Abstract
In the 2016 update of the World Health Organization (WHO) classification of myeloid neoplasms, acute undifferentiated leukemia (AUL) was defined by a lack of lineage-specific markers. AUL has very poor prognosis and no established therapies due to its rarity. We report a case of a 31-year-old man with AUL who showed complete molecular response to an acute lymphoblastic leukemia (ALL)-based regimen and received allogeneic hematopoietic stem cell transplantation. The patient’s blast cells were CD7-positive and localized to lymph nodes in the neck and to a large mediastinal mass; there was also rearrangement of the T-cell receptor delta locus. Although the tumor showed characteristics of T-cell lymphoblastic lymphoma, it was categorized as AUL based on WHO classification. This case suggests that a high-intensity conditioning regimen could be effective for rare cases of AUL that present only in the extramedullary mass, and chemotherapy for AUL should be selected based on the characteristics of the blasts.
Keywords: acute undifferentiated leukemia, rare leukemia, allogeneic hematopoietic stem cell transplantation, conditioning regimen
INTRODUCTION
In the 2016 update of the World Health Organization (WHO) classification of myeloid neoplasms, acute leukemias of ambiguous lineage (ALAL) are a rare subtype of acute leukemia that lack evidence of myeloid or lymphoid lineage or show evidence of more than one lineage. Acute undifferentiated leukemia (AUL) is listed as a subcategory of ALAL, which lacks the major immunophenotypic lineage markers including myeloperoxidase (MPO), cCD3, CD19, cCD22, and CD79a, and usually expresses only one surface lineage marker such as CD13, CD33, and CD7. Furthermore, due to its immature characteristics, AUL commonly expresses stem cell markers including CD34, HLA-DR, and terminal deoxynucleotidyl transferase (TdT).1-3
Because of the rarity of AUL, an optimal treatment strategy has not yet been established. Some clinicians suggest treating AUL with acute myeloid leukemia (AML)-based regimens because AUL sometimes has genetic and cytogenetic mutations observed in AML, such as IDH1 mutations del5q, del7, and del17p.4,5 In contrast, other clinicians suggest treating AUL with an acute lymphoblastic leukemia (ALL)-based regimen, which is effective for mixed phenotype acute leukemia (MPAL), which is also classified as ALAL.2,6,7 No firm conclusion has been reached over which induction and consolidation chemotherapy - an AML-based regimen or an ALL-based regimen - should be administered to patients with AUL. On the other hand, most of clinicians agree the necessity of receiving allogeneic hematopoietic stem cell transplantation (Allo-HSCT) to achieve long-term survival because of their poor prognosis.6,8 However, AUL occurs in older patients compared to AML and ALL, and most of the patients cannot be treated with chemotherapy.9
Herein, we report a rare case of AUL in a young patient who presented with abnormal lymphocytes only in lymph nodes of the neck and in a large mediastinal mass, thus resembling a T-lymphoblastic lymphoma (T-LBL). The patient achieved complete molecular response to an ALL-based regimen and underwent Allo-HSCT.
CASE
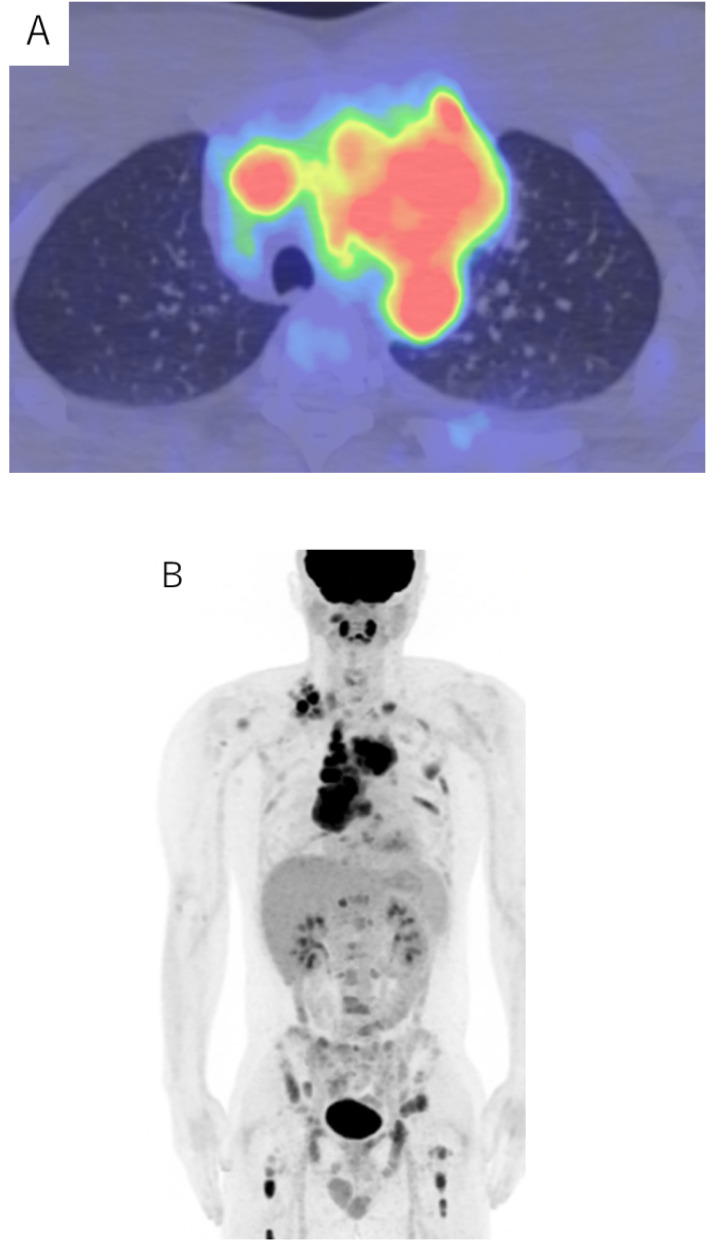
A 31-year-old man presented to the Japanese Red Cross Kochi Hospital with breathing difficulties. Chest radiography revealed a large mediastinal mass and positron emission tomography-computed tomography (PET-CT) showed high fluorodeoxyglucose accumulation in the mediastinal mass and multiple neck lymph nodes (SUVmax 13.9) (Fig. 1). Neck lymph node biopsies showed that the mass consisted of medium to large lymphocyte-like blast cells. Immunohistochemical staining revealed that the blast cells were positive for CD7, CD34, and CD99(dim), and negative for CD1a, CD3 (clone F7.2.38), CD4, CD5, CD8, CD10, CD15, CD20, CD30, CD56, CD79a, CD117, TdT, anaplastic lymphoma kinase (ALK), and myeloperoxidase (MPO) (Fig. 2). The blast cells were negative for Epstein-Barr virus-encoded small RNA (EBER) by in situ hybridization. Flow cytometric analysis of cells from the lymph nodes confirmed expression of CD7, CD34, and HLA-DR. The blasts did not express CD2, CD3, CD5, CD8, CD10, CD14, CD19, CD20, CD22, CD56, KORSA, and MPO (Fig. 3). The results of conventional G-banding chromosomal analysis of the lymph node revealed 92, XXYY, del(5)(q22q31)×2, add(6)(p21.3)×2, add(7)(p15)×2, t(9;15)(p13;q11.2)×2, i(17)(q10)×2 [20/20]. The peripheral blood film and bone marrow aspirate demonstrated no evidence of blast cells. Because the blast cells were not positive for any lineage-specific markers, the patient was diagnosed with AUL based on the 2016 updated WHO classification of hematological malignancies. He was initially treated with induction therapy usually used for AML patients (idarubicin 12 mg/m2 for 3 days and cytarabine 100 mg/m2 for 7 days). However, due to a rearrangement in the T cell receptor (TCR) delta locus detected after two days, the therapy was changed to an ALL-based induction therapy (cyclophosphamide 1000 mg/m2 for 1 day, daunorubicin 50 mg/m2 for 1 days, vincristine 2 mg for 4 days, L-asparaginase 6000 U/m2 for 6 days, and prednisolone 100 mg for 21 days).
Fig. 1.
Positron emission tomography-computed tomography (PET-CT) scan of neck lymph nodes and a large mediastinal mass (SUVmax 13.9).
Fig. 2.
Analysis of neck lymph node biopsy by Hematoxylin-Eosin (HE) staining and CD7, CD34, CD99, CD1a, CD3, CD4, CD8, MPO, and TdT immunohistochemistry.
Fig. 3.
Flow cytometric analysis of the blast cells.
After induction therapy, the patient was transferred to Kochi health sciences center for further evaluation and management of the acute leukemia. PET-CT revealed no accumulation, and peripheral blood film and bone marrow aspirate also showed absence of blast cells. Based on these findings, he was in complete remission and we assumed that the ALL-based therapy was effective. Next, the patient started receiving ALL-based consolidation therapy. After two course of consolidation therapies, the patient maintained complete molecular response, and underwent allogeneic peripheral blood stem cell transplantation from an HLA fully matched brother. The conditioning regimen was medium-dose etoposide (ETP, 15 mg/kg for 2 days), high-dose cyclophosphamide (CY, 60 mg/kg for 2 days), and total body irradiation (TBI, 12 Gy in 6 fractions). Graft versus host disease (GVHD) prophylaxis consisted of cyclosporine (3 mg/kg/day from day-1), short-term methotrexate (10 mg/m2 day 1, 7 mg/m2 day 3, 6), and anti-thymocyte globulin (1 mg/kg for 2 days). Regimen-related toxicities and grade 1 acute skin GVHD were controllable. Neutrophil engraftment occurred on day 12, full donor chimerism was established on day 27, and he was discharged on day 56. Currently, he maintains remission more than 9 months from transplantation.
DISCUSSION
AUL is a very rare leukemia with poor prognosis and no established treatment. In this case, the blast cells were positive for CD34, HLA-DR, and CD7, which are usually observed in AUL.8,10-12 However, the blast cells formed a large mediastinal mass and showed rearrangement of the TCR delta locus. In addition, the blast cells were slightly positive for CD99. CD99 is expressed in both immature myeloid-lineage and B-lineage cells, but immature thymic T-lineage cell shows highest levels of expression.13 Although our patient was diagnosed with AUL on the basis of the 2016 WHO classification, the blast cells had similar characteristics to T-LBL. Based on the clinical characteristics, he was treated with an ALL-based regimen, and achieved complete remission. Furthermore, he underwent allogeneic peripheral blood stem cell transplantation using a high intensity conditioning regimen which is an effective regimen for high risk ALL, and he maintains remission.
Since the 2008 WHO classification, MPO, cCD3, CD19, cCD22, and CD79a are defined as lineage markers. Before the revision of the classification, some cases of leukemia, which were only positive for CD7 and had no lineage markers like this case were reported; they were called CD7-positive stem cell leukemia.14-16 CD7-positive stem cell leukemias frequently exhibit bone marrow invasion, mediastinal masses, and lymphadenopathy. These leukemias are characterized by rearrangements of the TCR or immunoglobulin heavy chain and show at relapse myeloid or lymphoid lineages. Like AUL, CD7-positive stem cell leukemia is considered to originate from pluripotent hematopoietic stem cells based on the evidence that CD7-positive/CD4/8-negative blast cells have the ability to differentiate into myeloid and lymphoid lineages.15,16 In the presented case, we presumed that the blast cells originated from pluripotent hematopoietic stem cells prior to the prothymocyte stage because of the lack of CD3 which is usually observed in the process of differentiation into prothymocytes.14
Chromosomal analysis from the patient’s G-banding patterns revealed the presence of tetraploidy (92, XXYY). One report suggested that chromosome abnormalities are a poor prognostic factor for AUL. In that report, five in ten patients of AUL had chromosomal abnormalities, and all of these patients experienced primary induction failure. Three patients could not achieve complete response until HSCT and two patients experienced relapse post-HSCT (the others died as a result of sepsis or acute respiratory distress syndrome).8 In addition, there are case reports that AUL with near tetraploidy was resistant to induction and re-induction therapy.17 Similarly for AML, in one trial, less than half of near-tetraploid patients (11 of 23 cases) showed a complete response to induction therapy.18 In contrast to AUL and AML, near-tetraploidy is a favorable prognostic factor for childhood ALL.19 As already mentioned above, our case had characteristics of T cell lineage, and did not have poor prognostic chromosome abnormalities such as -7, -5, t(9;22), t(4;11), and t(1;19). These factors could have contributed to a favorable response to treatment.
Our patient was treated with conventional conditioning regimen (CY and TBI) plus medium-dose ETP. This conditioning regimen adding medium-dose ETP is known to decrease the rate of ALL relapse.20 Considering his general medical condition and the risk of recurrence, we selected this high-intensity conditioning regimen. He has been well without relapse since Allo-HSCT.
In conclusion, we report a rare case of AUL localized to the neck lymph nodes and a large mediastinal mass. The patient showed complete response to an ALL-based regimen and received Allo-HSCT. This case suggests that chemotherapy for AUL should be selected based on the characteristics of the blast cells. More data are needed to understand the mechanism, pathogenesis, and best treatment, including targeted therapy for AUL.
ACKNOWLEDGMENTS
The authors would like to thank the patient and medical staff for their contributions to this case report.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Lee HG, Baek HJ, Kim HS, et al. Biphenotypic acute leukemia or acute leukemia of ambiguous lineage in childhood: clinical characteristics and outcome. Blood Res. 2019; 54: 63-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurzer JH, Weinberg OK. Acute leukemias of ambiguous lineage: clarification on lineage specificity. Surg Pathol Clin. 2019; 12: 687-697. [DOI] [PubMed] [Google Scholar]
- 3.Béné MC, Porwit A. Acute leukemias of ambiguous lineage. Semin Diagn Pathol. 2012; 29: 12-18. [DOI] [PubMed] [Google Scholar]
- 4.Lao ZT, Ding LW, An O, et al. Mutational and transcriptomic profiling of acute leukemia of ambiguous lineage reveals obscure but clinically important lineage bias. Haematologica. 2019; 104: e200-e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel SH, Vasu S, Guo L, et al. Molecular complete remission following Ivosidenib in a patient with an acute undifferentiated leukemia. J Natl Compr Canc Netw. 2020; 18: 6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heesch S, Neumann M, Schwartz S, et al. Acute leukemias of ambiguous lineage in adults: molecular and clinical characterization. Ann Hematol. 2013; 92: 747-758. [DOI] [PubMed] [Google Scholar]
- 7.Duong VH, Begna KH, Kashanian S, et al. Favorable outcomes of acute leukemias of ambiguous lineage treated with hyperCVAD: a multi-center retrospective study. Ann Hematol. 2020; 99: 2119-2124. [DOI] [PubMed] [Google Scholar]
- 8.Kurosawa S, Toya T, Kishida Y, et al. Outcome of patients with acute undifferentiated leukemia after allogeneic hematopoietic stem cell transplantation. Leuk Lymphoma. 2018; 59: 3006-3009. [DOI] [PubMed] [Google Scholar]
- 9.Qasrawi A, Gomes V, Chacko CA, et al. Acute undifferentiated leukemia: data on incidence and outcomes from a large population-based database. Leuk Res. 2020; 89: 106301. [DOI] [PubMed] [Google Scholar]
- 10.Youk HJ, Cho CH, Lee JH, et al. A rare case of polycythemia vera following acute undifferentiated leukemia remission. Ann Lab Med. 2014; 34: 469-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berg HE, Blackburn PR, Baughn LB, et al. Identification of a novel KMT2A / GIMAP8 gene fusion in a pediatric patient with acute undifferentiated leukemia. Genes Chromosomes Cancer. 2021; 60: 108-111. [DOI] [PubMed] [Google Scholar]
- 12.Cannizzo E, Carulli G, Del Vecchio L, et al. Prethymic cytoplasmic CD3 negative acute lymphoblastic leukemia or acute undifferentiated leukemia: a case report. Case Rep Hematol. 2011; 2011: 230568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dworzak MN, Fritsch G, Buchinger P, et al. Flow cytometric assessment of human MIC2 expression in bone marrow, thymus, and peripheral blood. Blood. 1994; 83: 415-425. [PubMed] [Google Scholar]
- 14.Nagano M, Kimura N, Akiyoshi T, et al. T-stem cell leukemia/lymphoma with both myeloid lineage conversion and T-specific delta recombination. Leuk Res. 1997; 21: 763-773. [DOI] [PubMed] [Google Scholar]
- 15.Katsuno M, Abe Y, Taguchi F, et al. CD7+ stem cell leukemia/lymphoma. Features of a subgroup without circulating blast cells. Cancer. 1993; 72: 99-104. [DOI] [PubMed] [Google Scholar]
- 16.Karube K, Ohshima K, Tsuchiya T, et al. Non-B, non-T neoplasms with lymphoblast morphology: further clarification and classification. Am J Surg Pathol. 2003; 27: 1366-1374. [DOI] [PubMed] [Google Scholar]
- 17.Kim BH, Kim HR, Lee MK, Chi H. Two cases of near-tetraploidy in acute leukemias of ambiguous lineage. Ann Lab Med. 2013; 33: 371-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Béné MC, Castoldi G, Derolf A, et al. Near-tetraploid acute myeloid leukemias: an EGIL retrospective study of 25 cases. Leukemia. 2006; 20: 725-728. [DOI] [PubMed] [Google Scholar]
- 19.Lemež P, Attarbaschi A, Béné MC, et al. Childhood near-tetraploid acute lymphoblastic leukemia: an EGIL study on 36 cases. Eur J Haematol. 2010; 85: 300-308. [DOI] [PubMed] [Google Scholar]
- 20.Shigematsu A, Kondo T, Yamamoto S, et al. Excellent outcome of allogeneic hematopoietic stem cell transplantation using a conditioning regimen with medium-dose VP-16, cyclophosphamide and total-body irradiation for adult patients with acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2008; 14: 568-575. [DOI] [PubMed] [Google Scholar]