Summary
Here, we describe a fast and cost-effective procedure to generate a large array of mutant proteins and immediately screen for those with altered protein function. This protocol is a modification from three existing approaches: fusion PCR, Saccharomyces cerevisiae in-yeast recombination, and semi-quantitative growth assays. We also describe a mating step to reduce the occurrence of false positive findings due to ectopic mutations. The only requirement is that the protein elicits a phenotype in Saccharomyces cerevisiae.
Subject areas: Cell-based Assays, Model Organisms, Molecular Biology, Biotechnology and bioengineering
Graphical abstract

Highlights
-
•
Fast and low-cost generation of a series of mutant proteins using yeast as host
-
•
Rapid functional screening of mutant proteins
-
•
A mating procedure helps prevent false results due to ectopic mutations
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Here, we describe a fast and cost-effective procedure to generate a large array of mutant proteins and immediately screen for those with altered protein function. This protocol is a modification from three existing approaches: fusion PCR, Saccharomyces cerevisiae in-yeast recombination, and semi-quantitative growth assays. We also describe a mating step to reduce the occurrence of false positive findings due to ectopic mutations. The only requirement is that the protein elicits a phenotype in Saccharomyces cerevisiae.
Before you begin
-
1.
It must already be known which amino acids should be investigated, i.e., targeted for mutation in the gene-of-interest (GOI).
-
2.
The sequence of the GOI must be known.
-
3.
The DNA of the GOI must be available.
-
4.
The sequence of the vector or plasmid used should be verified.
-
5.
A promoter must be available for the gene. If the mutations are to be placed in a small region of the gene, the gene must be under the control of a yeast promoter and on a plasmid that can be maintained in the yeast Saccharomyces cerevisiae. If the mutations are to be placed throughout the gene, an empty vector is acceptable that can be maintained in the yeast Saccharomyces cerevisiae. The yeast promoter must be either present in the vector, or the GOI can be PCR amplified along with its promoter, as long as it is a yeast promoter.
-
6.
This protocol assumes that the researcher is familiar with conducting restriction digests, DNA electrophoresis, and setting up PCR reactions.
-
7.
This protocol assumes that the researcher is familiar with the basics of handling the yeast Saccharomyces cerevisiae.
-
8.
A yeast strain is needed that lacks the chromosomal GOI. If not available, such a strain can be purchased. See under “key resources table”, heading ‘Experimental models: Organisms/strains’, ‘Yeast strain deleted for Gene-of-Interest, in BY4741 background’.
-
9.
A yeast mating partner is needed that contains the GOI or not, as applicable. If the strain in point 8 above is in the BY4741 background, a suitable mating strain is the yeast H1511.
Preparation: Primer design
Institutional permissions
Any experiments involving genetically modified organisms may need prior approval. The procedures and the regulations differ between countries.
Timing: 1 day for primer design, 1–2 weeks for commercial synthesis of primers
The type of primers depends on where in the gene-of-interest (GOI) the mutations are to be made. The procedure depends on whether the mutations are located within a small region of the GOI, or whether they are to be introduced throughout the gene, i.e., choose preparation procedure I or II.
-
I.Mutations are located within a small region of the GOI.
-
a.The GOI must already be cloned in an expression vector, and under the control of a yeast promoter. We used an expression vector (vector pES128-9) in which the gene is expressed from a galactose inducible promoter (Sattlegger and Hinnebusch, 2000)..
-
b.Define the region in which site directed mutagenesis shall be performed (Figure 1A, indicated with blue arrowheads).
-
c.Identify unique restriction sites immediately flanking the region in which mutagenesis will be performed; one site upstream and one site downstream of this region (Figure 1A).
-
d.Design the following primers for PCR, with annealing temperatures of 55°C.Note: While there are many software programs used for primer design and determining the annealing temperature, we are using a much simpler approach which has worked equally well for us: for determining the annealing temperature count 4°C for every G or C, and count 2°C for every T or A, and then from the sum subtract 5°C.
-
i.Flanking primer pair that anneals in the area containing the unique restriction site (Figure 1A).
-
ii.Extension primer pair that contains the same sequence as the flanking primers, plus at their 5′ end at least 50 bases of sequence homologous to the sequences beyond the restriction site (Figure 1A).
-
iii.Mutagenesis primer pair: For each single amino acid substitution, design a sense and a reverse mutagenesis primer that each contains the desired point mutation.
 CRITICAL: For each primer, 3′ of the point mutation, add bases that are homologous to the gene's sequence such that it results in an annealing temperature of 55°C. For each primer pair, 5′ to the point mutation, add bases homologous to the DNA sequence so that the 5′ ends of the mutagenesis primers can anneal to each other with an annealing temperature of 55°C. (Figure 1A).Note: We usually add a silent mutation in the region of the point mutation that either generates or removes a restriction site, that can be used as a diagnostic restriction site for the presence of the respective point mutation.
CRITICAL: For each primer, 3′ of the point mutation, add bases that are homologous to the gene's sequence such that it results in an annealing temperature of 55°C. For each primer pair, 5′ to the point mutation, add bases homologous to the DNA sequence so that the 5′ ends of the mutagenesis primers can anneal to each other with an annealing temperature of 55°C. (Figure 1A).Note: We usually add a silent mutation in the region of the point mutation that either generates or removes a restriction site, that can be used as a diagnostic restriction site for the presence of the respective point mutation. -
iv.It is recommended to design verification primers pairs to check for the successful generation of the plasmid, for more see under ‘step-by-step method details’, step 5× (Figure 2F).
-
i.
-
a.
-
II.Mutations to be introduced throughout the gene.
-
a.Choose an expression vector (which has a multiple cloning site) into which you wish to clone your mutated GOI.
 CRITICAL: Either the GOI must be cloned along with a yeast promoter, or use a vector that contains a yeast promoter. We used a vector that contains a galactose inducible promoter (pES128-9).
CRITICAL: Either the GOI must be cloned along with a yeast promoter, or use a vector that contains a yeast promoter. We used a vector that contains a galactose inducible promoter (pES128-9). -
b.Identify a restriction site in the multiple cloning site that can be used to linearize the plasmid.
-
c.Design the following primers for PCR, with annealing temperatures of 55°C.
-
i.Design flanking primers that anneal at the 3′ and 5′ end of your GOI with an annealing temperature of 55°C (Figure 1B).Note: We added the restriction site used to linearize the vector to the 5′ ends of the flanking primers as well, to allow cloning via ligation if that becomes necessary. For both primers, add an additional 4–6 bases 5′ to the restriction site, to ensure the restriction enzyme is able to cut efficiently.
-
ii.Design a pair of extension primers that contain the same sequence as the flanking primers, plus at their 5′ end at least 50 bases that anneal to the vector just adjacent to the restriction sites used to linearize the vector (Figure 1B).
-
iii.For each single amino acid substitution to be made, design a sense and a reverse mutagenesis primer that contain the desired point mutation, in the same manner as done above under I.d.iii (Figure 1B).
-
iv.Take note of step I.d.iv.
-
i.
-
a.
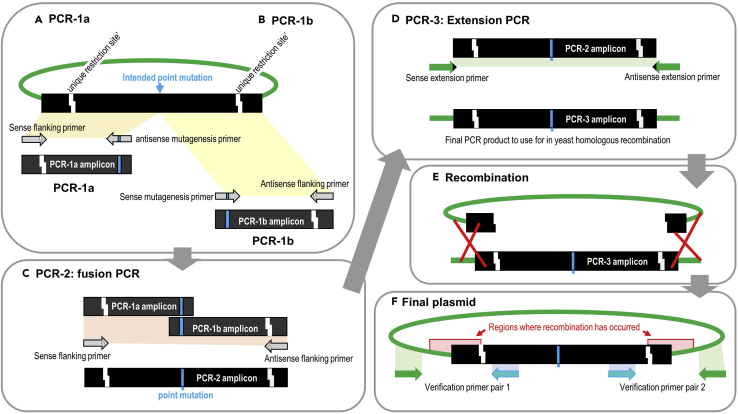
Figure 1.
Generation of DNA fragments containing desired single amino acid substitutions
(A) If in the Gene-of-Interest (GOI) only a restricted area is to be subjected to site directed mutagenesis, a plasmid borne GOI is necessary that is under the control of a yeast promoter. For each amino acid substitution, a mutagenesis primer pair needs to be designed, the image only shows a primer pair for one amino acid substitution. For more see text.
(B) If amino acid substitutions are to be placed throughout the GOI, the flanking primers need to be designed such that they amplify the entire gene including the promoter (unless the plasmid harbors a promoter). In this case, the starting PCR template does not need to be a plasmid-borne GOI. Otherwise, the primer design is similar to that in (A). For more see text.
(C) Plasmid titration, sample result. Uncut plasmid, and linearized plasmid were diluted as indicated, and then transformed into yeast following the protocol in step 3, and the number of transformants determined. In this case, the 1:80 dilution gives 5–10 transformants, and therefore would be the desired working concentration to be used for plasmid generation (step 5).
Figure 2.
Overview of PCR reactions for the generation of DNA fragments containing the desired amino acid substitution
(A and B) First, the DNA upstream and downstream of the intended point mutation is amplified separately.
(C) Then, these amplicons are fused together via fusion PCR.
(D) The 3′ and 5′ ends of the resulting amplicon are extended via an extension PCR.
(E and F) This leads to the addition of sequences that allow (E) recombination events to occur in yeast, between the PCR amplicon and the linearized plasmid (red crosses), resulting into (F) the final circular plasmid containing the GOI with the desired mutation. For more see text.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals | ||
| 10 mM dNTP mix | New England Biolabs | Cat#N0447L |
| Proofreading DNA polymerase 5 U/μL, Pfu DNA polymerase, and supplied 10× buffer | Promega, USA | Cat#7741 |
| Non-proofreading DNA polymerase 5 U/μL, Taq DNA polymerase, and supplied 10× buffer | Merck, USA | Cat#D1806 |
| Restriction enzymes and appropriate 10× reaction buffers, type of enzyme depends on procedure. See under ‘preparation: primer design’ | New England Biolabs, USA | Cat# depends on enzyme needed |
| Tris(hydroxymethyl)aminomethane (Tris) | Formedium, UK | Cat#TRIS05 |
| Ethylenediaminetetraacetic acid disodium salt dihydrate (EDTA) | Merck, USA | Cat#E5134 |
| Glacial acetic acid | Merck, USA | Cat#A6283 |
| Hydrochloric acid (HCl) | Fisher Scientific, USA | Cat#10316380 |
| Bromophenol blue | Sigma, USA | Cat#B5525 |
| Ethidium bromide 10 mg/mL, or | Merck, USA | Cat#E1510 |
| Non-ethidium bromide DNA stain, Novel Juice | Merck, USA | Cat#07371-1ML |
| Agarose | Merck, USA | Cat#A4718 |
| Lithium acetate dihydrate | Sigma, USA | Cat#L4158 |
| Polyethylene glycol CRITICAL REAGENT: do not substitute with another reagent |
Merck, USA | Cat#P4338 |
| Herring sperm DNA, 10 mg/mL | Thermo Fisher Scientific, USA | Cat#15634017 |
| Glycerol | Merck, USA | Cat#G5516 |
| Yeast extract | Formedium, UK | Cat#YEA03 |
| Peptone | Formedium, UK | Cat#PEP03 |
| Bacteriological grade agar | Pure Science, Australia | Cat#N1083942 |
| Glucose | Merck, USA | Cat#GLU03 |
| Galactose | Formedium, UK | Cat#GAL03 |
| Yeast Nitrogen Base without amino acids and without ammonium sulfate | Formedium, UK | Cat#CYN0502 |
| Ammonium sulfate | Merck, USA | Cat#A4418 |
| Isoleucine, supplement to cover yeast auxotrophy | Formedium, UK | Cat#DOC0152 |
| Leucine, supplement to cover yeast auxotrophy | Formedium, UK | Cat#DOC0156 |
| Valine, supplement to cover yeast auxotrophy | Formedium, UK | Cat#DOC0196 |
| Tryptophan, supplement to cover yeast auxotrophy | Formedium, UK | Cat#DOC0188 |
| Methionine, supplement to cover yeast auxotrophy | Formedium, UK | Cat#DOC0168 |
| Histidine, supplement to cover yeast auxotrophy | Formedium, UK | Cat#DOC0144 |
| Lysine, supplement to cover yeast auxotrophy | Formedium, UK | Cat#DOC0160 |
| Compounds to elicit a desired phenotype, if necessary, in our sample study it was 3-Amino-1,2,4-triazole (3AT) | Formedium, UK | Cat#3AT025 |
| Experimental models: Organisms/strains | ||
| Saccharomyces cerevisiae strain H2556 (MATα ura3-52 trp1-63 leu2-3,112 GAL2+gcn1Δ) | Alan Hinnebusch, NIH, USA, see reference | (Sattlegger and Hinnebusch, 2000) |
| Saccharomyces cerevisiae strain H1511 (MATα ura3-52 trp1-63 leu2-3,112 GAL2+) | Alan Hinnebusch, NIH, USA, see reference | (Foiani et al., 1991) |
| Saccharomyces cerevisiae strain BY4741 (MATa his3Δ1 leu2Δ0 ura3Δ0 met15Δ0) | Fisher Scientific, USA | Cat#10119542 |
| Saccharomyces cerevisiae strain BY4742 (MATa his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0) | Fisher Scientific, USA | Cat#10602384 |
| Saccharomyces cerevisiae strain deleted for Gene-of-Interest, in BY4741 background | Fisher Scientific, USA | Cat# depends on the strain needed for the study |
| Recombinant DNA | ||
| pES128-9, contains a galactose inducible promoter, and a GST tag, the yeast selectable marker is URA3 | Evelyn Sattlegger, Massey University, New Zealand | (Sattlegger and Hinnebusch, 2000) |
| Deposited data | ||
| Data S1: A file is provided that contains grids to be used as guides for transferring cells with an 8-channel pipette to solid medium | This paper | https://doi.org/10.17632/x83kktjrfd.1 |
| Data S2: Sample calculations, to determine the growth rate of strains in Figure 7A. | This paper | https://doi.org/10.17632/cfjgjnpxmt.1 |
| Other | ||
| Sterile filtration unit | Fisher Scientific, USA | Cat#FB12566502 |
| Replica plater, for mating on solid medium | VWR, USA | Cat#25395-380 |
| Glass beads, 3–5 mm diameter | Fisher Scientific, USA | Cat#11411545 |
| Salt and pepper shaker, made of glass and a metal cap | Any dollar shop, or Briscoes, New Zealand | no Cat# available |
| Round toothpicks | Any grocery store | n/a |
| Metal caps for 16 × 125 mm Borosilicate glass culture tubes | Bio-Strategy, USA, or Marienfeld, Germany | Cat#6602008 https://www.marienfeld-superior.com/test-tube-caps-labocap.html |
| Borosilicate glass test tubes 16 mm diameter | VWR, USA | Cat#KIML73500-16125 |
| Velvets, 15 × 15 cm, made of cotton | Any home store selling fabrics, or VWR, USA | Cat#89033-116 |
| Flat bottom 96 well microtiter plates, with lid. Purchasing non-sterile plates is fine, as long as they have not been removed from their bags yet (they are sterile essentially). | Merck, USA | Cat#CLS3300 |
| Vacuum pump | Lab Supply, New Zealand | Cat#ROC167400-22 |
| Autoclave, the autoclaving conditions are 121°C, at least 15 psi, for 20 min (wet heat). Autoclave is a standard lab equipment, hence a source and order number will not be listed here. Can also use pressure cooker. | Farmers, New Zealand | Cat#CPE300 |
| PCR thermal cycler | Bio-Rad, USA | Cat#T100Thermal Cycler |
| Gel electrophoresis apparatus | Lab Supply, New Zealand | Cat#CLEMSMIDI10 |
| Power supply for Gel electrophoresis apparatus | Bio-Rad, USA | Cat#1645050EDU |
| Light box to visualize DNA in the gel, such as a UV illuminator for ethidium bromide stained DNA, or alternative light source for non-ethidium bromide stained DNA (e.g., blue light (470 nm) for Novel Juice DNA stain) Hazard: ethidium bromide is carcinogenic, handle with gloves only |
Thermo Fisher Scientific, USA | Cat#LB0100 |
| Test tube rotator to grow yeast in glass tubes, or alternatively place the glass tubes in a rack and a 30°C incubator shaking | DKSH, New Zealand | Cat# SB3/5 |
| 30°C incubators, static and shaking | Many suppliers | Many suppliers |
| Microtube centrifuge, 4°C | Thermo Fisher Scientific, USA | Cat# 75002401 |
| 50 mL Falcon tube centrifuge, 4°C | Thermo Fisher Scientific, USA | Cat#THR75009750 |
| Heating block at 42°C | Thermo Fisher Scientific, USA | Cat# 88870004 |
| Heating block at 100°C | Thermo Fisher Scientific, USA | Cat# 88870004 |
| Document scanner connected to a computer | PB Tech, New Zealand | Cat# SCAEPP2914685 |
| Spectrophotometer able to measure absorbance at 600 nm | VWR, USA | Cat#UV-1600PC |
| Tips for pipettes, 5–200 μL | Lab Supply, New Zealand | Cat#MUL7231MUL7231 |
| Tips for pipettes, 200–1000 μL | Thermo Fisher Scientific, USA | Cat#axyt1000B |
| Standard sterile plastic petri dishes | Thermo Fisher Scientific, USA | Cat#LBS60002 |
| 1.5 mL microfuge tubes | Merck, USA | Cat#AXYMCT175C |
| 2 mL screw cap tubes, skirted, conical base, with cap | Sarstedt, Germany | Cat#72.694.300 |
| Sterile 50 mL Falcon tubes | Lab Supply, New Zealand | Cat#GRE227261 |
| Microfuge tubes that fit in the PCR thermal cycler | Merck | Cat#AXYPCR02C |
| 25 mL sterile serological pipettes | Lab Supply, New Zealand | Cat#JET JGSP010025 |
| 10 mL sterile serological pipettes | Lab Supply, New Zealand | Cat#JET JGSP010010 |
| 5 mL sterile serological pipettes | Lab Supply, New Zealand | Cat#JET JGSP010005 |
| 250 mL glass flasks | Lab Supply, New Zealand | Cat# MAR4110207 |
| Microfuge racks for 1.5 mL and 0.2 mL tubes | Interlab New Zealand | Cat#251020-Y |
| Rack for 50 mL tubes | Interlab, New Zealand | Cat#202113-M |
| Rack for tubes with 16 mm diameter (15 mL Falcon tubes have a 16 mm diameter) | Interlab, New Zealand | Cat#4050-4636 |
| A set of pipettes (0.5–2 μL, 2–20 μL, 20–200 μL, 100–1000 μL) | Gilson, USA | Cat# F167370 |
| Pipette aid for serological pipettes | Lab Supply, New Zealand | Cat#SCI740300038888 |
| 8 channel multichannel pipettes for pipetting 5 μL, 20 μL | LabsSupply, New Zealand | Cat#SCI713112069999 |
| 8 channel multichannel pipettes for pipetting 180 μL | Lab Supply, New Zealand | Cat#SCI713112129999 |
| Household sieve, at least 20 cm diameter, preferable stainless steel | Any dollar shop or grocery store | |
| Vortex mixer | Merck, USA | Cat#CLS6776 |
| Bunsen burner and source for gas, alternatively a camping gas burner with gas bottle can be used | Fisher Scientific, USA | Cat# 03-962Q |
Note: Do make sure that the purchased equipment is suitable for the voltage available in your country.
Materials and equipment
50× TAE DNA gel electrophoresis buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| TRIS | 2 M | 242 g |
| Glacial acetic acid Hazard: This is an acid, use gloves |
1 M | 57.1 mL |
| EDTA disodium salt dihydrate | 50 mM | 18.6 g |
| ddH2O | n/a | To final of 1L |
| Total | n/a | 1 L |
Store at room temperature (20°C–25°C), can be stored for several years.
10× TE buffer pH 7.4, for yeast transformation
| Reagent | Final concentration | Amount |
|---|---|---|
| TRIS | 100 mM | 1.211 g |
| EDTA disodium salt dihydrate | 10 mM | 372 mg |
| HCl Hazard: This is an acid, wear gloves and handle solution in chemical hood |
Adjust pH to 7.4 | As required |
| ddH2O | n/a | To final of 100 mL |
| Total | n/a | 100 mL |
Autoclave, store at room temperature (20°C–25°C), can be stored for several years.
1 M Lithium acetate stock
| Reagent | Final concentration | Amount |
|---|---|---|
| lithium acetate dihydrate | 1 M | 10.2 g |
| 10× TE buffer (pH 7.4) | 1× | 10 mL |
| ddH2O | n/a | 90 mL |
| Total | n/a | 100 mL |
Sterile filter, store at room temperature (20°C–25°C), can be stored for several years.
Solution I (0.1 M Lithium Acetate solution):
| Reagent | Final concentration | Amount |
|---|---|---|
| ddH2O | n/a | 160 mL |
| 10× TE buffer (pH 7.4), sterile | 1× | 20 mL |
| 1 M lithium acetate, sterile | 100 mM | 20 mL |
| Total | n/a | 200 mL |
Autoclave 160 mL of double deionized H2O (ddH2O) in a bottle, then add the other components, aseptically.
Store at room temperature (20°C–25°C), can be stored for several years.
44% PEG stock
| Reagent | Final concentration | Amount |
|---|---|---|
| polyethylene glycol | 44% (w/v) | 44 g |
| 10× TE buffer (pH 7.4) | 1× | 10 mL |
| ddH2O | n/a | Adjust to 100 mL |
| Total | n/a | 100 mL |
Autoclave, store at room temperature (20°C–25°C), can be stored for several years.
Solution II (40% PEG solution)
| Reagent | Final concentration | Amount |
|---|---|---|
| 44% PEG stock | 40% | 9 mL |
| 1 M lithium acetate | 100 mM | 1 mL |
| Total | n/a | 10 mL |
Pipette aseptically into a sterile Falcon tube, mix well, store at room temperature (20°C–25°C), can be stored for several years.
40% glucose solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Glucose | 40% | 80 g |
| ddH2O | n/a | Adjust to 200 mL |
| Total | n/a | 200 mL |
Autoclave, store at room temperature (20°C–25°C), can be stored for several years.
40% galactose solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Galactose | 40% | 80 g |
| ddH2O | n/a | Adjust to 200 mL |
| Total | n/a | 200 mL |
Autoclave, store at room temperature (20°C–25°C), can be stored for several years.
YPD solid medium
| Reagent | Final concentration | Amount |
|---|---|---|
| yeast extract | 1% (w/v) | 10 g |
| Peptone | 2% (w/v) | 20 g |
| Agar | 2% (w/v) | 20 g |
| ddH2O | n/a | Adjust to 950 mL |
| Glucose 40%, sterile, add last and after autoclaving | 2% | 50 mL |
| Total | n/a | 1 L |
Autoclave, let cool to 40°C–50°C (can hold bottle with bare hands without burning the skin), add glucose, pour into petri plates, let solidify, store at room temperature (20°C–25°C) for 2 days, then seal in a bag and store at 4C.
Can be stored for a year.
YPD liquid medium
| Reagent | Final concentration | Amount |
|---|---|---|
| yeast extract | 1% (w/v) | 10 g |
| Peptone | 2% (w/v) | 20 g |
| ddH2O | n/a | Adjust to 950 mL |
| Glucose 40%, sterile, add last and after autoclaving | 2% | 50 mL |
| Total | n/a | 1 L |
Autoclave, store at room temperature (20°C–25°C), add glucose just before usage, store unused medium at 4°C.
Can be stored for a year.
Synthetic dextrose (SD) solid medium
| Reagent | Final concentration | Amount |
|---|---|---|
| Yeast nitrogen base | 0.19% (w/v) | 1.9 g |
| Ammonium sulfate | 0.5% (w/v) | 5 g |
| Agar | 2% (w/v) | 20 g |
| ddH2O | n/a | Adjust to 950 mL∗ |
| Glucose 40%, sterile, add last and after autoclaving | 2% | 50 mL |
| Total | n/a | 1 L |
∗ the volume needs to be adjusted to accommodate additional volume for amino acid solutions and supplements.
Autoclave, let cool to 40°C–50°C (can hold bottle with bare hands without burning the skin), add glucose, pour into petri plates, let solidify, store at room temperature (20°C–25°C) for 2 days, then seal in a bag and store at 4°C.
Can be stored for 6 months.
Note: Make sure the yeast nitrogen base powder is dissolved quickly in ddH2O, otherwise it will clump together, and it will be hard to get it fully dissolved.
Note: Additional supplements need to be added depending on the auxotrophies of the strains, before pouring the medium.
Note: Additional supplements need to be added to trigger a phenotype, in our sample study (see expected results) it was 3-amino-1,2,4-triazole (3AT).
Synthetic dextrose (SD) liquid medium
| Reagent | Final concentration | Amount |
|---|---|---|
| Yeast nitrogen base | 0.19% (w/v) | 1.9 g |
| Ammonium sulfate | 0.5% (w/v) | 5 g |
| ddH2O | n/a | Adjust to 950 mL∗ |
| Glucose 40%, sterile, add last and after autoclaving | 2% | 50 mL |
| Total | n/a | 1 L |
∗ the volume needs to be adjusted to accommodate additional volume for amino acid solutions and supplements.
Autoclave, store at room temperature (20°C–25°C), add glucose just before usage, store unused medium at 4°C, can be stored for 6 months.
Note: Make sure the yeast nitrogen base powder is dissolved quickly in ddH2O, otherwise it will clump together, and it will be hard to get it fully dissolved.
Note: Additional supplements need to be added depending on the auxotrophies of the strains.
Synthetic Galactose (SGal) solid medium
| Reagent | Final concentration | Amount |
|---|---|---|
| Yeast nitrogen base | 0.19% (w/v) | 1.9 g |
| Ammonium sulfate | 0.5% (w/v) | 5 g |
| Agar | 2% (w/v) | 20 g |
| ddH2O | n/a | Adjust to 950 mL∗ |
| Galactose 40%, sterile, add last and after autoclaving | 2% | 50 mL |
| Total | n/a | 1 L |
∗ the volume needs to be adjusted to accommodate additional volume for amino acid solutions and supplements.
Autoclave, let cool to 40°C–50°C (can hold bottle with bare hands without burning the skin), add galactose, pour into petri plates, let solidify, store at room temperature (20°C–25°C) for 2 days, then seal in a bag and store at 4°C, can be stored for 6 months.
Note: Make sure the yeast nitrogen base powder is dissolved quickly in ddH2O, otherwise it will clump together, and it will be hard to get it fully dissolved.
Note: Additional supplements need to be added depending on the auxotrophies of the strains, before pouring the medium.
Note: Additional supplements need to be added to trigger a phenotype, in our sample study it was 3-amino-1,2,4-triazole (3AT).
ILV stock, media supplement to cover yeast Leucine auxotrophy
| Reagent | Final concentration | Amount |
|---|---|---|
| Leucine | 100 mM | 1.31 g |
| Isoleucine | 25 mM | 0.656 g |
| Valine | 25 mM | 0.586 |
| ddH2O | n/a | 200 mL |
| Total | n/a | 200 mL |
Autoclave, store at room temperature (20°C–25°C), can be stored for a year.
Use 20 mL in 1 L SD or SGal medium.
Note that if a strain is Leu auxotrophic, Leu along with Val and Ile need to be supplemented.
Trp stock, media supplements to cover yeast Tryptophane auxotrophy
| Reagent | Final concentration | Amount |
|---|---|---|
| Tryptophane | 40 mM | 0.8 g |
| ddH2O | n/a | 100 mL |
| Total | n/a | 100 mL |
Filter sterilize, store in a light-proof bottle at 4°C, when it turns yellow (in about 6 months) make fresh solution.
Use 10 mL in 1 L SD or SGal medium.
Met stock, media supplements to cover yeast Methionine auxotrophy
| Reagent | Final concentration | Amount |
|---|---|---|
| Methionine | 50 mM | 0.746 g |
| ddH2O | n/a | 100 mL |
| Total | n/a | 100 mL |
Autoclave, store at room temperature (20°C–25°C), can be stored for a year.
Use 20 mL in 1 L SD or SGal medium.
His stock, media supplements to cover yeast Histidine auxotrophy
| Reagent | Final concentration | Amount |
|---|---|---|
| Histidine | 100 mM | 1.55 g |
| ddH2O | n/a | 100 mL |
| Total | n/a | 100 mL |
Autoclave, store in a light-proof bottle at 4°C, can be stored for 3 months or until it turns yellow.
Use 3 mL in 1 L SD or SGal medium.
Lys stock, media supplements to cover yeast Lysine auxotrophy
| Reagent | Final concentration | Amount |
|---|---|---|
| Lysine | 100 mM | 1.827 g |
| ddH2O | n/a | 100 mL |
| Total | n/a | 100 mL |
Sterile filter, store in a light-proof bottle at 4°C, can be stored for 3 months or until it turns yellow.
Use 10 mL in 1 L SD or SGal medium.
3-amino-1,2,4-triazole (3AT) stock, drug that elicits His starvation
| Reagent | Final concentration | Amount |
|---|---|---|
| 3AT | 2 M | 0.8408 g |
| ddH2O | n/a | 5 mL |
| Total | n/a | 5 mL |
Make fresh each time, using a sterile Falcon tube and sterile ddH2O, no need to sterile filter.
Add to SD or SGal medium just before pouring into petri plates.
For 30 mM final concentration use 1.5 mL in 100 mL.
3AT is a Hazard, wear a face mask or use a chemical hood, while weighing and dissolving 3AT.
Note: The yeast strain must be His prototrophic.
30% Glycerol
| Reagent | Final concentration | Amount |
|---|---|---|
| Glycerol | 30% | 30 mL |
| ddH2O | n/a | 70 mL |
| Total | n/a | 100 mL |
Make fresh each time, mix well.
Sterile toothpicks:
-
•
Use glass salt-and-pepper shakers with metal lid.
-
•
Enlarge the holes in the lid with a nail and a hammer (Figure S1A).
-
•
Add toothpicks, only fill the shaker up to 2/3 of its volume (Figure S1A).
-
•
Close the shaker, place an aluminum foil cap (double layer aluminum foil) on the metal cap (Figure S1B).
-
•
Wrap shaker in aluminum foil (Figure S1C).
-
•
Autoclave.
-
•
For usage, take off the aluminum foil wrapping.
-
•
Take off the aluminum foil cap, and place next to Bunsen burner to keep its inside sterile (Figure S1D).
-
•
Turn salt-and-pepper shaker up-side-down and gently shake, to make toothpicks emerge through the holes.
-
•
With one hand take a toothpick for your experiments (without touching any other toothpicks).
-
•
Place the shaker close to the Bunsen burner until the next toothpick is needed.
-
•
When done, place down the salt-and-pepper shaker, and place the aluminum foil cap back onto the shaker to keep the remaining toothpicks sterile.
Sterile velvets.
-
•
Brush velvets (15 × 15 cm, made of cotton only) to remove any lint.
-
•
Lay out a double layer of aluminum foil, 40 × 40 cm.
-
•
Place 5 velvets up-side down on the foil.
-
•
Close aluminum foil, making sure the velvets remain flat and are fully enclosed.
-
•
Wrap package in another layer of aluminum foil.
-
•
Autoclave, let dry in a warm oven, this may take a few days.
-
•
Alternatively, sterilize at 180°C for 2 h in an oven (dry heat).
Cryogenic tubes for permanent yeast storage.
-
•
Place 2 mL screw-cap tubes in a cardboard microfuge tube box.
-
•
Add 500 μL of 30% glycerol to each tube.
-
•
Close tubes with lid, and open up again a quarter turn.
-
•
Autoclave.
Note: Do not turn box sideways or up-side-down.
-
•
Let cool to room temperature (20°C–25°C).
-
•
Close lids.
-
•
Store at room temperature (20°C–25°C), can be stored for a year.
Sterilize the following items
Sterilization is performed in an autoclave at 121°C, at least at 15 psi, for 20 min, or at dry heat at 180°C for 2 h, as indicated below. When removing items from the autoclave, place them in a warm room (30°C–60°C) for 1–2 days for drying.
-
•
Sterile tips for pipettes: Place pipet tips in pipette tip box, secure lid with autoclave tape, and then autoclave.
-
•
Sterile 1.5 mL microfuge tubes: Place tubes in glass jar, close lid loosely, secure lid with autoclave tape, then autoclave. After drying, close the lids tightly.
-
•
Sterile 250 mL glass flasks: Cover opening with double layer of aluminum foil, add autoclave tape, then either autoclave or subject to dry heat.
-
•
Sterile glass tubes with lid: Place glass tubes in metal rack, add metal caps, tape autoclave tape on top of ∼two lids, and either autoclave or subject to dry heat.
-
•
Sterile glass beads: Wash glass beads (3–5 mm diameter) with tap water for 10 min in a sieve while moving them around with your hand, then rinse with ddH2O, and dry beads at 30°C–60°C. Then fill 50 mL Falcon tubes half-way with glass beads, loosely close the lid, secure lid with autoclave tape, place tubes in rack, and then autoclave. After drying, close the lids tightly.
-
•
Sterile double deionized water (ddH2O): Fill 100 mL bottle with ddH2O, close lid loosely, secure with autoclave tape, then autoclave. After autoclaving tighten the lid.
Step-by-step method details
Generation of DNA fragments containing the desired mutation
Timing: 1–2 days depending on the size of PCR amplicons
Timing: For setting up each PCR reaction: about 30 min
Timing: For running each DNA gel: 1–2 h
Using a series of PCR reactions, a range of DNA fragments will be generated each containing a desired mutation.
Note: Steps 1 and 2 can be conducted in parallel.
-
1.Generate DNA fragments of the gene-of-interest (GOI) that each contain a specific mutation. The approach depends on where in the GOI the mutations are to be made, i.e., choose option a or b.
-
a.Mutations are located within a small region of the GOI.Your GOI must already be available in a plasmid suitable for expression in yeast. Generate DNA fragments harboring the desired point mutations, using PCR. Use a proofreading polymerase (such as Promega Pfu DNA polymerase) and perform PCR reactions following the manufacturer’s protocol (link to protocol is HERE). The individual PCR reactions are as follows:
-
i.For each mutagenesis, perform two PCR reactions in parallel, using as template the uncut plasmid harboring your GOI. These are PCR reactions PCR-1a and PCR-1b, as outlined in the next two steps.
-
ii.One PCR reaction will contain the sense flanking primer and antisense mutagenesis primer (Figure 2A: PCR-1a).
-
iii.The second PCR reaction will contain the antisense flanking primer, and sense mutagenesis primer (Figure 2B, PCR-1b).
-
iv.Validate successful PCR amplification via DNA gel electrophoresis.Troubleshooting #1: PCR reaction (PCR-1a or PCR-1b) leads to no amplification, or to PCR products of different sizes.
-
v.For the subsequent fusion PCR reaction, PCR-2, use the flanking primer pair, and as a template add 1/10th – 1/100th of the reaction volume of each of the two PCR reactions, PCR-1a, and PCR-1b (Figure 2C, PCR-2) (purification of the amplicon is not necessary).
-
vi.Validate successful PCR amplification via DNA gel electrophoresis.Troubleshooting #2: Fusion PCR leads to no amplification, or to multiple bands.
-
vii.Using PCR-2 as a template (1/10th - 1/100th of the reaction volume), conduct an extension PCR reaction using the extension primers (Figure 2D, PCR-3).
-
viii.Validate successful PCR amplification via DNA gel electrophoresis.Troubleshooting #2: Extension PCR leads to no amplification, or to multiple bands
-
i.
-
b.Mutations to be introduced throughout the gene.
-
i.Choose an expression vector (which has a multiple cloning site) into which you wish to clone your mutated GOI.
 CRITICAL: Either the GOI must be cloned along with a yeast promoter, or a vector must be used that contains a yeast promoter. We used a vector that contains a galactose inducible promoter (pES128-9).
CRITICAL: Either the GOI must be cloned along with a yeast promoter, or a vector must be used that contains a yeast promoter. We used a vector that contains a galactose inducible promoter (pES128-9). -
ii.Generate DNA fragments harboring the desired point mutations using PCR, as done above under steps 1aI–1aviii, except that in the first PCR (PCR-1a and PCR-1b) the starting template is your GOI, whether cloned into a plasmid or not (Figures 2A–2D).
-
iii.In order to also generate a plasmid containing the unmutated GOI, conduct PCR-2 using as template your GOI, followed by PCR-3.
-
iv.Validate successful PCR amplification via DNA gel electrophoresis.
 CRITICAL: The amplicon from PCR-3 (dubbed PCR-3 amplicon) is needed for step 5. Do not discard.
CRITICAL: The amplicon from PCR-3 (dubbed PCR-3 amplicon) is needed for step 5. Do not discard. Pause point: Completed PCR reactions can be kept frozen without any time limit.
Pause point: Completed PCR reactions can be kept frozen without any time limit.
-
i.
-
a.
Prepare vector or plasmid
Timing: 2 days, plus 2–3 days waiting time, for the entire procedure
If not available, streak out yeast on solid YPD medium to obtain single colonies (Step 3bi): day -2 or -3 [time needed: 10 min]
Inoculate liquid medium with yeast, to generate fresh overnight culture (Step 3bi): day -1 [time needed: 15–30 min]
Cut plasmid and test for complete digestion (Step 2): day 0 [time needed: 4–5 hrs including waiting times]
Make plasmid dilutions (Step 3a): day 0 [time needed: 10 min]
Transform plasmid dilutions into yeast (Steps 3bii–3c): day +1 [time needed: the whole day including waiting times]
Waiting time: 2–3 days
Evaluate numbers of transformants (Step 3d–3e): day +3 or +4 [time needed: 15 min]
Here the vector or plasmid will be linearized (step 2), and the appropriate working concentration of the linearized plasmid determined (step 3) for the subsequent in-yeast recombination step (steps 4–5).
Note: Steps 1 and 2 can be conducted in parallel.
-
2.
Cut plasmid with restriction enzymes.
Use 5 μg of plasmid DNA in a 200 μL reaction volume. The restriction digest must be complete. This is to be validated through DNA gel electrophoresis. The procedure differs depending on whether mutagenesis occurs in a small region of the GOI or throughout the GOI, i.e., perform step 2a or 2b.-
a.Mutations to be introduced within a small region in the GOI:
-
i.Linearize plasmid harboring your un-mutated GOI, at the unique restriction sites identified under Preparation I.c., using standard procedures. See table.
 CRITICAL: In parallel to the double digest reaction, perform single digest reactions. These will allow you to investigate whether each restriction enzyme did a complete digest.
CRITICAL: In parallel to the double digest reaction, perform single digest reactions. These will allow you to investigate whether each restriction enzyme did a complete digest.Sample
A
B
C
D
Double digest Single digest controls No digest control Plasmid 5 μg 0.5 μg 0.5 μg 0.5 μg 10× reaction buffer, compatible for both restriction enzymes 20 μL 2 μL 2 μL 2 μL Restriction enzyme 1 2 μL 0.2 μL n/a n/a Restriction enzyme 2 2 μL n/a 0.2 μL n/a ddH2O, to final volume of 200 μL 20 μL 20 μL 20 μL -
ii.Validate via DNA electrophoresis whether the restriction digest with each enzyme was complete. For this use 20 μL from each restriction digest samples A – D.
-
iii.If the digest was complete, store the remainder of sample A at −20°C or −80°C. It will be needed for steps 3 and 5.
-
i.
-
b.Mutations to be introduced throughout the GOI:
-
i.Cut the chosen vector in the multiple cloning sites, using the restriction enzyme identified in Preparation II.b., a single digest is sufficient, see table.
Sample
A
D
Digest No digest control Plasmid 5 μg 0.5 μg 10× reaction buffer 20 μL 2 μL Restriction enzyme 2 μL n/a ddH2O, to final volume of 200 μL 20 μL -
ii.Validate via DNA electrophoresis whether the restriction digest was complete, using 20 μL of each restriction digest sample.
-
iii.If the digest was complete, store the remainder of sample A at −20°C or −80°C. It will be needed for steps 3 and 5.
 Pause point: The linearized plasmid (Restriction digest sample A) can be kept frozen without any time limit.
Pause point: The linearized plasmid (Restriction digest sample A) can be kept frozen without any time limit.
-
i.
-
a.
-
3.Determine the appropriate working concentration for the linearized plasmid.Note: The digested plasmid from 2 does not need to be purified.
 CRITICAL: All steps need to be conducted aseptically.
CRITICAL: All steps need to be conducted aseptically.-
a.Dilute the linearized plasmid 10, 20, 40, and 80-fold in double deionized sterile water.
-
b.Generate competent yeast cells.
 CRITICAL: All steps need to be conducted aseptically.
CRITICAL: All steps need to be conducted aseptically.-
i.Inoculate a single yeast colony in YPD medium (4 mL, in sterile glass tube with cap) and grow overnight for 20–40 h at 30°C, using the test tube rotator.
-
ii.Transfer 1 mL of the overnight culture to each of two sterile 250 mL flasks containing 50 mL of YPD medium (Figure 3, point 1).
-
iii.Grow at 30°C, shaking at 160 rpm until OD600 of 0.8, but not higher than 1 (this takes approximately 3 h depending on your strain) (Figure 3, point 2).
-
iv.Transfer each culture into a sterile 50 mL Falcon tube aseptically.
-
v.Centrifuge at 1,500–2,000 × g (4,000 rpm for MultifugeX1R Pro, Thermo Fisher Scientific) for 5 min, discard supernatant (Figure 3, point 3).
-
vi.Resuspend each pellet in 8 mL Solution I (0.1 M Li-Acetate solution) (Figure 3, point 4).
-
vii.Either incubate for 30 min shaking at 30°C for same day transformations, or incubate at 4°C (not shaking) until the next day (Figure 3, point 5).
-
viii.Pellet cells by centrifugating at 1,500–2,000 × g (4,000 rpm for MultifugeX1R Pro, Thermo Fisher Scientific) for 5 min, discard the supernatant.
-
ix.Resuspend each pellet in 500 μL of solution I, (Figure 3, point 6), and combine the content of the two Falcon tubes into one tube.
-
x.Keep at 4°C until usage (same or next day).
-
i.
-
c.Transform the plasmid dilutions into yeast. In parallel, as controls, transform 1 μL of uncut plasmid (0.1–0.3 μg, positive control, sample 1) and no plasmid DNA (negative control, sample 6), respectively, as outlined next:Modified from (Gietz et al., 1995; Ma et al., 1987).
 CRITICAL: All steps need to be conducted aseptically.
CRITICAL: All steps need to be conducted aseptically.-
i.Heat an aliquot of herring sperm DNA (100 μL is sufficient for 20 transformations) for 10 min at 100°C, quickly cool at 4°C (Figure 3, point 7).
-
ii.Lay out aseptically six sterile 1.5 mL microfuge tubes, one tube for each sample (see below table) (Figure 3, point 8). Below is a table showing the samples required for the transformation:
Sample numberHerring sperm DNA Circular plasmid Linearized plasmid
5 μL1 5 μL 1 μL n/a 2 5 μL n/a 1:10 diluted plasmid 3 5 μL n/a 1:20 diluted plasmid 4 5 μL n/a 1:40 diluted plasmid 5 5 μL n/a 1:80 diluted plasmid 6 5 μL n/a n/a -
iii.Aliquot 5 μL of herring sperm DNA into each tube. (Figure 3, point 8).
-
iv.Add circular plasmid (uncut plasmid) and the dilutions of linearized plasmid in the respective tubes, as outlined in the table above (Figure 3, point 9).
-
v.Slowly pipet up and down the competent cells (from step 3bx) to resuspend the cells.
-
vi.Then add 100 μL of competent cells to each tube, and slowly pipet up and down to mix the sample.
-
vii.Incubate for 30 min at 30°C (Figure 3, point 10).
-
viii.Add 700 μL of Solution II (40% PEG-Li-Acetate solution) and mix (Figure 3, point 11).Note: Make sure the sample is mixed well. Best is to pipet solution II into the bottom of the tube, close the tube and shake tubes vigorously back and forth while horizontal.Note: Pipet slowly as solution II is viscous and easily gets drawn into the pipette.
-
ix.Incubate for 45 min at 30°C, shaking (Figure 3, point 12).
-
x.Subject to heat shock treatment at 42°C for 15 min, then transfer samples to ice for 2–5 min (Figure 3, point 13).
-
xi.Move to the next step or pause.
 Pause point: Samples can be stored for up to 20 h at 4°C.
Pause point: Samples can be stored for up to 20 h at 4°C. -
xii.Pellet cells at ∼100 × g (1,000 rpm for Pico centrifuge, Thermo Fisher Scientific) for 3 min and discard the supernatant.
-
xiii.Repeat the centrifugation for 1 min.
-
xiv.Take off the remainder of the supernatant using a 20–100 μL pipette (Figure 3, point 14).
-
xv.Add 10–20 sterile glass beads (diameter 3–5 mm) to each plate containing media that selects for transformants (Figure 3, point 15).
-
xvi.Resuspend the cell pellet in 50–100 μL of SD liquid medium lacking supplements and lacking a carbon source.
-
xvii.Transfer each sample to a plate containing the appropriate selective medium and the glass beads (Figure 3, point 16).
-
xviii.Shake plates back and forth to make the beads move around on the solid medium, thereby spreading the cells on the plate.
-
xix.Tip off the glass beads into a container with 70% ethanol solution (the beads can be washed, dried, autoclaved and re-used again) (Figure 3, point 17).
-
xx.Incubate plates at 30°C for 2–3 days (Figure 3, point 18).
-
i.
-
d.The control transformation with uncut plasmid (sample 1) should yield at least 1000 colonies.Note: High transformation efficiency is critical for step 5.Troubleshooting #3: The transformation with circular plasmid leads to a low number of transformants.
-
e.Determine the number of transformants obtained for each plasmid dilution.
-
f.Determine which linearized plasmid dilution leads to no more than 5–10 transformants. This is the optimal working concentration for step 5. In our example the optimal working concentration would be a 1:80 dilution (Figure 1C).Troubleshooting #4: Linearized plasmid, even the 1:80 dilution, leads to a lot of transformants.
-
a.
Figure 3.
Graphic illustration of yeast transformation for the generation of plasmid borne GOI with desired point mutations
For more detail see image and text (steps 4 and 5).
Generate circularized plasmid containing the GOI with a desired point mutation
Timing: 1 day, plus 3–4 days waiting time, for the entire procedure
Generate fresh liquid yeast overnight culture (Step 4a): day -1
[time needed: 15–30 min]
Yeast transformation (Steps 4b–4k, and Steps 5a–5s): day 0
[time needed: the whole day including waiting times]
Waiting time: 2–3 days
Select transformants (Steps 5t–5w): day +2 or +3
[time needed: 15 min–2 hrs depending on number of transformations]
Retrieval and storage of mutation transformants (Step 5w): day +3 or +4
[time needed: 5 min]
Colony PCR (Step 5x): day +3, +4, or +5
[time needed for setting up PCR reaction: 1–3 hrs depending on number of colony PCRs]
Here the plasmids will be generated that contain the GOI with the desired mutation. For this, the DNA fragment generated with PCR-3 in step 1 (dubbed PCR-3 amplicon) along with the linearized plasmid from steps 2–3 will be transformed into yeast, where recombination events will lead to the DNA-fragment being introduced into the plasmid, to generate the final circular plasmid that contains the GOI.
-
4.Generation of competent yeast cells.
 CRITICAL: All steps need to be conducted aseptically.Note: If recombination events can occur between sequences of the chromosomal GOI and the plasmid or the DNA fragment, it is important to use a yeast strain that is deleted for the GOI.
CRITICAL: All steps need to be conducted aseptically.Note: If recombination events can occur between sequences of the chromosomal GOI and the plasmid or the DNA fragment, it is important to use a yeast strain that is deleted for the GOI.-
a.Inoculate a single yeast colony in YPD medium (4 mL, in sterile glass tube with cap).
-
b.Grow overnight (for 20–40 h) at 30°C, using the test tube rotator.
-
c.Transfer 1 mL of the overnight culture to a sterile 250 mL flask containing 50 mL of YPD medium (Figure 3, point 1).
-
d.Grow at 30°C, shaking at 160 rpm until OD600 of 0.8, but not higher than 1 (this takes approximately 3 h depending on your strain) (Figure 3, point 2).
-
e.Transfer culture into a sterile 50 mL Falcon tube aseptically.
-
f.Centrifuge at 1,500–2,000 × g (4,000 rpm for MultifugeX1R Pro, Thermo Fisher Scientific) for 5 min, discard supernatant (Figure 3, point 3).
-
g.Resuspend pellet in 8 mL Solution I (0.1 M Li-Acetate solution) (Figure 3, point 4).
-
h.Either incubate for 30 min shaking at 30°C for same day transformations, or incubate at 4°C (not shaking) until the next day (Figure 3, point 5).
-
i.Pellet cells by centrifugating at 1,500–2,000 × g (4,000 rpm for MultifugeX1R Pro, Thermo Fisher Scientific) for 5 min, discard the supernatant.
-
j.Resuspend pellet in 500 μL of solution I, (Figure 3, point 6).
-
k.Keep at 4°C until usage (same or next day).
-
l.Note that for each individual transformation, 100 μL of competent cells are required.
-
a.
-
5.
Yeast transformation and homologous recombination.
Modified from (Gietz et al., 1995; Ma et al., 1987). CRITICAL: All steps need to be conducted aseptically.
CRITICAL: All steps need to be conducted aseptically.-
a.Heat an aliquot of herring sperm DNA (100 μL is sufficient for 20 transformations) for 10 min at 100°C, quickly cool at 4°C (Figure 3, point 7).
-
b.Lay out aseptically sterile 1.5 mL microfuge tubes, one tube for each sample (see below table) (Figure 3, point 8). Below is a table showing the samples required for the transformation:
Sample numberHerring sperm DNA Circular plasmid Linearized plasmid optimal dilution, as determined in step 3 PCR-3 amplicon∗ 1 5 μL 1 μL n/a n/a 2 5 μL n/a 5 μL n/a 3 5 μL n/a n/a 5 μL PCR-3 amplicon #1 4 5 μL n/a 5 μL 5 μL PCR-3 amplicon #1 5 5 μL n/a n/a 5 μL PCR-3 amplicon #2 6 5 μL n/a 5 μL 5 μL PCR-3 amplicon #2 . . . etc x-2 5 μL n/a n/a 5 μL PCR-3 amplicon #n x-1 5 μL n/a 5 μL 5 μL PCR-3 amplicon #n Final tube x 5 μL n/a n/a n/a ∗ The amplicons were generated in step 1. Each amplicon (numbered from #1 to #n) represents an amplicon with a specific mutation, as well as the un-mutated amplicon if required. -
c.Aliquot 5 μL of herring sperm DNA into each tube.
-
d.Add 5 μL of linearized plasmid DNA, PCR-3 amplicon, and uncut plasmid, in the respective tubes, as outlined in the table above (Figure 3, point 9).
-
e.Slowly pipet up and down the competent cells (from step 4) to resuspend the cells.
-
f.Then add 100 μL of competent cells to each tube, and slowly pipet up and down to mix the sample.
-
g.Incubate for 30 min at 30°C (Figure 3, point 10).
-
h.Add 700 μL of Solution II (40% PEG-Li-Acetate solution) and mix (Figure 3, point 11).Note: Make sure the sample is mixed well. Best is to pipet solution II into the bottom of the tube, close the tube and shake tubes vigorously back and forth while horizontal.Note: Pipet slowly as solution II is viscous and easily gets drawn into the pipette.
-
i.Incubate for 45 min at 30°C, shaking (Figure 3, point 12).
-
j.Subject to heat shock treatment at 42°C for 15 min, then transfer samples to ice for 2–5 min (Figure 3, point 13). Move to the next step or pause.
 Pause point: Samples can be stored for up to 20 h at 4°C.
Pause point: Samples can be stored for up to 20 h at 4°C. -
k.Pellet cells at ∼100 × g (1,000 rpm for Pico centrifuge, Thermo Fisher Scientific) for 3 min and discard the supernatant.
-
l.Repeat the centrifugation for 1 min.
-
m.Take off the remainder of the supernatant using a 20–200 μL pipette (Figure 3, point 14).
-
n.Add 10–20 sterile glass beads (diameter 3–5 mm) to each plate containing media that selects for transformants (Figure 3, point 15).
-
o.Resuspend the cell pellet in 50–100 μL of SD medium lacking supplements and lacking a carbon source.
-
p.Transfer each sample to a plate containing the appropriate selective medium and the glass beads (Figure 3, point 16).
-
q.Shake plates back and forth to make the beads move around on the solid medium, thereby spreading the cells on the plate.
-
r.Tip off the glass beads into a container with 70% ethanol solution (the beads can be washed, dried, autoclaved and re-used again) (Figure 3, point 17).
-
s.Incubate plates at 30°C for 2–3 days (Figure 3, point 18).
-
t.The control transformation with uncut plasmid (sample 1) should yield at least 1000 colonies.Troubleshooting #3: The transformation with circular plasmid leads to a low number of transformants.
-
u.Determine the number of transformants of the linearized plasmid-alone control (usually we obtain about 10 transformants).Note: It should have far less transformants than the plates growing transformants with the newly assembled circular plasmid with insert (usually we obtain 200–300 transformants).
 Pause point: Transformation plates can be stored for 3 days in the fridge. Do make sure the plates do not freeze.
Pause point: Transformation plates can be stored for 3 days in the fridge. Do make sure the plates do not freeze. -
v.Using sterile toothpicks, pick single colonies, and patch them on a fresh plate. These transformants contain the circularized plasmid (Figures 2E and 2F).Note: Picking 10 colonies is plenty, as 8–10 of these will contain the desired newly assembled plasmid.
-
w.Incubate plate for 1 day.Note: These transformants harbor the newly formed plasmids expressing the GOI with the desired mutation. For simplicity, from now onwards these transformants are called mutation transformants.Note: It is highly recommended to use some of these cells for permanent storage at −80°C. For this, for each mutation transformant, use a sterile toothpick to aseptically transfer cells directly from the plate into the sterile cryogenic tube containing 30% glycerol, vortex, and then transfer to −80°C.
 Pause point:This plate can be stored in the fridge for 2 weeks. Do make sure the plates do not freeze.
Pause point:This plate can be stored in the fridge for 2 weeks. Do make sure the plates do not freeze. -
x.A PCR based assay, i.e., colony PCR, can be used to test that the insert has been integrated into the plasmid. We recommend non-proofreading Taq polymerase for colony PCR.
-
i.For this, for each site of recombination, design primers that anneal to DNA flanking the area of recombination, yielding a PCR amplicon that preferably is 200–400 bp long (Figure 2F).
-
ii.Set up a standard PCR reaction, 20 μL volume.
-
iii.Grab the back part of a sterile yellow pipette tip with your thumb and index finger, making sure that the tip’s tip remains sterile. Then use the tip’s tip to aseptically transfer a tiny amount of yeast cells (hardly visible with the bare eye) from the plate into the PCR reaction mix.
 CRITICAL: Do not use toothpicks.
CRITICAL: Do not use toothpicks. CRITICAL: The yeast cells must be freshly grown, and not taken from a plate that was stored in the fridge.
CRITICAL: The yeast cells must be freshly grown, and not taken from a plate that was stored in the fridge. CRITICAL: Keep the tip’s tip emerged in the PCR reaction mix while rolling it back and forth against the wall of the tube, to ensure the cells have been transferred from the tip into the liquid.
CRITICAL: Keep the tip’s tip emerged in the PCR reaction mix while rolling it back and forth against the wall of the tube, to ensure the cells have been transferred from the tip into the liquid. -
iv.Place the samples into the PCR cycler, and start the machine.
 CRITICAL: The first denaturation event of the PCR reaction should be at 95°C for 5 min to ensure cell lysis, then continue with the PCR cycles according to the manufacturer's instructions (these can be found HERE).
CRITICAL: The first denaturation event of the PCR reaction should be at 95°C for 5 min to ensure cell lysis, then continue with the PCR cycles according to the manufacturer's instructions (these can be found HERE). CRITICAL: Do not centrifuge the PCR reactions once the cells have been added.
CRITICAL: Do not centrifuge the PCR reactions once the cells have been added.
-
i.
-
y.If the endogenous GOI needs to be re-introduced into the strain (recommended), go to step 6, otherwise go to step 7.
-
a.
Re-introducing endogenous GOI / generating diploids to cover ectopic mutations
Timing: 3–4 days for the entire procedure
For mating on solid medium:
Prepare master plates (Steps 6ai–6av): day -1 [time needed: 30 min (time is per master plate)]
Replica plating (Steps 6avi–6ax) day 0 [time needed: 15 min]
Waiting time: 2–3 days
Select diploid strains (Steps 6axi–6axii): day +2 or +3 [time needed: 15 min]
Retrieval of diploid strains (Steps 6axiii–6axiv): days +3 or +4 [time needed: 15–30 min]
Storage of diploid strains (Step 6axiv): day +4 or +5 [time needed: 5 min] [time needed for transferring yeast to permanent storage: 30 min]
For mating in liquid medium: Prepare master plate (Steps 6bi–6bvi): day -1 to -3 [time needed: 1–2 hrs (time is per mating plate)]
Prepare overnight culture of mating strain (Step 6bvii): day -1 [time needed: 10 min]
Set up mating (Steps 6bviii–6bxiii): day 0 [time needed: 30-60 min]
Waiting time: 1–2 days
Select for diploid strains (Steps 6bxiv–6bxvi): day +1 or +2 [time needed: 30–60 min]
Waiting time: 1–2 days
Retrieval of diploid strains (Step 6bxvi–6bxviii): day +3 or +4 [time needed: 30–60 min]
Storage of diploid strains (Step 6bxviii): day +4 or +5 [time needed: 5 min] [time needed for transferring yeast to permanent storage: 1–2 hrs]
The advantage of generating diploids is the fact that ectopic mutations that may occur during transformation (which are predominantly recessive), are complemented for in the diploid cell, reducing the risk of obtaining false positive or negative results in the subsequent phenotypic screen (steps 7–15).
To generate diploids, a mating strain is required that lacks the GOI and that is of the opposite mating type to the mutation transformants. Also, the mutation transformants and the mating strain each need to have an auxotrophy that can be complemented by the other strain, to enable the selection for the diploid strains (Figure S2).
The mating step may also be required if the endogenous GOI must be present in the cell for the phenotypic screen. Such a scenario would be when the plasmid-borne GOI elicits a dominant effect over the endogenous GOI. For example, in our sample study the plasmid borne truncated GOI was generated following steps 1–5. The expressed truncated protein disrupts the protein-protein interaction between the endogenous GOI (Gcn1) and its interaction partner (Gcn2) (see Figure 7B), thereby preventing growth on starvation medium (Figure 7A, rows 1,2 vs 7,8).
Figure 7.
Sample result
(A) Following procedures from steps 1–5, yeast cells were generated that express from a galactose inducible promoter the RWDBD of Gcn1, each containing an amino acid substitution (labeled a – h in the image). Substitution g is R2259A, shown previously to be required for Gcn2 binding (Sattlegger and Hinnebusch, 2000). Mutation transformants were then subjected to semiquantitative growth assays, using media containing glucose or galactose as carbon source, and a plate containing galactose and 3-amino-1,2,4-triazole (starvation). The growth on plates containing glucose or galactose was similar, suggesting the overexpression of the RWDBD did not affect cell growth. Shown in the figure are the plates containing galactose as carbon source (control). 3AT causes His starvation, and only cells able to activate Gcn2 can overcome starvation and grow (row 1,2,9,10). It has been shown previously that the Gcn1-Gcn2 interaction is critical for Gcn2 activation, mediated by a region in Gcn1 called RWDBD (Rakesh et al., 2017; Sattlegger and Hinnebusch, 2000). Overexpression of the RWDBD itself disrupts the Gcn1-Gcn2 interaction, and the concomitant inhibition of Gcn2 is visible by reduced growth on 3AT (rows 7,8,15,16). Single amino acid substitutions in the GOI leading to cell growth comparable to that of cells overexpressing wild-type (unmutated) GOI, would suggest that the mutated RWDBD are still able to bind and inhibit Gcn2. Hence the respective amino acids are not required for Gcn2 binding (rows 3,5,11,12,14). Single amino acid substitutions b, d, g, allowed better growth on 3AT, suggesting that the respective amino acids are relevant for Gcn2 binding (rows 4,6,13). The more Gcn2 function is impacted, the weaker the growth on 3AT, hence cell growth is indicative of the level of Gcn2 activity. Based on that, the importance of amino acids is ranked g > d > b.
(B) Illustration of protein-protein interactions occurring in the respective strains. See text for more detail (see under Anticipated Results).
(C) Growth scores used for quantitatively determining the growth of strains in (A) (step 16a).
(D) Relative growth rates for strains in (A) were calculated, following step 16. The values and calculations are provided in Data S2. The growth rates relative to the strain expressing GST alone were then plotted in a bar graph.
To re-introduce the GOI, a strain is required that contains the desired GOI and is of the opposite mating type to the mutation transformants. Also, the mutation transformants and the mating strain each need to have an auxotrophy that can be complemented by the other strain, to enable the selection for the diploid strains (Figure S2).
-
6.Mating. This can be done on solid medium or in liquid medium, as outlined next, i.e., choose step 6a or 6b. While the mating on solid medium requires less time on the bench, the mating in liquid medium allows the processing of a larger number of mating events (12 vs 50 mating events per plate).
 CRITICAL: All steps need to be conducted aseptically.
CRITICAL: All steps need to be conducted aseptically.-
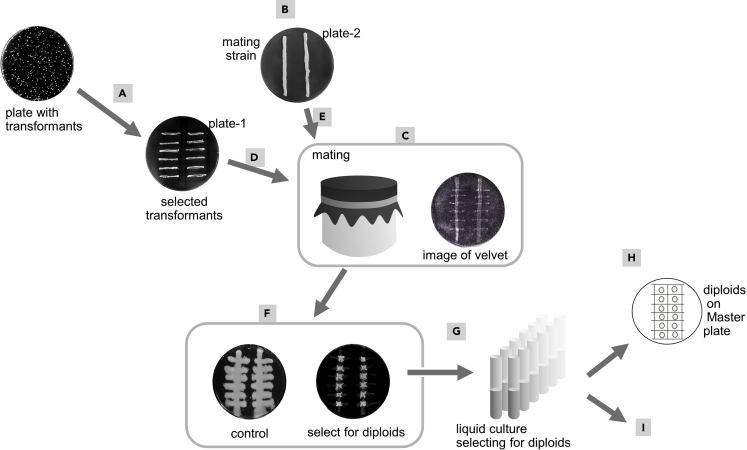
a.Mating on solid medium.
-
i.Draw a grid on the back of a plate containing appropriate selective medium to maintain the plasmid in the mutation transformants.
-
ii.Patch the mutation transformants on the plate minding the borders given by the grid (Figure 4A). Also include a transformant harboring the unmutated plasmid. This is plate-1.
-
iii.On another plate containing YPD medium, draw two lines on the back of the plate. This is plate-2.
-
iv.Patch the mating strain on plate-2 along the drawn lines (Figure 4B). This is plate-2.
-
v.Incubate plate-1 and plate-2 up-side-down for 15–20 h at 30°C. Make sure the cells have just grown up and are not overgrown.
-
vi.Under sterile conditions, mount a sterile velvet onto the replica plater. Secure the velvet cloth using the supplied ring (Figure 4C, left panel).
-
vii.Transfer cells from plate-1 onto the velvet (Figure 4D).
- viii.
-
ix.Now transfer the cells from the velvet onto fresh plates.Note: The first two plates harbor medium selecting for diploids [in our case it was SD medium with ILV]. The final plate contains YPD medium which serves as the control for even and efficient transfer of cells.
-
x.Incubate replica plates at 30°C for 2–3 days.Note: Only diploids should be growing on the plate selecting for diploids, and neither parental strain should grow (Figure 4F, plate on the right). In other words, there should be only growth at the intersections of the patches from both parents. On the YPD control plate the diploid as well as haploid cells have grown (Figure 4F, plate on the left).Troubleshooting #5: The haploid strains grow on medium selecting for diploid strains.
-
xi.With a sterile toothpick, take cells from the middle of the grown patch of the diploid cells, and transfer each to a sterile glass tube with lid, containing liquid medium that selects for diploids (Figure 4G, lids not shown in the image).
-
xii.Grow cultures until saturation at 30°C, agitating. This takes 1–2 days.
-
xiii.For each culture, aseptically transfer a drop (about 5 μL) to solid medium selecting for diploids (Figure 4H).
-
xiv.Let the drop dry in, incubate overnight (about 20 h) up-side-down at 30°C. This is your master plate for future experiments. Store at 4°C.Note: Diploid cells do not stay viable for a long time. It is highly recommended to use some of these cells for permanent storage at −80°C. For this, for each diploid strain use a sterile toothpick to aseptically transfer cells directly from the plate into a sterile cryogenic tube containing 30% glycerol, vortex, and then transfer to −80°C.
-
xv.Using the saturated culture from step 6axii, commence with the phenotypic screening starting with step 7 (Figure 4I).Note: The saturated liquid cultures need to be used within 1–2 days for the screening procedure (steps 7–15). Alternatively, make fresh liquid cultures, using cells from the master plate (step 6axiv).
 Pause point: The master plate harboring the diploids (step 6axiv) can be kept in the fridge for up to 1 week, do make sure the plate does not freeze.
Pause point: The master plate harboring the diploids (step 6axiv) can be kept in the fridge for up to 1 week, do make sure the plate does not freeze.
-
i.
-
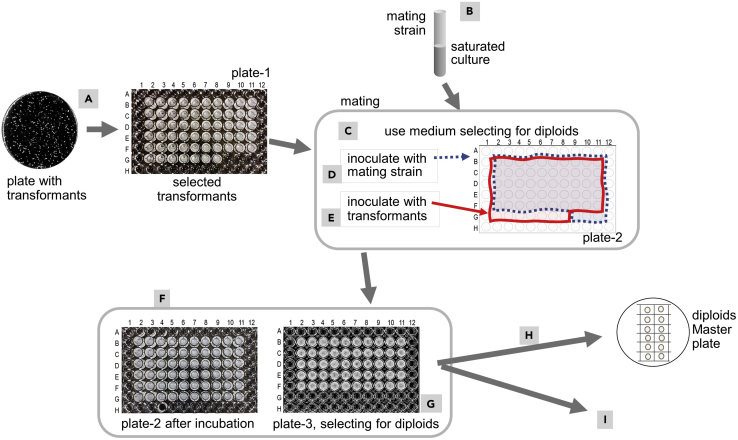
b.Mating in liquid medium.This procedure allows the mating of a larger number of mutation transformants.
-
i.In a 96 flat-well microtiter plate add to all wells 180 μL of SD liquid medium suitable for maintaining the plasmid present in the mutation transformants [in our case it was SD medium with Trp, ILV], this is plate-1.
-
ii.Inoculate wells B2-11, C2-11, D3-11, E3-11, F2-11 with mutation transformants (Figure 5A).Note: Use some of the wells for controls, such as transformants harboring the unmutated plasmid.
-
iii.Inoculate wells G2-6 with transformants that only serve as a control for mating.
-
iv.Cover the microtiter plate-1 with its lid.
-
v.Place plate-1 into a zip lock bag that contains a few holes to allow air exchange.Note: This prevents excessive evaporation of the media during incubation.
-
vi.Incubate plate-1 at 30°C until the bottom of the wells is covered with cells. This takes 1–3 days depending on how many cells were used for inoculation.Note: Agitation of the plate during incubation is not necessary.Note: Wells in row A and H, and column 1 and 12, only contain SD medium, to help reduce evaporation of liquid from wells containing cells.
-
vii.Generate a fresh overnight culture of the mating strain (Figure 5B).Note: The culture should not have entered the stationary phase for too long. For this, inoculate a sterile glass tube containing 4 mL of YPD medium with a colony of the mating strain, and grow for 15–20 h at 30°C, using the test tube rotator.
-
viii.In a fresh 96 flat-well microtiter plate, add 180 μL of liquid medium suitable for selecting for diploids as well as for the plasmid [in our case it was SD medium with ILV] (Figure 5C). This is plate-2.
-
ix.Transfer 10 μL of the mating strains’ fresh overnight culture to the wells B2-11, C2-11, D3-11, E3-11, F2-11, and G9-11 (Figure 5D, indicated with blue dotted lines).
-
x.Using an 8-channel pipette, pipet up and down the content of the wells in column 2 of plate-1, to resuspend the cells. Then transfer 10 μL from the wells in column 2 of plate-1 to the wells in column 2 of plate-2.
-
xi.Using new tips each time, repeat the process in step 6bx for each, wells 3–11 (Figure 5E).
-
xii.Cover the plate with its lid.
-
xiii.Place plate-2 in a zip-lock bag with holes, and incubate at 30°C until cells have covered the bottom of the well. This takes about 1–2 days.Note: At this stage haploid cells are still able to grow to some extent (Figure 5F).
-
xiv.In a fresh 96 flat-well microtiter plate add 180 μL of liquid medium suitable for selecting for diploids. This is plate-3.
-
xv.Using the 8-channel pipette, transfer 10 μL from each well in plate-2 to its respective well in plate-3 (Figure 5G), similarly to how it was done in steps 6bx–6bxi. Cover the plate with its lid.
-
xvi.Incubate plate-3 in a zip-lock bag with holes at 30°C until visible growth is seen, this takes 1–3 days.Note: Only diploid cells will be able to grow, while the wells containing haploid cells should not show any growth, i.e., the liquid should be clear (Figure 5G).Troubleshooting #5: The haploid strains grow on medium selecting for diploid strains.
-
xvii.If a master plate for future experiments is needed, for each culture, transfer a drop (about 5 μL) onto solid medium selecting for diploids (Figure 5H).
-
xviii.Let the drop dry in, incubate overnight (15–20 h) up-side-down at 30°C. Then use directly or store at 4°C.Note: Diploid cells do not stay viable for a long time. It is highly recommended to use some of these cells for permanent storage at −80°C. For this, for each diploid strain use a sterile toothpick to aseptically transfer cells directly from the plate into a sterile cryogenic tube containing 30% glycerol, vortex, and then transfer to −80°C.
-
xix.The saturated cultures in the wells of plate-3, step 6bxvi, can be directly used for phenotypic screening as outlined in steps 7–15.Note: The saturated liquid cultures from step 6bxvi (plate-3) need to be used within 1–2 days for the screening procedure (steps 7–15). Alternatively, make fresh liquid cultures, using cells from the master plate (step 6bxviii).
 CRITICAL: The master plate harboring the diploids (steps 6axiv or 6bxviii) can be kept in the fridge for 1 week, do make sure the plate does not freeze. We highly recommend to immediately use some of these cells for permanent storage at −80°C. For this, for each diploid strain use a sterile toothpick to aseptically transfer cells directly from the plate into a sterile cryogenic tube containing 30% glycerol, vortex, and then transfer to −80°C. This is because diploid cells cannot be stored at room temperature (20°C–25°C) or at 4°C for very long.
CRITICAL: The master plate harboring the diploids (steps 6axiv or 6bxviii) can be kept in the fridge for 1 week, do make sure the plate does not freeze. We highly recommend to immediately use some of these cells for permanent storage at −80°C. For this, for each diploid strain use a sterile toothpick to aseptically transfer cells directly from the plate into a sterile cryogenic tube containing 30% glycerol, vortex, and then transfer to −80°C. This is because diploid cells cannot be stored at room temperature (20°C–25°C) or at 4°C for very long.
-
i.
-
a.
Figure 4.
Introducing the genomic GOI into the mutation transformants via mating on solid medium
(A–I) For more detail see image and text (step 6a). The concept of mating and selecting for diploid cells is explained in Figure S2.
Figure 5.
Introducing the genomic GOI into the mutation transformants via mating in liquid medium
For more detail see image and text (step 6b). The concept of mating and selecting for diploid cells is explained in Figure S2.
Screening for mutations eliciting a desired phenotypic effect
Timing: 1 day, plus monitoring growth for 2–3 weeks, for the entire procedure
Prepare fresh overnight cultures (Step 6axv, 6bxix, or 7): day -1 [time needed: 30 min]
Set up growth assay (Steps 8–14): day 0 [time needed: 30 min – 2 hrs per assay, depending on number of plates]
Monitor growth (Step 15): weeks 1 to 2, or longer, depending on how long it takes to see growth differences [time needed for each monitoring event: 10 –30 min depending on number of plates]
Here, the yeast transformants containing the mutated GOI will be investigated for a change in phenotype, as compared to the yeast transformants containing the un-mutated GOI.
CRITICAL: All steps need to be conducted aseptically.
-
7.
Depending on the phenotypic growth assay, generate liquid overnight cultures either of the diploid strains (steps 6axiv or 6bxviii), using medium selecting for the plasmid and for the diploids, or generate overnight cultures of the mutant transformants (step 5w) using medium selecting for the plasmid.
CRITICAL: The overnight cultures must be saturated and must be well in the stationary phase. For this, inoculate each sterile glass tube containing 4 mL of the appropriate liquid medium with a colony of the respective strain. Grow the cultures for 39–48 h at 30°C, using the test tube rotator. To test whether the cultures are in the stationary phase, check the OD600nm of the cultures after 33–42 h of incubation, and 6–12 h later. The OD should be the same at both time points. If not, continue growing the cultures until there is no OD change.
CRITICAL: Strains used for the same growth assay need to be inoculated into separate sterile glass tubes containing the same batch of liquid medium, inoculated with similar starting densities, and grown for the same time period and under the same condition (e.g., temperature).
-
8.
In a microtiter plate, add to the wells in columns 2–5, 180 μL of SD medium lacking glucose as carbon source and lacking supplements (Figure 6A).
-
9.
In column 1 add 100–200 μL of the strains’ overnight cultures (Figure 6B).
Note: Eight strains can be investigated per plate.
-
10.Conduct 10-fold serial dilutions as follows (Figure 6C):
-
a.Using an 8-channel pipette set to 20 μL, pipet up and down the content of the wells in column 1, to mix the cells.
-
b.Then transfer 20 μL from column 1 to column 2.
-
c.Pipet up and down several times the content of the wells in column 2, to mix the cells.
-
d.Then transfer 20 μL from column 2 to column 3.
-
e.Repeat the process (steps 10c–10d), i.e., transfer cells from column 3–4, then column 4–5.Note: The same pipette tips can be used for steps 10a–10e.
-
f.Place the lid on the microtiter plate to keep the samples sterile.
-
a.
-
11.Prepare plates with solid medium suitable for the growth assay:
-
a.Select petri plates containing solid medium for scoring for phenotypic effects, i.e., for scoring differences in growth rates (for simplicity here dubbed growth-assay plates), and plates with solid medium that do no elicit phenotypic effects (control plates).
- b.
-
a.
-
12.Transfer cells from the microtiter plate to the solid medium:
-
a.Using an 8-channel pipette set to 50 μL, pipet up and down the content of the highest dilution (column 5) (Figure 6E).Note: This is to resuspend the cells which due to their size sediment over time.
-
b.Then - using an 8-channel pipet set to 5 μL - transfer 5 μL of the highest dilution (column 5) to each plate (Figures 6E and 6F).
-
c.Repeat the process for microtiter plate column 4, then 3, then 2, and finally column 1.Note: Each time when transferring samples to the plates with an 8-channel pipette, best is to first focus on aligning the 2 middle tips with the respective intersection of the grid (Figure 6F, pink arrows and dotted lines). This will help best with aligning all tips with the respective intersections of the grid, without puncturing the surface of the medium.Note: If a frogger / pin-replicator is available in the laboratory, this can be used instead of the multichannel pipette for transferring cells from the microtiter plate to the agar plates. Do determine how many μL the frogger transfers (this depends on the diameter of the pins, usually the volume transferred by a pin is 1 μL). The dilutions were optimized for the transfer of 5 μL, but 1 μL likely is still suitable for the growth assays.
-
a.
-
13.
Let the samples fully dry in.
Note: Freshly poured plates stored for 2-3 days at room temperature (20°C–25°C) on the bench (and not in a bag), are optimal for the growth assay, as the surface has slightly dried, making the samples dry in quickly and giving the best growth images.
-
14.
Incubate plates at 30°C, up-side-down.
CRITICAL: To prevent the media from drying out during long-term growth experiments, place the plates into a bag with small holes. Addition of a wet paper towel inside the bag is recommended.
-
15.Monitor the growth until all the highest dilutions have generated colonies of at least 1 mm diameter, this may take 2–3 weeks depending on the medium used. Use a document scanner to document the growth as follows:
-
a.Place the plates right-side up on the scanner.
-
b.Take off the lids, as otherwise the lids will reflect the light when scanning. Usually, a black background is placed over the plates.
-
c.Scan the plates.
-
d.Place the lids on the plates again, and immediately return the experiment back to 30°C.
-
a.
CRITICAL: Plates should be immediately returned to 30°C as otherwise the growth difference may not become as apparent.
Note: We experienced that the best results were obtained when the lid of the scanner was left open, the plates were not covered, and no bright light was directly shining into the scanner. The scanner should be located on a bench with little airflow (low foot-traffic). Even though the plates were opened while scanning, i.e., in a non-sterile environment, we observed hardly any contamination on the plates.
-
16.In order to quantify and gauge more objectively any observed growth differences between strains, growth rates can be calculated for each strain and plotted in a bar graph. This requires converting the amount of growth seen for each strain into a numerical value, by eye. An example for the quantitation of the semiquantitative growth assay in Figure 7A is shown in Data S2, and the below instructions refer to this example in square brackets. Steps for this evaluation are as follows:
-
a.For the growth-assay plate, assign growth scores for each strain at each of the five serial dilutions [Data S2, cells D9 -H16 and D21 – H28].Note: The growth-assay plate is the plate containing the medium where the mutated GOI potentially alters the growth rate of the cell [in our example this is the starvation medium, Figure 7A, right images labeled ‘starvation’].Note: Growth scores range from 0 for no growth whatsoever, to 10 for full growth. The assigned growth scores may differ between experimentalists, however, if the same criteria are applied to the entire experiment and by the same experimentalist, this will cancel itself out [Figure 7C shows an example for growth score criteria].
-
b.For each strain determine the sum of the five scores, to obtain the overall growth score [Data S2, cells J9-16 and J21-28].
- c.
-
d.Then determine the overall growth score for the control plates as done in step 16b [Data S2, cells T9 -16 and T21 – 28].Note: The control plates contain medium where the mutated GOI does not affect strain growth.
-
e.Determine the adjusted growth score for each individual strain, by dividing the overall growth score from the growth-assay plate by that of the control plate [Data S2, cells V9 -16 and V21 - 28].Note: This allows for consideration of any growth defects that are unrelated to the effects elicited by the medium on the growth-assay plate.
-
f.Determine the relative growth rate of each strain by dividing the adjusted growth score of each strain by that of the reference strain [Data S2, cells X9 -16 and X21 – 28, reference strain was the strain expressing GST alone].Note: This allows for the growth of each strain to be displayed relative to that of the reference strains, i.e., a growth rate of 1 means that the growth is equivalent to that of the reference strain grown on the same plate. This also allows for the comparison between different growth assays, as these calculations correct for potential growth differences between assays [Figure 7A shows two growth assays, the one in the top panel and the one in the bottom panel].
-
g.Assemble the data in the order desired for display in a bar graph [Data S2, columns Z-AD].
-
h.If more than one replicate was used, determine the average value for the relative growth rate [Data S2, cells AE9-18].
- i.
-
j.(not shown in this protocol) For any study, for each mutated GOI, at least 2 independent mutation transformants should be subjected to semi-quantitate growth assays, to test for reproducibility / variability of results. This then allows the determination of standard errors, which can be shown in the bar graph as error bars. A student t-test can be employed to test whether the mutated GOI significantly alters the growth rate as compared to the control strain.
-
a.
Figure 6.
Semi-quantitative growth assay to screen for amino acid substitutions eliciting a particular phenotype
For the semiquantitative growth assay, cells were grown to saturation, and then 10-fold serially diluted in a microtiter plate (for more see text, steps 7–10). Then, using an 8-channel pipette, 5 μL of the dilutions was transferred to solid medium (steps 11–12). For this, a grid was taped to the back of the petri plates, to aid in the transfer of cells onto petri plates, as shown in the right image. For grids see Data S1. To prevent puncturing of the agar surface by any of the 8 tips of the multichannel pipette, best is to first align the two middle tips with the respective intersections of the grid as shown, while slowly moving closer to the agar surface, and then aligning the outer tips with their respective intersections of the grid. When the tips are 1–2 mm close to the surface, slowly press the button of the pipette until the resistance point, so that the content of the tips will hang on the tips as they drop. Once the drops touch the agar surface, they will transfer to the plate. At this point, slowly press through the resistance point of the pipette to eject any residual content of the tips. Then remove the pipette.
Expected outcomes
The anticipated result is the fast generation of a collection of yeast strains, each expressing from a plasmid the GOI with a specific amino acid substitution. These strains can be immediately used in a semi-quantitative screen to identify mutations that affect a specific function of the GOI. The advantage is the fast narrowing down of interesting amino acids, for subsequent in-depth studies. The plasmid can be retrieved from the yeast cells via plasmid rescue (Zymo Research, order No D2001, Zymoprep Yeast Plasmid Rescue Miniprep I), and sequenced by Sanger sequencing to ensure no other mutations are present.
In our sample study, we intended to identify amino acids in Gcn1 required for binding to the RWD domain of Gcn2 (Castilho et al., 2014). Gcn2 must bind to Gcn1 to detect amino acid shortage, and subsequently elicit the general amino acid control signaling pathway (GAAC) to allow cells to cope and overcome starvation. A fragment in Gcn1 called the RWD binding domain (RWDBD) encompasses the region required for Gcn2 binding (Rakesh et al., 2017; Sattlegger and Hinnebusch, 2000). We had shown previously, that overexpression of the RWDBD from a galactose inducible promoter competes with Gcn2 for Gcn1 binding (Sattlegger and Hinnebusch, 2000). The subsequent disruption of Gcn1-Gcn2 interaction impairs Gcn2 activation, and as a consequence, these cells are unable to cope with starvation. The ability of cells to activate Gcn2 can be easily scored on medium containing 3-amino-1,2,4-triazole (3AT), a drug causing His starvation. While cells able to activate Gcn2 can grow in the presence of 3AT, cells unable to activate Gcn2 are less able to grow since they cannot overcome starvation. Accordingly, cells overexpressing the RWDBD cannot grow on 3AT, while cells overexpressing GST alone are able to grow on 3AT (Figure 7A, rows 7&8 vs 1&2, 15&16 vs 9&10; Figure 7B top vs middle panel). The R2259A substitution in the RWDBD has been shown previously to prevent binding to Gcn2. As a consequence, endogenous Gcn1-Gcn2 interaction is not affected, and cells are able to grow again on 3AT (Figure 7A, row 13, Figure 7B, bottom panel).
Our aim was to screen for additional amino acids in the RWDBD that are required for Gcn2 binding. Using our Rapid Screening for Functionally Relevant Amino Acids in a protein (RS-FRAA), we introduced single amino acid mutations individually into the RWDBD, followed by a semi-quantitative growth assay to determine which mutant transformants regained growth on 3AT medium (Figure 7A). In our sample result, we found that three of the eight transformants grew better on 3AT than the cells overexpressing unmutated RWDBD (Figure 7A, rows 4,6,13), suggesting the mutated amino acid is required for Gcn2 binding.
In this semi-quantitative growth assay, the stronger the reversion of the 3ATS phenotype, the more important an amino acid is for Gcn2 binding. In our sample result, the order of importance would be amino acid g > d > b. Surely, further studies are necessary to validate that the difference in 3ATS reversion is not due to differences in expression levels, and that the mutated amino acid in fact is required for Gcn2 binding per se. Nevertheless, this screening has helped in narrowing down and prioritizing amino acids for the subsequent – more laborious – in-depth studies.
The transformants generated in this procedure can be immediately used for further indicative studies such as a) immunoblotting to validate that the difference in growth on 3AT is not due to differences in RWDBD expression levels; and b) co-precipitation studies to investigate protein-protein interactions.
We have used our assay successfully in several other studies, for example one study aimed to identify amino acids in Yih1 relevant for Gcn2 binding (Sattlegger et al., 2011). In another example, we have screened fragments of the eEF1A protein for their ability to interfere with Gcn2 function, in a positive or negative fashion (Ramesh and Sattlegger, 2020).
Limitations
The desired mutations must affect protein function, and the alteration in protein function must elicit a phenotype in yeast, preferably a scorable growth phenotype in yeast. In case of protein-protein interactions, it is also possible to utilize the yeast-2-hybrid assay in our RS-FRAA procedure to elicit a scorable phenotype.
Troubleshooting
Problem 1
PCR reaction (PCR-1a or PCR-1b) leads to no amplification, or to PCR products of different sizes (step 1aiv).
Potential solution
Template has not been added, or too much template was used. Double check the concentration of the template.
Problem 2
Fusion or extension PCR leads to a) no amplification, or b) to multiple bands (steps 1avi or 1aviii).
Potential solution
For a) the template may not have been added. Alternatively, the templates are not able to anneal well to each other. Repeat the PCR reaction but reduce the annealing temperature by 2°C, 4°C, or even more until an amplification has occurred. For b) too much template may have been used, try using 10 or 100-fold less template. Alternatively, the primers may anneal at a 2nd position, try to increase the annealing temperature by 2°C or 4°C.
Problem 3
The transformation with circular plasmid leads to a low number of transformants (steps 3d or 5t).
Potential solution
Yeast transformation is not efficient. Double check the pH of the TE buffer (it has to be exactly at the desired pH). Double check that the heating block is truly at 42°C (steps 3cx or 5bj). Ensure that when growing yeast cells the OD600nm is below 1 (steps 3biii or 4d).
Problem 4
Linearized plasmid, even the 1:80 dilution, leads to a lot of transformants (step 3e).
Potential solution
The plasmid may not have been cut completely (step 2). Even a very small amount of uncut plasmid will lead to high numbers of transformants. Double check again via DNA electrophoresis whether the plasmid has been completely cut.
Problem 5
The haploid strains grow on medium selecting for diploid strains (steps 6ax or 6bxvi).
Potential solution
Too many haploid cells were transferred, leading to high background growth. If the procedure of mating on solid medium was used (step 6a), use as master plate the replica plate selecting for diploids that also showed growth of the haploid strains (instead of using plate-1), and repeat step 6avi Then transfer cells only from that replica plate to the velvet as explained in step 6avii (do not transfer cells from plate-1 or plate-2). After that, consecutively transfer cells to 5 plates selecting for diploids, followed by one YPD plate. Continue with step 6ax, one of these five plates will then show growth of diploids only. If the procedure of mating in liquid medium was used (step 6b), repeat step 6bxiv, but transfer cells from plate-3 instead of from plate-2, to this new plate. Cover plate with its lid and continue with step 6bxvi.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Evelyn Sattlegger (e.sattlegger@massey.ac.nz).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
This work was supported by funds from the School of Natural Sciences, Massey University. R.A.A. was supported by a Massey University Māori Doctoral Scholarship and funds from Massey University Foundation donors Bryce and Anne (née Percival) Carmine, S.G. by a Massey University Doctoral Scholarship and the School of Natural Sciences, A.A.G. by funds from Massey University Foundation donors Bryce and Anne (née Percival) Carmine.
Author contributions
E.S. conceived the idea, A.A.G., S.G., R.A.A., S.M.B.M.J.K., and C.D. conducted experiments, E.S., A.A.G., R.A.A., and S.G. drafted figures and the manuscript, with contributions from S.M.B.M.J.K., C.D., and A.H.S.. E.S. and A.H.S. finalized the figures and the manuscript, with contributions from A.A.G., C.D., S.G., and R.A.A.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2022.101545.
Supplemental information
Data and code availability
Supplemental files are available online as follows: Data S1: Mendeley data: https://doi.org/10.17632/x83kktjrfd.1; Data S2: Mendelet data: https://doi.org/10.17632/cfjgjnpxmt.1.
References
- Castilho B.A., Shanmugam R., Silva R.C., Ramesh R., Himme B.M., Sattlegger E. Keeping the eIF2 alpha kinase Gcn2 in check. Biochim. Biophys. Acta Mol. Cell Res. 2014;1843:1948–1968. doi: 10.1016/j.bbamcr.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Gietz R.D., Schiestl R.H., Willems A.R., Woods R.A. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast. 1995;11:355–360. doi: 10.1002/yea.320110408. [DOI] [PubMed] [Google Scholar]
- Ma H., Kunes S., Schatz P.J., Botstein D. Plasmid construction by homologous recombination in yeast. Gene. 1987;58:201–216. doi: 10.1016/0378-1119(87)90376-3. [DOI] [PubMed] [Google Scholar]
- Foiani M., Cigan A., Paddon C.J., Paddon C.J., Harashima S., Hinnebusch A. 'GCD2, a translational repressor of the GCN4 gene, has a general function in the initiation of protein synthesis in Saccharomyces cerevisiae. Mol. Cell Biol. 1991;11:3203–3216. doi: 10.1128/mcb.11.6.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakesh R., Krishnan R., Sattlegger E., Srinivasan N. Recognition of a structural domain (RWDBD) in Gcn1 proteins that interacts with the RWD domain containing proteins. Biol. Direct. 2017;12:12. doi: 10.1186/s13062-017-0184-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh R., Sattlegger E. 'Domain II of the translation elongation factor eEF1A is required for Gcn2 kinase inhibition. FEBS Lett. 2020;594:2266–2281. doi: 10.1002/1873-3468.13803. [DOI] [PubMed] [Google Scholar]
- Sattlegger E., Barbosa J.A.R.G., Moraes M.C.S., Martins R.M., Hinnebusch A.G., Castilho B.A. 'Gcn1 and actin binding to Yih1: implications for activation of the eIF2 kinase GCN2. J. Biol. Chem. 2011;286:10341–10355. doi: 10.1074/jbc.M110.171587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattlegger E., Hinnebusch A.G. Separate domains in GCN1 for binding protein kinase GCN2 and ribosomes are required for GCN2 activation in amino acid-starved cells. EMBO J. 2000;19:6622–6633. doi: 10.1093/emboj/19.23.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supplemental files are available online as follows: Data S1: Mendeley data: https://doi.org/10.17632/x83kktjrfd.1; Data S2: Mendelet data: https://doi.org/10.17632/cfjgjnpxmt.1.