Abstract
Cardiovascular disease (CVD) remains the leading cause of morbidity and mortality worldwide, ranking first in the global disease burden. Evidence on association between temperature and cardiovascular disease is insufficient and inconsistent in developing countries. In this study, a distributed lag nonlinear model (DLNM) was used to determine the association between daily mean temperature and cardiovascular diseases (CVD) related admission in Lanzhou 2015–2019. We included 41,389 patients with CVD in this study. The relative risk (RR) of CVD admission increased significantly with temperature in lag 5–10 days, and we found harvesting effect of temperature in the study, shown as decreased RR in lag 15–30 days. The maximum RR was 1.15 (95% confidence interval [CI]: 1.03–1.30), corresponding to 24 °C. Both cold and heat effects of temperature could impact the CVD admission. Compared with the 25th percentile of temperature (2 °C), the cumulative relative risk (cumRR) of extreme cold (−5 °C, the 2.5th percentile of the temperature) was 0.69 (95% CI: 0.51–0.94) in lag 0–14, whereas the cumRR of moderate cold (−2 °C, the 10th percentile) was 0.83 (95% CI:0.71–0.97). Compared with the 75th percentile of temperature (20-°C), the cumRR of extreme heat (27 °C, the 97.5th percentile) was 0.93 (95% CI: 0.78–1.10) in lag 0, whereas the cumRR of moderate heat (24 °C, the 90th percentile) was 1.01 (95% CI: 0.94–1.08). In the stratified analysis, cold decreased RR significantly in female and ≥65 years, whereas heat increased it more obviously in male and ≥65 years. Ambient temperature and CVD admissions were positively associated, with the harvesting effect. Our findings demonstrate the adaption of residents in Lanzhou to cold temperature. Public and environmental policies and measures aimed at moderate heat may minimize CVD burden effectively.
Keywords: Cardiovascular diseases, Mean temperature, Hospital admission, Adaptation, Harvesting effect, Distributed lag nonlinear model
Highlights
-
•
Evidence of the association between temperature and CVD related admission is scarce in Lanzhou City.
-
•
Temperature and daily CVD related admissions were found to be positively correlated.
-
•
People in Lanzhou are more susceptible to heat and adaptive to cold.
-
•
Harvesting effect was found in lag 15–30 days.
1. Introduction
Influence of ambient temperature on health outcomes is of increasing concern, given the loss of life and property caused by global climate change and extreme weather events [1]. Numerous studies report the impact of heat or cold or both on cardiovascular and cardiorespiratory diseases [2,3]. The more the changes in meteorological factors and their combinations, the more observable the biological effects induced by the weather-related factors [4].
Currently, most studies focused on the temperature-mortality link [[5], [6], [7], [8], [9]], and some have identified the harvesting effect [10–13]. Studies on temperature and CVD related admissions, however, are limited and inconsistent in developing countries [[14], [15], [16]], and positive, negative, or no relationships were reported between temperature and CVD related admissions [[16], [17], [18], [19]]. A J-shaped relationship was found in the study of temperatures on hospital admissions related to cardiovascular and cerebrovascular diseases in Shanghai [20]. Both cold and heat led to an increase in CVD related clinical visits in the rural villages in Ningxia, China [21]. However, the incidence of acute myocardial infarction (AMI) was negatively and linearly related to temperature in Kaunas residents, except those <44 years of age [4]. Sun et al. reported a decreased risk of MI hospitalization due to cold exposure in the meta-analysis study [22].
Moreover, the pattern of effects of high and low temperatures may also differ. The relative risk increased at low temperatures and decreased at high temperatures [16,23,24], while in Rasht, the cumulative effect of heat on ACS admissions was statistically significant, with a relative risk of 2.04 (95% CI: 1.06–4.16). The effect of temperature on CVD may be modified by individual characteristics (age, gender, general health status, etc.) [25]. Many studies have reported that high temperatures can significantly increase the risk of hospital admissions in the elderly and women [16,[26], [27], [28]]. In contrast, the risk of CVD admissions among the elderly in Hong Kong increased only in extreme cold conditions [29]. The MINAP registry of the United Kingdom, on the other hand, found that the population aged 75–84 years were more susceptible to AMI due to cold temperature and high temperature had no deleterious effect on them [30]. Additionally, studies have reported adaptation of humans to low temperatures [9,31].
As far as we know, the study of health effect of meteorological factors is uneven in China. There are few studies in the northwest of China, and scarcely in Lanzhou City. This study aimed to investigate the association between ambient temperature and CVD related admission in Lanzhou City, and to provide a plausible biological mechanism to elucidate the impact of temperature on cardiovascular disease. This study will be a powerful supplement to such studies in Lanzhou, and will present scientific basis for policy makers to formulate public policy conforming to local conditions.
2. Materials and methods
2.1. Study region
Lanzhou is in the central part of the Gansu Province and has an area of 13,100 square kilometers (Figure S1). It had a permanent population of approximately 4,359,400 in the year 2020. It has the geographical characteristics of a belt-shaped basin city and a typical temperate semi-arid continental monsoon climate, with dry and cold winters and sunny summers. The average annual temperature is 10 °C, and the average annual precipitation is 360 mm.
2.2. Data collection
Daily CVD inpatient data were obtained from the three largest public local hospitals (Lanzhou University First Hospital, Lanzhou University Second Hospital, and Gansu Provincial People' Hospital) between January 1, 2015 and December 31, 2019. The daily number of hospital admissions was pooled based on the date of admission. Hospitalization data included gender, age, current residence, date of admission, date of discharge, and diagnosis at discharge. The 10th Edition of the International Classification of Diseases was used to identify the reasons for CVD related hospitalizations, which mainly included ischemic heart disease (ICD-10: I20–I25), heart failure (ICD-10: I50.0-I50.1; I50.9), hypertension (ICD10: I10–I15). Referring to previous local studies of carbon monoxide and CVD related admissions [32], we divided the study population into subgroups according to gender (male, female) and age (<65 years,≥65 years).
Meteorological data for the period were obtained from the Lanzhou Meteorological Bureau and included the daily maximum temperature (°C), daily minimum temperature (°C), daily mean temperature (°C), daily mean pressure (hPa), daily average relative humidity (%) and wind speed (m/s). The daily temperature difference (DTR) was calculated based on the daily maximum and minimum temperatures. Information on air pollutants was obtained from the Lanzhou Environmental Monitoring Center, including SO2, NO2 and PM2.5. The daily concentration of each pollutant was determined based on the average of the existing results obtained from monitoring the four fixed stations in Lanzhou City.
2.3. Statistical analysis
A distributed lag nonlinear model (DLNM) using quasi-Poisson regression [20,27,33] was used to examine the association between mean temperature and CVD related hospitalizations after adjusting for confounding factors (air pollutants, relative humidity, windspeed, time trends, holidays, and day of the week). The DLNM model, characterized by the cross basis, is a two-dimensional function space composed of two sets of basic functions that can simultaneously describe the shape of the relationship as well as the predictor space and lag dimension in which it occurs [34].
We built the following model:
| Log(μt) = α+βTempt,l + ns(Time, df)+ns(Weather, df)+ns(windspeed, df)+ns(Pollutants, df)+DOWt + Holidayt |
Where,
Subscript t denotes the date of observation during the study period, μt is the expected number of CVD related admissions on day t; α is the intercept; β is the coefficient vector of the cross basis; ns is the natural cubic spline function; Tempt,l is the cross basis in the DLNM model, which is defined by natural cubic spline function for both exposure-response dimension with the three internal nodes placed at the 10th, 75th and 90th percentiles, and for the lag dimension with three equally spaced knots along its logarithmic scale [19]; time variable is used to control the long-term and seasonal trends; weather, windspeed and pollutants are covariates in the model. DOWt and Holidayt are dummy variables for “day of the week” and “holiday”, respectively, to adjust for differences in daily baseline hospitalization rates [33]. We chose 7° of freedom (dfs) for the time variable in the model per year [24], and 3 dfs for humidity, windspeed, and contaminants (PM2.5 and NO2) [2,[35], [36], [37]]. Based on previous research and the literature [20,38], we set the maximum lag to 30 days to fully assess the overall temperature effect and identify the possible harvesting effects.
Initially, we chose the median value of the mean temperature (12.6 °C) as the reference value to fit the model, and plotted the overall exposure-response curve. Based on the nearly positive linear nature of the obtained curve, the minimal morbidity temperature (MMT) was determined at −12 °C and adopted as the new reference value for further prediction and analysis [6,39]. Subsequently, we evaluated the effect of temperature on CVD related admissions in lag 0–30 and plotted the overall exposure-response curves along the whole temperature range. We also evaluated the relative risk and cumulative relative risk of extreme cold (2.5 th percentile of the temperature distribution), moderate cold (10th percentile), extreme heat (97.5th percentile), and moderate heat (90th percentile) temperatures versus the 25th and 75th percentiles of temperature as previously reported, to explore the effects of low and high temperatures. The selection of cutoffs above was based on previous reports [40]. The association was considered statistically significant if the 95% CI of relative risk did not include a relative risk = 1.0 [24]. Subgroup analyses were also performed by age (<65 years, ≥65 years) and gender (male, female) to identify the susceptible groups [24].
Sensitivity analysis was performed to assess the robustness of our main results: (1) using lags of 21 days or 14 days; (2) changing the pollutants in the model; (3) using different dfs (5 or 8 per year) for time trend, and different dfs for humidity (2 or 5) and pollutants (1 or 5). The descriptive statistics were done using SPSS 26. The statistical analyses were performed using R software (version 4.1.1). The DLNM model was fitted with the dlnm package. All statistical tests were two sided, and p < 0.05 were considered statistically significant.
3. Results
3.1. Descriptive summary
Table 1 shows the descriptive statistics for daily hospital admissions, daily meteorological variables and daily air pollutants in Lanzhou City, China, from January 1, 2015 to December 31, 2019. There were 41,389 CVD hospitalizations in total, among which 26,515 were males and 14,874 were females, with a male-to-female ratio of 1.78:1. The median value of daily CVD admission was 21; 13 were males, 8 were females, 12 were <65 years, and 9 were ≥65 years. The medians of daily mean temperature, DTR, relative humidity, and wind speed were 11.3 °C, 12.1 °C, 50.8%, and 1.12 m/s, respectively. The medians of daily averages of SO2, NO2 and PM2.5 were 19.87 μg/m3, 53.54 μg/m3 and 47.57 μg/m3, respectively.
Table 1.
Summary statistics of daily hospital admissions, weather conditions, and air pollutants between 2015 and 2019 in Lanzhou, China.
| Min | 2.5th | 10th | 25th | M | 75th | 90th | 97.5th | Max | |
|---|---|---|---|---|---|---|---|---|---|
| Daily admissions | |||||||||
| CVD | 0 | 3 | 5 | 9 | 21 | 32 | 42 | 59 | 86 |
| Male | 0 | 1 | 3 | 6 | 13 | 20 | 28 | 40 | 60 |
| Female | 0 | 0 | 1 | 3 | 8 | 12 | 16 | 22 | 35 |
| <65 years | 0 | 1 | 2 | 5 | 12 | 18 | 25 | 35 | 55 |
| ≥65years | 0 | 1 | 2 | 4 | 9 | 14 | 19 | 27 | 41 |
| Weather | |||||||||
| Temperature (°C) | −12.28 | −5.30 | −2.70 | 2.00 | 12.60 | 20.00 | 23.60 | 27.30 | 30.78 |
| DTR (°C) | 1.40 | 4.30 | 6.40 | 9.10 | 12.20 | 15.00 | 17.60 | 20.00 | 25.80 |
| Relative humidity (%) | 12.30 | 24.39 | 31.26 | 39.29 | 50.80 | 61.80 | 70.80 | 81.00 | 95.80 |
| Wind speed (m/s) | 0 | 0.3 | 0.5 | 0.8 | 1.1 | 1.4 | 1.9 | 2.6 | 6.0 |
| Air pollution (μg/m³) | |||||||||
| SO2 | 4.00 | 5.00 | 7.00 | 10.00 | 15.00 | 26.00 | 41.00 | 55.00 | 84.00 |
| NO2 | 13.00 | 22.00 | 29.00 | 38.00 | 50.00 | 64.00 | 84.00 | 104.22 | 150.00 |
| PM2.5 | 9.00 | 16.78 | 22.00 | 30.00 | 42.00 | 57.00 | 82.00 | 116.23 | 278.00 |
3.2. Correlation analysis
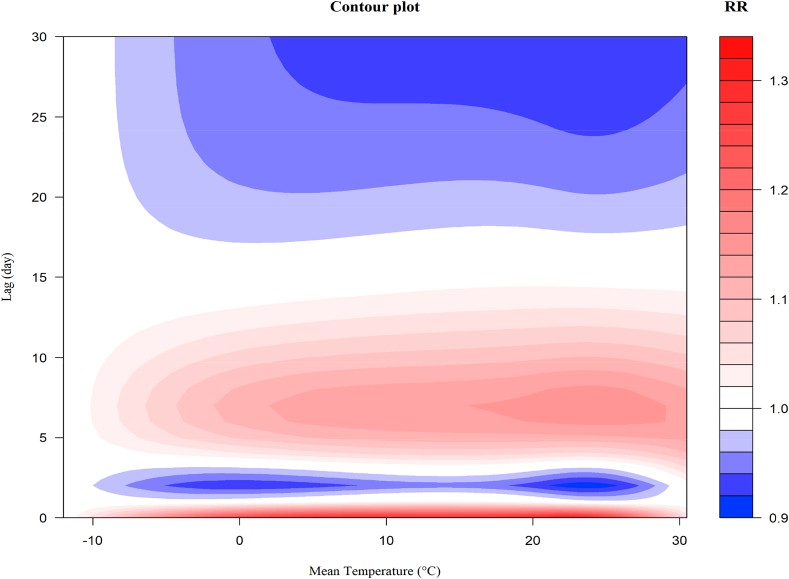
Fig. 1 shows a 3D plot of temperature versus CVD related admissions within a 30 days lag, with a referent temperature of −12 °C. The estimated effects of temperature were nonlinear and showed hysteresis. The relative risk increased with temperature, with a significant difference in lag 5–10, and then decreased gradually in lag 15–30. Fig. 2 depicts a more intuitive contour map indicating the relationship between temperature and CVD related admission. The temperature effect was evident, especially the heat temperature. The maximum effect of temperature emerged in the range of 16 °C–28 °C.
Fig. 1.
Three-dimensional plot of the association between temperature and hospitalizations along 30 lags, with reference at −12 °C.
Fig. 2.
The contour plot of the association between temperature and hospitalizations along 30 lags, with reference at −12 °C.
Table 2 shows the cumulative effect of cold and heat on hospitalization at different lags. The cumulative relative risk (cumRR) of extreme cold was 0.80 (95% CI: 0.66–0.99) in lag 0–7 and 0.69 (95% CI: 0.51–0.94) in lag 0–14, comparing to the 25th percentile of temperature (2 °C), whereas the cumRR of moderate cold was 0.89 (95% CI: 0.80–0.99) and 0.83 (95% CI: 0.71–0.97), respectively. The cumRR of extreme heat was 0.93 (95% CI: 0.78–1.10) in lag 0, 0.91 (95% CI: 0.55–1.53) in lag 0–30, which compared with the 75th percentile of temperature (20 °C), while the cumRR of moderate heat was 1.01 (95% CI: 0.94–1.08) and 0.93 (95% CI: 0.69–1.26), respectively. The maximum cumRR of extreme cold, moderate cold, extreme heat and moderate heat was 0.69 (95% CI: 0.50–0.95) in lag 15, 0.83 (95% CI: 0.69–0.98) in lag 16, 0.91 (95% CI: 0.75–1.11) in lag 2, and 1.01 (95% CI: 0.94–1.08) in lag 0, respectively (See also Table S1).
Table 2.
Cumulative relative risks and their 95% confidence intervals of cold and heat effects of low and high temperatures on CVD related hospitalizations over multiple lag days, with reference at −12 °C.
| Lag | Extreme colda | Moderate coldb | Moderate heatc | Extreme heatd |
|---|---|---|---|---|
| 0 | 0.92 (0.78–1.08) | 0.96 (0.89–1.04) | 1.01 (0.94–1.08) | 0.93 (0.78–1.10) |
| 0–3 | 0.93 (0.79–1.09) | 0.95 (0.88–1.04) | 0.96 (0.87–1.05) | 0.93 (0.76–1.14) |
| 0–7 | 0.80 (0.66–0.99)* | 0.89 (0.80–0.99)* | 0.96 (0.85–1.08) | 0.94 (0.75–1.19) |
| 0–14 | 0.69 (0.51–0.94)* | 0.83 (0.71–0.97)* | 0.98 (0.83–1.16) | 0.94 (0.69–1.27) |
| 0–21 | 0.72 (0.48–1.09) | 0.84 (0.68–1.04) | 0.96 (0.77–1.20) | 0.92 (0.62–1.37) |
| 0–30 | 0.86 (0.51–1.46) | 0.91 (0.69–1.20) | 0.93 (0.69–1.26) | 0.91 (0.55–1.53) |
*p < 0.05.
The 2.5th percentile of temperature (−5 °C) relative to the 25th percentile of temperature (2 °C).
The 10th percentile of temperature (−2 °C) relative to the 25th percentile of temperature (2 °C).
The 90th percentile of temperature (24 °C) relative to the 75th percentile of temperature (20 °C).
The 97.5th percentile of temperature (27 °C) relative to the 75th percentile of temperature (20 °C).
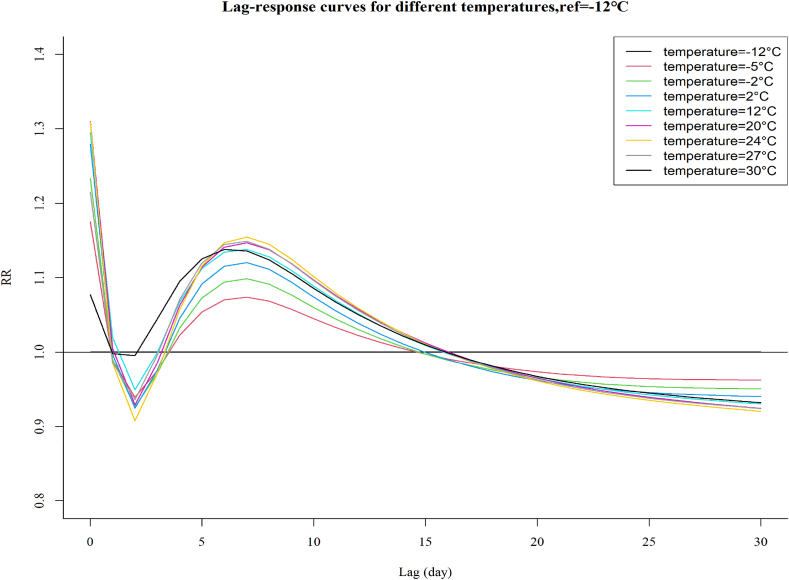
Fig. 3 illustrates the exposure-response curves of various temperatures on CVD related admissions in lag 0–30. Similar to that presented in Fig. 1, the relative risks increased from lag 2 and showed an apparent increase in lag 4–15, with a significant difference in lag 5–10, followed by a decrease in lag 15–30, indicating a possible morbidity displacement. The maximum relative risk emerged at lag 7. In line with that presented in Fig. 2, the temperature corresponding to the maximum relative risk was 24 °C, followed by 27 °C and 20 °C, rather than 30 °C. Temperatures with higher risk increment corresponded to more deficits of risk in lag 15–30.
Fig. 3.
Exposure-response curves of temperatures over 30 days of lag, with reference at −12 °C.
Fig. 4 shows the overall cumulative relative risk of CVD related admissions across the temperature range. The risk increased with temperature and peaked slightly in the range of 14 °C–20 °C; the curve seemed to approximate a positive, close-to-linear relationship. Table 3 shows that the maximum cumRRs of 14 °C, 16 °C, 18 °C and 20 °C were 3.16 (95% CI: 1.20–8.27), 3.18 (95% CI:1.19–8.53), 3.19 (95% CI:1.16–8.75), and 3.16 (95% CI:1.12–8.97), respectively, in lag 0–14.
Fig. 4.
Overall cumulative exposure–response associations of temperature and CVD related hospitalizations in Lanzhou, China, with reference at −12 °C.
Table 3.
Cumulative relative risks and their 95% confidence intervals of different temperature levels (12 °C–20 °C) on CVD related hospitalizations over multiple lag days, with reference at −12 °C.
| Temp (°C) | Lag 0-7 | Lag 0-14 | Lag 0-21 | Lag 0-30 |
|---|---|---|---|---|
| 12 | 1.92 (1.03–3.57) | 3.11 (1.21–7.95) | 2.72 (0.77–9.59) | 1.57 (0.31–7.85) |
| 14 | 1.93 (1.02–3.65) | 3.16 (1.20–8.27) | 2.79 (0.77–10.16) | 1.60 (0.30–8.46) |
| 16 | 1.93 (1.01–3.67) | 3.18 (1.19–8.53) | 2.83 (0.75–10.61) | 1.62 (0.29–8.93) |
| 18 | 1.91 (0.98–4.42) | 3.19 (1.16–8.75) | 2.83 (0.73–10.99) | 1.60 (0.28–9.26) |
| 20 | 1.87 (0.94–3.72) | 3.16 (1.12–8.97) | 2.80 (0.69–11.32) | 1.15 (0.25–9.48) |
3.3. Subgroup analyses by individual characteristics
The relative risk of low and high temperatures on admission, stratified by gender and age is shown in Table 4. The relative risk of cold decreased in all groups, compared with the 25th percentile of temperature, and decreased significantly in females and those ≥65 years of age, and was 0.94 (95% CI: 0.90–0.98) and 0.95 (95% CI: 0.91–0.98) in extreme cold, and 0.97 (95% CI: 0.95–0.99) and 0.97 (95% CI: 0.96–0.99) in moderate cold, respectively. The harmful effects of high temperature appeared to be higher among those ≥65 years of age and males than among females and those <65 years, when compared with the 75th percentile of temperature, and the relative risk was 1.02 (95% CI: 0.93–1.12) and 1.02 (95% CI: 0.94–1.11), respectively; however, the difference was not significant.
Table 4.
Relative risks and their 95% confidence intervals of cold and heat effects of low and high temperatures on admissions by gender and age, with reference at −12 °C.
| Variable | Extreme colda | Moderate coldb | Moderate heatc | Extreme heatd |
|---|---|---|---|---|
| Male | 0.93 (0.79–1.09) | 0.98 (0.97–1.00) | 1.00 (0.93–1.08) | 1.02 (0.94–1.11) |
| Female | 0.94 (0.90–0.98)* | 0.97 (0.95–0.99)* | 1.01 (0.99–1.04) | 1.01 (0.97–1.06) |
| <65 years | 0.95 (0.80–1.13) | 0.98 (0.90–1.06) | 1.01 (0.99–1.04) | 1.01 (0.97–1.05) |
| ≥65 years | 0.95 (0.91–0.98)* | 0.97 (0.96–0.99)* | 1.01 (0.92–1.11) | 1.02 (0.93–1.122) |
*p < 0.05.
The 2.5th percentile of temperature (−5 °C) relative to the 25th percentile of temperature (2 °C).
The 10th percentile of temperature (−2 °C) relative to the 25th percentile of temperature (2 °C).
The 90th percentile of temperature (24 °C) relative to the 75th percentile of temperature (20 °C).
The 97.5th percentile of temperature (27 °C) relative to the 75th percentile of temperature (20 °C).
Fig. 5 shows the overall cumRR of CVD and the subgroups across the whole temperature range. The curves of subgroups showed similar characteristics to that of total CVD, such that the risk increased with temperature; however, the cumRR was <1 for males in the range of −12 °C–0 °C and ranged from −12 °C to 2 °C in those <65 years of age. Females had the maximum overall cumulative relative risk during the entire temperature range among the total CVD and subgroups.
Fig. 5.
Overall cumulative exposure-response curves of temperatures for CVD and subgroups.
Sensitivity analyses showed no significant changes in the primary outcome when the maximum lag was changed to 21 or 14 days (See also Figure S2). When the particulate matter variables in the model varied, the results remain robust (See also Figure S3). Moreover, the results remained reliable after changing the degrees of freedom for time variable, humidity, and pollutants (See also Figures S4, S5, S6).
4. Discussion
To the best of our knowledge, this is the first study to investigate the association between ambient temperature and CVD based admissions in Lanzhou, China. We found a positive correlation between ambient temperature and CVD related admissions. Heat increased the relative risk of CVD admissions, while cold decreased it. Moderate heat was found to be associated with higher risk of admission rather than extreme heat. Additionally, we observed the harvesting effect in this study [11,13,41,42]. Our result underscores the importance of heat exposure control on hospital admissions from CVD.
The positive correlation between ambient temperature and CVD related admission indicated that the relative risk increased with temperature in this study. Our findings were in accordance with the temperature and CVD related admission study in Queensland, which reported the increased impact of high temperatures and adaptation to the impact of cold temperatures [9]. A study in North Sweden found that a 1 °C increase was associated with an increase in the number of nonfatal AMIs by 1.5% [43]. Our findings that low temperatures decreased the risk of admission compared with 25th percentile temperature also comply with the results from a previous study in Hefei, a region with subtropical monsoons and a humid climate [16]. However, the association between high temperature and CVD related admissions was not significant in our study. The finding has been reported in previous studies too. In the southwest of China, non-accidental deaths burden due to heat was found non-significant (0.67%; 95%CI: −2.44%–3.64%) [44], and the cumulative relative risk of extreme heat and moderate heat increased, but with no significance in Hefei city, comparing to 75th percentile temperature [16]. In Queensland, high temperature was reported not significantly associated with the increased risks of hospitalizations for cardiovascular diseases [9]. Naturally, previous studies have also demonstrated contradictory conclusions. No association was found between high temperature and acute myocardial infarction (AMI) in Beijing [45]. The highest temperature had lowest risk of acute myocardial infarction onset at the national level in a 324 Chinese cities study [46].
For extremely high temperatures, the cumRR was 0.91 (95% CI: 0.55–1.53) in lag 0–30, with no significance. Similarly, in Jinan, the relative risk of heat for ischemic stroke is 0.43 (95% CI: 0.31–0.59) [47]. For moderately high temperature, the cumRR was 1.01 (95%CI: 0.94–1.08) at lag 0. The result was consistent with previous studies and suggested that moderate temperature, rather than extreme temperature, led to most of the CVD related hospitalization burden [21,39]. However, most current studies reported that extremely high temperatures increased the risk of CVD admission. For example, in Beijing, the cumulative effect of high apparent temperature on ST segment elevation myocardial infarction related admissions reached a peak relative risk of 2.55 (95% CI: 1.02–6.38) in lag 0–6 days [48]. A 272 main Chinese cities time serials study found a relative risk of mortality from cardiovascular disease associated with extreme high temperature was 1.22 (95% CI: 1.16–1.28) [49]. For extremely low temperatures, the cumRR was 0.69 (95% CI: 0.51–0.94) for lag 0–14 days, which is consistent with previous research [16,23]. For moderately low temperatures, the cumRR was 0.83 (95% CI: 0.71–0.97) in lag 0–14 days. The result was consistent with Michelozzi et al. on temperature and CVD related admission in 12 European cities [50]. The decrease in risk with temperature was also demonstrated in Rasht for the association between apparent temperature and acute coronary syndrome [18]. However, numerous studies have found that extremely low temperatures can significantly increase hospital admissions and mortality [20,21,39,45,51], and most current study on low temperature and CVD related admissions are centered around extreme cold.
Consistent with the previous studies, we found that individual characteristics (such as gender, and age) could modify the impact of temperatures. The relative risk of cold decreased more significantly in female and individuals ≥65 years than in male and those <65 years, with RR of 0.94 (95% CI: 0.90–0.98) and 0.95 (95% CI: 0.91–0.98), respectively. Similarly, in Hefei, cold decreased the risk of admission from CVDs in female and individuals ≥65 years more obviously than male and those <65 years [16]. The cumRR of cold from cardiovascular and cerebrovascular diseases was 0.83 (95% CI: 0.36–1.90) for female and 1.16 (95% CI: 0.60–1.74) for male in Shanghai [20]. However, Tian et al. reported female and ≥65 years were more sensitive to extreme cold in the temperature and coronary heart disease mortality study in Beijing, China [52]; male and ≥65 years were reported as susceptive groups by Liu and colleagues in Beijing, China, 2013–2016 [45]. The relative risk of heat appeared more pronounced in male and individuals ≥65 years compared with female and those <65 years, however, with no significance. Similar to our findings, aged ≥65 years accounted for more attribution of mortality burden from heat than younger in Chengdu city, although cold was responsible for a higher proportion of deaths [53]; also, those aged over 65 years old presented higher relative risks of the extremely high temperature on cause-specific cardiovascular disease mortality in Beijing [54]. It is noteworthy that several studies reported the temperature effect does not differ between men and women in Hong Gong and in England and Wales [29,30]. Gronlund found no association for CVD in study of heat and hospitalization in the elderly [55].
Differences in the results may be related to the following underlying pathological mechanisms: Cold can incite direct cardiovascular stress due to the physiological increase in blood pressure and heart rate secondary to vasoconstriction after exposure to cold, and lead to an increase in cardiac hypertrophy, platelet counts and plasma fibrinogen [20,24]. Cold is associated with increased low-density lipoprotein cholesterol and decreased high-density lipoprotein cholesterol levels, which are considered critical factors in the increased incidence of CVDs [56]. Heat can cause complex physiological changes including an increase in the heart rate, cardiac output, blood viscosity, and platelet and red blood cell counts [39]. Moreover, dehydration in heat environments leads to fluid and electrolyte disturbances, hypotension, and even endothelial cell damage [57]. At high temperatures, blood vessel dilation and blood flow from vital organs to skin surface for cooling may also increase cardiac strain [58]. Our study as well as previous ones were completed in different districts with different climatic conditions, demographic and socioeconomic characteristics, and inhabitant infrastructure is also an important reason.
In daily life, human beings are exposed to different temperature environments. The discrepancy in individual physiques and behavioral characteristics may also impact the effect of ambient temperature. The age group of <65 years and male were found to have significantly more outdoor exposure than the ≥65 years group and female with respect to work, social interaction, and physical activity during periods of cold or heat [16,51,59]; Simultaneously, the ≥65 years group shows an active reduction in outdoor exposure during the temperature warnings; Also, female and the elderly are susceptible to CVDs that are affected by temperature [27,58,60]. Studies indicated that female was more susceptible to high temperatures due to their inadequate heat dissipation capacity, and greater core, skin, and active muscle temperatures [61,62]. Winter heating safeguards, air conditioner usage and differences in peoples’ ability to adapt to local climates [63] also have an impact on the temperature-health effect.
The “harvesting effect” observed in previous studies [12,13,64,65], characterized by mortality or morbidity displacement after an increase in risk induced by a temperature extreme and air pollution [41], was also observed in our study. The presence of the harvesting effect suggests that temperature events primarily affect the subgroup of individuals who are already susceptible to chronic health conditions [13]. The occurrence of harvesting effect was impacted by the baseline health conditions of the population, social-economic status, study design and the lag structure. For example, 14 days lag and 21days lag set would omit the risk displacement during lag 15–30 in this study. However, due to a lack of detailed individual data, we could not evaluate the effect of baseline health conditions of the population and social-economic status on harvesting effect.
5. Strengths and limitation of the study
The study has some strengths worth of stating. First, it investigated the association between CVD and ambient temperature with the mean temperature, which representing the average exposure of temperature throughout the 24 h period. Second, the DLNM used in our study is a validated statistical method. It controlled the long-term trend and the seasonal trend, and adjusted for differences in daily baseline hospitalization rates. We captured the harvesting effect with maximum lag of 30 days. Moreover, we found a nearly positive linear curve of the exposure-response relation, which representing the unique health effect of temperature in Lanzhou city. The study provides additional evidence for the diversity of healthy effects of meteorological variables. As well, the study was conducted in the undeveloped northwest region in China, with great benefit to public health.
Our study has some limitations. First, the study data were obtained from a single city, making it difficult to generalize the findings to other cities and climates. Second, we failed to obtain complete personal information of patients, including marital status, occupation, income, outdoor exposure, and ethnic origin. Third, meteorological variables and air pollutant data were extracted from appropriate government agencies rather than from individual exposure, resulting in unavoidable measurement deviations [39]. Forth, these three hospitals providing inpatient data were in the same district, that limiting the representativeness of our study. Finally, we discovered a slight peak of overall cumulative relative risk in the temperature range of 14 °C–20 °C, deficit of individual outdoor exposure restricting further research on it. Therefore, further studies are required to investigate the adverse effects of mild temperature to extend the understanding of the whole range of temperature effect.
6. Conclusions
Our study found a positive and nearly linear correlation between ambient temperature and CVD related admissions. The maximum relative risk corresponded to 24 °C, other than 30 °C. Male and those ≥65 years were susceptive groups to high temperature. Our findings suggest that public health plans and preventive measures aimed at moderating heat would have a more significant impact on decreasing the risk of CVD related admissions.
Author contribution statement
Zheng Zhang: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Dingxiong Xie: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Jianjian Jin: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Xiaoxue Meng; Dongmei Wang: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Bing Han; Tingting Wu; Qi Zhang; Jing Xie: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
Xiaoxue Meng was supported by Natural Science Foundation of Gansu Province [20JR10RA702].
Data availability statement
The authors are not permitted to share the hospitalization, meteorological and pollutant data used in the analyses publicly. For information on data access, readers with research needs are asked to contact Mr. Zheng Zhang (zhanglzu@yeah.net), who is the lead contact. All original code used in the research were included in the supplementary materials.
Acknowledgements
The authors would like to thank Lanzhou University First Hospital, Lanzhou University Second Hospital, Gansu Provincial People’s Hospital, Lanzhou Meteorological Bureau, and Lanzhou Environmental Monitoring Center for this study’s user data.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2023.e12997.
Contributor Information
Dingxiong Xie, Email: xiedingxiong45@163.com.
Zheng Zhang, Email: zhanglzu@yeah.net.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Bhaskaran K., Hajat S., Haines A., Herrett E., Wilkinson P., Smeeth L. Effects of ambient temperature on the incidence of myocardial infarction. Heart. 2009;95(21):1760–1769. doi: 10.1136/hrt.2009.175000. [DOI] [PubMed] [Google Scholar]
- 2.Shen Y., Zhang X., Chen C., Lin Q., Li X., Qu W., et al. The relationship between ambient temperature and acute respiratory and cardiovascular diseases in Shenyang. China Environ. Sci. Pollut. Res. Int. 2021;28(16):20058–20071. doi: 10.1007/s11356-020-11934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner L.R., Barnett A.G., Connell D., Tong S. vol. 23. 2012. pp. 594–606. (Ambient Temperature and Cardiorespiratory Morbidity: A Systematic Review and Meta-Analysis Epidemiology). [DOI] [PubMed] [Google Scholar]
- 4.Radišauskas R., Vaičiulis V., Ustinavičienė R., Bernotienė G. The effect of atmospheric temperature and pressure on the occurrence of acute myocardial infarction in Kaunas. Medicina. 2013;49(10):447–452. doi: 10.1186/s12940-017-0238-0. [DOI] [PubMed] [Google Scholar]
- 5.Phung D., Thai P.K., Guo Y., Morawska L., Rutherford S., Chu C. vol. 550. 2016. pp. 1084–1102. (Ambient Temperature and Risk of Cardiovascular Hospitalization: an Updated Systematic Review and Meta-Analysis Science of the Total Environment). [DOI] [PubMed] [Google Scholar]
- 6.Gasparrini A., Guo Y., Hashizume M., Lavigne E., Zanobetti A., Schwartz J., et al. Mortality risk attributable to high and low ambient temperature: a multicountry observational study. Lancet. 2015;386(9991):369–375. doi: 10.1016/s0140-6736(14)62114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMichael A.J., Wilkinson P., Kovats R.S., Pattenden S., Hajat S., Armstrong B., et al. International study of temperature, heat and urban mortality: the 'ISOTHURM' project. Int. J. Epidemiol. 2008;37:1121–1131. doi: 10.1093/ije/dyn086. [DOI] [PubMed] [Google Scholar]
- 8.Chan E.Y.Y., Goggins W.B., Kim J.J., Griffiths S.M. A study of intracity variation of temperature-related mortality and socioeconomic status among the Chinese population in Hong Kong. J. Epidemiol. Community Health. 2012;66:322–327. doi: 10.1136/jech.2008.085167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu P., Xia G., Zhao Q., Xu R., Li S.Y. Guo Temporal trends of the association between ambient temperature and hospitalisations for cardiovascular diseases in Queensland, Australia from 1995 to 2016: a time-stratified case-crossover study. PLoS Med. 2020;17(7):e1003176. doi: 10.1371/journal.pmed.1003176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lubczynska M.J., Christophi C.A., Lelieveld J. Heat-related cardiovascular mortality risk in Cyprus: a case-crossover study using a distributed lag non-linear model. Environ. Health. 2015;14:39. doi: 10.1186/s12940-015-0025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gasparrini A., Armstrong B., Kovats S., Wilkinson P. The effect of high temperatures on cause-specific mortality in England and Wales Occup. Environ. Med. 2012;69:56–61. doi: 10.1136/oem.2010.059782. [DOI] [PubMed] [Google Scholar]
- 12.Bhaskaran K., Gasparrini A., Hajat S., Smeeth L., Armstrong B. Time series regression studies in environmental epidemiology. Int. J. Epidemiol. 2013;42:1187–1195. doi: 10.1093/ije/dyt092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kouis P., Kakkoura M., Ziogas K., Paschalidou A.Κ. vol. 647. Greece Science of The Total Environment; 2019. pp. 1351–1358. (Papatheodorou the Effect of Ambient Air Temperature on Cardiovascular and Respiratory Mortality in Thessaloniki). S.I. [DOI] [PubMed] [Google Scholar]
- 14.Ponjoan A., Blanch J., Alves-Cabratosa L., Marti-Lluch R., Comas-Cufi M., Parramon D., et al. Effects of extreme temperatures on cardiovascular emergency hospitalizations in a Mediterranean region: a self-controlled case series study Environ. Health. 2017;16(1):32. doi: 10.1186/s12940-017-0238-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J., Mauzerall D.L., Zhu T., Liang S., Ezzati M., Remais J.V. Environmental health in China: progress towards clean air and safe water. Lancet. 2010;375:1110–1119. doi: 10.1016/S0140-6736(10)60062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui L., Geng X., Ding T., Tang J., Xu J., Zhai J. Impact of ambient temperature on hospital admissions for cardiovascular disease in Hefei City. China Int. J. Biometeorol. 2019;63(6):723–734. doi: 10.1007/s00484-019-01687-0. [DOI] [PubMed] [Google Scholar]
- 17.Nia H.S., Gorgulu O., Naghavi N., Froelicher E.S., Fomani F.K., Goudarzian A.H., et al. A time-series prediction model of acute myocardial infarction in northern of Iran: the risk of climate change and religious mourning. BMC Cardiovasc. Disord. 2021;21(1):563. doi: 10.1186/s12872-021-02372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moghadamnia M.T., Ardalan A., Mesdaghinia A., Naddafi K., Yekaninejad M.S. Association between apparent temperature and acute coronary syndrome admission in Rasht. Iran Heart Asia. 2018;10(2):e011068. doi: 10.1136/heartasia-2018-011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silveira I.H., Oliveira B.F.A., Cortes T.R., Junger W.L. The effect of ambient temperature on cardiovascular mortality in 27 Brazilian cities. Sci. Total Environ. 2019;691:996–1004. doi: 10.1016/j.scitotenv.2019.06.493. [DOI] [PubMed] [Google Scholar]
- 20.Xiong J., Lan L., Lian Z., Lin Y. Effect of different temperatures on hospital admissions for cardiovascular and cerebrovascular diseases. Case Study Indoor Built Environ. 2016;26(1):69–77. doi: 10.1177/1420326X15604492. [DOI] [Google Scholar]
- 21.Zhao Q., Zhao Y., Li S., Zhang Y., Wang Q., Zhang H., et al. Impact of ambient temperature on clinical visits for cardio-respiratory diseases in rural villages in northwest China. Sci. Total Environ. 2018;612:379–385. doi: 10.1016/j.scitotenv.2017.08.244. [DOI] [PubMed] [Google Scholar]
- 22.Sun Z., Chen C., Xu D., Li T. Effects of ambient temperature on myocardial infarction: a systematic review and meta-analysis. Environ. Pollut. 2018;241:1106–1114. doi: 10.1016/j.envpol.2018.06.045. [DOI] [PubMed] [Google Scholar]
- 23.Bijelović S., Dragić N., Bijelović M., Kovačević M., Jevtić M., Mrđenovački O.N. Impact of climate conditions on hospital admissions for subcategories of cardiovascular diseases. Med. Pr. 2017;68(2):189–197. doi: 10.13075/mp.5893.00606. [DOI] [PubMed] [Google Scholar]
- 24.Lam H.C.Y., Chan J.C.N., Luk A.O.Y., Chan E.Y.Y., Goggins W.B. Short-term association between ambient temperature and acute myocardial infarction hospitalizations for diabetes mellitus patients: a time series study. PLoS Med. 2018;15(7):e1002612. doi: 10.1371/journal.pmed.1002612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akioka H., Yufu K., Hara M., Abe I., Kondo H., Saito S., et al. Impact of age on gender differences in the acute myocardial infarction onset-weather association- oita ami registry Circ. For. Rep. 2020;2(3):152–157. doi: 10.1253/circrep.CR-19-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin S., Luo M., Walker R.J., Liu X., Hwang S.A., Chinery R. vol. 20. 2009. pp. 738–746. (Extreme High Temperatures and Hospital Admissions for Respiratory and Cardiovascular Diseases Epidemiology). [DOI] [PubMed] [Google Scholar]
- 27.Kwon B.Y., Lee E., Lee S., Heo S., Jo K., Kim J., et al. Vulnerabilities to temperature effects on acute myocardial infarction hospital admissions in South Korea. Int. J. Environ. Res. Publ. Health. 2015;12(11):14571–14588. doi: 10.3390/ijerph121114571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medina-Ramon M., Zanobetti A., Cavanagh D.P., Schwartz J. Extreme temperatures and mortality: assessing effect modification by personal characteristics and specific cause of death in a multi-city case-only analysis Environ. Health Perspect. 2006;114(9):1331–1336. doi: 10.1289/ehp.9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan E.Y., Goggins W.B., Yue J.S., Lee P. Hospital admissions as a function of temperature, other weather phenomena and pollution levels in an urban setting in China. Bull. World Health Organ. 2013;91:576–584. doi: 10.2471/BLT.12.113035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhaskaran K., Hajat S., Haines A., Herrett E., Wilkinson P., Smeeth L. Short term effects of temperature on risk of myocardial infarction in England and Wales: time series regression analysis of the Myocardial Ischaemia National Audit Project. MINAP) registry BMJ. 2010;341:c3823. doi: 10.1136/bmj.c3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang C., Meng X., Chen R., Cai J., Zhao Z., Wan Y., et al. Long-term variations in the association between ambient temperature and daily cardiovascular mortality in Shanghai. China Sci. Total Environ. 2015;538:524–530. doi: 10.1016/j.scitotenv.2015.08.097. [DOI] [PubMed] [Google Scholar]
- 32.Cheng J., Xu Z., Zhang X., Zhao H., Hu W. vol. 682. Scientific evidence for policy making Science of the Total Environment; China: 2019. Estimating Cardiovascular Hospitalizations and Associated Expenses Attributable to Ambient Carbon Monoxide in Lanzhou; pp. 514–522. [DOI] [PubMed] [Google Scholar]
- 33.Tian Y., Liu H., Zhao Z., Xiang X., Li M., Juan J., et al. Association between ambient air pollution and daily hospital admissions for ischemic stroke: a nationwide time-series analysis. PLoS Med. 2018;15(10):e1002668. doi: 10.1371/journal.pmed.1002668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gasparrini A., Armstrong B., Kenward M.G. Distributed lag non-linear models. Stat. Med. 2010;29(21):2224–2234. doi: 10.1002/sim.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gasparrini A., Leone M. Attributable risk from distributed lag models. BMC Med. Res. Methodol. 2014;14:55. doi: 10.1186/1471-2288-14-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li H., Wu J., Wang A., Li X., Chen S., Wang T., et al. Effects of ambient carbon monoxide on daily hospitalizations for cardiovascular disease: a time-stratified case-crossover study of 460,938 cases in Beijing, China from 2013 to 2017. Environ. Health. 2018;17(1):82. doi: 10.1186/s12940-018-0429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo P., Zheng M., Wang Y., Feng W., Wu J., Deng C., et al. Effects of ambient temperature on stroke hospital admissions: results from a time-series analysis of 104,432 strokes in Guangzhou. China Sci. Total Environ. 2017;580:307–315. doi: 10.1016/j.scitotenv.2016.11.093. [DOI] [PubMed] [Google Scholar]
- 38.Guo Y., Barnett A.G., Pan X., Yu W., Tong S. The impact of temperature on mortality in Tianjin, China: a case-crossover design with a distributed lag nonlinear model. Environ. Health Perspect. 2011;119:1719–1725. doi: 10.1289/ehp.1103598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bai L., Li Q., Wang J., Lavigne E., Gasparrini A., Copes R., et al. Increased coronary heart disease and stroke hospitalisations from ambient temperatures in. Ontario Heart. 2018;104(8):673–679. doi: 10.1136/heartjnl-2017-311821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferreira L.C.M., Nogueira M.C., Pereira R.V.B., de Farias W.C.M., Rodrigues M.M.S., Teixeira M.T.B., et al. Ambient temperature and mortality due to acute myocardial infarction in Brazil: an ecological study of time-series analyses. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-50235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwartz J. Is there harvesting in the association of airborne particles with daily deaths and hospital admissions? Epidemiology. 2001;12:55–61. doi: 10.1097/00001648-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Tsangari H., Paschalidou A., Vardoulakis S., Heaviside C., Konsoula Z., Christou S., et al. Human mortality in Cyprus: the role of temperature and particulate air pollution. Reg. Environ. Change. 2016;16(7):1905–1913. doi: 10.1007/s10113-015-0793-2. [DOI] [Google Scholar]
- 43.Messner T. Environmental variables and the risk of disease. Int. J. Circumpolar Health. 2005;64(5):523–533. doi: 10.3402/ijch.v64i5.18033. [DOI] [PubMed] [Google Scholar]
- 44.Deng C., Ding Z., Li L., Wang Y., Guo P., Yang S., et al. Burden of non-accidental mortality attributable to ambient temperatures: a time series study in a high plateau area of southwest China BMJ. Open. 2019;9(2):e024708. doi: 10.1136/bmjopen-2018-024708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu X., Kong D., Fu J., Zhang Y., Liu Y., Zhao Y., et al. Association between extreme temperature and acute myocardial infarction hospital admissions in Beijing, China: 2013-2016. PLoS One. 2018;13(10):e0204706. doi: 10.1371/journal.pone.0204706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang Y., Hu J., Peng L., Li H., Ji J.S., Fang W., et al. Non-optimum temperature increases risk and burden of acute myocardial infarction onset: a nationwide case-crossover study at hourly level in 324 Chinese cities. EClinicalMedicine. 2022;50 doi: 10.1016/j.eclinm.2022.101501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wichmann J., Andersen Z.J., Ketzel M., Ellermann T. Loft Apparent temperature and cause-specific mortality in Copenhagen, Denmark: a case-crossover analysis. Int. J. Environ. Res. Publ. Health. 2011;8:3712–3727. doi: 10.3390/ijerph8093712. S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li N., Ma J., Liu F., Zhang Y., Ma P., Jin Y., et al. Associations of apparent temperature with acute cardiac events and subtypes of acute coronary syndromes in Beijing, China. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-94738-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen R., Yin P., Wang L., Liu C., Niu Y., Wang W., et al. Association between ambient temperature and mortality risk and burden: time series study in 272 main Chinese cities. BMJ. 2018;363:k4306. doi: 10.1136/bmj.k4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Michelozzi P., Accetta G., De Sario M., D'Ippoliti D., Marino C., Baccini M., et al. High temperature and hospitalizations for cardiovascular and respiratory causes in 12 european cities. Am. J. Respir. Crit. Care Med. 2009;179(5):383–389. doi: 10.1164/rccm.200802-217OC. [DOI] [PubMed] [Google Scholar]
- 51.Luo Y., Li H., Huang F., Van Halm-Lutterodt N., Xu Q., Wang A., et al. The cold effect of ambient temperature on ischemic and hemorrhagic stroke hospital admissions: a large database study in Beijing, China between years 2013 and 2014-Utilizing a distributed lag non-linear analysis Environ. Pollut. 2018;232:90–96. doi: 10.1016/j.envpol.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 52.Tian Z., Li S., Zhang J., Jaakkola J.J.K., Guo Y. Ambient temperature and coronary heart disease mortality in Beijing, China: a time series study Environ. Health. 2012;11:56. doi: 10.1186/1476-069X-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cui Y., Yin F., Deng Y., Volinn E., Chen F., Ji K., et al. Heat or cold: which one exerts greater deleterious effects on health in a basin climate city? Impact of ambient temperature on mortality in Chengdu. China Int. J. Environ. Res. Public Health. 2016;13(12) doi: 10.3390/ijerph13121225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X., Li G., Liu L., Westerdahl D., Jin X., Pan X. Effects of extreme temperatures on cause-specific cardiovascular mortality in China. Int. J. Environ. Res. Publ. Health. 2015;12(12):16136–16156. doi: 10.3390/ijerph121215042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gronlund C.J., Zanobetti A., Schwartz J.D., Wellenius G.A., O'Neill M.S. Heat, heat waves, and hospital admissions among the elderly in the United States, 1992–2006 Environ. Health Perspect. 2014;122(11):1187–1192. doi: 10.1289/ehp.1206132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hong Y.C., Kim H., Oh S.Y., Lim Y.H., Kim S.Y., Yoon H.J., et al. Association of cold ambient temperature and cardiovascular markers. Sci. Total Environ. 2012;435–436:74–79. doi: 10.1016/j.scitotenv.2012.02.070. [DOI] [PubMed] [Google Scholar]
- 57.Cheng X., Su H. Effects of climatic temperature stress on cardiovascular diseases. Eur. J. Intern. Med. 2010;21:164–167. doi: 10.1016/j.ejim.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 58.Basu R. High ambient temperature and mortality: a review of epidemiologic studies from 2001 to 2008. Environ. Health. 2009;8:40. doi: 10.1186/1476-069X-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wichmann J., Ketzel M., Ellermann T. Loft Apparent temperature and acute myocardial infarction hospital admissions in Copenhagen, Denmark: a case-crossover study Environ. Health. 2012;11(1):1–12. doi: 10.1186/1476-069X-11-19. S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Basu R., Dominici F. Samet Temperature and mortality among the elderly in the United States: a comparison of epidemiologic methods. Epidemiology. 2005;16(1):58–66. doi: 10.1097/01.ede.0000147117.88386.fe. J.M. [DOI] [PubMed] [Google Scholar]
- 61.Gagnon D., Kenny G.P. Does sex have an independent effect on thermoeffector responses during exercise in the heat? J. Physiol. 2012;590(23):5963–5973. doi: 10.1113/jphysiol.2012.240739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gagnon D., Dorman L.E., Jay O., Hardcastle S., Kenny G.P. Core temperature differences between males and females during intermittent exercise: physical considerations. Eur. J. Appl. Physiol. 2009;105:453–461. doi: 10.1007/s00421-008-0923-3. [DOI] [PubMed] [Google Scholar]
- 63.Mercer J.B. Cold—an underrated risk factor for health. Environ. Res. 2003;92:8–13. doi: 10.1016/s0013-9351(02)00009-9. [DOI] [PubMed] [Google Scholar]
- 64.Rabl A. Air pollution mortality: harvesting and loss of life expectancy. J. Toxicol. Environ. Health, Part A. 2005;68:1175–1180. doi: 10.1080/15287390590936049. [DOI] [PubMed] [Google Scholar]
- 65.Kinney P.L., O’Neill M.S., Bell M.L. J. Schwartz Approaches for estimating effects of climate change on heat-related deaths: challenges and opportunities. Environ. Sci. Pol. 2008;11(1):87–96. doi: 10.1016/j.envsci.2007.08.001. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors are not permitted to share the hospitalization, meteorological and pollutant data used in the analyses publicly. For information on data access, readers with research needs are asked to contact Mr. Zheng Zhang (zhanglzu@yeah.net), who is the lead contact. All original code used in the research were included in the supplementary materials.