Abstract
Escherichia coli K-12 possesses two versions of Trk/Ktr/HKT-type potassium ion (K+) transporters, TrkG and TrkH. The current paradigm is that TrkG and TrkH have largely identical characteristics, and little information is available regarding their functional differences. Here, we show using cation uptake experiments with K+ transporter knockout mutants that TrkG and TrkH have distinct ion transport activities and physiological roles. K+-transport by TrkG required Na+, whereas TrkH-mediated K+ uptake was not affected by Na+. An aspartic acid located five residues away from a critical glycine in the third pore-forming region might be involved in regulation of Na+-dependent activation of TrkG. In addition, we found that TrkG but not TrkH had Na+ uptake activity. Our analysis of K+ transport mutants revealed that TrkH supported cell growth more than TrkG; however, TrkG was able to complement loss of TrkH-mediated K+ uptake in E. coli. Furthermore, we determined that transcription of trkG in E. coli was downregulated but not completely silenced by the xenogeneic silencing factor H-NS (histone-like nucleoid structuring protein or heat-stable nucleoid-structuring protein). Taken together, the transport function of TrkG is clearly distinct from that of TrkH, and TrkG seems to have been accepted by E. coli during evolution as a K+ uptake system that coexists with TrkH.
Keywords: Trk/Ktr/HKT, potassium transport, gene transfer, Escherichia coli, membrane protein, molecular evolution
Abbreviations: H-NS, histone-like nucleoid structuring protein; K+, potassium ion; qRT–PCR, quantitative RT–PCR
Potassium ion (K+) is an essential cation for most living organisms. It has significant roles in cytoplasmic ion homeostasis, enzymatic activation, membrane potential formation, and osmoregulation (1, 2, 3). The presence of a K+ uptake system in the membrane is crucial in prokaryotic cells and nonanimal eukaryotes because they lack the Na+/K+ pump present in animal cells. K+ uptake systems are divided into K+ channels and three classes of K+ transporters, Kdp, Kup/HAK/KT, and Trk/Ktr/HKT (4, 5). Which types and how many transporters are present varies between organisms. The Escherichia coli genome contains genes encoding a K+ channel homolog, kch, and all three classes of K+ uptake transporters, kdp, kup, and trk. K+ transport function of Kch has not been shown, but activities of the other three systems ensure that E. coli can satisfy its K+ requirements under different environmental conditions (1).
Under K+-limited conditions (<1 mM K+), the ATP-hydrolyzing K+ transporter, Kdp, is induced and functions as the primary K+ uptake route for E. coli survival (6). Kdp exhibits high affinity and high specificity for K+ (7, 8, 9). The transporter consists of the multiple subunits of the KdpFABC complex. Expression of the kdpFABC operon is controlled by a two-component regulatory system comprising KdpD and KdpE (10), which sense low intracellular K+ concentrations and high external osmolarity (6). Kdp expression is rapidly downregulated upon high K+ concentrations (>5 mM) (6).
When K+ concentrations are sufficient for the cells, Kup and Trk, which are constitutively expressed, are the main contributors to maintenance of intracellular K+ levels instead of Kdp. In E. coli, Kup has been proposed to function as a K+–H+ symporter (11, 12), forming the main K+ influx passage during hyperosmotic stress at low pH (13). Moreover, Kup has the ability to uptake Cs+ and Rb+, which allows E. coli to use Cs+ and/or Rb+ as a substitute for K+ under low K+ conditions (14, 15, 16). E. coli K-12 contains two Trk/Ktr/HKT homologs, TrkG and TrkH, which share 41% identity and have the same membrane topology (17, 18). Early studies have identified differences between TrkG and TrkH with regard to kinetics and SapD protein (TrkE) dependence (17, 19). In contrast, both activities depend on a cytosolic protein, TrkA, which contains an RCK domain to control K+ flux in E. coli (20, 21). The crystal structure of Vibrio parahemolyticus TrkH–TrkA (21, 22) reveals that a TrkH dimer forms a complex with a TrkA-tetramer ring. K+ transport activity of TrkH is activated by ATP binding to TrkA, which then induces conformational changes in TrkA and thereby opens the channel in TrkH (23). A similar architecture is also found in the closely related Bacillus subtilis KtrA–KtrB (24). Trk/Ktr/HKT transporters are present in prokaryotes as well as in eukaryotes including yeast and plants (5, 25, 26). All Trk/Ktr/HKT transporters are characterized by a common ancestral K+ channel structure (18, 27, 28, 29). Although they are classified as K+ transporters, some of them possess Na+-related activities, such as Na+-activated K+ transport properties (Synechocystis KtrABE (30) and Vibrio alginolyticus KtrAB (29)), K+–Na+ cotransport activity (wheat HKT1) (31), or strong Na+ over K+ selectivity for transport (Arabidopsis thaliana HKT1) (32). TrkG and TrkH in E. coli are considered ancestral Trk/Ktr/HKT-type transporters in terms of evolution, but their individual transport characteristics and potentially unique roles remain to be elucidated. Interestingly, trkG and trkH are predicted to have a different evolutionary history (33). While trkH is recognized to be the part of E. coli core genome, trkG is most likely a “foreign gene” that was acquired by E. coli via horizontal gene transfer, through bacteriophage transduction (33). This hypothesis is supported by two observations: (1) trkG has significantly lower GC content than trkH (38% versus 53%) and (2) trkG is located in the rac cryptic prophage. The expression of such foreign genes is usually silenced or repressed under normal conditions by H-NS (histone-like nucleoid structuring protein or heat-stable nucleoid-structuring protein), also known as xenogeneic silencing (34, 35, 36). Even though these differences are known, TrkG function has generally been extrapolated from information available for TrkH, mostly since only limited research has been done specifically on TrkG. Thus, there is a clear need to study TrkG and TrkH characteristics individually.
In this study, we characterized the ion transport properties, gene regulation, and physiological role of TrkG and TrkH in E. coli. TrkG exhibited Na+-dependent K+ uptake as well as Na+ uptake activity, whereas TrkH showed Na+-independent K+ uptake and no Na+ uptake activity. We confirmed that trkG expression was modulated by H-NS; however, this did not lead to gene silencing. Under sufficient K+ and Na+ conditions, both TrkG and TrkH functioned as important K+ uptake systems. These data suggest that during the course of bacterial evolution, horizontally transferred trkG contributed to the expansion of functional diversity in E. coli.
Results
TrkH has higher K+ uptake activity than TrkG
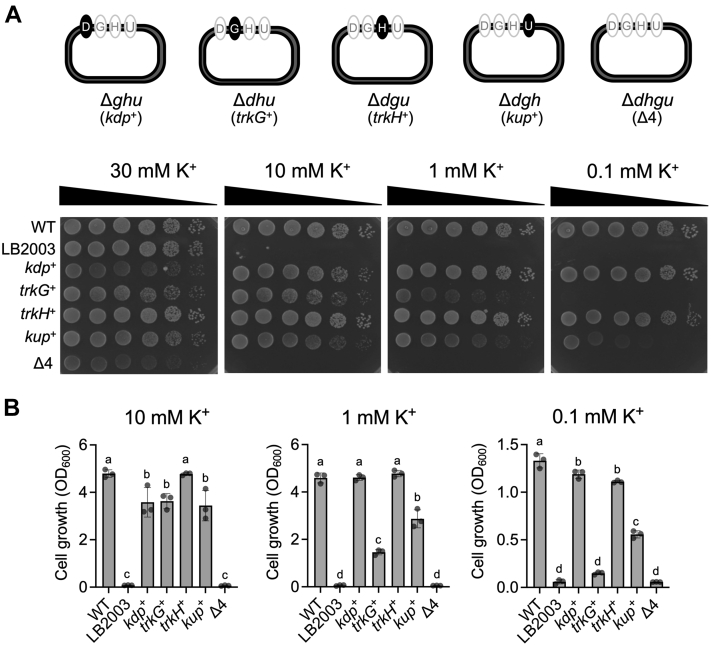
E. coli K-12 have two homologous Trk-type K+ transporters, TrkG and TrkH, whose functional properties and physiological roles have not been fully characterized (17, 33). To evaluate the functions of TrkG and TrkH in K+ uptake, we constructed a series of triple knockout mutants, each retaining only one of the four K+ transporters, Δdhu (trkG+), Δdgu (trkH+), Δghu (kdp+), and Δdgh (kup+), as well as a quadruple knockout mutant, Δdghu (Δ4). To simplify the naming of mutants, [d] represents kdpA, [g] represents trkG, [h] represents trkH, and [u] represents kup, and the alternative name in bracket describes the remaining K+ uptake gene in the strain. Each of the triple mutants, therefore, allowed us to assay the sole activity of one K+ transport system in E. coli (Table 1 and Fig. 1A, top panel). To compare how the K+ uptake ability of either TrkG or TrkH supported growth in E. coli, cells were grown with minimal medium containing varying concentrations of K+, both on plates (Fig. 1A, lower panels) or in liquid culture (Fig. 1B). The Δdgu (trkH+) mutant, lacking kdpA, trkG, and kup, was able to grow well in medium with low K+ (0.1 mM), similar to the Δghu (kdp+) mutant, which still contained the ATP-driven high-affinity K+ uptake transporter (37). The growth of the Δdhu (trkG+) and Δdgh (kup+) strains was very low on media with 0.1 mM and 1 mM K+, compared with the growth of Δdgu (trkH+) and Δghu (kdp+) (Fig. 1B). Furthermore, we determined growth curves for the strains under these K+ concentrations (Fig. S1). In comparison, the Δdghu (Δ4) mutant, lacking all four major K+ transport systems, was only able to grow in medium supplemented with 30 mM K+ (Fig. 1A, lower panels). This was similar to the growth of the widely used K+ transport–defective E. coli mutant, LB2003 (38). The growth in liquid culture generally showed the same tendency as that on agar medium (Fig. 1, A and B). These results suggested that TrkH was the dominant K+ uptake system, and TrkG and Kup were minor contributors to K+ uptake in E. coli at 0.1 mM K+.
Table 1.
Bacterial strains used in this study
| Strain name | Genotype | Reference |
|---|---|---|
| BW25113 | F−Δ(araD-araB)567 Δ(rhaD-rhaB)568 ΔlacZ4787(::rrnB-3) hsdR514 rph-1 | (78) |
| LB2003 | F−kup1 ΔkdpABC5 ΔtrkA rpsL metE thi rha gal | (38) |
| TO114 | W3110 (F−λ−rpoS(Am) rph-1 Inv(rrnD-rrnE)) nhaA::KmrnhaB::EmrchaA::Cmr | (41) |
| ΔtrkG | BW25113 ΔtrkG | This study |
| ΔtrkH | BW25113 ΔtrkH | This study |
| ΔtrkGΔtrkH | BW25113 ΔtrkGΔtrkH | This study |
| Δghu (kdp+) | BW25113 ΔtrkGΔtrkHΔkup | This study |
| Δdhu (trkG+) | BW25113 ΔtrkHΔkdpAΔkup | This study |
| Δdgu (trkH+) | BW25113 ΔkdpAΔkupΔtrkG | This study |
| Δdgh (kup+) | BW25113 ΔtrkGΔtrkHΔkdpA | This study |
| Δdghu (Δ4) | BW25113 ΔtrkGΔtrkHΔkupΔkdpA | This study |
| Δabc | BW25113 ΔnhaAΔnhaBΔchaA | This study |
| Δabcg | BW25113 ΔnhaAΔnhaBΔchaAΔtrkG::Kmr | This study |
| Δabch | BW25113 ΔnhaAΔnhaBΔchaAΔtrkH::Kmr | This study |
| W3110 | F− λ− rph-1 IN(rrnD-rrnE)1 | (86) |
| Δhns | W3110 Δhns | (55) |
| ΔhnsΔstpA | W3110 Δhns::KmrΔstpA::Cmr | (55) |
| ΔhnsΔydgT | W3110 Δhns::KmrΔydgT | (55) |
| ΔhnsΔhha | W3110 Δhns::KmrΔhha | (55) |
Abbreviations: Cm, chloramphenicol; Em, erythromycin; Km, kanamycin.
Figure 1.
Growth of Escherichia coli K+uptake transporter mutants in K+limited medium.A, schematic showing E. coli triple and quadruple knockout mutants lacking K+ uptake transporters in different combinations, d, kdpA; g, trkG; h, trkH; and u, kup (top). Growth of these mutants on K+-limited agar medium supplemented with different K+ concentrations. LB2003; Δghu (kdp+), Δdhu (trkG+), Δdgu (trkH+), Δdgh (kup+), and Δdghu (Δ4) (lower). Ten-fold serial dilutions of the cells were spotted on agar medium and then incubated at 30 °C for 2 days. The experiment was repeated three times; representative results are shown. B, growth of the same mutants in liquid culture. Cell growth was measured after overnight incubation (t = 15 h) at 30 °C. Mean ± SD. n = 3, biological replicates. One-way ANOVA, Tukey test, p < 0.05. Different letters above the bar in graph means statistically different. K+, potassium ion.
Na+ activates TrkG-mediated K+ uptake
Some members of the Trk/Ktr/HKT family of transporters have Na+-dependent K+ uptake activity (30, 39, 40). However, it remains unclear whether K+ transport mediated by TrkG and TrkH is also influenced by Na+. The Na+ dependency of TrkG and TrkH was examined by comparing the K+ uptake of Δdhu (trkG+) and Δdgu (trkH+) in high-Na+ buffer (Hepes–NaOH, 149.7 mM Na+ measured by atomic absorption spectrometry) versus low-Na+ buffer (Hepes–triethanolamine, 7.7 μM Na+ measured by atomic absorption spectrometry). K+ uptake activity of Δdhu (trkG+) was significantly higher in high-Na+ buffer, compared with that in low-Na+ buffer, whereas the K+ uptake activity of Δdgu (trkH+) was similar in both buffers (Fig. 2A). To further corroborate Na+ dependence of TrkG, we measured the K+ uptake rate of Δdhu (trkG+) in low-Na+ buffer while adding K+ followed by Na+. The Δdhu (trkG+) strain accumulated K+ very slowly in the presence of 0.1 mM K+, but the subsequent addition of 1 mM Na+ increased intracellular K+ accumulation dramatically (Fig. 2B, left). In contrast, the Δdgu (trkH+) strain showed high K+ uptake once 0.1 mM K+ was added, independently of Na+ (Fig. 2B, center). Next, we examined the effect of other cations on the activation of TrkG (Fig. 2C, left). Rb+, Li+, Cs+, and Ca2+ did not activate K+ uptake in Δdhu (trkG+). Therefore, Na+ was the only monovalent cation involved in TrkG activation. The half-activation constant for Na+ activation of TrkG-mediated K+ uptake was calculated to be 2.1 mM (Fig. 2C, right). Since high concentrations of NaCl also increase the osmotic pressure, we also considered the possibility that osmotic pressure may be involved in TrkG activation. We therefore examined the effect of high osmolarity on TrkG (Fig. 2D). Activation after addition of 200 mM sorbitol was smaller than activation by 100 mM NaCl, indicating that Na+ itself enhanced TrkG-mediated K+ transport.
Figure 2.
Na+dependency of TrkG-mediated K+uptake activity.A, initial velocity of K+ uptake was measured during the first minute after the addition of 0.2 to 5 mM KCl to cells resuspended in 200 mM Hepes–NaOH (pH 7.5) (closed symbols) (containing 149.7 mM Na+ measured by atomic absorption spectrometry) or Hepes–triethanolamine (pH 7.5) (open symbols) (containing 7.7 μM Na+ measured by atomic absorption spectrometry). Δdhu (trkG+) (circles), Δdgu (trkH+) (squares), and Δdghu (Δ4) (triangles) were used to measure the K+ uptake activity of TrkG, TrkH, and negative control, respectively. B, K+ content determined in cells suspended in 200 mM Hepes–triethanolamine. Addition of 0.1 mM KCl (t = 0 min) to the cells was followed by the addition of 1 mM NaCl (t = 10 min). C, initial velocity of K+ uptake was measured during the first minute after the addition of 1 mM KCl to the cell resuspended in 200 mM Hepes–triethanolamine buffer (pH 7.5) supplemented with 1 mM NaCl, RbCl, LiCl, CsCl, or CaCl2 (left) or with 0.2 to 20 mM NaCl (right). One-way ANOVA, Tukey test, p < 0.05. Different letters above the bar in graph means statistically different. D, the K+ content was measured in cells treated by adding 100 mM NaCl (black symbols), 200 mM sorbitol (gray symbols), or water (white symbols) 10 min before the addition of 0.1 mM KCl (t = 0 min). Data obtained from three experiments, biological replicates. Mean ± SD. N = 3. Solid line (A, B, and D) connects means, whereas the dotted line (C) shows the Michaelis–Menten nonlinear regression curve fit. K+, potassium ion; Na+, sodium ion.
To evaluate the effect of Na+ on TrkG and TrkH in vivo, growth of Δdhu (trkG+) and Δdgu (trkH+) was compared in phosphoric acid–based medium containing 0.1 mM KCl and 0 to 1000 mM NaCl (Fig. 3). The basal medium already contained trace concentrations of 17 μM K+ and 33 μM Na+ as measured by an atomic absorption spectrometry. The Δdghu (Δ4) mutant was unable to grow in any of these media because of the low K+ concentration. The growth of Δdhu (trkG+) increased with increasing Na+ in the medium and peaking at 10 mM Na+. These data supported the conclusion that TrkG-mediated K+ uptake was enhanced by Na+. However, concentrations of Na+ above 10 mM inhibited growth. It is likely that this impairment of cell growth is due to excess Na+ taken up by TrkG.
Figure 3.
Stimulation of growth of Δdhu (trkG+) through addition of Na+. Growth assay of Escherichia coli BW25113 WT, Δdhu (trkG+), Δdgu (trkH+), and Δdghu (Δ4) in phosphoric acid–based medium (containing K+ 17 μM K+ and 33 μM Na+ measured by atomic absorption spectrometry) supplemented with 0.1 mM KCl and various concentrations of NaCl. Cell growth was determined after an overnight incubation (t = 15 h) at 30 °C. Mean ± SD. n = 3, biological replicates. One-way ANOVA, Tukey test, p < 0.05. Different letters above the bar in graph means statistically different. K+, potassium ion; Na+, sodium ion.
TrkG mediates Na+ uptake but not Na+ efflux
We explored the ability of TrkG to transport Na+ by measuring of Na+ accumulation inside the cells of E. coli TO114 (41), a strain lacking the activity of three Na+ efflux–Na+/H+ antiporters, NhaA, NhaB, and ChaA. The cells expressing TrkG accumulated more intracellular Na+ than those expressing TrkH or containing an empty vector (Fig. 4A), indicating that TrkG had Na+ uptake activity. Since the background activity of Na+ uptake in TO114 was high, it is likely that endogenous TrkG contributed to this background activity. To confirm this hypothesis, we constructed quadruple knockout mutants of ΔnhaAΔnhaBΔchaAΔtrkG (Δabcg) as well as ΔnhaAΔnhaBΔchaAΔtrkH (Δabch) in the BW25113 background as well as ΔnhaAΔnhaBΔchaA (Δabc) as a control (Table 1). Na+ uptake activity was significantly lower in Δabcg, compared with the control strain Δabc or Δabch (Fig. 4B). Reintroduction of trkG into Δabcg successfully restored Na+ uptake activity of the mutant (Fig. 4C). This result suggested that TrkG conferred most of the Na+ uptake activity in Δabc background strains, whereas TrkH did not conduct Na+ influx. To re-examine the effect of Na+ uptake by TrkG on the growth of E. coli, we compared the growth of Δabc and Δabcg at low K+ medium with added Na+. Growth of the Δabcg strain was less sensitive to higher Na+ concentrations (50–100 mM) (Fig. 4D). This result is consistent with the interpretation that TrkG-mediated Na+ influx led to increased Na+ toxicity at high Na+. The growth of Δabc increased with increasing Na+ concentrations and showed optimum at 25 mM Na+, which was not observed in the wildtype. This seems unlikely to be related to TrkG, but the actual reasons remain unclear. We further examined whether TrkG had Na+ efflux activity using a plate assay. If TrkG or TrkH mediated Na+ efflux, transformed cells should become more Na+ tolerant and therefore able to grow in medium with higher Na+. The positive controls, E. coli NhaA (42) and Synechocystis NhaS3 (43), grew well on Na+-supplemented medium. The growth of the cells expressing TrkG and TrkH was inhibited as more Na+ was added to the medium (Fig. 4E). None of our results indicated that TrkG was involved in alleviation of salinity stress.
Figure 4.
Mediation of Na+uptake by TrkG.A–C, to measure Na+ content, cells were incubated in 200 mM Hepes–triethanolamine (pH 7.5) supplemented with 10 mM glucose, followed by the addition of 1 mM NaCl (t = 0 min). A, Escherichia coli strain TO114 transformed with TrkG (closed circles), TrkH (closed squares), and empty vector (closed triangles). B, E. coli mutant strains Δabcg (open circles), Δabch (open squares), and Δabc (open triangles). C, Δabcg transformed with same plasmids as in (A). D, growth assay of E. coli Δabc, Δabcg, and WT in phosphoric acid–based medium supplemented with 0.01 mM KCl and various concentrations of NaCl. Cell growth was determined after an overnight incubation (t = 15 h) at 30 °C. Mean ± SD. n = 3, biological replicates. One-way ANOVA, Tukey test, p < 0.05. Different letters above the bars in the graph means statistically different. E, growth test of TO114 expressing E. coli TrkG, TrkH, NhaA, or Synechocystis sp. PCC6803 NhaS3 on KLB medium (control) supplemented with various NaCl concentrations. Ten-fold serial dilutions of the cells were spotted onto agar medium and then incubated at 26 °C for 1 day. The experiment was repeated three times; representative results are shown. Na+, sodium ion.
D327 might be important for Na+ dependency of TrkG
Our data (Fig. 2) show that TrkG required Na+ for its K+ transport activity, whereas TrkH did not. Such different characteristics related to Na+ independence are not uncommon in Trk/Ktr/HKT-type transporters (40, 44, 45), but the specific structures responsible for these differences remain unknown. To identify which part of the TrkG protein was responsible for its Na+ dependency, we tested several combinations of TrkG and TrkH variants and several chimeras, but all of them showed loss of K+ uptake ability (see Discussion section and Fig. S3B). While comparing the amino acid sequence of Na+-activated Trk/Ktr/HKT transporters (Synechocystis KtrB (30), Triticum aestivum or wheat HKT1 (40), V. alginolyticus KtrB (39)) and Na+-independent transporters (Vibrio parahaemolyticus TrkH (22) and Trypanosoma brucei HKT1 (45)) (Fig. 5A), we focused on three negatively charged residues (D121, E231, and D327) that are located five or six residues away from the most important glycine in the first, second, and third K+ selectivity filter in the pore regions (29, 46, 47) and are conserved in Na+-activated transporters but missing in Na+-independent transporters. The model structure of TrkG (obtained from the AlphaFold Protein Structure Database: AF-P23849-F1; https://alphafold.ebi.ac.uk (48)) revealed that these three residues are located near the selectivity filter, facing the periplasmic region (Fig. 5B). We replaced the negatively charged residues with the neutral amino acid, serine, to create three TrkG variants, TrkGD121S, TrkGE231S and TrkGD327S. Some variants had very low K+ uptake activity and low amounts of the protein, which made it difficult to assess their Na+ dependency. Thereby, as an alternative approach, the viability of Δdghu (Δ4) cells expressing individual variants was examined in phosphoric acid–based medium (Fig. 5C). TrkGD121S and TrkGE231S grew as well as the wildtype. In contrast, the growth of cells expressing TrkGD327S was much lower than that of the wildtype at low Na+ but was similar to the wildtype at 10 to 100 mM Na+. This reflected that replacing D327 with serine increased the Na+ requirement for TrkG-containing cells, suggesting that D327 was crucial for Na+-dependent activation of TrkG-mediated K+ transport.
Figure 5.
Negatively charged residues near conserved glycines of TrkG.A, schematic illustration showing E. coli TrkG structure with the location of the negative residues (red dots) near the first, second, and third conserved glycine. Green indicates M2c1/D3M2a, and blue indicates M2c3/D3M2b. The conserved glycines that function as K+ selectivity filters are indicated by an asterisk. The insets show amino acid sequence alignments of the P-loop area of Escherichia coli TrkG (NP_415881.1), Synechocystis sp PCC6803 KtrB (BAL35525.1), Triticum aestivum HKT1 (Q41515), Vibrio alginolyticus KtrB (O87953), E. coli TrkH (YP_026273.1), Vibrio parahaemolyticus TrkH (Q87TN7) and Trypanosoma brucei HKT1 (Tb927.10.4300). Conserved residues are shaded with black. The line separates the Na+-activated Trk/Ktr/HKT transporters (top) from Na+-independent Trk/Ktr/HKT transporters (bottom). B, model structure of TrkG (AF-P23849). The negatively charged residues (D121, E231, and D327) and other residues at the periplasmic gate are represented by red and gray spheres, respectively, in both the side (left) and the top (right) view. The putative motion of M2c3/D3M2b is indicated by the arrow, and the inset illustrates the interactions of D327. The K+ ion (purple sphere) was associated with the critical glycine. C, growth assay of E. coli BW25113 Δdghu (Δ4) overexpressing empty vector (EV), WT TrkG, TrkGD121, TrkGE231S, and TrkGD327S in phosphoric acid–based medium supplemented with 1 mM KCl and different concentrations of NaCl. Cell growth was determined after an overnight incubation (t = 15 h) at 30 °C. Mean ± SD, n = 3, biological replicates. Na+, sodium ion; K+, potassium ion.
Regulation of trkG expression
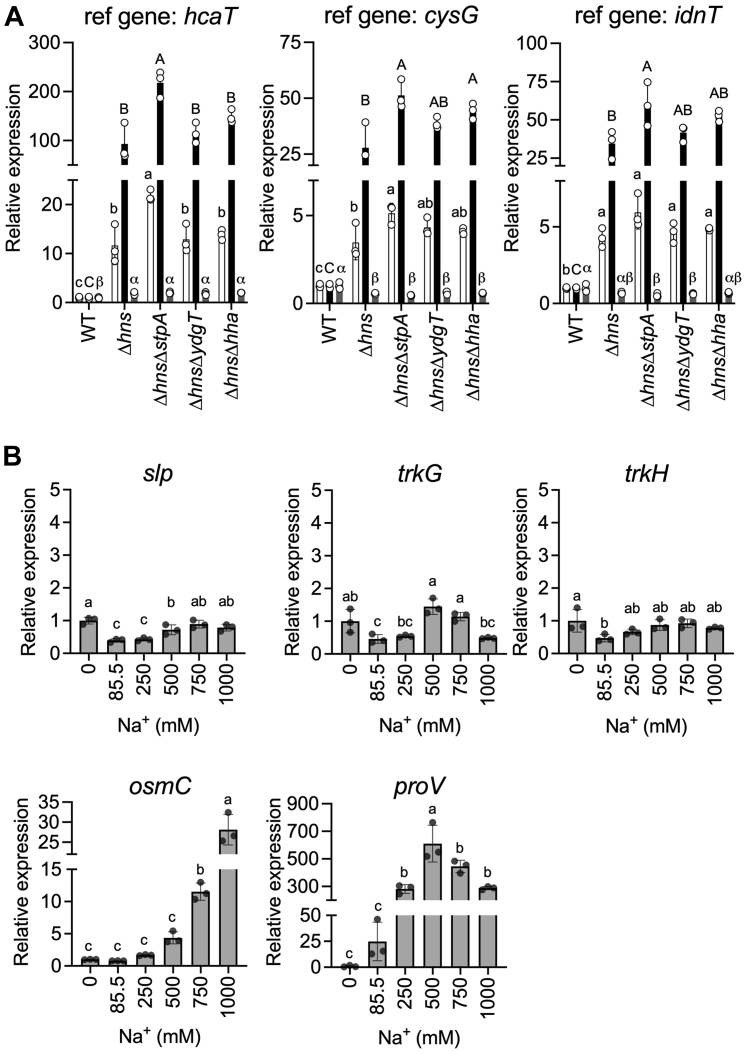
Because trkG has been proposed to be a “foreign” gene in E. coli and may be negatively regulated by H-NS (33, 36), we compared the level of trkG transcript in Δhns and wildtype by quantitative RT–PCR (qRT–PCR) using three reference genes, hcaT, cysG, and idnT whose expression is stable under various conditions (49). We included slp whose expression is under the control of H-NS as an experiment control (50, 51). Expression of slp was higher in the Δhns strain compared with the wildtype, regardless of the reference gene used (Fig. 6A). The trkG expression was significantly higher in Δhns, compared with the wildtype, suggesting that H-NS negatively regulated trkG expression (Fig. 6A). In contrast, trkH expression remained unchanged in all strains, confirming that trkH expression was not regulated by H-NS. These results were in agreement with data obtained by ChIP-chip analysis (36). E. coli has an H-NS paralog, StpA (52), which plays a role as a molecular backup for H-NS (53). Hha and YdgT proteins interact with either H-NS or StpA to modulate expression in E. coli (54, 55). As expected, trkG expression was increased in double knockout mutants of hns and stpA (ΔhnsΔstpA), indicating that stpA may also repress trkG expression (Fig. 6A). H-NS targeted genes are often regulated by environmental factors including osmotic stress (56, 57). In addition, some Trk/Ktr/HKT family proteins are involved in high osmolarity stress adaptation (30, 58). Therefore, we measured the transcript level of trkG in E. coli grown with varying concentrations of NaCl, using LB medium devoid of NaCl as a based medium (Fig. 6B). We included osmC and proV in the experiments because both genes are not only modulated by H-NS but also induced by osmotic stress (56, 59). We confirmed that the level of osmC and proV transcripts increased at high concentrations of NaCl in the medium, indicating that the cells adapted to high osmotic stress (Fig. 6B). However, the expression levels of trkG, slp, and trkH transcripts were not significantly affected by the concentration of NaCl (0–1000 mM). These results indicated that trkG expression was not induced by high osmotic stress although it was in part regulated by H-NS.
Figure 6.
trkG expression is repressed by H-NS and not induced by osmotic stress.A, relative expression levels of slp (white bars), trkG (black bars), and trkH (gray bars) normalized to the indicated reference genes in Escherichia coli W3110 WT, Δhns, ΔhnsΔstpA, ΔhnsΔydgT, and ΔhnsΔhha grown in the minimal medium supplemented with 30 mM KCl. B, relative expression levels of slp, trkG, trkH, osmC, and proV normalized to hcaT in E. coli W3110 WT grown in LB containing NaCl at the listed concentrations (85.5 mM NaCl was included because it is the approximate NaCl concentration in widely used LB medium). The relative expression level for each gene was calculated in relation to WT (A) or 0 mM NaCl medium (B), set at an arbitrary value of 1. Mean ± SD. n = 3, biological replicates. One-way ANOVA, Tukey test, p < 0.05. Different letters above the bar in graph means statistically different. H-NS, histone-like nucleoid structuring protein or heat-stable nucleoid-structuring protein.
Role of TrkG in E. coli
To gain insight into the role of TrkG in E. coli, we measured its promoter activity in E. coli using a bioluminescent reporter system (Fig. 7A) and determined the level of mRNA transcripts in the wildtype by qRT–PCR (Fig. 7B). The intensity of luminescence produced by trkG promoter–controlled luciferase decreased in high K+ medium (Fig. 7A, left). However, the trkG transcript level did not change significantly, regardless of the K+ concentration in the medium (Fig. 7B, left). On the other hand, for TrkH, both promoter activity and transcript level remained relatively stable at various K+ concentrations (Fig. 7, A and B, center). The luciferase activity controlled by the kdpA promoter was only significantly higher when cells were grown in medium containing 0.1 mM K+ or less. At K+ concentrations (1 and 10 mM), the kdpA promoter activity was very low (approximately 44.44 for 1 mM K+ and 14.5 for 10 mM K+), indicating there would be some basal expression of kdpA at sufficient K+ conditions (Fig. 7A, right). This result was consistent with the data obtained by qRT–PCR (Fig. 7B, right). To understand what potential benefit TrkG may provide for E. coli, we compared the growth of wildtype E. coli, ΔtrkG, and ΔtrkH under various stress conditions but were unable to identify specific conditions where the growth of ΔtrkG was either inhibited or improved. To further elucidate the connection of TrkG with TrkH, we constructed mutants expressing the bacterial luciferase gene under control of the kdpA promoter. We then determined the kdp gene expression level in the mutants as a way to monitor the intracellular K+ content. When the cytosolic K+ content is low, expression of kdpA is induced to replenish K+ from the external space (60). E. coli wildtype, ΔtrkG, ΔtrkH, and ΔtrkGΔtrkH showed similar growth in a phosphoric acid–based medium supplemented with 1 mM K+ regardless of the presence/absence of Na+ (Fig. 7C, top panels). The bioluminescence intensity of the luciferase remained at a relatively low level in the wildtype and ΔtrkG (Fig. 7C, bottom panels) at 3 h. TrkH probably functioned in both strains under these conditions; therefore, activation of the kdp promoter was not needed. In ΔtrkH, bioluminescence intensity was reduced to 36% at 3 h (47% at 6 h) in medium with added Na+, compared with medium without added Na+, indicating that in the presence of Na+, the Na+-stimulated TrkG activity was able to contribute to the replenishment of K+ (Fig. 7C). In the double mutant, ΔtrkHΔtrkG, bioluminescence increased both in medium with and without Na+. These results indicated that TrkH was the major Trk transporter in E. coli, and that TrkG could support replenishment of K+ when Na+ was present in the medium.
Figure 7.
Contribution of TrkG to K+uptake in the presence of Na+.A, promoter activity of trkG, trkH, and kdpA, measured by luciferase reporter assay. Transformants of Escherichia coli wildtype were grown in phosphoric acid–based medium supplemented with various concentrations of KCl. Promoter activity is shown as luciferase luminescence normalized to cell growth (absorbance at 600 nm) after an overnight incubation (t = 15 h) at 30 °C. B, relative expression levels of trkG, trkH, and kdpA normalized to hcaT in E. coli BW25113 wildtype grown in phosphoric acid–based medium supplemented with various concentrations of KCl. The relative expression level for each gene was calculated in relation to the 0.1 mM KCl medium, set at an arbitrary value of 1. Mean ± SD. n = 3, biological replicates. One-way ANOVA, Tukey test, p < 0.05. Different letters above the bar in graph means statistically different. C, cell growth (top) and normalized promoter activity (lower) of kdpA′-lux in E. coli wildtype, ΔtrkG, ΔtrkH, and ΔtrkGΔtrkH. Transformants were grown in phosphoric acid–based medium supplemented with 1 mM KCl with (gray bars) or without (white bars) addition of 10 mM NaCl. Mean ± SD. n = 3, biological replicates. Unpaired t test, Holm–Šídák method, p < 0.05. Asterisk shows p < 0.05, ns means not significant. K+, potassium ion; Na+, sodium ion.
Discussion
This study comprehensively investigated the function, physiological role, and genetic background of Trk transporters in E. coli, yielding these new results: (1) differences in Na+ requirement and Na+ permeability of TrkG and TrkH, (2) TrkH as the major K+ uptake system and TrkG and Kup as minor K+ uptake systems before having to use the energy-consuming Kdp-mediated K+ absorption, (3) identification of an aspartic acid residue (D327) involved in Na+-dependent activation of TrkG, (4) differences in gene regulation of trkG and trkH by H-NS (summarized in Fig. 8). Our results contribute to a fundamental understanding of Trk/Ktr/HKT-type transporters from prokaryotes to eukaryotes, which are related by common ancestry. Animal and plant cells contain a number of functional K+ channels (61, 62), whereas in E. coli, the central role of K+ uptake is performed by TrkG, TrkH, Kup, and Kdp instead of K+ channels. Previously, little attention had been paid to functional differences between TrkG and TrkH. In fact, TrkG and TrkH share basically the same transmembrane topology and the same cytosolic TrkA for their transport activity (18, 20, 23). In B. subtilis, which like E. coli K-12 also has two Trk/Ktr/HKT-type K+ transporters, KtrAB and KtrCD, these are involved in adaptation to osmotic stress (58). However, no differences in the function and physiological roles of KtrAB and KtrCD have been reported. This study revealed that Na+-dependent activation of TrkG was one notable feature that was different from TrkH (Fig. 2). The half-activation constant for Na+ activation of TrkG was approximately 2.1 mM Na+ (Fig. 2C). Na+-dependent K+ uptake of Trk/Ktr/HKT family transporters has been reported for Synechocystis KtrABE (30), V. alginolyticus KtrAB (39), and wheat HKT1 (40).
Figure 8.
Differentcharacteristics of Escherichia coli K-12 TrkG and TrkH. The trkG gene is presumed to be a “foreign” gene that was acquired through bacteriophage transduction (in the dashed circle). H-NS partially represses the expression of trkG but not trkH. TrkG shows Na+-dependent K+ uptake and Na+ uptake activity while TrkH shows Na+-independent K+ uptake and no Na+ uptake activity. H-NS, histone-like nucleoid structuring protein or heat-stable nucleoid-structuring protein; K+, potassium ion; Na+, sodium ion.
To narrow down which part of TrkG was responsible for its Na+-dependent activity, we introduced targeted mutations into the pore region and created various chimera variants (Fig. S3). TrkG and TrkH each have four sets of the pore loop between two transmembrane domains (D1–D4). Each pore loop contains a conserved glycine, which is the equivalent of the first glycine of the GYG signature sequence of K+ channels (29, 46, 47). We predicted that the amino acid sequence adjacent to the glycine might be involved in Na+ dependency (Figs. S2 and S3A). We therefore swapped these four residues including the glycine between TrkG and TrkH and tested the K+ transport activity of these variants. However, none of them showed any K+ uptake activity. We also constructed several chimeras by swapping domains between TrkG and TrkH (Fig. S3B), but none of the cultures expressing these chimeras were able to grow in low K+ medium (Fig. S3C). Thereby, as an alternative approach, we compared the amino acid sequences of Trk/Ktr/HKT-type K+ transporters with different properties and noticed negatively charged residues conserved in the transporters, which were absent from Trk/Ktr/HKT with Na+-independent activity (Fig. 5A). Conversion of one of those residues, D327, located in the third pore region of TrkG, to serine, an amino acid with neutral charge, resulted in a transporter that required higher Na+ concentrations than the wildtype (Fig. 5B). D327 is located close to a transmembrane domain (referred to as M2C/D3M2) (22, 27), which is responsible for gating control (63, 64). From a structural point of view, D327 is interacting with the residues Y301, T325, S330, and W331 that are all located in the vicinity of the N-terminal region of M2C/D3M2 (Fig. 5B, inset). These interactions might affect Na+-dependent gating through the motion of the M2C3/D3M2b helix (21, 63, 64). Association of Na+ with D327 may regulate K+ conductance through TrkG. Na+ activation is also found in K+ channels, for example, Slo2.2, a neuronal Na+-activated K+ channel (65). It has been reported that a negatively charged residue, aspartic acid, is important for Na+-dependent activation of G protein–gated inwardly rectifying K+ channels (66, 67). Na+ may function as a regulator of some classes of K+ transport systems in general (5).
Another distinct property of TrkG was its Na+ transport activity (Fig. 4, A–C). Some members of the Trk/Kup/HKT family are known to be Na+ permeable, and these may be more directly ancestral to TrkG than to TrkH (26, 31, 32). Several earlier reports have discussed physiological advantages conferred by the Na+ transport activity of Trk/Ktr/HKT transporters. Nutritional Na+ uptake by the Trk homolog from rice, OsHKT2;1 occurs in roots under K+ starvation conditions (68). Other plant Trk/Ktr/HKT transporters (e.g., TaHKT1 and AtHKT1) also provide Na+ uptake routes (26, 31, 32, 69). Under K+-limited conditions, Na+ can function as a K+ substitute, and Na+ accumulates in a special compartment (e.g., vacuole) to promote cell expansion (70). Na+ supports optimal growth in cyanobacteria (71, 72). Here, we only observed negative effects of Na+ toxicity because of TrkG-mediated Na+ uptake (Fig. 4D). However, this was reminiscent of the way that E. coli growth can be supported through uptake of the nonessential and cytotoxic cations Rb+ and Cs+ under K+-limited conditions, thereby allowing E. coli to expand its habitat (15).
Genetic exchanges between bacterial species or via lateral gene transfer from more evolutionarily distant organisms creates functional diversity and provides plasticity in the adaptation to various environments on an evolutionary timescale (73). In E. coli K-12, identified foreign genes account for approximately 13% of the genome. The trkH gene is native to E. coli; however, trkG is located within a phage-derived sequence in the E. coli genome (33). Because such foreign genes can potentially disrupt physiological functions of the host cell when they are first introduced, the host can suppress the expression of such foreign genes with the help of a transcription factor, H-NS (74). Consequently, expression of trkG was negatively regulated by H-NS, whereas trkH was not affected (Fig. 6A). However, trkG was not completely silenced in E. coli, and its expression level was similar to that of trkH (Fig. 6, A and B). TrkG and TrkH also share the same auxiliary cytosolic protein, TrkA, which further reflects the successful integration of trkG in E. coli (14). A foreign gene that is successfully adopted into the genome and the functioning of the cell often contributes to cellular stress tolerance. H-NS has been proposed to be an osmotic stress sensor, and salt concentrations affect the DNA-binding properties of H-NS (75). Excessive salt induces dissociation of the H-NS and DNA bridge complex, thus allowing RNA polymerase to proceed with the transcription process (75). Therefore, we evaluated whether osmotic stress influenced trkG expression. Our data showed that trkG expression remained relatively constant regardless of the NaCl concentrations, different from osmC and proV, whose expression is under the control of H-NS (Fig. 6B). Our data in Figure 4D suggest that the Na+ uptake activity of TrkG does not improve Na+ salinity tolerance. Therefore, it is likely that E. coli partially represses trkG expression through H-NS to avoid the excess “unwanted” Na+ uptake mediated by TrkG. Because it was difficult to assess directly how much TrkH or TrkG individually contributed to K+ supply of the cells, we instead estimated the expression level of kdp, whose expression is controlled by intracellular K+ concentrations (37) (Fig. 7). The expression level of kdp in both single mutants was lower than that of kdp in the ΔtrkGΔtrkH double mutant. Therefore, TrkG and TrkH compensated for each other's loss of activity, which could be the reason why E. coli has maintained two homologs of Trk-type transporters over the course of evolution. Our analysis also suggested that TrkH exhibited a strong K+ uptake system, compared with TrkG and Kup, and likely acted as a major K+ uptake system when Kdp was absent (Fig. 1).
This study added to our understanding of the four kinds of K+ uptake system in E. coli; Kdp is highly specific to K+ (76), whereas Kup also allows Cs+ transport across the membrane (14, 15). Here, we showed that TrkH is the major K+ uptake system, whereas TrkG transports Na+ in addition to K+. E. coli has therefore acquired powerful uptake systems for different alkaline cations (Na+, K+, Rb+, and Cs+). The diversity of these four types of transporters enhances the resilience and adaptability of E. coli to various environmental conditions.
Experimental procedures
Construction of knockout mutants
The E. coli strains used in this study are listed in Table 1. For K+ uptake experiments, multiple knockout mutants of the genes encoding the transmembrane protein (kdpA [symbolized as d], trkG [g], trkH [h], and kup [u]) of the E. coli K-12 K+ uptake systems were constructed using strains from the Keio collection (77). The kanamycin-resistant cassette was removed by using the FLP helper plasmid (pCP20). The marker-less mutant was then used as a parental strain for the next gene deletion. In-frame gene deletions were generated by PCR-based mutagenesis (78). Briefly, an FRT-flanked chloramphenicol resistance cassette was amplified using a set of primers carrying a sequence with homology to a region of the target gene. The parental strain expressing the λ Red recombinase from pKD46 was transformed with the PCR product. Transformants were selected on chloramphenicol-containing medium (25 μg/ml). The chloramphenicol resistance cassette was then removed to create a marker-less mutant. The process was repeated until all desired multiple mutations were accomplished. To analyze the Na+ uptake ability of TrkG and TrkH, a Na+ efflux transporter mutant (ΔnhaAΔnhaBΔchaA or Δabc) was constructed, and trkG or trkH mutations were introduced into this background (Δabcg and Δabch, respectively). The mutants were constructed using either the same method described previously or using a version of P1 transduction genome modification (79). In the latter approach, the kanamycin cassette region from the appropriate Keio collection strain was transferred into recipient strains to create multiple mutations with the help of the P1 phage. All gene deletions were confirmed by PCR or whole-genome sequencing.
Plasmid construction
Plasmids and primers used in this study are listed in Table S1 and S2. To measure K+ and Na+ uptake, E. coli trkG and trkH were amplified by PCR using a BamHI site–containing forward primer and a PstI site–containing reverse primer. The resulting 1.4 Kb PCR fragment was purified by gel extraction. The purified PCR fragment was cloned into BamHI- and PstI-digested pPAB404 using an In-Fusion HD cloning kit (Takara Bio USA, Inc), yielding plasmids pPAB404-TrkG and pPAB404-TrkH. Construction of selective pore variants and chimera variants was performed by combining PCR fragments using the same In-Fusion technique. Gene expression in pPAB404 was controlled by the tac promoter, and protein expression was induced by the addition of 0.1 mM isopropyl-β-d-1-thiogalactoside (76). For the luciferase reporter promoter assay, the promoter DNA fragment was amplified by PCR with genomic DNA of BW25113 as a template using the following primer pairs: trkH_LUX_F and trkH_LUX_R for pLux-trkH; trkG_LUX_F and trkG_LUX_R for pLux-trkG; and kdpA_LUX_F and kdpA_LUX_R for pLux-kdpA. The PCR fragments were cloned into the pLUX vector as previously described (80). The resulting plasmids were verified by DNA sequencing.
Growth conditions and mediums
E. coli BW25113 and its derivative mutants were routinely grown in KLB medium (0.5% yeast extract, 0.5% KCl, and 1% hypopolypeptone). Growth test at different K+ concentrations was essentially conducted as previously described with some modifications (15, 81). For growth tests on plates, E. coli K+ transporter mutants were first pregrown in K+ minimal medium (46 mM Na2HPO4, 23 mM NaH2PO4, 8 mM (NH4)2SO4, 0.4 mM MgSO4, 6 mM FeSO4, 10 μg/ml thiamine, and 1% glucose) supplemented with 30 mM KCl. These precultures were incubated at 30 °C, 150 rpm overnight (15 h). Cells were harvested by centrifugation, washed with K+-free buffer (46 mM Na2HPO4, 23 mM NaH2PO4, and 8 mM (NH4)2SO4) and then resuspended with the same buffer. The absorbance at 600 nm of the cell suspension was measured and adjusted to 0.5. The cell suspension was serially diluted with K+-free buffer, and 5 μl aliquots were spotted onto plates (minimal medium plus 1.5% agar) containing varying concentrations of KCl. The plates were incubated at 30 °C for 48 h. When comparing the cell growth of Δdghu (Δ4) transformants, the minimal medium was supplemented with 50 μg/ml ampicillin and 0.1 mM isopropyl β-d-1-thiogalactopyranoside. For the liquid growth test, the resuspended preculture cells were inoculated into minimal medium at an initial absorbance of 0.05 at 600 nm. Cell growth was measured as absorbance at 600 nm after incubation at 30 °C for 15 h. For growth tests at different K+ and Na+ concentrations, a phosphoric acid–based medium was created, a modification of the defined medium of Collins and Thune (82). This phosphoric acid–based medium contained 8 mM H3PO4, 8 mM (NH4)2SO4, 0.4 mM MgSO4, 6 μM FeSO4, 29 μM MnSO4.5H2O, 12 μM CaCl2.2H2O, 17 μM ZnSO4.7H2O, 0.4 μM CuSO4.5H2O, 0.4 μM CoCl2.6H2O, 0.73 μM p-aminobenzoic acid, 4.9 μM pyridoxine HCl, 1.5 μM thiamine, 1% (w/v) glucose, and 0.2 to 3.0 mM amino acid mixture (2.2 mM l-alanine, 1.4 mM l-arginine, 3 mM l-asparagine, 0.75 mM l-aspartic acid, 0.41 mM l-cysteine, 2 mM l-glutamic acid, 1.3 mM glycine, 0.39 mM l-histidine, 1.9 mM l-isoleucine, 1.9 mM l-leucine, 1.4 mM l-lysine, 0.67 mM dl-methionine, 0.61 mM l-phenylalanine, 0.87 l-proline, 0.48 mM l-serine, 1.7 mM l-threonine, 0.2 mM l-tryptophan, 0.55 mM l-tyrosine, and 2.1 mM l-valine), the pH was adjusted to 7.4 with Tris.
Measurement of K+ uptake
Cation uptake was determined as described previously with some modifications (30, 39). Briefly, E. coli mutants were cultured in a minimal medium containing 20 mM KCl at 30 °C, 150 rpm until absorbance at 578 nm reached ±0.6 to 0.9. The culture was harvested by centrifugation and treated with 1 mM EDTA in 120 mM Tris–HCl (pH 8.0) for 30 min at 37 °C. The cells were washed two times using 200 mM Hepes–NaOH or Hepes–triethanolamine (pH 7.5), then resuspended with the same buffer. Note that 200 mM Hepes–NaOH buffer contains 22.6 μM K+ and 149.7 mM Na+ and 200 mM Hepes–triethanolamine contains 11 μM K+ and 7.7 μM Na+ as measured by atomic absorption spectrometer. After 20 min of incubation at room temperature, the cell suspension was transferred into a new flask at an absorbance of 3 at 578 nm. About 10 mM of glucose was added into the suspensions prior to K+ addition, and K+ was added at the indicated final concentration. At designated time points, 1 ml samples were taken and centrifuged through silicone oil (Sigma–Aldrich) to separate the buffer and cells. The buffer was removed, and the cells were disrupted with 5% trichloroacetic acid and heated at 100 °C for 5 min. The K+ content extracted from the cells was determined using an atomic absorption spectrometer (iCE 3500 Thermo Fisher Scientific AA Spectrometer), and the total protein content in the cell pellets was measured using a BCA protein assay (Thermo Scientific). The intracellular concentration of K+ was calculated as nanomoles of K+/mg cellular protein. K+ uptake was calculated by subtracting the intracellular K+ concentration before K+ addition from the concentration at the indicated time after K+ addition.
Measurement of Na+ uptake
Na+ uptake was determined using the Na+ transporter knockout mutant strain (TO114 or Δabc transformants) and its derivatives. The procedure was similar to the one used for the K+ uptake experiments except the growth medium used was KLB supplemented with 30 μg/ml ampicillin and 0.1 mM isopropyl-β-d-1-thiogalactoside to induce protein expression, and the buffer used was 200 mM Hepes–triethanolamine (pH 7.5).
qRT–PCR
RNA was extracted from early exponential cultures of E. coli W3110 wildtype and Δhns (unless otherwise indicated) (absorbance at 600 nm = 0.4–0.6) using TRI reagent (Molecular Research Centre, lnc), followed by genomic DNA removal and complementary DNA synthesis using Revertra ace qPCR RT Master Mix with gDNA Remover (TOYOBO CO, LTD). The KAPA SYBR FAST qPCR Master Mix (2×) Kit (KAPA Biosystems) was used for qRT–PCR. The StepOnePlus Real-Time PCR system (Applied Biosystems) was used as the detection system. The composition of the reaction was 0.5 ng complementary DNA, 1× KAPA SYBR FAST qPCR master mix, 200 nM forward primer, and 200 nM reverse primer (Table S2). The reaction conditions were 95 °C for 3 min (95 °C for 3 s and 60 °C for 20 s) repeated 50 times. The temperature was then increased by 1 °C from 60 °C to 95 °C, and the melting temperature (Tm value) of the amplified fragment DNA was determined. hcaT, cysG, and idnT were used as reference genes (49).
Luciferase assay
E. coli BW25113 wildtype, ΔtrkG, ΔtrkH, and ΔtrkGΔtrkH were transformed with pLUX containing the bacterial luciferase gene under control of the kdp promoter. Transformants were grown in phosphoric acid–based medium containing 10 mM KCl and 50 μg/ml kanamycin. These precultures were incubated at 30 °C, 150 rpm overnight (15 h). Cells were harvested by centrifugation, washed with K+-free phosphoric acid–based buffer (8 mM H3PO4, 8 mM (NH4)2SO4, pH adjusted to 7.4 by Tris) and then resuspended with the same buffer. The resuspended preculture cells were inoculated into new medium at an initial absorbance of 0.05 at 600 nm. Cell growth (absorbance at 600 nm) and luciferase emission intensity were measured after 3 h and 6 h incubation at 30 °C. The measurements were performed using a multiplate reader (Synergy H1 Microplate Reader; BioTek). Promoter activity was calculated as luciferase emission intensityabsorbance at 600 nm.
Data availability
All data are contained within the article and the supporting information.
Supporting information
This article contains supporting information (22, 76, 78, 83, 84, 85).
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We thank Dr Taku Oshima of Toyama Prefectural University for providing W3110, Δhns, ΔhnsΔstpA, ΔhnsΔydgT, and ΔhnsΔhha strains. We thank Anke Reinders for critical reading of the manuscript.
Author contributions
K. Y. and N. U. conceptualization; E. T. and N. U. methodology; E. T. formal analysis; E. T., N. H., K. I., and T. F. investigation; K. Y. resources; E. T. and N. U. writing–original draft; E. T. and N. U. writing–review & editing; E. T. visualization; N. U. project administration; M. T., Y. I., and N. U. funding acquisition.
Funding and additional information
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (grant nos.: 20KK0127, 21H04543 and 21K19060; to N. U.; 21H05266, 21KK0268, and 22K19121 to Y. I.; and 21K14780 to M. T., respectively), Moonshot R&D (grant no.: JPMJMS2033) from Japan Science and Technology Agency to N. U.
Edited by Mike Shipston
Supporting information
References
- 1.Epstein W. The roles and regulation of potassium in bacteria. Prog. Nucleic Acid Res. Mol. Biol. 2003;75:293–320. doi: 10.1016/s0079-6603(03)75008-9. [DOI] [PubMed] [Google Scholar]
- 2.Rodríguez-Navarro A., Rubio F. High-affinity potassium and sodium transport systems in plants. J. Exp. Bot. 2006;57:1149–1160. doi: 10.1093/jxb/erj068. [DOI] [PubMed] [Google Scholar]
- 3.Danchin A., Nikel P.I. Why nature chose potassium. J. Mol. Evol. 2019;87:271–288. doi: 10.1007/s00239-019-09915-2. [DOI] [PubMed] [Google Scholar]
- 4.Hänelt I., Tholema N., Kröning N., Vor der Brüggen M., Wunnicke D., Bakker E.P. KtrB, a member of the superfamily of K+ transporters. Eur. J. Cell Biol. 2011;90:696–704. doi: 10.1016/j.ejcb.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Uozumi N., Dreyer I. Structure-function correlates in plant ion channels. Compr. Biophys. 2012;6:234–245. [Google Scholar]
- 6.Roe A.J., McLaggan D., O'Byrne C.P., Booth I.R. Rapid inactivation of the Escherichia coli Kdp K+ uptake system by high potassium concentrations. Mol. Microbiol. 2000;35:1235–1243. doi: 10.1046/j.1365-2958.2000.01793.x. [DOI] [PubMed] [Google Scholar]
- 7.Huang C.-S., Pedersen B.P., Stokes D.L. Crystal structure of the potassium-importing KdpFABC membrane complex. Nature. 2017;546:681–685. doi: 10.1038/nature22970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pedersen B.P., Stokes D.L., Apell H.J. The KdpFABC complex–K+ transport against all odds. Mol. Membr. Biol. 2019;35:21–38. doi: 10.1080/09687688.2019.1638977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heermann R., Jung K. The complexity of the “simple” two-component system KdpD/KdpE in Escherichia coli. FEMS Microbiol. Lett. 2010;304:97–106. doi: 10.1111/j.1574-6968.2010.01906.x. [DOI] [PubMed] [Google Scholar]
- 10.Ballal A., Basu B., Apte S.K. The Kdp-ATPase system and its regulation. J. Biosci. 2007;32:559–568. doi: 10.1007/s12038-007-0055-7. [DOI] [PubMed] [Google Scholar]
- 11.Zakharyan E., Trchounian A. K+ influx by Kup in Escherichia coli is accompanied by a decrease in H+ efflux. FEMS Microbiol. Lett. 2001;204:61–64. doi: 10.1111/j.1574-6968.2001.tb10863.x. [DOI] [PubMed] [Google Scholar]
- 12.Tascón I., Sousa J.S., Corey R.A., Mills D.J., Griwatz D., Aumüller N., et al. Structural basis of proton-coupled potassium transport in the KUP family. Nat. Commun. 2020;11:626. doi: 10.1038/s41467-020-14441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trchounian A., Kobayashi H. Kup is the major K+ uptake system in Escherichia coli upon hyper-osmotic stress at a low pH. FEBS Lett. 1999;447:144–148. doi: 10.1016/s0014-5793(99)00288-4. [DOI] [PubMed] [Google Scholar]
- 14.Bossemeyer D., Schlosser A., Bakker E.P. Specific cesium transport via the Escherichia coli Kup (TrkD) K+ uptake system. J. Bacteriol. 1989;171:2219–2221. doi: 10.1128/jb.171.4.2219-2221.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanudjaja E., Hoshi N., Su Y.-H., Hamamoto S., Uozumi N. Kup-mediated Cs+ uptake and Kdp-driven K+ uptake coordinate to promote cell growth during excess Cs+ conditions in Escherichia coli. Sci. Rep. 2017;7:2122. doi: 10.1038/s41598-017-02164-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi D., Uozumi N., Hisamatsu S., Yamagami M. AtKUP/HAK/KT9, a K+ transporter from Arabidopsis thaliana, mediates Cs+ uptake in Escherichia coli. Biosci. Biotechnol. Biochem. 2010;74:203–205. doi: 10.1271/bbb.90638. [DOI] [PubMed] [Google Scholar]
- 17.Schlosser A., Meldorf M., Stumpe S., Bakker E.P., Epstein W. TrkH and its homolog, TrkG, determine the specificity and kinetics of cation transport by the Trk system of Escherichia coli. J. Bacteriol. 1995;177:1908–1910. doi: 10.1128/jb.177.7.1908-1910.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato Y., Sakaguchi M., Mori Y., Saito K., Nakamura T., Bakker E.P., et al. Evidence in support of a four transmembrane-pore-transmembrane topology model for the Arabidopsis thaliana Na+/K+ translocating AtHKT1 protein, a member of the superfamily of K+ transporters. Proc. Natl. Acad. Sci. U. S. A. 2001;98:6488–6493. doi: 10.1073/pnas.101556598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harms C., Domoto Y., Celik C., Rahe E., Stumpe S., Schmid R., et al. Identification of the ABC protein SapD as the subunit that confers ATP dependence to the K+-uptake systems TrkH and TrkG from Escherichia coli K-12. Microbiology. 2001;147:2991–3003. doi: 10.1099/00221287-147-11-2991. [DOI] [PubMed] [Google Scholar]
- 20.Bossemeyer D., Borchard A., Dosch D.C., Helmer G.C., Epstein W., Booth I.R., et al. K+-transport protein TrkA of Escherichia coli is a peripheral membrane protein that requires other trk gene products for attachment to the cytoplasmic membrane. J. Biol. Chem. 1989;264:16403–16410. [PubMed] [Google Scholar]
- 21.Cao Y., Pan Y., Huang H., Jin X., Levin E.J., Kloss B., et al. Gating of the TrkH ion channel by its associated RCK protein TrkA. Nature. 2013;496:317–322. doi: 10.1038/nature12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao Y., Jin X., Huang H., Derebe M.G., Levin E.J., Kabaleeswaran V., et al. Crystal structure of a potassium ion transporter, TrkH. Nature. 2011;471:336–340. doi: 10.1038/nature09731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H., Pan Y., Hu L., Hudson M.A., Hofstetter K.S., Xu Z., et al. TrkA undergoes a tetramer-to-dimer conversion to open TrkH which enables changes in membrane potential. Nat. Commun. 2020;11:547. doi: 10.1038/s41467-019-14240-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vieira-Pires R.S., Szollosi A., Morais-Cabral J.H. The structure of the KtrAB potassium transporter. Nature. 2013;496:323–328. doi: 10.1038/nature12055. [DOI] [PubMed] [Google Scholar]
- 25.Gambale F., Uozumi N. Properties of shaker-type potassium channels in higher plants. J. Membr. Biol. 2006;210:1–19. doi: 10.1007/s00232-006-0856-x. [DOI] [PubMed] [Google Scholar]
- 26.Corratgé-Faillie C., Jabnoune M., Zimmermann S., Véry A.A., Fizames C., Sentenac H. Potassium and sodium transport in non-animal cells: the Trk/Ktr/HKT transporter family. Cell. Mol. Life Sci. 2010;67:2511–2532. doi: 10.1007/s00018-010-0317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durell S.R., Guy H.R. Structural models of the KtrB, TrkH, and Trk1,2 symporters based on the structure of the KcsA K+ channel. Biophys. J. 1999;77:789–807. doi: 10.1016/S0006-3495(99)76932-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato N., Akai M., Zulkifli L., Matsuda N., Kato Y., Goshima S., et al. Role of positively charged amino acids in the M2D transmembrane helix of Ktr/Trk/HKT type cation transporters. Channels. 2007;1:161–171. doi: 10.4161/chan.4374. [DOI] [PubMed] [Google Scholar]
- 29.Tholema N., Vor der Brüggen M., Mäser P., Nakamura T., Schroeder J.I., Kobayashi H., et al. All four putative selectivity filter glycine residues in KtrB are essential for high affinity and selective K+ uptake by the KtrAB system from Vibrio alginolyticus. J. Biol. Chem. 2005;280:41146–41154. doi: 10.1074/jbc.M507647200. [DOI] [PubMed] [Google Scholar]
- 30.Matsuda N., Kobayashi H., Katoh H., Ogawa T., Futatsugi L., Nakamura T., et al. Na+-dependent K+ uptake Ktr system from the cyanobacterium Synechocystis sp. PCC 6803 and its role in the early phases of cell adaptation to hyperosmotic shock. J. Biol. Chem. 2004;279:54952–54962. doi: 10.1074/jbc.M407268200. [DOI] [PubMed] [Google Scholar]
- 31.Rubio F., Gassmann W., Schroeder J.I. Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science. 1995;270:1660–1663. doi: 10.1126/science.270.5242.1660. [DOI] [PubMed] [Google Scholar]
- 32.Uozumi N., Kim E.J., Rubio F., Yamaguchi T., Muto S., Tsuboi A., et al. The Arabidopsis HKT1 gene homolog mediates inward Na+ currents in Xenopus laevis oocytes and Na+ uptake in Saccharomyces cerevisiae. Plant Physiol. 2000;122:1249–1259. doi: 10.1104/pp.122.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlosser A., Kluttig S., Hamann A., Bakker E.P. Subcloning, nucleotide sequence, and expression of trkG, a gene that encodes an integral membrane protein involved in potassium uptake via the Trk system of Escherichia coli. J. Bacteriol. 1991;173:3170–3176. doi: 10.1128/jb.173.10.3170-3176.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Navarre W.W., Porwollik S., Wang Y., McClelland M., Rosen H., Libby S.J., et al. Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science. 2006;313:233–236. doi: 10.1126/science.1128794. [DOI] [PubMed] [Google Scholar]
- 35.Navarre W.W., McClelland M., Libby S.J., Fang F.C. Silencing of xenogeneic DNA by H-NS - facilitation of lateral gene transfer in bacteria by a defense system that recognizes foreign DNA. Genes Dev. 2007;21:1456–1471. doi: 10.1101/gad.1543107. [DOI] [PubMed] [Google Scholar]
- 36.Oshima T., Ishikawa S., Kurokawa K., Aiba H., Ogasawara N. Escherichia coli histone-like protein H-NS preferentially binds to horizontally acquired DNA in association with RNA polymerase. DNA Res. 2006;13:141–153. doi: 10.1093/dnares/dsl009. [DOI] [PubMed] [Google Scholar]
- 37.Altendorf K., Siebers A., Epstein W. The KDP ATPase of Escherichia coli. Ann. N. Y. Acad. Sci. 1992;671:228–243. doi: 10.1111/j.1749-6632.1992.tb43799.x. [DOI] [PubMed] [Google Scholar]
- 38.Stumpe S., Bakker E.P. Requirement of a large K+-uptake capacity and of extracytoplasmic protease activity for protamine resistance of Escherichia coli. Arch. Microbiol. 1997;167:126–136. [PubMed] [Google Scholar]
- 39.Tholema N., Bakker E.P., Suzuki A., Nakamura T. Change to alanine of one out of four selectivity filter glycines in KtrB causes a two orders of magnitude decrease in the affinities for both K+ and Na+ of the Na+ dependent K+ uptake system KtrAB from Vibrio alginolyticus. FEBS Lett. 1999;450:217–220. doi: 10.1016/s0014-5793(99)00504-9. [DOI] [PubMed] [Google Scholar]
- 40.Gassmann W., Rubio F., Schroeder J.I. Alkali cation selectivity of the wheat root high-affinity potassium transporter HKT1. Plant J. 1996;10:869–882. doi: 10.1046/j.1365-313x.1996.10050869.x. [DOI] [PubMed] [Google Scholar]
- 41.Ohyama T., Igarashi K., Kobayashi H. Physiological role of the chaA gene in sodium and calcium circulations at a high pH in Escherichia coli. J. Bacteriol. 1994;176:4311–4315. doi: 10.1128/jb.176.14.4311-4315.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taglicht D., Padan E., Schuldiner S. Overproduction and purification of a functional Na+/H+ antiporter coded by nhaA (ant) from Escherichia coli. J. Biol. Chem. 1991;266:11289–11294. [PubMed] [Google Scholar]
- 43.Tsunekawa K., Shijuku T., Hayashimoto M., Kojima Y., Onai K., Morishita M., et al. Identification and characterization of the Na+/H+ antiporter NhaS3 from the thylakoid membrane of Synechocystis sp. PCC 6803. J. Biol. Chem. 2009;284:16513–16521. doi: 10.1074/jbc.M109.001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zulkifli L., Akai M., Yoshikawa A., Shimojima M., Ohta H., Guy H.R., et al. The KtrA and KtrE subunits are required for Na+-dependent K+ uptake by KtrB across the plasma membrane in Synechocystis sp. strain PCC 6803. J. Bacteriol. 2010;192:5063–5070. doi: 10.1128/JB.00569-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mosimann M., Goshima S., Wenzler T., Lüscher A., Uozumi N., Mäser P. A Trk/HKT-type K+ transporter from Trypanosoma brucei. Eukaryot. Cell. 2010;9:539–546. doi: 10.1128/EC.00314-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Durell S.R., Hao Y., Nakamura T., Bakker E.P., Guy H.R. Evolutionary relationship between K+ channels and symporters. Biophys. J. 1999;77:775–788. doi: 10.1016/S0006-3495(99)76931-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maser P., Hosoo Y., Goshima S., Horie T., Eckelman B., Yamada K., et al. Glycine residues in potassium channel-like selectivity filters determine potassium selectivity in four-loop-per-subunit HKT transporters from plants. Proc. Natl. Acad. Sci. U. S. A. 2002;99:6428–6433. doi: 10.1073/pnas.082123799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Varadi M., Anyango S., Deshpande M., Nair S., Natassia C., Yordanova G., et al. AlphaFold protein structure database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022;50:D439–D444. doi: 10.1093/nar/gkab1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou K., Zhou L., Lim Q., Zou R., Stephanopoulos G., Too H.P. Novel reference genes for quantifying transcriptional responses of Escherichia coli to protein overexpression by quantitative PCR. BMC Mol. Biol. 2011;12:18. doi: 10.1186/1471-2199-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krin E., Danchin A., Soutourina O. Decrypting the H-NS-dependent regulatory cascade of acid stress resistance in Escherichia coli. BMC Microbiol. 2010;10:273. doi: 10.1186/1471-2180-10-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamanaka Y., Winardhi R.S., Yamauchi E., Nishiyam S.I., Sowa Y., Yan J., et al. Dimerization site 2 of the bacterial DNA-binding protein H-NS is required for gene silencing and stiffened nucleoprotein filament formation. J. Biol. Chem. 2018;293:9496–9505. doi: 10.1074/jbc.RA117.001425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang A., Belfort M. Nucleotide sequence of a newly-identified Escherichia coli gene, stpA, encoding an H-NS-like protein. Nucleic Acids Res. 1992;20:6735. doi: 10.1093/nar/20.24.6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang A., Rimsky S., Reaban M.E., Buc H., Belfort M. Escherichia coli protein analogs StpA and H-NS: regulatory loops, similar and disparate effects on nucleic acid dynamics. EMBO J. 1996;15:1340–1349. [PMC free article] [PubMed] [Google Scholar]
- 54.Madrid C., Balsalobre C., García J., Juárez A. The novel Hha/YmoA family of nucleoid-associated proteins: use of structural mimicry to modulate the activity of the H-NS family of proteins. Mol. Microbiol. 2007;63:7–14. doi: 10.1111/j.1365-2958.2006.05497.x. [DOI] [PubMed] [Google Scholar]
- 55.Ueda T., Takahashi H., Uyar E., Ishikawa S., Ogasawara N., Oshima T. Functions of the Hha and YdgT proteins in transcriptional silencing by the nucleoid proteins, H-NS and StpA, in Escherichia coli. DNA Res. 2013;20:263–271. doi: 10.1093/dnares/dst008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lucht J.M., Dersch P., Kempf B., Bremer E. Interactions of the nucleoid-associated DNA-binding protein H-NS with the regulatory region of the osmotically controlled proU operon of Escherichia coli. J. Biol. Chem. 1994;269:6578–6586. [PubMed] [Google Scholar]
- 57.Suzuki T., Ueguchi C., Mizuno T. H-NS regulates OmpF expression through micF antisense RNA in Escherichia coli. J. Bacteriol. 1996;178:3650–3653. doi: 10.1128/jb.178.12.3650-3653.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holtmann G., Bakker E.P., Uozumi N., Bremer E. KtrAB and KtrCD: two K+ uptake systems in Bacillus subtilis and their role in adaptation to hypertonicity. J. Bacteriol. 2003;185:1289–1298. doi: 10.1128/JB.185.4.1289-1298.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gutierrez C., Devedjian J.C. Osmotic induction of gene osmC expression in Escherichia coli K12. J. Mol. Biol. 1991;220:959–973. doi: 10.1016/0022-2836(91)90366-e. [DOI] [PubMed] [Google Scholar]
- 60.Laimins L.A., Rhoads D.B., Epstein W. Osmotic control of kdp operon expression in Escherichia coli (potassium transport/lac fusion/turgor pressure) Proc. Natl. Acad. Sci. U. S. A. 1981;78:464–468. doi: 10.1073/pnas.78.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tian C., Zhu R., Zhu L., Qiu T., Cao Z., Kang T. Potassium channels: structures, diseases, and modulators. Chem. Biol. Drug Des. 2014;83:1–26. doi: 10.1111/cbdd.12237. [DOI] [PubMed] [Google Scholar]
- 62.Mäser P., Thomine S., Schroeder J.I., Ward J.M., Hirschi K., Sze H., et al. Phylogenetic relationships within cation transporter families of arabidopsis 1. Plant Physiol. 2001;126:1646–1667. doi: 10.1104/pp.126.4.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hänelt I., Löchte S., Sundermann L., Elbers K., Vor Der Brüggen M., Bakker E.P. Gain of function mutations in membrane region M2C2 of KtrB open a gate controlling K+ transport by the KtrAB system from Vibrio alginolyticus. J. Biol. Chem. 2010;285:10318–10327. doi: 10.1074/jbc.M109.089870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hänelt I., Wunnicke D., Müller-Trimbusch M., Vor Der Brüggen M., Kraus I., Bakker E.P., et al. Membrane region M2C2 in subunit KtrB of the K+ uptake system KtrAB from Vibrio alginolyticus forms a flexible gate controlling K+ flux: an electron paramagnetic resonance study. J. Biol. Chem. 2010;285:28210–28219. doi: 10.1074/jbc.M110.139311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hite R.K., MacKinnon R. Structural titration of Slo2.2, a Na+-dependent K+ channel. Cell. 2017;168:390–399.e11. doi: 10.1016/j.cell.2016.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ho I.H.M., Murrell-Lagnado R.D. Molecular determinants for sodium-dependent activation of G protein gated K+ channels. J. Biol. Chem. 1999;274:8639–8648. doi: 10.1074/jbc.274.13.8639. [DOI] [PubMed] [Google Scholar]
- 67.Zhang H., He C., Yan X., Mirshahi T., Logothetis D.E. Activation of inwardly rectifying K+ channels by distinct PtdIns(4,5)P2 interactions. Nat. Cell Biol. 1999;1:183–188. doi: 10.1038/11103. [DOI] [PubMed] [Google Scholar]
- 68.Horie T., Costa A., Kim T.H., Han M.J., Horie R., Leung H.-Y., et al. Rice OsHKT2;1 transporter mediates large Na+ influx component into K+-starved roots for growth. EMBO J. 2007;26:3003–3014. doi: 10.1038/sj.emboj.7601732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schachtman D., Schroeder J. Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature. 1994;370:655–658. doi: 10.1038/370655a0. [DOI] [PubMed] [Google Scholar]
- 70.Subbarao G.V., Ito O., Berry W.L., Wheeler R.M. Sodium—a functional plant nutrient. Crit. Rev. Plant Sci. 2003;22:391–416. [Google Scholar]
- 71.Miller A.G., Turpin D.H., Canvin D.T. Na+ requirement for growth, photosynthesis, and pH regulation in the alkalotolerant cyanobacterium Synechococcus leopoliensis. J. Bacteriol. 1984;159:100–106. doi: 10.1128/jb.159.1.100-106.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Billini M., Stamatakis K., Sophianopoulou V. Two members of a network of putative Na+/H+ antiporters are involved in salt and pH tolerance of the freshwater cyanobacterium Synechococcus elongatus. J. Bacteriol. 2008;190:6318–6329. doi: 10.1128/JB.00696-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ochman H., Lawrence J.G., Groisman E.A. Lateral gene transfer and the nature of bacterial innovation. Nature. 2000;405:299–304. doi: 10.1038/35012500. [DOI] [PubMed] [Google Scholar]
- 74.Higashi K., Tobe T., Kanai A., Uyar E., Ishikawa S., Suzuki Y., et al. H-NS facilitates sequence diversification of horizontally transferred DNAs during their integration in host chromosomes. PLoS Genet. 2016;12 doi: 10.1371/journal.pgen.1005796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qin L., Bdira F.B., Sterckx Y.G.J., Volkov A.N., Vreede J., Giachin G., et al. Structural basis for osmotic regulation of the DNA binding properties of H-NS proteins. Nucleic Acids Res. 2020;48:2156–2172. doi: 10.1093/nar/gkz1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buurman E.T., Kim K.-T., Epstein W. Genetic evidence for two sequentially occupied K+ binding sites in the Kdp transport ATPase. J. Biol. Chem. 1995;270:6678–6685. doi: 10.1074/jbc.270.12.6678. [DOI] [PubMed] [Google Scholar]
- 77.Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., Baba M., et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2006;2 doi: 10.1038/msb4100050. 2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Datsenko K.A., Wanner B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thomason L.C., Costantino N., Court D.L. E. coli genome manipulation by P1 transduction. Curr. Protoc. Mol. Biol. 2007;Chapter 1:1.17.1–1.17.8. doi: 10.1002/0471142727.mb0117s79. [DOI] [PubMed] [Google Scholar]
- 80.Yamanaka Y., Watanabe H., Yamauchi E., Miyake Y., Yamamoto K. Measurement of the promoter activity in Escherichia coli by using a luciferase reporter. Bio Protoc. 2020;10 doi: 10.21769/BioProtoc.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sato Y., Nanatani K., Hamamoto S., Shimizu M., Takahashi M., Tabuchi-Kobayashi M., et al. Defining membrane spanning domains and crucial membrane-localized acidic amino acid residues for K+ transport of a Kup/HAK/KT-type Escherichia coli potassium transporter. J. Biochem. 2014;155:315–323. doi: 10.1093/jb/mvu007. [DOI] [PubMed] [Google Scholar]
- 82.Collins L.A., Thune R.L. Development of a defined minimal medium for the growth of Edwardsiella ictaluri. Appl. Environ. Microbiol. 1996;62:848–852. doi: 10.1128/aem.62.3.848-852.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Madeira F., Pearce M., Tivey A.R.N., Basutkar P., Lee J., Edbali O., et al. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res. 2022;50:W276–W279. doi: 10.1093/nar/gkac240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Burton N.A., Johnson M.D., Antczak P., Robinson A., Lund P.A. Novel aspects of the acid response network of E. coli K-12 are revealed by a study of transcriptional dynamics. J. Mol. Biol. 2010;401:726–742. doi: 10.1016/j.jmb.2010.06.054. [DOI] [PubMed] [Google Scholar]
- 85.Cherepanov P.P., Wackernagel W. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene. 1995;158:9–14. doi: 10.1016/0378-1119(95)00193-a. [DOI] [PubMed] [Google Scholar]
- 86.Jensen K.F. The Escherichia coli K-12 “wild types” W3110 and MG1655 have an rph frameshift mutation that leads to pyrimidine starvation due to low pyrE expression levels. J. Bacteriol. 1993;175:3401–3407. doi: 10.1128/jb.175.11.3401-3407.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained within the article and the supporting information.