Abstract
Mosquito-borne diseases are responsible for significant human morbidity and mortality throughout the world. Efforts to control mosquito-borne diseases have been impeded, in part, by the development of drug-resistant parasites, insecticide-resistant mosquitoes, and environmental concerns over the application of insecticides. Therefore, there is a need to develop novel disease control strategies that can complement or replace existing control methods. One such strategy is to generate pathogen-resistant mosquitoes from those that are susceptible. To this end, efforts have focused on isolating and characterizing genes that influence mosquito vector competence. It has been known for over 70 years that there is a genetic basis for the susceptibility of mosquitoes to parasites, but until the advent of powerful molecular biological tools and protocols, it was difficult to assess the interactions of pathogens with their host tissues within the mosquito at a molecular level. Moreover, it has been only recently that the molecular mechanisms responsible for pathogen destruction, such as melanotic encapsulation and immune peptide production, have been investigated. The molecular characterization of genes that influence vector competence is becoming routine, and with the development of the Sindbis virus transducing system, potential antipathogen genes now can be introduced into the mosquito and their effect on parasite development can be assessed in vivo. With the recent successes in the field of mosquito germ line transformation, it seems likely that the generation of a pathogen-resistant mosquito population from a susceptible population soon will become a reality.
Mosquitoes are unquestionably the most medically important arthropod vectors of disease. The maintenance and transmission of the pathogens that cause malaria, lymphatic filariasis, and numerous viral infections are absolutely dependent on the availability of competent mosquito vectors. Human malaria, caused primarily by the protozoan species Plasmodium falciparum and Plasmodium vivax, affects nearly 500 million people annually and is responsible for nearly 3 million deaths annually (262). The nematodes Wuchereria bancrofti and Brugia malayi are the principal etiologic agents of lymphatic filariasis, causing morbidity in over 100 million individuals (18). Hundreds of thousands of humans also are infected with mosquito-borne viruses, with yellow fever and dengue fever being two of the more important mosquito-transmitted viral diseases (263). Although the medical community has known for over a century the role mosquitoes play in the transmission of malaria and lymphatic filariasis, these diseases continue to have a devastating influence on less privileged populations throughout the tropical and subtropical regions of the world. In fact, the current problems with controlling malaria are much more severe than those facing public health officials 30 years ago (117). Previous control efforts led to the rapid selection of insecticide-resistant strains of mosquitoes and drug-resistant strains of parasites that have contributed greatly to the present situation. However, numerous other environmental, economic, sociological, political, and demographic factors also have played significant roles in the reemergence of malaria and other infectious and parasitic diseases. Although new approaches to drug therapy show tremendous potential for the control of lymphatic filariasis (18), there are no promising strategies on the horizon for the control of malaria. New drug development faces the economic hurdle of limited returns for companies based in the developed world that are willing to invest the fiscal resources necessary to bring a new drug to market. Vaccine development remains a viable approach (157) but undoubtedly will require many more years of intensive research and will face continually the problems associated with a genetically plastic pathogen. Mosquito control, through environmental perturbation and pesticide application, used to be the primary strategy for controlling mosquito-borne diseases, but environmental and human health concerns, as well as the development of pesticide resistance, limit the usefulness of these traditional approaches in our modern-day world. In fact, there are efforts to enact a global ban on the insecticide DDT (United Nations Environment Programme Persistent Organic Pollutants Website; http://www.chem.unep.ch/pops), which has been used effectively in house-spraying campaigns in many tropical areas to reduce the incidence of malaria. As a consequence, the tools of molecular biology and genomics are being applied to new avenues of research in vector biology, the ultimate goal of which is the development of novel disease control strategies.
In the past, only limited resources have been devoted to understanding the details of the relationships between mosquito vectors and the pathogens they transmit, especially compared to the resources committed to understanding the vertebrate host-parasite relationship. This discrepancy probably was justified because of the often urgent need to treat people in affected areas. In addition, the small size of mosquitoes severely limited the quantity of materials that could be obtained for cellular and biochemical studies, and scientists lacked the ability to genetically manipulate mosquitoes or parasites in an efficient and meaningful way. Development of new technologies in recent years has essentially removed these limitations, and consequently there is a renewed interest in research of mosquito-pathogen systems. One of the driving forces used to justify present-day studies in vector biology is the working hypothesis that we can genetically engineer mosquitoes for resistance to parasite development and, with the appropriate driving mechanism, can drive parasite resistance genes into wild, susceptible vectors in areas of endemic infection to reduce the intensity of transmission (49). Obviously there are numerous scientific and nonscientific problems to be overcome before such a control strategy could be implemented, but this ultimate goal has fostered a tremendous amount of research and the development of new tools in a very short period that are beginning to provide us with a better understanding of the factors controlling vector competence. It is reasonable to propose that these new findings eventually will provide innovative control methods that are unrelated to the release of genetically engineered organisms.
During the last several years there have been significant advances in our understanding of the cellular and biochemical intricacies of mosquito-parasite relationships and of the genetic control of these phenotypes. This review will summarize what we believe are important advancements that have occurred in vector biology in recent years and, where appropriate, will attempt to point out gaps in our knowledge and potential experimental approaches that might be taken to fill these gaps. Although the aim of this review is not to detail how we can produce transgenic mosquitoes for release in control programs, we will discuss progress in the research areas that must be developed more fully if this strategy is to be technically feasible.
Although the terms “vectorial capacity” and “vector competence” often are used interchangeably to describe the ability of a mosquito to serve as a disease vector, vectorial capacity is defined quantitatively and is influenced by such variables as vector density and longevity as well as vector competence (17, 142). Estimates of vectorial capacity take into account all of the environmental, behavioral, cellular, and biochemical factors that influence the association between a vector, the pathogen transmitted by the vector, and the vertebrate host to which the pathogen is transmitted (17, 261). Both behavioral and environmental factors can play decisive roles in determining vectorial capacity. For example, a particular mosquito species might be genetically and biochemically compatible for the complete development of a particular pathogen, but if this species does not coexist temporally and spatially with a vertebrate host that harbors the parasite, or if the preferred blood source for this species does not include that vertebrate, this mosquito is not a suitable vector for the pathogen. Vector competence, as a component of vectorial capacity, is governed by intrinsic (genetic) factors that influence the ability of a vector to transmit a pathogen (17, 91, 261). Any trait, for example, host feeding preferences or susceptibility to pathogen infection, that has a genetic component will affect vector competence. This review is limited to an assessment of our present knowledge of the genetic factors of the mosquito that influence the vector-pathogen relationship from the time the pathogen is ingested in a blood meal until the infective stage of the pathogen is transmitted to the vertebrate host. When one considers the present interest in the generation of a parasite-resistant phenotypic mosquito from a parasite-susceptible genotypic mosquito, it is not surprising that a greater research effort has gone into gaining a better understanding of how incompetent vectors kill ingested parasites compared to factors that contribute to compatibility. Consequently, this review emphasizes our present understanding of the immune response of mosquitoes, including the means whereby mosquitoes are able to kill invading pathogens and what we know about the genetic basis of these responses.
PATHOGEN DEVELOPMENT IN MOSQUITO VECTORS
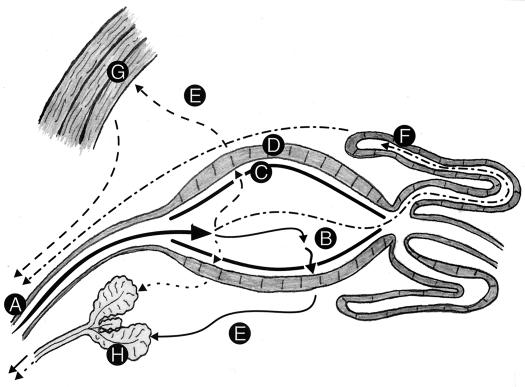
Figure 1 summarizes the major routes of migration and developmental sites within the mosquito for the three major pathogen groups transmitted to humans. Also included are the migration route and developmental site for the dog heartworm, Dirofilaria immitis, because this filarial worm has been used extensively in numerous laboratories as a model system for filariasis. Although the life cycle of each pathogen is distinct, they all face the common events of being ingested, exposed to the midgut environment, and traversing the hemolymph-filled hemocoel to reach their tissue site of development and/or suitable site for transmission back to a new vertebrate host. Each of these migratory steps presents potential barriers that might be manipulated to interfere with normal pathogen migration and/or development.
FIG. 1.
Migratory routes and developmental sites within the mosquito for viruses, malaria parasites, and filarial worms. Developmental sites within the mosquito are defined by the letters A to H, and migratory routes are represented by lines. Following ingestion in a blood meal (A), all the pathogens enter the midgut (B). Viruses (represented by ——) then enter the midgut epithelial cells (D), replicate, exit the cells, and travel through the hemolymph-filled hemocoel (E) to the salivary glands (H), where they again replicate and reside until injected into a vertebrate host. Malaria parasites (designated by · · · · · ·) remain in the midgut for several hours, where they undergo syngamy and ookinete formation before they migrate through the formed peritrophic matrix (C). They then pass through the midgut epithelium and lodge between this epithelial layer and the basal membrane of the midgut, where they undergo sporogony in oocysts to produce sporozoites. At maturity, the sporozoites rupture the oocyst, travel through the hemocoel, and penetrate the salivary glands, where they reside until injected into a host. When the filarial worms responsible for human disease (––––) and dog heartworm (–·–·–) are ingested in a blood meal, the former penetrate the midgut epithelium and migrate to their developmental site in the thoracic musculature (G) and the latter travel through the midgut lumen, migrate up the lumen of the Malpighian tubules, and enter the distal cells of the tubules, where they develop intracellularly (F). Following a period of development, infective third-stage filarial larvae break out of the thoracic musculature or the Malpighian tubules and enter the hemocoel, where they migrate thorough the open circulatory system to the head region. Unlike malaria sporozoites and viruses, which are directly injected into a host when a blood meal is ingested, infective-stage filarial worms actively emerge from the head region of the mosquito and are deposited on the surface of the vertebrate skin, which they enter through the wound made by the mosquito bite.
All pathogens transmitted by mosquitoes are acquired with a blood meal (Fig. 1, site A), and the biochemical makeup of this ingested blood can have a significant influence on vector competence. Mosquitoes and other blood-feeding arthropods have evolved various mechanisms that ensure the unimpeded flow of blood from host organisms, which, likewise, have evolved effective antihemostatic mechanisms to prevent blood loss (195). The antihemostatic factors present in mosquito saliva allow these insects to blood feed efficiently, but various mosquito species can differ markedly in the potency of these activities (231). Consequently, the fluid consistency of ingested blood may vary in different mosquitoes. The coagulation of blood within the midgut can inhibit ingested pathogens from migrating out of this environment as is required for further development. This potential barrier to pathogen development varies in significance depending on the length of time a pathogen spends within the midgut, but it clearly is evident that the consistency of ingested blood can significantly influence both the prevalence and intensity of infection for all mosquito-borne parasites (112).
Following ingestion by mosquitoes in a blood meal, pathogens encounter the pharyngeal or cibarial armature that, depending upon pathogen size, can affect infection parameters. In certain mosquito species, the pharyngeal armature can cause physical damage to large parasites, like microfilariae (∼250 to 300 μm), that can effectively prevent further development (149). However, this structure has no influence on the microscopic malaria parasites or arboviruses.
Within the midgut environment (Fig. 1, site B), ingested pathogens are exposed to proteolytic enzymes that are secreted into the lumen for the purpose of blood digestion. These digestive enzymes can have a negative or positive impact on the pathogen and therefore can influence vector competence (79, 218). The process of blood feeding also initiates within mosquitoes the formation of a chitinous structure called the peritrophic matrix (site C), which eventually surrounds the blood meal and physically separates it from the midgut epithelium. For pathogens spending more than a few hours within the midgut lumen, the peritrophic matrix presents a barrier that must be traversed if they are to penetrate the midgut epithelium during migration to their developmental site.
Developmental sites, including the midgut (Fig. 1, site D), Malpighian tubules (site F), thoracic musculature (site G), and salivary glands (site H), can provide the appropriate environment to initiate parasite gene expression or permit posttranslational modifications of previously expressed gene products that are required for development. Consequently, the composition of these tissues within specific species and strains of mosquitoes plays a major role in vector competence. However, little is known about the molecular “cross talk” that must occur between host cells and parasites at these developmental sites. In many incompetent vectors, parasites successfully reach the appropriate developmental site but then fail to develop or are killed by a defense response expressed by the mosquito (42).
At some time during their life cycle within the mosquito, all pathogens must travel through the hemolymph-filled hemocoel (Fig. 1, site E) when migrating to their developmental site or to their site for transmission to the vertebrate host. Within the hemolymph reside the primary immune components involved in the recognition of nonself and in the initiation of defense responses designed to kill foreign invaders (43, 183). Hemocytes (blood cells) that circulate within the hemolymph play major roles in recognition, phagocytosis, encapsulation, and the production of specific enzymes and other molecules required for parasite killing. The mechanisms used by the mosquito immune system to repel parasites, as well as the strategies used by parasites to avoid recognition and destruction, are perhaps the most thoroughly studied and important determinants of vector competence.
These various barriers to parasite development within mosquitoes may or may not function to protect vectors from parasite development. The genetic makeup of a particular mosquito species, or strain of a single species, to a large extent determines the success of a specific parasite-mosquito relationship. Likewise, the genetics of the parasite can play a major role in the ability or inability of a parasite to successfully adapt to a particular mosquito. Consequently, there has been a major effort during the last several years to develop the appropriate tools to investigate more accurately the genetic basis of vector competence in select mosquito species. However, little effort has been invested by biologists in studies designed to understand more fully the genetic components of parasites that enable them to effectively avoid destruction within certain mosquito vectors.
GENETIC BASIS OF VECTOR COMPETENCE
Clay Huff demonstrated 70 years ago that the susceptibility of Culex pipiens to an avian malaria parasite could be increased through selective mating strategies (99, 100), and subsequent studies with filarial worms and Plasmodium spp. verified the genetic basis for the susceptibility of several mosquito species to these parasites (41). The availability of a genetic linkage map for Aedes aegypti (168), based on isozyme and morphological mutant markers, enabled a number of investigators to determine the chromosomal regions of genes with a major influence on the susceptibility of this mosquito species to Plasmodium gallinaceum (113), Brugia spp. (143, 144, 145), and D. immitis (150). A recessive gene(s), located on chromosome 1, was shown to control susceptibility to the filarial worms B. malayi and Brugia pahangi (designated fm) and D. immitis (designated ft), and a dominant allele (pls) on chromosome 2 controlled susceptibility to P. gallinaceum. It also was clear from these studies that other genes, in addition to f and pls, must be involved in determining parasite susceptibility in this mosquito. However, the large number of segregating populations required to identify linkage associations with relevant genes when using a mutant marker map prevented the identification of other loci that contribute to the parasite susceptibility phenotype. Before studies could be conducted to identify quantitative trait loci (QTL) influencing vector competence, molecular marker genetic linkage maps, where markers delineate the entire genome, had to be constructed.
Recent advances in genomics, spawned primarily by the human genome project and plant genetics research, made it possible to develop these molecular maps for several mosquito species. This has become an active area of research in vector biology, and several laboratories have taken different approaches in developing physical and linkage maps for different mosquito species, primarily A. aegypti (211) and Anopheles gambiae (270).
A. aegypti is an excellent model organism for vector biology studies. Although this species is a natural vector for the avian parasite P. gallinaceum and for a group of flaviviruses that cause dengue fever and yellow fever, strains also have been selected that support the complete development of the filarial worms B. malayi (143, 144), B. pahangi (145), and D. immitis (150). Numerous subspecies and strains are available that differ in their geographic origins, morphological and physiological characteristics, and competence for pathogen development. This species is easy to maintain in the laboratory and is amenable to pairwise mating strategies, and eggs can be stored for relatively long periods. Although a large database on the genetics of this species has been available for many years (167), there is a disadvantage in that it is extremely difficult to obtain high-quality preparations of polytene chromosomes from A. aegypti. However, more data on mosquito physiology, genetics, and vector competence have been derived from studies of A. aegypti than from studies of any other species. In addition, the technology for germ line transformation has been developed for this mosquito species (see “Introduction of exogenous gene products in mosquitoes” below).
A. gambiae is more difficult to rear in the laboratory, eggs cannot be stored (they hatch within a few days of being oviposited), and relatively few geographic or morphological mutant strains are available. However, this species is the most important vector of human malaria in sub-Saharan Africa and probably is responsible for the majority of malaria transmission and subsequent mortality resulting from this disease. The medical importance of this species has spurred a tremendous research effort in recent years to develop the requisite tools and techniques necessary for investigating the mechanisms controlling the competence of A. gambiae for P. falciparum, the deadliest of the four human malaria parasites. Progress on the genetics of A. gambiae has been impressive, due in part to easily obtained polytene chromosomes for physical mapping (118).
The first molecular genetic linkage map was constructed for A. aegypti and used restriction fragment length polymorphism (RFLP) markers derived primarily from random cDNA clones (211). These efforts used a multiple mutant marker strain of A. aegypti (Red) that allowed the partial integration of the RFLP marker genetic linkage map into the classical genetic linkage map developed previously by Munstermann and Craig (168). The RFLP genetic linkage map for A. aegypti contains over 100 loci that cover approximately 165 centimorgans (cM) of the genome. Disadvantages associated with the construction of this type of map include the limited amount of DNA that can be obtained from an individual mosquito and the total number of Southern blots that can be performed on DNA from individuals in any given population. The advantage of using cDNAs as markers in a RFLP map, however, is that the genes they represent tend to be conserved in their location in the genome between different populations and species of mosquitoes (synteny); consequently, they can be used to construct usable linkage maps for other species in a relatively short time and they also serve as valuable tools to address questions of gene order within the family Culicidae. A high degree of synteny was apparent when a RFLP genetic linkage map was constructed for Aedes albopictus using A. aegypti cDNA markers (210). In addition, the prediction of whole-arm conservation of mosquito genomes (147) generally has been upheld when A. aegypti cDNA markers have been used to construct RFLP linkage maps for the mosquitoes C. pipiens (159) and Armigeres subalbatus (71). Data concerning genetic maps for A. aegypti, as well as other mosquito species, are continually updated and available at the Mosquito Genomics World Wide Website (http://klab.agsci.colostate.edu).
A robust genetic linkage map based on random amplified polymorphic DNA from PCR (RAPD-PCR) also has been constructed for A. aegypti (1). Using 10 RAPD primers, 94 markers were mapped, covering a recombinational map length of 168 cM. The cDNA map and RAPD map also agree regarding estimates of map length for individual chromosomes, i.e., 49, 60, and 56 cM (RAPD map) and 49, 60, and 57 cM (cDNA map) for chromosomes 1, 2, and 3, respectively. Although the tendency of RAPD markers to segregate as dominant characters has been considered problematic for use in QTL mapping (209), the RAPD map construction for A. aegypti used single-strand conformation polymorphism analysis in conjunction with RAPD-PCR. This approach significantly increased the number of polymorphisms observed, including an increase in those that were codominant (1). Another limitation of a RAPD-based linkage map is that RAPD markers might be limited to use in families and strains from which the map was derived. However, it also is apparent that maps based on RAPD polymorphisms for specific populations can be generated quite rapidly using limited manpower (1).
A physical map for A. aegypti that can be correlated with these genetic linkage maps is being developed and relies on fluorescence in situ hybridization (FISH) in concert with digital imaging microscopy (20, 21). Metaphase chromosome preparations are hybridized with a fluorescently-labeled nucleic acid fragment, for example, a cosmid clone, and are positioned on the chromosome as a fractional distance of the total chromosome length. Probes on metaphase chromosomes can be resolved to within approximately 1 Mb, but the use of interphase chromosomes permits resolution to within a few kilobases. To date, over 200 clones (primarily cosmid clones) have been mapped to the three chromosomes with an average spacing of approximately 1 Mb. Integration of this FISH-based physical map with the RFLP genetic linkage map has used sequence-tagged site (STS) markers using a PCR approach. Expressed sequence tags and STSs have been generated from both random cDNA clones and clones representing known genes previously mapped as RFLP markers for A. aegypti (214). Primers representing a specific STS then have been used with PCR to screen clones from an A. aegypti cosmid library; subsequently, positive clones have been mapped using FISH to begin integrating the physical and linkage maps for this mosquito.
Chromosomal maps of A. gambiae have been available for some time because of the ability to obtain high-quality polytene chromosomes (118), but the biological problems with laboratory mating strategies and the limited availability of morphological mutant strains have not been conducive for the development of an RFLP-based genetic linkage map similar to that developed for A. aegypti. Instead, Zheng et al. (270, 271) used simple sequence repeats (microsatellite markers) that are identifiable by PCR. This approach provides a mapping system whereby progeny resulting from a genetic cross can be analyzed numerous times for a large number of markers because only a very small amount of DNA (<0.002% of the DNA from an individual mosquito) is required for any single analysis. This approach produced a linkage map with 131 markers spanning the three chromosomes at an average spacing of 1.6 cM (48.9, 72.4, and 93.7 cM total length for chromosomes 1, 2, and 3 respectively [270]). Using polytene chromosomes from ovarian nurse cells, the cytogenetic locations of 47 marker genes also have been determined via in situ hybridization (270). Similar to the RAPD map for A. aegypti, the microsatellite linkage map is limited for use with A. gambiae, although it seems that some of these markers can be used in Anopheles arabiensis, a sibling species of A. gambiae (119); however, the medical importance of A. gambiae fully warrants the efforts required to generate a species-specific map.
The availability of molecular genetic linkage maps has allowed researchers to begin to address the genetic complexity of certain aspects of vector competence. The first such study evaluated the genetic control of susceptibility of A. aegypti to infection with the human pathogen B. malayi (212). Results from crosses involving three separate populations revealed linkage associations between parasite susceptibility and cDNA markers at two loci. Susceptibility seems to be controlled by a QTL on chromosome 1 (fsb[1, LF178]) that functions as a recessive allele (verifying the location and characteristics of fm) and a QTL on chromosome 2 (fsb[2, LF98]) that exerts an effect on fsb[1, LF178] in an additive manner (212). Subsequently, QTL mapping of the susceptibility of A. aegypti to the avian malaria parasite P. gallinaceum revealed that, again, the dual action of two QTL influenced the phenotypic trait of parasite susceptibility (213). The QTL on linkage group 2 (pgs[2, LF98]) has the greatest influence on susceptibility (65 and 49% of phenotypic variance in two independent populations) and undoubtedly represents the pls locus of Kilama and Craig (113). A second QTL on chromosome 3 (pgs[3, MalI]) has a lesser effect (14 and 10% of observed variance). Both of these genome regions show a partial dominance effect on susceptibility, with no detectable epistasis.
Previous studies suggested that the susceptibility of A. aegypti to yellow fever virus is influenced by a gene(s) in the same region of chromosome 2 that contains loci influencing susceptibility to B. malayi and P. gallinaceum (237). Recently, quantitative genetic studies at the Arthropod-Borne & Infectious Disease Laboratory at Colorado State University evaluated dengue fever (DEN) transmission in two subspecies of A. aegypti (19). These studies focused on two quantitative variables: (i) the dengue fever virus titer in the midgut as a measure of midgut infection, and (ii) the DEN virus titer in the head as a measure of disseminated infection. Multiple half-sib families were established for A. aegypti aegypti, originally collected in San Juan, Puerto Rico, and for A. aegypti formosus, originally collected in Ibo, Nigeria, and viral titers were measured in all F2 offspring. The study suggested that at least two genes control vector competence for DEN in A. aegypti. One gene or set of genes controls a midgut infection barrier, and the other controls a midgut escape barrier that prevents the infection from disseminating. Understanding the complexity of the genetic control of vector competence for any mosquito-borne pathogen could require an assessment of the particular vector species and pathogen responsible for disease maintenance in a particular geographic area.
Development of the microsatellite genetic linkage map enabled workers to assess the genetic background controlling the resistance of A. gambiae to the primate parasite Plasmodium cynomolgi B (51, 272). In this model, the resistance mechanism involves the melanotic encapsulation and destruction of the ookinete on the hemocoel side of the midgut epithelium (50) (see “Pathogen-tissue encounters within the mosquito vector” below). Genetic mapping of several backcross families determined that three QTL influence melanotic encapsulation of P. cynomolgi B. Pen1 (QTL1), located on the right arm of chromosome 2, accounted for ∼54% of the phenotypic variance, and Pen2, on the left arm of chromosome 3, accounted for ∼13% of the trait. A third QTL, Pen3, also mapped to the right arm of chromosome 1 approximately 45 cM from Pen1. Pen2 and Pen3 have an additive effect on Pen1, and together the three QTL accounted for ∼70% of the variance for melanotic encapsulation of ookinetes.
Earlier studies revealed that the melanization response of A. gambiae to intrathoracically inoculated CM-Sephadex beads mimicked the response to Plasmodium ookinetes (84, 185). Subsequent QTL mapping studies using a combined RFLP and microsatellite linkage map identified Pen1 on the same region of chromosome 2 (85). It seems that the primary genetic control of ookinete melanotic encapsulation might be the same as that controlling melanization of abiotic materials (83, 85).
With both the A. aegypti and A. gambiae experimental models, the requisite information now exists to isolate the gene(s) controlling the susceptibility of these vectors for several different pathogens. The identification of molecular marker loci linked to genes that influence pathogen susceptibility provides the necessary starting points for map-based cloning. Additional markers, targeted to these chromosomal regions containing QTL, need to be developed with additional crosses to increase the resolution of these QTL. Presently, markers on either side of the identified QTL probably flank many megabases of DNA, and identification of the gene(s) of interest will not be an easy task. However, the gene knockout techniques (178) and the development of an efficient and stable germ line transformation system (105) provide reliable methods for testing the influence on vector competence of candidate genes contained within the DNA (see “Introduction of exogenous gene products in mosquitoes” below).
The use of molecular marker technologies in efforts to define genes controlling vector competence have, to date, involved only model systems and do not necessarily reflect natural vector-parasite interactions that occur in areas where these mosquito-borne diseases are endemic. A. aegypti is not a natural vector of B. malayi, and P. gallinaceum and P. cynomolgi are not human pathogens. This does not reduce the importance of these studies, but it is possible that each individual vector-parasite system functioning in disease transmission will have to be evaluated or at least that the genes identified in model systems will have to be assessed for their potential influence on natural systems (15).
The genetic plasticity of the parasite and its potential influence on the dynamics of the vector-parasite interaction have not been considered in any of these systems. Undoubtedly, the isolation and characterization of a gene that imparts susceptibility or refractoriness of a mosquito to a parasite would be a tremendous accomplishment and would provide a significant improvement of our understanding of the mechanisms controlling vector competence. However, if the genetic contributions of the parasite are totally ignored, these new understandings could be seriously compromised. For example, 30 years ago Laurence and Pester (120) were able to use selective breeding strategies to adapt a strain of the filarial worm Brugia patei to a laboratory colony of Aedes togoi that initially was highly refractory. Following four generations of parasite selection, without any selection of the mosquito colony, susceptibility increased from ∼44 to ∼90% with a concomitant increase in intensity of infection from ∼2.5 to 11.7 infective-stage larvae/infected mosquito. This obviously raises a question concerning the use of laboratory strains of parasites that in many cases have been passed through the same mosquito colony populations for many generations or, in the case of Plasmodium, often serially passaged through vertebrate hosts or cell culture with only limited exposure to the mosquito vector. In an A. gambiae strain selected for resistance to several species of Plasmodium (50), ookinetes of New World and Asian strains of P. falciparum are killed within melanotic capsules but strains of African origin generally develop normally. Because the geographic range of A. gambiae is limited to Africa, it becomes obvious that the coevolutionary history of a parasite and its vector in one geographic area could well be distinct from vector-parasite relationships operating in another area. This relationship also is illustrated in a New World vector of P. falciparum. In work being conducted by the Parasitology group at the University of Edinburgh, it has been shown that Anopheles albimanus, a New World vector of P. falciparum, is susceptible to a parasite strain that originated from Central America but is not permissive to the strain of P. falciparum believed to have originated from Africa. These investigators currently are conducting genetic crosses with these parasite strains in an effort to characterize the genetic basis of the parasite differences (L. Ranford-Cartwright, personal communication). It also would be interesting and valuable to determine if selection strategies applied in the laboratory could produce parasite strains capable of developing in the refractory strains of mosquitoes used previously in genetic mapping studies. If these selections were successful, one would predict that different or additional loci within the mosquito genome might be identified following QTL mapping for susceptibility to the new strain of parasite.
With the development of molecular markers that enable researchers to scan the entire genome of specific mosquitoes, it now is possible to search for genes controlling parasite susceptibility in natural populations of mosquitoes. Family pedigrees of wild mosquitoes can be established from the progeny of individual, field-collected females because they normally mate only once in nature. Exposure of these progeny to local parasites and subsequent statistical analysis for linkage associations between the marker genotype and infection phenotype would provide QTL data for comparison with data generated using mosquito-parasite models in the laboratory. This type of evaluation is being conducted in Mali in sub-Saharan Africa, where preliminary studies provided strong evidence for family-specific differences in the susceptibility of A. gambiae to infection with P. falciparum in this region (K. D. Vernick, personal communication).
The genetic control of vector competence can operate at different developmental stages of the pathogen and in different tissue sites within the mosquito. Identification of molecules that might influence parasite development within these tissues, and the isolation of the genes that code for these molecules, is another approach in efforts to clarify the mechanisms controlling mosquito vector competence. Pathogen encounters with the salivary glands, midgut, hemolymph, thoracic musculature, and Malpighian tubules of the mosquito vector create specific opportunities for genetic interplay between the parasite and the vector that determines whether the association will be compatible. The following section assesses our present knowledge of gene expression in these specific sites and the ways in which this might influence vector competence.
PATHOGEN-TISSUE ENCOUNTERS WITHIN THE MOSQUITO VECTOR
Salivary Glands
The salivary glands of mosquitoes synthesize and secrete powerful antihemostatic agents that facilitate hematophagy by counteracting the effects of vertebrate wounding responses (195). When a probing mosquito pierces the skin and lacerates the vasculature, the major responses to wounding, which include vasoconstriction, platelet aggregation, and coagulation, are initiated in the vertebrate host. Consequently, components of mosquito saliva contain potent antihemostatic molecules to enable the mosquito to feed quickly and efficiently, thereby avoiding the defensive responses of the host. In A. aegypti, these antihemostatic molecules include apyrases, sialokinins, and anticoagulants that prevent platelet aggregation, vasoconstriction, and coagulation, respectively (104). Apyrases prevent platelet aggregation by converting ATP and ADP to AMP, eliminating the signal (ADP) for aggregation (30, 146, 199, 225). Vasodilation is promoted by the secretion of sialokinins, tachykinin-like molecules, in A. aegypti and catechol oxidase/salivary peroxidase in A. albimanus (7, 29, 194, 197, 198, 200). In A. aegypti, the AFXa gene encodes a serine protease inhibitor-like molecule that is directed against factor Xa of the coagulation pathway, thereby acting as an anticoagulant (230, 233). In Anopheles stephensi, a thrombin inhibitor is responsible for preventing coagulation (255). Moreover, of the mosquitoes examined, all of the Culicinae had anticoagulants directed against factor Xa and the Anophelinae targeted thrombin (232). Because mosquitoes feed rapidly on blood, coagulation is not likely to hinder the feeding process; rather, it has been proposed that the anticoagulants may aid in the digestion of blood and keep mouthparts free of coagulated blood (231).
The expression patterns of the antihemostatic molecules reflect their significance in blood feeding. These molecules are secreted principally by female mosquitoes and are synthesized in specialized regions (the distal lateral and medial lobes) of the salivary glands (7, 104, 225). Male mosquitoes, which are incapable of blood feeding and feed only on nectar, lack these specialized regions. Furthermore, the salivary gland lobes responsible for the synthesis of apyrase are also the regions of the glands where Plasmodium sporozoites reside until injected into the vertebrate host during blood feeding (234). It has been shown that sporozoite-infected mosquitoes exhibit a decrease in apyrase activity with a concomitant increase in intradermal probing time, thereby increasing the likelihood of sporozoite transmission (206).
Although the role of mosquito salivary gland constituents in preventing vertebrate hemostasis has been relatively well documented, the role of such components in facilitating parasite infection of the vertebrate host has not been described well. Although the salivary gland lysate from the sand fly Lutzomyia longipalpis has been shown to facilitate infection of the protozoan parasite Leishmania major in mice (242, 243), similar studies assessing the role of mosquito salivary gland lysates in promoting, for example, infection of Plasmodium species in vertebrate hosts are lacking. Osorio et al. (179) reported that deer and chipmunks developed higher and longer viremias following exposure to La Crosse virus via infected Aedes triseriatus than following exposure to the virus via needle and syringe inoculation. Also, mosquito feeding induced an enhancement of Cache Valley virus infection in mice (63). However, the mechanism(s) responsible for the enhanced viremias was not determined.
As a final destination for viruses and malaria sporozoites before injection into a vertebrate host, the mosquito salivary glands play a critical role in the transmission of pathogens (Fig. 1). Although the specific mechanisms by which such pathogens infect mosquito salivary glands are not particularly well detailed on a genetic or molecular level, a number of experiments have been interpreted to indicate that a receptor-ligand-like interaction mediates pathogen recognition and invasion of the salivary glands. Sporozoites can be found in association with other tissues, but the vast majority of sporozoites released from the oocysts are found in the glands, indicating that sporozoites can recognize salivary glands as distinct from other tissues (82). Furthermore, it was demonstrated that sporozoites would invade the glands of a competent host, even when the parasites matured in the body of an incompetent host, if the competent glands were transplanted (204). Pimenta et al. (187) morphologically described the invasion of the mosquito salivary glands by sporozoites, using the P. gallinaceum-A. aegypti model system. This process is initiated when the sporozoite attaches to and crosses the basal lamina of the salivary glands, continues as the sporozoite traverses the secretory cell, and ends as bundles of sporozoites await injection into the next vertebrate host. The specific parasite and host molecules involved in this process are unknown, but Barreau et al. (6) demonstrated that polyclonal antibodies raised against A. aegypti salivary glands block penetration by P. gallinaceum sporozoites, suggesting that the antibodies may recognize a receptor and, through competitive blocking, prevent sporozoites from binding. A subsequent study using monoclonal antibodies against salivary gland membranes and basal lamina identified at least four antigens on the salivary glands that may potentially serve as receptor molecules for sporozoite invasion (5). Additional evidence of a receptor-ligand-mediated interaction is provided by Sidjanski et al. (223), who demonstrated that recombinant P. falciparum circumsporozoite (CS) protein, the major surface protein of sporozoites, binds preferentially to A. stephensi salivary glands compared to other tissues. This report also showed that a peptide incorporating region I, a highly conserved sequence found in all rodent and primate Plasmodium CS proteins, prevents binding of CS to the salivary glands (223).
Concomitant with efforts to isolate salivary gland receptors for sporozoites (5) are efforts to isolate sporozoite ligands involved in salivary gland invasion (B. T. Beerntsen and A. A. James, unpublished data). Initial attempts to isolate novel sporozoite ligands were based on the hypothesis that differential gene expression was responsible for the observed differences in sporozoite infectivity for mosquito salivary glands and vertebrate host tissue. It has been demonstrated that Plasmodium berghei salivary gland sporozoites are 10,000-fold more infectious for the appropriate vertebrate tissue than are sporozoites isolated from oocysts (250). Touray et al. (244) confirmed this observation when they demonstrated that a 100% infection efficiency resulted when P. gallinaceum sporozoites were isolated from salivary glands and inoculated into chicken hosts but only a 20% efficiency resulted when oocyst sporozoites were inoculated. These authors (244) also showed that the infectivity of P. gallinaceum sporozoites for mosquito salivary glands was developmentally regulated. Sporozoites isolated from the oocyst and inoculated into mosquitoes invaded the salivary glands, but sporozoites isolated from salivary glands did not reinvade glands after intrathoracic inoculation. Based on these observations, cDNA libraries representing P. gallinaceum sporozoites isolated from oocysts or salivary glands were constructed and used in a subtraction hybridization protocol to obtain genes that are expressed differentially during the oocyst sporozoite stage (Beerntsen and James, unpublished). Using the subtraction protocol and a more traditional heterologous screening technique, three novel sporozoite genes were isolated that seem to be expressed at different levels between the two sporozoite stages. Experiments are under way to determine the cellular location of the gene products of these novel genes and determine by in vivo competitive-blocking assays if any of the three gene products are responsible for the invasion of mosquito salivary glands (Beerntsen and James, unpublished). Molecules that prevent sporozoite invasion of salivary glands will be candidate molecules for use in strategies to construct parasite-resistant mosquitoes (see “Introduction of exogenous gene products in mosquitoes” below).
Once in the salivary glands, immune peptides also may influence pathogen transmission. Dimopoulos et al. (61) used a reverse transcription-PCR (RT-PCR) protocol to assess expression levels of six immunoresponsive genes in the salivary glands of P. berghei-infected A. gambiae. No up-regulation of the six genes was noted in the salivary glands until sporozoites emerged from oocysts on the midguts and then invaded the salivary glands. It was hypothesized that salivary gland expression of immune system molecules may serve to limit bacterial and fungal proliferation in this tissue, but this remains to be determined.
Midgut
The majority of mosquito species require a blood meal for egg production (termed anautogeny), and this blood, mixed with saliva, is pumped through the foregut and into the midgut. The process of blood feeding and distension of the midgut initiates a number of physiological processes associated with digestion and the initiation of egg development. The blood meal also is the source of pathogens transmitted by mosquitoes: consequently, specific defense mechanisms against or responses to these foreign invaders must be considered in relation to the initiation of these other physiological events (see “Hemolymph and hemocoel” and “Fitness costs and the immune response” below).
The midgut is a potentially hostile environment for pathogens, because the blood within the midgut is different from the blood circulating in a homeothermic organism. Within the midgut, the temperature and pH change abruptly, proteolytic enzymes begin the process of blood digestion, ingested blood may lose its fluid nature, and shortly after ingestion a chitinous peritrophic matrix is produced that isolates the blood meal from the midgut epithelium (Fig. 1). Exiting the midgut as quickly as possible would seem to be a viable strategy for most mosquito-borne pathogens. Filarial worms and viruses do exit quite rapidly, but malaria parasites, which may spend over 24 h in the midgut, take advantage of this environment as the stimulus for sexual reproduction (16, 77, 78). As a result, Plasmodium spp. face different obstacles, such as extended exposure to proteolytic enzymes, from those faced by filarial worms or viruses. For example, an in vivo study has implicated trypsin-like proteinases in the destruction of P. gallinaceum ookinetes in the midgut of A. aegypti (79). Subsequent in vitro studies using P. gallinaceum ookinetes showed that the addition of crude midgut extracts or purified enzyme preparations from A. aegypti damaged or destroyed the ookinetes (79, 80, 266). Undoubtedly, the proteolytic environment of the midgut contributes to the attrition rate observed during the development of Plasmodium sexual stages, but other factors, as yet undetermined, also are involved (31).
In mosquito species in which the blood clots rapidly, the intensities of infections with filarial worms are significantly reduced compared with the intensities in species that clot blood more slowly, but these differences are abrogated when mosquitoes are exposed to parasites in blood containing an exogenous anticoagulant (112). Recent studies with substrains of A. aegypti selected from the black-eyed Liverpool strain for high susceptibility (LVPsbm) or high refractoriness (LVPrbm) to B. malayi revealed that the efficiency of midgut penetration by microfilariae in LVPrbm was significantly reduced compared with that in LVPsbm (12). In ∼17% of the mosquitoes examined, none of the ingested parasites migrated out of the midgut; therefore, the permissiveness of the midgut to parasite penetration influenced not only the intensity but also the prevalence of infection. QTL mapping of the number of parasites ingested and penetration efficiency revealed a locus on chromosome 2 (idb[2, LF181]) (idb = intensity determinant for Brugia) that is linked to QTL for Brugia susceptibility (fsb[2, LF98]) and P. gallinaceum susceptibility (pgs[2, LF98]). It is possible that the permissiveness of the midgut for penetration by parasites is a principal determinant of vector competence in A. aegypti for different parasites. Data suggesting that this region of chromosome 2 also influences susceptibility to yellow fever virus add credibility to this hypothesis (237). It has not been determined if differences in salivary gland components, midgut secretions, or the makeup of the midgut itself between these substrains are responsible for this midgut barrier to infection. Experiments could be designed, however, to determine if chromosome 2 QTL influence Brugia susceptibility by modification of the midgut environment. Mosquitoes can be exposed to infection via intrathoracic inoculation of microfilariae, thereby bypassing the midgut entirely. If fsb[2, LF98] imparts its influence on susceptibility through midgut factors, then QTL mapping of Brugia susceptibility in mosquitoes exposed by inoculation should not exhibit fsb[2, LF98] but only the QTL on chromosome 1.
Shortly after a blood meal is ingested, midgut cells secrete a peritrophic matrix composed of proteins embedded within chitin that separates the blood bolus from the midgut epithelium (189). It has been proposed that the peritrophic matrix provides protection against pathogens, keeps protease inhibitors within the lumen, and functions as a solid support as well as a semipermeable filter for digestive enzymes and blood proteins (14; M. Jacobs-Lorena, personal communication).
Relatively little is known about the biochemistry or genetic regulation of peritrophic matrix formation in any mosquito species. Anopheline mosquitoes secrete granules from the apical end of the midgut during feeding, and these form a matrix containing N-acetylgalactosamine and galactose, whereas in culicine mosquitoes the peritrophic matrix forms from gut secretions and is composed of N-acetylglucosamine and glucose (13). Although numerous proteins have been reported to be part of the matrix (162), only one protein from A. gambiae, designated adult peritrophin 1 (Ag-Aper1), has been identified to date (221). Recent experiments have shown that blood feeding induces a midgut-specific glutamine synthetase gene in A. aegypti (224). Transcription begins within 1 h of blood feeding and persists through 18 h, when the enzyme activity reaches its highest level. These authors propose that glutamine biosynthesis within the midgut is required for production of glucosamine-6-phosphate, which is converted to N-acetylglucosamine for use in chitin synthesis in peritrophic matrix formation. Biological evidence supports this hypothesis, because inhibition of glutamine synthetase in vivo prevents matrix formation in A. aegypti (224).
Complete formation of the peritrophic matrix takes 12 to 30 h (44); therefore, it does not serve as a formidable barrier to filarial worms or viruses that generally enter or migrate through the midgut epithelium within a few hours, although it has been suggested that viruses that fail to exit the blood bolus within a few hours become trapped by the developing peritrophic matrix (259). Differential infectivity of Plasmodium to different mosquitoes has been attributed to differences in the rate of peritrophic matrix formation and ookinete development (189, 191), but in general, Plasmodium parasites have developed specific mechanisms for traversing the peritrophic matrix following ookinete formation.
P. gallinaceum ookinetes traverse the peritrophic matrix by secreting a prochitinase that, upon activation, enables the ookinete to penetrate this chitinous barrier (98, 220). The parasite apparently relies on mosquito trypsins, enzymes present in the protease-rich milieu of the A. aegypti midgut, to activate the prochitinase, thereby aiding in its survival (218). P. falciparum also seems to secrete a chitinase to aid in peritrophic matrix penetration, but P. vivax, another human malaria parasite, apparently crosses the peritrophic matrix before it is complete, eliminating the need for a chitinase (191). Additional studies using antitrypsin antibodies have shown that they block the development of P. gallinaceum in the mosquito, suggesting that mosquito trypsin is a potential candidate molecule in malaria transmission-blocking strategies (215, 216). The results of the chitinase studies discussed above indicate that generalizations are not applicable and that each Plasmodium-mosquito species interaction is unique and likely will require individual assessment.
Recently, a decreased oocyst infection in A. stephensi was attributed to the susceptibility of P. berghei midgut stages to nitric oxide (NO) (138), a molecule involved in the destruction of macroparasites in vertebrates (180). In this study, elevated transcriptional levels of NO synthase (NOS), an enzyme critical for the production of NO, were noted in the midguts of P. berghei-infected A. stephensi on days 1 to 3 postinfection compared with control mosquitoes. Likewise, transcriptional activity of NOS also was assessed in A. gambiae during critical stages of P. berghei development and found to be up-regulated in abdominal tissue and the midgut at 24 h following infection (61). Although the production of NO may be induced by the presence of the parasite in the mosquito, this production also could be due to tissue damage or stress created by the invading parasite. However, regardless of the NO induction trigger, the parasite burdens in the mosquito, although not eliminated, seem to be negatively affected by NO production.
The methods by which pathogens penetrate the midgut epithelium are varied and not particularly well studied at the molecular level. Filarial worms reportedly traverse the midgut by means of a cephalic hook that enables them to migrate out of the midgut by mechanically disrupting the tissues of the midgut (64). In mosquito-borne arboviruses, a receptor-ligand interaction appears to mediate the attachment and entry of viruses into midgut cells. Monoclonal antibodies to viral envelope glycoproteins block viral invasion of mosquito cell lines (88, 139, 140) or midguts (140), and a putative receptor for Venezuelan equine encephalitis virus has been identified from a mosquito cell line (141). Penetration of the midgut epithelium by Plasmodium ookinetes also is likely to be receptor mediated, although specific details are lacking. Only recently has an ultrastructural investigation of ookinete adhesion to midgut epithelium been undertaken to describe the interactions that occur between these two entities (273). When purified P. gallinaceum ookinetes and isolated A. aegypti midguts were combined in vitro, the ookinetes initially adhered to and migrated through a microvillus-associated network that covers the surface of the midgut microvilli. The ookinetes then directly bound the microvilli and probably penetrated vesiculated and blebbed cell surfaces of the midgut epithelium via a carbohydrate ligand on the surface of the midgut (273, 274). Furthermore, a specific cell type in A. aegypti midguts, which is located at the posterior region of the posterior midgut and expresses vesicular ATPase, is invaded preferentially by P. gallinaceum ookinetes (48, 219). Efforts to understand at a molecular level the interactions that promote pathogen attachment to the midgut and enable the parasite to traverse the midgut will probably identify novel sites of intervention to prevent pathogen transmission.
Once within the midgut cells, parasites such as Plasmodium spp. are still susceptible to destruction. Vernick et al. (254) described a lytic mechanism of ookinete destruction that is responsible for refractoriness to the bird malaria parasite P. gallinaceum in the African vector of human malaria, A. gambiae. Melanotic encapsulation of ookinetes within the midgut epithelial cell is another means by which the mosquito destroys parasites. A strain of A. gambiae was selected that prevents development of the simian parasite P. cynomolgi by melanotically encapsulating the ookinete stage (50, 182). Feldmann and Ponnudurai (67) report another refractory mechanism in which P. falciparum ookinetes develop but are inefficiently transformed into oocysts in A. stephensi. The basis for this reduction in oocyst formation is unknown, but recent efforts have demonstrated an induction of novel proteins following a blood meal in the fully susceptible lines compared to the refractory line, suggesting that the refractory condition results from the absence of mosquito proteins required for transformation and development (190). These three refractory mechanisms seem to be genetically independent, and each apparently has a small number of genes contributing to the phenotype (50, 68, 253, 254).
Analysis of antimicrobial gene expression within mosquito midguts has demonstrated differences in immune system activation between A. gambiae and A. aegypti. An RT-PCR protocol detected an up-regulation in mRNA transcripts for antimicrobial genes, such as defensin, in the midguts of A. gambiae mosquitoes exposed to either bacteria or a P. berghei-infected blood meal (60, 202). However, in A. aegypti, RT-PCR detected low levels of defensin transcripts in the midguts of naive mosquitoes and of mosquitoes that had ingested a P. gallinaceum-infected or noninfected blood meal, but no up-regulation was noted (136). A more detailed analysis of defensin production in the midgut indicated that only defensin C was expressed but defensin isoforms A and B were not, suggesting a tissue specificity among the three defensin isoforms (136). Although defensin transcriptional activity in the midgut has been assessed, levels of protein expression for defensin and other antimicrobial peptides still need to be assayed in this critical tissue site. Furthermore, investigations into antimicrobial gene expression have been limited to the genera Anopheles and Aedes. Relatively little information concerning immune system peptide activation or synthesis exists for such genera as Culex and Mansonia, despite their importance as human disease vectors.
Hemolymph and Hemocoel
Within the hemocoel of the mosquito, tissues and organs are bathed in hemolymph, the medium that transports nutrients, hormones, and immune system effector molecules (Fig. 1). Immune system molecules provide the mosquito with an innate defense system against invading pathogens that is both discriminatory and efficient. Although this immune system lacks the typical antibody and immune system memory responses seen in vertebrate immune responses, it has an arsenal of humoral and cellular weapons, including encapsulation, melanization, and immune system peptide production, that limit or prevent pathogen development (Fig. 2) (183).
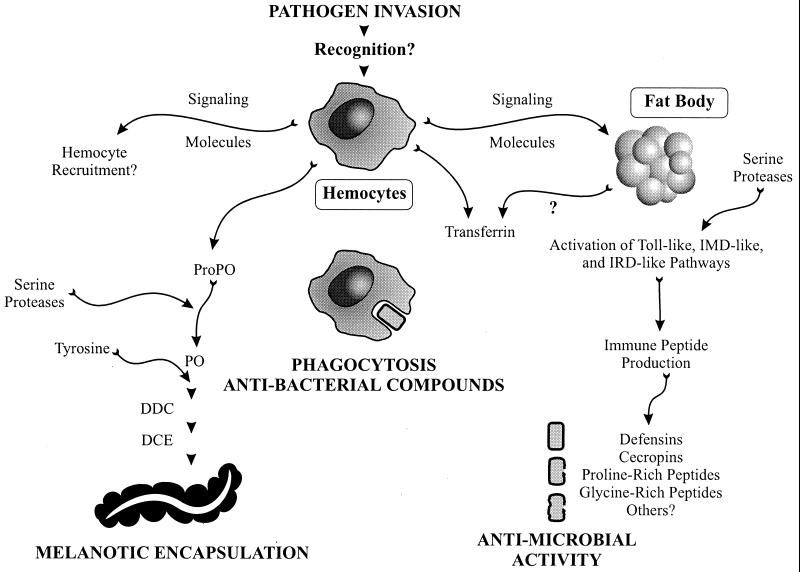
FIG. 2.
Mosquito immune responses to pathogens include melanotic encapsulation, phagocytosis, and production of antibacterial compounds and immune peptides. The hemocyte is a multifaceted cell that is probably involved in pathogen recognition, cell signaling, production of enzymes and immune system-associated molecules (e.g., transferrin), and phagocytosis. Invasion by bacteria results in phagocytosis by hemocytes and in the production of antibacterial compounds. If the pathogen is too large to be phagocytosed (e.g., filarial worms), mosquito hemocytes may recognize the pathogen as foreign and recruit other hemocytes to participate in the melanization response. Activated hemocytes also produce transferrin, which is a melanization-associated molecule, and a prophenoloxidase (ProPO), which is activated by a serine protease to become phenoloxidase (PO). After a phenoloxidase-catalyzed hydroxylation of tyrosine, other enzymes like dopa decarboxylase (DDC) and dopachrome conversion enzyme (DCE) catalyze other critical steps, ultimately resulting in melanin production. Also depicted is a proposed pathway for immune system peptide production. This pathway is probably induced when hemocytes recognize a foreign pathogen and relay a signal to the fat body, where signaling pathways, like Toll, IMD, and IRD, are activated by a serine protease. Following transcriptional activation, the fat body then produces defensin, cecropin, and proline-rich and glycine-rich peptides that have antimicrobial activity. Although the humoral immune response of mosquitoes does depend upon the fat body for immune system peptide production, other tissues, such as the midgut and salivary glands, do transcribe immune system peptides following activation by a pathogen (61).
The recognition of foreign/nonself molecules is critical for the initiation of the mosquito immune response. However, very little is known for insects, let alone mosquitoes, concerning specific host recognition molecules (receptors), the nonself molecules triggering the immune response, or the signaling pathways ultimately responsible for the production of immune effector molecules (Fig. 2). The detection of structural patterns on the surface of pathogens has been termed pattern recognition (108), and this detection system may initiate the innate immune response in insects. Although the surface molecules or attributes of Plasmodium spp. or filarial worm spp. that are responsible for initiating immune responses in mosquitoes are unknown, common structural components of the outer membranes and walls of bacteria and fungi, like lipopolysaccharide, peptidoglycan or β1,3 glucans, induce the synthesis of hemolymph peptides associated with antimicrobial responses (81). Recently, Kang et al. (111) cloned a protein, designated peptidoglycan recognition protein, that had been induced in the lepidopteran Trichoplusia ni following challenge with bacteria and discovered that the peptidoglycan recognition protein was conserved in mice and humans. Investigations of binding proteins or receptor molecules in mosquitoes have been limited to the observation that transcripts for a putative gram-negative bacterium-binding protein are up-regulated in A. gambiae infected with P. berghei (60, 61, 202).
Currently, considerably more information is available concerning the effector arms of the mosquito immune response. Since the days of Ross' black spores, which represent melanized ookinetes and oocysts (205), it has long been known that mosquitoes can melanize pathogens. This melanization response usually is mediated by hemocytes (34, 39, 74, 265) and culminates with the deposition of melanotic materials around the pathogen. This response has been observed in all mosquitoes studied to date, including mosquitoes susceptible to parasite development (185). It is a specific immune response and the extent and effectiveness of the response may be influenced by the pathogen (40, 50). For example, natural and laboratory populations of A. subalbatus melanotically encapsulate B. malayi microfilariae but are unable to melanize the closely related microfilariae of B. pahangi. Although Ross' black spores have long been observed in natural mosquito populations (205), only in selected laboratory strains can mosquitoes melanize every ookinete/oocyst of a particular strain of parasite (50). In these selected strains of A. gambiae, the influence of the malaria parasite on the immune response of the mosquito is readily apparent as sympatric strains of P. falciparum develop in these strains but strains of the parasite from other regions are melanized and destroyed (50). Likewise, A. albimanus supports the development of sympatric strains of P. falciparum (Ranford-Cartwright, personal communication).
Melanization involves a complex series of reactions requiring tyrosine precursors and phenol oxidases to produce a capsule, consisting of melanin polymers cross-linked with proteins, that sequesters the foreign invader (Fig. 3) (43, 269). This response is related to cuticular sclerotization, egg chorion tanning, and wound-healing pathways; consequently, a number of the substrates and enzymes are shared among these diverse biochemical events (22, 56, 128). Both the dopa and dopamine oxidation pathways can be involved in the production of melanin, which is required for defense responses (269). It is likely that the dopamine pathway is involved in melanization, because the intermediates are longer-lived and more stable, thereby making them more readily available for cross-linking with proteins involved in melanotic capsule formation (126).
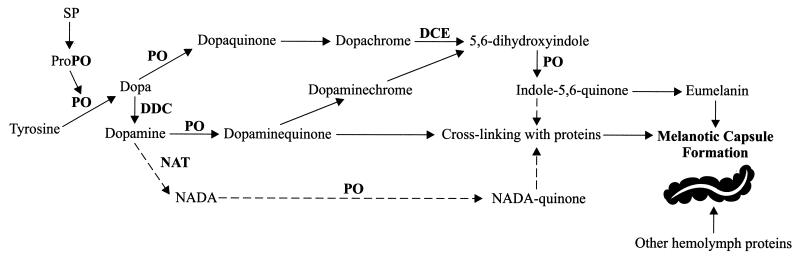
FIG. 3.
In the proposed melanotic encapsulation pathway of mosquitoes, a serine protease (SP) proteolytically cleaves an inactive prophenoloxidase (ProPO) to form an active phenoloxidase (PO). Tyrosine, the initial substrate, then is hydroxylated by the activated PO to form dopa, a key branchpoint substrate. Next, dopa is oxidized by PO to form dopaquinone, which then forms a dopachrome intermediate. A dopachrome conversion enzyme (DCE) converts this intermediate to 5,6-dihydroxyindole, which subsequently is oxidized by PO to form indole-5,6-quinone. This latter compound forms eumelanin or cross-links with proteins to eventually produce a melanized capsule. Dopa also can be decarboxylated by dopa decarboxylase (DDC) to form dopamine, which forms another branch point. A PO-based oxidation of dopamine produces dopaminequinone, which can cross-link with proteins or form melanin via the indole pathway. Dopamine also may be acetylated by N-acetyltransferase (NAT) to form N-acetyldopamine (NADA). PO oxidation of NADA then produces NADA-quinone, which cross-links with other proteins to form a melanotic capsule. Solid arrows designate likely major pathways, and dashed arrows denote probable minor pathways. Adapted from reference 269.
Although many of the initial studies investigating melanization were related to its role in cuticular sclerotization or eggshell tanning in other insects (22, 56, 128), substrates and enzyme activity levels have been assessed in immune-activated mosquitoes. Tyrosine and tyrosine-derived compounds were detected in A. aegypti (166) and A. subalbatus (269) that were melanotically encapsulating (i.e., immune system activated) the microfilariae of D. immitis and B. malayi, respectively. Increased enzyme activity levels of phenol oxidase were documented in immune system-activated A. aegypti (129) and A. subalbatus (269), and a significantly elevated amount of dopa decarboxylase (269) was observed in A. subalbatus in the initial hours following immune system activation. Recently, cDNAs encoding prophenoloxidase (ProPO), the inactive form of phenol oxidase, have been characterized from A. gambiae (110, 121, 165), A. subalbatus (37), and A. aegypti (A. Taft, C. C. Chen, and B. M. Christensen, unpublished data). Dopa decarboxylase from A. aegypti also has been molecularly characterized (70, 72), and dopachrome conversion enzyme has been purified and its characterization has begun (130; J. Johnson, J. Li, and B. M. Christensen, unpublished data). Although the biochemistry of melanization has been relatively well studied (Fig. 3), the genetic control of this complex biochemical pathway still needs to be delineated.
Efforts to understand the control mechanisms responsible for melanotic encapsulation in mosquitoes are made more difficult by the presence of multiple phenol oxidases, a critical enzyme in the melanization pathway, and serine proteases, enzymes implicated in both the activation of ProPO and the signaling pathways leading to immune peptide production. To date, six cDNAs representing different ProPO genes have been isolated from larvae, pupae, and cell culture of the malaria vector A. gambiae (110, 121, 165) and two different cDNAs have been isolated from A. aegypti (A. Taft, C. C. Chen, and B. M. Christensen, unpublished data). In A. subalbatus, a ProPO gene associated with the melanization of D. immitis has been isolated and cloned (37) and a second ProPO that has other functions has been isolated (C. C. Chen, personal communication). mRNA expression profiles for these multiple ProPO genes indicate a wide range of gene expression. Some of the A. gambiae ProPO genes are transcriptionally active during specific larval stages, and others are active predominantly in the pupal and adult stages (165). Association of particular ProPOs with immune system activation has been assessed in some experimental studies, with varying results. In an A. gambiae cell line, six ProPO transcripts were constitutively expressed, but none was up-regulated following immune system induction even though the cell line did show bacterium-induced immune transcripts for defensin (165). In A. subalbatus, ProPO transcripts were observed in hemocytes 3 days after immune system activation with D. immitis microfilariae (37), suggesting a replenishment of ProPO mRNA following depletion of the enzyme required earlier for the melanotic encapsulation of filarial worms. In A. aegypti, transcripts for one ProPO have been observed in embryos and transcripts for a second ProPO were found in third-stage larvae. No up-regulation could be detected in either bacterium-inoculated or microfilaria-inoculated A. aegypti (Taft, Chen, and Christensen, unpublished).
The ProPO cascade is initially activated by serine proteases that are functionally diverse enzymes involved in a number of important processes including digestion (3, 164), hormone activation (97), and vertebrate blood coagulation (59). Siden-Kiamos et al. (222) reported the isolation of three serine proteases from A. gambiae with no apparent identity to the digestive serine proteases, and transcripts for these genes are expressed at low levels during various developmental stages. Han et al. (89) characterized another serine protease from A. gambiae, AgSp24D, that exhibits more abundant transcripts in adults than in larvae or pupae. Transcripts for AgSp24D are not induced in response to septic wounding, bacterial injection, or CM-Sephadex bead injection, but more abundant transcripts for this gene are found in a strain of A. gambiae refractory to Plasmodium development than in a susceptible strain (89). Another serine protease from A. gambiae, AgSp14D1, is transcriptionally up-regulated following wounding or bacterial challenge (184), maps to the polytene chromosome region that has been implicated in the melanization phenotype, and is one of a few hemolymph proteins that is differentially expressed between the refractory and susceptible strains of A. gambiae (S. Paskewitz, personal communication). It contains conserved residues typically found in serine proteases including a clip (i.e., disulfide knot) domain characteristic of a subfamily of regulatory serine proteases that includes the Drosophila proteins Easter and Snake (184). Five additional serine proteases are expressed in A. gambiae following immune system challenge, and one of these genes, AgSp22D, is expressed after bacterial challenge but not after wounding. Interestingly, this serine protease contains a scavenger receptor cysteine-rich domain, a mucin/chitin-binding domain, a histidine-rich domain, and an epidermal growth factor-like and protease domain (Paskewitz, personal communication). To date, a large number of phenol oxidases and serine proteases have been identified, and it seems probable that more will be identified in the future. Consequently, the challenge is to identify and further characterize the genes that are involved directly or indirectly in mosquito immunity. Although genes that are up-regulated following immune system activation are strong candidates for involvement in mosquito immunity, constitutively expressed genes also might play key roles. Therefore, the development of a cell transfection assay to test functional significance may be required. In this assay, a serine protease or phenol oxidase gene would be linked to a reporter gene and then the production of the reporter molecule would be assayed following immune system activation of the cells. Another option for determining the relevance of a particular enzyme for mosquito immunity would be to use a gene knockout (e.g., antisense) strategy or transformation strategy to confirm the biological function (see “Introduction of exogenous gene products in mosquitoes” below).
Immune system peptide production is another means whereby insects protect themselves against pathogens (Fig. 2). The insect immune system peptides have been classified according to their physical structure and include cysteine-rich defensins, proline-rich and glycine-rich compounds, and α-helix-containing cecropins (23, 47, 93). In mosquitoes, defensins and, to a lesser extent, cecropins have been well described, but little specific information is currently available concerning proline- and glycine-rich compounds.
Mosquito defensins are peptides, 40 amino acids in length, containing six cysteine residues that form intramolecular bridges (27, 36, 132, 137, 201). The defensins are active primarily against gram-positive bacteria and are the predominant immune system peptides produced in bacterially challenged A. aegypti (27, 132). Consequently, they have been relatively well studied, and information is available concerning isoforms, genomic organization, expression profiles, and activity against eukaryotic organisms.
Defensins from A. aegypti exist as three isoforms (A, B, and C) (132, 137), but in A. gambiae only one has been characterized to date (201). As a group, defensins in A. aegypti are expressed rapidly and in large amounts, with concentrations of up to 45 μM mature peptide observed in the hemolymph 24 h following immune system activation (132, 136, 137). Mosquito defensins exist as preprodefensins, with significant variability observed in the signal peptide and prodefensin sequences, particularly between isoform C and the other two isoforms (137, 201). Recent studies of defensin genomic organization in A. aegypti suggest that two genes encode the three isoforms, with isoforms A and B likely to be allelic variants of the same gene (137). Additional differences between isoform C and the other two isoforms have been noted at the transcriptional level and in the levels of mature protein in the hemolymph (132, 136). Isoform C was observed at lower levels in the hemolymph of bacterially challenged A. aegypti than were isoforms A and B (132). As noted previously, only isoform C was transcriptionally active in A. aegypti midguts (136); however, exposure of mosquitoes to a P. gallinaceum-infected or uninfected blood meal did not induce an up-regulation of the isoform C transcript (136). These transcriptional and translational differences, coupled with the differences in signal peptide sequences, probably reflect tissue-specific expression patterns (136, 137).
Cecropins, which are small peptides (4 kDa) with broad-spectrum activity against gram-positive and gram-negative bacteria, were induced and then isolated from a bacterially challenged A. albopictus cell line, with the resulting peptide sequence exhibiting 36% identity to cecropin from Drosophila melanogaster (92, 235). Recently, a novel member of the cecropin family, devoid of both a customary tryptophan residue and C-terminal amidation, was isolated and characterized from the hemolymph of bacterially challenged A. aegypti (133). Expression of this mosquito cecropin was not detected in any immature stage or in naive adults by either Northern or RT-PCR analysis, but Northern analysis did detect expression in immune system-activated adults, beginning 6 h postinoculation and continuing for 7 to 10 days. Because of its unique structural features, its activity against bacteria, fungi, and yeast was compared to that of Drosophila cecropin A, which contains the usual C-terminal amidation and initial tryptophan residue. The two cecropins had similar activity profiles against bacteria and filamentous fungi, but the mosquito cecropin was generally less active against these pathogens than was its Drosophila counterpart (133). However, the A. aegypti molecule was active against yeast, unlike the Drosophila cecropin (133).
Although produced in response to bacterial infection, immune system peptides have been assayed for their ability to prevent or limit infection of mosquitoes by eukaryotic organisms, e.g., filarial worms and malaria parasites. Gwadz et al. (87) demonstrated that when magainins and cecropins, derived from the skin of frogs or giant silk moths, respectively, were injected into Anopheles infected with Plasmodium spp., malaria transmission was prevented due to the disruption of normal oocyst development. Subsequently, a study suggested that the coinjection of B. pahangi microfilariae with cecropins into A. aegypti also significantly reduced the numbers of developing larvae observed compared with those in controls (28). Shahabuddin et al. (217) showed that defensins from the dragonfly and flesh fly differentially killed some of the mosquito stages of P. gallinaceum in A. aegypti. In vitro studies indicated that the defensins had no detectable effects on the early stages, zygotes and ookinetes, but were toxic to the sporozoite stages (217). Injection of defensins into P. gallinaceum-infected A. aegypti significantly reduced oocyst density or damaged developing oocysts depending upon the timing of the injections (217). Bacterial challenge of mosquitoes followed by an infectious blood meal containing either the filarial worm B. malayi or the malaria parasite P. gallinaceum or P. berghei also resulted in a significantly reduced prevalence and mean intensity of infection compared with that in controls with no immune system activation (135, 136). In these studies, it is suggested that defensins played a significant role in the observed parasite reductions due to the predominance of this peptide in immunologically activated hemolymph (136). However, the role of other, as yet unidentified, immune system proteins cannot be discounted, because immune system activation with bacteria elicits the expression of a number of immune system peptides (P. Bulet and C. A. Lowenberger, unpublished data). The development of an orally infectious Sindbis virus expression system for A. aegypti (208a), in which defensin or other immune system molecules can be specifically expressed in the hemolymph of A. aegypti, circumvents the need for immune system activation by injection of bacteria. Using the double subgenomic Sindbis virus system, mosquitoes can be engineered to transiently express defensin or other immune system peptides in the hemolymph. These mosquitoes then can be challenged with a parasite-infected blood meal, and the influence of a specific peptide on parasite development can be evaluated by measuring the prevalence and mean intensity of infection at appropriate times following exposure. If specific immune system peptides have a significant impact on these parameters, they would be candidate molecules for use in a germ line transformation protocol where the peptides would be induced or constitutively expressed in a mosquito population prior to challenge with the parasite (see “Introduction of exogenous gene products in mosquitoes” below).
Transferrin is another protein that is up-regulated in mosquitoes after immune system activation. An 84-kDa protein, later identified as transferrin, was detected in hemocytes and preferentially expressed in the hemolymph of A. aegypti mosquitoes that were melanotically encapsulating D. immitis microfilariae (8, 9, 267). Transferrin also is up-regulated in the A. subalbatus-B. malayi model system, where ingested microfilariae are melanized rapidly, thereby reflecting a melanization phenomenon that occurs naturally in the field. In A. subalbatus, transferrin is transcriptionally up-regulated during melanization of B. malayi following ingestion via a blood meal, but blood feeding alone does not significantly up-regulate transferrin transcription (S. Falk, C. A. Lowenberger, and B. M. Christensen, unpublished data). In the absence of a blood meal but following injection of bacteria, transferrin mRNA levels also increase (Falk et al., unpublished). In addition, transferrin is up-regulated in A. aegypti and A. albopictus cell cultures following treatment with heat-killed bacteria (267). Collectively, these data suggest that transferrin is involved both in antibacterial responses and during melanization reactions.
The molecular characterization of mosquito transferrin has demonstrated that, like transferrin from two other insect species, it has conserved iron-binding residues in the N-terminal lobe but, unlike vertebrate transferrins, it lacks iron-binding residues in the C-terminal lobe (267). It has been hypothesized that the N-terminal lobe allows transferrin to sequester iron from invading organisms, and the absence of the C-terminal lobe may be a mechanism to prevent the act of “iron piracy” (53), whereby bacteria preferentially pirate iron from receptors located on the C terminus of the transferrin molecule (267).
Although the study of immune system proteins in mosquitoes has blossomed in the last 5 years, there are undoubtedly many additional proteins whose involvement in the recognition, signal transduction, and effector mechanisms of the immune response still need to be determined (Fig. 2). In preliminary studies using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, approximately 13 peptides were up-regulated in A. aegypti following bacterial exposure (Bulet and Lowenberger, unpublished). This powerful technology already has been used to identify peptides associated with the immune response in Drosophila exposed to bacteria (249). Furthermore, detailed studies investigating the role of immune system proteins in other medically important genera such as Culex and Mansonia need to be undertaken. Toward this end, preliminary studies have employed a PCR strategy, based upon sequence identity with cecropin and defensin from A. aegypti, to isolate these immune system proteins from C. pipiens (C. A. Lowenberger, H. Farid, and B. M. Christensen, unpublished data).
Genetic Regulation
Currently, investigations into the genetic control of insect immune system proteins are most advanced for Drosophila, due primarily to previous advances in the study of fruit fly genetics and developmental biology. It is apparent that signaling mechanisms like the Toll pathway, which has been identified as a critical component in the embryonic development of Drosophila (160), are also important in the immune response of Drosophila to bacteria and fungi (94, 152). A number of components of the Toll pathway have amino acid sequence identity to mammalian innate, acute-phase response proteins, suggesting a common ancestry for these immune system molecules (94, 152). Similar to the cytokine-induced acute-phase response molecules (e.g., interleukin-1 receptor, IκB, and NF-κB) in mammals, the insect homologues, e.g., Toll, Cactus, and Dorsal, respectively, seemingly also play a significant role in the regulation of immune responses (94, 95, 152). It is proposed that in the Toll pathway, a serine protease activates the ligand Spaetzle, which in turn binds to the Toll receptor. Following Toll activation and a subsequent cascade of events, a Rel family transcriptional activator, like Dorsal, Dif, or Relish, is released from its inhibitor, Cactus, and translocates to the nucleus. Once in the nucleus, the transcriptional activator binds to its appropriate DNA motif, and transcription of specific immune system peptides occurs (94, 95, 152). Other pathways in Drosophila that direct antimicrobial gene expression are the recently discovered but as yet molecularly uncharacterized immune deficiency (Imd) (122) and immune response-deficient (Ird) (264) pathways, which control the expression of antibacterial genes (94, 95, 152). It is of particular interest that not only can the immune response of Drosophila discriminate between various classes of microorganisms (antifungal and antibacterial) (124) but also specific signaling pathways are responsible for specific antimicrobial responses. For example, the Toll pathway controls the systemic expression of the antifungal peptide drosomycin (123) but not its local expression in barrier epithelial tissues (73). Moreover, the Imd pathway controls the expression of the antibacterial peptides diptericin and drosocin, while both pathways apparently direct the expression of the antibacterial peptides cecropin, attacin, and defensin and the dual-activity peptide Metchnikowin, which is active against bacteria and fungi (95, 122, 123, 125).
Although progress has been made in understanding the genetic control mechanisms responsible for immune system protein production in Drosophila, knowledge of the genetic regulation of the immune response in mosquitoes is limited. It seems reasonable to predict that pathways similar to those in Drosophila (e.g., Toll, Imd, and Ird) will be identified in mosquitoes in the future. Toward that end, a serine protease that is transcriptionally up-regulated following injection of bacteria has been isolated and shown to have significant identity to Easter, a potential activator of Spaetzle (184). However, it remains to be determined whether this serine protease is involved in the production of immune system peptides. Determination of its involvement will require a functional assay of some sort, for example, a cell transfection assay using a serine protease promoter-reporter transgene, or a knockout/transformation protocol (see “Introduction of exogenous gene products in mosquitoes” below).
Transcriptional activators with sequence identity to Drosophila immune response-related transcription factors also have been isolated from mosquitoes. Recently, Barillas-Mury et al. cloned from A. gambiae the transcription factors Ag-STAT (4) and Gambif1 (gambiae immune factor 1) (2), which are novel members of the STAT (signal transducers and activators of transcription) and Rel families, respectively. Gambif1 is related to Dorsal and Dif, and Ag-STAT belongs to a family of transcription factors that have previously been shown to regulate aspects of the vertebrate immune response (155, 239). Both Gambif1 and Ag-STAT are translocated to the nucleus in fat body cells, the site of immune system protein production in mosquitoes, and Gambif binds to κb-like regulatory elements in vitro; however, its in vivo role has not been determined. In mosquitoes, promoter regions containing regulatory sequences or motifs for transcription factor binding have been identified only for the A. aegypti defensin gene. Cho et al. (35) showed that the upstream region of the gene encoding A. aegypti defensin A contains motifs for the binding of GATA factors that have been implicated in fat body gene expression. In addition, numerous cis-regulatory sequences previously identified in immune system protein genes from other insects (153) have been identified in the A. aegypti defensin gene. These regulatory sequences include binding motifs for NF-κB, nuclear factor interleukin-6, and nuclear factor endothelial leukocyte adhesion molecule 1. However, the involvement of these and other potential regulatory sequences in the control of the mosquito immune response has yet to be confirmed by functional assays.
Immune System Suppression or Evasion by Parasites
Despite the best efforts of the mosquito, many pathogens are able to evade or suppress the immune response and successfully develop within the insect. However, the mechanisms by which the pathogens avoid the immune response are relatively unknown. At what step in the process leading to production of immune proteins, melanin deposition, or induction of lytic mechanisms does the successful pathogen block or avoid the response? Is it at the level of recognition, signal transduction, or production of immune system effector molecules? It has been proposed that pathogens may avoid recognition as nonself by acquiring host antigens or by expressing, on their surface, molecules that exhibit identity to host antigens (57). Parasites also may produce secretory/excretory products that suppress the immune response of the mosquito (40). It has been demonstrated that A. subalbatus recognizes and melanizes B. malayi microfilariae (11, 265) but fails to melanize microfilariae of the closely related species B. pahangi. In another study, melanization of inoculated B. pahangi was significantly (28 to 47%) decreased in A. aegypti harboring a developing B. pahangi infection compared with that in noninfected controls (40). Also, as previously mentioned, A. gambiae and A. albimanus can melanotically encapsulate many different strains of P. falciparum but are unable to melanize sympatric strains of the parasite (50; Ranford-Cartwright, personal communication). Collectively, these results suggest that the parasites that develop successfully in the mosquito may lack immune system-stimulatory surface molecules, share host antigens, or produce secretory/excretory products that inhibit the immune response by interfering with either the recognition process, signaling pathways, or effector mechanisms.
Fitness Costs and the Immune Response
Parasitism has been shown to be physiologically and reproductively costly to mosquitoes (96, 103), but defense reactions that result in the melanotic encapsulation of parasites also can prove reproductively costly to the mosquito. Ferdig et al. (69) demonstrated a significant reduction in tyrosine levels, a decrease in ovarian development, and a delay in mean time to oviposition for A. subalbatus mosquitoes that were melanotically encapsulating B. malayi microfilariae. Because both melanotic encapsulation of parasites and egg tanning require tyrosine (128), a competition for limited resources ensued, ultimately resulting in a reproductive cost (i.e., lower fecundity) for the mosquito (69). Consequently, this fitness cost could be a detrimental factor to efforts to genetically engineer mosquitoes that prevent disease transmission by melanotically encapsulating pathogens (69).
Thoracic Musculature and Malpighian Tubules as Developmental Sites
The thoracic musculature is the developmental site for B. malayi and B. pahangi, and the Malpighian tubules are the developmental site for D. immitis (Fig. 1). In susceptible mosquitoes, filarial worms develop in these tissues and, upon maturation, break out of these sites. They then travel through the hemocoel to the head region, from which they actively emerge onto the surface of the vertebrate host's skin when the mosquito takes a blood meal. In some refractory strains, the filarial worms migrate to the developmental site but fail to develop, probably due to a physiological incompatibility that is independent of active immune responses (42). McGreevy et al. (150) postulated that the genes controlling the susceptibility of A. aegypti to filarial worms directly influence the host tissue that supports parasite development. Support for this hypothesis was provided by reciprocal transplantation studies using D. immitis-infected Malpighian tubules (172). These studies indicated that the genotype of the tubules (susceptible or refractory) controlled parasite developmental success or failure but that the genotype of the hemolymph bathing the tubules had no effect on filarial worm development (172). In contrast, however, studies of hemolymph transfer between susceptible and refractory strains of A. aegypti demonstrated that refractory hemolymph inhibited the development of B. malayi within the thoracic musculature of susceptible mosquitoes (115).
Despite the importance of the thoracic musculature and Malpighian tubules as developmental sites, efforts to discover the gene(s) or gene product(s) responsible for susceptibility or refractoriness to filarial worm infection in these sites have not been extensive. In vivo studies by Wattam and Christensen (257, 258) assessed radiolabeled polypeptide synthesis in the thoracic tissues of A. aegypti strains susceptible and refractory to B. malayi infection. Using sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by two-dimensional gel electrophoresis, six different polypeptide differences were observed only in the refractory strains after exposure to a blood meal (257), supporting the hypothesis that genes influencing susceptibility and refractoriness are present in the tissues in which parasites develop. However, no studies have confirmed that these polypeptides play a direct or indirect role in the refractory condition. With the technology now available, such as differential display and subtractive hybridization, it should be possible to assess differences in gene expression, at the molecular level, between parasite-susceptible and refractory strains of mosquitoes.
INTRODUCTION OF EXOGENOUS GENE PRODUCTS IN MOSQUITOES
Transformation Systems
The concept of permanently altering vector competence by creating a parasite-refractory mosquito from a susceptible population was proposed in 1968 by Chris Curtis of the London School of Tropical Medicine and Hygiene (54). However, not until the relatively recent advances in molecular biology and genetic engineering had taken place could research in this area progress much beyond the hypothesis stage. In 1996, proof-of-principle for this idea was provided when a susceptible strain of A. aegypti was rendered refractory to dengue virus using the Sindbis virus to express an antisense RNA complementary to part of the dengue virus genome (178). Moreover, the Sindbis virus system can be a powerful tool to evaluate in vivo the effectiveness of antipathogen candidate genes, for example, those encoding immune system peptides and serine proteases. These molecules can be overexpressed and their antipathogen activity can be assessed by examination of key parameters such as prevalence and mean intensity of infection. However, the Sindbis virus-mediated expression system is transient and not heritable. A germ line transformation system was needed whereby exogenous DNA could be introduced into mosquitoes and expressed in a stable and heritable manner. This was not a particularly easy task, but mosquito transformation was recently accomplished (45, 109). This achievement now forms the foundation of efforts to make a parasite-resistant mosquito by introducing genes whose expression will prevent parasite development and/or transmission.
Initial efforts to transform mosquitoes focused on the use of the P-element, a mobile genetic element originally isolated from D. melanogaster (207, 229), to integrate foreign DNA into the mosquito genome (148, 158, 161). Although foreign DNA did integrate into the genome in these experiments, work with the P-element did not progress because the integrated DNA did not contain sequences specifically defined by the P-element termini and the frequency of integration was low. In fact, the P-element system has proved useful only for other drosophilid species (174, 176). Consequently, there was a need to find and assess other transposable elements that could mediate the integration of exogenous DNA in nondrosophilid species. Elements that were found to fulfill this requirement include Minos, Hermes, mariner, and piggyBac, which were isolated initially from Drosophila hydei (75), Musca domestica (177), Drosophila mauritiana (151), and Trichoplusia ni (76), respectively.
Initially, an in vivo plasmid-based excision assay was used to determine if the Hermes element was capable of cut-and-paste transposition in A. aegypti embryos. In this assay, the excision of a marked Hermes element from a donor plasmid to a target plasmid was measured following injection of embryos with both of these plasmids and a helper plasmid that supplied the required transposase (208). Hermes transposition was estimated at 0.286 transposition per 10,000 donor plasmids, suggesting that Hermes could be a suitable transformation vector in mosquitoes. A year later, the Hermes and mariner elements were independently used in successful mosquito transformation experiments (45, 109). In these studies, the transposable element was linked to a genomic clone of the Drosophila cinnabar (cn) gene, which is a morphological marker coding for kynurenine 3-hydroxylase, and then injected into embryos of A. aegypti deficient in this enzyme (khw) (52). The presence of colored eyes in the offspring of the injected embryos and the detection of an insertion into their genome by Southern blot analysis indicated a successful transformation event (Fig. 4).
FIG. 4.
Eye color phenotypes in Hermes-transformed adult A. aegypti. Transformation of the khw (white-eye) strain of Aedes aegypti with a Hermes transposon carrying a wild-type copy of the D. melanogaster cinnabar gene (encoding kynurenine hydroxylase) restores eye color. Counterclockwise from the top left: head of a wild-type mosquito showing deep purple eyes; head of a khw/khw mosquito showing white eyes; three heads of transformed mosquitoes from independent Hermes insertions showing different eye colors. The variability in the eye color among transformed lines presumably results from insertion site effects that modulate the expression of the transgene.
Investigations into additional markers and transposable elements are in progress in order to make the transformation process more efficient and streamlined. Although the mutant khw strain has been used successfully for transformation studies, it is not a particularly robust mosquito (Beerntsen and James, unpublished). Therefore, there is a need for visible markers, such as green fluorescent protein (GFP) (26) or yellow fluorescent protein (YFP) (156), that, because they do not need to be introduced into a particular recipient strain, can facilitate the assessment of a target gene in any strain of mosquito. Toward this end, Peter Atkinson and his research group at the University of California—Riverside have been investigating GFP as a visible marker for transformation. They have used the Hermes element to successfully transform A. aegypti with GFP and have observed expression in transformed offspring in all developmental stages from the embryo to the adult (188). YFP also has been used successfully as a marker in transformation experiments of A. aegypti, with expression patterns similar to those observed with GFP (D. A. O'Brochta, personal communication). Other transposable elements, including Minos and piggyBac, also are being tested as possible transformation vectors. Each is effective in mediating transposition in the Mediterranean fruit fly, Ceratitis capitata (90, 131), and they now need to be assessed for their ability to integrate into mosquito genomes.
Currently, transformation of the A. gambiae genome is an area of intense research due to its importance as a vector of human malaria. Although success in this arena has been slower, Zhao and Eggleston (268) recently demonstrated that the Hermes element mediated the stable transformation of an A. gambiae cell line. It is now probably only a matter of time before Anopheles spp. are successfully transformed.
With the success of transformation in A. aegypti, it becomes possible to genetically engineer a pathogen-resistant mosquito. Genes with the potential to inhibit parasite development can now be introduced into the A. aegypti genome, expressed in specific locations, and assayed for their ability to block pathogen transmission. The antipathogen gene would be a part of a chimeric gene that also would include the transposable element, a marker gene to identify transformants, and a promoter to direct expression of the antipathogen gene in specific tissues. Depending upon the developmental sites of a particular parasite or virus, the antipathogen gene would be expressed specifically in salivary glands, hemolymph, midgut, Malpighian tubules, or thoracic musculature. Recently, tissue-specific gene expression has been demonstrated in transformed mosquitoes using a promoter/reporter construct (46). In these studies, salivary gland promoters for the Maltase-like I (Mal1) (106) and Apyrase (Apy) (225) genes were able to direct the expression of recombinant firefly luciferase in Hermes-transformed A. aegypti in a developmentally specific and sex- and tissue-specific manner (i.e., in adult female salivary glands) that was identical to the expression of the endogenous genes.
There are a number of candidate molecules that could serve as antipathogen gene products. Each molecule has advantages and disadvantages that must be critically assessed to find an antipathogen molecule whose production creates the least possible physiological stress on the mosquito (105). Antipathogen molecules could be endogenous mosquito genes that would be expressed and then subsequently limit parasite transmission. Candidate molecules include mosquito defensins and cecropins that usually are not produced in response to infection with filarial worms or malaria parasites but are effective against eukaryotic parasites (28, 135, 136). Serine proteases and phenoloxidases that are critical enzymes in the melanotic encapsulation pathway may be other potential antipathogen molecules. Antipathogen molecules also might include a mosquito receptor that facilitates tissue invasion by binding to a particular pathogen ligand. In this scenario, an overexpressed soluble receptor would bind competitively to the pathogen ligand and, in turn, prevent it from recognizing and binding to tissue-bound receptors. Likewise, an overexpressed soluble form of the pathogen ligand could competitively bind to the mosquito receptor and prevent binding of the pathogen-bound ligand. In either situation, the required pathogen-tissue interaction does not occur and pathogen transmission is prevented. A resistant phenotype also can be created by expressing recombinant single-chain (ScFv) antibodies (260) designed from monoclonal antibodies that block pathogen invasion of mosquito tissues. For example, monoclonal antibodies to the P. gallinaceum circumsporozoite protein, which is the most abundant surface protein on sporozoites, block sporozoite invasion of salivary glands (256) and vertebrate host tissue (116, 192, 203). Studies now are in progress to express anti-circumsporozoite protein recombinant ScFvs in the hemolymph and salivary glands of mosquitoes in an effort to prevent malaria transmission (105).
Engineering a parasite-resistant mosquito population will no doubt depend upon not only selecting a suitable antipathogen effector molecule but also linking it to a suitable promoter such that the resulting chimeric resistance gene minimizes the fitness cost to the recipient mosquito. Promoters that are induced only following ingestion of a blood meal might lessen the fitness cost compared with a constitutive promoter. Examples of inducible promoters associated with blood feeding would be midgut genes like glutamine synthetase (224) or the late trypsin gene (3). However, the timing of gene expression is a critical feature. The antipathogen gene product must be present at the time the pathogen is present in the developmental site and in sufficient amounts to negatively affect parasite transmission. Therefore, a constitutive promoter, like those directing the expression of a number of salivary gland genes, might be ideal because of its continual expression in the salivary glands. Another option would be to use a promoter from a gene that is constitutively expressed but then is up-regulated following ingestion of a blood meal. Lipophorin is an example of a gene exhibiting this type of expression profile (251). Regardless of the promoter type, the selection of an appropriate promoter will require that its expression occur at the developmental site of the pathogen and with a minimal fitness cost to the mosquito.
Spreading Genes into Wild Populations
Laboratory strains resistant to parasites must be developed before the appropriate genes can be used in wild mosquitoes. A number of problems must be solved before such genes can be introduced into wild populations. The first obstacle is to determine the mechanism that will spread a gene through the populations. As discussed by Collins and James (49), and O'Brochta and Atkinson (175), Mendelian transmission of an antipathogen gene is expected to take too long to establish an introduced gene. In addition, such transmission always provides a chance that heterozygous animals would mate with each other and produce homozygous offspring that are competent to transmit pathogens. These individuals, even at a small percentage of the total population, may be sufficient to maintain the transmission cycle. Therefore, other mechanisms for moving genes into populations are required.
Two classes of active drive elements have been proposed for moving genes into wild populations, the first of which comprises the aforementioned transposable elements. These elements spread via mating, during which time they move from chromosomes that have elements to those that do not. Replicative transposition events increase the copy number of elements in a genome and result in circumstances where either all or most of the progeny arising from a mating of an animal carrying elements and an animal lacking elements have the potential to contain integrated elements in their genome. Replicative transposition circumvents the problem of heterozygosity and rapidly increases transposon frequencies in populations. In addition, this type of gene spreading does not require a fitness advantage of the transposing element to see an increase in frequency in the population. Mobility and a positive reproductive rate are the major factors required to see an increase in the frequency of the element (196). Experiments with P elements in caged D. melanogaster have shown that it is possible to increase the frequency of marker genes linked to the transposing element (25, 154). The worldwide dissemination of the P element through D. melanogaster populations represents the best contemporary example of the rapid spread of a transposable element occurring in insects in nature. The P element has spread throughout global populations of this fruit fly within the last 70 to 90 years (58). A similar spread of an engineered transposable element through a vector population could be enhanced by releases of mosquitoes carrying a linked antipathogen gene.
Alternative approaches to spread an antipathogen gene include the use of symbionts or other infectious agents. Antipathogen genes inserted into the genomes or plasmids of these microorganisms can be used to infect the arthropod host. Ben Beard and his colleagues have transformed the bacterium Rhodococcus rhodnii, which colonizes the midguts of vector bugs of the genus Triatoma, with a plasmid that expresses and secretes cecropins (62). Bugs infected with transformed endosymbionts are resistant to infection by Trypanosoma cruzi, the etiological agent of Chagas' disease. Similar approaches have been proposed to exploit Wolbachia intracellular symbionts, which are present in many dipteran species, including mosquitoes (186, 247). Movements of Wolbachia through natural populations of D. melanogaster have been documented (246), providing support for the hypothesis that these microorganisms can be exploited to move genes rapidly through populations.
Regardless of the specific element that forms the basis for genetic drive, a number of important factors must be taken into account in the overall design of the control program. For example, it is important that linkage be maintained between the antipathogen gene and the element or agent that is used to spread it through the target population. A loss of coupling of the antipathogen gene with the vector will reduce the effectiveness of the strategy. To circumvent this problem, elements or microorganisms containing antipathogen genes could be engineered so that they are disabled or self-destruct should they lose the antipathogen gene. It should be anticipated that elements or other drive agents will eventually lose the antipathogen gene; however, the key here is that the coupling of the two must remain in effect only long enough to have the desired effect on the target pathogen.
Major vector species may differ among geographical regions or may change seasonally, making it difficult to determine which population is actually transmitting the infective pathogen (55). This means that there may be multiple targets into which the element must be introduced. Additionally, it will be necessary to restrict specific genetic drive agents to a limited range of target species. Release programs with indiscriminate vector targets risk unanticipated consequences of introducing novel genes into untargeted species. Therefore, the host specificity of the drive mechanism must be thoroughly evaluated.
It is possible that pathogens will be selected for resistance to antipathogen genes. Effective measures to counteract resistance include incorporating more than one gene into each drive construct. This makes it less likely that resistant parasites will emerge and prolongs the efficacy of the construct.
Complete transformation of the wild population probably will not be necessary to interrupt the transmission of the pathogen (107). There are theoretical threshold levels of incidence and prevalence below which pathogen transmission fails. The frequency of an antipathogen gene must push incidence and prevalence below this threshold level before transmission will cease. This is an interesting area of research that requires additional work.
Research has to be done to define the populations in the wild that are the targets of genetic intervention. Transmission of a specific pathogen may occur with different vector species in different geographical regions, or the vector species may vary seasonally. The local distribution of vector animals within a transmission zone may affect gene dispersal (114). Therefore, it is important to know the number and distribution of genetically distinct vector populations in an area being considered for genetic intervention, because each target could require a specific intervention to affect control.
It is important to know the size of the target vector population. The rate at which an introduced gene moves through a population depends on the number of animals in the population, as well as the specific properties of the drive mechanism and the number of the initially released animals carrying antipathogen genes (196). Knowing the size of the population also is important in monitoring the frequency of the introduced gene in the population. The rate of change of the frequency and sampling sizes will be based on the estimated maximum size of the target population.
Because most of the anticipated drive mechanisms rely upon mating to spread the antipathogen genes, the breeding structure of the population must be determined (66). Species complexes have been defined for many of the vector species, and although there may be limited gene flow among the members of the complex, a certain level of inbreeding will allow an introduced element to move into another member of the complex.
Migration among vector populations also is important. Gene flow studies have shown that even the migration of a small number of individuals can have an effect on subsequent gene frequency in populations (236). Migration also can work in favor of a genetic control strategy by moving the antipathogen genes to new vector populations that are removed from the original release site.
Finally, we must have relevant measurements of the effectiveness of a genetic release program. Although it will be important to measure the change in gene frequency in target populations as the antipathogen gene is spread, the ultimate measure will be a reduction in disease and deaths in the human populations. This impact may be complicated by the concomitant use of vaccines, improved medical care, and a general improvement in the health and well-being of people living in disease-endemic areas. Careful analysis of each component may be difficult in this multicomponent strategy, but it will be essential for maintaining support and enthusiasm for these approaches.
In addition to the biological concerns associated with driving foreign genes into mosquito populations, other aspects of transmission dynamics need to be addressed (227). Although vector control strategies, e.g., DDT spraying, have had success in the past and novel vector control methods hold promise for the future, there is concern that reducing transmission without eradication may have little effect on mortality rates (226, 245) or be worse than no intervention at all (228). Snow and Marsh (226) have evaluated transmission and mortality data for P. falciparum and have suggested that even a 100-fold decrease in transmission may not significantly reduce mortality in African children younger than 5 years. Likewise, Trape and Rogier (245) suggest that reductions in the number of infective bites per person per year, which is also known as the entomological inoculation rate, will not decrease the long-term impact on mortality and morbidity from malaria unless this rate can be reduced to less than one infective bite per person every 10 years. Despite these predictions, studies investigating the impact of insecticide-treated bednet usage have demonstrated dramatic decreases in malaria-induced mortality (86), indicating that treated bednets are effective in decreasing transmission. It remains to be determined, however, if these reductions in mortality can be sustained in the long term.
Furthermore, transgenic mosquitoes created in the laboratory will have to be rigorously tested to ensure that they not only are unable to transmit a particular pathogen but also do not have the ability to transmit a different, undesired pathogen. Locations where releases of transgenic mosquitoes are to occur also need to be well studied so that the transgenic mosquito population can be introduced without undue disruption of the surrounding natural environment. Furthermore, educating the local residents as to what is planned for their community and enlisting their support for the release also are critical areas to be addressed, because community involvement and approval will facilitate the activities of the transgenic release program.
CONCLUDING REMARKS
Undoubtedly, the genetic makeup of the mosquito significantly contributes to vector competence. Although the specific nature of any one gene controlling susceptibility or refractoriness to pathogen infection has yet to be determined, a number of genes, like the NOS (61, 138), transferrin (9, 267), and Ag-STAT (4) genes, have been identified that seem to be involved in, or at least correlated with, mosquito infection status. Several QTL, containing genes influencing vector competence, have been identified in mosquitoes (12, 212, 213, 272), and although these QTLs represent many megabases of DNA containing many genes, fine-scale mapping should make it easier to isolate the specific genes responsible for susceptibility or refractoriness to parasite infection.
In recent years, the development of molecular tools has greatly enhanced the ability to investigate mosquito-pathogen interactions. The limited quantity of biological material generally available from parasite stages and mosquito tissues now can be subjected to molecular analysis that would have been extremely difficult 10 to 20 years ago. Research is no longer limited to descriptive reports detailing the vector competence of mosquitoes for particular pathogens but can now begin to address questions of vector competence at a mechanistic level (38). Mosquito and parasite genes are routinely isolated and characterized, and gene knockout and transformation systems for both parasites and mosquitoes are becoming available and can be used to evaluate the influence of particular gene products on vector competence. The advances in the development of molecular tools and protocols also have made it possible to use existing genes in novel ways or to develop novel gene products in an effort to prevent pathogen transmission by mosquitoes.
Furthermore, the advent of genome-sequencing projects for parasites (241; http://www.ncbi.nlm.nih.gov) and mosquitoes (http://www.niaid.nih.gov/facts/minutes/0599min1.htm; http://www.niaid.nih.gov/ncn/microbes.htm) has provided and will continue to provide a wealth of information. Computational biology and bioinformatics are becoming increasingly sophisticated in their capacity to mine the massive databases and to tie gene sequence to structure and function (33, 169, 240). However, an important caveat is that biological data must guide the interpretation of sequence data, because sequence data alone will not automatically lead to genes and function.
An important feature of these genome-sequencing projects is that they can interact powerfully with genetic mapping and linkage analysis by directly tying the statistically defined loci to underlying sequence and genes. A database of linear sequences will enable a “positional candidate” approach, in which sequence analysis will define possible candidate genes in a crossover-defined genetic interval that, in turn, can be evaluated for their specific contribution to variations in a particular phenotype.
Recent developments in the field of DNA microarray and oligonucleotide chip technologies also should make it possible to define the repertoire of gene transcription and expression in parasites and mosquitoes under different biological circumstances (101). With this technology, each gene in the genome can be represented as a “spot” on duplicate slides that are differentially probed with entire complements of mRNA to identify gene transcription that is specific to one or the other experimental state (e.g., pre- versus postinfection, different strains, developmental stage).
With the many revolutionary advances in molecular biology and their tremendous impact on vector biology, the future of research in mosquito vector competence is very bright. The field of mosquito vector biology research has attracted many enthusiastic and talented researchers, yet with so many questions still to be answered, there is a need for even more researchers. Many of the genes involved in pathogen recognition, signal transduction, and effector molecule production still need to be determined, and the influence of the parasites themselves on mosquito vector competence needs to be assessed. Furthermore, other vector-pathogen interactions, e.g., sand fly-Leishmania, tsetse fly-Trypansoma brucei, reduviid bug-Trypanosoma cruzi, and blackfly-Onchocerca, that are responsible for millions of clinical cases of disease are likely to be fruitful areas of research because studies of vector competence at a molecular level in these systems have been limited.
ACKNOWLEDGMENTS
We thank C. J. Coates, M. T. Ferdig, C. A. Lowenberger, and C. T. Smartt for their review of the manuscript. We also thank researchers who graciously allowed us to cite their work as a personal communication or unpublished data.
This work was supported in part by grants from the National Institutes of Health to B.M.C. (AI19769 and AI44461), to B.T.B. (F32AI09731), and to A.A.J. (AI29746 and AI44238); from the Burroughs-Wellcome Fund to B.M.C. and A.A.J.; and from the John D. and Catherine T. MacArthur Foundation to A.A.J.
REFERENCES
- 1.Antolin M F, Bosio C F, Cotton J, Sweeney W, Strand M R, Black W C., IV Intensive linkage mapping in a wasp (Bracon hebetor) and a mosquito (Aedes aegypti) with single-strand conformation polymorphism analysis of random amplified polymorphic DNA markers. Genetics. 1996;143:1727–1738. doi: 10.1093/genetics/143.4.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barillas-Mury C, Charlesworth A, Gross I, Richman A, Hoffman J A, Kafatos F C. Immune factor Gambif1, a new rel family member from the human malaria vector, Anopheles gambiae. EMBO J. 1996;15:4691–4701. [PMC free article] [PubMed] [Google Scholar]
- 3.Barillas-Mury C, Graf R, Hagedorn H H, Wells M A. cDNA and deduced amino acid sequence of a blood meal-induced trypsin from the mosquito, Aedes aegypti. Insect Biochem. 1991;21:825–831. [Google Scholar]
- 4.Barillas-Mury C, Han Y S, Seeley D, Kafatos F C. Anopheles gambiae Ag-STAT, a new insect member of the STAT family, is activated in response to bacterial infection. EMBO J. 1999;18:959–967. doi: 10.1093/emboj/18.4.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barreau C, Conrad J, Fischer E, Lujan H D, Vernick K D. Identification of surface molecules on salivary glands of the mosquito, Aedes aegypti, by a panel of monoclonal antibodies. Insect Biochem Mol Biol. 1999;29:515–526. doi: 10.1016/s0965-1748(99)00025-9. [DOI] [PubMed] [Google Scholar]
- 6.Barreau C, Touray M, Pimenta P F, Miller L H, Vernick K D. Plasmodium gallinaceum: sporozoite invasion of Aedes aegypti salivary glands is inhibited by anti-gland antibodies and by lectins. Exp Parasitol. 1995;81:332–343. doi: 10.1006/expr.1995.1124. [DOI] [PubMed] [Google Scholar]
- 7.Beerntsen B T, Champagne D E, Coleman J L, Campos Y A, James A A. Characterization of the Sialokinin I gene encoding the salivary vasodilator of the yellow fever mosquito, Aedes aegypti. Insect Mol Biol. 1999;8:459–468. doi: 10.1046/j.1365-2583.1999.00141.x. [DOI] [PubMed] [Google Scholar]
- 8.Beerntsen B T, Christensen B M. Dirofilaria immitis: effect on hemolymph protein synthesis in Aedes aegypti during melanotic encapsulation reactions against inoculated microfilariae. Exp Parasitol. 1990;71:406–414. doi: 10.1016/0014-4894(90)90066-l. [DOI] [PubMed] [Google Scholar]
- 9.Beerntsen B T, Christensen B M. Aedes aegypti: characterization of a hemolymph polypeptide expressed during melanotic encapsulation of filarial worms. Exp Parasitol. 1994;79:312–321. doi: 10.1006/expr.1994.1094. [DOI] [PubMed] [Google Scholar]
- 10.Reference deleted.
- 11.Beerntsen B T, Luckhart S, Christensen B M. Brugia malayi and Brugia pahangi: inherent difference in immune activation in the mosquitoes Armigeres subalbatus and Aedes aegypti. J Parasitol. 1989;75:76–81. [PubMed] [Google Scholar]
- 12.Beerntsen B T, Severson D W, Klinkhammer J A, Kassner V A, Christensen B M. Aedes aegypti: a QTL influencing filarial worm intensity is linked to QTL for susceptibility to other mosquito-borne pathogens. Exp Parasitol. 1995;81:355–362. doi: 10.1006/expr.1995.1126. [DOI] [PubMed] [Google Scholar]
- 13.Berner R, Ruden W, Hecker H. Peritrophic membranes and protease activity in the midgut of the malaria mosquito, Anopheles stephensi (Liston) (Insecta: Diptera) under normal and experimental conditions. J Ultrastruct Res. 1983;83:195–204. doi: 10.1016/s0022-5320(83)90077-1. [DOI] [PubMed] [Google Scholar]
- 14.Billingsley P F. The midgut ultrastructure of hematophagous insects. Annu Rev Entomol. 1990;35:219–248. [Google Scholar]
- 15.Billingsley P F, Sinden R E. Determinants of malaria-mosquito specificity. Parasitol Today. 1997;13:297–301. [Google Scholar]
- 16.Billker O, Lindo V, Panico M, Etienne A E, Paxton T, Dell A, Rogers M, Sinden R E, Morris H R. Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature. 1998;392:289–292. doi: 10.1038/32667. [DOI] [PubMed] [Google Scholar]
- 17.Black W C, IV, Moore C G. Population biology as a tool for studying vector-borne diseases. In: Beaty B J, Marquardt W C, editors. The biology of disease vectors. Niwot, Colo: University Press of Colorado; 1996. pp. 393–416. [Google Scholar]
- 18.Bockarie M J, Alexander N D, Hyun P, Dimber Z, Bockarie F, Ibam E, Alpers M P, Kazura J W. Randomised community-based trial of annual single-dose diethylcarbamzine with or without ivermectin against Wuchereria bancrofti infection in human beings and mosquitoes. Lancet. 1998;351:162–168. doi: 10.1016/S0140-6736(97)07081-5. [DOI] [PubMed] [Google Scholar]
- 19.Bosio C F, Beaty B J, Black W C., IV Quantitative genetics of vector competence for dengue-2 virus in Aedes aegypti. Am J Trop Med Hyg. 1998;59:965–970. doi: 10.4269/ajtmh.1998.59.965. [DOI] [PubMed] [Google Scholar]
- 20.Brown S E, Knudson D L. FISH landmarks for Aedes aegypti chromosomes. Insect Mol Biol. 1997;6:197–202. doi: 10.1111/j.1365-2583.1997.tb00088.x. [DOI] [PubMed] [Google Scholar]
- 21.Brown S E, Menninger J, Difillipantonio M, Beaty B J, Ward D C, Knudson D L. Toward a physical map of Aedes aegypti. Insect Mol Biol. 1995;4:161–167. doi: 10.1111/j.1365-2583.1995.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 22.Brunet P C J. The metabolism of the aromatic amino acids concerned in the cross-linking of insect cuticle. Insect Biochem. 1980;10:467–500. [Google Scholar]
- 23.Bulet P, Hetru C, Dimarcq J L, Hoffmann D. Antimicrobial peptides in insects: Structure and function. Dev Comp Immunol. 1999;23:329–344. doi: 10.1016/s0145-305x(99)00015-4. [DOI] [PubMed] [Google Scholar]
- 24.Reference deleted.
- 25.Carareto C M, Kim W, Wojciechowski M F, O'Grady P, Prokchorova A V, Silva J C, Kidwell M G. Testing transposable elements as genetic drive mechanisms using Drosophila P element constructs as a model system. Genetica. 1997;101:13–33. doi: 10.1023/a:1018339603370. [DOI] [PubMed] [Google Scholar]
- 26.Chalfie M, Tu Y, Euskirchin G, Ward W W, Prasher D C. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–806. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 27.Chalk R, Albuquerque C M, Ham P J, Townson H. Full sequence and characterization of two insect defensins: immune peptides from the mosquito Aedes aegypti. Proc R Soc London Ser B. 1995;261:217–221. doi: 10.1098/rspb.1995.0139. [DOI] [PubMed] [Google Scholar]
- 28.Chalk R, Townson H, Ham P J. Brugia pahangi: the effects of cecropins on microfilariae in vitro and in Aedes aegypti. Exp Parasitol. 1995;80:401–406. doi: 10.1006/expr.1995.1052. [DOI] [PubMed] [Google Scholar]
- 29.Champagne D E, Ribeiro J M. Sialokinin I and II: vasodilatory tachykinins from the yellow fever mosquito Aedes aegypti. Proc Natl Acad Sci USA. 1994;91:138–142. doi: 10.1073/pnas.91.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Champagne D E, Smartt C T, Ribeiro J M, James A A. The salivary gland-specific apyrase of the mosquito Aedes aegypti is a member of the 5′-nucleotidase family. Proc Natl Acad Sci USA. 1995;92:694–698. doi: 10.1073/pnas.92.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chege G M, Pumpuni C B, Beier J C. Proteolytic enzyme activity and Plasmodium falciparum sporogonic development in three species of Anopheles mosquitoes. J Parasitol. 1996;82:11–16. [PubMed] [Google Scholar]
- 32.Reference deleted.
- 33.Chervitz S A, Aravind L, Sherlock G, Ball C A, Koonin E V, Dwight S S, Harris M A, Dolinski K, Mohr S, Smith T, Weng S, Cherry J M, Botstein D. Comparison of the complete protein sets of worm and yeast: orthology and divergence. Science. 1998;282:2022–2028. doi: 10.1126/science.282.5396.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chikilian M L, Bradley T J, Nayar J K, Knight J W. Ultrastructural comparison of extracellular and intracellular encapsulation of Brugia malayi in Anopheles quadrimaculatus. J Parasitol. 1994;80:133–140. [PubMed] [Google Scholar]
- 35.Cho W L, Fu T F, Chiou J Y, Chen C C. Molecular characterization of a defensin gene from the mosquito, Aedes aegypti. Insect Biochem Mol Biol. 1997;27:351–358. doi: 10.1016/s0965-1748(97)00017-9. [DOI] [PubMed] [Google Scholar]
- 36.Cho W L, Fu Y C, Chen C C, Ho C M. Cloning and characterization of cDNAs encoding the antibacterial peptide, defensin A, from the mosquito, Aedes aegypti. Insect Biochem Mol Biol. 1996;26:395–402. doi: 10.1016/0965-1748(95)00108-5. [DOI] [PubMed] [Google Scholar]
- 37.Cho W L, Liu H S, Lee C H, Kuo C C, Chang T Y, Liu C T, Chen C C. Molecular cloning, characterization and tissue expression of prophenoloxidase cDNA from the mosquito Armigeres subalbatus inoculated with Dirofilaria immitis microfilariae. Insect Mol Biol. 1998;7:31–40. doi: 10.1046/j.1365-2583.1998.71049.x. [DOI] [PubMed] [Google Scholar]
- 38.Christensen B M, Ferdig M T. The field of vector biology: a perspective on the first 100 years. Parasitol Today. 1997;13:295–297. [Google Scholar]
- 39.Christensen B M, Forton K F. Hemocyte-mediated melanization of microfilariae in Aedes aegypti. J Parasitol. 1986;72:220–225. [PubMed] [Google Scholar]
- 40.Christensen B M, LaFond M M. Parasite-induced suppression of the immune response in Aedes aegypti by Brugia pahangi. J Parasitol. 1986;72:216–219. [PubMed] [Google Scholar]
- 41.Christensen B M, Severson D W. Biochemical and molecular basis of mosquito susceptibility to Plasmodium and filarioid nematodes. In: Beckage N E, Thompson S N, Federici B A, editors. Parasites and pathogens of insects. San Diego, Calif: Academic Press, Inc.; 1993. pp. 245–266. [Google Scholar]
- 42.Christensen B M, Sutherland D R, Gleason L N. Defense reactions of mosquitoes to filarial worms: comparative studies on the response of three different mosquitoes to inoculated Brugia pahangi and Dirofilaria immitis microfilariae. J Invertebr Pathol. 1984;44:267–274. doi: 10.1016/0022-2011(84)90024-7. [DOI] [PubMed] [Google Scholar]
- 43.Christensen B M, Tracy J W. Arthropod-transmitted parasites: mechanisms of immune interaction. Am Zool. 1989;29:387–389. [Google Scholar]
- 44.Clements A N. Development, nutrition, and reproduction. Vol. 1. London, United Kingdom: Chapman & Hall; 1992. Adult digestion. The biology of mosquitoes. [Google Scholar]
- 45.Coates C J, Jasinskiene N, Miyashiro L, James A A. Mariner transposition and transformation of the yellow fever mosquito, Aedes aegypti. Proc Natl Acad Sci USA. 1998;95:3748–3751. doi: 10.1073/pnas.95.7.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coates C J, Jasinskiene N, Pott G, James A A. Promoter-directed expression of recombinant fire-fly luciferase in the salivary glands of Hermes-transformed Aedes aegypti. Gene. 1999;226:317–325. doi: 10.1016/s0378-1119(98)00557-5. [DOI] [PubMed] [Google Scholar]
- 47.Cociancich S, Bulet P, Hetru C, Hoffmann J A. The inducible antibacterial peptides of insects. Parasitol Today. 1994;10:132–139. doi: 10.1016/0169-4758(94)90260-7. [DOI] [PubMed] [Google Scholar]
- 48.Cociancich S O, Park S S, Fidock D A, Shahabuddin M. Vesicular ATPase-overexpressing cells determine the distribution of malaria parasite oocysts on the midguts of mosquitoes. J Biol Chem. 1999;274:12650–12655. doi: 10.1074/jbc.274.18.12650. [DOI] [PubMed] [Google Scholar]
- 49.Collins F H, James A A. Genetic modification of mosquitoes. Sci Med. 1996;3:52–61. [Google Scholar]
- 50.Collins F H, Sakai R K, Vernick K D, Paskewitz S, Seeley D C, Miller L H, Collins W E, Campbell C G, Gwadz R W. Genetic selection of a Plasmodium-refractory strain of the malaria vector Anopheles gambiae. Science. 1986;234:607–610. doi: 10.1126/science.3532325. [DOI] [PubMed] [Google Scholar]
- 51.Collins F H, Zheng L, Paskewitz S M, Kafatos F C. Progress in the map-based cloning of the Anopheles gambiae genes responsible for the encapsulation of malarial parasites. Ann Trop Med Parasitol. 1997;91:517–521. doi: 10.1080/00034989760888. [DOI] [PubMed] [Google Scholar]
- 52.Cornel A J, Benedict M Q, Rafferty C S, Howells A J, Collins F H. Transient expression of the Drosophila melanogaster cinnabar gene rescues eye color in the white (WE) strain of Aedes aegypti. Insect Biochem Mol Biol. 1997;27:993–997. doi: 10.1016/s0965-1748(97)00084-2. [DOI] [PubMed] [Google Scholar]
- 53.Cornelissen C N, Sparling P F. Iron piracy: acquisition of transferrin-bound iron by bacterial pathogens. Mol Microbiol. 1994;14:843–850. doi: 10.1111/j.1365-2958.1994.tb01320.x. [DOI] [PubMed] [Google Scholar]
- 54.Curtis C F. Possible use of translocations to fix desirable genes in insect pest populations. Nature. 1968;218:368–369. doi: 10.1038/218368a0. [DOI] [PubMed] [Google Scholar]
- 55.Curtis C F, Pates H V, Takken W, Maxwell C A, Myamba J, Priestman A, Akinpel O, Yayo A M, Hu J T. Biological problems with the replacement of a vector population by Plasmodium-refractory mosquitoes. Parassitologia. 1999;41:479–481. [PubMed] [Google Scholar]
- 56.Czapla T H, Hopkins T L, Kramer K J. Catecholamines and related o-diphenols in the hemolymph and cuticle of the cockroach Leucophaea maderae (F.) during sclerotization and pigmentation. Insect Biochem. 1989;19:509–515. [Google Scholar]
- 57.Damian R T. Parasite immune evasion and exploitation: reflections and projections. Parasitology. 1997;115:S169–175. doi: 10.1017/s0031182097002357. [DOI] [PubMed] [Google Scholar]
- 58.Daniels S B, Peterson K R, Strausbaugh L D, Kidwell M G, Chovnick A. Evidence for horizontal transmission of the P transposable element between Drosophila species. Genetics. 1990;124:339–355. doi: 10.1093/genetics/124.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davie E W. Introduction to the blood coagulation cascade and the cloning of blood coagulation factors. J Protein Chem. 1986;5:247–253. [Google Scholar]
- 60.Dimopoulous G, Richman A, Müller H M, Kafatos F C. Molecular immune responses of the mosquito Anopheles gambiae to bacteria and malaria parasites. Proc Natl Acad Sci USA. 1997;94:11508–11513. doi: 10.1073/pnas.94.21.11508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dimopoulos G, Seeley D, Wolf A, Kafatos F C. Malaria infection of the mosquito Anopheles gambiae activates immune-responsive genes during critical transition stages of the parasite life cycle. EMBO J. 1998;17:6115–6123. doi: 10.1093/emboj/17.21.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Durvasula R V, Gumbs A, Panackal A, Kruglov O, Askoy S, Merrifield R B, Richards F F, Beard C B. Prevention of insect-borne disease: an approach using transgenic symbiotic bacteria. Proc Natl Acad Sci USA. 1997;94:3274–3278. doi: 10.1073/pnas.94.7.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Edwards J F, Higgs S, Beaty B J. Mosquito feeding-induced enhancement of Cache Valley virus (Bunyaviridae) infection in mice. J Med Entomol. 1998;35:261–265. doi: 10.1093/jmedent/35.3.261. [DOI] [PubMed] [Google Scholar]
- 64.Esslinger J H. Behavior of microfilariae of Brugia pahangi in Anopheles quadrimaculatus. Am J Trop Med Hyg. 1962;11:749–758. [Google Scholar]
- 65.Reference deleted.
- 66.Favia G, della Torre A, Bagayoko M, Lanfroncotti A, Sagnon N, Toure Y T, Coluzzi M. Molecular identification of sympatric chromosomal forms of Anopheles gambiae and further evidence of their reproductive isolation. Insect Mol Biol. 1997;6:377–383. doi: 10.1046/j.1365-2583.1997.00189.x. [DOI] [PubMed] [Google Scholar]
- 67.Feldmann A M, Ponnudurai T. Selection of Anopheles stephensi for refractoriness and susceptibility to Plasmodium falciparum. Med Vet Entomol. 1989;3:41–52. doi: 10.1111/j.1365-2915.1989.tb00473.x. [DOI] [PubMed] [Google Scholar]
- 68.Feldmann A M, van Germert G J, van de Vegte-Bolmer M, Jansen R. Genetics of refractoriness to Plasmodium falciparum in the mosquito Anopheles stephensi. Med Vet Entomol. 1998;12:302–312. doi: 10.1046/j.1365-2915.1998.00122.x. [DOI] [PubMed] [Google Scholar]
- 69.Ferdig M T, Beerntsen B T, Spray F J, Li J, Christensen B M. Reproductive cost associated with resistance in a mosquito-filarial worm system. Am J Trop Med Hyg. 1993;49:756–762. doi: 10.4269/ajtmh.1993.49.756. [DOI] [PubMed] [Google Scholar]
- 70.Ferdig M T, Li J, Severson D W, Christensen B M. Mosquito dopa decarboxylase cDNA characterization and blood-meal-induced ovarian expression. Insect Mol Biol. 1996;5:119–126. doi: 10.1111/j.1365-2583.1996.tb00046.x. [DOI] [PubMed] [Google Scholar]
- 71.Ferdig M T, Taft A S, Severson D W, Christensen B M. Development of a comparative genetic linkage map for Armigeres subalbatus using Aedes aegypti RFLP markers. Genome Res. 1998;8:41–47. doi: 10.1101/gr.8.1.41. [DOI] [PubMed] [Google Scholar]
- 72.Ferdig, M. T., A. S. Taft, C. T. Smartt, C. A. Lowneberger, J. Zhang, J. Li, and B. M. Christensen. Aedes aegypti dopa decarboxylase: gene structure and regulation. Insect Mol. Biol., in press. [DOI] [PubMed]
- 73.Ferrandon D, Jung A C, Criqui M, Lemaitre B, Uttenweiler-Joseph S, Michaut L, Reichhart J, Hoffman J A. A drosomycin-GFP reporter transgene reveals a local immune response in Drosophila that is not dependent on the Toll pathway. EMBO J. 1998;10:1217–1227. doi: 10.1093/emboj/17.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Forton K F, Christensen B M, Sutherland D R. Ultrastructure of the melanization response of Aedes trivittatus against inoculated Dirofilaria immitis microfilariae. J Parasitol. 1985;71:331–341. [PubMed] [Google Scholar]
- 75.Franz G, Savakis C. Minos, a new transposable element from Drosophila hydei, is a member of the Tc1-like family of transposons. Nucleic Acids Res. 1991;19:6646. doi: 10.1093/nar/19.23.6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fraser M J, Ciszczon T, Elick T, Bauser C. Precise excision of TTAA-specific lepidopteran transposons piggyBac (IFP2) and tagalong (TFPE) from the baculovirus genome in cell lines from two species of Lepidoptera. Insect Mol Biol. 1996;5:141–151. doi: 10.1111/j.1365-2583.1996.tb00048.x. [DOI] [PubMed] [Google Scholar]
- 77.Garcia G E, Wirtz R A, Barr J R, Woolfitt A, Rosenberg R. Xanthurenic acid induces gametogenesis in Plasmodium, the malaria parasite. J Biol Chem. 1998;273:12003–12005. doi: 10.1074/jbc.273.20.12003. [DOI] [PubMed] [Google Scholar]
- 78.Garcia G E, Wirtz R A, Rosenberg R. Isolation of a substance from the mosquito that activates Plasmodium fertilization. Mol Biochem Parasitol. 1997;88:127–135. doi: 10.1016/s0166-6851(97)00086-8. [DOI] [PubMed] [Google Scholar]
- 79.Gass R F. Influences of blood digestion on the development of Plasmodium gallinaceum (Brumpt) in the midgut of Aedes aegypti (L) Acta Trop. 1977;34:127–140. [PubMed] [Google Scholar]
- 80.Gass R F, Yeates R A. In vitro damage of cultured ookinetes of Plasmodium gallinaceum by digestive proteinases from susceptible Aedes aegypti. Acta Trop. 1979;36:243–252. [PubMed] [Google Scholar]
- 81.Gillespie J P, Kanost M R, Trenczek T. Biological mediators of insect immunity. Annu Rev Entomol. 1997;42:611–643. doi: 10.1146/annurev.ento.42.1.611. [DOI] [PubMed] [Google Scholar]
- 82.Golenda C F, Starkweather W H, Wirtz R A. The distribution of circumsporozoite protein (CS) in Anopheles stephensi mosquitoes infected with Plasmodium falciparum malaria. J Histochem Cytochem. 1990;38:475–481. doi: 10.1177/38.4.2181019. [DOI] [PubMed] [Google Scholar]
- 83.Gorman M J, Cornel A J, Collins F H, Paskewitz S M. A shared genetic mechanism for melanotic encapsulation of CM-Sephadex beads and a malaria parasite, Plasmodium cynomolgi B, in the mosquito, Anopheles gambiae. Exp Parasitol. 1996;84:380–386. doi: 10.1006/expr.1996.0126. [DOI] [PubMed] [Google Scholar]
- 84.Gorman M J, Paskewitz S M. A genetic study of a melanization response to Sephadex beads in Plasmodium-refractory and susceptible strains of Anopheles gambiae. Am J Trop Med Hyg. 1997;56:446–451. doi: 10.4269/ajtmh.1997.56.446. [DOI] [PubMed] [Google Scholar]
- 85.Gorman M J, Severson D W, Cornel A J, Collins F H, Paskewitz S M. Mapping a quantitative trait locus involved in melanotic encapsulation of foreign bodies in the malaria vector, Anopheles gambiae. Genetics. 1997;146:965–971. doi: 10.1093/genetics/146.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Greenwood B M. What's new in malaria control? Ann Trop Med Parasitol. 1997;91:523–531. doi: 10.1080/00034989760897. [DOI] [PubMed] [Google Scholar]
- 87.Gwadz R W, Kaslow D, Lee J Y, Maloy W L, Zasloff M, Miller L H. Effects of magainins and cecropins on the sporogonic development of malaria parasites in mosquitoes. Infect Immun. 1989;57:2628–2633. doi: 10.1128/iai.57.9.2628-2633.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hacker J K, Hardy J L. Absorptive endocytosis of California encephalitis virus into mosquito and mammalian cells: a role for G1. Virology. 1997;235:40–47. doi: 10.1006/viro.1997.8675. [DOI] [PubMed] [Google Scholar]
- 89.Han Y S, Salazar C E, Reese-Stardy S R, Cornel A, Gorman M J, Collins F H, Paskewitz S M. Cloning and characterization of a serine protease from the human malaria vector, Anopheles gambiae. Insect Mol Biol. 1997;6:385–395. doi: 10.1046/j.1365-2583.1997.00193.x. [DOI] [PubMed] [Google Scholar]
- 90.Handler A M, McCombs S D, Fraser M J, Saul S H. The lepidopteran transposon vector, piggyBac, mediates germ-line transformation in the Mediterranean fruit fly. Proc Natl Acad Sci USA. 1998;95:7520–7525. doi: 10.1073/pnas.95.13.7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hardy J L, Houk E J, Kramer L D, Reeves W C. Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Annu Rev Entomol. 1983;28:229–262. doi: 10.1146/annurev.en.28.010183.001305. [DOI] [PubMed] [Google Scholar]
- 92.Hernandez V P, Gerenday A, Fallon A M. Secretion of an inducible cecropin-like activity by cultured mosquito cells. Am J Trop Med Hyg. 1994;50:440–447. doi: 10.4269/ajtmh.1994.50.440. [DOI] [PubMed] [Google Scholar]
- 93.Hetru C, Hoffmann D, Bulet P. Antimicrobial peptides from insects. In: Brey P T, Hultmark D, editors. Molecular mechanisms of immune responses in insects. London, United Kingdom: Chapman & Hall; 1998. pp. 40–60. [Google Scholar]
- 94.Hoffmann J A, Kafatos F C, Janeway C A, Jr, Ezekowitz R A B. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 95.Hoffmann J A, Reichhart J M. Drosophila immunity. Trends Cell Biol. 1997;7:309–316. doi: 10.1016/S0962-8924(97)01087-8. [DOI] [PubMed] [Google Scholar]
- 96.Hogg J C, Hurd H. Malaria-induced reduction of fecundity during the first gonotrophic cycle of Anopheles stephensi mosquitoes. Med Vet Entomol. 1995;9:176–180. doi: 10.1111/j.1365-2915.1995.tb00175.x. [DOI] [PubMed] [Google Scholar]
- 97.Hook V Y H, Azaryan A V, Hwang S R, Tezapsidis N. Proteases and the emerging role of protease inhibitors in prohormone processing. FASEB J. 1994;8:1269–1278. doi: 10.1096/fasebj.8.15.8001739. [DOI] [PubMed] [Google Scholar]
- 98.Huber M, Cabib E, Miller L H. Malaria parasite chitinase and penetration of the mosquito peritrophic membrane. Proc Natl Acad Sci USA. 1991;88:2807–2810. doi: 10.1073/pnas.88.7.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huff C G. The effects of selection upon susceptibility to bird malaria in Culex pipiens Linn. Ann Trop Med Parasitol. 1929;23:427–442. [Google Scholar]
- 100.Huff C G. The inheritance of natural immunity to Plasmodium cathemerium in two species of Culex. J Prev Med. 1931;5:249–259. [Google Scholar]
- 101.Iyer V R, Eisen M B, Ross D T, Schuler G, Moore T, Lee J C F, Trent J M, Staudt L M, Hudson J, Jr, Boguski M S, Lashkari D, Shalon D, Botstein D, Brown P O. The transcriptional program in the response of human fibroblasts to serum. Science. 1999;283:83–87. doi: 10.1126/science.283.5398.83. [DOI] [PubMed] [Google Scholar]
- 102.Reference deleted.
- 103.Jahan N, Hurd H. The effects of infection with Plasmodium yoelii nigeriensis on the reproductive fitness of Anopheles stephensi. Ann Trop Med Parasitol. 1997;91:365–369. doi: 10.1080/00034989760987. [DOI] [PubMed] [Google Scholar]
- 104.James A A. Molecular and biochemical analyses of the salivary glands of vector mosquitoes. Bull Inst Pasteur. 1994;92:133–150. [Google Scholar]
- 105.James A A, Beerntsen B T, de L. Capurro M, Coates C J, Coleman J L, Jasinskiene N J, Krettli A U. Controlling malaria transmission with genetically-engineered, Plasmodium-resistant mosquitoes: milestones in a model system. Parassitologia. 1999;41:461–471. [PubMed] [Google Scholar]
- 106.James A A, Blackmer K, Racioppi J V. A salivary gland-specific, maltase-like gene of the vector mosquito, Aedes aegypti. Gene. 1989;75:73–83. doi: 10.1016/0378-1119(89)90384-3. [DOI] [PubMed] [Google Scholar]
- 107.James A A, Peloquin J J. Engineering resistance to malaria parasite development in mosquitoes. In: Sherman I W, editor. Malaria: parasite biology, pathogenesis, and protection. Washington, D.C.: American Society for Microbiology; 1998. pp. 63–69. [Google Scholar]
- 108.Janeway C A. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harbor Symp Quant Biol. 1989;54:1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 109.Jasinskiene N, Coates C J, Benedict M Q, Cornel A J, Salazar-Rafferty C, James A A, Collins F H. Stable, transposon-mediated transformation of the yellow fever mosquito, Aedes aegypti, using the Hermes element from the housefly. Proc Natl Acad Sci USA. 1998;95:3743–3747. doi: 10.1073/pnas.95.7.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jiang H, Wang Y, Korochkina S E, Benes H, Kanost M R. Molecular cloning of cDNAs for two pro-phenol oxidase subunits from the malaria vector, Anopheles gambiae. Insect Mol Biol. 1997;27:693–699. doi: 10.1016/s0965-1748(97)00045-3. [DOI] [PubMed] [Google Scholar]
- 111.Kang D, Liu G, Lundstrom A, Gelius E, Steiner H. A peptidoglycan recognition protein in innate immunity conserved from insects to humans. Proc Natl Acad Sci USA. 1998;95:10078–10082. doi: 10.1073/pnas.95.17.10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kartman L. Factors influencing infection of the mosquito with Dirofilaria immitis (Leidy, 1856) Exp Parasitol. 1953;2:27–78. [Google Scholar]
- 113.Kilama W L, Craig G B., Jr Monofactorial inheritance of susceptibility to Plasmodium gallinaceum in Aedes aegypti. Ann Trop Med Parasitol. 1969;63:419–432. doi: 10.1080/00034983.1969.11686645. [DOI] [PubMed] [Google Scholar]
- 114.Kiszewski A E, Spielman A. Spatially explicit model of transposon-based genetic drive mechanisms for displacing fluctuating populations of anopheline vector mosquitoes. J Med Entomol. 1998;35:584–590. doi: 10.1093/jmedent/35.4.584. [DOI] [PubMed] [Google Scholar]
- 115.Kobayashi M, Ogura N, Yamamoto H. Studies of filariasis X: a trial to analyse refractory mechanisms of the mosquito Aedes aegypti to the filarial larvae Brugia malayi by means of parabiotic twinning. Dokkyo J Med Sci. 1986;13:61–67. [Google Scholar]
- 116.Krettli A U, Rocha E M M, Lopes J D, Carneiro C R W, Kamboj K K, Cochrane A H, Nussenzweig R S. Circumsporozoite protein of Plasmodium gallinaceum characterized by monoclonal antibodies. Parasite Immunol. 1988;10:523–533. doi: 10.1111/j.1365-3024.1988.tb00240.x. [DOI] [PubMed] [Google Scholar]
- 117.Krogstad D J. Malaria as a reemerging disease. Epidemiol Rev. 1996;18:77–89. doi: 10.1093/oxfordjournals.epirev.a017918. [DOI] [PubMed] [Google Scholar]
- 118.Kumar V, Cornel A J, Mukabayire O. In situ hybridization to Anopheles polytene chromosomes. In: Crampton J M, Beard C B, Louis C, editors. The molecular biology of insect disease vectors: a methods manual. New York, N.Y: Chapman & Hall; 1997. pp. 337–345. [Google Scholar]
- 119.Lanzaro G C, Toure Y T, Carnahan J, Zheng L, Dolo G, Traore S, Petrarca V, Vernick K D, Taylor C E. Complexities in the genetic structure of Anopheles gambiae populations in West Africa as revealed by microsatellite DNA analysis. Proc Natl Acad Sci USA. 1998;95:14260–14265. doi: 10.1073/pnas.95.24.14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Laurence B R, Pester F R. Adaptation of a filarial worm, Brugia patei, to a new mosquito host, Aedes togoi. J Helminthol. 1967;41:365–392. doi: 10.1017/s0022149x00021908. [DOI] [PubMed] [Google Scholar]
- 121.Lee W J, della Torre A, Kobayashi A, Ashida M, Brey P T. Molecular cloning and chromosomal localization of a prophenoloxidase cDNA from the malaria vector Anopheles gambiae. Insect Mol Biol. 1998;7:41–50. doi: 10.1046/j.1365-2583.1998.71047.x. [DOI] [PubMed] [Google Scholar]
- 122.Lemaitre B, Kromer-Metzger E, Michaut L, Nicolas E, Meister M, Georgel P, Reichhart J M, Hoffmann J A. A recessive mutation, immune deficiency (imd), defines two distinct control pathways in the Drosophila host defense. Proc Natl Acad Sci USA. 1995;92:9465–9469. doi: 10.1073/pnas.92.21.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lemaitre B, Nicolas E, Michaut L, Reichhart J M, Hoffmann J A. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 124.Lemaitre B, Reichhart J M, Hoffmann J A. Drosophila host defense: differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc Natl Acad Sci USA. 1997;94:14614–14619. doi: 10.1073/pnas.94.26.14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Levashina E A, Ohresser S, Lemaitre B, Imler J L. Two distinct pathways can control expression of the gene encoding the Drosophila antimicrobial peptide Metchnikowin. J Mol Biol. 1998;278:515–527. doi: 10.1006/jmbi.1998.1705. [DOI] [PubMed] [Google Scholar]
- 126.Li J. Egg chorion tanning in Aedes aegypti mosquito. Comp Biochem Physiol Ser A. 1994;109:835–843. doi: 10.1016/0300-9629(94)90231-3. [DOI] [PubMed] [Google Scholar]
- 127.Reference deleted.
- 128.Li J, Christensen B M. Involvement of L-tyrosine and phenol oxidase in the tanning of Aedes aegypti eggs. Insect Biochem Mol Biol. 1993;23:739–748. [Google Scholar]
- 129.Li J, Tracy J W, Christensen B M. Phenol oxidase activity in hemolymph compartments of Aedes aegypti during melanotic encapsulation reactions against microfilariae. Dev Comp Immunol. 1992;16:41–48. doi: 10.1016/0145-305x(92)90050-m. [DOI] [PubMed] [Google Scholar]
- 130.Li J, Zhao X, Christensen B M. Dopachrome conversion activity in Aedes aegypti: significance during melanotic encapsulation of parasites and cuticular tanning. Insect Biochem Mol Biol. 1994;24:1043–1049. doi: 10.1016/0965-1748(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 131.Loukeris T G, Livadaras I, Arca B, Zabalou S, Savakis C. Gene transfer into the medfly, Ceratitis capitata, with a Drosophila hydei transposable element. Science. 1995;270:2002–2005. doi: 10.1126/science.270.5244.2002. [DOI] [PubMed] [Google Scholar]
- 132.Lowenberger C A, Bulet P, Charlet M, Hetru C, Hodgeman B, Christensen B M, Hoffmann J A. Insect immunity: isolation of three novel inducible antibacterial defensins from the vector mosquito, Aedes aegypti. Insect Biochem Mol Biol. 1995;25:867–873. doi: 10.1016/0965-1748(95)00043-u. [DOI] [PubMed] [Google Scholar]
- 133.Lowenberger C A, Charlet M, Vizioli M, Kamal S, Richman A, Christensen B M, Bulet P. Antimicrobial activity spectrum, cDNA cloning, and mRNA expression of a newly isolated member of the cecropin family from the mosquito vector Aedes aegypti. J Biol Chem. 1999;274:20092–20097. doi: 10.1074/jbc.274.29.20092. [DOI] [PubMed] [Google Scholar]
- 134.Unpublished data.
- 135.Lowenberger C A, Ferdig M T, Bulet P, Khalili S, Hoffman J A, Christensen B M. Aedes aegypti: induced antibacterial proteins reduce the establishment and development of Brugia malayi. Exp Parasitol. 1996;83:191–201. doi: 10.1006/expr.1996.0066. [DOI] [PubMed] [Google Scholar]
- 136.Lowenberger C A, Kamal S, Chiles J, Paskewitz S, Bulet P, Hoffman J A, Christensen B M. Mosquito-Plasmodium interactions in response to immune activation of the vector. Exp Parasitol. 1999;91:59–69. doi: 10.1006/expr.1999.4350. [DOI] [PubMed] [Google Scholar]
- 137.Lowenberger C A, Smartt C T, Bulet P, Ferdig M T, Severson D W, Hoffman J A, Christensen B M. Insect immunity: molecular cloning, expression, and characterization of cDNAs and genomic DNA encoding three isoforms of insect defensin in Aedes aegypti. Insect Mol Biol. 1999;8:107–118. doi: 10.1046/j.1365-2583.1999.810107.x. [DOI] [PubMed] [Google Scholar]
- 138.Luckhart S, Vodovotz Y, Cui L, Rosenberg R. The mosquito Anopheles stephensi limits malaria parasite development with inducible synthesis of nitric oxide. Proc Natl Acad Sci USA. 1998;95:5700–5705. doi: 10.1073/pnas.95.10.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ludwig G V, Israel B A, Christensen B M, Yuill T M, Schultz K T. Monoclonal antibodies directed against the envelope glycoproteins of La Crosse virus. Microb Pathog. 1991;11:411–421. doi: 10.1016/0882-4010(91)90037-b. [DOI] [PubMed] [Google Scholar]
- 140.Ludwig G V, Israel B A, Christensen B M, Yuill T M, Schultz K T. Role of La Crosse virus glycoproteins in attachment of virus to host cells. Virology. 1991;181:564–571. doi: 10.1016/0042-6822(91)90889-j. [DOI] [PubMed] [Google Scholar]
- 141.Ludwig G V, Kondig J P, Smith J F. A putative receptor for Venezuelan equine encephalitis virus from mosquito cells. J Virol. 1996;70:5592–5599. doi: 10.1128/jvi.70.8.5592-5599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Macdonald G. The epidemiology and control of malaria. London, United Kingdom: Oxford University Press; 1957. [Google Scholar]
- 143.Macdonald W W. The selection of a strain of Aedes aegypti susceptible to infection with semi-periodic Brugia malayi. Ann Trop Med Parasitol. 1962;56:368–372. doi: 10.1080/00034983.1963.11686200. [DOI] [PubMed] [Google Scholar]
- 144.Macdonald W W. The genetic basis of susceptibility of infection with semi-periodic Brugia malayi in Aedes aegypti. Ann Trop Med Parasitol. 1962;56:373–382. [Google Scholar]
- 145.Macdonald W W, Ramachandran C P. The influence of the gene fm (filarial susceptibility, Brugia malayi) on the susceptibility of Aedes aegypti to seven strains of Brugia, Wuchereria and Dirofilaria. Ann Trop Med Parasitol. 1965;59:64–73. doi: 10.1080/00034983.1965.11686284. [DOI] [PubMed] [Google Scholar]
- 146.Marinotti O, deBrito M, Moreira C K. Apyrase and alpha-glucosidase in the salivary glands of Aedes albopictus. Comp Biochem Physiol Ser B. 1996;113:675–679. doi: 10.1016/0305-0491(95)02035-7. [DOI] [PubMed] [Google Scholar]
- 147.Matthews T C, Munstermann L E. Chromosomal repatterning and linkage group conservation in mosquito karyotypic evolution. Evolution. 1994;48:146–154. doi: 10.1111/j.1558-5646.1994.tb01301.x. [DOI] [PubMed] [Google Scholar]
- 148.McGrane V, Carlson J O, Miller B R, Beaty B J. Microinjection of DNA in Aedes triseriatus ova and detection of integration. Am J Trop Med Hyg. 1988;39:502–510. doi: 10.4269/ajtmh.1988.39.502. [DOI] [PubMed] [Google Scholar]
- 149.McGreevy P B, Bryan J H, Oothuman P, Kolstrup N. The lethal effects of the cibarial and pharyngeal armatures of mosquitoes on microfilariae. Trans R Soc Trop Med Hyg. 1978;72:361–368. doi: 10.1016/0035-9203(78)90128-1. [DOI] [PubMed] [Google Scholar]
- 150.McGreevy P B, McClelland G A H, Lavoipierre M M J. Inheritance of susceptibility to Dirofilaria immitus infection in Aedes aegypti. Ann Trop Med Parasitol. 1974;68:97–109. doi: 10.1080/00034983.1974.11686929. [DOI] [PubMed] [Google Scholar]
- 151.Medhora M, Maruyama K, Hartl D L. Molecular and functional analysis of the mariner mutator element Mos1 in Drosophila. Genetics. 1991;128:311–318. doi: 10.1093/genetics/128.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Medzhitov R, Janeway C A., Jr An ancient system of host defense. Curr Opin Immunol. 1998;10:12–15. doi: 10.1016/s0952-7915(98)80024-1. [DOI] [PubMed] [Google Scholar]
- 153.Meister M, Braun A, Kappler C, Reichhart J M, Hoffmann J A. Insect immunity. A transgenic analysis in Drosophila defines several functional domains in the diptericin promoter. EMBO J. 1994;13:5958–5966. doi: 10.1002/j.1460-2075.1994.tb06941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Meister G A, Grigliatti T A. Rapid spread of a P element/Adh gene construct through experimental populations of Drosophila melanogaster. Genome. 1993;36:1169–1175. doi: 10.1139/g93-155. [DOI] [PubMed] [Google Scholar]
- 155.Meraz M A, White J M, Sheehan K C, Bach E A, Rodig S J, Dighe A S, Kaplan D H, Riley J K, Greenlund A S, Campbell D, Carver-Moore K, DuBois R N, Clark R, Aguet M, Schreiber R D. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- 156.Miller D M, III, Desai N S, Hardin D C, Piston D W, Patterson G H, Fleenor J, Xu S, Fire A. Two-color GFP expression system for C. elegans. Bio/Techniques. 1999;26:914–918. doi: 10.2144/99265rr01. , 920–921. [DOI] [PubMed] [Google Scholar]
- 157.Miller L H, Hoffman S L. Research toward vaccines against malaria. Nat Med. 1998;4(Suppl. 5):520–524. doi: 10.1038/nm0598supp-520. [DOI] [PubMed] [Google Scholar]
- 158.Miller L H, Sakai R K, Romans P, Gwadz R W, Kantoff P, Coon H G. Stable integration and expression of a bacterial gene in the mosquito Anopheles gambiae. Science. 1987;237:779–781. doi: 10.1126/science.3039658. [DOI] [PubMed] [Google Scholar]
- 159.Mori A, Severson D W, Christensen B M. Comparative linkage maps for the mosquitoes (Culex pipens and Aedes aegypti) based on common RFLP loci. J Hered. 1999;90:160–164. doi: 10.1093/jhered/90.1.160. [DOI] [PubMed] [Google Scholar]
- 160.Morisato D, Anderson K V. Signaling pathways that establish the dorsal-ventral pattern of the Drosophila embryo. Annu Rev Genet. 1995;29:371–399. doi: 10.1146/annurev.ge.29.120195.002103. [DOI] [PubMed] [Google Scholar]
- 161.Morris A C, Eggleston P, Crampton J M. Genetic transformation of the mosquito Aedes aegypti by microinjection of DNA. Med Vet Entomol. 1989;3:1–7. doi: 10.1111/j.1365-2915.1989.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 162.Moskalyk L A, Oo M M, Jacobs-Lorena M. Peritrophic matrix proteins of Anopheles gambiae and Aedes aegypti. Insect Mol Biol. 1996;5:261–268. doi: 10.1111/j.1365-2583.1996.tb00100.x. [DOI] [PubMed] [Google Scholar]
- 163.Reference deleted.
- 164.Müller H M, Crampton J M, Torre A D, Sinden R, Crisanti A. Members of a trypsin gene family in Anopheles gambiae are induced in the gut by blood meal. EMBO J. 1993;12:2891–2900. doi: 10.1002/j.1460-2075.1993.tb05951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Müller H M, Dimopoulos G, Blass C, Kafatos F C. A hemocyte-like cell line established from the malaria vector Anopheles gambiae expresses six prophenoloxidase genes. J Biol Chem. 1999;274:11727–11735. doi: 10.1074/jbc.274.17.11727. [DOI] [PubMed] [Google Scholar]
- 166.Munkirs D D, Christensen B M, Tracy J W. High-pressure liquid chromatographic analysis of hemolymph plasma catecholamines in immune-reactive Aedes aegypti. J Invertebr Pathol. 1990;56:267–279. doi: 10.1016/0022-2011(90)90110-r. [DOI] [PubMed] [Google Scholar]
- 167.Munstermann L E. Linkage map of the yellow fever mosquito, Aedes aegypti. In: O'Brian S J, editor. Genetic maps: locus maps of complex genomes. Vol. 5. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1990. pp. 3.179–3.183. [Google Scholar]
- 168.Munstermann L E, Craig G B., Jr Genetics of Aedes aegypti: updating the linkage map. J Hered. 1979;70:291–296. [Google Scholar]
- 169.Reference deleted.
- 170.Reference deleted.
- 171.Reference deleted.
- 172.Nayar J, Knight J W, Bradley T J. Further characterization of refractoriness in Aedes aegypti (L.) to infection by Dirofilaria immitis (Leidy) Exp Parasitol. 1988;66:124–131. doi: 10.1016/0014-4894(88)90057-4. [DOI] [PubMed] [Google Scholar]
- 173.Reference deleted.
- 174.O'Brochta D A, Atkinson P W. Transposable elements and gene transformation in non-drosophilid insects. Insect Biochem Mol Biol. 1996;26:739–753. doi: 10.1016/s0965-1748(96)00022-7. [DOI] [PubMed] [Google Scholar]
- 175.O'Brochta D A, Atkinson P W. Building the better bug. Sci Am. 1998;279:20–95. doi: 10.1038/scientificamerican1298-90. [DOI] [PubMed] [Google Scholar]
- 176.O'Brochta D A, Gomez S P, Handler A M. P element excision in Drosophila melanogaster and related drosophilids. Mol Gen Genet. 1991;225:387–394. doi: 10.1007/BF00261678. [DOI] [PubMed] [Google Scholar]
- 177.O'Brochta D A, Warren W D, Saville K J, Atkinson P W. Hermes, a functional non-drosophilid insect gene vector from Musca domestica. Genetics. 1996;142:907–914. doi: 10.1093/genetics/142.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Olson K E, Higgs S, Gaines P J, Powers A M, Davis B S, Kamrud K I, Carlson J O, Blair C D, Beaty B J. Genetically engineered resistance to dengue-2 virus transmission in mosquitoes. Science. 1996;272:884–886. doi: 10.1126/science.272.5263.884. [DOI] [PubMed] [Google Scholar]
- 179.Osorio J E, Godsey M S, Defoliart G R, Yuill T M. La Crosse viremias in white-tailed deer and chipmunks exposed by injection or mosquito bite. Am J Trop Med Hyg. 1996;54:338–342. doi: 10.4269/ajtmh.1996.54.338. [DOI] [PubMed] [Google Scholar]
- 180.Oswald I P, Wynn T A, Sher A, James S L. NO as an effector molecule of parasite killing: modulation of its synthesis by cytokines. Comp Biochem Physiol Ser C. 1994;108:11–18. doi: 10.1016/1367-8280(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 181.Reference deleted.
- 182.Paskewitz S M, Brown M R, Lea A O, Collins F H. Ultrastructure of the encapsulation of Plasmodium cynomolgi (B strain) on the midgut of a refractory strain of Anopheles gambiae. J Parasitol. 1988;74:432–439. [PubMed] [Google Scholar]
- 183.Paskewitz S M, Christensen B M. Immune responses of vectors. In: Beaty B J, Marquardt W C, editors. The biology of disease vectors. Niwot, Colo: University Press of Colorado; 1996. pp. 371–392. [Google Scholar]
- 184.Paskewitz S M, Reese-Stardy S, Gorman M J. An easter-like serine protease from Anopheles gambiae exhibits changes in transcript abundance following immune challenge. Insect Mol Biol. 1999;8:329–337. doi: 10.1046/j.1365-2583.1999.83124.x. [DOI] [PubMed] [Google Scholar]
- 185.Paskewitz S M, Riehle M A. Response of Plasmodium refractory and susceptible strains of Anopheles gambiae to inoculated Sephadex beads. Dev Comp Immunol. 1994;18:369–375. doi: 10.1016/0145-305x(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 186.Pettigrew M M, O'Neill S L. Control of vector-borne disease by genetic manipulation of insect populations: Technological requirements and research priorities. Aust J Entomol. 1997;36:309–317. [Google Scholar]
- 187.Pimenta P F, Touray M, Miller L. The journey of malaria sporozoites in the mosquito salivary gland. J Eukaryot Microbiol. 1994;41:608–624. doi: 10.1111/j.1550-7408.1994.tb01523.x. [DOI] [PubMed] [Google Scholar]
- 188.Pinkerton, A. C., K. Michel, D. A. O'Brochta, and P. W. Atkinson. Green fluorescent protein as a genetic marker in transgenic Aedes aegypti. Insect Mol. Biol., in press. [DOI] [PubMed]
- 189.Ponnudurai T, Billingsley P F, Rudin W. Differential infectivity of Plasmodium for mosquitoes. Parasitol Today. 1988;4:319–321. doi: 10.1016/0169-4758(88)90114-7. [DOI] [PubMed] [Google Scholar]
- 190.Prevot G I, Laurent-Winter C, Feldmann A M, Rodhain F, Bourgouin C. Two-dimensional gel analysis of midgut proteins of Anopheles stephensi lines with different susceptibility to Plasmodium falciparum infection. Insect Mol Biol. 1998;7:375–383. doi: 10.1046/j.1365-2583.1998.740375.x. [DOI] [PubMed] [Google Scholar]
- 191.Ramasamy M S, Kulasekera R, Wanniarachchi I C, Srikrishnaraj A A M, Ramasamy R. Interactions of human malaria parasites, Plasmodium vivax and P. falciparum with the midgut of Anopheles mosquitoes. Med Vet Entomol. 1997;11:290–296. doi: 10.1111/j.1365-2915.1997.tb00409.x. [DOI] [PubMed] [Google Scholar]
- 192.Ramirez A D, Rocha E M, Krettli A U. Antisporozoite antibodies with protective and nonprotective activities: in vitro and in vivo correlations using Plasmodium gallinaceum, an avian model. J Eukaryot Microbiol. 1995;42:705–708. doi: 10.1111/j.1550-7408.1995.tb01620.x. [DOI] [PubMed] [Google Scholar]
- 193.Reference deleted.
- 194.Ribeiro J M. Characterization of a vasodilator from the salivary glands of the yellow fever mosquito Aedes aegypti. J Exp Biol. 1992;165:61–71. doi: 10.1242/jeb.165.1.61. [DOI] [PubMed] [Google Scholar]
- 195.Ribeiro J M C. Blood-feeding arthropods: live syringes or invertebrate pharmacologists? Infect Agents Dis. 1995;4:143–152. [PubMed] [Google Scholar]
- 196.Ribeiro J M, Kidwell M G. Transposable elements as population drive mechanisms: specification of critical parameter values. J Med Entomol. 1994;31:10–16. doi: 10.1093/jmedent/31.1.10. [DOI] [PubMed] [Google Scholar]
- 197.Ribeiro J M, Nussenzvieg R H. The salivary catechol oxidase/peroxidase activities of the mosquito Anopheles albimanus. J Exp Biol. 1993;179:273–287. doi: 10.1242/jeb.179.1.273. [DOI] [PubMed] [Google Scholar]
- 198.Ribeiro J M, Nussenzvieg R H, Tortorella G. Salivary vasodilators of Aedes triseriatus and Anopheles gambiae (Diptera: Culicidae) J Med Entomol. 1994;31:747–753. doi: 10.1093/jmedent/31.5.747. [DOI] [PubMed] [Google Scholar]
- 199.Ribeiro J M, Sarkis J J, Rossignol P A, Spielman A. Salivary apyrase of Aedes aegypti: characterization and secretory fate. Comp Biochem Physiol Ser B. 1984;79:81–86. doi: 10.1016/0305-0491(84)90081-6. [DOI] [PubMed] [Google Scholar]
- 200.Ribeiro J M, Valenzuela J G. Purification and cloning of the salivary peroxidase/catechol oxidase of the mosquito Anopheles albimanus. J Exp Biol. 1999;202:809–816. doi: 10.1242/jeb.202.7.809. [DOI] [PubMed] [Google Scholar]
- 201.Richman A M, Bulet P, Hetru C, Barillas-Mury C, Hoffmann J A, Kafatos F C. Inducible immune factors of the vector mosquito Anopheles gambiae: biochemical purification of a defensin antibacterial peptide and molecular cloning of preprodefensin cDNA. Insect Mol Biol. 1996;5:203–210. doi: 10.1111/j.1365-2583.1996.tb00055.x. [DOI] [PubMed] [Google Scholar]
- 202.Richman A M, Dimopoulos G, Seeley D, Kafatos F C. Plasmodium activates the innate immune response of Anopheles gambiae mosquitoes. EMBO J. 1997;16:6114–6119. doi: 10.1093/emboj/16.20.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Rocha E M, Hollingdale M R, Gwadz R, Krettli A U. Exoerythrocytic development of Plasmodium gallinaceum sporozoites in a chicken fibroblast cell line and inhibition of the cell invasion by specific anti-sporozoite monoclonal antibodies. J Eukaryot Microbiol. 1993;40:64–66. doi: 10.1111/j.1550-7408.1993.tb04883.x. [DOI] [PubMed] [Google Scholar]
- 204.Rosenberg R. Inability of Plasmodium knowlesi sporozoites to invade Anopheles freeborni salivary glands. Am J Trop Med Hyg. 1985;34:687–691. doi: 10.4269/ajtmh.1985.34.687. [DOI] [PubMed] [Google Scholar]
- 205.Ross R. Report on the cultivation of Proteosoma labbe in grey mosquitoes. Calcutta, India: Government Press; 1898. pp. 401–451. [PMC free article] [PubMed] [Google Scholar]
- 206.Rossignol P A, Ribeiro J M, Spielman A. Increased intradermal probing time in sporozoite-infected mosquitoes. Am J Trop Med Hyg. 1984;33:17–20. doi: 10.4269/ajtmh.1984.33.17. [DOI] [PubMed] [Google Scholar]
- 207.Rubin G M, Spradling A C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- 208.Sarkar A, Yardley K, Atkinson P W, James A A, O'Brochta D A. Transposition of the Hermes element in embryos of the vector mosquito, Aedes aegypti. Insect Biochem Mol Biol. 1997;27:359–363. doi: 10.1016/s0965-1748(97)00018-0. [DOI] [PubMed] [Google Scholar]
- 208a.Seabaugh R C, Olson K E, Higgs S, Carlson J O, Beaty B J. Development of a chimeric Sindbis virus with enhanced per os infection of Aedes aegypti. Virology. 1998;243:99–112. doi: 10.1006/viro.1998.9034. [DOI] [PubMed] [Google Scholar]
- 209.Severson D W. Applications of molecular marker analysis to mosquito vector competence. Parasitol Today. 1994;10:336–340. doi: 10.1016/0169-4758(94)90243-7. [DOI] [PubMed] [Google Scholar]
- 210.Severson D W, Mori A, Kassner V A, Christensen B M. Comparative linkage maps for the mosquitoes, Aedes albopictus and Ae. aegypti, based on common RFLP loci. Insect Mol Biol. 1995;4:41–45. doi: 10.1111/j.1365-2583.1995.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 211.Severson D W, Mori A, Zhang Y, Christensen B M. Linkage map for Aedes aegypti using restriction fragment length polymorphisms. J Hered. 1993;84:241–247. doi: 10.1093/oxfordjournals.jhered.a111333. [DOI] [PubMed] [Google Scholar]
- 212.Severson D W, Mori A, Zhang Y, Christensen B M. Chromosomal mapping of two loci affecting filarial worm susceptibility in Aedes aegypti. Insect Mol Biol. 1994;3:67–72. doi: 10.1111/j.1365-2583.1994.tb00153.x. [DOI] [PubMed] [Google Scholar]
- 213.Severson D W, Thathy V, Mori A, Zhang Y, Christensen B M. Restriction fragment length polymorphism mapping of quantitative trait loci for malaria parasite susceptibility in the mosquito Aedes aegypti. Genetics. 1995;139:1711–1717. doi: 10.1093/genetics/139.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Severson D W, Zhang Y. Generation of expressed sequence tags and sequence-tagged sites as physical landmarks in the mosquito, Aedes aegypti, genome. Genome. 1996;39:224–229. doi: 10.1139/g96-030. [DOI] [PubMed] [Google Scholar]
- 215.Shahabuddin M. Plasmodium ookinete development in the mosquito midgut: a case of reciprocal manipulation. Parasitology. 1998;116:S83–S93. doi: 10.1017/s0031182000084973. [DOI] [PubMed] [Google Scholar]
- 216.Shahabuddin M, Cociancich S, Zieler H. The search for novel malaria transmission-blocking targets in the mosquito midgut. Parasitol Today. 1998;14:493–497. doi: 10.1016/s0169-4758(98)01348-9. [DOI] [PubMed] [Google Scholar]
- 217.Shahabuddin M, Fields I, Bulet P, Hoffman J A, Miller L. Plasmodium gallinaceum: differential killing of some mosquito stages of the parasite by insect defensin. Exp Parasitol. 1998;89:103–112. doi: 10.1006/expr.1998.4212. [DOI] [PubMed] [Google Scholar]
- 218.Shahabuddin M, Lemos F J A, Kaslow D C, Jacobs-Lorena M. Antibody-mediated inhibition of Aedes aegypti midgut trypsins blocks sporogonic development of Plasmodium gallinaceum. Infect Immun. 1996;64:739–743. doi: 10.1128/iai.64.3.739-743.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Shahabuddin M, Pimenta P F P. Plasmodium gallinaceum preferentially invades vesicular ATPase-expressing cells in Aedes aegypti midgut. Proc Natl Acad Sci USA. 1998;95:3385–3389. doi: 10.1073/pnas.95.7.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Shahabuddin M, Toyoshima T, Aikawa M, Kaslow D C. Transmission-blocking activity of a chitinase inhibitor and activation of malarial parasite chitinase by mosquito protease. Proc Natl Acad Sci USA. 1993;90:4266–4270. doi: 10.1073/pnas.90.9.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Shen Z, Jacobs-Lorena M. A type-I peritrophic matrix protein from the malaria vector Anopheles gambiae binds to chitin. J Biol Chem. 1998;273:17665–17670. doi: 10.1074/jbc.273.28.17665. [DOI] [PubMed] [Google Scholar]
- 222.Siden-Kiamos I, Skavdis G, Rubio J, Papagiannakis G, Louis C. Isolation and characterization of three serine protease genes in the mosquito Anopheles gambiae. Insect Mol Biol. 1996;5:61–71. doi: 10.1111/j.1365-2583.1996.tb00041.x. [DOI] [PubMed] [Google Scholar]
- 223.Sidjanski S P, Vanderberg J P, Sinnis P. Anopheles stephensi salivary glands bear receptors for region I of the circumsporozoite protein of Plasmodium falciparum. Mol Biochem Parasitol. 1997;90:33–41. doi: 10.1016/s0166-6851(97)00124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Smartt C T, Chiles J, Lowenberger C, Christensen B M. Biochemical analysis of a blood meal-induced Aedes aegypti glutamine synthetase gene. Insect Biochem Mol Biol. 1998;28:935–945. doi: 10.1016/s0965-1748(98)00073-3. [DOI] [PubMed] [Google Scholar]
- 225.Smartt C T, Kim A P, Grossman G L, James A A. The apyrase gene of the vector mosquito, Aedes aegypti, is expressed specifically in the adult female salivary glands. Exp Parasitol. 1995;81:239–248. doi: 10.1006/expr.1995.1114. [DOI] [PubMed] [Google Scholar]
- 226.Snow R W, Marsh K. Will reducing Plasmodium falciparum alter malaria mortality among African children? Parasitol Today. 1995;11:188–190. doi: 10.1016/0169-4758(95)80025-5. [DOI] [PubMed] [Google Scholar]
- 227.Spielman A. Why entomological antimalaria research should not focus on transgenic mosquitoes. Parasitol Today. 1994;10:374–376. doi: 10.1016/0169-4758(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 228.Spielman A, Kitron U, Pollack R J. Time limitation and the role of research in the worldwide attempt to eradicate malaria. J Med Entomol. 1993;30:6–19. doi: 10.1093/jmedent/30.1.6. [DOI] [PubMed] [Google Scholar]
- 229.Spradling A C, Rubin G M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982;218:341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- 230.Stark K R, James A A. A factor Xa-directed anticoagulant from the salivary glands of the yellow fever mosquito Aedes aegypti. Exp Parasitol. 1995;81:321–331. doi: 10.1006/expr.1995.1123. [DOI] [PubMed] [Google Scholar]
- 231.Stark K R, James A A. Anticoagulants in vector arthropods. Parasitol Today. 1996;12:430–437. doi: 10.1016/0169-4758(96)10064-8. [DOI] [PubMed] [Google Scholar]
- 232.Stark K R, James A A. Salivary gland anticoagulants in Culicine and Anopheline mosquitoes (Diptera: Culicidae) J Med Entomol. 1996;33:645–650. doi: 10.1093/jmedent/33.4.645. [DOI] [PubMed] [Google Scholar]
- 233.Stark K R, James A A. Isolation and characterization of the gene encoding a novel factor Xa-directed anticoagulant from the yellow fever mosquito, Aedes aegypti. J Biol Chem. 1998;273:20802–20809. doi: 10.1074/jbc.273.33.20802. [DOI] [PubMed] [Google Scholar]
- 234.Sterling C R, Aikawa M, Vanderberg J P. The passage of Plasmodium berghei sporozoites through the salivary glands of Anopheles stephensi: an electron microscope study. J Parasitol. 1973;59:593–605. [PubMed] [Google Scholar]
- 235.Sun D, Eccleston E D, Fallon A M. Peptide sequence of an antibiotic cecropin from the vector mosquito, Aedes albopictus. Biochem Biophys Res Commun. 1998;19:410–415. doi: 10.1006/bbrc.1998.9150. [DOI] [PubMed] [Google Scholar]
- 236.Tabachnick W J. Microgeographic and temporal genetic variation in populations of the bluetongue virus vector Culicoides variipennis (Diptera: Ceratopogonidae) J Med Entomol. 1992;29:384–394. doi: 10.1093/jmedent/29.3.384. [DOI] [PubMed] [Google Scholar]
- 237.Tabachnick W J, Wallis G P, Aitken T H, Miller B R, Amato G D, Lorenz L, Powell J R, Beaty B J. Oral infection of Aedes aegypti with yellow fever virus: geographic variation and genetic considerations. Am J Trop Med Hyg. 1985;34:1219–1224. doi: 10.4269/ajtmh.1985.34.1219. [DOI] [PubMed] [Google Scholar]
- 238.Reference deleted.
- 239.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 240.Tatusov R L, Koonin E V, Lipman D J. A genomic perspective on protein families. Science. 1997;278:631–637. doi: 10.1126/science.278.5338.631. [DOI] [PubMed] [Google Scholar]
- 241.Tetteh K. Exploring parasite genomes: the way forward. Parasitol Today. 1999;15:88–90. doi: 10.1016/s0169-4758(99)01390-3. [DOI] [PubMed] [Google Scholar]
- 242.Theodos C M, Ribeiro J M, Titus R G. Analysis of enhancing effect of sand fly saliva on Leishmania infection in mice. Infect Immun. 1991;59:1592–1598. doi: 10.1128/iai.59.5.1592-1598.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Titus R G, Ribeiro J M. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science. 1988;239:1306–1308. doi: 10.1126/science.3344436. [DOI] [PubMed] [Google Scholar]
- 244.Touray M, Warburg A, Laughinghouse A, Krettli A U, Miller L H. Developmentally regulated infectivity of malaria sporozoites for mosquito salivary glands and the vertebrate host. J Exp Med. 1992;175:1607–1612. doi: 10.1084/jem.175.6.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Trape J F, Rogier C. Combating malaria morbidity and mortality by reducing transmission. Parasitol Today. 1996;12:236–240. doi: 10.1016/0169-4758(96)10015-6. [DOI] [PubMed] [Google Scholar]
- 246.Turelli M, Hoffmann A A. Rapid spread of an inherited incompatibility factor in California Drosophila. Nature. 1991;353:440–442. doi: 10.1038/353440a0. [DOI] [PubMed] [Google Scholar]
- 247.Turelli M, Hoffmann A A. Microbe-induced cytoplasmic incompatability as a mechanism for introducing transgenes into arthropod populations. Insect Mol Biol. 1999;8:243–255. doi: 10.1046/j.1365-2583.1999.820243.x. [DOI] [PubMed] [Google Scholar]
- 248.Reference deleted.
- 249.Uttenweiler-Joseph S, Moniatte M, Lagueux M, Van Dorsselaer A, Hoffmann J A, Bulet P. Differential display of peptides induced during the immune response of Drosophila: a matrix-assisted laser desorption ionization time-of-flight mass spectrometry study. Proc Natl Acad Sci USA. 1998;95:11342–11347. doi: 10.1073/pnas.95.19.11342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Vanderberg J. Development of infectivity by the Plasmodium berghei sporozoite. J Parasitol. 1975;61:43–50. [PubMed] [Google Scholar]
- 251.van Huesden M C, Thompson F, Dennis J. Biosynthesis of Aedes aegypti lipophorin and gene expression of its apolipoproteins. Insect Biochem Mol Biol. 1998;28:733–738. doi: 10.1016/s0965-1748(98)00068-x. [DOI] [PubMed] [Google Scholar]
- 252.Reference deleted.
- 253.Vernick K D, Collins F H, Gwadz R W. A general system of resistance to malaria infection in Anopheles gambiae controlled by two main genetic loci. Am J Trop Med Hyg. 1989;40:585–592. doi: 10.4269/ajtmh.1989.40.585. [DOI] [PubMed] [Google Scholar]
- 254.Vernick K D, Fujioka H, Seeley D C, Tandler B, Aikawa M, Miller L H. Plasmodium gallinaceum: A refractory mechanism of ookinete killing in the mosquito, Anopheles gambiae. Exp Parasitol. 1995;80:583–595. doi: 10.1006/expr.1995.1074. [DOI] [PubMed] [Google Scholar]
- 255.Waidhet-Kouadio P, Yuda M, Ando K, Chinzei Y. Purification and characterization of a thrombin inhibitor from the salivary glands of a malarial vector mosquito, Anopheles stephensi. Biochim Biophys Acta. 1998;1381:227–233. doi: 10.1016/s0304-4165(98)00026-9. [DOI] [PubMed] [Google Scholar]
- 256.Warburg A, Touray M, Krettli A U, Miller L H. Plasmodium gallinaceum: antibodies to circumsporozoite protein prevent sporozoites from invading the salivary glands of Aedes aegypti. Exp Parasitol. 1992;75:303–307. doi: 10.1016/0014-4894(92)90215-v. [DOI] [PubMed] [Google Scholar]
- 257.Wattam A R, Christensen B M. Induced polypeptides associated with filarial worm refractoriness in Aedes aegypti. Proc Natl Acad Sci USA. 1992;89:6502–6505. doi: 10.1073/pnas.89.14.6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Wattam A R, Christensen B M. Further evidence that the genes controlling susceptibility of Aedes aegypti to filarial parasites function independently. J Parasitol. 1992;78:1092–1095. [PubMed] [Google Scholar]
- 259.Whitfield S G, Murphy F A, Sudia W D. St. Louis encephalitis virus: An ultrastructural study of infection in a mosquito vector. Virology. 1973;56:70–87. doi: 10.1016/0042-6822(73)90288-2. [DOI] [PubMed] [Google Scholar]
- 260.Whitlow M, Filpula D. Single-chain Fv proteins and their fusion proteins. Methods Companion Methods Enzymol. 1991;2:97–105. [Google Scholar]
- 261.Woodring J L, Higgs S, Beaty B J. Natural cycles of vector-borne pathogens. In: Beaty B J, Marquardt W C, editors. The biology of disease vectors. Niwot, Colo: University Press of Colorado; 1996. pp. 51–72. [Google Scholar]
- 262.World Health Organization. Fact sheet 94. Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
- 263.World Health Organization. Disease statistics. The World Health Report. Geneva, Switzerland: World Health Organization; 1999. [Google Scholar]
- 264.Wu L P, Anderson K V. Regulated nuclear import of Rel proteins in the Drosophila immune response. Nature. 1998;392:93–97. doi: 10.1038/32195. [DOI] [PubMed] [Google Scholar]
- 265.Yamamoto H, Kobayashi M, Ogura N, Tsuruoka H, Chigusa Y. Studies on filariasis. VI. The encapsulation of Brugia malayi and B. pahangi larva in the mosquito, Armigeres subalbatus. Jpn J Sanit Zool. 1985;36:106. [Google Scholar]
- 266.Yeates R A, Steiger S. Ultrastructural damage of in vitro cultured ookinetes of Plasmodium gallinaceum (Brumpt) by purified proteinases of susceptible Aedes aegypti (L.) Parasitology. 1981;66:93–97. doi: 10.1007/BF00941949. [DOI] [PubMed] [Google Scholar]
- 267.Yoshiga T, Hernandez V P, Fallon A M, Law J H. Mosquito transferrin, an acute-phase protein that is up-regulated upon infection. Proc Natl Acad Sci USA. 1997;94:12337–12342. doi: 10.1073/pnas.94.23.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Zhao Y, Eggleston P. Stable transformation of an Anopheles gambiae cell line mediated by the Hermes mobile genetic element. Insect Biochem Mol Biol. 1998;28:213–219. [Google Scholar]
- 269.Zhao X, Ferdig M T, Li J, Christensen B M. Biochemical pathway of melanotic encapsulation of Brugia malayi in the mosquito, Armigeres subalbatus. Dev Comp Immunol. 1995;19:205–215. doi: 10.1016/0145-305x(95)00005-e. [DOI] [PubMed] [Google Scholar]
- 270.Zheng L, Benedict M Q, Cornel A J, Collins F H, Kafatos F C. An integrated genetic map of the African human malaria vector mosquito, Anopheles gambiae. Genetics. 1996;143:941–952. doi: 10.1093/genetics/143.2.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Zheng L, Collins F H, Kumar V, Kafatos F C. A detailed genetic map for the X chromosome of the malaria vector, Anopheles gambiae. Science. 1993;30:605–608. doi: 10.1126/science.8342025. [DOI] [PubMed] [Google Scholar]
- 272.Zheng L, Cornel A J, Wang R, Erfle H, Voss H, Ansorge W, Kafatos F C, Collins F H. Quantitative trait loci for refractoriness of Anopheles gambiae to Plasmodium cynomolgi B. Science. 1997;276:425–428. doi: 10.1126/science.276.5311.425. [DOI] [PubMed] [Google Scholar]
- 273.Zieler H, Garon C F, Fischer E R, Shahabuddin M. Adhesion of Plasmodium gallinaceum ookinetes to the Aedes aegypti midgut: sites of parasite attachment and morphological changes in the ookinete. J Eukaryot Microbiol. 1998;45:512–520. doi: 10.1111/j.1550-7408.1998.tb05110.x. [DOI] [PubMed] [Google Scholar]
- 274.Zieler H, Nawrocki J P, Shahabuddin M. Plasmodium gallinaceum ookinetes adhere specifically to the midgut epithelium of Aedes aegypti by interaction with a carbohydrate ligand. J Exp Biol. 1999;202:485–495. doi: 10.1242/jeb.202.5.485. [DOI] [PubMed] [Google Scholar]