Abstract
Neurofibromatosis type 2 (NF2) is a brain tumor predisposing syndrome caused by inactivating alterations of the NF2 gene mapped at chromosome 22q. Currently, no genetic information exists on medulloblastomas occurring in NF2 patients. We herein report on the genetic alterations observed in a girl in which the NF2 gene was de novo altered due to a constitutional translocation: t(5;22)(q35.1;q11.2). This girl had a particularly aggressive disease course. At the age of 4, she had already been diagnosed with three lesions classified as schwannomas and a meningioma. At 10 years old, she developed a medulloblastoma. She died at the age of 14 due to a refractory acute myeloid leukemia (AML). From the genetic point of view, we observed that (1) the NF2 gene was rearranged in all patient samples: blood, tumor, and leukemic cells; (2) loss of 3′ region of NF2 and the downstream regions of chromosome 22 were only detected in medulloblastoma cells; (3) the known cancer AML-related gene: NPM1 which is mapped at 5q35.1 was not the target of any alteration in our patient. Our data suggest that inactivation of the NF2 gene was relevant for the medulloblastoma pathogenesis. Furthermore, we know that malignant cancers are the result of a multi-epi-genetic sequence of events, and although, unquestionably limited to the genetic findings in one case. We may hypothesize, that as described for a fraction of medulloblastomas, the alteration of a gene mapped at 5q might also have been relevant for medulloblastoma development in our patient.
Keywords: Constitutional translocation, Translocation, NF2, Medulloblastoma
Introduction
Cancers arise due to the accumulation of genetic and epigenetic alterations in a group of genes that are regulators of cell homeostasis and are broadly classified as oncogenes and tumor suppressor genes. For each cancer type, it was evidenced that tumor development and progression are dependent upon the interplay of many factors: the category of genes involved, number, type of genetic and/or epigenetic alterations, specific cell of origin, time and order of occurrence of the alterations, relations established with the other surrounding cell types, and the effect of certain environmental factors. The demonstration therefore, that in neoplasia there is a specific association between cell type – the gene and epigenetic changes – the evolutionary pattern of these changes, has allowed that in clinical practice, cancer-associated alterations can be used as diagnostic and prognostic markers. Most importantly, it uncovered that these changes could be explored as potential targets for anticancer therapy. At present, major clinical advances have been obtained in various hematological neoplasias by targeting fusion oncoproteins. The upmost example is still chronic myeloid leukemia, where target therapies for the BCR-ABL fusion transcript turned out a once fatal neoplasia in a “chronic disease” [1–4]. In pediatric brain cancer, active investigation of genomic and cell signals pathways in neurofibromatosis type 1-related neoplasms has also been most valuable. Indeed, mitogen-activated protein kinases inhibitors became the first FDA-approved target therapies for inoperable brain neurofibromatosis type 1 tumors [5]. In contrast, the advantages brought by target therapies in other brain pediatric tumors, namely, in medulloblastomas, the most common malignant brain cancer in children, or in neurofibromatosis type 2 (NF2) are rather limited and further studies are needed [6, 7].
NF2 is a brain tumor predisposing syndrome. It occurs due to alterations in the NF2 tumor suppressor gene located at chromosome 22q [8]. Approximately 50% of the patients inherit a germline mutation of one of the alleles and the remaining others acquire a de novo mutation. Studies in NF2-related tumors demonstrated that in these neoplasms development is linked to inactivation of both alleles of the NF2 gene [7]. The NF2 gene codes for a protein named merlin or neurofibromin 2. Functional studies performed in the most common NF2-associated tumors showed that biallelic NF2 inactivation abrogates neurofibromin 2 activity and that this loss has a pivotal role in neoplastic development by (1) inducing loss of contact growth inhibition; (2) modifying the degradation of YAP/tafazzin complex by the proteosome; (3) controlling the levels of availability of the neurogenic locus notch homolog protein receptors; (4) regulating the availability of β-catenin at the nucleus; (5) modifying transforming growth factor beta, platelet-derived growth factor receptor, and epidermal growth factor receptor signaling activation; and (6) by disturbing the oxidative milieu [9].
The major tumor types arising in individuals with NF2 are usually classified as benign lesions and are by order of frequency: vestibular schwannomas present in approximately 95% of the patients, followed by meningiomas and ependymomas that occur in approximately 50% and 30–50% of persons with NF2. NF2 gene analysis revealed that genotype alterations influenced patient phenotype. Accordingly, patients with nonsense and splice-site mutations develop vestibular schwannomas at adult age in the 3rd decade of life (Gardner’s phenotype). Contrastingly, truncating mutations and structural alterations such as translocations were observed to be linked to early age onset, development of multiple tumors, and an increased risk of mortality (Wistar phenotype) [7].
We herein report on the genetic alterations observed in a girl in which the NF2 gene was de novo altered due to a translocation. This girl had a particularly aggressive disease course. At the age of 4, she was diagnosed with four concurrent tumors, three lesions classified as schwannomas and a meningioma of the right optic nerve. At 10 years old, she developed a medulloblastoma and died at the age of 14 due to a refractory acute myeloid leukemia (AML). Our case is as far as we are aware the second report of a medulloblastoma developing in a child with NF2 [10] and the first in which genetic analysis is described. Our findings thus represent the first genetic insight into the investigation of a particularly aggressive and rare association: medulloblastoma in a NF2 context.
Case Presentation
Patient Clinical Features and Follow-Up
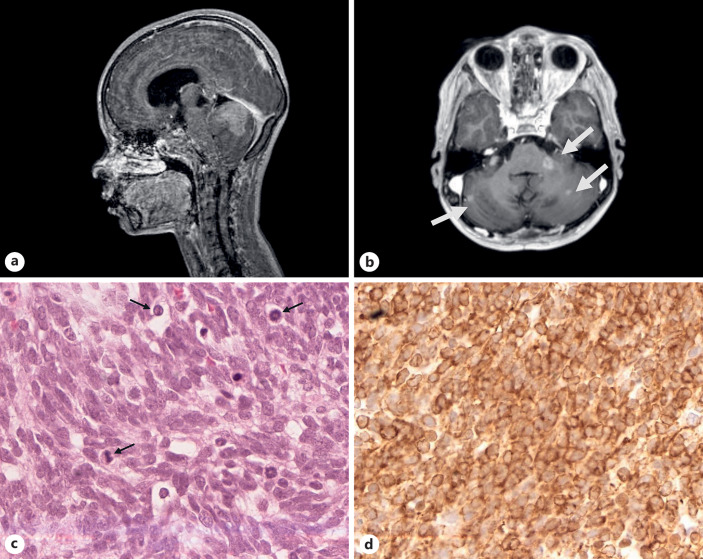
The patient, a 10-year-old girl, was referred to the pediatric neuro-oncology of the Portuguese Cancer Institute in Lisbon after a macroscopical total surgical resection of a cerebellar vermis tumor with leptomeningeal dissemination (shown in Fig. 1a, b) at the neurosurgery department of Lisbon Hospital Center (CHULN). The tumor was diagnosed at the neuropathology department of Lisbon Hospital Center (CHULN) as a medulloblastoma classical pattern (shown in Fig. 1c, d). This girl was then recommended to be treated at the Portuguese Cancer Institute in Lisbon. Adjuvant therapy protocol followed was irradiation with tumor bed boost and chemotherapy (lomustine, cisplatin, and vincristine). This girl had a clinical history of NF2. Accordingly, at the age of 4 she had started with signs of right facial palsy and had a paretic esotropia which led to clinical investigation and visualization by magnetic resonance imaging of four lesions. These lesions were identified as a meningioma of the right optic nerve, a left schwannoma of the facial nerve (7), a right schwannoma of the vestibulocochlear nerve (8), and a schwannoma of the root of C5. She was the second child of three in a family, with no remarkable family history of NF2 noted, either in the parents or brothers. At our institution after the end of adjuvant treatment for medulloblastoma, the patient entered in remission. She had a stable clinical condition during 2 years. However, at the age of 12 an intracranial leptomeningeal recurrence was observed on magnetic resonance imaging. She underwent treatment with etoposide for 11 months with partial response. Treatment was stopped due to severe hematological toxicity. She deceased 5 months later, at 14 years of age due to refractory AML.
Fig. 1.
a MRI (sagittal T1-weighted image with gadolinium) showing a contrast-enhanced solid cerebellar lesion. b MRI (axial T1-weighted image with gadolinium) showing leptomeningeal dissemination in cerebellum and left cerebellopontine angle (arrows). c Medulloblastoma of the histological defined classic form: poorly differentiated round and polyhedric embryonal cells with high mitotic activity (arrows) (H&E ×40). d Immunoreactivity for neurofilament antibody (×40).
Material and Methods
Samples for Genetic Analysis
At the Portuguese Cancer Institute in Lisbon, a surgical fragment of the medulloblastoma specimen was genetically evaluated by conventional cytogenetics, high-resolution comparative genomic hybridization, and fluorescence in situ hybridization (FISH). In order to confirm the diagnosis of NF2, conventional cytogenetic studies were performed at the peripheral blood samples of the patients and parents. FISH analysis was also performed in the girl peripheral blood metaphases. The patient refractory AML was also genetically characterized by cytogenetic, molecular, and FISH analysis. All studies in this patient were conducted according to the ethical rules of World Medical Association of Helsinki. A CARE Checklist can be consulted as supplemental data to this manuscript (for all online suppl. material, see www.karger.com/doi/10.1159/000527564).
Conventional Cytogenetics
Conventional cytogenetic analysis on peripheral blood samples of the patients and parents was performed according to standard protocols. Bone marrow patient karyotype was established on 24 h unstimulated cultures as to standard protocol. The karyotype evaluation of the medulloblastoma was performed on a sterile fragment of the surgical obtained specimen as previously described [11].
HR-CGH Analysis
High-resolution comparative genomic analysis was performed on the tumor sample as previously described [11].
Fluorescence in situ Hybridization
Analysis by FISH with the NF2 and NPM1 Dual Color Break Apart probes (Empire Genomics, Inc.) was performed according to the manufacture’s protocols on (1) the patient tumor metaphases, (2) the patient blood metaphases, and (3) at the metaphases of the refractory AML sample.
Sanger Sequencing
PCR amplification and Sanger sequencing with primers defined to NPM1 and FTL3 genes was completed as described by Damm-Welk C et al. [12].
Results
Conventional Cytogenetics
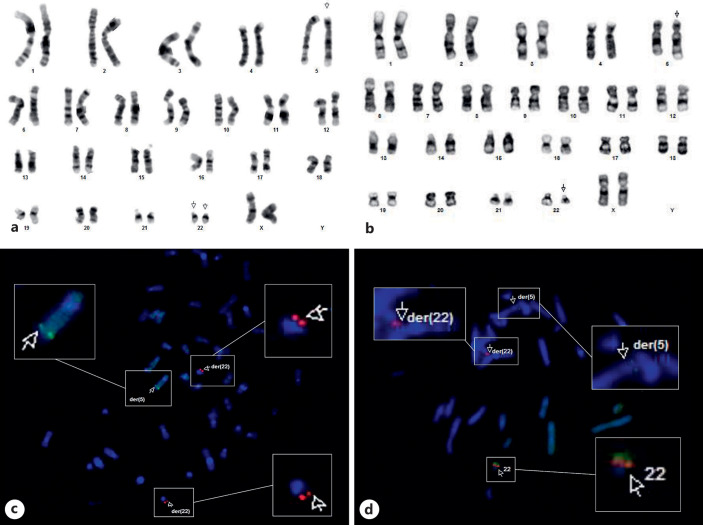
Analysis of the medulloblastoma cells revealed the following karyotype in all tumor cells: 46,XX,der(5)t(5;22)(q35.1; q11.2), der(22)t(5;22)(q35.1;q11.2) ×2 (shown in Fig. 2a). Patient peripheral blood karyotype revealed that she was a carrier of a balanced translocation involving chromosomes 5 and 22 with the same breakpoints as observed in medulloblastoma cells. The established patient constitutional karyotype was 46,XX,t(5;22)(q35.1; q11.2)c (shown in Fig. 2b). Both parents had normal karyotypes: 46,XX, the mother, and 46,XY, the father. In leukemia cells the observed karyotype in all analyzed metaphases was 45,XX,(5;22)(q35.1;q11.2)c,-7,-10,add(21)(q22),+ mar.
Fig. 2.
a Tumor metaphase showing the two der(22)t(5;22)(q35.1;q11.2) chromosomes. b Peripheral blood metaphase of the patient showing the constitutional t(5;22)(q35.1; q11.2). cNF2 FISH in a tumor metaphase; the 5′ NF2 BAC probe was labelled with a red fluorochrome (spectrum orange) and the 3′ NF2 BAC probe was labelled with a green fluorochrome (spectrum green). dNF2 FISH in a peripheral metaphase of the patient, as previously mentioned; the 5′ NF2 BAC probe was labelled with a red fluorochrome (spectrum orange) and the 3′ NF2 BAC probe was labelled with a green fluorochrome (spectrum green).
HR-CGH Analysis
Medulloblastoma evaluation by this methodology revealed a highly unbalanced genome with multiple gains and losses. The observed genetic alterations are depicted in Table 1 and online supplementary Figure 1.
Table 1.
Summary of the HR-CGH imbalances observed in the medulloblastoma cells
| HR-CGH gains at chromosomal regions | 1q21-q44, 2p25-q37, 3q21-q29, 5p15-q35, 6p12-q25, 7p22-q36, 8q13-q24.1, 9p24-p13, 10q21, 11p15-p11.2, 11q13-q23, 12p12, 12q14-q24.1, 14q11.2-q24, 15q11.2-q22, 18q11.2-q23, 21q11.2-q22, Xp22.1-p11.2, Xq13-q24 |
| HR-CGH losses at chromosomal regions | 1p36.3-p13, 3p26-p12, 4p16-4q35, 8p23-p11.2, 22q12-q13 |
HR-CGH, high-resolution comparative genomic hybridization.
Fluorescence in situ Hybridization
Analysis with an NF2 Dual Color Break Apart probe (Empire Genomics, Inc.) revealed that this gene was rearranged. In the tumor, no normal copy of chromosome 22 was observed, and loss of the 3′- green NF2 probe occurred at both derivatives chromosomes 22 (shown in Fig. 2c). In the patient’s lymphocytes, both copies of the 3′- green NF2 probe were detected: one at the normal chromosome 22 and the other at the derivative chromosome 5 (shown in Fig. 2d). At the leukemic cells, the NF2 gene was also rearranged, but no deletions occurred (shown in online suppl. Fig. 2). Analysis with the NPM1 (nucleophosmin) Dual Color Break Apart probe (Empire Genomics, Inc.) showed that this gene mapped at 5q35.1 was not affected by the translocation in any of the evaluated samples of the patient.
Sanger Sequencing
Sanger sequencing for the NPM1 and the FTL3 genes on refractory AML sample revealed no mutations in this case.
Conclusion
Evaluation of the genetic alterations present in several cancer types such as leukemias helped to understand the pathogenesis of each cancer type and paved the way to identify new therapeutic strategies [1–4]. Regarding pediatric brain tumors although cure rates have risen over the years, they still are the leading cause of childhood deaths in Europe and North America, representing, medulloblastoma, the most common malignant pediatric brain tumor. Furthermore, children with medulloblastomas who survive cancer usually suffer for long-term sequel including mental disabilities, organ toxicities, and secondary cancer. To define more specific, efficient, and less-damaging therapies, large cooperative consortiums like the International Pediatric Pan-Cancer Consortium (http://.pedcan.com) were built to unravel the complete genetic repertoire of pediatric malignancies. The data obtained by this consortium indicated that nearly 50% of the pediatric neoplasms harbored a potentially druggable event, but it also highlighted the need for a personalized profiling for each patient [13]. According to data contained in public databases (http://.pedcan.com; https://www.tumorfusions.org; and https://www.ccsmuth.edu), our case is as far as we are aware the first report on the genetic abnormalities present in a medulloblastoma associated to NF2.
Karyotype and FISH analysis of the patient’s peripheral blood lymphocytes revealed that the child was a carrier of a de novo t(5;22) (q35.1;q11.2) that caused the rearrangement of the NF2 gene at chromosome 22q. Previous reports in patients with translocations affecting the NF2 gene [7] show that patients affected by this type of abnormalities present a more aggressive NF2 phenotype, namely, the development at early age of multiple schwannomas and meningiomas, such as it occurred in our patient. Also, in keeping with data observed in common associated NF2 tumors [7], our findings indicate that in the patient medulloblastoma cells the NF2 gene acted as a tumor suppressor gene. Indeed, in tumor cells no normal copy of chromosome 22 was present. FISH analysis demonstrated loss of the two green fluorescent bacterial artificial chromosomes containing the DNA sequences of the 3′ region of NF2 at chromosome 22, and by CGH analysis a wide-ranging loss of genetic material at the long arm of chromosome 22 was also observed. Furthermore, suggesting the NF2 gene-specific role in the pathogenesis of medulloblastoma, we could verify that in the patient refractory AML cells, NF2 was rearranged but not deleted.
It is important when reflecting about the putative role of NF2 in the genesis of medulloblastoma that we know that a malignant cancer is the result of time and environmental multi-epi-genetic sequence of events. Additionally, it has been defined that structural variants and structural copy number alterations play a relevant role in the pathogenesis of medulloblastomas sub-groups 3 and 4 [14]. Unquestionably, we are limited to the genetic findings in this case, but it is highly suggestive that one of the other crucial events for the malignant transformation of the cerebellum cells in our patient involved the disruption of a gene located at chromosome 5q. Previous studies performed in medulloblastoma support this hypothesis [6, 14]. Likewise, Northcott et al. [14] demonstrated that (1) PR/SET domain 6 (PRMD6) gene was a target of enhancer hijacking in 17% of group 4 medulloblastomas and that (2) the synphilin-1 (SNCAIP) gene that is mapped 600 Kb upstream of PRDM6 is a recurrent target of tandem duplications in the same medulloblastoma group.
In our patient, the breakpoint at chromosome 5 did not seem to disrupt region 5q23 but, a downstream region, 5q35.1 [15]. Nucleophosmin (NPM1), a cancer-related gene, is mapped at 5q35.1. To us, NPM1 was a fundamental gene to be considered in the genetics and biology since our patient developed a refractory AML and the NPM1 gene is reported to be most frequently mutated in AML. Moreover, a differential clinical course is known to be linked to NPM1-altered AML. Characteristically, a favorable prognosis is noted in NPM1 AML mutated without a FLT3 internal tandem duplication, whereas an intermediate prognosis is associated with a NPM1 AML mutated with FLT3 internal tandem duplication. Also, NPM1 has been implicated in the control of ploidy and DNA repair [15]. In keeping with this NPM1 role, we observed a largely unbalanced CGH in medulloblastoma cells.
In our case, a rearrangement or mutation was not observed at the NPM1 gene. These findings, nevertheless, do not exclude the hypothesis that, as in other previously reported medulloblastoma cases [14], in this patient, a gene mapped at 5q might have a central role in the medulloblastoma development and the more aggressive NF2 phenotype. Moreover, our data also corroborate the impression already noted [13] that in pediatric neoplasms a personalized profiling is needed for each patient. In this way, further studies are needed in order to identify the gene that either directly or by inducing enhancer hijacking might have contributed to the medulloblastoma development.
Acknowledgments
We thank Carla Pereira and Sandra Gonçalves for all the technical assistance.
Statement of Ethics
This study was conducted ethically in accordance with the World Medical Association of Helsinki. A written informed consent was obtained from the legal guardian of the patient to publish this case and any accompanying images. This document will be available to the editor if requested. The herein reported study was approved by the Ethical Committee of the Portuguese Cancer Institute in Lisbon (committee’s reference number: UIC/971).
Conflict of Interest Statement
The authors have no conflict of interest to declare.
Funding Sources
This study was funded by Instituto Português de Oncologia de Lisboa (IPO), Lisbon, Portugal, and by Fundação para a Ciência e Tecnologia/Ministério da Ciência, Tecnologia e Ensino Superior (FCT/MCTES, Portugal), through national funds to iNOVA4Health (UIDB/04462/2020 and UIDP/04462/2020) and the Associated Laboratory LS4Future (LA/P/0087/2020).
Author Contributions
All authors read and approved the final manuscript. This research has not been and will not be submitted simultaneously to another journal, in whole or in part. The paper reports previously unpublished work. All those named as authors have made a sufficient contribution to the work and have obtained all the necessary consent from their employers or funding bodies. Sofia Nunes was the pediatric neuro-oncologist responsible for the patient, helped in the draft of the first version of the manuscript, and critically revised the final manuscript. Claudia Faria performed the neuro-surgery, helped in the draft of the first version of the manuscript, and critically revised the final manuscript. José Pimentel performed the histological analysis, helped in the draft of the first version of the manuscript, and critically revised the final manuscript. Rafael Roque performed the histological review of the case and critically revised the final manuscript. Helena Alaiz, Isabel Salazar, and Teresa Pereira performed the cytogenetic analysis in the hematological samples of the patient and critically revised the final manuscript. Filipa Ferreira performed Sanger sequencing analysis and critically revised the final manuscript. Lúcia Roque performed the cytogenetic analysis in the tumor sample, performed the fluorescent in situ hybridization and comparative genomic hybridization experiments, and was responsible for the revision of the literature, draft, critical revision, and submission of the manuscript.
Funding Statement
This study was funded by Instituto Português de Oncologia de Lisboa (IPO), Lisbon, Portugal, and by Fundação para a Ciência e Tecnologia/Ministério da Ciência, Tecnologia e Ensino Superior (FCT/MCTES, Portugal), through national funds to iNOVA4Health (UIDB/04462/2020 and UIDP/04462/2020) and the Associated Laboratory LS4Future (LA/P/0087/2020).
Data Availability Statement
No digital data were generated in this study. All data produced and analyzed during this study are included in this article and in online supplementary Figures 1 and 2.
Supplementary Material
Supplementary Material
Supplementary Material
References
- 1. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011 Mar;144(5):646–74. 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2. Asnafi AA, Deris Zayeri Z, Shahrabi S, Zibara K, Vosughi T. Chronic myeloid leukemia with complex karyotypes: prognosis and therapeutic approaches. J Cell Physiol. 2019 May;234(5):5798–806. 10.1002/jcp.27505. [DOI] [PubMed] [Google Scholar]
- 3. Zayeri Z, Rasras S, Zibara K, Vosughi T. Genetics and epigenetics of glioblastoma: therapeutic challenges. Clin Cancer Investig J. 2018 Mar-Apr;7(2):43. 10.4103/ccij.ccij_82_17. [DOI] [Google Scholar]
- 4. Deris Zayeri Z, Tahmasebi Birgani M, Mohammadi Asl J, Kashipazha D, Hajjari M. A novel infram deletion in MSH6 gene in glioma: conversation on MSH6 mutations in brain tumors. J Cell Physiol. 2019 Jul;234(7):11092–102. 10.1002/jcp.27759. [DOI] [PubMed] [Google Scholar]
- 5. Fangusaro J, Onar-Thomas A, Young Poussaint T, Wu S, Ligon AH, Lindeman N, et al. Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial. Lancet Oncol. 2019 Jul;20(7):1011–22. 10.1016/S1470-2045(19)30277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hovestadt V, Ayrault O, Swartling FJ, Robinson GW, Pfister SM, Northcott PA. Medulloblastomics revisited: biological and clinical insights from thousands of patients. Nat Rev Cancer. 2020 Jan;20(1):42–56. 10.1038/s41568-019-0223-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coy S, Rashid R, Stemmer-Rachamimov A, Santagata S. An update on the CNS manifestations of neurofibromatosis type 2. Acta Neuropathol. 2020 Apr;139(4):643–65. 10.1007/s00401-019-02029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rouleau GA, Merel P, Lutchman M, Sanson M, Zucman J, Marineau C, et al. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature. 1993 Jun;363(6429):515–21. 10.1038/363515a0. [DOI] [PubMed] [Google Scholar]
- 9. Mota M, Shevde LA. Merlin regulates signaling events at the nexus of development and cancer. Cell Commun Signal. 2020 Apr;18(1):63. 10.1186/s12964-020-00544-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kalimullah J, Sohail AMAH, Shahjehan RD, Siddique S, Bari ME. Neurofibromatosis type 2 patient presenting with medulloblastoma. Surg Neurol Int. 2015 Oct;6(Suppl 17):S440–3. 10.4103/2152-7806.166771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Faria CC, Miguens J, Antunes JL, Barroso C, Pimentel J, Martins MDC, et al. Genetic alterations in a papillary glioneuronal tumor. J Neurosurg Pediatr. 2008 Jan;1(1):99–102. 10.3171/ped-08/01/099. [DOI] [PubMed] [Google Scholar]
- 12. Damm-WelK C, Busch K, Burkhardt B, Schieferstein J, Viehmann S, Oschlies I, et al. Prognostic significance of circulating tumor cells in bone marrow or peripheral blood as detected by qualitative and quantitative PCR in pediatric NPM-ALK-positive anaplastic large-cell lymphoma. Blood. 2007 Jul;110(2):670–7. 10.1182/blood-2007-02-066852. [DOI] [PubMed] [Google Scholar]
- 13. Gröbner SN, Worst BC, Weischenfeldt J, Buchhalter I, Kleinheinz K, Rudneva VA, et al. The landscape of genomic alterations across childhood cancers. Nature. 2018 Mar;555(7696):321–7. 10.1038/nature25480. [DOI] [PubMed] [Google Scholar]
- 14. Northcott PA, Buchhalter I, Morrissy AS, Hovestadt V, Weischenfeldt J, Ehrenberger T, et al. The whole-genome landscape of medulloblastoma sub-types. Nature. 2017 Jul;547(7663):311–7. 10.1038/nature22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grisendi S, Mecucci C, Falini B, Pandolfi PP. Nucleophosmin and cancer. Nat Rev Cancer. 2006 Jul;6(7):493–505. 10.1038/nrc1885. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No digital data were generated in this study. All data produced and analyzed during this study are included in this article and in online supplementary Figures 1 and 2.