Abstract
Background
Peripherally inserted central catheters (PICCs) and midlines are commonly used devices for reliable vascular access. Infection and thrombosis are the main adverse effects of these catheters. We aimed to evaluate the relative risk of complications from midlines and PICCs.
Methods
We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) and observational studies. The primary outcomes were catheter-related bloodstream infection (CRBSI) and thrombosis. Secondary outcomes evaluated included mortality, failure to complete therapy, catheter occlusion, phlebitis, and catheter fracture. The certainty of evidence was assessed using the GRADE approach.
Results
Of 8368 citations identified, 20 studies met the eligibility criteria, including 1 RCT and 19 observational studies. Midline use was associated with fewer patients with CRBSI compared with PICCs (odds ratio [OR], 0.24; 95% CI, 0.15–0.38). This association was not observed when we evaluated risk per catheter. No significant association was found between catheters when evaluating risk of localized thrombosis and pulmonary embolism. A subgroup analysis based on location of thrombosis showed higher rates of superficial venous thrombosis in patients using midlines (OR, 2.30; 95% CI, 1.48–3.57). We did not identify any significant difference between midlines and PICCs for the secondary outcomes.
Conclusions
Our findings suggest that patients who use midlines might experience fewer CRBSIs than those who use PICCs. However, the use of midline catheters was associated with greater risk of superficial vein thrombosis. These findings can help guide future cost-benefit analyses and direct comparative RCTs to further characterize the efficacy and risks of PICCs vs midline catheters.
Keywords: PICC, catheter, infection, midline, thrombosis
PICC and midlines are commonly used devices in hospitalized patients. We performed a systematic review and meta-analysis to evaluate the complications of the use of midline catheter vs. PICC, focusing on catheter-related bloodstream infection and thrombosi
Peripherally inserted central catheters (PICCs) have become ubiquitous in the care of hospitalized patients. These lines are associated with low insertion risk and low rates of complications and allow for durable outpatient intravenous (IV) access, thereby facilitating timely dismissal of patients requiring prolonged IV infusions and frequent blood draws [1]. A systematic review by Chopra and colleagues in 2013 demonstrated a lower risk of central line–associated bloodstream infection (CLABSI) with PICC lines when compared with central venous catheters (CVCs) [2]. The convenience of these devices, however, has led to misuse and overuse, including the utilization of PICC lines in patients with reliable peripheral access and no need for centrally administered medications. The increasing use of PICCs has made the drawbacks of these devices obvious; long-term lines are associated with thrombotic events and risk of luminal occlusion and pose risk of infection similar to other CVCs [2–4].
The midline catheter has emerged as an alternative to PICC lines. It is a shorter catheter inserted into the arm, like a PICC, but terminating at the basilic or axillary vein rather than the central venous circulation. These devices provide the benefit of durable access, but with shorter length and lower surface area, reducing the theoretical risk of thrombosis and contamination, as well as possibly lowering rates of infection [5]. Midline catheters have a shorter indwell time (up to 4 weeks) compared with PICC lines (weeks to months) but are considerably more durable than peripheral IVs. They represent an attractive option for short- to medium-term venous access in the inpatient and outpatient settings and are preferred for this indication by the Michigan Appropriateness Guide for Intravenous Catheters (MAGIC) [6, 7].
As midline catheters are not considered CVCs, their infection rates are not routinely reported as part of CLABSI metrics [8]. Increased use of midline catheters should lead to a reduction in reported CLABSIs, but it is unclear whether this is attributable to a true decrease in the risk of infection vs underreporting of metrics. The increasing use of midline catheters makes it important to critically assess complications of these devices and ensure that these apparent improvements are not artifacts of definitions [7, 9–11].
Despite emerging data regarding the efficacy of these devices, there is limited evidence comparing device outcomes and risks, specifically the risk of catheter-related bloodstream infection (CRBSI) and thrombosis. For this reason, we conducted a systematic review to evaluate the relative risk of complications from midline catheters and PICC lines.
METHODS
This review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statements. The study protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO ID: 42018088270).
Literature Search
We searched Embase, Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE, Cochrane Central Registrar of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus from inception to June 24, 2022. The literature search strategy was developed by an experienced medical librarian with input from the senior researchers. The detailed search strategy is available in Supplementary Table 1.
Study Selection
Eligible studies (1) were randomized controlled trials (RCTs) and comparative observational studies; (2) included adult patients (≥18 years old) requiring venous access using PICC lines or midline catheters for >24 hours; and (3) were published in English. We excluded studies with >50% of patients on chemotherapy, total parenteral nutrition, or dialysis in any group (PICC or midline). Other comparators, such as port devices, implanted devices, tunnel catheters, dialysis catheters, short-term CVCs, femoral catheters, internal jugular catheters, Hickman catheters, and palindrome catheters, were not considered for outcome analysis.
Titles and abstracts of all citations were screened in pairs. Studies included by either reviewer were retrieved for full-text screening. Independent reviewers, working in overlapping duplicates, screened the full-text version of eligible studies. In this phase, any disagreements between the reviewers were harmonized by a third senior investigator. These activities were conducted using the online software DistillerSR (Evidence Partners, Ottawa, ON, Canada).
Data Extraction and Quality Assessment
A standardized data extraction form was developed to extract relevant study characteristics and outcome data. Reviewers worked independently to extract study data. We assessed the risk of bias of the included RCTs using the Cochrane Collaboration's Risk of Bias 2 tool, which evaluates the randomization process, deviations from the intended interventions, missing outcome data, measurement of the outcome, and selection of the reported results [12]. For observational studies, we adopted selected items from the Newcastle-Ottawa Scale assessing selection, comparability, outcome [13].
The certainty of evidence was graded using the GRADE approach, categorized as “high,” “moderate,” “low,” and “very low.” RCTs without important limitations provide high-quality evidence, and observational studies without special strengths or important limitations provide low-quality evidence. Factors that downrate the quality of evidence are risk of bias, inconsistency, indirectness, imprecision, and publication bias. The process of rating followed the GRADE handbook [14], and the evidence profile table was generated using the software GRADEpro GDT [15].
The 2 primary outcomes were CLABSI (in the case of PICCs) or CRBSI (in the case of midlines) and thrombosis. We also evaluated mortality, failure to complete therapy, catheter occlusion, phlebitis, and catheter fracture. Outcomes were reported and analyzed per patient or per catheter in the individual studies because they are considered different units of analysis (ie, 1 patient could have had >1 catheter in a study). The reported definitions of the primary outcomes were provided by each individual study (Supplementary Table 2 ). The authors judged whether a given set of studies could be pooled in the outcome analysis based on these definitions.
Data Synthesis and Analysis
All statistical analyses were based on the “intention-to-treat” (ITT) principle for RCTs and on the number of patients who received the intervention at the beginning of the study for observational studies. For this study, the ITT principle considered all randomized participants. We calculated odds ratios (ORs) for binary outcomes. Meta-analyses were conducted using the random-effects model. We evaluated statistical heterogeneity between studies using the I2 estimate, where values closer to 100% represent considerable statistical heterogeneity [16]. Additionally, we conducted subgroup analyses based on thrombosis localization: deep venous thrombosis (DVT) or superficial venous thrombosis (SVT). A 2-sided P value of <.05 was deemed statistically significant. We estimated the optimal information size (OIS) of the main outcomes using 0.05 for type 1 error (α). We repeated the analysis using fixed values for type 2 error (β) of 80%, 85%, and 90%. The control event rates were calculated, and the minimally important clinical differences were set at 0.5%, 1%, and 1.5%.
Analysis was performed using OpenMeta analysis software and R (4.2.0).
RESULTS
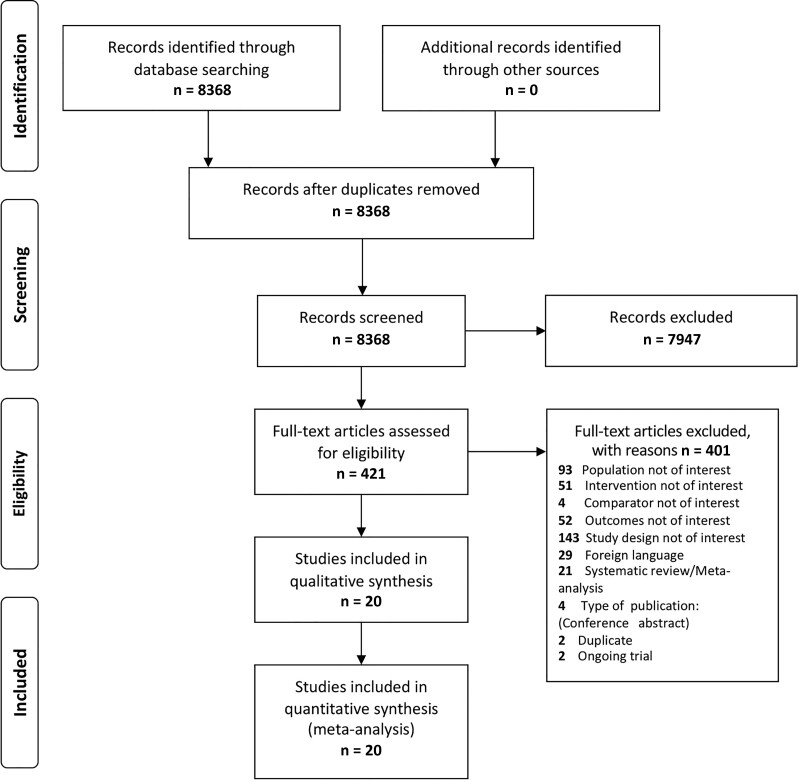
The literature search identified 8368 records. A total of 7947 studies were excluded at the title or abstract screening level, and 421 were eligible for full-text review, of which 20 were selected for inclusion in the analysis. The flow diagram of the systematic literature review is illustrated in Figure 1.
Figure 1.
Selection of trials for inclusion in the review and meta-analysis.
Study Characteristics
We included 1 RCT [7] and 19 observational studies [9–11, 17–32] published between 1997 and 2022. Twelve studies were conducted in the United States, 1 in the United States and Canada, 2 in the United Kingdom, 2 in Australia, 2 in Italy, and 1 in Korea. Thirteen studies were conducted in an inpatient setting, 6 in an outpatient setting, and 1 in a palliative care unit. Additional information and characteristics of each study are presented in Table 1. Risk of bias assessment is presented in Supplementary Tables 3 and 4. GRADE evidence profile is depicted in Supplementary Table 5.
Table 1.
Study Characteristics
| Author, y | Study Design, Country | Setting | Intervention | Population Characteristics | Device Characteristic |
|---|---|---|---|---|---|
| Bahl, 2019 [9] | Retrospective cohort, USA | Inpatient (noncritical) | Midline | 1094 patients aged 63.70 ± 17.80 y; 65.50% female | Place of insertion: 59.80% basilic, 32.60% brachial, 6.50% cephalic, 1.10% other; 36.20% antibiotic therapy; 52.30% poor vascular access; 0% TPN; 11.50% other |
| PICC | 1483 patients aged 63.70 ± 16.90 y; 47.50% female | Place of insertion: 69.80% basilic, 26.60% brachial, 2.60% cephalic, 1% other; 62.40% antibiotic therapy; 6.50% poor vascular access; 12.80% TPN; 18.20% other | |||
| Barr, 2012 [17] | Retrospective cohort, United Kingdom | Outpatient | Midline | 648 catheters | 100% antibiotic therapy |
| PICC | 43 catheters | 100% antibiotic therapy | |||
| Bing, 2022 [29] | Retrospective cohort, USA | Inpatient and ICU | Midline | 1772 patients (2049 catheters) aged 57 ± 17.7 y; 54% female; race: Hispanic 2%, non-Hispanic 98% | NA |
| PICC | 1636 patients (2502 catheters) aged 57 ± 16.29 y; 51% female; Hispanic 2%, non-Hispanic 98% | NA | |||
| Caparas, 2014 [7] | RCT, USA | Inpatient (noncritical) | Midline | 29 patients (30 catheters) aged 72 y; 69% female | 100% basilic, brachial, and cephalic; catheter dwell time 5.80 ± 2.75 d; 100% antibiotic therapy |
| PICC | 25 patients (28 catheters) aged 69 y; 48% female | 100% basilic, brachial, and cephalic; catheter dwell time 6.30 ± 6 d; 100% antibiotic therapy | |||
| Caserta, 2022 [30] | Retrospective cohort, Italy | ICU | Midline | 42 patients (42 catheters; midline) | 34% antibiotic therapy; 66% poor vascular access; 0% TPN |
| PICC | 61 patients (61 catheters; PICC); total population: aged 74 ± 10 y; 53% female | 58.50% antibiotic therapy; 54.70% poor vascular access; 26.80% TPN | |||
| Dickson, 2019 [18] | Retrospective cohort, Australia | Outpatient | Midline | 38 patients; 31.60% female | Catheter dwell time 25.20 ± 17.90 d; 100% antibiotic therapy |
| PICC | 33 patients; 45.50% female | Catheter dwell time 35.40 ± 28.01 d; 100% antibiotic therapy | |||
| Khalidi, 2009 [19] | Prospective cohort, USA | Inpatient (noncritical) | Midline | 44 patients (midline) 116 patients (PICC); total population: 43.10% female; race: 75.60% White, 13.10% African American, 11.30% other | Place of insertion: 54.40% basilic, 24.40% cephalic, 20.60% other; 82% antibiotic therapy, 18% other |
| PICC | |||||
| Kim, 2022 [31] | Retrospective cohort, Korea | Inpatient (noncritical) | Midline | 20 patients (20 catheters) aged 57 ± 18.40 y; 40% female | Catheter dwell time 15.10 ± 18.75 d; 65% poor vascular access; 10% TPN; 25% other |
| PICC | 10 patients (10 catheters) aged 67.7 ± 20.70 y; 70% female | Catheter dwell time 28.70 ± 38.50 d; 70% poor vascular access; 20% TPN; 10% other | |||
| Lescinskas, 2020 [20] | Prospective cohort, USA | Inpatient (noncritical) | Midline | 58 patients aged 49.10 ± 12.90 y; 62% female; race: 48% White, 46% African American, 40% Asian, American Indian, Hispanic, and other | 12% antibiotic therapy; 52% poor vascular access; 28% other |
| PICC | 63 patients aged 45.50 ± 13.90 y; 27% female; race: 68.30% White, 28.60% African American, 58.70% Asian, American Indian, Hispanic, and other | 11.10% antibiotic therapy; 11.10% poor vascular access; 12.70% other | |||
| Magnani, 2019 [21] | Prospective cohort, Italy | Palliative care unit | Midlinea | 8 patients (midline), 24 patients (PICC); total population: aged 73 ± 13 y; 47.80% female | Place of insertion: 87.50% basilic, 12.50% brachial, 0% cephalic |
| PICCa | Place of insertion: 75% basilic, 25% brachial, 0% cephalic | ||||
| Moureau, 2002 [22] | Retrospective cohort, USA | Outpatient | Midline | 5397 patients (5423 catheters); 48% female | NA |
| PICC | 25 590 patients (25 707 catheters); 45% female | ||||
| Mushtaq, 2018 [23] | Retrospective cohort, USA | Inpatient and ICU | Midline | 411 patients aged 58.79 ± 17.72 y; 55.20% female | 19.40% antibiotic therapy; 76.60% poor vascular access; 0% TPN; 2.90% other |
| PICCb | 282 patients aged 56.62 ± 17.76 y; 45.70% female | 23.70% antibiotic therapy; 48.90% poor vascular access; 1.70% TPN; 25.10% other | |||
| Sargent, 1997 [24] | Retrospective cohort, United Kingdom | Inpatient (noncritical) | Midline | 12 catheters | Catheter dwell time 7 d |
| PICC | 18 catheters | Catheter dwell time 21 d | |||
| Seo, 2019 [31] | Retrospective cohort, USA | Outpatient | Midline | 82 patients aged 66.39 ± 21.23 y; 43.90% female | Place of insertion: 29.30% basilic, 22% brachial, 24.40% cephalic; catheter dwell time 10.65 ± 7.43 d |
| PICC | 50 patients aged 61 ± 20 y; 60% female | Place of insertion: 66% basilic, 30% brachial, 4% cephalic; catheter dwell time 29 ± 15.50 d | |||
| Sharp, 2014 [11] | Retrospective cohort, Australia | Outpatient | Midline | 231 patients; 44% female | NA |
| PICC | 97 patients; 39% female | ||||
| Swaminathan, 2022 [32] | Retrospective cohort, USA | Inpatient and ICU | Midline | 5105 patients aged 64.80 ± 16.81 y; 58.20% female; race: 56.40% White, 39.20% African American, 0.60% Asian | Place of insertion: 48.70% basilic, 37.10% brachial, 14.20% other; catheter dwell time 6 ± 6.66 d; 40.10% antibiotic therapy; 72.40% poor vascular access; 0.10% TPN; 1.30% other |
| PICC | 5758 patients aged 64.90 ± 16 y; 48.10% female; race: 75.90% White, 18.50% African American, 0.50% Asian | Place of insertion: 60.60% basilic, 32.50% brachial, 6.80% other; catheter dwell time 14 ± 14.81 d; 71.20% antibiotic therapy; 40.10% poor vascular access; 3.80% TPN; 5.50% other | |||
| Tokars, 1999 [26] | Prospective cohort, USA and Canada | Outpatient | Midlinec | 155 catheters (midline), 324 catheters (PICC); total population: aged 52 y; 41.50% female | Total population: 67.50% antibiotic therapy; 10.20% TPN; 4.20% chemotherapy |
| PICCc | |||||
| Tso, 2017 [27] | Retrospective cohort, USA | Inpatient (noncritical) | Midline | 100 catheters (midline), 205 catheters (PICC); total population: aged 38 ± 12.16 y; 74% female | NA |
| PICC | |||||
| Vanek, 1997 [28] | Case series, USA | Inpatient (noncritical) | Midline | 2169 catheters | NA |
| PICC | 61 catheters | ||||
| Xu, 2016 [10] | Retrospective cohort, USA | Inpatient and ICU | Midline | 172 patients (200 catheters) aged 62.50 ± 13.16 y; 54.70% female | 0% antibiotic therapy; 97% poor vascular access |
| PICC | 185 patients (206 catheters) aged 60 ± 11.33 y; 38.40% female | 63.60% antibiotic therapy; 35.40% poor vascular access |
Abbreviations: CVC, central venous catheter; ICU, intensive care unit; NA, not available; PICC, peripherally inserted central catheter; RCT, randomized controlled trial; TPN, total parenteral nutrition.
Total population includes patients with midline, PICC, and “short” midline insertion.
Included patients with CVC lines (peripherally inserted central catheter, internal jugular, subclavian, or femoral).
These numbers belong to the total population (n = 827), not only PICC and midline patients.
Primary Outcomes: CLABSI/CRBSI and Thrombosis
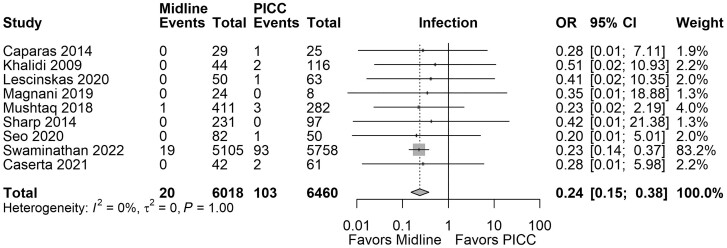
Sixteen studies reported the number of patients with CLABSI and/or the number of catheters with an associated bloodstream infection. Overall, midline use was associated with fewer patients with CRBSI compared with PICC (OR, 0.24; 95% CI, 0.15–0.38; I2 = 0.00%; 9 studies; 12 478 patients; very low certainty); the results of the meta-analyses are summarized in Figure 2. We estimated that we would need a sample of 926 (with 80% power and α = 0.05) to detect a plausible difference in treatment effect for midline compared with PICC on catheter-related bloodstream infections, corresponding to a relative risk reduction of 1.5% (Supplementary Table 6). No association was observed when we evaluated risk of bloodstream infection per catheter (OR, 0.70; 95% CI, 0.39–1.27; I2 = 81.99%; 9 studies; 49 426 catheters; very low certainty).
Figure 2.
Forest plot comparing rates of CRBSI in patients with midlines vs PICCs. Abbreviations: CRBSI, catheter-related bloodstream infection; PICCs, peripherally inserted central catheters.
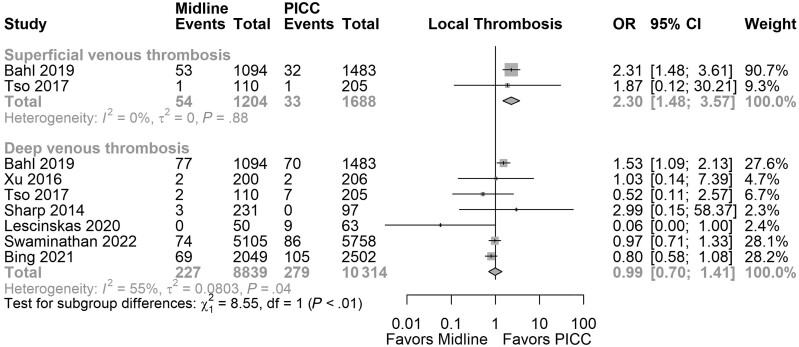
Thrombosis was reported as either localized thrombosis (SVT, DVT, any other thrombosis) or pulmonary embolism (PE). No association was noted when we evaluated localized thrombosis per catheter (OR, 1.05; 95% CI, 0.69–1.57; I2 = 74.02%; 6 studies; 48 177 catheters; very low certainty) and per patient (OR, 1.31; 95% CI, 0.74–2.30; I2 = 60.24%; 9 studies; 14 555 patients; very low certainty). Inconsistency in the results and heterogeneity in the reporting of the outcomes were noted. Our estimates showed that we would need a sample of 28 321 (with 80% power and α = 0.05) to detect a relative risk reduction of 0.5% for midlines compared with PICCs for localized thrombosis (Supplementary Table 6). A subgroup analysis based on type of localized thrombosis revealed higher rates of SVT in patients using midlines (OR, 2.30; 95% CI, 1.48–3.57; I2 = 0.00%; very low certainty), but no significant difference was observed in rates of DVT (OR, 0.99; 95% CI, 0.70–1.41; I2 = 55%; very low certainty). Figure 3 presents the results of the subgroup analysis.
Figure 3.
Subgroup analysis based on thrombosis localization.
We did not observe any correlation between catheter type and risk of pulmonary embolism (OR, 0.87; 95% CI, 0.53–31.44; I2 = 0.00%; 2 studies that included 13 440 patients; very low certainty; and OR, 0.80; 95% CI, 0.37–1.72; I2 = 0.00%; 2 studies that included 12 464 catheters; very low certainty).
Secondary Outcomes
Mortality was reported in 1 study, which did not observe any difference in this outcome between catheter type (OR, 1.90; 95% CI, 0.82–4.41; 406 patients; very low certainty).
The use of PICC lines was associated with more patients failing to complete therapy compared with midlines (OR, 1.92; 95% CI, 1.01–3.66; I2 = 32.08%; 6 studies, 13 653 patients; very low certainty). No difference was observed between catheter type and failure to complete therapy when analyzed by catheter (OR, 2.08; 95% CI, 0.53–8.15; I2 = 69.27%; 3 studies, 31 071 catheters; very low certainty).
No difference was observed between catheter type and catheter occlusion when evaluated per patient (OR, 0.46; 95% CI, 0.18–1.18; I2 = 34.06%; 5 studies, 11 515 patients; very low certainty) or per catheter (OR, 2.28; 95% CI, 0.19–27.58; I2 = 98.08%; 4 studies, 43 220 catheters; very low certainty).
Rates of phlebitis were similar among patients who used midline vs PICC catheters (OR, 0.91; 95% CI, 0.39–2.15; I2 = 0%; 5 studies, 659 patients; very low certainty), as well as when evaluated per catheter (OR, 1.74; 95% CI, 0.41–7.36; 1 study, 406 catheters; very low certainty).
Lastly, we did not note any differences between midline and PICC with respect to proportion of patients with a fractured catheter (OR, 0.84; 95% CI, 0.08–9.36; 1 study, 328 patients; very low certainty) or in the proportion of fractured catheters (OR, 1.11; 95% CI, 0.88–1.40; 1 study, 30 987 catheters; very low certainty).
DISCUSSION
Summary of Findings
This meta-analysis identified 20 studies that compared primary outcomes including risk of CRBSI and thrombosis between midline catheters and PICC. Our findings suggest lower rates of bloodstream infection in patients who used midlines in comparison to PICC lines. However, the use of a midline catheter was associated with greater risk of SVT, albeit similar rates of overall localized thrombosis (SVT and DVT) and pulmonary embolism. Use of a PICC line was associated with more patients failing to complete therapy. We did not find any significant difference between midlines and PICCs for the secondary outcomes assessed including mortality, phlebitis, and rates of fractured catheter.
Implications to Clinical Practice
CRBSI and catheter-related thrombosis are the main adverse events associated with vascular catheters and have been widely investigated [33]. Catheter-related infection is a serious and frequent complication that varies according to the device used [34]. The risk can be influenced by setting, experience of proceduralist, frequency of catheter access and care, duration of placement, and patient-specific characteristics. A prior meta-analysis has demonstrated the risk of CRBSI for several catheter types at 0.1% for peripheral, 0.4% for midline, 2.4% for PICC, 4.4% for CVC [1]. Historically, PICC lines have been associated with reduced risk of CLABSI relative to CVC [2].
Interestingly, midline catheters may have an even lower infection rate compared with PICC lines [1]. In our study, rates of CRBSI per patient were lower with midline catheters when compared with PICC lines. This association was not present when the outcome was analyzed per catheter. It is important to note that CLABSI rates being higher in PICCs is largely being driven by 1 cohort [32]. A prior meta-analysis by Lu and colleagues found no difference in rates of CRBSI between PICCs and midlines (relative risk, 0.77; 95% CI, 0.50–1.17) [5].
Catheter-related venous thrombosis is a second significant complication of catheter insertion. This outcome can be categorized into minor complications like superficial thrombophlebitis and major complications such as DVT and PE. PICCs have been associated with an increased risk of thrombosis in several studies. A prior meta-analysis by Chopra and colleagues comparing PICCs with other CVCs reported an increased risk of PICC-associated deep vein thrombosis (OR, 2.55; 95% CI, 1.54–4.23) [35]. The difference in risk of thrombosis between midlines and PICCs is not clear. Our study demonstrated lower rates of SVT with PICCs when compared with midlines. However, we did not identify any significant difference in rates of DVT or PE between catheter types. This is consistent with a prior cohort study comparing midlines with PICCs, which did not find a significant difference in the risk of DVT or PE (OR, 0.93; 95% CI, 0.63–1.37; and OR, 1.29; 95% CI, 0.46–3.61; for DVT and PE, respectively) [32]. In contrast, a prior small prospective trial by Lescinskas et al. (n = 113) found that 14.5% of patients with PICCs developed DVT compared with no patients in the midline group [20]. Part of the difference observed in rates of DVT and PE between our meta-analysis and prior prospective trials may be secondary to significant heterogeneity and imprecision of outcomes as noted.
The number of patients included in the analysis is only 51% of the 28 321 we calculated was required, which is the number of patients needed to reliably reject a difference in effect of midline and PICC on localized thrombosis based on a relative risk reduction of 0.5% and a control event rate of ∼2.7%. Our analysis showed that the current evidence for a clinically relevant difference of midlines and PICCs for localized thrombosis is still inconclusive. Although more head-to-head data are needed to compare risk of thrombosis between midlines and PICCs, the overall risk of serious thrombotic events including PE appears to be low with both vascular catheters.
Strengths and Limitations
This study included a comprehensive search strategy of relevant medical databases such as OVID, MEDLINE, and Scopus, with systematic screening to facilitate identification, assessment, and synthesis of the body of current evidence relevant to the study question. The majority of the included studies had a prospective, longitudinal design, which facilitated correlation of sequence of events, starting from catheter insertion to observation of outcomes of interest. The primary and secondary outcomes were analyzed by rate of events per patient or per catheter according to reporting within the original study. This strategy allowed us to be inclusive of all studies that reported the outcomes of interest.
This study has several limitations. First, most of the data came from observational studies and only 1 small RCT (n = 54) at high risk of bias, which ultimately accounted for overall very low certainty in the evidence. While observational studies have proven useful when assessing risk factors, RCTs are better accepted as an optimal design to compare the efficacy and effectiveness of medical interventions, offering less heterogeneity between studies and lower risk of bias due to confounding and overestimation of treatment effects. Second, not many head-to-head prospective studies were included. These types of trials are critical in evaluating direct comparisons of outcomes and providing insight into shared decision-making among all the available options. Third, we did not exclude any records by publication year, thus including some studies from ≥20 years ago (1997–2002). This may falsely skew toward worse outcomes as newer catheters, medical equipment, and procedural techniques likely offer better safety profiles. Fourth, many included studies were at high risk of bias. The most frequent source of bias was the comparability between interventions due to lack of matching, which introduced risk of critical confounders such as catheter usage time. Finally, imprecision was a concern for certain important primary outcomes including rates of pulmonary embolism, likely driven by low event rates.
Future Research
Further head-to-head RCTs in patients who are candidates to receive either a PICC or a midline are needed. Clinically important outcomes including infection and thrombosis (both local and systemic) should be considered by trialists. Furthermore, the relative contribution of SVT and DVT to overall risk of thrombosis needs to be further delineated, as our data support that the higher rates of thrombosis with midlines may largely be SVT.
These RCTs should ideally consider evaluating treatment subgroups by setting (inpatient vs outpatient), site of insertion of midline (ie, basilic vs brachial vs cephalic), expertise of the proceduralist, and duration of placement, as well as patient-, device-, and health care–related characteristics. Furthermore, cost-benefit analyses are needed to further guide decision-making for clinicians and patients when deciding on the type of catheter that best fits the patient’s needs.
CONCLUSIONS
Bloodstream infections and thrombosis are important health care consequences of vascular catheters. This meta-analysis compared the rates of these primary outcomes between PICCs and midlines. Our findings suggest that patients who use midlines might experience fewer CRBSIs than those who use PICC lines. However, the use of a midline catheter was associated with greater risk of thrombosis, with more patients having SVT. The relative risk of DVT and PE remains unknown. These findings warrant future cost-benefit analyses and direct comparative RCTs to further characterize the efficacy and risks of PICC vs midline catheters.
Supplementary Material
Acknowledgments
Financial support. This project was not funded.
Potential conflicts of interest. V.C. has received book royalties from Oxford University Press, royalties from Wolters Kluwer for UpToDate chapters, and grant funding from the Agency for Healthcare Research and Quality. The rest of the authors do not report conflicts of interest.
Patient consent. The present study does not include factors necessitating patient consent.
Contributor Information
Meritxell Urtecho, Mayo Clinic Evidence-based Practice Center, Rochester, Minnesota, USA; Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, Mayo Clinic, Rochester, Minnesota, USA.
Victor D Torres Roldan, Mayo Clinic Evidence-based Practice Center, Rochester, Minnesota, USA; Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, Mayo Clinic, Rochester, Minnesota, USA.
Tarek Nayfeh, Mayo Clinic Evidence-based Practice Center, Rochester, Minnesota, USA; Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, Mayo Clinic, Rochester, Minnesota, USA.
Nataly R Espinoza Suarez, Knowledge and Evaluation Research Unit (KER), Mayo Clinic, Rochester, Minnesota, USA.
Nischal Ranganath, Division of Public Health, Infectious Diseases and Occupational Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Priya Sampathkumar, Division of Public Health, Infectious Diseases and Occupational Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Vineet Chopra, Division of Hospital Medicine, University of Michigan, Ann Arbor, Michigan, USA.
Nasia Safdar, Division of Infectious Diseases, University of Wisconsin, Madison, Wisconsin, USA.
Larry J Prokop, Department of Library-Public Services, Mayo Clinic, Rochester, Minnesota, USA.
John C O’Horo, Division of Public Health, Infectious Diseases and Occupational Medicine, Mayo Clinic, Rochester, Minnesota, USA; Division of Pulmonary and Critical Care Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc 2006; 81:1159–71. [DOI] [PubMed] [Google Scholar]
- 2. Chopra V, O'Horo JC, Rogers MA, Maki DG, Safdar N. The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta-analysis. Infect Control Hosp Epidemiol 2013; 34:908–18. [DOI] [PubMed] [Google Scholar]
- 3. Turcotte S, Dubé S, Beauchamp G. Peripherally inserted central venous catheters are not superior to central venous catheters in the acute care of surgical patients on the ward. World J Surg 2006; 30:1605–19. [DOI] [PubMed] [Google Scholar]
- 4. Periard D, Monney P, Waeber G, et al. . Randomized controlled trial of peripherally inserted central catheters vs. peripheral catheters for middle duration in-hospital intravenous therapy. J Thromb Haemost 2008; 6:1281–8. [DOI] [PubMed] [Google Scholar]
- 5. Lu H, Hou Y, Chen J, et al. . Risk of catheter-related bloodstream infection associated with midline catheters compared with peripherally inserted central catheters: a meta-analysis. Nursing Open 2021; 8:1292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chopra V, Flanders SA, Saint S, et al. . The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med 2015; 163(6 Suppl):S1–40. [DOI] [PubMed] [Google Scholar]
- 7. Caparas JV, Hu J-P. Safe administration of vancomycin through a novel midline catheter: a randomized, prospective clinical trial. J Vasc Access 2014; 15:251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cawcutt KA, Hankins RJ, Micheels TA, Rupp ME. Optimizing vascular-access device decision-making in the era of midline catheters. Infect Control Hosp Epidemiol 2019; 40:674–80. [DOI] [PubMed] [Google Scholar]
- 9. Bahl A, Karabon P, Chu D. Comparison of venous thrombosis complications in midlines versus peripherally inserted central catheters: are midlines the safer option? Clin Appl Thromb Hemost 2019; 25:1076029619839150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu T, Kingsley L, DiNucci S, et al. . Safety and utilization of peripherally inserted central catheters versus midline catheters at a large academic medical center. Am J Infect Control 2016; 44:1458–61. [DOI] [PubMed] [Google Scholar]
- 11. Sharp R, Esterman A, McCutcheon H, Hearse N, Cummings M. The safety and efficacy of midlines compared to peripherally inserted central catheters for adult cystic fibrosis patients: a retrospective, observational study. Int J Nurs Stud 2014; 51:694–702. [DOI] [PubMed] [Google Scholar]
- 12. Sterne JAC, Savovic J, Page MJ, et al. . Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366:l4898. [DOI] [PubMed] [Google Scholar]
- 13. Wells G, Shea B, O’connell D, et al. . The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000. Available at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 22 November 2022.
- 14. Schunemann H. GRADE handbook for grading quality of evidence and strength of recommendation. Version 3.2. 2008. Available at:http://www.cc-ims.net/gradepro. Accessed 22 November 2022.
- 15. GRADEpro GDT: GRADEpro Guideline Development Tool [Software] . McMaster University and Evidence Prime, 2022. Available at: https://www.gradepro.org/. Accessed 22 November 2022.
- 16. Higgins JP, Thomas J, Chandler J, et al. . Cochrane Handbook for Systematic Reviews of Interventions. Version 6.3. John Wiley & Sons; 2022. [Google Scholar]
- 17. Barr DA, Semple L, Seaton RA. Self-administration of outpatient parenteral antibiotic therapy and risk of catheter-related adverse events: a retrospective cohort study. Eur J Clin Microbiol Infect Dis 2012; 31:2611–9. [DOI] [PubMed] [Google Scholar]
- 18. Dickson HG, Flynn O, West D, Alexandrou E, Mifflin N, Malone M. A cluster of failures of midline catheters in a hospital in the home program: a retrospective analysis. J Infus Nurs 2019; 42:203–8. [DOI] [PubMed] [Google Scholar]
- 19. Khalidi N, Kovacevich DS, Papke-O'Donnell LF, Btaiche I. Impact of the positive pressure valve on vascular access device occlusions and bloodstream infections. J Assoc Vasc Access 2009; 14:84–91. [Google Scholar]
- 20. Lescinskas EH, Trautner BW, Saint S, et al. . Use of and patient-reported complications related to midline catheters and peripherally inserted central catheters. Infect Control Hosp Epidemiol 2020; 41:608–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Magnani C, Calvieri A, Giannarelli D, Espino M, Casale G. Peripherally inserted central catheter, midline, and “short” midline in palliative care: patient-reported outcome measures to assess impact on quality of care. J Vasc Access 2019; 20:475–81. [DOI] [PubMed] [Google Scholar]
- 22. Moureau N, Poole S, Murdock MA, Gray SM, Semba CP. Central venous catheters in home infusion care: outcomes analysis in 50,470 patients. J Vasc Interv Radiol 2002; 13:1009–16. [DOI] [PubMed] [Google Scholar]
- 23. Mushtaq A, Navalkele B, Kaur M, et al. . Comparison of complications in midlines versus central venous catheters: are midlines safer than central venous lines? Am J Infect Control 2018; 46:788–92. [DOI] [PubMed] [Google Scholar]
- 24. Sargent J, Nixon E. I.V. access options for AIDS patients with cytomegalovirus disease. Br J Nurs 1997; 6:543–53. [DOI] [PubMed] [Google Scholar]
- 25. Seo H, Altshuler D, Dubrovskaya Y, et al. . The safety of midline catheters for intravenous therapy at a large academic medical center. Ann Pharmacother 2020; 54:232–8. [DOI] [PubMed] [Google Scholar]
- 26. Tokars JI, Cookson ST, McArthur MA, Boyer CL, McGeer AJ, Jarvis WR. Prospective evaluation of risk factors for bloodstream infection in patients receiving home infusion therapy. Ann Intern Med 1999; 131:340–7. [DOI] [PubMed] [Google Scholar]
- 27. Tso AR, Patniyot IR, Gelfand AA, Goadsby PJ. Increased rate of venous thrombosis may be associated with inpatient dihydroergotamine treatment. Neurology 2017; 89:279–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vanek VW, Kupensky DT, Thomson DJ. Hypersensitivity-like reactions related to insertion of aquavene-based midline and PICC catheters. J Intraven Nurs 1997; 20:23–7. [PubMed] [Google Scholar]
- 29. Bing S, Smotherman C, Rodriguez RG, Skarupa DJ, Ra JH, Crandall ML. PICC versus midlines: comparison of peripherally inserted central catheters and midline catheters with respect to incidence of thromboembolic and infectious complications. Am J Surg 2022; 223:983–7. [DOI] [PubMed] [Google Scholar]
- 30. Caserta D, Pico V, Frascarelli S, Sbrana F, Formichi B. Peripherally inserted central catheter (PICC) and midline: experience in a cardiopulmonary critical care unit. J Vasc Access 2022; 23:485–7. [DOI] [PubMed] [Google Scholar]
- 31. Kim SH, Hur S, Lee M, et al. . Outcomes of venoplasty-assisted, peripherally inserted central catheter placement in patients with upper-arm venous stenosis: comparison with midlines and contralateral placement. J Vasc Interv Radiol 2022; 33:189–96. [DOI] [PubMed] [Google Scholar]
- 32. Swaminathan L, Flanders S, Horowitz J, Zhang Q, O'Malley M, Chopra V. Safety and outcomes of midline catheters vs peripherally inserted central catheters for patients with short-term indications: a multicenter study. JAMA Intern Med 2022; 182:50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Adams DZ, Little A, Vinsant C, Khandelwal S. The midline catheter: a clinical review. J Emerg Med 2016; 51:252–8. [DOI] [PubMed] [Google Scholar]
- 34. Mermel LA, Allon M, Bouza E, et al. . Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49:1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chopra V, Anand S, Hickner A, et al. . Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet 2013; 382:311–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.