Abstract
Non-polar oil and polar short-chain alcohols, used as reactants in the transesterification reaction, are immiscible. Transesterification reactions can only occur on the phase boundary and they are therefore diffusion-limited. Several methods are employed to overcome the limitation of mass transfer by increasing miscibility and thereby accelerating the reaction. Co-solvents are additional solvents that should be soluble in the oil and alcohol phase; this could lead to an increase in the reaction rate and a reduction in the temperature and the reaction time. This work aims to provide a comprehensive literature review on the influence of co-solvents on the processes of catalysed methanolysis for the biodiesel production. Most authors have not systematically determined and justified the effects of cosolvents. So far it seems impossible to establish which cosolvents are the most suitable for which methanolysis systems. The purpose of this work is to highlight and justify the differences or similarities in co-solvent impacts among the various publications by examining the chemical structure of the respective co-solvents, including the functional groups and the resulting physicochemical properties such as the dielectric constant or the log P value. Besides biodiesel, co-solvents like THF and acetone seems to be the best choices for alkaline methanolysis systems due to successful broadly applications with different oils, catalysts and reaction conditions. Moreover, THF and n-hexane are essentially advisable for in-situ methanolysis.
Keywords: Biodiesel biofuel, Catalysis, Co-solvent, Mass transfer, Methanolysis
Highlights
-
•
Effect of co-solvent on the catalysed methanolysis.
-
•
Co-solvents to overcome the mass transfer limitation.
-
•
Advances in the use of co-solvents for the biodiesel production.
List of abbreviations
- BSFL
black soldier fly larvae
- DEE
diethyl ether
- DME
dimethyl ether
- FAAE
fatty acid alkyl ester
- FAME
fatty acid methyl ester
- FFA
free fatty acid
- MeOH
methanol
- MTBE
methyl tert-butyl ether
- N/S
not specified
- NIA
no information available
- PFAD
palm fatty acid distillate
- PTSA
p-toluenesulfonic acid
- RT
Room temperature
- STR
stirred tank reactor
- T
temperature
- THF
tetrahydrofuran
- WCO
waste cooking oil
- &epsiv
dielectric constant
1. Introduction
Biodiesel is a non-petroleum-based fuel for use in compression ignition engines that comprises of mono-alkyl esters of long chain fatty acids derived from vegetable oils or animal fats [1,2]. Transesterification is the most commonly-used chemical conversion process to produce biodiesel, reducing the viscosity of the oil or fat [3,4]. Transesterification involves three consecutive reversible steps. Overall, 1 mol of triglycerides react with 3 mol of short-chain alcohols to form 3 mol of fatty acid alkyl esters (FAAE) as the desired product and 1 mol of glycerine as a by-product. The respective reaction intermediates are consequently di- and monoglycerides. Methanol (MeOH) is the most commonly-used alcohol due to its low cost and overall availability. When methanol is used, the resulting esters are known as fatty acid methyl esters (FAME) [5]. The EN 14214 standard includes the minimum content of FAME with 96.5 wt%.
The transesterification can run without any catalysts [4], but they are essential for an economically feasible production. Catalysts include homogeneous and heterogeneous catalysts.
Homogeneous catalysts include acidic and alkaline catalysts [6,7]. These are solved in the alcohol phase prior to transesterification [8], so it is a two-phase reaction system including immiscible oil and alcohol phase. Heterogeneous catalysts include acidic and alkaline catalysts and immobilised enzymes. These are solids and form a separate phase to the methanol and oil phase [4,9]; as a result, the reaction system is a three-phase system.
Due to non-polar oil and polar short-chain alcohols are immiscible, the transesterification reaction can only occur at the limit of the phase and thus their diffusion is limited. This narrows the reaction rate, especially in the initial phase. Later the di- and monoglycerides formed act as surfactants, increasing the solubility and thus the reaction rate [10]. Towards the end of the reaction, the reaction rate slows down again because the biodiesel and glycerol products are likewise immiscible [5]. Resistance to mass transfer resistance is enhanced by the use of heterogeneous catalysts [11]. Several methods are applied to overcome the limitation of mass transfer by increasing miscibility. Possible ways include heating and mixing, co-solvent addition, alternative solvents (ionic liquids), phase-transfer catalysts, as well as the use of supercritical alcohol [3].
Co-solvents are additional solvents that should be soluble in the oil and alcohol phase, and do not react with the reactants nor the catalyst [9,12]. Co-solvents dissolve the phase boundary between alcohol and oil, by which a three-phase systems turns into a two-phase system [13], and a two-phase system becomes a single phase system [14]. This should lead to an increased in the reaction rate and therefore have a positive influence on the parameters which influence the transesterification, such as the reduction of the temperature and the necessary reaction time needed [15]. After about two decades of co-solvent application, since the research team around David Boocock started to describe the phase homogenisation with THF [16,17], it is evident that different positive impacts in catalysed methanolysis systems are possible. However, most of the publications have not determined and justified the effects of co-solvents in a systematic way. So far it seems impossible to establish which co-solvents are the most suitable for which methanolysis systems.
This review includes a comprehensive literature study on the effects of co-solvents on the processes of catalysed methanolysis for the biodiesel production. The purpose of this work is to highlight and justify the differences or similarities in co-solvent impacts among the various publications by examining the chemical structure of the respective co-solvents, including the functional groups and the resulting physicochemical properties such as the dielectric constant or the log P value.
2. Properties of co-solvents
In Table 1 the physicochemical properties of the more frequently used co-solvents for the biodiesel production are listed. Both the chemical structure of the molecules and a selection of physicochemical properties are shown, including two parameters for assessing the co-solvent polarity. The chemical structure as well as the log P value and the dielectric constant (ϵ) are listed to examine the amphiphilic co-solvent character for effective emulsification of MeOH and oil. These propertieswere chosen for a short, concise and differentiated discussion from others, including the Hildebrand solubility parameter, surface and interfacial tension as well as the hydrogen bond donor density and hydrogen bond acceptor density [18]. Information about the miscibility of the respective co-solvents in MeOH, glycerol and fat/oil are also listed in Table 1. This experimental information may support theory-based discussion approaches.
Table 1.
Physicochemical properties of selected co-solvents.
| Substance class |
Co-solvent | Chemical structure |
Properties |
Price [€/L] | Ref. (properties [17] and price) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ϵ (20 °C) | log P (25 °C) |
Boiling point (°C) |
Dynamic viscosity [mPa·s] (25 °C) |
Density [g/cm3] (20 °C) |
Miscibility (20 °C) |
|||||||
| Methanol | Glycerol | Fat | ||||||||||
| Alcohol | Methanol |  |
33 | −0.74 | 64.4 | 0.544 | 0.7914 | + | + | - | 6.40a | [21,22] |
| 1-propanol |  |
20.8 | 0.05 | 97.2 | 1.945 | 0.800 (25 °C) |
+ | + | + | 23.88a | [11,23] | |
| 2-propanol |  |
20.18 | 0.25 | 82.3 | 2.04 | 0.7809 (25 °C) |
+ | + | + | 11.00a | [11,24] | |
| t-butanol |  |
12.47 | 0.35 | 82.4 | 4.31 | 0.7887 | NIA | NIA | NIA | 14.40a | [25] | |
| Alkane | n-hexane | 1.8865 | 4 | 68.73 | 0.300 | 0.6606 (25 °C) |
- | - | + | 33.24a,b | [11,26] | |
| Cyclohexane |  |
2.0243 | 0.81 | 80.73 | 0.894 | 0.7738 | - | NIA | NIA | 19.08a | [27,28] | |
| Aromatic hydrocarbons |
Toluene | 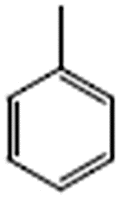 |
2.379 | 2.73 | 110.63 | 0.560 | 0.8668 | + | NIA | NIA | 14.80a | [29,30] |
| Ester | Ethyl acetate |  |
6.0814 | 0.73 | 77.11 | 0.423 | 0.9003 | + | NIA | NIA | 17.86c | [30,31] |
| Ether | DME |  |
NIA | 0.1 | −24.8 | 0.122 (25 °C) |
0.67d (N/S) |
NIA | NIA | NIA | NIA | [32] |
| DEE |  |
4.2666 | 0.89 | 34.5 | 0.224 | 0.7138 | + | - | + | 20.32a | [21,33] | |
| MTBE |  |
NIA | 0.94 | 55 | 0.27 (20 °C) |
0.7353 (25 °C) |
+ | NIA | NIA | 18.52a | [30,32,34] | |
| Petroleum ether | – | NIA | NIA | >50 | NIA | 0.666 (15 °C) |
NIA | NIA | NIA | 25.96e | [35,36] | |
| THF |  |
7.52 | 0.46 | 65 | 0.456 | 0.8833 (25 °C) |
+ | - | + | 28.24a | [11,37] | |
| Ketone | Acetone |  |
21.01 | −0.24 | 56.05 | 0.365 | 0.7845 (25 °C) |
+ | - | + | 9.80a | [11,38] |
Based on the prices for 25 L containers. Supelco – EMPLURA.
Supelco- EMSURE.
5 L plastic canister; Supelco – EMPLURA.
Liquid form.
25 L stainless steel drum; Supelco for denaturation.
The log P value (octanol-water partition coefficient P) describes the distribution of a substance in a system that contains a water phase and a 1-octanol phase in equilibrium under a set temperature [19]. P is the relationship between therespective equilibrium concentration of the substance in the octanol-rich phase and the water-rich phase. A high log P value means a high attraction to the octanol phase due to non-polar molecule structures, whereas small values means more polar functional groups, which favour the accumulation in the water phase [19]. Although it is possible to make a statement about the hydrophilicity or hydrophobicity, in the present work this parameter is primarily used to estimate how well the co-solvents can interact with the oil phase.
In order to estimate the ability for hydrogen bonding and consequently to interact with the polar MeOH phase, the dielectric constant was chosen over the permanent dipole moment, expressed in Debye, and the hydrogen bond donor density and hydrogen bond acceptor density. Rubino and Yalkowsky [18] defined the latter two as the product of the number of proton donor groups or non-bonding electron pairs with the co-solvent density and the factor 1.000, divided by the co-solvent molecular weight. This is why MeOH can be both an acceptor as well as a donor molecule with its hydroxyl group. Therefore, with the two free electron pairs from the oxygen atom in the hydroxyl group, a hydrogen bond acceptor density of about 49 and one partial positively-charged proton, a hydrogen bond donor density of about 26 results [18]. Accordingly, a suitable co-solvent should be more of a hydrogen bond donator to better interact with the MeOH.
The ϵ was chosen as this parameter is mathematically related to the permanent dipole moment and polarizability as individual molecular properties. A correlation between the solubility and the ϵ has been proven, especially for molecules with similar bonding characteristics [18]. Water molecules could develop more hydrogen bonds on average, the higher the respective ϵ [20]. The solubility parameter developed by Hildebrand – which represents all attractive forces surrounding a molecule in one value [18] – was not chosen due to the limited availability of suitable sources.
Tables 2–5 show data on the use of co-solvents in homogeneous alkaline, homogeneous acidic, heterogeneous alkaline and heterogeneous acidic catalysed methanolysis, respectively. The test that achieved yields higher than 90 wt% were included. Publications that found no influences, negative influences or did not provide sufficient information are discussed in the text. All specifications regarding the dosage of co-solvents used in this work were recalculated to v.% regarding the MeOH volume to facilitate the overall comparability. The original information regarding the co-solvent amount stated from the respective publication is always in parentheses besides or underneath the recalculated value.
Table 2.
Use of co-solvents in homogeneous alkaline catalysed methanolysis.
| Co- solvent |
Catalyst | Reactor type | Feedstock | Pre- treatment |
Reaction conditions |
Yield (wt.%d) |
Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Co-solvent amount (wt.%a, v.%b, mol%c,d) |
Catalyst amount (wt.%a, mol%d) |
MeOH/ oil molar ratio |
Time (min) |
T (°C) |
Stirrer (rpm) |
Special conditions |
|||||||
| Acetone | KOH | STR | Canola oil | – | 154 v.% (25 wt%) |
1 | 4.5:1 | 30 | 25 | N/S | – | 98.30 | [39] |
| Catfish oil | – | 0.5 | 60 | 25 | 97.50 | [21] | |||||||
| – | 1 | 30 | 35 | 97.02 | [39] | ||||||||
| Jatropha curcas L. oil | – | 1 | 30 | 25 | 97.91 | [39] | |||||||
| – | 0.5 | 60 | 25 | 97.80 | [21] | ||||||||
| 1 | 92 v.% (20 wt%) |
1 | 6:1 | 30 | 40 | 90 | [40] | ||||||
| Acetone | KOH | STR | WCO | – | 154 v.% (25 wt%) |
1 | 4.5:1 | 30 | 25 | N/S | – | 98 | [39] |
| Biodiesel | KOH | STR | Soybean oil | – | 20 v.% (5 wt%) |
0.8 | 6:1 | 60 | 70 | 100 | – | ∼95e | [14] |
| DEE | KOH | STR | Rapseed oil | – | 256 v.% (100 mol%c) |
0.7 | 9:1 | 120 | 30 | 700 | – | 97.6 | [15] |
| [41] | |||||||||||||
| Microtube | Sunflower oil | – | 187 v.% (73 mol%c) |
1 | 8:1 | 93 s | 25 | -f | 92.8g | [32] | |||
| NaOH | STR | Soybean oil | – | 144 v.% (56 mol%c) |
1.07 | 5.65:1 | 30 | 34 | 500 | 98.35 | [42] | ||
| DME | KOH | Oscillating zylinder | Sunflower oil | – | 100 mol%c | 1 | 6:1 | 20 | 25 | 2.6 Hz shaking |
500 kPa | ∼99e,g | [32] |
| n-hexane | CH3ONa | STR | Castor oil | – | 15 v.% | 1 | 6:1 | 120 | 60 | 600 | – | 95.5 | [43] |
| KOH | STR |
Cyprinus carpio fish oil (fish waste) |
150 v.% | 0.60 | 5:1 | 30 | 50 | 600 | 97.24 | [44] | |||
| Pork lard | 214 v.% (65 wt%) |
2.0 | 10:1 | 120 | 50–60 | 600 | 98.2 | [45] | |||||
| Microreactor | Soybean oil (used) |
45 vol% | 1 | 33.3 v.%g | 9.05 s | 57.2 | -f | 98.8 | [41] | ||||
| NaOH | STR | Jatropha curcas L. oil | 2 | 40 v.% | 1 | 8:1 | 7 | 60 | N/S | 96.50 | [47] | ||
| Rapeseed oil soybean oil (1:1) |
3 | 5 g | 0.80 | 5:1 | 120 | 55 | 1600 | 94 | [48] | ||||
| MTBE | KOH | STR | Rapeseed oil | – | 296 v.% (100 mol%c) |
0.7 | 9:1 | 120 | 30 | 700 | – | 97.5 | [15] |
| NaOH | Microwave cell (batch) |
Rapeseed oil | 165 v.% (no ratio given) |
1.3 | 12:1 | 1 | 60 | 400 | 2.45 kHz | 95 | [49] | ||
| Soybean oil | 97 | [49] | |||||||||||
| Microwave cell (continuous) |
Soybean oil | 97 | [49] | ||||||||||
| Petro- Diesel |
NaOH | Sonication vessel | Soybean oil | – | 265 v.% (30 wt%) |
1.0 | 3:1 | 30 | 50 | -f | 20 KHz 750 W 2 s On 2 s Off |
92 | [50] |
| THF | NaCl | Electrolysis cell | Corn oil | – | 50 v.% (25 mol%c) |
0.56 | 24:1 | 120 | N/SL | -f | 18.6 V 2 wt% water |
97 | [51] |
| KOH | STR | Mahua oil | 4 | 125 v.% | 1 | 6:1 | 180 | 45 | N/S | – | 99g | [52] | |
| STR | Rapeseed oil | – | 202 v.% (100 mol%c) |
0.7 | 9:1 | 120 | 30 | 700 | 98.3 | [15] | |||
| [41] | |||||||||||||
| Oscillating bottle |
Thespesia populnea biomass |
- | 25 v.% | 1.5 | 5.5:1h | 120 | 60 | 500 | 97.8 | [53] | |||
| NaOH | STR | Jatropha curcas L. oil | 1 | 100 v.% | 0.5 | 4:1 | 10 | 40 | 200 | 98.04 | [54] | ||
| KM Micromixer | WCO | 5 | 30 v.% | 1 | 12:1 | – | 70 | -f | 60 ml/h | 97 | [55] | ||
h regarding the oil volume.
1: esterification.
2: degumming, esterification and drying.
3: neutralisation, purification and heating.
4: ethyl acetate and saturated sodium bicarbonate solution were shaken with the oil followed by settling period.
5: filtration and esterification.
regarding the oil weight.
regarding the MeOH volume.
regarding mole MeOH.
%FAME yield.
estimated from diagram.
no agitation.
%conversion.
MeOH volume to biomass weight ratio.
Table 5.
Use of co-solvents in heterogeneous acidic catalysed methanolysis.
| Co- solvent |
Catalyst | Reactor type |
Feedstock | Pre- treatment |
Reaction conditions |
Yield (wt.%c) |
Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Co-solvent amount (wt.%a, v.%b) |
Catalyst amount (wt.%a) | MeOH/oil molar ratio | Time (min) |
T (°C) |
Stirrer (rpm) |
Special conditions |
|||||||
| Biodiesel | Sulphated tin oxide supported with silica |
STR | WCO | – | 50 v.% | 6 | 15:1 | 90 | 150 | 350 | 10 bar | 88.2 | [94] |
| THF | Cellulose (carbonated and sulfonated) |
STR + ultrasonic horn |
PFAD | 1 | 0.3 v.% (0.2 wt%) |
3 | 6:1 | 20 | 60 | 1200 | 20 kHz 120 W 8 s ON/ 2 s OFF |
86 | [82] |
| Toluene | WO3–ZrO2 | Vessel | PFAD | 2 | 10 v.% | 0.5 | 5:1 | 120 | 80 | -d | – | 90.3 | [95] |
1: hydrolysis.
2: melted previously at 60°C.
regarding the oil weight.
regarding the MeOH volume.
%FAME yield.
No agitation.
3. Co-solvents in homogeneously alkaline catalysed methanolysis
The co-solvents used in homogeneously alkaline catalysed methanolysis are shown in Table 2. Influence of acetone, 2-propanol, ethers, n-hexane and bio-/petroleum diesel are discussed.
3.1. Influence of acetone and 2-propanol
Maeda et al. [21] applied different co-solvents in the methanolysis of catfish oil and Jatropha curcas L. oil. Acetone, 2-propanol, tetrahydrofuran (THF), diethyl ether (DEE) and acetonitrile positively affected the reaction system. The co-solvents tested effectively transferred the two-phase system into a single phase. Accordingly, the reaction rate increased in the first 3 min of reaction and yields near the equilibrium were reached after only 10 min in the different co-solvent systems. By comparison, in the co-solvent free system, the yield increased even after 60 min and almost reached the yield of the methanolysis with acetonitrile at this point, which had the lowest maximum yield of the co-solvent systems. The highest yields occurred under the use of 2-propanol and acetone (>97 wt%).
Moreover, the use of 2-propanol had the feature that only a MeOH/oil molar ratio of 3:1 was sufficient for a similar yield in the same reaction time as with acetone. An excess of MeOH (typically 6:1) for a complete conversion of oil in a short reaction time is no longer needed with 2-propanol. This may reduce chemical costs, thermal energy for evaporation and product purification time, and thus partially offset the increased costs by the additional co-solvent. An high initial reaction rate, a higher maximum yield and consequently a reduced reaction time were checked by Thanh et al. [39] with the additional information that a minimum of 123 v.% (20 wt% regarding the oil weight) of acetone was required for a homogenous phase formation. The results presented by Luu et al. [40] underline the increased yield. A difference between the application of acetone and 2-propanol was that acetone is not miscible with glycerol, whereby this by-product is separated, whereas with 2-propanol it remains in the single phase reaction mixture. Glycerol as a separate phase involves that the catalyst and the polar MeOH are dissolved in it and therefore they are not present for the methanolysis reaction [21,39]; and consequently, the reaction rate decreases. On the other hand, the glycerol separation means a product withdrawal and thus supports an increased product formation. These two effects are counteracting. However, because the methanolysis with 2-propanol needs less MeOH compared with the acetone system, its withdrawal seems to affect the conversion predominantly. Nonetheless, even with acetone, a MeOH/oil molar ratio of 4.5:1 is lower than the typically-applied MeOH amount in co-solvent free systems. Because the reaction takes place on a molecular level in the homogenous phase [39], the excess of MeOH can be reduced to reach an adequate contact frequency between the reactants. Besides, with an excess of co-solvents miscible with glycerol like 2-propanol, the solubility of glycerol in biodiesel will increase. In this case, glycerol acts as an impurity and is suspected to favour the backwards reaction of FAME [15].
Acetone showed a further advantage in terms of how the separation time for glycerol and biodiesel is decreased from usually 24 h down to 30 min. The co-solvent accumulates in the biodiesel phase due to the immiscibility with glycerol. According to the EN 14214 standar, biodiesel has a density of 860–900 kg/m3, whereas the density of acetone is only around 785 kg/m3 (Table 1). Together with the lower viscosity of 0.365 mPa s (3.5–5 mPa s for biodiesel), both characteristics support the separation process [21,42]. Thanh et al. [39] added that with an increase of the acetone amount from 123 v.% up to 185 v.% (20 wt% to 30 wt%), the separation time increased from 37 min to 50 min. The stated reason was that with less acetone, the MeOH and glycerol concentration is higher and therefore the collision frequency increases, which supports a rejection of glycerol in a separate phase. One final advantage of acetone refers to the acceptable water content. Maeda et al. [21] determined that 5 wt% of water did not negatively interfere with the methanolysis, whereas without the co-solvent the FAME yield dropped to 15 wt% after 60 min. Perhaps acetone diluted the water [19], which decreased the contact of the catalyst with it and therefore the formation of soap.
3.2. Influence of different ethers
A variety of ether compounds like THF, DEE, methyl tert-butyl ether (MTBE), dimethyl ether (DME), isopropyl ether and petroleum ether have been tested in different methanolysis processes. Although publications often refer to THF as a broadly- and successfully-used co-solvent concerning the early works of Boocock et al. [16,17], which introduced THF for phase homogenisation, MTBE and DEE also count among the most fruitfully-applied co-solvents in homogeneously and heterogeneously catalysed methanolysis (Tables 3–5). Consistent with the chemical ether structures and the corresponding log P values listed in Table 1, it is evident that the hydrophobicity and therefore the solubility in the oil phase increase together with the degree of methyl group branching from DME over DEE and MTBE to isopropyl ether. On the other hand, researchs have supported the assumption of a correlation between the degree of molecular branching and the solubility in oil [56,57]. By contrast, THF combines a comparably high ability to interact with polar MeOH (ϵ = 7.52) with a log P value of 0.46, which is in between DME and DEE in a spatially compact molecule form (Table 1).
Table 3.
Use of co-solvents in homogeneous acidic catalysed methanolysis.
| Co- solvent |
Catalyst | Reactor type | Feedstock | Pre- treatment |
Reaction conditions |
Yield (wt.%e) |
Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Co-solvent amount (wt.%a,b, v.%c, mol%d) |
Catalyst amount (wt.%a) | MeOH/oil molar ratio | Time (min) |
T (°C) |
Stirrer (rpm) |
Special conditions |
|||||||
| DME | PTSA | Oscillated vial | Corn oil | – | 176 v.% | 4 | 10:01 | 120 | 80 | shaking 2.6 Hz |
500 kPa | 97.1 | [64] |
| MTBE | H2SO4 | STR | PFAD | – | 39 v.% (25 wt%a) |
10 | 7:1 | 70 | 55 | 300 | – | 96.80 | [65] |
| n-hexane | H2SO4 | STR | BSFL powder | 1 | 50 v.% | 1.2 ml | 12 ml MeOH | 90 | 120 | N/S | – | 94.14 | [66] |
|
Jatropha curcas L. seeds |
2 | 10 v.% | 15 | 7.5 ml/g | 24 h | 60 | 99.80 | [67] | |||||
| THF | AlCl3 | STR | Canola oil | – | 90 v.% (100 wt%b) |
5 | 24:1 | 18 h | 110 | N/S | – | 98f | [68] |
| H2SO4 | Vial |
Chlorella vulgaris microalgae lipid |
50 v.% (25 mol%) |
21 | 60:1 | 180 | 60 | -g | 95 | [69] | |||
| Oscillated flask | Coconut meat | 2 | 40 v.% | 25% (v/wt.) | 60:1 | N/S | 60 | shaking | 96.7 | [70] | |||
Reduction in particle size.
1. (<0.25 mm).
2. (<0.355 mm).
regarding the oil weight.
regarding methanol weight.
regarding the MeOH volume.
regarding mole MeOH.
%FAME yield.
% oil conversion.
no agitation.
DEE has been applied successfully, among others with different determined positive influences on the respective methanolysis systems [15,32,41,42]. In a direct comparison with an earlier work, Wu et al. [42] were able to demonstrate a reduction in activation energy through the use of DEE from 31.03 kJ/mol to 23.73 kJ/mol.
Besides, the role of agitation in the methanolysis changes with the use of co-solvents. Encinar et al. [41] stated in an earlier work that agitation no longer has a significant influence in the homogeneous phase generated by DEE in the range of 500–1100 rpm. In a later work, this outcome was underlined with exact yield values, which vary between 95.1 and 97.6 wt% in the same stirring speed range. Mohammed-Dabo et al. [54] verified that agitation had no significant influence with THF and concluded in another work that with cyclohexane only 100 rpm was sufficient, compared with 600 rpm without a co-solvent [58]. Wu et al. [42] estimated the influence of agitation over 500 rpm as only slightly significant. A co-solvent can homogenise the oil and MeOH phase within the first seconds or minutes of the reaction, whereby increasing the phase solubility by agitation in this initial phase becomes neglectable [21,39]. The mission of stirring is to disperse the methanol and oil phase, thus increasing the phase boundary. Since co-solvents increase the solubility at the molecular level, this effect seems to be more critical than the effect of stirring. However, stirring may initially disperse the co-solvent in both phases, which increases the effect on phase solubility by the co-solvent.
Guan et al. [32] determined a reduction in the biodiesel viscosity with DME, DEE, MTBE and THF in this order, which corresponds with the order of the respective co-solvent viscosities. For DEE, high yields were achieved in methanolysis systems with temperatures of 34 °C or lower (Table 2), which is because the boiling point of DEE is only 34.5 °C. Besides the low temperature, Wu et al. [42] could determine – in comparison with their previous work without DEE – that the optimal MeOH/oil molar ratio could be diminished from 7.41:1 down to 5.65:1. The maximal yield of the co-solvent free system was achieved with DME about 10 min earlier. As a gaseous co-solvent, DME offered the ability to be separated from the reaction mixture by depressurisation, which is a more appropriate process than thermal separation process. An excessive application of DME resulted in a dilution effect of reactants [32]. Most of the literature attributes a decrease in yield, above a certain co-solvent quantity, to a dilution effect on the reactants.
Many authors have found yield increases and elevated reaction rates in the initial phase with the use of THF [15,32,41,51,53,55]. The yield increases were around 30 wt% [51,53,55] (Table 2). Bhargavi et al. [53] applied THF in an in-situ methanolysis of Thespesia populnea seeds and could determine that the extraction rate was the highest with THF over that of chloroform, toluene and benzene compared with a conventional n-hexane extraction. With a log P value of 0.46 compared with −0.74 of MeOH, THF is more able to solve and therefore extract oil out of the seeds. Moreover, with the moderate ability to interact with polar substances, it is able to extract both polar and neutral lipids. The time needed for a phase homogenisation with 50 v.% THF was 30 min. Kumar et al. [52] established a comprehensive kinetic study with the use of THF (125 v.%) in the methanolysis of Mahua oil and Jatropha curcas L. oil; for both oils, the increase in the reaction rate was higher at a low temperature of 28 °C compared with 45 °C. The reaction rate itself with Jatropha curcas L. oil was significantly higher even without THF, so that the improvement with this co-solvent was smaller. Consequently, a 99 wt% yield was achieved after 30 min at 45 °C, with the conclusion that no THF was needed, while with Mahua oil 99 wt% could only be achieved after 180 min at 45 °C and with THF. In this system, the triglyceride conversion increase about 300% at room temperature in the first minute. After 30 min, the increase was only 5%, which highlights the idea that co-solvents have only a minor effect after phase homogenisation.
Encinar et al. [15,41] and Guan et al. [32] also tested MTBE. The resulting yields were very similar to the FAME contents achieved with THF and DEE in each case (Table 2). Hernando et al. [49] could show that microwave as a heating source for batch and continous systems has a significant influence on the reaction rate.
3.3. Influence of n-hexane
It is evident, from the polarity parameters and the chemical structure listed in Table 1, that n-hexane is a hydrophobic substance, with a high orientation to the oil phase and a limited ability to form hydrogen bonds over the weak dipole resulting from the low electronegativity difference of the C–H bonds. Latter ones are orientated very symmetrically in the molecule, which also diminishes the dipole moment by mutual cancellation. This makes it well suited to act as a solvent for non-polar substances but makes it inappropriate to interact with polar molecules like MeOH. Based on this theoretical view, it seems unsuitable to increase the overall solubility between methanol and oil with n-hexane as a co-solvent. However, authors have reported a yield increase by using n-hexane as the co-solvent in the range of 7 wt% up to 35 wt% [[43], [44], [45], [46], [47], [48],59].
The authors reported that a phase homogenisation occurred. Todorovic et al. [60] examined the exact molar ratios of different co-solvents for a phase homogenisation with sunflower oil at 60 °C per volumetric titration of the respective co-solvents in an oil-MeOH mixture until the point at which the system changed from turbid to transparent. It was indeed possible to achieve a single phase with n-hexane. The necessary amount (190 v.%) was higher than for other substances like DEE (90 v.%) or THF (60 v.%). In most of the research works (Table 2), the amount of n-hexane applied was 45 v.% or lower with expectations of 214 v.% (64 wt%). However, the co-solvent amount researched by Todorovic et al. [60] seems to be the point where the system turns instantly miscible. Therefore, even smaller amounts can support the phase homogenisation. Besides, temperature and catalyst concentration – which influence the phase homogenisation– are similar in most works listed in Table 2 for n-hexane. The only explanatory approach given why n-hexane is suitable for increasing the miscibility of oil and MeOH was given by Escobar et al. [47]. The co-solvent molecules arrange between the oil molecules and thus reduce the cohesive forces between them, whereby small MeOH molecules can more frequently interact with the oil compounds with the development of a pseudo-homogeneous phase. Moreover, with the viscosity lower than MeOH and oil, it also improves the miscibility [44].
A further advantage of n-hexane is the reduced reaction time to achieve the highest yield of the co-solvent system compared with the respective maximal yield of the co-solvent free system [44,47]. Fadhil et al. [44] determined that the influence of n-hexane is high in combination with a low temperature (30 °C). Rahimi et al. [46] indicate that the temperature in higher ranges (45 °C) has a more decisive influence than that of the co-solvent. These results validate the kinetic study presented for THF [41]. Escobar et al. [47] validated the notion that the separation time of glycerol and biodiesel is diminished due to the immiscibility of n-hexane and glycerol. In addition, in the methanolysis with n-hexane were significantly less soap existent, which promotes the separation of glycerol and biodiesel [43].
3.4. Influence of bio-/petroleum diesel
The idea to use the product biodiesel itself as a co-solvent is since a separation process is not needed after the reaction, in the step of product purification. The FAAE molecules comprise a small polar region made up from the ester connection at one end of the molecule and a long non-polar carbon chain to the opposite direction. The length and degree of unsaturation influence the polarity of the non-polar region and the ability to interact with the oil components via Van der Waals forces by the resulting curvature. With this amphiphilic structure, FAME molecules can interact with the MeOH and the oil in the initial phase of the methanolysis reaction [61]. Moreover, unreacted mono- and diglycerides in the biodiesel can also effectively act as emulsifiers with the hydroxyl group(s) and fatty acid chain(s) [62]. Within the natural spatial orientation of a di- or monoglyceride, the hydroxyl group(s) and partly the double-bonded oxygen atom of the ester connection(s) are orientated to the same side, while the fatty acid(s) are orientated contrary [63].
Park et al. [14] – who applied biodiesel – and Parida et al. [50] – who used petroleum diesel – verified a yield increase and an elevated reaction rate in the initial phase. In the case of Parida et al. [50], the co-solvent free system only reached an equilibrium yield that was around 47 wt% lower than that with 265 v.% (30 wt%) co-solvent. Both systems reached the respective maximal yield after 30 min [50]. The co-solvent free methanolysis system of Park et al. [14] was able to develop a similar yield to that of the conversion with biodiesel [20 v.% (5 wt%)], although 45 min later (at 60 min of reaction time). However, the methanolysis with biodiesel was pursued until 60 min due to a small ongoing yield increase. A distinctive lag phase (about 35 min) in which the yield only grew by a few percent, was observable due to the phase mixing only by application of heat and slow stirring. With the biodiesel addition, the lag phase was no longer existent; rather, the reaction started immediately. After the first 5 min, the reaction rate strongly increased. Park et al. [14] deliberately applied a slow agitation in the co-solvent free system to show this long lag phase. With biodiesel, the time to reach a complete phase homogenisation was diminished from 30 to 20 min and the reaction rate slowed strongly down after 20 min, where the co-solvent no longer had any effect [14]. The authors stated that the homogenisation time further depends on the catalyst concentration and the temperature. This emphasizes the argument that the agitation is not a significant influence factor on the use of a co-solvent. Besides, Park et al. [14] determined that over a catalyst concentration of 0.55 wt%, the influence of the co-solvent was inferior to the impact of the catalyst to form a single phase.
Parida et al. [50] proved that with a MeOH/oil molar ratio of 6:1 and 9:1, the impact of petroleum diesel on the methanolysis was almost non-existent, whereas with a ratio of 3:1 the maximal effect of 46 wt% yield increase was observed. It was concluded that with the higher MeOH amounts, the ultrasound applied was sufficient to achieve a yield higher than 90 wt% in the first few minutes of reaction. These results support the assumption that a reduction in MeOH can be achieved when co-solvent is used. The 265 v.% of petroleum diesel applied [50] seems uneconomical if it is possible to achieve related results with a small amount of biodiesel, apart from the fact that petroleum diesel does not fit with the idea of eco-friendliness or renewability.
4. Co-solvents in homogeneously acidic catalysed methanolysis
Few publications were found on the use of co-solvents in a homogeneously acidic catalysed methanolysis. Table 3 provides a concise overview of the results. In all of them, the application of co-solvents led to an improvement of the individual methanolysis process.
Guan et al. [64] investigated the effect of the co-solvent DME in the methanolysis of corn oil (0.45 mg KOH/g) with p-toluenesulfonic acid (PTSA) as catalyst. In order to imitate a high FFA (free fatty acid) content, oleic acid was added in a range of 2–15 v.% (regarding to the oil volume). Moreover, the amount of water was changed from 0.5 to 5 wt% (regarding the oil weight). The maximum yield was 97.1 wt% with the reaction parameters stated in Table 3. Without the use of DME, the yield reached only 66.3 wt%. The observation of the reaction system revealed that the co-solvent amount was sufficient to form a single phase. The authors stated that the transfer to a homogeneous phase was the main reason for the accelerated reaction rate. With the addition of water, the yield reduced. This effect was attributed to the water, which destabilised the single-phase system in the presence of DME. The successful application of DME as a co-solvent requires a water content less than 0.5 wt%. Water promotes the hydrolysis of glycerides and FAME, resulting in a yield reduction. In contrast to acetone, DME does not appear to be polar enough to dilute the water to such an extent that hydrolysis is reduced. Besides the water, an increase in the FFA content also led to a diminished yield. The authors specified that esterification occurs homogeneously even in a two-phase system. It seems to be logical that FFA are more soluble in MeOH than triglycerides due to their amphiphilic nature with a polar carboxylic group and a non-polar carbon chain together with the smaller molecular weight.
Soriano et al. [68] used AlCl3 as acid catalyst and THF as the co-solvent in the methanolysis of canola oil. The authors reported that a volume ratio of 1:1 of THF to MeOH is sufficient to form a single phase in case of MeOH/oil molar ratios of 24:1 and higher. Below a molar ratio of 6:1, the system occurred heterogeneously, even in the presence of the co-solvent. In the two-phase system, the catalyst tends to augment in the polar MeOH phase together with THF. Therefore, the catalyst cannot activate the carbonyl carbon of the triglycerides molecules, which hinders the reaction rate, besides the fact that the reaction can only occur at the phase boundary. The degree of oil conversion without THF under the same reaction conditions was only half of the maximum conversion (98%).
Nguyen et al. [66], Shuit et al. [67] and Khang et al. [70] performed an in-situ methanolysis with H2SO4 as the acidic catalyst. Shuit et al. [67] used 10 v.% n-hexane and achieved a yield of 99.8 wt% after 24 h at 60 °C while processing Jatropha curcas L. seeds. An oil extraction of more than 90% was possible with the combination of methanol and n-hexane. With its high lipophilicity (log P = 4), the non-polar n-hexane is a suitable extraction reagent for oil. Thus, it supports the oil extraction process and simultaneously accelerates the transesterification rate by increasing the miscibility with the surrounding MeOH. Ngyuen et al. [66] – who also used n-hexane as the co-solvent (50 v.%) – processed the powder of black soldier fly larvae (BSFL) in the methanolysis and obtained a 94.14 wt% yield in only 90 min (Table 3). Compared with Shuit et al. [67], the reaction temperature was 120 °C, twice as high. Acetone, chloroform and petroleum ether were also tested, and the corresponding yields were 54.83 wt%, 48.50 wt% and 35,67 wt%, respectively. It was emphasised that n-hexane is eco-friendly and cheap. Khang et al. [70] used ground coconut meat as the feedstock and THF as the co-solvent (40 v.%). The reaction rate in the first 5 h was substantially increased by increasing the THF to MeOH volume ratio.
Lam and Lee [69] evaluated the influence of THF on the methanolysis of crude microalgae lipid with H2SO4 as catalyst. The lipid had a high viscosity with 243.4 mPa s (40 °C) and an acid value of 63.9 mg KOH/g. Besides the mass transfer resistance between alcohol and triglycerides, the viscosity also hinders the miscibility. In order to attain a 95 wt% yield in a two-phase system, a MeOH/oil molar ratio of 180:1 was needed to reduce the viscosity, increase the overall miscibility and promote the product formation. Besides, 35 wt% catalyst in combination with a reaction time of 6 h were the other required reaction conditions to fulfil the production with a temperature of 60 °C. The addition of 50 v.% THF significantly improved this costly and energy-intensive methanolysis. It reduced the reaction time by half and the MeOH/oil molar ratio by a factor of three. Beyond that, 21 wt% catalyst was sufficient in the co-solvent system.
Other co-solvents like toluene and n-hexane worked less suitably than THF regarding the yield; toluene performed better than n-hexane in the work of Lam and Lee [69]. Both are suitable to extract oil but are by contrast less suitable to interact with MeOH compared with THF (Table 1). On the other hand, methyl acetate, ethyl acetate, chloroform and ethanol had no significant effect. It should be noted that chloroform has a higher ϵ than toluene (4.8 vs 2.378), while they show similar log P values (1.97 vs 2.73) [19]. Therefore, chloroform should favour a phase homogenisation more effectively with a resulting yield increase. As previously reported, in the case of Nguyen et al. [66], chloroform again could not support the methanolysis system in a similar effective way than n-hexane and functioned even inferior to the polar acetone in terms of increasing the biodiesel yield. Additionally, methyl acetate and ethyl acetate have similar dielectric constants to THF (7.07, 6.08 and 7.52, respectively) with log P values surrounding that of THF (0.18, 0.73 and 0.46, respectively) [19]. From this perspective, these co-solvents should show a higher impact as determined. The co-solvent comparison was made after 60 min, so perhaps a sufficient homogenisation did not occur by then. Finally, ethanol can react with the glycerides by itself, so only a negligible amount of FAME can occur.
5. Co-solvents in heterogeneously alkaline catalysed methanolysis
The co-solvents used in heterogeneously alkaline catalysed methanolysis are shown in Table 4. Impact of acetone, propanol, n-hexane, THF and biodiesel are discussed.
Table 4.
Use of co-solvents in heterogeneous alkaline catalysed methanolysis.
| Co-solvent | Catalyst | Reactor type | Feedstock | Pre- treatment |
Reaction conditions |
Yield (wt.%d) |
Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Co-solvent amount (wt.%a, v.%b, mol%c) |
Catalyst amount (wt.%a) | MeOH/oil molar ratio | Time (min) |
T (°C) |
Stirrer (rpm) |
Special conditions |
|||||||
| Acetone | CaO (River snail shells) |
STR | Palm oil | – | 10 v.% | 5 | 12:1 | 120 | 65 | 300 | – | 95.28 | [72] |
| CaO (scallop shells) |
Flask | Beef tallow/ soybean oil (1:1) |
36 v.% | 150 | -e | 85.3 | [11] | ||||||
| Ca2Al2O5 | STR | WCO | 1 | 92 v.% (20 wt%) |
1.2 | 6:1 | 25 | 55 | N/S | 97.98f | [73] | ||
| Waste cement (calcined with K2CO3; 1:4) |
Electrolysis cell | WCO | – | 40 v.% (10 wt%) |
12 | 7:1 | 120 | 25 | 300 | 30 V 2 wt% water |
95.5 | [74] | |
| Zeolite/chitosan/ KOH composite |
Electrolysis cell | WCO | 40 v.% (10 wt%) |
1 | 180 | RT | 100 | 40 V 2 wt% water |
93 | [75] | |||
| Crude biodiesel |
CaO | STR | Sunflower oil | – | 34 vol% (10 wt%) |
41.5 g/Ltotal volume |
7.1:1 | 90 | 52 | N/S | – | 98.9 | [77] |
| n-hexane | CaO | STR | Sunflower oil | – | 110 v.% (20 wt%) |
5.82 | 6:1 | 240 | 60 | N/S | – | 98.8 | [60] |
| CaO | Microreactor | WCO | 3 | 58 v.% | 8.17 | 51.3 v.%g (1:1.95) |
10 | 62 | -e | 97.03 | [78] | ||
| Na/NaOH/ γ-Al2O3 |
STR | Soybean oil | – | 55 v.% | 2.17 | 9:1 | 120 | 60 | 300 | 94 | [79] | ||
| 2-propanol | CaO | Packed- microchannel |
Palm oil | – | 20 v.% (14.5 wt%) |
30 mg | 20:1 | 6.5 | 65 | -e | – | 98.3 | [71] |
| 1-propanol | CaO (River snail shells) |
STR | Palm oil | – | 10 v.% | 5 | 12:1 | 120 | 65 | 300 | – | 95.69 | [72] |
| 90 | 94.44 | ||||||||||||
| THF | CaO (waste crab shells) |
STR | Fish oil | 3 | 100 v.% | 2.5 | 10:1 | 90 | 65 | 650 | – | 95 | [80] |
| CaO (River snail shells) |
STR | Palm oil | – | 10 v.% | 5 | 12:1 | 120 | 65 | 300 | 96.68 | [72] | ||
| CaO | STR | Rapeseed oil | 62 v.% (30 wt%) |
2.5 | 12:1 | 360 | 60 | 600 | 97 | [81] | |||
| ZnO–Sr(NO3)2 | STR | Soybean oil | 91 v.% (44.5 wt%) |
5 | 12:1 | 240 | 65 | N/S | 96.8f | [82] | |||
| CaO | STR | Sunflower oil | 82 v.% (20 wt%) |
5.82 | 6:1 | 240 | 60 | N/S | 98.1 | [60] | |||
| Limescale | Microreactor | WCO | 3 | 62 v.% | 8.13 | 50.8 v.%g (1:1.97) |
10 | 64.5 | -e | 95.21 | [78] | ||
| Zeolite/chitosan/ KOH composite |
Electrolysis cell | WCO | – | 35 v.% (10 wt%) |
1 | 7:1 | 180 | RT | 100 | 40 V 2 wt% water |
93 | [75] | |
1: filtrated and dried.
2: filtration, drying and esterification.
3: esterification.
regarding the oil weight.
regarding the MeOH volume.
regarding mole MeOH.
%FAME yield.
no agitation.
% oil conversion.
regarding the oil volume.
5.1. Impact of acetone
Besides THF, acetone is a broadly-used co-solvent in heterogeneously alkaline catalysed methanolysis (Table 4). In most of these works, acetone led to an improvement in the biodiesel yield, even if other tested co-solvents partly achieved greater yields [11,[72], [73], [74], [75],83]. By contrast, a minority of works presented negative results by using acetone [80,81].
Soares Dias et al. [11] analyzed the catalyst surface after the co-solvent supported methanolysis of soybean oil mixed with beef tallow. Several positive effects of acetone were highlighted. For instance, acetone prevented the formation of calcium diglyceroxide, which can inactivate the catalyst due to the leaching of calcium species and therefor contaminate the glycerol phase [84]. Due to the immiscibility in glycerol, acetone can replace it from the catalyst surface directly after the formation; consequently, the co-solvent prevented a further reaction to an inhibiting substance. Furthermore, due to the accelerated oil conversion, the contact time of the reactants with the catalyst is diminished; as a result, the oil is unable to be adsorbed on the catalyst surface. The authors also related the increased yield to the effect of the immiscibility of acetone with glycerol. Moreover, Singh et al. [73] could observe that the density difference between biodiesel and glycerol at the end of the process was increased, thus the subsequent separation was improved. Besides the positive impact of acetone on the reaction system and the reactants, it is a considerably cheap co-solvent (Table 1) and readily available [75].
Other researchers doubt the suitability of acetone as a co-solvent or were unable to verify the reported positive influences. One argument concerns the polarity of acetone, since it is one of the most polar co-solvents (Table 1). Accordingly, it is more suitable soluble in MeOH than in oil, which reduces the function as an oil carrier to the MeOH phase [72]. The log P value of −0.24 supports the description as a hydrophilic substance with the tendency to accumulate in the polar phase. Galli et al. [81] and Madhu et al. [80] determined a yield decrease with the application of acetone. It was speculated that the methoxide ions performed a nucleophilic attack on the carbonyl group of acetone [81]. This side reaction means an elimination of the co-solvent and methoxide as a catalyst.
5.2. Impact of propanol
Chueluecha et al. [71], as well as Roschat et al. [72], tested 2-propanol as a co-solvent, among others. In comparison with THF and ethyl acetate, 2-propanol achieved a similarly high yield as the other two, but in a shorter residence time and/or a smaller quantity [71]. Without 2-propanol as co-solvent, the same yield was reached within a longer residence time (2.4 min) [71]. Chueluecha et al. [71] determined a reduction in the MeOH amount (from 24:1 M ratio to 20:1), a slightly increased yield (from 98.1 wt% to 98.3 wt%) and an increased productivity when 2-propanol was applied. Moreover, an energy analysis revealed a reduced total amount of energy (155.68 MJ–142.63 MJ), which also means an elevated energy efficiency. A cost analysis highlighted that the total costs increased due to the additional costs for the co-solvent. Compared with the homogeneous alkaline catalysed methanolysis, in this particular case a MeOH/oil molar ratio of 20:1 is far from the 3:1 M ratio determined by Maeda et al. [21]. It is possible that because the catalyst forms a separate phase and the reaction can therefore only take place on the surface, considerably more MeOH is still required.
It was related that the higher diffusion coefficient of 2-propanol in contrast to THF and ethyl acetate supported a faster homogenisation of the reaction mixture. The chemical structure of 2-propanol is very similar to acetone (Table 1). The polarity is slightly lower estimated from the ϵ, although the log P value indicates that it is more suitable to accumulate in the non-polar phase. In conclusion, 2-propanol should be a more effective co-solvent than acetone. The work of Roschat et al. [72] supports this idea, because besides THF as the most effective co-solvent, 2-propanol covered the third place behind 1-propanol but after acetone. While the authors verified the inertness of 2-propanol, they did not check the same for 1-propanol. As a non-branched acyl acceptor, 1-propanol could react in the transesterification to the respective propyl ester form. It is stated that a branched methyl structure provides a steric hindrance for the oil conversion [57]. However, the higher FAME yield in the presence of 1-propanol showed that MeOH was still a preferred reactant as the formation of propyl esters would otherwise diminish the FAME yield. Besides, with an increased branched co-solvent structure, the log P value rises and therefore the miscibility in oil increases (Table 1). Consequently, 1-propanol should perform less effective than 2-propanol. Nevertheless, an insufficient number of publications are available to evaluate the performance of 1-propanol accurately. No negative examples for the use of propanol species were found in the literature.
5.3. Impact of n-hexane
In the field of heterogeneously alkaline catalysed methanolysis, non-homogeneous results are presented for n-hexane. A slight majority of authors determined positive influences [60,[78], [79], [80],85,86], while others reported negative impacts [11,82,83,87]. First of all, n-hexane is a suitable co-solvent for an in-situ transesterification as it plays a double role with its suitable extraction properties with a simultaneous phase homogenisation [85]. Unlike n-hexane, MeOH as a very polar substance is a poor solvent for oil. However, the functionality of n-hexane as a co-solvent is controversially discussed. Listed arguments include the unsuitable polarity described in 3.3 section [87]. Sahani et al. [83] claims that because oil would dissolve in n-hexane, the backward reaction increased to balance the withdrawal of educt. A disadvantage of n-hexane in combination with CaO is that it can lead to amorphisation of the catalyst and thus reduce the catalytic activity [11].
Regarding the yield, Kim et al. [79] reported a yield increase of10 wt%, increase which is higher than the increases reported by the other researchers which was mostly marginal [60,80,86]. Besides, Aghel et al. [78] concluded that n-hexane led to a reduction in the MeOH amount.
5.4. Impact of THF
Like in homogeneously alkaline catalysed methanolysis, THF is a broadly-applied co-solvent in the heterogeneously alkaline catalysed conversion. The majority of researchers described positive impacts on the methanolysis [11,60,71,72,75,78,[80], [81], [82],86]; nonetheless, negative impacts have also been determined [79,[87], [88], [89], [90]]. Galli et al. [81] – who achieved around 90 wt% yield even without co-solvents under the applied reaction conditions (Table 4) – attained a slight yield increase with THF and other co-solvents. Besides, the reaction rate was elevated up to 30%. The main effect observed was the ability of THF to transfer the oil phase and MeOH effectively to a single phase and thus raise the reaction rate, along with increased yield [60,75,78,82,86,91]. Roschat et al. [72] and Madhu et al. [80] tested a variety of co-solvents, and in both cases THF reached the highest yields. In case of Roschat et al. [72], THF was superior over very polar co-solvents like acetone as well as propanol variants. THF significantly improved the methanolysis of palm oil with CaO as the catalyst. The time to reach the maximal yield was reduced from 180 min down to 90 min, and there was no lag phase detected. The reaction constant was more than twice as high compared with the co-solvent free system (4.99·10−2 min−1 instead of 1.84·10−2 min−1). Moreover, the activation energy was reduced from 67,6 kJ/mol to 57.79 kJ/mol. THF was – as well as acetone – able to prevent the fat adsorption on the catalytic surface by increasing the reaction rate [11]. Besides, Aghel et al. [78] noticed that THF reduced the amount of MeOH.
Some authors have used both THF and acetone, among others with different results, THF or acetone being better. In the case of acetone, it was attributed to the higher miscibility to MeOH due to its higher polarity [11]. In case THF led to a further increase in yield, the explanation given was exactly the opposite, namely that the polar co-solvents have been solved in the MeOH phase, so contact with the oil phase is not sufficient [72]. From the physicochemical perspective, THF seems more suitable because it tends to enter into contact with the oil phase (log P value 0.46) more easily and it also has the ability to act as a hydrogen bond acceptor with the free electron pairs of the oxygen atom.
Zieba et al. [88] thought that the tested co-solvents supported the addition of glycerol or glycerides to the catalyst surface. However, because THF is not miscible in glycerol, it should favour the rejection from the catalyst surface instead of promoting the addition [88].
5.5. Impact of biodiesel
Todorovic et al. [77] includes the use of biodiesel as a co-solvent (Table 4). The results showed a clear lag phase (were no reaction occurred) in the co-solvent free system. However, with the use of biodiesel, the oil conversion starts directly with an increased reaction rate and the maximum yield was attained after 120 min, whereas without the co-solvent and a higher temperature (60 °C) the same yield was achieved after 180 min. By optimising the reaction conditions, the time could further be reduced to 90 min. Besides the increased reaction rate, another positive impact of biodiesel was a reduced separation time at the end of the methanolysis (from 24 h to 90 min). The added biodiesel increased the total FAME content, which resulted in a viscosity decrease and a higher density difference. Both supported a fast separation. However, subjoined biodiesel also brings unreacted di- and monoglycerides into the reaction mixture, which means more educts. Thus, the determined yield growth can perhaps be attributed to the conversion of these other substrates into FAME [92]. López Granados et al. [93] needed only 5 v.% (3 wt%) of biodiesel in comparison with 34 v.% (10 wt%) by Todorovic et al. [77]. With this low amount, the authors observed a paste formation in combination with activated CaO. As a positive effect, this paste protected the catalyst against poisoning with CO2 and H2O.
6. Co-solvents in heterogeneously acidic catalysed methanolysis
Heterogeneous acidic catalysts play only a minor role in methanolysis in comparison with heterogeneous alkaline catalysts due to the lower catalytic activity and often necessarily harsh reaction conditions. The number of works in this case is even further limited. Table 5 provides a concise overview of the results.
Lam and Lee [94] – who applied sulphated tin oxide supported on silica as a solid-acidic catalyst in the methanolysis of waste cooking oil (WCO) with the addition of biodiesel as a co-solvent – could achieve a yield increase of around 30 wt%. Besides, the optimal yield was already set after 90 min instead of 180 min without the co-solvent. The co-solvent become mixed with the catalyst before the transesterification for 10 h to gain the improved yield within a reduced reaction time, besides the formation of a single phase in the subsequent methanolysis. However, the necessary reaction conditions are comparatively high (Table 5).
Mongkolbovornkij et al. [95] and Gaikwad and Gogate [82] reported over the methanolysis of PFAD (palm fatty acid distillate), one with THF [82] and the other with toluene [85] as the co-solvent. Mongkolbovornkij et al. [95] determined a decrease in the amount of MeOH needed. While a MeOH/oil molar ratio of 5:1 was sufficient with a fixed amount of 10 v.% of toluene to reach a yield of 90.3 wt%, a ratio of 9:1 was necessary to achieve a yield of 87.7 wt% without a co-solvent. At ratios of 5:1 and below, the effect of toluene was most explicit. The yield increased around 10–15 wt% at fixed ratios with the use of toluene in this range. At 9:1, the difference was negligible under consideration of the error bars. This result seems to support the described effect that MeOH in excess not only shifts the reaction equilibrium to the product side but also elevate the contact probability with the oil and the catalyst. With benzene, the yields showed the same trend regarding the MeOH/oil molar ratios as described for toluene, but the respective yields were lower, whereas with n-hexane the determined yields were even lower as in the co-solvent free system.
Gaikwad and Gogate [76] applied an ultrasound extension to the reactor in addition to the use of THF. The feedstock was pre-treated with sulfuric acid (Table 5). Because THF is characterised by a higher polarity (ϵ = 7.52) than toluene (ϵ = 2.378), it is suitable to be more miscible in MeOH by forming hydrogen bonds [96]. The optimal reaction conditions (Table 5) are competitive compared with those of a homogeneously alkaline catalysed methanolysis (Table 2). However, with an additional device to apply the ultrasound in combination with THF as an additional chemical substance, it does not seem competitive given to the extra investment and production costs. Especially, with the extra esterification process prior to the actual use of the heterogeneous acid catalyst for transesterification which appears unnecessary as this type of catalyst can catalyse both reaction types.
By contrast, a negative influence of toluene was determined by Jiménez-Morales et al. [97] with mesoporous tantalum phosphate as catalyst in the methanolysis of sunflower oil. The yield under the conditions of 5 wt% catalyst, a 12:1 M ratio of MeOH to oil, 200 °C and 2 h was 88.9 wt%. With 10 v.% of toluene and 3 wt% catalyst, the yield remained under 60 wt%. The authors stated that the toluene diluted the reactants; however, the boiling point of toluene is around 110 °C (Table 1), so the co-solvent should evaporate under a reaction temperature of 200 °C.
An application of a co-solvent to a heterogeneously acidic catalysed methanolysis can support milder reaction conditions to at least similar levels as listed for heterogeneous alkaline catalysed reaction systems with the advantage of processing acidic low-value feedstocks or algae oils.
7. Conclusions and future perspectives
This review reveals that the co-solvents are mostly used in the field of homogeneously alkaline catalysed methanolysis. In this context, a phase homogenisation of oil and MeOH was mentioned explicitly for acetone, 2-propanol, n-hexane, DEE, biodiesel and petroleum diesel as co-solvents. It can be assumed that a single phase was formed sufficiently, even if not mentioned explicitly.
The methanolysis with co-solvents and homogeneous catalysts resulted in increased biodiesel yields and/or reduced time to achieve a similar or higher yield as the co-solvent free system. Co-solvents that are immiscible with glycerol led to a significantly diminished time reduction in the step of product separation. The impact of agitation – especially in the initial reaction phase – was not significant after the application of co-solvent. After the co-solvents established the single phase, they no longer had an impact on the reaction rate. The time needed to achieve a one-phase system was mostly not determined. If it was stated, mostly a period of seconds or a few minutes was sufficient but partly up to 30 min.
Similar positive influences were established in the field of acidic catalysed methanolysis, however the extent of publications is considerably small. For DEE, a water content above 0.5 wt% can dismantle the established single phase. Besides, n-hexane and THF improved the in-situ methanolysis by increasing the oil extraction with a subsequent phase homogenisation. THF and toluene were successfully-applied. The benefits include yield increases and a time reduction as well as a reduced MeOH amount.
In the field of heterogeneously alkaline catalysed methanolysis, acetone is a widely-researched co-solvent. Acetone prevented the formation of calcium diglyceroxide as well as oil adsorption on the catalyst surface. Besides, a reduced product separation time was stated. Nevertheless, due to the solubility in MeOH, acetone can not act sufficiently as an oil carrier. The research results regarding n-hexane were mixed with slightly more positive recorded impacts, including mostly little yield increases together with a suitability for in-situ processes. For THF, determined effects included yield increases, increased reaction rates, reduced reaction times regarding the yield, higher reaction rate constants, as well as a reduced activation energy. Moreover, THF prevented the fat adsorption on the catalyst surface. With biodiesel, increased yields within a shorter reaction time together with a faster product separation time were among the observed impacts.
Most of the publications have not determined and justified the effects of co-solvents in a systematic way. So far it seems impossible to establish which co-solvents are the most suitable for which methanolysis systems. Therefore, the necessary research to assess the suitability of co-solvents more precisely will be highlighted in the following.
Works focusing on investigating co-solvents should systematically break down the effects occurring with respect to each reaction parameter. It is essential to provide a direct comparison with a co-solvent free system to determine the effect of the co-solvent. Additional parameters include the time to achieve the single phase under the given optimal reaction conditions as well as the minimum co-solvent amount for a phase homogenisation.
The discussion of the results should be more detailed, especially with respect to possible interactions of the co-solvent used with the different oil compounds, the catalyst and the polar MeOH molecules. Considering the above points, it could be possible to systematically gain an understanding as to why specific co-solvents perform more or less effectively in different methanolysis systems.
This work has tried to emphasise and justify the differences or similarities of the co-solvent impacts between the different publications by looking at the chemical structure of the respective co-solvents, including the functional groups and the resulting physicochemical properties like the ϵ or the log P value. However, several other polarity parameters exist like the Hildebrand solubility or the three-dimensional Hansen solubility (especially the sub-parameter for hydrogen bonding), which are possibly more accurate for investigating the co-solvent interactions with the oil and MeOH phase. In this way, it seems crucial not to correlate the parameters individually with the yield but rather to consider them in connection with each other regarding the co-solvent ability to emulsify the reaction mixture. Comprehensive studies are generally missing that deal with a broad variety of different co-solvents.
There is also a lack of kinetic studies dealing with the effect of co-solvents. More studies should be undertaken focusing on the activation energy and the reaction rate constants. With the help of these studies, it would be possible to identify the oils and/or catalysts for which the use of co-solvents is particularly useful. The reaction rate constants are slow in the initial phase of the reaction if no co-solvent is applied. In addition, it would be possible to determine which co-solvents are especially effective at low temperatures and low methanol quantities.
Biodiesel itself appears to be a very promising cosolvent. It could provide yield increases in shorter reaction times and reduced product separation times. Nonetheless, more works are needed to verify biodiesel as an overall suitable co-solvent.
Besides biodiesel, co-solvents like THF and acetone seems to be the best choices for alkaline methanolysis systems due to successful broadly applications with different oils, catalysts and reaction conditions and the previously discussed benefits. Moreover, THF and n-hexane are essentially advisable for in-situ methanolysis.
Probably the most important point to evaluate the suitability of co-solvents is the resulting total production costs. It is essential that the costs for co-solvents do not increase the overall production costs since competitiveness with petroleum diesel production is one of the main objectives in the development of biodiesel. Suitable cost analysis should include all factors related to the use of the respective co-solvent. Thus, a possibly lower amount of methanol, a lower catalyst amount, lower energy costs, e.g. due to a lower reaction temperature as well as the price of the co-solvent and the increased amount of energy required to separate the co-solvent from the product phases, must be taken into account.
Nowadays, deep eutectic solvents (DESs), a sub-class of ionic liquids have gained wide popularity in various fields of biodiesel production because of process competence and environmental benevolence. DESs are inexpensive, non-toxic and environmentally friendly and their various industrial applications have received much attention in recent years. Mamtani et al. [98] and Najaf-Abadi et al. [99] studied the application of DESs as a catalyst, cosolvent and extracting solvent in biodiesel production and, the authors suggested that a fully DESs-based biodiesel production scheme is possible; however, deep eutectic solvents require more research and identify future possibilities.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
L.A. González was supported by FEDER funds INTERREG MAC 2014-2020 programme within the ACLIEMAC project (MAC2/3.5b/380).
L.A. González was supported by Fundación CajaCanarias and Fundación Bancaria "la Caixa" through the “Convocatoria Proyectos Investigación 737 2019”.
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare no competing interests.
References
- 1.Shereena K.M., Thangaraj T. Biodiesel: an alternative fuel produced from vegetable oils by transesterification. Electron J. Biol. 2009;5(3):67–74. ISSN 1860-3122. [Google Scholar]
- 2.Ataya F., Dubé M.A., Ternan M. Single-phase and two-phase base-catalyzed transesterification of canola oil to fatty acid methyl esters at ambient conditions. Eng. Chem. Res. 2006;45(15):5411–5417. doi: 10.1021/ie060152o. [DOI] [Google Scholar]
- 3.Tabatabaei M., et al. Reactor technologies for biodiesel production and processing: a review. Prog. Energy Combust. Sci. 2019;74:239–303. doi: 10.1016/j.pecs.2019.06.001. [DOI] [Google Scholar]
- 4.Abbaszaadeh A., Ghobadian B., Omidkhah M.R., Najafi G. Current biodiesel production technologies: a comparative review. Energy Convers. Manag. 2012;63:138–148. doi: 10.1016/j.enconman.2012.02.027. [DOI] [Google Scholar]
- 5.Moser B.R. Biodiesel production, properties, and feedstocks. Vitro Cell Dev. Biol. Plant. 2009;45(3):229–266. doi: 10.1007/s11627-009-9204-z. [DOI] [Google Scholar]
- 6.Lam M.K., Lee K.T., Mohamed A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: a review. Biotechnol. Adv. 2010;28(4):500–518. doi: 10.1016/j.biotechadv.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Lin Y.-C., Yang P.-M., Chen S.-C., Lin J.-F. Improving biodiesel yields from waste cooking oil using ionic liquids as catalysts with a microwave heating system. Fuel Process. Technol. 2013;115:57–62. doi: 10.1016/j.fuproc.2013.04.004. [DOI] [Google Scholar]
- 8.Leung D.Y., Wu X., Leung M. A review on biodiesel production using catalyzed transesterification. Appl. Energy. 2010;87(4):1083–1095. doi: 10.1016/j.apenergy.2009.10.006. [DOI] [Google Scholar]
- 9.Lourinho G., Brito P. Advanced biodiesel production technologies: novel developments. Rev. Environ. Sci. Biotechnol. 2015;14(2):287–316. doi: 10.1007/s11157-014-9359-x. [DOI] [Google Scholar]
- 10.Meher L., Vidyasagar D., Naik S. Technical aspects of biodiesel production by transesterification—a review. Renew. Sustain. Energy Rev. 2006;10(3):248–268. doi: 10.1016/j.rser.2004.09.002. [DOI] [Google Scholar]
- 11.Soares Dias A.P., Ramos M., Catarino M., Puna J., Gomes J. Waste Biomass Valor; 2019. Solvent Assisted Biodiesel Production by Co-processing Beef Tallow and Soybean Oil over Calcium Catalysts. [DOI] [Google Scholar]
- 12.Banković-Ilić I.B., Stamenković O.S., Veljković V.B. Biodiesel production from non-edible plant oils. Renew. Sustain. Energy Rev. 2012;16(6):3621–3647. doi: 10.1016/j.rser.2012.03.002. [DOI] [Google Scholar]
- 13.Wu S., Song L., Sommerfeld M., Hu Q., Chen W. Optimization of an effective method for the conversion of crude algal lipids into biodiesel. Fuel. 2017;197:467–473. doi: 10.1016/j.fuel.2017.02.040. [DOI] [Google Scholar]
- 14.Park J.-Y., Kim D.-K., Wang Z.-M., Lee J.-S. Fast biodiesel production with one-phase reaction. Appl. Biochem. Biotechnol. 2009;154(1–3):67–73. doi: 10.1007/s12010-008-8421-y. [DOI] [PubMed] [Google Scholar]
- 15.Encinar J.M., Pardal A., Sánchez N. An improvement to the transesterification process by the use of co-solvents to produce biodiesel. Fuel. 2016;166:51–58. doi: 10.1016/j.fuel.2015.10.110. [DOI] [Google Scholar]
- 16.Boocock D.B.G., Konar S.K., Mao V., Sidi H. Fast one-phase oil-rich processes for the preparation of vegetable oil methyl esters. Biomass Bioenergy. 1996;11(1):43–50. doi: 10.1016/0961-9534(95)00111-5. [DOI] [Google Scholar]
- 17.Boocock David G.B., Konar Samir K., Mao V., Lee C., Buligan S. Fast formation of high-purity methyl esters from vegetable oils. J. Am. Oil Chem. Soc. 1998;75(9):1167–1172. doi: 10.1007/s11746-998-0130-8. [DOI] [Google Scholar]
- 18.Rubino J.T., Yalkowsky S.H. Cosolvency and cosolvent polarity. Pharm. Res. (N. Y.) 1987;4(3):220–230. doi: 10.1023/a:1016456127893. [DOI] [PubMed] [Google Scholar]
- 19.Haynes W.M., Lide D.R., Bruno T.J. 95th ed. CRC Press; Taylor & Francis Group, LLC; Boca Raton, FL: 2014. CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data. [Google Scholar]
- 20.Seehusen J. Rheinischen Friedrich-Wilhelms-Universität; Bonn: 2010. Schwingungsdynamik von intra- und intermolekularen Wasserstoffbrückenbindungen,” Dissertation. [Online]. Available: https://d-nb.info/1013348982/34. [Google Scholar]
- 21.Maeda Y., et al. New technology for the production of biodiesel fuel. Green Chem. 2011;13(5):1124. doi: 10.1039/c1gc15049a. [DOI] [Google Scholar]
- 22.Merck KGaA, Darmstadt, Germany and/or its affiliates, methanol CAS 67-56-1 | 822283. [Online]. Available: https://www.merckmillipore.com/DE/de/product/Methanol, MDA_CHEM-822283 (accessed: Jan. 25 2022).
- 23.Merck KGaA, Darmstadt, Germany and/or its affiliates, 1-propanol CAS 71-23-8 | 100996. [Online]. Available: https://www.merckmillipore.com/DE/de/product/1-Propanol,MDA_CHEM-100996 (accessed: Jan. 25 2022).
- 24.Merck KGaA, Darmstadt, Germany and/or its affiliates, 2-propanol CAS 67-63-0 | 818766. [Online]. Available: https://www.merckmillipore.com/DE/de/product/2-Propanol,MDA_CHEM-818766 (accessed: Jan. 25 2022).
- 25.Merck KGaA, Darmstadt, Germany and/or its affiliates, tert-Butanol CAS 75-65-0 | 822264. [Online]. Available: https://www.merckmillipore.com/DE/de/product/tert-Butanol,MDA_CHEM-822264 (accessed: Jan. 25 2022).
- 26.Merck KGaA, Darmstadt, Germany and/or its affiliates, n-Hexan CAS 110-54-3 | 104374. [Online]. Available: https://www.merckmillipore.com/DE/de/product/n-Hexane,MDA_CHEM-104374 (accessed: Jan. 25 2022).
- 27.Merck KGaA, Darmstadt, Germany and/or its affiliates, cyclohexan CAS 110-82-7 | 102832. [Online]. Available: https://www.merckmillipore.com/DE/de/product/Cyclohexane,MDA_CHEM-102832 (accessed: Jan. 25 2022).
- 28.Meyer V.R. tenth ed. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim: 2009. Praxis der Hochleistungs-Flüssigchromatographie. [Google Scholar]
- 29.Merck KGaA, Darmstadt, Germany and/or its affiliates, Toluol CAS 108-88-3 | 108323. [Online]. Available: https://www.merckmillipore.com/DE/de/product/Toluene,MDA_CHEM-108323 (accessed: Jan. 25 2022).
- 30.Xu R., Mi Y. Simplifying the process of microalgal biodiesel production through in situ transesterification technology. J. Am. Oil Chem. Soc. 2011;88(1):91–99. doi: 10.1007/s11746-010-1653-3. [DOI] [Google Scholar]
- 31.Merck KGaA, Darmstadt, Germany and/or its affiliates, ethylacetat CAS 141-78-6 | 822277. [Online]. Available: https://www.merckmillipore.com/DE/de/product/Ethyl-acetate,MDA_CHEM-822277 (accessed: Jan. 25 2022).
- 32.Guan G., Sakurai N., Kusakabe K. Synthesis of biodiesel from sunflower oil at room temperature in the presence of various cosolvents. Chem. Eng. J. 2009;146(2):302–306. doi: 10.1016/j.cej.2008.10.009. [DOI] [Google Scholar]
- 33.Merck KGaA, Darmstadt, Germany and/or its affiliates, diethylether CAS 60-29-7 | 100923. [Online]. Available: https://www.merckmillipore.com/DE/de/product/Diethyl-ether,MDA_CHEM-100923 (accessed: Jan. 25 2022).
- 34.Merck KGaA, Darmstadt, Germany and/or its affiliates, tert-Butylmethylether CAS 1634-04-4 | 101843. [Online]. Available: https://www.merckmillipore.com/DE/de/product/tert-Butyl-methyl-ether,MDA_CHEM-101843 (accessed: Jan. 25 2022).
- 35.Merck KGaA, Darmstadt, Germany and/or its affiliates, petrolether | 101769. [Online]. Available: https://www.merckmillipore.com/DE/de/product/Petroleum-ether,MDA_CHEM-101769 (accessed: Jan. 25 2022).
- 36.Merck, Safety data sheet: petroleum ether (101769). [Online]. Available: https://www.merckmillipore.com/DE/de/product/msds/MDA_CHEM-101769?Origin=PDP.
- 37.Merck KGaA, Darmstadt, Germany and/or its affiliates, tetrahydrofuran CAS 109-99-9 | 108114. [Online]. Available: https://www.merckmillipore.com/DE/de/product/Tetrahydrofuran,MDA_CHEM-108114 (accessed: Jan. 25 2022).
- 38.Merck KGaA, Darmstadt, Germany and/or its affiliates, aceton CAS 67-64-1 | 822251. [Online]. Available: https://www.merckmillipore.com/DE/de/product/Acetone,MDA_CHEM-822251 (accessed: Jan. 25 2022).
- 39.Le Thanh T., Okitsu K., Sadanaga Y., Takenaka N., Maeda Y., Bandow H. A new co-solvent method for the green production of biodiesel fuel – optimization and practical application. Fuel. 2013;103:742–748. doi: 10.1016/j.fuel.2012.09.029. [DOI] [Google Scholar]
- 40.Luu P.D., et al. Production of biodiesel from Vietnamese Jatropha curcas oil by a co-solvent method. Bioresour. Technol. 2014;173:309–316. doi: 10.1016/j.biortech.2014.09.114. [DOI] [PubMed] [Google Scholar]
- 41.Encinar J.M., González J.F., Pardal A., Martínez G. Third International Symposium on Energy from Biomass and WasteVenice; Italy: 2010. Transesterification of Rapeseed Oil with Methanol in Presence of Various Co-solvents” Proceedings Venice. [Google Scholar]
- 42.Wu L., Huang K., Wei T., Lin Z., Zou Y., Tong Z. Process intensification of NaOH-catalyzed transesterification for biodiesel production by the use of bentonite and co-solvent (diethyl ether) Fuel. 2016;186:597–604. doi: 10.1016/j.fuel.2016.08.106. [DOI] [Google Scholar]
- 43.Peña R., Romero R., Martínez S.L., Ramos M.J., Martínez A., Natividad R. Transesterification of Castor oil: effect of catalyst and Co-solvent. Ind. Eng. Chem. Res. 2009;48(3):1186–1189. doi: 10.1021/ie8005929. [DOI] [Google Scholar]
- 44.Fadhil A.B., Al-Tikrity E.T., Albadree M.A. Transesterification of a novel feedstock, Cyprinus carpio fish oil: influence of co-solvent and characterization of biodiesel. Fuel. 2015;162:215–223. doi: 10.1016/j.fuel.2015.09.001. [DOI] [Google Scholar]
- 45.Janchiv A., Oh Y., Choi S. High quality biodiesel production from pork lard by high solvent additive. Sci. Asia. 2012;38(1):95. doi: 10.2306/scienceasia1513-1874.2012.38.095. [DOI] [Google Scholar]
- 46.Rahimi M., Mohammadi F., Basiri M., Parsamoghadam M.A., Masahi M.M. Transesterification of soybean oil in four-way micromixers for biodiesel production using a cosolvent. J. Taiwan Inst. Chem. Eng. 2016;64:203–210. doi: 10.1016/j.jtice.2016.04.023. [DOI] [Google Scholar]
- 47.Escobar E.C., Demafelis R.B., Pham L.J., Florece L.M., Borines M.G. Biodiesel production from jatropha curcas L. Oil by transesterification with hexane as cosolvent. Philipp. J. Crop Sci. 2008;33(3) [Google Scholar]
- 48.Qiu F., Li Y., Yang D., Li X., Sun P. Biodiesel production from mixed soybean oil and rapeseed oil. Appl. Energy. 2011;88(6):2050–2055. doi: 10.1016/j.apenergy.2010.12.070. [DOI] [Google Scholar]
- 49.Hernando J., Leton P., Matia M.P., Novella J.L., Alvarez-Builla J. Biodiesel and FAME synthesis assisted by microwaves: homogeneous batch and flow processes. Fuel. 2007;86(10–11):1641–1644. doi: 10.1016/j.fuel.2006.11.003. [DOI] [Google Scholar]
- 50.Parida S., Sahu D.K., Misra P.K. Optimization of transesterification process by the application of ultrasound energy coupled with diesel as cosolvent. J. Energy Inst. 2017;90(4):556–562. doi: 10.1016/j.joei.2016.05.006. [DOI] [Google Scholar]
- 51.Guan G., Kusakabe K. Synthesis of biodiesel fuel using an electrolysis method. Chem. Eng. J. 2009;153(1–3):159–163. doi: 10.1016/j.cej.2009.06.005. [DOI] [Google Scholar]
- 52.Kumar G.R., Ravi R., Chadha A. Kinetic studies of base-catalyzed transesterification reactions of non-edible oils to prepare biodiesel: the effect of Co-solvent and temperature. Energy Fuel. 2011;25(7):2826–2832. doi: 10.1021/ef200469u. [DOI] [Google Scholar]
- 53.Bhargavi G., Nageswara Rao P., Renganathan S. Production of Biodiesel from Thespesiapopulnea seed oil through rapid in situ transesterification - an optimization study and assay of fuel properties. IOP Conf. Ser. Mater. Sci. Eng. 2018;330 doi: 10.1088/1757-899X/330/1/012046. [DOI] [Google Scholar]
- 54.Mohammed-Dabo I.A., Ahmad M.S., Hamza A., Muazu K., Aliyu A. Cosolvent transesterification of Jatropha curcas seed oil. J. Pet Technol. Altern. Fuels. 2012;4(3):42–51. doi: 10.5897/JPTAF11.038. [DOI] [Google Scholar]
- 55.Elkady M.F., Zaatout A., Balbaa O. Production of biodiesel from waste vegetable oil via KM micromixer. J. Chem. 2015:1–9. doi: 10.1155/2015/630168. 2015. [DOI] [Google Scholar]
- 56.Yu D., et al. Ultrasonic irradiation with vibration for biodiesel production from soybean oil by Novozym 435. Process Biochem. 2010;45(4):519–525. doi: 10.1016/j.procbio.2009.11.012. [DOI] [Google Scholar]
- 57.Fu B., Vasudevan P.T. Effect of organic solvents on enzyme-catalyzed synthesis of biodiesel. Energy Fuel. 2009;23(8):4105–4111. doi: 10.1021/ef900187v. [DOI] [Google Scholar]
- 58.Mohammed-Dabo I.A. Single phase (Cyclohexane-Assisted) transesterification of jatropha curcas seed oil using full factorial design of experiment” int. J. Sustain. Green Energy. 2013;2(5):191. doi: 10.11648/j.ijrse.20130205.11. [DOI] [Google Scholar]
- 59.Yusuf M., Athar M. Vth International Symposium on Fusion of Science & Technology; 2016. Biodiesel Production Using Hexane as Co Solvent; pp. 159–161. [Google Scholar]
- 60.Todorović Z.B., et al. The effects of cosolvents on homogeneously and heterogeneously base-catalyzed methanolysis of sunflower oil. Fuel. 2013;107:493–502. doi: 10.1016/j.fuel.2012.11.049. [DOI] [Google Scholar]
- 61.Asif S., et al. In: Rastegari A., Yadav A., Gupta A., editors. vol. 10. Springer; Cham: 2019. Chemical conversion in biodiesel refinery. (Prospects of Renewable Bioprocessing in Future Energy Systems. Biofuel and Biorefinery Technologies). [DOI] [Google Scholar]
- 62.Vaclavik V., Christian E.W. third ed. Springer; New York NY: 2008. Essentials of Food Science. [Google Scholar]
- 63.Ebermann R., Elmadfa I. second ed. Springer; Wien: 2008. Lehrbuch Lebensmittelchemie und Ernährung. [Google Scholar]
- 64.Guan G., Kusakabe K., Sakurai N., Moriyama K. Transesterification of vegetable oil to biodiesel fuel using acid catalysts in the presence of dimethyl ether. Fuel. 2009;88(1):81–86. doi: 10.1016/j.fuel.2008.07.021. [DOI] [Google Scholar]
- 65.Nikhom R., Mueanmas C., Suppalakpanya K. Enhancement of biodiesel production from palm fatty acid distillate using methyl t-butyl ether co-solvent: process optimization. Int. J. Renew. Energy Resour. 2019;9(3):1319–1327. [Google Scholar]
- 66.Nguyen H.C., et al. Direct transesterification of black soldier fly larvae (Hermetia illucens) for biodiesel production. J. Taiwan Inst. Chem. Eng. 2018;85:165–169. doi: 10.1016/j.jtice.2018.01.035. [DOI] [Google Scholar]
- 67.Shuit S.H., Lee K.T., Kamaruddin A.H., Yusup S. Reactive extraction and in situ esterification of Jatropha curcas L. seeds for the production of biodiesel. Fuel. 2010;89(2):527–530. doi: 10.1016/j.fuel.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 68.Soriano N.U., Venditti R., Argyropoulos D.S. Biodiesel synthesis via homogeneous Lewis acid-catalyzed transesterification. Fuel. 2009;88(3):560–565. doi: 10.1016/j.fuel.2008.10.013. [DOI] [Google Scholar]
- 69.Lam M.K., Lee K.T. Catalytic transesterification of high viscosity crude microalgae lipid to biodiesel: effect of co-solvent. Fuel Process. Technol. 2013;110:242–248. doi: 10.1016/j.fuproc.2012.12.021. [DOI] [Google Scholar]
- 70.Khang D.S., Razon L.F., Madrazo C.F., Tan R.R. In situ transesterification of coconut oil using mixtures of methanol and tetrahydrofuran. Chem. Eng. Res. Des. 2014;92(8):1512–1518. doi: 10.1016/j.cherd.2014.01.002. [DOI] [Google Scholar]
- 71.Chueluecha N., Kaewchada A., Jaree A. Enhancement of biodiesel synthesis using co-solvent in a packed-microchannel. J. Ind. Eng. Chem. 2017;51:162–171. doi: 10.1016/j.jiec.2017.02.028. [DOI] [Google Scholar]
- 72.Roschat W., Siritanon T., Kaewpuang T., Yoosuk B., Promarak V. Economical and green biodiesel production process using river snail shells-derived heterogeneous catalyst and co-solvent method. Bioresour. Technol. 2016;209:343–350. doi: 10.1016/j.biortech.2016.03.038. [DOI] [PubMed] [Google Scholar]
- 73.Singh V., Yadav M., Sharma Y.C. Effect of co-solvent on biodiesel production using calcium aluminium oxide as a reusable catalyst and waste vegetable oil. Fuel. 2017;203:360–369. doi: 10.1016/j.fuel.2017.04.111. [DOI] [Google Scholar]
- 74.Fereidooni L., Khalilnezhad R., Pirkaramic A. Evaluation of methyl ester production using cement as a heterogeneous catalyst. Phys. Chem. Res. 2018;6(2):323–333. doi: 10.22036/pcr.2018.110984.1441. [DOI] [Google Scholar]
- 75.Fereidooni L., Mehrpooya M. Experimental assessment of electrolysis method in production of biodiesel from waste cooking oil using zeolite/chitosan catalyst with a focus on waste biorefinery. Energy Convers. Manag. 2017;147:145–154. doi: 10.1016/j.enconman.2017.05.051. [DOI] [Google Scholar]
- 76.Gaikwad N.D., Gogate P.R. Synthesis and application of carbon based heterogeneous catalysts for ultrasound assisted biodiesel production. Green Process. Synth. 2015;4(1) doi: 10.1515/gps-2014-0079. [DOI] [Google Scholar]
- 77.Todorović Z.B., et al. Optimization of CaO-catalyzed sunflower oil methanolysis with crude biodiesel as a cosolvent. Fuel. 2019;237:903–910. doi: 10.1016/j.fuel.2018.10.056. [DOI] [Google Scholar]
- 78.Aghel B., Mohadesi M., Sahraei S. Effect of different cosolvents on transesterification of waste cooking oil in a microreactor. Chem. Eng. Technol. 2018;41(3):598–605. doi: 10.1002/ceat.201700025. [DOI] [Google Scholar]
- 79.Kim H.-J., et al. Transesterification of vegetable oil to biodiesel using heterogeneous base catalyst. Catal. Today. 2004;93–95:315–320. doi: 10.1016/j.cattod.2004.06.007. [DOI] [Google Scholar]
- 80.Madhu D., Arora R., Sahani S., Singh V., Sharma Y.C. Synthesis of high-quality biodiesel using feedstock and catalyst derived from fish wastes. J. Agric. Food Chem. 2017;65(10):2100–2109. doi: 10.1021/acs.jafc.6b05608. [DOI] [PubMed] [Google Scholar]
- 81.Galli F., et al. Heterogeneous oil transesterification in a single-phase liquid mixture using a Co-solvent for improved biofuels production. Energy Technol. 2015;3(12):1170–1173. doi: 10.1002/ente.201500186. [DOI] [Google Scholar]
- 82.Yang Z., Xie W. Soybean oil transesterification over zinc oxide modified with alkali earth metals. Fuel Process. Technol. 2007;88(6):631–638. doi: 10.1016/j.fuproc.2007.02.006. [DOI] [Google Scholar]
- 83.Sahani S., Banerjee S., Sharma Y.C. Study of 'co-solvent effect' on production of biodiesel from Schleichera Oleosa oil using a mixed metal oxide as a potential catalyst. J. Taiwan Inst. Chem. Eng. 2018;86:42–56. doi: 10.1016/j.jtice.2018.01.029. [DOI] [Google Scholar]
- 84.Soares Dias A.P., Puna J., Gomes J., Neiva Correia M.J., Bordado J. Biodiesel production over lime. Catalytic contributions of bulk phases and surface Ca species formed during reaction. Renew. Energy. 2016;99:622–630. doi: 10.1016/j.renene.2016.07.033. [DOI] [Google Scholar]
- 85.Lian S., Li H., Tang J., Tong D., Hu C. Integration of extraction and transesterification of lipid from jatropha seeds for the production of biodiesel. Appl. Energy. 2012;98:540–547. doi: 10.1016/j.apenergy.2012.04.029. [DOI] [Google Scholar]
- 86.Brito A., Borges M.E., Arvelo R., Garcia F., Diaz M.C., Otero N. Reuse of fried oil to obtain biodiesel: zeolites Y as a catalyst. Int. J. Chem. React. 2007;5 doi: 10.2202/1542-6580.1548. [DOI] [Google Scholar]
- 87.Ilgen O., Akin A.N., Boz N. Investigation of biodiesel production from canola oil using amberlyst-26 as a catalyst. Turk. J. Chem. 2009;33:289–294. doi: 10.3906/kim-0809-30. [DOI] [Google Scholar]
- 88.Ziȩba A., Pacuła A., Drelinkiewicz A. Transesterification of triglycerides with methanol catalyzed by heterogeneous zinc hydroxy nitrate catalyst. Evaluation of variables affecting the activity and stability of catalyst. Energy Fuels. 2010;24(1):634–645. doi: 10.1021/ef900780q. [DOI] [Google Scholar]
- 89.Putra R.S., Hartono P., Julianto T.S. Conversion of methyl ester from used cooking oil: the combined use of electrolysis process and chitosan. Energy Proc. 2015;65:309–316. doi: 10.1016/j.egypro.2015.01.057. [DOI] [Google Scholar]
- 90.Guan G., Kusakabe K., Yamasaki S. Tri-potassium phosphate as a solid catalyst for biodiesel production from waste cooking oil. Fuel Process. Technol. 2009;90(4):520–524. doi: 10.1016/j.fuproc.2009.01.008. [DOI] [Google Scholar]
- 91.Gryglewicz S. Rapeseed oil methyl esters preparation using heterogeneous catalysts. Bioresour. Technol. 1999;70(3):249–253. doi: 10.1016/S0960-8524(99)00042-5. [DOI] [Google Scholar]
- 92.Ketcong A., et al. Production of fatty acid methyl esters over a limestone-derived heterogeneous catalyst in a fixed-bed reactor. J. Ind. Eng. Chem. 2014;20(4):1665–1671. doi: 10.1016/j.jiec.2013.08.014. [DOI] [Google Scholar]
- 93.López Granados M., Martín Alonso D., Alba-Rubio A.C., Mariscal R., Ojeda M., Brettes P. Transesterification of triglycerides by CaO: increase of the reaction rate by biodiesel addition. Energy Fuel. 2009;23(4):2259–2263. doi: 10.1021/ef800983m. [DOI] [Google Scholar]
- 94.Lam M.K., Lee K.T. Accelerating transesterification reaction with biodiesel as co-solvent: a case study for solid acid sulfated tin oxide catalyst. Fuel. 2010;89(12):3866–3870. doi: 10.1016/j.fuel.2010.07.005. [DOI] [Google Scholar]
- 95.Mongkolbovornkij P., Champreda V., Sutthisripok W., Laosiripojana N. Esterification of industrial-grade palm fatty acid distillate over modified ZrO2 (with WO3–, SO4 –and TiO2–): effects of co-solvent adding and water removal. Fuel Process. Technol. 2010;91(11):1510–1516. doi: 10.1016/j.fuproc.2010.05.030. [DOI] [Google Scholar]
- 96.Karmee S.K., Chadha A. Preparation of biodiesel from crude oil of Pongamia pinnata. Bioresour. Technol. 2005;96(13):1425–1429. doi: 10.1016/j.biortech.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 97.Jiménez-Morales I., Santamaría-González J., Maireles-Torres P., Jiménez-López A. Mesoporous tantalum phosphate as acidic catalyst for the methanolysis of sunflower oil. Appl. Catal. B Environ. 2012;123–124:316–323. doi: 10.1016/j.apcatb.2012.04.023. [DOI] [Google Scholar]
- 98.Mamtani K., Shahbaz K., Farid M.M. Deep eutectic solvents–Versatile chemicals in biodiesel production. Fuel. 2021;295 doi: 10.1016/j.fuel.2021.120604. 120604. [DOI] [Google Scholar]
- 99.Najaf-Abadi M.K., Ghobadian B., Dehghani-Soufi M. A review on application of deep eutectic solvents as green catalysts and co-solvents in biodiesel production and purification processes. Biomass Conv. Bioref. 2022 doi: 10.1007/s13399-022-02644-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


