Abstract
Though mass vaccination programs helped to reduce the severity of the ongoing pandemic, various unwanted effects were reported in Turkey and Bangladesh after taking vaccines. The purpose of this study was to evaluate and compare the adverse effects of several vaccines in Turkey and Bangladesh and how the population of both countries prioritizes the continuation of vaccination compared to the side effects. An online survey with a pretest was conducted to gather data over the research period from July 10, 2021 to December 10, 2021. Finally, the questionnaire was shared with the mass population of Turkey and Bangladesh who have received at least one or two doses of the COVID-19 vaccines. The quality of the questionnaire was evaluated with Cronbach’s alpha test. The study consisted of 1508 respondents from Bangladesh and 602 respondents from Turkey. Among the total 2110 respondents, 50.0% were male 66.8% were from the 18–30 years age range, and 77.5% reported living in the city area. Among all the respondents, 64.99% of those vaccinated in Bangladesh and 67.28% of those vaccinated in Turkey reported side effects after vaccinations. Participants receiving mRNA vaccines (Pfizer and Moderna) experienced the most side effects, with many reporting pain at the injection site in both nations. Following that, fever, body pain, and headache were common in Bangladesh, whereas body pain, fatigue, and arm numbness were common in Turkey. The study found no significant adverse events reported in Turkey and Bangladesh following the first and second doses of COVID-19 vaccination. These COVID-19 vaccines showed similar patterns of efficacy and safety during the short period of analysis. Vaccines from different manufacturers showed a non-significant level of adverse events during this binational AEFI approach to COVID-19 vaccines. More studies are recommended on the efficacy and safety of several vaccines to discover unexpected effects.
Keywords: COVID-19 vaccine; Vaccine side effect, After Events following Immunization (AEFI), Turkey, Bangladesh
Introduction
The novel coronavirus disease 2019 (COVID-19) is primarily caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and causes public health issues as well as substantial economic implications across the world (Hossain et al. 2020). In December 2019, the first case of COVID-19 was detected, and in the latter week of January 2020, it was proclaimed a public health emergency, and in the middle of March 2020, it was designated a pandemic (Wiersinga et al. 2020; Zhu et al. 2020). This pandemic has continued till now due to the virus’s fast mutation and spread around the world (Hatmal et al. 2021). In order to slow down the global spread of COVID-19, several preventative and control measures at varying levels have been developed in country territories. Individually, they include things like keeping at least three feet of distance from each other, washing hands often, using hand sanitizers, covering mouth while coughing or sneezing, not shaking hands or kissing strangers, staying away from those who are showing the symptoms of different respiratory diseases, and wearing a face mask in public places (Al-Tammemi 2020; Lai et al. 2020). At least 186 nations have instituted varied degrees of population movement restrictions in order to curb the spread of the coronavirus and keep health services from being overburdened; these restrictions have resulted in lockdowns in 82 countries (Lai et al. 2020).
The rapid development of vaccines and anti-viral medicine against the SARS-CoV-2 virus prevents the acceleration of the explosive nature of the pandemic (Harmukh 2020; Felsenstein et al. 2020; Wu et al. 2021). Prevention of diseases from life-threatening infections is made simpler with the use of vaccinations which assist the body’s adaptive immunity (Lee 2021; Fritsche et al. 2010). Moreover, this novel discovery also faces challenges in regard to keeping promises of ensuring optimal immune response against the extremely infectious and lethal strains of SARS-CoV-2 (Harrison and Wu 2020). Multiple advanced technological approaches in structural biology and genomics present a new era of COVID-19 vaccines with very non-significant adverse effects (Sharma et al. 2020).
By the beginning of 2021, many international health authorities had declared the licensing of several vaccine candidates for emergency use (Alhazmi et al. 2021). The COVID-19 worldwide pandemic problem has generated socioeconomic and public health challenges that are part of the ongoing debate (Ardito et al. 2021). Yet, the success rate of disease prevention and management across countries is correlated not only with the quick distribution of COVID-19 vaccines to the mass population but also with strong governance, relatively high health expenditures, and universal healthcare systems (Coccia 2021). Since November 11, 2020, 259 vaccine development projects have been initiated worldwide with the objective to provide safe and effective vaccines for COVID-19 (Saeed et al. 2021), there are several vaccines that get authorized approval for worldwide use. Expansion of vaccine production got the priority to meet the urge in the time of the explosion of various strains of SARS-CoV-2 (Shahcheraghi et al. 2021). Increased availability of vaccine supply provides mass immunization efforts and offers a promise in expanding vaccination capacity and increasing vaccination rates — a critical step toward ending the COVID-19 global pandemic (Goralnick et al. 2021). Consequently, both Turkey and Bangladesh initiated a mass vaccination program on January 14, 2021 (COVID, 19 C.E.) and February 7, 2021 (Abedin et al. 2021), respectively. Already, Turkey has deployed four vaccines (Tokuç and Varol 2020), and Bangladesh has deployed five vaccines (Khatun 2021). However, certain common and uncommon adverse events have been observed after the deployment of a widespread vaccination program (El-Shitany et al. 2021; Riad et al. 2021; Solomon et al. 2021; Tissot et al. 2021). Though according to “The Centers for Disease Control and Prevention (CDC),” the majority of these symptoms should subside after a few days, some unusual effects were reported to stay longer than expected (Gee et al. 2021; Hartert and Sockrider 2021). For instance, suspected severe allergic reactions and anaphylaxis following vaccination were reported in the Vaccine Adverse Event Reporting System (VAERS), the national passive surveillance (spontaneous reporting) system for adverse events after immunization after the administration of Pfizer-BioNTech vaccine (Shimabukuro and Nair 2021).
Therefore, collecting pharmacovigilance data is critical for recognizing adverse events and comprehending their nature, frequency, and potential risk factors to the degree feasible (Hossain and Amran 2019). Besides, it is important to monitor side effects and perceptions following immunization in order to better understand vaccine efficacy and to combat vaccine pessimism and rumors (Sallam et al. 2021). However, it is pretty much possible that the prevalence and severity of side effects may vary with age, gender, or even geographic location. Thus, the primary aim of this study is to picture a comparative assessment of the adverse effects that patients reported after receiving different COVID-19 vaccines in Bangladesh and Turkey. In particular, this study can provide potential answers to various questions and logical inferences, such as (a) Is vaccination safe?; (b) What is the perception of people of two countries towards the mass vaccination process?; (c) Which vaccine is relatively more effective?; (d) Which variables influence the function of vaccines?; (e) Do the environments of two nations have an influence on the effects of vaccines?; and (f) Has any vaccination demonstrated negative impacts or interactions with body components? Besides, this study has also provided a glimpse of the management patterns of people from two countries before mass vaccination started. This study could therefore provide a clear idea of the differences in disease prevalence according to demographic characteristics, perception of people towards vaccines, distribution of vaccines, patterns, and management of side effects after taking different COVID-19 vaccines between Turkey and Bangladesh, which may help to decide effective management systems to terminate COVID-19 pandemic.
Materials and methods
Sample and data
This retrospective and cross-sectional survey on AEFIs of the COVID-19 vaccination was conducted online. Following a thorough assessment of COVID-19 data and surveillance from the CDC, the survey questionnaire was developed (Sultana et al. 2021). The questionnaire was finalized after an extensive review of relevant literature on the related adverse effects of different COVID-19 vaccines (Hatmal et al. 2021) (Alhazmi et al. 2021; Saeed et al. 2021) (Khan et al. 2020) and group discussion. The Ethical Review Committee of the Faculty of Biological Sciences, University of Dhaka gave its clearance for this survey (Reference No. 159/Biol. Scs.), and all users provided consent for the non-commercial use of their data. The online questionnaire was then circulated via social and electronic media (Email, Facebook, Messenger, Twitter, and WhatsApp) using a snowball sampling method. We pre-tested the questionnaire by sending it to 50 primary recipients at the start of the COVID-19 immunization program. The pre-testing was conducted to ensure that the questionnaire was clear and unambiguous. These individuals were thereafter urged to share the survey questionnaire on their social networking sites.
Sample selection
The intended participants were Bangladeshi and Turkish persons who can read and comprehend Bangla, Turkish, or English, and received either a single dose or a double dose of any of the COVID-19 vaccines. Because of the limitations of employing face-to-face procedures during an active outbreak, all data were collected only through the use of the Google Forms platform. We have removed any participants who declined to take part in the study or who were not immunized against COVID-19 from consideration. Throughout Bangladesh and Turkey, this online form was widely circulated on social media and electronic websites and included people from various socioeconomic levels as well as across all age groups.
Measures of variables
The survey questionnaire was created using Google Forms in English and evaluated by an expert panel who offered input on the survey’s various components, which were subsequently revised based on their ideas. Prior to testing and distribution, the survey was translated into Bangla and Turkish for greater comprehension.
The survey form was divided into eight sections, each of which contained information about the vaccination, the participant’s health prior to and following the vaccination, the associated side effects of the first and second doses of the vaccine, and any symptom management steps taken by the participant. The first section provided background information about the study and requested approval. All respondents were required to respond to this section in order to proceed with the survey. The second section of the questionnaire requested information about the respondent's age, gender, nationality, area of residence, educational qualifications, and occupation. Additionally, this part included a 5-point Likert scale remark stating that “Taking COVID-19 vaccination can successfully prevent COVID-19 infection.” The third section of the questionnaire was designated for women exclusively. It included four questions on the female’s pregnancy and lactation status, as well as information about tetanus vaccination. The next part Section 4 addressed numerous issues about the individuals’ health status before immunization. This portion included questions about the participant’s current COVID-19 status, allergic problems, chronic diseases with current treatment routines, and past immunization history. The majority of the questions in this section have a binary “yes” or “no” structure. Section 5 of the questionnaire was prepared specifically for responders who had been infected with COVID-19 prior to immunization. It included some questions containing the complications related to COVID-19 infection, pharmacological and non-pharmacological management of the infection and recovery time. Section 6 was specially designed for the chronic disease condition (comorbidity) before vaccination. The respondents who were suffering from different types of chronic diseases were asked two questions containing the name of the disease(s) and medicines taken for their disease(s). Sections seven and eight were addressed as “After Events Following Vaccination” for the 1st and the 2nd doses, respectively. These sections contained some questions concerning the vaccination information of the current individual, including vaccine name, vaccination date, vaccination center, and any specific information given after vaccination. In our segment on side effects, we included the most frequently reported adverse effects from prior studies (Alhazmi et al. 2021; Gee et al. 2021; Hatmal et al. 2021; Menni et al. 2021; Saeed et al. 2021; Zhu et al. 2020), including pain and irritation at the site of injection, body and joint pain, headache, fever, nausea, vomiting, diarrhea, sore throat, decreased appetite, fatigue, anaphylaxis, and drowsiness. Additionally, we included a space for reporting any additional unlisted adverse events that our trial participants may have encountered. These sections discussed the duration and severity of side effects, as well as the management and treatment plan for physical discomfort experienced following vaccination. There were also two questions where the respondents were asked if they were affected by COVID-19 or had COVID-like symptoms after taking the 1st or the 2nd doses of the vaccine.
Duration of the study
The research was carried out between July 10, 2021, and December 10, 2021. To gather responses from COVID-19 vaccine recipients, a total of 5 months was allotted for response collection.
Data analysis procedure
The data collected from Turkey and Bangladesh were first analyzed for consistency. Inconsistent data in the data set and the participants who filled out the questionnaire even though they were not vaccinated were excluded from the study. Frequency and percentage distributions of the demographic data of the participants were examined on the data set. In addition, the questions about the vaccination status of the participants, the symptoms that are seen after the vaccination, the hospitalization status, side effects, the intensity and duration of the side effects, and the treatments applied were compared using the chi-square test of independence in the samples of Turkey and Bangladesh. The Monte Carlo p-value was used for the variables for which there were not enough observations in the distributions. Analyses were conducted on SPSS 22 software package. Internal consistency between multiple survey items of the questionnaire was evaluated through Cronbach’s alpha analysis (Sultana et al. 2021). In this analysis, Cronbach’s alpha describes the coherence between the descriptors of the survey study. Internal consistency among the demographical data, COVID-19 vaccine-related side effects, and severity of it has been explained with the reliability indicator Cronbach’s alpha value of 0.89. This value indicates a higher interrelatedness in the assessment of the questionnaire (Taber 2018).
Results
The study consisted of 602 people from Turkey and 1508 people from Bangladesh who had at least one dose of the COVID-19 vaccines. Among them, 561 individuals from Turkey and 806 individuals from Bangladesh took the second dose of the COVID-19 vaccines. Table 1 showed the demographic data of individuals from Turkey and Bangladesh who participated in the research.
Table 1.
Demographic data of participants from Turkey and Bangladesh who have taken at least one dose of COVID-19 vaccine
| Sociodemographic parameters | Turkey | Bangladesh | Total | ||||
|---|---|---|---|---|---|---|---|
| F | % | F | % | F | % | ||
| Gender | Male | 125 | 20.8 | 931 | 61.7 | 1056 | 50.0 |
| Female | 474 | 78.7 | 574 | 38.1 | 1048 | 49.7 | |
| Not specified | 3 | 0.5 | 3 | 0.2 | 6 | 0.3 | |
| Age | < 18 | 29 | 4.8 | 9 | 0.6 | 38 | 1.8 |
| 18–30 | 457 | 75.9 | 953 | 63.2 | 1410 | 66.8 | |
| 31–40 | 38 | 6.3 | 210 | 13.9 | 248 | 11.8 | |
| 41–50 | 53 | 8.8 | 153 | 10.1 | 206 | 9.8 | |
| 51–60 | 20 | 3.3 | 115 | 7.6 | 135 | 6.4 | |
| 61–70 | 3 | 0.5 | 53 | 3.5 | 56 | 2.7 | |
| > 70 | 2 | 0.3 | 15 | 1.0 | 17 | 0.8 | |
| Place of residence | Village | 38 | 6.3 | 352 | 23.3 | 390 | 18.5 |
| City | 557 | 92.5 | 1079 | 71.6 | 1636 | 77.5 | |
| Abroad | 7 | 1.2 | 77 | 5.1 | 84 | 4.0 | |
| Total | 602 | 100 | 1508 | 100 | 2110 | 100 | |
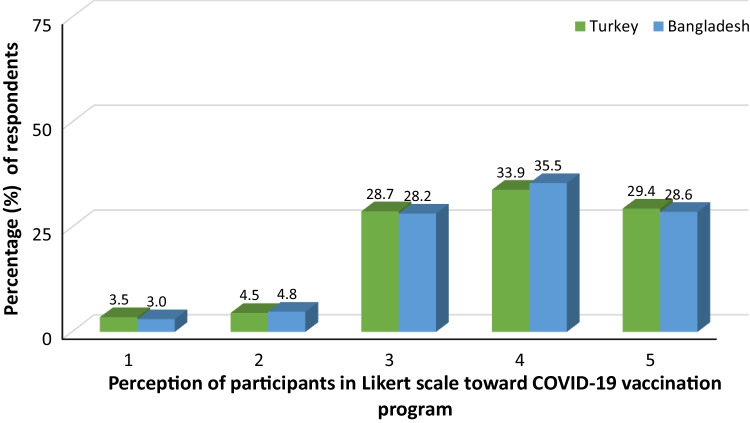
Figure 1 shows the distributions of perceptions regarding the prevention of infection by COVID-19 vaccines. The participants both from Turkey and Bangladesh agreed that vaccines would prevent infection.
Fig. 1.
Perceptions regarding the prevention of infection by COVID-19 vaccines (1: Totally disagree, 2: Disagree, 3: Neutral, 4: Agree 5: Totally agree)
The rate of women who were vaccinated during pregnancy was 0.42% in Turkey and 0.87% in Bangladesh. Two out of seven women (28.57%) who were vaccinated during pregnancy stated that there was an abnormality in their pregnancy.
Table 2 included the COVID-19 status and treatments applied before vaccination. Before they were vaccinated, 23.26% of respondents in Turkey and 13.53% of respondents in Bangladesh had COVID-19. While 66.86% of these people who had COVID-19 before they were vaccinated received drug treatment, 55.23% used herbal supplements and alternative treatment.
Table 2.
Contracting COVID-19 and treatments before getting vaccinated
| Turkey | Bangladesh | Total | χ2 | p | |||||
|---|---|---|---|---|---|---|---|---|---|
| F | % | F | % | F | % | ||||
| Contracting COVID-19 before getting vaccinated | No | 462 | 76.74 | 1304 | 86.47 | 1766 | 83.70 | 28.838 | < 0.001 |
| Yes | 140 | 23.26 | 204 | 13.53 | 344 | 16.30 | |||
| Plasma treatment | No | 137 | 97.86 | 202 | 99.02 | 339 | 98.55 | 0.783 | 0.652a |
| Yes | 3 | 2.14 | 2 | 0.98 | 5 | 1.45 | |||
| Herbal support/alternative treatment | No | 70 | 50.00 | 84 | 41.18 | 154 | 44.77 | 2.614 | 0.106 |
| Yes | 70 | 50.00 | 120 | 58.82 | 190 | 55.23 | |||
| Hospital | No | 116 | 82.86 | 175 | 85.78 | 291 | 84.59 | 0.546 | 0.460 |
| Yes | 24 | 17.14 | 29 | 14.22 | 53 | 15.41 | |||
| Medicine | No | 36 | 25.71 | 78 | 38.24 | 114 | 33.14 | 5.874 | 0.015 |
| Yes | 104 | 74.29 | 126 | 61.76 | 230 | 66.86 | |||
aMonte Carlo p-value
Due to COVID-19 infection, 2.49% of individuals in Turkey and 5.31% of individuals in Bangladesh were hospitalized before vaccination. Numerous complexities were shown when they were suffering from the infection. While upper respiratory tract infection was observed with a maximum of 60% in these hospitalized people in Turkey, low oxygen saturation, and other symptoms were observed with a maximum of 37.50% in Bangladesh.
Table 3 showed the distributions of vaccines for the first and second doses. For the first dose, the most applied vaccine in Turkey was Pfizer-BioNTech (78.41%) and Sinovac (20.93%). In Bangladesh, the most applied vaccines were Sinopharm (49%), Oxford-AstraZeneca (22.68%), and Moderna (17.51%). For the second dose, the most applied vaccines in Turkey were Pfizer-BioNTech with 78.43% and Sinovac with 20.86%. In Bangladesh, the most applied vaccines were Sinopharm (38.96%), Oxford-AstraZeneca (34.49%), Moderna (14.02%), and Pfizer-BioNTech (11.29%) for the second dose.
Table 3.
Distributions of the vaccines for first and second doses in Turkey and Bangladesh
| Name of the vaccine | First dose | Second dose | ||||||
|---|---|---|---|---|---|---|---|---|
| Turkey (n = 602) | Bangladesh (n = 1508) | Turkey (n = 561) | Bangladesh (n = 806) | |||||
| F | % | F | % | F | % | F | % | |
| Moderna | 2 | 0.33 | 264 | 17.51 | 2 | 0.36 | 113 | 14.02 |
| Pfizer-BioNTech | 472 | 78.41 | 148 | 9.81 | 440 | 78.43 | 91 | 11.29 |
| Sinovac | 126 | 20.93 | 8 | 0.53 | 117 | 20.86 | 7 | 0.87 |
| Sputnik V | 1 | 0.17 | 6 | 0.40 | 1 | 0.18 | 2 | 0.25 |
| Turkovac | 1 | 0.17 | 0 | 0.00 | 1 | 0.18 | 0 | 0.00 |
| Oxford-AstraZeneca | 0 | 0.00 | 342 | 22.68 | 0 | 0.00 | 278 | 34.49 |
| Sinopharm | 0 | 0.00 | 739 | 49 | 0 | 0.00 | 314 | 38.96 |
| Johnsons | 0 | 0.00 | 1 | 0.07 | 0 | 0.00 | 1 | 0.12 |
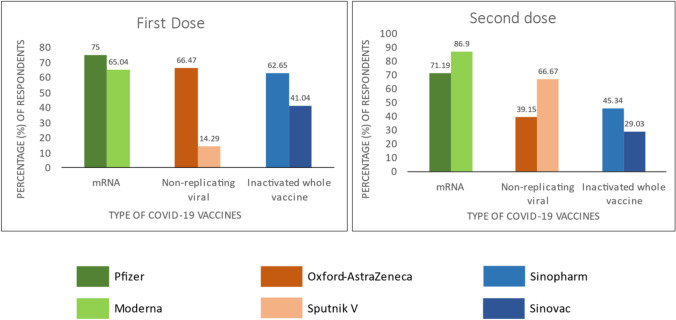
Table 4 shows the results of the comparison of physical discomfort experienced after the first and second doses of vaccination according to the vaccine types. As a result of the analysis, for the first dose, there was a significant difference in the state of experiencing physical discomfort according to the vaccine types (χ2 = 83,909, p < 0.0001). Johnsons and Turkovac were taken by a limited number of participants, but Pfizer-BioNTech, Sinopharm, and Oxford-AstraZeneca were taken by large populations. When more vaccines were examined, the rate of physical discomfort was at most 75% in the Pfizer-BioNTech vaccine. Then, there was Oxford-AstraZeneca with 66.47%, Moderna with 65.04%, and Sinopharm with 62.65%. The vaccines with the lowest rate of physical discomfort were Sputnik V with 14.29%. For the second dose, there was a significant difference in physical discomfort according to vaccine types (χ2 = 180,754, p < 0.0001). The Johnsons and Turkovac vaccine types were administered to very few people, and none of them experienced physical discomfort. When more vaccines were examined, the rate of physical discomfort was the Moderna vaccine with the highest rate of 86.09%. Then, Pfizer-BioNTech with 71.19% and Sputnik V vaccine with 66.67%. The vaccines with the lowest rate of physical discomfort were Sinovac at 29.03%, Oxford-AstraZeneca at 39.15%, and Sinopharm at 45.34%.
Table 4.
Prevalence of physical discomfort after taking the first and second doses
| Name of the vaccine | Physical discomforts | |||||||
|---|---|---|---|---|---|---|---|---|
| First dose (n = 2110) | Second dose (n = 1367) | |||||||
| Yes | No | Yes | No | |||||
| F | % | F | % | F | % | F | % | |
| Johnsons | 1 | 100 | 0 | 0 | 0 | 0 | 1 | 100 |
| Moderna | 173 | 65.04 | 93 | 34.96 | 99 | 86.09 | 16 | 13.91 |
| Oxford-AstraZeneca | 228 | 66.47 | 115 | 33.53 | 110 | 39.15 | 171 | 60.85 |
| Pfizer-BioNTech | 465 | 75.00 | 155 | 25.00 | 378 | 71.19 | 153 | 28.81 |
| Sinopharm | 463 | 62.65 | 276 | 37.35 | 141 | 45.34 | 170 | 54.66 |
| Sinovac | 55 | 41.04 | 79 | 58.96 | 36 | 29.03 | 88 | 70.97 |
| Sputnik V | 1 | 14.29 | 6 | 85.71 | 2 | 66.67 | 1 | 33.33 |
| Turkovac | 0 | 0 | 1 | 100 | 0 | 0 | 1 | 100 |
Figure 2 illustrated that among the major classes, mRNA vaccines exerted maximum side-effects after the 1st dose (Pfizer 75% and Moderna 65.04%) and after the 2nd dose (Pfizer 71.19% and Moderna 86.09%).
Fig. 2.
Side-effects according to vaccine type after getting the first and second doses
Table 5 shows the distribution of physical discomfort experienced after the first and second doses. In the case of the first dose, post-vaccination physical discomfort occurred in 67.28% of those who got vaccinated in Turkey and 64.99% of those who got vaccinated in Bangladesh. In Turkey, 75.06% of the participants with physical discomfort had pain at the injection site; 32.35% had body pain; and 31.36% had symptoms of fatigue. On the other hand, 75.51% of participants with physical discomfort in Bangladesh had pain at the injection site; 41.12% had a fever; and 38.67% had body pain. For the second dose, post-vaccination physical discomfort occurred in 60.96% of those vaccinated in Turkey and 52.61% in Bangladesh. In Turkey, 76.90% of the participants with physical discomfort had pain at the injection site; 42.40% had body pain; 33.04% had a headache; and 30.99% had joint pain symptoms. In Bangladesh, 70.52% of the participants with physical discomfort had pain at the injection site; 37.50% had a fever; and 33.73% had body pain.
Table 5.
Physical discomforts experienced after the first and second doses
| First dose | Second dose | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Turkey (n = 602) | Bangladesh (n = 1508) | Turkey (n = 561) | Bangladesh (n = 806) | ||||||
| F | % | F | % | F | % | F | % | ||
| Experiencing physical discomfort after the first dose | No | 197 | 32.72 | 528 | 35.01 | 219 | 39.04 | 382 | 47.39 |
| Yes | 405 | 67.28 | 980 | 64.99 | 342 | 60.96 | 424 | 52.61 | |
| Pain at the injection site | 304 | 75.06 | 740 | 75.51 | 263 | 76.90 | 299 | 70.52 | |
| Irritation/ rash at the injection site | 24 | 5.93 | 129 | 13.16 | 23 | 6.73 | 59 | 13.92 | |
| Fever | 79 | 19.5 | 403 | 41.12 | 25 | 7.31 | 159 | 37.50 | |
| Body pain | 131 | 32.35 | 379 | 38.67 | 145 | 42.40 | 143 | 33.73 | |
| Joint pain | 94 | 23.21 | 137 | 13.98 | 106 | 30.99 | 49 | 11.56 | |
| Arm numbness | 117 | 28.89 | 150 | 15.31 | 87 | 25.44 | 49 | 11.56 | |
| Headache | 96 | 23.7 | 237 | 24.18 | 113 | 33.04 | 93 | 21.93 | |
| Nausea | 38 | 9.38 | 62 | 6.33 | 43 | 12.57 | 27 | 6.37 | |
| Diarrhea | 10 | 2.47 | 13 | 1.33 | 15 | 4.39 | 7 | 1.65 | |
| Sore throat | 10 | 2.47 | 21 | 2.14 | 14 | 4.09 | 7 | 1.65 | |
| Shortness of breath | 11 | 2.72 | 21 | 2.14 | 15 | 4.39 | 4 | 0.94 | |
| Appetite reduction | 22 | 5.43 | 28 | 2.86 | 16 | 4.68 | 10 | 2.36 | |
| Fatigue | 127 | 31.36 | 116 | 11.84 | 116 | 33.92 | 40 | 9.43 | |
| Complaints about ear | 3 | 0.74 | 3 | 0.31 | 6 | 1.75 | 2 | 0.47 | |
| Heartburn | 2 | 0.49 | 16 | 1.63 | 5 | 1.46 | 10 | 2.36 | |
| Itching | 7 | 1.73 | 29 | 2.96 | 6 | 1.75 | 10 | 2.36 | |
| Stroke | 0 | 0 | 5 | 0.51 | 0 | 0.00 | 1 | 0.24 | |
| Intestinal blockage problem | 0 | 0 | 2 | 0.2 | 1 | 0.29 | 2 | 0.47 | |
| Hypersensitivity | 4 | 0.99 | 3 | 0.31 | 8 | 2.34 | 1 | 0.24 | |
| Muscle pain | 48 | 11.85 | 9 | 0.92 | 48 | 14.04 | 6 | 1.42 | |
| Swelling | 16 | 3.95 | 17 | 1.73 | 15 | 4.39 | 10 | 2.36 | |
| Dizziness | 52 | 12.84 | 89 | 9.08 | 55 | 16.08 | 43 | 10.14 | |
| Vertigo | 20 | 4.94 | 88 | 8.98 | 17 | 4.97 | 46 | 10.85 | |
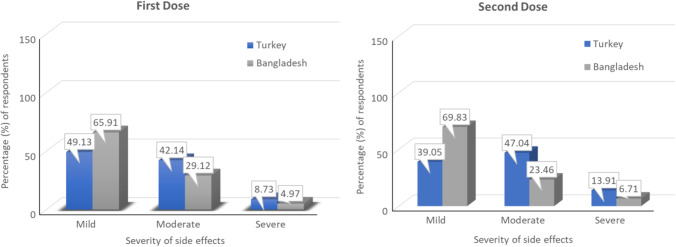
Figure 3 shows the opinions of the participants about the severity of the side effects experienced after the first dose and second dose of COVID-19 vaccines. The majority of participants both from Turkey and Bangladesh experienced the side effects at a mild level after taking the first dose. In the case of the second dose, while the majority of the participants from Turkey experienced the side effects at a moderate level, the majority of the participants from Bangladesh experienced them at a mild level.
Fig. 3.
The severity of side effects experienced after getting the vaccines (both for the first dose and second dose)
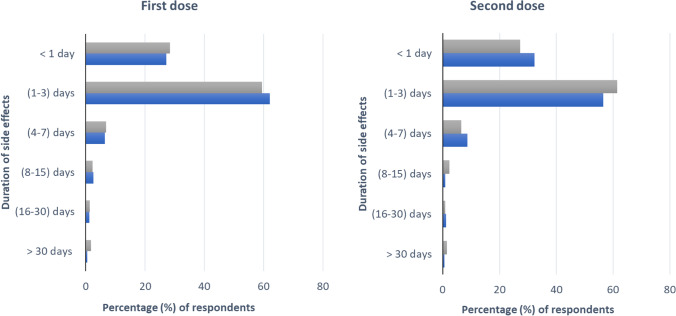
Figure 4 shows the distribution of the duration of side effects after the first dose and second dose of vaccines. For both doses, the side effects lasted between 1 and 3 days for the majority of participants from Turkey and Bangladesh.
Fig. 4.
Duration of side effects after taking the first and second doses of COVID-19 vaccines
After the first and second doses, it was observed that the percentages of the medication use to treat the side effects of the participants from Bangladesh were significantly higher (p < 0.05).
There was no significant difference (p > 0.05) after the first and second vaccine doses of the participants from Turkey according to the presence of chronic disease. On the other hand, while there was no significant difference (p > 0.05) after the first dose of the participants from Bangladesh according to the presence of chronic disease, a significant difference (p < 0.05) was found after the second dose of the vaccine. While 60.73% of the participants with chronic diseases from Bangladesh experienced side effects after the first dose, the rate of those who experienced side effects after the second dose was 42.31%. For Turkey, it was 73.85% for the first dose and 63.93% for the second dose, which is slightly greater than the data obtained from Bangladesh.
Table 6 shows the distribution of COVID-19-like symptoms experienced after vaccines. The majority of the participants both from Bangladesh and Turkey did not experience any COVID-like symptoms after either dose. However, while there was no significant difference between Turkey and Bangladesh in terms of the symptoms experienced after the first dose, there was a significant difference after the second dose. While the rate of experiencing symptoms similar to COVID-19 after the second dose was 10.34% in Turkey, it was 4.34% in Bangladesh.
Table 6.
The distribution of COVID-19-like symptoms experienced after vaccines
| Turkey | Bangladesh | Total | χ2 | p | |||||
|---|---|---|---|---|---|---|---|---|---|
| F | % | F | % | F | % | ||||
| First dose | No | 532 | 88.37 | 1320 | 87.53 | 1852 | 87.77 | 1.988 | 0.216a |
| Yes | 70 | 11.63 | 84 | 5.57 | 154 | 7.30 | |||
| Unknown | 0 | 0.00 | 104 | 6.90 | 104 | 4.93 | |||
| Second dose | No | 461 | 82.17 | 744 | 92.31 | 1205 | 88.15 | 13.796 | 0.001 |
| Yes | 58 | 10.34 | 35 | 4.34 | 93 | 6.80 | |||
| Unknown | 42 | 7.49 | 27 | 3.35 | 69 | 5.05 | |||
aMonte Carlo p-value
Discussion
Our study aimed at pooling the local and systemic adverse effect differences among COVID-19 vaccines in a binational approach. The reasons behind choosing Turkey and Bangladesh for the comparison are the geographic and socioeconomical differences between the two countries along with their disease management system and different distribution patterns of different COVID-19 vaccines. To lessen the severity of this pandemic, different vaccines were manufactured and administered worldwide (Callaway 2021; Mallapaty and Ledford 2020). This study’s demographic information indicated that the majority of respondents were female. In our survey, 77.5% of vaccination recipients reside in urban regions whereas 29.37% reside in rural areas. Consequently, there was a discernible disparity in awareness among rural residents. The biggest number of responders (66.8%) belonged to the 18–30 age group.
Our previous research showed the AEFI of Oxford-AstraZeneca vaccines with a non-replicating viral vector against COVID-19 and its severity (Sultana et al. 2021). The present study revealed that the mRNA vaccines Pfizer and Moderna have shown a higher percentage of adverse events, compared to inactivated whole virus vaccines Sinopharm and Sinovac, in both countries. Some previous studies also support this data (Kadali et al. 2021; Navar et al. 2021; Braun-Moscovici et al. 2021; Cohen 2021; Krammer et al. 2021).
The mRNA vaccines showed a higher percentage of occurrence of local and systemic side effects such as fever, pain, irritation at the site of injection, headache, and body pain in both countries. It is also noticeable that Sinopharm, Sinovac, and Oxford-AstraZeneca vaccines showed a significant reduction in adverse events after the second dose of overall immunization.
In some previous studies, the Moderna vaccine showed injection-site urticarial and injection-site maculopapular dermatitis. Immediate injection site reaction and delayed inflammatory reaction after the first dose were reported during the phase III clinical trial (Paterlini 2021). After receiving the Pfizer-BioNTech vaccine, a 55-year-old woman visited the Oral Medicine Department at the Policlinic of Bari, where she reported experiencing severe sores on her lips, oral mucosa, hands, knees, and feet (Petruzzi et al. 2022). Oxford-AstraZeneca and Sputnik-V showed allergic skin reactions, dermatitis, alopecia, eczema, and most concerning, four deaths in the phase III clinical trial (Kounis et al. 2021; Munavalli et al. 2021; Rice et al. 2021; Wise 2021). When listing the potential side effects of the Oxford-AstraZeneca vaccine, it is important to include superficial vein thrombosis (SVT). The potential for thromboses is outweighed by the benefits of the vaccination in preventing the further spread of COVID-19 (Chavda et al 2022).
Administering Johnson and Johnson vaccine has been paused due to blood clotting problems in vaccinated people. Six cases of blood clots were reported with low platelet counts in the US till April 13, 2021 (Fansher et al. 2022; Mahase 2021).
It has been determined that COVID-19 infection can lead to a variety of cardiovascular complications, including thrombosis (particularly in the coronary arteries), acute coronary syndrome, cardiac arrest, and myocarditis. Similar cardiovascular side effects are linked to a number of COVID-19 vaccines, according to data from regulatory surveillance and self-reporting systems like the Vaccine Adverse Events Reporting System (VAERS) in the United States (US), the Yellow Card System in the UK, and the EudraVigilance system in Europe (Sun et al. 2022).
In this study, we determined the severity of AEFI based on its duration. Different COVID-19 vaccine side effects generally stayed for 0–3 days, and most adverse events were mild to moderate in both countries. However, the Bangladeshi population faced 15% more mild side effects and 10% fewer side effects, compared to the Turkish population. The trend for the severity was surprisingly similar for both the 1st and 2nd doses in both countries. For both doses, the most taken vaccine was Pfizer-BioNTech in Turkish participants and Sinopharm in Bangladeshi participants. Turkish population showed almost two times higher severe side effects, compared to the Bangladeshi population, but the fatality ratio is 0.89% and 1.77% for Turkey and Bangladesh, respectively (Tazerji et al. 2022). According to CDC recommendations, the majority of participants were directed to remain at the vaccination center for longer than 15 min following injection (CDC, 2021). During this period, participants also received guidance on how to manage mild to moderate side effects.
In our study, 60.73% of the participants from Bangladesh experienced side effects after the first dose and 42.31% after the second dose who had chronic disease conditions. Results obtained from Turkey showed an almost similar pattern. A previous study showed that individuals with no prior comorbid disorders had a case fatality rate of 0.9%; it was much higher for those with diabetes, cardiovascular diseases, chronic respiratory diseases, systemic hypertension, and cancer, making these demographic groups high-risk and more susceptible to severe COVID-19 (Varghese et al. 2020).
In both countries, the population experiences a lower percentage of COVID-like symptoms after the second vaccination, and the possible reason could be the boost of the immune system (Mancuso et al. 2021). Various literature studies suggest that the COVID-19 vaccines can hold up to half of the antibodies every 3.5 months (CDC 2022; Dolgin 2021). During the surge of delta variants of SARS-CoV-2, the second jab was deployed to the adolescent to elderly mass population in Turkey and Bangladesh. The young population aged from 18 to 30 years is the majority of the recipient of COVID-19 vaccines with the percentages of 75.9% and 63.2%, respectively, for Turkey and Bangladesh. This indicates that the awareness of this pandemic in both countries is prominent to the young generation compared to the elder. Tendency to explore different mass media and the use of logical thought to imagine the worst consequences of COVID-19 drives them to be alert to this pandemic. Senior citizens in both countries aged above 60 years are the minority who were included in this survey (Turkey 4.1%, Bangladesh 9.9%). The possible reason behind this could be less internet use by elderly people in both countries. However, strict regulation of mass vaccination programs has an impact on this worldwide vaccination rate variation among the elderly population (Callaway 2021). In the beginning, people had hesitancy to take the vaccines due to their uncertain severity, but it overcame over time (Sallam 2021; Troiano and Nardi 2021). In our study, we found that the majority of people (Turkey 63.5% and Bangladesh 64%) agreed that vaccines would help to prevent the pandemic. Although there has been a neutral thought about the vaccine efficacy in a similar percentage of 28.7% and 28.2% for Turkey and Bangladesh, respectively, these perception statistics regarding vaccination efficacy help us understand people’s interaction with the medicinal treatment approach to eradicate a pandemic. Additionally, we discovered in our study that the COVID-19 vaccination was related to a decreased percentage of any type of allergic reaction. Thus, our review of the COVID-19 vaccine-associated adverse effects will be useful in dispelling misconceptions surrounding these vaccinations and providing a clear scenario of the outcomes of the COVID-19 vaccination rollout in Turkey and Bangladesh.
Conclusion
After the systematic analysis of the associated side effects and severity of COVID-19 vaccines, we can summarize that the COVID-19 vaccines are safe. People gave preferences and values to the effectiveness of COVID-19 vaccines more than the minor side effects. These vaccines have shown proof of immune response through non-serious adverse events. Binational assessment between Turkey and Bangladesh also showed a similar pattern of non-serious adverse events in healthy adults along with the elderly population with different comorbid diseases. The study possesses multiple strengths and a few limitations. Considering different possible variables including age, perception about vaccination, comorbid conditions, the inclusion of a wide range of possible side effects due to COVID-19 vaccination, the binational approach of the survey, and the role of social networking are the remarkable strengths of our COVID-19 vaccine AEFI study. Regarding limitations, our online-based survey study could not approach the mass population with face-to-face interactions. Another limitation was that the participation of the elder population was not as high as the participation of the young adult population due to technological retrogression. The effectiveness of existing vaccines in controlling and preventing SARS-CoV-2 infection in Turkey and Bangladesh should be evaluated by defining the appropriate vaccination strategy in the context of a large-scale follow-up study. Longitudinal surveys and pharmacovigilance analyses should also be conducted to investigate vaccination adverse effects over time. As the COVID-19 pandemic crisis continues to unfold, it is becoming increasingly urgent to assess the level of precautions taken by nations to face this pandemic and to explain the crucial qualities that might facilitate more effective policy responses to limit and/or avoid the detrimental consequences of future pandemic crises on people’s health and the economy.
Acknowledgements
We express our gratitude to all the participants of the study who patiently answered all the questions and shared their valuable responses. We would like to thank the authors of the articles we cited. We also express our gratitude to the authority of the Department of Pharmaceutical Chemistry for use of their computer at the Molecular Pharmacology and Herbal Drug Research Laboratory established under the HEQEP Project.
Author contribution
MSA, SMB, and MSR have conceived the original idea. AS (Arifa Sultana), SRM, SMB, and MSA constructed the questionnaire in Google form. AS (Arifa Sultana), SRM, AS (Ananya Saha), FY, RT, NBB, KRF, SS, KMRP, FR, FN, MS, QAS, MIU, JF, MAHM, TA, MMR, MRK, FA, and SMB extensively collected the survey data by using electronic and social media. AS (Arifa Sultana), SRM, AS (Ananya Saha), and NBB prepared the initial manuscript with references. SMB, SRM, SS, and KMRP did the statistical analysis. MMRS, SMB, JAC, AAC, and SK critically reviewed the overall activities. SMB, MSR, and MSA supervised the whole activity. All the authors have read and agreed to the published version of the manuscript.
Funding
This work was self-financed.
Data and material/code availability
The data used for this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval
Ethics approval was obtained from the Ethical Review Committee of the Faculty of Biological Sciences, University of Dhaka (Ref. No. 159/Biol. Scs.).
Consent to participate
Participants gave their implied consent to participate in the study by voluntarily completing the survey.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Disclaimer
The views expressed in this article are the personal views of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abedin M, Islam MA, Rahman FN, Reza HM, Hossain MZ, Hossain MA, Arefin A, Hossain A. Willingness to vaccinate against COVID-19 among Bangladeshi adults: understanding the strategies to optimize vaccination coverage. PLoS ONE. 2021;16(4):e0250495. doi: 10.1371/journal.pone.0250495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhazmi A, Alamer E, Daws D, Hakami M, Darraj M, Abdelwahab S, Maghfuri A, Algaissi A. Evaluation of side effects associated with COVID-19 vaccines in Saudi Arabia. Vaccines. 2021;9(6):674. doi: 10.3390/vaccines9060674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Tammemi AB. The battle against COVID-19 in Jordan: an early overview of the Jordanian experience. Front Public Health. 2020;8:188. doi: 10.3389/fpubh.2020.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardito L, Coccia M, Messeni Petruzzelli A. Technological exaptation and crisis management: evidence from COVID-19 outbreaks. R&d Management. 2021;51(4):381–392. [Google Scholar]
- Braun-Moscovici Y, Kaplan M, Braun M, Markovits D, Giryes S, Toledano K, Tavor Y, Dolnikov K, Balbir-Gurman A. Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the Pfizer mRNA vaccine against SARS-CoV-2. Ann Rheum Dis. 2021;80(10):1317–1321. doi: 10.1136/annrheumdis-2021-220503. [DOI] [PubMed] [Google Scholar]
- Callaway E. COVID vaccine boosters: the most important questions. Nature. 2021;596(7871):178–180. doi: 10.1038/d41586-021-02158-6. [DOI] [PubMed] [Google Scholar]
- CDC (2022) Understanding how COVID-19 vaccines work. Centers for Disease Control and Prevention. Last accessed: 25 January, 2022. Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/how-they-work.html
- Chavda VP, Vihol DR, Solanki HK, Apostolopoulos V. The vaccine world of COVID-19: India’s Contribution. Vaccines. 2022;10(11):1943. doi: 10.3390/vaccines10111943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M (2021) Preparedness of countries to face COVID-19 pandemic crisis: strategic positioning and underlying structural factors to support strategies of prevention of pandemic threats. Environ Res 111678 [DOI] [PMC free article] [PubMed]
- Cohen J (2021) What went wrong with CureVac’s mRNA vaccine? American Association for the Advancement of Science. Available at: https://www.science.org/content/article/what-went-wrong-curevac-s-highly-anticipated-new-mrna-vaccine-covid-19
- Dolgin E. COVID vaccine immunity is waning-how much does that matter. Nature. 2021;597(7878):606–607. doi: 10.1038/d41586-021-02532-4. [DOI] [PubMed] [Google Scholar]
- El-Shitany NA, Harakeh S, Badr-Eldin SM, Bagher AM, Eid B, Almukadi H, Alghamdi BS, Alahmadi AA, Hassan NA, Sindi N. Minor to moderate side effects of Pfizer-BioNTech COVID-19 vaccine among Saudi residents: a retrospective cross-sectional study. International Journal of General Medicine. 2021;14:1389. doi: 10.2147/IJGM.S310497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fansher M, Adkins TJ, Lalwani P, Boduroglu A, Carlson M, Quirk M, Lewis RL, Shah P, Zhang H, Jonides J. Icon arrays reduce concern over COVID-19 vaccine side effects: a randomized control study. Cognitive Research: Principles and Implications. 2022;7(1):1–9. doi: 10.1186/s41235-022-00387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein S, Herbert JA, McNamara PS, Hedrich CM. COVID-19: immunology and treatment options. Clin Immunol. 2020;215:108448. doi: 10.1016/j.clim.2020.108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche PJ, Helbling A, Ballmer-Weber BK. Vaccine hypersensitivity-update and overview. Swiss Med Wkly. 2010;140(17–18):238–246. doi: 10.4414/smw.2010.12980. [DOI] [PubMed] [Google Scholar]
- Gee J, Marquez P, Su J, Calvert GM, Liu R, Myers T, Nair N, Martin S, Clark T, Markowitz L. First month of COVID-19 vaccine safety monitoring—United States, December 14, 2020–January 13, 2021. Morb Mortal Wkly Rep. 2021;70(8):283. doi: 10.15585/mmwr.mm7008e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goralnick E, Kaufmann C, Gawande AA. Mass-vaccination sites—an essential innovation to curb the COVID-19 pandemic. N Engl J Med. 2021;384(18):e67. doi: 10.1056/NEJMp2102535. [DOI] [PubMed] [Google Scholar]
- Harmukh N. Medicinal plants and their uses in natural immunity improvement with special reference to COVID-19. Research Interventions and Advancements in Plant Sciences. 2020;1:169–174. [Google Scholar]
- Harrison EA, Wu JW. Vaccine confidence in the time of COVID-19. Eur J Epidemiol. 2020;35(4):325–330. doi: 10.1007/s10654-020-00634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartert TV, Sockrider M. What are COVID-19 vaccines? Am J Respir Crit Care Med. 2021;203(9):P22–P23. doi: 10.1164/rccm.2021C2. [DOI] [PubMed] [Google Scholar]
- Hatmal MM, Al-Hatamleh MAI, Olaimat AN, Hatmal M, Alhaj-Qasem DM, Olaimat TM, Mohamud R. Side effects and perceptions following COVID-19 vaccination in Jordan: a randomized, cross-sectional study implementing machine learning for predicting severity of side effects. Vaccines. 2021;9(6):556. doi: 10.3390/vaccines9060556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MA, Amran MS. A Cross-Sectional Pilot Study on Pharmacovigilance to Improve the Drug Safety in Bangladesh. Biomedical and Pharmacology Journal. 2019;12(3):1039–1049. [Google Scholar]
- Hossain MF, Hasana S, al Mamun A, Uddin MS, Wahed MII, Sarker S, Behl T, Ullah I, Begum Y, Bulbul IJ. COVID-19 outbreak: pathogenesis, current therapies, and potentials for future management. Front Pharmacol. 2020;11(11):563478. doi: 10.3389/fphar.2020.563478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadali RAK, Janagama R, Peruru S, Malayala S, v. Side effects of BNT162b2 mRNA COVID-19 vaccine: a randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int J Infect Dis. 2021;106:376–381. doi: 10.1016/j.ijid.2021.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan YH, Mallhi TH, Alotaibi NH, Alzarea AI, Alanazi AS, Tanveer N, Hashmi FK. Threat of COVID-19 vaccine hesitancy in Pakistan: the need for measures to neutralize misleading narratives. Am J Trop Med Hyg. 2020;103(2):603. doi: 10.4269/ajtmh.20-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatun F (2021) An impressive start to COVID vaccination in Bangladesh. Daily Star. Available at: https://www.thedailystar.net/opinion/macro-mirror/news/impressive-start-covid-vaccination-bangladesh-2044789
- Kounis NG, Koniari I, de Gregorio C, Velissaris D, Petalas K, Brinia A, Assimakopoulos SF, Gogos C, Kouni SN, Kounis GN. Allergic reactions to current available COVID-19 vaccinations: pathophysiology, causality, and therapeutic considerations. Vaccines. 2021;9(3):221. doi: 10.3390/vaccines9030221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F, Srivastava K, Alshammary H, Amoako AA, Awawda MH, Beach KF, Bermúdez-González MC, Bielak DA, Carreño JM, Chernet RL. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384(14):1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C-C, Shih T-P, Ko W-C, Tang H-J, Hsueh P-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GM (2021) The importance of context in COVID-19 vaccine safety. In New England Journal of Medicine (Vol. 385, Issue 12, pp. 1138–1140). Mass Medical Soc [DOI] [PMC free article] [PubMed]
- Mahase E (2021) Covid-19: US suspends Johnson and Johnson vaccine rollout over blood clots. BMJ 2021, 373:n970. Available at: https://www.bmj.com/content/373/bmj.n970 [DOI] [PubMed]
- Mallapaty S, Ledford H (2020) COVID-vaccine results are on the way-and scientists’ concerns are growing. Nature 16–17 [DOI] [PubMed]
- Mancuso M, Eikenberry SE, Gumel AB. Will vaccine-derived protective immunity curtail COVID-19 variants in the US? Infectious Disease Modelling. 2021;6:1110–1134. doi: 10.1016/j.idm.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, Sudre CH, Nguyen LH, Drew DA, Merino J. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21:939–949. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munavalli GG, Knutsen-Larson S, Lupo MP, Geronemus RG. Oral angiotensin-converting enzyme inhibitors for treatment of delayed inflammatory reaction to dermal hyaluronic acid fillers following COVID-19 vaccination-a model for inhibition of angiotensin II–induced cutaneous inflammation. JAAD Case Reports. 2021;10:63–68. doi: 10.1016/j.jdcr.2021.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navar AM, McNally E, Yancy CW, O’Gara PT, Bonow RO. Temporal associations between immunization with the COVID-19 mRNA vaccines and myocarditis: the vaccine safety surveillance system is working. JAMA Cardiology. 2021;6(10):1117–1118. doi: 10.1001/jamacardio.2021.2853. [DOI] [PubMed] [Google Scholar]
- Paterlini M (2021) Covid-19: Sweden, Norway, and Finland suspend use of Moderna vaccine in young people “as a precaution”. BMJ 2021, 375:n2477. Available at: https://www.bmj.com/content/375/bmj.n2477 [DOI] [PubMed]
- Petruzzi M, Galleggiante S, Messina S, della Vella, F. Oral erythema multiforme after Pfizer-BioNTech COVID-19 vaccination: a report of four cases. BMC Oral Health. 2022;22(1):1–8. doi: 10.1186/s12903-022-02124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riad A, Pokorná A, Attia S, Klugarová J, Koščík M, Klugar M. Prevalence of COVID-19 vaccine side effects among healthcare workers in the Czech Republic. J Clin Med. 2021;10(7):1428. doi: 10.3390/jcm10071428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice SM, Ferree SD, Mesinkovska NA, Kourosh AS. The art of prevention: COVID-19 vaccine preparedness for the dermatologist. International Journal of Women’s Dermatology. 2021;7:209–212. doi: 10.1016/j.ijwd.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed BQ, Al-Shahrabi R, Alhaj SS, Alkokhardi ZM, Adrees AO. Side effects and perceptions following Sinopharm COVID-19 vaccination. Int J Infect Dis. 2021;111:219–226. doi: 10.1016/j.ijid.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallam M. COVID-19 vaccine hesitancy worldwide: a concise systematic review of vaccine acceptance rates. Vaccines. 2021;9(2):160. doi: 10.3390/vaccines9020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallam M, Dababseh D, Eid H, Al-Mahzoum K, Al-Haidar A, Taim D, Yaseen A, Ababneh NA, Bakri FG, Mahafzah A. High rates of COVID-19 vaccine hesitancy and its association with conspiracy beliefs: a study in Jordan and Kuwait among other Arab countries. Vaccines. 2021;9(1):42. doi: 10.3390/vaccines9010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahcheraghi SH, Ayatollahi J, Aljabali AAA, Shastri MD, Shukla SD, Chellappan DK, Jha NK, Anand K, Katari NK, Mehta M. An overview of vaccine development for COVID-19. Ther Deliv. 2021;12(3):235–244. doi: 10.4155/tde-2020-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma O, Sultan AA, Ding H, Triggle CR. A review of the progress and challenges of developing a vaccine for COVID-19. Front Immunol. 2020;11:2413. doi: 10.3389/fimmu.2020.585354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimabukuro T, Nair N. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine. JAMA. 2021;325(8):780–781. doi: 10.1001/jama.2021.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon Y, Eshete T, Mekasha B, Assefa W. COVID-19 vaccine: side effects after the first dose of the Oxford AstraZeneca vaccine among health professionals in low-income country: Ethiopia. J Multidiscip Healthc. 2021;14:2577. doi: 10.2147/JMDH.S331140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana A, Shahriar S, Tahsin M, Mim SR, Fatema KR, Saha A, Yesmin F, Bahar NB, Samodder M, Mamun M. A retrospective cross-sectional study assessing self-reported adverse events following immunization (AEFI) of the COVID-19 vaccine in Bangladesh. Vaccines. 2021;9(10):1090. doi: 10.3390/vaccines9101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CLF, Jaffe E, Levi R. Increased emergency cardiovascular events among under-40 population in Israel during vaccine rollout and third COVID-19 wave. Sci Rep. 2022;12(1):1–12. doi: 10.1038/s41598-022-10928-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber KS. The use of Cronbach’s alpha when developing and reporting research instruments in science education. Res Sci Educ. 2018;48(6):1273–1296. [Google Scholar]
- Tazerji SS, Shahabinejad F, Tokasi M, Rad MA, Khan MS, Safdar M, Filipiak KJ, Szarpak L, Dzieciatkowski T, Jurgiel J (2022) Global data analysis and risk factors associated with morbidity and mortality of COVID-19. Gene Rep 101505 [DOI] [PMC free article] [PubMed]
- Tissot N, Brunel A-S, Bozon F, Rosolen B, Chirouze C, Bouiller K. Patients with history of COVID-19 had more side effects after the first dose of COVID-19 vaccine. Vaccine. 2021;39(36):5087–5090. doi: 10.1016/j.vaccine.2021.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuç B, Varol G. Medical education in Turkey in time of COVID-19. Balkan Med J. 2020;37(4):180. doi: 10.4274/balkanmedj.galenos.2020.2020.4.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiano G, Nardi A. Vaccine hesitancy in the era of COVID-19. Public Health. 2021;194:245–251. doi: 10.1016/j.puhe.2021.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese GM, John R, Manesh A, Karthik R, Abraham OC. Clinical management of COVID-19. Indian J Med Res. 2020;151(5):401. doi: 10.4103/ijmr.IJMR_957_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- Wise J (2021) COVID-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. BMJ 2021, 372:n699. Available at: https://www.bmj.com/content/372/bmj.n699 [DOI] [PubMed]
- Wu Q, Dudley MZ, Chen X, Bai X, Dong K, Zhuang T, Salmon D, Yu H. Evaluation of the safety profile of COVID-19 vaccines: a rapid review. BMC Med. 2021;19(1):1–16. doi: 10.1186/s12916-021-02059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu FC, Li YH, Guan XH, Hou LH, Wang WJ, Li JX, Wu SP, Wang BS, Wang Z, Wang L. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. The Lancet. 2020;395(10240):1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used for this study are available from the corresponding author upon reasonable request.