Abstract
We analyzed immune response to SARS-CoV-2 vaccination by measuring specific IgG titers and T-cell reactivity to different SARS-CoV-2 peptides in multiple sclerosis patients taking different disease-modifying treatments. Of the 88 patients included, 72 developed any kind of immune response after vaccination. Although DMTs such as fingolimod and anti-CD20+ treatments prevented patients from developing a robust humoral response to the vaccine, most of them were still able to develop a cellular response, which could be crucial for long-term immunity. It is probably advisable that all MS patients take additional/booster doses to increase their humoral and/or cellular immune response to SARS-CoV-2.
1. Introduction
Over 450 million people around the world have been infected with SARS-CoV-2 and around 6 million people have died from this infection (COVID-19) [1]. Multiple sclerosis (MS) is an inflammatory autoimmune disease of the CNS that is often treated with immunomodulatory medications, which in some cases increase the risk of opportunistic infection and infection-related mortality rates [2]. Thus, there have been concerns about the severity of disease in infected patients and the effectiveness of vaccines against SARS-CoV-2.
However, various studies have shown that patients with MS are not at greater risk of infection or severe COVID-19 than the general population [3,[4]. Worse outcomes are more likely in older MS patients and those that suffer from comorbid conditions, such as obesity, which would also be expected in the general population.
Furthermore, the use of disease-modifying therapies (DMTs) that modulate or suppress the immune system is not associated with increased COVID-19 severity [3,5,6], with the possible exception of anti-CD20 therapies, which can reduce the SARS-CoV-2 antibody response due to B cell depletion [7].
Live or attenuated vaccines are contraindicated with almost all MS treatments because of the risk of reactivation of the inoculated microorganism. Inactive vaccines may also be less effective, which is why administration is recommended before the start of DMT treatment. If this is not possible, administration should be carried out during treatment, even if the response is not optimal [8,9]. Careful analysis of the humoral and cellular immune responses elicited by SARS-CoV-2 vaccination in MS patients treated with immunomodulatory drugs is key to understanding the degree of protection conferred and determining the optimal vaccination strategy.
The antibody-driven (humoral) response to SARS-CoV-2 vaccines, dependent on B cells, can wane over a 3–10-week period after a second dose [10], but the cellular response, mediated by T lymphocytes, is thought to be longer lasting, as has been observed in patients who have recovered from SARS and SARS-CoV-2 [11]. The cellular response could also be more resistant to virus mutations in emerging variants than antibodies [12].
Recent studies have shown significant levels of protective cellular immunity to SARS-CoV-2 in MS patients exposed to the virus and after vaccination, even if the vaccine-specific humoral response was impaired [13,14]. For example, there is evidence that patients on anti-CD20 therapies could mount a robust T cell response after SARS-CoV-2 vaccination despite having a reduced humoral response [15,16].
The aim of this study was to investigate the humoral and cellular immunity developed in MS patients taking different DMTs between 4 and 6 weeks after the last dose of a SARS-CoV2 vaccine.
2. Material and methods
2.1. Study design and patients
This prospective, longitudinal study analyzed the antibody titer and cellular immunity in MS patients taking different DMTs and followed at the MS centre in the Princesa University Hospital (Madrid) The samples were taken between 4 and 6 weeks after they received the last dose of the SARS-CoV2 vaccine.
The patients included in this study met the following criteria: (1) MS diagnosed according to the reviewed McDonald criteria 2010 [17] and taking teriflunomide, dimethyl fumarate (DMF), fingolimod, cladribine, natalizumab, ocrelizumab, rituximab or alemtuzumab; (2) Age ≥ 18 years; (3) Fully vaccinated against COVID-19; (4) Signed written informed consent.
Treatment was not suspended in any patient prior to vaccination. All patients taking ocrelizumab and rituximab were vaccinated at least 3 months after the last treatment dose. All patients taking cladribine had an absolute lymphocyte count (ALC) above 600 cells/µl before vaccination. All patients taking alemtuzumab had received the last drug dose at least a year prior to vaccination and none of them had lymphopenia.
This study was completed in accordance with the principles in the Declaration of Helsinki and received approval from the local Research Ethics Committee of La Princesa University Hospital.
Demographic and laboratory data described in Table 1 were collected from electronic clinical records and included in an anonymized database.
Table 1.
Patient data. EDSS, Expanded Disability Status Scale; naïve, patients who have not received any other drugs previously for the treatment of MS; SD, standard deviation; yr, years.
| N | 88 | ||
|---|---|---|---|
| Sex, female | 61% | ||
| Age - mean (SD) | 47.6 (SD 10.8) | ||
| MS duration (yr) - mean (SD) | 11.3 (SD 7.3) | ||
| EDSS - mean (SD) | 3.5 (SD 2.1) | ||
| Years of treatment - mean (SD) | 3.7 (SD 2.4) | ||
| NAIVE | 39% | ||
| MS treatment number - mean (SD) | 1 (SD 1.1) | ||
| DMT | Age – mean | Years of treatment – mean | MS treatment number - mean |
| Teriflunomide (N = 10) | 51.3 | 3.8 | 0.3 |
| Dimethyl-fumarate (N = 9) | 41.9 | 5 | 0.3 |
| Cladribine (N = 13) | 52.6 | 1.8 | 1 |
| Fingolimod (N = 9) | 44.3 | 5.2 | 1 |
| Natalizumab (N = 10) | 41 | 6.3 | 0.8 |
| Ocrelizumab (N = 27) | 47 | 2.5 | 1.2 |
| Rituximab (N = 5) | 62.6 | 4 | 0.6 |
| Alemtuzumab (N = 5) | 45.6 | 4.2 | 3 |
2.2. Baseline evaluation of lymphocyte counts
The distribution of the different leukocyte subsets was characterized by multiparametric flow cytometry 0–10 days before the first vaccine dose, following the Guidelines for the use of flow cytometry and cell sorting in immunological studies. To this end, 200 µl of whole fresh blood were stained with anti-CD45 Pacific Orange, anti-CD3 APC, anti-CD4 FITC, anti-CD8 APC H7, anti-CD19 PerCP, anti-CD27 PE, anti IgD FITC and anti-IgM APC (BD Becton Dickinson). Lymphocyte populations were identified as follows: CD4+ lymphocytes: CD45+ CD3+, CD4+, CD8-; naïve B cells: CD45+ CD3- CD19+ CD27- IgM+ IgD+. Absolute cell numbers were calculated from white blood cell counts obtained with an XN-10 Hematology System (Sysmex, Kobe, Japan- Roche, Basel, Switzerland).
2.3. Humoral response assay
Antibody titers were obtained by SARS CoV-2 IgG II QUANTAlinity (Abbott®, USA). This assay is an automated, two-step immunoassay for the quantitative determination of IgG antibodies against the receptor-binding domain (RBD) of the spike protein S1 subunit of SARS-CoV-2 in human serum, using chemiluminescent microparticle immunoassay (CMIA).
Antibody results were expressed in Binding Antibody Unit/mL (BAU/mL), based on the WHO International Standard study for anti-SARS-CoV-2 immunoglobulin [18]. Results were interpreted following the criteria of the manufacturer, considering <7.1 BAU/mL as negative and ≥7.1 BAU/mL as positive.
The system has an analytical measurement range of 2.98–5680 BAU/mL. When quantification is >5870 BAU/mL, the system reports as >5870 BAU/mL. The company notifies a sensitivity of 99.3% and a specificity of 99.5%.
2.4. T cell response assay
For T cell activation assays, peripheral blood mononuclear cells (PBMCs) were isolated by density-gradient sedimentation using Ficoll-Paque (Panbiotech). Isolated PBMCs were kept at −80 °C or in liquid nitrogen for long-term storage.
For flow cytometry assays, 2 × 105 PBMCs per well were cultured for 16 h in the presence of six different SARS-CoV-2 peptide pools (PepMixTM; JPT Peptide Technologies). Pools included peptides from S1, S2 and RBD from spike (S) protein, VME1 (membrane protein), NCAP (nucleoprotein) and Mpro (Cys-like protease, nsp5) (1 µg/ml). Cells were stimulated with the staphylococcal enterotoxin B (SEB; Sigma Aldrich) (1 µg/ml) or an actin peptide pool (PepMixTM; JPT Peptide Technologies) (1 µg/ml) as positive and negative controls, respectively. We evaluated T cell response in peptide pools different from those derived from S protein in order to assess whether any of the patients had been in contact with SARS-CoV-2 virus.
After stimulation, cells were incubated with a combination of four monoclonal antibodies: anti-human CD69-FITC, CD25-PE, CD5-PE-Cy7 and CD4-Pacific Blue (BD Becton Dickinson) for 30 min. 7-AAD (eBioscience) was added following manufacturer´s instructions.
All the samples were acquired on a BD FACSCanto II flow cytometer (BD Becton Dickinson). CD4+ T cells were identified as positive for CD4 and CD5 markers. Activated cells were defined as double positive for CD69 and CD25 markers [19]. SARS-CoV-2-responsive Th1, Th2, Th17, Th17-derived Th1 and Tfh CD4+ T lymphocytes were defined as CD25+CD69+ cells within CXCR5-CXCR3+CCR6-, CXCR5-CXCR3-CCR6-, CXCR5- CXCR3-CCR6+, CXCR5- CXCR3+CCR6+ and CXCR5+ subsets respectively. Flow cytometry data were analyzed using FlowJo software (BD Becton Dickinson).
Specific T cell response was determined by subtracting the percentage of double positive cells in the presence of actin peptide pool from that obtained with SEB/peptides (specific percentage). A positive result was considered for samples with specific percentage equal or above the median two-fold standard deviation of all negative controls (1.57%). All the data about immune response are showed in supplemental Table 1.
2.5. Statistical analysis
Graphs and statistical analyses were performed with Graph Pad Prism 8 Software (GraphPad Software, USA) and with IBM Statistical Package for Social Science (SPSS, IBM Corp 25.0). Quantitative variables were represented as mean ± standard deviation (SD). For comparison between populations, analysis of variance was performed. For correlation analyses, Pearson's r and Spearman's rho were calculated. P values <0.05 were considered to be statistically significant.
3. Results
3.1. Patient population
Eighty-eight patients with MS (61% female) were evaluated. The mean age was 47.6 years (SD 10.8 years) and the mean disease duration was 11.3 years (SD 7.3). The characteristics of the study cohort are shown in Table 1.
The distribution of treatment was as follows: 10 were taking teriflunomide, 9 dimethyl fumarate (DMF), 13 cladribine, 9 fingolimod, 10 natalizumab, 27 ocrelizumab, 5 alemtuzumab and 5 rituximab.
Six patients were vaccinated with ChAdOx1 nCoV-19 (Oxford-AstraZeneca), 6 with mRNA-1273 (Moderna) and 68 with BNT162b2 (Pfizer/BioNTech). All patients received a complete vaccination schedule before the humoral and cellular immunity were examined.
There were no serious adverse events or relapses related to vaccination. Two patients experienced a pseudo-relapse secondary to post-vaccination fever.
3.2. Pre-vaccination lymphocyte count
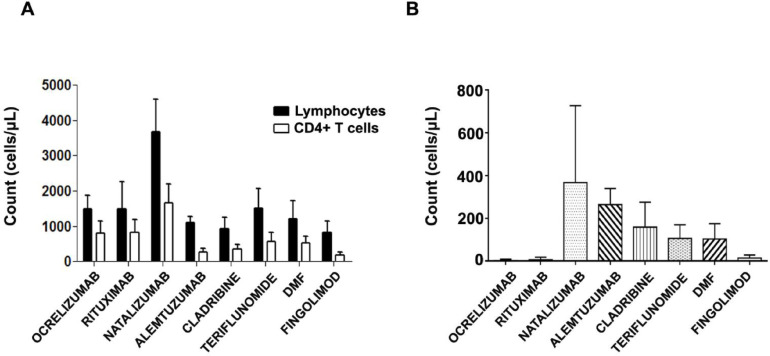
Before vaccination, the ALC, the CD4+T cell count and the CD19+ naïve B cell count were calculated for each patient. The average counts according to treatment are shown in Fig. 1 .
Fig. 1.
Lymphocyte counts in MS patients prior to vaccination according to treatment. A. The graph shows mean+SD of absolute lymphocyte count and CD4+ T-cell count in each treatment group. B. Graphic shows median+SD of naïve B cell count in each treatment group.
Most patients had normal total lymphocyte (between 1000 and 4800 cells/µl) and normal CD4+T cell counts (between 400 and 1400 cells/µl). Lymphopenia was detected among patients taking cladribine, fingolimod and DMF. The number of CD4+T cells in patients taking alemtuzumab was also below the lowest normal limit (277 cells/µl) (Fig. 1A).
The highest total count of naïve B cells was found in patients taking natalizumab (median 390.5 cells/µl) and alemtuzumab (median 235 cells/µl). As expected, the lowest total count of naïve B cells was found in patients taking rituximab and ocrelizumab (Fig. 1B).
3.3. Humoral response to vaccine
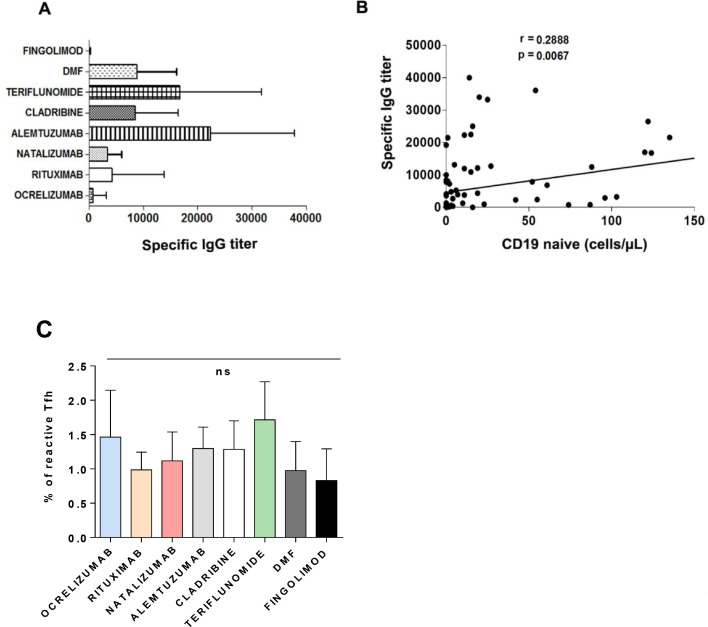
The highest antibody titers were observed in patients taking alemtuzumab (22,432.9 UI/ml), teriflunomide (18,509.5 UI/ml), dimethyl fumarate (8828.36 UI/ml) and cladribine (8571 UI/ml) (Fig. 2 A). Very low specific IgG titers were observed in patients taking fingolimod (95.8 UI/ml), and ocrelizumab (818.3 UI/ml)
Fig. 2.
Humoral response in MS patients after vaccination. A. The graph shows mean+SD of specific IgG titers according to treatment. B. Global correlation between the absolute count of naïve B cells and antibody titers. Pearson's r coefficient is shown. C. Percentages of reactive Tfh cells in all the treatment groups. Graph represents mean+SD; ns, non-significant.
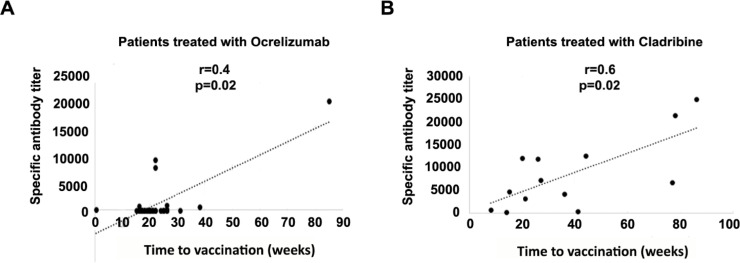
We found a moderately significant positive correlation between the time from ocrelizumab treatment to vaccination (weeks) and the antibody titer (rs=0.403, p = 0.022) (Fig. 3 A), and a highly significant positive correlation between the time from cladribine treatment to vaccination (weeks) and the antibody titer (r = 0.626; p = 0.022) (Fig. 3B).
Fig. 3.
Correlation between specific antibody titer and time from last dose of treatment to vaccination. A. Correlation between specific antibody titer and time from last dose of ocrelizumab treatment to vaccine administration (Time to vaccination). B. Correlation between specific antibody titer and time from last dose of cladribine to vaccine administration (Time to vaccination). Pearson's r coefficient is shown.
Overall, we found a moderately significant correlation (r = 0.2888; p = 0.0067) between the count of CD19+ naïve cells and specific IgG titers (Fig. 2B).
T-follicular helper cells (Tfh) constitute a specialized subset of CD4+ T cells that collaborates in the generation of high-affinity antibodies. We found a SAR-CoV-2 specific circulating Tfh (cTfh) in all the groups with no significant differences among them (Fig. 2C).
When we analyzed possible clinical factors related to the humoral response, we found no correlation between the antibody titers against SARS-CoV-2 production and age (r=−0.015; p = 0.890), disease duration (r=−0.104; p = 0.34) or number of previous DMTs (r=−0.036; p = 0.74). In the multivariate analysis performed including these three factors and current treatment, only the ongoing DMT was independently related to the antibody titers against SARS-CoV-2 (p<0.05) (Data not shown)
3.4. T cell response to vaccine
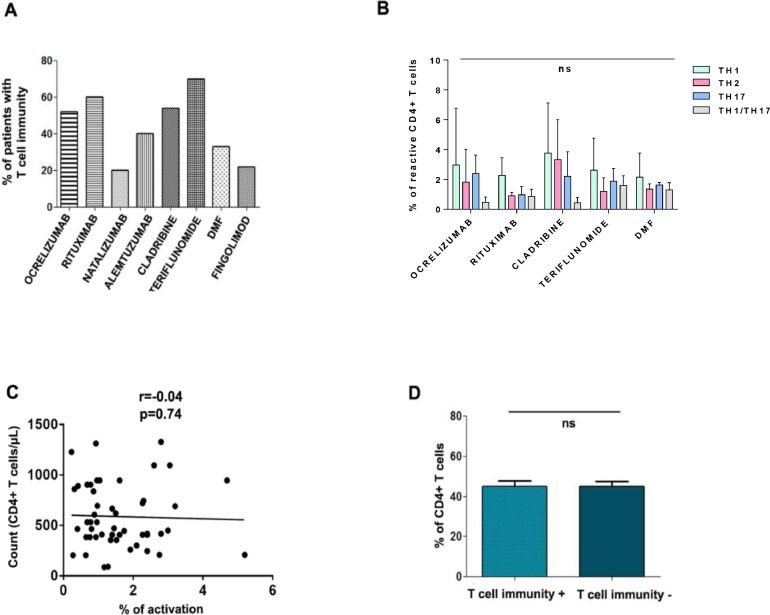
T cell responses against SARS-CoV-2 are mediated mainly by CD4+ T lymphocytes [20,[21], so the development of T cell immunity to vaccination was analyzed specifically in this population. The percentage of patients with T cells that are reactive to different SARS-CoV-2 peptides was calculated 4–6 weeks after the last dose of the vaccine (Fig. 4 A). The highest percentages were observed in patients taking teriflunomide (70%), rituximab (67%) and cladribine (54%). The lowest percentages were observed in those taking natalizumab (20%) and fingolimod (22%). To determine whether these differences were due to a general impairment in CD4+ activation capacity in vitro, the CD4+ cell response to SEB positive control was analyzed. We found no significant differences between treatment groups (Supplementary Figure 1).
Fig. 4.
T-cell reactivity against SARS-CoV-2 in MS patients after vaccination. A. The graph shows the percentage of patients with SARS-CoV-2-reactive T cells 4–6 weeks after the last dose of the vaccine according to treatment type. B. Percentage of reactive Th1, Th2, Th17 and Th1/Th17 SARS-CoV-2-specific CD4+ cells according to treatment type. C. Global correlation between the percentage of CD4+ cells and the percentage of SARS-CoV-2-activated CD4+ cells. Pearson's r coefficient is shown. D. Bars show mean+SD of percentages of CD4+ cells in patients with and without T-cell immunity after vaccination. ns, non-significant.
Because SARS-CoV-2 mRNA vaccines predominantly promote a Th1 response in the general population [22], we examined if there were any differences in the percentage of reactive Th1,Th2, Th-17 and Th-1/Th-17 CD4+ cells in any of the patient groups in which there were more than two patients with a cellular response. Despite a tendency to develop a predominant Th1 response, we found no significant differences between these subsets in any treatment (Fig. 4B).
Further analyses showed that there is no correlation (r=−0.04; p = 0.74) between the CD4+ cell count and the percentage of activated CD4+ (Fig. 4C), and that the percentage of CD4+ cell prior to vaccination does not influence the development of a cellular response (Fig. 4D).
The reactivity of CD4+ T cells to different SARS-CoV-2 peptide pools according to treatment is shown in Supplementary Figure 2. Within treatment groups, different T-cell reactivity profiles are observed after vaccination, with a predominant response towards S1, alone or in combination with other SARS-CoV-2 proteins. Of note, several patients show CD4+ specific response against VME1, which reflects SARS-CoV-2 infection prior to vaccine administration.
3.5. Overall immune response to SARS-CoV-2 vaccination
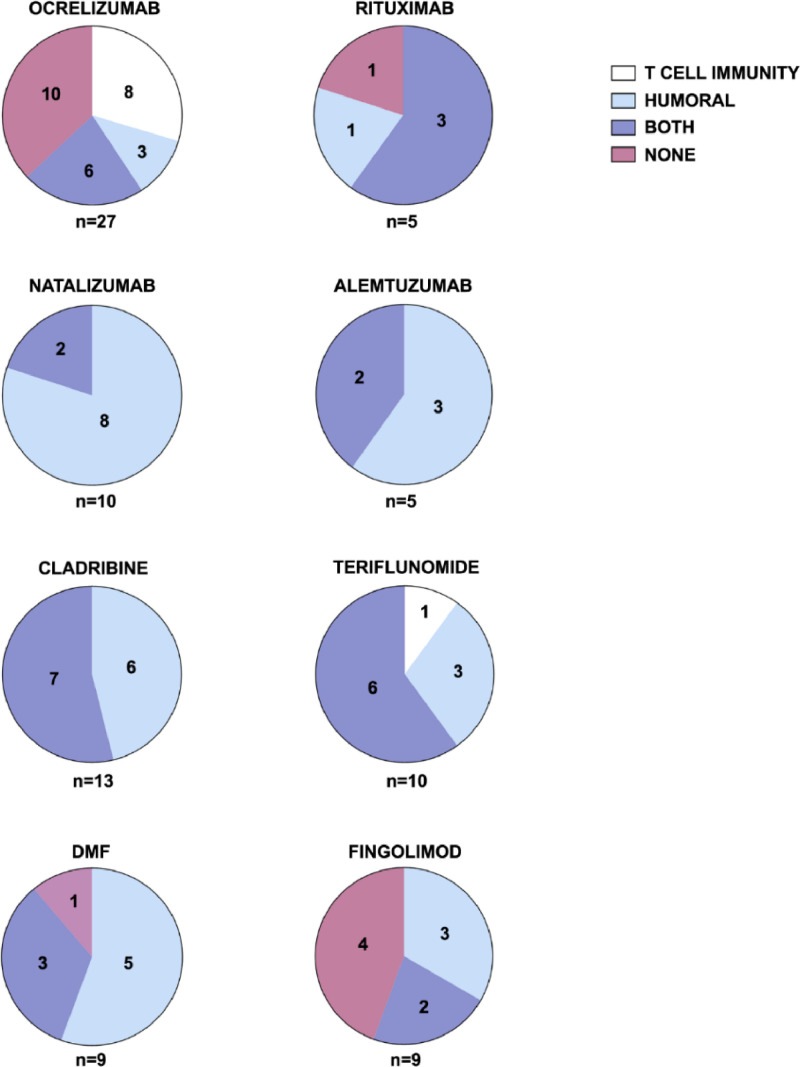
SARS-CoV-2 vaccines elicit different types of immune response in MS patients according to the treatment they are taking (Fig. 5 ). Overall, 72/88 patients (81.8%) developed an immune response (humoral, cellular or both) after vaccination. Nine of the 88 patients (10.2%) developed only a cellular response, 32/88 (36.4%) developed only a humoral response and 16/88 (18.2%) did not develop any type of immune response.
Fig. 5.
Types of immune response that develop following vaccination against SARS-CoV-2 in MS patients according to treatment. Pie charts show absolute counts of patients from each treatment group, which either develop or not cellular immunity, humoral immunity or both after vaccination.
In patients taking ocrelizumab, 14/27 (51%) developed a cellular response and 9/27 (33.3%) developed a humoral response (albeit with very low antibody titers, Fig. 2A). In patients taking rituximab, 3/5 (60%) developed cellular immunity and 4/5 (80%) developed humoral immunity (also with very low antibody titers, Fig. 2A). After a full vaccination course, 10/27 patients taking ocrelizumab (37%) and 1/5 patients taking rituximab (20%) did not develop either humoral or cellular immunity. In the case of patients treated with ocrelizumab, the time between the last drug dose and the administration of the vaccine is a significant factor in the development of antibodies (Fig. 3A).
In patients taking natalizumab, 8/10 (80%) developed a humoral response and 2/10 (20%) developed both a humoral and a cellular response.
Three of the ten patients taking teriflunomide (30%) developed a humoral response and 6/10 (60%) developed both a humoral and cellular response. One patient taking teriflunomide (10%) developed only cellular immunity.
In patients taking DMF, 5/9 (55.5%) developed a humoral response and 3/9 (33.3%) developed both a humoral and cellular response. One patient taking DMF (11.1%) did not develop any type of immune response.
All patients taking cladribine (13) developed a humoral response, with over 50% of them developing coth cellular and humoral response, with a high correlation between the antibody titer and the time between the last drug and the administration.
In patients taking alemtuzumab, 3/5 (60%) developed a humoral response and 2/5 (40%) developed both a cellular and humoral response.
The worst response was found in patients taking fingolimod, with 4/9 (44.4%) not developing humoral or cellular immunity. Five of the nine patients (55.5%) developed a humoral response, albeit with very low antibody titers (Fig. 2A). Only 2/9 (22.2%) of patients developed both a humoral and cellular response.
4. Discussion
In this study we have shown that most MS patients develop an immune response to SARS-CoV-2 shortly after vaccination, however, the response varies according to treatment type.
Sixty percent of patients (6/10) taking teriflunomide developed both humoral and cellular responses after vaccination; one patient (10%) developed only a cellular response and three (30%) developed only a humoral response (Fig. 5). Teriflunomide is known to impair lymphocyte proliferation [25], a process that is not evaluated in our short-term T cell activation assay. Therefore, we cannot rule out an impaired T cell response in a higher proportion of teriflunomide-treated patients.
Thirty three percent of patients taking DMF developed cellular and humoral response and over 55% developed humoral immunity. The absence of cellular immunity in these patients could be due to the presence of lymphopenia.
Patients taking fingolimod had a very low humoral and cellular response. This is consistent with other studies showing that fingolimod can lead to attenuated humoral responses to other vaccines such as the seasonal influenza vaccine and tetanus toxoid boosters [29], [30], [31]. The reduced humoral and cellular immunity is not associated with a worse evolution of patients infected with SARS-CoV-2 [32], suggesting that an attenuated humoral immune response is effective at conferring protection and that a small cellular response is present but may be difficult to detect due to lymphopenia.
The effect of this drug could be explained with its mechanism of action, which involves the sequestration of naïve T and B cells in secondary lymphoid organs. Fingolimod often leads to lymphopenia, limiting the development of an adequate cellular response against a microorganism that the individual has not been exposed to before. It is also possible that our assay is not sensitive enough to detect a cellular response with such a low number of lymphocytes in the samples. Despite these findings, it is not advisable to stop treatment with fingolimod to increase the immune response to the vaccine due to the risk of disease rebound [26]. In new starters, delaying the commencement of treatment to allow time for vaccination could be considered. A third dose of vaccine could also help boost protection in those that are already taking fingolimod.
In patients taking anti-CD20 DMTs (ocrelizumab and rituximab) the humoral response, which is dependent on the CD19+ naïve B cell count, was low. This fact has also been described in other studies [31]. In addition, this response is likely to vary depending on the time of administration of the vaccine with respect to the drug, as well as the repopulation capacity of each patient. Ideally, patients should wait more than 3 months after a course of ocrelizumab before having a vaccine [23]. Accordingly, we found a positive correlation between time from the last dose of ocrelizumab to vaccination, and antibody titers (Fig. 3A), probably due to the repopulation of CD20+ cells.
Despite developing a weak or no humoral response to the SARS-CoV-2 vaccine, we found a cellular response (independent of CD4+ lymphocyte count) in over 50% of patients (Fig. 4). Suppoting this, Apostolidis and colleagues have shown that vaccine-specific T cell responses are comparable between patients with MS treated with ocrelizumab and healthy controls [34]. These findings suggest that vaccinating B cell-deficient patients provides some measure of immunity to SARS-CoV-2, which could confer protection against emerging variants of concern with antibody escape mutations [35].
Interestingly, many of the patients taking anti-CD20 DMTs who did not have cellular response had switched from other treatments like fingolimod. The lack of a cellular response could be a ‘carry over’ effect due to fingolimod's long-half-life and its effects on reversing the CD4+/CD8+ ratio [24]. Our results support the use of vaccines in patients taking anti-CD20 DMTs, and not delaying vaccine administration to wait for B cell repopulation, as this could increase the risk of disease activation. We speculate that even if a third vaccine dose does not stimulate the production of antibodies (if CD19+ have not repopulated to normal levels), it could boost cellular immunity in patients who have not developed it.
A good humoral and cellular response was also observed in patients taking cladribine, with 7/13 (53%) developing both, and 6/13 (46%) developing a humoral response. The timing of vaccination and the presence of lymphopenia in these patients does not seem to affect the development of cellular immunity, but we found a significant positive correlation between antibody titer and time from the last dose of cladribine to vaccine administration (Fig. 3B).
Patients taking alemtuzumab also developed humoral and cellular immunity to SARS-CoV-2 after vaccination. All of them had received the second dose of alemtuzumab at least 12 months prior to vaccination, which could explain the good response.
All patients taking natalizumab developed a humoral response with high antibody titers, but only 20% developed a cellular response. Natalizumab targets α4β1 integrin (VLA-4), which in addition to mediating lymphocyte transmigration across the blood-brain barrier into the central nervous system, has been shown to localize in immune synapses and participate in the development of the Th1 response [27]. Thus, the blockade of VLA-4 by natalizumab could prevent T cell activation by SARS-CoV-2 vaccination. In addition, natalizumab could decrease the functional activity of antigen presenting cells, thus affecting T cell activation [28].
Our findings are also in agreement with a recent study looking at the humoral response in MS patients up to 6 months after the last dose of PfizerBNT162b2 vaccine showing that only 22.8% (22/114) of patients treated with ocrelizumab and 9.5% (4/42) of patients treated with fingolimod developed an anti-SARS-CoV-2 IgG humoral immune response [33].
Anti-SARS-CoV-2 mRNA vaccines primarily generate a Th1 response [22]. In the patients we have studied, despite a tendency towards a predominant Th1 response, there is no significant difference between the percentage of reactive Th1 and Th2 cells. This may be due to the low number of patients in some treatment groups, or to the fact that some treatments such as DMF cause a switch towards Th2 that could be interfering with the post-vaccination response in this regard [36].
Due to the small number of patients enrolled in this study, we were not able to examine whether the immune response varies according to vaccine type (mRNA or viral vector) or previous SARS-CoV-2 infection.
In this study we have not carried out parallel studies in healthy donors. However, we have previously evaluated cellular and humoral immune responses to SARS-CoV-2 vaccination in a comparable cohort of healthy (HD; n = 22) and COVID-19 convalescent (CD; n = 23) donors, using similar experimental settings [37]. In this analysis, we found positive CD4+ T cell responses in up to 60% HD and 81% CD, and specific IgG against RBD in 100% of both HD and CD after vaccination. Thus, although our results in MS patients should be interpreted with caution due the reduced number of patients in some treatment groups, some immunomodulatory therapies seem to mainly affect to T cell response (natalizumab, alemtuzumab, DMF), humoral response (rituximab), or both (ocrelizumab, fingolimod). Conversely, patients treated with cladribine and teriflunomide show little impairment of immune response development after SARS-CoV-2 vaccination.
In addition to the small sample size and the lack of a healthy control group, other limitations of this study are the fact that some cell subtype counts are missing for some patients and the fact that our assay may not have been sufficiently sensitive to detect cellular immunity in cases of lymphopenia.
5. Conclusions
In summary, we have shown that DMTs can affect the response to vaccination against SARS-CoV-2. Although patients taking fingolimod and ocrelizumab are less likely to develop a robust humoral response to the vaccine, they are still able to develop a cellular response. For this reason, vaccination is useful in these patients and booster doses could induce or enhance protection [38].
There are no studies associating a higher antibody titer with greater protection or greater cellular immunity with greater protection. Further longer-term studies are required to understand the changes in cellular and humoral immune responses over time, and their contribution, together or separately, towards lasting protection against infection. Given the need to continue DMTs in many patients, it will be interesting to determine the effects of changing the spacing between vaccine doses and of additional doses to achieve adequate protection against infection whilst keeping MS under control.
5.1. Funding
This work was supported by grants to A.A. from Fondo de Investigación Sanitaria del Instituto de Salud Carlos III [FIS PI22/01542], and Sociedad Cooperativa de Viviendas Buen Suceso, S. Coop. Mad. To A.A. and F.S-M. from CIBER Cardiovascular from the Instituto de Salud Carlos III (Fondo de Investigación Sanitaria del Instituto de Salud Carlos III with co-funding from the Fondo Europeo de Desarrollo Regional; FEDER). To F.S-M. from Banco de Santander and CRUE [“Fondos Supera COVID19”] and Comunidad de Madrid [“Ayuda Covid 2019″ and “Inmunovacter” REACT-UE].
Declaration of Competing Interest
L.E-P., N.R., F.S-M, and A.A. declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We'd like to thank the patients participating in the study for their collaboration and MS patient associations for their daily work, commitment to MS and help. We thank MW and Mónica Hoyos for medical writing assistance [Springer Healthcare Communications] funded by Merck. We also want to thank all health professionals for their tireless work during this pandemic.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.clicom.2023.02.001.
Appendix. Supplementary materials
Data availability
No data was used for the research described in the article.
References
- 1.WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/ (accessed 14th March 2022).
- 2.Klotz L., Havla J., Schwab N., et al. Ther. Adv. Neurol. Disord. 2019;12 doi: 10.1177/1756286419836571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louapre C., Collongues N., Stankoff B., et al. JAMA Neurol. 2020;77:1079–1088. doi: 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaudhry F., Jageka C., Levy P.D., et al. J. Cell Immunol. 2021;3:68–77. doi: 10.33696/immunology.3.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreno-Torres I., Meca Lallana V., Costa-Frossard L., et al. Eur. J. Neurol. 2021;28:3712–3721. doi: 10.1111/ene.14990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salter A., Fox R.J., Newsome S.D., et al. JAMA Neurol. 2021;78:699–708. doi: 10.1001/jamaneurol.2021.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louapre C., Ibrahim M., Maillart E., et al. J. Neurol. Neurosurg. Psychiatry. 2022;93:24–31. doi: 10.1136/jnnp-2021-326904. [DOI] [PubMed] [Google Scholar]
- 8.Riva A., Barcella V., Benatti S.V., et al. Mult. Scler. 2021;27:347–359. doi: 10.1177/1352458520952310. [DOI] [PubMed] [Google Scholar]
- 9.Otero-Romero S., Ascherio A. Curr. Opin. Neurol. 2021;34:322–328. doi: 10.1097/WCO.0000000000000929. [DOI] [PubMed] [Google Scholar]
- 10.Shrotri M., Navaratnam A.M.D., Nguyen V., et al. Lancet. 2021;398:385–387. doi: 10.1016/S0140-6736(21)01642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Bert N., Tan A.T., Kunasegaran K., et al. Nature. 2020;584:457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 12.Skelly D.T., Harding A.C., Gilbert-Jaramillo J., et al. Nat. Commun. 2021;12:5061. doi: 10.1038/s41467-021-25167-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asplund Högelin K., Ruffin N., Pin E., et al. iScience. 2021;24 doi: 10.1016/j.isci.2021.103078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonifacius A., Tischer-Zimmermann S., Dragon A.C., et al. Immunity. 2021;54:340–354. doi: 10.1016/j.immuni.2021.01.008. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gadani S.P., Reyes-Mantilla M., Jank L., et al. EBioMedicine. 2021;73 doi: 10.1016/j.ebiom.2021.103636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sormani M.P., Inglese M., Schiavetti I., et al. EBioMedicine. 2021;72 [Google Scholar]
- 17.Polman C.H., Reingold S.C., Banwell B., et al. Ann. Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.HO/BS.2020.2403. Establishment of the WHO International Standard and Reference Panel for anti-SARS-CoV-2 antibody. https://www.who.int/publications/m/item/WHO-BS-2020.2403 (accessed 19th January 2022).
- 19.Esparcia-Pinedo L., Martínez-Fleta P., Ropero N., Vera-Tomé P., Reyburn H.T., Casasnovas J.M., Rodríguez Frade J.M., Valés-Gómez M., Vilches C., Martín-Gayo E., Muñoz-Calleja C., Sanchez-Madrid F., Alfranca A. Front. Immunol. 2022;12 doi: 10.3389/fimmu.2021.755891. Jan 19eCollection 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grifoni A., Weiskopf D., Ramirez S.I., et al. Cell. 2020;181:1489–1501. doi: 10.1016/j.cell.2020.05.015. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelde A., Bilich T., Heitmann J.S., et al. Nat. Immunol. 2021;22:74–85. doi: 10.1038/s41590-020-00808-x. [DOI] [PubMed] [Google Scholar]
- 22.Sadarangani M., Marchant A., Kollmann T.R. Nat. Rev. Immunol. 2021;21:475–484. doi: 10.1038/s41577-021-00578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buttari F., Bruno A., Dolcetti E., et al. Mult. Scler. Relat. Disord. 2021;52 doi: 10.1016/j.msard.2021.102983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kappos L., Antel J., Comi G., et al. N. Engl. J. Med. 2006;355:1124–1140. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- 25.Bar-Or A., Pachner A., Menguy-Vacheron F., et al. Drugs. 2014;74:659–674. doi: 10.1007/s40265-014-0212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.B Barry, Erwin A.A., Stevens J., Tornatore C. Neurol. Ther. 2019;8:241–250. doi: 10.1007/s40120-019-00160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mittelbrunn M., Molina A., Escribese M.M., et al. Proc. Natl. Acad. Sci. U. S. A. 2004;101(30):11058–11063. doi: 10.1073/pnas.0307927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Andrés C., Teijeiro R., Alonso B., et al. PLoS One. 2012;7(4):e34103. doi: 10.1371/journal.pone.0034103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kappos L., Mehling M., Arroyo R., et al. Neurology. 2015;84:872–879. doi: 10.1212/WNL.0000000000001302. [DOI] [PubMed] [Google Scholar]
- 30.Metze C., Winkelmann A., Loebermann M., et al. CNS Neurosci. Ther. 2019;25:245–254. doi: 10.1111/cns.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bar-Or A., Calkwood J.C., Chognot C., et al. Neurology. 2020;95:e1999–e2008. doi: 10.1212/WNL.0000000000010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sullivan R., Kilaru A., Hemmer B., et al. Neurol. Neuroimmunol. Neuroinflamm. 2021;9:e1092. [Google Scholar]
- 33.Achiron A., Mandel M., Dreyer-Alster S., et al. J. Neuroimmunol. 2021;361 doi: 10.1016/j.jneuroim.2021.577746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Apostolidis S.A., Kakara M., Painter M.M., et al. Nat. Med. 2021;27(11):1990–2001. doi: 10.1038/s41591-021-01507-2. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goel R.R., Painter M.M., Apostolidis S.A., et al. Science. 2021:eabm0829. doi: 10.1126/science.abm0829. Oct 14Epub ahead of print. PMID: 34648302. [DOI] [Google Scholar]
- 36.Wu Q., Wang Q., Mao G., et al. J. Immunol. 2017;198(8):3069–3080. doi: 10.4049/jimmunol.1601532. Apr 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esparcia-Pinedo L., Martínez-Fleta P., Ropero N., et al. Front. Immunol. 2022;12 doi: 10.3389/fimmu.2021.755891. Jan 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mbaeyi S., Oliver S.E., Collins J.P., et al. MMWR Morb. Mortal. Wkly. Rep. 2021;70:1545–1552. doi: 10.15585/mmwr.mm7044e2. PMID: 34735422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.