Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) affects multiple body systems, including the nervous system. Cerebrovascular accidents can also occur. Patients with comorbid illnesses have severe manifestations and poor outcomes. Despite the proper mechanism of SARS-CoV-2 infection-associated stroke having not yet been settled, various possible mechanisms have been hypothesized. One possibility is that the virus causes endothelial dysfunction and immune-mediated injury. Another possibility is that the trans-neuronal spread of the virus affects brain tissue. In addition, hypercoagulability caused by SARS-CoV-2 infection could lead to a stroke. A virus-induced dysfunction of the renin-angiotensin system could also lead to a stroke. The immune response and vasculitis resulting from SARS-CoV-2 infection are also possible causes via a cytokine storm, immune dysfunction, and various inflammatory responses. SARS-CoV-2 infection may affect calcitonin gene-related peptides and cerebral blood flow and may lead to stroke. Finally, SARS-CoV-2 may cause hemorrhagic strokes via mechanisms stimulated by its interaction with angiotensin-converting enzyme 2 (ACE2), leading to arterial wall damage and blood pressure changes. In this article, we will present seven cases of stroke-associated SARS-CoV-2 infection.
Keywords: Severe acute respiratory syndrome coronavirus 2, Stroke, Hemorrhagic stroke, Cytokine storm, Immune dysfunction
Introduction
Coronavirus disease 2019 (COVID-19) is a major pandemic disease of rising public concern [1]. COVID-19 primarily presents with respiratory symptoms; however, several central nervous system (CNS) manifestations have also been reported. According to a recent systematic review, commonly reported CNS manifestations include strokes, hyposmia, headaches, loss of taste sensation [2], and polyneuropathy, e.g., GBS, may also occur as well [3]. The actual incidence of cerebrovascular events in COVID-19 patients is variable. The incidence is higher in studies that include severely affected patients than in studies where patients have a laboratory-confirmed infection and are hospitalized. This variability is thought to be due to differences in populations and the variability in each country’s diagnostic procedures [4]. Mao et al. [5] reported that the incidence of cardiovascular and cerebrovascular disorders in COVID-19 patients which had been done at 3 designated special care centers for COVID-19 in Wuhan, China, reached 5.7%. In addition, the incidence of stroke has recently been more evident in COVID-19 patients under 50 years of age with no risk factors [6]. There also seems to be an association between the development of severe pneumonia and the incidence of stroke; 31% of COVID-19 patients with pneumonia were found to have strokes and stroke-related symptoms [7, 8]. The underlying mechanism responsible for strokes in COVID-19 patients is still unclear. Varga et al. [9] reported that the receptor of the causative virus (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]) is angiotensin-converting enzyme 2 (ACE2). ACE2 is expressed on the cell membrane of various types of cells, including endothelial cells. Therefore, one of the possibilities is that the virus causes strokes by inducing inflammatory reactions in the endothelium of CNS blood vessels. Another central mechanism that may explain the increased incidence of stroke is the disruption of the coagulation pathway [10]. COVID-19 patients are more likely to develop a vascular hypercoagulable state. This is widely supported by the fact that COVID-19 patients express higher levels of factor VIII, fibrinogen, and D-dimer, which are directly involved in clot formation [8, 11]. In a retrospective study, 13 patients of the observed 219 participants developed cerebrovascular disorders, and 11 of them experienced cerebrovascular accidents including ten ischemic strokes and one intracerebral hemorrhage [12]. Moreover, case studies have reported an associated increase in the prevalence of large vessel occlusion stroke in COVID-19 patients [4]. A recent study found evidence of megakaryocyte clusters and large megakaryocytes in brain autopsies. These cells can cause blood vessel occlusions that, in turn, cause ischemic alterations and neurological impairment [13].
In this article, we describe seven cases of SARS-CoV-2 infection associated with cerebrovascular accidents and emphasize their clinical presentations and some important abnormalities in their laboratory test results. The CARE Checklist has been completed by the authors for this case report, available as online supplementary material at www.karger.com/doi/10.1159/000529122.
Case Series Presentations
Case One
A 31-year-old male presented to our hospital with no comorbid illnesses. He was complaining of headache and dizziness associated with persistent vomiting for 4-day duration. On examination, the patient was conscious, alert, oriented, hypothermic, and hypotensive (due to excessive vomiting). Neurological examination showed no signs of meningeal irritation. The motor power of the right upper and lower limbs was equally affected (grade 4/5) and associated with right-side hemihypesthesia that included the face. Also, there was cerebellar dysfunction in the form of bilateral horizontal nystagmus associated with bilateral unsteady gait; other cerebellar functions were intact. The magnetic radiology imaging (MRI) showed acute ischemic infarction within the left cerebellar hemisphere (Fig. 1). The patient’s echocardiography and the computerized tomography angiography results were normal. COVID-19 screen upon admission was positive for COVID-19. The laboratory test results are presented in Table 1. Also, routine stroke workup showed normal HBA1C and lipid profiles with normal electrocardiogram (ECG), Holter findings. The echocardiographic picture showed normal ejection fraction with no source of cardiac emboli such as no left ventricular thrombus or atrial myxomas. The patient was diagnosed with acute cerebellar infarction with COVID-19. He was treated with anti-ischemic drugs and antiviral therapy according to Ministry of Health (MOH) protocols. He was discharged well after 5 days of hospital stay without further complications for the continuation of physiotherapy.
Fig. 1.
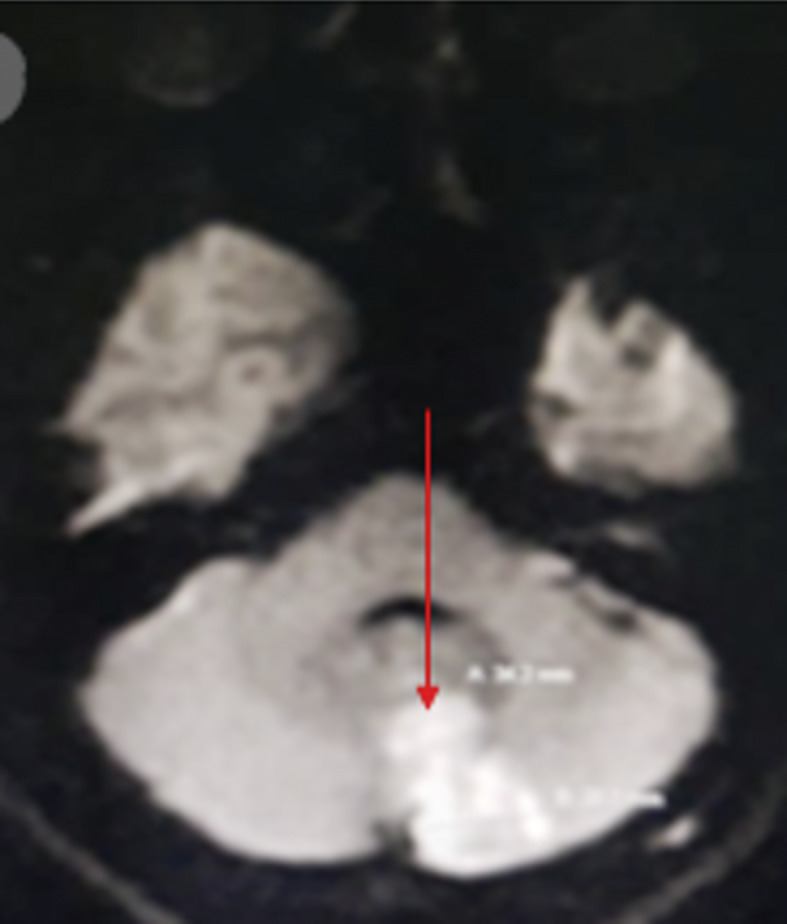
MRI brain coronal view showed left cerebellar infarction (red arrow).
Table 1.
Laboratory test results of the seven included cases
| Laboratory/case number | Reference range | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|---|
| WBCS | (4–10) × 103 cells/cmm3 | 18.27 | 13.01 | 13.55 | 16.6 | 8.13 | 11.83 | 12.86 |
| Neutrophils | (40–75%) | 81.6 | 62 | 80.8 | 70.1 | 87.5 | 83.6 | 91.3 |
| Lymphocytes | (20–45%) | 12.4 | 2.77 | 11.8 | 18.9 | 8.2 | 12.3 | 3.5 |
| Platelet count | (150–450) × 103 cells/cmm3 | 181 | 345 | 610 | 511 | 322 | 461 | 434 |
| RBC | (4.5–5.5) × 1012 cells/L | 5.53 | 5.01 | 3.56 | 6.14 | 4.86 | 3.9 | 3.02 |
| HB | (13–17) gm/dL | 15.8 | 15.5 | 11.1 | 16.8 | 14.8 | 10.6 | 8 |
| INR | (0.9–1.2) | 1.3 | 1.16 | 1 | 1 | 1.4 | 1.4 | 1.1 |
| D-dimer | (0–500) ng/mL | 4,705 | 1,656 | 1,402 | 2,401 | 1,505 | 6,828 | 2,303 |
| Ferritin | (15–235) ng/mL | 343.1 | 904 | 711 | 526.9 | 499 | 924 | 350 |
| ESR | (0–10) MM/HR | 27 | 136 | 57 | 25 | 119 | 66 | 25 |
| Creatinine | (60–106) Umol/L | 7 | 146 | 158 | 72.55 | 158 | 38 | 56 |
| LDH | (85–225) U/L | 461 | 227 | 570 | 219 | 280 | 180 | 200 |
| Glucose random | (4.1–11.1) mmol/L | 4.4 | 6.7 | 6.7 | 14.6 | 6.5 | 7.1 | 6.1 |
| APPT | (26–44) SEC. | 57.4 | 26 | 27.6 | 34.5 | 34.3 | 41.5 | 38.9 |
| CK | (0–190) U/L | 654 | 527 | 1,241 | 163 | 175 | 102 | 2,805 |
WBCs, white blood cells; RBCs, red blood cells; HB, hemoglobin; INR, international normalized ratio; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase; APPT, activated partial thromboplastin time; CK, creatine kinase.
Case Two
An 84-year-old bedbound male with a past medical history of ischemic stroke presented to the hospital due to ischemic cerebrovascular strokes. The patient was brought to the emergency room due to the sudden onset of a decreased level of consciousness associated with aphasia and right-side weakness. A person accompanying the patient provided a history of fever and cough a few days prior to the stroke symptoms. On examination, the patient was on mechanical ventilation and sedated. There were no signs of meningeal irritation. There was no motor movement in response to painful stimuli (quadriplegic) and a positive Parinaud sign with equivocal plantar response bilaterally. The brain CT scan showed age-related changes with suspicion of lacunar infarction at the brainstem and a hypodense lesion in the left cerebellum and left parieto-occipital area (Fig. 2). The stroke workup was normal including echocardiogram, lipid profile, HBA1C, carotid Doppler, ECG, and telemetry. In a PCR test for COVID-19, the patient returned a positive result, and his laboratory test results are presented in Table 1. The patient was diagnosed with ischemic stroke in setting of COVID-19 infection. He was admitted to the intensive care unit (ICU). He was treated with supportive care and medications, including anti-ischemic drugs and antiviral therapy, according to MOH protocols. On following, the patient died after 7 days.
Fig. 2.
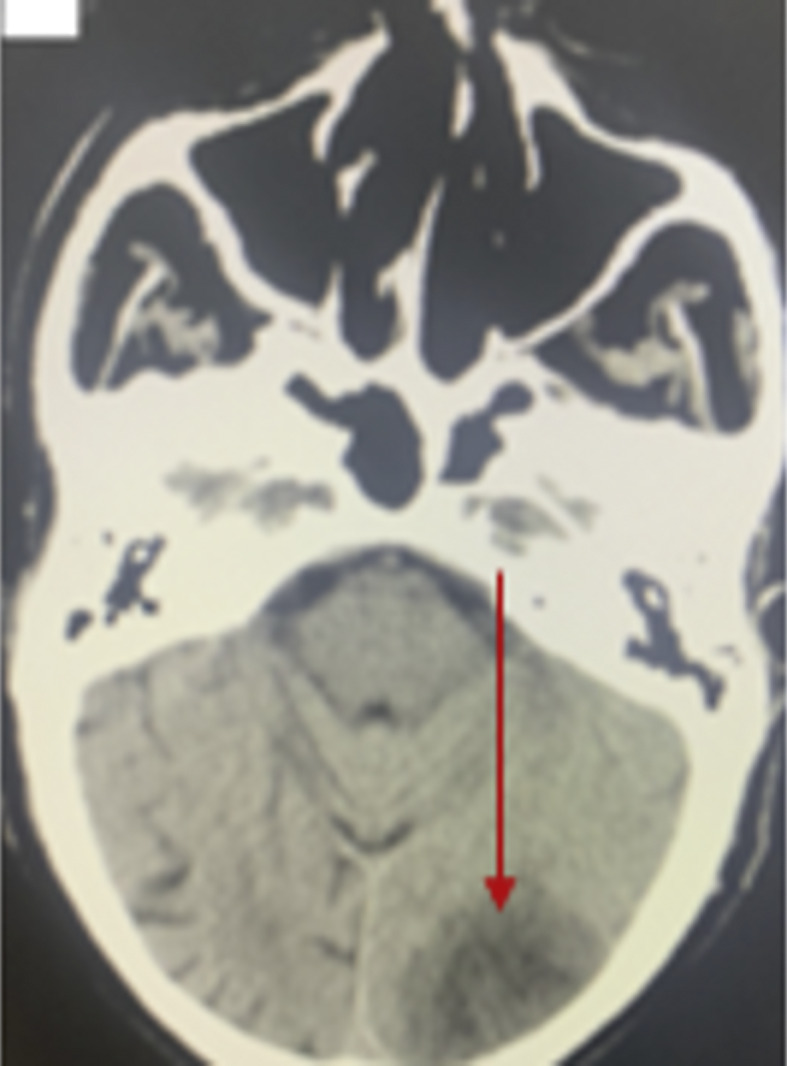
CT brain without contrast showed left parieto-occipital infarction (red arrow).
Case Three
A 45-year-old male patient with diabetes (controlled with treatment and last HbA1C = 5.7%) presented with a prolonged attack of generalized tonic-clonic seizures associated with upward rolling of both eyes, tongue biting, frothy mouth secretions, and loss of consciousness lasting for a few minutes. These fits were controlled with a loading dose of phenytoin. The patient’s routine laboratory test results were normal, including the serum glucose level and serum electrolytes. On examination, the patient was drowsy (postictal state) and had no fever or signs of meningeal irritation. There was quadriparesis, more so on the left side. The patient could slightly move his right side (grade 2 power), while his left side had grade 0 power, and there was a bilateral equivocal plantar response. This weakness was associated with a right-side deviation of the mouth. An urgent brain CT scan was performed and showed a right parietal hypodense area with old left subcortical lacunar infarction (Fig. 3). The stroke workup was negative for any cardioembolic or atherosclerotic source. COVID-19 screening by PCR test showed that the patient had COVID-19, and his laboratory test results are shown in Table 1. The patient was diagnosed with status epilepticus due to ischemic stroke and COVID-19 infection. For COVID-19 infection, remdesivir was administered as part of the patient’s medical management for his stroke. In the meantime, he continued his physical therapy at home after being discharged home after 6 days of hospital stay.
Fig. 3.
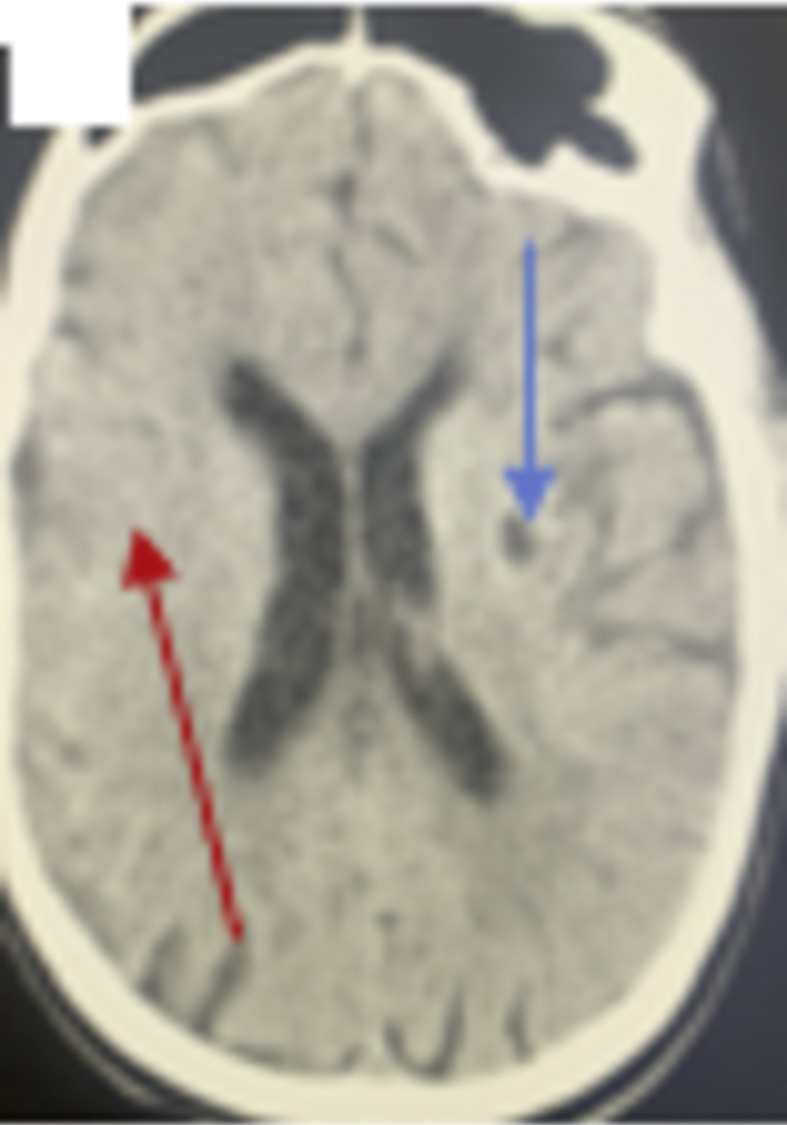
CT brain showed old left subcortical lacunar infarction with right-side effacement and ribboning of the greater sulcus (blue arrow) and small hypodense area at right parietal (red arrow).
Case Four
This is a case of a 36-year-old male patient with type 2 diabetes and hypertension, which were controlled with medication. The patient was diagnosed with COVID-19 4 days before the onset of neurological illness. The patient was referred by the COVID-19 clinic due to acute onset of left-side weakness. On examination, the patient was fully conscious, alert, oriented, and had no fever. He had dysarthria with a right-side deviation of the mouth angle. The patient also had left-side weakness in both the left upper and lower limbs (grade 0 power). There were no sensory or cerebellar affections. A brain CT scan showed an ill-defined hypodense area in the right temporoparietal lobe with cortical and subcortical distribution in the region of the right middle cerebral artery and signs of hyperdense vessels associated with effacement of related cortical sulci (Fig. 4). The patient’s laboratory test results are presented in Table 1. A routine stroke workup revealed elevated random blood sugar (RBS) but normal HBA1C and lipid profiles along with normal ECG and Holter results. On echocardiography, there were no signs of cardiac emboli, such as left ventricular thrombus or atrial myxomas. The patient was diagnosed with COVID-19 plus acute ischemic stroke. The patient was managed medically for his stroke and received remdesivir for COVID-19 infection. He was discharged home after 5 days of hospital stay and continued physical therapy at home.
Fig. 4.
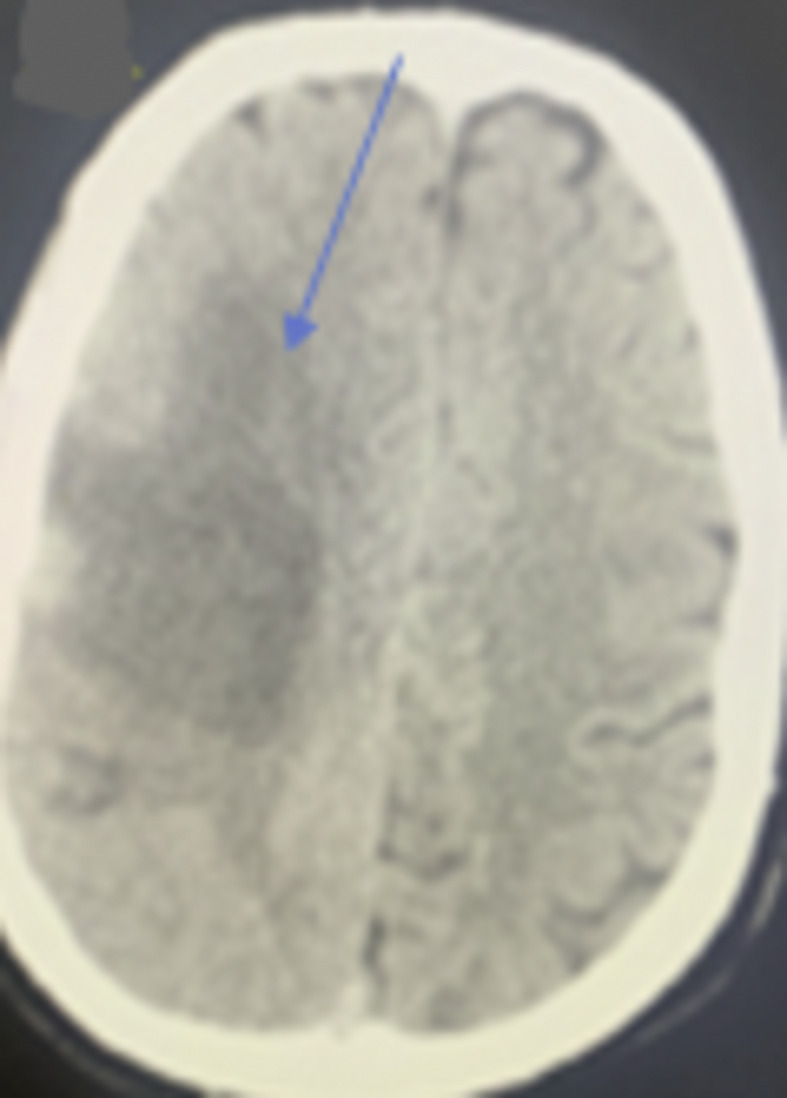
CT brain showed an ill-defined hypodense area in the right temporoparietal lobe with cortical and subcortical distribution in the region of the right middle cerebral artery and signs of hyperdense vessels associated with effacement of related cortical sulci (blue arrow).
Case Five
A 74-year-old male patient with controlled diabetes and hypertension was admitted 3 days prior to the onset of stroke symptoms due to typical chest pain, which was diagnosed as non-ST segment elevation myocardial infarction (NSTEMI), for further coronary angiography. The patient was also diagnosed with COVID-19 due to a positive PCR test result. He presented with slurred speech. On examination, he was confused, had mildly slurred speech, and had a slight deviation of the mouth angle to the right side. There were no signs of meningeal irritation, had normal motor strength, intact sensory responses, except for a bilateral equivocal plantar response. The brain MRI scan (Fig. 5) showed small left basal ganglia areas of restricted diffusion, denoting recent ischemic insult. The patient’s laboratory test results are presented in Table 1. As part of a routine stroke workup, the RBS was elevated, but the HBA1C and lipid profiles were normal, as well as the ECG and Holter results were normal. There were no signs of cardiac emboli, such as left ventricular thrombus or atrial myxomas, on echocardiography. The patient was diagnosed with COVID-19 with NSTEMI and ischemic infarction. He was admitted to the ICU. He was treated with medication, including anti-ischemic drugs and antiviral therapy, according to MOH protocols, and further carotid angiography was planned.
Fig. 5.
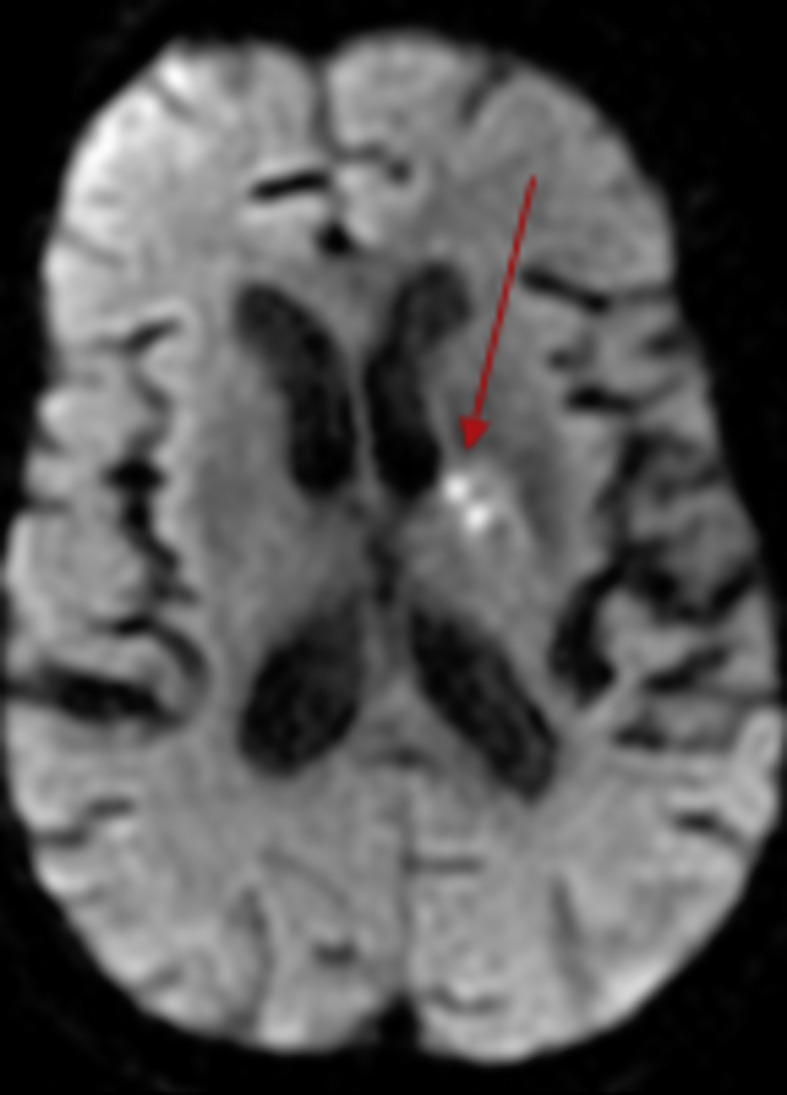
MRI brain showed small left basal ganglia areas of restricted diffusion, denoting recent ischemic insult (red arrow).
Case Six
A 54-year-old male patient with controlled hypertension presented with severe COVID-19 pneumonia, respiratory failure, and NSTEMI (carotid angiography was planned for a later date). Ten days later, the patient developed a sudden onset of slurred speech and confusion. On examination, the patient was confused and noncooperative and had incoherent speech. There was left-side lateralization in the form of decreased movement on the left side with a left extensor plantar response. The patient’s brain CT scan (Fig. 6) showed a right frontoparietal hypodense area with perifocal edema and a left hypodense area that indicated bilateral subacute ischemic stroke. The patient’s laboratory test results are presented in Table 1. Routine stroke testing revealed elevated RBS but normal HBA1C, lipids, ECG, and Holter results as well. An echocardiogram did not detect any cardiac emboli, such as left ventricular thrombuses or atrial myxomas. The patient was admitted to the ICU and treated with medication, including anti-ischemic drugs and antiviral therapy, according to MOH protocols.
Fig. 6.
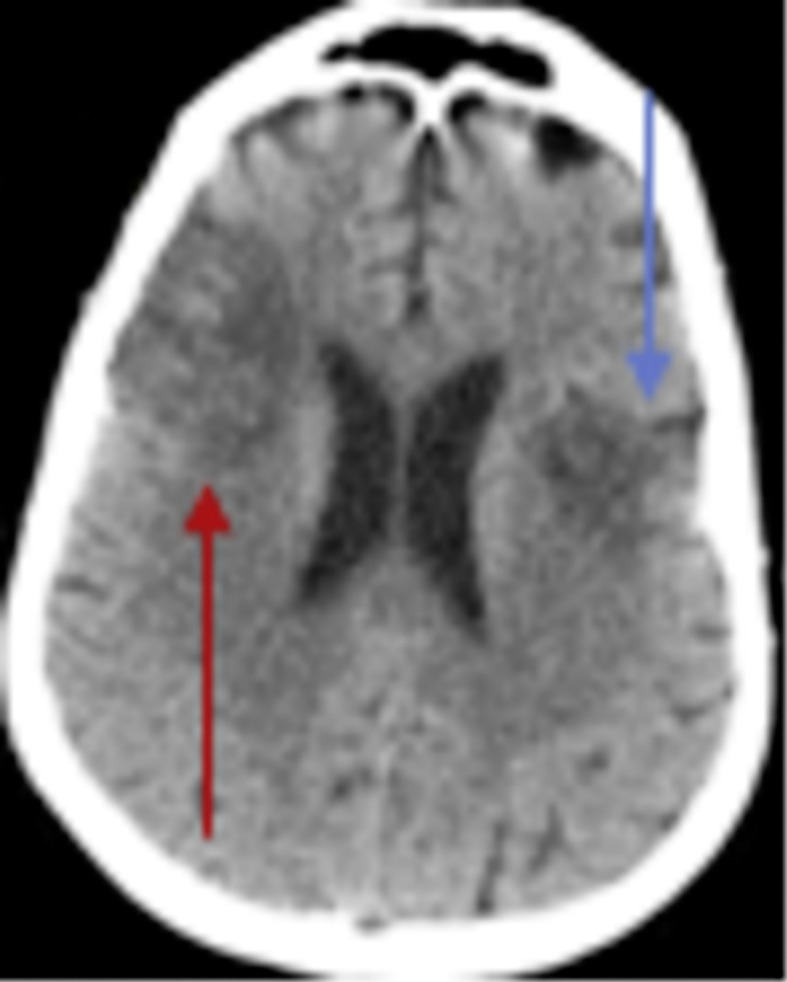
CT brain showed a right frontoparietal hypodense area with perifocal edema (red arrow) and a left hypodense area that indicated bilateral subacute ischemic stroke (blue arrow).
Case Seven
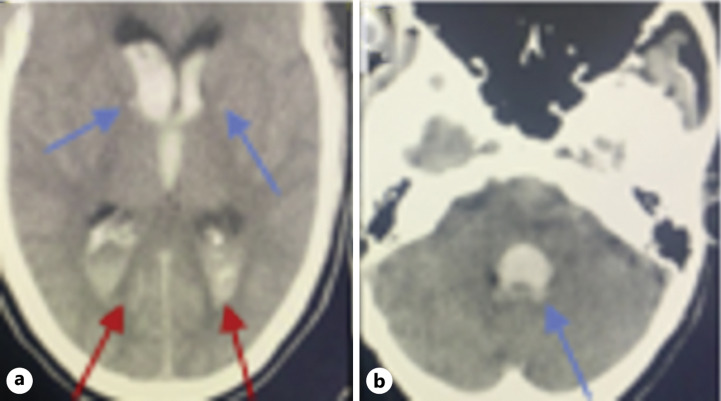
A 39-year-old male patient with no comorbid illnesses presented with an acute onset of a disturbed level of consciousness associated with a history of fever for several days. He had high blood pressure, a positive COVID-19 result, and a high temperature (39°C). On examination, the patient was deeply comatose with an unreactive pupillary reaction, a bilateral extensor plantar response, and no signs of meningeal irritation. A brain CT scan (Fig. 7a, b) showed intracerebral hemorrhage in the basal ganglia with intraventricular extension. The septic screen was negative, and the patient’s laboratory test results are presented in Table 1. Stroke workup revealed no cardioembolic or atherosclerotic sources. The computerized tomography angiography and diagnostic angiogram were normal with no aneurysm rupture or vascular malformation observed. The patient was medically managed with antihypertensives, dehydrating measures, and anti-COVID-19 measures; however, he died after 7 days of hospital stay.
Fig. 7.
Noncontrast CT brain showed (a) intracerebral hemorrhage in the basal ganglia (blue arrows) with lateral and third ventricle intraventricular extension (red arrows) (b) the fourth ventricle intraventricular extension (blue arrow).
Discussion
COVID-19 is a multisystem disorder with respiratory involvement being the most common. The CNS could be affected, and the patient can present with varying symptoms, e.g., encephalitis [14], headache, strokes, and smell and taste affection [2]. In a study of 58,104 COVID-19 patients, the prevalence of acute ischemic stroke was estimated to be 1.11% [15]. In this case series, neurological manifestations of the patients’ post-SARS-CoV-2 ischemic strokes were varied and included aphasia, hypesthesia, status epilepticus, and hemiparetic cerebral dysfunction. SARS-CoV-2 infection was likely the cause of these complications, given that the patients’ inflammation profiles included elevated white blood cells with a mean of 13.6 × 103 cells/cmm3 (6 out of 7 cases), high lactate dehydrogenase (3 out of 7 cases), and lymphopenia (all cases). Such profiles are common among COVID-19 patients [16] and frequently found in COVID-19-associated stroke [17]. Increases in other inflammatory biomarkers, such as ferritin and ESR, are also evidence of an inflammatory mechanism being involved in stroke events [18, 19].
A hypercoagulable state, indicated by a high D-dimer level, is evidence of a thrombotic event in ischemic stroke [18]. In this study, increased platelet levels in some cases (3 out of 7 cases) suggest the involvement of thrombocytosis and megakaryocyte-related thrombosis. This aligns with the findings of a study that reported megakaryocyte clusters and large megakaryocytes being present in the brain tissue of SARS-CoV-2 patients during autopsy [13]. These large cells can cause ischemic alteration and blood vessel occlusions, leading to ischemic events and neurological impairment [13].
Regarding the severity of the disease, 3 cases were severe (with two deaths) and 4 cases were moderate. The patients’ cerebral artery lesions were large artery infarctions, as diagnosed based on clinical and cranial CT and MRI findings (four anterior circulation infarctions, two posterior circulation infarctions, and one cerebral hemorrhage). These findings are consistent with those of Kihira et al. [20], who found that SARS-CoV-2 predominantly affects the large vessels of the circle of Willis, with large vessel occlusion present in 31.7% of COVID-19 patients and small vessel occlusion present in 5.9% of COVID-19 patients.
The majority of our patients (4 out of 7 cases) had preexisting cardiovascular risk factors. This finding is similar to that of Qureshi et al. [18], who reported that most of their COVID-19 patients who developed acute ischemic stroke had preexisting cardioembolic risk factors and cardiovascular risk factors for large vessel atherosclerosis and small vessel disease.
In another study, the prevalence of hemorrhagic stroke was 0.46% among 67,155 COVID-19 patients [15]. Here, 1 patient was found to have an intracerebral hemorrhage in the basal ganglia with intraventricular extension. This deep location is an interesting common feature shared by the majority of hemorrhagic conditions associated with COVID-19 [21]. Additionally, hemorrhage in the posterior circulation or intraventricular region is also frequently seen [21]. Collectively, these areas might be considered vulnerable to the selective vascular invasion exhibited by SARS-CoV-2 [21].
Despite the lack of clarity about the pathogenic mechanisms responsible for the cerebrovascular events associated with COVID-19, there are multiple mechanisms that are potentially involved. The most popular of these among the research community include vascular remodeling, direct or immune-mediated endothelial injury, impairment of physiological fibrinolysis, and hypercoagulability [22–27]. Understanding the transneural spread of SARS-CoV-2 is important for elucidating the putative mechanisms responsible for COVID-19 cerebrovascular manifestations. In fact, a quantitative analysis of several published case series, case reports, and clinical trials found that most patients from whom SARS-CoV-2 proteins/mRNA were isolated from their cerebrospinal fluid had symptoms suggestive of encephalitis or demyelinating diseases, and only a few had respiratory symptoms. Furthermore, these proteins were found in the brain, olfactory bulb/nerve or olfactory mucosa, brainstem, cerebellum, and cerebrum [28]. Given that multiple studies have reported that ACE2 is expressed in nerve terminals and that SARS-CoV-2 proteins have been found in cerebrospinal fluid samples, it is reasonable to assume that SARS-CoV-2 enters the CNS via binding to ACE2 [29–31]. SARS-CoV-2 causes intracerebral hemorrhage via its interaction with ACE2. SARS-CoV-2 binding to ACE2 on the surface of endothelial and arterial smooth muscle cells may damage cerebral arteries and cause arterial wall dissection or rupture with hemorrhage [32].
In addition, there is accumulating evidence that COVID-19 can elicit a multifactorial hypercoagulable state. Specifically, higher levels of D-dimer [33–36], fibrinogen, factor VIII [37], von Willebrand factor [38], and tissue factor [39] are associated with SARS-CoV-2 infection. One of the most popular theories for explaining the pathogenesis of COVID-19 is that there is an imbalance in the renin-angiotensin system [40, 41], which is also thought to play a significant role in disease severity [42]. Angiotensinogen II has pro-inflammatory and hypertrophic effects on the cerebral vasculature. Through its receptor AT1R, Ang II increases the expression of adhesion molecules, such as intercellular adhesion molecule 1 (ICAM-1), which promotes macrophage infiltration and inflammation in cerebral vessels [43]. It also induces a cytokine storm [44]. A study of eighty-eight (88) patients infected with SARS-CoV-2 (confirmed by PCR test) found high levels of IFN-γ, IL-6, IL-8, and MCP-1 in the patient’s serum samples [45]. An observational study of patients infected with SARS-CoV-2 showed high amounts of IL-1, IFN-γ, and MCP-1, reflecting the presence of a cytokine storm [46]. Increased levels of these cytokines lead to widespread vascular damage [47] and coagulation disorders [48]. It is also worth noting that decreased levels of calcitonin gene-related peptide in COVID-19 patients [49] may influence the occurrence of strokes since this peptide has a protective role against cerebral ischemia [50].
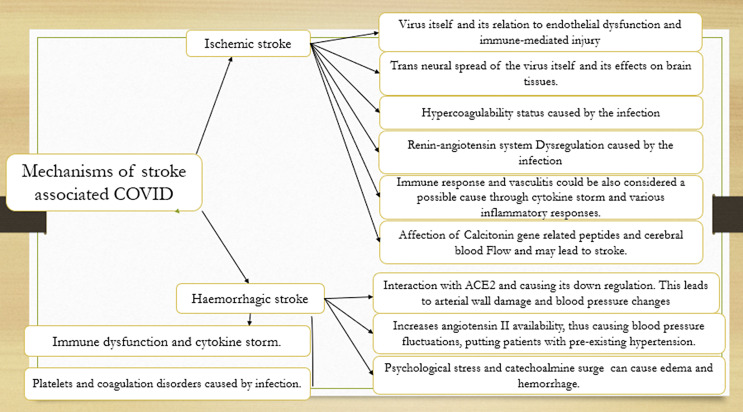
Finally, the COVID-19 pandemic has caused considerable anxiety and stress in individuals all over the world. Psychological distress has been demonstrated to have a strong positive association with stroke and myocardial infarction in men and women in a prospective study, despite adjustment for confounders [51]. Catecholamines seem to be involved in stress reactions [52], and catecholamine surges in pheochromocytoma patients can lead to endothelial injury, vasogenic edema, and hemorrhage [53](Fig. 8).
Fig. 8.
Possible mechanisms of stroke associated with COVID-19.
Conclusion
SARS-CoV-2 infection can cause various neurological problems, including strokes. Ischemic strokes are more frequently associated with SARS-CoV-2 infection than hemorrhagic strokes. Early diagnosis and specific management may improve the final prognosis and outcomes. Further research is needed to determine the exact sequence of events that lead to cerebrovascular manifestations of SARS-CoV-2 infection.
Statement of Ethics
The article describes a case series. Therefore, no additional permission from our Ethics Committee was required. Ethical approval is not required for this study in accordance with local or national guidelines. Written informed consent was obtained from the patient’s next of kin for publication of the details of their medical case and any accompanying images (Cases 2 and 7). Written informed consent was obtained from patients (Cases 1, 3, 4, 5, 6) for publication of the details of their medical case and accompanying images.
Conflict of Interest Statement
The authors declare that they have no competing interests.
Funding Sources
Open cccess funding was provided by the Qatar National Library; no other funding received.
Author Contributions
Mostafa Mahmoud Meshref, Ibrahim M. Hewila, Yahia Khlidj, Rafik Korissi, Nour Shaheen, Abdulqadir J. Nashwan, Yassamine Ouerdane, Yara Amro, Khaled M. Taher, and Mahmoud Galal Ahmed: data collection, literature search, and manuscript preparation. All authors read and approved the final manuscript.
Funding Statement
Open cccess funding was provided by the Qatar National Library; no other funding received.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Supplementary Material
References
- 1. Li X, Zai J, Zhao Q, Nie Q, Li Y, Foley BT, et al. Evolutionary history, potential intermediate animal host, and cross-species analyses of SARS-CoV-2. J Med Virol. 2020 Jun 11;92(6):602–11. 10.1002/jmv.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Montalvan V, Lee J, Bueso T, De Toledo J, Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. 2020 Jul;194:105921. 10.1016/j.clineuro.2020.105921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meshref M, A Alshammari H, Khairat SM, Khan R, Khan I. Guillain-barre syndrome associated with COVID-19 infection: a case report with review of literature. Cureus. 2021 Feb 3;13(2):e13096. 10.7759/cureus.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tan YK, Goh C, Leow AST, Tambyah PA, Ang A, Yap ES, et al. COVID-19 and ischemic stroke: a systematic review and meta-summary of the literature. J Thromb Thrombolysis. 2020 Oct 13;50(3):587–95. 10.1007/s11239-020-02228-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 Jun 1;77(6):683. 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, et al. Large-vessel stroke as a presenting feature of covid-19 in the young. N Engl J Med. 2020 May 14;382(20):e60. 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020 Jul;191:145–7. 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morassi M, Bagatto D, Cobelli M, D’Agostini S, Gigli GL, Bnà C, et al. Stroke in patients with SARS-CoV-2 infection: case series. J Neurol. 2020 Aug 20;267(8):2185–92. 10.1007/s00415-020-09885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020 May;395(10234):1417–8. 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and antiphospholipid antibodies in patients with covid-19. N Engl J Med. 2020 Apr 23;382(17):e38. 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020 Jul 24;18(7):1738–42. 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li Y, Li M, Wang M, Zhou Y, Chang J, Xian Y, et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. 2020 Sep;5(3):279–84. 10.1136/svn-2020-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nauen DW, Hooper JE, Stewart CM, Solomon IH. Assessing brain capillaries in coronavirus disease 2019. JAMA Neurol. 2021 Feb 12;78(6):760–2. 10.1001/jamaneurol.2021.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meshref M, Hewila IM, Abdel Mageed S, Morra ME. COVID-19 associated with encephalitis: case report and review of literature. Neurologist. 2021 Nov;26(6):268–70. 10.1097/NRL.0000000000000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Syahrul S, Maliga HA, Ilmawan M, Fahriani M, Mamada SS, Fajar JK, et al. Hemorrhagic and ischemic stroke in patients with coronavirus disease 2019: incidence, risk factors, and pathogenesis: a systematic review and meta-analysis. F1000Res. 2021 Jan;10:34. 10.12688/f1000research.42308.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao Q, Meng M, Kumar R, Wu Y, Huang J, Deng Y, et al. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a systemic review and meta-analysis. Int J Infect Dis. 2020 Jul;96:131–5. 10.1016/j.ijid.2020.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Katz JM, Libman RB, Wang JJ, Filippi CG, Sanelli P, Zlochower A, et al. COVID-19 severity and stroke: correlation of imaging and laboratory markers. AJNR Am J Neuroradiol. 2021 Feb;42(2):257–61. 10.3174/ajnr.A6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qureshi AI, Baskett WI, Huang W, Shyu D, Myers D, Raju M, et al. Acute ischemic stroke and COVID-19: an analysis of 27,676 patients. Stroke. 2021 Mar;52(3):905–12. 10.1161/STROKEAHA.120.031786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kappert K, Jahić A, Tauber R. Assessment of serum ferritin as a biomarker in COVID-19: bystander or participant? Insights by comparison with other infectious and non-infectious diseases. Biomarkers. 2020 Nov;25(8):616–25. 10.1080/1354750X.2020.1797880. [DOI] [PubMed] [Google Scholar]
- 20. Kihira S, Schefflein J, Mahmoudi K, Rigney B, N Delman B, Mocco J, et al. Association of coronavirus disease (COVID-19) with large vessel occlusion strokes: a case-control study. AJR Am J Roentgenol. 2021 Jan;216(1):150–6. 10.2214/AJR.20.23847. [DOI] [PubMed] [Google Scholar]
- 21. Mishra S, Choueka M, Wang Q, Hu C, Visone S, Silver M, et al. Intracranial hemorrhage in COVID-19 patients. J Stroke Cerebrovasc Dis. 2021 Apr;30(4):105603. 10.1016/j.jstrokecerebrovasdis.2021.105603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler Thromb Vasc Biol. 2004 Jun;24(6):1015–22. 10.1161/01.ATV.0000130465.23430.74. [DOI] [PubMed] [Google Scholar]
- 23. Ramadan SM, Kasfiki EV, Kelly CW, Ali I. An interesting case of small vessel pathology following coronavirus infection. BMJ Case Rep. 2020 Sep;13(9):e237407. 10.1136/bcr-2020-237407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kapiotis S, Besemer J, Bevec D, Valent P, Bettelheim P, Lechner K, et al. Interleukin-4 counteracts pyrogen-induced downregulation of thrombomodulin in cultured human vascular endothelial cells. Blood. 1991 Jul;78(2):410–5. 10.1182/blood.v78.2.410.bloodjournal782410. [DOI] [PubMed] [Google Scholar]
- 25. Moore KL, Esmon CT, Esmon NL. Tumor necrosis factor leads to the internalization and degradation of thrombomodulin from the surface of bovine aortic endothelial cells in culture. Blood. 1989 Jan;73(1):159–65. 10.1182/blood.v73.1.159.bloodjournal731159. [DOI] [PubMed] [Google Scholar]
- 26. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529–39. 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M, et al. Immunology of COVID-19: current state of the science. Immunity. 2020 Jun;52(6):910–41. 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li Y, Zhang Y, Tan B. What can cerebrospinal fluid testing and brain autopsies tell us about viral neuroinvasion of SARS-CoV-2. J Med Virol. 2021 Mar;93(7):4247–57. 10.1016/jmv.26943-jmv.26943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu J, Lazartigues E. Expression of ACE2 in human neurons supports the neuro-invasive potential of COVID-19 virus. Cell Mol Neurobiol. 2022;42(1):305–9. 10.1007/s10571-020-00915-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020 Mar;323(18):1843–4. 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Serrano GE, Walker JE, Arce R, Glass MJ, Vargas D, Sue LI, et al. Mapping of SARS-CoV-2 brain invasion and histopathology in COVID-19 disease. medRxiv. 2021 Feb. [Google Scholar]
- 32. Vogrig A, Gigli GL, Bnà C, Morassi M. Stroke in patients with COVID-19: clinical and neuroimaging characteristics. Neurosci Lett. 2021 Jan;743:135564. 10.1016/j.neulet.2020.135564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar;395(10229):1054–62. 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 Apr 30;382(18):1708–20. 10.1056/nejmoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jin X, Duan Y, Bao T, Gu J, Chen Y, Li Y, et al. The values of coagulation function in COVID-19 patients. PLoS One. 2020 Oct;15(10):e0241329. 10.1371/journal.pone.0241329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020 Jun;7(6):e438–40. 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martín-Rojas RM, Pérez-Rus G, Delgado-Pinos VE, Domingo-González A, Regalado-Artamendi I, Alba-Urdiales N, et al. COVID‐19 coagulopathy: an in-depth analysis of the coagulation system. Eur J Haematol. 2020 Dec;105(6):741–50. 10.1111/ejh.13501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mancini I, Baronciani L, Artoni A, Colpani P, Biganzoli M, Cozzi G, et al. The ADAMTS13-von Willebrand factor axis in COVID-19 patients. J Thromb Haemost. 2021 Feb;19(2):513–21. 10.1111/jth.15191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rosell A, Havervall S, von Meijenfeldt F, Hisada Y, Aguilera K, Grover SP, et al. Patients with COVID-19 have elevated levels of circulating extracellular vesicle tissue factor activity that is associated with severity and mortality: brief report. Arterioscler Thromb Vasc Biol. 2021 Feb;41(2):878–82. 10.1161/ATVBAHA.120.315547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Braga CL, Silva‐Aguiar RP, Battaglini D, Peruchetti DB, Robba C, Pelosi P, et al. The renin–angiotensin–aldosterone system: role in pathogenesis and potential therapeutic target in COVID-19. Pharmacol Res Perspect. 2020 Aug;8(4):e00623. 10.1002/prp2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sparks MA, South AM, Badley AD, Baker-Smith CM, Batlle D, Bozkurt B, et al. Severe acute respiratory syndrome coronavirus 2, COVID-19, and the renin-angiotensin system: pressing needs and best research practices. Hypertension. 2020 Nov;76(5):1350–67. 10.1161/HYPERTENSIONAHA.120.15948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mehrabadi ME, Hemmati R, Tashakor A, Homaei A, Yousefzadeh M, Hemati K, et al. Induced dysregulation of ACE2 by SARS-CoV-2 plays a key role in COVID-19 severity. Biomed Pharmacother. 2021 May;137:111363. 10.1016/j.biopha.2021.111363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ando H, Zhou J, Macova M, Imboden H, Saavedra JM. Angiotensin II AT 1 receptor blockade reverses pathological hypertrophy and inflammation in brain microvessels of spontaneously hypertensive rats. Stroke. 2004 Jul;35(7):1726–31. 10.1161/01.STR.0000129788.26346.18. [DOI] [PubMed] [Google Scholar]
- 44. Mahmudpour M, Roozbeh J, Keshavarz M, Farrokhi S, Nabipour I. COVID-19 cytokine storm: the anger of inflammation. Cytokine. 2020 Sep;133:155151. 10.1016/j.cyto.2020.155151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huang KJ, Su IJ, Theron M, Wu YC, Lai SK, Liu CC, et al. An interferon-γ-related cytokine storm in SARS patients. J Med Virol. 2005;75(2):185–94. 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb;395(10223):497–506. 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bhaskar S, Sinha A, Banach M, Mittoo S, Weissert R, Kass JS, et al. Cytokine storm in COVID-19-immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM consortium position paper. Front Immunol. 2020;11:1648. 10.3389/fimmu.2020.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Levi M, van der Poll T. Coagulation and sepsis. Thromb Res. 2017 Jan;149:38–44. 10.1016/j.thromres.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 49. Ochoa-Callejero L, García-Sanmartín J, Villoslada-Blanco P, Íñiguez M, Pérez-Matute P, Pujadas E, et al. Response to letter to the editor from abobaker and darrat: “circulating levels of calcitonin gene-related peptide are lower in COVID-19 patients”. J Endocr Soc. 2021 Mar;5(10):bvab053. 10.1210/jendso/bvab053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Du Z, Zhang H, Chen Q, Gao Y, Sun B. Intranasal calcitonin gene-related peptide protects against focal cerebral ischemic injury in rats through the wnt/β-catenin pathway. Med Sci Monit. 2018 Dec;24:8860–9. 10.12659/MSM.913777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jackson CA, Sudlow CLM, Mishra GD. Psychological distress and risk of myocardial infarction and stroke in the 45 and up study. Circ Cardiovasc Qual Outcomes. 2018;11(9):e004500. 10.1161/CIRCOUTCOMES.117.004500. [DOI] [PubMed] [Google Scholar]
- 52. Lader M. The peripheral and central role of the catecholamines in the mechanisms of anxiety. Int Pharmacopsychiatry. 1974;9(3):125–37. 10.1159/000468126. [DOI] [PubMed] [Google Scholar]
- 53. Madhok J, Kloosterboer A, Venkatasubramanian C, Mihm FG. Catecholamine-induced cerebral vasospasm and multifocal infarctions in pheochromocytoma. Endocrinol Diabetes Metab Case Rep. 2020 Aug;2020:20-0078. 10.1530/EDM-20-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.