Abstract
Ferroptosis is a recently identified iron-dependent form of intracellular lipid peroxide accumulation-mediated cell death. Different from other types of cell death mechanisms, it exhibits distinct biological and morphological features characterized by the loss of lipid peroxidase repair activity caused by glutathione peroxidase 4, the presence of redox-active iron, and the oxidation of phospholipids-containing polyunsaturated fatty acids. In recent years, studies have shown that ferroptosis plays a key role in various liver diseases such as alcoholic liver injury, non-alcoholic steatohepatitis, liver cirrhosis, and liver cancer. However, the mechanism of ferroptosis and its regulation on chronic liver disease are controversial among different types of cells in the liver. Herein, we summarize the current studies on mechanism of ferroptosis in chronic liver disease, aiming to outline the blueprint of ferroptosis as an effective option for chronic liver disease therapy.
Keywords: ferroptosis, iron metabolism, liver disease, oxidative stress, cell death
Introduction
Ferroptosis is a type of programmed cell death caused by iron-dependent lipid peroxidation and overproduction of reactive oxygen species (ROS).1 When a disorder of intracellular iron metabolism increases the level of free iron, the latter catalyzes the Fenton reaction, which produces ROS that subsequently induces lipid peroxidation and leads to the accumulation of lipid peroxides.2 Above process not only depletes glutathione (GSH) but also reduces the activity of glutathione peroxidase 4 (GPX4), thereby further preventing lipid peroxide from being metabolized through the GPX4-catalyzed glutathione reductase reaction. Eventually, this disrupts the integrity of cell membranes, leading to ferroptosis.3 Ferroptosis shows specific characteristics such as the disappearance of mitochondrial cristae or even an increase in the membrane density of mitochondria without significant changes in nuclear morphology, with these morphological changes making ferroptosis distinct from other types of cell death.4 In terms of biochemical features, ferroptosis is thought to be characterized by a greater release of polyunsaturated fatty acids (PUFAs), the inhibition of GPX4 activity, the depletion of GSH, and an accumulation of ROS.5 Briefly, there are two main mechanisms involved in ferroptosis: lipid peroxidation6 and unstable iron and genes associated with iron metabolism7 (Figure 1). GPX4, a major neutralizing enzyme of peroxisomal phospholipids (PLOOH),8 is involved in ferroptosis induced by erastin/RSL3, thus inhibiting cystine transfer, promoting cell accumulation of more cysteine and promoting GSH production.9,10 Meanwhile, under certain pathological conditions, persistent parenchymal cell injury or even iron overload in the tissues and organs, an imbalance in iron homeostasis can occur.11 In the presence of excessive Fe2+, lipid peroxidation induces oxidative stress through the Fenton reaction catalyzed by free Fe2+, and this eventually increases cells’ sensitivity to ferroptosis.12
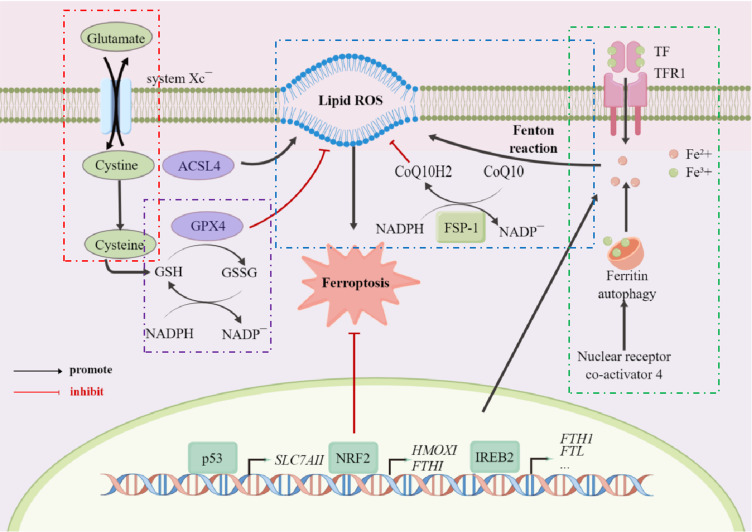
Figure 1.
Schematic illustration of the main mechanisms of ferroptosis.
Notes: The mechanism of ferroptosis is divided into the following parts, namely the transport of cystine (red box), the function of GPX4 (purple box), the transport of iron ions (green box), and the Fenton reaction (blue box). The first is the transport of cystine, which will be transported into the membrane by cystine/glutamate transferase, while glutamate will be transported out. After entering the membrane, cystine, first converted to cysteine, will then be with glutamic acid and glycine, forming reduced glutathione (GSH). GSH forms oxidized glutathione (glutathione disulfide, GSSG) via glutathione peroxidase 4 (GPX4). This process will assist GPX4 in removing the peroxidation of polyunsaturated fatty acids, which form plasma membrane oxygen radicals. At the same time, trivalent iron ions enter the cell membrane as ferritin through TFR1 and are reduced to divalent iron ions after entering the membrane. Divalent iron ions combine with a series of peroxides to produce the Fenton reaction, which in turn produces hydroxyl radicals with strong oxidative properties that promote lipid peroxidation and ultimately lead to the development of ferroptosis.
Chronic liver disease (CLD), such as hepatitis, alcoholic liver disease, and non-alcoholic fatty liver disease, is a global public health concern, causing considerable morbidity and mortality.13 With more and more attention on basic research and clinical research, the important regulatory status and regulatory mechanism of ferroptosis in CLD are gradually revealed.14–16 However, the role of ferroptosis appears to be varied, which may be caused by the different types and characteristics of cells (Figure 2). Therefore, mechanism involved in ferroptosis needs to be detailed and targeting ferroptosis in patients with AH may be a potential hepatoprotective strategy. An updated and concise review of the relevant literature on ferroptosis in CLD caused by different factors is presented here.
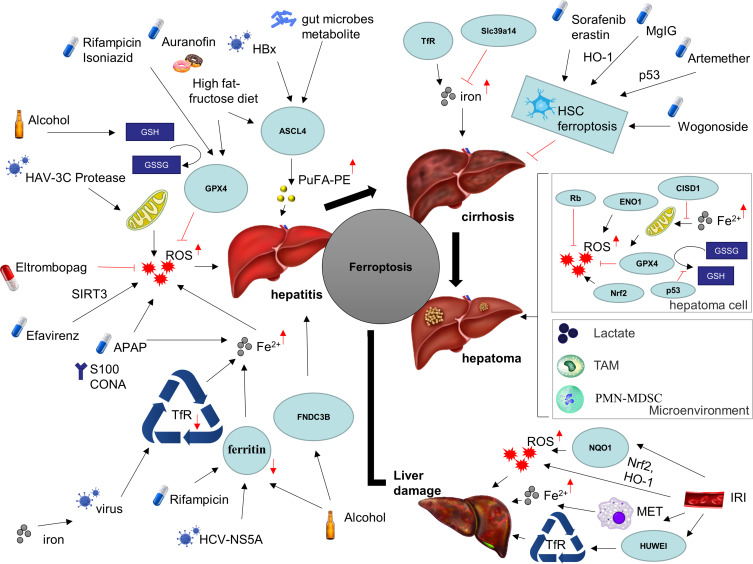
Figure 2.
Ferroptosis in chronic liver diseases.
Note: Ferroptosis has been implicated in a variety of chronic liver diseases, including various etiologies of hepatitis, ischemic liver injury, cirrhosis, and liver cancer.
Abbreviations: HBx, hepatitis B virus X protein; HAV, hepatitis A virus; HCV, hepatitis C virus; ASCL4, achaete-scute family BHLH transcription factor 4; PuFA-PE, polyunsaturated phosphatidylethanolamines; GSH, glutathione; GSSG, oxidized glutathione disulfide; GPX4, glutathione peroxidase 4; ROS, reactive oxygen species; SIRT3, sirtuin 3; APAP, acetaminophen; CONA, Concanavalin A; TfR, transferrin; FNDC3B, fibronectin type III domain containing 3B; Slc39a14, solute carrier family 39 member 14; HO-1, heme oxygenase 1; MgIG, magnesium isoglycyrrhizinate; HSC, hepatic stellate cell; Rb, retinoblastoma gene; ENO1, alpha-enolase 1; CISD1, CDGSH iron sulfur domain 1; Nrf2, nuclear factor erythroid 2-related factor; 2TAM, tumor-associated macrophage cell; PMN-MDSC, pathologically activated neutrophils-termed myeloid-derived suppressor cell.
Ferroptosis in Viral Hepatitis
Serum ferritin and iron metabolism-related indicators, such as hepcidin, are important indicators to evaluate the severity of chronic hepatitis B or chronic hepatitis C.17–20 Mitochondrial potential loss and lipid peroxidation were observed in hepatocytes infected with hepatitis A virus 3C protease,21 while reduction of ferritin was observed in hepatocytes infected with hepatitis C virus NS5A.22 Changes in hepcidin levels in hepatocytes, enterocytes, and macrophages in the presence and absence of HCV were observed, indicating that iron-assisted innate immune responses facilitated viral spreading.23 As an important process of iron metabolism, transferrin binding was decreased by prevention of TfR recycling.24 Previous experiments have also confirmed that SLC7A11 was inhibited by hepatitis B virus X protein.25 Eltrombopag, recommended as a first-line agent for chronic hepatitis B patients, mediated reactive oxygen species production by iron chelation, inhibiting the production of IFN-stimulated genes in monocytes in the antiviral response.26 However, the correlation between viral hepatitis and ferroptosis and the specific treatment strategies related to ferroptosis still remain to be further explored.
Ferroptosis in Drug-Induced Liver Injury
Fenton reactions and ROS accumulation cause ferroptosis in liver when ferrous iron (Fe2+) accumulates in the body.27 Prior research generally confirms that drugs may induce ferroptosis by mitochondrial homeostasis and lipid deposition. Cyproterone acetate, an antitumor drug, caused liver cirrhosis with iron overload.28 Efavirenz,29 rifampicin,30 and isoniazid31 are found to impair ATP biosynthesis by regulating ferritin or ferroptosis-related genes. High doses of auranofin can cause liver lipid peroxidation and ferroptosis by inhibiting the activity of thioredoxin reductase.32 In addition, upregulation of hepatic toxicity, lipid peroxidation, and iron death marker genes happened in the APAP-induced liver,33 which may be responsible for Obvious increasement in Fe2+.34
Ferroptosis in Alcoholic Fatty Liver Disease
Alcoholic liver disease (ALD) is a complex disease progression caused when alcohol is excessively consumed, and it includes conditions such as alcoholic cirrhosis, alcoholic liver fibrosis, alcoholic hepatitis, simple steatosis, or even liver cancer.35 Serum iron and ferritin levels are higher in patients with ALD,36,37 which may be a hallmark of ALD.38 Possible mechanisms include ethanol-enhanced reactive oxygen species accumulation and unstable mitochondrial iron pools39,40 in hepatocyte injury. In addition, alcohol-treated mice were even found to have a significantly lower level of NADPH, a coenzyme of GSH reductase, in their livers.41 In iron overload, the depletion of GSH, followed by the subsequent peroxidation of lipids and inflammation are thought to be characteristic of ferroptosis.42 Besides, deletion of hepatocyte-specific FNDC3B, the role of which in ferroptosis was verified by the administration of the ferroptosis inhibitor ferrostatin-1,43 increased liver steatosis induced by alcohol through inhibition of the AMP-activated protein kinase (AMPK). Taking into account the fact that in ALD, both lipid and iron metabolism are disrupted, ferroptosis is likely to be involved in its pathogenesis.
Ferroptosis in Metabolism-Related Fatty Liver Disease
Metabolism-related fatty liver disease (MAFLD), previously known as non-alcoholic fatty liver disease (NAFLD), is a commonly encountered chronic liver disease that encompasses different conditions ranging from simple hepatic steatosis to non-alcoholic steatohepatitis (NASH) or even cirrhosis.43 A plethora of research work has explored the role and mechanism of iron metabolism, iron deposition, and ferroptosis in MAFLD, and researchers have reviewed and discussed this in considerable detail.44–46 Biopsies confirm poorer long-term outcomes in patients with NAFLD and iron overload,47 which may be due to elevated insulin resistance, excess hepatic lipid peroxidation, and iron overload contributing to the progression of liver fibrosis. GPX4-reduced ferroptosis is a crucial trigger for MAFLD progression to NASH.48 Using sodium selenite as a GPX4 activator attenuated RSL3-induced lipid peroxidation and cell death to reduce the severity of NASH.47 Pan et al analyzed single nucleotide polymorphism in Chinese patients with MAFLD, finding that MAFLD susceptibility may be influenced by the rs4434553 polymorphism in TFR2.49
Moreover, distant organ-liver axis is involved in ferroptosis in MAFLD. Iron is absorbed by duodenal intestinal epithelial cells and then entered into the blood, bound by transferrin, and arrived liver through hepatic portal circulation. Liu et al50 found that bromoacetic acid induced abnormal lipid metabolism. Further investigation using lipidomics and microbial metabolomics unveiled that gut microbiota metabolite glycochenodeoxycholate activated TFR-ACSL4-mediated ferroptosis. Mouth-liver axis was getting in on the act. After oral administration with Porphyromonas gingivalis, amino acid biosynthesis, TCA cycling, and fatty acid and lipid degradation were observed, which are metabolic pathways associated with ferroptosis.51
The above findings suggest that ferroptosis inhibition could be specifically targeted in the treatment of MAFLD.
Ferroptosis in Autoimmune Hepatitis
Autoimmune hepatitis (AIH), an inflammatory lesion of the liver, is characterized by hyperglobulinemia, multiple autoantibodies, and necrotic debris in the portal venous confluence area. It is mainly caused by injury mediated by the immune system and, in the absence of quick and effective treatment, it increases the risk for developing end-stage liver disease.52 The morphological features of autoimmune hepatitis are often variable, but the latter is known for its interface hepatitis on pathological sections as well as the absence of changes typical of other types of liver disease, such as hypergammaglobulinemia, which is more common in women.53 However, the pathogenesis of autoimmune hepatitis, especially the specific processes involved in the death of hepatocytes, are yet to be uncovered. Microarray also suggested the presence of ferroptosis in AIH mice.54 S100-induced autoimmune hepatitis in a mouse model showed that the knockdown of GPX4 promoted the S100-induced accumulation of the lipid peroxide MDA and Fe2+ in liver tissues, hence significantly exacerbating ferroptosis in AIH. Fer-1 is one of the most recognized and typical ferroptosis inhibitors known to reverse S100-induced AIH and subsequent ferroptosis to reduce liver injury.55 Intervention of another typical inhibitor of ferroptosis such as indoleamine 2,3-dioxygenase 1 (IDO1), an intracellular heme enzyme and immune-related modulator involved in the production of Fe2+, also attenuates immune-mediated ferroptosis in mice with liver injury.56 Studies57 have shown that gene knockout of caveolin-1 significantly promoted the massive accumulation of ROS and reactive nitrogen species induced by ConA while Fer-1, a ferroptosis inhibitor, could alleviate ConA-induced AIH.58 Those studies highlight the relevance of ferroptosis to immune-mediated hepatitis, its possible involvement in disease progression in autoimmune hepatitis as an initiator or intermediate mediator, as well as the potential of targeting ferroptosis for improving AIH treatment, thereby providing new perspectives for disease treatment.
Ferroptosis in Hepatic Ischemia-Reperfusion Injury
Hepatic ischemia-reperfusion injury (HIRI) is the result of temporary decrease in tissue blood supply due to vascular reconstruction.59 In 2014, Angeli et al60 proved that ferroptosis was a mechanism included in HIRI. Yamada et al61 analyzed clinical data from pediatric liver transplantation patients and found that iron overload was closely associated with poor prognosis, suggesting the potential effect of ferroptosis in HIRI. Further investigations suggested that nuclear factor erythroid 2-related factor 2 (Nrf2)62 and heme oxygenase 1(HO-1)63 were involved in. In addition, the HECT domain-containing ubiquitin E3 ligase was identified as a negative modulator through TfR in HIRI.64 High-iron diet in HIRI intensified macrophage extracellular trap (MET)65 and iron overload were alleviated after inhibiting METs. Tanshinone, functioning as a coenzyme of NAD(P)H: Quinone oxidoreductase 1 also detoxified lipid peroxy radicals and inhibited ferroptosis in HIRI.66 In liver surgery, the resection of liver lesions requires the blockage of liver blood flow, resulting in different degrees of IRI. The in-depth study of ferroptosis is of great significance for the diagnosis and treatment of IRI.
Ferroptosis in Liver Cirrhosis
For liver cirrhosis treatment, whether ferroptosis is to be inhibited or promoted is in dispute. Gao et al67 found that liver cells secreted iron through external vesicles leading to intracellular iron deficiency, and iron overload existed in hepatic stellate cells (HSC). After iron accumulation, HSC fibrogenic activated as a result of the overproduction of ROS. Yu et al68 also detected accumulation of non-transferrin binding iron in the liver and pathological features of hepatic fibrosis in liver Trf knockout mice. In this regard, possibility of inhibiting ferroptosis for hepatic fibrosis was put forward.
A key characteristic of liver cirrhosis is the excessive deposition of extracellular matrix (ECM) due to the activation of hepatic stellate cell (HSC).69 There was evidence that some drugs could alleviate cirrhosis by promoting HSC ferroptosis. Sorafenib and erastin could promote the expression of autophagy-related gene 16L1 protein, induce the binding of nuclear receptor coactivator 4 to ferritin heavy chain 1, target the degradation of ferritin, and excessive release of iron ions in activated HSC.70 Heme oxygenase 1-induced iron death of HSC is required for magnesium isoglycyrrhizinate to improve liver fibrosis.71 Wogonoside72 and Artemether73 could eventually induce ferroptosis in HSC through P53 pathway.
Ferroptosis in Hepatocellular Carcinoma (HCC)
During tumorigenesis, ferroptosis plays a dual role in promoting and inhibiting tumor,74 which is dependent on the release of damage-associated molecular patterns in the tumor microenvironment and activation of immune responses triggered by iron death injury.
Targeted induction of ferroptosis in HCC cells is a treatment for liver cancer. The first type of drug used to treat advanced hepatocellular carcinoma is usually the multikinase inhibitor Sorafenib, with Louandre et al75 demonstrating that the drug could induce ferroptosis. Since then, the role of ferroptosis in the occurrence and development of HCC has attracted the attention of scholars. The p62-Keap1-NRF2 antioxidant signaling pathway is a key negative regulator involved in the transcriptional activation of ROS and iron-death related genes in HCC cells.76 The results showed that p62-mediated Keap1 degradation led to the activation of Nrf2, and Nrf2 regulatory genes NQO1, HO-1 and FTH1 are inhibited. Jiang et al77 reported that p53 can inhibit the transcription of SLC7A11 and enhanced ferroptosis through the p53 reaction element at 5’ end. Louandre et al78 confirmed that the expression level of Rb was significantly decreased in HCC cells after being treated with sorafenib. Sorafenib could induce ferroptosis by producing mitochondrial ROS, while the inactivation of Rb will increase mitochondrial ROS and enhance oxidative stress. Yuan et al79 treated HepG2 and Hep3B with Alastin, a classical iron death inducer, and found that it could promote the expression of CDGSH iron sulfur domain 1 (CISD1) in an iron-dependent manner. In contrast, the treatment of HCC cells with pioglitazone, an iron-sulfur cluster stabilizer of CISD1, showed that pioglitazone inhibited mitochondrial iron uptake and lipid peroxidation. Alpha-enolase 1 (ENO1) acts as an RNA-binding protein to degrade mRNA, indicating that ENO1 binds and degrades the mRNA of iron regulatory protein 1 gene, thereby regulating the metabolic homeostasis of iron in cells. These results gradually shed light on the mechanism of ferroptosis in liver cancer. The research and development of ferroptosis inducers for tumor targeted therapy, such as arginine-modified vesicular manganese silicate nanobubbles76 and nanobubble-based oxygen-boosted sonodynamic therapy,80 can cause tumor cell death by targeting different ferroptosis-related pathways, which is expected to open up a new picture of precision therapy.
Tumor microenvironment is involved in the process of tumor ferroptosis. The work of Zhao et al81 further revealed the regulatory role of oncometabolite lactate in the tumor microenvironment in the process of ferroptosis in tumor cells. It was confirmed that lactic acid can induce monounsaturated fatty acid formation, which can help HCC cells resist lipid peroxidation induced by oxidative stress. Innate immune cells, including macrophages and neutrophils, are the main components of the tumor microenvironment and promote the dysregulation of iron metabolism in tumor cells.82 On the other hand, ferroptosis of immune cells promotes tumor development. The latest study conducted by Kim et al83 showed that ferroptosis of pathologically activated neutrophils-termed myeloid-derived suppressor cells (PMN-MDSC) in immunologically normal hosts promoted tumor growth by limiting anti-tumor immunity and inducing ferritin hypertrophy promoted tumor growth. Ferritin deposition is a unique immunosuppressant mechanism of PMN-MDSCs in the tumor microenvironment, which can be regulated by targeted drugs to limit tumor development. APOC1 inhibits the development of HCC cancer cells by reversing the M2 phenotype to the M1 phenotype via the iron death pathway of tumor-associated macrophage cells.
Conclusion and Future Research Perspectives
As one of the main organs responsible for iron processing, liver is the first to suffer once iron homeostasis is disrupted. As studies on the mechanism of ferroptosis in the pathogenesis of CLD gradually emerged, diagnosis and treatment strategies of CLD based on ferroptosis are on the agenda.
Ferroptosis is a threat to the survival of any cell, including hepatocytes, stellate cells, Kupffer cells, and cancer cells. However, various properties and roles of different cells in CLD make the clinical feasibility of treating CLD by inhibiting ferroptosis become questionable. Targeted inhibition of ferroptosis was reported to be important for preventing lipid peroxidation-mediated liver injury, inflammatory infiltration, and immune disorders in the development of these diseases, while resistance to targeted therapy needs to be reversed by inducing ferroptosis. There are still many problems worth discussing. What role ferroptosis plays in the evolution from hepatitis to cirrhosis or HCC is still required for further basic and clinical research breakthrough. In addition, how iron transport between intracellular and extracellular compartments affects liver status remains mysterious, due to the constant variation of iron between reduced and oxidized forms.
In conclusion, with the ongoing development of new projects dedicated to basic research on ferroptosis, a full picture of intracellular interactions after ferroptosis is expected, and the development of new diagnostic system and therapeutic strategies based on ferroptosis will become a hot spot of liver disease research in the future.
Funding Statement
This research was supported by a grant from Zhejiang Province Public Welfare Fund Research Project (LGD22H030007), and Open Fund of Zhejiang Provincial Key Laboratory of Precision Diagnosis, Treatment and Translation of Chronic Liver Diseases (No.2020E10014-003 and No.2020E10014-007), and the Wenzhou Science and Technology Bureau basic medical and health science and technology projects (No. Y20210147 and No. 2021Y1691).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Hirschhorn T, Stockwell BR. The development of the concept of ferroptosis. Free Radic Biol Med. 2019;133:130–143. doi: 10.1016/j.freeradbiomed.2018.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finn AV, Nakano M, Polavarapu R, et al. Hemoglobin directs macrophage differentiation and prevents foam cell formation in human atherosclerotic plaques. J Am Coll Cardiol. 2012;59(2):166–177. doi: 10.1016/j.jacc.2011.10.852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou N, Bao J. FerrDb: a manually curated resource for regulators and markers of ferroptosis and ferroptosis-disease associations. Database. 2020;2020. doi: 10.1093/database/baaa021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31(2):107–125. doi: 10.1038/s41422-020-00441-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battaglia AM, Chirillo R, Aversa I, Sacco A, Costanzo F, Biamonte F. Ferroptosis and cancer: mitochondria meet the “iron maiden” cell death. Cells. 2020;9(6):1505. doi: 10.3390/cells9061505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kose T, Vera-Aviles M, Sharp PA, Latunde-Dada GO. Curcumin and (-)- epigallocatechin-3-gallate protect murine MIN6 pancreatic beta-cells against iron toxicity and erastin-induced ferroptosis. Pharmaceuticals. 2019;12(1). doi: 10.3390/ph12010026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song X, Xie Y, Kang R, et al. FANCD2 protects against bone marrow injury from ferroptosis. Biochem Biophys Res Commun. 2016;480(3):443–449. doi: 10.1016/j.bbrc.2016.10.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang W, Green M, Choi JE, et al. CD8 T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569(7755):270–274. doi: 10.1038/s41586-019-1170-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang HM, Tang HL. Cell recovery by reversal of ferroptosis. Biol Open. 2019;8(6). doi: 10.1242/bio.043182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Li J, Kang R, Klionsky DJ, Tang D. Ferroptosis: machinery and regulation. Autophagy. 2021;17(9):2054–2081. doi: 10.1080/15548627.2020.1810918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu J, Eckard J, Chen H, Costa M, Frenkel K, Huang X. Altered iron homeostasis involvement in arsenite-mediated cell transformation. Free Radic Biol Med. 2006;40(3):444–452. doi: 10.1016/j.freeradbiomed.2005.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canli Ã, Alankuåÿ YB, Grootjans S, et al. Glutathione peroxidase 4 prevents necroptosis in mouse erythroid precursors. Blood. 2016;127(1):139–148. doi: 10.1182/blood-2015-06-654194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pimpin L, Cortez-Pinto H, Negro F, et al. Burden of liver disease in Europe: epidemiology and analysis of risk factors to identify prevention policies. J Hepatol. 2018;69(3):718–735. doi: 10.1016/j.jhep.2018.05.011 [DOI] [PubMed] [Google Scholar]
- 14.Gao W, Zhao J, Gao Z, Li H. Synergistic interaction of light alcohol administration in the presence of mild iron overload in a mouse model of liver injury: involvement of triosephosphate isomerase nitration and inactivation. PLoS One. 2017;12(1):e0170350. doi: 10.1371/journal.pone.0170350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi J, Kim J-W, Zhou Z, Lim C-W, Kim B. Ferroptosis affects the progression of nonalcoholic steatohepatitis via the modulation of lipid peroxidation-mediated cell death in mice. Am J Pathol. 2020;190(1):68–81. doi: 10.1016/j.ajpath.2019.09.011 [DOI] [PubMed] [Google Scholar]
- 16.Yamane D, Hayashi Y, Matsumoto M, et al. FADS2-dependent fatty acid desaturation dictates cellular sensitivity to ferroptosis and permissiveness for hepatitis C virus replication. Cell Chem Biol. 2022;29(5):799–810.e4. doi: 10.1016/j.chembiol.2021.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang R, Zhao S, Du J, Qiao J, Li W, Nan Y. A correlation analysis of the serum hepcidin concentrations and viral loads in HCV-infected patients. Am J Transl Res. 2021;13(6):6297–6304. [PMC free article] [PubMed] [Google Scholar]
- 18.Chang M-L, Hu J-H, Yen C-H, et al. Evolution of ferritin levels in hepatitis C patients treated with antivirals. Sci Rep. 2020;10(1):19744. doi: 10.1038/s41598-020-76871-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bian Z, Hann H-W, Ye Z, et al. Ferritin level prospectively predicts hepatocarcinogenesis in patients with chronic hepatitis B virus infection. Oncol Lett. 2018;16(3):3499–3508. doi: 10.3892/ol.2018.9099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo J, Liang X, Xin J, et al. Serum ferritin diagnosis and prediction of hepatitis B virus-related acute-on-chronic liver failure. J Med Virol. 2022;95:e28183. [DOI] [PubMed] [Google Scholar]
- 21.Komissarov AA, Karaseva MA, Roschina MP, et al. Individual expression of hepatitis A virus 3C protease induces ferroptosis in human cells in vitro. Int J Mol Sci. 2021;22(15):7906. doi: 10.3390/ijms22157906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dimitriadis A, Foka P, Kyratzopoulou E, et al. The Hepatitis C virus NS5A and core proteins exert antagonistic effects on HAMP gene expression: the hidden interplay with the MTF-1/MRE pathway. FEBS Open Bio. 2021;11(1):237–250. doi: 10.1002/2211-5463.13048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foka P, Dimitriadis A, Karamichali E, et al. HCV-induced immunometabolic crosstalk in a triple-cell co-culture model capable of simulating systemic iron homeostasis. Cells. 2021;10(9):2251. doi: 10.3390/cells10092251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haberger V, Elgner F, Roos J, Bender D, Hildt E. Regulation of the transferrin receptor recycling in hepatitis C virus-replicating cells. Front Cell Dev Biol. 2020;8:44. doi: 10.3389/fcell.2020.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu G-Z, Xu X-W, Tao S-H, Gao M-J, Hou Z-H. HBx facilitates ferroptosis in acute liver failure via EZH2 mediated SLC7A11 suppression. J Biomed Sci. 2021;28(1):67. doi: 10.1186/s12929-021-00762-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma S, Liu A, Hu X, et al. Eltrombopag inhibits Type I interferon-mediated antiviral signaling by decreasing cellular iron. Biochem Pharmacol. 2021;186:114436. doi: 10.1016/j.bcp.2021.114436 [DOI] [PubMed] [Google Scholar]
- 27.Xing G, Meng L, Cao S, et al. PPARα alleviates iron overload-induced ferroptosis in mouse liver. EMBO Rep. 2022;23(8):e52280. doi: 10.15252/embr.202052280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bessone F, Lucena MI, Roma MG, et al. Cyproterone acetate induces a wide spectrum of acute liver damage including corticosteroid-responsive hepatitis: report of 22 cases. Liver Int. 2016;36(2):302–310. doi: 10.1111/liv.12899 [DOI] [PubMed] [Google Scholar]
- 29.Imaizumi N, Kwang Lee K, Zhang C, Boelsterli UA. Mechanisms of cell death pathway activation following drug-induced inhibition of mitochondrial complex I. Redox Biol. 2015;4:279–288. doi: 10.1016/j.redox.2015.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou J, Tan Y, Hu L, et al. Inhibition of HSPA8 by rifampicin contributes to ferroptosis via enhancing autophagy. Liver Int. 2022;42(12):2889–2899. doi: 10.1111/liv.15459 [DOI] [PubMed] [Google Scholar]
- 31.Pan Y, Tang P, Cao J, et al. Lipid peroxidation aggravates anti-tuberculosis drug-induced liver injury: evidence of ferroptosis induction. Biochem Biophys Res Commun. 2020;533(4):1512–1518. doi: 10.1016/j.bbrc.2020.09.140 [DOI] [PubMed] [Google Scholar]
- 32.Yang L, Wang H, Yang X, et al. Auranofin mitigates systemic iron overload and induces ferroptosis via distinct mechanisms. Signal Transduct Target Ther. 2020;5(1):138. doi: 10.1038/s41392-020-00253-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niu B, Lei X, Xu Q, et al. Protecting mitochondria via inhibiting VDAC1 oligomerization alleviates ferroptosis in Acetaminophen-induced acute liver injury. Cell Biol Toxicol. 2022;38(3):505–530. doi: 10.1007/s10565-021-09624-x [DOI] [PubMed] [Google Scholar]
- 34.Wu L, Ding Q, Wang X, et al. Visualization of dynamic changes in labile iron(II) pools in endoplasmic reticulum stress-mediated drug-induced liver injury. Anal Chem. 2020;92(1):1245–1251. doi: 10.1021/acs.analchem.9b04411 [DOI] [PubMed] [Google Scholar]
- 35.Martin SR, Alvarez F, Anand R, Song C, Yin W. Outcomes in children who underwent transplantation for autoimmune hepatitis. Liver Transpl. 2011;17(4):393–401. doi: 10.1002/lt.22244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki Y, Saito H, Suzuki M, et al. Up-regulation of transferrin receptor expression in hepatocytes by habitual alcohol drinking is implicated in hepatic iron overload in alcoholic liver disease. Alcohol Clin Exp Res. 2002;26(8Suppl):26S–31S. doi: 10.1111/j.1530-0277.2002.tb02698.x [DOI] [PubMed] [Google Scholar]
- 37.Dostalikova-Cimburova M, Balusikova K, Kratka K, et al. Role of duodenal iron transporters and hepcidin in patients with alcoholic liver disease. J Cell Mol Med. 2014;18(9):1840–1850. doi: 10.1111/jcmm.12310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li L-X, Guo -F-F, Liu H, Zeng T. Iron overload in alcoholic liver disease: underlying mechanisms, detrimental effects, and potential therapeutic targets. Cell Mol Life Sci. 2022;79(4):201. doi: 10.1007/s00018-022-04239-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu J, He H, Wang J, et al. Oxidative stress-dependent frataxin inhibition mediated alcoholic hepatocytotoxicity through ferroptosis. Toxicology. 2020;445:152584. doi: 10.1016/j.tox.2020.152584 [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Zhao S, Fu Y, et al. Computational repositioning of dimethyl fumarate for treating alcoholic liver disease. Cell Death Dis. 2020;11(8):641. doi: 10.1038/s41419-020-02890-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu M-J, Cai Y, Wang H, et al. Fat-specific protein 27/CIDEC promotes development of alcoholic steatohepatitis in mice and humans. Gastroenterology. 2015;149(4):1030–1041.e6. doi: 10.1053/j.gastro.2015.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou Z, Ye TJ, Bonavita G, et al. Adipose-specific lipin-1 overexpression renders hepatic ferroptosis and exacerbates alcoholic steatohepatitis in mice. Hepatol Commun. 2019;3(5):656–669. doi: 10.1002/hep4.1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.You Y, Liu C, Liu T, et al. FNDC3B protects steatosis and ferroptosis via the AMPK pathway in alcoholic fatty liver disease. Free Radic Biol Med. 2022;193:808–819. doi: 10.1016/j.freeradbiomed.2022.10.322 [DOI] [PubMed] [Google Scholar]
- 44.Ma C, Han L, Zhu Z, Heng Pang C, Pan G. Mineral metabolism and ferroptosis in non-alcoholic fatty liver diseases. Biochem Pharmacol. 2022;205:115242. doi: 10.1016/j.bcp.2022.115242 [DOI] [PubMed] [Google Scholar]
- 45.Wang S, Liu Z, Geng J, Li L, Feng X. An overview of ferroptosis in non-alcoholic fatty liver disease. Biomed Pharmacother. 2022;153:113374. doi: 10.1016/j.biopha.2022.113374 [DOI] [PubMed] [Google Scholar]
- 46.Feng G, Byrne CD, Targher G, Wang F, Zheng M-H. Ferroptosis and metabolic dysfunction-associated fatty liver disease: is there a link? Liver Int. 2022;42(7):1496–1502. doi: 10.1111/liv.15163 [DOI] [PubMed] [Google Scholar]
- 47.Eder SK, Feldman A, Strebinger G, et al. Mesenchymal iron deposition is associated with adverse long-term outcome in non-alcoholic fatty liver disease. Liver Int. 2020;40(8):1872–1882. doi: 10.1111/liv.14503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsurusaki S, Tsuchiya Y, Koumura T, et al. Hepatic ferroptosis plays an important role as the trigger for initiating inflammation in nonalcoholic steatohepatitis. Cell Death Dis. 2019;10(6):449. doi: 10.1038/s41419-019-1678-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pan X, Peng H, Zhang J, Wu Y, Hu Z, Peng X-E. Genetic variants in promoter region of is associated with the risk of non-alcoholic fatty liver disease in a Chinese Han population: a case-control study. Gastroenterol Rep. 2022;10:goac060. doi: 10.1093/gastro/goac060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu S, Gao Z, He W, et al. The gut microbiota metabolite glycochenodeoxycholate activates TFR-ACSL4-mediated ferroptosis to promote the development of environmental toxin-linked MAFLD. Free Radic Biol Med. 2022;193(Pt 1):213–226. doi: 10.1016/j.freeradbiomed.2022.10.270 [DOI] [PubMed] [Google Scholar]
- 51.Yao C, Lan D, Li X, Wang Y, Qi S, Liu Y. Porphyromonas gingivalis is a risk factor for the development of nonalcoholic fatty liver disease via ferroptosis. Microbes Infect. 2022;25:105040. doi: 10.1016/j.micinf.2022.105040 [DOI] [PubMed] [Google Scholar]
- 52.Montano-Loza AJ, Ronca V, Ebadi M, et al. Risk factors and outcomes associated with recurrent autoimmune hepatitis following liver transplantation. J Hepatol. 2022;77(1):84–97. doi: 10.1016/j.jhep.2022.01.022 [DOI] [PubMed] [Google Scholar]
- 53.Hajoui O, Martin S, Alvarez F. Study of antigenic sites on the asialoglycoprotein receptor recognized by autoantibodies. Clin Exp Immunol. 1998;113(3):339–345. doi: 10.1046/j.1365-2249.1998.00673.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Y, Chen H, Hao J, Li Z, Hou T, Hao H. Characterization and functional prediction of the microRNAs differentially expressed in a mouse model of concanavalin A-induced autoimmune hepatitis. Int J Med Sci. 2020;17(15):2312–2327. doi: 10.7150/ijms.47766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu L, Chen D, Zhu Y, et al. GPX4-regulated ferroptosis mediates S100-induced experimental autoimmune hepatitis associated with the Nrf2/HO-1 signaling pathway. Oxid Med Cell Longev. 2021;2021:6551069. doi: 10.1155/2021/6551069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeng T, Deng G, Zhong W, et al. Indoleamine 2, 3-dioxygenase 1enhanceshepatocytes ferroptosis in acute immune hepatitis associated with excess nitrative stress. Free Radic Biol Med. 2020;152:668–679. doi: 10.1016/j.freeradbiomed.2020.01.009 [DOI] [PubMed] [Google Scholar]
- 57.Selmi C, Generali E, Gershwin ME. Rheumatic manifestations in autoimmune liver disease. Rheum Dis Clin North Am. 2018;44(1):65–87. doi: 10.1016/j.rdc.2017.09.008 [DOI] [PubMed] [Google Scholar]
- 58.Deng G, Li Y, Ma S, et al. Caveolin-1 dictates ferroptosis in the execution of acute immune-mediated hepatic damage by attenuating nitrogen stress. Free Radic Biol Med. 2020;148:151–161. doi: 10.1016/j.freeradbiomed.2019.12.026 [DOI] [PubMed] [Google Scholar]
- 59.Gracia-Sancho J, Casillas-RamÃ-rez A, Peralta C. Molecular pathways in protecting the liver from ischaemia/reperfusion injury: a 2015 update. Clin Sci. 2015;129(4):345–362. doi: 10.1042/CS20150223 [DOI] [PubMed] [Google Scholar]
- 60.Friedmann Angeli JP, Schneider M, Proneth B, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16(12):1180–1191. doi: 10.1038/ncb3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamada N, Karasawa T, Wakiya T, et al. Iron overload as a risk factor for hepatic ischemia-reperfusion injury in liver transplantation: potential role of ferroptosis. Am J Transplant. 2020;20(6):1606–1618. doi: 10.1111/ajt.15773 [DOI] [PubMed] [Google Scholar]
- 62.Ye J, Peng J, Liu K, Zhang T, Huang W. MCTR1 inhibits ferroptosis by promoting NRF2 expression to attenuate hepatic ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2022;323(3):G283–G293. doi: 10.1152/ajpgi.00354.2021 [DOI] [PubMed] [Google Scholar]
- 63.Li X, Wu L, Tian X, et al. miR-29a-3p in exosomes from heme oxygenase-1 modified bone marrow mesenchymal stem cells alleviates steatotic liver ischemia-reperfusion injury in rats by suppressing ferroptosis via iron responsive element binding protein 2. Oxid Med Cell Longev. 2022;2022:6520789. doi: 10.1155/2022/6520789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu Y, Jiao H, Yue Y, et al. Ubiquitin ligase E3 HUWE1/MULE targets transferrin receptor for degradation and suppresses ferroptosis in acute liver injury. Cell Death Differ. 2022;29(9):1705–1718. doi: 10.1038/s41418-022-00957-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu S, Yang J, Sun G, et al. Macrophage extracellular traps aggravate iron overload-related liver ischaemia/reperfusion injury. Br J Pharmacol. 2021;178(18):3783–3796. doi: 10.1111/bph.15518 [DOI] [PubMed] [Google Scholar]
- 66.Wang T-X, Duan K-L, Huang Z-X, et al. Tanshinone functions as a coenzyme that confers gain of function of NQO1 to suppress ferroptosis. Life Sci Alliance. 2023;6(1):e202201667. doi: 10.26508/lsa.202201667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gao H, Jin Z, Bandyopadhyay G, et al. Aberrant iron distribution via hepatocyte-stellate cell axis drives liver lipogenesis and fibrosis. Cell Metab. 2022;34(8):1201–1213.e5. doi: 10.1016/j.cmet.2022.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu Y, Jiang L, Wang H, et al. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood. 2020;136(6):726–739. doi: 10.1182/blood.2019002907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang YM, Noureddin M, Liu C, et al. Hyaluronan synthase 2-mediated hyaluronan production mediates Notch1 activation and liver fibrosis. Sci Transl Med. 2019;11:496. doi: 10.1126/scitranslmed.aat9284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Z, Guo M, Li Y, et al. RNA-binding protein ZFP36/TTP protects against ferroptosis by regulating autophagy signaling pathway in hepatic stellate cells. Autophagy. 2020;16(8):1482–1505. doi: 10.1080/15548627.2019.1687985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sui M, Jiang X, Chen J, Yang H, Zhu Y. Magnesium isoglycyrrhizinate ameliorates liver fibrosis and hepatic stellate cell activation by regulating ferroptosis signaling pathway. Biomed Pharmacother. 2018;106:125–133. doi: 10.1016/j.biopha.2018.06.060 [DOI] [PubMed] [Google Scholar]
- 72.Liu G, Wei C, Yuan S, et al. Wogonoside attenuates liver fibrosis by triggering hepatic stellate cell ferroptosis through SOCS1/P53/SLC7A11 pathway. Phytother Res. 2022;36(11):4230–4243. doi: 10.1002/ptr.7558 [DOI] [PubMed] [Google Scholar]
- 73.Wang L, Zhang Z, Li M, et al. P53-dependent induction of ferroptosis is required for artemether to alleviate carbon tetrachloride-induced liver fibrosis and hepatic stellate cell activation. IUBMB Life. 2019;71(1):45–56. doi: 10.1002/iub.1895 [DOI] [PubMed] [Google Scholar]
- 74.Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021;18(5):280–296. doi: 10.1038/s41571-020-00462-0 [DOI] [PubMed] [Google Scholar]
- 75.Louandre C, Ezzoukhry Z, Godin C, et al. Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib. Int J Cancer. 2013;133(7):1732–1742. doi: 10.1002/ijc.28159 [DOI] [PubMed] [Google Scholar]
- 76.Wang S, Li F, Qiao R, et al. Arginine-rich manganese silicate nanobubbles as a ferroptosis-inducing agent for tumor-targeted theranostics. ACS Nano. 2018;12(12):12380–12392. doi: 10.1021/acsnano.8b06399 [DOI] [PubMed] [Google Scholar]
- 77.Jiang L, Kon N, Li T, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520(7545):57–62. doi: 10.1038/nature14344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Louandre C, Marcq I, Bouhlal H, et al. The retinoblastoma (Rb) protein regulates ferroptosis induced by sorafenib in human hepatocellular carcinoma cells. Cancer Lett. 2015;356(2Pt B):971–977. doi: 10.1016/j.canlet.2014.11.014 [DOI] [PubMed] [Google Scholar]
- 79.Yuan H, Li X, Zhang X, Kang R, Tang D. CISD1 inhibits ferroptosis by protection against mitochondrial lipid peroxidation. Biochem Biophys Res Commun. 2016;478(2):838–844. doi: 10.1016/j.bbrc.2016.08.034 [DOI] [PubMed] [Google Scholar]
- 80.Chen Y, Shang H, Wang C, et al. RNA-seq explores the mechanism of oxygen-boosted sonodynamic therapy based on all-in-one nanobubbles to enhance ferroptosis for the treatment of HCC. Int J Nanomedicine. 2022;17:105–123. doi: 10.2147/IJN.S343361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao Y, Li M, Yao X, et al. HCAR1/MCT1 regulates tumor ferroptosis through the lactate-mediated AMPK-SCD1 activity and its therapeutic implications. Cell Rep. 2020;33(10):108487. doi: 10.1016/j.celrep.2020.108487 [DOI] [PubMed] [Google Scholar]
- 82.Liang W, Ferrara N. Iron metabolism in the tumor microenvironment: contributions of innate immune cells. Front Immunol. 2020;11:626812. doi: 10.3389/fimmu.2020.626812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim R, Hashimoto A, Markosyan N, et al. Ferroptosis of tumour neutrophils causes immune suppression in cancer. Nature. 2022;612:338–346. doi: 10.1038/s41586-022-05443-0 [DOI] [PMC free article] [PubMed] [Google Scholar]