Background
Plasma renin activity (PRA) level at admission is reported to be a prognostic predictor of acute decompensated heart failure (ADHF) patients. Although PRA is affected during hospitalization by several factors including fluid volume and drug titration, whether the changes in PRA levels during hospitalization (ΔPRA) are associated with prognosis of ADHF patients are largely unknown. Purpose: Investigate the predictive impact of ΔPRA on the prognosis of ADHF patients with reduced ejection fraction (HFrEF) and mildly reduced ejection fraction (HFmrEF). Methods: Retrospectively analyzed consecutive 116 HFrEF and HFmrEF patients admitted for ADHF. PRA measurements were acquired at admission and at discharge. The primary outcome was a composite of cardiovascular death and HF re-hospitalization. Results: Out of 116 patients, 85 had PRA measurements both at admission and at discharge. Compared to admission, PRA level was significantly higher at discharge (0.8 (IQR 0.3–2.2) to 2.8 (IQR 1.0–7.2), p < 0.001). Tertiary groups ranked by PRA level on admission showed trend of poor prognosis in order of high, mid, and low PRA level (p = 0.07). On the contrary, PRA level at discharge significantly differentiated the prognosis and was poor in order of high, low, and mid (p = 0.026). Next, when the participants were divided into tertiary groups ranked by ΔPRA, prognosis worsened in the order of “minimal”, “decreasing”, and the “increasing” tier. Cubic splines analysis also indicate a similar tendency. Conclusions: In ADHF patients with HFrEF and HFmrEF, patients with minimal ΔPRA showed the better prognosis over the those with either increasing or decreasing.
Keywords: Renin, ADHF, Prognosis
Highlights
-
•
In HFmrEF or HFrEF patients admitted for AHF, PRA level is significantly elevated at discharge compared to admission.
-
•
This is the first report indicating that amount change in PRA level during hospitalization are associated with prognosis.
-
•
Minimal change in PRA level during hospitalization was associated with better outcome.
1. Introduction
Today, the number of new heart failure (HF) cases is increasing dramatically around the world, which has been termed the HF pandemic [1]. Re-hospitalization for HF is very common [2,3]. Biomarkers that predict HF re-hospitalization have been studied to prevent repeated re-hospitalizations that hasten HF death [4,5]. Association with plasma NT-proBNP levels, HF re-hospitalization, and death has been extensively investigated. NT-proBNP has proven to be an important prognostic marker in HF patients [6,7], and NT-proBNP levels may be useful in selecting patients who need more intensive treatment. However, NT-proBNP is modified by factors such as renal failure [8,9], obesity [9,10], age [9,11,12], and gender [11,12,13], which limits its predictive accuracy alone. Hence, further development of biomarkers that enable accurate prediction of HF prognosis development is warranted.
Various biomarkers, including NT-proBNP levels, are known to change significantly during the exacerbation of HF, reflecting impaired hemodynamics, and are used or studied to predict HF prognosis [14]. However, these biomarkers fluctuate, reflecting changes in fluid volume status and other interventions for acute decompensated heart failure (ADHF) treatment, such as relieving of congestion. In a clinical trial comparing NT-proBNP at admission and discharge reported that the change in values before and after treatment for ADHF allowed stratification of HF prognosis [6,15,16].
The renin–angiotensin–aldosterone system (RAAS) plays a central role in HF with reduced ejection fraction (HFrEF) [17]. RAAS activation is an important physiological mechanism for maintaining organ perfusion by stimulating peripheral vasoconstriction and renal reabsorption of sodium and water [18]. But in HF patients, such activation is known to cause maladaptation and exacerbation of HF disease status [19]. Prolonged RAAS activation causes myocardial remodeling and induces a decline in cardiac function. This further activates the RAAS, forming a vicious cycle in the pathophysiology of HF. In addition, angiotensin-converting enzyme inhibitors (ACEi) [20,21], angiotensin receptor blocker (ARB) including angiotensin receptor-neprilysin inhibitor (ARNi) [22,23,24], and mineralocorticoid receptor antagonist (MRA) [25] have all been shown to improve long-term outcomes in chronic HF patients by suppressing RAAS, indicating that RAAS plays a major role in the pathophysiology of HF progression.
Renin is the most upstream component of RAAS, and it has been reported that ADHF patients who have high plasma renin activity (PRA) level on admission have poor prognosis [26,27,28]. Similar to NT-proBNP, PRA fluctuates significantly with changes in fluid volume and renal blood flow following HF treatment [29,30], and with the initiation or up-titration of guideline directed medical therapies (GDMTs) [31,32,33]. Recent study reported that PRA is a prognostic predictor in stabilized HF patients with LVEF<50%, but change in PRA in this population has not been investigated [34]. Currently, there are no reports focused on changes in PRA level at admission and discharge to evaluate prognosis of HF.
2. Materials and methods
A retrospective cohort study was conducted in 116 consecutive HF with reduced ejection fraction (HFrEF) or HF with mildly reduced ejection fractions (HFmrEF) patients, who were admitted for ADHF to the Division of Cardiovascular Medicine in Hyogo College of Medicine between December 2017 and July 2019. Salt-restricted diet containing 6 g/day of NaCl were served for all patients during admission. ADHF was diagnosed according to Framingham criteria. Patients were included if they were ≥20 years old, had New York Heart Association (NYHA) class > II, and had collected blood samples for neurohumoral factors within 24 h of admission. Patients were excluded if they had hemodialysis (n = 9); active malignancy (n = 9); in-hospital death (n = 13). As a result, 85 patients were eligible for the present study.
All data were retrospectively collected from the electronic medical record system. Demographic data, vital signs, medical histories, laboratory values, and echocardiographic findings were collected to investigate the influence of neurohumoral factors in patients with ADHF. Left ventricular ejection fraction (LVEF) was obtained using Simpson or Teichholz method, as appropriate. A composite endpoint was defined as all cause death or unplanned hospital admission for ADHF. Oral loop diuretic doses were converted to furosemide equivalents, with 60 mg of azosemide = 8 mg of torasemide = 40 mg of furosemide. In addition, oral beta blocker doses were converted to carvedilol equivalents, with 5 mg bisoprolol = 20 mg carvedilol. This study complies with the Declaration of Helsinki and was approved by the institutional review board at Hyogo College of Medicine, Hyogo, Japan (approval number 3281). Written informed consent was waived because this was a retrospective study.
On admission, the blood samples were collected at the earliest possible time point no later than 24 h from admission. At discharge, the blood samples were collected before taking breakfast and medication in the morning of the day after the attending physician decided to allow discharge. Blood sampling at both time points were performed after 30 min of supine rest or in the sitting position if orthopnea was strong. The samples were prepared by following previous reports [34,35]. Briefly, the whole blood sample was placed on ice and plasma was obtained within 18 h followed by immediate freezing. Frozen plasma was thawed just before the measurement. PRA, PAC, and ADH levels were measured by radioimmunoassay using Plasma Renin Activity FR kit with proteasome inhibitor (Fujirebio, Osaka, Japan) which contains protease inhibitor, SPAC-S aldosterone RIA kit (Fujirebio, Osaka, Japan) and AVP kit Yamasa (Yamasa Shoyu Corporation, Choshi, Japan), respectively. Epinephrine levels were measured by high performance liquid chromatography method using CA test (Tosoh Bioscience, Tokyo, Japan). Plasma creatinine was measured with Cobas Cygnus auto CRE (Rosch Diagnostics, Germany) and NT-proBNP was measured with the electro-chemiluminescence assay (Elecsys 2010, Roche Diagnostics, Germany). PRA levels which were undetectable (≤0.1 ng/mL/h) were recorded as a PRA of 0.1 ng/mL/h. All measurements were performed by central core laboratory (SRL, Inc.,Tokyo, Japan).
All continuous variables are expressed as the mean ± SD if they fit a normal distribution; alternatively, values are expressed as the median and interquartile range (IQR). Categorical variables are expressed as the number (%). Parameters fitting a normal distribution were analyzed by unpaired t-test or paired t-test. If the data did not fit a normal distribution, Mann–Whitney U test or Wilcoxon signed-rank test was performed. Categorical variables were analyzed by performing chi-square, Fisher's exact test, or McNemar's test as appropriate. Kaplan–Meier analysis survival curves and log-rank tests were used to evaluate the composite outcome of all-cause mortality or re-hospitalization for worsening HF. We adjusted for RAAS inhibitors (RAASi) such as ACEi, ARB, and MRA, and beta blockers (BB) to determine the factors for the composite outcome in our multivariable Cox proportional hazards regression analysis. Results are expressed as hazard ratio (HRs) with 95% confidence intervals (95% CI) and p-values. In addition, the non-adjusted Cox proportional hazard model with five knots restricted cubic splines were used to examine nonlinear association between ΔPRA and composite outcome [37]. All reported p-values were two-sided and were considered as statistically significant at a value < 0.05. Statistical analyses were performed using JMP version 15.2.0 software (SAS Institute, Cary, NC) and R software, version 4.1.0 (The R Foundation for Statistical Computing, Vienna, Austria).
3. Results
Eighty-five patients who had PRA measurements both at admission and at discharge were analyzed. Baseline characteristics of these patients are shown in Table 1. The median age was 78 years (IQR 72–85) and 63% were male. Serum creatinine and NT-pro BNP on admission was 1.09 mg/dL (IQR 0.88–1.68) and 6839 pg/mL (IQR 3293–20,016), respectively. The etiology of HF was ischemia in 26 (31%) and hypertension in 58 (68%). Prior hospitalization due to HF was seen in 42 (49%). Median LVEF and left ventricular diastolic diameter (LVDd) was 34% (IQR 27–40) and 58 mm (IQR 51–66), respectively. Oral medication on admission was as follow: ACEi or ARB 62%; BB 71%; loop diuretics 52%; MRA 18%. BB and loop diuretics equivalents were 7.5 mg for carvedilol (IQR 0–11.25) and 10 mg for furosemide (IQR 0–40), respectively. A comparison between on admission and at discharge showed that body weight and NT-proBNP were significantly decreased, while prescription rate of loop diuretics, MRA, RAASi, and BB increased.
Table 1.
Baseline characteristics.
| All patient (n = 85) |
Decreasing tier (n = 28) |
Minimal tier (n = 28) |
Increasing tier (n = 29) |
|||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Discharge | Baseline | Discharge | Baseline | Discharge | Baseline | Discharge | |
| Age (y) | 78 (72–85) | 78 (70–85) | 78 (68–83) | 78 (74–87) | ||||
| Male, n (%) | 54 (63) | 13 (46) | 21 (75) | 20 (69) | ||||
| NYHA class, n (%) | ||||||||
| II | 2 [2] | 37 [44] * | 0 (0) | 15 (54) * | 2 [7] | 12 [43] | 0 (0) | 10 [34] |
| III | 43 (51) | 16 [19] * | 14 (50) | 4 [14] * | 11 [39] | 4 [14] | 18 (62) | 8 [28] * |
| IV | 40 (47) | 0 (0) * | 14 (50) | 0 (0) | 15 (54) | 0 (0) * | 11 [38] | 0 (0) |
| Pleural effusion, n (%) | 65 (76) | 24 [28] * | 21 (75) | 13 (46) * | 21 (75) | 7 [25] * | 23 (79) | 4 [14] *☨ |
| BW (kg) | 58.1 (48.0–68.3) | 54.5 (43.5–61.3) * | 54.7 (44.0–61.0) | 49.0 (42.6–61.3) * | 62.8 (50.8–69.7) | 55.1 (43.0–62.0) * | 58.0 (48.1–68.2) | 55.0 (44.1–59.1) * |
| BW change (kg) | −6.3 (−9.4–−3.7) | −4.5 (−9.5–−2.5) | −7.4 (−9.2–−4.0) | −6.0 (−9.8–−3.5) | ||||
| SBP (mmHg) | 137 (118–162) | 111 (99–125) * | 140 (125–168) | 115 (101–128) * | 131 (112–146) | 113 (99–122) * | 124 (110–154) | 103 (96–126) * |
| HR (bpm) | 91 (72–112) | 70 (62–80) * | 97 (77–112) | 71 (62–83) * | 80 (68–102) | 70 (59–80) * | 83 (64–102) | 70 (62–79) |
| prior HF hospitalization, n (%) | 42 (49) | 11 [39] | 13 (46) | 18 (62) | ||||
| Hemoglobin (g/dL) | 11.1 (9.4–12.8) | 11.6 (9.5–13.1) * | 10.9 (9.2–12.4) | 10.8 (9.2–12.5) | 11.1 (9.7–13.3) | 11.7 (10.3–12.9) * | 11.2 (9.3–12.8) | 11.9 (9.5–13.9) |
| BUN (mg/dL) | 25 [18–39] | 31 (23–46) * | 29 (18–49) | 24 (15–50) | 24 [17–31] | 29 (23–45) * | 23 [18–36] | 35 (26–48) |
| Creatinine (mg/dL) | 1.09 (0.88–1.68) | 1.28 (0.92–1.88) * | 1.07 (0.75–1.85) | 1.12 (0.80–2.18) | 1.07 (0.89–1.45) | 1.17 (1.01–1.48) | 1.43 (0.93–1.76) | 1.47 (0.93–2.06) * |
| Plasma Na (mEq/L) | 141 (138–143) | 139 (136–141) * | 140 (137–143) | 139 (137–142) | 142 (140–143) | 140 (137–141) * | 140 (138–143) | 137 (135–139) *☨ |
| Plasma K (mEq/L) | 4.1 (3.8–4.6) | 4.4 (4.0–4.7) | 4.1 (3.8–4.7) | 4.2 (3.8–4.9) * | 4.0 (3.7–4.6) | 4.4 (4.0–4.7) | 4.3 (3.7–4.7) | 4.4 (4.2–4.7) |
| Plasma Cl (mEq/L) | 106 (103–108) | 101 (99–104) * | 104 (99–108) | 102 (99–104) | 107 (104–108) | 102 (101–104) * | 105 (102–108) | 100 (97–103) * |
| BNP (pg/mL) | 881 (475–1570) | 323 (168–564) * | 1100 (498–1598) | 502 (220–736) * | 788 (516–1743) | 312 (209–439) * | 881 (458–1565) | 231 (140–420) *☨ |
| NT-pro BNP (pg/mL) | 6839 (3293–20016) | 2779 (1127–5766) * | 10,195 (4257–29303) | 5370 (2060–9457) * | 6909 (3219–20016) | 2339 (1083–4029) * | 5268 (3038–10770) | 1724 (1034–3188) *☨ |
| PRA (ng/mL/h) | 0.8 (0.3–2.2) | 2.8 (1.0–7.2) | 0.9 (0.3–3.4) | 0.6 (0.3–1.6) * | 0.6 (0.3–1.5) | 2.0 (1.3–3.3) * | 1.4 (0.6–2.4) | 12 [7–18] *☨ |
| LLoQ of PRA, n (%) | 8 [9] | 3 [4] | 2 [7] | 3 [11] | 3 [11] | 0 (0) | 3 [10] | 0 (0) ☨ |
| Aldosterone (pg/mL) | 83 (47–142) | 113 (68–186) * | 64 (44–165) | 72 (42–137) | 84 (36–130) | 97 (67–143) | 88 (65–150) | 183 (130–233) *☨ |
| WRF, n (%) | 20 [24] | 7 [25] | 5 [19] | 8 [29] | ||||
| LVDd (mm) | 58 (51–66) | 58 (51–65) * | 56 (50–61) | 56 (49–62) | 61 (53–66) | 58 (52–62) | 61 (51–71) | 62 (52–67) * |
| LVEF (%) | 34 [27–40] | 33 [25–40] * | 35 (26–48) | 33 [24–44] | 32 [26–38] | 33 [26–38] | 34 [28–41] | 34 [24–41] |
| E/e prime | 23 [18–32] | 19 [13–23] * | 22 [18–36] | 19 [14–24] | 23 [19–31] | 20 [13–22] * | 23 [16–34] | 18 [13–24] * |
| LAVI (ml/m2) | 69 (50–81) | 54 (42–71) * | 71 (48–78) | 56 (46–70) | 71 (54–97) | 53 (41–72) * | 62 (45–86) | 54 (41–75) |
| AF or AFL, n (%) | 29 [34] | 8 [29] | 10 [36] | 11 [38] | ||||
| Ischemic etiology, n (%) | 26 [31] | 9 [32] | 7 [25] | 10 [34] | ||||
| Hypertention, n (%) | 58 (68) | 23 (82) | 20 (71) | 15 (52) ☨ | ||||
| Diabates mellitus, n (%) | 32 [38] | 12 [43] | 10 [36] | 10 [34] | ||||
| Oral medications, n (%) | ||||||||
| ACE-inhibitors or ARBs, n (%) | 53 (62) | 66 (78) * | 17 (61) | 19 (68) | 16 (57) | 21 (75) | 20 (69) | 26 (90) |
| BB, n (%) | 60 (71) | 76 (89) * | 16 (57) | 25 (89) * | 23 (82) | 25 (89) | 21 (72) | 26 (90) |
| Carvedilol equivalent, mg | 7.5 (0–11.25) | 10 [5–15] * | 6.25 (0–11.875) | 10 [5–20] * | 8.75 (3.125–11.875) | 10 [5–15] | 5 (0–12.5) | 10 (2.5–10) |
| Loop diuretics, n (%) | 44 (52) | 63 (74) * | 9 [32] | 17 (61) | 15 (54) | 22 (79) | 20 (69) ☨ | 24 (83) |
| Furosemide equivalent, mg | 10 (0–40) | 20 (0–40) * | 0 (0–40) | 20 (0–40) * | 5 (0–40) | 20 [10–40] | 30 (0–40) | 20 [20–40] |
| MRA, n (%) | 15 [18] | 39 (46) * | 7 [25] | 10 [36] | 2 [7] | 12 [43] * | 6 [21] | 17 (59) * |
| Hospitalization length (days) | 17 [11–26] | 20 [11–33] | 15 [10–22] | 18 [11–26] | ||||
Data are presented median (25th to 75th percentiles) for continuous variables, or n (%). *p < 0.05 vs on admission data. †<0.05 showed significant differences between the tiers at the same phase. ACE, angiotensin-converting enzyme; AF, atrial fibrillation; AFL, atrial flutter; ARB, angiotensin receptor blocker; BB, beta-blocker; BNP, B-type natriuretic peptide; E/e’, the ratio between early mitral inflow velocity and mitral annular early diastolic velocity; eGFR, estimated glomerular filtration rate; HF, heart failure; HR, heart rate; LAVI, left arterial volume index; LLoQ: Lower Limit of Quantification, LVDd, left ventricular end-diastolic dimension; LVEF left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-termial pro B-type natriuretic peptide; PRA, plasma renin activity; NYHA, New York Heart Association; SBP, systolic blood pressure; WRF, worsening renal failure.
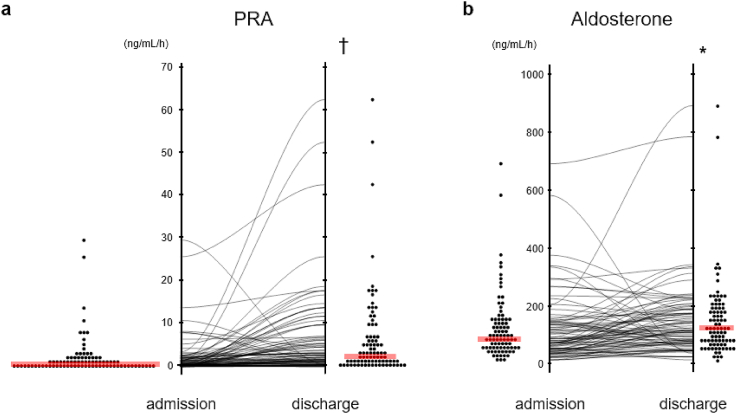
Fig. 1 shows changes of PRA (ΔPRA) and PAC (ΔPAC) during hospitalization. The number of the samples whose PRA level were undetectable (≤0.1 ng/mL/h) so that recorded as a PRA of 0.1 ng/mL/h were 8 on admission and 3 at discharge where 1 patient showed PRA level below lower limit of quantification (Table 1). Median PRA and PAC on admission was 0.8 (IQR 0.3–22) ng/mL/h and 83 (IQR 47–142) pg/mL, respectively. In contrast, median PRA and PAC at discharge was 2.8 (IQR 1.0–7.2) ng/mL/h and 113 (IQR 68–186) pg/mL, respectively. PRA was significantly elevated at discharge compared to admission (p < 0.001), as was PAC (p < 0.05). ΔPRA during hospitalization was 1.1 (IQR 0.1–6.1) ng/mL/h and ΔPAC was 26 (IQR -25 - 75) pg/mL, respectively. 79% of patients exhibited increased PRA, and 67% of patients exhibited increased PAC.
Fig. 1.
The change in PRA and aldosterone during hospitalization.
The level of PRA(a) and aldosterone (b) on admission and at discharge in each case was plotted. Both the level of PRA and aldosterone at discharge were higher than those on admission. Red line indicates median. Statistical analysis was performed using paired t-test. Statistically significant difference from baseline: * = p < 0.05 and † = p < 0.001. PRA; plasma renin activity. . (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
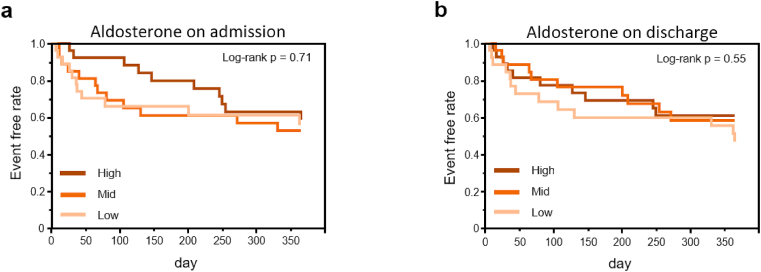
During follow-up of 365 days, cardiac event occurred in 33 patients (39%). On the Kaplan-Meier curves, the tertiary groups ranked by PRA level on admission tend to show worse prognosis in order of high, mid, and low PRA level, although prognostic value of singular PRA and PAC measurement did not reach statistical significance (p = 0.07 by log-rank test) (Fig. 2A). On the other hand, tertiary groups ranked by PRA level at discharge were significantly differentiated at the primary outcome, and the prognosis was worse in order of high, low, and mid (p = 0.034 by log-rank test) (Fig. 2B). Interestingly, on the Kaplan-Meier curves, the order of prognosis according to the tertiary groups ranked by PRA level was different between admission and discharge. On the other hand, the tertiary groups ranked by PAC levels at admission and at discharge showed no significant difference in prognosis (Supplementary Figure 1).
Fig. 2.
Kaplan-Meier event-free survival curves for cardiovascular death and re-hospitalization due to heart failure in the tertile groups for PRA on admission (a) or PRA at discharge (b). Log-rank test was used for statistical analysis.
Solid line, dashed line, and dotted line represent the high, mid, and low groups of the tertile, respectively.
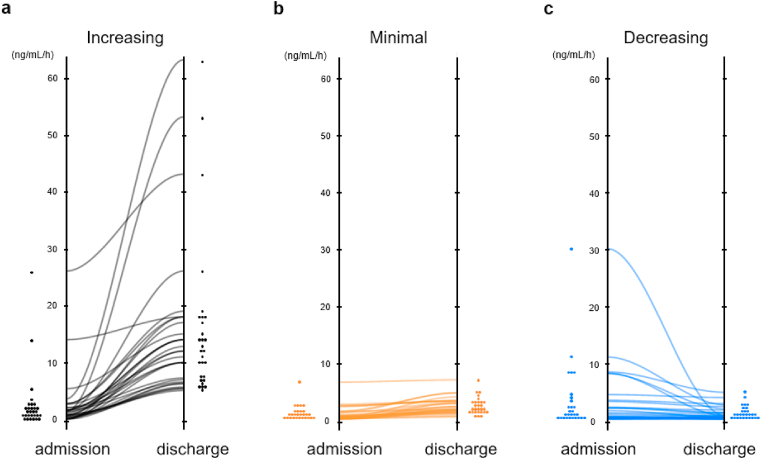
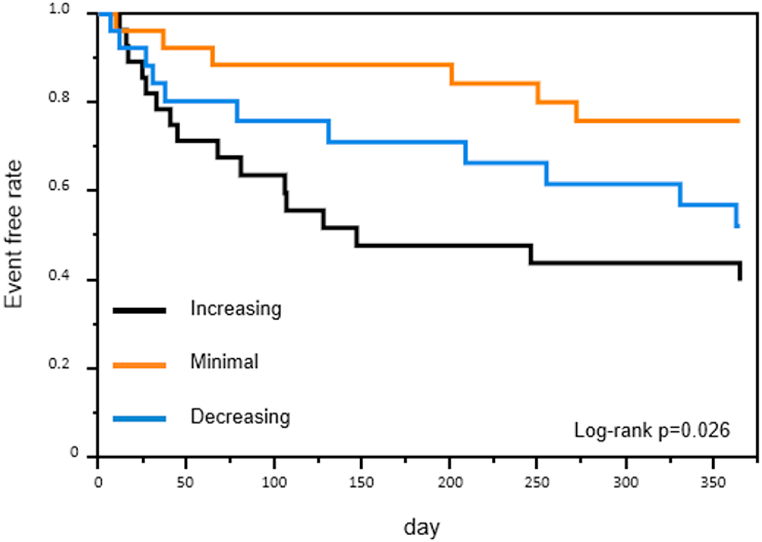
As has been previously reported, the higher the PRA on admission, the worse the prognosis [26,27,29]. However, this was not the expected order for PRA at discharge. Hence, we analyzed prognostic value of dual PRA measurement. Participants were divided into tertiary groups according to the ΔPRA levels during hospitalization. The ΔPRA levels categorized as Increasing, Minimal, and Decreasing tiers were 9.9 (IQR 5.6–15.7) pg/mL, 1.1 (IQR 0.6–1.8) pg/mL, and −0.3 (−0.9–0.1) pg/mL, respectively. Most of patients in the minimal group maintained low PRA level at admission and discharge (Fig. 3). Predicted prognosis progressively worsened in order of Minimal, Decreasing, Increasing tiers (p = 0.026 by log-rank test) (Fig. 4). Cox proportional hazard model with restricted cubic splines showed that PRA level at admission was associated with prognosis, but not in simple direct proportion (Fig. 5a). But when we analyzed using ΔPRA, the hazard ratio tended to be smaller when the ΔPRA was minimal (Fig. 5b). Of note, even after discarding the patients whose PRA level was below lower limit of quantification, comparable result was obtained (Supplementary figure 2).
Fig. 3.
ΔPRA comparison in the three tiers.
Black (a), orange (b), and blue line (c) represent the increasing, minimal, and decreasing groups of ΔPRA, respectively. PRA; plasma renin activity. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 4.
Kaplan-Meier event-free survival curves for cardiovascular death and re-hospitalization due to heart failure in the tertile groups for ΔPRA.
Log-rank test was used for statistical analysis. Black, orange, and blue line represent the increasing, minimal, and decreasing groups of the tertile for PRA change, respectively. PRA; plasma renin activity. . (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 5.
Hazard ratio (95% CI) for primary outcome based on the Cox proportional hazard model with five knots restricted cubic splines. (a) The curve represents the association between PRA on admission and the primary outcome. (b) The curve represents the association between ΔPRA and the primary outcome. The solid red line indicates a hazard ratio of 1. The solid black line shows the hazard ratio and the gray area shows its confidence interval. PRA; plasma renin activity. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Since renin is secreted from juxtaglomerular apparatus, physiological PRA level is affected by renal function. To better understand the impact of eGFR on PRA in our study, we examined the level of PRA as well as the ΔPRA according to the eGFR level. As shown in the Supplementary figure S3, among the patients with eGFR<30, 30 ≤ eGFR <60, and eGFR ≤60 mL/min/1.73 m2, PRA level on admission nor ΔPRA did not show significant difference (p-value was 0.20 and 0.11, respectively). We also examined the Cox proportional hazard model after discarding the patients with eGFR <30 mL/min/1.73 m2 and obtained comparable results to Fig. 4 with log-rank p = 0.0505 (Supplementary figure 4).
4. Discussion
The major findings of this study are: 1) in HFmrEF or HFrEF ADHF patients, PRA level are elevated at discharge compared to that of admission; 2) Singular measurement of PRA level at admission was not sufficient to predict prognosis 3) ΔPRA level were associated with prognosis; and 4) Minimal ΔPRA level during hospitalization was associated with better outcome. This study focused on change in PRA level during hospitalization as biomarker and revealed its value for predicting prognosis of HF patients. And since renin is located upstream of the RAAS signaling cascade deeply involved in the pathophysiology of heart failure, we consider this study as clinically important and highly relevant.
Renin is the most upstream regulator of RAAS signaling cascade and is involved in regulation of neurohumoral factors. Renin is secreted from kidneys, particularly by the juxtaglomerular apparatus, and its secretion is stimulated when renal inflow is decreased due to impaired cardiac output [38,39]. In fact, it has been reported that PRA level elevates as HF stage advances [17]. And since excessive RAAS activity is known to cause progressive myocardial remodeling RAASi are the fundamental treatment of HF. Although RAASi have shown to improve heart failure outcomes, their use has also been reported to increase PRA level [31,33]. This fact seems inconsistent with previous reports that concluded high PRA level are associated with poor prognosis [26,27,28]. Vaduganathan et al. reported a substudy of ASTRONAUT trial [40]. They analyzed 1306 cases of patients hospitalized for HF with LVEF <40% with available PRA level at baseline. Although it should be noted that they did not select patients with high PRA level nor analyzed confounding factors such as co-administered HF therapies, the result showed that the primary composite outcome of cardiovascular mortality and HF hospitalization was lowest in the quartile of lowest baseline PRA whilst change in PRA level from baseline to 1 month was not predictive of primary endpoint at 12 months. Therefore we believe that neither PRA nor renin is an effector regulating the pathophysiology of HF. Furthermore, if elevated PRA level truly indicates overall RAAS activity, there should be a simple association between PRA level and prognosis, but in our analysis, PRA level at admission was associated with prognosis, but not in simple direct proportion (Fig. 5a).
In the setting of worsening HF or congestion, neurohumoral factors, including RAAS, are considered to be temporarily activated to ensure the organ perfusion. And their activation is generally believed or accepted to cease after the relief of congestion or successful treatment of ADHF. Thus their temporary activation is considered a reflection of congestion. Taking these into account, in HF patients, PRA may not be a direct effector regulating the pathophysiology of HF but may play a greater role as a biomarker reflecting hemodynamic status and degree of RAAS inhibition achieved by HF GDMTs.
Although there have been various studies on biomarkers to predict prognosis of HF patients, most studies have only analyzed the data at admission. However, changes in these biomarkers after therapeutic intervention for HF are not always uniform. Studies examining NT-proBNP, which is the most frequently used biomarker of HF prognosis, report a better prognosis in the group with a 30% or greater decrease in NT-proBNP after HF treatment [6]. In another study examining HF prognosis using dual point serum chloride levels, at admission and at discharge, prognosis was worse in the group with low chloride both at admission and at discharge; and normal chloride at admission but low chloride at discharge [41]. As for PRA, although there have been several reports indicating the high PRA levels at admission is associated with a poor prognosis, but to the best of our knowledge, no comparisons between PRA at admission and at discharge have been reported. There is one previous report that measured renin concentrations, not PRA, at admission and at discharge in ADHF patients, but there was no correlation between the change of renin concentration and HF prognosis [42]. Our study showed that even in patients who had low levels PRA at admission, when these patient's PRA levels at discharge elevates, they had a poor prognosis. This suggests that PRA at admission alone is not sufficient to predict the prognosis of HF.
As mentioned above, previous studies have reported a correlation between high PRA level at admission and poor HF prognosis. Also in our study, tertiary groups ranked by PRA level on admission showed trend of poor prognosis in order of high, mid, and low PRA level (Fig. 2a). However, when PRA level at discharge was examined, prognosis was poor in order of high, low, and mid PRA level group (Fig. 2b). Of the patients who were in the high PRA level group at admission, 47% remained in the high PRA level group at discharge, while 37% changed to mid PRA level group, and 17% changed to low PRA level group. Thus, PRA values do change by HF treatment, and since PRA at discharge is later, it makes sense to update prognostic predictions according to PRA levels at discharge. In the present study, we compared PRA level between admission and discharge and used the ΔPRA to evaluate HF prognosis. Prior to this analysis, we anticipated that prognosis would be worse in order of Increasing, Minimal and Decreasing tiers. But interestingly, our analysis revealed that the prognosis was actually worse in the order of Increasing, Decreasing and Minimal tiers. What caused this difference in the change of PRA level? Since influence of ARB on PRA elevation in HF is reported in VaL-HeFT study 31, we examined difference of PRA with treatment of HF. Baseline usage of RAASi was associated with higher PRA levels at admission (1.2 (0.5–2.7) vs 0.5 (0.1–1.3), p = 0.003) which was consistent with VaL-HeFT. But we found no differences of initiation or titration of RAASi, BB, or loop diuretics in Increasing, Minimal, and Decreasing tiers (Supplementary Table 1 and Supplementary figure 5). There were also no differences between groups in dose change of RAASi, BB or loop diuretics. As for the association of PRA and renal function, we could not find the difference in PRA level on admission as well as in ΔPRA during admission amongst patients with eGFR<30, those with 30 ≤ eGFR <60, and those with eGFR ≤60 mL/min/1.73 m2. The Cox proportional hazard model showed similar results after discarding the patients with eGFR <30, suggesting that the change in PRA during admission may predicts the prognosis across HF patients with broad range of renal function. In addition, no significant differences were found in the rate of worsening renal failure during the course of hospitalization, nor in changes in systolic blood pressure, heart rate or body weight (Table 1). So the difference of changes in PRA level could not be explained by HF treatment, vital signs, or physical findings.
In addition to RAAS, we also analyzed other neurohumoral factors, such as ADH and epinephrine, but found no significant differences in each group (Supplementary Table 2). No significant differences were found in changes of NT-proBNP and BNP among each group, but NT-proBNP and BNP were significantly higher at discharge in the Decreasing group than in the other groups. As mentioned above, high NT-proBNP level at discharge is a predictor of poor prognosis, but residual pleural effusion at discharge has also been reported as a poor prognostic factor [43]. In the present study, significantly high prevalence of residual pleural effusion was observed in Decreasing group. And this might suggest that further need for dehydration or inadequate volume control. In this present analysis, we cannot determine whether the elevated PRA at admission was to maintain hemodynamic in the setting of ADHF, and/or a temporary response due to congestion, or an increase in the baseline level of PRA due to stage progression of HF, but these aforementioned factors may have contributed to the worse prognosis in Decreasing group. On the other hand, patients in Increasing group had significantly lower serum sodium level at discharge. It has been reported that as the stage of HF progresses, serum sodium level declines and is an independent predictor of poor prognosis [44]. Also, PRA level at discharge was 0.6 (IQR 0.3–1.6) ng/mL/h in Decreasing group and 2.0 (IQR 1.3–3.3) ng/mL/h in Minimal group but was 12.0 (IQR 7.0–18.0) ng/mL/h in Increasing group, which was significantly higher than other two groups (Table 1). Therefore, we can speculate that excessive dehydration, until PRA level is elevated, was needed to relieve the congestion in Increasing group. Given this perspective, this Increasing group may include patients with potentially severe HF who cannot be assessed by conventional HF biomarkers such as NT-proBNP or BNP (Supplementary Table 2).
The reason for the better prognosis in the Minimal group also could not be explained by etiology or severity of HF, blood pressure, pulse rate, renal function or HF drug induction rate at admission and discharge (Table 1). Considering that PRA levels are altered by hemodynamic changes, signals from sympathetic nerve activity, and feedback from factors downstream of the RAAS signaling cascade, it may be possible to consider that patients who are able to maintain low level of PRA even during HF exacerbations are the ones who have the better prognosis. This study indicates that it may be possible to further stratify the prognosis of HF patients by comparing PRA level at admission and discharge and analyzing its change or trajectory.
4.1. Limitations
There are several limitations to our study. First, this was single center, retrospective study. There was no significant difference in the commonly accepted association between PRA at admission and HF prognosis. Secondly, the number of patients in this study was small. Thirdly, each patient's treatment is different, the changes in PRA levels are not uniform. Fourthly, this study was conducted in the period before ARNI was launched in Japan, so PRA values may be different from current standards. Fifthly, the time between admission and blood collection varies from patient to patient. Because the half-life of PRA is 40–120 min, PRA level may have changed due to HF treatment with rest and oxygenation. Sixthly, it is known that PRA is affected by various regulating factors of RAAS, such as emotional stress, nutrition, and renal perfusion but we could not analyze these factors in the current study. Seventhly, the blood sample on admission was collected as early as possible within 24 h from admission for ADHF. Therefore, the time of sampling and the length of the time from the last meal or the last medication etc. was not standardized in this study. Lastly, this is an observational clinical study and do not provide mechanistic insight that links regulation of PRA and the pathophysiology of HF, suggesting the need for further studies including animal research.
5. Conclusion
In ADHF patients with ejection fraction less than 50%, patients with minimal change of PRA during hospitalization showed better prognosis over those with either increasing or decreasing PRA levels. These results suggest that measuring and comparing PRA level at admission and discharge may allow for more detailed stratification of HF prognosis. Further research on PRA in HF is desirable.
Author contribution statement
Masanori Asakura and Masaharu Ishihara: Conceived and designed the experiments. Kohei Azuma, Koichi Nishimura and Kyung-Duk Min: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper. Kanae Takahashi: Analyzed and interpreted the data. Yuki Matsumoto, Akiyo Eguchi, Yoshitaka Okuhara, Yoshiro Naito and Shinichiro Suna: Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest's statement
The authors declare no competing interests.
Additional information
No additional information is available for this paper.
Acknowledgement
We appreciate the professional advice about cardio-renal interaction from Dr Yasuyuki Nagasawa of Hyogo Medical University, Hyogo, Japan.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2023.e13181.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Savarese G., Becher P.M., Lund L.H., Seferovic P., Rosano G.M.C., Coats A.J.S. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc. Res. 2022;1–16 doi: 10.1093/cvr/cvac013. [DOI] [PubMed] [Google Scholar]
- 2.Dharmarajan K., Rich M.W. Epidemiology, pathophysiology, and prognosis of heart failure in older adults. Heart Fail. Clin. 2017;13:417–426. doi: 10.1016/j.hfc.2017.02.001. Available at: [DOI] [PubMed] [Google Scholar]
- 3.Bottle A., Newson R., Faitna P., Hayhoe B., Cowie M.R. Changes in heart failure management and long-term mortality over 10 years: observational study. Open Hear. 2022;9 doi: 10.1136/openhrt-2021-001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin A.H., Chin J.C., Sicignano N.M., Evans A.M. Repeat hospitalizations predict mortality in patients with heart failure. Mil. Med. 2017;182:e1932–e1937. doi: 10.7205/MILMED-D-17-00017. [DOI] [PubMed] [Google Scholar]
- 5.Demissei B.G., Postmus D., Cleland J.G., O'Connor C.M., Metra M., Ponikowski P., Teerlink J.R., Cotter G., Davison B.A., Givertz M.M., Bloomfield D.M., Veldhuisen DJ van, Dittrich H.C., Hillege H.L., Voors A.A. Plasma biomarkers to predict or rule out early post-discharge events after hospitalization for acute heart failure. Eur. J. Heart Fail. 2017;19:728–738. doi: 10.1002/ejhf.766. [DOI] [PubMed] [Google Scholar]
- 6.Bettencourt P., Azevedo A., Pimenta J., Friões F., Ferreira S., Ferreira A. N-terminal-pro-brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation. 2004;110:2168–2174. doi: 10.1161/01.CIR.0000144310.04433.BE. [DOI] [PubMed] [Google Scholar]
- 7.Olsson L.G., Swedberg K., Cleland J.G.F., Spark P.A., Komajda M., Metra M., Torp-Pedersen C., Remme W.J., Scherhag A., Poole-Wilson P. Prognostic importance of plasma NT-pro BNP in chronic heart failure in patients treated with a β-blocker: results from the Carvedilol or Metoprolol European Trial (COMET) trial. Eur. J. Heart Fail. 2007;9:795–801. doi: 10.1016/j.ejheart.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Takase H., Dohi Y. Kidney function crucially affects B-type natriuretic peptide (BNP), N-terminal proBNP and their relationship. Eur. J. Clin. Invest. 2014;44:303–308. doi: 10.1111/eci.12234. [DOI] [PubMed] [Google Scholar]
- 9.Bachmann K.N., Gupta D.K., Xu M., Brittain E., Farber-Eger E., Arora P., Collins S., Wells Q.S., Wang T.J. Unexpectedly low natriuretic peptide levels in patients with heart failure. JACC Hear Fail. 2021;9:192–200. doi: 10.1016/j.jchf.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parcha V., Patel N., Kalra R., Suri S.S., Arora G., Wang T.J., Arora P. Obesity and serial nt-probnp levels in guided medical therapy for heart failure with reduced ejection fraction: insights from the guide-it trial. J. Am. Heart Assoc. 2021:10. doi: 10.1161/JAHA.120.018689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hess G., Runkel S., Zdunek D., Hitzler W.E. Reference interval determination for N-terminal-B-type natriuretic peptide (NT-proBNP): a study in blood donors. Clin. Chim. Acta. 2005;360:187–193. doi: 10.1016/j.cccn.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 12.Braisch U., Koenig W., Rothenbacher D., Denkinger M., Friedrich N., Felix S.B., Ittermann T., Dörr M., Dallmeier D. N-terminal pro brain natriuretic peptide reference values in community-dwelling older adults. ESC Hear Fail. 2022:1703–1712. doi: 10.1002/ehf2.13834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daubert M.A., Yow E., Barnhart H.X., Piña I.L., Ahmad T., Leifer E., Cooper L., Desvigne-Nickens P., Fiuzat M., Adams K., Ezekowitz J., Whellan D.J., Januzzi J.L., O’connor C.M., Felker G.M. Differences in nt-probnp response and prognosis in men and women with heart failure with reduced ejection fraction. J. Am. Heart Assoc. 2021:10. doi: 10.1161/JAHA.120.019712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oremus M., Don-Wauchope A., McKelvie R., Santaguida P.L., Hill S., Balion C., Booth R., Brown J.A., Ali U., Bustamam A., Sohel N., Raina P. BNP and NT-proBNP as prognostic markers in persons with chronic stable heart failure. Heart Fail. Rev. 2014;19:471–505. doi: 10.1007/s10741-014-9439-6. [DOI] [PubMed] [Google Scholar]
- 15.Salah K., Kok W.E., Eurlings L.W., Bettencourt P., Pimenta J.M., Metra M., Verdiani V., Tijssen J.G., Pinto Y.M. Competing risk of cardiac status and renal function during hospitalization for acute decompensated heart failure. JACC Hear Fail. 2015;3:751–761. doi: 10.1016/j.jchf.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Salah K., Stienen S., Pinto Y.M., Eurlings L.W., Metra M., Bayes-Genis A., Verdiani V., Tijssen J.G.P., Kok W.E. Prognosis and NT-proBNP in heart failure patients with preserved versus reduced ejection fraction. Heart. 2019;105:1182–1189. doi: 10.1136/heartjnl-2018-314173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan R.D., Mehta R.M., Tripathi R., Reed G.L., Gladysheva I.P. Renin activity in heart failure with reduced systolic function—new insights. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20133182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robert W., Schrier M.D., William T., Abraham M.D. Hormones and hemodynamics in heart failure. N. Engl. J. Med. 1999:577–585. doi: 10.1056/NEJM199908193410806. [DOI] [PubMed] [Google Scholar]
- 19.Braunwald E. Heart failure. JACC Hear Fail. 2013;1:1–20. doi: 10.1016/j.jchf.2012.10.002. Available at: [DOI] [PubMed] [Google Scholar]
- 20.Group T.C.T.S. Effects of enalapril on mortality in severe congestive heart failure. N. Engl. J. Med. 1987;316(23):1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 21.Inhibitor A.E., Packer M., Poole-wilson P a, Armstrong P.W., Cleland J.G.F., Horowitz J.D., Massie B.M., Ryde L., Thygesen K. Clinical investigation and reports comparative effects of low and high doses of the. Heart Fail. 1999:2312–2318. doi: 10.1161/01.cir.100.23.2312. [DOI] [PubMed] [Google Scholar]
- 22.Granger C.B., McMurray J.J.V., Yusuf S., Held P., Michelson E.L., Olofsson B., Östergren J., Pfeffer M.A., Swedberg K. CHARM-Alternative main. Lancet. 2003;362:772–776. doi: 10.1016/S0140-6736(03)14284-5. [DOI] [PubMed] [Google Scholar]
- 23.Pitt B., Poole-Wilson P., Segal R., Martinez F.A., Dickstein K., Camm A.J., Konstam M.A., Riegger G., Klinger G.H., Neaton J., Sharma D., Thiyagarajan B. Effects of losartan versus captopril on mortality in patients with symptomatic heart failure: rationale, design, and baseline characteristics of patients in the Losartan Heart Failure Survival Study - ELITE II. J. Card. Fail. 1999;5:146–154. doi: 10.1016/s1071-9164(99)90037-4. [DOI] [PubMed] [Google Scholar]
- 24.John J.V., McMurray M.D., Milton Packer M.D., Akshay S., Desai M.D., H M.P., Jianjian Gong PhD., Martin P., Lefkowitz M.D., Adel R., Rizkala PharmD., Jean L., Rouleau M.D., Victor C., Shi M.D., Scott D., Solomon M.D., Karl Swedberg M.D., PhD. M MD for the P-HI and C. Angiotensin-neprilysin inhibition versus enalapril in heart failure - supplementary appendix. N. Engl. J. Med. 2014;371(11):993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 25.Pitt B. Effectiveness of Spironolactone added to an angiotensin-converting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure (The Randomized Aldactone Evaluation Study [RALES]) Am. J. Cardiol. 1996;78:902–907. doi: 10.1016/s0002-9149(96)00465-1. [DOI] [PubMed] [Google Scholar]
- 26.Nakada Y., Takahama H., Kanzaki H., Sugano Y., Hasegawa T., Ohara T., Amaki M., Funada A., Yoshida A., Yasuda S., Ogawa H., Anzai T. The predictability of renin–angiotensin–aldosterone system factors for clinical outcome in patients with acute decompensated heart failure. Heart Ves. 2016;31:925–931. doi: 10.1007/s00380-015-0688-7. [DOI] [PubMed] [Google Scholar]
- 27.Ueda T., Kawakami R., Nishida T., Onoue K., Soeda T., Okayama S., Takeda Y., Watanabe M., Kawata H., Uemura S., Saito Y. Plasma renin activity is a strong and independent prognostic indicator in patients with acute decompensated heart failure treated with renin-angiotensin system inhibitors. Circ. J. 2015;79:1307–1314. doi: 10.1253/circj.CJ-14-1203. [DOI] [PubMed] [Google Scholar]
- 28.Vergaro G., Emdin M., Iervasi A., Zyw L., Gabutti A., Poletti R., Mammini C., Giannoni A., Fontana M., Passino C. Prognostic value of plasma renin activity in heart failure. Am. J. Cardiol. 2011;108:246–251. doi: 10.1016/j.amjcard.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 29.Poletti R., Vergaro G., Zyw L., Prontera C., Passino C., Emdin M. Prognostic value of plasma renin activity in heart failure patients with chronic kidney disease. Int. J. Cardiol. 2013;167:711–715. doi: 10.1016/j.ijcard.2012.03.061. [DOI] [PubMed] [Google Scholar]
- 30.Sim J.J., Shi J., Calara F., Rasgon S., Jacobsen S., Kalantar-Zadeh K. Association of plasma renin activity and aldosterone-renin ratio with prevalence of chronic kidney disease: the Kaiser Permanente Southern California cohort. J. Hypertens. 2011;29:2226–2235. doi: 10.1097/HJH.0b013e32834bbc8a. [DOI] [PubMed] [Google Scholar]
- 31.Masson S., Solomon S., Angelici L., Latini R., Anand I.S., Prescott M., Maggioni A.P., Tognoni G., Cohn J.N. Elevated plasma renin activity predicts adverse outcome in chronic heart failure, independently of pharmacologic therapy: data from the Valsartan heart failure trial (Val-HeFT) J. Card. Fail. 2010;16:964–970. doi: 10.1016/j.cardfail.2010.06.417. Available at: [DOI] [PubMed] [Google Scholar]
- 32.Ferreira J.P., Santos M., Almeida S., Marques I., Bettencourt P., Carvalho H. High-dose spironolactone changes renin and aldosterone levels in acutely decompensated heart failure. Cor Vasa. 2014;56:e463–e470. [Google Scholar]
- 33.Nijst P., Verbrugge F.H., Martens P., Bertrand P.B., Dupont M., Francis G.S., Tang W.H.W., Mullens W. Plasma renin activity in patients with heart failure and reduced ejection fraction on optimal medical therapy. JRAAS - J. Renin-Angiotensin-Aldosterone Syst. 2017;18 doi: 10.1177/1470320317729919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aimo A., Prontera C., Passino C., Emdin M., Vergaro G. Norepinephrine, plasma renin activity and cardiovascular mortality in systolic heart failure. Heart. 2021;107:989–995. doi: 10.1136/heartjnl-2020-318791. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi M., Stienen S., Maaten JM ter, Dickstein K., Samani N.J., Lang C.C., Ng L.L., Anker S.D., Metra M., Preud’homme G., Duarte K., Lamiral Z., Girerd N., Rossignol P., Veldhuisen DJ van, Voors A.A., Zannad F., Ferreira J.P. Clinical determinants and prognostic implications of renin and aldosterone in patients with symptomatic heart failure. ESC Hear Fail. 2020;7:953–963. doi: 10.1002/ehf2.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maaten JM ter, Voors A.A., Damman K., Meer P van der, Anker S.D., Cleland J.G., Dickstein K., Filippatos G., Harst P., van der, Hillege H.L., Lang C.C., Metra M., Navis G., Ng L., Ouwerkerk W., Ponikowski P., Samani N.J., Veldhuisen DJ van, Zannad F., Zwinderman A.H., Borst MH de. Fibroblast growth factor 23 is related to profiles indicating volume overload, poor therapy optimization and prognosis in patients with new-onset and worsening heart failure. Int. J. Cardiol. 2018;253:84–90. doi: 10.1016/j.ijcard.2017.10.010. Available at: [DOI] [PubMed] [Google Scholar]
- 37.Harrell F.E., Jr. second ed. Springer Series in Statistics.; 2015. Regression Modeling Strategies with Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. [Google Scholar]
- 38.Skott O.L.E., Briggs J.P. Direct demonstration of mac ula densa-mediated. Renin Secretion. 1987;237:5–8. doi: 10.1126/science.3306925. [DOI] [PubMed] [Google Scholar]
- 39.Persson A.E.G., Ollerstam A., Liu R., Brown R. 2004. Mechanisms for Macula Densa Cell Release of Renin; pp. 471–474. [DOI] [PubMed] [Google Scholar]
- 40.Vaduganathan M., Cheema B., Cleveland E., Sankar K., Subacius H., Fonarow G.C., Solomon S.D., Lewis E.F., Greene S.J., Maggioni A.P., Böhm M., Zannad F., Butler J., Gheorghiade M. Plasma renin activity, response to aliskiren, and clinical outcomes in patients hospitalized for heart failure: the ASTRONAUT trial. Eur. J. Heart Fail. 2018;20:677–686. doi: 10.1002/ejhf.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kondo T., Yamada T., Tamaki S., Morita T., Furukawa Y., Iwasaki Y., Kawasaki M., Kikuchi A., Ozaki T., Sato Y., Seo M., Ikeda I., Fukuhara E., Abe M., Nakamura J., Sakata Y., Fukunami M. Serial change in serum chloride during hospitalization could predict heart failure death in acute decompensated heart failure patients. Circ. J. 2018;82:1041–1050. doi: 10.1253/circj.CJ-17-0938. [DOI] [PubMed] [Google Scholar]
- 42.Biegus J., Nawrocka-Millward S., Zymliński R., Fudim M., Testani J., Marciniak D., Rosiek-Biegus M., Ponikowska B., Guzik M., Garus M., Ponikowski P. Distinct renin/aldosterone activity profiles correlate with renal function, natriuretic response, decongestive ability and prognosis in acute heart failure. Int. J. Cardiol. 2021;345:54–60. doi: 10.1016/j.ijcard.2021.10.149. [DOI] [PubMed] [Google Scholar]
- 43.Yaku H., Seko Y., Kato T., Morimoto T., Kimura T. Prognostic implications of residual pleural effusions at discharge in patients with acute decompensated heart failure. Eur. J. Intern. Med. 2021;85:133–135. doi: 10.1016/j.ejim.2020.11.023. [DOI] [PubMed] [Google Scholar]
- 44.Abebe T.B., Gebreyohannes E.A., Tefera Y.G., Bhagavathula A.S., Erku D.A., Belachew S.A., Gebresillassie B.M., Abegaz T.M. The prognosis of heart failure patients: does sodium level play a significant role? PLoS One. 2018;13:1–14. doi: 10.1371/journal.pone.0207242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.