Abstract
Cholesterol uptake via LDL receptor (LDLR) is increased in some malignant tumors, and incorporated LDL contribute to lipid droplet formation. Burkitt’s lymphoma is known to have a large number of vacuoles in the cytoplasm, however, intracellular vacuoles are also seen in high-grade lymphomas such as adult T-cell leukemia/lymphoma, diffuse large B-cell lymphoma and primary central nervous system lymphoma. Recent studies have shown that esterified cholesterol is the main component of these vacuoles and the expression of cholesterol metabolism-related molecules such as LDLR, acetyl-CoA acetyltransferase 1 (ACAT1) which esterifies free cholesterol, and scavenger receptor class B type I (SR-BI) which effluxes free cholesterol, was significantly upregulated in lymphoma cells. Moreover, negative feedback of LDLR was not regulated even under cholesterol-rich conditions in lymphoma cells. We found that cytoplasmic free cholesterol was increased by ACAT and SR-BI inhibitors (CI-976 and BLT-1, respectively), and the accumulation of free cholesterol induced lymphoma cell apoptosis. In addition, overexpression of lipid droplet surface proteins has been correlated with poor prognosis in several malignant tumor such as ovarian cancer and clear cell renal cell carcinoma, and it is important to evaluate lipid droplet formation in malignant tumors including lymphomas.
Keywords: LDLR, SR-BI, ACAT1, lymphoma, lipid metabolism
INTRODUCTION
Cholesterol is an essential component of cell membranes and the backbone of steroid hormones. Low-density lipoprotein (LDL), a source of cholesterol, is taken up by the LDL receptor (LDLR) on cellular membranes.1 LDLR is expressed in all cells and its expression is increased in malignant tumors.2 Incorporated LDL sequentially aggregate and fuse, thereby contributing to lipid droplet formation.3 It is well known that numerous lipid droplets are observed in the cytoplasm of foamy macrophages during atherosclerosis. Lipid droplet accumulation is also observed in various tumors as a result of increased lipid metabolism.4,5 Moreover, vacuoles of lipid origin are observed in malignant lymphomas such as Burkitt’s lymphoma.6 In addition, increased expression of LDLR is observed in Burkitt’s lymphoma cells,7 suggesting that lipid metabolism may be enhanced; however, few studies have examined lipid metabolism in malignant lymphomas in detail. Therefore, this review focuses on the mechanisms of lipid metabolism in malignant lymphomas and the usefulness of lipid metabolism-related factor inhibitors.
Burkitt’s lymphoma is known to contain lipid-derived vacuoles,6 and intracytoplasmic vacuoles are observed in other malignant lymphomas.8,9 In our study using malignant lymphoma cell lines, LDL stimulation resulted in an increase in lipid droplet vacuoles and an increase in intracellular cholesterol content, indicating that the major component of these vacuoles is LDL-derived cholesterol.10 In addition, the expression of cholesterol metabolism-related factors such as LDLR, acetyl-CoA acetyltransferase 1 (ACAT1), and scavenger receptor class B type I (SR-BI) is increased in malignant lymphoma cells and plays an important role in lymphoma lipid metabolism.10
Cholesterol metabolism in malignant lymphoma
LDLR expression is increased in many malignant tumors, including malignant lymphomas.2 The expression of LDLR in normal cells is regulated by the intracellular cholesterol concentration, which is sensed by sterol regulatory element binding protein-2 (SREBP-2) in the endoplasmic reticulum membrane.11 When intracellular cholesterol levels are high, activation of the transcription factor SREBP-2 is suppressed and expression of a group of genes involved in cholesterol synthesis and uptake, including LDLR, is negatively regulated. However, we have reported that in malignant lymphoma cells, downregulation of SREBP-2 and LDLR does not occur even under cholesterol-rich conditions, indicating that the negative feedback mechanism is disrupted.10
ACAT1 is an enzyme belonging to the membrane-bound O-acyltransferase (MBOAT) family, which esterifies free cholesterol to produce cholesterol esters that are stored as lipid droplets. Overexpression of ACAT1 has been reported in some malignant tumors, including pancreatic cancer12 and clear cell renal cell carcinoma (ccRCC).13 We have reported that in malignant lymphomas, ACAT1 expression is increased in response to the uptake of large amounts of cholesterol and protects cells from cholesterol toxicity by esterifying excess cholesterol, thereby maintaining intracellular cholesterol homeostasis.10
SR-BI is a high-density lipoprotein (HDL) receptor first identified by sequence homology to cluster determinant 36 (CD36) and is a member of the class B scavenger receptor family.14,15 SR-BI expressed in hepatocytes mediates reverse cholesterol transport by uptake of HDL cholesterol for routing to bile. Conversely, SR-BI expressed in macrophages suppresses the progression of arteriosclerosis by effluxing intracellular cholesterol into HDL and minimizing the formation of foam cells in arteriosclerotic lesions. We reported that SR-BI expressed in malignant lymphoma cells is mainly involved in cholesterol efflux, since blocking its function with SR-BI inhibitor (BLT-1) increased the amount of free cholesterol in the cells.10
Targeting the cholesterol metabolism of malignant lymphoma
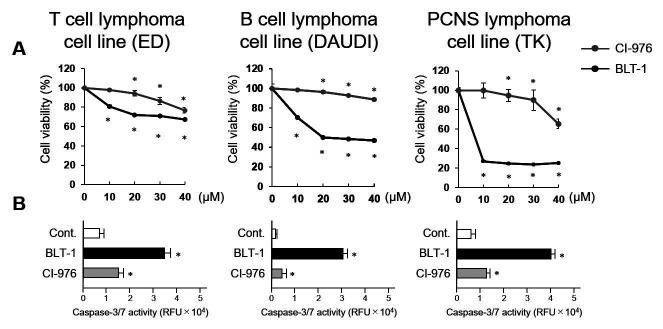
Statins (HMG-CoA reductase inhibitor) have previously been reported to induce apoptosis in some malignant lymphoma cells. Cholesterol depletion by high-dose statins has been shown to disrupt lipid rafts and alter the localization and function of proteins in the lipid rafts of cell membranes.16,17 Simvastatin induces apoptosis of EBV virus-infected lymphoma cells by dissociating Latent Membrane Protein 1 (LMP-1) from lipid rafts in the cell membrane and inhibiting NF-κB activation.18 Studies on cell death due to excessive accumulation of free cholesterol have also been conducted. Excess free cholesterol is harmful to cells.19,20 It has been reported that in pancreatic cancer cells, an ACAT inhibitor increases the amount of intracellular free cholesterol, and the resultant cholesterol toxicity induces apoptosis.12 In addition, we have reported that SR-BI inhibitor blocks the efflux of free cholesterol to HDL, resulting in excessive accumulation of free cholesterol, which in turn induces apoptosis in malignant lymphomas10 (Fig. 1). In malignant lymphoma cells, negative feedback of LDLR is unregulated even under cholesterol-rich conditions, so inhibition of cholesterol efflux by SR-BI inhibitor readily leads to cholesterol over-accumulation, which in turn induces apoptosis (Fig. 2).
Fig. 1.
Effects of ACAT and SR-BI inhibitors on lymphoma cell proliferation. Proliferation of lymphoma cells following incubation with the indicated concentrations of CI-976 or BLT-1 for 24 h (A). Caspase-3/7 activation of lymphoma cells following incubation with CI-976 (10 µM) or BLT-1 (10 µM) for 24 h (B). CI-976: ACAT inhibitor, BLT-1: SR-BI inhibitor. *p-value <0.05 compared with the control. PCNS, primary central nervous system; RFU, relative fluorescence unit
Fig. 2.
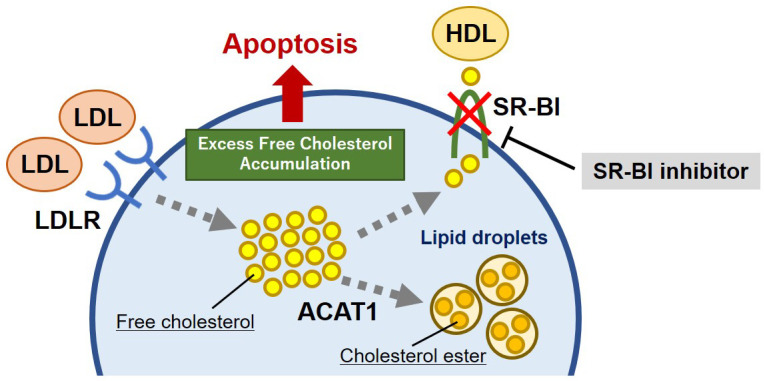
Schema of the lipid metabolism in malignant lymphoma. Malignant lymphomas have unregulated the negative feedback of LDL receptor (LDLR) and increased LDL uptake. Scavenger receptor class B type I (SR-BI) inhibitor blocks cholesterol efflux to HDL, leading to intracellular over-accumulation of free cholesterol. As a result, apoptosis is induced in malignant lymphoma cells due to cholesterol toxicity.
Lipid droplet vacuole in malignant lymphoma
Lipid droplet accumulation in non-adipocytic tissues represents a novel characteristic of cancer. Glioblastoma (GBM) is a malignant tumor associated with dysfunction of lipid metabolism.21,22 It is the most aggressive brain cancer and is a grade IV astrocytoma. Large amounts of lipid droplets are observed in tumor tissue from patients with GBM, but are undetectable in low-grade gliomas and normal brain tissue.23 Therefore, lipid droplet may be developed as a promising diagnostic biomarker for GBM. We have reported that lipid droplet vacuoles are frequently found in adult T-cell leukemia/lymphoma, diffuse large B-cell lymphoma (DLBCL), and primary central nervous system lymphoma, which are considered high-grade lymphomas (vacuole-positive rate of more than 50%). In contrast, lipid droplet vacuoles are less frequently detected in follicular lymphomas, which are classified as low-grade lymphomas (vacuole-positive rate of about 13%). Similarly, in cell lines, high-grade lymphoma cells tended to show more lipid droplets10 (Fig. 3). In addition, patients with lipid droplet vacuole-positive DLBCL had significantly shorter survival than those lacking vacuoles.10 Therefore, lipid droplets may represent a new prognostic and grade-predictive factor in malignant lymphomas.
Fig. 3.
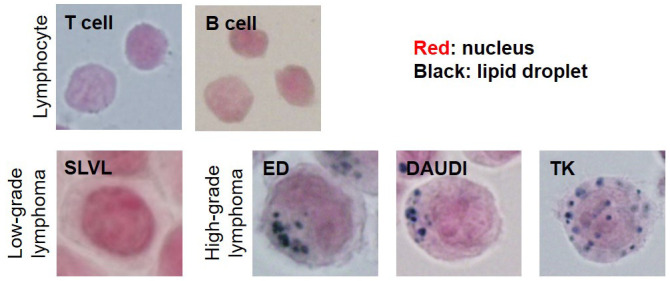
Lipid stained (Sudan Black B staining) images of lymphocytes and Malignant lymphoma cell lines.
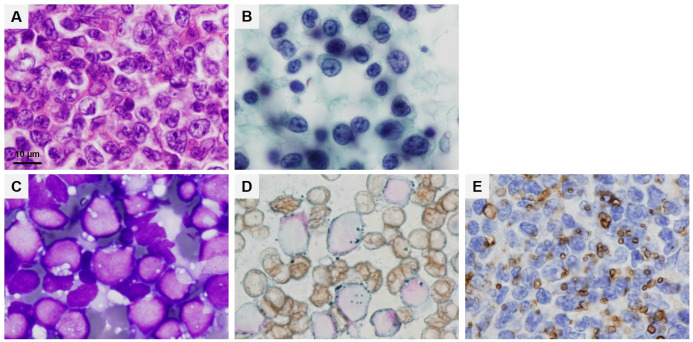
In addition to being cellular organelles that store lipids, lipid droplets also have diverse functions including, but not limited to, lipid metabolism, energy production, and escape from lipotoxicity and endoplasmic reticulum stress. Thus, lipid droplets are advantageous for the survival of malignant cells and have been reported to play a role in chemotherapy resistance.24 In malignant lymphomas, the presence of lipid droplet vacuoles is positively correlated with malignancy. Therefore, lipid droplet vacuole formation is expected to be a marker for aggressive lymphoma as well as a maker for predicting grade and prognosis. It has also been shown that SR-BI inhibitors tend to have a greater antitumor effect on higher-grade lymphoma cells.10 Therefore, it is suggested that observation of lipid droplet vacuoles is important for determining the therapeutic efficacy of lipid metabolism inhibitors. However, lipid droplet vacuoles in malignant lymphomas cannot be observed in hematoxylin and eosin-stained or Papanicolaou-stained specimens. In specimens prepared for routine examination, lipid droplet vacuoles can only be observed with Giemsa staining for cytology. Therefore, it is important to prepare cytology specimens using the stamp preparation before formalin fixation and observe them with Giemsa staining. It may also be important to perform lipid staining (Oil Red O staining or Sudan Black B staining) to distinguish degenerated vacuoles (Fig. 4).
Fig. 4.
Specimens from same adult T-cell lymphoma (ATL) case. Hematoxylin and eosin staining (A). Papanicolaou staining (B). May-Grünwald Giemsa staining (C). Lipid staining (Sudan Black B staining) (D). Immunohistostaining (adipophilin) (E). Scale bar, 10 µm.
Expression of lipid droplet surface proteins in malignant tumors
In recent years, several malignant tumors have indirectly been shown to exhibit lipid droplet formation by immunostaining for lipid droplet surface proteins (Perilipin 1, Perilipin 2: adipophilin, Perilipin 3, Perilipin 4, Perilipin 5). Furthermore, it has been reported that high expression of adipophilin predicts poor prognosis in high-grade serous ovarian cancer25 and overexpression of Perilipin 3 is a poor prognostic factor in clear cell renal cell carcinoma.26 The association between lipid membrane proteins and prognosis in malignant lymphoma has not been reported and should be investigated in the future.
CONCLUSION
In conclusion, the formation of lipid droplet vacuoles in malignant lymphoma cells can be not only a biomarker of malignancy and prognosis, but also a morphological characteristic that predicts the effect of lipid metabolism inhibitors. This also applies to other malignant tumors in which lipid metabolism is enhanced. Therefore, it is important to observe specimen with attention to lipid droplet vacuoles during pathological and cytological diagnosis.
ACKNOWLEDGMENT
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 20H03459). Samples were prepared from specimens obtained from patients diagnosed as lymphoma at Izumi General Hospital (Izumi, Kagoshima, Japan). Written informed consent was obtained from all patients, and the study design was approved by the review board (#57).
Footnotes
CONFLICT OF INTEREST
All authors have no financial competing interests to declare.
REFERENCES
- 1.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986; 232: 34-47. [DOI] [PubMed] [Google Scholar]
- 2.Tatidis L, Masquelier M, Vitols S. Elevated uptake of low density lipoprotein by drug resistant human leukemic cell lines. Biochem Pharmacol. 2002; 63: 2169-2180. [DOI] [PubMed] [Google Scholar]
- 3.Lu M, Gursky O. Aggregation and fusion of low-density lipoproteins in vivo and in vitro. Biomol Concepts. 2013; 4: 501-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Accioly M, Pacheco P, Maya-Monteiro CM, et al. Lipid bodies are reservoirs of cyclooxygenase-2 and sites of prostaglandin-E2 synthesis in colon cancer cells. Cancer Res. 2008; 68: 1732-1740. [DOI] [PubMed] [Google Scholar]
- 5.Nardi F, Fitchev P, Brooks KM, et al. Lipid droplet velocity is a microenvironmental sensor of aggressive tumors regulated by V-ATPase and PEDF. Lab Invest. 2019; 99: 1822-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright DH. Lipid content of malignant lymphomas. J Clin Pathol. 1968; 21: 643-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yen CF, Kalunta CI, Chen FS, et al. Regulation of low-density lipoprotein receptors and assessment of their functional role in Burkitt’s lymphoma cells. Biochim Biophys Acta. 1995; 1257: 47-57. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z, Liu J, Zhang T, et al. Distinct BTK inhibitors differentially induce apoptosis but similarly suppress chemotaxis and lipid accumulation in mantle cell lymphoma. BMC Cancer. 2021; 21: 732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luanpitpong S, Janan M, Thumanu K, et al. Deciphering the Elevated Lipid via CD36 in Mantle Cell Lymphoma with Bortezomib Resistance Using Synchrotron-Based Fourier Transform Infrared Spectroscopy of Single Cells. Cancers (Basel). 2019; 11: 576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yano H, Fujiwara Y, Horlad H, et al. Blocking cholesterol efflux mechanism is a potential target for antilymphoma therapy. Cancer Sci. 2022; 113: 2129-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radhakrishnan A, Goldstein JL, McDonald JG, Brown MS. Switch-like control of SREBP-2 transport triggered by small changes in ER cholesterol: a delicate balance. Cell Metab. 2008; 8: 512-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Gu D, Lee SSY, et al. Abrogating cholesterol esterification suppresses growth and metastasis of pancreatic cancer. Oncogene. 2016; 35: 6378-6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsumoto K, Fujiwara Y, Nagai R, Yoshida M, Ueda S. Expression of two isozymes of acyl-coenzyme A: cholesterol acyltransferase-1 and -2 in clear cell type renal cell carcinoma. Int J Urol. 2008; 15: 166-170. [DOI] [PubMed] [Google Scholar]
- 14.Calvo D, Vega MA. Identification, primary structure, and distribution of CLA-1, a novel member of the CD36/LIMPII gene family. J Biol Chem. 1993; 268: 18929-18935. [PubMed] [Google Scholar]
- 15.Acton SL, Scherer PE, Lodish HF, Krieger M. Expression cloning of SR-BI, a CD36-related class B scavenger receptor. J Biol Chem. 1994; 269: 21003-21009. [PubMed] [Google Scholar]
- 16.Gubina E, Chen T, Zhang L, Lizzio EF, Kozlowski S. CD43 polarization in unprimed T cells can be dissociated from raft coalescence by inhibition of HMG CoA reductase. Blood. 2002; 99: 2518-2525. [DOI] [PubMed] [Google Scholar]
- 17.Hansen GH, Niels-Christiansen LL, Thorsen E, Immerdal L, Danielsen EM. Cholesterol depletion of enterocytes. Effect on the Golgi complex and apical membrane trafficking. J Biol Chem. 2000; 275: 5136-5142. [DOI] [PubMed] [Google Scholar]
- 18.Katano H, Pesnicak L, Cohen JI. Simvastatin induces apoptosis of Epstein-Barr virus (EBV)-transformed lymphoblastoid cell lines and delays development of EBV lymphomas. Proc Natl Acad Sci USA. 2004; 101: 4960-4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown MS, Ho YK, Goldstein JL. The cholesteryl ester cycle in macrophage foam cells. Continual hydrolysis and re-esterification of cytoplasmic cholesteryl esters. J Biol Chem. 1980; 255: 9344-9352. [PubMed] [Google Scholar]
- 20.Warner GJ, Stoudt G, Bamberger M, Johnson WJ, Rothblat GH. Cell toxicity induced by inhibition of acyl coenzyme A:cholesterol acyltransferase and accumulation of unesterified cholesterol. J Biol Chem. 1995; 270: 5772-5778. [DOI] [PubMed] [Google Scholar]
- 21.Guo D, Bell EH, Chakravarti A. Lipid metabolism emerges as a promising target for malignant glioma therapy. CNS Oncol. 2013; 2: 289-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng C, Ru P, Geng F, et al. Glucose-mediated N-glycosylation of SCAP is essential for SREBP-1 activation and tumor growth. Cancer Cell. 2015; 28: 569-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geng F, Cheng X, Wu X, et al. Inhibition of SOAT1 suppresses glioblastoma growth via blocking SREBP-1-mediated lipogenesis. Clin Cancer Res. 2016; 22: 5337-5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rak S, De Zan T, Stefulj J, et al. FTIR spectroscopy reveals lipid droplets in drug resistant laryngeal carcinoma cells through detection of increased ester vibrational bands intensity. Analyst (Lond). 2014; 139: 3407-3415. [DOI] [PubMed] [Google Scholar]
- 25.Iwahashi N, Ikezaki M, Fujimoto M, et al. Lipid Droplet Accumulation Independently Predicts Poor Clinical Prognosis in High-Grade Serous Ovarian Carcinoma. Cancers (Basel). 2021; 13: 5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang K, Ruan H, Song Z, et al. PLIN3 is up-regulated and correlates with poor prognosis in clear cell renal cell carcinoma. Urol Oncol. 2018; 36: 343.e9-343.e19. [DOI] [PubMed] [Google Scholar]