Abstract
The urinary system, comprising the kidneys, ureters, bladder, and urethra, has a unique mechanical and fluid microenvironment, which is essential to the urinary system growth and development. Microfluidic models, based on micromachining and tissue engineering technology, can integrate pathophysiological characteristics, maintain cell-cell and cell-extracellular matrix interactions, and accurately simulate the vital characteristics of human tissue microenvironments. Additionally, these models facilitate improved visualization and integration and meet the requirements of the laminar flow environment of the urinary system. However, several challenges continue to impede the development of a tissue microenvironment with controllable conditions closely resemble physiological conditions. In this review, we describe the biochemical and physical microenvironment of the urinary system and explore the feasibility of microfluidic technology in simulating the urinary microenvironment and pathophysiological characteristics in vitro. Moreover, we summarize the current research progress on adapting microfluidic chips for constructing the urinary microenvironment. Finally, we discuss the current challenges and suggest directions for future development and application of microfluidic technology in constructing the urinary microenvironment in vitro.
Keywords: Urinary system, Microfluidic chip, Urinary microenvironment, Tissue engineering
Graphical abstract
1. Introduction
The urinary system is involved in waste excretion and regulation of blood volume in the body [1]. It is a soft tissue system comprising the proximal tubule, ureters, and urethra, and is covered by the urothelium [2], which serves as a barrier to the urinary system (Fig. 1). Urothelial cells, interstitial cells, cytokines, the extracellular matrix (ECM), and various physical and biochemical factors constitute the microenvironment of the urinary system. This microenvironment contributes to the peristaltic movement of the ureter, expansion and contraction of the bladder, and complex urinary flow [3]. Maintenance of the dynamic equilibrium within this unique microenvironment is critical in ensuring normal physiological activities such as growth, proliferation, and differentiation, as well as in maintaining the structure and function of the cells [4,5]. Hence, it is imperative that in vitro models designed to study this complex system can accurately simulate the cellular microenvironment [6].
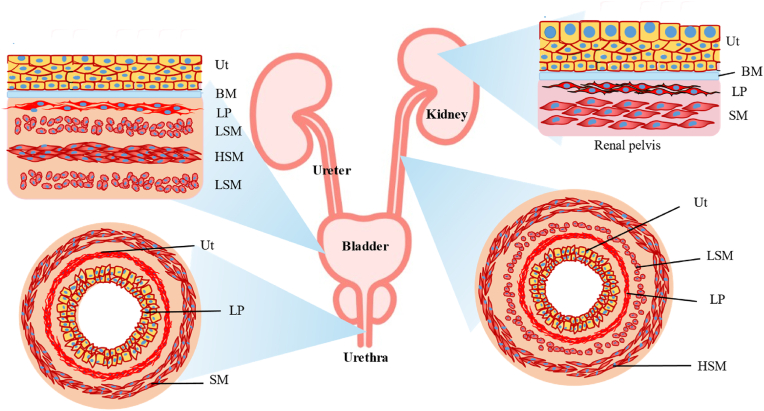
Fig. 1.
Urinary tract system. Cartoon depicting the organization of the urothelium and underlying tissues in the renal pelvis, ureter, bladder, and proximal urethra. Ut, urothelium, LP, lamina propria; SM, Smooth muscle layers; HSM, Horizontal smooth muscle layers; LSM, Longitudinal smooth muscle layers.
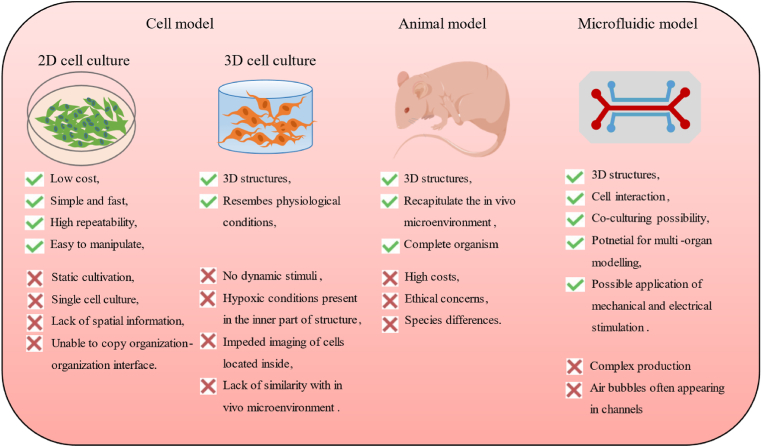
Traditional studies on urinary diseases are based on two-dimensional (2D) cell cultures and animal models [7]. However, these models can only assess single factors and are often incapable of simulating the physiological or pathological microenvironment [8]. In particular, in vitro cell culture models often lack the ability to characterize cell-cell and cell-ECM interactions and, thus, do not accurately reflect the physiological conditions [[9], [10], [11]]. Although animal models can overcome these shortcomings, they can be expensive, tedious, and time-consuming [12,13]. Moreover, they are unsuitable for monitoring and visualizing biological changes occurring within live organs in real-time. Additionally, the ethical concerns surrounding the use of animal models that reflect human physiology continue to limit their application [14,15]. Hence, there exists an urgent need exists for the development of new novel research models that integrate the physical-biochemical microenvironment and mimic the in vivo environment of cells, thus facilitating the comprehensive and in-depth analysis of various diseases of the urinary system (Fig. 2).
Fig. 2.
Advantages and disadvantages of current in vitro and in vivo models for the urology system.
A microfluidic chip, also known as an organ chip, a lab-on-chip, or a microphysiological system, is a micron channel structure constructed using micromachining technology that can integrate a variety of experimental operations into one chip [16]. This structure facilitates favorable characteristics, such as flexible design, easy integration, real-time monitoring, high throughput, and low cost [17,18]. Moreover, microfluidic models can realize multicellular co-culture and regulate the cellular microenvironment in time and space to reconstruct the biomimetic environment [[19], [20], [21]]. Therefore, they have become valuable tools for simulating the physiological microenvironment of various tissues [22,23]. Compared with macroscopic models, microfluidic devices can integrate controllable physical or chemical stimuli and maintain cell-cell crosstalk and cell-ECM interactions, thus maximizing the restoration of physiological microenvironments [24]. Over the past decade, microfluidic platforms have been widely used to simulate the physiological microenvironment of different organs, including the lungs [[25], [26], [27]], kidneys [[28], [29], [30], [31], [32]], heart [[33], [34], [35]], and bones [36,37].
However, compared with these organs, the urinary system involves multiple organs and is highly complex. Existing studies mainly focus on aspects such as nephrotoxicity, detection of tumors, and applications of three-dimensional (3D) printing in the urinary system. However, the role of the microenvironment has not been factored into the construction of microfluidic chips [38,39]. Consequently, the study of other urinary physiological models remains in its infancy, and the application of microfluidic chips in the urinary system microenvironment has not been reviewed.
As the microenvironment is the basis of the urinary system microfluidic model design, we collated the microenvironment characteristics of various organs in the urinary system, summarized their common and unique characteristics, and discussed the latest developments and challenges in this field, to elucidate the physiological microenvironment and diseases of the urinary system using microfluidic models. First, we described the microenvironment of the urinary system and the microfluidic models developed to understand the progression of urinary diseases. We then discussed the feasibility of microfluidic technology in simulating the urinary microenvironment in vitro based on the key characteristics of the physiological parameters. We summarized the current research results regarding microfluidic models for constructing the urinary microenvironment. Finally, we discuss the limitations of the current research and consider the development directions and application prospects of microfluidic technology in constructing models reflecting the urinary microenvironment in vitro.
2. Microfluidic technology
A microfluidic chip consists of cells, external control devices, and microfluidic devices. Common microfluidic chip fabrication methods mainly include soft lithography, laser, and chemical etching, micromilling, molding, and 3D printing [40]. The choice of the microfluidic fabrication process determines the materials which will be used for the chip.
Polydimethylsiloxane (PDMS) is the most commonly used material and is fabricated via soft lithography. It has good biocompatibility, high gas permeability, transparency, and low cost [41]. However, it has a high degree of hydrophobicity, which is not conducive to the adhesion of cells, and requires treatment with a coating material (such as fibronectin or laminin) [42]. In addition, its small molecule adsorption properties also limit its application in drug screening [43]. Thermoplastic chips represented by polymethylmethacrylate can overcome the aforementioned drawbacks. They are simple and inexpensive to prepare, can be mass-produced, and are already commercialized [44]. However, they have certain disadvantages such as easy deformation by heating and airtightness. Other common materials include glass, polyurethane, and polystyrene [45,46]. Additionally, the development of 3D printing technology provides more options for microfluidic materials, such as hydrogels (natural or synthetic), including collagen, alginate, and gelatin.
3. Microenvironments in the microfluidic models of the urinary system
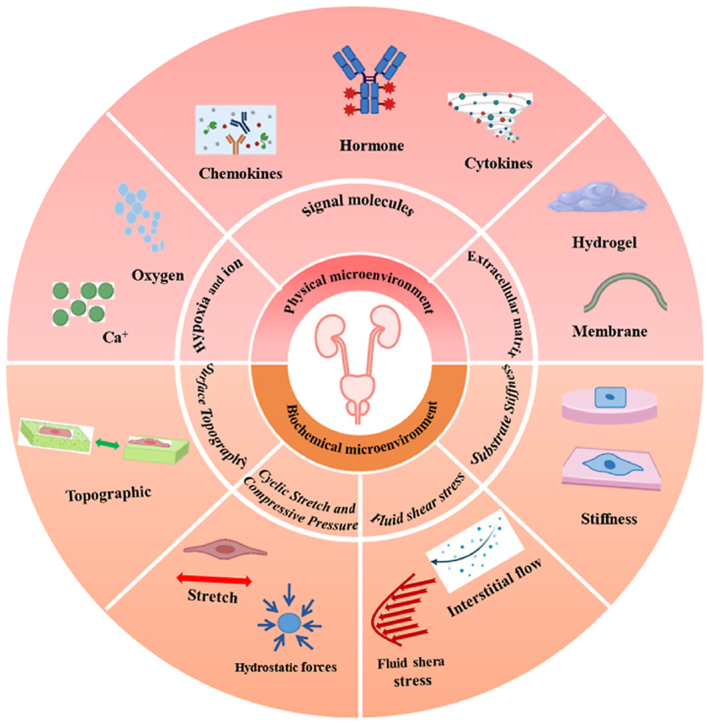
3.1. Biochemical microenvironment
The biochemical microenvironment of the urinary system is primarily composed of cytokines, hormones, ions, and other biochemical factors that regulate the interaction between cells and the ECM. The urinary system contains over ten renal cell types, surrounded by the ECM and complex vascular system [47]. Cells interact with each other via various cytokines, which are critical for the study of the pathology and physiology of the urinary system. Cell-cell interactions in microfluidic chips are primarily studied by cell co-culture, which can be divided into membrane-based 2D and hydrogel-based 3D cultures [48]. Microfluidic models of the urinary system (MMUSs) primarily focus on epithelial-mesenchymal interactions and urinary system tumors [49], such as induction of kidney damage following inflammation, recruitment of immune cells, renal fibrosis, or urinary tumors [50]. Owing to differences in various cell culture conditions, the design of effective multicellular co-cultures remains a substantial challenge in constructing microfluidic models. In microfluidic chips, membranes, microcolumns, 3D printing, as well as other materials and methods are often used in cell co-culture to limit the infiltration of each culture medium and maintain the role of cytokines between cells [51,52] (Fig. 3).
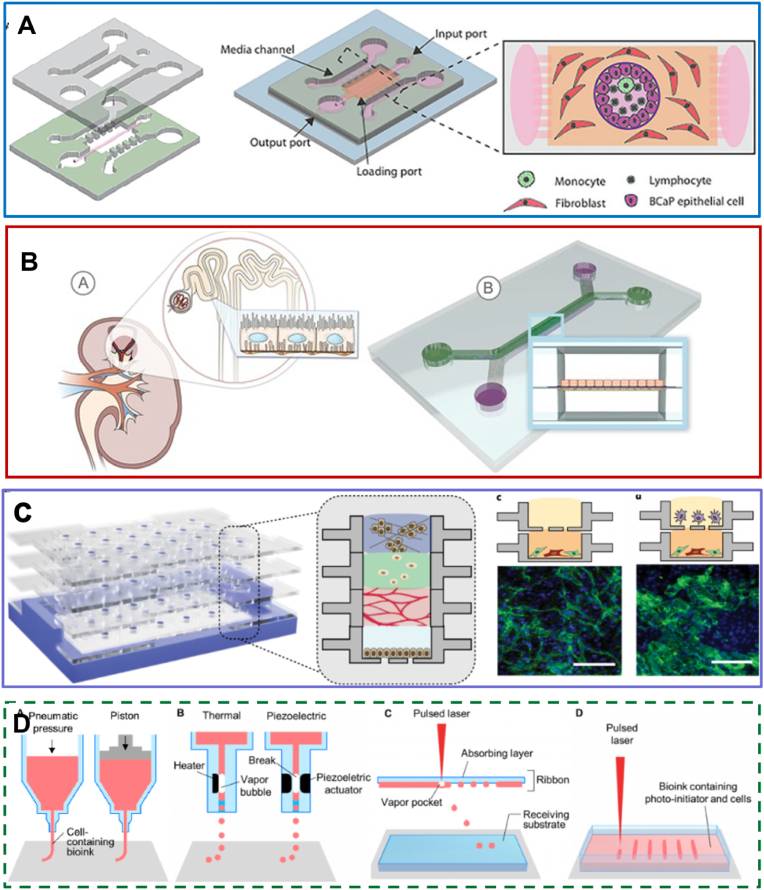
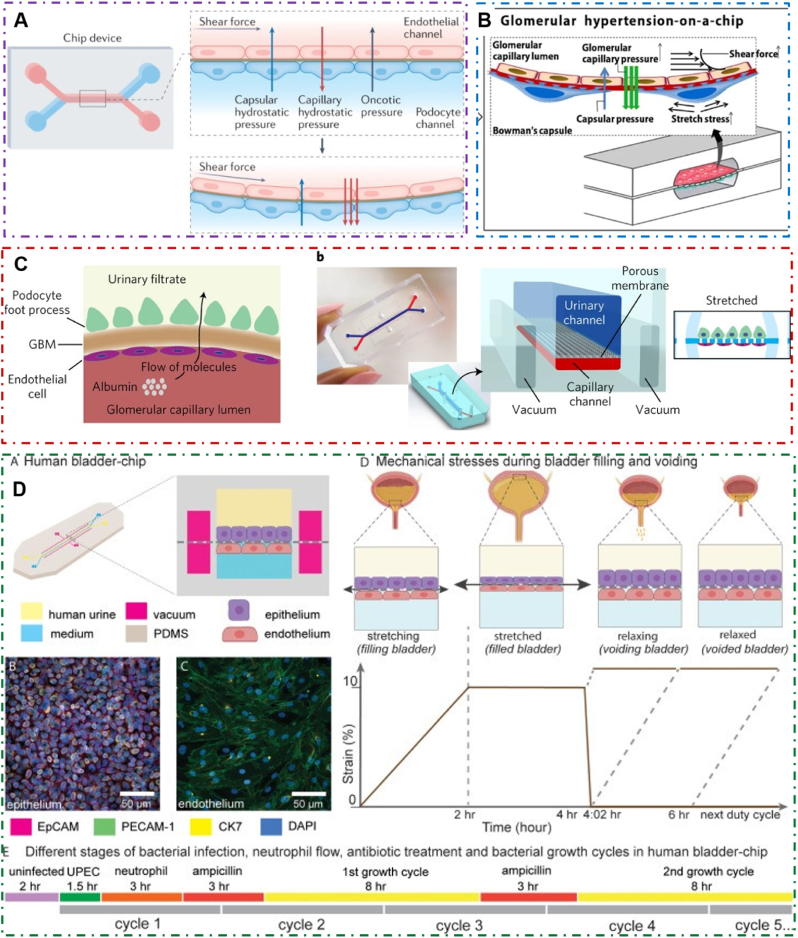
Fig. 3.
Microfluidic models of cell co-culture with different structures in urinary system. (A) Microfluidic model of a prostate duct. The two PDMS layers are bonded together around a rod allowing creation of a lumen structure molded from a collagen hydrogel (orange). Lumens are lined with benign or metastatic BCaP epithelial cells (purple). PBMCs (green) are added inside the lumen. Media channels allow exchange of cell culture media (pink) from the side channels without perturbing the immune cells in the lumen [58]. Reproduced with permission [58]. Copyright 2020, Royal Society of Chemistry. (B) Proximal tubule on-a-chip model based on membrane. The microfluidic channels overlap to create a filtrate channel (green) in communication with a vascular channel (purple). The cross-sectional architecture (inset) mimics in vivo epithelial-endothelial barrier and generates cell-mediated transport through the membrane [52]. Reproduced with permission [52]. Copyright 2017, Public Library of Science. (C) A reconfigurable multilayer suspended microfluidic system. Layers can be vertically assembled into stacks using a holder (blue). The bottom layer containing a mixed culture of endothelial cells and fibroblasts is removed from the stack, and immunocytochemistry for the endothelial marker CD31 (green) and DAPI staining (blue) is performed to visualize endothelial structures [67]. Reproduced with permission [67]. Copyright 2019, Springer Nature. (D) Schematic illustrations of (a) microextrusion, (b) inkjet, (c) laser-assisted printing, and (d) stereolithography techniques [141]. Reproduced under terms of the CC-BY license [141]. Copyright 2016, American Chemical Society.
3.1.1. ECM
The urinary system, a soft tissue system, is in the urinary flow environment, and its ECM must withstand the impact of hydrostatic pressure and fluid shear stress (FSS). The ECM is typically composed of laminin, fibronectin, collagen, and other proteins; however, their proportion varies at different locations in the urinary tract. In addition to facilitating the attachment of urothelial cells, the ECM of the urinary system also provides the 3D microenvironment required by the interstitial cells. The key to simulating the ECM is its porous structure that facilitates penetration of culture medium and intercellular communication. Currently, simulation of the ECM in a microfluidic chip can be achieved by constructing a membrane or hydrogel. However, membranes, are more commonly used owing to their simplicity and high success rate. Notably, polycarbonate membranes are bonded between two PDMS layers and co-cultured. For example, the human kidney proximal tubule microfluidic chip constructed by Ingber et al. comprises two PDMS channels, similar to proximal tubules and stroma, separated by an ECM-coated porous membrane [53].
The glomerular basement membrane (GBM) within the urinary system is the most commonly simulated membrane structure. The basement membrane (BM) is a component of the ECM connected to all epithelial and endothelial cells and can, therefore, participate in structural changes, filtration, and signal transduction [54,55]. Progress has been made in assembling microporous biocompatible membranes via electrospinning to replicate the GBM [56]. This technique makes it possible to accurately simulate the development of thin layers of interlaced nanoscale-diameter GBM fibers. For instance, human immortalized endothelial cells and podocytes were co-cultured on both sides of these membranes to construct a microfluidic glomerular model. In addition, a collagen-coated anodic aluminum oxide membrane exposed to transmembrane pressure was prepared and used to mimic the GBM [57]. However, to further improve cell adhesion and the ECM microenvironment, it is often necessary to coat the membrane with fibronectin or ECM [58].
Currently, membrane-based MMUSs cannot achieve 3D culture and only act as a physical barrier. Hence, an increasing number of studies are focusing on multicellular co-culture and the 3D spatial distribution of cells, which aid in the design and construction of complex tissue models. Hydrogels are widely utilized in these studies as they play the role of the ECM in a microfluidic model, with a wide range of applications, ranging from proximal tubule microfluidic models to prostate tumor models. They make it possible to co-culture of over two cell types in the same environment and ensure flexibility in their spatial arrangement. Kerr et al. simulated the prostate tumor microenvironment model in vitro by mixing rat tail type I collagen with fibroblasts [59]. In the glomerular model, Matrigel has been applied as the GBM to maintain the vital functions of the glomerulus [60]. In addition, Moll et al. inoculated human renal proximal tubular epithelial cells (HPTECs) into the collagen gel layer containing fibroblasts to study the response of fibroblasts to cisplatin. They demonstrated that epithelial cells modulate fibroblast gene expression and phenotype [61]. In addition to the construction of the 3D environment, ECM has proven superior and physiologically relevant to 2D cultures in terms of cellular functional expression and pathophysiological reactions [62]. This was observed in a study on the effect of ifosfamide on MadinDarby canine kidney (MDCK) cells, in which ifosfamide had no effect in 2D culture; however, it reduced the inflammatory response in the microfluidic model. Thus, the microfluidic model was deemed superior to the 2D cell culture model in predicting drug-induced nephrotoxicity [48].
With an increased understanding of the spatial structure of cells and the development of material science, a biomimetic ECM has been developed for MMUSs. A 3D tubular network was created in the sodium alginate-collagen-I hydrogel to simulate the epithelial tubules and adjacent endothelial vessels of the nephron [63]. Meanwhile, Ng et al. developed a hollow fiber model in which monolayer HPTECs were successfully cultured in a fibrin-coated hollow fiber membrane [64]. Although introducing hydrogel facilitates the construction of a 3D microenvironment, no urinary tissue-specific ECM (such as the bladder acellular matrix) has been successfully used in urinary microfluidic 3D culture.
3.1.2. Autocrine- and paracrine-derived signaling molecules
Jiang et al. constructed a prostate microfluidic model by co-culturing prostate epithelial cells and stromal cells on a polyester porous membrane [65]. Paracrine and endocrine crosstalk between the two cell types were evaluated by adjusting the flow rate, thereby simulating the functional development of human prostate cancer in vivo. Moreover, a multi-compartment co-culture model of mouse macrophages and engineered human embryonic kidney cells was used to demonstrate the cell-paracrine interaction, whereby the signaling molecule, lipopolysaccharide, diffused through the gel barrier and induced a cell-cell response between the two cell types (Fig. 4) [66].
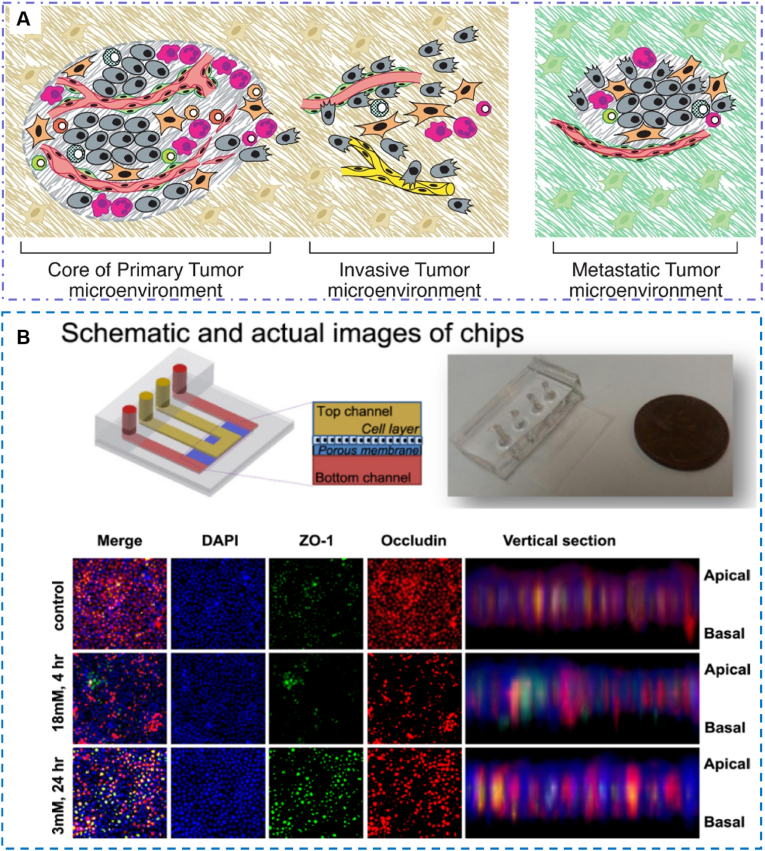
Fig. 4.
A microfluidic model for simulating the biochemical microenvironment of urinary system. (A) The distinctive microenvironments of tumors. The multiple stromal cell types create a succession of tumor microenvironments that change as tumors invade normal tissue and thereafter seed and colonize distant tissues. The abundance, histologic organization, and phenotypic characteristics of the stromal cell types, as well as of the ECM (hatched background), evolve during progression, thereby enabling primary, invasive, and then metastatic growth [68]. Reproduced with permission [68]. Copyright 2011, Cell Press. (B) Kidney-on-a-chip is developed for monitoring nephrotoxicity. Schematic design and actual image of a kidney-on-a-chip. Junctional protein expression of each group. The static and shear groups are measured before exposure to gentamicin. All groups show improved polarization compared to Transwell cultures [112]. Reproduced with permission [112]. Copyright 2016, IOP Publishing Ltd.
The microfluidic platform significantly improves our understanding of cellular signal transduction, particularly, paracrine signaling between cells and the release of nutritional factors. However, microfluidic devices often lack the flexibility to add or remove multiple cell types or subsets at precise times during the culture period, while also enabling flexibility of the culture environment [67]. To address this issue, Yu et al. described a reconfigurable microfluidic cell culture system that promotes the assembly of 3D tissue models by combining open and suspended microfluidic models and stacking layers containing preconditioned microenvironments [67]. In this way, paracrine signaling events can be modeled by providing space and time flexibility in 2D and 3D multicellular culture analysis.
3.1.3. Hypoxia and ion concentration
Vormann et al. constructed a co-culture model of renal proximal tubule-endothelial vascular perfusion to simulate ischemia-reperfusion injury by controlling oxygen levels, availability of nutrients, and perfusion settings [68]. Hypoxia and interruption of blood flow exerted evident interference effects on proximal tubules. Wei et al. [69] constructed a microfluidic device comprising a circular cross-section (400 μm) channel for culturing submandibular immortalized epithelial cells. The intracellular calcium gradient increased with elevated calcium concentration within the lumen. Moreover, calcium phosphate stones formed following the addition of CaCl2 and Na3PO4 to the channel.
3.2. Physical microenvironment
3.2.1. Fluid shear stress
The urinary system is where urine is filtered, concentrated, and excreted within a unique urine flow microenvironment. This includes various ions in the urine, as well as FSS and hydrostatic pressure caused by urine flow (Fig. 5A).
Fig. 5.
A microfluidic model for simulating the physical microenvironment of urinary system. (A) Glomerulus-on-a-chip microfluidic: podocytes and endothelial cells are cultured on opposite sides of an artificial membrane. Endothelial cells are also affected by blood flow shear force. This chip device can recreate capillary pressure by supplying perfusion flow in the upper microchannel and introducing mechanical forces [137]. Reproduced with permission [137]. Copyright 2022, Springer Nature. (B) A pathological glomerular microenvironment was established by perfusion flow regulating mechanical forces [81]. Reproduced under terms of the CC-BY license [81]. Copyright 2016, Springer Nature. (C) Modelling the human glomerular capillary wall with an organ-on-a-chip microfluidic device. Arrow shows directional flow of molecules from the capillary lumen to urinary space. Cyclic mechanical strain was applied to cell layers by stretching the flexible PDMS membrane using vacuum [82]. Reproduced with permission [82]. Copyright 2017, Springer Nature. (D) Human Bladder-chip model of UTI recapitulates the physiology of bladder filling and voiding. Human bladder epithelial cell line (epithelium, top) and primary human bladder microvascular endothelial cells (endothelial, bottom) on either side of the stretchable and porous membrane. Pooled human urine diluted in PBS and endothelial cell medium were perfused in the apical and vascular channels respectively to mimic bladder physiology. A negative pressure in the ‘vacuum’ channels (magenta) on either side of the main channel was applied to stretch the porous membrane to mimic stretching of the bladder [83]. Reproduced under terms of the CC-BY license [83]. Copyright 2021, eLife Sciences Publications.
Laminar flow represents the predominant urinary flow type and includes pulse and periodic flow. Microfluidic chips can accurately control fluid flow via an external pump to ensure precise simulation of the urinary system flow environment. Moreover, flowing microfluidics can excrete metabolites and help achieve long-term culture while providing mechanical cues for cell growth and development. Furthermore, several studies have consistently reported an improvement in the physiological behavior of the urinary system cells in response to FSS [70,71]. Jang et al. described a similar microfluidic device that simulates the function of proximal tubules in the human kidney [53]. The renal epithelial monolayer was exposed to FSS. Compared with the traditional Transwell culture system, the epithelial cells became polarized to form primary cilia under FSS. Moreover, their albumin transport and glucose reabsorption were enhanced, and alkaline phosphatase activity at the brush border was increased. Additionally, compared with static culture, the rearrangement of the actin cytoskeleton and expression of tight junction proteins was increased under FSS. Functionally, the microfluidic system was used to apply FSS to the proximal renal tubular epithelial cell line HK-2 [72]. The results showed that FSS can affect HK-2 cell morphology and upregulate the level of megalin and clathrin, thus improving the uptake efficiency of HK-2 cells for energy-driven carriers, such as macromolecular and albumin nanoparticles [72].
A pump-less microfluidic device was used to evaluate the effect of FSS on mouse ureteral bud (UB) cells cultured in vitro [73]. Exposure to FSS was shown to cause enrichment in UB tip cells. Indeed, some studies have focused on assessing the correlation between the fluid dynamic process and the deposition of encrusting particles in the ureteral stents [74,75]. The related hydrodynamic environment of a ureter with a stent was simulated by establishing a microfluidic model. An inverse correlation was defined between the deposition of encrusting bodies and local wall shear stress in a stented ureter model [74]. Moreover, the findings of this study confirmed that the critical areas of encrusting body deposition include the side hole of the stent, and the cavity formed by ureteral occlusion, thus, providing essential insights into the design of improved stents via hydrodynamic optimization. De Grazia et al. used a similar model to evaluate the attachment of bacteria to ureteral stents in a mobile environment, thus providing a reference for the design of new ureteral stents to better resist the formation of biofilms [75].
In addition to urine flow, interstitial flow is also an important stimulating factor. Tissue interstitial flow provides nutrients and material circulation for cells. Low shear stress exerted by interstitial flow can affect the proliferation and invasion of tumor cells, induce stem cell differentiation, and promote the maturation of renal tubular epithelial cells [[76], [77], [78]]. One study adopted an experimental platform that combines traction force-measurable technology with microfluidic chips [79]. They reported that interstitial flow inhibits MDCK cell migration and alters the physical dynamics of the cell island.
Although progress has been made in the application of microfluidics to simulate the urinary system fluid environment, MMUSs often apply a single fluid type, which differs from the physiological urinary flow. Furthermore, differences in flow velocity have been noted between studies owing to the lack of physiological parameters.
3.2.2. Compressive pressure and cyclic stretch
The pressure in the urinary system primarily arises from glomerular filtration pressure, circulating hydrostatic pressure, and urinary hydrostatic pressure. The pressure gradient can affect filtration rate, cell polarization, and the functions of the capillaries and filter barriers [80]. In the microfluidic models, the physiological parameters of transcapillary hydraulic pressure and filtration pressure for glomerular filtration rate can be simulated by adjusting the flow rate. In addition, the creation of multi-layer systems makes it possible to add filter barriers between channels, thus providing appropriate permeability simulation. In a glomerulus-on-chip, mouse endothelial cells and podocytes were co-cultured on the opposite side of a porous polycarbonate membrane [81]. The diameter of the microfluidic channel could be altered to establish pressure and simulate the mechanism of glomerular hypertension and glomerulosclerosis (Fig. 5B). In this model, high pressure can alter podocyte shape and the filtration barrier permeability, which provides a reference for establishing a GFB-on-a-chip. In the four-channel chip developed by Wei et al., two central channels were separated by a PDMS membrane with a 7-μm hole, and cyclic suction was applied to the two lateral channels to replicate the cyclic stress experienced by glomerular cells [69]. Podocytes derived from human primary endothelial cells and induced pluripotent stem cells (iPSCs) were implanted on the opposite side of the PDMS membrane in the central channel. Podocytes exhibited enhanced foot processes under the action of fluid flow and cyclic mechanical strain, and the expression of specific mature podocyte markers increased with increasing fluid stimulation.
To simulate the dynamic mechanical strain observed in the living glomerulus caused by circulatory pulsation of the renal blood flow, Musah et al. added two hollow chambers on either side of the central microfluidic channel and applied cyclic suction to generate cyclic tension (10% strain) [82]. The mechanical strain increased nephrin expression and vascular endothelial growth factor-α secretion in podocytes (Fig. 5C). The same design has also been applied to the human bladder-on-chip model to mechanically simulate bladder filling and urination by applying and releasing linear strain, as well as to explore the dynamic response of Escherichia coli-induced urinary tract infection to host stress and antibiotic therapy [83]. Moreover, a microfluidic platform was applied to elucidate the physical factors associated with cancer-stromal interactions, in particular to study the effect of mechanical stimulation on human prostate fibroblasts (Fig. 5D) [84]. Stretched normal tissue-associated fibroblasts reportedly produced a matrix with a more organized and linear structure, accompanied by increased expression of platelet-derived growth factor receptor α, that effectively guided the migration of co-cultured cancer cells.
3.2.3. Surface topography
Topographic patterns affect morphology and cellular function [85], particularly via contact guidance, and lead to directional cell morphology, migration, and structural reorganization [86,87]. The response of cells to topography depends on a myriad of factors, including cell type and topographic geometry [88]. Compared with other parts of the urinary system, the GBM has a unique surface topography. The BM surface topography is formed by the network structure of proteins and proteoglycan sulfate. It is characterized by pores, ridges, and fibers with sizes ranging from the nanometer to the submicron scale [89,90]. Experimental evidence has revealed that topography is essential to the attached cells and may enable the regulation of barrier function and transport.
Frohlich et al. used a hot-embossing method to model submicron topographic features onto a track-etched porous membrane, thus creating membranes with isolated transmembrane pores and controllable topography [91]. Xie et al. combined microfluidic technology, 3D biological printing, and biomaterials to create extruded terrain hollow fibers. They established the dynamic culture of primary venous endothelial cells and podocytes of Lewis rats, thus ensuring that endothelial cells and podocytes were co-cultured with a microconvex topographic map [92]. Frohlich et al. also cultured HK-2 cells in a microfluidic model with a topographical patterned substrate, and simultaneously exposed the cells to different forms and levels of FSS [71]. Results suggested that surface topography and FSS exert a synergistic effect. That is, in the presence of FSS, the submicron ridge/groove feature created a more realistic in vitro model of human renal tissue by eliciting cell arrangement and influencing tight junction formation.
3.2.4. Substrate stiffness
Substrate stiffness is a mechanical property of ECM that can regulate cell proliferation, migration, and differentiation, particularly in glomerular diseases [[93], [94], [95]]. For instance, ECM hardness increases in chronic kidney disease and diabetic nephropathy [[96], [97], [98]]. Substrate stiffness is regulated by adjusting the hydrogel concentration and type. Biomaterials, such as collagen, fibronectin, and gelatin hydrogel have been successfully applied to analyze the effect of matrix stiffness [99,100]. Meanwhile, Garcia et al. designed a microfluidic device with different soluble chemical factors and matrix stiffness, and observed MDCK scattering [101]. Although the matrix stiffness of other urinary organs has not yet been reported with microfluidic models, they have been studied in 2D and 3D models.
4. Microfluidic chips of urinary system diseases with microenvironment imbalance
An out-of-balance microenvironment induces various diseases. Microfluidic chips of urinary system diseases with microenvironment imbalance can be divided into two categories: (1) to be used as diseased cells, such as urinary system tumors and (2) to simulate the pathological microenvironment by constructing pathological parameters, such as hypertensive nephropathy.
4.1. Urinary system tumor-on-chips
Tumor microenvironment (TME) consists of malignant and nonmalignant cells (cancer-associated fibroblasts, cancer-associated immune cells), ECM, blood vessels, and various physiological environments (such as mechanical stress, fluid shear, oxygen, and drug concentration gradients) [102,103] (Fig. 6A and B). The diversity of TME may affect tumor behavior, including tumor invasion and metastasis. Stromal fibroblasts are essential for the proliferation and invasion of urinary tumors. Xu et al. used microfluidic technology to establish a 3D co-culture system of bladder tumor cells and fibroblasts to explore the characteristics of energy metabolism of bladder cancer cells [104]. Shi et al. [105] proposed a 3D microfluidic co-culture device to study the interaction between cancer-associated fibroblasts (CAF) and bladder cancer cells. The results indicated that the cytokines secreted by bladder cancer cell line T24 could effectively transform fibroblasts into CAF. However, CAF after aerobic glycolysis exhibited a higher ability to produce lactic acid and provide energy for the proliferation and invasion of bladder cancer cells.
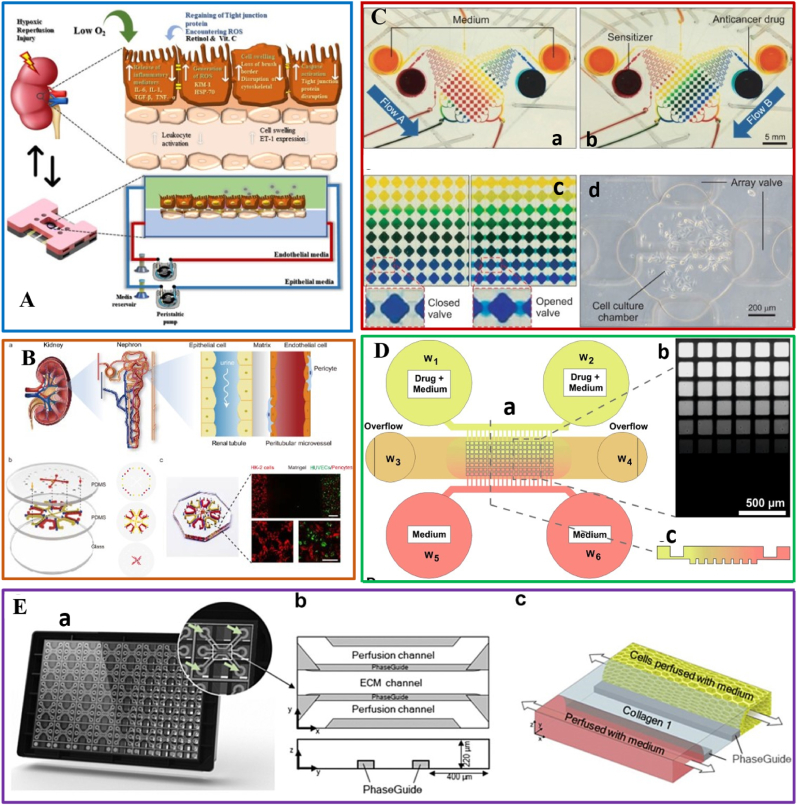
Fig. 6.
Disease and high-throughput drug screening-on-chips in urinary system. (A) Schematic of renal hypoxia-reperfusion injury-on-chip model [102]. Reproduced with permission [102]. Copyright 2022, American Chemical Society; (B) Construction of an integrated functional tubule-vascular microfluidic chip. (a) An illustration of the characteristic physiological structures of the renal interstitial microenvironment. (b) Schematic image of a microfluidic renal interstitium-on-a-chip device with microchannels replicating the renal tubules and peritubular microvessels. (c) Photograph of the microfluidic chip (left), and the representative image of the co-culture of HK-2 cells, HUVECs, and pericytes in the chip (right) [103]. Reproduced with permission [103]. Copyright 2022, Elsevier BV; (C) Demonstration of concentration gradient in microfl uidic system using color dye solution (a) Trapping of 8 different concentration gradient of a sensitizer through a diffusive mixer by driven micropump from two reservoirs (sensitizer and medium are represented by red and yellow respectively) (b) The second trapping of 8 different concentration gradient of drug (drug was represented by blue color dye) (c) Concentration gradient of color dye was maintained 4 h after closing valves without perfusion of reagent (d) Representative cell culture chamber with PC3 cells trapped and grown for 24 h [118]. Reproduced under terms of the CC-BY license [118]. Copyright 2014, Korean Society of Applied Pharmacology; (D) Microfluidic device layout and functions. (a) Schematic representation of the device structure. (b) Fluorescent image showing a calcein concentration gradient over the micro-well array. (c) Schematic cross-section of the gradient generated along a column of the spheroid array [119]. Reproduced under terms of the CC-BY license [119]. Copyright 2018, Springer Nature; (E) The human proximal renal tubule-on-chip for the study of nephrotoxicity and drug interaction. (a) Image of the back side of the OrganoPlate 3-lane. The microfluid network is positioned in-between a glass sandwich of two microscope grade glass plates which are attached to the bottom of a standard 384 titer well plate. (b) Schematic of one chip presenting two perfusion channels and the extracellular matrix (ECM) channel in the middle. (c) Artist impression of one chip. The chip was loaded with collagen 1 (blue) to the ECM channel and proximal tubule cells (yellow) were seeded to the top channel [117]. Reproduced under terms of the CC-BY license [117]. Copyright 2021, Elsevier.
Models based on microfluidic devices can also be used to study metastatic cancer and tumor immunity [106]. Hsiao et al. [107] designed a 3D metastatic prostate cancer model to simulate the bone metastatic microenvironment, including the type of cells in the skeletal microenvironment where the metastatic prostate cancer cells are located. In this model, 3D multicellular spheres of metastatic prostate cancer cells (PC-3 cell line), osteoblasts, and endothelial cells were cultured using a two-layer microfluidic system. This model considerably reduced the proliferation rate of PC-3 cells without reducing the cell survival rate, and can more truly reproduce the in vivo growth behavior of cancer cells in the microenvironment of prostate cancer with bone metastasis. Kerr et al. [59] reported an in vitro model of prostate TME based on microfluidic control, which simulated the structure of the prostate duct and related immune cells and stromal cells. Immune cells were exposed to benign prostate TME or metastatic prostate TME, and their metabolism, gene, and cytokine expression were studied. This platform could provide a valuable tool for studying immune cell phenotypes in vitro TME.
Hypoxia is a known key feature of the tumor microenvironment. Hanahan conducted a specific study on the microenvironment of prostate cancer with a focus on analyzing the effects of hypoxia on prostate cancer cells [108]. They used a microfluidic device to test the sensitivity of cancer cells to the chemotherapeutic agent staphylococcin.
4.2. Urinary system non-tumor disease-on-chips
Studies have investigated the glomerular microstructure to facilitate the construction of glomerular models. These models have been employed to characterize the pathological response induced by high glucose in diabetic nephropathy. For example, Wang et al. [60] constructed glomerular models. More specifically, the glomerular microstructure channel simulates the capillary lumen side of the glomerulus; 3D Matrigel is injected into the middle gel channel to simulate the GBM to support the adhesion and growth of the separated glomeruli. The results showed that hyperglycemia plays a key role in increasing the permeability of the albumin barrier, and the development of glomerular dysfunction, leading to albuminuria. Based on the glomerulus-on-chips with physiological characteristics, Zhou et al. [81] studied the hypertensive nephropathy model by controlling the flow of culture medium, evaluating the permeability of the small, medium and high molecular weight proteins, and observing the injury markers induced by hypertension. Moll et al. [61] used adrenal epithelial cells in cisplatin-induced microfluidic chip to construct renal fibrosis model, and studied the pathological role of renal epithelial cells in cisplatin-induced nephrotoxicity and renal fibrosis.
Chethikkattuveli et al. [109] established a microfluidic renal hypoxia-reperfusion (RHR) model using hypoxia and oxygenated cell culture medium, in which primary HPTECs and human endothelial cells were cultured on the top and basal side of the porous membrane. The disease model was validated by detecting its specific hypoxia biomarkers. In addition, retinol, ascorbic acid and combined doses were tested to design a therapeutic solution for RHR.
Liu et al. [110] developed a renal tubule-vascular chip to create a proteinuria model. The results suggested that it was involved in the crosstalk between renal tubules and peritubular microvessels in renal interstitial fibrosis induced by proteinuria, and inhibition of receptor fucosyltransferase 8 could reverse the injury of peritubular microvessels and renal interstitial fibrosis. The chip can provide a reference for studying the mechanism of renal disease. De Grazia et al. [75] used a similar model to evaluate the attachment of bacteria to ureteral stents in a mobile environment. This study provides a reference for the design of new ureteral stents to better resist biofilm formation.
5. Drug toxicity and drug screening
The kidney is a vital organ responsible for drug metabolism and excretion. Animal models are considered the gold standard for drug safety testing and screening. However, drugs usually metabolize faster in animals than in humans, which underestimates the nephrotoxicity of drugs [111]. The renal cell model lacks sufficient physiological correlation. Therefore, the existing preclinical studies cannot better predict nephrotoxicity. Simultaneously, the mode and dose of administration also affect drug safety [112]. Microfluidic chips have numerous prospective applications in drug toxicity and drug screening because of their high throughput, low reagent consumption, and high similarity with the physiological environment. Evidence suggests that the pharmacodynamics and pathophysiology of cells are more accurate in a microfluidic system than in a 2D culture.
Kim et al. analyzed the gentamicin-induced nephrotoxicity using membrane-based microfluidic chips [112], where MDCK cells were inoculated into the upper channel and cultured under physiological FSS to simulate urine flow in vivo. Using fluid movement regulation, they demonstrated that once-a-day injection of gentamicin was less harmful than continuous infusion under the same dose. Qu et al. [113] constructed a microfluidic platform simulating the nephron, which consists of glomerulus, Bowman's capsule, proximal tubular lumen and peritubular capillary. The results indicated that the model made renal cells more sensitive to the nephrotoxicity of cisplatin and doxorubicin. In addition, the nephrotoxicity induced by cadmium, ammonia and ifosfamide has been reported in the microfluidic chip models [114,115].
The microfluidic system also provides the ability to integrate multiple cell types in different tissues. In fact, studies have successfully integrated several tissues into a multiple-organs-on-chip through a microcolumn structure to study multiple organ responses, including those of the kidneys and liver [116], or four organs on a single chip [117]. Multiple-organs-on-chips can be used to evaluate the interaction between organs, to study the metabolic process of drugs in the kidney, and to better explain the pharmacokinetics [118] (Fig. 6C). Choucha-Snouber et al. used a liver-kidney combined organ-on-chip to simulate the interaction between the liver and kidney in the context of drug metabolism and drug-induced nephrotoxicity [48]. Ifosfamide was found to substantially reduce the number of MDCK cells in this model, thus simulating its nephrotoxicity. Additionally, Zhang et al. [119] designed a 3D microfluidic cell culture system comprising hepatocytes, lung cells, kidney cells, and adipocytes to assess the effects of drugs on each organ cell type, and evaluate organ-to-organ interactions (Fig. 6D). Chang et al. [120] used a combination of hepatocytes and kidney cells on the chip platform to study the nephrotoxicity of Aristolochia acid. Maschmeyer et al. [117] developed a multiple-organs-on-chip comprising four culture chambers, including intestinal epithelial cells, hepatocytes, skin cells, and renal proximal tubule cells. The functions of the four organs were connected to identify the pathways related to absorption, distribution, metabolism, and excretion of certain drugs (Fig. 6E). Overall, the microfluidic kidney chip model with a physiological microenvironment can enhance the prediction of drug nephrotoxicity, accurately reflect the real human physiological and pathological response, and reproduce the process of drug filtration and reabsorption.
High-throughput screening is one of the latest technologies for drug design and discovery [121]. In recent years, microfluidic models that can be used for high-throughput screening have been developed to enable researchers to rapidly screen drug efficacy or evaluate toxicity. Shaughnessey et al. [122] utilized a high-throughput microfluidic platform with rapid transepithelial electrical resistance measurement capability and multi-flow control function to evaluate cisplatin-induced toxicity in human primary proximal tubule models. However, this study contained no further experiments on high-throughput drug screening. Jing et al. [123] constructed a high-throughput human renal proximal tubule model based on an integrated biomimetic array chip. The results showed that primary HPTECs cultured on this microfluidic platform could form a tighter barrier and better protein transport function than static Transwell. Polymyxin B, doxorubicin, and sunitinib were also used to evaluate the nephrotoxicity.
Vormann et al. [124] designed a high-throughput 3D microfluidic platform (NephroScreen) for the detection of drug-induced kidney injury (DIKI). This system was established with four model nephrotoxic drugs (cisplatin, tenofovir, tobramycin and cyclosporin A) and tested with eight pharmaceutical compounds. A variety of detection indicators confirmed that the method of evaluating DIKI by NephroScreen is reliable, compatible with automatic fluid transfer, and can be used in long-term experiments. An et al. [125] established a microfluidic high-throughput drug screening platform composed of eight different concentrations of two chemotherapeutic agents. The platform was used to explore the best therapeutic concentration for the effect of prostate cancer cells. Advantageously, it is cost effective and does not require continuous perfusion. In addition to drug screening for tumor cells, microfluidic technology is also used for drug screening in the tumor tissues of the patients. Mulholland et al. [126] presented a microfluidic platform that enables drug screening of cancer cell-enriched multicellular spheroids from tumor biopsies. The model can form repeatable drug concentration gradients on a series of spheres without an external fluid drive, which can meet the requirements of personalized treatment.
6. Challenges and future directions
In recent years, considerable progress has been achieved in utilizing microfluidic technology in the research of urinary tumor drug screening, tumor metastasis, and glomerulus-on-chip development [[127], [128], [129], [130]]. Microfluidic models of the urinary systems have enriched the understanding of microenvironments from single-cell cultures to co-culture involving two types of cells based on membranes to multicellular co-culture based on microcolumns or 3D biological printing technology. Microfluidic chips have been developed from a single channel to an integrated, high-throughput channel, and their practicability and standardization are increasing. Compared with traditional cell research methods, microfluidic technology integrates physical, biological, and chemical factors with a low test dose [131], laminar flow distribution, and biology-related length scale [132]. It facilitates the precise spatiotemporal regulation of the cellular microenvironment. Based on microfluidic models, the interaction between cells and cell-ECM can be studied in a specific urinary system (Fig. 7).
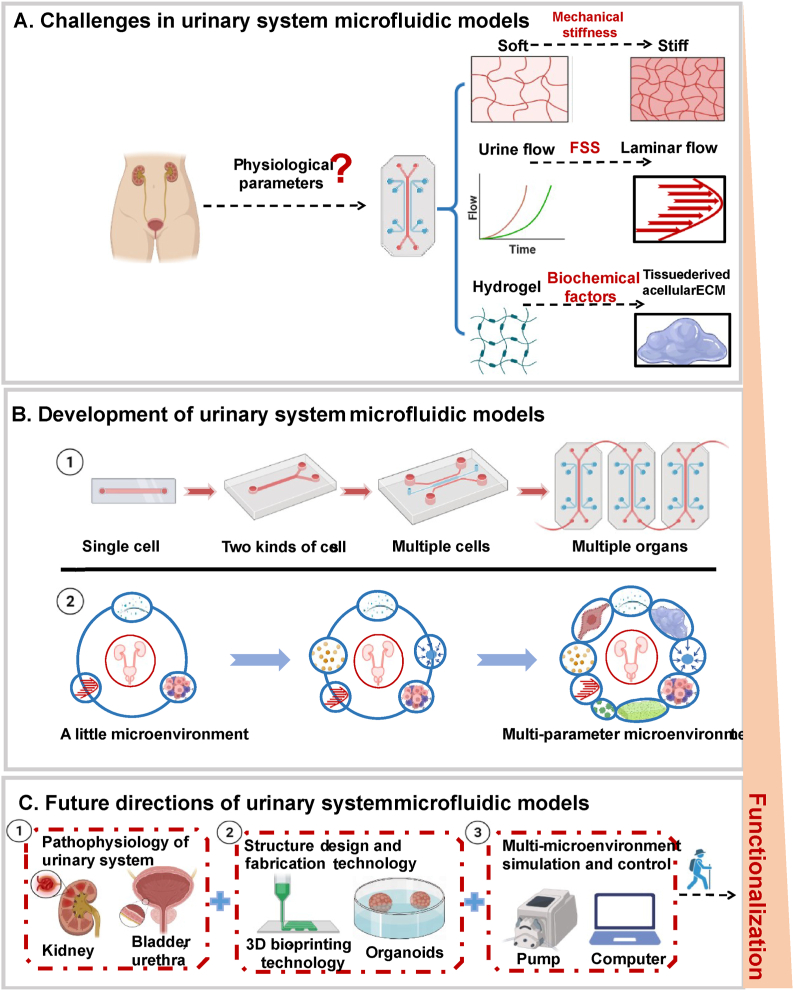
Fig. 7.
Challenges, development and future directions in urinary system microfluidic models. (A) The challenge of microfluidic technology in simulating real microenvironment lies in the lack of physiological parameters, including urine flow velocity, stiffness of ECM, porosity and so on. (B) The urinary system microfluidic model has developed from a single channel to an integrated, high-throughput channel, and the practicability and standardization are increasing, and the connotation of its microenvironment is also being enriched. (C) With the further study of pathophysiology of urinary system, the improvement of chip structure design and fabrication process, and multi-parameter microenvironment simulation and control will help to promote the development of urinary system microfluidic models.
However, this model still has certain limitations. Microfluidic technology does not reconstruct the whole organ system but only comprises the smallest cells or tissues needed to simulate the specific functions of the representative organs; hence, its ability to replicate multi-functional organs is limited [133]. Another challenge in building a urinary microfluidic model is the choice of cell types. Primary cells can maintain cell phenotype and have the advantages of individualization and tissue specificity. However, their use is limited by their long culture cycles, difficult cultivation, and ethical issues [134]. Although cell lines can overcome these limitations, their genes and phenotypes often differ from those of primary cells [135]. Moreover, immortalized cell lines, which are easy to culture in vitro, are difficult to culture under a relatively closed microfluidic system. Nevertheless, human iPSCs provide a new cell source for urinary system microfluidic chips. Indeed, podocytes and endothelial cells derived from iPSCs have been successfully applied to construct glomerulus-on-chips (Table 1) [82].
Table 1.
Comparison of urinary system microfluidic models.
| Organ | Cell types | Hydrogel/Coating material | Fabrication technique | Microenvironment | Design | Reference/Authors |
|---|---|---|---|---|---|---|
| Glomerular |
iPSC; Cell line: human glomerular endothelial cells |
Porous flexible PDMS membrane | Stereolithography, PDMS | FSS and cyclic mechanical strain | Hollow chambers on either side of the central microfluidic channels | Musah Samira [38] |
| Glomerular | Primary cell: glomerular microtissues | Matrigel | Soft lithography and micromolding, PDMS | FSS, Basement membrane | Crescent microstructures, capillary channels, | Li Wang [60] |
| Renal proximal Tubule/Blood Vessel-on-a-Chip | Cell line: Human RPTEC, HUVEC | Type 1 collagen | Commercial: OrganoPlate 3-lane | Oxygen concentration, perfusion flow, and nutrients | Capillary pinning | Vormann MK [68] |
| Renal proximal tubule |
Cell line: HK-2; Primary cell: RPTEC |
Collagen IV | Hot-embossing meth, photolithography and reactive ion etching | Topography | Polycarbonate membrane | Else M Frohlich [71] |
| Prostate |
Cell line: BCaP-NT, BCaP-M1; Primary cell: fibroblast. |
Rat-tail collagen type 1 | Soft-lithography, PDMS | Tumor microenvironment | Microcolumn | Kerr SC [59] |
| Prostate | Cell line: PrECs, HPrS1s | Polyester porous membrane | Soft-lithography, PDMS | FSS | Polyester porous membrane | L Jiang [65] |
| Bladder | Cell line: CAFs and bladder cancer cells | Metrigel | Lithography | Cytokines | Microcolumn | Haoqing Shi [105] |
Abbreviations: BCaP, BPH-1-derived Cancer Progression (BCaP) cells; PrECs, prostate basal epithelial cells; HPrS1s, human prostate stromal cells; CAFs, cancer-associated fibroblasts; Human RPTEC, Kidney PTEC Control Cells; HUVEC, Human umbilical vein endothelial cells; HK-2, human kidney proximal tubule epithelial cell line; RPTEC, renal proximal tubule epithelial cells; PDMS, Polydimethylsiloxane.
With the introduction and realization of organoids, cell self-assembly has become a new solution to simulate in vivo conditions [136]. Compared to cell-based organ chips, organoids can differentiate into a variety of organ-specific cell types, thus, simulating a specific organ function or spatial structure. However, the lack of vasculature can limit their application [137]. Hence, researchers have recently combined organoid culture with microfluidic technology [138], such as kidney organoids cultured in microfluidic chips, to circumvent the limitations of each model.
In addition, the microenvironments within different body parts are complex and diverse. Simulation of the urinary system ECM using microfluidic technology remains insufficient. The urinary system comprises soft tissues loaded with all types of cells. The ECM provides adhesion for cells and generates physical, biological, and chemical signals to affect their maturation and development. In MMUSs, the hydrogel serves as a substitute for the ECM, primarily collagen and fibrin. These are the most critical components of the ECM and have been widely used due to their viscoelasticity and availability, as well as their ease of integration into microfluidic devices. However, hydrogels cannot guarantee that the matrix stiffness, porosity, and carrier cytokines are similar to the real ECM. Simultaneously, the lack of relevant pathophysiological parameters limits the further application of microfluidic technology in urology. Therefore, researchers continue to explore the means to extract the design variables of microfluidic chips appropriately. Despite their shortcomings, a variety of natural and synthetic hydrogels have been developed based on biocompatibility, hydrophilicity, and structural similarity, including photo-crosslinked gelatin methacrylate with multiple cytokines [139].
The emerging 3D printing technology, with sub-millimeter and submicron accuracy, also provides a new direction for the development of MMUSs [140]. It can reproduce the shape and structure of tissues and organs. In the field of microfluidic technology, it can also accurately control the composition, structure, and spatial distribution of chip materials. Compared with the traditional hydrogel mixed cells to achieve 3D culture, 3D biological printing provides the ability to pattern, has more flexibility and structural specificity, and provides a more suitable method for constructing complex tissues [141,142]. In addition, to better utilize microfluidic chips for simulating the cellular microenvironment, improvement in the chip structure design and fabrication process, as well as the addition of multi-parameter microenvironment simulations, is necessary. We believe that with continued analysis and characterization of the pathophysiology of the urinary system, as well as improvements in chip structure design and fabrication, microfluidic chip technology will become an invaluable tool for the in-depth study of the urinary system and its associated diseases. However, this will undoubtedly require the close cooperation of cell biologists, pharmacologists, and bioengineers.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NO. 81974085), the Natural Science Foundation of Shanghai (NO. 18ZR1429200), the International Cooperation Fund of the Science and Technology Commission of Shanghai Municipality (NO. 19410741700) and the Talent Plan of Shanghai Municipal Health Commission (NO. 2022XD015). We acknowledge BioRender.com for providing icons of illustrations.
Contributor Information
Xiang Chen, Email: xiangchen@sjtu.edu.cn.
Lujie Song, Email: ljsong@sjtu.edu.cn.
Data availability
No data was used for the research described in the article.
References
- 1.Jones-Freeman B., Chonwerawong M., Marcelino V.R., Deshpande A.V., Forster S.C., Mr S. The microbiome and host mucosal interactions in urinary tract diseases. Mucosal Immunol. 2021;14(4):779–792. doi: 10.1038/s41385-020-00372-5. [DOI] [PubMed] [Google Scholar]
- 2.Dalghi M.G., Montalbetti N., Carattino M.D., G. A The urothelium: life in a liquid environment. Physiol. Rev. 2020;100:1621–1705. doi: 10.1152/physrev.00041.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wa rrick J.W., Murphy W.L., B.b. DJ Screening the cellular microenvironment: a role for microfluidics. Ieee Reviews In Biomedical Engineering. 2008;1:75∼93. doi: 10.1109/RBME.2008.2008241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma Y., Li Y., Yang Y., L. P. The microenvironment that regulates vascular wall stem/progenitor cells in vascular injury and repair. BioMed Res. Int. 2022;2022 doi: 10.1155/2022/9377965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makkar H., Zhou Y., Tan K.S., Lim Ct S.G. Modelling crevicular fluid flow and host-Oral microbiome interactions in a gingival crevice-on-Chip. Adv Healthc Mater. 2022;17 doi: 10.1002/adhm.202202376. [DOI] [PubMed] [Google Scholar]
- 6.Laplane L., Duluc D., Larmonier N., Pradeu T., B. A The multiple layers of the tumor environment. Trends Cancer. 2018;4(12):802–809. doi: 10.1016/j.trecan.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Wan L., Neumann C.A., LeDuc P. Tumor-on-a-chip for integrating a 3D tumor microenvironment: chemical and mechanical factors. Lab Chip. 2020;20:873–888. doi: 10.1039/c9lc00550a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yi C.Q., Li C.W., Ji S.L., Y. MS Microfluidics technology for manipulation and analysis of biological cells. Analytica Chemica Acta. 2006;560(1–2):1∼23. doi: 10.1016/j.aca.2018.06.054. [DOI] [PubMed] [Google Scholar]
- 9.Sung J.H., Esch M.B., Prot J.-M., Long C.J., Smith A., Hickman J.J., Ml S. Microfabricated mammalian organ systems and their integration into models of whole animals and humans. Lab Chip. 2013;13:1201–1212. doi: 10.1039/c3lc41017j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang K., Dong C., Xu Y W.L. Microfluidic-based biomimetic models for life science research. RSC Adv. 2016;6:26863–26873. [Google Scholar]
- 11.Edmondson R., Broglie J.J., Adcock A.F., Y. L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014;12:207–218. doi: 10.1089/adt.2014.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Lamki R.S., Bradley P.J.S., JR. Human organ culture: updating the approach to bridge the gap from in vitro to in vivo in inflammation, cancer, and stem cell biology. Front. Med. 2017;4:148. doi: 10.3389/fmed.2017.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang K., Man K., Liu J., Liu Y., Chen Q., Zhou Y., Y. Y Microphysiological systems: design, fabrication, and applications. ACS Biomater. Sci. Eng. 2020;6(6):3231–3257. doi: 10.1021/acsbiomaterials.9b01667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sertkaya A., Wong H.-H., Jessup A., B. T Key cost drivers of pharmaceutical clinical trials in the United States. Clin. Trials. 2016;13:117–126. doi: 10.1177/1740774515625964. [DOI] [PubMed] [Google Scholar]
- 15.Hachey S.J., Ccw H. Applications of tumor chip technology. Lab Chip. 2018;18:2893–2912. doi: 10.1039/c8lc00330k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar V., V. S. Ex vivo tumor-on-a-chip platforms to study intercellular interactions within the tumor microenvironment. Adv Healthc Mater. 2019;8(4) doi: 10.1002/adhm.201801198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang B., Korolj A., Lai Bfl R.M. Advances in organ-on-a-chip engineering. Nat. Rev. Mater. 2018;3:257–278. [Google Scholar]
- 18.Zhang Y.S., Zhang Y.-N.Z.W. Cancer-on-a-chip systems at the frontier of nanomedicine. Drug Discov. Today. 2017;22:1392–1399. doi: 10.1016/j.drudis.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahadian S., Civitarese R., Bannerman D., Mohammadi M.H., Lu R., Wang E., Davenport-Huyer L., Lai B., Zhang B., Zhao Y., Mandla S., Korolj A R.M. Organ-on-A-chip platforms: a convergence of advanced materials, cells, and microscale technologies. Adv Healthc Mater. 2018;7(2) doi: 10.1002/adhm.201700506. [DOI] [PubMed] [Google Scholar]
- 20.Z.B.a.R. M Organ-on-a-chip devices advance to market. Lab Chip. 2017;17(14):2395–2420. doi: 10.1039/c6lc01554a. [DOI] [PubMed] [Google Scholar]
- 21.de Jongh R., Spijkers X.M., Pasteuning-Vuhman S., Vulto P.P.R.J. Neuromuscular junction-on-a-chip: ALS disease modeling and read-out development in microfluidic devices. J. Neurochem. 2021;157:393–412. doi: 10.1111/jnc.15289. [DOI] [PubMed] [Google Scholar]
- 22.Jiang W., Li M., Chen Z., Kw L. Cell-laden microfluidic microgels for tissue regeneration. Lab Chip. 2016;16(23):4482–4506. doi: 10.1039/c6lc01193d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Natarajan A., Sethumadhavan A.K.U.M. Toward building the neuromuscular junction: in vitro models to study synaptogenesis and neurodegeneration. ACS Omega. 2019;4:12969–12977. doi: 10.1021/acsomega.9b00973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.M. P. Microfluidics—downsizing large-scale biology. Nat. Biotechnol. 2001;19(8):717–721. doi: 10.1038/90754. [DOI] [PubMed] [Google Scholar]
- 25.Stucki A.O., Stucki J.D., Hall S.R., Felder M., Mermoud Y., Schmid R.A., Geiser T., Ot G. A lung-on-a-chip array with an integrated bio-inspired respiration mechanism. Lab Chip. 2015;15(5):1302–1310. doi: 10.1039/c4lc01252f. [DOI] [PubMed] [Google Scholar]
- 26.Huh D., Matthews B.D., Mammoto A., Montoya-Zavala M., Hsin Hy I.D.E. Reconstituting organ-level lung functions on a chip. Science. 2010;328(5986):1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan J., Guo Q., Tian L., Pei Z., Li D., Wu M., Zhang J., G. X Biomimetic lung-on-a-chip to model virus infection and drug evaluation. Eur. J. Pharmaceut. Sci. 2022 doi: 10.1016/j.ejps.2022.106329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gijzen L., Yousef Yengej F.A., Schutgens F., Vormann M.K., Ammerlaan C.M.E., K.D. Nicolas A., Vulto P R.M., Lanz H.L., Verhaar Mc C.H. Culture and analysis of kidney tubuloids and perfused tubuloid cells-on-a-chip. Nat. Protoc. 2021;16(4):2023–2050. doi: 10.1038/s41596-020-00479-w. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen V.V.T., Ye S., Gkouzioti V., van Wolferen M.E., Yengej F.Y., Melkert D., Siti S., de Jong B., Besseling P.J., Spee B., van der Laan L.J.W., Horland R., Verhaar M.C., Bwm v.B. A human kidney and liver organoid-based multi-organ-on-a-chip model to study the therapeutic effects and biodistribution of mesenchymal stromal cell-derived extracellular vesicles. J. Extracell. Vesicles. 2022;22 doi: 10.1002/jev2.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roye Y., Bhattacharya R., Mou X., Zhou Y., Burt M.A., M. S A personalized glomerulus chip engineered from stem cell-derived epithelium and vascular endothelium. Micromachines. 2021;12(8):967. doi: 10.3390/mi12080967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen W.Y., Evangelista E.A., Yang J., Kelly E.J., Ck Y. Kidney organoid and microphysiological kidney chip models to accelerate drug development and reduce animal testing. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.695920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myram S., Venzac B., Lapin B., Battistella A., Cayrac F., Cinquin B., Cavaniol C., Gropplero G., Bonnet I., Demolombe S., Descroix S., C. S A multitubular kidney-on-chip to decipher pathophysiological mechanisms in renal cystic diseases. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.624553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y.S., Arneri A., Bersini S., Shin S.R., Zhu K., Goli-Malekabadi Z., Aleman J., Colosi C., Busignani F., Dell'Erba V., Bishop C., Shupe T., Demarchi D., Moretti M., Rasponi M., Dokmeci M.R., Atala A K.A. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials. 2016;110:45–59. doi: 10.1016/j.biomaterials.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doherty E.L., Aw WY. Hickey A.J., Wj P. Microfluidic and organ-on-a-chip approaches to investigate cellular and microenvironmental contributions to cardiovascular function and pathology. Front. Bioeng. Biotechnol. 2021;4(9) doi: 10.3389/fbioe.2021.624435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider O., Moruzzi A., Fuchs S., Grobel A., Schulze H.S., Mayr T., L. P Fusing spheroids to aligned μ-tissues in a heart-on-chip featuring oxygen sensing and electrical pacing capabilities. Mater Today Bio. 2022;15 doi: 10.1016/j.mtbio.2022.100280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang Q., Li X., Lai C., Li L., Wu H., Wang Y S.X. Fabrication of a hydroxyapatite-PDMS microfluidic chip for bone-related cell culture and drug screening. Bioact. Mater. 2020;6(1):169–178. doi: 10.1016/j.bioactmat.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jahn P., Karger R.K., Soso Khalaf S., Hamad S., Peinkofer G., Sahito R.G.A., Pieroth S., Nitsche F., Lu J., Derichsweiler D., Brockmeier K., Hescheler J., Schmidt A M., P. K. Engineering of cardiac microtissues by microfluidic cell encapsulation in thermoshrinking non-crosslinked PNIPAAm gels. Biofabrication. 2022;14(3) doi: 10.1088/1758-5090/ac73b5. [DOI] [PubMed] [Google Scholar]
- 38.Musah S., Dimitrakakis N., Camacho D.M., Church G.M., de I. Directed differentiation of human induced pluripotent stem cells into mature kidney podocytes and establishment of a Glomerulus Chip. Nat. Protoc. 2018;13(7):1662–1685. doi: 10.1038/s41596-018-0007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nascimento-Gonçalves E., Ferreira R., Oliveira P.A., Bja C. An overview of current alternative models for use in the context of prostate cancer research. Altern Lab Anim. 2020;48(2):58–69. doi: 10.1177/0261192920929701. [DOI] [PubMed] [Google Scholar]
- 40.Puryear J.R., Iii, Yoon Jk K.Y. Advanced fabrication techniques of microengineered physiological systems. Micromachines. 2020;11(8):730. doi: 10.3390/mi11080730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ashammakhi N., Wesseling-Perry K., Hasan A., Elkhammas E., Ys Z. Kidney-on-a-chip: untapped opportunities. Kidney Int. 2018;94(6):1073–1086. doi: 10.1016/j.kint.2018.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Novak R., Ingram M., Marquez S., al e. Robotic fluidic coupling and interrogation of multiple vascularized organ chips. Nat Biomed Eng. 2020;4(4):407–420. doi: 10.1038/s41551-019-0497-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gokaltun A., Yarmush M.L., Asatekin A., U. OB. Recent advances in nonbiofouling PDMS surface modification strategies applicable to microfluidic technology, Technology. Singap World Sci). 2017;5(1):1–12. doi: 10.1142/S2339547817300013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ren K., Zhou J W.H. Materials for microfluidic chip fabrication. Acc. Chem. Res. 2013;46(11):2396–2406. doi: 10.1021/ar300314s. [DOI] [PubMed] [Google Scholar]
- 45.Bhattacharjee N., Urrios A., Kang S., F. A. The upcoming 3D-printing revolution in microfluidics. Lab Chip. 2016;16(10):1720–1742. doi: 10.1039/c6lc00163g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campbell S.B., Wu Q., Yazbeck J., Liu C., Okhovatian S R.M. Beyond Polydimethylsiloxane: alternative materials for fabrication of organ-on-a-chip devices and microphysiological systems. ACS Biomater. Sci. Eng. 2021;7(7):2880–2899. doi: 10.1021/acsbiomaterials.0c00640. [DOI] [PubMed] [Google Scholar]
- 47.Tiong H.Y., Huang P., Xiong S., Li Y., Vathsala A., Z. D Drug-induced nephrotoxicity: clinical impact and preclinical in vitro models. Mol. Pharm. 2014;11(7):1933–1948. doi: 10.1021/mp400720w. [DOI] [PubMed] [Google Scholar]
- 48.Choucha-Snouber L., Aninat C., Grsicom L., Madalinski G., Brochot C., Poleni P.E., Razan F., Guillouzo C.G., Legallais C., Corlu A., L. E Investigation of ifosfamide nephrotoxicity induced in a liver-kidney co-culture biochip. Biotechnol. Bioeng. 2013;110(2):597–608. doi: 10.1002/bit.24707. [DOI] [PubMed] [Google Scholar]
- 49.Xu X.D., Shao S.X., Cao Y.W., Yang X.C., Shi H.Q., Wang Y.L., Xue S.Y., Wang Xs N.H.T. The study of energy metabolism in bladder cancer cells in co-culture conditions using a microfluidic chip. Int. J. Clin. Exp. Med. 2015;8 [PMC free article] [PubMed] [Google Scholar]
- 50.Liu P.F., Cao Y.W., Zhang S.D., Zhao Y., Liu X.G., Shi H.Q., Hu K.Y., Zhu G.Q., Ma B., Ht N. A bladder cancer microenvironment simulation system based on a microfluidic co-culture model. Oncotarget. 2015;6:37695–37705. doi: 10.18632/oncotarget.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Au A.K., Huynh W., Horowitz L.F., Folch A. 3D-printed microfluidics. Angew. Chem. Int. Ed. 2016;55:3862–3881. doi: 10.1002/anie.201504382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vedula E.M., Alonso J.L., Arnaout M.A., Jl C. A microfluidic renal proximal tubule with active reabsorptive function. PLoS One. 2017;12(10) doi: 10.1371/journal.pone.0184330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jang K.-J., Mehr A.P., Hamilton G.A., McPartlin L.A., Chung S., Suh K.-Y., Ingber D.E. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment, Integr. Biol. 2015;5:1119. doi: 10.1039/c3ib40049b. [DOI] [PubMed] [Google Scholar]
- 54.LeBleu V.S., Macdonald B K.R. Structure and function of basement membranes. Exp. Biol. Med. 2007;232(9):1121–1129. doi: 10.3181/0703-MR-72. [DOI] [PubMed] [Google Scholar]
- 55.Erickson Ac C., JR. Still more complexity in mammalian basement membranes. J. Histochem. Cytochem. 2000;48(10):1291–1306. doi: 10.1177/002215540004801001. [DOI] [PubMed] [Google Scholar]
- 56.Slater S.C., Beachley V., Hayes T., Zhang D., Welsh G.I., Saleem M.A., Mathieson P.W., Wen X., Su B., Sc S. An in vitro model of the glomerular capillary wall using electrospun collagen nanofibres in a bioartificial composite basement membrane. PLoS One. 2011;6(6) doi: 10.1371/journal.pone.0020802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen T.H., Chen J.S., Ko Y.C., Chen J.W., Chu H.Y., Lu C.S., Chu C.W., Hsu H.H., Fg T. A microfluidic platform for investigating transmembrane pressure-induced glomerular leakage. Micromachines. 2018;9(5):228. doi: 10.3390/mi9050228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schophuizen C.M., De Napoli I.E., Jansen J., Teixeira S., Wilmer M.J., Hoenderop J.G., Van Den Heuvel L.P., Masereeuw R., Stamatialis D. Development of a living membrane comprising a functional human renal proximal tubule cell monolayer on polyethersulfone polymeric membrane. Acta Biomater. 2015;14:22–32. doi: 10.1016/j.actbio.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 59.Kerr S.C., Morgan M.M., Gillette A.A., Livingston M.K., Lugo-Cintron K.M., Favreau P.F., Florek L., Johnson B.P., Lang J.M., Skala M.C., B. DJ A bioengineered organotypic prostate model for the study of tumor microenvironment-induced immune cell activation. Integr Biol (Camb). 2020;12(10):250–262. doi: 10.1093/intbio/zyaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang L., Tao T., Su W., Yu H., Yu Y Q.J. A disease model of diabetic nephropathy in a glomerulus-on-a-chip microdevice. Lab Chip. 2017;17(10):1749–1760. doi: 10.1039/c7lc00134g. [DOI] [PubMed] [Google Scholar]
- 61.Moll S., Ebeling M., Weibel F., Farina A., Araujo Del Rosario A., Hoflack J.C., Pomposiello S., Prunotto M. Epithelial cells as active player in fibrosis: findings from an in vitro model. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0056575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shen C., Meng Q., Z. G. Increased curvature of hollow fiber membranes could up-regulate differential functions of renal tubular cell layers. Biotechnol. Bioeng. 2013;110(8):2173–2183. doi: 10.1002/bit.24874. [DOI] [PubMed] [Google Scholar]
- 63.Mu X., Zheng W., Xiao L., Zhang W., J. X. Engineering a 3D vascular network in hydrogel for mimicking a nephron. Lab Chip. 2013;13(8):1612–1618. doi: 10.1039/c3lc41342j. [DOI] [PubMed] [Google Scholar]
- 64.Ng C.P., Zhuang Y., Lin A.W.H., Teo J.C.M. A fibrin-based tissue-engineered renal proximal tubule for bioartificial kidney devices: development, characterization and in vitro transport study. Int. J. Tissue Eng 2013. 2013;1–10 [Google Scholar]
- 65.Jiang L., Ivich F., Tahsin S., Tran M., Frank S.B., Miranti C.K., Z. Y. Human stroma and epithelium co-culture in a microfluidic model of a human prostate gland. Biomicrofluidics. 2019;13(6) doi: 10.1063/1.5126714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Byrne M.B., Trump L., Desai A.V., Schook L.B., Gaskins Hr K.P.J. Microfluidic platform for the study of intercellular communication via soluble factor-cell and cell-cell paracrine signaling. Biomicrofluidics. 2014;8(4) doi: 10.1063/1.4887098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu J., Berthier E., Craig A., de Groot T.E., Sparks S., Ingram P.N., Jarrard D.F., Huang W., Beebe D.J., Ab T. Reconfigurable open microfluidics for studying the spatiotemporal dynamics of paracrine signalling. Nat Biomed Eng. 2019;3(10):830–841. doi: 10.1038/s41551-019-0421-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vormann M.K., Tool L.M., Ohbuchi M., Gijzen L., van Vught R., Hankemeier T., Kiyonaga F., Kawabe T., Goto T., Fujimori A., Vulto P., Lanz H.L., T. K Modelling and prevention of acute kidney injury through ischemia and reperfusion in a combined human renal proximal tubule/blood vessel-on-a-chip. Kidney. 2021;3(2):217–231. doi: 10.34067/KID.0003622021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wei Z., Amponsah P.K., Al-Shatti M., Nie Z., Bandyopadhyay B.C. Engineering of polarized tubular structures in a microfluidic device to study calcium phosphate stone formation. Lab Chip. 2012;12(20):4037–4040. doi: 10.1039/c2lc40801e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang H.C., Chang Y.J., Chen W.C., Harn H.I., Tang M.J., W. CC Enhancement of renal epithelial cell functions through microfluidic-based coculture with adipose-derived stem cells. Tissue Eng. 2013;19(17):2024–2034. doi: 10.1089/ten.tea.2012.0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frohlich E.M., Zhang X., Jl C. The use of controlled surface topography and flow-induced shear stress to influence renal epithelial cell function. Integr Biol (Camb). 2012;4(1):75–83. doi: 10.1039/c1ib00096a. [DOI] [PubMed] [Google Scholar]
- 72.Xu Y., Qin S., Niu Y., Gong T., Zhang Z., F. Y. Effect of fluid shear stress on the internalization of kidney-targeted delivery systems in renal tubular epithelial cells. Acta Pharm. Sin. B. 2020;10(4):680–692. doi: 10.1016/j.apsb.2019.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kimura H., Nishikawa M., Yanagawa N., Nakamura H., Miyamoto S., Hamon M., Hauser P., Zhao L., Jo O.D., Komeya M., Ogawa T., Y. N. Effect of fluid shear stress on in vitro cultured ureteric bud cells. Biomicrofluidics. 2018;12(4) doi: 10.1063/1.5035328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mosayyebi A., Yue Q.Y., Somani B.K., Zhang X., Manes C., C. D Particle accumulation in ureteral stents is governed by fluid dynamics: in vitro study using a "Stent-on-Chip" model. J. Endourol. 2018;32(7):639–646. doi: 10.1089/end.2017.0946. [DOI] [PubMed] [Google Scholar]
- 75.De Grazia A., LuTheryn G., Meghdadi A., Mosayyebi A., Espinosa-Ortiz E.J., Gerlach R., C. D A microfluidic-based investigation of bacterial attachment in ureteral stents. Micromachines. 2020;11(4):408. doi: 10.3390/mi11040408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hofmann M., Guschel M., Bernd A., Bereiter-Hahn J., Kaufmann R., Tandi C., Wiig H.K.S. Lowering of tumor interstitial fluid pressure reduces tumor cell proliferation in a xenograft tumor model. Neoplasia. 2006;8:89–95. doi: 10.1593/neo.05469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boardman K.C., S. M.A. Interstitial flow as a guide for lymphangiogenesis. Circ. Res. 2003;92:801–808. doi: 10.1161/01.RES.0000065621.69843.49. [DOI] [PubMed] [Google Scholar]
- 78.Homan K.A., Gupta N., Kroll K.T., Kolesky D.B., Skylar-Scott M., Miyoshi T., Mau D., Valerius M.T., Ferrante T., B. J.V Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat. Methods. 2019;16:255–262. doi: 10.1038/s41592-019-0325-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim M., Jang H., P. Y. Study on the expansion dynamics of MDCK epithelium by interstitial flow using a traction force-measurable microfluidic chip. Materials. 2021;14(4):935. doi: 10.3390/ma14040935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pollak M.R., Quaggin S.E., Hoenig Mp D.L.D. The glomerulus: the sphere of influence. Clin. J. Am. Soc. Nephrol. 2014;9(8):1461–1469. doi: 10.2215/CJN.09400913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou M., Zhang X., Wen X., Wu T., Wang W., Yang M., Wang J., Fang M., Lin B., L. H Development of a functional glomerulus at the organ level on a chip to mimic hypertensive nephropathy. Sci. Rep. 2016;6 doi: 10.1038/srep31771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Musah S., Mammoto A., Ferrante T.C., Jeanty S.S.F., Hirano-Kobayashi M., Mammoto T., Roberts K., Chung S., Novak R., Ingram M., Fatanat-Didar T., Koshy S., Weaver J.C., Church G.M., de I. Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat Biomed Eng. 2017;1:69. doi: 10.1038/s41551-017-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sharma K., Dhar N., Thacker V.V., Simonet T.M., Signorino-Gelo F., Knott G.W., Jd M. Dynamic persistence of UPEC intracellular bacterial communities in a human bladder-chip model of urinary tract infection. Elife. 2021;10 doi: 10.7554/eLife.66481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ao M., Brewer B.M., Yang L., Franco Coronel O.E., Hayward S.W., Webb D.J., L. D Stretching fibroblasts remodels fibronectin and alters cancer cell migration. Sci. Rep. 2015;5:8334. doi: 10.1038/srep08334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bettinger C.J., Langer R., B. JT. Engineering substrate topography at the micro- and nanoscale to control cell function. Angew Chem. Int. Ed. Engl. 2009;48(30):5406–5415. doi: 10.1002/anie.200805179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yim Ek L.K.W. Significance of synthetic nanostructures in dictating cellular response. Nanomedicine. 2005;1(1):10–21. doi: 10.1016/j.nano.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 87.Dalby M.J., Gadegaard N., Tare R., Andar A., Riehle M.O., Herzyk P., Wilkinson C.D., Ro O. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater. 2007;6(12):997–1003. doi: 10.1038/nmat2013. [DOI] [PubMed] [Google Scholar]
- 88.Y. PD. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harbor Perspect. Biol. 2011;3(2) doi: 10.1101/cshperspect.a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ferrell N., Groszek J., Li L., Smith R., Butler R.S., Zorman C.A., Roy S., F. WH Basal lamina secreted by MDCK cells has size- and charge-selective properties. Am. J. Physiol. Ren. Physiol. 2011;300(1):F86–F90. doi: 10.1152/ajprenal.00484.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Teixeira A.I., Abrams G.A., Bertics P.J., Murphy C.J., Pf N. Epithelial contact guidance on well-defined micro- and nanostructured substrates. J. Cell Sci. 2003;116(10):1881–1892. doi: 10.1242/jcs.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Frohlich E.M., Alonso J.L., Borenstein J.T., Zhang X., Arnaout M.A., Jl C. Topographically-patterned porous membranes in a microfluidic device as an in vitro model of renal reabsorptive barriers. Lab Chip. 2013;13(12):2311–2319. doi: 10.1039/c3lc50199j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xie R., Korolj A., Liu C., Song X., Lu R.X.Z., Zhang B., Ramachandran A., Liang Q., R. M. H-FIBER: microfluidic topographical hollow fiber for studies of glomerular filtration barrier. ACS Cent. Sci. 2020;6(6):903–912. doi: 10.1021/acscentsci.9b01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bruns J., Egan T., Mercier P., Sp Z. Glioblastoma spheroid growth and chemotherapeutic responses in single and dual-stiffness hydrogels. Acta Biomater. 2022;(22):S1742–S7061. doi: 10.1016/j.actbio.2022.05.048. 00324-5. [DOI] [PubMed] [Google Scholar]
- 94.Afthinos A., Bera K., Chen J., Ozcelikkale A., Amitrano A., Choudhury M.I., Huang R., Pachidis P., Mistriotis P., Chen Y K.K. Migration and 3D traction force measurements inside compliant microchannels. Nano Lett. 2022;22(18) doi: 10.1021/acs.nanolett.2c01261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jiang S., Alisafaei F., Huang Y.Y., Hong Y., Peng X., Qu C., Puapatanakul P., Jain S., Miner J.H., Genin G.M., Hy S. An ex vivo culture model of kidney podocyte injury reveals mechanosensitive, synaptopodin-templating, sarcomere-like structures. Sci. Adv. 2022;8(35) doi: 10.1126/sciadv.abn6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wyss H.M., Henderson J.M., Byfield F.J., Bruggeman L.A., Ding Y., Huang C., Suh J.H., Franke T., Mele E., Pollak M.R., Miner J.H., Janmey P.A., Weitz D.A., Rt M. Biophysical properties of normal and diseased renal glomeruli. Am. J. Physiol. Cell Physiol. 2011;300:C397–C405. doi: 10.1152/ajpcell.00438.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Janmey P.A., M. R.T. Mechanisms of mechanical signaling in development and disease. J. Cell Sci. 2011;124:9–18. doi: 10.1242/jcs.071001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Embry A.E., Liu Z., Henderson J.M., Byfield F.J., Liu L., Yoon J., Wu Z., Cruz K., Moradi S., G. C.B. Similar biophysical abnormalities in glomeruli and podocytes from two distinct models. J. Am. Soc. Nephrol. 2018;29:1501–1512. doi: 10.1681/ASN.2017050475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Osório L.A., Silva E.M.R.E. A review of biomaterials and scaffold fabrication for organ-on-a-chip (OOAC) systems. Bioengineering (Basel). 2021;8:113. doi: 10.3390/bioengineering8080113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu H., Wang Y., G.Y. Cui K., Zhang X., Q. J. Advances in hydrogels in organoids and organs-on-a-chip. Adv. Mater. 2019;31 doi: 10.1002/adma.201902042. [DOI] [PubMed] [Google Scholar]
- 101.Garcia S., Sunyer R., Olivares A., Noailly J., Atencia J., T. X. Generation of stable orthogonal gradients of chemical concentration and substrate stiffness in a microfluidic device. Lab Chip. 2015:2606–2614. doi: 10.1039/c5lc00140d. [DOI] [PubMed] [Google Scholar]
- 102.Shih H.C., Lee T.A., Wu H.M., Ko P.L., Liao W.H., Yc T. Microfluidic collective cell migration assay for study of endothelial cell proliferation and migration under combinations of oxygen gradients, tensions, and drug treatments. Sci. Rep. 2019;9(1):8234. doi: 10.1038/s41598-019-44594-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Doherty M., Wang T., Lamprou D.A., Ja C. Microfluidic technologies in tumour metabolism. Int. J. Pharm. 2022;629 doi: 10.1016/j.ijpharm.2022.122370. [DOI] [PubMed] [Google Scholar]
- 104.Xu X.D., Shao S.X., Cao Y.W., Yang X.C., Shi H.Q., Wang Y.L., Xue S.Y., Wang Xs N.H.T. The study of energy metabolism in bladder cancer cells in co-culture conditions using a microfluidic chip. Int. J. Clin. Exp. Med. 2015;8(8):12327–12336. [PMC free article] [PubMed] [Google Scholar]
- 105.Shi H., Jiang H., Wang L., Cao Y., Liu P., Xu X., Wang Y., Sun L., N. H. Overexpression of monocarboxylate anion transporter 1 and 4 in T24-induced cancer-associated fibroblasts regulates the progression of bladder cancer cells in a 3D microfluidic device. Cell Cycle. 2015;14(19):3058–3065. doi: 10.1080/15384101.2015.1053666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu P.F., Cao Y.W., Zhang S.D., Zhao Y., Liu X.G., Shi H.Q., Hu K.Y., Zhu G.Q., Ma B., Ht N. A bladder cancer microenvironment simulation system based on a microfluidic co-culture model. Oncotarget. 2015;6(35):37695–37705. doi: 10.18632/oncotarget.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hsiao A.Y., Torisawa Y.S., Tung Y.C., Sud S., Taichman R.S., Pienta K.J., T. S Microfluidic system for formation of PC-3 prostate cancer co-culture spheroids. Biomaterials. 2009;30(16):3020–3027. doi: 10.1016/j.biomaterials.2009.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hanahan D., W. RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 109.Chethikkattuveli Salih A.R., Asif A., Samantasinghar A., Umer Farooqi H.M., Kim S., Kh C. Renal hypoxic reperfusion injury-on-chip model for studying combinational vitamin therapy. ACS Biomater. Sci. Eng. 2022;8(9):3733–3740. doi: 10.1021/acsbiomaterials.2c00180. [DOI] [PubMed] [Google Scholar]
- 110.Liu A., Wang X., Hu X., Deng Y., Wen X., Lin B., Zhou M., Wang W., Luo Y., Deng J., Tang Q., Du X., Qin B., Song H., L. H. Core fucosylation involvement in the paracrine regulation of proteinuria-induced renal interstitial fibrosis evaluated with the use of a microfluidic chip. Acta Biomater. 2022;142:99–112. doi: 10.1016/j.actbio.2022.02.020. [DOI] [PubMed] [Google Scholar]
- 111.Kim S., Takayama S. Organ-on-a-chip and the kidney. Kidney Res. Clin. Pract. 2015;34(3):165–169. doi: 10.1016/j.krcp.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim S., LesherPerez S.C., Kim B.C., Yamanishi C., Labuz J.M., Leung B., T. S. Pharmacokinetic profile that reduces nephrotoxicity of gentamicin in a perfused kidney-on-a-chip. Biofabrication. 2016;8(1) doi: 10.1088/1758-5090/8/1/015021. [DOI] [PubMed] [Google Scholar]
- 113.Qu Y., An F., Luo Y., Lu Y., Liu T., Zhao W., L. B. A nephron model for study of drug-induced acute kidney injury and assessment of drug-induced nephrotoxicity. Biomaterials. 2018;155:41–53. doi: 10.1016/j.biomaterials.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 114.Li Z., Jiang L., Tao T., Su W., Guo Y., Yu H Q.J. Assessment of cadmium-induced nephrotoxicity using a kidney-on-a-chip device. Toxicol. Res. 2017;6(3):372–380. doi: 10.1039/c6tx00417b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shintu L., Baudoin R., Navratil V., Prot J.M., Pontoizeau C., Defernez M., Blaise B.J., Domange C., Péry A.R., Toulhoat P., Legallais C., Brochot C., Leclerc E., Me D. Metabolomics-on-a-chip and predictive systems toxicology in microfluidic bioartificial organs. Anal. Chem. 2012;84(4):1840–1848. doi: 10.1021/ac2011075. [DOI] [PubMed] [Google Scholar]
- 116.Li Z., Su W., Zhu Y., Tao T., Li D., Peng X., Q. J. Drug absorption related nephrotoxicity assessment on an intestine-kidney chip. Biomicrofluidics. 2017;11(3) doi: 10.1063/1.4984768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Maschmeyer I., Lorenz A.K., Schimek K., Hasenberg T., Ramme A.P., Hübner J., Lindner M., Drewell C., Bauer S., Thomas A., Sambo N.S., Sonntag F., Lauster R., M. U. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip. 2015;15(12):2688–2699. doi: 10.1039/c5lc00392j. [DOI] [PubMed] [Google Scholar]
- 118.Kinstlinger I.S., Miller J.S. 3D-printed fluidic networks as vasculature for engineered tissue. Lab Chip. 2016;16(11):2025–2043. doi: 10.1039/c6lc00193a. [DOI] [PubMed] [Google Scholar]
- 119.Zhang C., Zhao Z., Abdul R., N.A. Van Noort D., Yu H. Towards a human-on-chip: culturing multiple cell types on a chip with compartmentalized microenvironments. Lab Chip. 2009;9(22):3185–3192. doi: 10.1039/b915147h. [DOI] [PubMed] [Google Scholar]
- 120.Chang S.Y., Weber E.J., Sidorenko V.S., Chapron A., Yeung C.K., Gao C., Mao Q., Shen D., Wang J., Rosenquist T.A., Dickman K.G., Neumann T., Grollman A.P., Kelly E.J., Himmelfarb J., Dl E. Human liver-kidney model elucidates the mechanisms of Aristolochia acid nephrotoxicity. JCI Insight. 2017;2(22) doi: 10.1172/jci.insight.95978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Szymanski P., Markowicz M., Mikiciuk-Olasik E. Adaptation of high-throughput screening in drug discovery-toxicological screening tests. Int. J. Mol. Sci. 2012;13(1):427–452. doi: 10.3390/ijms13010427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shaughnessey E.M., Kann S.H., Azizgolshani H., Black L.D., Charest J.L., V. EM. Evaluation of rapid transepithelial electrical resistance (TEER) measurement as a metric of kidney toxicity in a high-throughput microfluidic culture system. Sci. Rep. 2022;12(1) doi: 10.1038/s41598-022-16590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jing B., Yan L., Li J., Luo P., Ai X., T. P. Functional evaluation and nephrotoxicity assessment of human renal proximal tubule cells on a chip. Biosensors. 2022;12(9):718. doi: 10.3390/bios12090718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vormann M.K., Vriend J., Lanz H.L., Gijzen L v., an den Heuvel A., Hutter S., Joore J., Trietsch S.J., Stuut C., Nieskens T.T.G., Peters J.G.P., Ramp D., Caj M., Russel F.G.M., Jacobsen B., Roth A., Lu S., Polli J.W., Naidoo A.A., Vulto P., Masereeuw R., Wilmer M.J., S.-D. L Implementation of a human renal proximal tubule on a chip for nephrotoxicity and drug interaction studies. J Pharm Sci. 2021;110(4):1601–1614. doi: 10.1016/j.xphs.2021.01.028. [DOI] [PubMed] [Google Scholar]
- 125.An D., Kim K., K. J. Microfluidic system based high throughput drug screening system for curcumin/TRAIL combinational chemotherapy in human prostate cancer PC3 cells. Biomol Ther (Seoul) 2014;22(4):355–362. doi: 10.4062/biomolther.2014.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mulholland T., McAllister M., Patek S., Flint D., Underwood M., Sim A., Edwards J., Z. M. Drug screening of biopsy-derived spheroids using a self-generated microfluidic concentration gradient. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-33055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ishahak M., Hill J., Amin Q., Wubker L., Hernandez A., Mitrofanova A., Sloan A., Fornoni A., A. A. Modular microphysiological system for modeling of biologic barrier function. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.581163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sethakorn N., Heninger E., Breneman M.T., Recchia E., Ding A.B., Jarrard D.F., Hematti P., Beebe D.J., K. D. Integrated analysis of the tumor microenvironment using a reconfigurable microfluidic cell culture platform. Faseb. J. 2022;36(10) doi: 10.1096/fj.202200684RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wu Y., Zhou Y., Paul R., Qin X., Islam K., L. Y. Adaptable microfluidic vessel-on-a-chip platform for investigating tumor metastatic transport in bloodstream. Anal. Chem. 2022;94(35):12159–12166. doi: 10.1021/acs.analchem.2c02556. [DOI] [PubMed] [Google Scholar]
- 130.Azizipour N., Avazpour R., Weber M.H., Sawan M., Ajji A., R. DH. Uniform tumor spheroids on surface-optimized microfluidic biochips for reproducible drug screening and personalized medicine. Micromachines. 2022;13(4):587. doi: 10.3390/mi13040587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tan W.H., T. S. A trap-and-release integrated microfluidic system for dynamic microarray applications. Proc. Natl. Acad. Sci. U.S.A. 2007;104(4):1146–1151. doi: 10.1073/pnas.0606625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sochol R.D., Higa A.T., Janairo R.R.R., Li S., L.L. Effects of micropost spacing and stiffness on cell motility. Micro & Nano Lett. 2011;6(5):323–326. [Google Scholar]
- 133.Esch E.W., Bahinski A., H. D. Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discov. 2015;14(4):248–260. doi: 10.1038/nrd4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zeilinger K., Freyer N., Damm G., Seehofer D., K. F. Cell sources for in vitro human liver cell culture models. Exp. Biol. Med. 2016;241(15):1684–1698. doi: 10.1177/1535370216657448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kuna L., Bozic I., Kizivat T., Bojanic K., Mrso M., Kralj E., Smolic R., Wu Gy S.M. Models of drug induced liver injury (DILI) - current issues and future perspectives. Curr. Drug Metabol. 2018;19(10):830–838. doi: 10.2174/1389200219666180523095355. 2018;19(10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.N. R. Human kidney organoids: progress and remaining challenges. Nat. Rev. Nephrol. 2019;15(10):613–624. doi: 10.1038/s41581-019-0176-x. [DOI] [PubMed] [Google Scholar]
- 137.Valverde M.G., Mille L.S., Figler K.P., Cervantes E., Li V.Y., Bonventre J.V., Masereeuw R., Ys Z. Biomimetic models of the glomerulus. Nat. Rev. Nephrol. 2022;18(4):241–257. doi: 10.1038/s41581-021-00528-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Homan K.A., Gupta N., Kroll K.T., Kolesky D.B., Skylar-Scott M., Miyoshi T., Mau D., Valerius M.T., Ferrante T., Bonventre J.V., Lewis Ja M.R. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat. Methods. 2019;16(3):255–262. doi: 10.1038/s41592-019-0325-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang Y., Chen H., L. J. Recent advances on gelatin methacrylate hydrogels with controlled microstructures for tissue engineering. Int. J. Biol. Macromol. 2022;221:91–107. doi: 10.1016/j.ijbiomac.2022.08.171. [DOI] [PubMed] [Google Scholar]
- 140.Murphy Sv A.A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014;32(8):773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 141.Jang J., Yi H.G., Dw C. 3D printed tissue models: present and future. ACS Biomater. Sci. Eng. 2016;2(10):1724. doi: 10.1021/acsbiomaterials.6b00129. [DOI] [PubMed] [Google Scholar]
- 142.Gungor-Ozkerim P.S., Inci I., Zhang Y.S., Khademhosseini A., D. MR. Bioinks for 3D bioprinting: an overview. Biomater. Sci. 2018;6(5):915–946. doi: 10.1039/c7bm00765e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.