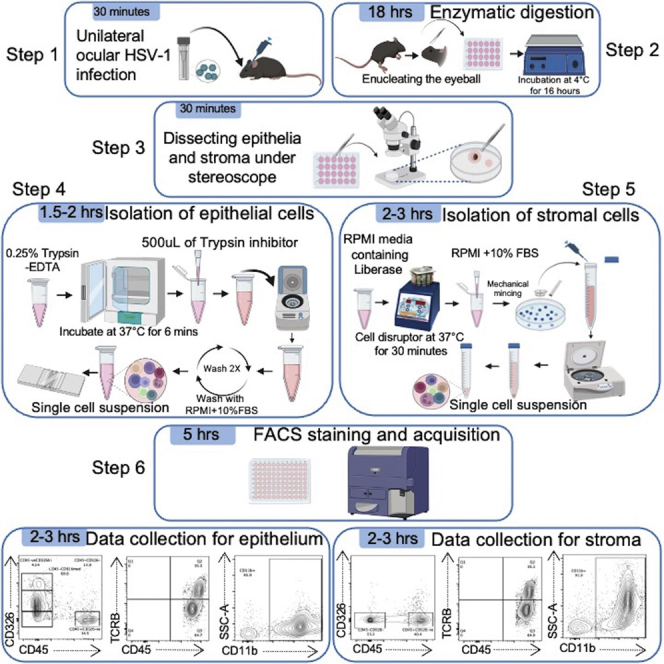
Summary
Existing flow cytometry approaches identify immune cells using the whole infected/inflamed cornea, which limits its ability to distinguish the immune cells infiltrating the corneal epithelium from the corneal stroma. Here, we present a protocol to analyze immune cells in the separated epithelium and stroma from naïve and herpes simplex virus-1 (HSV-1)-infected mouse corneas. We describe steps for viral infection, separation of corneal epithelium from stroma, preparation of a single-cell suspension of the individual epithelium and stroma, and flow cytometry assay.
Subject areas: Flow Cytometry/Mass Cytometry, Health Sciences, Immunology, Microbiology
Graphical abstract
Highlights
-
•
Separation of intact epithelium and stroma from naïve and HSV-1-infected mouse corneas
-
•
Preparation of single cell suspension from the epithelium and stroma
-
•
Flow cytometry to enumerate immune cells in the individual corneal epithelium and stroma
-
•
The protocol can be applied to other corneal inflammation models
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Existing flow cytometry approaches identify immune cells using the whole infected/inflamed cornea, which limits ability to distinguish the immune cells infiltrating the corneal epithelium from the corneal stroma. Here, we present a protocol to analyze immune cells in the separated epithelium and stroma from naïve and herpes simplex virus-1 (HSV-1)-infected mouse corneas. We describe steps for viral infection, separation of corneal epithelium from stroma, preparation of a single-cell suspension of the individual epithelium and stroma, and flow cytometry assay.
Before you begin
Mice handling and biohazard training
Before working on this protocol, the individuals must have received the appropriate training for mice handling, intraperitoneal injection of the mouse, and safety training for proper handling and disposal of the hazardous biological materials.
The protocol below shows the separation of intact epithelium and stroma from an individual mouse cornea with herpes stromal keratitis (HSK) lesion and the preparation of a single cell suspension of separated epithelium and stroma for flow cytometry. We have also used this method to separate the corneal epithelium from uninfected corneas and prepared the single-cell suspension described in detail in step-by-step method details.
Institutional permissions
All experimental procedures described below are reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Wayne State University.
Preparation on the day of corneal HSV-1 infection
Timing: 30 min
-
1.
Prepare Anesthesia cocktail prior to mouse corneal HVS-1 infection as described under materials and equipment.
-
2.
Use stock concentration of 100 mg/mL of Ketamine and 20 mg/mL of Xylazine to prepare the cocktail.
-
3.
Prepare the cocktail in DPBS.
CRITICAL: Keep DPBS at 37°C for 30 min before preparation.
-
4.
For each mouse, add 20 μL of Ketamine HCl and 6.6 μL of Xylazine in 173.4 μL of PBS. Use 200 μL of the cocktail solution to anesthetize one mouse.
CRITICAL: The final concentrations of Ketamine and Xylazine in a single intraperitoneal injection are 10 mg/mL and 0.66 mg/mL, respectively. Scale up the volume accordingly when more than one mouse is being anesthetized.
Note: We usually work with 8–12 weeks old female mice weighing 20g. Our dosage is 0.01 mL/g. 200 μL of the cocktail solution is injected to anesthetize one mouse weighing 20g.
If using a 25g mouse, 250uL of the cocktail solution should effectively anesthetize the mouse.
-
5.
Prepare sterile microisolator mouse cages. Use 1 cage for maximum of 5 mice.
Preparation on the day of mice euthanasia
Timing: 1 h
-
6.Prepare the following solutions as described under materials and equipment.
-
a.Dispase® II digestion media: One eyeball requires 1 mL of digestion media.Once prepared, dispase digestion media can be stored at 4°C for 1 h.
-
b.Complete supplemented RPMI media with 10% FBS: Store the media at 4°C for maximum of one month.
-
c.FACS Buffer: It can be stored at 4°C for one month.
 CRITICAL: Prepare all working solutions under sterile conditions in a biosafety cabinet.
CRITICAL: Prepare all working solutions under sterile conditions in a biosafety cabinet.
-
a.
Preparation on the day of FACS staining
Timing: 1–1.5 h
-
7.Prepare the stroma digestion media and the following working solutions on the day of FACS staining as described under materials and equipment.
-
a.0.25% Trypsin EDTA.
-
b.Soybean Trypsin Inhibitor.
-
a.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| APC/Cyanine7 anti-mouse CD326 (Ep-CAM) (1:100) | BioLegend | Clone G8.8, Cat# 118218; RRID: AB_2098648 |
| BV605 Rat Anti-mouse CD45 (1:100) | BD Biosciences | Clone 30-F11, Cat# 563053; RRID: AB_2737976 |
| V450 Hamster Anti-mouse TCR- β chain (1:100) | BD Biosciences | Clone H57-597, Cat# 560706; RRID: AB_1727576 |
| PerCP-CyTM5.5 Rat Anti-CD11b (1:100) | BD Biosciences | Clone M1/70, Cat# 550993; RRID: AB_394002 |
| PE-CyTM7 Rat Anti-Mouse Ly-6G (1:100) | BD Biosciences | Clone 1A8, Cat# 560601; RRID: AB_1727562 |
| Alexa Fluor® 700 Rat Anti-Mouse Ly-6C (1:100) | BD Biosciences | Clone AL-21, Cat# 561237; RRID: AB_10612017 |
| PE-CF594 Rat Anti-Mouse CD31 (1:100) | BD Biosciences | Clone MEC 13.3, Cat# 563616; RRID: AB_2738320 |
| Rat anti-mouse CD16/32 (Fc block) (1:100) | BD Biosciences | Clone 2.4G2, Cat# 553142; RRID: AB_394656 |
| Bacterial and virus strains | ||
| Herpes simplex virus-1 (McKrae) strain | Rana et al.1 | Available upon request |
| Chemicals, peptides, and recombinant proteins | ||
| DMEM/F-12 | Thermo Fisher Scientific | Cat# 11320033 |
| Dispase® II (neutral protease, grade II) | MilliporeSigma | Cat# 04942078001 |
| HEPES Buffered Saline Solution | MilliporeSigma | Cat# C-40020 |
| D-sorbitol | MilliporeSigma | Cat# S3889 |
| Antibiotic/ Antimycotic | Thermo Fisher Scientific | Cat# 15240062 |
| PBS, pH 7.2 | Thermo Fisher Scientific | Cat# 20012050 |
| Trypsin-EDTA (0.25%), phenol red | Thermo Fisher Scientific | Cat# 25200056 |
| Trypsin Inhibitor from soybean | MilliporeSigma | Cat# C10109886001 |
| DPBS, calcium, magnesium | Thermo Fisher Scientific | Cat# 14040133 |
| Liberase™ TL Research Grade | Sigma-Aldrich | Cat# 5401020001 |
| RPMI 1640 medium, HEPES | Thermo Fisher Scientific | Cat# 2240001 |
| Fetal Bovine Serum | Thermo Fisher Scientific | Cat# A47688-01 |
| L-Glutamine solution, 200mM | Sigma-Aldrich | Cat# G7513 |
| Penicillin-Streptomycin | Sigma-Aldrich | Cat# P0781 |
| Sodium Pyruvate solution, 100mM | Sigma-Aldrich | Cat# S8636 |
| Sodium Bicarbonate (Powder/Certified ACS) | Fisher scientific | Cat# S233 |
| 2-Mercaptoethanol | Sigma-Aldrich | Cat# M3148 |
| Ketathesia-Ketamine hydrochloride injection | Henry Schein Animal Health | NDC code: 11695-0701-1 |
| Xylazine Sterile Solution (20 mg/mL) | Animal Health International | Cat# 20101547 Mfr# 59399-0110-20 |
| Critical commercial assays | ||
| LIVE/DEAD™ Fixable Aqua Dead Cell Stain Kit for 405 nm excitation | Invitrogen | Cat# L34966 |
| UltraComp eBeads™ Compensation Beads | Invitrogen | Cat# 01-2222-42 |
| Experimental models: Organisms/strains | ||
| Mouse: C57BL/6J (female), 8–12 weeks old | The Jackson Laboratory | Stock# 000664 |
| Software and algorithms | ||
| FlowJo Version v10.4 | Tree Star | https://www.flowjo.com |
| BD FACSDiva Software | BD Biosciences | https://www.bdbiosciences.com/enus/products/software/instrument-software/bd-facsdiva-software |
| Other | ||
| 50 mL tubes | Corning | Cat# 352098 |
| 15 mL tubes | Corning | Cat# 352099 |
| 1.7 mL tubes | Thomas Scientific | Cat# 1149K01 |
| 1.5 mL tubes | Fisher Scientific | Cat# 05-408-129 |
| 0.22 μm syringe filters | Fisher Scientific | Cat# 09-720-004 |
| 3 mL syringes | Fisher Scientific | Cat# 14-823-435 |
| 10 mL syringes | Fisher Scientific | Cat# 14-823-2A |
| 24-well plates | Corning | Ref# 3524 |
| 96-well U-bottom FACS plates | Corning | Ref# 353077 |
| Medium Petri dish | Corning | Ref# 351007 |
| 27G (1/2 in.) needle | BD | Cat# 305109 |
| Cell strainers 70 μm | Fisher scientific | Cat# 22363548 |
| Curved forceps | Integra Miltex | Cat# 18-782 |
| Straight forceps | Integra Miltex | Cat# 18-780 |
| Castroviejo scissors | Integra Miltex | Cat# 18-1576 |
| Eppendorf 5424/5424R centrifuge | Eppendorf | Rotor Cat# FA-45-24-11 |
| Eppendorf 5810R centrifuge | Eppendorf | Rotor Cat# S-4-104 |
| Inverted microscope | Leica DMi1 | N/A |
| Stereo microscope | Leica S8APO | N/A |
| Slit lamp microscope | Kowa SL-15 | N/A |
| Orbital shaker | Fisher scientific | N/A |
| Disruptor Genie | Scientific Industries | N/A |
| Incubator (Heratherm™) | Thermo Fisher Scientific | N/A |
| CO2 incubator (Heracell™ 150i) | Thermo Fisher Scientific | N/A |
| Class II Biosafety Cabinet | Labconco | N/A |
| BD LSRFortessa flow cytometer | BD Biosciences | N/A |
Materials and equipment
Flow cytometer
A 14 color (5-Blue, 3-Red, and 6-Violet) BD LSRFortessa flow cytometer was used to acquire the fluorochrome stained cell samples. The configuration of the flow cytometer is as follows: 5 channels for Blue 488 nm laser (Alexa Fluor 488, PerCP-Cy5.5, PE, PE-CF594, and PE-Cy7), 3 channels for the Red 640 nm laser (APC-H7, APC, and Alexa Fluor 700), 6 channels for the Violet 405 nm laser (Brilliant Violet 605, Qdot 565, Brilliant Violet 421, Brilliant Violet 510, Brilliant Violet 650, and Qdot 585).
Anesthesia cocktail
| Reagent | Final concentration | Amount |
|---|---|---|
| Ketamine HCl (100 mg/mL) | 10 mg/mL | 20 μL |
| Xylazine (20 mg/mL) | 0.66 mg/mL | 6.6 μL |
| DPBS | N/A | 173.4 μL |
| Total | N/A | 200 μL |
Note: Anesthesia cocktail should be freshly prepared prior to ocular HSV-1 infection in mice.
Dispase® II digestion media
| Reagent | Final concentration | Amount |
|---|---|---|
| DMEM F-12 | N/A | 9.1 mL |
| Dispase® II | 15 mg/mL | 750 μL |
| D-sorbitol | 100 mM | 182 mg |
| Antibiotic-Antimycotic (100X) | 1X | 100 μL |
| Total | N/A | 10 mL |
Note: Sterile filter the media using a syringe filter. Use freshly prepared Dispase II Digestion media. This media can be stored at 4°C for upto one h.
Corneal stroma digestion media
-
•
Reconstitute Liberase™ TL Research Grade with RPMI medium to get a final concentration of 2.5 mg/mL.
-
•
Add 20 μL of this media in each tube containing 200 μL of RPMI medium with dissected stromas.
CRITICAL: Do not add serum in the corneal stroma digestion media as it may affect Liberase activity.
Note: Corneal stroma digestion media should be freshly prepared prior to stroma digestion.
Working solutions
0.25% trypsin EDTA
Add 500 μL of 0.25% Trypsin EDTA for each eyeball in each of 1.7 mL ice-cold Eppendorf tubes.
Note: Thaw 0.25% Trypsin EDTA in water bath set at 37°C.
Alternative: TrypLE Express can be used as an alternative for 0.25% Trypsin EDTA.
Soybean Trypsin Inhibitor
Prepare 2 mg/mL of Soybean Trypsin Inhibitor in PBS, pH 7.2 and add 500 μL in each tube.
Note: Prepare fresh and store at 4°C for up to one h.
Complete supplemented RPMI+ 10% FBS media
| Reagent | Final concentration | Amount |
|---|---|---|
| RPMI media | N/A | 845 mL |
| L-glutamine (200 mM) | 2 mM | 10 mL |
| Penicillin-Streptomycin (100 X) | 1X | 10 mL |
| Sodium pyruvate (100 mM) | 1 mM | 10 mL |
| 7.5% Sodium Bicarbonate | 0.18% | 25 mL |
| Fetal Bovine Serum (FBS) | 10% | 100 mL |
| 2-Mercaptaethanol | N/A | 3.4 μL |
| Total | N/A | 1000 mL |
Note: FBS should be heat inactivated at 56°C for 30 min to eliminate complement activity. Filter the media using a 0.22 μm filter. Store at 4°C for up to one month.
FACS buffer
A mixture of 2% FBS in DPBS. Store at 4°C for up to one month.
Step-by-step method details
Corneal herpes simplex virus-1 (HSV-1) infection
Timing: 30 min to carry out the infection and 16-days for the development of full-blown herpes stromal keratitis after corneal HSV-1 infection
This step describes how to carry out HSV-1 infection of the mouse cornea and monitor the development of herpes stromal keratitis (HSK) until the euthanization of infected mice.
-
1.
Before carrying out the infection, inject each C57BL/6J mouse intraperitoneally with 200 μL of anesthesia cocktail as provided under Anesthesia cocktail.
-
2.
Check the toe reflex response by gently pinching the hind limb to ensure the mouse is fully anesthetized.
Note: No toe reflex response is considered as the sign of fully anesthetized mice.
-
3.
Once fully anesthetized, use ½ inch 27G needle to mildly scratch the corneal surface of the right eye in all four quadrants.
Note: Give a total of 12 scratches (three in each quadrant) as depicted in Figure 1.
CRITICAL: Too harsh scratching of the corneal surface may cause corneal epithelium debridement, failure in establishing productive viral infection in the epithelium, and the inability to develop HSK.
-
4.
Apply HSV-1 McKrae (1×103 plaque forming unit) topically in 3 μL PBS using a 0.5–10 μL pipette.
CRITICAL: During the topical application, ensure the pipette tip touches the corneal surface.
-
5.
After viral inoculation, lay down the anesthetized mice sideways in a disposable microisolator cage with infected eye facing up.
-
6.
Keep the cage with anesthetized mice on a heating pad.
Note: The warmth from the heating pad prevents inadvertent hypothermia in anesthetized mice.
-
7.
Provide the corncob bedding and a water bottle along with feed pellets in the microisolator cages following mice recovery from anesthesia (recovery time: 90–120 min).
-
8.
House infected mice in AAALAC-accredited pathogen-free animal facility.
-
9.
Monitor the development of HSK in infected eyes every other day using a hand-held slit lamp microscope under the class II Biosafety Cabinet.
Note: Assign the corneal opacity and hemangiogenesis score as reported.2
-
10.
Use the mouse with severe HSK to separate the corneal epithelium and corneal stroma followed by the preparation of a single-cell suspension for flow cytometry assay.
CRITICAL: The corneal opacity score >3.0 and angiogenesis score >12 in infected eyes is considered as severe HSK.
Figure 1.
Primary corneal HSV-1 infection
Prior to the corneal HSV-1 infection, the smoothness of the corneal surface before and after scratching at 40X magnification. Scale bars, 1mm.
Separation of corneal epithelium and single cell preparation for flow cytometry
Timing: 1.5hfor media preparation and mice euthanasia, 16 hovernight incubation- a day prior to staining
Timing: 2.5–3 h on the day of staining
This step enables the successful peeling of mouse corneal epithelial sheets and describes how to make single-cell suspensions from the individual epithelial sheet for FACS staining. See Figure 2 and Methods Video S1.
-
11.
Euthanize the mouse with overexposure to Isoflurane followed by cervical dislocation.
-
12.
Enucleate the eyeballs with curved forceps.
-
13.
Place the eyeballs in a 24-well plate containing 1 mL of Dispase® II digestion media/well as stated under materials and equipment (One well for one eyeball).
-
14.
Incubate the plate at 4°C on an orbital shaker set at orbital speed of 33 rpm for 16 h.
Pause Point: Ensure the orbital shaker stays ON during the entire incubation period.
-
15.
At the end of incubation, place the eyeball in 150–200 μL of Dispase® II digestion media in a 60 mm × 15 mm petri dish with the cornea facing sideways.
-
16.
Under the stereo microscope, peel off the epithelial sheet with straight forceps.
Note: The epithelial sheet should be peeled off without exerting excessive force. It takes around a min to peel off a single epithelial sheet.
CRITICAL: If working with more than five eyeballs, take the eyeballs out of the digestion media and dip them in 1 mL of PBS to prevent over-digestion while processing other samples.
-
17.
Carefully add individual epithelial sheet in 500 μL of 0.25% Trypsin-EDTA in 1.7mL Eppendorf tubes (already kept on ice).
-
18.
Incubate the tubes at 37°C in a CO2 incubator for 6 min.
-
19.
Pipette up and down to homogenize the solution.
CRITICAL: Proceed to the next step once all clumps are homogenized. This step is crucial for making a single-cell suspension.
-
20.
Add 500 μL of ice-cold 2 mg/mL Soybean Trypsin Inhibitor in each tube to deactivate trypsin.
-
21.
Centrifuge the tubes at 2500 × g for 5 min at 4°C in a 5424/5424R centrifuge.
-
22.
After centrifugation, gently aspirate the supernatant and wash the pellet twice with 1mL of complete supplemented RPMI media as stated under working solutions.
-
23.
Finally, resuspend the pellet in 250 μL of complete supplemented RPMI media. Pipette up and down 5–6 times.
-
24.
Vortex the tubes and proceed with counting.
-
25.
Use 20 μL of the cell suspension and dilute 1:5 with 0.4% solution of Trypan Blue in PBS.
Note: From the naïve epithelium, we can get around 1×105–1.5×105 cells while in the HSV-1 infected epithelium, these numbers increase to 2.5×105–3.0×105 cells per corneal epithelium.
-
26.
Place the tubes on ice until ready to plate.
CRITICAL: Be cautious while peeling the epithelial sheet and transferring it to Trypsin-EDTA. It is easier to lose the epithelial sheet because of its transparency. The tubes containing Trypsin-EDTA should be kept on ice to delay trypsin digestion, as it will take some time to peel off the epithelial sheets from all the samples.
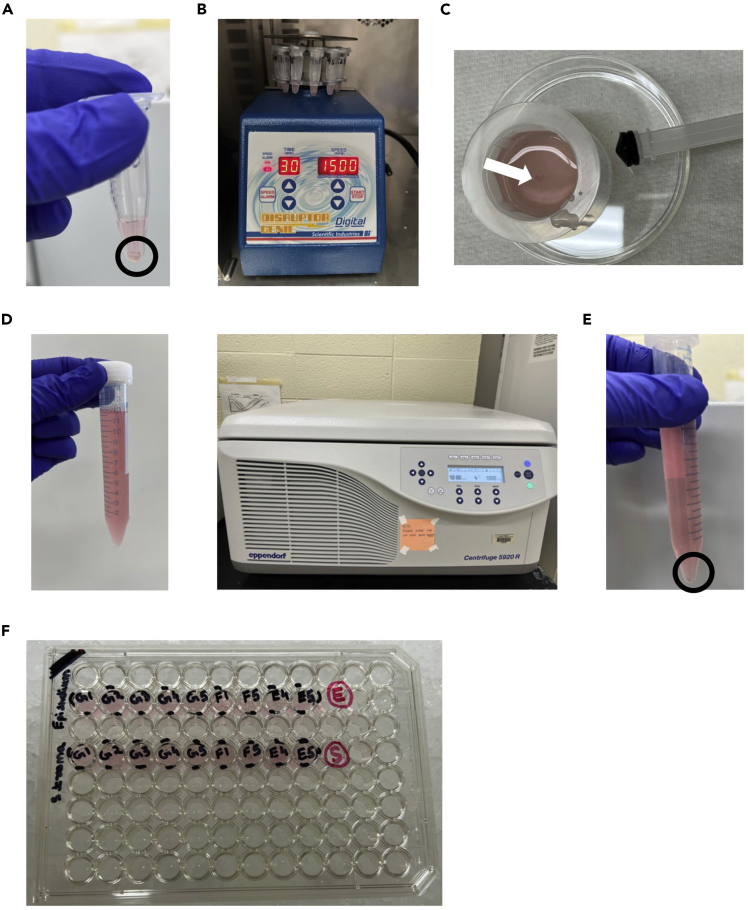
Figure 2.
Enzymatic digestion and preparation of single cell suspension from epithelium
(A) Representative eye image for 16 DPOI at 25X. Scale bars, 1mm.
(B) Mice euthanasia and enucleating eyeball with forceps.
(C) Eyeballs in 24-well plate containing 1 mL of digestion media/well.
(D) 24-well plate kept on orbital shaker for 16-h incubation at 4°C.
(E) The eyeball under stereoscope after overnight enzymatic digestion. The epithelium is peeled off and separated from the eyeball followed by stroma dissection. Scale bars, 1mm.
(F) The peeled off intact epithelial sheet in 500 μL of 0.25% Trypsin-EDTA in 1.7 mL Eppendorf tube. The black outline shows the epithelial sheet. Incubation at 37°C In CO2 incubator for 6 min.
(G) Epithelial single cell suspension after pipetting and addition of 500 L of Soybean trypsin inhibitor following incubation. Spin at 2500 × g for 5 min at 4°C.
(H) The pellet of epithelial cells after spinning. The triangular shaped pellet is shown within the black outline.
(I) Counting of epithelial cells with 1:5 dilution with Trypan blue.
Dissection and digestion of corneal stroma for flow cytometry
Timing: 2–3 h on the day of staining depending on the number of samples
This step enables the successful dissection of mouse corneal stroma from enucleated eyeballs after peeling off the corneal epithelial sheets. This step also describes how to make single cell suspensions from an individual dissected corneal stroma for FACS staining. See Figure 3.
-
27.
After gently peeling off the epithelial sheet from the enucleated eyeball under a stereo microscope, dissect the corneal stroma using a Castroviejo scissor.
Note: Hold the back of the eyeball with straight forceps to make the initial cut at scleral rim .
CRITICAL: Since the eyeballs become soft, sticky, and easy to disintegrate after an overnight incubation in the digestion media, an extra caution is needed while removing iris from the dissected corneal stroma.
-
28.
Carefully add the individually dissected stroma in 200 μL of RPMI medium in 1.7 mL Eppendorf tubes already kept on ice.
-
29.
Add 20 μL of corneal stroma digestion media as stated under materials and equipment to the tubes containing dissected stroma in RPMI medium.
CRITICAL: Secure the tubes with Parafilm before placing them for digestion.
-
30.
Place these tubes on a Genie tissue disruptor set at 1500 rpm for 30 min, which is kept inside an incubator set at 37°C for stroma digestion.
-
31.
At the end of incubation, take out the tubes and immediately place it on ice.
-
32.
Add 1 mL of ice cold complete supplemented RPMI media to each tube.
-
33.
In a medium petri dish (60 mm × 15 mm), place a 70 μm cell strainer.
Note: Add 1 mL of complete supplemented RPMI media through the cell strainer. This step will moisten the cell strainer.
-
34.
Empty the Eppendorf tube containing the enzyme digested stroma on the cell strainer.
-
35.
Use a 3 mL-syringe plunger to gently homogenize the tissue to make a single cell suspension.
Note: Rinse the strainer thoroughly with complete supplemented RPMI media.
-
36.
Transfer the contents of the Petri dish to a 15 mL conical tube.
-
37.
Add 1 mL of complete supplemented RPMI media to the Eppendorf tube and repeat step 35 through 37, three times.
CRITICAL: The digested stroma after 30 min may have some visible tissue pieces left depending on the severity of HSK. An extra caution is needed to prevent the loss of any tissues while transferring the contents of the tubes to cell strainer placed inside the petri dish.
-
38.
Centrifuge the 15 mL tubes at 211 × g for 10 min at 4°C in a 5810R centrifuge.
-
39.
Take out the tubes and carefully discard the supernatant leaving about 500 μL media at the bottom.
-
40.
Gently vortex the tube to break the cell pellet at the bottom.
-
41.
Place the tubes on ice until ready to plate.
Figure 3.
Corneal stroma processing and preparation of single cell suspension
(A) Stroma (shown within black outline) with corneal stroma digestion media in 1.7 mL of Eppendorf tube.
(B) Genie disruptor placed inside incubator set at 37°C with stroma tubes displaying the program used to digest the stromal tissue.
(C) The partially digested stromal tissue shown by white arrow is added on to the cell strainer and is minced with 3 mL syringe plunger.
(D) The single cell suspension from stroma after complete homogenization and rinsing of cells strainer and petri dish with media.
(E) The pellet after spinning stromal cells in 15 mL tube.
(F) Plating of epithelial and stromal cells in 96 well plate for FACS staining.
Cell surface staining for flow cytometry and acquisition
Timing: 5 h on the day of staining
This step describes how to perform FACS staining of freshly prepared corneal epithelial and stromal cells for flow cytometry using LIVE/DEAD™ Fixable Aqua Dead Cell Stain and fluorochrome conjugated cell-surface antibodies.
-
42.
Plate single cell suspension of corneal epithelial and stromal cells in 96 well U-bottom plate on ice.
-
43.
Centrifuge plate for 4 min at 475 × g at 4°C.
-
44.
Remove/flick off supernatant and add 200 μL of ice-cold DPBS.
-
45.
Centrifuge plate for 4 min at 475 × g at 4°C.
-
46.
Remove/flick off supernatant and add LIVE/DEAD™ Fixable Aqua Dead Cell Stain diluted in 100 μL DPBS per well. (1:100 dilution).
-
47.
Incubate the plate in dark for 30 min on ice.
-
48.
At the end of incubation, add 100 μL of DPBS. Centrifuge the plate for 4 min at 475 × g at 4°C.
-
49.
Remove/flick off supernatant and give one more wash with 200 μL of DPBS.
-
50.
Next, add anti-mouse CD16/CD32 antibody diluted in 100 μL FACS Buffer per well (1:100 dilution) to block Fc receptors.
-
51.
Incubate the plate in dark for 30 min on ice.
-
52.
Add 100 μL FACS buffer per well and centrifuge the plate at 475 × g for 4 min to wash off the unbound antibody.
-
53.
For cell surface staining, prepare a cocktail mixture of fluorochrome-conjugated antibodies at the dilutions stated in key resources table.
-
54.
Add 100 μL/well of the antibodies cocktail mixture.
-
55.
Incubate in dark for 30 min on ice.
-
56.
Add 100 μL FACS Buffer, centrifuge at 475 × g at 4°C for 4 min and remove/flick off supernatant.
-
57.
Give two more washes with 200 μL of FACS Buffer.
-
58.
Finally, fix the cells with 1:1 of 100 μL FACS buffer and 100 μL 2% paraformaldehyde (200 μL total per well).
-
59.
Store the cells for 14–16 h at 4°C.
Note: The cells can also be fixed for 30 min followed by the acquisition on the flow cytometer.
Pause Point: For 14–16 h incubation, ensure the FACS plate with cells is incubated in dark at 4°C.
-
60.
Next day, acquire samples on the medium flow rate using BD LSRFortessa flow cytometer.
Data collection on flow cytometer
Timing: 5–6 h on the day of acquisition
Epithelial and stromal cells acquisition
-
61.
Acquire corneal epithelial and stromal cell samples on BD LSRFortessa™.
-
62.
Set the appropriate PMT voltage.
CRITICAL: Set the Forward Scatter Area (FSC-A) -180 and Side Scatter Area (SSC-A)- 250 for epithelial cells, and FSC-A 216 and SSC-A 280 for corneal stromal cells.
Note: The PMT voltage settings for the fluorochromes remains the same for corneal epithelial and corneal stromal cells.
-
63.
Use compensation beads for reliable and precise gating, thereby minimizing fluorescence spillover.
-
64.
Acquire the maximum possible events from each sample and analyze data using FlowJo version 10.
Expected outcomes
Immune and non-immune cells in corneal epithelium
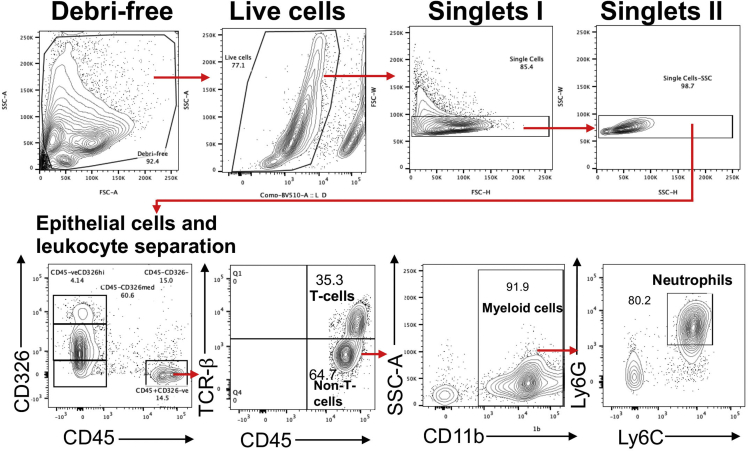
The antibody panel used for staining the single cell suspension of the corneal epithelium can differentiate between epithelial cells and different subsets of leukocytes in HSK corneas. The gating strategy employed to separate corneal epithelial cells from leukocytic subsets is shown in Figure 4. The debris-removal gate was employed first. This was followed by removing dead cells using aqua live/dead cell staining. Next, the singlets are gated using FSC-W versus FSC-H and then SSC-W versus SSC-H parameters. The singlet population is used to separate corneal epithelial cells from the leukocytes by opening the FACS plots between CD326 versus CD45. CD326 marker (EpCAM) is known to express on corneal epithelial cells.3 CD45 is the pan-leukocyte marker. Our data show that CD326-expressing cells lack the expression of CD45 leukocytic marker, demonstrating them as corneal epithelial cells. Moreover, CD326 expressing cells are subdivided into CD326hi and CD326medium epithelial cells. Among CD45 expressing cells, a significant proportion of these leukocytes are T cells, as evident from TCR-beta stained CD45+ cells. Most TCR-beta-veCD45+ cells are of myeloid cell origin as they are stained for CD11b molecule. Furthermore, most myeloid cells in the corneal epithelium are neutrophils as they get stained for both Ly6G and Ly6C. A smaller proportion of non-T cells and non-myeloid leukocytes are also found in the corneal epithelium of the HSK cornea.
Figure 4.
Flow cytometry analysis of cells from the corneal epithelium of HSK corneas
Gating strategies employed showed first the removal of debris from the stained cells followed by the separation of singlets based on height and width parameters. The gating strategy showed the presence of leukocytes (CD45+CD326-ve) in the corneal epithelium of the HSK cornea. Corneal epithelial cells are subdivided based on the expression of EpCAM (epithelial cell adhesion molecule) as CD45-ve CD326hi and CD45-ve CD326medium cells. Most CD45+ leukocytes in the corneal epithelium are either T cells (TCR-beta+) or CD11b expressing Ly6Ghi neutrophils.
Immune and non-immune cells in corneal stroma
The antibody panel used to stain the single cell suspension of the corneal stroma can differentiate between vascular endothelial cells and leukocytic subsets in HSK corneas. Stromal neovascularization is the hallmark of HSK.4 Our flow cytometry approach can enumerate stromal neovascularization by estimating the frequency of CD31+ CD45-ve vascular endothelial cells. The gating strategy employed to distinguish between vascular endothelial cells and different leukocytic subsets is shown in Figure 5. When analyzing FACS data, the Debris-free gating is employed, followed by removing dead cells using the aqua stain. Doublets are removed from the live cells using FSC-W (width) versus FSC-H (height) gating, followed by SSC-W versus SSC-H gating. Singlets are separated into CD45+ and CD45-ve cell populations. Among CD45-ve cells, a small subset of cells expresses CD31, also known as platelet endothelial cell adhesion molecule 1 (PECAM-1), which is considered a specific marker for vascular endothelial cells.5 CD45+ leukocytes are sub-categorized into T cells (TCR-beta+) or non-T cells (TCR-beta-ve) subsets. The majority of non-T cells leukocytes express CD11b molecule and are categorized as myeloid cells. Among myeloid cells, neutrophils and monocytes are the major cell subsets in the corneal stroma, as is evident from the expression of Ly6G and Ly6C.
Figure 5.
Flow cytometry analysis of cells from the corneal stroma of HSK corneas
Gating strategies employed showed first the removal of debris from the stained cells followed by the separation of singlets based on height and width parameters. Next, leukocytes in the corneal stroma are separated from the non-leukocytes based on CD45 expression. Non-leukocytic CD45-ve cells are gated for CD31 expression to enumerate the percentage of vascular endothelial cells (CD45-veCD31+ve) contributing to stromal neovascularization. Stromal leukocytes are subdivided into TCR-beta + T cells and TCR-beta-ve CD11b+ myeloid cells. Most CD11b+ myeloid cells are either neutrophils (Ly6GhiLy6C+) or monocytes (Ly6G-veLy6C+). A subset of myeloid cells in the corneal stroma neither express Ly6G nor Ly6C. The identification of these cells is uncertain from the current antibody staining panel.
Quantification and statistical analysis
FACSDiva software was used to acquire the FACS samples. FlowJo version 10 (FlowJo, LLC https://www.flowjo.com/) was used for data analysis.
Limitations
The flow cytometry panel used in this procedure is meant to enumerate the frequency of major immune cell types and non-immune cells in the corneal epithelium versus corneal stroma of HSK corneas. More detailed immune cell subset analysis can be performed by optimizing the fluorochrome conjugated antibody panel.
There is a possibility that certain surface molecule levels are reduced due to Dispase digestion.
Troubleshooting
Problem 1
Difficulty in peeling intact epithelial sheet at step 16.
Potential solution
The Dispase® II digestion media should be prepared fresh with the same concentrations of media components as mentioned under Digestion media to ease the peeling. Ensure the orbital shaker stays ON during the entire incubation period of 16 h. Longer than 16 h incubation with Dispase is not recommended, as this may cause the degradation of cell surface molecules.
Problem 2
No visible epithelial cells pellet after centrifugation at step 21.
Potential solution
There is a high probability that epithelial sheet never got inside the tube and remained stuck with the peeling forceps. To overcome this, ensure epithelial sheet floats inside the 0.25% Trypsin-EDTA tube at step 17.
Problem 3
Low epithelial cell counts or visible clumps under microscope while counting at step 25.
Potential solution
Low cell counts arise due to clumping of epithelial cells. To prevent this, make sure to pipette up and down as described in step 23 and briefly vortex the tube before proceeding for counting. Also, do not over incubate with 0.25% Trypsin-EDTA at step 18.
Problem 4
Increased epithelial cell death evident by Trypan Blue staining at step 25.
Potential solution
Over incubation with Trypsin will result in low cell viability. It is recommended to not incubate cells for more than 15 min in Trypsin-EDTA (step 18). Additionally, keep the tubes on ice as soon as the Trypsin-EDTA incubation is over and add ice cold Soybean Trypsin Inhibitor to neutralize Trypsin activity.
Problem 5
Decreased cell yield and increased cell death in Stroma due to over incubation with Liberase enzyme (steps 61–64).
Potential solution
Immediately placing the Eppendorf tubes on ice after enzymatic Liberase digestion and adding the ice-cold media will slow down enzymatic reaction (step 31, 32). Also, it is important to carefully process the stroma through the cell strainer in the petri dish. The tube, strainer and petri dish should be rinsed thoroughly to increase the cell yield (step 34, 35).
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Susmit Suvas (ssuvas@med.wayne.edu).
Materials availability
No new materials are generated in this protocol.
Acknowledgments
This study was supported by NIH grants R01 EY030129 (S.S.) and R01 EY029690 (S.S.) and core grant P30EY004068 (LDH) and by a Research to Prevent Blindness unrestricted grant to the Department of Ophthalmology, Wayne State University School of Medicine.
Author contributions
M.S., P.K.S., and S.S. designed the experiments. M.S. and P.K.S. carried out the experiments. M.S., P.K.S., and S.S. analyzed the data. M.S. selected the journal and created the work flow for images. S.S., M.S., and P.K.S. wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2023.102056.
Contributor Information
Mizumi Setia, Email: mizumi.setia@med.wayne.edu.
Susmit Suvas, Email: ssuvas@med.wayne.edu.
Data and code availability
The study did not generate new datasets or code.
References
- 1.Rana M., Setia M., Suvas P.K., Chakraborty A., Suvas S. Diphenyleneiodonium treatment inhibits the development of severe herpes stromal keratitis lesions. J. Virol. 2022;96:e0101422. doi: 10.1128/jvi.01014-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaddipati S., Estrada K., Rao P., Jerome A.D., Suvas S. IL-2/anti-IL-2 antibody complex treatment inhibits the development but not the progression of herpetic stromal keratitis. J. Immunol. 2015;194:273–282. doi: 10.4049/jimmunol.1401285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Royer D.J., Elliott M.H., Le Y.Z., Carr D.J.J. Corneal epithelial cells exhibit myeloid characteristics and present antigen via MHC class II. Invest. Ophthalmol. Vis. Sci. 2018;59:1512–1522. doi: 10.1167/iovs.17-23279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giménez F., Suryawanshi A., Rouse B.T. Pathogenesis of herpes stromal keratitis--a focus on corneal neovascularization. Prog. Retin. Eye Res. 2013;33:1–9. doi: 10.1016/j.preteyeres.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeLisser H.M., Christofidou-Solomidou M., Strieter R.M., Burdick M.D., Robinson C.S., Wexler R.S., Kerr J.S., Garlanda C., Merwin J.R., Madri J.A., Albelda S.M. Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am. J. Pathol. 1997;151:671–677. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study did not generate new datasets or code.