Abstract
Heated tobacco products (HTP) have become increasingly common in many countries worldwide. The principle of heating tobacco, without combustion, to produce a nicotine-containing aerosol with remarkably reduced levels of other known toxins, compared to combusted tobacco cigarettes, is now well established. As these products are intended as alternatives to traditional combusted products, during the early stages of their development, it is important for manufacturers to ensure that the design of the product does not lead to any unintentionally increased or new risk for the consumer, compared to the traditional products that consumers seek to replace. There is limited guidance from tobacco product regulations concerning the requirements for performing such preliminary toxicological assessments. Here, we review the published literature on studies performed on HTPs in the pursuit of such data, outline a proposed approach that is consistent with regulatory requirements, and provide a logical approach to the preliminary toxicological assessment of HTPs.
Keywords: Heated tobacco products, Product assessment, Product stewardship, Toxicological assessment
Highlights
-
•
Heated tobacco products have become increasingly common in many countries worldwide.
-
•
Heating tobacco reduces levels of toxins, compared to combusted tobacco cigarettes.
-
•
Limited guidance from tobacco product regulations to assess their toxicity.
-
•
This review proposes an approach to perform preliminary toxicological assessment.
-
•
The proposed approach is consistent with regulatory requirements.
1. Introduction
Alternative nicotine and tobacco products, such as e-cigarettes and heated tobacco products (HTP), which are marketed as alternatives to cigarettes, have become increasingly prominent in many markets. In HTPs, the processed tobacco is heated, instead of being combusted, to produce an aerosol containing nicotine. The principle underlying the design of such products is to reduce the temperature of heating tobacco below the combustion point to substantially reduce the levels of toxic substances in the aerosol. This is based on the understanding that most of the harmful or potentially harmful compounds (HPHC) found in tobacco smoke are formed through chemical transformations occurring only at temperatures above the point at which self-sustaining combustion is initiated. It is worthy of note, therefore, that establishing the absence of combustion is a defining quality for HTPs.
Several different HTP designs are currently available on the market and can be categorized into three types [23]:
-
1.
Electrically heated tobacco products (eHTP): A processed tobacco insert or article is heated, without combustion, using an electrical tobacco heating device (THD) to release nicotine-containing flavored aerosol for inhalation (e.g., glo™ and IQOS™).
-
2.
Carbon-heated tobacco products (cHTP): A tobacco substrate is heated by smoldering carbon to produce a nicotine-containing aerosol (e.g., Eclipse™).
-
3.
Aerosol-heated tobacco products (aHTP): The tobacco component is heated by the warmed aerosol from an electrical THD without combustion of the tobacco to produce a nicotine-containing aerosol (e.g., iFUSE™ and Ploom™).
Scientific evaluation to substantiate the harm reduction potential of these products in comparison to smoking cigarettes requires rigorous non-clinical and clinical assessments, as described, for example, by the US Food and Drug Administration (FDA) guidance for applications for modified risk tobacco products (MRTP) [68]. In addition to comparisons with conventional combustible products, it is necessary to understand the individual toxicological risk profiles of emerging or new variants of these products, independent of their potential risk-reduction profile. The focus of these assessments is to establish that, compared to combusted cigarettes, these products have no new or increased hazards. Several scientific bodies, including the German Institute for Risk Assessment [42], National Institute for Public Health and the Environment in the Netherlands [6], Public Health England [45], and WHO Study Group on Tobacco Product Regulation [74], have reviewed the scientific data available on alternative tobacco products. Specific challenges have been recognized, particularly, relating to some customizable elements of electronic cigarettes [77] or the potential for the transfer of metals from the coils to the aerosol [25]. However, most of the emphasis has been on e-cigarettes, which are different from HTPs in many ways.
Although e-cigarette aerosols are produced by heating a liquid containing nicotine (extracted from tobacco or synthetic), HTPs contain a substrate reconstituted from natural tobacco [49]. Nicotine is released from tobacco by heating, along with various other components of the tobacco leaf that add taste and flavor to the aerosol. Although HTPs closely resemble conventional cigarettes, the physical and chemical processes involved in releasing aerosols from the former are considerably different from the combustion processes that produce smoke in the latter. Furthermore, to achieve consistent aerosol production, a complex system is required that creates a controlled heat source and exposes appropriately processed tobacco. This often involves compartmentalizing the product, including a consumable part containing tobacco, and either a separate heating device (such as an electronic heater) or an integrated heating element (such as a carbon tip). Thus, each group of products provides different contexts in which risk assessment must be performed.
In a regulatory context, these products are treated differently. Several countries include alternative tobacco products specifically in their tobacco product regulations (such as Canada [30], USA [67], and European Union countries [20]). Specific regulations for HTPs are rare and generally only cover specific tax categorization for the products [32]. Where specific references to novel or alternative products are made in regulations, the emphasis has been on establishing the reduced risk potential of such products.
Philip Morris International (PMI) has previously described the overall strategy for product assessment of an early version of HTP—the heatbar [58]. These studies formed the foundation for future product assessments in PMI and helped establish the approach described by Smith et al. in 2016 as a generally applicable assessment program integrating seven steps to complete in order to demonstrate that a candidate modified risk tobacco product (MRTP) [59]. In the Smith et al., approach, the three first steps involve evaluation of the product design and control principles; evaluation of aerosol chemistry and physics; and standard toxicological assessment of the products. According to this approach, the main objective of these three first stages is to establish reduced formation of HPHCs and reduced toxicity in laboratory models, before going further to establish reduced risk in laboratory models, (step 6), reduced exposure and risk in individuals (steps 5 and 6) and finally reduced population harm (step 7).
However, before any full evaluation of the reduced risk status can proceed, there are some basic standards to which all products should be held. For example, for HTPs, this might involve establishing that no combustion occurs during the use of the product, there is an overall reduction in HPHCs, there is some indication of reduced toxicity in in vitro systems, and all components and ingredients of both consumables and devices are appropriate for intended use and will not present new or increased toxicity to the final product. We term this phase of assessment “preliminary toxicological assessment”, to distinguish it from reduced risk assessment. Much of this phase is captured under steps 1–3 of the Smith et al. approach.
The purpose of this paper, therefore, is to describe an approach for preliminary toxicological assessment of HTPs that could be seen as a standard approach for future emerging products in this category. We have previously published a toxicological assessment of ingredients for cigarettes [14]. In this paper we compared the requirements for toxicological assessment of ingredients and materials specifically in the context of HTPs. This approach is supported by a review of the literature identifying common standard practice for toxicological risk assessment of HTPs, thereby ensuring the approach is practical and accessible for most manufacturers, as well as providing consistent information to facilitate regulatory review and product comparisons.
To our knowledge this is the first publication building on a review of the literature on current and best practice for HTP assessment to articulate a strategy that can be easily and consistently applied for the preliminary stages of HTP toxicology assessment. This strategy can help provide appropriate data to manufacturers and regulators for evaluating the product toxicological profile and determining the appropriateness to proceed to further assessment of reduced risk potential. This could be a pre-requisite to market release.
We recognize this is not sufficient to establish the appropriateness of placing such products on the market or to meet regulatory requirements for such placements. Rather, it should be seen as a preliminary step to ensure that HTPs are unlikely to present novel or increased risks in comparison to smoking cigarettes. Nevertheless, we believe it is of value to separate out this step and describe it in full in order to provide manufacturers and regulators with a common approach to this early phase of assessment.
2. Methods
We reviewed the US FDA guidance on Premarket Tobacco Product Applications for Electronic Nicotine Delivery Systems as the starting point for establishing a standardized approach to the preliminary toxicological assessment of HTPs [69]. A literature search was performed in PubMed to capture papers publishing strategies, methods, or data, that could be relevant to satisfying the non-clinical assessment requirements outlined by the US FDA for HTPs. The papers identified from the literature search were screened to identify methods that were commonly applied by different institutions. The identification of common methods, if sufficient to address the needs of a preliminary toxicological assessment, provide the advantage of being likely to be easily accessible to manufacturers, regulators and other interested parties. Once again it is important to point out that though this may be sufficient and valid criteria for preliminary toxicological assessment, it should be emphasized that other methods will be much more important in the later stages of risk assessment for such products. Thus, exclusion from this literature collection is by no means intended to imply that other novel assessment methods should be ignored for other assessment purposes.
An initial search was performed in April 2021, with the search terms: ((electronic cigarettes) OR (heated tobacco products) OR (novel tobacco products) OR (electronic nicotine delivery systems) OR (IQOS) OR (PLOOM) OR (electrically heated cigarette)) AND ((risk assessment) OR (toxicology assessment) OR (safety assessment)) in all fields. Whilst recognizing that no search strategy is likely to capture all relevant publications, this strategy was developed based on our prior knowledge of the literature. As the products of interest are emerging products, the terminology used to describe them in the literature has varied considerably over the years. We therefore sought to identify a range of different terms for the products and evaluated the completeness of the search results against our own databases. The literature search was also supplemented with personal knowledge of relevant studies and by snowball-searching the references in the relevant papers identified. Snowball search was performed on all reviews, and on all papers selected for inclusion in the final analysis.
The results of the literature search were transferred to an excel file where they were manually curated to identify the relevant articles. These relevant articles were further classified into those that discussed the general principles of some form or preliminary product assessment, and those presenting original data from products tested in standardized tests. The first group were used to evaluate the level of consensus on methods for preliminary toxicological assessment. The second to identify if there were specific tests that were commonly applied that would cover all the requirements for preliminary toxicological assessment.
Articles that referred only to product use behavior, marketing, perceptions, physical injury, or clinical trials were excluded. Non-English language were also excluded, as were conference proceedings or abstracts where sufficient information on the assays being applied were not provided. The remaining abstracts were screened to identify studies showing original data on standardized tests that could be considered relevant to the ingredient, material, or product assessment of HTPs. Studies that used novel and non-validated assays, such as non-OECD protocol studies and systems biology approaches were excluded. Although many of these may provide valuable information for overall product risk assessment, they are of limited value in a regulatory context where standardized and validated assays are required to provide reliable data from which regulatory conclusions can be drawn. The US FDA’s response to non-validated methods in the recent MRTP authorization for IQOS, stating that “methods that were non-validated towards understanding risk; were non-standardized; and are unknown as to their reliability and, consequently, their applicability towards regulatory use” corroborates this point [70].
In general, studies that were specific to e-cigarettes were also excluded; however, studies that used e-cigarettes as models for ingredient assessment or transfer from devices were included as they provided potentially relevant information for HTP assessment.
For the papers reporting original data, articles published prior to 2010 were also excluded, as earlier HTP models may differ in many aspects from the more recent and commercially successful versions. A 10-year coverage for the methods applied seems reasonable, given that publications prior to this date would have been considered by the more recent articles, and the main learnings from them would have been captured sufficiently [58]. The search was run on a weekly basis since the first execution through to February 2022, and new articles were added to the review as appropriate.
3. Results
3.1. Regulatory requirements for preliminary toxicological assessment
Owing to the relative recency of commercialized HTPs, there are relatively few guidelines on specific toxicological testing that meet regulatory requirements. In the US, HTPs are considered tobacco products and are regulated by the FDA according to the Family Smoking Prevention and Tobacco Control Act (FSPTCA) of 2009 [67]. This was the first regulation that required FDA authorization for any new product or product variant introduced into the market and for which authorization was only granted if the FDA concluded that, based on sufficient scientific data, the introduction of the product to the market would be appropriate for the protection of public health (APPH). In addition, FDA is probably the only body that provides specific guidance on the scientific evidence required to establish the status of APPH [69]. Although this guidance applies to electronic nicotine delivery systems (ENDS) rather than HTPs specifically, much of the guidance is equally applicable or at least adaptable to HTPs.
With respect to the preliminary toxicological assessment of products, the FDA Premarket Tobacco Product Application guidance outlines several requirements namely:
-
•
a full statement of the components, ingredients, additives, and properties;
-
•
a full review of toxicology data from the literature, with a particular focus on oral, inhalation, dermal, and ocular routes of exposure and endpoints such as cytotoxicity, genotoxicity, carcinogenicity, respiratory toxicity, cardiotoxicity, or developmental and reproductive toxicity;
-
•
analysis of constituents, including HPHCs and other toxicants, under both intense and non-intense use conditions;
-
•
in vitro toxicology studies, such as genotoxicity and cytotoxicity studies, based on potential human exposure, as recommended for ICH or OECD guidance protocols;
-
•
computational modeling of toxicants in the product (to estimate the toxicity of the product); and
-
•
in vivo toxicological studies (to address unique toxicological issues that cannot be addressed using alternative approaches).
In Canada, heated tobacco products are considered smokeless tobacco products and must comply with the tobacco reporting regulations (SOR/2000–273) under which tobacco constituent data must be provided (26 constituents). Health Canada also requires companies to submit all research related to toxicity, health effects, ingredients, taste and flavor, product modifications, marketing, and usage.
For all member states of the European Union, the 2014 European Union Tobacco Product Directive (EU TPD) specifically refers to novel tobacco products as those that consist, even partly, of tobacco, which are not cigarettes, roll-your-own tobacco, pipe tobacco, waterpipe tobacco, cigars, cigarillos, chewing tobacco, nasal tobacco, or tobacco for oral use, and are placed on the market after May 19, 2014 [20]. According to Article 19 of the EU TPD, member states should require manufacturers to provide notification of any product prior to its introduction to the market. The content requirements for these notifications are not precisely defined in the directive but should generally include detailed description of the products, information on ingredients and emissions, and “available scientific studies on toxicity, addictiveness, and attractiveness…” The EU TPD also allows member states to introduce a system of authorization of novel tobacco products, and several member states, including Austria, Belgium, Denmark, Luxembourg, Poland, and Portugal, formally require such authorization prior to marketing.
For most other countries, regulations provide little guidance for the preliminary toxicological assessment of modified tobacco products and HTPs. For example, in South Korea, HTPs are regulated as tobacco products under the Tobacco Business Act and National Health Promotion Act. This involves licensing, health warnings, sales, and restrictions on public use. Similarly, in Japan, HTPs are regulated by the Health Promotion Act, which mainly refers to the limitations on where products may be used. Further regulations in Japan refer to taxation and sales to minors. The general absorption of HTPs into other tobacco laws seems common in many countries [32].
3.2. Literature review on preliminary toxicological assessment of HTPs
The initial literature search yielded 735 papers. After full screening of the results from the literature searches, 39 papers were identified with relevant information for preliminary toxicological assessment of HTPs. These were categorized into papers that discussed the general principles of such a preliminary assessment and those that presented original data from products tested in standardized tests.
3.2.1. Papers proposing general principles of preliminary toxicological assessment for HTPs
Table 1 includes 10 publications that describe generalized principles, strategies, and frameworks for the overall product assessment of MRTPs [2], [29], [4], [8],[58], [59],[33], [60], [61], [73]. These include three from tobacco-industry authors [58], [59], [8], two from academic institutes [2], [29], and four representing commissioned work on request from the official organizations World Health Organization [73], the US Institute of Medicine as commissioned by the FDA [33,61], and the Tobacco Product Assessment Consortium (TobPRAC) commissioned by the US National Cancer Institute [4]. The other publication was from the Life Sciences Research Office (LSRO) and commissioned by PMI [60]. All the assessment approaches proposed by these publications recognize the need for some preliminary toxicological assessments prior to the marketing of such products. They also recognize that the tools available for such assessments have limitations for overall risk assessment and should be viewed as informative to identify particularly risky products and ensure that those proceeding to further stages of development have a reasonable chance of success. Each of these approaches also includes recommendations for the more advanced stages of risk assessment, which encompass the evaluation of product use and clinical studies. In this review, we focus only on the preliminary assessment stages, for which there is general agreement, at least on the principles that should be included in such a preliminary assessment.
Table 1.
Published risk assessment frameworks and strategies for risk assessment of novel tobacco products.
| Author (Affiliation) | Productsa | Framework / Strategy Objective | Elements relevant to preliminary toxicological assessment (PTA) |
|---|---|---|---|
| [61] (IOM) | Potentially reduced exposure products (PREPs) | To provide a scientific basis for the achievement of tobacco harm reduction based on modified tobacco products. | Concluded that harm reduction was feasible and recommended 11 regulatory principles to achieve this, including: - Reg. Principle 1:. quantitative analytical data on ingredients; Reg. Principle 2: …yields of nicotine and other tobacco toxicants… Reg. Principle 3: …appropriate toxicological testing in preclinical laboratory and animal models… Reg. Principle 8: …all added ingredients…subject to a comprehensive toxicological review |
| WHO SACTob, 2003 | New or modified tobacco products | To provide guidance on issues identified by the scientific community that may form the basis for regulatory and other decisions about these products. | “First logical step … is to examine the characteristics of the product.” Including ingredient quantities and toxicology; emissions compared to conventional products, and under conditions of actual use. |
| [60] (LSRO) | Potentially reduced risk tobacco products (PRRTPs) | To identify the types of scientific information needed to assess risk reduction; establish criteria to evaluate the scientific information, and define a review process. | Recommend a weight of evidence approach prior to marketing including preclinical studies such as product characterizations, smoke chemistry studies, cytotoxicity and genotoxicity and animal studies. |
| [29] | Tobacco and other nicotine containing products | To identify research opportunities to develop empirically based and comprehensive methods and measures for testing tobacco and other nicotine-containing products so that the best science is available when decisions are made about products or policies. | The first stage of the approach discusses toxicity, toxicant exposure, and potential health risks. The phase, which is described as the pre-human phase, has elements of PTA. These include constituent analysis and toxicological analysis in vitro and in vivo. |
| [58] (PMI) | Electrically heated cigarette smoking system (EHCSS) | To describe the testing strategy applied to the evaluation of reduced exposure to HPHCsc | "Product level testing" is described as part of the overall reduced exposure assessment, with one of the objectives being to establish that the product meets the minimum criteria of presenting no increased or new hazard in comparison to conventional cigarettes. |
| [33] | Modified risk tobacco products (MRTP) | To provide guidance on the design and conduct of studies for the assessment and ongoing review of an MRTP applicant. | Describes the first stage as “preclinical” requiring assurance of manufacturing quality control; significant and substantial reduction in toxicant and carcinogen content in product; significant reduction in exposure to toxicants and carcinogens in limited human study; no significant evidence for offsetting increases in content of or exposure to other toxicants. |
| [4] (TobPRAC)b | New tobacco products | To develop a comprehensive scientific framework to guide the evaluation of new tobacco products and health-related claims | Provides a four-phase framework for overall tobacco product assessment. The first of these, "pre-market evaluation", includes (1) assessment of the tobacco product design, physical performance, characteristics and contents; and (2) laboratory chemical and toxicological analysis. |
| [59] (PMI) | Tobacco heating system 2.2 (THS2.2) | Overall MRTP assessment program | The first step in the overall process designed to ensure appropriate quality standards and to establish product specification maintained through strict change control process. |
| [2] | Electronic cigarettes (e-cigarettes) | Overall assessment of e-cigarettes for public health impact. | Proposes a 7 step approach of which steps 1 (characterization of the product) and step 5 (evaluation of direct health effects) parallel PTA. |
| [8] (BAT) | Electronic cigarettes (EC) | Overall assessment of e-cigarettes for public health impact.exposure | 6 step process for product stewardship includes purity standards; exclusion of CMRd and respiratory allergens; toxicological risk assessment on e-liquid and aerosol, with potential bridging approach. |
Products as described within the publication; b TobPRAC = Tobacco Product Assessment Consortium; c HPHC = harmful and potentially harmful constituents; d CMR = carcinogenicity, mutagenicity, and reproductive toxicity
Common to all these papers is the requirement to characterize the products and establish standards for key ingredients, such as flavors and humectants. Similarly, a review of published toxicity data on all materials and ingredients, including the potential for degradation, reaction, and transfer to aerosols for all components, is recommended. In addition to ingredient assessment, all 10 of these studies recommended chemical and toxicological assessments of the product. Specifically, aerosol chemistry analysis and in vitro assays for cytotoxicity and genotoxicity were commonly recommended. Several studies have also noted the necessity to perform these assessments under conditions of actual use, with reference to either selecting an appropriate puffing regimen for the generation of the aerosol [8], or to compare activities using a range of different aerosol generation regimes to evaluate the potential for different chemical or toxicological effects to be noted under extreme conditions [58], [59],[60], [61], [73].
3.2.2. Studies reporting original data
Twenty-nine studies were identified as those reporting original data from established assessment methods on HTPs. Details of these studies are presented in Table 2. Nineteen of these studies were published by the tobacco industry, with the remaining ten from academia or public health institutes. Most studies were on eHTPs, such as IQOS™ and glo™, or their prototypes. Only a few studies have examined cHTPs [44], [66] or aHTPs [52], [62], perhaps reflecting the maturity of commercialization of these products.
Table 2.
Studies reporting original data from preliminary toxicological assessment of heated tobacco products (HTP) according to the types of studies performed, categories of HTPs, and comparator reference products.
| Author-date | Aerosol Chemistry | In Vitro | In Vivo | Other | HTP typea |
Reference cigaretteb | Test material generatione | Analytes / Assaysp |
|---|---|---|---|---|---|---|---|---|
| Zenzen 2012 | X | X | E | 2R4F CC |
ISO HPBf |
2) 49 HPHCs 3) Ames / NRU |
||
| Schaller 2016a | X | X | X | E | 3R4F | HCI ISO APRg |
2) 58 HPHCs 3) Ames / NRU / MLA 5) aerosol droplet size - MMAD |
|
| Oviedo 2016 | X | E | 3R4F | HCI | 4) OECD Test No. 413 /Systems toxicology endpoints | |||
| Schaller 2016b | X | E | 3R4F | HCI | 2) 58 HPHCs | |||
| Wong 2016 | X | E | 3R4F | HCI | 4) OECD Test No. 413 /Systems toxicology endpoints | |||
| Auer 2017 | X | E | CC; PDc |
ISO | 2) 8 VOCs; 16 PAHs; 3 inorganic compounds; Nicotine | |||
| Bekki 2017 | X | E | 1R5F; 3R4F |
HCI | 2) Nicotine; Tar; CO; 4 TSNAs | |||
| Farsalinos 2017 | X | E | CC | HCI LPVh |
2) Nicotine | |||
| Jaccard 2017 | X | E | 3R4F; CC |
HCI | 2) 44 compounds (Health Canada list) | |||
| Poynton 2017 | X | A | 3R4F | HCI CRM81i |
2) Untargeted GC scans; 113 compounds (FDA / HC) |
|||
| Jaunky 2018 | X | E | 3R4F | HCI | 3) NRU | |||
| Takahashie 2018 | X | X | A | 3R4F | HCI | 2) 43 Hoffmann analytes; propylene glycol, glycerol, triacetin, TPM, Nicotine, CO; 3) Ames / MN / NRU |
||
| Thorne 2018 | X | E | 3R4F | HCI | 3) Ames / NRU / MLA / Bhas cell transformation | |||
| Eaton 2018 | X | E | 3R4F | nak | 1) TGAq | |||
| Farsalinos 2018 | X | E | CC | HCI + 2 Intensel |
2) 5 carbonyls (acetaldehyde, acrolein, formaldehyde, propionaldehyde and crotonaldehyde) | |||
| Forster 2018 | X | X | E | 3R4F | HCI CRM81i |
2) 126 HPHCs 5) Physical analysis: particle diameter and number |
||
| Li 2018 | X | X | E | 3R4F | HCI ISO |
1) Simulated pyrolysis 2) TPM; water, tar, nicotine, propylene glycol, glycerin, CO, VOCs, aromatic amines, HCN, ammonia, TSNAs, phenol, PAHs |
||
| Mallock 2018 | X | E | PDd | HCI | 2) TPM; Nicotine, Water, Aldehydes (Acetaldehyde; acrolein; formaldehyde; crotonaldehyde) VOCs (1,3-Butadiene; Benzene; Isoprene; Styrene; Toluene) | |||
| Pacitto 2018 | X | E | PD | (-/2/10)m | 5) physical characteristics of the aerosol (Particle size distribution; particle volatilitiy) | |||
| Savareear 2018 | X | E | 3R4F | HCI | 2) Non targeted screening of VOCs from PP fraction of aerosols | |||
| Titz 2018 | X | C | 3R4F | HCI | 4) OECD413 / Systems toxicology endpoints | |||
| Godec, 2019 | X | E | 3R4F | HCI | 3) Ames / MLA | |||
| Thorne 2019a | X | E | 3R4F | HCI | 3) MLA | |||
| Thorne 2019b | X | E | 3R4F | HCI | 3) MN | |||
| McAdam 2019 | X | C, E | CC | Multiplen | 2) Total aerosol mass (AM); smoke particulate matter (TPM); Water; Nicotine; Glycerol | |||
| Salman 2019 | X | X | E | CC | HCI ISO |
2) Carbonyl compounds and total nicotine (free-base and protonated) and Reactive oxygen species (ROS) | ||
| Savareear 2019 | X | E | 3R4F | HCI ISO° |
2) non-targeted screening for PP components | |||
| Caruso 2021 | X | E | IR6F | HCI | 3) Cytotoxicity tests in Human H292 cells via ALI (Air-liquid interface) exposure: NRU; MTT; Annexin V apoptosis; |
|||
| Dusautoir 2021 | X | X | E | 3R4F | HCI (WA) | 2) Nicotine; Aldehydes, PAHs 3) BEAS-2B cells cell viability; Glutathione Content assay; Gene expression; inflammatory mediators (GM-CSF; GRO-α IL-1ẞ; IL-6; IL-8; IL-13; MCP-1; MIP-1 α, RANTES and INF-γ) |
a: HTP category as defined by CORESTA [23]: E = electrically heated tobacco product (eHTP); A = aerosol-heated tobacco product (aHTP); C = carbon-heated tobacco product (cHTP).
b: CC = marketed conventional cigarettes, PD = published data.
c: Published data based on reference [71].
d: Published data based on reference [12]
e: Test material generation as defined by the puffing regimen applied to produce the testing material characterized by puff volume (mL)/puff duration(s)/puff interval(s): ISO = International Organization for Standardization (35/2/60); HCI = Health Canada Intense (55/2.0/30). Other regimens specified by relevant footnotes: CRM81 = CORESTA recommended method (55/3/30); test material exposure; TPM = total particulate matter; GVP = gas–vapor phase; WA = whole aerosol; ALI = air–liquid interface.
f: HPB = human puffing behavior—nine different puffing regimens compared based on observed human topography studies (see [76] for details).
g: APR—test material also generated using five alternative puffing regimens based on human puffing behavior (see [56] for details).
h: LPV includes a long puff variant of the HCI regimen (55/4/30) in addition to the standard HCI regimen.
i: Reference cigarette 3R4F generation using HCI regimen; CRM81 for HTPs.
k: na = not applicable. A pyrolysis study was conducted based on 10 g of tobacco with 10 g wrapping paper heated in air or nitrogen in a platinum crucible up to 900 °C (see [16] for experimental details).
l: Products were tested using HCI and two more intense regimens (80/3/30 and 90/3/25).
m: Each test was performed with four puff profiles of five puffs for 2 s and an inter-puff time of 10 s. The puff volume was not specified; however, the flow rate was 1 L/min.
n: Five different regimens used with fixed 30-s puff interval and 20 puffs per device. The puff volume ranged from 20 mL to 150 mL and the duration from 2 s to 5 s
o: HCI regimen used for the HTPs; ISO for 3R4F.
p:1) Brief description of the pyrolysis method applied; 2) analytes measured in aerosol chemistry studies represented as the total number of analytes; plus, details of specific lists as given by the authors; 3) in vitro assays used: NRU = neutral red uptake, MLA = mammalian mouse lymphoma assay, MN = micronucleus, other tests as described; 4) in vivo protocol applied: OECD413 = Organization for Economic Cooperation and Development Test Guideline 413: Repeated dose inhalation toxicity; 5) other assays are briefly described. Please refer to the original papers for full details.
q: TGA = thermogravimetric analysis with Pyris 1 TGA system in air or nitrogen: 5 °C/min from ambient temperature to 240 °C, held for 5 min at 240 °C, and continued ramping to 900 °C.
Aerosol chemistry was the most common assessment reported in these studies (18 studies), with in vitro and in vivo studies being reported in 10 and 5 papers, respectively. Individual studies that reported pyrolysis [16], [39], reactive oxygen species generation, distribution of nicotine free-base and protonated forms [53], and physical characteristics of the aerosol [24], [48], [56] were also identified.
In 24 of the 29 studies listed in Table 2, HTP-aerosols derived using the Health Canada Intense smoking regimen were used. Exceptions to this are two studies where specific generation methods adjusted for the products were used [24], [52], and one that applied the ISO method [1]. One study used aerosols derived from a range of different smoking regimens based on measured human puffing behavior [76], and one study used a continuous flow rate to collect aerosols for physical parameter measurements only [48]. In addition to standardized puffing regimens, nine studies compared aerosols from HTPs that had been produced over two or more different puffing regimes to determine how stable or varied the assessment results would be under different usage conditions [21], [22], [39], [44], [48], [53], [55], [56], [57], [76].
Of the 18 studies on aerosol chemistry, 7 measured a range of analytes represented as established HPHC lists, although the actual list used varied [24], [34], [52], [56], [57], [62], [76]. The other eleven looked at a subset of analytes, such as nicotine, aldehydes, polyaromatic hydrocarbons [1], [15], [22], [39], [42], [44], [53], [54], [55], nicotine, carbon monoxide, and tobacco-specific nitrosamines [3] with one paper looking at nicotine delivery [21]. In addition, four studies added new compounds to these lists based on the presence of high vegetable glycerin or propylene glycol levels in HTPs [24], [39], [44], [62]. To ensure that these relatively recent product designs do not result in the production of novel, unexpected, and potentially toxic aerosol constituents, three groups of investigators have recently reported non-targeted analysis methods as a more general, semi-quantitative approach to identify all compounds found in aerosols [52], [54], [55].
Neutral red uptake (NRU) assay is the most common in vitro assay reported in six papers [10], [35], [56], [62], [63], [76]. For genotoxicity, the Ames assay was the most reported (five studies) [28], [56], [62], [63], [76], with four reporting on mammalian cell mutation assays [28], [56], [63], [64], and two reporting on in vitro micronucleus assays [62], [65]. In all cases, HTPs showed considerably reduced activity, and no novel activity was reported. In most of the studies, cells were exposed to aerosol fractions, e.g., total particulate matter (TPM) or gas–vapor phase, although three of them used air–liquid interface (ALI) exposure systems. The three papers reporting on in vivo studies were all part of longer-term assessment programs that ultimately aimed to support clinical studies and the overall weight of evidence for risk reduction [47], [66], [75].
Other analyses have included studies on physical characteristics, such as mass median aerodynamic diameter and particle number, density, and distribution [24], [48], [56]. One study reported pyrolysis products from an HTP [16].
4. Discussion
Our analysis indicates:
-
i)
Regulators acknowledge a requirement for preliminary toxicological assessment of HTP products but provide only limited guidance for the methods that should be used; The most comprehensive guidance is provided by the FDA which requires listing of all components; a toxicological review of all the components; constituent analysis (e.g., aerosol chemistry); in vitro toxicology studies; computational modeling of toxicants, and in vivo toxicological studies.
-
ii)
Several groups, including manufacturers; academia and official organizations, have reported on general principles for preliminary toxicological risk assessment in the context of the overall assessment of MRTPs, though without specific guidance for HTPs. The common recommendation from these includes standards for ingredients and a review of toxicity data on all components, as well as chemical and toxicological evaluation.
-
iii)
The most common assessment methods reported in the literature as applied to HTPs specifically include aerosol chemistry, neutral red uptake, bacterial mutagenicity with the Ames assay, and mammalian cell mutation assays.
Several previous studies have examined some aspects of preliminary toxicological risk assessment focused on e-cigarettes [38], [46], [72]. Although some of these processes for e-cigarettes may be relevant for HTPs, there exist different considerations for HTPs that would argue for establishing a distinct process for HTPs. A case in point is related to nicotine quality. For e-cigarettes, where nicotine is extracted from tobacco and concentrated before addition to the e-liquid, it is appropriate to define a quality standard, such as pharmaceutical grade purity. This is not relevant for HTPs, where nicotine is derived directly from the natural nicotine levels in tobacco.
Although flavor ingredients are used in both products, differences exist in the matrix to which the flavors are applied (e-liquid versus tobacco substrate) as well as likely differences in the levels of flavors used in different products. The mechanism of transfer may also affect the nature of the ingredients transferred into the aerosol; e-cigarette aerosol formation is usually based on evaporation from a wick or membrane, whereas the aerosol is formed from the heating of the tobacco substrate for HTPs. Thus, it is important to consider the potential impact of flavor compounds in the context of the product itself. For example, there may be relevant differences in the components of flavors transferred to the aerosols of e-cigarettes, HTPs, or combusted cigarettes due to degradation or thermal decomposition at different temperatures. Although arguably, in a combustible cigarette, all processes occur, it is just a matter of distance from the burning coal that makes the difference. In e-liquids, the transfer is because of aerosolization, although some thermal degradation may occur. For HTPs, the flavors are transferred at a much lower temperature than for combustible cigarettes, although they are likely higher than for e-cigarettes. In all cases, the amount and nature of transfer of any given ingredient should be evaluated in the context of the product itself.
One approach to address this is to evaluate the potential toxicity of mixtures of flavor compounds in a similar exposure system. This approach was explored in an in vivo study that examined the impact of a mixture of 26 flavor compounds in a 90-day-long rat inhalation study (OECD Test No. 413) [31]. The flavors were selected to represent a potential worst-case scenario for the addition of flavors to e-liquids in e-cigarettes or tobacco in HTPs, thereby providing supporting data for the inclusion of a range of flavors in these products from a toxicological perspective.
In addition to the flavor ingredients, both e-cigarettes and HTPs commonly incorporate the humectants propylene glycol or vegetable glycerin to facilitate aerosol formation. Concerns have been raised regarding the potential formation of toxic compounds from the thermal degradation of humectants [17], [26], [27], [36], [43], [5]. Thus, it is necessary to establish that these reactions do not occur when the products are used.
A recent comprehensive review on the methods and devices for the generation, exposure, and collection of aerosols from HTPs examined the challenges arising from the differences in aerosol formations between cigarettes and HTPs [7]. Various adaptations to testing methods for addressing specific product attributes were identified, and the authors concluded a need to harmonize the methods used to avoid the difficulties in interpreting comparative data.
The Cooperation Centre for Scientific Research Relative to Tobacco (CORESTA) has published a technical report on HTPs and recommended methods for the generation and collection of emissions from different HTP types. This technical report recommends the Health Canada method as the basis for aerosol generation for eHTPS and cHTPs. However, adaptation of the ISO method has been suggested to be more appropriate for aHTPs [23].
4.1. Proposed preliminary toxicological assessment approach
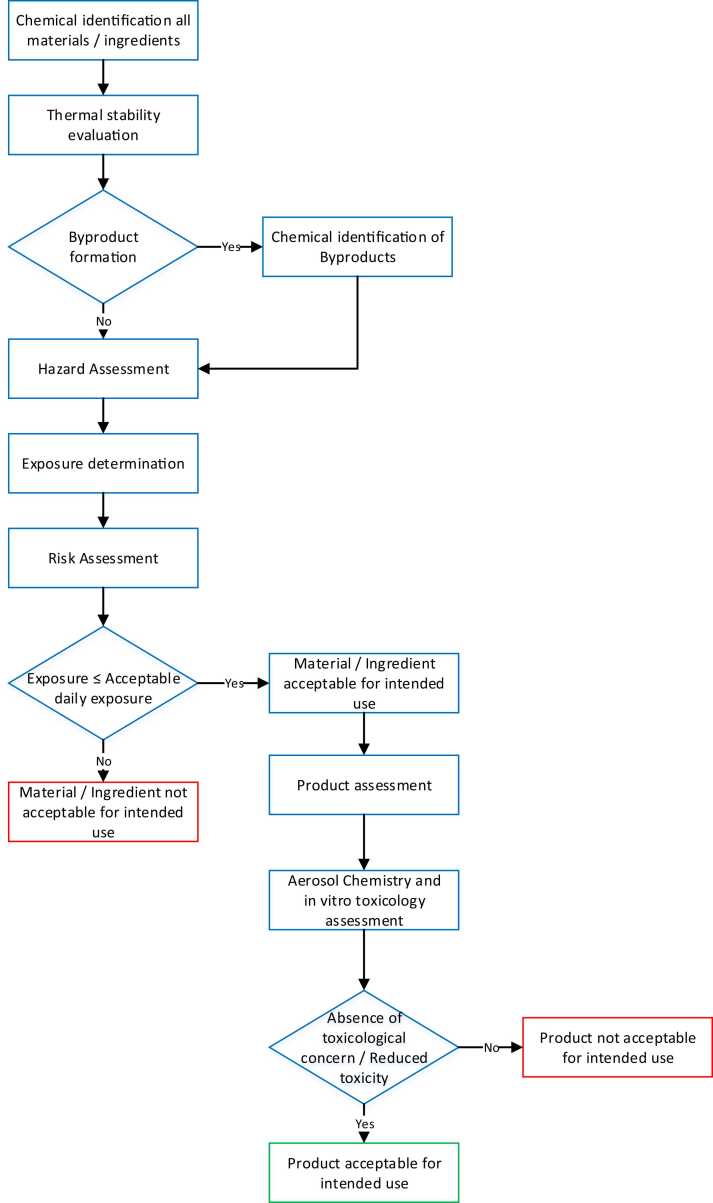
Fig. 1 describes a preliminary toxicological risk assessment strategy for the development of HTPs. The strategy adopts a classic toxicological risk assessment approach to address specific challenges or concerns that may arise from heating tobacco (rather than heating e-liquids or burning tobacco).
Fig. 1.
Decision tree flow chart describing a preliminary toxicology assessment process for heated tobacco products. Please refer to the main text for full description of the steps.
First, all materials and substances used or applied to the tobacco substrate (e.g., flavors; diluents such as propylene glycol, glycerin, water, or ethanol; and additives) must be chemically identified. In addition, the thermal stability of substances at the temperatures to which they will be exposed needs to be evaluated to identify further degradation or reaction products that may impact toxicity. Similarly, any material that could potentially be transferred from the non-substrate parts of the product or from the heating device must be identified. Hazard assessment for each substance is performed based on prior knowledge from toxicological databases and literature review, with an emphasis on information relating to the inhalation exposure route. In the absence of adequate toxicity data, quantitative structure–activity relationship (QSAR) models and read-across from well-characterized surrogates are applied.
Second, the expected or estimated exposure dose is determined by considering the observed usage data for the product, when available, and/or worst-case assumptions, for example, by modeling predicted transfer rates based on intense puffing conditions. These hazard and exposure assessments provide a basis for risk assessments. The acceptability of the substance for its intended use is determined based on the derivation of an acceptable daily exposure (ADE) via the inhalation route using appropriate Modifying Factors (MF). For example, the inhalation Derived No Effect Level (DNEL) for general population established by the European Chemical Agency (ECHA) or the Reference Concentration (RfC) from the American Environmental Protection Agency (US. EPA) could be used without additional uncertainty factors. In absence of such exposure limits, Occupational Exposure Limits (OELs) for inhalation exposure could be used as surrogate, however with specific MFs as they have been developed for workers, meaning addressed to a specific population for a specific exposure time (generally 8 h). For example, DNEL for workers, Recommended Exposure Limit (REL) from the National Institute for Occupational Safety and Health (NIOSH), or Permissible Exposure Limit (PEL) from the Occupational Safety and Health Administration (OSHA) could be considered applying different MFs. A first MF of 10 appears reasonably sufficient to protect the larger part of the population on account of individual susceptibility and absence of controlled environment. A second MF needs to be applied. Effectively, breathed air volume per day is slightly different for workers and general population. ECHA considers 10 m3 for 8 h in workers and 20 m3 for 24 h (i.e., 5/6 or 0.83 m3/h) in general population. A third MF needs to be introduced, as OELs are determined for 8 h exposure whereas ADEs apply to 24 h exposure. A last MF should be considered as workers are exposed 5 days per week whereas general population is exposed 7 days per week. Where the inhalation data is not available, an ADE may be derived from published toxicological reference values, thereby applying modifying factors to consider differences in routes of exposure and other considerations. Reference values like oral DNEL, Acceptable Daily Intake (ADI) from the Joint FAO/WHO Expert Committee on Food Additives (JECFA) or the European Food Safety Authority (EFSA), or Reference Dose (RfD) from the US. EPA can be considered. To these values, appropriate MFs are applied as advised by ECHA i.e., a default factor of 2 in the case of oral to inhalation extrapolation. European Chemical Agency) [19]. Nevertheless, it should be noted whereas route-to route extrapolation may help to determine ADE for systemic effects in absence of relevant data, such ADEs will however not protect against potential local effects like irritation. For substances with known local effects on the respiratory tract, the route-to-route extrapolation should be conducted with caution relying on expert judgment. In absence of human data, animal studies could be considered to derive appropriate ADE proven that they are adequate, reliable, and pertinent (animal choice). In addition, duration should be aligned with the intended period of human exposure for marketed products. The use of studies with limitations, like the absence of effective dose or incomplete datasets, should be validated by expert judgment. Once the study is judged acceptable, Uncertainty Factors (UF) are considered to perform human health risk assessment from animal studies. The methodology used to determine the appropriate UFs is derived from the ECHA guidance [19]. Alternatively, a threshold of toxicological concern approach adapted to inhalation exposure may be applied. In reviewing the available related publications [11], [18], [40], [9], it appears acceptable to consider the following thresholds, i.e., 1800 µg/day for Cramer Class I compounds, 90 µg/day for Cramer Class II and III compounds, 18 µg/day for organophosphates, and 0.15 µg/day for substances presenting structural alerts for genotoxicity. The outcome of this risk assessment is a decision on the acceptability of the substance, along with the maximum use level at which the substance is deemed acceptable.
Last, aerosol chemistry analysis and in vitro toxicology assessment of the final product or flavor mixture is performed. This final assessment will help identify potential interactions or unintended consequences of the overall product design. The in vitro assessment utilizes a battery of assays, including cytotoxicity, bacterial mutagenicity, and mammalian cell genotoxicity assays, according to the recommendations from the CORESTA In Vitro Toxicity Testing subgroup [37]. These assays are evaluated with reference to a comparator product, usually a reference combustible cigarette such as the Kentucky reference 3R4F or 1R6F, as well as to a key reference HTP, which has undergone more in-depth non-clinical and clinical studies. These comparisons first ensure that the new products offer at least a reduction in toxicity in these assays compared to the reference combustible product and should be within the range of values expected from the comparator reference HTP.
This approach to preliminary toxicological assessment satisfies most of the criteria that appear in the regulations described above, particularly for FDA guidance for non-clinical assessment of ENDS products [69]. However, the FDA guidance also requires data from in vivo toxicological studies to address unique toxicology issues that cannot be addressed through alternative approaches, while also supporting the need to reduce, replace, and/or refine the use of animal testing. In lieu of in vivo testing for each new product design, PMI has performed multiple studies on finished products [47], [50], [66], [75] or on ingredients used in the products [31], [51]. Extrapolation from these studies allows us to infer the appropriateness of the use of these ingredients and flavors in HTPs of similar designs and functionalities, thus minimizing the need to perform additional animal studies for each product modification.
Nevertheless, new methods and analyses will be developed, and as products become more widely commercialized, they will be scrutinized by different laboratories using different techniques. Therefore, manufacturers such as PMI should continuously monitor the literature on components, ingredients, and finished products and critically review any new data when identifying unintentional toxicities that may not have been picked up in the preliminary toxicological risk assessment. A recent report identifying formaldehyde cyanohydrin released from heatsticks in the IQOS product provides an example of such a case [13]. This compound of concern was not previously identified during the PMI assessment process; therefore, a reanalysis was performed to investigate the findings reported by Davis et al. Subsequent studies by PMI indicated that the peak identified by Davis et al. as formaldehyde cyanohydrin was meso-lactide—a known condensation product of lactic acid—which was identified in PMI’s initial analysis [41].
5. Conclusions
Over the last two decades, several new HTPs have emerged for marketing from the product-development process. The regulation of these products varies worldwide, with many countries encompassing HTPs in regular tobacco regulations, often without providing specific guidance appropriate for the principles of HTPs. The absence of specific guidance on the testing that should be performed on these products is likely to lead to regulators receiving diverse data sets making comparison between products difficult.
A limitation of this research has been to restrict the review to studies using standardized methods. This may provide a false impression of the consistency of the approach. However, it seems that most manufacturers of these products have used the same standardized methods as a basic approach, with additional methods applied in an exploratory manner. The application of many new alternative methodologies to the assessment of these products will likely provide improved understanding of these products in the future, but to date these are too varied to afford useful comparisons between products. Acceptance of the best new approaches by regulators will likely be facilitated in the near future when comparative studies by different laboratories on different products could provide insights into standardization of the methods and interpretation of the data.
In our literature review, we highlight many consistencies in the way manufacturers and other institutions have assessed these products. Considering both the general regulatory guidance and published studies, a logical preliminary toxicological assessment process can be defined. This involves verification of the suitability of all materials used in the product, stability under the temperature ranges likely to be experienced during the use of the product, and toxicological assessment of the finished product through aerosol chemistry and standard in vitro studies to characterize the final product. Repeated in vivo studies on individual products are deemed unnecessary and therefore inappropriate, based on sufficient data being readily available from in vivo assessments of the main components to allow extrapolation to individual product designs using these components. Continued follow-up of the products in the market and insights that might be gained from other studies on the products should be deemed an ongoing step in the assessment process.
Statements and Declarations
GR, FV, IG, MB and ME are employees of Philip Morris International. RD was an employee of Philip Morris international and is contracted and paid by Philip Morris International.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The research described in this article was supported by Philip Morris International.
Handling Editor: Dr. L.H. Lash
Contributor Information
Ruth Dempsey, Email: ruth.dempsey@bluewin.ch.
Gregory Rodrigo, Email: gregory.rodrigo@pmi.com.
Data availability
Data will be made available on request.
References
- 1.Auer R., Concha-Lozano N., Jacot-Sadowski I., Cornuz J., Berthet A. Heat-not-burn tobacco cigarettes: smoke by any other name. JAMA Intern. Med. 2017;177(7):1050–1052. doi: 10.1001/jamainternmed.2017.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banks E., Martin M., Harris M. Framework for the public health assessment of electronic cigarettes. Tob. Control. 2021 doi: 10.1136/tobaccocontrol-2020-056271. [DOI] [PubMed] [Google Scholar]
- 3.Bekki K., Inaba Y., Uchiyama S., Kunugita N. Comparison of chemicals in mainstream smoke in heat-not-burn tobacco and combustion cigarettes. J. UOEH. 2017;39(3):201–207. doi: 10.7888/juoeh.39.201. [DOI] [PubMed] [Google Scholar]
- 4.Berman M.L., Connolly G., Cummings M.K., et al. Providing a science base for the evaluation of tobacco products. Tob. Regul. Sci. 2015;1(1):76–93. doi: 10.18001/TRS.1.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertrand P., Bonnarme V., Piccirilli A., et al. Physical and chemical assessment of 1,3 Propanediol as a potential substitute of propylene glycol in refill liquid for electronic cigarettes. Sci. Rep. 2018;8(1):10702. doi: 10.1038/s41598-018-29066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bos P.M.J., Soeteman-Hernández L.G., Talhout R. Risk assessment of components in tobacco smoke and e-cigarette aerosols: a pragmatic choice of dose metrics. Inhal. Toxicol. 2021:1–15. doi: 10.1080/08958378.2021.1909678. [DOI] [PubMed] [Google Scholar]
- 7.Boué S., Goedertier D., Hoeng J., et al. State-of-the-art methods and devices for the generation, exposure, and collection of aerosols from heat-not-burn tobacco products. Toxicol. Res. Appl. 2020;4:1–40. doi: 10.1177/2397847319897869. [DOI] [Google Scholar]
- 8.Camacho O.M., Ebajemito J.K., Coburn S., Prasad K., Costigan S., Murphy J.J. Evidence from the scientific assessment of electronic cigarettes and their role in tobacco harm reduction. Contrib. Tob. Nicotine Res. 2021;30(2):63–108. doi: 10.2478/cttr-2021-0007. [DOI] [Google Scholar]
- 9.Carthew P., Clapp C., Gutsell S. Exposure based waiving: the application of the toxicological threshold of concern (TTC) to inhalation exposure for aerosol ingredients in consumer products. Food Chem. Toxicol. 2009;47(6):1287–1295. doi: 10.1016/j.fct.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 10.Caruso M., Emma R., Rust S., et al. Screening of different cytotoxicity methods for the assessment of ENDS toxicity relative to tobacco cigarettes. Regul. Toxicol. Pharmacol. 2021:125. doi: 10.1016/j.yrtph.2021.105018. [DOI] [PubMed] [Google Scholar]
- 11.Costigan S., Meredith C. An approach to ingredient screening and toxicological risk assessment of flavours in e-liquids. Regul. Toxicol. Pharmacol. 2015;72(2):361–369. doi: 10.1016/j.yrtph.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 12.Counts M., Morton M., Laffoon S., Cox R., Lipwicz P. Smoke composition and predicting relationships for international commercial cigarettes smoked with three machine-smoking conditions. Regul. Toxicol. Pharmacol. 2005;41:185–227. doi: 10.1016/j.yrtph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Davis B., Williams M., Talbot P. iQOS: evidence of pyrolysis and release of a toxicant from plastic. Tob. Control. 2019;28(1):34–41. doi: 10.1136/tobaccocontrol-2017-054104. [DOI] [PubMed] [Google Scholar]
- 14.Dempsey R., Coggins C.R.E., Roemer E. Toxicological assessment of cigarette ingredients. Regul. Toxicol. Pharmacol. 2011;61(1):119–128. doi: 10.1016/j.yrtph.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Dusautoir R., Zarcone G., Verriele M., et al. Comparison of the chemical composition of aerosols from heated tobacco products, electronic cigarettes and tobacco cigarettes and their toxic impacts on the human bronchial epithelial BEAS-2B cells. J. Hazard. Mater. 2021:401. doi: 10.1016/j.jhazmat.2020.123417. [DOI] [PubMed] [Google Scholar]
- 16.Eaton D., Jakaj B., Forster M., et al. Assessment of tobacco heating product THP1.0. Part 2: product design, operation and thermophysical characterisation. Regul. Toxicol. Pharmacol. 2018;93:4–13. doi: 10.1016/j.yrtph.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Erythropel H.C., Jabba S.V., DeWinter T.M., et al. Formation of flavorant-propylene glycol adducts with novel toxicological properties in chemically unstable e-cigarette liquids. Nicotine Tob. Res. 2019;21(9):1248–1258. doi: 10.1093/ntr/nty192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Escher S.E., Tluczkiewicz I., Batke M., et al. Evaluation of inhalation TTC values with the database RepDose. Regul. Toxicol. Pharmacol. 2010;58(2):259–274. doi: 10.1016/j.yrtph.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 19.European Chemical Agency (2012) Guidance on information requirements and chemical safety assessment Chapter R.8: Characterisation of dose (concentration)-response for human health. European Chemicals Agency, Finland.
- 20.European Union (2014) Directive on the approximation of the laws, regulations and administrative provisions of the Member States concerning the manufacture, presentation and sale of tobacco and related products. In: Official Journal of the European Union (ed). vol Directive 2014/40/EU,
- 21.Farsalinos K.E., Yannovits N., Sarri T., Voudris V., Poulas K. Nicotine delivery to the aerosol of a heat-not-burn tobacco product: comparison with a tobacco cigarette and e-cigarettes. Nicotine Tob. Res. 2017;20(8):1004–1009. doi: 10.1093/ntr/ntx138. [DOI] [PubMed] [Google Scholar]
- 22.Farsalinos K.E., Yannovits N., Sarri T., Voudris V., Poulas K., Leischow S.J. Carbonyl emissions from a novel heated tobacco product (IQOS): comparison with an e-cigarette and a tobacco cigarette. Addiction. 2018;113(11):2099–2106. doi: 10.1111/add.14365. [DOI] [PubMed] [Google Scholar]
- 23.Flora J., Digard H., Sinclair C., Belushkin M. (2020) Heated Tobacco Products (HTPs): Standardized Terminology and Recommendations for the Generation and Collection of Emissions. CORESTA Technical Report. CORESTA.
- 24.Forster M., Fiebelkorn S., Yurteri C., et al. Assessment of novel tobacco heating product THP1.0. Part 3: comprehensive chemical characterisation of harmful and potentially harmful aerosol emissions. Regul. Toxicol. Pharmacol. 2018;93:14–33. doi: 10.1016/j.yrtph.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Gaur S., Agnihotri R. Health effects of trace metals in electronic cigarette aerosols-a systematic review. Biol. Trace Elem. Res. 2019;188(2):295–315. doi: 10.1007/s12011-018-1423-x. [DOI] [PubMed] [Google Scholar]
- 26.Geiss O., Bianchi I., Barrero-Moreno J. Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours. Int. J. Hyg. Environ. Health. 2016;219(3):268–277. doi: 10.1016/j.ijheh.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Gillman I.G., Pennington A.S.C., Humphries K.E., Oldham M.J. Determining the impact of flavored e-liquids on aldehyde production during Vaping. Regul. Toxicol. Pharmacol. 2020;112 doi: 10.1016/j.yrtph.2020.104588. [DOI] [PubMed] [Google Scholar]
- 28.Godec T.L., Crooks I., Scott K., Meredith C. In vitro mutagenicity of gas-vapour phase extracts from flavoured and unflavoured heated tobacco products. Toxicol. Rep. 2019;6:1155–1163. doi: 10.1016/j.toxrep.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hatsukami D.K., Biener L., Leischow S.J., Zeller M.R. Tobacco and nicotine product testing. Nicotine Tob. Res. 2012;14(1):7–17. doi: 10.1093/ntr/ntr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Health Canada (2020) Health Canada: Tobacco and Vaping Products Act: An Act to regulate the manufacture, sale, labelling and promotion of tobacco products and vaping products. In: Justice CMo (ed) SC, 1997, c 13.
- 31.Ho J., Sciuscio D., Kogel U., et al. Evaluation of toxicity of aerosols from flavored e-liquids in Sprague–Dawley rats in a 90-day OECD inhalation study, complemented by transcriptomics analysis. Arch. Toxicol. 2020;94(6):2179–2206. doi: 10.1007/s00204-020-02759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Institute for Global Tobacco Control (2020) Countries that Regulate Heated Tobacco. In: Johns Hopkins, Bloomberg School of Public Health,. https://globaltobaccocontrol.org/en/resources/countries-regulate-heated-tobacco Accessed 2021–08-11 2021.
- 33.IOM (Institute of Medicine) The National Academies Press; Washington, D.C: 2012. Scientific Standards for Studies on Modified Risk Tobacco Products. [Google Scholar]
- 34.Jaccard G., Tafin Djoko D., Moennikes O., Jeannet C., Kondylis A., Belushkin M. Comparative assessment of HPHC yields in the Tobacco Heating System THS2.2 and commercial cigarettes. Regul. Toxicol. Pharmacol. 2017;90:1–8. doi: 10.1016/j.yrtph.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Jaunky T., Adamson J., Santopietro S., et al. Assessment of tobacco heating product THP1.0. Part 5: in vitro dosimetric and cytotoxic assessment. Regul. Toxicol. Pharmacol. 2018;93:52–61. doi: 10.1016/j.yrtph.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 36.Jin L., Lynch J., Richardson A., et al. Electronic cigarette solvents, pulmonary irritation, and endothelial dysfunction: role of acetaldehyde and formaldehyde. Am. J. Physiol. Heart Circ. Physiol. 2021;320(4):H1510–h1525. doi: 10.1152/ajpheart.00878.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jordan K., Wieczorek R., Moennikes O., et al. (2019) The CORESTA In Vitro Toxicity Testing Sub-Group: The Rationale and Strategy for In Vitro toxicity Testing of Combustible Tobacco Products CORESTA.
- 38.Kaur G., Pinkston R., McLemore B., Dorsey W.C., Batra S. Immunological and toxicological risk assessment of e-cigarettes. Eur. Respir. Rev. 2018;27(147) doi: 10.1183/16000617.0119-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X., Luo Y., Jiang X., et al. Chemical analysis and simulated pyrolysis of tobacco heating system 2.2 compared to conventional cigarettes. Nicotine Tob. Res. 2018;21(1):111–118. doi: 10.1093/ntr/nty005. [DOI] [PubMed] [Google Scholar]
- 40.Lovsin Barle E., Winkler G.C., Glowienke S., Elhajouji A., Nunic J., Martus H.J. Setting occupational exposure limits for genotoxic substances in the pharmaceutical industry. Toxicol. Sci. 2016;151(1):2–9. doi: 10.1093/toxsci/kfw028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maeder S., Peitsch M.C. (2018) Response to Davis et al. "IQOS: evidence of pyrolysis and release of a toxicant from plastic.": experimental demonstration of the absence of formaldehyde cyanohydrin emission from PLA using a reference standard. Tobacco Control 28(1). [DOI] [PubMed]
- 42.Mallock N., Böss L., Burk R., et al. Levels of selected analytes in the emissions of “heat not burn” tobacco products that are relevant to assess human health risks. Arch. Toxicol. 2018;92(6):2145–2149. doi: 10.1007/s00204-018-2215-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Margham J., McAdam K., Forster M., et al. Chemical composition of aerosol from an e-cigarette: a quantitative comparison with cigarette smoke. Chem. Res. Toxicol. 2016;29(10):1662–1678. doi: 10.1021/acs.chemrestox.6b00188. [DOI] [PubMed] [Google Scholar]
- 44.McAdam K., Davis P., Ashmore L., et al. Influence of machine-based puffing parameters on aerosol and smoke emissions from next generation nicotine inhalation products. Regul. Toxicol. Pharmacol. 2019;101:156–165. doi: 10.1016/j.yrtph.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 45.McNeill A., Brose L.S., Calder R., Bauld L., Robson D. Evidence review of e-cigarettes and heated tobacco products 2018: a report commissioned by Public Health England. 2018:2018. [Google Scholar]
- 46.Murphy J., Gaca M., Lowe F., et al. Assessing modified risk tobacco and nicotine products: description of the scientific framework and assessment of a closed modular electronic cigarette. Regul. Toxicol. Pharmacol. 2017;90:342–357. doi: 10.1016/j.yrtph.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 47.Oviedo A., Lebrun S., Kogel U., et al. Evaluation of the Tobacco Heating System 2.2. Part 6: 90-day OECD 413 rat inhalation study with systems toxicology endpoints demonstrates reduced exposure effects of a mentholated version compared with mentholated and non-mentholated cigarette smoke. Regul. Toxicol. Pharmacol. 2016;81:S93–S122. doi: 10.1016/j.yrtph.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Pacitto A., Stabile L., Scungio M., Rizza V., Buonanno G. Characterization of airborne particles emitted by an electrically heated tobacco smoking system. Environ. Pollut. 2018;240:248–254. doi: 10.1016/j.envpol.2018.04.137. [DOI] [PubMed] [Google Scholar]
- 49.Philip Morris International (2021) OUR SCIENCE: Heated tobacco products: How are they different from and similar to, e-cigarettes? In. https://www.pmi.com/our-science/difference-between-heated-tobacco-products-and-ecigarettes Accessed 2021–12-15 2021.
- 50.Phillips B., Szostak J., Titz B., et al. A six-month systems toxicology inhalation/cessation study in ApoE−/− mice to investigate cardiovascular and respiratory exposure effects of modified risk tobacco products, CHTP 1.2 and THS 2.2, compared with conventional cigarettes. Food Chem. Toxicol. 2019;126:113–141. doi: 10.1016/j.fct.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 51.Phillips B., Titz B., Kogel U., et al. Toxicity of the main electronic cigarette components, propylene glycol, glycerin, and nicotine, in Sprague-Dawley rats in a 90-day OECD inhalation study complemented by molecular endpoints. Food Chem. Toxicol. 2017;109(Pt 1):315–332. doi: 10.1016/j.fct.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 52.Poynton S., Sutton J., Goodall S., et al. A novel hybrid tobacco product that delivers a tobacco flavour note with vapour aerosol (Part 1): product operation and preliminary aerosol chemistry assessment. Food Chem. Toxicol. 2017;106:522–532. doi: 10.1016/j.fct.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 53.Salman R., Talih S., El-Hage R., et al. Free-base and total nicotine, reactive oxygen species, and carbonyl emissions from IQOS, a heated tobacco product. Nicotine Tob. Res. 2019;21(9):1285–1288. doi: 10.1093/ntr/nty235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Savareear B., Escobar-Arnanz J., Brokl M., et al. Comprehensive comparative compositional study of the vapour phase of cigarette mainstream tobacco smoke and tobacco heating product aerosol. J. Chromatogr. A. 2018;1581–1582:105–115. doi: 10.1016/j.chroma.2018.10.035. [DOI] [PubMed] [Google Scholar]
- 55.Savareear B., Escobar-Arnanz J., Brokl M., et al. Non-targeted analysis of the particulate phase of heated tobacco product aerosol and cigarette mainstream tobacco smoke by thermal desorption comprehensive two-dimensional gas chromatography with dual flame ionisation and mass spectrometric detection. J. Chromatogr. A. 2019;1603:327–337. doi: 10.1016/j.chroma.2019.06.057. [DOI] [PubMed] [Google Scholar]
- 56.Schaller J.P., Keller D., Poget L., et al. Evaluation of the Tobacco Heating System 2.2. Part 2: chemical composition, genotoxicity, cytotoxicity, and physical properties of the aerosol. Regul. Toxicol. Pharmacol. 2016;81(Suppl 2):S27–S47. doi: 10.1016/j.yrtph.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 57.Schaller J.P., Pijnenburg J.P.M., Ajithkumar A., Tricker A.R. Evaluation of the Tobacco Heating System 2.2. Part 3: influence of the tobacco blend on the formation of harmful and potentially harmful constituents of the Tobacco Heating System 2.2 aerosol. Regul. Toxicol. Pharmacol. 2016;81(Suppl 2):S48–S58. doi: 10.1016/j.yrtph.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 58.Schorp M.K., Tricker A.R., Dempsey R. Reduced exposure evaluation of an Electrically Heated Cigarette Smoking System. Part 1: non-clinical and clinical insights. Regul. Toxicol. Pharmacol. 2012;64(2Suppl 1):S1–S10. doi: 10.1016/j.yrtph.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 59.Smith M.R., Clark B., Lüdicke F., et al. Evaluation of the Tobacco Heating System 2.2. Part 1:description of the system and the scientific assessment program. Regul. Toxicol. Pharmacol. 2016;81(Suppl 2):S17–S26. doi: 10.1016/j.yrtph.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 60.St Hilaire C.L., Scientific Methods to Evaluate Potential Reduced-Risk Tobacco Products: Executive Summary, 2007. In. http://www.lsro.org/articles/rrrvw_report_042407_execsum.pdf Accessed 2022–04-26.
- 61.Stratton K., Shetty P., Wallace R., Bondurant S. Clearing the smoke: the science base for tobacco harm reduction--executive summary. Tob. Control. 2001;10(2):189–195. doi: 10.1136/tc.10.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takahashi Y., Kanemaru Y., Fukushima T., et al. Chemical analysis and in vitro toxicological evaluation of aerosol from a novel tobacco vapor product: a comparison with cigarette smoke. Regul. Toxicol. Pharmacol. RTP. 2018;92:94–103. doi: 10.1016/j.yrtph.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 63.Thorne D., Breheny D., Proctor C., Gaca M. Assessment of novel tobacco heating product THP1.0. Part 7: comparative in vitro toxicological evaluation. Regul. Toxicol. Pharmacol. 2018;93:71–83. doi: 10.1016/j.yrtph.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 64.Thorne D., Leverette R., Breheny D., et al. Genotoxicity evaluation of tobacco and nicotine delivery products: Part One. Mouse lymphoma assay. Food Chem. Toxicol. 2019:132. doi: 10.1016/j.fct.2019.110584. [DOI] [PubMed] [Google Scholar]
- 65.Thorne D., Leverette R., Breheny D., et al. Genotoxicity evaluation of tobacco and nicotine delivery products: Part Two. In vitro micronucleus assay. Food Chem. Toxicol. 2019:132. doi: 10.1016/j.fct.2019.05.054. [DOI] [PubMed] [Google Scholar]
- 66.Titz B., Kogel U., Martin F., et al. A 90-day OECD TG 413 rat inhalation study with systems toxicology endpoints demonstrates reduced exposure effects of the aerosol from the carbon heated tobacco product version 1.2 (CHTP1.2) compared with cigarette smoke. II. Systems toxicology assessment. Food Chem. Toxicol. 2018;115:284–301. doi: 10.1016/j.fct.2018.02.058. [DOI] [PubMed] [Google Scholar]
- 67.U.S. Food and Drug Administration (2009) Family Smoking Prevention and Tobacco Control Act - an Overview. In: US FDA. https://www.fda.gov/tobacco-products/rules-regulations-and-guidance/family-smoking-prevention-and-tobacco-control-act-overview Accessed 2021–08-12.
- 68.U.S. Food and Drug Administration (2012) Guidance for Industry: Modified Risk Tobacco Product Applications. (Draft Guidance). In: US FDA. https://www.fda.gov/media/83300/download Accessed 2022–04-25.
- 69.U.S. Food and Drug Administration (2019) Premarket Tobacco Product Applications for Electronic Nicotine Delivery Systems (ENDS). In: FDA. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/premarket-tobacco-product-applications-electronic-nicotine-delivery-systems-ends Accessed 2021–05-05.
- 70.U.S. Food and Drug Administration (2020) Scientific Review of Modified Risk Tobacco Product Application (MRTPA) Under Section 911(d) of the FD&C Act - Technical Project Lead. In. https://www.fda.gov/media/139796/download Accessed 2021–10-06.
- 71.Vu A.T., Taylor K.M., Holman M.R., Ding Y.S., Hearn B., Watson C.H. Polycyclic aromatic hydrocarbons in the mainstream smoke of popular U.S. cigarettes. Chem. Res Toxicol. 2015;28(8):1616–1626. doi: 10.1021/acs.chemrestox.5b00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang G., Liu W., Song W. Toxicity assessment of electronic cigarettes. Inhal. Toxicol. 2019;31(7):259–273. doi: 10.1080/08958378.2019.1671558. [DOI] [PubMed] [Google Scholar]
- 73.WHO Scientific Advisory Committee on Tobacco Product Regulation . World Health Organisation (WHO); Geneva, Switzerland: 2003. Statement of Principles Guiding the Evaluation of New or Modified Tobacco Products. [Google Scholar]
- 74.WHO study group on tobacco product regulation (2021) Report on the scientific basis of tobacco product regulation: Eighth report of a WHO study group. WHO Technical Report Series. [PubMed]
- 75.Wong E.T., Kogel U., Veljkovic E., et al. Evaluation of the Tobacco Heating System 2.2. Part 4: 90-day OECD 413 rat inhalation study with systems toxicology endpoints demonstrates reduced exposure effects compared with cigarette smoke. Regul. Toxicol. Pharmacol. 2016;81:S59–S81. doi: 10.1016/j.yrtph.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 76.Zenzen V., Diekmann J., Gerstenberg B., Weber S., Wittke S., Schorp M.K. (2012) Reduced exposure evaluation of an Electrically Heated Cigarette Smoking System. Part 2: smoke chemistry and in vitro toxicological evaluation using smoking regimens reflecting human puffing behavior. Regul. Toxicol. Pharmacol. 64(2 Suppl 1) S11-S34 doi:https://doi.org/10.1016/j.yrtph.2012.08.004. [DOI] [PubMed]
- 77.Zhao J., Nelson J., Dada O., Pyrgiotakis G., Kavouras I.G., Demokritou P. Assessing electronic cigarette emissions: linking physico-chemical properties to product brand, e-liquid flavoring additives, operational voltage and user puffing patterns. Inhal. Toxicol. 2018;30(2):78–88. doi: 10.1080/08958378.2018.1450462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.