Abstract
Background
Red blood cell distribution width (RDW) and albumin level were considered to be related to the prognosis of patients with acute myocardial infarction (AMI). This study aims to investigate the correlation between RAR and 90-day mortality in AMI patients.
Methods
Data of AMI patients were obtained from the Medical Information Mart for Intensive Care III (MIMIC-III) database. According to the median, RAR < 4.32 was regarded as low RAR level group, and RAR ≥ 4.32 as high RAR level group; low RDW level group was defined as < 14.00%, and high RDW level group as ≥ 14.00%; albumin < 3.30 g/dL was low level group, and albumin ≥ 3.30 g/dL as high level group. The outcome was the mortality rate within 90 days after admission to ICU. Univariate and multivariate Cox models were performed to determine the relationship between RAR and 90-day mortality in AMI patients with hazard ratio (HR) and 95% confidence interval (CI). Stratification analyses were conducted to explore the effect of RAR on 90-day mortality in different subgroups of age, gender, simplified acute physiology score II (SAPS II), elixhauser comorbidity index (ECI) score, treatment modalities and white blood cell.
Results
Of the total 2081 AMI patients, 543 (26.09%) died within 90-day follow-up duration. The results showed that high RAR (HR = 1.65, 95% CI 1.34–2.03) and high RDW levels (HR = 1.31, 95% CI 1.08–1.61) were associated with an increased risk of death in AMI patients, and that high albumin level was related to a decreased risk of death (HR = 0.77, 95%CI 0.64–0.93). The relationship of RAR level and the mortality of AMI patients was also observed in the subgroup analysis. Additionally, the finding indicated that RAR might be a more effective biomarker for predicting 90-day mortality of AMI patients than albumin, RDW.
Conclusion
RAR may be a potential marker for the prognostic assessment of AMI, and a high RAR level was correlated with increased risk of 90-day mortality of AMI patients.
Keywords: The red blood cell distribution width-to-albumin ratio, Acute myocardial infarction, 90-day mortality, MIMIC-III, Relationship
Background
Acute myocardial infarction (AMI) is a type of myocardial necrosis caused by acute coronary artery occlusion [1], which is the leading cause of hospitalization and death in the world [2]. Despite advances in some treatments, the mortality rate from AMI still remains high, with a 90-day mortality rate of 12.2% [3, 4]. Timely identification of influencing factors associated with death in patients with AMI is of great significance to improve prognosis and provide better guidance for treatment.
Red blood cell distribution width (RDW) is considered as an indicator of the heterogeneity of red blood cell (RBC) volume in peripheral blood [5, 6]. Existing evidence suggests that RDW has been a novel biomarker of inflammation and oxidative stress [6, 7]. Previous studies have reported that higher RDW value was independent risk factor for poor prognosis in many diseases, such as AMI [5], atrial fibrillation [8], ischaemic stroke [9]. Recently, some recommendations have indicated that combining RDW with some biomarkers may be more valuable for disease prognosis. Albumin, an important protein that can reflect the nutritional and inflammatory status [10, 11], has been shown to be associated with anti-inflammatory activity [12] and reduction of oxidative stress [13]. A cohort study in 2,305 patients with first-onset AMI showed that low serum albumin levels at admission was related to an increased risk of long-term all-cause, cardiovascular, and cardiac mortality in patients [14]. Several studies have examined the relationship between prognosis of circulation system diseases and the combined marker-RDW/albumin ratio (RAR). In the study of Zhao et al., they concluded that a high RAR level was associated with an increased mortality among intensive care unit (ICU) patients with stroke [15]. Furthermore, an elevated RAR level was also regarded as an independent risk factor of all-cause mortality in patients with thoracic or abdominal aortic aneurysm [16]. However, to our knowledge, there are few relevant studies investigating the association between RAR and the prognosis of AMI patients to date.
Herein, the aim of this study was to explore the correlation between RAR and 90-day mortality in patients with AMI.
Methods
Study population
This cohort study collected the information of participants from the Medical Information Mart for Intensive Care III (MIMIC-III) database 2001–2012. This large, single-center database records the medical information over forty thousand patients admitted to ICU between 2001 and 2012 [17], which includes some information, such as demographic data, vital signs, laboratory test, and survival data.
International Classification of Diseases-9 (ICD-9) diagnosis codes (41000–41092) were used to identify patients with AMI in the MIMIC-III database. The inclusion criteria were as follows: (1) patients diagnosed with AMI at ICU admission; (2) patients aged ≥ 18 years old at diagnosis; (3) patients with measurement of RDW and albumin. The exclusion criteria were as follows: (1) length of ICU stay < 24 h; (2) patient’s individual data with missing. This study was conducted in accordance with the Declaration of Helsinki. The MIMIC-III database was approved by the Institutional Review Boards of the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center. https://mimic.mit.edu/docs/iii/. Informed consent has been obtained from all participants.
Data collection
The data of patients were extracted from the MIMIC-III database: (1) demographics: age, gender, race and marital status; (2) vital signs: respiratory rate (times/min), heart rate (times/min), systolic blood pressure (SBP, mmHg), diastolic blood pressure (DBP, mmHg) and pulse oximetry-derived oxygen saturation (SpO2, %); (3) laboratory parameters: sodium (mEq/L), potassium (mEq/L), calcium (mEq/L), creatine kinase isoenzymes (CK-MB, ng/mL), pH, white blood cell (WBC, K/uL), glucose (mg/dL), creatinine (mg/dL), blood urea nitrogen (BUN, mg/dL), bicarbonate (mEq/L), hemoglobin (g/dL), albumin (g/dL), RDW (%); (4) scoring systems: sequential organ failure assessment (SOFA), simplified acute physiology score II (SAPS II), glasgow coma scale (GCS), elixhauser comorbidity index (ECI). (5) Treatment modalities: percutaneous coronary intervention (PCI), coronary artery bypass graft (CABG), and thrombolysis. (6) Vasopressor use and ICU length (days). Vital signs and laboratory parameters recorded within 24 h of initial admission were used for analysis.
Assessment of RAR
RAR was defined as the ratio of the RDW (%) to albumin (g/dL). We divided RAR levels into two groups based on the median: RAR < 4.32 was regarded as low RAR level group, and RAR ≥ 4.32 was regarded as high RAR level group. Similarly, albumin levels were divided into low albumin level group (albumin < 3.30 g/dL) and high albumin level group (≥ 3.30 g/dL) based on median. Low RDW level group was defined as < 14.00%, and high RDW level group as ≥ 14.00%.
Outcome and follow-up
The endpoint event of this study was the mortality of AMI patients within 90 days after admission to ICU. The maximum follow-up duration was 90 days, and the median follow-up time was 90 (75, 90) days.
Statistical analysis
Measurement data with normal distribution was described by the mean ± standard deviation (Mean ± SD), and measurement data with non-normal distribution by the median and quartile spacing [M (Q1, Q3)]. The categorical data was depicted by the number of cases and composition ratio n (%). Baseline characteristics of participants were compared between survival group and death group by using either Mann–Whitney U rank-sum test or χ2 test.
Univariate and multivariate Cox models were used for assessing the relationship between RAR and 90-day mortality in patients with AMI, and hazard ratio (HR) with 95% confidence interval (CI) was calculated. Model 1 was the univariate Cox model (unadjusted); covariates in model 2 were adjusted for age, vasopressor use, SOFA score, SAPS II score, GCS score and ECI score; covariates in model 3 were adjusted for age, marital status, respiratory rate, heart rate, DBP, WBC, potassium, calcium, pH, creatinine, bicarbonate, hemoglobin, SpO2, vasopressor use, SOFA score, SAPS II score, GCS score ECI score, PCI, CABG and ICU length. Stratification analyses were conducted to explore the effect of RAR between different subgroups (age, gender, SAPS II score, ECI score, treatment modalities and WBC). Box plots and t-test were constructed to observe the positive correlation between RAR and ECI score, SAPS II score and SOFA score. We adopted Kaplan–Meier survival curve to analyze survival status in both the low and high RDW level groups, both the low and high albumin level groups, and both the low and high RAR level groups. A restricted cubic spline (RCS) model was used to observe whether there was a linear relationship between RAR and 90-day mortality of patients with AMI. Lastly, the total samples were randomly split into training set and testing set with the ratio of 7:3. A prediction model by using the RAR was established in the training set for the prediction of 90-day mortality of patients with AMI. The C-index was adopted to compare the predicting performance between developed RAR model and RDW-model, albumin-model in the testing set. All statistical analyses were conducted by using Rstudio (version 4.0.3) and SAS (version 9.4). P < 0.05 was considered statistically significant.
Results
Baseline characteristics
We excluded some patients who did not measure RDW and albumin (n = 1731) or whose baseline data were incomplete (n = 909) or whose ICU stay was less than 24 h (n = 244) or who were lost to follow-up (n = 52), 2081 AMI patients were included in the final analysis. They were divided into survival group (n = 1538) and death group (n = 543). The 90-day mortality of patients with AMI was 26.09%. As shown in Table 1, baseline characteristics of participants were compared between survival group and death group. We found that there were more AMI patients with high levels of albumin, low levels of RDW and RAR in the survival group than in the death group.
Table 1.
Baseline characteristics of the study population
| Characteristics | Total (n = 2081) | Survival group (n = 1538) | Death group (n = 543) | P |
|---|---|---|---|---|
| Gender, n (%) | 0.108 | |||
| Female | 823 (39.5%) | 592 (38.5%) | 231 (42.5%) | |
| Male | 1258 (60.5%) | 946 (61.5%) | 312 (57.5%) | |
| Age, years, n (%) | < 0.001 | |||
| < 65 | 631 (30.3%) | 559 (36.3%) | 72 (13.3%) | |
| ≥ 65 | 1450 (69.7%) | 979 (63.7%) | 471 (86.7%) | |
| Race, n (%) | 0.207 | |||
| Asian | 34 (1.63%) | 25 (1.63%) | 9 (1.66%) | |
| Black | 114 (5.48%) | 89 (5.79%) | 25 (4.60%) | |
| Other# | 422 (20.3%) | 296 (19.2%) | 126 (23.2%) | |
| White | 1511 (72.6%) | 1128 (73.3%) | 383 (70.5%) | |
| Marital status, n (%) | 0.035 | |||
| Married | 1157 (55.6%) | 867 (56.4%) | 290 (53.4%) | |
| Other* | 924 (44.4%) | 671 (43.6%) | 253 (46.6%) | |
| Respiratory rate, times/min, Mean ± SD | 19.0 ± 6.80 | 18.3 ± 6.45 | 21.2 ± 7.29 | < 0.001 |
| Heart rate, times/min, Mean ± SD | 88.3 ± 19.3 | 87.3 ± 18.6 | 91.1 ± 20.9 | < 0.001 |
| SBP, mmHg, Mean ± SD | 122 ± 25.8 | 122 ± 25.0 | 120 ± 27.9 | 0.052 |
| DBP, mmHg, Mean ± SD | 62.8 ± 17.1 | 63.5 ± 16.5 | 60.8 ± 18.6 | 0.003 |
| SPO2, %, Mean ± SD | 96.7 ± 6.96 | 97.1 ± 6.09 | 95.7 ± 8.90 | 0.001 |
| Sodium, mEq/L, Mean ± SD | 138 ± 4.79 | 138 ± 4.42 | 138 ± 5.70 | 0.907 |
| Potassium, mEq/L, Mean ± SD | 4.36 ± 0.84 | 4.30 ± 0.81 | 4.53 ± 0.90 | < 0.001 |
| Calcium, g/dL, Mean ± SD | 8.54 ± 0.97 | 8.58 ± 0.95 | 8.43 ± 0.99 | 0.002 |
| CK-MB, ng/mL, Mean ± SD | 77.1 ± 152 | 82.5 ± 147 | 63.6 ± 165 | 0.058 |
| pH, Mean ± SD | 7.36 ± 0.11 | 7.36 ± 0.10 | 7.34 ± 0.12 | < 0.001 |
| WBC, K/uL, Mean ± SD | 12.6 ± 8.08 | 12.2 ± 7.49 | 13.9 ± 9.44 | < 0.001 |
| Glucose, mg/dL, Mean ± SD | 179 ± 116 | 181 ± 120 | 175 ± 104 | 0.322 |
| Creatinine, mg/dL, Mean ± SD | 1.80 ± 1.96 | 1.70 ± 2.02 | 2.09 ± 1.74 | < 0.001 |
| BUN, mg/dL, Mean ± SD | 33.0 ± 23.9 | 30.1 ± 21.9 | 41.3 ± 27.0 | < 0.001 |
| Bicarbonate, mEq/L, Mean ± SD | 23.2 ± 4.94 | 23.4 ± 4.76 | 22.6 ± 5.39 | 0.005 |
| Hemoglobin, g/dL, Mean ± SD | 11.9 ± 2.16 | 12.0 ± 2.19 | 11.3 ± 1.96 | < 0.001 |
| Albumin, g/dL, n (%) | < 0.001 | |||
| Low level | 930 (44.7%) | 599 (38.9%) | 331 (61.0%) | |
| High level | 1151 (55.3%) | 939 (61.1%) | 212 (39.0%) | |
| RDW, n (%) | < 0.001 | |||
| Low level | 1013 (48.7%) | 831 (54.0%) | 182 (33.5%) | |
| High level | 1068 (51.3%) | 707 (46.0%) | 361 (66.5%) | |
| RAR, n (%) | < 0.001 | |||
| Low level | 1041 (50.0%) | 885 (57.5%) | 156 (28.7%) | |
| High level | 1040 (50.0%) | 653 (42.5%) | 387 (71.3%) | |
| SAPS II, Mean ± SD | 41.9 ± 13.8 | 39.1 ± 12.7 | 49.6 ± 13.7 | < 0.001 |
| SOFA total score, Mean ± SD | 6.06 ± 3.47 | 5.63 ± 3.32 | 7.29 ± 3.60 | < 0.001 |
| GCS score, Mean ± SD | 13.7 ± 2.81 | 13.9 ± 2.66 | 13.3 ± 3.17 | < 0.001 |
| ECI score, Mean ± SD | 9.84 ± 8.02 | 8.55 ± 7.55 | 13.5 ± 8.18 | < 0.001 |
| PCI, n (%) | 0.006 | |||
| No | 1567 (75.3%) | 1134 (73.7%) | 433 (79.7%) | |
| Yes | 514 (24.7%) | 404 (26.3%) | 110 (20.3%) | |
| CABG, n (%) | < 0.001 | |||
| No | 1510 (72.6%) | 1083 (70.4%) | 427 (78.6%) | |
| Yes | 571 (27.4%) | 455 (29.6%) | 116 (21.4%) | |
| Thrombolysis, n (%) | 0.370 | |||
| No | 1961 (94.2%) | 1454 (94.5%) | 507 (93.4%) | |
| Yes | 120 (5.77%) | 84 (5.46%) | 36 (6.63%) | |
| Vasopressor use, n (%) | < 0.001 | |||
| No | 1901 (91.4%) | 1453 (94.5%) | 448 (82.5%) | |
| Yes | 180 (8.65%) | 85 (5.53%) | 95 (17.5%) | |
| ICU length, days, Mean ± SD | 6.64 ± 7.40 | 5.97 ± 6.91 | 8.55 ± 8.35 | < 0.001 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; SpO2, pulse oximetry-derived oxygen saturation; CK-MB, creatine kinase isoenzymes; WBC, white blood cell; BUN, blood urea nitrogen; RDW, red blood cell distribution width; RAR, the ratio of the RDW to albumin; SAPS, simplified acute physiology score; SOFA, sequential organ failure assessment; GCS, glasgow coma scale; ECI, elixhauser comorbidity index; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; ICU, intensive care unit. Other# include Hispanic or Latino, American Indian/Alaska, unknown and multirace ethnicity; Other* include divorce, separate, single and widowed
Relationship between RAR and 90-day mortality of patients with AMI
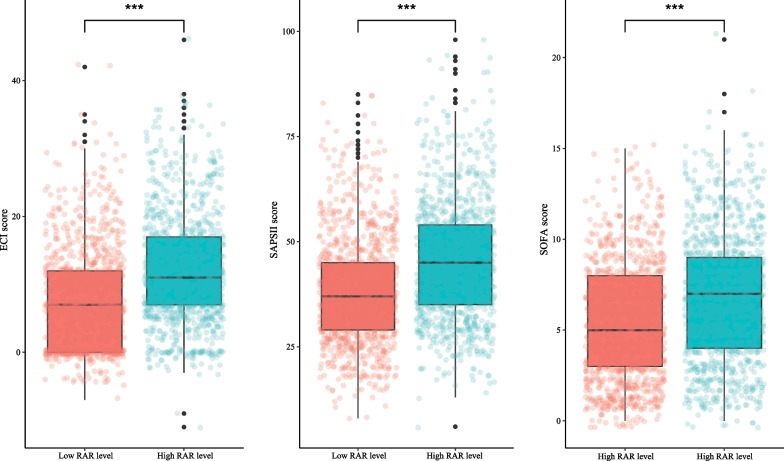
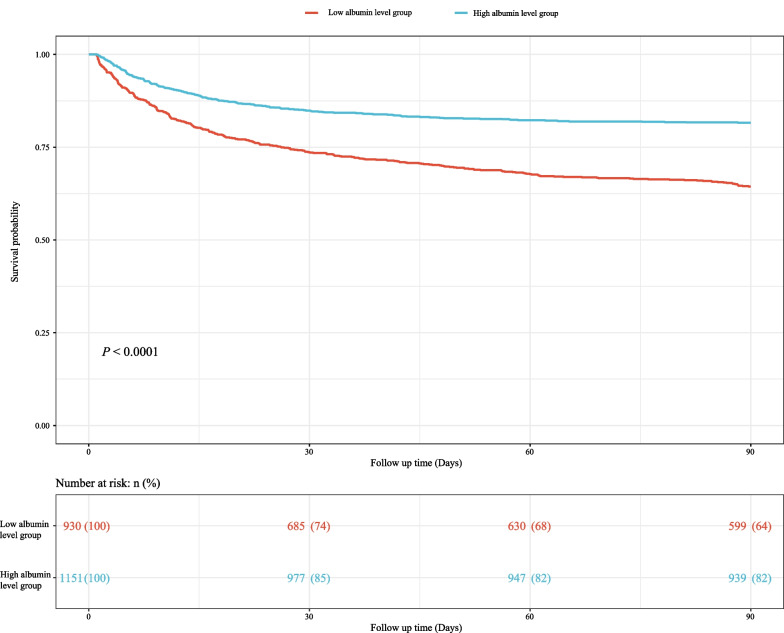
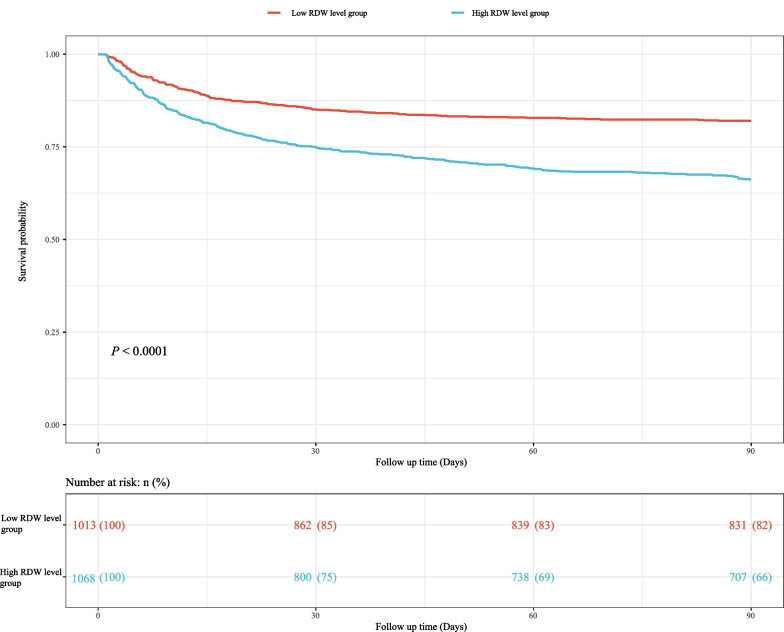
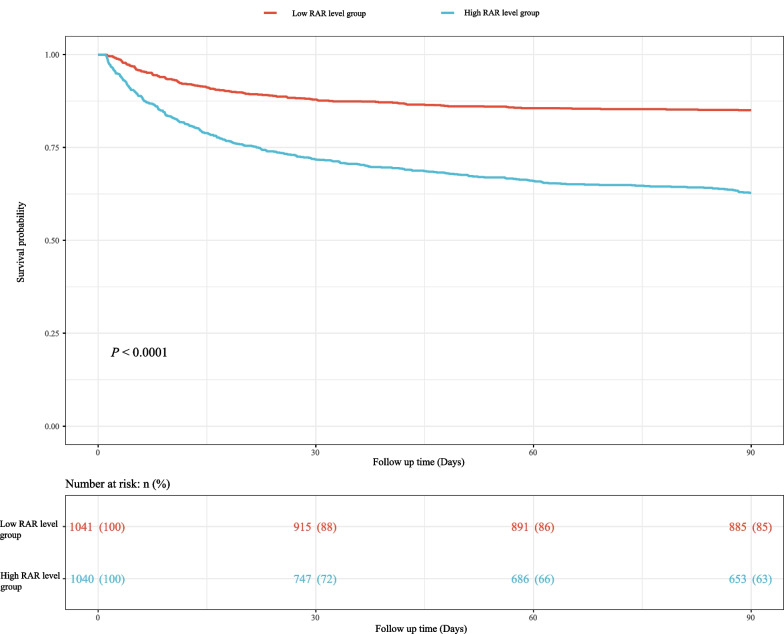
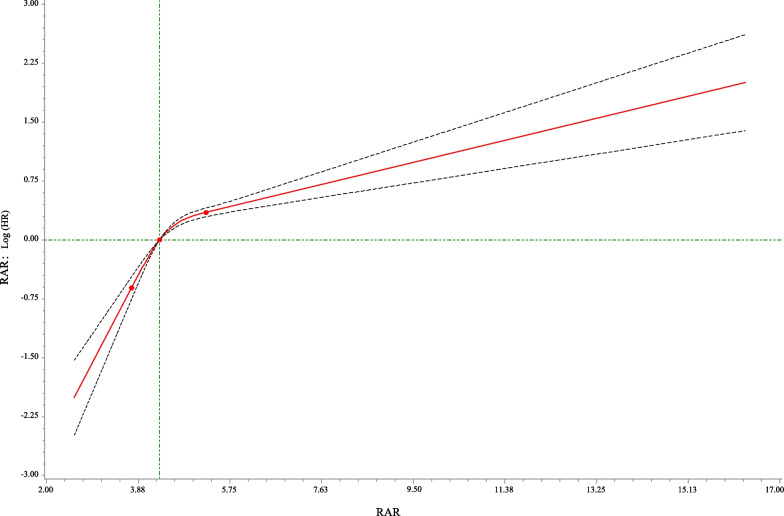
We used Cox models to determine the association between RAR and 90-day mortality of patients with AMI. As illustrated in Table 2, the results suggested that the risk of death in AMI patients with high RAR level group was higher than that in patients with low RAR level group (Model 1: HR = 2.84, 95% CI 2.36–3.42, P < 0.001; Model 2: HR = 1.76, 95% CI 1.45–2.13, P < 0.001; Model 3: HR = 1.65, 95% CI 1.34–2.03, P < 0.001). Simultaneously, the relationship of RDW, albumin and 90-day mortality of patients with AMI were also investigated in the Table 2. These results indicated that high RDW level (Model 3: HR = 1.31, 95% CI 1.08–1.61, P = 0.007) was associated with an increased risk of death in AMI patients, and that high albumin level was related to a decreased risk of death (Model 3: HR = 0.77, 95% CI 0.64–0.93, P = 0.007). Figure 1 shows that RAR level was positively correlated with ECI score, SAPS II score and SOFA score, which suggested that RAR might was related to 90-day mortality of patients with AMI. Additionally, Fig. 2 presents the survival curves of both the low and high RDW level groups, both the low and high albumin level groups, and both the low and high RAR level groups, and the result indicated that the higher the RDW level or the lower the albumin level or the higher the RAR level, the worse the survival probability of AMI patients. The RCS model showed that the relationship between RAR and 90-day mortality of patients with AMI was nonlinear (Fig. 3).
Table 2.
The association between albumin, RDW, RAR and 90-day mortality of patients with AMI by univariate and multivariate Cox models
| Variables | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Albumin | ||||||
| Low level | Ref | Ref | Ref | |||
| High level | 0.47 (0.40–0.56) | < 0.001 | 0.71 (0.60–0.85) | < 0.001 | 0.77 (0.64–0.93) | 0.007 |
| RDW | ||||||
| Low level | Ref | Ref | Ref | |||
| High level | 2.05 (1.72–2.45) | < 0.001 | 1.34 (1.11–1.62) | 0.002 | 1.31 (1.08–1.61) | 0.007 |
| RAR | ||||||
| Low level | Ref | Ref | Ref | |||
| High level | 2.84 (2.36–3.42) | < 0.001 | 1.76 (1.45–2.13) | < 0.001 | 1.65 (1.34–2.03) | < 0.001 |
Model 1 was the univariate Cox model (unadjusted); Model 2: adjusted for age, vasopressor use, sequential organ failure assessment score, simplified acute physiology score II score, glasgow coma scale score and elixhauser comorbidity index score; Model 3: adjusted for age, marital status, respiratory rate, heart rate, diastolic blood pressure, white blood cell, potassium, calcium, pH, creatinine, bicarbonate, hemoglobin, pulse oximetry-derived oxygen saturation, intensive care unit length, vasopressor use, sequential organ failure assessment score, simplified acute physiology score II score, glasgow coma scale score, elixhauser comorbidity index score, percutaneous coronary intervention and coronary artery bypass graft
HR, hazard ratio; CI, confidence interval; Ref, reference; RDW, red blood cell distribution width; RAR, the ratio of the RDW to albumin; AMI: acute myocardial infarction
Fig. 1.
Box plots was constructed to observe the correlation between RAR and ECI score, SAPS II score and SOFA score, *** represents P < 0.001. RDW, red blood cell distribution width; RAR, the ratio of the RDW to albumin; ECI, elixhauser comorbidity index; SAPS, simplified acute physiology score; SOFA, sequential organ failure assessment
Fig. 2.
The survival curves of a both the low and high albumin level groups, b both the low and high RDW level groups and c both the low and high RAR level groups. RDW, red blood cell distribution width; RAR, the ratio of the RDW to albumin
Fig. 3.
Association between the RAR and 90-day mortality of patients with AMI based on the restricted cubic spline model. RAR, the ratio of the RDW to albumin; AMI, acute myocardial infarction
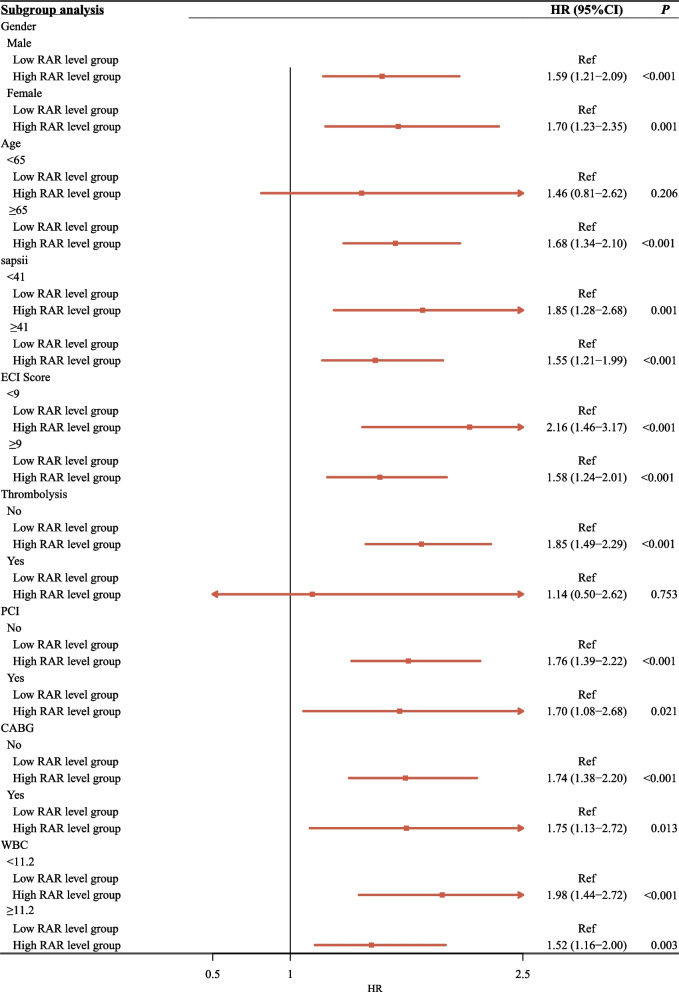
Subgroup analyses based on the age, gender, SAPS II score, ECI score, treatment modalities and WBC
In the subgroup analyses conducted for age, gender, SAPS II score, ECI score, treatment modalities and WBC (Fig. 4), the results implied that AMI patients with the high RAR level had higher risk of 90-day mortality than those with low RAR level in subgroup analyses, except for those patients with aged < 65 years and those receiving thrombolysis.
Fig. 4.
The subgroup analyses based on the age, gender, SAPS II score, ECI score, thrombolysis, PCI, CABG and WBC. SAPS, simplified acute physiology score; ECI, elixhauser comorbidity index; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; WBC, white blood cell.
Predicting value
In addition, we also developed a RAR-model for predicting the 90-day mortality of AMI patients, and compared the predicting performance of RAR-model and RDW-model and albumin-model (Table 3). The C-indexes of the RAR-model were 0.610 (95% CI 0.586–0.634) in the training set, and 0.629 (95% CI 0.593–0.665) in the testing set, which were higher than RDW model and albumin model, respectively.
Table 3.
The predicting performance of RAR-model and RDW-model and albumin-model
| Models | Training set (n = 1457) | Testing set (n = 624) | ||
|---|---|---|---|---|
| C-index (95%CI) | P | C-index (95%CI) | P | |
| RDW-model | 0.594 (0.569–0.619) | Ref | 0.592 (0.555–0.629) | Ref |
| Albumin-model | 0.574 (0.548–0.599) | < 0.001 | 0.606 (0.569–0.643) | 0.074 |
| RAR-model | 0.610 (0.586–0.634) | < 0.001 | 0.629 (0.593–0.665) | < 0.001 |
HR, hazard ratio; CI, confidence interval; RDW, red blood cell distribution width; RAR, the ratio of the RDW to albumin; Ref, reference
Discussion
In the present study, we found that elevated RAR level was related to an increased risk of 90-day mortality of patients with AMI. Additionally, the finding also indicated that RAR might be a more effective biomarker in predicting 90-day mortality of patients with AMI than albumin and RDW.
RDW, commonly measured by using a blood analyzer, plays a role in the states of inflammation, poor nutrition, and microcirculation impairment [18, 19]. Previous studies have shown that RDW level were associated with the risk of mortality of AMI patients: the higher the RDW level, the higher the mortality of AMI patients [5, 20]. The mechanisms underlying the association may be as follows: some studies have pointed out that increased levels of RDW indicate reduced RBC deformability and impaired microcirculatory blood flow, which may lead to reduced oxygen supply at the tissue level, resulting in an imbalance between oxygen supply and demand that increases the risk of adverse events [21, 22]. Moreover, for patients with AMI, inflammation and oxidative stress may increase RDW level by compromising iron metabolism and shortening the lifespan of RBC, thereby modulating the response to erythropoietin through bone marrow [23, 24]. Similarly, albumin, as an inflammation biomarker, has been reported to be related to the prognosis of coronary artery disease [25] and AMI [14]. Djoussé et al. have proposed that after adjusting for age, total cholesterol, and hypertension, low albumin level was associated with an increased risk of myocardial infarction [26]. Low albumin level plays a proinflammatory role and was associated with increased oxidative stress, platelet activation and aggregation, raising all-cause mortality risk in AMI [27]. Similar to these results, our study showed that high RDW level was interconnected with an increased risk of death for AMI patients, and high albumin level might be a protective factor of death risk in patients with AMI. In the study of Li et al., they reported that RAR (RDW-to-albumin Ratio) was an independent predictor for 30-day mortality of patients with first-onset AMI [28]; however, they did not consider the effect of severity score as well as treatment modality. Remarkably, this study indicated that after adjusted for age, marital status, respiratory rate, heart rate, DBP, WBC, potassium, calcium, pH, creatinine, bicarbonate, hemoglobin, SpO2, vasopressor use, SOFA score, SAPS II score, GCS score ECI score, PCI, CABG and ICU length, high RAR level was a risk factor of the 90-day mortality for AMI patients, which may be related to the fact that both RDW and albumin were independent predictors of AMI. In addition, the subgroup analysis also displayed that the relationship of RAR level and the mortality of AMI patients was stable.
Although albumin levels and RDW level could be used as predictors of AMI death, this present study suggested that the RAR level may have a better predicting performance of 90-day mortality in AMI patients than albumin and RDW. In addition, RAR can also be obtained quickly and simply from the admission laboratory. Therefore, RAR may be a simple and readily available biomarker for prediction of death risk in AMI patients.
Nevertheless, there are also some drawbacks in this study. Firstly, since all data of participants in this study were from MIMIC-III database which is a retrospective study conducted in a single-center, the results may have a potential selection bias. Secondly, only patients with AMI in the ICU were included in our study, therefore, the relationship of RAR level and AMI patients who were not admitted to the ICU could not be determined. Thirdly, therapeutic strategies for AMI have evolved further over the decade, such as the evolution of antiplatelet drugs and drug eluting stents and the change of Dual Anti-platelet Therapy (DAPT) duration [29]. But patients’ information on the evolution of antiplatelet drugs and drug eluting stents, as well as DAPT duration was lacking in the MIMIC-III database. this may also be confounding factors in this study. Lastly, patients’ information on Killip classification [30], Thrombolysis in Myocardial Infarction (TIMI) risk score [31], peak creatine kinase (CK) values [30], left ventricular ejection fraction [31], and revascularization [32] was not collected in the MIMIC-III database, which may also be confounding factors. More prospective with multi-center studies will be conducted in the future to validate our findings.
Conclusion
We found that RAR was a potential prognostic marker for AMI patients, and a high RAR level was correlated with increased risk of 90-day mortality for patients with AMI. In the future, RAR may be a simple and readily available biomarker for prediction of death risk in AMI patients.
Acknowledgements
Not applicable.
Abbreviations
- AMI
Acute myocardial infarction
- RDW
Red blood cell distribution width
- RBC
Red blood cell
- RAR
RDW/albumin ratio
- ICU
Intensive care unit
- MIMIC-III
Medical Information Mart for Intensive Care III
- ICD-9
International Classification of Diseases-9
- SBP
Systolic blood pressure
- SpO2
Oximetry-derived oxygen saturation
- CK-MB
Creatine kinase isoenzymes
- WBC
White blood cell
- BUN
Blood urea nitrogen
- SOFA
Sequential organ failure assessment
- SAPS II
Simplified acute physiology score II
- GCS
Glasgow coma scale
- ECI
Elixhauser comorbidity index
- PCI
Percutaneous coronary intervention
- CABG
Coronary artery bypass graft
- Mean ± SD
Mean ± standard deviation
- HR
Hazard ratio
- CI
Confidence interval
- RCS
Restricted cubic spline
Author contributions
HL and YX designed the study. HL wrote the manuscript. HL and YX collected, analyzed and interpreted the data. YX critically reviewed, edited and approved the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in the MIMIC-III database, https://mimic.physionet.org/iii/.
Declarations
Ethics approval and consent to participate
The MIMIC-III database was approved by the Institutional Review Boards of the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center. Informed consent has been obtained from all participants. This study was conducted in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hsu PC, Lee WH, Chiu CA, Chen YC, Chang CT, Tsai WC, et al. Usefulness of ankle-brachial index calculated using diastolic blood pressure for prediction of mortality in patients with acute myocardial infarction. J Clin Hypertens (Greenwich) 2020;22:2044–2050. doi: 10.1111/jch.14032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castro-Dominguez Y, Dharmarajan K, McNamara RL. Predicting death after acute myocardial infarction. Trends Cardiovasc Med. 2018;28:102–109. doi: 10.1016/j.tcm.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Di Chiara A, Clagnan E, Valent F. Epidemiology and mortality in an Italian region after the adoption of the universal definition of myocardial infarction. J Cardiovasc Med (Hagerstown) 2020;21:34–39. doi: 10.2459/JCM.0000000000000893. [DOI] [PubMed] [Google Scholar]
- 4.Zhang T, Guan YZ, Liu H. Association of acidemia with short-term mortality of acute myocardial infarction: a retrospective study base on MIMIC-III database. Clin Appl Thromb Hemost. 2020;26:1076029620950837. doi: 10.1177/1076029620950837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen M, Liao L, Yan J, Lin FQ. Predictive value of red blood cell distribution width for 1-year all-cause mortality in critically ill patients with acute myocardial infarction. Int J Gen Med. 2022;15:465–471. doi: 10.2147/IJGM.S345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Go H, Ohto H, Nollet KE, Sato K, Ichikawa H, Kume Y, et al. Red cell distribution width as a predictor for bronchopulmonary dysplasia in premature infants. Sci Rep. 2021;11:7221. doi: 10.1038/s41598-021-86752-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu XS, Chen ZQ, Hu YF, Chen JX, Xu WW, Shu J, et al. Red blood cell distribution width is associated with mortality risk in patients with acute respiratory distress syndrome based on the Berlin definition: a propensity score matched cohort study. Heart Lung. 2020;49:641–645. doi: 10.1016/j.hrtlng.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Shao Q, Korantzopoulos P, Letsas KP, Tse G, Hong J, Li G, et al. Red blood cell distribution width as a predictor of atrial fibrillation. J Clin Lab Anal. 2018;32:e22378. doi: 10.1002/jcla.22378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng GH, Li HP, Li QL, Fu Y, Huang RB. Red blood cell distribution width and ischaemic stroke. Stroke Vasc Neurol. 2017;2:172–175. doi: 10.1136/svn-2017-000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rencuzogullari I, Karabağ Y, Çağdaş M, Karakoyun S, Seyis S, Gürsoy MO, et al. Assessment of the relationship between preprocedural C-reactive protein/albumin ratio and stent restenosis in patients with ST-segment elevation myocardial infarction. Rev Port Cardiol (Engl Ed) 2019;38:269–277. doi: 10.1016/j.repc.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Karabağ Y, Çağdaş M, Rencuzogullari I, Karakoyun S, Artaç İ, İliş D, et al. Usefulness of the C-reactive protein/albumin ratio for predicting no-reflow in ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. Eur J Clin Investig. 2018;48:e12928. doi: 10.1111/eci.12928. [DOI] [PubMed] [Google Scholar]
- 12.Arques S. Human serum albumin in cardiovascular diseases. Eur J Intern Med. 2018;52:8–12. doi: 10.1016/j.ejim.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Sitar ME, Aydin S, Cakatay U. Human serum albumin and its relation with oxidative stress. Clin Lab. 2013;59:945–952. doi: 10.7754/Clin.Lab.2012.121115. [DOI] [PubMed] [Google Scholar]
- 14.Xia M, Zhang C, Gu J, Chen J, Wang LC, Lu Y, et al. Impact of serum albumin levels on long-term all-cause, cardiovascular, and cardiac mortality in patients with first-onset acute myocardial infarction. Clin Chim Acta. 2018;477:89–93. doi: 10.1016/j.cca.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Zhao N, Hu W, Wu Z, Wu X, Li W, Wang Y, et al. The red blood cell distribution width-albumin ratio: a promising predictor of mortality in stroke patients. Int J Gen Med. 2021;14:3737–3747. doi: 10.2147/IJGM.S322441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long J, Xie X, Xu D, Huang C, Liu Y, Meng X, et al. Association between red blood cell distribution width-to-albumin ratio and prognosis of patients with aortic aneurysms. Int J Gen Med. 2021;14:6287–6294. doi: 10.2147/IJGM.S328035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Y, Hao C, Bo X, Lu Z, Qian H, Chen L. The prognostic value of admission lymphocyte-to-monocyte ratio in critically ill patients with acute myocardial infarction. BMC Cardiovasc Disord. 2022;22:308. doi: 10.1186/s12872-022-02745-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinho J, Silva L, Quintas-Neves M, Marques L, Amorim JM, Reich A, et al. Red cell distribution width is associated with 30-day mortality in patients with spontaneous intracerebral hemorrhage. Neurocrit Care. 2021;34:825–832. doi: 10.1007/s12028-020-01103-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye WY, Li J, Li X, Yang XZ, Weng YY, Xiang WW, et al. Predicting the one-year prognosis and mortality of patients with acute ischemic stroke using red blood cell distribution width before intravenous thrombolysis. Clin Interv Aging. 2020;15:255–263. doi: 10.2147/CIA.S233701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan L, Xu Q, Shi R. A nomogram for predicting hospital mortality in intensive care unit patients with acute myocardial infarction. Int J Gen Med. 2021;14:5863–5877. doi: 10.2147/IJGM.S326898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin G, Dai C, Xu K, Wu M. Predictive value of neutrophil to lymphocyte ratio and red cell distribution width on death for ST segment elevation myocardial infarction. Sci Rep. 2021;11:11506. doi: 10.1038/s41598-021-91082-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arbel Y, Weitzman D, Raz R, Steinvil A, Zeltser D, Berliner S, et al. Red blood cell distribution width and the risk of cardiovascular morbidity and all-cause mortality. A population-based study. Thromb Haemost. 2014;111:300–307. doi: 10.1160/TH13-07-0567. [DOI] [PubMed] [Google Scholar]
- 23.Li N, Zhou H, Tang Q. Red blood cell distribution width: a novel predictive indicator for cardiovascular and cerebrovascular diseases. Dis Mark. 2017;2017:7089493. doi: 10.1155/2017/7089493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierce CN, Larson DF. Inflammatory cytokine inhibition of erythropoiesis in patients implanted with a mechanical circulatory assist device. Perfusion. 2005;20:83–90. doi: 10.1191/0267659105pf793oa. [DOI] [PubMed] [Google Scholar]
- 25.Çağdaş M, Rencüzoğullari I, Karakoyun S, Karabağ Y, Yesin M, Artaç I, et al. Assessment of relationship between C-reactive protein to albumin ratio and coronary artery disease severity in patients with acute coronary syndrome. Angiology. 2019;70:361–368. doi: 10.1177/0003319717743325. [DOI] [PubMed] [Google Scholar]
- 26.Djoussé L, Rothman KJ, Cupples LA, Levy D, Ellison RC. Serum albumin and risk of myocardial infarction and all-cause mortality in the Framingham Offspring Study. Circulation. 2002;106:2919–2924. doi: 10.1161/01.CIR.0000042673.07632.76. [DOI] [PubMed] [Google Scholar]
- 27.Plakht Y, Gilutz H, Shiyovich A. Decreased admission serum albumin level is an independent predictor of long-term mortality in hospital survivors of acute myocardial infarction. Soroka Acute Myocardial Infarction II (SAMI-II) project. Int J Cardiol. 2016;219:20–24. doi: 10.1016/j.ijcard.2016.05.067. [DOI] [PubMed] [Google Scholar]
- 28.Li D, Ruan Z, Wu B. Association of red blood cell distribution width-albumin ratio for acute myocardial infarction patients with mortality: a retrospective cohort study. Clin Appl Thromb Hemost. 2022;28:10760296221121286. doi: 10.1177/10760296221121286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan SU, Singh M, Valavoor S, Khan MU, Lone AN, Khan MZ, et al. Dual antiplatelet therapy after percutaneous coronary intervention and drug-eluting stents: a systematic review and network meta-analysis. Circulation. 2020;142:1425–1436. doi: 10.1161/CIRCULATIONAHA.120.046308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taguchi E, Konami Y, Inoue M, Suzuyama H, Kodama K, Yoshida M, et al. Impact of Killip classification on acute myocardial infarction: data from the SAIKUMA registry. Heart Vessels. 2017;32:1439–1447. doi: 10.1007/s00380-017-1017-0. [DOI] [PubMed] [Google Scholar]
- 31.Yildiz I, Rencüzoğulları I, Karabağ Y, Karakayali M, Artac I, Gurevin MS. Predictors of left ventricular ejection function decline in young patients with ST-segment elevation myocardial infarction. Rev Assoc Med Bras. 1992;2022(68):802–807. doi: 10.1590/1806-9282.20220033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saito Y, Oyama K, Tsujita K, Yasuda S, Kobayashi Y. Treatment strategies of acute myocardial infarction: updates on revascularization, pharmacological therapy, and beyond. J Cardiol. 2023;81:168–178. doi: 10.1016/j.jjcc.2022.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the MIMIC-III database, https://mimic.physionet.org/iii/.