espín D, et al. A versatile drug delivery system targeting senescent cells. EMBO Mol. Med. 2018;10:e9355. doi: 10.15252/emmm.201809355. [DOI] [PMC free article] [PubMed] [Google Scholar]