Key Points
-
•
Treatment with IL-15 before alemtuzumab was safe and led to decreased number and proliferative activity of T-cell leukemic cells.
-
•
IL-15 was associated with increased activation and proliferation of patient-derived NK and CD8+ T cells, increasing their ADCC capacity.
Visual Abstract
Abstract
Interleukin-15 (IL-15) monotherapy substantially increases the number and activity of natural killer (NK) cells and CD8+ T cells but has not produced clinical responses. In a xenograft mouse model, IL-15 enhanced the NK cell–mediated antibody-dependent cell cytotoxicity (ADCC) of the anti-CD52 antibody alemtuzumab and led to significantly more durable responses than alemtuzumab alone. To evaluate whether IL-15 potentiates ADCC in humans, we conducted a phase 1 single-center study of recombinant human IL-15 and alemtuzumab in patients with CD52-positive mature T-cell malignances. We gave IL-15 subcutaneously 5 days per week for 2 weeks in a 3 + 3 dose escalation scheme (at 0.5, 1, and 2 μg/kg), followed by standard 3 times weekly alemtuzumab IV for 4 weeks. There were no dose-limiting toxicities or severe adverse events attributable to IL-15 in the 11 patients treated. The most common adverse events were lymphopenia (100%), alemtuzumab-related infusion reactions (90%), anemia (90%), and neutropenia (72%). There were 3 partial and 2 complete responses, with an overall response rate of 45% and median duration of response 6 months. Immediately after 10 days of IL-15, there was a median 7.2-fold increase in NK cells and 2.5-fold increase in circulating CD8+ T cells, whereas the number of circulating leukemic cells decreased by a median 38% across all dose levels. Treatment with IL-15 was associated with increased expression of NKp46 and NKG2D, markers of NK-cell activation, and increased ex vivo ADCC activity of NK cells, whereas inhibitory receptors PD1 and Tim3 were decreased. This trial was registered at www.clinicaltrials.gov as #NCT02689453.
Introduction
Adult T-cell leukemia/lymphoma (ATL) is a highly aggressive malignancy caused by the human T-lymphotropic virus-1 retrovirus.1 Although patients with the indolent smoldering and chronic subtypes have median overall survival (OS) of 6 to 8 years, those with the more common acute and lymphoma subtypes, along with the more recently delineated high-risk chronic and aggressive cutaneous subtypes, have median OS of less than a year from diagnosis.2 Allogeneic stem cell transplantation (alloSCT) is the only known curative treatment for ATL, with a 30% to 40% cure rate described in Japan3; however, most patients treated in North America have chemotherapy-refractory disease and have no clear survival benefit from alloSCT.4 Mogamulizumab, an immunoglobulin G4 monoclonal antibody targeting C-C chemokine receptor type 4, has been approved in Japan for treatment of relapsed/refractory ATL, but the overall response rate in the global phase 2 study was less than 20%.5
Our group had conducted a single-center phase 2 study of alemtuzumab (CAMPATH) 12-week monotherapy in patients with aggressive subtypes of ATL, in which the overall response rate (ORR) was 40% but the median duration of response was only 3 months.6 Prolonged treatment with alemtuzumab is also associated with an increased incidence of opportunistic infections and viral reactivation.7 The infection rfisk is further increased by ATL itself, as the malignant cells have an immunosuppressive T-regulatory cell phenotype and function.8 Alemtuzumab is an immunoglobulin G1 monoclonal antibody that targets the cell surface glycoprotein CD52, also known as the CAMPATH-1 antigen, expressed by most lymphoid malignancies including more than 90% of cases of ATL and peripheral T-cell lymphoma not otherwise specified (PTCL-NOS).9, 10, 11, 12 It achieves its tumor-killing effect chiefly through antibody-dependent cell-mediated cytotoxicity (ADCC), which is mediated by natural killer (NK) cells and macrophages.13
To enhance the ADCC activity of alemtuzumab we tested it in combination with interleukin-15 (IL-15) in an animal model of ATL.13 IL-15 is a cytokine of the α-helix bundle family that shares 2 receptor components: IL-2 receptor-β (IL-2/15R-α or CD122) and the common γ chain with IL-2.14, 15, 16, 17 In a multicenter phase 1 study of subcutaneous recombinant human (rh)IL-15 monotherapy for patients with solid tumors, it did not lead to tumor responses but did achieve an eightfold increase in circulating NK cells and a threefold increase in CD8+ T cells.18 We hypothesized that the increase of NK-cell count would lead to enhanced ADCC activity of antitumor antibodies. With this in mind, we tested the combination of IL-15 and alemtuzumab in nonobese, diabetic, SCIDγ (NSG) mice injected with the ATL cell line MT-119 and demonstrated superior responses and survival of mice receiving the combination compared with either agent alone, in support of a human trial.
Here we present the results of the single-center phase 1 study of subcutaneous rhIL-15 in combination with alemtuzumab for patients with CD52-positive T-cell lymphomas including ATL and PTCL-NOS.
Materials and methods
Study design and patients
Patients with the diagnosis of acute and high-risk chronic subtype ATL, PTCL-NOS, angioimmunoblastic T-cell lymphoma (AITL), mycosis fungoides/Sezary syndrome, and T-prolymphocytic leukemia who had persistent or relapsed disease after 1 or more lines of systemic therapy, including alloSCT, were eligible. Other requirements included appropriate bone marrow function (hemoglobin > 9 g/dL, absolute neutrophil count > 1000 cells/μL, platelet count > 100 000/μL), liver and kidney function (creatinine ≤ 1.5 g/dL, liver function tests < 3 times upper limit of normal), performance status (Eastern Cooperative Oncology Group Performance Status [ECOG] ≤ 1), and absence of other malignancies, autoimmune disorders, and conditions requiring the use of more than replacement dose steroids (greater than prednisone 10 mg daily or equivalent). See supplemental Appendix 1 for full eligibility criteria.
The protocol was registered at clinicaltrials.gov (#NCT02689453) and was conducted in accordance with the principles of the Declaration of Helsinki. The treatment study was approved by the National Cancer Institute institutional review board, and all participants signed informed consent. For the de-identified analysis of circulating tumor deoxyribonucleic acid (ctDNA) we obtained exemption from institutional review board review.
Treatment and response assessment
Treatment was limited to 6 weeks: subcutaneous rhIL-15 was given at doses of 0.5, 1, and 2 μg/kg per day (dose levels 1, 2, and 3) Monday through Friday of the first 2 weeks, followed by titration of IV alemtuzumab from 3 to 30 mg during week 3, and administration at full 30-mg dose on Mondays, Wednesdays, and Fridays of weeks 4 through 6 (supplemental Figure 1A). Dose escalation of rhIL-15 occurred in cohorts of 3 to 6 participants until the maximum tolerated dose was reached, defined as the highest dose of rhIL-15 at which ≤1 of 6 participants developed a dose-limiting toxicity (DLT). There was no intrapatient dose escalation or other modification of rhIL-15. We used standard dose adjustment of alemtuzumab based on severity of initial and subsequent infusion reactions and other toxicities (supplemental Figure 1B).
Participants received acetaminophen before each rhIL-15 dose and acetaminophen and diphenhydramine before each alemtuzumab dose. Everyone received antiviral prophylaxis with acyclovir 400 mg twice daily or equivalent, and Pneumocystis jirovecii pneumonia prophylaxis with Bactrim DS or equivalent. Only participants with documented history of fungal infections received prophylactic fluconazole. After 2 participants developed asymptomatic venous thromboembolism (VTE) during treatment, mandatory prophylaxis with oral rivaroxaban or equivalent was instituted.
Restaging by computerized tomography (CT) occurred at the end of week 3 and week 6 during treatment, then every 60 days for 6 months, and every 90 days for up to 2 years after finishing treatment. Additionally, positron emission tomography (PET) scans were performed at the end of treatment and at scheduled posttreatment follow-up. Response and progression were evaluated using the international consensus criteria for response assessment for participants with ATL1 and Response Evaluation Criteria in Solid Tumors guideline version 1.1 for all other participants.20 Leukemic cells as percentage of peripheral blood mononuclear cells (PBMCs) and as absolute numbers were followed by flow cytometry in a Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory.
Toxicity criteria
We used the revised National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 for toxicity reporting. See supplemental Appendix 1 for definitions of serious adverse events (SAEs) and DLTs.
Correlative assays
Quantification of immune cell subsets, ADCC analysis, cytokine measurements, and detection of anti–rhIL-15 antibodies were performed as previously described.21,22
Results
Eleven participants were enrolled at the Clinical Center of the National Institutes of Health between January 2017 and June 2020 (Table 1). Median age was 46 years (range, 18-71 years), and 9 of 11 participants (82%) were male. Median number of prior treatments was 2 (range, 1-7), with 9 participants having received prior anthracycline-based combination chemotherapy. Two participants, with acute subtype ATL and PTCL-NOS, had relapses after alloSCT.
Table 1.
Characteristic and outcomes of all enrolled patients
| ID | Age | Sex | Diagnosis | Number of prior treatments | Dose level | Best response | NK cell increase (fold) | CD8+ T-cell increase (fold) |
|---|---|---|---|---|---|---|---|---|
| 1 | 37 | Female | ATL-a | 7 | 1 | SD | 1.04 | 1.41 |
| 2 | 18 | Male | ATL-a | 1 | 1 | CR | 2.81 | 1.21 |
| 3 | 20 | Male | ATL-a | 2∗ | 1 | PD | 5.93 | 2.92 |
| 4 | 48 | Male | ATL-a | 1 | 2 | PD | 5.22 | 3.57 |
| 5 | 66 | Male | ATL-a | 3 | 2 | SD | 11.51 | 4.88 |
| 6 | 41 | Male | ATL-c | 2 | 2 | PR | 10.94 | 3.24 |
| 7 | 71 | Female | PTCL-NOS | 6 | 2 | PD | 8.43 | 0.97 |
| 8 | 51 | Male | PTCL-NOS | 4∗ | 3 | CR | 4.40 | 2.15 |
| 9 | 48 | Male | ATL-c | 1 | 3 | PR | 15.31 | 1.95 |
| 10 | 46 | Male | ATL-a | 1 | 3 | PD | — | — |
| 11 | 43 | Male | ATL-a | 1 | 3 | SD | 17.12 | 5.98 |
ATL-a, acute subtype adult T-cell leukemia/lymphoma; ATL-c, chronic subtype adult T-cell leukemia/lymphoma; PD, progressive disease; SD, stable disease.
Prior treatment includes allogeneic stem cell transplantation.
Ten of 11 participants were able to complete the 2-week course of rhIL-15. The 1 participant unable to complete the IL-15 pretreatment had acute subtype ATL with known liver involvement and had rapidly progressing disease even before treatment initiation; he stopped protocol treatment and received cytotoxic chemotherapy after a single dose of rhIL-15 because of acutely worsening intrahepatic biliary obstruction and jaundice. Another participant with acute subtype ATL did not complete the 4 weeks of alemtuzumab because of progressive disease and refractory hypercalcemia. In the remaining 9, we were able to administer all 90 planned rhIL-15 doses and 114 of the 117 planned alemtuzumab doses.
Adverse events and tolerability
We did not observe any DLTs among the participants, nor did administration of rhIL-15 lead to accelerated disease progression or acute worsening of alemtuzumab-related infusion reactions. There were 2 SAEs, both VTEs deemed possibly or probably related to alemtuzumab but not rhIL-15. In both cases, an end-of-treatment CT scan of the chest showed pulmonary emboli and a central line-associated thrombus, with normal blood oxygen saturation and no respiratory or cardiac symptoms. Both participants had resolution of VTE on follow-up scans after removal of the central line and 3 months of therapeutic anticoagulation. The most common grade ≥ 3 hematologic adverse events (AEs; Table 2) were lymphopenia (55%), and leukopenia (45%), whereas 82% of participants had treatment-emergent anemia of any grade and 36% had thrombocytopenia of any grade, all of which were attributed to alemtuzumab. All hematologic toxicities except for lymphopenia resolved within 4 weeks of therapy completion. Neither of the 2 patients after alloSCT experienced graft-versus-host disease.
Table 2.
Infectious, hematologic, and other adverse events possibly, probably or definitely related to research, excluding single occurrences of grade 1 events
| CTCAE v5.0 term | All dose levels, n = 11 |
Dose level 1 (0.5 μg/kg/d), n = 3 |
Dose level 2 (1 μg/kg/d), n = 4 |
Dose level 3 (2 μg/kg/d), n = 4 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade ≤ 2 | Grade 3 | Grade 4 | Grade ≤ 2 | Grade 3 | Grade 4 | Grade ≤ 2 | Grade 3 | Grade 4 | |
| Infections and infestations (CMV) | 4 | 4 | |||||||||||
| Herpes simplex reactivation | 1 | 1 | |||||||||||
| Shingles | 1 | 1 | |||||||||||
| Sinusitis | 1 | 1 | |||||||||||
| Skin infection | 1 | 1 | |||||||||||
| Lymphocyte count decreased | 3 | 2 | 2 | 4 | 1 | 1 | 3 | 4 | 1 | 1 | |||
| White blood cell decreased | 3 | 4 | 4 | 1 | 5 | 3 | 1 | 2 | 1 | ||||
| Neutrophil count decreased | 3 | 3 | 2 | 3 | 1 | 3 | 1 | ||||||
| Thromboembolic event | 2 | 1 | 1 | ||||||||||
| Anemia | 1 | 7 | 1 | 6 | 1 | 2 | |||||||
| Platelet count decreased | 2 | 1 | 1 | 1 | 1 | 2 | |||||||
| Infusion related reaction | 1 | 2 | 1 | 2 | 1 | 1 | |||||||
| Rash maculo-papular | 3 | 1 | 1 | 3 | |||||||||
| Urticaria | 2 | 1 | 1 | 1 | 1 | ||||||||
| Serum amylase increased | 1 | 1 | |||||||||||
| Fever | 9 | 2 | 2 | 8 | 1 | ||||||||
| Chills | 4 | 4 | 1 | ||||||||||
| Hyponatremia | 8 | 8 | |||||||||||
| Fatigue | 3 | 3 | 4 | 2 | |||||||||
| Aspartate aminotransferase increased | 3 | 2 | 3 | 2 | |||||||||
| Sinus bradycardia | 5 | ||||||||||||
| Hypophosphatemia | 1 | 3 | 1 | 2 | 1 | ||||||||
| Alanine aminotransferase increased | 3 | 1 | 3 | 1 | |||||||||
| Hypoalbuminemia | 2 | 1 | 3 | ||||||||||
| Hyperglycemia | 3 | 3 | |||||||||||
| Nausea | 3 | 2 | 1 | ||||||||||
| Bone pain | 1 | 1 | 2 | ||||||||||
| Gastroesophageal reflux disease | 1 | 1 | 2 | ||||||||||
| Anorexia | 2 | 2 | |||||||||||
| Creatinine increased | 2 | 2 | |||||||||||
| Hyperkalemia | 2 | 2 | |||||||||||
| Pruritus | 1 | 1 | |||||||||||
There were 4 grade 3 nonhematologic AEs reported among the 11 participants, 1 each of infusion-related reaction, maculopapular rash, urticaria, and increased serum amylase. The most common nonhematologic AE of any grade was fever, occurring in all, and chills in 8 of 11 participants, which were attributed to both drugs. The frequency and severity of alemtuzumab-associated infusion reactions were not above what was expected with alemtuzumab; however, several participants developed marked cutaneous reactions shortly after the initial infusions, which in 1 participant led to a permanent decrease of alemtuzumab dose to 10 mg. Anti–IL-15 antibodies were not detected in any of the participants.
Clinical efficacy
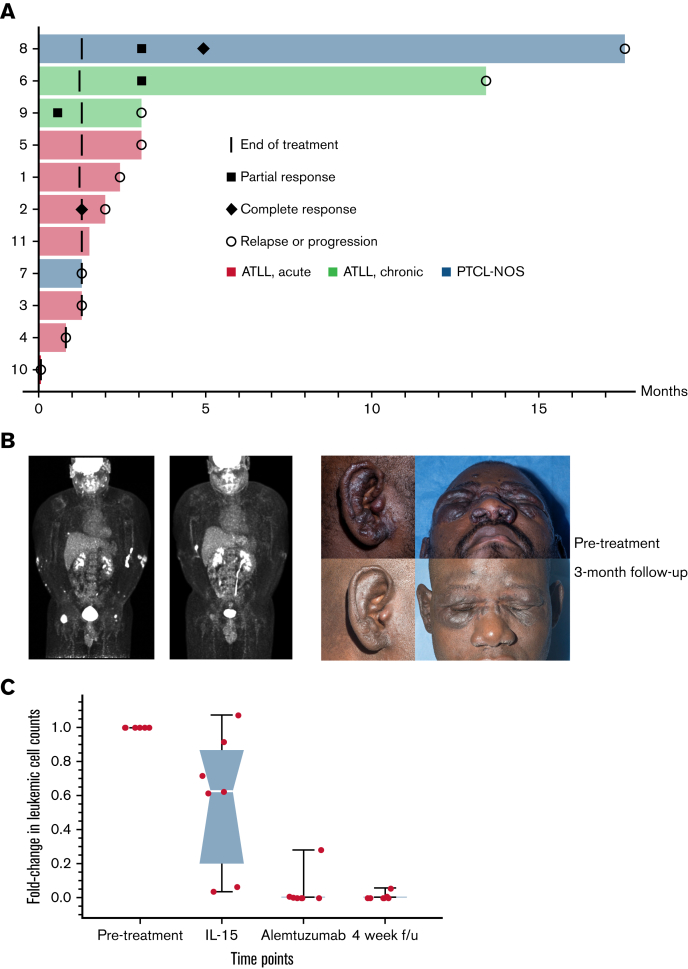
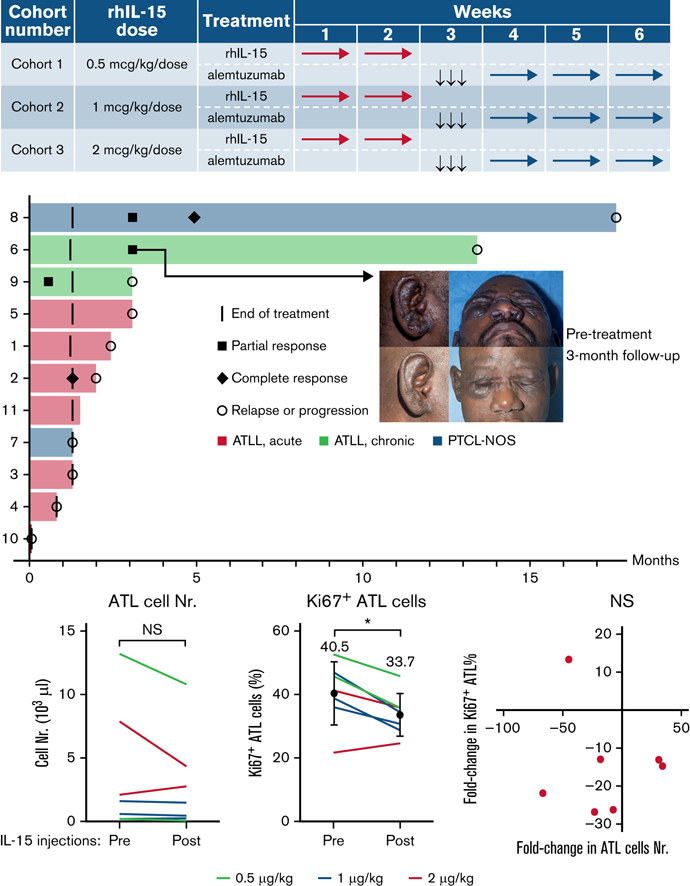
The overall response rate was 36%, with 2 complete responses (CRs) and 2 partial responses (PRs; Figure 1A). Median duration of response was 7 months (range, 4 weeks to 14 months). A participant whose PTCL-NOS relapsed after alloSCT at the site of his original disease (popliteal fossa) developed a CR that lasted for more than a year. The second CR was in a participant with acute subtype ATL who was initially treated with combination chemotherapy without intrathecal prophylaxis and had predominantly leukemic disease at relapse. The CR was noted after the first week of treatment with alemtuzumab, but the participant relapsed in the central nervous system 2 months later. Notably, the central nervous system remained the only site of disease until their death 8 months later. A participant with chronic subtype ATL deemed high risk because of tumor-stage cutaneous lesions and who was refractory to prior combination chemotherapy and radiotherapy had marked improvement in skin lesions with persistent residual fluorodeoxyglucose (FDG) uptake on PET even while the lesions continued shrinking and soluble CD25 normalized (Figure 1B). Low-level FDG uptake and continuing decrease in size persisted for several months after completion of treatment, despite no further treatment until progression 14 months later.
Figure 1.
Signs of clinical efficacy after treatment with IL-15 and alemtuzumab. (A) Swimmers’ plot of all trial participants during on-study follow-up. (B) FDG-PET and clinical photograph of a patient with chronic subtype ATL before and 3 months after treatment. (C) Leukemic cell count during and after treatment with IL-15 and alemtuzumab represented as fold-change from baseline in absolute number of ATL cells detected by flow cytometry. Images published with study participant’s consent.
Median number of prior therapies in the responding patients was 1.5 (range, 1-4), whereas the number of prior therapies in the nonresponding patients was 2 (range, 1-7). The small sample size precludes any conclusions on the possible relationship of prior therapy with response.
Contrary to the fear that administration of a cytokine would lead to rapid progression of T-cell lymphoma, most participants with detectable leukemic cells at baseline had decreased absolute leukemic cell counts after the 2-week treatment with rhIL-15 (Figure 1C). As expected, the absolute leukemic cell count decreased further with alemtuzumab administration, with 3 of 8 participants being minimal residual disease negative by peripheral blood flow cytometry at the end of treatment.
IL-15 injections alone are associated with decreased numbers of leukemic cells
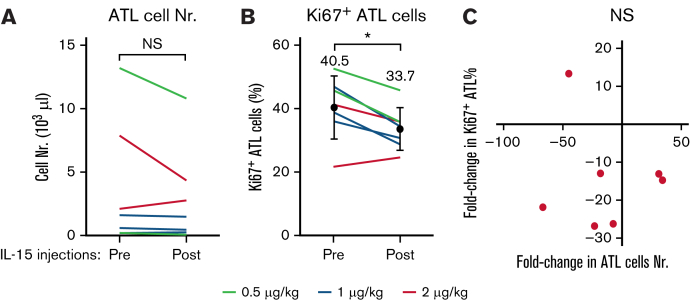
IL-15 supports the proliferation of T cells and could therefore also affect the growth of T leukemic cells. To investigate, we compared the numbers of leukemic ATL cells among PBMCs before and after the completion of the 2-week course of IL-15 injections. Of the 8 participants with ATL in this study, 7 harbored leukemic cells. IL-15 treatments were associated with decreases in the number of leukemic cells in 5 of those 7 participants (Figure 2A), although this difference did not prove significant because of the low number of participants. Leukemic cell numbers could have been affected by their rates of proliferation and/or cell death. We analyzed Ki67 expressions that enabled us to determine the rates of proliferation. Contrary to expectation, proliferation rates were significantly decreased after IL-15 treatments (Figure 2B). The average percentages of Ki67-expressing proliferating leukemic cells significantly decreased from 40.5% to 33.7% (P = .0246). However, the numbers of leukemic cells did not correlate with their rates of proliferation (Figure 2C), suggesting mechanisms other than cell proliferation contribute to the decreases of their numbers among PBMCs. These data suggest that IL-15 treatments are associated with both reduced ATL cell proliferation and decreases in numbers of circulating ATL cells through unknown mechanisms.
Figure 2.
IL-15 treatments are associated with decreased numbers and decreased proliferations of leukemic cells in participants with ATL. Leukemic cell counts are shown for 7 participants with ATL before and after 2-week courses of IL-15. Six of 8 patients had reduced leukemic cell counts (A), and 7 of these 8 patients also showed a significant reduction in their rate of proliferation as measured by Ki67 expression (B). (C) Cell counts and proliferation rates were not correlated. ∗P < .01; NS, not significant.
NK-cell populations increase in size after IL-15 treatments
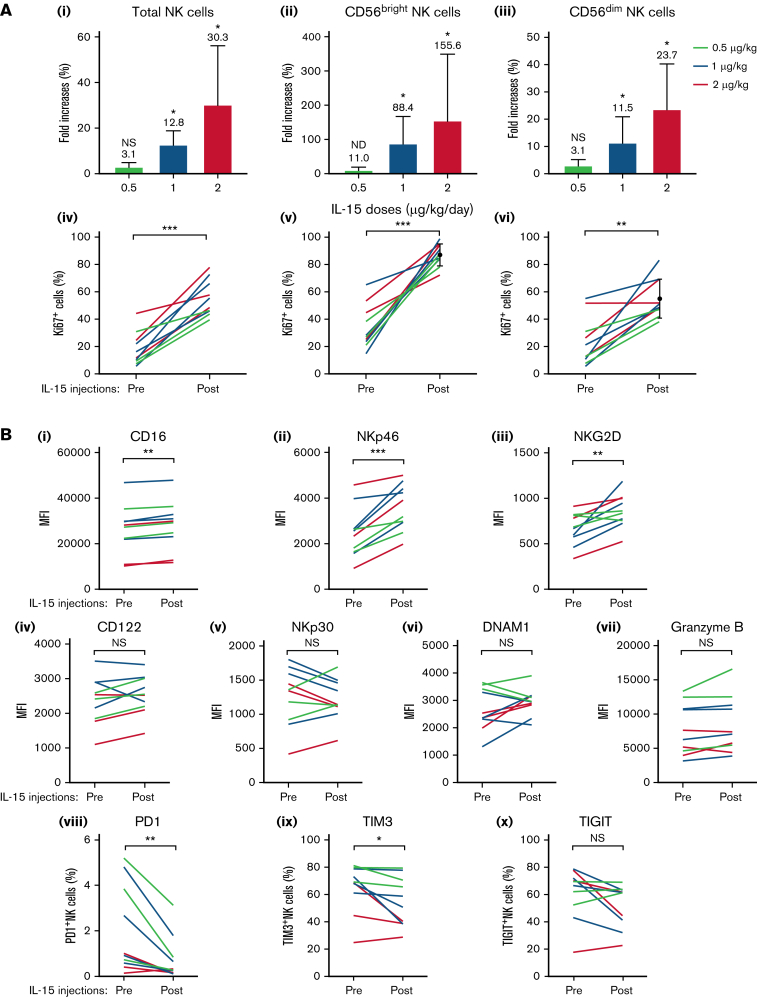
The concept of IL-15 treatments before alemtuzumab infusions is based on the effect of the cytokine on the number and activation state of NK cells.23 These cells are major mediators of ADCC, and their increased numbers and activation could increase the killing rates of antibody-coated tumor cells. Comparing PBMC samples before and after the 2-week course of subcutaneous IL-15 injections in all 8 participants with ATL and 2 with PTCL, we observed that total NK-cell numbers were indeed increased in a dose-dependent manner (Figure 3Ai), resulting in averages of 3.1-, 12.8-, and 30.3-fold increases for the 3 respective dose levels similar to previous observations.24 The CD56bright NK-cell subset showed the greatest expansion rates (11.0-, 88.4-, and 155.5-fold increases in response to 0.5, 1, and 2 μg/kg per day IL-15, respectively, Figure 3Aii), whereas increases among CD56dim NK cells were less pronounced (3.1-, 11.4-, and 23.7-fold increases, respectively; Figure. 3Aiii). Proliferation rates corresponded to these increases in cell numbers (Figure 3Aiv-vi) with CD56bright and CD56dim NK cells having average Ki67 expression rates of 87.2% vs 54.8% after IL-15 treatments, respectively. These data confirm previous reports of IL-15–induced NK-cell number increases.
It has been previously described that IL-15 treatments also increased the expressions of receptors necessary for NK-cell function that could suggest and increased readiness to perform cytolysis.24 We analyzed a number of different surface molecules that are involved in NK-cell function in all 10 participants. We observed that IL-15 treatments increased the expressions of the receptor CD16 that is essential for ADCC (Figure 3B).25 Increased surface amounts were also observed for NKp46 and NKG2D that are involved in other cytotoxic pathways (Figure 3B),25 whereas CD122, NKp30, DNAM1, and gramzyme B remained unchanged. In contrast, we found that the inhibitory receptors PD1 and Tim325 were decreased after IL-15 treatments, whereas TIGIT remained unchanged (Figure 3B). These data suggest that IL-15 treatments could also increase the NK-cell readiness to perform cytotoxicity.
Figure 3.
Numeric expansions and activations of NK cells in response to IL-15 injections in participants with ATL and PTCL. (A) Top graphs depict average increases + standard deviation of total NK cell numbers (i) and their CD56bright (ii) and CD56dim subsets (iii) among PBMCs compared with before treatments for each IL-15 dose. Stars denote significance comparing samples before and after IL-15. (Aiv-vi) Percentages of proliferating Ki67+ NK cells for each patient before and after treatment. (B) Expression levels of molecules involved in NK cell activation (i-vii) and inhibition (viii-x are shown for each participant before and after IL-15 treatments. Significant increases were observed for CD16, NKp46, and NKG2D, whereas PD1 and TIM3 were reduced. ∗P < .01; ∗∗P < .001; ∗∗∗P < .0001; NS, not significant.
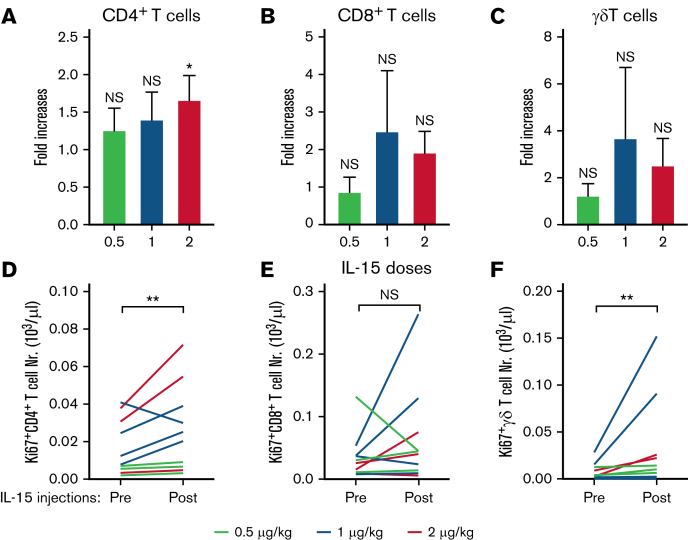
We furthermore analyzed the effects of IL-15 treatments on other IL-15–responsive lymphocyte subsets.21 The total numbers of CD4+ T cells, CD8+ T cells, and γδ+ T cells showed modest increases (Figure 4A-C) that correlated with their number of proliferating Ki67+ cells (Figure 4D-F). IL-15 treatments did not affect the relative percentages of naïve, effector, effector memory, and central memory among CD4+ T cells and CD8+ T cells (supplemental Figure 2).26 Percentages of CD8+ T cells that expressed PD1, TIM3, TIGIT, or DNMA1 also remained unchanged (supplemental Figure 3). We also studied whether IL-15 injections would affect the surface expression of CD52 that is targeted by alemtuzumab. We observed no CD52 expression changes on either lymphocyte or leukemic cells (supplemental Figure 4). In conclusion, IL-15 injections before alemtuzumab infusions caused expansions of NK-cell numbers and improved their activation status that could prime the patient’s immune system for augmented killing rates of antibody-coated tumor cells.
Figure 4.
Effects of IL-15 injections on additional IL-15–responsive lymphocyte subpopulation in participants with ATL and PTCL. (A-C) Average fold increases + standard deviation of CD4+ T, CD8+ T, and γδ+ T cells among PBMCs after IL-15 treatment are shown for each treatment group. (D-F) Total numbers of respective proliferating Ki67+ cells for each patient before and after treatment. ∗P < .01; ∗∗P < .001; NS, not significant.
IL-15 treatments increased NK cell–mediated ADCC activity
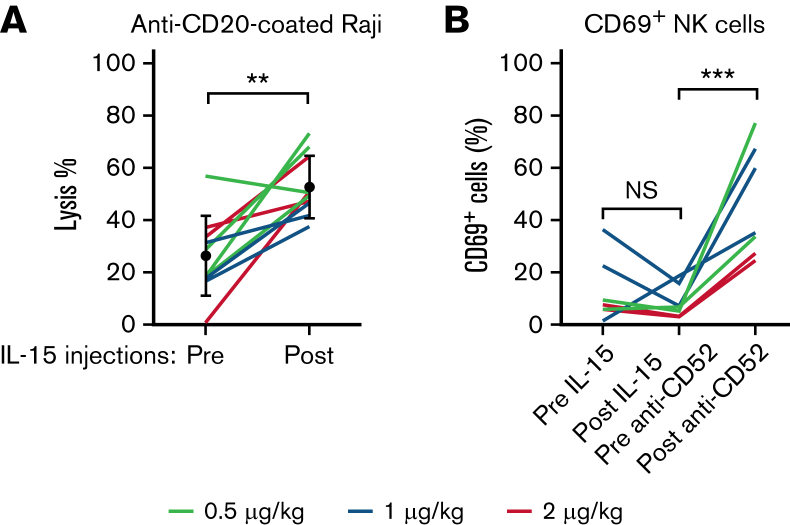
We determined whether IL-15 treatments, in addition to increasing their numbers, did enhance the killing abilities of NK cells via ADCC in a functional ex vivo assay using antibody-coated target cells. We observed that the average ability to lyse anti–CD20-coated Raji target cells approximately doubled in response to IL-15 injections when equal numbers of NK cells were present in the cytotoxicity assay (Figure 5A). We also determined whether NK cells responded in vivo to the presence of antibody-coated leukemic cells after alemtuzumab infusions. We observed the upregulation of the NK-cell activation marker CD69 18 hours after infusions in all participants with circulating ATL cells (Figure 5B). These data show that NK cells respond to IL-15 injections by enhancing their lysis abilities and that NK cells participate in alemtuzumab-induced cytotoxicity. Furthermore, significant augmentation was observed in lysis of leukemic cells in samples collected after IL-15 compared with before, whereas no difference was detected in rates of NK-cell disappearances before and after IL-15 injections (supplemental Figure 5). Altogether, this suggest that NK cells have acquired increased abilities to kill target cells but not themselves after IL-15 injections. However, the total number of circulating NK cells was affected by alemtuzumab injections, as lymphocyte count 2 days after antibody infusion was below 200 cells/μL. Both fratricide and migration may account for this observation.
Figure 5.
Functional ex vivo analysis of NK-cell lysis ability before and after IL-15 injections. (A) PBMCs from participants with ATL and PTCL before and after IL-15 treatment with equal number of NK cells mixed with CD20-coated Raji target cells and uncoated Raji control cells and incubated for 22 hours. We determined the percentages of lysis based on the ratios of surviving coated vs uncoated Raji cells. (B) PBMC samples from patients with detectable circulating ATL cells were stained for the activation marker CD69 on NK cells. Although percentages of activated NK cells were reduced by IL-15 treatments, their percentages were increased after alemtuzumab infusions. ∗∗P < .001; ∗∗∗P < .0001; NS, not significant.
Effects of IL-15 and alemtuzumab serum levels of interferon-γ and other cytokines
There was a significant increase in serum levels of interferon-γ seen after treatment with both IL-15 and alemtuzumab (supplemental Figure 6). There were no significant changes observed in serum levels of soluble CD25, tumor necrosis factor α, or IL-6 after treatment IL-15, whereas the latter 2 were transiently increased 4 hours after alemtuzumab infusion, returning to baseline levels within 24 hours (supplemental Figure 6).
Discussion
IL-15 is 1 of the most important cytokines that regulates the generation, persistence, and differentiation of NK cells and CD8 T cells. It was identified by our group and by Grabstein in culture supernatants of HuT-102 and Cv1/EBNA cell lines that stimulate proliferation of a cytokine-dependent T-cell line CTLL.27, 28, 29 IL-15 stimulated the proliferation of T cells, induced generation of cytotoxic lymphocytes and memory phenotype CD8 T cells, and stimulated the proliferation and maintenance of NK cells.30, 31, 32 Efficacy was observed with IL-15 in multiple murine immunotherapy trials including syngeneic TRAMP-C2 prostatic cancer, Pmel-1, B16 melanoma, and MC38 and CT26 colon carcinoma models, suggesting that IL-15 might be more efficacious than IL-2 in cancer therapy.33, 34, 35 We reported a first-in-human clinical trial with IL-15 by bolus, continuous IV, and subcutaneous administration.18,21,22,36 IL-15 by subcutaneous administration to patients with metastatic cancer yielded a 10.8-fold expansion of circulating NK cells, a 39.7-fold increase in CD56bright cells, and a 3-fold increase in the number of CD8 T cells.18 Although rhIL-15 yielded dramatic augmentation in the number of circulating NK cells, it was not effective as an anticancer agent, probably because of the inhibitory action of immunologic checkpoints and the lack of tumor-specific targeting by NK cells.23 Thus, IL-15 to be effective as an anticancer agent will have to be used in combination therapy with drugs that show efficacy as tumor therapeutics. To translate the dramatic increases in circulating NK-cell numbers and function, in preclinical trials, IL-15 preparations have been reported to be of value in combination with in vivo–administered anticancer monoclonal antibodies to increase their ADCC and anticancer tumor efficacy.13 The present trial of IL-15 in combination with alemtuzumab for patients with CD52-positive T-cell malignancies is our initial report of just such a combination IL-15 monoclonal antibody trial.
Treatment with subcutaneous rhIL-15 followed by 4 weeks of IV alemtuzumab was safe to administer and showed signs of clinical activity in participants with highly aggressive T-cell lymphomas, including a durable CR in a participant whose PTCL-NOS relapsed after alloSCT. There were no indications of rapid progression of ATL or PTCL-NOS after treatment with rhIL-15, whereas other forms of immunotherapy, most notably the immune checkpoint inhibitor nivolumab in ATL8,37 and AITL38 and pembrolizumab in PTCL,39, 40, 41 have led to rapid progression or development of new T-cell lymphomas in several participants. To the contrary, in participants with baseline leukemic disease, treatment with rhIL-15 was associated with a decrease in circulating leukemic cell count that was in stark contrast to the median 2.5-fold and 7-fold increase in circulating CD8+ T cells and NK cells, respectively. NK-cell numbers had 3.1-, 12.8-, and 30.3-fold increases, respectively, for the 3 dose levels (0.5, 1, and 2 μg/kg per day IL-15). Those fold increases appeared to be dose dependent and were similar to those obtained when IL-15 was injected subcutaneously in patients with solid tumors (3.3-, 4.4-, and 10.8-fold increases, respectively, for 0.5, 1, and 2 μg/kg per day IL-15).18 Although the initial decrease of leukemic cells after rhIL-15 administration may represent redistribution, the persistence of the decrease on days 8 and 15 of treatment even while the absolute counts of circulating nonmalignant lymphocytes increased, along with the decreased Ki67 of ATL cells compared with the increase in several immune cells subsets, indicates that rhIL-15 alone may have a direct or indirect antitumor effect.
ATL cells themselves show autocrine and paracrine signaling of multiple cytokines, including IL-15, and may depend on them for survival.42 Furthermore, ex vivo ATL cells demonstrated increased apoptosis after IL-2 and IL-15 blockade by BNZ-1, an inhibitor of selective γ-chain cytokines.43 However, administration of IL-15 did not lead to proliferation of leukemic cells and overt disease progression in this study. There are at least 2 explanations for the conflicting results. First, IL-15 may concurrently stimulate both ATL and immune cells, with the net effect being no change, or decrease, in circulating ATL cell numbers. Second, IL-15 signaling pathway may already be saturated by autocrine signaling in ATL cells but not in the normal immune cells, leading to no change in the former and proliferation and activation of the latter. Decrease in Ki67-positive ATL cells seen after rhIL-15 treatment favors the latter hypothesis.
Concerns have been raised that the continuous daily exposure to IL-15 may lead to NK-cell exhaustion.44 In our study, daily subcutaneous dosing of rhIL-15 led to increased absolute NK-cell counts, increase in the percentage of Ki-67–positive cells, increased ADCC capacity of ex vivo NK cells, and decreased expression of NK-cell inhibitors PD-1 and Tim3. This is similar to the experience with the 5- and 10-day continuous IV infusions of rhIL-15 in patients with metastatic solid tumors and unlike some in vitro observations.23,36 Although this increase in NK-cell activity does not by itself guarantee improved efficacy, it does provide the basis for further development of rhIL-15 in combination with monoclonal antibodies whose antitumor effect is mediated through ADCC. In addition to the combination with alemtuzumab in the present study, our group is investigating rhIL-15 in combination with obinutuzumab in chronic lymphocytic leukemia (#NCT03759184), mogamulizumab in ATL and cutaneous T-cell lymphoma (#NCT04185220), and avelumab in renal cell carcinoma (#NCT04150562) and in mature T-cell malignancies other than ATL, AITL, and PTCL-T follicular helper phenotype (PTCL-TFH) (#NCT03905135).
Clinical activity of the combination compares favorably to that seen after 12 weeks of alemtuzumab monotherapy, with the caveat of a small and heterogenous population in the present study. In our phase 2 study of single-agent alemtuzumab given over 12 weeks to patients with acute, chronic, and lymphoma subtype ATL, the response rate was 40%, and median duration of response was 3 months compared with the 36% response rate and 6-month median duration of response (range, 8 weeks to 12+ months) with the combination.6 Those treated with the combinations also had fewer infectious complications, although the occurrence of asymptomatic catheter-associated thrombi and pulmonary emboli in 2 of the 11 participants (18%) treated with the combination is notable. The reported incidence of VTE with single-agent alemtuzumab is 5%,45,46 and there were no VTE events with single-agent rhIL-15 given subcutaneously or IV.18,22,36 Lysis by alemtuzumab of rhIL-15–activated lymphocytes and the subsequent release of cytokines may be a contributing factor, although the precise mechanism of VTE in our 2 cases is unclear.
Interestingly, the only durable CR in the study was seen in a participant with PTCL-NOS who had relapsed several years after alloSCT. When an IL-15 “superagonist” N-803 (ALT-803) was given to 28 patients whose acute myeloid leukemia or myelodysplastic syndrome relapsed after alloSCT, 1 had CR and 1 had PR.47 In that study, NK cells had the greatest increase, followed by T effector memory and T memory cells. The altered post-alloSCT immune microenvironment may be more conducive for more pronounced CD8+ T-cell proliferation and activation, which in turn may be associated with improved clinical responses, but this hypothesis would need to be validated in future clinical trials.
In summary, we demonstrated the safety of combined subcutaneous rhIL-15 and short-course alemtuzumab administration in participants with ATL and PTCL-NOS. The combination shows signs of activity that may be the same or better than that of alemtuzumab given over 12 weeks, with decreased potential for long-term immunosuppression given the lower total dose of alemtuzumab. Future clinical trials will focus on combining rhIL-15 with other antitumor antibodies that work through ADCC and on the effects of single-agent rhIL-15 in patients who relapse or are at high risk of relapsing after alloSCT.
Conflict-of-interest disclosure: M.D.M. is an employee of Cartesian Therapeutics, Inc. The remaining authors declare no competing financial interests.
Thomas A. Waldmann died on 25 September 2021.
Acknowledgment
This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Authorship
Contribution: M.D.M. and S.P.D. designed and performed research, analyzed and interpreted data, and wrote the paper; J.R.M. analyzed and interpreted data; B.B. and E.M. collected data; K.C.C. designed and performed research; and T.A.W. designed research, analyzed data, and wrote the paper.
Footnotes
The clinical study protocol is available from the corresponding author, Milos D. Miljkovic (miljkovicm@mail.nih.gov).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Tsukasaki K, Hermine O, Bazarbachi A, et al. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. J Clin Oncol. 2009;27(3):453–459. doi: 10.1200/JCO.2008.18.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cook LB, Fuji S, Hermine O, et al. Revised adult T-cell leukemia-lymphoma international consensus meeting report. J Clin Oncol. 2019;37(8):677–687. doi: 10.1200/JCO.18.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katsuya H, Ishitsuka K, Utsunomiya A, et al. ATL-Prognostic Index Project Treatment and survival among 1594 patients with ATL. Blood. 2015;126(24):2570–2577. doi: 10.1182/blood-2015-03-632489. [DOI] [PubMed] [Google Scholar]
- 4.Malpica L, Pimentel A, Reis IM, et al. Epidemiology, clinical features, and outcome of HTLV-1-related ATLL in an area of prevalence in the United States. Blood Adv. 2018;2(6):607–620. doi: 10.1182/bloodadvances.2017011106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips AA, Fields PA, Hermine O, et al. Mogamulizumab versus investigator choice of chemotherapy regimen in relapsed/refractory adult T-cell leukemia/lymphoma. Haematologica. 2018;104(5):993–1003. doi: 10.3324/haematol.2018.205096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma K, Janik JE, O’Mahony D, et al. Phase II study of alemtuzumab (CAMPATH-1) in patients with HTLV-1-associated adult T-cell leukemia/lymphoma. Clin Cancer Res. 2017;23(1):35–42. doi: 10.1158/1078-0432.CCR-16-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demko S, Summers J, Keegan P, Pazdur R. FDA drug approval summary: alemtuzumab as single-agent treatment for B-cell chronic lymphocytic leukemia. Oncologist. 2008;13(2):167–174. doi: 10.1634/theoncologist.2007-0218. [DOI] [PubMed] [Google Scholar]
- 8.Rauch DA, Conlon KC, Janakiram M, et al. Rapid progression of adult T-cell leukemia/lymphoma as tumor infiltrating Treg cells after PD-1 blockade. Blood. 2019;134(17):1406–1414. doi: 10.1182/blood.2019002038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodig SJ, Abramson JS, Pinkus GS, et al. Heterogeneous CD52 expression among hematologic neoplasms: implications for the use of alemtuzumab (CAMPATH-1H) Clin Cancer Res. 2006;12(23):7174–7179. doi: 10.1158/1078-0432.CCR-06-1275. [DOI] [PubMed] [Google Scholar]
- 10.Piccaluga PP, Agostinelli C, Righi S, Zinzani PL, Pileri SA. Expression of CD52 in peripheral T-cell lymphoma. Haematologica. 2007;92(4):566–567. doi: 10.3324/haematol.10767. [DOI] [PubMed] [Google Scholar]
- 11.Geissinger E, Bonzheim I, Roth S, Rosenwald A, Müller-Hermelink HK, Rüdiger T. CD52 expression in peripheral T-cell lymphomas determined by combined immunophenotyping using tumor cell specific T-cell receptor antibodies. Leuk Lymphoma. 2009;50(6):1010–1016. doi: 10.1080/10428190902926981. [DOI] [PubMed] [Google Scholar]
- 12.Jiang L, Yuan CM, Hubacheck J, et al. Variable CD52 expression in mature T cell and NK cell malignancies: implications for alemtuzumab therapy. Br J Haematol. 2009;145(2):173–179. doi: 10.1111/j.1365-2141.2009.07606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang M, Wen B, Anton OM, et al. IL-15 enhanced antibody-dependent cellular cytotoxicity mediated by NK cells and macrophages. Proc Natl Acad Sci USA. 2018;115(46):E10915–E10924. doi: 10.1073/pnas.1811615115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waldmann TA, Dubois S, Miljkovic MD, Conlon KC. IL-15 in the combination immunotherapy of cancer. Front Immunol. 2020;11:868. doi: 10.3389/fimmu.2020.00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waldmann TA, Miljkovic MD, Conlon KC. Interleukin-15 (dys)regulation of lymphoid homeostasis: Implications for therapy of autoimmunity and cancer. J Exp Med. 2020;217(1):1. doi: 10.1084/jem.20191062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6(8):595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 17.Conlon KC, Miljkovic MD, Waldmann TA. Cytokines in the treatment of cancer. J Interferon Cytokine Res. 2019;39(1):6–21. doi: 10.1089/jir.2018.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller JS, Morishima C, McNeel DG, et al. A first-in-human phase I study of subcutaneous outpatient recombinant human IL15 (rhIL15) in adults with advanced solid tumors. Clin Cancer Res. 2018;24(7):1525–1535. doi: 10.1158/1078-0432.CCR-17-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinuma Y, Nagata K, Hanaoka M, et al. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci USA. 1981;78(10):6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Conlon KC, Lugli E, Welles HC, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol. 2015;33(1):74–82. doi: 10.1200/JCO.2014.57.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubois SP, Miljkovic MD, Fleisher TA, et al. Short-course IL-15 given as a continuous infusion led to a massive expansion of effective NK cells: implications for combination therapy with antitumor antibodies. J Immunother Cancer. 2021;9(4) doi: 10.1136/jitc-2020-002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dubois S, Conlon KC, Müller JR, et al. IL15 infusion of cancer patients expands the subpopulation of cytotoxic CD56bright NK cells and increases NK-cell cytokine release capabilities. Cancer Immunol Res. 2017;5(10):929–938. doi: 10.1158/2326-6066.CIR-17-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller JS, Morishima C, McNeel DG, et al. A first-in-human phase I study of subcutaneous outpatient recombinant human IL15 (rhIL15) in adults with advanced solid tumors. Clin Cancer Res. 2017;24(7):1525–1535. doi: 10.1158/1078-0432.CCR-17-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nat Immunol. 2016;17(9):1025–1036. doi: 10.1038/ni.3518. [DOI] [PubMed] [Google Scholar]
- 26.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naïve, central memory and effector memory CD4+ T cells. Pathol Biol (Paris) 2003;51(2):64–66. doi: 10.1016/s0369-8114(03)00098-1. [DOI] [PubMed] [Google Scholar]
- 27.Bamford RN, Grant AJ, Burton JD, et al. The interleukin (IL) 2 receptor beta chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci USA. 1994;91(11):4940–4944. doi: 10.1073/pnas.91.11.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burton JD, Bamford RN, Peters C, et al. A lymphokine, provisionally designated interleukin T and produced by a human adult T-cell leukemia line, stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci USA. 1994;91(11):4935–4939. doi: 10.1073/pnas.91.11.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grabstein KH, Eisenman J, Shanebeck K, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264(5161):965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 30.Lugli E, Goldman CK, Perera LP, et al. Transient and persistent effects of IL-15 on lymphocyte homeostasis in nonhuman primates. Blood. 2010;116(17):3238–3248. doi: 10.1182/blood-2010-03-275438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sneller MC, Kopp WC, Engelke KJ, et al. IL-15 administered by continuous infusion to rhesus macaques induces massive expansion of CD8+ T effector memory population in peripheral blood. Blood. 2011;118(26):6845–6848. doi: 10.1182/blood-2011-09-377804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waldmann TA, Lugli E, Roederer M, et al. Safety (toxicity), pharmacokinetics, immunogenicity, and impact on elements of the normal immune system of recombinant human IL-15 in rhesus macaques. Blood. 2011;117(18):4787–4795. doi: 10.1182/blood-2010-10-311456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu P, Steel JC, Zhang M, et al. Simultaneous inhibition of two regulatory T-cell subsets enhanced Interleukin-15 efficacy in a prostate tumor model. Proc Natl Acad Sci USA. 2012;109(16):6187–6192. doi: 10.1073/pnas.1203479109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu P, Steel JC, Zhang M, Morris JC, Waldmann TA. Simultaneous blockade of multiple immune system inhibitory checkpoints enhances antitumor activity mediated by interleukin-15 in a murine metastatic colon carcinoma model. Clin Cancer Res. 2010;16(24):6019–6028. doi: 10.1158/1078-0432.CCR-10-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies E, Reid S, Medina MF, Lichty B, Ashkar AA. IL-15 has innate anti-tumor activity independent of NK and CD8 T cells. J Leukoc Biol. 2010;88(3):529–536. doi: 10.1189/jlb.0909648. [DOI] [PubMed] [Google Scholar]
- 36.Conlon KC, Potter EL, Pittaluga S, et al. IL-15 by continuous i.v. infusion to adult patients with solid tumors in a Phase I trial induced dramatic NK cell subset expansion. Clin Cancer Res. 2019;25(16):4945–4954. doi: 10.1158/1078-0432.CCR-18-3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ratner L, Waldmann TA, Janakiram M, Brammer JE. Rapid progression of adult T-cell leukemia-lymphoma after PD-1 inhibitor therapy. N Engl J Med. 2018;378(20):1947–1948. doi: 10.1056/NEJMc1803181. [DOI] [PubMed] [Google Scholar]
- 38.Bennani NN. Blood. suppl 1. Vol. 134. 2019. 2019. A Phase II Study of Nivolumab in Patients with Relapsed or Refractory Peripheral T-Cell Lymphoma; p. 467. [Google Scholar]
- 39.Anand K, Ensor J, Pingali SR, et al. T-cell lymphoma secondary to checkpoint inhibitor therapy. J Immunother Cancer. 2020;8(1) doi: 10.1136/jitc-2019-000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malachowski SJ, Hatch LA, Sokol L, Messina J, Seminario-Vidal L. Pembrolizumab-associated tumor development in a patient with Sézary syndrome. JAAD Case Rep. 2019;6(1):16–18. doi: 10.1016/j.jdcr.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duke TC, Nair R, Torres-Cabala C, et al. Angioimmunoblastic T-cell lymphoma associated with immune checkpoint inhibitor treatment. JAAD Case Rep. 2020;6(12):1264–1267. doi: 10.1016/j.jdcr.2020.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen J, Petrus M, Bryant BR, et al. Autocrine/paracrine cytokine stimulation of leukemic cell proliferation in smoldering and chronic adult T-cell leukemia. Blood. 2010;116(26):5948–5956. doi: 10.1182/blood-2010-04-277418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang TT, Yang J, Zhang Y, et al. IL-2 and IL-15 blockade by BNZ-1, an inhibitor of selective γ-chain cytokines, decreases leukemic T-cell viability. Leukemia. 2019;33(5):1243–1255. doi: 10.1038/s41375-018-0290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Felices M, Lenvik AJ, McElmurry R, et al. Continuous treatment with IL-15 exhausts human NK cells via a metabolic defect. JCI Insight. 2018;3(3) doi: 10.1172/jci.insight.96219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fox EJ, Sullivan HC, Gazda SK, et al. A single-arm, open-label study of alemtuzumab in treatment-refractory patients with multiple sclerosis. Eur J Neurol. 2012;19(2):307–311. doi: 10.1111/j.1468-1331.2011.03507.x. [DOI] [PubMed] [Google Scholar]
- 46.Alemtuzumab. Package Insert. Millennium and ILEX Partners L. US Food and Drug Administration website. 2018. Created September 2003. Accessed September 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2001/alemmil050701LB.htm. [Google Scholar]
- 47.Romee R, Cooley S, Berrien-Elliott MM, et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood. 2018;131(23):2515–2527. doi: 10.1182/blood-2017-12-823757. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.