Abstract
This study examined the application of chemically synthesized apatite (CHAp) powder as a potential adsorbent for the elimination of Cd2+ in aqueous medium. The synthesized hydroxyapatite (HAp) powder before and after adsorption was elucidated by XRD, EDX, FT-IR, SEM, and TEM analytical techniques. The role of time, initial Cd2+ concentration, amount of CHAp used, temperature and solution pH on the adsorption process were investigated. Data from the adsorption process were subjected to Dubinin-Radushkevich, Langmuir, Freundlich, and Tempkin adsorption isotherms, while pseudo-first-order, pseudo-second-order, Elovich and intraparticle diffusion kinetic models were used for the kinetics investigation. Results from XRD confirmed that chief characteristic peaks of HAp powder were detected, while functional groups such as PO43−, CO32− and OH− matching pure HAp were displayed in the FT-IR spectra. Round shape morphology of the CHAp was confirmed by SEM and TEM analyses. Langmuir isotherm best described the adsorption process with ceiling adsorption capacity of 195.711 mg/g, whereas, the adsorption mechanism obeys the pseudo-first-order model which suggests a physical adsorption process. The value of entropy change (ΔS) of the adsorption of Cd2+ onto CHAp surface was obtained to be 0.610 kJ/mol, while the value of enthalpy change obtained was 175.591 kJ/mol. Results from free energy change obtained adjudged the adsorption process to be spontaneous and endothermic in character. Thus, the chemically synthesized HAp could be an excellent adsorbent for the elimination of Cd2+ in bioremediation applications.
Keywords: Adsorption, Isotherm, Equilibrium, Apatite, Kinetics, Cadmium
1. Introduction
The presence of huge amount of environmental contamination and pollution resulting from increased population and industrialization coupled with natural resources extraction is of a great concern with the deposition of toxic substances into contaminated sites and water across the universe [1]. Due to the adverse effect of toxicity and visibility of contaminated water, wastewater from dye industries had been the focal point in the field of wastewater management [2]. The release of these pollutants into the ecosystem endangers the human and animal health and also affects the general physiology of plants, as a result of their toxicity, mutagenicity, and their inability to be broken down into small components by biological actions [3].
Cadmium (Cd) which is without known biological function is considered to be one of the very toxic heavy metal. Cd presence as impurity in zinc galvanized pipes, water coolers and taps and water heaters are some of the contamination routs in drinking water [4]. The kidney has been reported as one of the organs that can accumulate Cd in the human body and this can lead to kidney malfunction [4,5]. Other chronic illnesses which could be associated with Cd exposure are neurodevelopmental disorder, and iron deficiency anaemia [5]. This had leads the US Environment Protection Agency to classified Cd into the group B1 carcinogen [4]. The ceiling amount of Cd expected in drinking water as recommended by World Health Organization and US EPA are 0.005 ppm and 0.005 ppm respectively [4,6].
Thus, the thorough screening of Cd in water purification is very essential and of a great importance.
Over the years, methods such as adsorption, coagulation by chemical agents, oxidations, ion-exchange, electrochemical method, membrane separation, and many more have been discussed for possible treatment of wastewater [2,7]. Reports have it that some of these methods are inefficient, very expensive to operate and do lead to the generation of secondary contaminants [2,7]. Among the available methods mentioned above, adsorption is widely acknowledged as the most economically and friendly favourable method for water treatment and this is in addition to its simple mode of operation [8,9]. Activated carbon is the widely-utilized adsorbent; however, it's expensive when compared with other adsorbents coupled with the fact that its usage depends largely on the availability of activated carbon and the degree of the required treatment process [10]. Furthermore, complexing agents which are also known as enhancer will be required to elevate the effectiveness of the activated carbon in elimination of inorganic pollutants [6].
Several adsorbents such as commercial activated carbon and cussel shells [8], calcined oyster shells [11], waste eggshell [12], modified maize straw [13], modified cantaloupe peel [14], orange peel derived biochar [15], aragonite shells-derived biosorbent [16], waste shells of golden apple snail [17] and orange peel [18] among others have been effectively deployed in the remediation of different kinds of pollutants in wastewater. Hydroxyapatite (HAp) is one of the apatite minerals constricting 60–70% of bone matrix containing high affinity for cations and thus enables it to be highly biocompatible, bioactive and as an adsorbent [9]. Besides, many applications of HAp such as bone and food supplements, drug delivery, carriers of genes, dental treatment, sorbent material in proteins and pollutants removal [[19], [20], [21], [22], [23]] have been well documented in literature. HAp was selected as adsorbent in this present work due to its well developed porous structure, low solubility, good ion-exchange ability and ease of preparation. In this study, our aim is to develop hydroxyapatite adsorbent for cadmium ion removal from aqueous solution. The HAp structure was analyzed with FT-IR, SEM, XRD, EDAX, and TEM. The effects of Cd2+ concentration, contact time, pH, and HAp quantity were investigated alongside with the equilibrium, kinetics, and thermodynamics properties.
2. Experimental
2.1. Preparation of the adsorbent
The chemically synthesized HAp was done by following Gross et al. [24] method with some adjustments. 0.1 M Ca(NO3)2∙6H2O was made and magnetically stirred for 10 min and added to 0.06 M solution of (NH4)H2PO4 in dropwise and keeping the pH at 11 with ammonia solution. The mixture was constantly stirred for 12 h, filtered and washed with deionized water. The resultant product was dried at 135 °C for 8 h and labeled as CHAp.
2.2. Characterization of adsorbent
A Hitachi, Japan, S–3000H model scanning electron microscope coupled with energy dispersive X-ray (EDX) was deployed for the elemental composition analysis of CHAp, while a PANalytical, X'Pert PRO, Netherland model of X-ray diffractometer was used to determine the phase purity and crystallinity in an incremental step size of 0.02 using CuKα (γ = 1.54178 °A) within the range of 10° to 60°. A TENSOR 27 Fourier transform infrared (Bruker, Germany) (FT-IR) was utilized for the functional group investigation from 400 to 4000 cm−1. A Tecnai 20 G2 FEI (Netherland) model of Transmission electron microscope (TEM) was used to further investigate the surface morphology of the adsorbent. The zero point charge (pHzpc) of CHAp was performed by using a Zetasizer Nano ZS instrument (Malvern, UK) from a solution of cadmium ion prepared by dissolving 0.1 g of CHAp in 25 ml of cadmium solution.
2.3. Cd2+ preparation
Cd2+ stock solutions were made from Cd(NO3)2. 4H2O (99%) obtained from Aldrich, Germany. 1 g of Cd(NO3)2. 4H2O was weighed and dissolved in 1 L de-ionized water and other standard solutions were thereafter made from this using dilution principle. In order to investigate the effect of solution pH on the adsorption process, 1.0 mol L−1 of HCl or NaOH solution was used to adjust the pH to a desired value prior to the addition of the metal ions.
2.3.1. Equilibrium study
Equilibrium study was performed in 250 ml Erlenmeyer flasks which consist of 25 mL of metal ions solution in the range of 40–240 mg/L and 0.004 g of CHAp. The contents were placed on a thermostated shaker at 150 rpm and 30 ± 1 °C for 120 min to attained equilibrium. A Varian Model SPECTRA 220 of Atomic Absorption Spectrophotometer was used to determine Cd(II) concentrations before and after adsorption process. The amount of Cd2+ sorbed by CHAp was obtained using equation (1) below:
| (1) |
The adsorbed amount of Cd2+ is denoted as Qe in mg/g, while the concentrations of Cd2+ (initial and equilibrium) in mg/L are given as Co and Ce respectively, volume (V) in L is the amount of Cd2+ used and the mass of the CHAp used is denoted as m in g.
2.4. Kinetics evaluation
The kinetics evaluations were performed at time intervals between 0 and 240 min. The quantity of the solutes adsorbed at certain t, Qt (mg g−1), was determined using equation (2) below:
| (2) |
Such that the liquid-phase initial concentration of Cd2+ is Co, (mg L−1) and Ct (mg L−1) is the amount of liquid-phase left at time t, volume V in L of the solution and the mass (g) of CHAp used is given as W.
2.5. Adsorption kinetics studies
Pseudo-first order, pseudo-second-order, Elovich and Intra-particle diffusion kinetic models as described by equations (3)–6) below were used for the evaluation of the data generated [[25], [26], [27]]:
| (3) |
| (4) |
| (5) |
| (6) |
All the parameters in these equations are defined by Adeogun et al. [25].
2.6. Statistical test
The sum square error function (SSE) was used to validate the bester fits between the first- and second-order models as given in equation (7):
| (7) |
2.7. Isotherms studies
Adsorption data generated the equilibrium investigation were analyzed with four common adsorption isotherm models namely, Langmuir, Freundlich, Dubinin–Radushkevich (D-R) and Tempkin isotherm as described in equations (8), (9), (10), (11) [25,[28], [29], [30], [31]].
2.8. Langmuir isotherms
| (8) |
2.9. Freundlich isotherm
| (9) |
2.10. Tempkin isotherm model
| (10) |
2.11. The Dubinin–Radushkevich isotherm
| (11) |
Details about the definitions of all the parameters are well documented by Adeogun et al. [25].
The adsorption's mean free energy per mole of the adsorbate is given in equation (12) as:
| (12) |
If E is within the range of 8 and 16 kJ/mol, it is chemisorption, but when the value is below 8 kJ/mol, it's a physical process [9,12].
2.12. Thermodynamics study
The distribution of the Cd2+ molecules in the solution at equilibrium is given in equation (13) as:
| (13) |
The equilibrium constant Kd is temperature dependent which was deployed to determine the thermodynamic parameters, ΔH◦, ΔS◦ and ΔG◦ using the equations listed in (14(14) & (15)(15) [9,12]:
| (14) |
| (15) |
2.13. Desorption studies
In other to assess the reusability of the prepared adsorbent, 0.1 L of 0.05 M HCl, 0.05 M NaOH, and distilled water were contacted with 0.2 g of the exhausted adsorbent and agitated for 100 min and thereafter analyzed using a AAS. Percentage desorption was computed using the equation below:
where Ca is the concentration of adsorbed pollutant (mg/g), and Cd is the concentration of the pollutant desorbed (mg/g).
3. Results and discussions
3.1. Characterizations
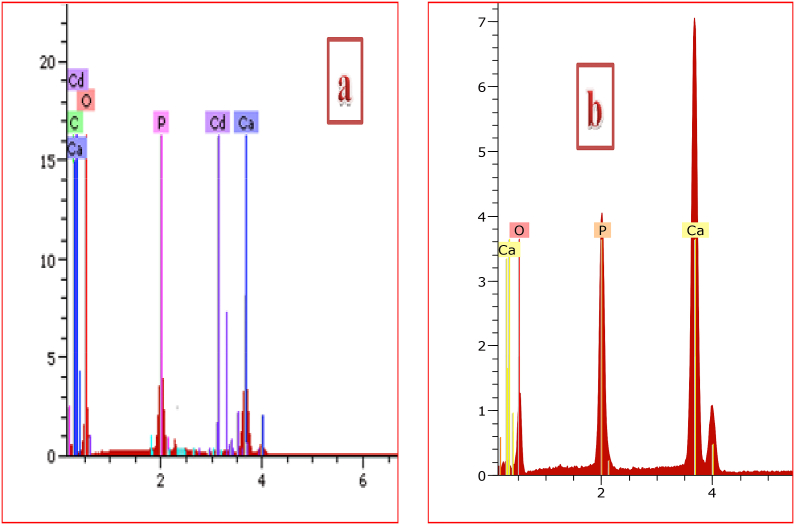
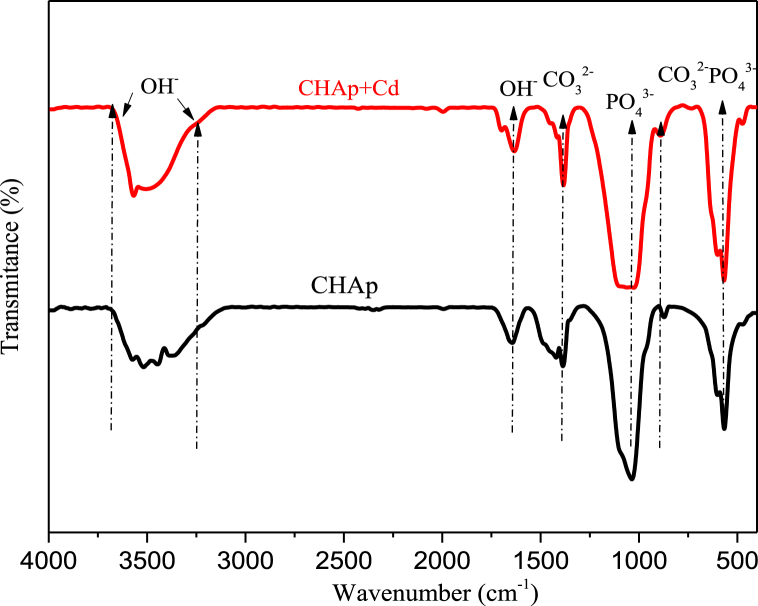
EDAX analyses of CHAp before and after adsorption of Cd2+ are as presented in Fig. 1a and b respectively indicating Ca, O and P as the main constituents of CHAp. However, Cd was detected after adsorption process thus suggesting that Cd2+ were sorbed onto the apatite surface. FT-IR spectra of the CHAp before and after Cd2+ uptake is shown in Fig. 2. Chief functional bands of CHAp were seen with slight dissimilarities observed after cadmium adsorption and this further corroborate the integration of Cd2+ onto the surface of the apatite. Bands in the range of 1028 and 1055 cm−1 were assigned to asymmetric mode of PO43−. The peaks observed at 886 cm−1 corresponds to CO32− which was adsorbed during the synthesis process from open air. Bands such as 1650, 1674, 3145 and 3154 cm−1 were attributed to water molecules adsorbed, while the bands notices between 3526 and 3625 cm−1 were assigned to the stretching of O–H group. Bands assigned to PO43− and OH− after cadmium adsorption became broadening which further suggest the uptake of the pollutant onto the apatite surface.
Fig. 1.
EDAX analysis of CHAp (a) before and (b) after adsorption of Cd2+.
Fig. 2.
FTIR spectra of CHAp before adsorption and after adsorption of Cd2+.
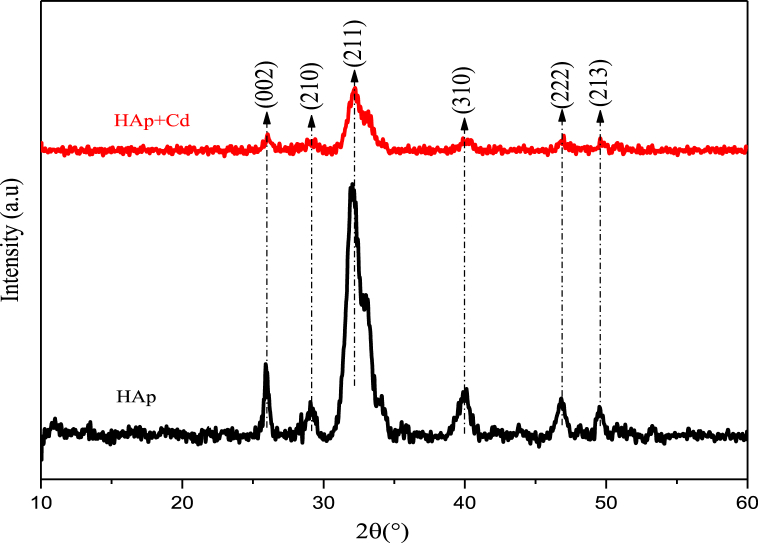
The prepared CHAp was examined for phase purity and stability by XRD technique and match up with JCPDS file no. 09–432 (see Fig. 3). Broad peaks were seen which is as a consequence of the poor crystallinity of the prepared CHAp. Most prominent diffraction peaks such as (002), (211), (310) and (213) planes correspond to pure HAp were detected. Broadening and slight changes in the peak positions after the uptake of Cd2+ were noticeable and this also corroborates the FT-IR result.
Fig. 3.
XRD spectra of CHAp before adsorption and after adsorption of Cd2+.
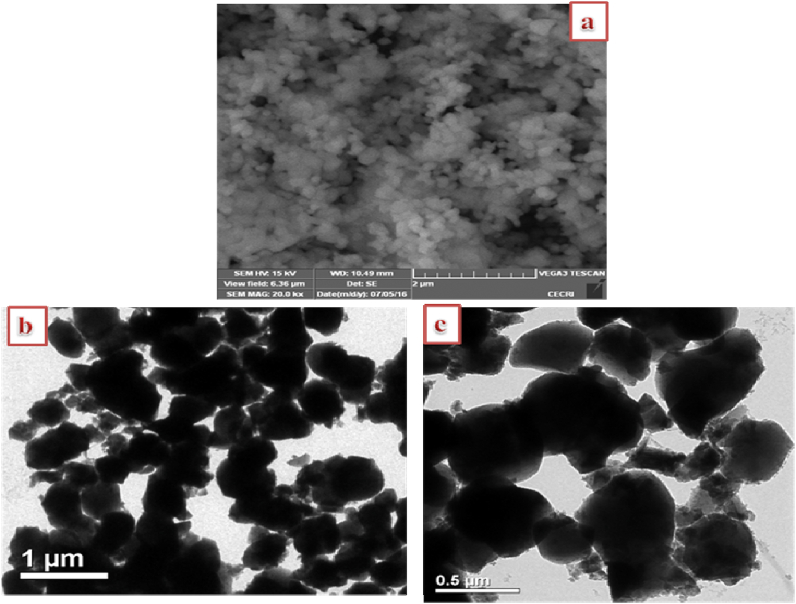
The SEM morphology of the synthesized CHAp is presented in Fig. 4a whereas, TEM images before and after the uptakes of Cd2+ are shown in Fig. 4b & c respectively. The SEM result depicts fine particles with round shapes and this was corroborated by the TEM micrograph. After the adsorption process, the particles appear bigger suggesting the incorporation of Cd(II) ions onto the apatite surface.
Fig. 4.
(a) SEM of CHAp, (b) TEM image of CHAp before and (c) TEM after adsorption.
3.2. Equilibrium studies
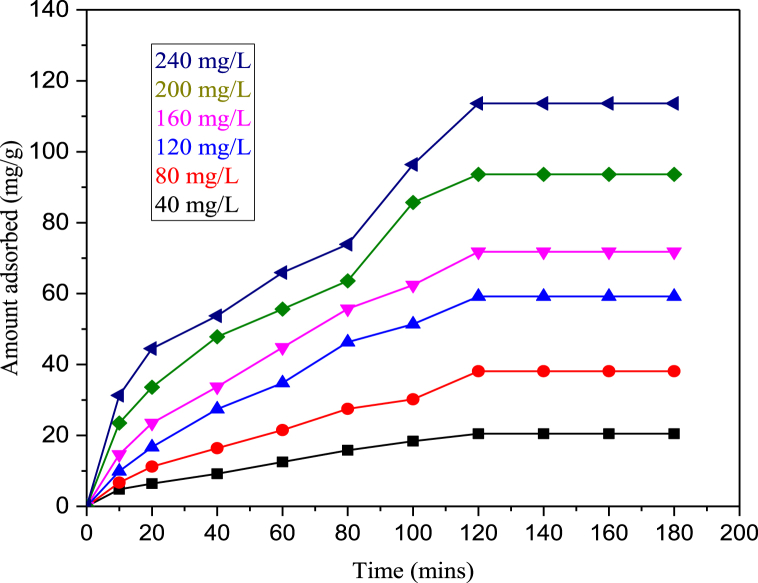
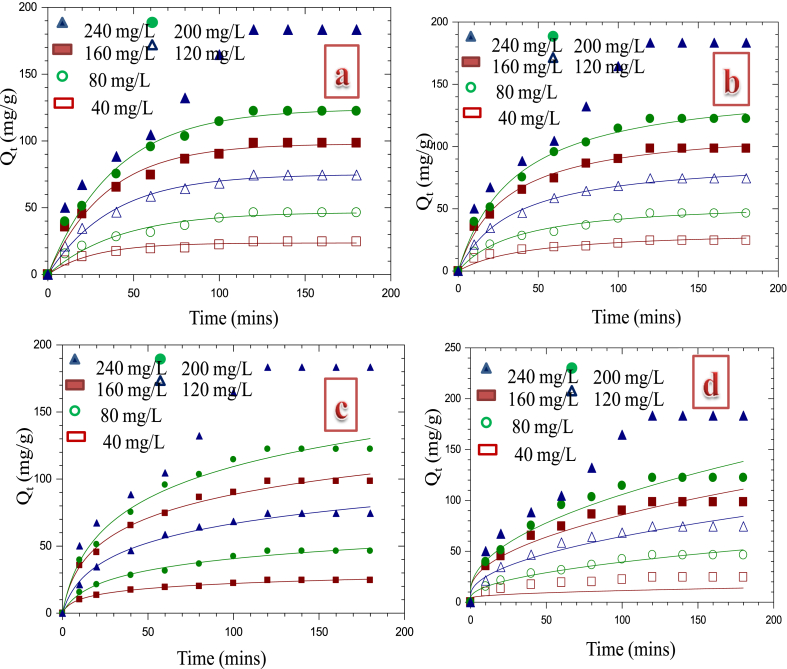
Uptake of Cd2+ was studied for initial adsorbate concentrations of 40, 80, 120, 160, 200 and 240 mg/L and at pH of 6.0 as presented in Fig. 5. It is evident from the plots that the reaction was rapid at the initial stage and slows down as the reaction approaches equilibrium stage. Highest adsorption potential of Cd2+ by CHAp was obtained at 120 min of contact time and 240 mg/L of adsorbate concentration. The amount of Cd2+ adsorbed increased from 10.2 to 24.5 mg/g when the contact time was move up from 10 to 120 min at 40 mg/L initial metal concentration. At an elevated metal concentration of 240 mg/L, an increase from 50.2 to 193.4 mg/g was observed. According to Maingi et al. [32], the extent of sorption increased expeditiously till optimum equilibrium was reached at 80 min for cadmium ions adsorption by geopolymers. In a similar work by Akinhanmi et al. [18], utmost uptake of Cd(II) ion was obtained at a contact time of 120 min. Ofudje et al. [9] reported a rapid uptake of the cadmium ions in the first 15 min, followed by a gradual increase for the next 120 min maximum adsorption capacity 134.3 mg/g.
Fig. 5.
Equilibrium studies showing the impacts of time and initial concentrations on the adsorption of Cd2+.
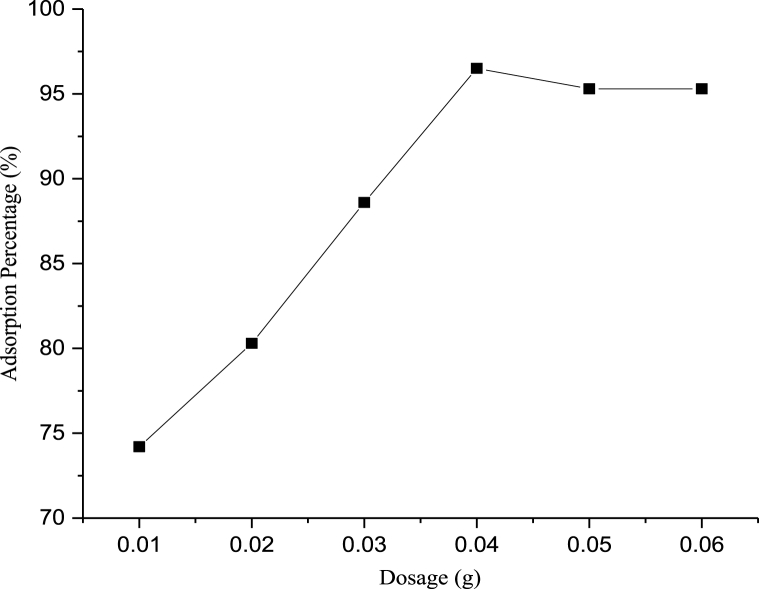
The relationship between CHAp dosage and the amount of Cd2+ adsorbed was evaluated and is presented graphically in Fig. 6. The results indicated that the percentage adsorption of Cd2+ increased alongside with increase in the amount of the synthesized HAp until saturation was attained at a dosage of 0.04 g of the adsorbent. When the dosage of the CHAp was adjusted from 0.01 g to 0.04 g, it was observed that the percentage adsorption of cadmium was found to have increased from 68.3 to 96.4% at 120 min, 240 mg/L of Cd2+ concentration, temperature of 45 °C and solution pH of 6.0 [25–28].
Fig. 6.
Equilibrium studies showing the impacts of CHAp dosage on the adsorption of Cd2+.
3.3. Study on pH
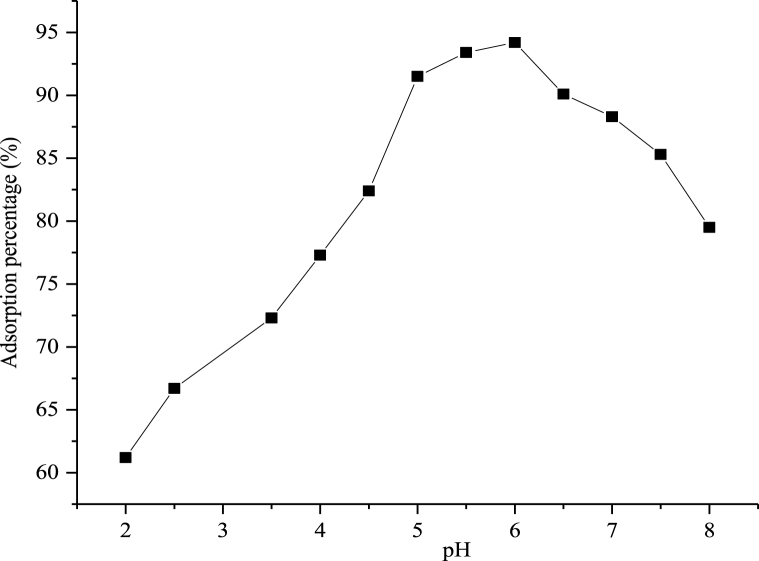
The study on the role of pH on Cd2+ adsorption was evaluated by varying the solution pH between 2.0 and 8.5 as represented graphically in Fig. 7. The elimination of Cd2+ rose significantly from 61.2 to 94.5% when the pH was adjusted from 2.0 to 6.0. Lower adsorption was noticed at a pH higher than 6.0. According to Gerçel and Gerçel [33], the competition between pollutant and H+ at low pH for the unoccupied sites and the positive charge density of the binding sites of the metal resulted in the low adsorption observed within this region. However, deprotonation which occurred on the metal binding sites as a result of the increase in the negative charge density on the surface of the adsorbent at higher pH improved the adsorption efficiency [33,34]. The precipitation of Cd2+ at higher pH may also be responsible for the low adsorption at higher solution pH. According to Schubert et al. [34], reduction in surface charge of metal ions could be due to charge screening on the surface and insufficient proton (pH). Thus, the occurrence of hydroxides depends on pH, redox potential and ion concentration. This implies that at a particular pH, the metal ion could be transformed into its hydroxides forms and subsequently results in insufficient surface charges responsible to the adsorption process [35].
Fig. 7.
Equilibrium studies showing the impacts of pH on the uptake of Cd2+.
The zero point charge (pHpzc) of CHAp was estimated to be 5.21 and it's expected that the surface of CHAp will be positive at pH less than the value of the pHpzc and negative at a pH higher than the pHpzc value [36]. Thus, the positive charges on the surface of CHAp at pH < pHpzc justifies the low adsorption of cadmium ions in high acidic medium but with the surface becoming negative at pH > Hpzc, the uptake of cadmium was greatly enhanced due to the strong electrostatic attraction between the positively charged cadmium ions and the negatively charged surface of the CHAp adsorbent [36]. Maingi et al. [32] reported that optimum adsorption of cadmium ion by geopolymer was achieved at pH 5. Literature reports from Akinhanmi et al. [18] showed that with a rise in the pH from 2 to 5.5, the sorption capacity of orange peel adsorbent for the elimination of Cd(II) ions rose from 24.62 to 44.42% with the highest adsorption accomplished at a solution pH of 5.5. It was reported further that with elevated pH, the negative charge network on orange peel increases which is due to the deprotonation of the binding sites and this enhances the uptake of Cd(II) ions. Ofudje et al. [9] reported that the uptake of Cd2+ by bone meal-derived apatite increased drastically from 60.3 to 84.3% as the solution pH was raised from 3.5 to 6. It was further reported that at lower pH, contest arising from metal ions and hydrogen ions for the empty sites coupled with the positive density charge on the adsorbent sites were responsible for the lower removal efficiency as observed, but that as the solution pH increases, there is less competition by the hydrogen ions for the adsorption sites due to deprotonation and this gave rise to better uptake of the metal ions at optimum solution pH of 6.
3.4. Adsorption isotherms studies
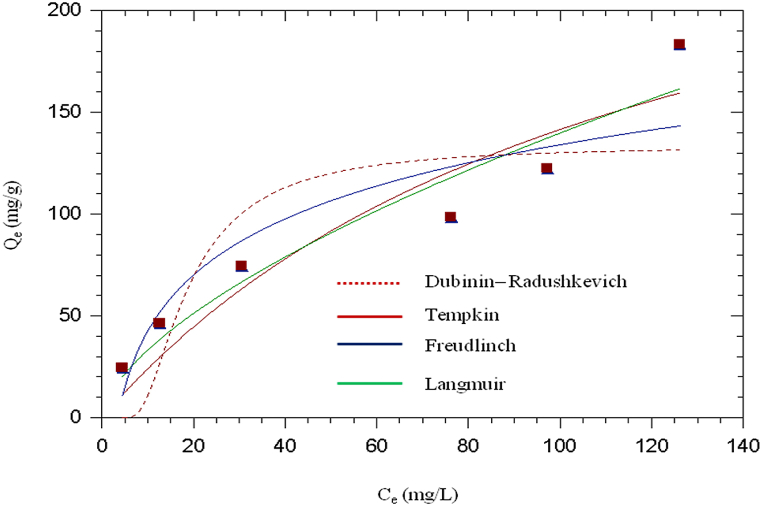
Fig. 8 shows Langmuir, Dubinin-Radushkevich, Freundlich and Tempkin isotherms plots for the adsorption of cadmium, while the isotherm parameters are given in Table 1. Taking the coefficient of correlation (R2) as a criterion for good fit of the system, the equilibrium adsorption data of cadmium fitted with Langmuir model, although other isotherms also showed good fitting. The utmost sorption capacity calculated from Langmuir equation is 195.711 mg/g, Freundlich isotherm is 6.703 mg/g) (mg/L)−1/2 while that of D-R isotherm is 98.924 mg/g in that order. Langmuir adsorption intensity (RL) were found to be in the range of 0–1; indicating favourable adsorption process; i.e when 0 < RL < 1; adsorption is favourable [25]. From the Tempkin adsorption isotherm, the values of Tempkin constant (αT) and heat of adsorption (bT) were obtained to be 0.292 (L/g) and 62.378 J/mol respectively. The D–R isotherm matches well with the data obtained experimentally. The value of E so calculated is 0.106 kJ/mol which indicates that the adsorption of Cd2+ onto CHAp adsorbent follows physical adsorption since E is less than 8 kJ/mol [37]. Table 2 showed that the adsorption capacity of CHAp for the uptake of Cd2+ compete favorably with other adsorbents in literature.
Fig. 8.
Isotherm fits for Cd2+ adsorption onto CHAp at metal conc: 40–240 mg/L, CHAp dosage: 0.04 g/L, Temp: 45 °C and pH: 6.0.
Table 1.
Isotherm constants Cd2+ adsorption by CHAp surface.
| Langmuir | Qmax(mg/g) | 195.711 |
| RL | 0.008 | |
| b (mg/L) | 0.517 | |
| R2 | 0.998 | |
| Freudlinch | KF(mg/g) (mg/L)−1/2 | 6.703 |
| 1/n | 0.624 | |
| R2 | 0.981 | |
| Tempkin | αT(L/g) | 0.292 |
| bT(J/mol) | 62.378 | |
| R2 | 0.959 | |
| Dubinin– Radushkevich | Q (mg/g) | 98.924 |
| E (kJmol−1) | 0.106 | |
| ε (mol/J)2 | 15.550 | |
| R2 | 0.921 |
Table 2.
Adsorption capacities of CHAp in comparism with others in literature.
| Adsorbent | Adsorbent capacity (qe) | References |
|---|---|---|
| Commercial activated carbon | 31.9 | Van et al. [8] |
| Mussel shells | 26.0 | Van et al. [8] |
| bone meal-derived apatite | 116.16 | Ofudje et al. [9] |
| calcined oyster shells | 29.5 | Alidoust et al. [11] |
| Waste eggshell | 23.4 | Baláž et al. [12] |
| modified maize straw | 196 | Guo et al. [13] |
| Modified cantaloupe peel | 30.4 | Tran and Chao [14] |
| orange peel derived biochar | 115 | Tran et al. [15] |
| aragonite shells-derived biosorbent | 189 | Veneu et al. [16] |
| waste shells of golden apple snail | 81.3 | Zhao et al. [17] |
| Orange peel | 128.23 | Akinhanmi et al. [18] |
4. Kinetics evaluations
Data from cadmium adsorption by the chemically synthesized hydroxyapatite (CHAp) were subjected to pseudo-first- and second-orders, intra-particle diffusion and Elovich models as shown in Fig. 9a–d, while physical parameters are as given in Table 3. Values of R2 obtained from pseudo-first and second-orders models were greater than 0.9 for all the initial metal concentrations. However, on the comparison of the values of experimental adsorption capacity (qe.exp.) of the pseudo-first-order model with computed adsorption capacity (qe.cal.), showed closeness than the pseudo-second-order model. Also, values from the sums of error squares computed in the case of pseudo-second-order were found to be higher than those of first order and it can therefore be deduced that the uptake of Cd2+ by CHAp followed pseudo-first-order kinetic model.
Fig. 9.
Kinetics plots for Cd2+ adsorption by CHAp using (a) pseudo-first order (b) pseudo-second-order (c) Elovich model & (d) intraparticle diffusion.
Table 3.
Kinetics constant values of Cd2+ adsorption by CHAp.
| Co(mg/L) | 40 | 80 | 120 | 160 | 200 | 240 | |
|---|---|---|---|---|---|---|---|
| First order | Qe(exp) (mg/g) | 24.500 | 46.300 | 74.500 | 98.400 | 122.300 | 183.400 |
| Qe(cal) (mg/g) | 23.621 | 46.669 | 75.086 | 98.002 | 124.046 | 201.942 | |
| k1(mins−1) | 0.017 | 0.024 | 0.027 | 0.029 | 0.036 | 0.036 | |
| R2 | 0.994 | 0.995 | 0.999 | 0.997 | 0.998 | 0.993 | |
| % SSE | 0.011 | 0.002 | 0.002 | 0.001 | 0.004 | 0.030 | |
| Second order | Qe(cal) (mg/g) | 32.987 | 56.705 | 91.366 | 116.896 | 151.544 | 267.324 |
| k2× 104(g/mg/min) | 6.802 | 4.824 | 3.200 | 2.904 | 1.831 | 5.323 | |
| R2 | 0.991 | 0.997 | 0.999 | 0.999 | 0.999 | 0.994 | |
| % SSE | 0.104 | 0.068 | 0.068 | 0.057 | 0.072 | 0.138 | |
| Elovich | α (mg/g/mins) | 2.868 | 2.527 | 4.284 | 7.087 | 6.758 | 4.891 |
| β (g/mg) | 0.179 | 0.073 | 0.045 | 0.037 | 0.027 | 0.013 | |
| R2 | 0.999 | 0.998 | 0.998 | 0.999 | 0.998 | 0.994 | |
| Intra particle diffusion | Kp(mg/g/mins1/2) | 1.752 | 3.547 | 5.798 | 7.433 | 9.533 | 14.891 |
| C (mg/g) | 3.971 | 3.941 | 6.631 | 11.428 | 10.511 | 0.742 | |
| R2 | 0.990 | 0.995 | 0.992 | 0.992 | 0.992 | 0.994 |
4.1. Thermodynamic studies
The role played by temperature during the uptake of Cd2+ was conducted at diverse temperatures (25–55 °C). When the temperature is increased, the rate of distribution of Cd2+ particles across the external border layers and the internal pores of CHAp increased, as a result of decline in the fluidity of the Cd2+ [37]. The plots of versus the reciprocal of temperature in Fig. 10 for Cd2+ adsorption was used to evaluate the thermodynamic constants given in Table 4. The distribution ratio (KD) values increased with temperature from 1.0028 to 1.0072 on adjusting the temperature from 298 to 318 K thus inferring the endothermic character of the process of adsorption. The free energy change, ΔG, was found to have increased from −5.937 to −20.035 kJ/mol with rise in temperature. The ΔH and ΔS for the sorption process were estimated as be 175.591 and 0.610 kJ/mol respectively. Increasing the temperature could generate a swelling effect in the internal structure of CHAp, thus causing huge penetration of adsorbate molecules [[38], [39], [40]].
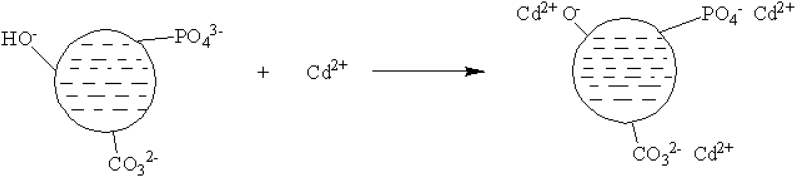
Fig. 10.
Schematic mechanism of Cd2+ adsorption by CHAp.
Table 4.
Thermodynamic constants for Cd2+ adsorption by CHAp.
| T (K) | KD | ΔG (kJ/mol) | ΔH (kJ/mol) | ΔS (kJ/mol) | R2 |
|---|---|---|---|---|---|
| 298 | 1.0028 | −5.937 | 175.591 | 0.610 | 0.968 |
| 303 | 1.0035 | −8.817 | |||
| 308 | 1.0044 | −11.261 | |||
| 313 | 1.0064 | −16.656 | |||
| 318 | 1.0072 | −20.035 |
4.2. Mechanism of Cd2+ adsorption by CHAp
Several mechanisms are involved during the adsorption process of heavy metal ions and some of them include electrostatic interaction, ion exchange, complexation, physical adsorption, chemical adsorption, and precipitation [36,41]. These mechanisms can be understood by exploiting the various information provide from structural elucidation techniques such as XRD, FT-IR, kinetics, isotherms, etc. The information from the isotherm analysis revealed that the mechanism of cadmium ion adsorption by CHAp is physical in nature due to the conformity of the equilibrium data with Langmuir isotherm. This was also corroborated by the kinetic studies which fitted perfectly with the Pseudo-first-order model. FT-IR analysis showed that the of the CHAp contained some negatively charged functional groups such as phosphate, carbonate, and hydroxyl which are attached to the positively charged Cd2+ thus indicating electrostatic attraction mechanism [36,41]. This was supported from the report on the zero point charge (pHpzc) of the adsorbent surface.
Maingi et al. [32] using kinetic analysis, proposed that Cd (II) ions adsorption on geopolymer adsorbents occurs through chemical processes involving the valence forces or the shared exchangeable electrons since pseudo-second-order kinetic mode is more predominant in the rate-controlling step for the cadmium system. Similarly, Akinhanmi et al. [18] through careful inspection of values of the Qecal from the first-order model which correspond well with the Qeexp, explained that the whole adsorption process of Cd(II) ion onto the surface of orange peel adsorbent was physisorption. Ofudje et al. [9] observed by FT-IR analysis that the mechanism of Cd(II) ions uptake by bone meal-derived apatite is as a result of the electrostatic attractions forces between the various negative charges such as CO32−, OH−, PO43− on the surface of the adsorbent and the positive pollutants.
5. Conclusion
In this study, we chemically prepared hydroxyapatite via precipitation technique and characterized by TEM, XRD, FT-IR and SEM. CHAp showed round shape as indicated by TEM and SEM images. FT-IR spectra revealed the presence of phosphate, hydroxyl and carbonate groups. The sorption potential of CHAp was investigated for the removal of Cd2+ from aqueous system. Adsorption kinetics fitted well with the pseudo-first-order model. Equilibrium models results showed that the Cd2+ adsorption data using CHAp followed the Langmuir model which inferred that monolayer and homogeneous surfaces are more effective when compared with heterogeneous surfaces. Furthermore, results obtained from n and RL values confirmed that the adsorption process is favourable. The study on the thermodynamic parameters indicated that Cd2+ adsorption process is spontaneous and endothermic. Conclusively, CHAp can be introduced as an effective and efficient adsorbent in removing Cd2+ from industrial wastewaters.
Author contribution statement
Edwin Andrew OFUDJE: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Ezekiel F. SODIYA: Contributed reagents, materials, analysis tools or data; Performed the experiments.
Olajire S. Olanrele: Contributed reagents, materials, analysis tools or data; Analyzed and interpreted data.
Fatai AKINWUNMI: Performed the experiments; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare no competing interests.
Acknowledgments
Technical staff of CSIR-CECRI, Central Instrumentation Facility (CIF) of Karaikudi, India are well appreciated for their supports during characterization.
References
- 1.Chavan B.B. Health and environmental hazards of synthetic dyes. 2013. http://www.fibre2fashion.com/industryarticle/printarticle.asp?article_id=4708&page=1 Available at: Accessed.
- 2.Asouhidou D.D., Triantafyllidis K.S., Lazaridis N.K., Matis K.A., Kim S.S., Pinnavaia T.J. Sorption of reactive dyes from aqueous solutions by ordered hexagonal and disordered mesoporous carbons. Micropor. Mesopor. Mater. 2009;117:257–267. doi: 10.1016/j.micromeso.2008.06.034. [DOI] [Google Scholar]
- 3.Abo-Farha S.A. Comparative study of oxidation of some azo dyes by different advanced oxidation processes: fenton, Fenton-like, photo-Fenton and photo-Fenton-like. J. American Sc. 2010;6:128–142. [Google Scholar]
- 4.Wang F.Y., Wang H., Ma J.W. Adsorption of cadmium (II) ions from aqueous solution by a new low-cost adsorbent—bamboo charcoal. J. Hazard Mater. 2010;177:300–306. doi: 10.1016/j.jhazmat.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 5.US EPA . Office of Science and Technology; 2002. National Recommended Water Quality Criteria. EPA–822-R-02–047. Office of Water. [Google Scholar]
- 6.Yusof M.S.M., Othman M.H.D., Mustafa A., Rahman M.A., Jaafar J., Ismail A.F. Feasibility study of cadmium adsorption by palm oil fuel ash (POFA) -based low-cost hollow fibre zeolitic membrane. Environ. Sci. Pollut. Res. 2018 doi: 10.1007/s11356-018-2286-6. [DOI] [PubMed] [Google Scholar]
- 7.Mohammadine E.H., Abdelmajid R., My-Rachid L., Rachid M., Nabil S. Use of Fenton reagent as advanced oxidative process for removing textile dyes from aqueous solutions. J. Mater. Environ. Sci. 2014;5:667–674. [Google Scholar]
- 8.Van H.T., Nguyen L.H., Nguyen V.D., Nguyen X.H., Nguyen T.H., Nguyen T.V., Vigneswaran S., Rinklebef J., Tran H.N. Characteristics and mechanisms of cadmium adsorption onto biogenic aragonite shells-derived biosorbent: batch and column studies. J. Environ. Manag. 2018 doi: 10.1016/j.jenvman.2018.09.079. [DOI] [PubMed] [Google Scholar]
- 9.Ofudje E.A., Adeogun I.A., Idowu M.A., Kareem S.O., Ndukwe N.A. Simultaneous removals of cadmium(II) ions and reactive yellow 4 dye from aqueous solution by bone meal-derived apatite: kinetics, equilibrium and thermodynamic evaluations. J. Anal. Sci. and Technol. 2020;11:7. doi: 10.1186/s40543-020-0206-0. [DOI] [Google Scholar]
- 10.Ali H.R., Hassaan M.A. Applications of bio-waste materials as green synthesis of nanoparticles and water purification. Adv. Mater. Chem. 2017;1:6–22. [Google Scholar]
- 11.Alidoust D., Kawahigashi M., Yoshizawa S., Sumida H., Watanabe M. Mechanism of cadmium biosorption from aqueous solutions using calcined oyster shells. J. Environ. Manag. 2015;150:103–110. doi: 10.1016/j.jenvman.2014.10.032. [DOI] [PubMed] [Google Scholar]
- 12.Baláž M., Ficeriová J., Briančin J. Influence of milling on the adsorption ability of eggshell waste. Chemosphere. 2016;146:458–471. doi: 10.1016/j.chemosphere.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Guo H., Zhang S., Kou Z., Zhai S., Ma W., Yang Y. Removal of cadmium(II) from aqueous solutions by chemically modified maize straw. Carbohydr. Polym. 2015;115:177–185. doi: 10.1016/j.carbpol.2014.08.041. [DOI] [PubMed] [Google Scholar]
- 14.Tran H.N., Chao H.P. Adsorption and desorption of potentially toxic metals on modified biosorbents through new green grafting process. Environ. Sci. Pollut. Res. 2018;25:12808–12820. doi: 10.1007/s11356-018-1295-9. [DOI] [PubMed] [Google Scholar]
- 15.Tran H.N., You S.J., Chao H.P. Effect of pyrolysis temperatures and times on the adsorption of cadmium onto orange peel derived biochar. Waste Manag. Res. 2016;34:129–138. doi: 10.1177/0734242X15615698. [DOI] [PubMed] [Google Scholar]
- 16.Veneu D.M., L Schneider C., de Mello Monte M.B., Cunha O.C.G., Yokoyama L. Cadmium removal by bioclastic granules (Lithothamnium calcareum): batch and fixed-bed column systems sorption studies. Environ. Technol. 2017:1–12. doi: 10.1080/09593330.2017.1336574. [DOI] [PubMed] [Google Scholar]
- 17.Zhao B., Zhang J.E., Yan W., Kang X., Cheng C., Ouyang Y. Removal of cadmium from aqueous solution using waste shells of golden apple snail. Desalination Water Treat. 2016;57:23987–24003. doi: 10.1080/19443994.2016.1140078. [DOI] [Google Scholar]
- 18.Akinhanmi T.F., Ofudje E.A., Adeogun A.I., Aina P., Joseph I.M. Orange peel as low-cost adsorbent in the elimination of Cd(II) ion: kinetics, isotherm, thermodynamic and optimization evaluations. Bioresour. Bioprocess. 2020;7:34. doi: 10.1186/s40643-020-00320-y. [DOI] [Google Scholar]
- 19.Neelakandeswari N., Sangami G., Dharmaraj N. Preparation and characterization of nanostructured hydroxyapatite using a biomaterial. Synth. React. Inorg., Met.-Org., Nano-Met. Chem. 2011;41:513–516. doi: 10.1080/15533174.2011.568434. [DOI] [Google Scholar]
- 20.Gunjan V., Barick K.C., Manoj N., Sahu A.K., Hassan P.A. Rod-like micelle templated synthesis of porous hydroxyapatite. Ceram. Int. 2013;39:8995–9002. doi: 10.1016/j.ceramint.2013.04.100. [DOI] [Google Scholar]
- 21.Kai L., Sie C.T. Preparation and characterization of isotactic polypropylene reinforced with hydroxyapatite nanorods. J. Macromol. Sci., Part B: Phys. 2011;50:1983–1995. doi: 10.1080/00222348.2010.549437. [DOI] [Google Scholar]
- 22.Kaygili O., Tatar C. The investigation of some physical properties and microstructure of Zn-doped hydroxyapatite bioceramics prepared by sol-gel method. J. Sol. Gel Sci. Technol. 2012;61:296–309. doi: 10.1007/s10971-011-2627-0. [DOI] [Google Scholar]
- 23.Mehdi S.S., Mohammad T.K., Ehsan D.K., Ahmad J. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013;9:7591–7621. doi: 10.1016/j.actbio.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Gross K.A., Komarovska L., Viksna A. Efficient zinc incorporation in hydroxyapatite through crystallization of an amorphous phase could extend the properties of zinc apatites. J. Australas. Ceram. Soc. 2013;49:129–135. [Google Scholar]
- 25.Adeogun A.I., Ofudje A.E., Idowu M.A., Ahmed S.A. Kinetic, thermodynamic and isothermal parameters of biosorption of Cr (VI) and Pb (II) ions from aqueous solution by biosorbent prepared from corncob biomass. Inorg Chem Ind J. 2012;7:119–129. [Google Scholar]
- 26.Lin G.H., Brusick D.J. Mutagenicity studies on two triphenylmethane dyes, bromophenol blue and tetrabromophenol blue. J. Appl. Toxicol. 1992;12:267–274. doi: 10.1002/jat.2550120410. [DOI] [PubMed] [Google Scholar]
- 27.Chen X., Wright J.V., Conca J.L., Peurrung L.M. Evaluation of heavy metal remediation using mineral apatite. Water, Air, Soil Pollut. 1997;98:57–78. doi: 10.1007/BF02128650. [DOI] [Google Scholar]
- 28.Langmuir I. The adsorption of gases on plane surfaces of glass, mica, and platinum. J. Am. Chem. Soc. 1918;40:1361–1403. doi: 10.1021/ja02242a004. [DOI] [Google Scholar]
- 29.Kundu S., Gupta A.K. Arsenic adsorption onto iron oxide-coated cement (IOCC), Regression analysis of equilibrium data with several isotherm models and their optimization. Chem. Eng. J. (Lausanne) 2006;122:93–106. [Google Scholar]
- 30.Dubinin M.M., Zaverina E.D., Radushkevich L.V. Sorption and structure of active carbons. I. Adsorption of organic vapors. Zh. Fiz Khim. 1947;21:1351–1362. [Google Scholar]
- 31.Dogan M., Alkan M. Adsorption kinetics of methyl violet onto perlite. Chemosphere. 2003;50:517–528. doi: 10.1016/s0045-6535(02)00629-x. [DOI] [PubMed] [Google Scholar]
- 32.Maingi F.M., Mbuvi H.M., Ng’ang’a M.M., Mwangi H. Adsorption of cadmium ions on geopolymers derived from ordinary clay and rice husk ash. Int. J. Mater. Chem. 2018;8:1–9. doi: 10.5923/j.ijmc.20180801.01. [DOI] [Google Scholar]
- 33.Gerçel O., Gerçel H.F. Adsorption of lead(II) ions from aqueous solutions by activated carbon prepared from biomass plant material of Euphorbia rigida. Chem. Eng. J. 2007;132:289–297. [Google Scholar]
- 34.Schubert J., Radeke C., Ferya A., Chanana M. The role of pH, metal ions and their hydroxides in charge reversal of protein-coated nanoparticles. Phys. Chem. Chem. Phys. 2019;21:11011–11018. doi: 10.1039/C8CP05946B. [DOI] [PubMed] [Google Scholar]
- 35.Kosmulski M. Isoelectric points, points of zero charge of metal (hydr)oxides: 50 years after Parks' review. Adv. Colloid Interface Sci. 2016;238:1–61. doi: 10.1016/j.cis.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Ofudje E.A., Adedapo A.E., Oladeji O.B., Sodiya E.F., Ibadin F.H., Zhang D. Nano-rod hydroxyapatite for the uptake of nickel ions: effect of sintering behaviour on adsorption parameters. J. Environ. Chem. Eng. 2021;9 doi: 10.1016/j.jece.2021.105931. [DOI] [Google Scholar]
- 37.Han K.L., Tjoon T.T., Mahamad H.I., Anees A., Hui T.C. Adsorption and removal of zinc (II) from aqueous solution using powdered fish bones. APCBEE Procedia. 2012;1:96–102. doi: 10.1016/j.apcbee.2012.03.017. [DOI] [Google Scholar]
- 38.Al-qodah Z. Adsorption of dyes using shale oil ash. Water Resour. 2000;34:4295–4303. doi: 10.1016/S0043-1354(00)00196-2. [DOI] [Google Scholar]
- 39.Dogan M., Alkan M. Adsorption kinetics of methyl violet onto perlite. Chemosphere. 2003;50:517–528. doi: 10.1016/s0045-6535(02)00629-x. [DOI] [PubMed] [Google Scholar]
- 40.Ofudje E.A., Sodiya E.F., Ibadin F.H., Ogundiran A.A., Alayande S.O., Osideko O.A. Mechanism of Cu2+ and reactive yellow 145 dye adsorption onto eggshell waste as low-cost adsorbent. Chem. Ecol. 2020 doi: 10.1080/02757540.2020.1855153. [DOI] [Google Scholar]
- 41.Inyang M.I., Gao B., Yao Y., Xue Y., Zimmerman A., Mosa A., Pullammanappallil P., Ok Y.S., Cao X. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol. 2016;46:406–433. doi: 10.1080/10643389.2015.1096880. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.