Abstract
Senna tora (L.) Roxb. is an ethno-medicinal herb used by rural and tribal people of the Satpura region of Madhya Pradesh in India and the Phatthalung Province of Thailand for treating rheumatism, bronchitis, ringworm, itches, leprosy, dyspepsia, liver disorders and heart disorders. It is also used in Chinese and Ayurvedic medicine. This study was conducted to investigate the potential of Senna tora (L.) Roxb. as a source of drug candidates against oxidants, inflammation, and bacterial infection. Preliminary phytochemical screening (PPS) and GC-MS were performed to identify the phytochemicals in the ethyl acetate extract of Senna tora (L.) Roxb. leaves (EAESTL). The in vitro antioxidant activity was assessed by 2,2-diphenyl-1-picrylhydrazyl (DPPH)- and H2O2-scavenging tests; the in vitro anti-inflammatory activity was determined by bovine serum albumin (BSA) denaturation and red blood cell (RBC) hemolysis inhibition; and the antibacterial activity was evaluated by agar-well diffusion methods. Cytotoxicity was estimated by Artemia salina larvae lethality, while acute toxicity was evaluated by oral delivery of the extract to mice. In silico antioxidant, anti-inflammatory, and antibacterial activities were predicted by the Prediction of Activity Spectra for Substances (PASS) program. The pharmacokinetics related to ADME and toxicity tests were determined by the admetSAR2 and ADMETlab2 web servers, and drug-able properties were assessed by the SwissADME server. GC-MS detected fifty-nine phytochemicals that support the types of compounds (phenols, flavonoids, tannins, terpenoids, saponins, steroids, alkaloids, glycosides and reducing sugar) identified by phytochemical screening. EAESTL exhibited dose-dependent antioxidant, anti-inflammatory, and antibacterial activities without any adverse effects or fluctuations in body weight. The PASS program predicted that the identified phytochemicals have antioxidant, anti-inflammatory and antibacterial activities. Among 51 phytochemicals, 16 showed good ADME, and 8 fulfilled drug-able properties without toxicity. Altogether, four phytochemicals, viz., benzyl alcohol, 3-(hydroxy-phenyl-methyl)-2,3-dimethyl-octan-4-one, phenylethyl alcohol and 2,6,6-trimethylbicyclo [3.1.1] heptane-3-ol, showed good pharmacokinetics and drug-able properties without toxicity, along with antioxidant, anti-inflammatory, and antibacterial activities. The obtained results suggest that Senna tora (L.) Roxb. leaves contain bioactive phytochemicals that have the potential to fight against oxidants, inflammation, and bacterial infection as potential drug candidates.
Keywords: Senna tora (L.) Roxb., Antioxidant activity, Anti-inflammatory activity, Antibacterial activity, Phytochemicals, PASS, ADMET, Drug-able properties
Highlights
-
•
The Senna tora leaves contained bioactive phytochemicals that had antioxidant, anti-inflammatory and antibacterial activities.
-
•
Benzyl alcohol had antioxidant, anti-inflammatory, and antibacterial activity. After this in another bullet point write: 3-(hydroxy-phenyl-methyl)-2,3-dimethyl-octan-4-one had anti-inflammatory activity.
-
•
Phenylethyl alcohol was found to have antioxidant and anti-inflammatory activity.
-
•
2,6,6-trimethylbicyclo [3.1.1] heptane-3-ol, also known as isopinocampheol/3-pinanol, could be an efficacious drug candidate against inflammation and bacterial infection.
1. Introduction
Humans receive medicinal plants as a gift from nature to aid in their quest for improved health. Since the dawn of humanity, mankind has recognized and utilized natural plant products as the prime source of therapeutic medications [1,2]. They are still a huge source of prominent and effective bioactive compounds that are capable of being employed as medications right away [2]. There are numerous examples of plant-based medications, like antimicrobial as nicotine, antioxidant as nordihydroguaiaretic acid (NDGA), anticancer as vinblastine, bleomycin, paclitaxin, and taxol, and anti-inflammatory as aescin and capsaicin, cardiotonic as acetyldigoxin, and antiparkinsonism as l-dopa, etc [1,3]. Their use is promoted in the Ayurvedic, Unani, Siddha, Homeopathic, and other frameworks of medication [1]. They pertain impact on huge number of human lives as customary medicines for different sicknesses like rheumatism, bronchitis, burns, itches, leprosy, colds, roundworm infection, wound bleeding, diarrhea and others [1,4]. Despite the discovery of numerous pharmaceuticals with plant origins, research into novel bioactive chemicals is still necessary to expand the available pharmacological options and look for less toxic medications with improved efficacy [5,6]. Therefore, the search for highly antioxidant, anti-inflammatory, and antimicrobial phytochemical compounds is one of the most intensive fields of research to minimize the risk of free radical-induced cellular damage or tissue injury, inflammation, bacterial infection, and associated diseases. Synthetic antioxidants are available for the treatment of oxidative stress-mediated oxidant-induced diseases and disorders but have associated health risks and severe adverse effects [7]. Nonsteroidal anti-inflammatory drugs (NSAIDs) are used for treating inflammation, but they have several detrimental consequences, including gastrointestinal ulcers, heartburn, kidney impairment, nausea, and vomiting [8,9]. Antimicrobials are losing their effectiveness, making it more difficult and occasionally impossible to cure diseases like pneumonia, TB, blood poisoning, gonorrhea, and foodborne diseases. New resistance mechanisms are emerging and spreading globally, threatening our ability to treat common infectious diseases [10]. In addition, the majority of currently available antibiotics have serious side effects, including life-threatening immune-related fallacious effects, anaphylaxis, dermatological cross-reactivity [11], hypersensitivity, and altering the body's normal microbiota, hence affecting normal physiological processes and making the lungs more vulnerable to viral diseases such as the flu [12]. The “back to nature” method has therefore gained significant acceptance in recent years. A large number of plant-based molecules have been developed and approved as antioxidants, anti-inflammatory agents and antibacterial potential therapeutics [[13], [14], [15]]. Hence, to find a feasible course of action that is safe and that lowers the adverse effects induced by chemotherapy, the continuous exploration of antioxidant, anti-inflammatory, and antibacterial phytochemicals plays a critical role.
Senna tora (L.) Roxb. Is a yearly foetid herb that belongs to the family Fabaceae and commonly propagates in barren land as a monsoon wild plant in humid countries (Bangladesh, India, Pakistan, and western China) [16–18]. Locally, it is recognized as chakramard in Ayurveda, chakunda/panevar/aranchi in Bengali, and foetid cassia/sickle pod/sickle senna in English [16–19]. It is also an ethno-medicinal herb used in traditional Chinese medicine and Ayurvedic medicine [[16], [17], [18],20]. Rural and tribal people and traditional healers use this plant to treat constipation, ulcers, ringworm, itch, appetizers, insomnia, liver disorders, leprosy, bronchitis, cough, dyspepsia, and heart diseases [[16], [17], [18],20]. The leaves have antirheumatic action in folklore preparation, and the leaf essence is used as a laxative [18]. Different parts of Senna tora (L.) Roxb. have been reported for their in vitro antioxidant and antibacterial activities and in vivo anti-inflammatory and analgesic activities due to the high content of different phytochemicals, such as tannins, saponins, reducing sugar, gum, steroids and alkaloids [21].
However, very little evidence related to Senna tora (L.) Roxb's phytochemical compounds is available, and the in vivo cytotoxicity and acute toxicity, pharmacokinetics (ADME), and drug-able properties have not been explored, though it is an important ethnomedicinal plant. No research has identified the specific bioactive phytochemicals in Senna tora (L.) Roxb. leaves that have biological activity against oxidants, inflammation, and bacterial infection and have good pharmacokinetic and drug-able properties without toxicity. Thus, this study focused on identifying the phytochemicals of the extract and assessing their in vitro antioxidant, anti-inflammatory, and antibacterial activities; evaluation of the cytotoxicity and acute toxicity; and prediction of the in silico probable biological activity, pharmacokinetics (ADME), in silico toxicity, and drug-able properties of identified phytochemicals extracted by ethyl acetate in an ideal drug development approach. The findings of this study will support further in vivo research into the potential therapeutic use of Senna tora (L.) Roxb. leaves against oxidants, inflammation, and bacterial infection, as well as the development of potential drugs for the treatment of associated diseases.
2. Materials and methods
2.1. Chemicals and reagents
BSA (CAS: 9048-46-8), DPPH (CAS: 1898-66-4), vincristine sulfate (CAS: 2068-78-2), and l-ascorbic acid (CAS: 50-81-7) were collected from Sigma-Aldrich, Chemie GmbH, Germany. Methanol (CAS: 67-56-1), ethyl acetate (CAS: 141-78-6), dimethyl sulfoxide (CAS: 67-68-5), ferric chloride (CAS: 10025-77-1), lead acetate (CAS: 6080-56-4), HCl (CAS: 7647-01-0), CuSO4 (CAS: 7758-99-8), H2SO4 (CAS: 7664-93-9), sodium nitroprusside (CAS: 13755-38-9), were purchased from Merck, Darmstadt, Germany. Pyridine (CAS: 110-86-1) and chloroform (CAS: 67-66-3) were from Alfa Aesar, Shore Road, Heysham, UK. NaCl (CAS: 7647-14-5), H2O2 (CAS: 7722-84-1) and NaOH (CAS: 1310-73-2) were purchased from Wako Pure Chemical Industries, Ltd., Japan. Mayer's solution and Fehling's solution were obtained from Central Drug House (P) Ltd., New Delhi, India. Nutrient broth and nutrient agar media were obtained from Liofilchem, Italy. Glacial acetic acid (CAS: 64-19-7) was acquired from PanReac AppliChem, Darmstadt, Germany. Erythromycin antibiotic discs were purchased from Bio-Rad, USA. Ibuprofen was gifted by Beximco Pharmaceuticals Ltd., Bangladesh.
2.2. Plant material
The Senna tora (L.) Roxb. leaves were taken in March 2019 from Churamonkathi, located at latitude 23.2238° N and longitude 89.1646° E in the Jashore District in Bangladesh. The plant material was botanically identified by Professor Dr. Shaikh Mizanur Rahman, Department of Genetic Engineering and Biotechnology, Jashore University of Science and Technology, Jashore-7408, Bangladesh. A voucher specimen was prepared and the plant's authentication was further confirmed by a taxonomist, Dr. Sardar Nasiruddin, at the National Herbarium, Dhaka, Bangladesh, and a voucher specimen (voucher number DACB 65146) was deposited at the National Herbarium, Dhaka, Bangladesh. The collected leaves were soaked and rinsed with water. An air-conditioned setting was utilized to dry the leaves at room temperature (∼25 °C). The leaves were dried and blended rigorously by a blender, and the obtained powder particles were stored in a sealed container.
2.3. Bacterial strains used
Both Gram-positive and Gram-negative bacteria were used in this study. From the Gram-positive category, Bacillus infantis, ATCC 15697 (B. infantis), Exiguobacterium sp., ATCC BAA-1283/AT1b (Exiguobacterium sp.), Staphylococcus aureus ATCC 23235 (S. aureus) and Enterococcus sp., ATCC 19952 (Enterococcus sp.) were tested in antibacterial experiments. Escherichia coli O157:H7 (E. coli), Vibrio cholerae O139 (V. cholerae), Salmonella typhi ATCC 13311 (S. typhi), Pseudomonas aeruginosa ATCC 27853 (P. aeruginosa), and Haemophilus influenzae ATCC 49247 (H. influenzae) represented the Gram-negative bacteria.
2.4. Experimental animals and ethical approval
The mice used in this study were collected from the Animal Center of Jahangirnagar University, Savar, Dhaka, Bangladesh. The mice were Swiss albino, male, 22–25 g in weight, and 6 weeks of age. The mice were accommodated in an animal house and the conditions were maintained in the animal house with a standard day-night (12-h) alternating light cycle at ambient temperature (23 ± 2 °C). Here, six mice were accommodated randomly in each of the polypropylene cages for the experiment. Free access to food and water, along with a normal supply, was arranged for the experimental mice. The animal experiment in this study was carried out in accordance with the ARRIVE guidelines [22]. The protocol (No. ERC/FBST/JUST/2021-91) was approved by the Institutional Ethical Committee of the Faculty of Biological Science of Jashore University of Science and Technology, Jashore-7408, Bangladesh. The use of human blood samples, such as the method of sample collection, privacy protection, data analysis, and decomposition plan, was validated by following the institutional standard.
2.5. Preparation of plant extract
The phytochemicals of Senna tora (L) Roxb. leaves were extracted by following the method explained previously with some small changes in the workup process [23]. The powdered plant product (500 g) was taken into five different conical flasks (1 L in size), and ethyl acetate (400 mL) was added to each flask. The conical flask was shaken for 72 h at 250 rpm by using a floor-standing shaking incubator (JSSI-300T, JSR, Republic of Korea), and the temperature was set at 37 °C. After this shaking, a few min of settling time were allowed, and clean cotton gauze was used to filter the mixture, followed by a second filtration by Whatman filter paper (no. 1). The sample was concentrated using a rotary evaporator (RE100-Pro, DLAB Scientific Inc., CA, USA), freeze-dried to obtain a powder, and stored in a sterile tube at 4 °C. The yield of extract from 500 g powder plant material was 20.55 g (dry weight, 4.11% w/w).
2.6. Qualitative phytochemical screening
The EAESTL solution was used in various qualitative analyses for phytochemicals. Alkaline reagent, FeCl3, and lead acetate tests were utilized to detect the existence of phenolic compounds (flavonoids and tannins). In an alkaline reagent test, a few drops of NaOH (5%) solution were added dropwise into the EAESTL solution (25 mg EAESTL dissolved in 2.5 mL methanol), which produced a deep-yellow color. Afterwards, a concentrated HCl (10%) solution was added to this alkaline solution and the color was lost just after adding the acid solution, which identified the presence of flavonoids [24]. To identify the presence of phenols, a few drops of FeCl3 (5%) solution were added to 2.0 mL of EAESTL solution (10 mg/mL) prepared in distilled water [25]. This test provides information regarding the presence of tannins in the EAESTL solution by developing a reddish-black color. A creamy-white color precipitation appeared after adding lead acetate solution (10%) to the 5 mg of EAESTL, indicating the availability of tannins. The Salkowski test was used to determine the presence of terpenoids and steroids [26]. In this test, 8 mg of EAESTL was shaken separately with 4 mL of chloroform, filtered to collect the clear part, and transferred to 2 test tubes evenly. Afterwards, H2SO4 (conc.) was added at the tube edge without shaking, and a bluish-brown layer at the interface suggested the presence of terpenoids. Concentrated H2SO4 was added to another tube of EAESTL and agitated; after settling, a black color appeared on the bottom face, indicating that steroids were present in the sample. In Mayer's test, 25 mg of EAESTL was dissolved in 10 mL of aqueous HCl (1%) with constant stirring in a water bath and then filtered off to collect the clear part. 1 mL of Mayer's reagent was mixed with 1.5 mL of this clear acid-treated EAESTL solution. A creamy-colored precipitate appeared at the edge, indicating the presence of alkaloids in EAESTL [25]. To detect cardiac glycosides, legal tests were utilized. 5 mg of EAESTL was treated with 3 mL of pyridine, and then 2 drops of nitroprusside and a drop of 20% sodium hydroxide solution were added to this solution. The appearance of a blood-red color demonstrated the existence of cardiac glycosides in EAESTL [24. Frothing and foaming tests were used to identify the presence of saponins in EAESTL [24]. Here, distilled water (3 mL) was added to 0.5 mg/mL of EAESTL in methanol and shaken for a few min before being allowed to stand. Even after 10 min of standing, the foam did not disappear, demonstrating the presence of saponins. In Fehling's test, 5 mg of EAESTL was added to an equal volume of Fehling's solutions A and B and boiled for a few min. Reducing sugar was identified by the sudden appearance of a yellowish color, which later turned into a brick-red precipitate [24].
2.7. Gas chromatography-mass spectrometry (GC-MS) analysis
GC-MS was conducted in accordance with a previously described method [27] with minor modifications. A gas chromatography-mass spectrometer (Shimadzu triple-quad GCMS-TQ8040) was used to identify the phytochemicals in EAESTL. Helium gas and an Rtx-5MS capillary column (30 m × 0.25 mm id, film thickness of 0.25 m) were utilized as the mobile and stationary phases, respectively. The column oven temperature was controlled by dedicated software, and graduated heating was set at 50, 200, and 300 °C at 1, 2, and 7 min, respectively. The temperature of the sample injector was 250 °C, with a 40-min experimental run time. This instrument was operated in splitless mode, where a 1 μL sample was injected. The constant flow rate was set at 1 mL/min. The detector parameters for GC–MS analyses were set as follows: 250 °C interface temperature, 230 °C ion source temperature, 50–600 (m/z) scanning range, and an ionization energy of 70 eV with a scan time of 0.3s. The phytochemical ingredients were identified by comparing the retention time and obtained pattern of the spectrum, which was then matched with the database of the National Institute of Standards and Technology (NIST). The individual peak area of an ingredient with respect to the total area of all other peaks was used to calculate the individual content (%) of an ingredient in EAESTL.
2.8. Antioxidant activity assay
2.8.1. In vitro antioxidant activity assay by scavenging DPPH free radicals
DPPH was used to determine the free radical-scavenging activity of EAESTL by following a previously described method [24]. Individual stock solutions of DPPH (1.5 mg/mL), EAESTL (10 mg/mL), and ascorbic acid (10 mg/mL) were prepared in methanol. A DPPH solution (60 μL at 1.5 mg/mL) was added to different concentrations (200, 180, 160, 80, 40, 20, 10, 5, and 2.5 μg/mL) of EAESTL and ascorbic acid separately to a final concentration of DPPH 30 μg/mL and a final volume of 3.0 mL with methanol. The control was a 3.0 mL DPPH solution of 30 μg/mL concentration in methanol. The reaction mixtures were properly mixed and shaken, followed by 30 min of preservation in the dark. The absorbance was measured at 517 nm using a UV–vis spectrophotometer. For each analysis, three independent samples were prepared, and the average absorbance was used for the calculation. The free radical-scavenging activity was estimated utilizing the following formula (Eq. (1)):
| (1) |
where Abscontrol and Abssample are the absorbance of the control and EAESTL-added samples, respectively. Absascorbic acid represents the absorbance of the standard sample, where ascorbic acid was added instead of EAESTL. Microsoft (MS) Excel was used to determine the IC50 of EAESTL and ascorbic acid, where the percent inhibition was plotted with the respective concentration of the sample. The formula y = ax + b was used in the final calculation, where x is the IC50 and was calculated at the y = 50 position of the curve.
2.8.2. Scavenging of hydrogen peroxide (H2O2)
The antioxidant activity of EAESTL was measured by H2O2-scavenging assay by following the method described earlier [28]. 1.0 M of H2O2 solution was prepared using phosphate-buffered saline (PBS, pH 7.4) and 10 mg/mL of EAESTL solution and 10 mg/mL of ascorbic acid solution were prepared in methanol as stock solutions. A total of 60 μL of a 1 M H2O2 solution was added to varying concentrations (200, 180, 160, 80, 40, 20, 10, 5, and 2.5 μg/mL) of EAESTL and ascorbic acid separately to obtain a final concentration of H2O2 of 20 mM and a final volume of 3.0 mL with 2880 μL of PBS and the rest of methanol. The control was 60 μL of 1 M H2O2 solution plus 2880 μL of PBS and the rest of the methanol for a final volume of 3.0 mL. Before measuring the absorbance data, the samples were kept for 10 min for settling purposes, and then the absorbance was recorded at a wavelength of 230 nm by utilizing a UV–vis spectrophotometer. The percent (%) H2O2-scavenging activity was estimated by the following equation (Eq. (2)):
| (2) |
where Abscontrol is the absorbance of the negative control sample, and Abssample is the absorbance of the sample, where EAESTL was added. Absascorbic acid is the absorbance of the ascorbic acid-added sample. The IC50 values for EAESTL and ascorbic acid were calculated by following a method similar to that described above in Section 2.5, where the H2O2-scavenging activity was plotted against the concentration of the solution.
2.9. In vitro anti-inflammatory activity
2.9.1. Heat-induced hemolysis of RBC assay
The RBC suspension solution (sparingly soluble) was prepared by following the previously explained method, with minor modifications [29]. The whole blood of a healthy human individual was collected in a heparinized centrifuge tube. After collection, the blood was centrifuged for 5 min at 3000 rpm and washed three times with an equal volume of normal saline (0.9% NaCl). The upper clear part was discarded from the top of the tube. The residue part was reconstituted as a 10% (v/v) RBC suspension with an isotonic sodium phosphate buffer solution (10 mM, pH 7.4). To prepare 1 L of a 10 mM sodium phosphate buffer solution, 1.379 g NaH2PO4, 1.419 g Na2HPO4 and 9.0 g NaCl were used. The stock solutions of EAESTL and ibuprofen were prepared by dissolving them separately into DMSO to a concentration of 10 mg/mL. The RBC suspension (0.05 mL) was added to the varying concentrations (200, 180, 160, 80, 40, 20, 10, 5, and 2.5 μg/mL) of EAESTL and ibuprofen separately to a final volume of 3.0 mL with an isotonic sodium phosphate buffer solution. The control samples contained 0.05 mL of RBC suspension plus the same amount of DMSO instead of the tested sample and an isotonic sodium phosphate buffer solution with a final volume of 3.0 mL. In a shaking water bath, these samples were incubated at 54 °C for 20 min with constant shaking. After centrifugation at 2500 rpm, the clear part was collected from the top of the centrifuge tube. The absorbance of these sample solutions was measured at a 540 nm wavelength by using a UV–vis spectrophotometer. The following equation (Eq. (3)) was used to estimate the percent inhibition of hemolysis:
| (3) |
Here, Abssample and Abscontrol are the absorbances in the presence and absence of the EAESTL sample in PBS, respectively. Similarly, the ibuprofen-added sample was also compared with the control sample. The IC50 value was calculated by following a similar method described in Section 2.5, where the percent inhibition of erythrocyte hemolysis was plotted against the concentration of the respective sample.
2.9.2. BSA protein denaturation assay
This experiment was performed by following a previously reported method with a slight modification [29]. 0.12 mL of 1% BSA was added to the varying concentrations (200, 180, 160, 80, 40, 20, 10, 5, and 2.5 g/mL) of EAESTL and ibuprofen separately to obtain a final concentration of BSA 0.04% and a final volume of 3.0 mL with phosphate buffered saline (PBS 1×, pH 7.4). The control samples were prepared by mixing 0.12 mL of 1% BSA with the same amount of DMSO instead of the tested sample and 1× phosphate buffered saline to a final volume of 3.0 mL and a final concentration of 0.04% BSA. All the samples were incubated in a water bath for 15 min at 37 °C, followed by an additional 5 min at 70 °C. The samples were allowed to cool, and the absorbance was recorded at a wavelength of 660 nm utilizing a UV–vis spectrophotometer. The following equation (Eq. (4)) was used to estimate the percent inhibition of protein denaturation:
| (4) |
Here, Abscontrol and AbsTest sample are the absorbance of the negative control and EAESTL or ibuprofen added sample at a wavelength of 660 nm, respectively. The IC50 of EAESTL and standard (ibuprofen) sample was calculated by following the same method described above in Section 2.5, where the percent inhibition of BSA denaturation was plotted against the concentration of the test sample.
2.10. Antibacterial activity assay
2.10.1. Agar well diffusion assay
The agar well diffusion technique [24] was used to evaluate the antibacterial activity of EAESTL. All the tested bacterial strains were sub-cultured first by the streak plate technique on nutrient agar petri-plates. A single colony was taken from each bacterial culture plate of an individual strain and incubated in 25 mL of nutrient broth medium at 37 °C in a shaking incubator at 250 rpm and cultured to the mid-log phase of absorbance at 0.4 at 600 nm wavelength. 100 μL of fresh mid-log phase bacterial culture of each strain was spread on an individual nutrient agar petri-plate by a glass spreader and dried for 20 min. The four wells were produced by utilizing a 6 mm sterile cork borer on each nutrient agar petri-plate containing bacterial inoculums. A stock solution of EAESTL (500 mg/mL) was dissolved in DMSO and serially diluted to prepare 250, 125, 62.5 mg/mL concentrations of EAESTL. 100 μL of EAESTL solution of an individual concentration was applied sequentially in an individual well of the nutrient agar petri-plate. As a positive control, an antibiotic disc (erythromycin) was placed in the middle of the nutrient agar petri plate. As a negative control, 100 μL of DMSO was also applied into the well of nutrient agar petri-plate containing bacterial inoculums. Then, the nutrient agar petri-plates were kept in a refrigerator at 4 °C for 30 min to allow the samples to diffuse properly in the nutrient agar medium, followed by incubation at 37 °C for 16 h. The antibacterial activity was estimated by the zone of inhibition (within a well diameter) on the agar plate. The antibacterial activity assay was performed in triplicate.
2.10.2. Minimum inhibitory concentration and minimum bactericidal concentration determination
The twofold serial dilution method was used to test the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of EAESTL [30]. A 500 mg/mL stock solution was placed in an autoclavable 15-mL glass tube and serially diluted with LB broth to achieve 250, 125, 62.5, 31.25, 15.625, 7.812, 3.906, and 1.953 mg/mL. A 50 μL bacterial culture in mid-log phase (absorption at 600 nm reached 0.4) was transferred to each tube. The control tube contained only bacterial strains and not the plant extract. The culture tubes were incubated at 37 °C for 24 h, and the turbidity of the tubes was examined. The lowest concentration of EAESTL that inhibited visible bacterial growth was considered the MIC. To determine the minimum bactericidal concentration of EAESTL, 50 μL of each culture was subcultured on the surface of an LB agar plate at 37 °C for 16 h. MBC was recorded as the lowest concentration of EAESTL that did not permit any visible bacterial colony growth on the LB agar plate after the incubation period. The MIC and MBC assays were performed three times.
2.11. Test of cytotoxicity by Artemia salina larvae lethality bioassay
The cytotoxicity of EAESTL was assessed using Artemia salina larvae as a test organism [31]. Artemia salina eggs were purchased from an aquarium shop in Dhaka, Bangladesh, and artificial seawater was used for hatching purposes. To prepare the artificial seawater, 38 g NaCl salt was dissolved in 1 L water followed by 48 h of hatching at 25 °C with constant aeration and irradiation of light. Active Artemia salina larvae were taken from this hatching and shifted to another vessel that contained fresh artificial seawater. The EAESTL and vincristine sulfate were dissolved separately into DMSO to a final concentration of 10 mg/mL. Each 0.1 mL of these solutions was mixed separately with 9.9 mL of artificial seawater in a total volume of 10 mL of 100 μg/mL concentration, from which serially diluted solutions were made to obtain a series of concentrations of 50, 25, 12.5, 6.25, and 3.125 μg/mL. An identical control was prepared without adding test samples but by diluting 0.05 mL of DMSO in 4.95 mL of artificial seawater to a final concentration of 1% DMSO, which is the DMSO concentration used in test samples. Ten (10) Artemia salina larvae per vial that contained either test or negative control samples were accommodated and examined after 24 h using a magnifying glass. The data were recorded based on the number of surviving Artemia salina larvae per vial. The following equation (Eq. (5)) was utilized for the estimation of the mortality of Artemia salina larvae for each concentration:
| (5) |
where NT and NL are the quantity of Artemia salina larvae taken and alive, respectively, after a 1-day incubation period. The LC50 was quantified using a mortality-versus-concentration curve, where the LC50 value was calculated at y = 50 by following a method similar to that described for IC50 in Section 2.5.
2.12. Acute oral toxicity study
The Organization for Economic Co-operation and Development (OECD) (Test No. 425) was followed during the assessment of the acute toxicity of EAESTL on Swiss albino mice [32]. An acute oral toxicity study was performed according to the method described previously [24]. Forty-two (42) male mice were divided into seven groups (6 in each), namely, the g-250, g-500, g-1000, g-2000, g-4000, g-5000, and control groups. Before starting the experiment, the mice were starved for 3–4 h, and only water was allowed during this time. A stock solution of EAESTL (500 mg/mL) was dissolved in 2% DMSO in a 0.9% saline solution. Each mouse in the g-250, g-500, g-1000, g-2000, g-4000, and g-5000 groups received a single oral dosage that contained 250, 500, 1000, 2000, 4000, and 5000 mg/kg EAESTL, respectively. A blank dosage containing only 0.25 mL DMSO (2% in 0.9% saline) was given to each of the mice in the control group. Following treatment, mice were allowed to feed ad libitum and were monitored for the development of any symptoms of toxicity (vomiting, diarrhea, ataxia, and death) after dosage administration. These monitoring data were recorded after 3 h of dosage administration and then every 4 h for 1 day. For the remaining 13 days, data was recorded once a day. The mortality of mice was calculated after 48 h of oral dosage administration using the following equation (Eq. (6)):
| (6) |
where NMT and NML are the number of mice taken at the beginning and alive 48 h after EAESTL administration, respectively. The LD50 was calculated by following a similar method described above in Section 2.5. Here, the mortality was plotted with respect to the dose amount (concentration) taken in the relevant test sample. At the end of the experiment, mice were placed in a new cage with corn cob bedding and immediately euthanized by displacement of air with 100% carbon dioxide, followed by being burial in soil at an approved landfill at our university. This experiment was repeated three times.
2.13. Bodyweight measurement
The body weight of the selected mice (six mice in each group) groups (g-Control, g-250, g-500, g-1000, and g-2000) was measured by following a reported method [33]. In summary, the initial body weight was recorded just before the extract was administered. The body weight of the mice was monitored for 2 weeks and weighed after 3, 6, 9, 12, and 14 days of extract administration. The cumulative weight change was estimated by using the following equation (Eq. (7)):
| (7) |
where CWINTV represents the cumulative weight for a particular group at a specific time and CWINT is the same parameter at the initial time of the experiment.
2.14. Computer-aided prediction of the biological activity of phytochemicals
The PASS online server assessed the possible biological functions of EAESTL phytochemicals in the context of biological activities [34]. Briefly, the name of each phytochemical was submitted to the PubChem server, and a canonical SMILES of this element was extracted into the PASS program (http://www.way2drug.com/PASSOnline/) to obtain the probable biological activity of a specific phytochemical. The outcome was obtained as a ratio of ‘probability of being active (Pa)’ and ‘probability of being inactive (Pi)’ in the context of Pa>0.3. A higher Pa for a phytochemical indicates a greater chance of having a particular pattern of biological activity.
2.15. Prediction of pharmacokinetics (ADME), toxicity and drug-able properties
Different properties of the identified phytochemicals were evaluated in terms of being ideal drug candidates. The pharmacokinetic properties were related to ADME (absorption, distribution, metabolism, and excretion), toxicity (hERG inhibitor, AMES toxicity, carcinogenicity, hepatotoxicity, and rat oral acute toxicity), and drug-able properties [physicochemical parameters (MW, HBAs, HBD, RTB, and TPSA), lipophilicity (cLogP), water solubility (LogS), drug-likeness (Lipinski's Rule of Five: RO5) and medicinal chemistry (synthetic accessibility)]. The AdmetSAR2 web server (http://lmmd.ecust.edu.cn/admetsar2/) [35] was utilized to anticipate absorption (human intestinal absorption = HIA, human oral bioavailability = HOB, p-glycoprotein inhibitors, p-glycoprotein substrates), distribution (BBB penetration), metabolism (CYP450 inhibitors and CYP450 substrates), and toxicity (hepatotoxicity). The canonical SMILES ID of each phytochemical was submitted into the admetSAR2 web server, and the result was recorded for a set of parameters. Plasma protein binding (PPB) (distribution), excretion (renal clearance), and toxicity were predicted by the ADMETlab2 server (https://admetmesh.scbdd.com/) [36]. To do this, the canonical SMILES ID of each phytochemical ingredient was submitted to the ADMETlab2 server, and the result was obtained for a selected set of parameters. However, the SMILES format data for identified phytochemicals were further uploaded to the SwissADME server (http://www.swissadme.ch/), and computational results for physicochemical properties, lipophilicity, water solubility, drug-likeness and a synthetic accessibility score were collected [37].
2.16. Statistical analysis
The experimental data are represented as the average ± STD of the replicates for all experiments except body weight measurements of mice after oral administration of different concentrations of extract. The statistical interpretation of tests was accomplished using one-way ANOVA by Origin Lab version 2018, and Bonferroni's and Tukey's post hoc tests were used for the tests where *p < 0.05 and **p < 0.01 are significant compared with control values.
3. Results
3.1. Phytochemical analysis of ethyl acetate extract of Senna tora (L.) Roxb. leaves (EAESTL)
Phytochemicals are indispensable in plant defense and have been used as important raw materials for pharmaceutical manufacturing for centuries. Therefore, we tested the existence of phytochemicals in the ethyl acetate extract of Senna tora (L.) Roxb. leaves (EAESTL) (Supplementary Figure S1). EAESTL was found to contain phenols (flavonoids and tannins), terpenoids, saponins, steroids, cardiac glycosides and reducing sugars, as listed in Table 1 and Supplementary Figure S2.
Table 1.
Preliminary phytochemical screening of the ethyl acetate extract of Senna tora (L.) Roxb. leaves.
| Test name | Observation | Result | Name of phytochemicals |
|---|---|---|---|
| Alkaline reagent | Colorless solution | + | Phenolic (Flavonoids) |
| Ferric chloride | Reddish-black coloration | + | Phenolic (Tannins) |
| Lead acetate | Creamy-white color precipitation | + | Phenolic (Tannins) |
| Salkowski's | Bluish-brown layer at upper interface | + | Terpenoids |
| Salkowski's | Black color in the bottom face | + | Steroids |
| Mayer's | Creamy-colored precipitation | + | Alkaloids |
| Legal's | Blood-red color | + | Cardiac glycosides |
| Foaming | Foam formation | + | Saponins |
| Fehling's | Brick-red precipitation | + | Reducing sugar |
“+” means presence of specific phytochemicals.
3.2. GC-MS analysis of phytochemicals and predicting their antioxidant, anti-inflammatory, and antibacterial properties using PASS program
EAESTL had 59 peaks in its GC–MS chromatogram (Supplementary Figure S3). Each peak represented the existence of individual phytochemicals based on retention time (RT), peak area (%), chemical formula, compound structure and in silico, in vitro and/or in vivo biological properties (Table 2). The major phytochemicals identified in EAESTL were esters [methyl hexadecanoate (PN53 in Table 2, 7.28%), 2-(2-(2-butoxyethoxy)ethoxy)ethyl 2-methylbutanoate (PN40, 3.65%), isopentyl acetate (PN34, 3.35%), methyl stearate (PN55, 1.34%), trimethylsilyl 3-methyl-4-[(trimethylsilyl)oxy] benzoate (PN15, 1.33%) and decyl (2-methylpentyl) fumarate (PN22, 1.03%)], phenolics [phenylethyl alcohol (PN37, 3.65%), resorcinol (PN43, 3.58%), benzyl alcohol (PN28, 2.78%) and 4-(methoxymethyl)phenol, (PN4, 2.11%)], aldehyde [3-furaldehyde (PN5, 4.82%), 5-(hydroxymethyl)furan-2-carbaldehyde (PN42, 4.03%), and 5-methylfuran-2-carbaldehyde (PN17, 2.76%)], silicon compounds [octamethyl cyclotetrasiloxane, (PN39, 3.49%), decamethyl cyclopentasiloxane, (PN38, 2.26%), octamethylcyclotetrasiloxane (PN20, 2.10%), dimethyl 2,2'-((1,1,3,3,5,5-hexamethyltrisiloxane-1,5-diyl)bis(oxy))diacetate (PN27, 1.94%) and 1,1,3,3,5,5,7,7,9,9-decamethyl-1,9-pentasiloxane (PN44, 1.03%)], terpenoids [neophytadiene (PN50, 4.17%), (2E,7R,11R)-3,7,11,15-tetramethyl-2-hexadecen-1-ol (PN52, 2.05%) and 3,7,11,15-tetramethyl-2-hexadecen-1-ol (PN51, 1.46%)], alkaloids [phenyl carbamate (PN19, 2.44%) and 2,3,4,6,7,8-hexahydropyrrolo[1,2-a]pyrimidine (32, 1.13%)], alcoholic compounds [tert-butyldimethyl((3-methylbenzyl)oxy)silane (PN8, 1.97%) and benzyl alcohol (PN28, 2.78%)], silyl ether [benzyl(benzyloxy) dimethylsilane (PN10, 1.20%)], cyclic amino acid [piperidine-2-carboxylic acid (PN1, 2.17%)], omega-3 fatty acid [methyl (8E,11E,14E)-docosa-8,11,14-trienoate (PN55, 1.88%)], alkane [tetradecane (PN47, 1.44%)], organosulfur [2-methyl-3-(methylthio)furan (PN29, 1.47%)], steroids [stigmasta-5,22-dien-3beta-ol, acetate (PN57, 1.34%)], organic peroxide [3-hydroperoxyhexane (PN18, 1.25%)] and an aromatic compound [7a-methyloctahydro-2,7,4-(epiethane [1,1,2]triyl)cyclopenta[b]pyran (PN16, 1.15%)]. The relative content of phytochemicals in EAESTL was determined based on the peak area (%), and the order of occurrence was esters > phenolics > aldehydes > silicon compounds > terpenoids > alkaloids > heterocyclic compounds > alcoholic compounds > silyl ethers > cyclic amino acids > omega-3 fatty acids > alkanes > organosulfur > steroids > organic peroxides > aromatic compounds. The biological activity of a phytoconstituent is mostly inherent in the chemical and structural nature of the compound. Applications of medicinal plants are generally based on their biological activity, which is a prerequisite for consideration as a drug candidate. PASS program analysis of 59 phytochemicals predicted that 51 of them have pharmacological activities in the context of antioxidant, anti-inflammatory, and antibacterial activities. However, eight phytochemicals [hexamethylcyclotrisiloxane (PN2), 3-furaldehyde (PN5), TBDMS derivative (PN8), 4,2,7-ethanylylidenecyclopenta[b]pyran (PN16), phenyl carbamate (PN19), 2-methyl-3-(methylthio) furan (PN29), N-isobutylbicyclo [2.2.1] heptane-2-carboxamide (PN33), and pentasiloxane, 1,1,3,3,5,5,7,7,9,9-decamethyl (PN44)] did not have any antioxidant, anti-inflammatory, or antibacterial activities. Among fifty-one phytochemicals, thirty (PN3, PN4, PN6, PN7, PN11, PN12, PN15, PN18, PN20, PN22, PN23, PN24, PN27, PN28, PN32, PN34, PN35, PN37, PN38, PN39, PN40, PN41, PN45, PN46, PN47, PN49, PN53, PN54, PN56, and PN57) showed only anti-inflammatory activity, thirteen (PN1, PN9, PN10, PN13, PN14, PN17, PN21, PN26, PN31, PN36, PN42, PN51, and PN55) exhibited dual activities (anti-inflammatory and antibacterial), three (PN43, PN52 and PN59) showed triple activities (anti-inflammatory, antibacterial and antioxidant), two (PN30 and PN50) showed only antibacterial activity, one (PN25) exerted antibacterial and antioxidant activities, and two (PN48 and PN58) showed anti-inflammatory and antioxidant activities. This finding suggests that the presence of these phytochemicals in the extract is responsible for the in vitro antioxidant, anti-inflammatory, and antibacterial properties that we have shown in this study.
Table 2.
GC–MS identified phytochemicals in the ethyl acetate extract of Senna tora (L.) Roxb. leaves. The phytochemical functionalities and their corresponding probable biological activities (PBAs) were predicted using the PASS program.
| Peak No | Retention time | Area % | Structure, name and formula of the compound | Nature of compound | Compound CID | In silico PBA | Pa > 0,3 |
In vitro and/or in vivo biological activity |
|
|---|---|---|---|---|---|---|---|---|---|
| Pa | Pi | ||||||||
| 1 | 3.620 | 2.17 |  |
Cyclic amino acid | 849 | AI | 0, 357 | 0, 038 | Antibacterial [38] and inhibitor against EAC tumor cells [39] |
| ABO | 0,380 | 0, 003 | |||||||
| 2 | 3.674 | 0.52 |  |
Organosilicon | 10914 | – | – | – | Antimicrobial & antioxidant [40] and antidiabetic [41] |
| 3 | 3.741 | 0.70 |  |
Diol/dihydric alcohol | 250594 | A | 0, 426 | 0, 083 | No activity reported |
| AI | 0, 409 | 0, 020 | |||||||
| AO | 0, 329 | 0, 044 | |||||||
| 4 | 3.815 | 2.11 |  |
Phenol | 79310 | A | 0, 345 | 0, 126 | Antibacterial [42] and neuroprotective [43] |
| AI | 0, 573 | 0, 004 | |||||||
| AO | 0, 375 | 0, 017 | |||||||
| 5 | 3.905 | 4.82 |  |
Aldehyde | 10351 | – | – | – | Nrf2-keap1 pathway activator in skin cells [44] |
| 6 | 3.951 | 0.67 |  |
Ketone | 70258 | A | 0, 367 | 0, 026 | Photosynthesis inhibitor [45] and anticancer (breast) [46] |
| AI | 0, 461 | 0, 011 | |||||||
| AO | 0, 420 | 0, 008 | |||||||
| 7 | 3.998 | 0.51 |  |
Esters | 91698641 | A | 0, 400 | 0, 095 | No activity reported |
| AI | 0, 321 | 0, 056 | |||||||
| AO | 0, 470 | 0, 005 | |||||||
| 8 | 4.040 | 1.97 |  |
Silyl ether | 22967275 | – | – | – | No activity reported |
| 9 | 4.075 | 0.88 |  |
Unsaturated aldehyde | 5283345 | A | 0, 391 | 0, 101 | Antifungal [47] and antioxidant [48] |
| AI | 0, 427 | 0, 017 | |||||||
| AB | 0, 334 | 0, 048 | |||||||
| 10 | 4.195 | 1.20 |  |
Silyl ether | 518982 | A | 0, 391 | 0, 101 | No activity reported |
| AI | 0, 427 | 0, 017 | |||||||
| AB | 0, 334 | 0, 048 | |||||||
| 11 | 4.275 | 0.68 |  |
Aromatic hydrocarbon | 7237 | A | 0, 375 | 0, 109 | Antibacterial [49] |
| AI | 0, 459 | 0, 011 | |||||||
| 12 | 4.490 | 0.72 |  |
Polyunsaturated hydrocarbon | 637866 | A | 0, 315 | 0, 147 | No activity reported |
| AI | 0, 519 | 0, 006 | |||||||
| AO | 0, 399 | 0, 010 | |||||||
| 13 | 4.685 | 0.75 |  |
Heterocyclic organic compound | 10341 | A | 0, 434 | 0, 079 | Antioxidant, anti-inflammatory, analgesic, antibacterial anticancer, anticonvulsant, antifungal, antiulcer and anti-TB [50] |
| AI | 0, 359 | 0, 036 | |||||||
| AO | 0, 326 | 0, 047 | |||||||
| AB | 0, 414 | 0, 027 | |||||||
| 14 | 4.805 | 0.57 |  |
Monoterpene | 99038 | AO | 0, 331 | 0, 042 | Antifungal [51] |
| AB | 0, 398 | 0, 030 | |||||||
| 15 | 5.150 | 1.33 |  |
Ester | 91740684 | AI | 0, 344 | 0, 043 | Protects human cells against oxidative damage and cancer [52] |
| 16 | 5.215 | 1.15 |  |
Aromatic compound | 565150 | – | – | – | No activity reported |
| 17 | 5.306 | 2.76 |  |
Aldehyde | 12097 | AI | 0, 306 | 0, 066 | Antioxidant [53] |
| AB | 0, 417 | 0, 026 | |||||||
| 18 | 5.360 | 1.25 |  |
Organic Peroxide |
141085 | AI | 0, 437 | 0, 015 | No activity reported |
| AO | 0, 345 | 0, 032 | |||||||
| 19 | 5.441 | 2.44 |  |
Alkaloids | 69322 | – | – | – | Acetylcholinesterase inhibitor [54] and antifungal [55] |
| 20 | 5.485 | 3.49 |  |
Organosilicon | 11169 | AI | 0, 342 | 0, 044 | Estrogenic [56] |
| 21 | 5.590 | 0.97 |  |
Esters | 137020 | A | 0, 765 | 0, 009 | No activity reported |
| AO | 0, 355 | 0, 025 | |||||||
| AB | 0, 427 | 0, 025 | |||||||
| 22 | 5.622 | 1.03 |  |
Esters | 91737497 | A | 0, 515 | 0, 053 | No activity reported |
| AI | 0, 343 | 0, 044 | |||||||
| 23 | 5.650 | 0.99 |  |
Alcoholic compound | 542357 | AI | 0, 377 | 0, 030 | No activity reported |
| AO | 0, 407 | 0, 009 | |||||||
| 24 | 5.725 | 1.39 |  |
Bromine compound | 98219 | AI | 0, 402 | 0, 022 | Antimicrobial [57] |
| 25 | 5.755 | 0.58 |  |
Vitamin-C | 54685836 | AOx | 0, 381 | 0, 014 | No activity reported |
| AB | 0, 381 | 0, 035 | |||||||
| 26 | 6.073 | 0.80 |  |
Haloalkane | 33574 | AI | 0, 425 | 0, 017 | No activity reported |
| AB | 0, 327 | 0, 050 | |||||||
| 27 | 6.109 | 1.94 |  |
Organosilicon | 91738767 | AI | 0, 320 | 0, 057 | No activity reported |
| 28 | 6.166 | 2.78 |  |
Aromatic alcohol/Pehnol | 244 | A | 0, 414 | 0, 089 | Analgesic [58], antioxidant and antibacterial [59] |
| AI | 0, 532 | 0, 005 | |||||||
| AO | 0, 363 | 0, 021 | |||||||
| 29 | 6.282 | 1.47 |  |
Aryl thioethers (organosulfur) | 526618 | – | – | – | No activity reported |
| 30 | 6.340 | 0.50 |  |
Ester | 607122 | AB | 0, 346 | 0, 044 | No activity reported |
| 31 | 6.570 | 0.53 | Fatty aldehyde | 5364438 | AI | 0, 427 | 0, 017 | Mosquito larvicidal [60] | |
| AB | 0, 400 | 0, 030 | |||||||
| 32 | 6.76 | 1.13 |  |
Amidine base/Alkaloids | 76349 | A | 0, 515 | 0, 052 | No activity reported |
| 33 | 6.81 | 3.6 |  |
Carboxamide (Amide) | 565668 | – | – | – | No activity reported |
| 34 | 6.9 | 3.35 |  |
Ester | 31276 | A | 0, 602 | 0, 031 | No activity reported |
| AI | 0, 323 | 0, 055 | |||||||
| AO | 0, 354 | 0, 026 | |||||||
| 35 | 7.075 | 0.49 |  |
Aromatic alcohol/Phenol | 559104 | A | 0, 415 | 0, 053 | No activity reported |
| AI | 0, 570 | 0, 004 | |||||||
| AO | 0, 305 | 0, 072 | |||||||
| 36 | 7.105 | 0.63 |  |
Unsaturated heterocyclic ketone | 699486 | A | 0, 875 | 0, 005 | No activity reported |
| AI | 0, 410 | 0, 020 | |||||||
| AO | 0, 450 | 0, 005 | |||||||
| AB | 0, 353 | 0, 042 | |||||||
| 37 | 8.025 | 3.65 |  |
Aromatic alcohol/Phenol | 6054 | AO | 0,450 | 0,005 | Antioxidant [61], antifungal [62] and anti-inflammatory [63] |
| AI | 0,410 | 0,020 | |||||||
| A | 0,305 | 0,156 | |||||||
| 38 | 7.218 | 2.26 |  |
Organosilicon | 10913 | AI | 0, 342 | 0, 044 | No activity reported |
| 39 | 7.946 | 2.10 |  |
Silicon compound | 6453921 | AI | 0, 342 | 0, 044 | Antioxidant and antimicrobial [64] |
| 40 | 8.025 | 3.65 |  |
Ester | 91693497 | A | 0, 531 | 0, 048 | No activity reported |
| AI | 0, 311 | 0, 062 | |||||||
| AO | 0,372 | 0, 018 | |||||||
| 41 | 8.185 | 3.90 |  |
Heterocyclic Compound |
10329 | A | 0, 521 | 0, 051 | Antileishmanial [65] |
| AI | 0, 353 | 0, 039 | |||||||
| AO | 0, 359 | 0, 023 | |||||||
| 42 | 8.248 | 4.03 |  |
Aromatic aldehyde | 237332 | AI | 0, 328 | 0, 052 | Anti-sickling [66], antioxidant and antiproliferative [67] |
| AB | 0, 407 | 0, 028 | |||||||
| 43 | 8.793 | 3.58 |  |
Phenol | 5054 | AOx | 0, 486 | 0, 007 | Antioxidative [68] and anticancer [69] |
| A | 0, 496 | 0, 058 | |||||||
| AI | 0, 537 | 0, 005 | |||||||
| AO | 0, 369 | 0, 019 | |||||||
| AB | 0, 310 | 0, 057 | |||||||
| 44 | 8.958 | 1.03 |  |
Organosilicon | 6329089 | – | – | – | No activity reported |
| 45 | 9.008 | 0.88 |  |
Organosilicon | 10911 | AI | 0, 342 | 0, 044 | No activity reported |
| 46 | 9.287 | 0.52 |  |
Oxo alcohol | 24847 | A | 0, 543 | 0, 045 | No activity reported |
| AI | 0, 440 | 0, 014 | |||||||
| AO | 0, 356 | 0, 025 | |||||||
| 47 | 10.059 | 1.44 |  |
Long chain alkane | 12389 | A | 0, 424 | 0, 084 | Antibacterial and antifungal [70] |
| AI | 0, 585 | 0, 004 | |||||||
| AO | 0, 378 | 0, 015 | |||||||
| 48 | 11.075 | 0.48 |  |
Aromatic alcohol/Phenol | 70825 | AOx | 0, 469 | 0, 008 | Antifungal [71] |
| A | 0, 610 | 0, 029 | |||||||
| AI | 0, 449 | 0, 013 | |||||||
| AO | 0, 313 | 0, 061 | |||||||
| 49 | 11.075 | 0.52 | Hydrocarbon | 12391 | A | 0, 424 | 0, 084 | No activity reported | |
| AI | 0, 585 | 0, 004 | |||||||
| AO | 0, 378 | 0, 015 | |||||||
| 50 | 14.898 | 4.17 |  |
Terpenoid | 10446 | AB | 0, 363 | 0, 040 | Antimicrobial and antioxidant [72] |
| 51 | 15.241 | 1.46 |  |
Diterpene | 5366244 | A | 0, 458 | 0, 070 | Anticarcinogenic and antitumor [73], antimicrobial, anti-inflammatory, anticancer and antiartrhitis [74] and antinociceptive and antioxidant [75] |
| AI | 0, 305 | 0, 067 | |||||||
| AB | 0, 417 | 0, 026 | |||||||
| 52 | 15.529 | 2.05 |  |
Diterpene | 5280435 | AOx | 0, 475 | 0, 008 | Antinociceptive and antioxidant [75] |
| A | 0, 458 | 0, 070 | |||||||
| AI | 0, 305 | 0, 067 | |||||||
| AB | 0, 417 | 0, 026 | |||||||
| 53 | 16.184 | 7.28 |  |
Fatty acid methyl ester | 8181 | A | 0, 510 | 0, 054 | Anti-inflammatory [76] |
| AI | 0, 758 | 0, 002 | |||||||
| AO | 0, 441 | 0, 005 | |||||||
| 54 | 18.905 | 0.48 |  |
Ester | 534619 | A | 0, 330 | 0, 135 | No activity reported |
| AI | 0, 380 | 0, 029 | |||||||
| AO | 0, 446 | 0, 005 | |||||||
| 55 | 19.008 | 1.88 |  |
Omega 3 fatty acid | 5364473 | A | 0, 728 | 0, 013 | No activity reported |
| AI | 0, 681 | 0, 003 | |||||||
| AO | 0, 407 | 0, 009 | |||||||
| AB | 0, 302 | 0, 059 | |||||||
| 56 | 19.435 | 1.34 |  |
Fatty acid ester | 8201 | A | 0, 510 | 0, 054 | Antibacterial, antioxidant and antitumor [77] and anticancer [78] |
| AI | 0, 758 | 0, 002 | |||||||
| AO | 0, 441 | 0, 005 | |||||||
| 57 | 33.568 | 1.34 |  |
Steroid | 6432445 | A | 0, 546 | 0, 044 | Antifungal [79] |
| AO | 0, 402 | 0, 010 | |||||||
| 58 | 34.901 | 0.65 |  |
Synthetic form of vitamin E | 2116 | AOx | 0, 967 | 0, 002 | Anticancer [80], anti-inflammatory, antioxidant, antimicrobial, radical scavenging and antispasmodic [81] |
| A | 0, 814 | 0, 006 | |||||||
| AO | 0, 587 | 0, 003 | |||||||
| 59 | 37.218 | 0.58 |  |
Carotene | 5366411 | AOx | 0, 456 | 0, 008 | Antioxidant, antiasthma, anticancer, immune enhancement, arthrosclerosis and gingivitis prevention [82] |
| A | 0, 561 | 0, 040 | |||||||
| AI | 0, 378 | 0, 029 | |||||||
| AB | 0, 336 | 0, 047 | |||||||
Compound's probable biological activity (PBA) was determined in the aspect of only antioxidant, anti-inflammatory, and antibacterial activity and Pa>0.3. Here, AOx = Antioxidant, A = Anti-inflammatory, AI = Anti-inflammatory intestinal, AO = Anti-inflammatory ophthalmic, AB = Anti-bacterial, ABO = Antibacterial ophthalmic.
3.3. DPPH and hydrogen peroxide radical scavenging activity
DPPH is a nitrogen-extractable oxidant that is conventionally used to determine the free radical-scavenging activities of antioxidants present in plant extracts or synthetic compounds. EAESTL at concentrations of 2.5, 5, 10, 20, 40, 80, 160, and 200 μg/mL exhibited dose-dependent antioxidant activity (Fig. 1A), with a range from 33.041 ± 1.166% to 91.068 ± 1.950% DPPH inhibition and an IC50 value of 24.425 μg/mL. Similar antioxidant activity was shown by ascorbic acid, ranging from 40.455 ± 2.019% to 98.190 ± 0.863% DPPH restraint with an IC50 value of 2.585 μg/mL. Although H2O2 is not a free radical, it plays a pivotal role in causing oxidative damage by transforming into a highly reactive hydroxyl radical (HO•). Therefore, we also performed an H2O2-scavenging assay for EAESTL and ascorbic acid. This analysis found concentration-dependent antioxidant activity for EAESTL by scavenging H2O2 with a range of 34.595 ± 1.104% to 93.734 ± 0.336% and an IC50 value of 17.434 μg/mL (Fig. 1B). Moreover, ascorbic acid showed H2O2-scavenging activity comparable with that of EAESTL, and the range was 39.238 ± 2.040 to 99.154 ± 0.115% with an IC50 value of 1.923 μg/mL. In summary, comparing the results obtained in different scavenging assays, EAESTL and a well-known antioxidant (ascorbic acid) showed similar antioxidant activity.
Fig. 1.
The antioxidant activity of EAESTL was compared with that of ascorbic acid. (A) DPPH free radical scavenging by EAESTL and ascorbic acid. (B) The H2O2-scavenging activity of EAESTL and ascorbic acid. The mean standard deviation (STD) of three individual tests was employed to calculate the results.
3.4. EAESTL exhibited potent anti-inflammatory activity
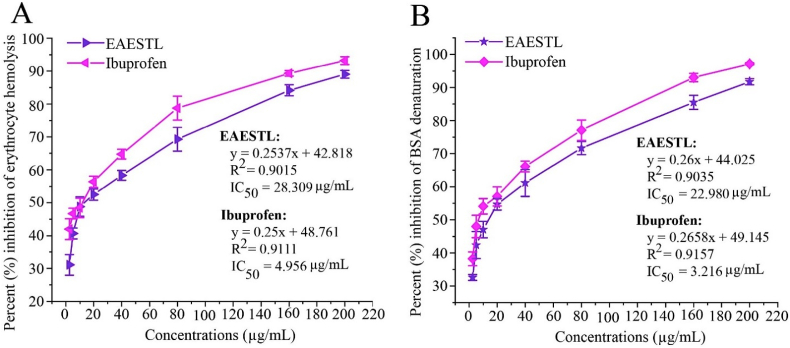
Inflammation is firmly related to pathophysiological processes. The quest for natural compounds and phytoconstituents that might prevent inflammation could be helpful for human health. In this study, EAESTL displayed dose-related anti-inflammatory activity by inhibiting RBC hemolysis and BSA denaturation. At different concentrations, EAESTL inhibited hemolysis of the RBC membrane within a range of 31.058 ± 3.145% to 89.029 ± 1.186%, where the IC50 value was 28.309 μg/mL (Fig. 2A). In addition, the BSA denaturation inhibition of EAESTL ranged from 32.617 ± 0.890% to 91.731 ± 0.949, and the IC50 value was 22.980 μg/mL (Fig. 2B). The anti-inflammatory activity of EAESTL was compared with ibuprofen, which commonly functions as an active ingredient in standard anti-inflammatory drugs. It showed anti-inflammatory activity with a range of 41.978 ± 1.634 to 93.194 ± 0.353% in RBC hemolysis inhibition (IC50 4.956 μg/mL) and 38.260 ± 2.081 to 97.116 ± 0.679 in BSA denaturation inhibition (IC50 3.216 μg/mL). Thus, similar efficiency for EAESTL as for a prominent anti-inflammatory drug, ibuprofen, can be noted.
Fig. 2.
The anti-inflammatory activity of EAESTL was contrasted with ibuprofen. The anti-inflammatory activity of EAESTL was ascertained by the heat-induced hemolysis of RBC repression (A) and BSA distortion inhibition (B). The mean ± STD of three individual tests was used to analyze the outcomes.
3.5. EAESTL exhibited potential antibacterial efficacy against a number of microorganisms that cause disease in humans
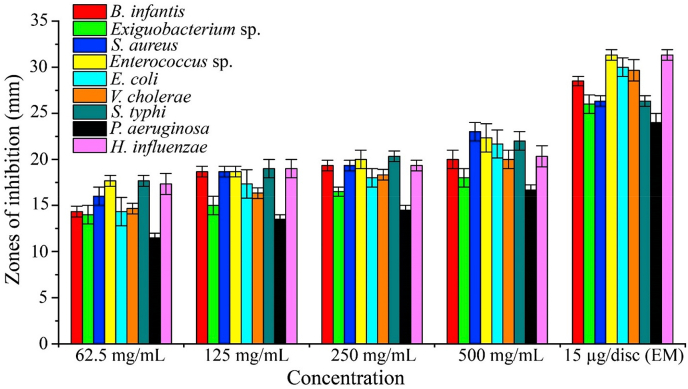
Medicinal plants containing bioactive phytochemicals have been used as a starting point for antibiotic synthesis to treat infectious diseases. Therefore, we assessed the antibacterial potential of EAESTL. Our findings revealed that EAESTL, at rates ranging from 62.5 to 500 mg/mL, can prevent the proliferation of bacteria, with inhibition zones ranging from 14 ± 1 to 23 ± 1 mm for Gram-positive bacteria and 11.5 ± 0.5 to 22 ± 1 mm for Gram-negative bacteria (Supplementary Figure S4). This finding demonstrates EAESTL's antibacterial effectiveness across a broad range of microorganisms. As shown in Fig. 3, against all the bacteria tested, a greater inhibition zone was obtained at a concentration of 500 mg/mL. EAESTL exhibited the highest antibacterial activity with a 23 ± 1 mm inhibition zone against S. aureus at 500 mg/mL, and the lowest antibacterial efficacy was 11.5 ± 0.5 mm against P. aeruginosa at 62.5 mg/mL. P. aeruginosa was always at the lowest level in terms of the zone of inhibition, while the growth of S. aureus was inhibited more than that of any other bacterial type studied. Erythromycin (EM) was used as a standard during this study and showed antibacterial activity with inhibition zones extending from 24 ± 1 to 31.333 ± 0.577 mm, which is comparable with EAESTL at a concentration of 500 mg/mL. The MIC and MBC values for the EAESTL tested on the nine bacterial strains are shown in Supplementary Table S1. The values recorded for MBC were greater than those for MIC, ranging from 3.270 ± 1.133 to 6.541 ± 2.266 mg/mL.
Fig. 3.
Antibacterial activity of EAESTL. Different concentrations of EAESTL and the commercial antibiotic erythromycin (EM) produced zones of inhibition against Gram-positive (B. infantis, Exiguobacterium sp., S. aureus, and Enterococcus sp.) and Gram-negative bacteria (E. coli, V. cholerae, S. typhi, P. aeruginosa, and H. influenzae). The proportions of inhibition zones were portrayed in millimeters with respect to the concentration. The data are represented by the mean ± STD (n = 3).
3.6. EAESTL has been found to be safe in cytotoxicity and acute toxicity tests
The cytotoxicity of EAESTL was tested using an Artemia salina larvae lethality bioassay. In this study, all Artemia salina larvae survived under control conditions after 24 h of EAESTL treatment. The mortality of Artemia salina larvae was proportionally dependent on the concentration of EAESTL up to 24 h of treatment time. Here, 100% of Artemia salina larvae died at a concentration of 100 μg/mL vincristine sulfate, while 90% died at the same concentration of EAESTL. As shown in Fig. 4A, the cytotoxic effect (LC50) of EAESTL was 35.246 μg/mL; however, under the same conditions for a standard anticancer drug (vincristine sulfate), it was 2.238 μg/mL, and up to 100 μg/mL is considered nontoxic. Therefore, the cytotoxic effect of EAESTL can be considered moderately cytotoxic. The general focus of the acute toxicity studies was on determining the safe acute doses. Acute toxicity is generally expressed by the LD50 value, which denotes the amount of potential poisoning material given at once for a short-term (usually less than 24 h) interval that causes the death of 50% of a group of test animals. In this work, the LD50 was determined for Swiss albino mice. Any behavioral changes were also monitored to identify additional symptoms. None of the mice died after 24 h (Fig. 4B); however, after 40–43 h of dose implementation, 11.11 ± 9.622%, 50 ± 16.666%, and 61.111 ± 9.622% of the mice died at doses of 2000, 4000, and 5000 mg/kg b. w., respectively. Mouse mortalities that appeared within 40–43 h and up to 14 days of EAESTL (>2000 mg/kg) administration were considered for computing the oral LD50. The EAESTL oral LD50 value for the mice was 4263.906 mg/kg b. w. (Fig. 4C), which is more than two times higher than the nontoxic limit (>2,000 mg/kg b. w.) [83] and can be employed for further evaluation as a therapeutic agent to examine the effect of EAESTL on life-related parameters. During a 14-day observation period, EAESTL at a dose of 2000 mg/kg b. w. caused no death, behavioral abnormalities, locomotor ataxia, or diarrhea. At a dose of 2000 mg/kg b. w. (Fig. 4D), no noteworthy effect on body weight was noticed, and weight loss did not appear with increasing doses. From the behavioral monitoring, the food intake or water consumption attitude was similar for both test and control mice. This result indicates that EAESTL does not change the expected behavior of participating animals.
Fig. 4.
The toxicity of EAESTL. (A) EAESTL cytotoxicity in Artemia salina larvae. (B) The effect of EAESTL dosage on mouse mortality. (C) The median lethal dose (LD50) of EAESTL in acute toxicity. (D) Effects of acute EAESTL treatment on body weight.
3.7. Sixteen phytochemicals exhibited good ADME to be possible drug candidates
The ADME profiles of 51 phytochemicals that showed individual antioxidant, anti-inflammatory, and antibacterial potential in the PASS program were investigated (Table 3). The bioavailability of a drug is greatly affected by the route of drug administration, such as human oral bioavailability (HOB) and human intestinal absorption (HIA). Therefore, HIA and HOB were calculated at different drug development stages. Among the 51 phytochemicals tested, 50 showed positive HIA, and 34 exhibited good HOB. In the gastrointestinal tract, P-glycoproteins (Pgp) affect drug absorption. Our findings demonstrated that 48 phytochemicals are noninhibitors and 51 phytochemicals are nonsubstrates of Pgp. Thus, the pharmacokinetic efficacy of phytochemicals is neither inhibited nor lowered by Pgp. Some medications pass through the BBB, while others do not. All of the tested phytochemicals (51) showed no BBB permeability, indicating non-CNS targets. The impact of plasma proteins on drug delivery is the largest. Only the free (unbound to plasma proteins) drug can reach the tissues and produce a therapeutic benefit. Drugs with limited PPB (<90%) are thought to be therapeutically effective. We determined that 23 out of 51 phytochemicals have optimal plasma protein-binding potential with less than 90% PPB. Drug metabolism reduces the bioavailability of a drug. The endoplasmic reticulum of the liver pertains to a group of enzymes called cytochrome P450 (CYP450) that are needed for xenobiotic detoxification. Most orally administered drugs were also metabolized by these enzymes. Drugs that repress CYPs can be hazardous because of the aggregation of coadministered medications or the actual medicine. We observed that 51 phytochemicals are noninhibitors of CYP4503A4, 45 are nonsubstrates of CYP4503A4, 51 are nonsubstrates of CYP4502D6, 48 are nonsubstrates of CYP4502D6, 51 are nonsubstrates of CYP4502C9 and 47 are nonsubstrates of CYP4502C9, indicating that these phytochemicals neither accumulate nor undergo metabolism in the liver and will be able to enter the systemic circulation for distribution throughout the body to exert their desired action. Pharmacokinetics also manifest how drugs reach their target sites of action and are excreted. One of the most important routes for eliminating a metabolized and unaltered chemical is through renal excretion. Substances that will become future drug-like compounds must have excretion qualities during the drug development process. We revealed that 30 phytochemicals have modest clearance potential and that 2 phytochemicals have a high clearance tendency. However, above all, among 51 phytochemicals, 16 phytochemicals (PN3, PN6, PN11, PN12, PN13, PN14, PN17, PN18, PN23, PN25, PN28, PN32, PN35, PN37, PN42 and PN43) showed good ADME.
Table 3.
The ADME profiles of the 51 phytochemicals that showed antioxidant, anti-inflammatory, and antibacterial activities in the PASS program.
| Peak No. | Compounds name | Compound CID | Canonical SMILES |
Absorption |
Distribution |
Metabolism |
Excretion |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CYP450 inhibitor |
CYP450 substrate |
|||||||||||||||
| HIA | HOB | p-glycoprotein inhibitor | p-glycoprotein substrate | BBB penetration | PPB (%) | CYP4502C9 | CYP4502D6 | CYP4503A4 | CYP4502C9 | CYP4502D6 | CYP4503A4 | Renal clearance (ml/min/kg) | ||||
| 3 | 1,1-Cyclohexanedimethanol | 250594 | C1CCC(CC1)(CO)CO | + | + | NI | NS | − | 19.524 | NI | NI | NI | NS | NS | NS | 6.556 (Moderate) |
| 6 | 4-Cyclopentene-1,3-dione | 70258 | C1C(=O)C]CC1=O | + | + | NI | NS | − | 57.697 | NI | NI | NI | NS | NS | NS | 8.913 (Moderate) |
| 11 | O-Xylene | 7237 | CC1=CC]CC]C1C | + | + | NI | NS | − | 89.002 | NI | NI | NI | NS | NS | NS | 10.483 (Moderate) |
| 12 | 1,3,5,7-Cyclooctatetraene | 637866 | C1=CC]CC]CC]C1 | + | + | NI | NS | − | 84.437 | NI | NI | NI | NS | NS | NS | 2.413 (Low) |
| 13 | 2-Furanone | 10341 | C1C]CC(=O)O1 | + | + | NI | NS | − | 79.219 | NI | NI | NI | NS | NS | NS | 14.220 (Moderate) |
| 14 | 2,6,6-Trimethylbicyclo [3.1.1] heptane-3-ol or Isopinocampheol or 3-Pinanol | 99038 | CC1C2CC(C2(C)C)CC1O | + | + | NI | NS | − | 54.637 | NI | NI | NI | NS | NS | NS | 15.208 (High) |
| 17 | 5-Methylfuran-2-carbaldehyde or 5-Methyl furfural | 12097 | CC1=CC]C(O1)C]O | + | + | NI | NS | − | 70.804 | NI | NI | NI | NS | NS | NS | 6.582 (Moderate) |
| 18 | 3-Hydroperoxyhexane or 3-Hexyl hydroperoxide | 141085 | CCCC(CC)OO | + | + | NI | NS | − | 59.289 | NI | NI | NI | NS | NS | NS | 8.870 (Moderate) |
| 23 | 3-Methoxy-2- (methoxymethyl)-2-methylpropan-1-ol | 542357 | CC(CO)(COC)COC | + | + | NI | NS | − | 11.982 | NI | NI | NI | NS | NS | NS | 5.628 (Moderate) |
| 25 | 5-(2-Ethyl-1,3,2-dioxaborolan-4-yl)-3,4-dihydroxyfuran-2(5H)-one | 54685836 | B1(OCC(O1)C2C(=C(C(=O)O2)O)O)CC | + | + | NI | NS | − | 85.147 | NI | NI | NI | NS | NS | NS | 11.526 (Moderate) |
| 28 | Benzyl alcohol | 244 | C1=CC]C(C]C1)CO | + | + | NI | NS | − | 51.396 | NI | NI | NI | NS | NS | NS | 9.037 (Moderate) |
| 32 | 2,3,4,6,7,8-Hexahydropyrrolo[1,2-a]pyrimidine | 76349 | C1CC2 = NCCCN2C1 | + | + | NI | NS | − | 45.148 | NI | NI | NI | NS | NS | NS | 12.065 (Moderate) |
| 35 | 3-(Hydroxy-phenyl-methyl)-2,3-dimethyl-octan-4-one | 559104 | CCCCC(=O)C(C)(C(C)C)C(C1=CC]CC]C1)O | + | + | NI | NS | − | 91.190 | NI | NI | NI | NS | NS | NS | 10.550 (Moderate) |
| 37 | Phenylethyl alcohol | 6054 | C1=CC]C(C]C1)CCO | + | + | NI | NS | − | 53.125 | NI | NI | NI | NS | S | NS | 10.085 (Moderate) |
| 42 | 5-(Hydroxymethyl)furan-2-carbaldehyde or 5-hydroxymethylfurfural | 237332 | C1=C(OC(=C1)C]O)CO | + | + | NI | NS | − | 50.357 | NI | NI | NI | NS | NS | NS | 7.185 (Moderate) |
| 43 | Resorcinol | 5054 | C1=CC(=CC(=C1)O)O | + | + | NI | NS | − | 44.189 | NI | NI | NI | NS | NS | NS | 16.027 (High) |
Here, “+” and “−” means present and absent, respectively; I = Inhibitor; NI = Noninhibitor; NS = Non-substrate; S = Substrate.
3.8. The drug-able properties of phytochemicals
A comprehensive evaluation of drug-able properties and toxicity is required for effective drug development. These properties greatly influence the ADME of the drug candidates. In this study, the SwissADME server was used to quantify these properties (Table 4) for the identified phytochemicals of EAESTL. Lipinski suggested RO5, which states that the four basic physicochemical properties are MW (<500 g/mol), HBA ≤ 10, HBD ≤ 5, cLogP 1> and ≤5. These are all correlated with higher HOB. Our computational study for EAESTL found that 49 phytochemicals have an optimal MW (<500 g/mol), and all 51 phytochemicals have an appropriate number of HBA (≤10) and HBD (≤5). In addition, 27 phytochemicals have a standard assortment of cLogP (1> and <5). The ideal RB range is 0–11, while the highest border for effective absorption is 13. Forty-four phytochemicals contain RB attributed to the standard range. Compound absorption is also connected to TPSA, and a TPSA of ≤140 is associated with high compound absorption. All identified phytochemicals have a TPSA value of <106.97, indicating that they have high permeability. The LogS value, which is always preferably low, impacts drug solubility. Twenty-eight phytochemicals have a suitable LogS value range of −4.0 to 0.5, indicating that they can cross the membrane bilayer. Toxicity prediction is essential in the current drug discovery process for assessing a compound's adverse effects on an animal and the environment. The hERG gene (human ether-a-go-go-related gene) generally codes for a potential-dependent potassium ion channel that controls heartbeat. Many drugs have been abandoned due to cardiotoxicity induced by hERG inhibition. Among the 51 phytochemicals, 49 are noninhibitors of hERG. An obstacle to medicinal agents is carcinogenicity, which becomes severe when linked with mutagenicity. The Ames test is the most popular way of determining mutagenicity. Forty-five phytochemicals of EAESTL were found to be nonmutagenic in an Ames test (Table 4). Liver toxicity is one of the most critical aspects of the medication development process. The hepatotoxicity test revealed that 50 phytochemicals are not hepatotoxic. Acute oral toxicity is an essential endpoint in medicinal research and environmental risk management. However, the experiments are tedious to conduct; therefore, in silico approaches have been developed as a replacement. The United States Environmental Protection Agency (EPA) has classified toxicity based on LD50, and we found that among 51 phytochemicals, 42 are not acutely toxic. One of the well-known methods of evaluating a drug's drug-likeness is RO5. In this study, the SwissADME server was used to assess the compounds' RO5, and 36 phytochemicals did not violate this set of rules, indicating that they are drug-like. The synthetic accessibility of a drug candidate is an important factor; for this reason, computational and medicinal chemists consider this to be a basic objective in the early drug development stage. Compounds with a synthetic accessibility score (SAscore) ≥6 are challenging to synthesize, and those with an SAscore ≤6 are easy to synthesize. Forty-eight phytochemicals showed a favorable synthetic accessibility score of ≤6 and the desired properties. However, only eight phytochemicals (PN7, PN14, PN24, PN28, PN34, PN35, PN37 and PN46) exhibited optimal druggable properties without toxicity. Taken together, four phytochemicals (PN14, PN28, PN35, and PN37) passed ADME and demonstrated optimal druggable properties without toxicity.
Table 4.
The drug-able properties (physicochemical, lipophilicity, water solubility, drug-likeness (RO5) and synthetic accessibility properties) and toxicity of 51 phytochemicals.
| Peak No. | Compound CID | Canonical SMILES | Physicochemical properties |
Lipophilicity (cLOGPo/w) |
Water solubility [LogS (ESOL)] |
Drug likeness |
Medicinal chemistry |
Toxicity |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MW (g/mol) | No. of HBA | No. of HBD | No. of rotatable bonds | TPSA | RO5 violation | Synthetic accessibility score |
hERG inhibitor |
AMES toxicity | Carcinogenicity | Hepatotoxicity | Rat oral acute toxicity | |||||
| 7 | 91698641 | CCOCCOC(=O)CC(C)C | 174.24 | 3 | 0 | 7 | 35.53 | 1.86 | −1.47 | 0 | 2.09 | NI | NT | NC | NT | NT |
| 14 | 99038 | CC1C2CC(C2(C)C)CC1O | 154.25 | 1 | 1 | 0 | 20.23 | 2.25 | −2.4 | 0 | 3.8 | NI | NT | NC | NT | NT |
| 24 | 98219 | CCCCCCCC(C)Br | 207.15 | 0 | 0 | 6 | 0 | 3.98 | −3.81 | 0 | 3.91 | NI | NT | NC | NT | NT |
| 28 | 244 | C1=CC]C(C]C1)CO | 108.14 | 1 | 1 | 1 | 20.23 | 1.41 | −1.69 | 0 | 1 | NI | NT | NC | NT | NT |
| 34 | 31276 | CC(C)CCOC(=O)C | 130.18 | 2 | 0 | 4 | 26.3 | 1.76 | −1.8 | 0 | 1.19 | NI | NT | NC | NT | NT |
| 35 | 559104 | CCCCC(=O)C(C)(C(C)C)C(C1=CC]CC]C1)O | 262.39 | 2 | 1 | 7 | 37.3 | 3.66 | −3.65 | 0 | 2.74 | NI | NT | NC | NT | NT |
| 37 | 6054 | C1=CC]C(C]C1)CCO | 122.16 | 1 | 1 | 2 | 20.23 | 1.64 | −1.82 | 0 | 1 | NI | NT | NC | NT | NT |
| 46 | 24847 | CCCCCC(CCC)CO | 158.28 | 1 | 1 | 7 | 20.23 | 3.08 | −2.72 | 0 | 2.1 | NI | NT | NC | NT | NT |
I = Inhibitor; NI = Non-inhibitor; NC = Noncarcinognic; NT = Non-toxic; T = Toxic.
4. Discussion
Plants generate secondary metabolites and utilize them to defend themselves against possible adversaries and environmental risks. These metabolites and their derivatives have been effective therapeutic agents from the beginning of human evolution and play a significant role in treating various illnesses [84]. Therefore, this study explored the in vitro antioxidant, anti-inflammatory, and antibacterial activities of EAESTL and identified the phytochemicals in the extract to discover phytochemical compounds by means of a computational study of pharmacokinetics (ADME), toxicity, drug-able properties, and the probability of biological activities and in vivo cytotoxicity and acute toxicity to aid in developing potential therapeutic agents against oxidants, inflammation, and bacterial infection.
In living organisms, ROS are created endogenously or exogenously. The external supply of antioxidants acts as either ROS scavengers or inducers of endogenous (enzymatic and nonenzymatic) antioxidative systems to fight against ROS. Natural antioxidants produced from medicinal and dietary plants are commonly used as therapeutic agents. Therefore, we determined the antioxidant activity of EAESTL by using DPPH free radical- and H2O2-scavenging activity methods. EAESTL solution converted purple-colored DPPH to a yellow-colored DPPH-H protonated substance. Thus, the absorbance of DPPH diminished with increasing EAESTL condensation, indicating the free radical-scavenging potency of EAESTL. Similar free radical-scavenging activity was reported for the ethyl acetate extract of Xylocarpus granatum leaves by Darmadi et al. [85]. H2O2 is not a free radical but it is an electrically neutral ROS. It can generate a highly reactive hydroxyl radical, which severely damages adjacent biomolecules [86]. In addition, it is hazardous due to its mobility, typically passing through cellular membranes and arriving at the cell compartments far from the site of its origination [87]. Thus, the removal of H2O2 is crucial to avoid oxidative damage. Our antioxidant activity results showed that EAESTL scavenges H2O2 in a dose-related manner, which is consistent with the known antioxidant capacities of the ethyl acetate extract of Fructus ligustri lucidi [88] and the methanol extract of Glinus oppositifolius [89]. The DPPH and H2O2 scavenging capacity of EAESTL may arise from the phenolics, terpenoids, vitamin C, vitamin E, and carotene in EAESTL.
During inflammation, leukocytes discharge lysosomal enzymes, and proteases are part of their protective roles, causing more damage to the tissue [90]. Preventing hemolysis of the RBC membrane can provide insight into anti-inflammation because the RBC membrane and the lysosome membrane are identical. The firmness of such a plasma membrane can prevent or postpone cytolysis, preventing the liberation of cytosolic elements and, consequently, preventing tissue injury and the inflammatory reaction. Protein denaturation is one additional reason for inflammation [91]. An aid that can inhibit protein damage could be helpful in the treatment of inflammatory disorders. The ability of EAESTL to prevent RBC lysis and BSA protein denaturation was assessed as part of the research into the process of treating symptoms of inflammation. EAESTL exhibited concentration-dependent anti-inflammatory activity by inhibiting RBC lysis and BSA protein denaturation. The results demonstrate that this anti-inflammatory effect is perhaps attributed to the presence of esters, phenolics, terpenes, hydrocarbons, ketones, and aldehydes in EAESTL. Similar anti-inflammatory activity has also been observed in the research conducted by Gunathilake et al. [29], which showed that extracts of Cassia auriculata, Passiflora edulis, Sesbania grandiflora, Olax zeylanica, Gymnema lactiferum, and Centella asiatica prevented RBC lysis and BSA protein denaturation.
The bacterial strains used in this study are infectious in nature and can induce diseases. For instance, B. infantis was found in a neonate with sepsis, and Exiguobacterium sp. promotes pneumonia (CAP) and bacteremia [92,93]. S. aureus is among the most prevalent human pathogens, exacerbating bacteremia, respiratory tract and soft tissue infections, and endocarditis [94]. Some Streptococcus sp. involved in the pathogenesis of upper respiratory infections [95], and virulent E. coli accelerate gastroenteritis and urinary tract infections [96]. V. cholerae is an enteric pathogen that releases cholera toxin and causes acute secretory diarrhea [97]. S. typhi is an MDR-resistant bacterial pathogen that usually causes typhoid and paratyphoid, and it has rapidly gained resistance to previously efficacious drugs, such as ciprofloxacin [98]. P. aeruginosa can induce significant life-threatening infections by preventing the immune systems of affected individuals [99]. H. influenzae is the main bacterial agent that spreads diseases in the respiratory and sensory systems of the body [100]. Therefore, the antibiotic activity of EAESTL was assessed against these bacteria. The presence of physiologically active chemicals such as phenolics, esters, terpenes, hydrocarbons, aldehydes, and ketones could account for this antibacterial action. Our findings are in accordance with the previous research data that plant extracts high in phenolic compounds [101], fatty acid methyl esters [102], terpenes, hydrocarbons [103], aldehydes, and ketones [104] have antibacterial activity. Our recent observations show that the ethyl acetate extract of Ruellia prostrata Poir. has significant antibacterial activity against B. infantis, Exiguobacterium sp., E. coli, and P. aeruginosa [24], suggesting a similar potential for inhibiting these pathogenic bacteria that share a common carnage mechanism. The study conducted by Saranraj et al. [105], in accordance with our results, found that an ethyl acetate extract of Acalypha indica plant leaves had antibacterial activity against bacterial strains of S. aureus, S. typhi, V. cholerae, and E. coli.
The unexpected side effects of a particular drug compound can be avoided by estimating the level of toxicity with respect to the dose. Therefore, the cytotoxicity and acute oral toxicity studies were performed in Artemia salina larvae and mice, and we found that the LC50 and LD50 of EAESTL were 35.246 μg/mL and 4263.906 mg/kg, respectively. The LC50 value of EAESTL is lower than the LC50 value of standard vincristine sulfate. The LD50 value of EAESTL is beyond 2,000 mg/kg, which indicates that it is nontoxic. In the acute toxicity study, if the LD50 value is above 2000 mg/kg, it is considered nontoxic [83]. Thereby, EAESTL can be a good source of potential therapeutic agents. Successful drug development entails an accurate evaluation and rationalization of pharmacokinetics (ADME) and drug-able properties. Therefore, we investigated these properties in silico and discovered that only four phytochemicals, namely, 2,6,6-trimethylbicyclo [3.1.1] heptane-3-ol (PN14), benzyl alcohol (PN28), 3-(hydroxy-phenyl-methyl)-2,3-dimethyloctan-4-one (PN35), and phenylethyl alcohol (PN37), passed ADME and demonstrated good drug-able properties without toxicity. Among these four phytochemicals, 2,6,6-trimethylbicyclo [3.1.1] heptane-3-ol also known as isopinocampheol or 3-pinanol, a terpenoid in nature, was found to have anti-inflammatory and antibacterial activities. In accordance with our results, in vitro activity investigations of essential oils from Hyssopus officinalis [51], Cedrelopsis grevei leaves [106] and the hexane-ether extract of Tanacetum santolinoides [107] have been reported to contain 2,6,6-trimethylbicyclo [3.1.1] heptane-3-ol, which has anti-inflammatory and antimicrobial properties. Benzyl alcohol exhibited anti-inflammatory activity. Consistent with our findings, the chloroform fraction of aerial parts of Isodon rugosus Wall. ex Benth contains benzyl alcohol along with other bioactive phytochemicals that have been reported to have anti-inflammatory and analgesic activity when analyzed in vivo [58]. Both the ethanol extract of Albizia coriaria (Welw ex. Oliver) leaves and the ethyl acetate extract of Albizia coriaria (Welw ex. Oliver) stem bark contain benzyl alcohol as a significant component and are reported to have in vitro antioxidant and antibacterial properties [59]. 3-(hydroxy-phenyl-methyl)-2,3-dimethyl-octan-4-one was found to have anti-inflammatory activity. Consistently, an ethyl acetate extract of Ruellia prostrata Poir. aerial parts contains 3-(hydroxy-phenyl-methyl)-2,3-dimethyl-octan-4-one, a phenolic molecule with anti-inflammatory properties [24]. Phenylethyl alcohol displayed anti-inflammatory activity. Our findings are also in good agreement with the results reported by Dadkhah et al. [59] and Amor et al. [63] who observed that phenylethyl alcohol is one of the main phytoconstituents of Rosa damascena Mill. and Phlomis species essential oils and exhibited antioxidant and anti-inflammatory activities, respectively. In addition, phenylethyl alcohol is a driven compound of Trichoderma virens 7b hexane extract, which showed antifungal activity against Ganoderma boninense, the causal agent of a devastating disease affecting oil palm in Southeast Asian countries [60]. Collectively, our results may contribute to the development of antioxidant, anti-inflammatory, and antibacterial agents for humans. Moreover, this work also shows a correlation between the ethnomedicinal uses of Senna tora (L.) Roxb leaves and their pharmacological activities.
5. Conclusion
The findings of this study concluded that Senna tora (L.) Roxb. leaves contain antioxidant, anti-inflammatory, and antibacterial activities due to the presence of some biologically active phytochemicals, viz., phenolics, terpenoids, vitamin C, vitamin E, carotene, esters, hydrocarbons, ketones, and aldehydes. Based on pharmacokinetics, drug-able properties, and toxicity, this study also identified four phytochemicals including 2,6,6-trimethylbicyclo [3.1.1] heptane-3-ol, benzyl alcohol, 3-(hydroxy-phenyl-methyl)-2,3-dimethyl-octan-4-one, and phenylethyl alcohol, as possible therapeutic candidates. Among the four phytochemical compounds, 2,6,6-trimethylbicyclo [3.1.1] heptane-3-ol is anti-inflammatory and antibacterial; benzyl alcohol is an antioxidant, anti-inflammatory, and antibacterial; 3-(hydroxy-phenyl-methyl)-2,3-dimethyl-octan-4-one is anti-inflammatory; and phenylethyl alcohol is both an antioxidant and an anti-inflammatory. Further research is needed for the isolation of these phytochemical compounds with fascinating in vitro and in vivo pharmacological properties and to assess their safety and bioavailability in in vivo animal models to aid in developing drugs against oxidants, inflammation, and bacterial infection.
Author contribution statement
Md. Mashiar Rahman and Shahina Akhter conceived and designed the experiments, contributed reagents, materials, analysis tools, or data, and wrote the paper. Md. Abdullah Al Noman, Shapla Khatun, Rahat Alam, Md. Mahade Hasan Shetu, Enamul Kabir Talukder, Raihan Rahman Imon, and Md. Yaman Biswas performed the experiments, analyzed and interpreted the data. K. M. Anis-Ul-Haque and Mohammad Jashim Uddin analyzed and interpreted the data and wrote the paper.
Funding
No external funding was received for this research work.
Declaration of competing interest
None.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2023.e12855.
Contributor Information
Mohammad Jashim Uddin, Email: j.uddin@just.edu.bd.
Shahina Akhter, Email: shahinabtge.med@gmail.com.
Abbreviations
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- BSA
Bovine Serum Albumin
- RBC
Red Blood Cell
- EAEST
Ethyl acetate extract of Senna tora (L.) Roxb. Leaves
- PASS
Prediction of Activity Spectra for Substances
- ADME
Absorption, Distribution, Metabolism, and Excretion
- PN
Peak Number
- NSAIDs
Nonsteroidal anti-inflammatory drugs
- COX-1
Cyclooxygenase 1
- COX-2
Cyclooxygenase 2
- EGCG
Epigallocatechin-3-gallate
- MeOH
Methanol
- DMSO
Dimethyl Sulfoxide
- PBS
Phosphate-Buffered Saline
- OECD
Organization for Economic Co-operation and Development
- GC-MS
Gas Chromatography Mass Spectrometry
- hERG
Human Ether-a-go-go-Related gene
- MW
Molecular Weight
- HBA
Hydrogen Bond Acceptor
- HBD
Hydrogen Bond Donor
- RTB
Rotatable Bond
- TPSA
Topological Polar Surface Area
- PPB
Plasma Protein Binding
- BBB
Blood Brain Barrier
- HIA
Human Intestinal Absorption
- HOB
Human Oral Bioavailability
- RO5
Lipinski's Rule of Five
- CYP450
Cytochrome P450
- LD50
Lethal Dose 50
- IC50
Inhibitory Concentration 50
- STD
Standard Deviation
- EM
Erythromycin
- Pgp
P-glycoproteins
- CNS
Central Nervous System
- EPA
Environmental Protection Agency
- CAP
Community-Acquired pneumonia
- GI
Gastrointestinal
- MIC
Minimum Inhibitory Concentration
- MBC
Minimum Bactericidal Concentration
Appendix B. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Adnan M., Siddiqui A.J., Hamadou W.S., Patel M., Ashraf S.A., Jamal A., Awadelkareem A.M., Sachidanandan M., Snoussi M., De Feo V. Phytochemistry, bioactivities, pharmacokinetics and toxicity prediction of Selaginella repanda with its anticancer potential against human lung, breast and colorectal carcinoma cell lines. Molecules. 2021;26:768. doi: 10.3390/molecules26030768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siddiqui A.J., Jahan S., Singh R., Saxena J., Ashraf S.A., Khan A., Choudhary R.K., Balakrishnan S., Badraoui R., Bardakci F., Adnan M. Plants in anticancer drug discovery: from molecular mechanism to chemoprevention. BioMed Res. Int. 2022;2022 doi: 10.1155/2022/5425485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kabir M., Al-Noman A., Dash B.K., Hasan M., Akhter S., Rahman M. Trema orientalis (Linn.) leaves promotes anticancer activity in Ehrlich ascites carcinoma (EAC) in Swiss albino mice. J. Basic Clin. Physiol. Pharmacol. 2020;31:1–12. doi: 10.1515/jbcpp-2019-0121. [DOI] [PubMed] [Google Scholar]
- 4.Elasbali A.M., Al-Soud W.A., Al-Oanzi Z.H., Qanash H., Alharbi B., Binsaleh N.K., Alreshidi M., Patel M., Adnan M. Cytotoxic activity, cell cycle inhibition, and apoptosis-inducing potential of Athyrium hohenackerianum (Lady Fern) with its phytochemical profiling. Evid. base Compl. Alternative Med. 2022;2022 doi: 10.1155/2022/2055773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alreshidi M., Noumi E., Bouslama L., Ceylan O., Veettil V.N., Adnan M., Danciu C., Elkahoui S., Badraoui R., Al-Motair K.A., Patel M., De Feo V., Snoussi M. Phytochemical screening, antibacterial, antifungal, antiviral, cytotoxic, and anti-quorum-sensing properties of Teucrium polium l. aerial parts methanolic extract. Plants. 2020;9:1–20. doi: 10.3390/plants9111418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Awadelkareem A.M., Al-Shammari E., Elkhalifa A.O., Adnan M., Siddiqui A.J., Patel M., Khan M.I., Mehmood K., Ashfaq F., Badraoui R., Ashraf S.A. Biosynthesized silver nanoparticles from Eruca sativa Miller leaf extract exhibits antibacterial, antioxidant, anti-quorum-sensing, antibiofilm, and anti-metastatic activities. Antibiotics. 2022;11:853. doi: 10.3390/antibiotics11070853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lourenco S.C., Moldão-Martins M., Alves V.D. Antioxidants of natural plant origins: from sources to food industry applications. Molecules. 2019;24:14–16. doi: 10.3390/molecules24224132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sostres C., Gargallo C.J., Arroyo M.T., Lanas A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract. Res. Clin. Gastroenterol. 2010;24:121–132. doi: 10.1016/j.bpg.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Von Philipsborn P., Biallas R., Burns J., Drees S., Geffert K., Movsisyan A., Pfadenhauer L.M., Sell K., Strahwald B., Stratil J.M., Rehfuess E. Adverse effects of non-steroidal anti-inflammatory drugs in patients with viral respiratory infections: rapid systematic review. BMJ Open. 2020;10:1–11. doi: 10.1136/bmjopen-2020-040990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lomazzi M., Moore M., Johnson A., Balasegaram M., Borisch B. Antimicrobial resistance - moving forward? BMC Publ. Health. 2019;19:1–6. doi: 10.1186/s12889-019-7173-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blumenthal K.G., Peter J.G., Trubiano J.A., Phillips E.J. Antibiotic allergy. Lancet. 2019;393:183–198. doi: 10.1016/S0140-6736(18)32218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradley K.C., Finsterbusch K., Schnepf D., Crotta S., Llorian M., Davidson S., Fuchs S.Y., Staeheli P., Wack A. Microbiota-driven tonic interferon signals in lung stromal cells protect from influenza virus infection. Cell Rep. 2019;28:245–256. doi: 10.1016/j.celrep.2019.05.105. [DOI] [PubMed] [Google Scholar]
- 13.Furst R., Zündorf I. Plant-derived anti-inflammatory compounds: hopes and disappointments regarding the translation of preclinical knowledge into clinical progress. Mediat. Inflamm. 2014;2014 doi: 10.1155/2014/146832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savoia D. Plant-derived antimicrobial compounds: alternatives to antibiotics. Future Microbiol. 2012;7:979–990. doi: 10.2217/fmb.12.68. [DOI] [PubMed] [Google Scholar]
- 15.Subramani R., Narayanasamy M., Feussner K.D. Plant-derived antimicrobials to fight against multi-drug-resistant human pathogens. 3 Biotech. 2017;7:1–15. doi: 10.1007/s13205-017-0848-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain S., Patil U.K. Phytochemical and pharmacological profile of Cassia tora Linn. An Overview. Indian J. Nat. Prod. Resour. 2010;1:430–437. [Google Scholar]
- 17.Maneenoon K., Khuniad C., Teanuan Y., Saedan N., Prom-in S., Rukleng N., Kongpool W., Pinsook P., Wongwiwat W. Ethnomedicinal plants used by traditional healers in Phatthalung Province, Peninsular Thailand. J. Ethnobiol. Ethnomed. 2015;11:1–20. doi: 10.1186/s13002-015-0031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pawar H.A., Lalitha K.G. Extraction, characterization, and molecular weight determination of Senna tora (L.) seed polysaccharide. Int. J. Biomater. 2015;2015 doi: 10.1155/2015/928679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mishra S.K., Moses A.S. Phytochemical screening and antifungal efficacy of closely related Senna obtusifolia and Senna tora on some phytopathogenic fungi. Proc. Natl. Acad. Sci. India B Biol. Sci. 2018;88:1169–1175. [Google Scholar]
- 20.Sultana B., Yaqoob S., Zafar Z., Bhatti H.N. Escalation of liver malfunctioning: a step toward herbal awareness. J. Ethnopharmacol. 2018;216:104–119. doi: 10.1016/j.jep.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Murshid G.M.M., Moniruzzaman M., Rahman A.A., Saifuzzaman M., Uddin S.N. Phytochemical and pharmacological screening of Senna tora Roxb. J. Pharmacol. Toxicol. 2007;2:386–390. [Google Scholar]
- 22.Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Osteoarthritis Cartilage. 2012;20:256–260. doi: 10.1016/j.joca.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Rahman M.M., Kabir M.M., AL Noman M.A., Islam M.R., Dash B.K., Akhter S., Uddin M.J., Rahman A. Mikania cordata leaves extract promotes activity against pathogenic bacteria and anticancer activity in EAC cell-bearing swiss albino mice. J. Appl. Pharmaceut. Sci. 2020;10:112–122. [Google Scholar]
- 24.Akhter S., Hossain M.W., Sultana S., Jharna J.F., Meghla N.S., Alam R., Anis-Ul-Haque K.M., Rahman M.M. Ruellia prostrata Poir. activity evaluated by phytoconstituents, antioxidant, anti-inflammatory, antibacterial activity, and in silico molecular functions. J. Saudi Chem. Soc. 2022;26 [Google Scholar]
- 25.Aziz M.A. Qualitative phytochemical screening and evaluation of anti-inflammatory, analgesic and antipyretic activities of Microcos paniculata barks and fruits. J. Integr. Med. 2015;13:173–184. doi: 10.1016/S2095-4964(15)60179-0. [DOI] [PubMed] [Google Scholar]
- 26.Singh K.L., Bag G.C. Phytochemical analysis and determination of total phenolics content in water extracts of three species of hedychium. Int. J. PharmTech Res. 2013;5:1516–1521. [Google Scholar]
- 27.He Y., Jia Y., Lu F. New products generated from the transformations of ferulic acid dilactone. Biomolecules. 2020;10:175. doi: 10.3390/biom10020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Badami S., Channabasavaraj K.P. In vitro antioxidant activity of thirteen medicinal plants of India's Western Ghats. Pharm. Biol. 2007;45:392–396. [Google Scholar]
- 29.Gunathilake K.D.P.P., Ranaweera K.K.D.S., Rupasinghe H.P.V. In vitro anti-inflammatory properties of selected green leafy vegetables. Biomedicines. 2018;6:1–10. doi: 10.3390/biomedicines6040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chandrasekaran M., Venkatesalu V. Antibacterial and antifungal activity of Syzygium jambolanum seeds. J. Ethnopharmacol. 2004;91:105–108. doi: 10.1016/j.jep.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Rahman M.M., Bairagi N., Kabir M., Uddin M.J. Antibacterial potentiality and brine shrimp lethality bioassay of the methanol extract of Trema orientalis leaves. South Asian Res. J. Nat. Prod. 2018;1:1–9. [Google Scholar]
- 32.Organization for Economic Co-operation and Development (OECD) OECD Publishing; Paris: 2008. Test No. 425: Acute Oral Toxicity: Up-And-Down Procedure; p. 27. [DOI] [Google Scholar]
- 33.Petreanu M., Guimaraes Á.A.A., Broering M.F., Ferreira E.K., Machado I.D., Gois A.L.T., de Carvalho J.E., Monache F.D., Niero R., Santin J.R. Antiproliferative and toxicological properties of methanolic extract obtained from Solanum capsicoides All. seeds and carpesterol. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2016;389:1123–1131. doi: 10.1007/s00210-016-1275-x. [DOI] [PubMed] [Google Scholar]
- 34.Lagunin A., Stepanchikova A., Filimonov D., Poroikov V. PASS: prediction of activity spectra for biologically active substances. Bioinformatics. 2000;16:747–748. doi: 10.1093/bioinformatics/16.8.747. [DOI] [PubMed] [Google Scholar]
- 35.Yang H., Lou C., Sun L., Li J., Cai Y., Wang Z., Li W., Liu G., Tang Y. AdmetSAR 2.0: web-service for prediction and optimization of chemical ADMET properties. Bioinformatics. 2019;35:1067–1069. doi: 10.1093/bioinformatics/bty707. [DOI] [PubMed] [Google Scholar]
- 36.Xiong G., Wu Z., Yi J., Fu L., Yang Z., Hsieh C., Yin M., Zeng X., Wu C., Lu A., Chen X., Hou T., Cao D. ADMETlab 2.0: an integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021;49 doi: 10.1093/nar/gkab255. W5–W14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daina A., Michielin O., Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017;7:1–13. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vogel-Adghough D., Stahl E., Návarová H., Zeier J. Pipecolic acid enhances resistance to bacterial infection and primes salicylic acid and nicotine accumulation in tobacco. Plant Signal. Behav. 2013;8:1–9. doi: 10.4161/psb.26366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mostafa S.I. Mixed ligand complexes with 2-piperidine-carboxylic acid as primary ligand and ethylene diamine, 2,2′-bipyridyl, 1,10-phenanthroline and 2(2′-pyridyl)quinoxaline as secondary ligands: preparation, characterization and biological activity. Transit. Met. Chem. 2007;32:769–775. [Google Scholar]
- 40.Krishna S.R., Hafza S., Proona C.G., Lekhya P.C., Bhaskara R.K.V. Pharmacological properties, phytochemical and GC-MS analysis of Bauhinia acuminata Linn. Artic. J. Chem. Pharm. Res. 2015;7:372–380. [Google Scholar]
- 41.Abdel-Karim O.H., Abo-Shady A.M., Ismail G.A., Gheda S.F. Potential effect of Turbinaria decurrens acetone extract on the biochemical and histological parameters of alloxan-induced diabetic rats. Int. J. Environ. Health Res. 2021:1–22. doi: 10.1080/09603123.2021.1888895. [DOI] [PubMed] [Google Scholar]
- 42.Raza C., Dildar A., Inam L., Parsa D., Muhammad S. Study of bioactivities of lipid content of fresh Lagenaria siceraria seeds pulp and identification of its chemical constituents. J. Med. Plants Res. 2014;8:1014–1020. [Google Scholar]
- 43.Hiep N.T., Kwon J., Kim D.W., Hong S., Guo Y., Hwang B.Y., Kim N., Mar W., Lee D. Neuroprotective constituents from the fruits of Maclura tricuspidata. Tetrahedron. 2017;73:2747–2759. [Google Scholar]
- 44.Ron-Doitch S., Soroka Y., Frusic-Zlotkin M., Barasch D., Steinberg D., Kohen R. Saturated and aromatic aldehydes originating from skin and cutaneous bacteria activate the Nrf2-keap1 pathway in human keratinocytes. Exp. Dermatol. 2021;30:1381–1387. doi: 10.1111/exd.14103. [DOI] [PubMed] [Google Scholar]
- 45.Varejao J.O.S., Barbosa L.C.A., Varejao E.V.V., Maltha C.R.A., King-Diaz, Lotina-Hennsen Cyclopent-4-ene-1,3-diones: a new class of herbicides acting as potent photosynthesis inhibitors. J. Agric. Food Chem. 2014;62:5772–5780. doi: 10.1021/jf5014605. [DOI] [PubMed] [Google Scholar]
- 46.Innett G.W., Agen D.V.A., Chrey M.S. Prostaglandin J2 metabolites inhibit aromatase activity by redox-sensitive mechanisms: potential implications for breast cancer therapy. Int. J. Cancer. 2003;103:600–605. doi: 10.1002/ijc.10878. [DOI] [PubMed] [Google Scholar]
- 47.Alves R., Dias E., Moura G., Pedrini N., Moraes L., Henrique P. Unveiling chemical defense in the rice stalk stink bug against the entomopathogenic fungus Metarhizium anisopliae. J. Invertebr. Pathol. 2015;127:93–100. doi: 10.1016/j.jip.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 48.Shahwar M.K., El-Ghorab A.H., Anjum F.M., Butt M.S., Hussain S., Nadeem M. Characterization of coriander (Coriandrum sativum L.) seeds and leaves: volatile and non volatile extracts. Int. J. Food Prop. 2012;15:736–747. [Google Scholar]
- 49.Chouitah O., Meddah B., Aoues A., Sonnet P. Essential oil from the leaves of Ajuga iva: chemical composition and antimicrobial activity. J. Essent. Oil-Bearing Plants. 2017;20:873–877. [Google Scholar]
- 50.Husain A., Khan S.A., Iram F., Iqbal M.A., Asif M. Insights into the chemistry and therapeutic potential of furanones: a versatile pharmacophore. Eur. J. Med. Chem. 2019;171:66–92. doi: 10.1016/j.ejmech.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 51.Letessier M.P., Svoboda K.P., Walters D.R. Antifungal activity of the essential oil of hyssop (Hyssopus officinalis) J. Phytopathol. 2001;149:673–678. [Google Scholar]
- 52.Marandi R.R., Britto S.J., Soreng P.K. Phytochemical profiling, antibacterial screening and antioxidant properties of the sacred tree (Shorea robusta Gaertn.) of Jharkhand. Int. J. Pharm. Sci. Res. 2016;7:2874–2888. [Google Scholar]
- 53.Yanagimoto K., Ochi H., Lee K.G., Shibamoto T. Antioxidative activities of fractions obtained from brewed coffee. J. Agric. Food Chem. 2004;52:592–596. doi: 10.1021/jf030317t. [DOI] [PubMed] [Google Scholar]
- 54.Weinstock M., Razin M., Chorev M., Enz A. Pharmacological evaluation of phenylcarbamates as CNS-selective acetylcholinesterase inhibitors. J. Neural. Transm. Suppl. 1994;43:219–225. [PubMed] [Google Scholar]
- 55.Hollomon D.W., Butters J.A., Barker H., Hall L. Fungal β-tubulin, expressed as a fusion protein, binds benzimidazole and phenylcarbamate fungicides. Antimicrob. Agents Chemother. 1998;42:2171–2173. doi: 10.1128/aac.42.9.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He B., Rhodes-Brower S., Miller M.R., Munson A.E., Germolec D.R., Walker V.R., Korach K.S., Meade B.J. Octamethylcyclotetrasiloxane exhibits estrogenic activity in mice via ERα. Toxicol. Appl. Pharmacol. 2003;192:254–261. doi: 10.1016/s0041-008x(03)00282-5. [DOI] [PubMed] [Google Scholar]
- 57.Jenecius A., Uthayakumari F., Mohan V. GC-Ms determination of bioactive components of Sauropus Bacciformis Blume (Euphorbiaceae) J. Curr. Chem. Pharm. Sci. 2012;2:347–358. [Google Scholar]
- 58.Zeb A., Ahmad S., Ullah F., Ayaz M., Sadiq A. Anti-nociceptive activity of ethnomedicinally important analgesic plant Isodon rugosus wall. ex Benth: mechanistic study and identifications of bioactive compounds. Front. Pharmacol. 2016;7:1–10. doi: 10.3389/fphar.2016.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Omara T., Kiprop A.K., Kosgei V.J. Isolation and characterization of compounds in ethanolic extract of Albizia coriaria (Welw ex. Oliver) leaves: a further evidence of its ethnomedicinal diversity. Bull. Natl. Res. Cent. 2022;46:1–15. [Google Scholar]
- 60.Rawani A., Ray A.S., Ghosh A., Sakar M., Chandra G. Larvicidal activity of phytosteroid compounds from leaf extract of Solanum nigrum against Culex vishnui group and Anopheles subpictus. BMC Res. Notes. 2017;10:1–8. doi: 10.1186/s13104-017-2460-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dadkhah A., Fatemi F., Malayeri M.R.M., Ashtiyani M.H.K., Noureini S.K., Rasooli A. Considering the effect of rosa damascena mill. essential oil on oxidative stress and cox-2 gene expression in the liver of septic rats. Turk. J. Pharm. Sci. 2019;16:416–424. doi: 10.4274/tjps.galenos.2018.58815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pei L., Angel L., Yusof M.T., Ismail I.S., Tay B., Ping Y., Kamarudin N.H., Sundram S. An in vitro study of the antifungal activity of Trichoderma virens 7b and a profile of its non-polar antifungal components released against Ganoderma boninense. J. Microbiol. 2016;54:732–744. doi: 10.1007/s12275-016-6304-4. [DOI] [PubMed] [Google Scholar]
- 63.Amor I.L., Boubaker J., Ben M., Skandrani I., Bhouri W., Neffati A., Kilani S., Bouhlel I., Ghedira K. Phytochemistry and biological activities of Phlomis species. J. Ethnopharmacol. 2009;125:183–202. doi: 10.1016/j.jep.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 64.Ismail G.A., Gheda S.F., Abo-shady A.M., Abdel-karim O.H. In vitro potential activity of some seaweeds as antioxidants and inhibitors of diabetic enzymes. Food Sci. Technol, Campinas. 2020;40:681–691. [Google Scholar]
- 65.de Castro Oliveira L.G., Brito L.M., de Moraes Alves M.M., Amorim L.V., Sobrinho-Junior E.P.C., de Carvalho C.E.S., da Franca Rodrigues K.A., Arcanjo D.D.R., das Gracas Lopes Cito A.M., de Amorim Carvalho F.F. In vitro effects of the neolignan 2,3-dihydrobenzofuran against Leishmania amazonensis. Basic Clin. Pharmacol. Toxicol. 2017;120:52–58. doi: 10.1111/bcpt.12639. [DOI] [PubMed] [Google Scholar]
- 66.Abdulmalik O., Safo M.K., Chen Q., Yang J., Brugnara C., Ohene-Frempong K., Abraham D.J., Asakura T. 5-Hydroxymethyl-2-furfural modifies intracellular sickle haemoglobin and inhibits sickling of red blood cells. Br. J. Haematol. 2005;128:552–561. doi: 10.1111/j.1365-2141.2004.05332.x. [DOI] [PubMed] [Google Scholar]
- 67.Zhao L., Chen J., Su J., Li L., Hu S., Li B., Zhang X., Xu Z., Chen T. In vitro antioxidant and antiproliferative activities of 5-hydroxymethylfurfural. J. Agric. Food Chem. 2013;61:10604–10611. doi: 10.1021/jf403098y. [DOI] [PubMed] [Google Scholar]
- 68.Ozyurek M., Bekdeşer B., Güҫlü K., Apak R. Resorcinol as a spectrofluorometric probe for the hypochlorous acid scavenging activity assay of biological samples. Anal. Chem. 2012;84:9529–9536. doi: 10.1021/ac302369p. [DOI] [PubMed] [Google Scholar]
- 69.Shahinozzaman M., Ishii T., Halim M.A., Hossain M.A., Islam M.T., Tawata S. Cytotoxic and anti-inflammatory resorcinol and alkylbenzoquinone derivatives from the leaves of Ardisia sieboldii. Z. Naturforsch., C: J. Biosci. 2019;26:11–12. doi: 10.1515/znc-2019-0114. [DOI] [PubMed] [Google Scholar]
- 70.Girija S., Duraipandiyan V., Kuppusamy P.S., Gajendran H., Rajagopal R. Chromatographic characterization and GC-MS evaluation of the bioactive constituents with antimicrobial potential from the pigmented ink of Loligo duvauceli. Int. Sch. Res. Notices. 2014;2014 doi: 10.1155/2014/820745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rathna J., Bakkiyaraj D., Pandian S.K. Anti-biofilm mechanisms of 3,5-di-tert-butylphenol against clinically relevant fungal pathogens. Biofouling. 2016;32:979–993. doi: 10.1080/08927014.2016.1216103. [DOI] [PubMed] [Google Scholar]
- 72.Swamy M.K., Arumugam G., Kaur R., Ghasemzadeh A., Yusoff M.M., Sinniah U.R. GC-MS Based Metabolite Profiling, Antioxidant and antimicrobial properties of different solvent extracts of Malaysian Plectranthus amboinicus leaves, Evidence-based Complement. Altern. Med. 2017;2017 doi: 10.1155/2017/1517683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Alencar M.V.O.B., Islam M.T., de Lima R.M.T., Paz M.F.C.J., dos Reis A.C., da Mata A.M.O.F., de Filho J.W.G.O., Cerqueira G.S., Ferreira P.M.P., de Sousa J.M.C., Mubarak M.S., de Melo-Cavalcante A.A.C. Phytol as an anticarcinogenic and antitumoral agent: an in vivo study in swiss mice with DMBA-induced breast cancer. IUBMB Life. 2019;71:200–212. doi: 10.1002/iub.1952. [DOI] [PubMed] [Google Scholar]
- 74.Gnanapriyanka Beulah G., Soris P.T., Mohan V.R. GC-MS determination of bioactive compounds of Dendrophthoe falcata (L.F) Ettingsh: an epiphytic plant. Int. J. Health Sci. Res. 2018;8:261–269. [Google Scholar]
- 75.de Santos C.C.M.P., Salvadori M.S., Mota V.G., Costa L.M., de Almeida A.A.C., de Oliveira G.A.L., Costa J.P., de Sousa D.P., de Freitas R.M., de Almeida R.N. Antinociceptive and antioxidant activities of phytol in vivo and in vitro models. Neurosci. J. 2013;2013:1–9. doi: 10.1155/2013/949452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Othman A.R., Abdullah N., Ahmad S., Ismail I.S., Zakaria M.P. Elucidation of in-vitro anti-inflammatory bioactive compounds isolated from Jatropha curcas L. plant root. BMC Compl. Alternative Med. 2015;15:1–10. doi: 10.1186/s12906-015-0528-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ashraf A., Sarfraz R.A., Anwar F., Shahid S.A., Alkharfy K.M. Chemical composition and biological activities of leaves of Ziziphus mauritiana L. native to Pakistan. Pakistan J. Bot. 2015;47:367–376. [Google Scholar]
- 78.Ukwubile C.A., Ahmed A., Ya’u U.A., Katsayal J., Mejida S. GC-MS analysis of bioactive compounds from Melastomastrum capitatum (Vahl)Fern. leaf methanol extract: an anticancer plant. Sci. African. 2019;3:10–17. [Google Scholar]
- 79.Prayitno T.A., Widyorini R., Lukmandaru G. Chemical variation of five natural extracts by non-polar solvent. Maderas Cienc. Tecnol. 2021;23:1–12. [Google Scholar]
- 80.Foote J.A., Ranger-Moore J.R., Einspahr J.G., Saboda K., Kenyon J., Warneke J., Miller R.C., Goldman R., Xu M.J., Roe D.J., Alberts D.S. Chemoprevention of human actinic keratoses by topical DL-α-tocopherol. Cancer Prev. Res. 2009;2:394–400. doi: 10.1158/1940-6207.CAPR-08-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mujeeb F., Bajpai P., Pathak N. Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of aegle marmelos. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/497606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khan S., Kaur H., Jhamta R., Khan C.S. Evaluation of antioxidant potential and phytochemical characterization using GC-MS analysis of bioactive compounds of Achillea filipendulina (L.) leaves. J. Pharmacogn. Phytochem. 2019;8:258–265. [Google Scholar]
- 83.Mohammed T., Erko B., Giday M. Evaluation of antimalarial activity of leaves of Acokanthera schimperi and Croton macrostachyus against Plasmodium berghei in Swiss albino mice. BMC Compl. Alternative Med. 2014;14:1–10. doi: 10.1186/1472-6882-14-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Erb M., Kliebenstein D.J. Plant secondary metabolites as defenses, regulators, and primary metabolites: the lurred functional trichotomy. Plant Physiol. 2020;184:39–52. doi: 10.1104/pp.20.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Darmadi J., Batubara R.R., Himawan S., Azizah N.N., Audah H.K., Arsianti A., Kurniawaty E., Ismail I.S., Batubara I., Audah K.A. Evaluation of Indonesian mangrove Xylocarpus granatum leaves ethyl acetate extract as potential anticancer drug. Sci. Rep. 2021;11:1–18. doi: 10.1038/s41598-021-85383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Valko M., Leibfritz D., Moncol J., Cronin M.T.D., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 87.Kurutas E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr. J. 2016;15:122. doi: 10.1186/s12937-016-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ju H.Y., Chen S.C., Wu K.J., Kuo H.C., Hseu Y.C., Ching H., Wu C.R. Antioxidant phenolic profile from ethyl acetate fraction of Fructus Ligustri Lucidi with protection against hydrogen peroxide-induced oxidative damage in SH-SY5Y cells. Food Chem. Toxicol. 2012;50:492–502. doi: 10.1016/j.fct.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 89.Asokkumar K., Umamaheswari M., Sivashanmugam A.T., Subhadradevi V., Subhashini N., Ravi T.K. Free radical scavenging and antioxidant activities of Glinus oppositifolius (carpet weed) using different in vitro assay systems. Pharm. Biol. 2009;47:474–482. [Google Scholar]
- 90.Erfle D.J., Santerre J.P., Labow R.S. Lysosomal enzyme release from human neutrophils adherent to foreign material surfaces: enhanced release of elastase activity. Cardiovasc. Pathol. 1997;6:333–340. doi: 10.1016/S1054-8807(97)00031-8. [DOI] [PubMed] [Google Scholar]
- 91.Oyedapo O.O., Famurewa A.J. Antiprotease and membrane stabilizing activities of extracts of fagara zanthoxyloides, olax subscorpioides and tetrapleura tetraptera. Pharm. Biol. 1995;33:65–69. [Google Scholar]
- 92.Chen X., Wang L., Zhou J., Wu H., Li D., Cui Y., Lu B. Exiguobacterium sp. A1b/GX59 isolated from a patient with community-acquired pneumonia and bacteremia: genomic characterization and literature review. BMC Infect. Dis. 2017;17:1–7. doi: 10.1186/s12879-017-2616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ko K.S., Oh W.S., Lee M.Y., Lee J.H., Lee H., Peck K.R., Lee N.Y., Song J.H. Bacillus infantis sp. nov. and Bacillus idriensis sp. nov., isolated from a patient with neonatal sepsis. Int. J. Syst. Evol. Microbiol. 2006;56:2541–2544. doi: 10.1099/ijs.0.64213-0. [DOI] [PubMed] [Google Scholar]
- 94.Guo Y., Song G., Sun M., Wang J., Wang Y. Prevalence and therapies of antibiotic-resistance in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2020;10:1–11. doi: 10.3389/fcimb.2020.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Davis K.L., Gonzalez O., Kumar S., Dick E.J. Pathology associated with Streptococcus spp. infection in Baboons (Papio spp.) Vet. Pathol. 2020;57:714–722. doi: 10.1177/0300985820941496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Garretto A., Miller-Ensminger T., Ene A., Merchant Z., Shah A., Gerodias A., Biancofiori A., Canchola Stacey, Canchola Stephanie, Castillo E., Chowdhury T., Gandhi N., Hamilton S., Hatton K., Hyder S., Krull K., Lagios D., Lam T., Mitchell K., Mortensen C., Murphy A., Richburg J., Rokas M., Ryclik S., Sulit P., Szwajnos T., Widuch M., Willis J., Woloszyn M., Brassil B., Johnson G., Mormando R., Maskeri L., Batrich M., Stark N., Shapiro J.W., Hernandez C.M., Banerjee S., Wolfe A.J., Putonti C. Genomic survey of E. coli from the bladders of women with and without lower urinary tract symptoms. Front. Microbiol. 2020;11:2094. doi: 10.3389/fmicb.2020.02094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Satitsri S., Pongkorpsakol P., Srimanote P., Chatsudthipong V., Muanprasat C. Pathophysiological mechanisms of diarrhea caused by the Vibrio cholerae O1 El Tor variant: an in vivo study in mice. Virulence. 2016;7:789–805. doi: 10.1080/21505594.2016.1192743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mannan A., Shohel M., Rajia S., Mahmud N.U., Kabir S., Hasan I. A cross sectional study on antibiotic resistance pattern of Salmonella typhi clinical isolates from Bangladesh. Asian Pac. J. Trop. Biomed. 2014;4:306–311. doi: 10.12980/APJTB.4.2014C770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moradali M.F., Ghods S., Rehm B.H.A. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front. Cell. Infect. Microbiol. 2017;7:39. doi: 10.3389/fcimb.2017.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wajima T., Anzai Y., Yamada T., Ikoshi H., Noguchi N. Oldenlandia diffusa extract inhibits biofilm formation by Haemophilus influenzae clinical isolates. PLoS One. 2016;11:1–10. doi: 10.1371/journal.pone.0167335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bahri-sahloul R., Fredj R., Boughalleb N., Shria J., Saguem S., Hilbert J., Trotin F., Ammar S., Bouzid S., Harzallah-skhiri F. Phenolic composition and antioxidant and antimicrobial activities of extracts obtained from Crataegus azarolus L. var. aronia (Willd.) Batt. ovaries calli. J. Bot., Le. 2014;2014 [Google Scholar]
- 102.Sati A., Sati S.C., Sati N., Sati O.P. Chemical composition and antimicrobial activity of fatty acid methyl ester of Quercus leucotrichophora fruits. Nat. Prod. Res. 2017;31:713–717. doi: 10.1080/14786419.2016.1217202. [DOI] [PubMed] [Google Scholar]
- 103.Badawy M.E.I., Marei G.I.K., Rabea E.I., Taktak N.E.M. Antimicrobial and antioxidant activities of hydrocarbon and oxygenated monoterpenes against some foodborne pathogens through in vitro and in silico studies. Pestic. Biochem. Physiol. 2019;158:185–200. doi: 10.1016/j.pestbp.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 104.Bendiabdellah A., Dib M.E.A., Meliani N., Muselli A., Nassim D., Tabti B., Costa J. Antibacterial activity of Daucus crinitus essential oils along the vegetative life of the plant. J. Chem. 2013;2013 [Google Scholar]
- 105.Saranraj P., Stella D., Sathiyaseelan K., Samuel S. Antibacterial potentiality of ethanol and ethyl acetate extract of Acalypha indica against human pathogenic bacteria. J. Ecobiotechnol. 2010;2:23–27. [Google Scholar]
- 106.Afoulous S., Ferhout H., Raoelison E.G., Valentin A., Moukarzel B., Couderc F., Bouajila J. Chemical composition and anticancer, antiinflammatory, antioxidant and antimalarial activities of leaves essential oil of Cedrelopsis grevei. Food Chem. Toxicol. 2013;56:352–362. doi: 10.1016/j.fct.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 107.El-shazly A., Dorai G., Wink M. Composition and antimicrobial activity of essential oil and hexane-ether extract of Tanacetum santolinoides (DC.) Feinbr. and Fertig. Z. Naturforsch. 2002;57c:620–623. doi: 10.1515/znc-2002-7-812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.