Abstract
Background
Regulation has not kept pace with the growth of the hemp-derived CBD market. We have evaluated the risk of Δ9-tetrahydrocannabinol (Δ9-THC) contamination in 80 unregulated products with comparison to a regulated control, Epidiolex®.
Methods
Local and national brands of hemp-derived oil products were purchased online and from local retailers in central Kentucky (which carry both national and local brands). These were extracted by solvent extraction and quantified by liquid-chromatography tandem mass-spectrometry (LC-MS/MS) using a validated method.
Results
Of the 80 unregulated products and Epidiolex®, Δ9-THC was detected above the limit of quantification (LOQ = 0.005 mg/mL) of the assay in 52 samples, ranging from 0.008 mg/mL to 2.071 mg/mL. Twenty-one of the products tested were labelled as “THC-Free”, and 5 of these products contained detectable levels of Δ9-THC ranging from 0.015 mg/mL to 0.656 mg/mL.
Conclusions
Consumers are taking hemp-derived CBD products without understanding the risks of unintentional consumption of Δ9-THC. This accidental use of Δ9-THC could have adverse effects on health and safety as well as potential legal consequences (e.g., child custody, impaired driving), as Δ9-THC drug test findings could impact employment, military, and sport eligibility status.
Keywords: Δ9-THC, CBD, contamination, regulatory, workplace drug testing, sport doping
1. Introduction
Hemp derived products are increasingly available in retail stores and through online retailers throughout the United States in part due to the passage of the Agricultural Improvement Act of 2018 (2018 Farm Bill) which permitted sales of hemp products, including cannabidiol (CBD). CBD, a cannabinoid with limited to no abuse potential (Babalonis et al., 2017; Schoedel et al., 2018; Viudez-Martínez et al., 2019), has been a driver of the market growth of the hemp-derived/CBD industry due to purported therapeutic benefits. There is one FDA-approved CBD product currently on the market (Epidiolex®) leaving the vast majority of the CBD products sold in the United States unregulated. These unregulated products are sold both online and in various marketplaces and are not currently considered drugs nor legal dietary supplements by the FDA (FDA 2021; Gurley et al., 2020). Although the FDA has utilized authority under the Federal Food, Drug and Cosmetic Act (FD&C Act) to enforce some regulation of hemp-derived CBD products including false marketing claims, (Wagoner et al., 2021) there is no regulation or oversight regarding the contents of these products. This has allowed CBD products to be sold to consumers that contain 1) no measurable CBD, 2) various concentrations of synthetic cannabinoids as well as other drugs, and 3) other contaminants including residual solvents and heavy metals (Bonn-Miller et al., 2017; Dubrow et al., 2021; Gurley et al., 2020; Liebling et al., 2020).
Recent reports indicate that many CBD products may contain appreciable concentrations of the psychoactive cannabinoid Δ9-tetrahydrocannabinol (Δ9-THC) (Bonn-Miller et al., 2017; Dubrow et al., 2021; Gurley et al., 2020; Hazekamp, 2018; Liebling et al., 2020; Pavlovic et al., 2018). This has likely occurred due to an imprecise definition of the federal legal limit of Δ9-THC that is permitted in CBD products, along with little to no regulatory oversight of their manufacture, sale, and distribution. For example, the 2018 Farm Bill defined hemp as Cannabis sativa L. plant/plant parts containing concentrations of Δ9-THC of no more than 0.3 percent of dry weight (Agricultural Improvement Act, 2018). This definition, based on the content of plant material from which the product is derived, offers no clear guidelines on how much Δ9-THC is permitted in finished products such as oils, gummies and salves (as the plant biomass has been removed). Additionally, the 2018 Farm Bill leaves further regulation of hemp and hemp-derived products, beyond the general definition, up to the individual states. As such, an inconsistent patchwork of laws has emerged leading to a lack of clear guidance for consumers and producers. The FDA released guidance on how to calculate Δ9-THC content in hemp-derived products for investigation of new drug (IND) applications and new drug applications (NDA) (FDA, 2020a); however, this guidance has not been adopted for unregulated hemp-derived CBD products that make up the vast majority of the current market.
Although previous studies reported on the range of Δ9-THC found across the CBD products or the number of products testing above the limit of quantification (LOQ), there is little information quantifying the amount (mg/mL) of Δ9-THC in each of the products and none were validated against a regulated control (Bonn-Miller et al., 2017; Dubrow et al., 2021; FDA 2020b; Gurley et al., 2020). The aim of the present study was to perform detailed quantitative analysis of the Δ9-THC content in unregulated hemp-derived CBD products validated against a regulated control. The current study randomly sampled hemp-derived products available for purchase online and at various stores in central Kentucky. The Δ9-THC content of each sample was determined by internally controlled LC-MS/MS analysis. None of the products tested reported the quantity of Δ9-THC content on the label nor did they suggest that the product had the possibility of containing Δ9-THC. The current study details the Δ9-THC concentrations for 80 unregulated products as well as Epidiolex®, a regulated CBD product derived from Cannabis sativa L.
2. Material and Methods
2.1. Sample Selection
Eighty unregulated hemp-derived CBD oil products were tested including 51 different brands (both national and local brands). These products were purchased from 21 online retailers and from 9 local (brick and mortar) retailer sources between April 2 to May 9, 2021. The inclusion of online retailers ensured the inclusion of a representative selection of products produced in a variety of locations outside of Kentucky. Epidiolex® (the FDA-approved CBD product) was also analyzed (UK Investigational Drug Service Pharmacy) to serve as regulated control.
Each product was randomly assigned a study identifier to blind researchers to product identification. Products were stored according to packaging instructions or in a cool, dry space if no instructions were provided. All products were tested immediately after opening and all were tested well before their stated expiration dates.
2.2. Reagents and standards
Reference materials were purchased from two different suppliers with ISO17025 and ISO17034 accreditation for the preparation of calibrator samples and quality control samples. Δ9-THC was purchased from Cayman Chemical (Ann Arbor, MI, USA) as a certified reference material for the preparation of calibrator samples and from Dr. Ehrenstorfer (LGC Standards, Manchester, NH, USA) as a certified reference material for the preparation of quality control samples. Δ9-tetrahydrocannabinol-d9 (Δ9-THC-d9) was sourced from Cayman Chemical. Reagents and solvents (LC/MS grade) for use during the extraction and analysis were purchased from Fisher Scientific (Hampton, NH, USA). Extra virgin olive oil, which was used as an analyte-free matrix was obtained from a local grocery retailer (Kroger, Cincinnati, OH, USA).
2.3. Method Validation
The validation of the method for the analysis of Δ9-THC included studies of linearity, accuracy, precision, recovery, and matrix effects. The accuracy and precision of the method was determined by the analysis of 3 calibrator and control sets with each set consisting of 8 calibrators, ranging from 0.005 to 1.500 mg/mL, and 6 control samples replicates prepared at each of 4 different quality control levels (0.005, 0.020, 0.600, and 1.200 mg/mL) across the calibration range. Recovery of Δ9-THC was determined by comparison of pre-extraction supplemented samples and post-extraction supplemented samples. In the same experiment, matrix effect was determined by the comparison of post-extraction supplemented samples to neat samples, containing no matrix.
2.4. Sample Preparation
Prior to analysis, all sample containers were inverted multiple times to ensure contents were thoroughly mixed. Sub-aliquots, 3 replicates of 50 μL, of products were taken and transferred to appropriately labelled containers where internal standard, 10 μL, was added for a concentration of 0.020 mg/mL. After mixing, a volume of 2.5 mL of acetonitrile was added and the samples were further mixed, then centrifuged (1811 × g, 20 mins). A 50 μl sub-portion of the supernatant and transferring it to an autosampler vial and diluting with solvent and water to form a sample within an appropriate concentration range and composition (nominally 50:50 acetonitrile: water v:v) for analysis. The samples were capped and briefly vortex mixed prior to analysis by liquid chromatography – tandem mass spectrometry (LC-MS/MS). Samples with analyte concentrations above the calibration range were re-analyzed with dilution in solvent (10-fold) prior to internal standard addition.
2.5. Instrumentation
Analysis of samples was carried out via LC-MS/MS using a Thermo Accela 1250 quaternary LC system coupled with a TSQ Vantage mass spectrometer (Waltham, MA, USA). Separations were carried out using a reversed phase (C8) Kinetex® analytical column (2.1 × 100 mm, 2.6 μm) purchased from Phenomenex (Torrance, CA, USA). A gradient solvent program was employed using mobile phases of 0.1% formic acid in water (A) and in acetonitrile (B). Briefly, from a starting composition of 50% B, the percentage of organic mobile phase (i.e., B) was increased to 65% B over 10 minutes, then an organic flush employed to remove residual matrix components before returning to the solvent starting composition. The solvent flow rate was 500 μL/min, and the total analytical run time was 14.25 min. Through the use of reference material, the method was demonstrated to chromatographically separate Δ9-THC from common interferences such as CBD and Δ8-tetrahydrocannabinol (Δ8-THC).
The mass spectrometer was equipped with an electrospray ionization (ESI) source operated in positive ion mode using selective reaction monitoring (SRM). Monitored transitions for Δ9-THC and its internal standard are listed in Table 1.
Table 1.
Selective reaction monitoring (SRM) transitions for Δ9-THC and Δ9-THC-d9 (internal standard).
| Analyte | Precursor Ion (m/z) | Product Ions (m/z) |
|---|---|---|
| Δ9-tetrahydrocannabinol (Δ9-THC) | 315.2 | 193.1* |
| 259.2 | ||
| 123.1 | ||
| Δ9-tetrahydrocannabinol -d9 (Δ9-THC-d9) | 324.2 | 202.1* |
| 268.2 | ||
| 122.9 |
quantifier ion
3. Results
3.1. Method Validation
The purpose of the validation study was to determine the method performance for the quantification of Δ9-THC in oil matrix. For the calibration range, 0.005 to 1.500 mg/mL, a linear calibration model was used with 1/x2 weighting and the coefficient of determination (R2) was greater than 0.99 for all batches. The inter-batch accuracy and precision of the 3 batches were calculated for each quality control sample level and the results are shown in Table 2. Recovery of Δ9-THC was 96%, and a minor matrix effect, ion enhancement, was observed of +1%. The method was demonstrated to be suitable for the quantitative analysis of Δ9-THC.
Table 2.
Summary of method validation studies characterizing Δ9-THC quantification
| Quality control samples | 0.005 mg/mL | 0.020 mg/mL | 0.600 mg/mL | 1.200 mg/mL |
| Mean concentration | 0.005 mg/mL | 0.021 mg/mL | 0.620 mg/mL | 1.158 mg/mL |
| Standard deviation | 0.0005 mg/mL | 0.0009 mg/mL | 0.0232 mg/mL | 0.0392 mg/mL |
| Relative error | 4.1 % | 6.4 % | 3.3 % | −3.5% |
| Inter-batch Coefficient of Variation | 10.4 % | 4.4 % | 3.7 % | 3.4 % |
3.2. Δ9-THC Determination in Products
The Δ9-THC content was determined from 81 products including Epidiolex®. Data are reported as the mean of 9 sample measurements from 3 extractions from the source product, each divided into 3 separate samples for individual analysis, ± standard error of mean (SEM). Of the 80 unregulated products analyzed in this study, Δ9-THC was not detected in 29 products (36%). As shown in Figure 1, the Δ9-THC concentration for the 51 products (64% of the unregulated products sampled) ranged from 0.008 mg/mL to 2.071 mg/mL. The mean concentration across these products was 0.620 mg/mL, and the median was 0.640 mg/mL. Epidiolex®, included as a regulated control for comparison, contained a Δ9-THC concentration of 0.022 mg/mL (± 0.001). The mean Δ9-THC concentration (± SEM) for each product is reported in Tables 3 – 5. Table 3 lists the Δ9-THC concentrations for the 11 products with a result greater than 1 mg/mL, while the results for the 17 products with a Δ9-THC concentration ranging from 1 mg/mL to 0.5 mg/mL are listed in Table 4. Table 5 details the results for the 24 products with a Δ9-THC concentration ranging from 0.5 mg/mL to 0.005 mg/mL. Of the products tested, 21 carried a label of “THC Free” - 5 of these products (24%) contained detectable levels of Δ9-THC (0.015 to 0.656 mg/mL) and are noted in Tables 4 and 5.
Figure 1.
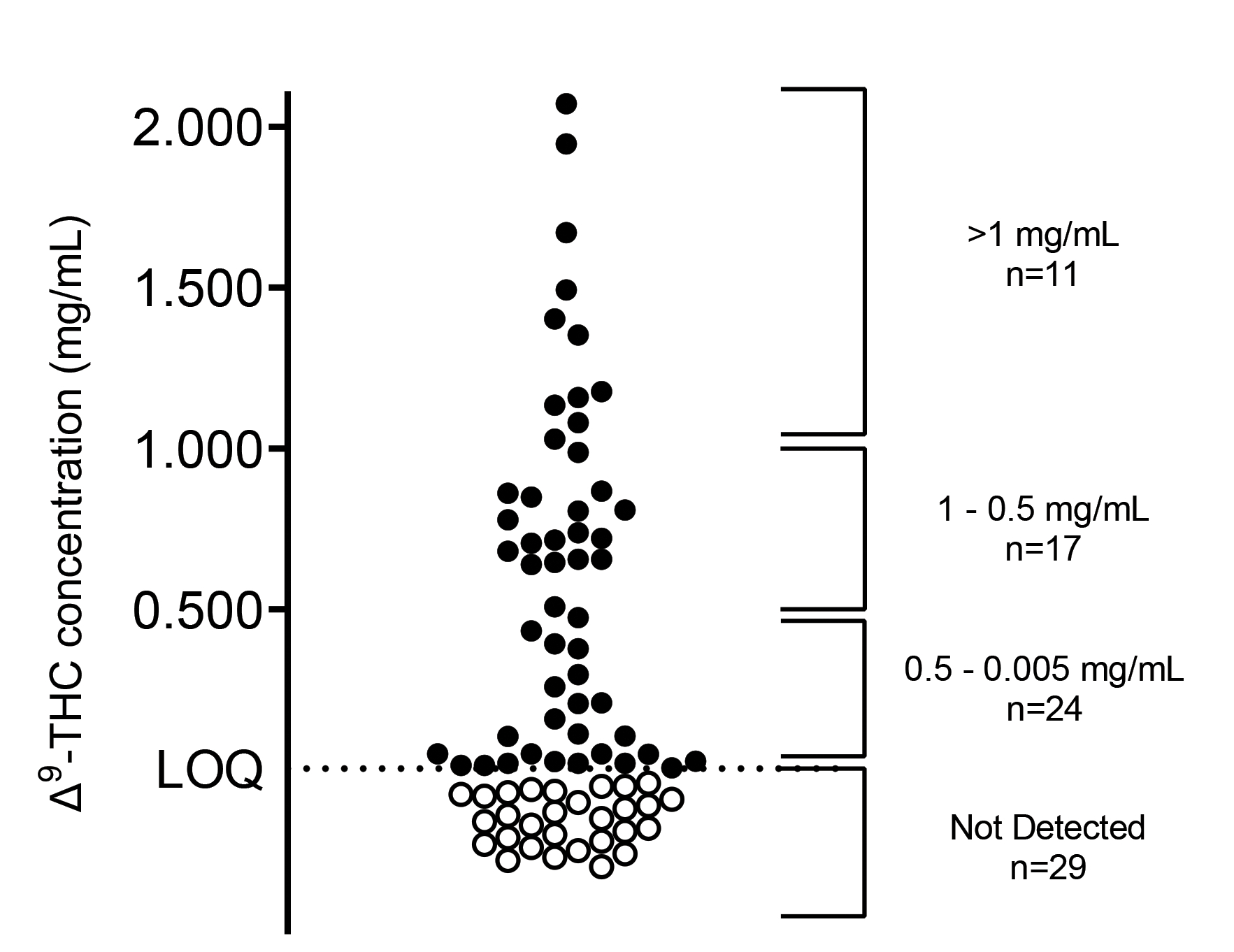
Δ9-THC concentrations in 80 commercially available hemp-derived oil products and Epidiolex®.
Table 3.
Δ9-THC concentration (± standard error of the mean) with a result greater than 1 mg/mL in the samples tested.
| Sample Identifier | Product Label | Δ9-THC concentration mg / mL (±SEM) |
|---|---|---|
| 34 | Full Spectrum | 2.071 (± 0.041) |
| 60 | Full Spectrum | 1.946 (± 0.029) |
| 82 | Full Spectrum | 1.671 (± 0.032) |
| 32 | Full Spectrum | 1.492 (± 0.015) |
| 43 | Full Spectrum | 1.403 (± 0.007) |
| 9 | Full Spectrum | 1.353 (± 0.011) |
| 44 | Full Spectrum | 1.176 (± 0.010) |
| 4 | Full Spectrum | 1.158 (± 0.007) |
| 33 | Full Spectrum | 1.134 (± 0.007) |
| 28 | Full Spectrum | 1.080 (± 0.008) |
| 47 | Full Spectrum | 1.029 (± 0.009) |
Table 5.
Δ9-THC concentration (± standard error of the mean) with a result between 0.5 mg/mL and 0.005 mg/mL in the samples tested.
| Sample Identifier | Product Label | Δ9-THC concentration mg / mL (±SEM) |
|---|---|---|
| 57 | Full Spectrum | 0.474 (± 0.003) |
| 19 | Full Spectrum | 0.433 (± 0.004) |
| 38 | Full Spectrum | 0.393 (± 0.004) |
| 39 | Full Spectrum | 0.378 (± 0.004) |
| 49 | Full Spectrum | 0.297 (± 0.003) |
| 11 | Full Spectrum | 0.259 (± 0.003) |
| 48 | Full Spectrum | 0.210 (± 0.002) |
| 13 | Full Spectrum | 0.207 (± 0.002) |
| 36 | Full Spectrum | 0.159 (± 0.001) |
| 55 | Broad Spectrum | 0.113 (± 0.003) |
| 37 | Full Spectrum | 0.106 (± <0.001) |
| 30 | Full Spectrum | 0.105 (± 0.003) |
| 5 | Full Spectrum | 0.051 (± 0.001) |
| 7 | Full Spectrum | 0.051 (± <0.001) |
| 17* | Broad Spectrum | 0.051 (± <0.001) |
| 64* | Broad Spectrum | 0.050 (± 0.002) |
| 74 | Broad Spectrum | 0.028 (± <0.001) |
| 54* | CBD Isolate | 0.027 (± <0.001) |
| 83 | Control | 0.022 (± 0.001) |
| 27 | Broad Spectrum | 0.022 (± <0.001) |
| 67 | Broad Spectrum | 0.022 (± 0.001) |
| 6 | CBD Isolate | 0.016 (± <0.001) |
| 12* | CBD Isolate | 0.015 (± <0.001) |
| 52 | CBD Isolate | 0.008 (± <0.001) |
‘No THC’ label claim
Table 4.
Δ9-THC concentration (± standard error of the mean) with a result between 1 mg/mL and 0.5 mg/mL in the samples tested.
| Sample Identifier | Product Label | Δ9-THC concentration mg / mL (±SEM) |
|---|---|---|
| 29 | Full Spectrum | 0.987 (± 0.011) |
| 35 | Full Spectrum | 0.867 (± 0.004) |
| 2 | Full Spectrum | 0.861 (± 0.008) |
| 22 | Full Spectrum | 0.849 (± 0.009) |
| 24 | Full Spectrum | 0.809 (± 0.012) |
| 58 | Full Spectrum | 0.806 (± 0.007) |
| 3 | Full Spectrum | 0.778 (± 0.005) |
| 56 | Full Spectrum | 0.738 (± 0.012) |
| 53 | Full Spectrum | 0.720 (± 0.007) |
| 21 | Full Spectrum | 0.715 (± 0.004) |
| 16 | Full Spectrum | 0.706 (± 0.006) |
| 1 | Full Spectrum | 0.680 (± 0.003) |
| 51* | Full Spectrum | 0.656 (± 0.006) |
| 31 | Full Spectrum | 0.655 (± 0.007) |
| 23 | Full Spectrum | 0.646 (± 0.010) |
| 20 | Full Spectrum | 0.640 (± 0.007) |
| 40 | Full Spectrum | 0.508 (± 0.005) |
‘No THC’ label claim
4. Discussion
The lack of clear regulations for hemp-derived products leaves consumers at risk of unintentional Δ9-THC exposure. The present study of 80 hemp-derived CBD oil products represents a cross section of nationally distributed brands as well as brands reported to be local to Kentucky. Epidiolex® was included as a regulated control to allow for a comparison of quality standards between an FDA approved drug to unregulated products. These data clearly demonstrate that with the lack of transparent and accurate label information stating a specific amount of Δ9-THC in the product by volume, consumers have no choice but to suspect the presence of Δ9-THC in hemp-derived CBD products.
The results of the current study align with several other recent studies that have reported many commercially available CBD products, readily available and sold over the counter, contain Δ9-THC (Bonn-Miller et al., 2017; Dubrow et al., 2021; Gurley et al., 2020; Pavlovic et al., 2018). Due to the possibility of intoxication or impairment, it is important for the consumer to understand the possibility for CBD products to contain Δ9-THC.
Here we report that 52 products contained detectable concentrations of Δ9-THC; 11 products had concentrations ≥ 1 mg/mL and one product contained ≥ 2 mg/mL. The regulated control, Epidiolex®, contained 0.022 mg/mL of Δ9-THC. Of the products that contained Δ9-THC, the mean concentration was 0.620 mg/mL (median = 0.640 mg/mL). The product labels suggest that consumers take between 1–3 doses per day (dose is usually defined as 1 mL); the available data suggest that consumers generally follow this advice (Corroon and Phillips, 2018). If a consumer takes 1mL of a product with an average amount of Δ9-THC (0.620 mg) three times per day, they would be exposed to 1.86 mg of Δ9-THC per day. If they took the product containing the highest concentration of CBD (2.071 mg) three times per day, they would be exposed to 6.213 mg of Δ9-THC per day. For comparison, the starting dose for dronabinol, synthetic oral Δ9-THC, is 2.5 mg (Solvay Pharamaceuticals, 2017) and the National Institute on Drug Abuse (NIDA) has established a 5 mg dose of inhaled Δ9-THC as the standard unit for research (NIDA, 2021). Although these examples are not perfect comparators (due to differences in bioavailability, route of administration, and oral product preparations), the overall concern is that consumers could be unknowingly exposed to relatively high doses of Δ9-THC when taking unregulated CBD products. In addition, it should be noted Δ9-THC exposure is predicted to be even greater with repeated daily dosing (versus acute dosing) due to drug accumulation. As Δ9-THC is highly lipophilic, bioaccumulation occurs in tissues such as brain, lung, heart, adipocytes, and liver and results in its slow released from body stores (Huestis, 2007). Recent studies support this and have reported the presence of urine 9-carboxy-11-nor- Δ9-THC (i.e., THC-COOH, an inactive Δ9-THC metabolite) in urine samples after multi-day administration of CBD oil products. (Dahlgren et al., 2021; Peters et al., 2021)
There are some data to suggest that this unintentional Δ9-THC exposure can have medical consequences, particularly for the paediatric population. For example, Herbst and Musgrave reported a case study of a 9-year-old child with refractory epilepsy presenting symptoms of an accidental Δ9-THC overdose after consuming a CBD oil product that unknowingly contained Δ9-THC. After evaluation in the emergency department, the child was admitted to the paediatric intensive care unit. The child’s toxicology report indicated a urine 9-carboxy-11-nor- Δ9-THC (i.e., THC-COOH, an inactive Δ9-THC metabolite) concentration of 123 ng/mL. (Herbst and Musgrave, 2020) By comparison, the THC-COOH urine threshold for a positive workplace drug test is 15 ng/mL. In general, Δ9-THC can produce more serious consequences in the paediatric population than seen in adults (Richards et al., 2017) and caution is warranted when administering unregulated CBD products to children. Overall, medical consequences of CBD ingestion are increasing, and the American Association of Poison Control Centres released an alert regarding accidental CBD-related poisonings. In 2021, there were 4,680 cases (adult and paediatric – up from 2,226 cases in 2020) related to CBD, and the agency has warned that unintentional Δ9-THC ingestion may be playing a role in these poisoning events (AAPCC, 2021).
In addition to safety concerns, consumers of CBD products must also consider the potential impact of unintentional Δ9-THC consumption on drug testing outcomes such as biospecimen testing in the workplace, limits for driving, criminal justice system testing, and sport doping. For workplace drug testing programs in the United States, the urinary thresholds for THC-COOH (inactive metabolite) are 50 ng/mL for immunoassay tests and often 15 ng/mL for confirmatory by gas chromatography-mass spectrometry (GC-MS) (SAMHSA, 2017). The World Anti-Doping Agency (WADA) and United States Anti-Doping Agency (USADA) have a threshold for THC-COOH of 150 ng/mL, (WADA, 2021). There is no widely accepted daily dose or total amount of Δ9-THC that an individual can consume and stay below these limits preventing a positive drug test. However, some studies have suggested that positive drug tests can occur with doses of less than 0.4 mg of Δ9-THC per day (Bosy and Cole, 2000; Gustafson et al., 2003; Leson et al., 2001; Schlienz et al., 2018) – well below the mean concentration of the products sampled here. A joint report from Centre for Medical Cannabis (CMC), Association of the Cannabinoid Industry (ACI), and Conservative Drug Policy Reform Group (CDPRG) recommends a Δ9-THC safety limit of 0.021 milligrams per day (King et al., 2021). In the current study, 30 products (37% of the samples tested) would exceed the 0.4 mg limit and 49 (60% of samples tested) would exceed the 0.021 mg limit including Epidiolex® with a 1 mL daily dose.
In an attempt to mitigate risk, some consumers seek out products labelled as “THC Free” and may assume that these products are safe. Here we report that 5 out of 21 (24%) of the products labelled as “THC Free” contained Δ9-THC ranging from 0.015 mg/mL to 0.656 mg/mL. Contamination of Δ9-THC in products specifically marketed as “THC Free” has resulted in workplace drug testing positive findings (Long, 2019; Miller, 2020). In one case, a hazardous material truck driver suffered a career ending workplace drug testing violation for Δ9-THC after consuming a CBD product labelled as THC Free (Miller, 2020). The FDA does allow “free from” claims for food products containing trace amount sodium, fat, and sugar, but a specified threshold of Δ9-THC has not been set for hemp-derived CBD products (Corroon et al., 2020). The inadequacy of labelling information clearly poses a risk to the consumer of unintended or overconsumption of Δ9-THC (Corroon et al., 2020).
Active-duty military and veterans are particularly vulnerable to consequences from contaminated products due to strict drug testing rules. Due to the purported benefits of CBD for conditions including pain, anxiety, and post-traumatic stress disorder, hemp-derived CBD products have been heavily marketed to this group. However, the Department of Defense (DoD) has explicitly prohibited service members and civilian employees from using hemp-derived products (other than a medical prescription for an FDA-approved drug) based on the potential for Δ9-THC contamination (Donovan, 2020). The DoD has issued guidance of a complete prohibition, regardless of stated or actual Δ9-THC content, to mitigate the risk of service members being charged for Δ9-THC violations stemming from consumption of hemp-derived products (Scarborough, 1997).
Similarly, athletes have increasingly turned to CBD products for their claimed benefits, and in 2018, WADA removed CBD from its list of banned substances to permit its use. However, athletes are subject to anti-doping testing, and the presence of Δ9-THC has led to suspensions and bans (Kasper et al., 2020; Mareck et al., 2021). Since 2018, there have been 60 CBD-related doping infringements reported, mostly involving Δ9-THC (Starling, 2021). This incudes Devin Logan, a US Ski and Snowboard athlete, who received a 3-month suspension after an adverse drug finding for Δ9-THC stemming from CBD product use (US Ski & Snowboard, 2019). Despite these risks, a study by Kasper et al. showed that male professional rugby players are turning to CBD products for pain management as an alternative to drugs such as opiates (Kasper et al., 2020). For similar reasons, the National Football League (NFL) has expressed interest in research on CBD and cannabinoids in pain management as an alternative to opiates (NFL, 2021; Associated Press, 2022). Despite the potential Δ9-THC contamination, athletes are using CBD products on the assumption that they have pharmacological benefits for injury and recovery and likely do not suspect Δ9-THC contamination.
It should be noted that this study could not evaluate product legality or compliance with the U.S. Farm Bill, which requires that source material/dry plant weight contain no more than 0.3% Δ9-THC. This study only evaluated end-product materials marketed for consumer use. We are aware that some manufacturers use the 0.3% criterion for these marketed products; however, this is not an appropriate interpretation of the Farm Bill. Gross calculations (not accounting for variations in carrier oil density) and using a denominator of 1 g/mL, would result in all products falling under the 0.3% threshold. However, applying the threshold in this manner suggests that products with as much as 3 mg/mL would still be legal/acceptable. It is likely that even low concentrations (0.25 – 0.5 mg/mL) could be problematic if taken multiple times per day on a daily basis. Thus, we respectfully suggest additional consideration and regulation of the allowable Δ9-THC present in marketed products to protect the health and safety of consumers.
Limitations of the presented data include: 1) the analysis of only hemp-derived oil products to the exclusion of other product types such as gummies, topicals, and vapes – at the time of purchase, oils were the most prevalent option available; 2) reporting only Δ9-THC concentrations but no other cannabinoid concentrations; and 3) not implementing a formal sampling protocol (i.e., a priori distribution of online, national and local brands; although all are represented in the current data). Nonetheless, this data suggests that 1) the presence of Δ9-THC is not uncommon in unregulated CBD oils, 2) products that contain Δ9-THC do not disclose its presence or quantity, and 3) the Δ9-THC concentration can range from trace levels to mg concentrations – multiple doses of which could result impairment/intoxication and/or positive urine drug test.
5. Conclusions
Hemp-derived products are increasingly available through online and local retailers. This study reports the quantification of Δ9-THC in 80 of these unregulated hemp-derived products as well as Epidiolex®, the highly purified CBD product approved by the FDA. A wide range of consumers are taking unregulated hemp-derived products without a clear understanding of the risks of unintended consumption of Δ9-THC. Here we report that many products labelled as being free of Δ9-THC are not. Considering that the majority of hemp-derived CBD products contain some amount of Δ9-THC, the unintended consumption of Δ9-THC carries a range of risks including adverse health effects, legal implications including child custody cases, driving while intoxicated laws, and risk to livelihood and military status. Carefully controlled research studies are needed on the specific Δ9-THC dose threshold to prevent workplace and sports positive blood and urine drug tests. The results of these studies indicate an urgent need to require accurate quantification and labelling of these products and to clarify the limits of Δ9-THC in marketed products to better ensure the safety of consumers.
References
- American Association of Poison Control Centers (2021). Cannabidiol Alert. https://www.aapcc.org/CBD-Alert
- Agricultural Improvement Act of 2018, in: Congress, U.S. (2018) H.R. 2 – 115-334. United States Government, p. 530. [Google Scholar]
- Associated Press. 2022. NFL awards $1 million for studies on cannabinoids’ effects on pain management in players. ESPN https://www.espn.com/nfl/story/_/id/33191830/nfl-awards-1-million-studies-cannabinoids-effects-pain-management (February 1, 2022)
- Babalonis S, Haney M, Malcolm RJ, Lofwall MR, Votaw VR, Sparenborg S, Walsh SL, 2017. Oral cannabidiol does not produce a signal for abuse liability in frequent marijuana smokers. Drug and alcohol dependence 172, 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn-Miller MO, Loflin MJE, Thomas BF, Marcu JP, Hyke T, Vandrey R, 2017. Labeling Accuracy of Cannabidiol Extracts Sold Online. JAMA 318(17), 1708–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosy TZ, Cole KA, 2000. Consumption and Quantitation of Δ9-Tetrahydrocannabinol in Commercially Available Hemp Seed Oil Products*. Journal of Analytical Toxicology 24(7), 562–566. [DOI] [PubMed] [Google Scholar]
- Corroon J, MacKay D, Dolphin W, 2020. Labeling of Cannabidiol Products: A Public Health Perspective. Cannabis and Cannabinoid Research 5(4), 274–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corroon J, Phillips JA, 2018. A Cross-Sectional Study of Cannabidiol Users. Cannabis and Cannabinoid Research 3(1), 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren MK, Sagar KA, Lambros AM, Smith RT, Gruber SA, 2021. Urinary Tetrahydrocannabinol After 4 Weeks of a Full-Spectrum, High-Cannabidiol Treatment in an Open-label Clinical Trial. JAMA Psychiatry 78(3), 335–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan MP, 2020. Adoption of Punitive General Orders to Address Use of Hemp Products, in: Department of Defense (Issued: February 26, 2020). [Google Scholar]
- Dubrow GA, Pawar RS, Srigley C, Fong Sam J, Talavera C, Parker CH, Noonan GO, 2021. A survey of cannabinoids and toxic elements in hemp-derived products from the United States marketplace. Journal of food composition and analysis 97, 103800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA. 2020a. Cannabis and Cannabis-Derived Compounds: Quality Considerations for Clinical Research Guidance for Industry. US Food and Drug Administration; https://www.fda.gov/media/140319/download [Google Scholar]
- FDA. 2020b. Report to the U.S. House “Sampling Study of the Current Cannabidiol Marketplace to Determine the Extent That Products are Mislabeled or Adulterated Report in Response to Further Consolidated Appropriations Act. US Food and Drug Administration; https://hempindustrydaily.com/wp-content/uploads/2020/07/CBD-Marketplace-Sampling_RTC_FY20_Final.pdf [Google Scholar]
- FDA. 2021. FDA Regulation of Cannabis and Cannabis-Derived Products, Including Cannabidiol (CBD). US Food and Drug Administration; (January 22, 2021) https://www.fda.gov/news-events/public-health-focus/fda-regulation-cannabis-and-cannabis-derived-products-including-cannabidiol-cbd#dietarysupplements [Google Scholar]
- Gurley BJ, Murphy TP, Gul W, Walker LA, ElSohly M, 2020. Content versus Label Claims in Cannabidiol (CBD)-Containing Products Obtained from Commercial Outlets in the State of Mississippi. Journal of dietary supplements 17(5), 599–607. [DOI] [PubMed] [Google Scholar]
- Gustafson RA, Levine B, Stout PR, Klette KL, George MP, Moolchan ET, Huestis MA, 2003. Urinary Cannabinoid Detection Times after Controlled Oral Administration of Δ9-Tetrahydrocannabinol to Humans. Clinical Chemistry 49(7), 1114–1124. [DOI] [PubMed] [Google Scholar]
- Hazekamp A, 2018. The Trouble with CBD Oil. Medical Cannabis and Cannabinoids 1(1), 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst J, Musgrave G, 2020. Respiratory depression following an accidental overdose of a CBD-labeled product: A pediatric case report. Journal of the American Pharmacists Association 60(1), 248–252. [DOI] [PubMed] [Google Scholar]
- Huestis MA, 2007. Human Cannabinoid Pharmacokinetics. Zürich, pp. 1770–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper AM, Sparks SA, Hooks M, Skeer M, Webb B, Nia H, Morton JP, Close GL, 2020. High Prevalence of Cannabidiol Use Within Male Professional Rugby Union and League Players: A Quest for Pain Relief and Enhanced Recovery. International Journal of Sport Nutrition and Exercise Metabolism 30(5), 315–322. [DOI] [PubMed] [Google Scholar]
- King D, Bhatarah P, Duffy P, Yates A, O’Sullivan S, 2021. Health Guidance Levels for THC in CBD products. Centre for Medical Cannabis (CMC), Association for the Cannabinoid Industry (ACI), and Conservative Drug Policy Reform Group (CDPRG). https://theaci.co.uk/aci-and-cmc-recommend-home-office-clarify-thc-limit-for-cbd-products/ [Google Scholar]
- Leson G, Pless P, Grotenhermen F, Kalant H, ElSohly MA, 2001. Evaluating the Impact of Hemp Food Consumption on Workplace Drug Tests. Journal of Analytical Toxicology 25(8), 691–698. [DOI] [PubMed] [Google Scholar]
- Liebling JPC, Nicholas James; Gibbs Blair William; Yates Andrew Stephen Yates; and O’Sullivan Saoirse Elizabeth, Forthcoming. An Analysis of Over-the-Counter Cannabidiol Products in the United Kingdom. Cannabis and Cannabinoid Research. (Epub April 1, 2020) doi: 10.1089/can.2019.0078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J, 2019. Pennsylvania woman sues CBD company after failed drug test. https://www.naturalproductsinsider.com/litigation/pennsylvania-woman-sues-cbd-company-after-failed-drug-test. (Accessed September 3, 2021.
- Mareck U, Fusshöller G, Geyer H, Huestis MA, Scheiff AB, Thevis M, 2021. Preliminary data on the potential for unintentional antidoping rule violations by permitted cannabidiol (CBD) use. Drug Testing and Analysis 13(3), 539–549. [DOI] [PubMed] [Google Scholar]
- Miller E, 2020. Fired Driver’s Civil Suit Against CBD Companies Set for Trial in October. https://www.ttnews.com/articles/fired-drivers-civil-suit-against-cbd-companies-set-trial-october. (Accessed September 3, 2021.
- NFL. 2021. NFL-NFLPA Pain Management Committee Accepting Applications for $1 Million in Research Funding. National Football League. (Issued: June 8, 2021) https://www.nfl.com/playerhealthandsafety/health-and-wellness/medical-research/nfl-nflpa-pain-management-committee-accepting-applications-for-1-million-in-rese [Google Scholar]
- NIDA. 2021. Notice of Information: Establishment of a Standard THC Unit to be used in Research, NOT-DA-21–049. National Institute on Drug Abuse. [Google Scholar]
- Pavlovic R, Nenna G, Calvi L, Panseri S, Borgonovo G, Giupponi L, Cannazza G, Giorgi A, 2018. Quality Traits of “Cannabidiol Oils”: Cannabinoids Content, Terpene Fingerprint and Oxidation Stability of European Commercially Available Preparations. Molecules 23(5), 1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters EN, Mosesova I, MacNair L, Vandrey R, Land MH, Ware MA, Turcotte C, Bonn-Miller MO, 2021. Safety, Pharmacokinetics and Pharmacodynamics of Spectrum Yellow Oil in Healthy Participants. Journal of Analytical Toxicology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JR, Smith NE, Moulin AK, 2017. Unintentional Cannabis Ingestion in Children: A Systematic Review. The Journal of pediatrics 190, 142–152. [DOI] [PubMed] [Google Scholar]
- SAMHSA. 2017. Mandatory Guidelines for Federal Workplace Drug Testing Programs, in: Health and Human Services. (Effective October 1, 2017). Federal Register, Mandatory Guidelines for Federal Workplace Drug Testing Program: Final Rule, Federal Register, 82 FR 7920 – 2017 [Google Scholar]
- Scarborough R, 1997. Health drink challenges military drug-testing program, The Washington Times. p. A3. [Google Scholar]
- Schlienz NJ, Cone EJ, Herrmann ES, Lembeck NA, Mitchell JM, Bigelow GE, Flegel R, LoDico CP, Hayes ED, Vandrey R, 2018. Pharmacokinetic Characterization of 11-nor-9-carboxy-Δ9-tetrahydrocannabinol in Urine Following Acute Oral Cannabis Ingestion in Healthy Adults. Journal of Analytical Toxicology 42(4), 232–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoedel KA, Szeto I, Setnik B, Sellers EM, Levy-Cooperman N, Mills C, Etges T, Sommerville K, 2018. Abuse potential assessment of cannabidiol (CBD) in recreational polydrug users: A randomized, double-blind, controlled trial. Epilepsy & Behavior 88, 162–171. [DOI] [PubMed] [Google Scholar]
- Solvay Pharmaceuticals. 2017. Marinol: Prescribing Information, in: US Food and Drug Administration. [Google Scholar]
- Starling S, 2021. The challenges with CBD for sports nutrition. NutraIngredients-USA. https://www.nutraingredients-usa.com/Article/2021/06/17/The-challenges-with-CBD-and-sports-nutrition?utm_source=copyright&utm_medium=OnSite&utm_campaign=copyright [Google Scholar]
- U.S. Ski & Snowboard. 2019. U.S. Ski & Snowboard Athlete Accepts USADA Sanction. https://usskiandsnowboard.org/news/us-ski-snowboard-athlete-accepts-usada-sanction
- Viudez-Martínez A, García-Gutiérrez MS, Medrano-Relinque J, Navarrón CM, Navarrete F, Manzanares J, 2019. Cannabidiol does not display drug abuse potential in mice behavior. Acta Pharmacologica Sinica 40(3), 358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADA. 2021. Decision Limits for the Confirmatory Quantification of Exogenous Threshold Substances by Chromatography-Based Analytical Methods (TD2021DL). World Anti-Doping Agency [Google Scholar]
- Wagoner KG, Lazard AJ, Romero-Sandoval EA, Reboussin BA, Forthcoming 2021. Health Claims About Cannabidiol Products: A Retrospective Analysis of U.S. Food and Drug Administration Warning Letters from 2015 to 2019. Cannabis and Cannabinoid Research. (Epub: June 17, 2021) doi: 10.1089/can.2020.0166 [DOI] [PMC free article] [PubMed] [Google Scholar]


