Abstract
Background
At present, many studies have confirmed that inflammation plays a central role in Parkinson's disease (PD). The inflammatory index is related to the prognosis of the disease, but a single inflammatory index has some limitations. The C-reactive protein-albumin ratio (CAR) is a better marker of inflammation or nutritional status than C-reactive protein (CRP) or albumin (Alb), but there is limited study on the association between CAR and the overall survival (OS) of PD.
Object
To study the association between CAR and OS in PD patients.
Methods
All of these data were obtained from the Dryad Digital Repository, based on which we conducted a secondary analysis. The study was conducted by the Department of Neurology, the National Regional Center for Neurological Disorders, and the National Hospital of Utano study between March 2004 to November 2007. The final analytic sample included 235 PD patients with the outcome of survival or all-cause death from the study registration to the endpoint. In this study, univariate and multivariate COX regression analyses were used to calculate the adjusted hazard ratio (HR), with a 95% confidence interval (CI). In addition, the association between CAR and OS in PD patients was explored by Kaplan-Meier curve and subgroup analysis.
Results
This study included 235 PD patients with an average age of 62.25 years, including 135 females and 100 males, and 45 died during the follow-up period. CAR was associated with gender, modified Hoehn-Yahr stages (mH-Y), and Mini-Mental State Examination (MMSE) of PD patients. In the COX multivariate regression model, after adjusting the age, gender, PD duration, mH-Y, MMSE, and the non-steroidal anti-inflammatory drugs, CAR was found to be associated with the OS in PD (HR = 1.54, 95% CI = 1.01–2.34, p = 0.044). Subgroup analysis showed that the subgroup did not play an interactive role in the association between the prognosis of patients with CAR and PD (p for interaction >0.05), and the results remained stable.
Conclusions
The all-cause mortality of PD patients with a high level of CAR is higher, which indicates that the poor overall survival of PD patients is associated with the increase of CAR. The CAR may be a reliable prognostic biomarker for PD patients.
Keywords: Parkinson's disease, C-reactive protein-albumin ratio, Biomarker, Prognosis, Overall survival
Abbreviations: PD, Parkinson's disease; CRP, C-reactive protein-albumin ratio; Alb, albumin; CAR, C-reactive protein; HR, Hazard ratio; CI, Confidence interval; mH-Y, Modified Hoehn-Yahr stages; MMSE, Mini-Mental State Examination; NSAIDs, Non-steroidal anti-inflammatory drugs; IQR, Interquartile range
1. Introduction
Parkinson's disease (PD) is the second neurodegenerative disease, accounting for 2–3% of the 65-year-old population [1]. With the increase of age, the mortality rate of PD did not increase in the first decade, but doubled since then [2], seriously affecting people's quality of life and bringing a huge burden to society and families. McGeer found the association between inflammation and PD by autopsy for the first time, and activated microglia appeared in the dense part of the substantia nigra of the midbrain [3], which was proved by subsequent clinical studies [4,5]. Recent studies have further shown that neuritis is closely associated with the poor prognosis of PD [6, 7, 8].
It is well known that C-reactive protein (CRP) is a biomarker reflecting the inflammatory state of the body, and it has been reported to be associated with the progress of PD [9]. Recent reports support the association between inflammation and PD and have found that hypersensitive CRP is associated with an increased risk of PD, especially in the elderly population [10]. In addition, albumin (Alb) can reflect the nutritional status of the body, and it has also been reported that the Alb in patients with advanced PD is higher than that in early patients, and the difference is significant [9]. C-reactive protein-albumin ratio (CAR) is based on CRP and Alb and is considered a better marker of inflammation than CRP [11], reflecting the balance between CRP and Alb levels and prognostic significance based on systemic inflammation [12]. The current studies showed that CAR can be used as a better predictive marker of tumor, cardiovascular and severe burn sepsis [11,13, 14, 15], but the association between CAR and overall survival (OS) in PD has not been studied in the existing literature. Therefore, the objective of this cohort study is to examine the association between CAR and OS in PD patients.
2. Methods
2.1. Data source
All of this data is obtained from Dryad Digital Repository (https://datadryad.org/). This website permitted users to freely download the raw data. According to Dryad Terms of Service, we cited the Dryad data package (Data from Baseline C-reactive protein level and life prognosis in PD, Dryad, Dataset, https://doi.org/10.5061/dryad.63Vc5) in the present study. Their results were published in 2015 [16].
2.2. Study design and participants
This is a retrospective cohort study. Data were drawn from the Department of Neurology, the National Regional Center for Neurological Disorders, and the National Hospital of Utano, which is located in Kyoto, Japan. The enrollment time of this study is March 2004 and the endpoint is May 2014. 313 PD patients who were not infected at the time of enrollment were included. The definition of “free of infection” included no use of antibiotics, no fever (body temperature >37.5 °C), and no findings of pneumonia upon chest X-ray. PD diagnosis was based on the United Kingdom PD Brain Bank Diagnostic Criteria.
2.3. Data collection and measurements
The baseline data included age, gender, observation time, PD duration, modified Hoehn-Yahr (mH-Y) stages, Mini-Mental State Examination (MMSE) scores, and non-steroidal anti-inflammatory drug (NSAIDs). The source of the variable in this article is referred to in the article of Hideyuki Sawada [16]. The registration date for this study is the date on which the blood was first collected for CRP and Alb measurement. There was no infection before and 28 days after blood collection. In the survival time analysis, the observation period represented the time from study enrollment to the endpoint (date of death or May 16, 2014). In this study, the calculation formula of CAR is . According to the CAR level at the time of registration, the patients were divided into three groups [G1 (≤0.0714), G2 (0.0714–0.1951), and G3 (≥0.1952)] on average.
According to previous literature, aging in PD patients is likely to accelerate after the age of 70, regardless of the duration of the disease or the age of onset [17]. Therefore, we divided the age into two groups based on the age of 70 (<70 years or ≥ 70 years). It is well known that mH-Y and MMSE are important scores for evaluating the severity of motor and cognitive impairment in patients, respectively. Studies have shown that progression to higher H–Y stage 4 or above is associated with dyskinesia and deterioration of quality of life [18,19]. Traditionally, cognitive impairment has been defined as an MMSE score of ≤24 [20,21]. Therefore, the mH-Y was divided into two groups (1–3 or 4–5), and the MMSE was also divided into two groups (≤24 or > 24). Meanwhile, it has been reported that the use of ibuprofen is associated with a reduced risk of PD [22,23]. Therefore, the use of NSAIDs at study enrollment was also collected in the current analysis. Those who did not use NSAIDs at enrollment are marked as “No”, and those who were using NSAIDs and frequently used NSAIDs are marked as “Yes”. All-cause death of PD was defined as pneumonia, asphyxia, fall fracture, dehydration, accidental sudden death, vascular death, and cancer death.
2.4. Statistical analysis
For continuous variables, the data is represented as the median (interquartile range [IQR]); for classification variables, the data is expressed as a frequency or percentage. For the analysis of baseline characteristics, the continuous variables were analyzed by one-way ANOVA, and the classified variables were tested by chi-square test to test the statistical differences between the CAR tertiles. Hazard ratio (HR) and 95% confidence interval (CI) were calculated for OS in PD patients associated with CAR by using Cox proportional hazards models. Both the unadjusted model and the multivariable adjustment model are used. We input the covariates into the Cox proportional hazard model in the basic model and compare the regression coefficients to evaluate the confusion. The Cox proportional hazard model was adjusted according to age, gender, PD duration, mH-Y, MMSE, and NSAIDs. A stratified Cox proportional hazard model was used to analyze the gender, mH-Y, MMSE, and NSAIDs. Survival curves were plotted by Kaplan–Meier and log-rank analyses. The interaction between subgroups was tested by the likelihood ratio test. All the analyses were performed with the statistical software packages R (http://www.R-project.org) and Free Statistics software versions 1.2. P-value <0.05 (two-sided) was considered statistically significant.
3. Result
3.1. Population
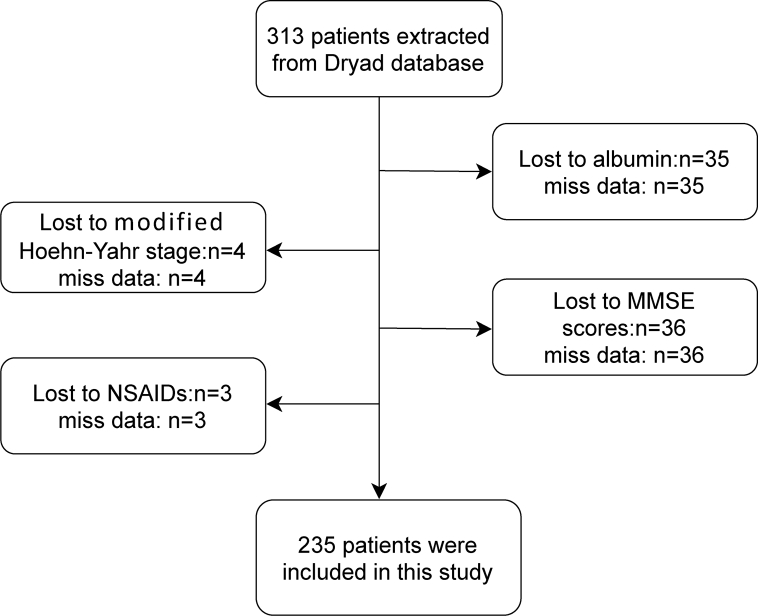
In this study, 313 PD patients were screened, and the final analysis samples included 235 PD patients. Another 78 participants were excluded because of missing data of covariates. We strictly excluded the following participants: 35 cases with missing Alb, 4 cases with missing mH-Y, 36 cases with missing MMSE, and 3 cases with missing NSAIDs. The flow chart of the study patients’ selection is presented in Fig. 1.
Fig. 1.
Flow chart of the screening of study participants. MMSE Mini-Mental Status Examination; NSAIDs non-steroidal anti-inflammatory drugs.
3.2. Baseline characteristics
The baseline characteristics of all participants are shown in Table 1. A total of 135 female and 100 male PD patients were enrolled. The median PD duration was 7 years (4−10). The majority of PD patients with mH-Y 1–3 were 148 (63.0%), while those with mH-Y 4–5 were 87 (37.0%). There were 100 (42.6%) PD patients with MMSE ≤24 and 135 (57.4%) PD patients with MMSE <24. In addition, 40 (17.0%) PD patients used NSAIDs, while the remaining 198 (83.0%) did not. The level of CAR was associated with gender, mH-Y, and MMSE of PD patients (p < 0.05), but not with age, PD duration, and NSAIDs (p > 0.05).
Table 1.
Baseline characteristics of patients.
| Variables | All participants | CAR Tertiles |
P | ||
|---|---|---|---|---|---|
| G1 (≤0.0714) | G2 (0.0714–0.1951) | G3 (≥0.1952) | |||
| Participants | n = 235 | n = 78 | n = 78 | n = 79 | |
| Age, n (%) | 0.773 | ||||
| <70 (year) | 114 (48.5) | 38 (48.7) | 40 (51.3) | 36 (45.6) | |
| ≥70 (year) | 121 (51.5) | 40 (51.3) | 38 (48.7) | 43 (54.4) | |
| Gender, n (%) | 0.040 | ||||
| Female | 135 (57.4) | 52 (66.7) | 46 (59) | 37 (46.8) | |
| Male | 100 (42.6) | 26 (33.3) | 32 (41) | 42 (53.2) | |
| PD duration (year) | 7.0 (4.0, 10.0) | 6.5 (3.2, 10.0) | 7.0 (3.0, 10.0) | 8.0 (4.5, 11.5) | 0.425 |
| mH-Y stage, n (%) | <0.001 | ||||
| 1–3 | 148 (63.0) | 63 (80.8) | 52 (66.7) | 33 (41.8) | |
| 4–5 | 87 (37.0) | 15 (19.2) | 26 (33.3) | 46 (58.2) | |
| MMSE score, n (%) | 0.017 | ||||
| ≤24 | 100 (42.6) | 32 (41) | 25 (32.1) | 43 54.4) | |
| >24 | 135 (57.4) | 46 (59) | 53 (67.9) | 36 (45.6) | |
| NSAIDs use, n (%) | 0.895 | ||||
| No | 195 (83.0) | 64 (82.1) | 66 (84.6) | 65 (82.3) | |
| Yes | 40 (17.0) | 14 (17.9) | 12 (15.4) | 14 (17.7) | |
Abbreviations: mH-Y modified Hoehn and Yahr classification, MMSE Mini-Mental Status Examination, NSAIDs non-steroidal anti-inflammatory drugs.
3.3. Univariate and multivariate Cox regression analyses of PD
The results of the univariate and multivariate Cox proportional hazards regression analyses are summarized in Table 2. In the univariate analysis, age (HR = 1.06, 95% CI = 1.02–1.10, p < 0.001), PD duration (HR = 1.09, 95% CI = 1.04–1.14, p < 0.001), mH-Y (HR = 5.10, 95% CI = 2.68–9.69, p < 0.001) and G3 group (HR = 3.20, 95% CI = 1.48–692, p = 0.003) had associated on the OS in PD patients. The HR of PD patients was a positive association with gender, MMSE, and NSAIDs (all p < 0.05).
Table 2.
Univariate and Multivariate Cox regression analyses of PD.
| Characteristics | Univariate analyses |
Multivariate analyses |
||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI adjusted) | P value adjusted | |
| Age | 1.06 (1.02–1.10) | 0.005 | 1.05 (1.01–1.10) | 0.042 |
| Male | 1.33 (0.73–2.41) | 0.360 | 1.34 (0.72–2.50) | 0.377 |
| PD duration | 1.09 (1.04–1.14) | <0.001 | 1.07 (1.01–1.12) | 0.043 |
| mH-Y (4–5) | 5.10 (2.68–9.69) | <0.001 | 3.05 (1.47–6.33) | 0.004 |
| MMSE>24 | 0.70 (0.38–1.28) | 0.254 | 1.94 (0.97–3.88) | 0.135 |
| NSAIDs (use) | 1.01 (0.45–2.26) | 0.989 | 0.85 (0.36–2.02) | 0.920 |
| CAR | ||||
| G1 (≤0.0714) | 1 | 1 | ||
| G2 (0.0714–0.1951) | 1.11 (0.45–2.73) | 0.826 | 1.03 (0.42–2.57) | 0.943 |
| G3 (≥0.1952) | 3.20 (1.48–6.92) | 0.003 | 2.17 (0.96–4.91) | 0.062 |
| Trend test | 1.54 (1.01–2.34) | 0.044 | ||
Abbreviations: mH-Y modified Hoehn and Yahr classification, MMSE Mini-Mental Status Examination, NSAIDs non-steroidal anti-inflammatory drugs, HR hazard ratio, 95% CI Confidence Interval.
In the multivariate analysis, HR is obtained after eliminating the interaction among the factors and correcting the confounders. Multivariate analysis showed that age (HR = 1.05, 95% CI = 1.01–1.10, p = 0.042), PD duration (HR = 1.07, 95% CI = 1.01–1.12, p = 0.043), mH-Y (HR = 3.05, 95% CI = 1.47–6.33, p = 0.004), and CAR (HR = 1.54, 95% CI = 1.01–2.34, p for trend = 0.044) were independently associated with OS in PD patients. There was no association between gender, MMSE, NSAIDs, and OS in PD patients (all p > 0.05).
3.4. The association between the level of CAR and OS of PD
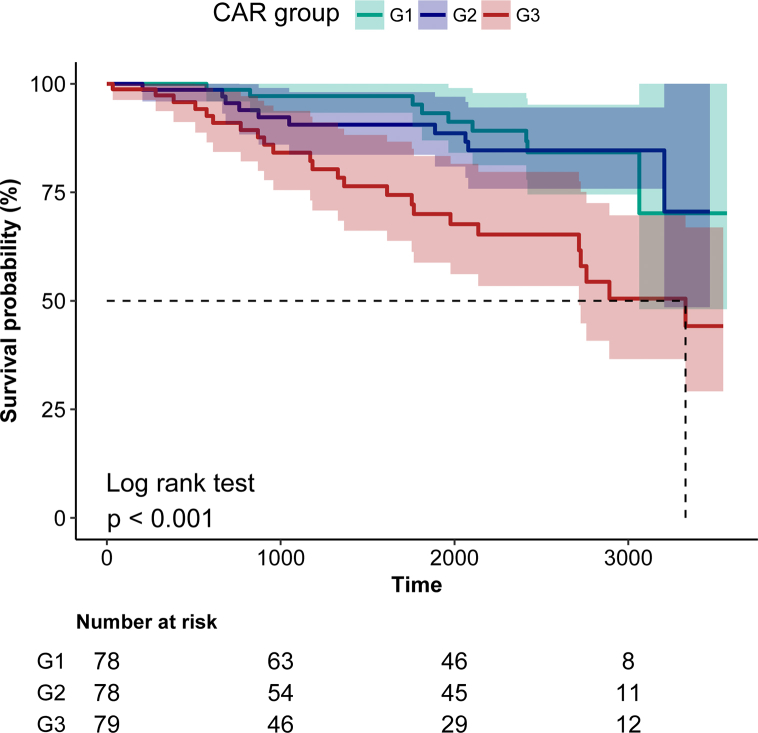
Table 3 showed the HRs and 95% CIs for risk of all-cause death in PD by CAR levels. In the unadjusted model, the risk of all-cause death in PD patients increased with the increase of CAR (HR = 1.93, 95% CI = 1.29–2.89, p for trend = 0.001). The G3 group had a threefold increase in the risk of all-cause death from PD compared with the G1 group. After adjustment in multivariable analyses, CAR was significantly associated with all-cause death of PD, so that all-cause death of PD was 0.03 or 1.17 higher with each 1 unit increase in the G2 group or G3 group compared with the G1 group. Kaplan-Meier curve showed there was higher all-cause death in PD patients with a high level of CAR (Log-rank test: p < 0.001, Fig. 2).
Table 3.
Association between CAR and OS in PD.
| Non adjusted | Adjust I | Adjust II | Adjust III | |
|---|---|---|---|---|
| CAR | 1.93 (1.29–2.89) | 1.92 (1.28–2.87) | 1.71 (1.13–2.59) | 1.54 (1.01–2.34) |
| CAR Tertiles | ||||
| G1 (≤0.0714) | 1 | 1 | 1 | 1 |
| G2 (0.0714–0.1951) | 1.11 (0.45–2.73) | 1.09 (0.44–2.69) | 1.05 (0.42–2.61) | 1.03 (0.42–2.57) |
| G3 (≥0.1952) | 3.20 (1.48–6.92) | 3.15 (1.45–6.83) | 2.59 (1.16–5.74) | 2.17 (0.96–4.91) |
| P for trend | 0.001 | 0.002 | 0.011 | 0.044 |
Data presented are HRs and 95% CIs. Adjust I model adjusts for age and gender; adjust II model adjusts for adjust I + PD duration, NSAIDs; adjust III model adjusts for adjust II + mH-Y, MMSE.
Fig. 2.
Kaplan-Meier curves of survival time of PD patients during follow-up. G1, G2, G3: Kaplan-Meier curves of different groups in PD patients grouped by CRA level. The shadow area represents the 95% CI.
3.5. Subgroup analyses
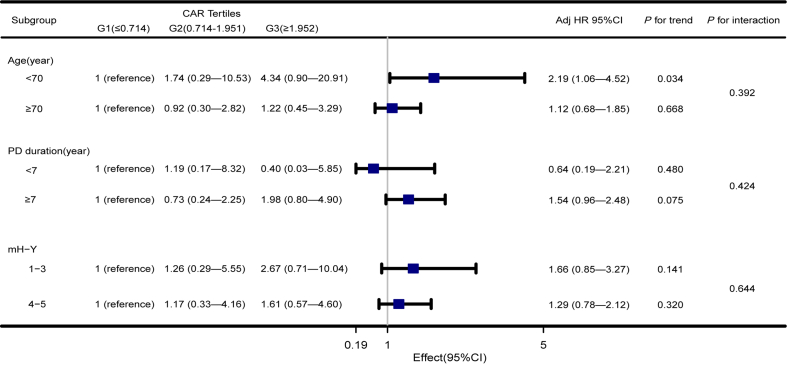
To understand whether the association between CAR level and OS of PD is stable, we performed subgroup analysis and interaction analysis (Fig. 3). According to the median (7 years), the PD duration is divided into two groups. The trend test was carried out for people under 70 years old. In addition, the study found that the HR of PD duration less than 7 years group (HR = 0.64, 95% CI = 0.19–2.21) and G3 group (HR = 0.40, 95% CI = 0.03–5.85) was lower. However, the results showed that the effect of CAR on the OS of PD was stable in the subgroup, while the multiplicative interaction of CAR × age (p for interaction = 0.392), CAR × PD duration (p for interaction = 0.424), and CAR × mH-Y (p for interaction = 0.644) on the OS of PD was not significant.
Fig. 3.
Association between CAR and OS in PD according to baseline characteristics. Each stratification adjusted for all the factors (age, gender, PD duration, mH-Y, MMSE, and NSAIDs).
4. Discussion
Through a literature search, we have not found any research on the association between CAR and OS in PD patients. In this retrospective cohort study, the high level of CAR indicates poor OS in PD patients. After subgroup analysis, the results were still stable.
In the previous literature, it is still controversial whether the use of NSAIDs can reduce the incidence and progression of Parkinson's disease [24, 25, 26, 27, 28]. However, we found that CAR levels were not associated with NSAIDs (Table 1), although they may change and affect inflammatory levels in the long-term clinical course, further studies are needed to find conclusions. In this study, COX univariate analysis showed that age, PD duration, mH-Y, and CAR were independently associated with OS in PD patients (p < 0.05) (Table 2). This result is consistent with previous reports, as it has been confirmed that age [17,29], PD duration [30], and mH-Y [19] are associated with PD progression. In addition, the survival curve showed that there was a significant difference in mortality among the three CAR groups (p < 0.001), and the OS in PD patients with high levels of CAR was lower (Fig. 2).
Subgroup analysis showed that except for the age <70 years old group, there was a stable interlayer association between CAR and OS in PD patients (Fig. 3). The results showed that CAR concentration was positively associated with OS in PD patients in the age <70 years old group (HR = 2.19, 95% CI = 1.06–4.52, p for trend = 0.034). Simultaneously, we noted that the HR of the G3 group under the age <70 years old was 4.34 times higher than that of the G1 group (HR = 4. 34, 95% CI = 0.90–20.91). However, the result is not statistically significant. Previous studies have shown that the effect of age on the progression of PD is significant [17,29], so the age variable may have an impact on the value of CAR in PD patients, which still needs to be studied with a larger sample size. Moreover, the study found that HR was lower in the PD duration <7 years group (HR = 0.64, 95% CI = 0.19–2.21), which may suggest that PD duration <7 years is a protective factor for all-cause death of PD, but the results are not statistically significant.
Recent clinical studies have found that higher levels of CAR are also associated with poor OS of other diseases, and CAR may be used as a better prognostic marker than single inflammatory markers such as CRP [31, 32, 33, 34]. CAR is not a simple addition of CRP or Alb. A study shows that CAR can reflect the dynamic changes of systemic inflammation and identify slight differences between patients [35], which suggests that CAR is likely to be more sensitive to predicting systemic [32]. As a marker of an acute reaction, CRP is widely used in the clinic. Similarly, the level of Alb is also an effective marker of mortality in many diseases [36]. Interestingly, the CRP response increased in the acute phase, while Alb decreased in both acute and chronic inflammatory conditions [37, 38, 39]. This suggests that CAR may increase both acute and chronic inflammation, which further reflects the advantage of CAR.
Although there is a certain understanding of the etiology of PD, and there are many efficient treatments, PD is still a refractory progressive disease that can lead to disability [1]. If the prognosis of PD patients can be predicted earlier and more accurately, it may provide a certain possibility for delaying the progress of PD and improving the life quality of patients. Most of the current studies on PD and inflammation focus on neuroinflammation [40]. Our results provide a good reference for clinical doctors to remind them that it is necessary to control systemic inflammation and ensure adequate nutrition in patients with PD. CAR can be used as a good indicator of prognosis. To some extent, this also guides the lives of PD patients. The strength of our study included the cohort study based on publicly available data, which provides a certain degree of data credibility. In addition, we strictly excluded the participants with missing covariates, and after adjusting the important variables associated with the progress of PD, such as age, PD duration, and mH-Y, we still obtained a stable association between CAR and the OS in PD.
Our study also has some limitations. Some studies have reported that PD may be affected by races, nationalities, genotypes, or environments [1,41, 42, 43], while our participants are from Japan in Asia. In addition, diseases with CRP, Alb, or CAR changes should also be considered to ensure the adequacy of baseline data. Thus, to exclude the influence of race on this study, the conclusions need to be further explored in a large multicentric clinical trial. However, the plasma values of Alb are 5000 to 10 000 times higher than those of CRP. The biological disproportion between these two proteins limits the importance of their ratio. And there is no cut-off value for “high-level CAR”, suggesting that more research may be needed to explore the definition of “high-level CAR” which can be widely used in the future.
5. Conclusion
The all-cause mortality of PD patients with a high level of CAR is higher, which indicates that the poor overall survival of PD patients is associated with the increase of CAR. The CAR may be a reliable prognostic biomarker for PD patients. This association is worthy of further study.
Funding
This study was supported by (1) National Traditional Chinese Medicine Clinical Characteristic Technology Inheritance Talent Project [Chinese Medicine People’s Education Letter (2019) No. 36]; (2) Major Public Relations Project of Scientific and technological Innovation Project of Chinese Academy of Chinese Medical Science (CI2021A01204) (3) Shandong Province Financial Special Fund Shandong Province famous 380 veteran traditional Chinese Medicine inheritance Studio Construction Project (grant no. Lu traditional 381 Chinese Medicine letter (2018) No. 5).
Data availability statements
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethic statements
The study was approved by the Bioethics Committee of National Hospital of Utano (approval no. 26–4). The Bioethics committee waived the need for informed consent due to the retrospective nature of the study and anonymity of the collected data.
Author contributions
Mengqi Gao, Chuanlong Zhang, Shanmei Sun, Lucheng Song, Shiwei Liu: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Lijie Gao: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Lucheng Song, Shiwei Liu: Conceived and designed the experiments; Wrote the paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Jie Liu for his guiding suggestions for this study. We thank Hideyuki Sawada et al. for providing us with available data. We are grateful for the helpful reviewer comments on this paper.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2022.e12671.
Contributor Information
Lucheng Song, Email: lucheng.s@163.com.
Shiwei Liu, Email: liushiwei1977@yeah.net.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Poewe W., Seppi K., Tanner C.M., Halliday G.M., Brundin P., Volkmann J., et al. Parkinson disease. Nat. Rev. Dis. Prim. 2017;3 doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- 2.van de Kamp J.M., Errami A., Howidi M., Anselm I., Winter S., Phalin-Roque J., et al. Mortality in Parkinson's disease: a 38-year follow-up study. Clin. Genet. 2015;87(2):141–147. doi: 10.1111/cge.12355. [DOI] [PubMed] [Google Scholar]
- 3.McGeer P.L., Itagaki S., Boyes B.E., McGeer E.G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38(8):1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 4.Taylor J.M., Main B.S., Crack P.J. Neuroinflammation and oxidative stress: co-conspirators in the pathology of Parkinson's disease. Neurochem. Int. 2013;62(5):803–819. doi: 10.1016/j.neuint.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Duke D.C., Moran L.B., Pearce R.K., Graeber M.B. The medial and lateral substantia nigra in Parkinson's disease: mRNA profiles associated with higher brain tissue vulnerability. Neurogenetics. 2007;8(2):83–94. doi: 10.1007/s10048-006-0077-6. [DOI] [PubMed] [Google Scholar]
- 6.Gu C., Hu Q., Wu J., Mu C., Ren H., Liu C.F., et al. P7C3 inhibits LPS-induced microglial activation to protect dopaminergic neurons against inflammatory factor-induced cell death in vitro and in vivo. Front. Cell. Neurosci. 2018;12:400. doi: 10.3389/fncel.2018.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luan Y.Y., Yao Y.M. The clinical significance and potential role of C-reactive protein in chronic inflammatory and neurodegenerative diseases. Front. Immunol. 2018;9:1302. doi: 10.3389/fimmu.2018.01302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirsch E.C., Hunot S. Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol. 2009;8(4):382–397. doi: 10.1016/s1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 9.Pisani V., Stefani A., Pierantozzi M., Natoli S., Stanzione P., Franciotta D., et al. Increased blood-cerebrospinal fluid transfer of albumin in advanced Parkinson's disease. J. Neuroinflammation. 2012;9:188. doi: 10.1186/1742-2094-9-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin H., Gu H.Y., Mao C.J., Chen J., Liu C.F. Association of inflammatory factors and aging in Parkinson's disease. Neurosci. Lett. 2020;736 doi: 10.1016/j.neulet.2020.135259. [DOI] [PubMed] [Google Scholar]
- 11.Yu Y., Wu W., Dong Y., Li J. C-reactive protein-to-albumin ratio predicts sepsis and prognosis in patients with severe burn injury. Mediat. Inflamm. 2021;2021 doi: 10.1155/2021/6621101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fairclough E., Cairns E., Hamilton J., Kelly C. Evaluation of a modified early warning system for acute medical admissions and comparison with C-reactive protein/albumin ratio as a predictor of patient outcome. Clin. Med. 2009;9(1):30–33. doi: 10.7861/clinmedicine.9-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao C.K., Yu Y.L., Lin Y.C., Hsu Y.J., Chern Y.J., Chiang J.M., et al. Prognostic value of the C-reactive protein to albumin ratio in colorectal cancer: an updated systematic review and meta-analysis. World J. Surg. Oncol. 2021;19(1):139. doi: 10.1186/s12957-021-02253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelesoglu S., Yilmaz Y., Elcik D. Relationship between C-reactive protein to albumin ratio and coronary collateral circulation in patients with stable coronary artery disease. Angiology. 2021 doi: 10.1177/00033197211004392. [DOI] [PubMed] [Google Scholar]
- 15.Cagdas M., Rencuzogullari I., Karakoyun S., Karabag Y., Yesin M., Artac I., et al. Assessment of relationship between C-reactive protein to albumin ratio and coronary artery disease severity in patients with acute coronary syndrome. Angiology. 2019;70(4):361–368. doi: 10.1177/0003319717743325. [DOI] [PubMed] [Google Scholar]
- 16.Sawada H., Oeda T., Umemura A., Tomita S., Kohsaka M., Park K., et al. Baseline C-reactive protein levels and life prognosis in Parkinson disease. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0134118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kempster P.A., O'Sullivan S.S., Holton J.L., Revesz T., Lees A.J. Relationships between age and late progression of Parkinson's disease: a clinico-pathological study. Brain. 2010;133(Pt 6):1755–1762. doi: 10.1093/brain/awq059. [DOI] [PubMed] [Google Scholar]
- 18.Goetz C.G., Poewe W., Rascol O., Sampaio C., Stebbins G.T., Counsell C., et al. Movement disorder society task force report on the Hoehn and Yahr staging scale: status and recommendations. Mov. Disord. 2004;19(9):1020–1028. doi: 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- 19.Liou H.H., Wu C.Y., Chiu Y.H., Yen A.M., Chen R.C., Chen T.F., et al. Mortality of Parkinson's disease by Hoehn-Yahr stage from community-based and clinic series [Keelung Community-based Integrated Screening (KCIS) no. 17)] J. Eval. Clin. Pract. 2009;15(4):587–591. doi: 10.1111/j.1365-2753.2008.01041.x. [DOI] [PubMed] [Google Scholar]
- 20.Dick J.P., Guiloff R.J., Stewart A., Blackstock J., Bielawska C., Paul E.A., et al. Mini-mental state examination in neurological patients. J. Neurol. Neurosurg. Psychiatry. 1984;47(5):496–499. doi: 10.1136/jnnp.47.5.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arevalo-Rodriguez I., Smailagic N., Roqué I.F.M., Ciapponi A., Sanchez-Perez E., Giannakou A., et al. Mini-Mental State Examination (MMSE) for the detection of Alzheimer's disease and other dementias in people with mild cognitive impairment (MCI) #N/A. 2015;2015(3) doi: 10.1002/14651858.CD010783.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao X.A., Chen H.L., Schwarzschild M.A., Ascherio A. Use of ibuprofen and risk of Parkinson disease. Neurology. 2011;76(10):863–869. doi: 10.1212/WNL.0b013e31820f2d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Driver J.A., Logroscino G., Lu L., Gaziano J.M., Kurth T. Use of non-steroidal anti-inflammatory drugs and risk of Parkinson's disease: nested case-control study. Br. Med. J. 2011;342:d198. doi: 10.1136/bmj.d198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen H., Zhang S.M., Hernan M.A., Schwarzschild M.A., Willett W.C., Colditz G.A., et al. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch. Neurol. 2003;60(8):1059–1064. doi: 10.1001/archneur.60.8.1059. [DOI] [PubMed] [Google Scholar]
- 25.Rees K., Stowe R., Patel S., Ives N., Breen K., Clarke C.E., et al. Non-steroidal anti-inflammatory drugs as disease-modifying agents for Parkinson's disease: evidence from observational studies. #N/A. 2011;(11) doi: 10.1002/14651858.CD008454.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Gao X., Chen H., Schwarzschild M.A., Ascherio A. Use of ibuprofen and risk of Parkinson disease. Neurology. 2011;76(10):863–869. doi: 10.1212/WNL.0b013e31820f2d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bornebroek M., de Lau L.M., Haag M.D., Koudstaal P.J., Hofman A., Stricker B.H., et al. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Neuroepidemiology. 2007;28(4):193–196. doi: 10.1159/000108110. [DOI] [PubMed] [Google Scholar]
- 28.Ton T.G., Heckbert S.R., Longstreth W.T., Jr., Rossing M.A., Kukull W.A., Franklin G.M., et al. Nonsteroidal anti-inflammatory drugs and risk of Parkinson's disease. Mov. Disord. 2006;21(7):964–969. doi: 10.1002/mds.20856. [DOI] [PubMed] [Google Scholar]
- 29.de Lau L.M., Breteler M.M. Epidemiology of Parkinson's disease. Lancet Neurol. 2006;5(6):525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 30.de Lau L.M., Schipper C.M., Hofman A., Koudstaal P.J., Breteler M.M. Prognosis of Parkinson disease: risk of dementia and mortality: the Rotterdam Study. Arch. Neurol. 2005;62(8):1265–1269. doi: 10.1001/archneur.62.8.1265. [DOI] [PubMed] [Google Scholar]
- 31.Cinier G., Hayiroglu M.I., Kolak Z., Tezen O., Yumurtas A.C., Pay L., et al. The value of C-reactive protein-to-albumin ratio in predicting long-term mortality among HFrEF patients with implantable cardiac defibrillators. Eur. J. Clin. Invest. 2021 doi: 10.1111/eci.13550. ARTN e13550. [DOI] [PubMed] [Google Scholar]
- 32.Yu Y.H., Wu W.W., Dong Y.Y., Li J.L. C-reactive protein-to-albumin ratio predicts sepsis an prognosis in patients with severe burn injury. Mediat. Inflamm. 2021:2021. doi: 10.1155/2021/6621101. Artn 6621101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang H.N., Pan B.H., Wang L., Zhu H.Y., Fan L., Xu W., et al. C-reactive protein-to-albumin ratio is an independent poor prognostic factor in newly diagnosed chronic lymphocytic leukaemia: a clinical analysis of 322 cases. Transl. Oncol. 2021;14(4) doi: 10.1016/j.tranon.2021.101035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Capkin S., Guler S., Ozmanevra R. C-reactive protein to albumin ratio may predict mortality for elderly population who undergo hemiarthroplasty due to hip fracture. J. Invest. Surg. 2020 doi: 10.1080/08941939.2020.1793038. [DOI] [PubMed] [Google Scholar]
- 35.Sun P., Chen C., Xia Y., Bi X., Liu P., Zhang F., et al. The ratio of C-reactive protein/albumin is a novel inflammatory predictor of overall survival in cisplatin-based treated patients with metastatic nasopharyngeal carcinoma. Dis. Markers. 2017;2017 doi: 10.1155/2017/6570808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akirov A., Masri-Iraqi H., Atamna A., Shimon I. Low albumin levels are associated with mortality risk in hospitalized patients. Am. J. Med. 2017;130(12):e11–e19. doi: 10.1016/j.amjmed.2017.07.020. 1465. [DOI] [PubMed] [Google Scholar]
- 37.Levitt D.G., Levitt M.D. Human serum albumin homeostasis: a new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int. J. Gen. Med. 2016;9:229–255. doi: 10.2147/IJGM.S102819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lemieux I., Pascot A., Prud'homme D., Alméras N., Bogaty P., Nadeau A., et al. Elevated C-reactive protein: another component of the atherothrombotic profile of abdominal obesity. #N/A. 2001;21(6):961–967. doi: 10.1161/01.atv.21.6.961. [DOI] [PubMed] [Google Scholar]
- 39.Quispe E.A., Li X.M., Yi H. Comparison and relationship of thyroid hormones, IL-6, IL-10 and albumin as mortality predictors in case-mix critically ill patients. Cytokine. 2016;81:94–100. doi: 10.1016/j.cyto.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Pajares M., IR A., Manda G., Bosca L., Cuadrado A. Inflammation in Parkinson's disease: mechanisms and therapeutic implications. Cells. 2020;9(7) doi: 10.3390/cells9071687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abbas M.M., Xu Z., Tan L.C.S. Epidemiology of Parkinson's disease-east versus west. Mov. Disord. Clin. Pract. 2018;5(1):14–28. doi: 10.1002/mdc3.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pitcher T.L., Myall D.J., Pearson J.F., Lacey C.J., Dalrymple-Alford J.C., Anderson T.J., et al. Parkinson's disease across ethnicities: a nationwide study in New Zealand. Mov. Disord. 2018;33(9):1440–1448. doi: 10.1002/mds.27389. [DOI] [PubMed] [Google Scholar]
- 43.Ball N., Teo W.P., Chandra S., Chapman J. Parkinson's disease and the environment. Front. Neurol. 2019;10:218. doi: 10.3389/fneur.2019.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.