Multicenter trials involve procedures, rules, and guidelines that require meticulous attention to details and strict adherence to compliance issues. It is widely accepted that the design, implementation, coordination and analysis of modern clinical trials require a multidisciplinary specialist approach [1]. At the same time, appropriate training and mastery of the competencies that characterize each role of the research process are essential to carry out high-quality clinical and translational research [2,3]. Usually research teams include the principal investigator (PI), sub-investigators (SI), clinical study nurses (CSNs), clinical research coordinators (CRC), also called study coordinators (SC) and other figure as data manager, statisticians, and clinical monitors also called clinical research associates (CRAs).
Although study coordinator figure exists for at least two decades, the CRCs position and their key role in the scenario of clinical trials, have only recently been defined [[4], [5], [6], [7]]. While the responsibilities of investigators, sponsors and clinical monitors are well defined by national regulations of clinical trials and international guidelines on Good Clinical Practice, the importance of the CRC position, their responsibilities and main tasks, have not yet been completely described. Moreover, there is limited available evidence on this topic, since most papers mainly address the required skills of a CRC or describe their responsibilities within a specific topic. Moreover, the term data manager and study coordinator have often been used as synonyms for many years and this may have contributed to confusion and delay in CRC precise definition.
Data for this review were identified by searches of MEDLINE, Current Contents, PubMed, and references from relevant articles, using the search terms “clinical research coordinator”, “study coordinator”, “research nurse”. Other search engines, as Google Scholar, have been considered in a second review of scientific literature, despite its limitations [8]. All found papers have been critically evaluated for review. Only article published in English were included; English abstract of other languages papers have been excluded from review when not sufficient to fully describe the study design.
The definition provided by the Association of Clinical Research Professionals states that "A Clinical Research coordinator, Study Site Research Nurse or Study Site Coordinator, works at a clinical research site under the immediate direction of a principal investigator, whose research activities are conducted under Good Clinical Practice regulations. Among other tasks, CRC's perform site preparation, patient screening and recruitment, patient enrolment, conduct and ensure the quality of case report forms, maintain source documents, and ensure site quality" [9].
This definition clearly separates the roles of CRCs and CSNs from those of data managers, whose activities represent only a part of the multiple tasks that belong to CRCs and nurses, as further described below and summarized in Table 1.
Table 1.
CRC activities, divided in different areas, modified from Rico-Villademoros F et al. 2004.
| Administrative activities |
|---|
| Feasibility questionnaire completion Management of IRB submission (protocol and amendments), periodical trial reports (AE/SAE communication, first patient enrollment, close-out visit) Collaborate with Sponsors and CRO for study documents Management of Hospital Director submission Scheduled protocol specified tests Scheduled patient's CT appointments Contract and budget revision |
|
Monitoring activities Patient registration/randomization procedures (IVRS/IWRS) To deal with Hospital Pharmacy and drug accountability IP dispensation (IVRS/IWRS) To deal with central lab and facilities (temperature log, calibration certificate …) Prepare and process lab kits for central lab Recruitment follow-up CRF completion To collaborate with the CRA during the study and site monitoring visits Queries resolution Reporting serious adverse events To handle the investigator file and source documents To prepare and/or attend audits Identification and revision of SOPs (if applicable) |
|
Data management and statistics Database set-up Data entry Statistical analysis |
|
Researcher-related activities Participation in protocol/CRF design Participation in protocol/CRF review Attending investigators meeting Participation in Final Report Participation in CT publication |
|
Clinical activities (if applicable) To identify potential eligible patients Assessment of inclusion/exclusion criteria Participation in informing of patients Participation in obtaining informed consent To assess response to therapy To assess toxicities Scales/questionnaires completion |
Even though recent literature has focused on the importance of these positions, the figure of CRCs is still likely to be neglected and undervalued in research practice. Moreover, it is possible to find an overlap of the professional figures, because many sites do not have the possibility to hire all the staff necessary to support the research. Therefore, it is frequent to find sub-inv and/or SN that fulfill the role and responsibilities of CRC and this may generate confusion between different roles.
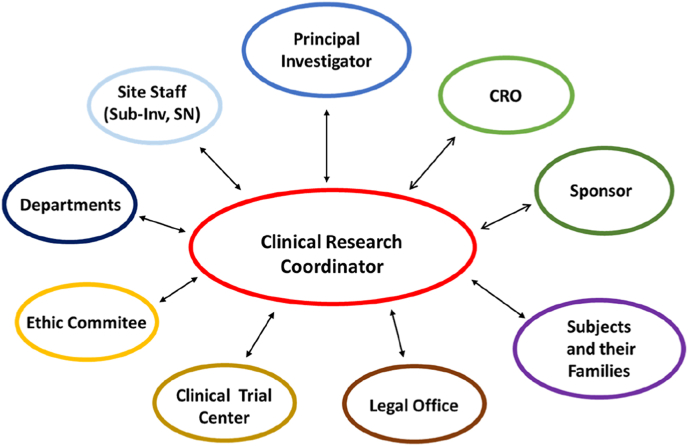
In 2002 a group of American authors referred to CRCs as the invisible hand in clinical research [10]. They underlined that the CRCs are crucial for the success of clinical research due to their wide range of responsibilities, representing the connection between study sponsor and patients and clinical, regulatory and administrative staff. CRCs’ central position is highlighted in Fig. 1 that shows their specific coordinating role.
Fig. 1.
Figure shows the central position of CRC and the connections with all the subjects involved in the research machine.
In 2004, a Spanish group performed a survey to elucidate the standard tasks performed by CRCs in oncology clinical trials, to assess their job satisfaction and training needs [11]. They found some inaccuracies, starting from the definition of their role. More than two-thirds of the respondents were called "Data Managers'' while the title "Clinical Research Coordinator" (i.e. titles that included both terms "research" and "coordinator") appeared in only 3 job titles. As for educational level and training, they generally had a university degree (Medicine, Pharmacy, Biology and Nursing as the most frequent) and received specific training (Oncology, Clinical Research, Clinical Trials, GCP, Statistics, Database preparation & management in the majority of cases). As for the tasks, overall, CRCs seemed to be devoted to what we call "monitoring activities'', including patient registration/randomization, recruitment follow-up, case report form (CRF) completion, collaboration with the CRA, serious adverse events (SAE) reporting, investigator file handling, and preparing the site for and/or attending audits. It was not uncommon for CRCs participating in that survey to deal with the hospital pharmacy. They maintained the required records of study activity, including drug accountability logs, regulatory documents as well as financial records. To a lesser extent, they were also involved in some administrative activities (such as Ethic committee submission and scheduling patient's visits), and various clinical activities (inclusion/exclusion criteria assessment and completion of scales/questionnaires) (Table 1). The CRC ensures that clinical trials run smoothly and preserve data quality, monitors study activity to guarantee protocol compliance and all relevant local, federal, and national regulatory and institutional policies. Although the PI is responsible for the overall conduct of the clinical trial, the CRC is responsible for the daily activities.
CRCs collaborate with the study nurse, but while the role of the clinical study nurse has been defined better during past years, CRCs’ role is still vague. The study nurse is essential in the management of everything related to research participants. The CRC is crucial in the management and coordination of the entire study protocol, from the early stages of the site selection to the close out visit.
Already many years ago, the addition of research nurses to clinical studies was found to have a positive effect on patient enrollment [12], showing that without a stable, knowledgeable research nurse workforce, the conduction of research is significantly affected. To address the need for educating clinical research nurses to support clinical trials, dedicated training programs were proposed [13], as the engagement of well-trained and motivated nurses in clinical trials is known to improve patient outcomes [14].
If the involvement of CSN in clinical trials is a crucial factor, studies have shown that adding a coordinator to a research team significantly improves subject recruitment numbers, subject retention and general study efficiency [11], increasing general quality in all study phases. A more active involvement of CRCs in the activities of clinical trials, including a more frequent participation to investigators meetings and a deeper contribution in protocol/case report form review and design, is advocated, since the CRC can provide a worthy perspective on the logistics of proposed study procedures. Finally, the involvement of CRCs in such activities will have a positive influence on their job satisfaction. A recent Italian survey, proposed by AIOM CRC Working Group and involving 319 oncology sites [15], confirmed a direct association between the number of clinical studies and the number of coordinators, whose contribution to the research activities was believed to be essential for trial conduction. More than 80% of participating sites associated the improvement of the quality of clinical research to the implementation of a coordinator as member of the team.
In 2018 a Japanese group examined the present status and the perspectives toward broader contribution of clinical research coordinators by a cross-sectional study [16]. More than 70% of the respondents were affiliated with medical institutions but were mainly involved in industry-initiated registration trials, mainly for funding issues. Half of the CRCs and other clinical research-related personnel viewed a broadening of CRCs’ involvement in research activities positively. Accordingly, a structured practical program aimed at encouraging such involvement may help expanding and strengthening their contribution into the future. Additionally, it is plausible that a greater involvement of CRCs in clinical research will help to ensure the reliability of investigator-initiated clinical research.
With the increase in regulatory oversight among clinical trials and upcoming novelties in regulatory procedures, the demands and expectations of CRCs have increased and therefore now require additional skills, training, and medical knowledge (Table 2). Even if the role is often considered as administrative, CRCs have, as mentioned above, university education and are highly qualified. However, CRC is still considered a transient position, probably for the lack of professional identity for these roles, leading to unclear training requirements and job criteria.
Table 2.
Table summarizes the most important requested skills for a CRC.
| CRC Skills |
|---|
| Experience in clinical trials |
| Research experiences |
| GCP knowledge |
| Ability to manipulate biological samples |
| Organization/Management/Planning Skills |
| Comunication Skills |
| Problem Solving |
| Mediation Skills |
| Precision |
| Flexibility |
In Italy in particular, the CRC professional profile is not formally recognized by specific certifications, and no formal CRC position exists in institutions’ staff, as required by national health care workforce regulations. This makes it difficult for institutions and policy makers to appraise the demand for CRCs and to respond by allocating the necessary resources [17].
Competency based training is critical to the task demands of CRCs. Nonetheless, staff turnover is high and opportunities for advancement limited, particularly at academic health centers. Although hiring a CRC represents a significant investment in time and training to properly carry out job responsibilities, turnover in this role is as much costly, mainly due to the time for education and development, marketing and recruitment, loss of productivity during orientation and training, and emotional costs of turnover on current staff. From this point of view, it is advantageous to retain individuals in this position for extended periods, positioning this role as a long-term career [18,19]. A recent paper by Buchanan DA and colleagues [20] focused on factors associated with job satisfaction and increased retention. These authors found that, although salary and compensation were a significant predictor of retention among this select population of clinical research staff, greater predictors of retention were related to the involvement and relationship with the PI, showing the importance to invest time in building genuine relationships with study staff.
The lack of a well-defined job position and scarce compensation on one hand, and the work overload generated by the continuous updating of regulatory procedures, without structured training and educational programs on the other, are probably the major causes of frustration and dissatisfaction reported from CRCs. In this point of view, some emerging evidences [21] indicated that the burnout phenomenon, that has been extensively investigated among health care professionals, particularly on physicians and nurses, does not even spare CRCs. Resilience, sleep dysfunction, stress, and incivility experienced from patients/family have been found significant predictors of burnout for oncology CRCs in a recent mixed-method American study [22].
1. Conclusion
Our review underlines that, being still not well defined, some crucial research roles as CRCs and study nurses are as heterogeneous as their tasks and job positions in different research contexts. As data management activities represent only a very small part of the multiple tasks usually performed by study coordinators, the term Clinical Research Coordinator is certainly more appropriate.
Emerging evidence [11,15,17,18] confirms that the establishment of a clear job description, the involvement as central positions in the complex research machine, and the standardization and regulation of their job titles are essential to avoid CRCs migration towards the private sector and guarantee academic successful and high-standard quality research.
Funding
No specific grant from any funding agency in the public, commercial, or not-for-profit sectors was received for this manuscript.
Author statement
All authors contributed to the concept of the manuscript. VM and SC conceptualized the idea, elaborated draft and revised versions of the manuscript. GW, MAP, RS and FMCP collaborated in bibliographic research and revised it critically, CF and AL edited and revised the manuscript and collaborated to built tables and figure, GI and AG coordinated and integrated work, providing their intellectual contribute to the manuscript. All authors approved this version to be published.
Declaration of competing interest
All authors declare no conflict of interest.
Acknowledgements
A special mention to Dr. D. Piacentini and Dr. R. Galluzzi (Direzione Risorse Umane, Fondazione Policlinico Universitario A. Gemelli, IRCCS) to contribute to define research profiles and job description within Fondazione and to Fondazione Roma (Via del Corso 239, 00187, Rome) for the relevant and continuous support to CEMAD in clinical research and its applications.
Contributor Information
Stefania Colantuono, Email: stefania.colantuono@guest.policlinicogemelli.it.
SC&RN group:
Pirozzoli Maria Celeste, Giannone Luciana, Spataro Cristina, Graziani Cristina, Capodrossi Anna, Teberino Maria Anna, Tolusso Barbara, Di Ciurcio Marica, Verdirosi Diana, Rotunno Serena, Finotti Ludovica, Turchini Laura, Amatucci Valeria, Schiavoni Elisa, Napolitano Daniele, Durini Eleonora, Strazzeri Martina, Lombardi Maria Teresa, and Schifano Elisabetta
References
- 1.Riley D., Ward L., Young T. For the British oncology data managers association (BODMA): oncology data management in the UK – BODMA's view. Br. J. Cancer. 1994;70:391–394. doi: 10.1038/bjc.1994.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hornung C.A., Jones C.T., Calvin-Naylor N.A., et al. Competency indices to assess the knowledge, skills and abilities of clinical research professionals. Int. J. Clinic. Trial. 2018;5(1):46–53. [Google Scholar]
- 3.Califf R.M., Filerman G.L., Murray R.K., et al. Envisioning a Transformed Clinical Trials Enterprise in the United States: Establishing an Agenda for 2020. National Academies Press; Washington, DC: 2012. Appendix D: discussion Paper–The clinical trials enterprise in the United States: a call for disruptive innovation. Institute of Medicine. [PubMed] [Google Scholar]
- 4.Warlow C. How to do it. Organise a multicentre trial. BMJ. 1990 Jan 20;300(6718):180–183. doi: 10.1136/bmj.300.6718.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cassidy J., Macfarlane D.K. The role of the nurse in clinical cancer research. Cancer Nurs. 1991 Jun;14(3):124–131. [PubMed] [Google Scholar]
- 6.Arrigo C., Gall H., Delogne A., Molin C. The involvement of nurses in clinical trials. Results of the EORTC Oncology Nurses Study Group survey. Cancer Nurs. 1994;17:429–433. [PubMed] [Google Scholar]
- 7.Brown J.M., Haining S.A., Hale J.M. Views on local data management in cancer clinical trials. Clin. Oncol. 1997;9:403–406. doi: 10.1016/s0936-6555(97)80138-0. [DOI] [PubMed] [Google Scholar]
- 8.Jacsó P. Google Scholar: the pros and the cons. Online Inf. Rev. 2005;29(2):208–214. [Google Scholar]
- 9.https://acrpnet.org/, 2021/11/9
- 10.Davis A.M., Hull S.C., Grady C., Wilfond B.S., Henderson G.E. The invisible hand in clinical research: the study coordinator's critical role in human subjects protection. J. Law Med. Ethics. 2002 Fall;30(3):411–419. doi: 10.1111/j.1748-720x.2002.tb00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rico-Villademoros F., Hernando T., Sanz J.L., et al. The role of the clinical research coordinator--data manager--in oncology clinical trials. BMC Med. Res. Methodol. 2004 Mar 25;4:6. doi: 10.1186/1471-2288-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isaacman D.J., Reynolds E.A. Effect of a research nurse on patient enrollment in a clinical study. Pediatr. Emerg. Care. 1996 Oct;12(5):340–342. doi: 10.1097/00006565-199610000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Showalter B.L., Cline D., Yungclas J., Frentz K., Stafford S.R., Maresh K.J. Clinical research nursing: development of a residency program. Clin. J. Oncol. Nurs. 2017 Oct 1;21(5):633–636. doi: 10.1188/17.CJON.633-636. [DOI] [PubMed] [Google Scholar]
- 14.Scala E., Patterson B.J., Stavarski D.H., Mackay P. Engagement in research: a clinical nurse profile and motivating factors. J. Nurses Prof. Dev. 2019 May/Jun;35(3):137–143. doi: 10.1097/NND.0000000000000538. [DOI] [PubMed] [Google Scholar]
- 15.Cinefra M, Cagnazzo C, McMahon L et al, The Critical Role of the Clinical Research Coordinator for Clinical Trials: a Survey in Oncology doi.org/10.5301/maapoc.0000015.
- 16.Yanagawa H., Nokihara H., Yokoi H., et al. Present status and perspectives on future roles of Japanese clinical research coordinators. J. Clin. Med. Res. 2018 Dec;10(12):877–882. doi: 10.14740/jocmr3602w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caminiti C., Maglietta G., Frau I., et al. Presence and activities of clinical research coordinators at Italian Health Care Institutions: a national cross-sectional survey. J. Clinic. Trans. Sci. 2022;6(1):E1. doi: 10.1017/cts.2021.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snyder D.C., Brouwer R.N., Ennis C.L., et al. Retooling institutional support infrastructure for clinical research. Contemp. Clin. Trials. 2016 May;48:139–145. doi: 10.1016/j.cct.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Causey M. ACRP Blog; 2017. Professional Pathways Boost Staff Retention in Clinical Research Settings.www.acrpnet.org Retrieved from. [Google Scholar]
- 20.Buchanan D.A., Goldstein J., Pfalzer A.C., Lin Y.C., Kang H., Claassen D.O. Empowering the clinical research coordinator in academic medical centers. Mayo Clin. Proc. Innov. Qual. Outcomes. 2020 Dec 25;5(2):265–273. doi: 10.1016/j.mayocpiqo.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cagnazzo C., Filippi R., Zucchetti G., et al. vol. 22. 2021 Mar 12. p. 205. (Clinical Research and Burnout Syndrome in Italy - Only a Physicians' Affair? Trials). 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mascaro J.S., Palmer P.K., Ash M.J., et al. Incivility is associated with burnout and reduced compassion satisfaction: a mixed-method study to identify causes of burnout among oncology clinical research coordinators. Int. J. Environ. Res. Publ. Health. 2021 Nov 12;18(22) doi: 10.3390/ijerph182211855. [DOI] [PMC free article] [PubMed] [Google Scholar]