TO THE EDITOR:
Anaplastic large T-cell lymphoma (ALCL) is an aggressive disease that presents in pediatric or adult patients with advanced stage disease, extranodal involvement, and constitutional symptoms.1 Approximately 50% of ALCLs are characterized by constitutive activity of the anaplastic lymphoma kinase (ALK) gene because of chromosomal translocations, most frequently a t(2;5) leading to an NPM1-ALK gene fusion.2 ALK alterations can be detected also in other lymphoma histotypes, histiocytosis, and nonhematological cancers.2,3 In rare cases, ALK fusions with NPM1 or other partners can be driver events also in diffuse large B-cell lymphoma (DLBCL) and plasmablastic lymphoma (PBL),4,5 but the optimal management of these rare diseases is unknown. In ALK+ ALCLs, anthracycline-based chemotherapy regimens are associated with an overall response rate (ORR) of 80% to 85%1 and a 3-year overall survival (OS) of 82% is reported in patients attaining complete remission (CR). However, more than 50% of patients relapse and need further therapies.6 Platinum-based chemotherapy and the anti-CD30 immunoconjugate brentuximab vedotin (BV) achieve CR in 50% to 60% of cases in patients with relapsed/refractory (R/R) disease.1,6, 7, 8 However, 75% of patients treated with BV develop severe peripheral neuropathy that requires discontinuing treatment.8 Nevertheless, a significant proportion of patients eventually relapse, even after autologous stem cells transplantation (SCT), with rapidly progressive disease and death. Crizotinib is an ALK inhibitor that showed a rapid antitumoral effect on ALK+ ALCLs with acceptable toxicity and a 2-year OS and PFS of 66% and 65%9 and was recently approved by the US Food and Drug Administration in ALK+ ALCL in pediatric and young adult patients; in Italy, crizotinib obtained reimbursement for all ALK+ ALCL, regardless of age, in 2022. Our center at Monza’s San Gerardo Hospital has been using crizotinib for the treatment of ALK+ lymphomas since 2010. We present here a long-term efficacy and safety analysis of crizotinib in the 27 patients affected by ALK+ lymphomas at this center.
We collected baseline clinical, radiological, molecular data on 27 patients with ALK+ lymphomas relapsed/refractory to at least 1 prior chemotherapy. The study population included patients who received at least 1 dose of crizotinib at our institution: 2 patients received crizotinib for compassionate use, 24 patients were enrolled in 2 different phase 2 studies from 2010 to 2019 (NCT02419287 and EudraCT 2010-022978-14), and 3 patients were treated outside protocols. Radiologic response was assessed through full-body computed tomography/positron emission tomography scan or full body computed tomography scan at 4 weeks and 12 weeks after crizotinib start, and then every 12 weeks. Radiological response was evaluated according to Response Evaluation Criteria in Lymphoma 2017.10 Molecular response was evaluated through qualitative real-time polymerase chain reaction (RT-PCR) assay for NPM-ALK transcript in peripheral blood after 4 and 12 weeks of therapy, and then every 12 weeks. ORR, defined as the achievement of CR or partial remission after crizotinib initiation, median follow-up (FU), PFS, OS, and crizotinib’s toxicity profile were evaluated according to an intention-to-treat strategy. Descriptive statistics were used to summarize patient characteristics, treatment administration, antitumor activity, and safety. PFS and OS were calculated using GraphPad Prism 8 (GraphPad Software, San Diego, CA). Additional information about patients and pathological and molecular diagnosis is reported in supplemental materials. San Gerardo Hospital (ASST Monza) provided the institutional review board approval to our study in accordance with the Declaration of Helsinki.
Twenty-five patients were affected by ALK+ ALCL, 1 by ALK+ DLBCL, and 1 by ALK+ PBL. Median age at diagnosis was 24 years (range, 15-82). Twenty-one patients had advanced stage disease (3-4) at diagnosis, no patient had active central nervous system disease before starting crizotinib. Median previous lines of chemotherapy were 2 (range, 1-6). Six patients were treated with BV before crizotinib start. Seven patients underwent autologous SCT, and 1 patient underwent allogeneic SCT before crizotinib. Patients’ baseline characteristics are summarized in supplemental Table 1.
Crizotinib was started at standard dose of 250 mg twice per day, by oral administration. One patient died of septic shock 4 hours after the first dosing. Only 1 patient developed signs of tumor lysis syndrome a few days from crizotinib start.
Of the 27 patients analyzed, 15 (56%; 95% confidence interval [CI], 37-72) are still alive today in radiological and molecular CR on crizotinib treatment, 1 (3%) received allogeneic transplantation after 2 months of therapy and thus was censored from further survival analyses, and 11 (41%; 95% CI, 24-59) eventually died of progressive disease.
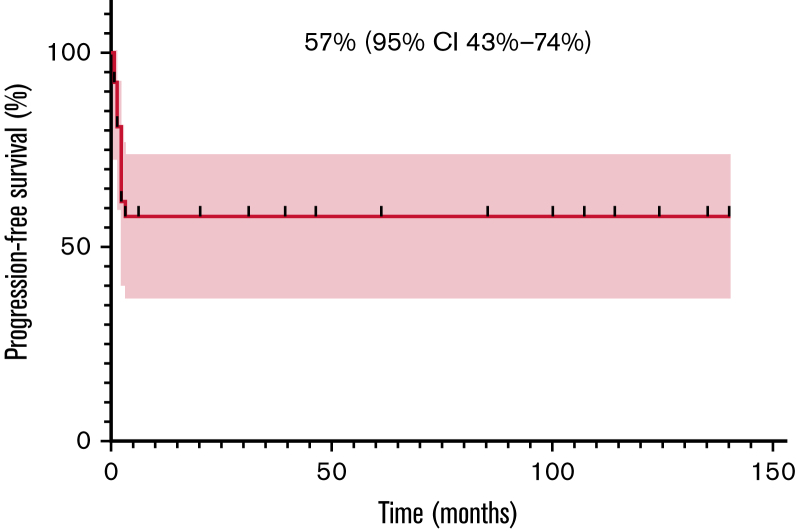
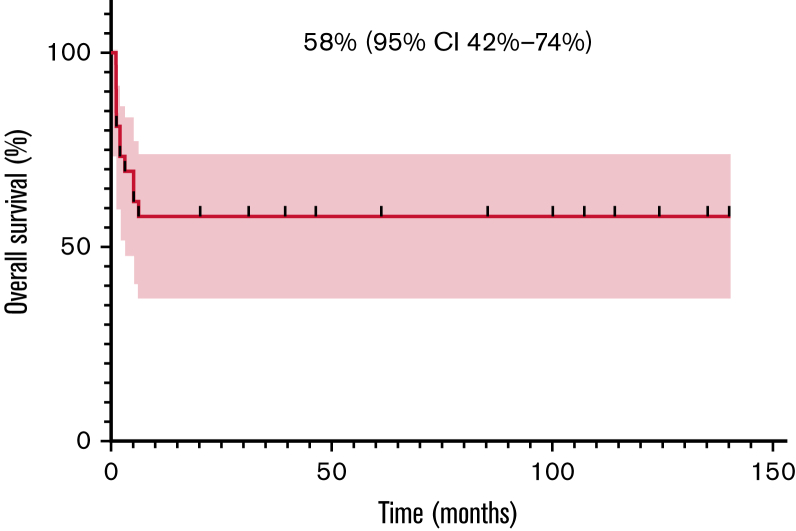
Among 25 evaluable patients, ORR after 1 month of therapy was 80% (20/25; 95% CI, 61-91). Relapses always presented within 3 months from treatment start, as previously reported.9 Most of these patients had a very high tumor burden at baseline and were in poor clinical conditions. Six of the 11 patients who eventually died obtained an initial but transient radiological response and then progressed. Three patients developed central nervous system (CNS) disease, isolated in 2 cases and in the context of systemic relapse in 1 case. The patient affected by DLBCL was treated with crizotinib for 2 months, but eventually progressed and died. The patient affected by PBL achieved a prompt CR and is still on treatment after 124 months. Two-year PFS and OS were 57% (95% CI, 36-74) and 58% (95% CI, 37-74), respectively. The same value is calculated at the last available follow-up since all progressions developed within 3 months and all deaths happened within 6 months after crizotinib start (Figures 1 and 2).
Figure 1.
Progression-free survival (PFS) in 27 R/R ALK ± patients with lymphoma.
Figure 2.
Overall survival (OS) in 27 R/R ALK ± patients with lymphoma.
Twenty-five patients were evaluable for molecular assessment at baseline: 7 patients (28%; 95% CI, 14-48) were negative and 18 patients (72%; 95% CI, 52-85) were positive by RT-PCR for the known ALK fusion transcripts. Among the 18 patients with a positive RT-PCR before treatment, 10 (56%; 95% CI, 33-76) converted to negativity after 1 month of therapy. Only 1 patient with negative PCR at the 1-month assessment eventually progressed. On the contrary, 5 of 7 patients with positive RT-PCR at the 1-month assessment eventually progressed. After 3 months of therapy, 9 patients had a negative RT-PCR (50%; 95% CI, 29-71) and are still in stable CR. On the contrary, 4 patients had a positive RT-PCR after 3 months of therapy: 3 were in overt clinical and radiological progression and 1 patient converted to negative PCR after 6 months of therapy and is still in CR. This confirms that molecular assessment in peripheral blood is a reliable method to predict long-term survival and to monitor.11 Data on crizotinib radiological and molecular response are summarized in supplemental Table 2.
Ten of 27 patients (37%; 95% CI, 22-56) developed a drug-related hematological adverse event (AE), particularly neutropenia (2 patients G1/2, 8 patients G3/4). Most of these neutropenias resolved within few weeks, spontaneously or with a short course of oral steroids because they can increase neutrophil count. Neutropenia usually developed within 6 months from crizotinib start, probably representing a late effect of cytotoxic treatment rather than a direct effect of crizotinib. A drug-induced neutropenia mediated by the immune system in patients treated with ALK inhibitors was hypothesized in some studies. Short courses of steroids resulted in complete resolution of neutropenia in these cases.12,13 Only 2 late neutropenia were reported 8 and 60 months after therapy introduction; these 2 cases required temporary therapy interruption. The most common nonhematological AEs were gastrointestinal disturbances (nausea and/or vomiting), visual disturbances, peripheral edema, paraesthesia, muscle cramps, mostly G1/2, usually within few weeks from treatment introduction and self-limiting. Some patients developed transient G3/4 elevation of creatinine kinase and transaminases of unknown relationship with crizotinib, requiring a temporary therapy interruption. The most frequently reported long-term AEs are peripheral edema, abdominal disturbances, usually well-controlled with symptomatic therapy or with dose reductions. At the current time, no patients discontinued crizotinib for any AE. Six patients are assuming a dose of 250 mg/d, 5 patients are at 400 mg/d, and 4 patients remain on the starting dose of 500 mg/d of crizotinib. More data about adverse events and dose reductions are listed in detail in supplemental Table 3.
Overall, by considering the whole cohort, analyzed using intention-to-treat strategy, the median FU is 31 months (range, 0-140). However, by considering only alive patients on crizotinib, the median FU is 61 months (range, 6-140); this represents to our knowledge the longest follow-up available in the literature.
The results of this study indicate that crizotinib represents an effective therapeutic option for long term therapy of patients with R/R ALK+ aggressive lymphomas, a condition that generally carries a very poor prognosis. The present results represent the longest follow-up to our knowledge. Crizotinib showed a good tolerability profile, with only mild collateral effects, easily manageable with symptomatic therapies short treatment interruptions or dose reductions down to 250 mg/d. This dosage was tried in 6 patients and no disease relapse was observed. This is particularly important, as duration of treatment is still unknown and some cases of early and abrupt disease relapses after crizotinib discontinuation were reported.14 Further studies are needed to understand if it will be possible to interrupt crizotinib therapy permanently.
As we previously observed, patients who developed early crizotinib molecular response then obtained stable long-term remission, whereas patients who eventually progressed and died generally did not obtained early molecular remission. The introduction of crizotinib early in the treatment appears to increase the possibility to achieve an early radiological and molecular response that is predictive of long-term remission.
In conclusion, crizotinib was able to obtain durable remissions of R/R ALK+ lymphomas in almost 2/3 of treated patients, with negligible adverse events. Future directions of research will involve either the combination of crizotinib with other drugs, the use of more potent ALK inhibitors such as lorlatinib (NCT03505554), and the early use of crizotinib to treat this condition. In our series, 3 patients developed CNS relapse. Because other ALK inhibitors, such as alectinib and lorlatinib, have typically a better penetration in CNS, they might be considered to treat patients with CNS relapse. Specific studies are needed to establish the potential role of crizotinib as first-line treatment for ALK+ lymphomas.
Conflict-of-interest disclosure: The authors declare no competing financial conflicts.
Acknowledgments
Acknowledgments: This work was supported by Fondazione AIRC per la Ricerca sul Cancro (grant IG 2017 Id.20112 project; principal investigator, Gambacorti-Passerini Carlo) and by National Institutes of Health, National Cancer Institute grant 5R01CA196703 (to R.C.).
Contribution: G.R., E.B., A.A., L.V., G.Z., S.T., R.P., L.M., R.C., and C.G.-P. collected data; G.R. analyzed results and made the figures; and G.R. and C.G.-P. designed the research and wrote the paper.
Footnotes
For original data, please contact Carlo Gambacorti Passerini (carlo.gambacorti@unimib.it).
The full-text version of this article contains a data supplement.
Contributor Information
Giovanni Rindone, Email: g.rindone1@campus.unimib.it.
Carlo Gambacorti-Passerini, Email: carlo.gambacorti@unimib.it.
Supplementary Material
References
- 1.Shustov A, Soma L. Anaplastic large cell lymphoma: contemporary concepts and optimal management. Cancer Treat Res. 2019;176:127–144. doi: 10.1007/978-3-319-99716-2_6. [DOI] [PubMed] [Google Scholar]
- 2.Andraos E, Dignac J, Meggetto F. NPM-ALK: a driver of lymphoma pathogenesis and a therapeutic target. Cancers (Basel) 2021;13(1):1–17. doi: 10.3390/cancers13010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang KTE, Tay AZE, Kuick CH, et al. ALK-positive histiocytosis: an expanded clinicopathologic spectrum and frequent presence of KIF5B-ALK fusion. Mod Pathol. 2019;32(5):598–608. doi: 10.1038/s41379-018-0168-6. [DOI] [PubMed] [Google Scholar]
- 4.De Paepe P, Baens M, van Krieken H, et al. ALK activation by the CLTC-ALK fusion is a recurrent event in large B-cell lymphoma. Blood. 2003;102(7):2638–2641. doi: 10.1182/blood-2003-04-1050. [DOI] [PubMed] [Google Scholar]
- 5.Onciu M, Behm FG, Downing JR, et al. ALK-positive plasmablastic B-cell lymphoma with expression of the NPM-ALK fusion transcript: report of 2 cases. Blood. 2003;102(7):2642–2644. doi: 10.1182/blood-2003-04-1095. [DOI] [PubMed] [Google Scholar]
- 6.Gritti G, Boschini C, Rossi A, et al. Primary treatment response rather than front line stem cell transplantation is crucial for long term outcome of peripheral T-cell lymphomas. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0121822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horwitz S, O’Connor OA, Pro B, et al. ECHELON-2 Study Group Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet. 2019;393(10168):229–240. doi: 10.1016/S0140-6736(18)32984-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broccoli A, Pellegrini C, Di Rocco A, et al. Italian real-life experience with brentuximab vedotin: results of a large observational study of 40 cases of relapsed/refractory systemic anaplastic large cell lymphoma. Haematologica. 2017;102(11):1931–1935. doi: 10.3324/haematol.2017.171355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bossi E, Aroldi A, Brioschi FA, et al. Phase two study of crizotinib in patients with anaplastic lymphoma kinase (ALK)-positive anaplastic large cell lymphoma relapsed/refractory to chemotherapy. Am J Hematol. 2020;95(12):E319–E321. doi: 10.1002/ajh.25967. [DOI] [PubMed] [Google Scholar]
- 10.Younes A, Hilden P, Coffier B, et al. International Working Group consensus response evaluation criteria in lymphoma (RECIL 2017) Ann Oncol. 2017;28(7):1436–1447. doi: 10.1093/annonc/mdx097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damn Welk C, Mussolin L, Zimmermann M. Early assessment of minimal residual disease identifies patients at very high relapse risk in NPM-ALK positive anaplastic large cell lymphoma. Blood. 2014;123(3):334–337. doi: 10.1182/blood-2013-09-526202. [DOI] [PubMed] [Google Scholar]
- 12.Osugi J, Owada Y, Yamaura T, et al. Jun at al. Successful management of crizotinib-induced neutropenia in a patient with anaplastic lymphoma kinase-positive non-small cell lung cancer: a case report. Case Rep Oncol. 2016;9(1):51–55. doi: 10.1159/000443662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toyota Y, Sakugawa M, Bessho A, et al. Therapeutic use of low-dose corticosteroids to treat neutropenia resulting from crizotinib administration in a patient with anaplastic lymphoma kinase gene translocation-positive lung cancer. Haigan. 2014;54(2):78–83. [Google Scholar]
- 14.Gambacorti-Passerini C, Mussolin L, Brugieres L. Abrupt relapse of ALK-positive lymphoma after discontinuation of crizotinib. N Engl J Med. 2016;374(1):94–95. doi: 10.1056/NEJMc1511045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.