Abstract
Research on bacterial membrane vesicles (BMVs) is an emerging topic, and the goal is to address whether BMVs can bring translational tools to improve current therapies. In this review, we provided the updated studies on BMVs including their production, their types, and therapeutic regimens for treating infectious diseases and cancers. We described several platforms of BMVs, such as outer membrane vesicles (OMVs), inner membrane vesicles (IMVs) and double membrane vesicles (DMVs), and those structures were produced from Gram-negative or Gram-positive bacteria. We also discussed how to engineer and formulate new and novel BMVs using chemical, physical, and genetic methods. For therapies, we analyzed current methods for loading drugs in BMVs and discussed their limitations. Finally, we reviewed several therapeutic platforms of BMVs that have been exploited in improving the treatments of infectious diseases and cancers. Although BMVs offer the promising biomedical applications, it is needed to develop rigorous approaches and methods to generate reproducible and scalable drug delivery systems for translation.
Keywords: Bacterial membrane vesicles, Outer membrane vesicles, Anti-infection therapy, Anti-cancer therapy
1. Introduction
Living cells secrete membrane-formed vesicles (MVs) that contain biologically active molecules, and these vesicles play a central role in mediating intercellular communications and cellular functions [1]. Both eukaryotic and procaryotic cells can actively or passively produce MVs. Membrane-formed vesicles from eukaryotic cells have been investigated [2–4]. Based on their biogenesis and sizes, there are three types of membrane vesicles: exosomes, microvesicles and apoptotic cell bodies. It is challenging to distinguish these three types of vesicles because they have similar sizes and lipid membrane properties. Therefore, they are usually called extracellular vehicles (EVs). EVs can be produced from a wide range of eukaryotic cells, such as immune cells (neutrophils [5], monocytes [6] or macrophages [7]), red blood cells [8,9] and stem cells [10,11]. EVs also exist in a variety of tissues [12] and circulation systems [13]. EVs can be engineered and loaded with cargos for treating acute lung inflammation [14–17], stroke [18], sepsis [19], and cancer [20]. Several reviews have covered this topic [4,21,22]. MVs derived from procaryotic cells become an emerging research topic and they show the unique features different from EVs derived from mammal cells [23]. This review is focused on discussing bacterial membrane-derived vesicles (BMVs).
The first discovery of BMVs produced by Gram-negative bacteria was traced back more than 50 years ago [24] (Fig. 1). Ten years later, this observation was further confirmed and the interest in BMVs dramatically increased [25,26]. The studies found that Gram-negative and Gram-positive bacteria spontaneously release BMVs in culture media, but interestingly, the production of BMVs is increased when bacteria are stimulated or activated [27].
Fig. 1.
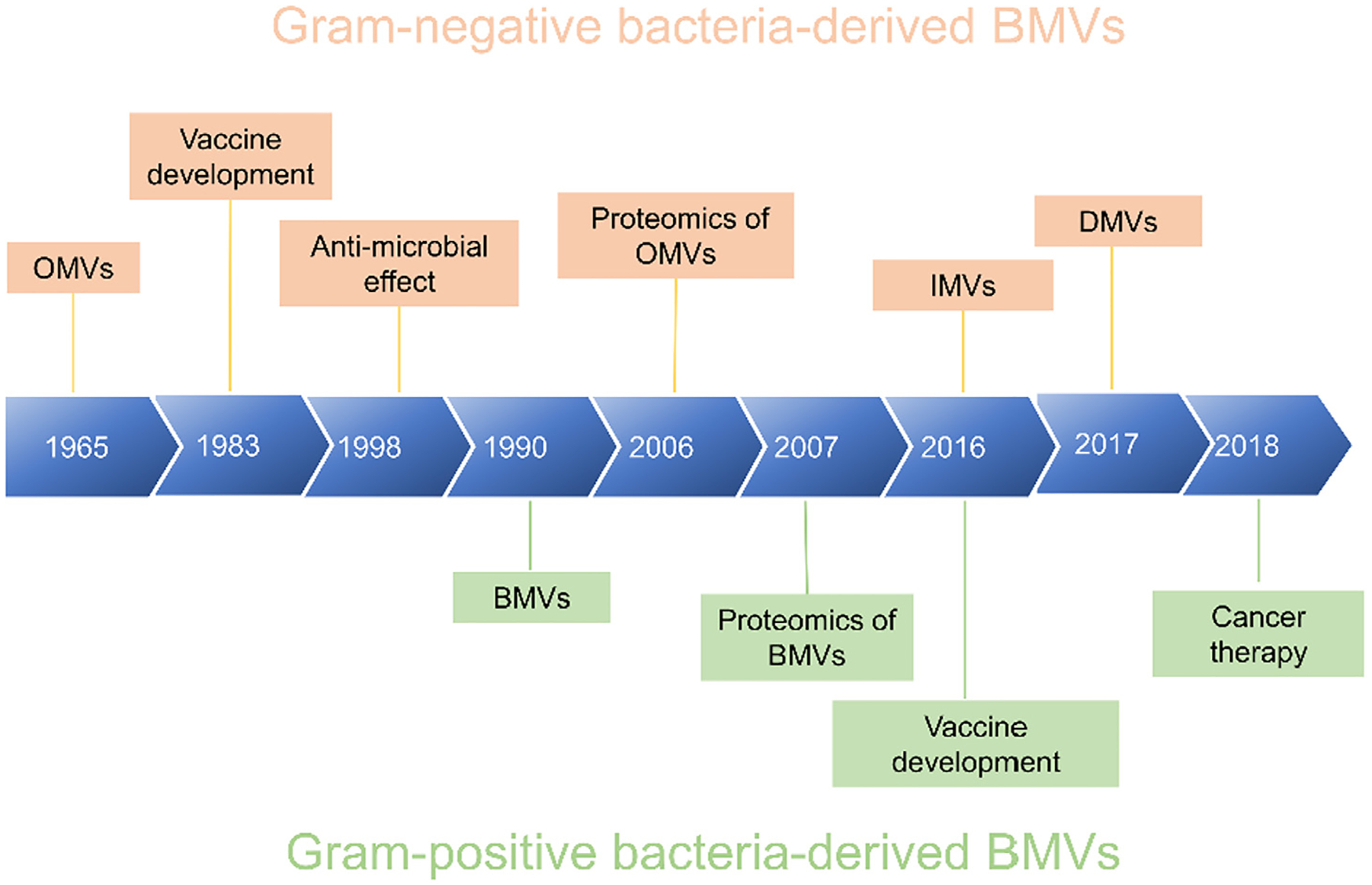
Milestones on BMVs derived from Gram-negative and Gram-positive bacteria. BMVs, bacterial membrane vesicles; OMVs, outer membrane vesicles; IMVs, inner membrane vesicles; DMVs, double membrane vesicles.
BMVs were initially considered as cellular debris or artifacts because they were made of bacterial membrane, unlikely possessing biological functions [28]. Recent studies and advances in microscopy suggest that BMVs are important mediators in regulating interactions between the host and bacteria. For example, BMVs remove pathogen-stimulating molecules released from bacteria in antimicrobial effects. In other hand, BMVs may support bacterial survival through transfer of genes and sequestering of nutrients to assist the biofilm formation. Interestingly, BMVs also support the development of antimicrobial resistance [29–32]. Fig. 1 shows the milestones of BMVs made from Gram-negative or Gram-positive bacteria. BMVs derived Gram-negative bacteria have been intensively studied, such as the discovery and biogenesis of OMVs, and creating inner membrane vesicles (IMVs) and double membrane vesicles (DMVs). The proteomics shows the unique properties of BMVs, and these properties enable to develop a wide range of biomedical applications, such as targeted drug delivery. In contrast, BMVs made from Gram-positive bacteria are less studied, but it is an emerging area to expand the biomedical applications of BMVs.
Advances in microbiology, immunology and microscopy have revealed many roles of BMVs in bacterial ecology, physiology, and host–microbe interactions. BMVs are also strongly correlated to disease development, so BMVs have demonstrated the potential applications in treating infectious diseases and inflammatory disorders. Furthermore, advances in nanotechnology, bioengineering and genetic modifications allow us to design and fabricate varied types of bacterial membrane structures for targeted drug delivery and vaccine development. There are several reviews on outer membrane vesicles (OMVs) and their biomedical applications [33–35]. The growth on studying BMVs is very rapid and there are many emerging technologies and approaches used to engineer BMVs.
In this review, we expand a scope from the discovery of BMVs to their biogenesis and engineering. We also discuss their applications in improved therapies to infectious diseases and cancers. Furthermore, we describe several studies to illustrate the concept on designing and engineering BMVs for vaccine development and targeting tumor microenvironments for improved cancer treatment. Finally, we discuss the barriers to translate the technologies of BMVs in targeted drug delivery and we also offer our views on opportunities for BMVs.
2. Generation of BMVs
Bacteria bud their membrane and release them into surrounding environments and this process is a spontaneous event. After these components are isolated and purified, they show lipid bilayer-enclosed structures that are morphologically similar to eukaryotic EVs [14,15,36]. Bacteria release BMVs with sizes with 20 to 400 nm in diameter [37,38]. Both Gram- negative and Gram-positive bacteria can spontaneously secrete BMVs. Their secretion is also dependent on surrounding environments, affecting diverse biological processes, such as virulence, horizontal gene transfer, export of cellular metabolites, phage infection and cell-to-cell communications [39] (Fig. 2).
Fig. 2.

The components and functions of BMVs. BMVs are composed of phosphatidylglycerol, phosphatidylethanolamine, and membrane of proteins and lipopolysaccharides. Nucleic acids, metabolites and metal ions are incorporated in BMVs during their formation. The functions of secretion of BMVs are for bacterial growth (such as bacterial survival and nutrition acquisition) and for immune responses (signal communication and material transfer).
There are two types of bacteria based on gram staining, such as, Gram-negative, and Gram-positive. Their structural difference is that Gram-negative bacteria have a thin peptidoglycan layer and an outer lipid membrane while Gram-positive bacteria have a thick peptidoglycan layer and no outer lipid membrane. Therefore, the formation and structures of BMVs depend on bacterial types. Fig. 3 shows the different types of BMVs made from Gram-negative or Gram-positive bacteria.
Fig. 3.

Schematic illustration of varied types of BMVs made from Gram-negative or Gram-positive bacteria and their applications in infections and cancers. The outer membrane of Gram-negative bacteria forms OMVs. IMVs (inner membrane vesicles) and DMVs (double membrane vesicles) are created using biological or physical methods. BMVs are also produced from genetically engineered Gram-negative and Gram-positive bacteria.
2.1. BMVs formed from Gram-negative bacteria
2.1.1. OMVs
BMVs derived from Gram-negative bacteria have been extensively investigated [21,40,41]. Gram-negative bacteria possess a distinctive structure made of lipopolysaccharide (LPS) and an outer lipid membrane. Gram-negative bacteria spontaneously release the outer lipid membrane to form membrane vesicles. This structure is called OMVs (outer membrane vesicles) [38,39]. The production process of OMVs is comprised of the following steps: 1) The membrane proteins connect an outer lipid membrane to peptidoglycan to form a stable Gram-negative envelope. The proteins begin to destabilize during the formation of OMVs. 2) The linking proteins between the outer lipid membrane and peptidoglycans are disassociated by either relocation of the linking proteins or by breaking of the connection between outer lipid membrane and peptidoglycans. 3) OMVs are released after the breaking of linking proteins. The formation of OMVs strongly depends on local environments, so OMVs may contain different cargos, such as proteins or genetic molecules. Proteins linking the outer lipid membrane and peptidoglycans may be also present in OMVs.
The mechanisms of formation of OMVs are complex so that OMVs are heterogeneous in their sizes and compositions [31,38]. OMVs are comprised of phospholipids, proteins, nucleic acids, proteins, and virulence factors (such as LPS) [42]. Sometimes, metal ions, signaling molecules and metabolites are also present in OMVs [43,44] (Fig. 2). Since proteins play an important role in OMVs for tissue targeting and signal transduction, the proteomics have been performed to analyze proteins in OMVs. There are several approaches and methods, for example, sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) [45,46], 2D-electrophoresis [47] followed by in situ mass spectrometry (MS) [45] or direct trypsin digestion of electrophoresis gels followed by liquid chromatography (LC)-MS/MS [46,48]. The proteomics show that OMVs are mainly composed of outer membrane-associated proteins rather than from either the inner membrane or cytoplasm. The findings strongly support the hypothesis of OMVs biogenesis.
Formation of OMVs from Gram-negative bacteria strongly depend on local cues, such as stress [49], and stimulators [50] (Table 1). Therefore, the optimization of culture media and conditions may increase the production of OMVs and control their compositions.
Table 1.
Current technologies used to enhance the production of BMVs.
| Types | Methods | Genes/Agents | Strains | Key Results | Ref. |
|---|---|---|---|---|---|
| OMVs | Biological | Mutation of tolR and galU | Shigella sonnei | Increased vesiculation | [94] |
| Mutation of tolR gene | Helicobacter pylori | Increased vesiculation | [93] | ||
| T4 phage infection | Escherichia coli | Enhanced generation of OMVs | [79] | ||
| Chemical | IPTG | Escherichia coli | Enhanced generation of OMVs | [49] | |
| Gentamicin | Pseudomonas aeruginosa | Production of OMVs increased by 3 times | [78] | ||
| Sodium bicarbonate | Vibrio cholerae | Increased production of OMVs | [82] | ||
| Arabinose | Escherichia coli | Increased production of OMVs and concentration dependent | [49] | ||
| Cysteine | Neisseria meningitidis | Depletion of cysteine increased OMVs | [77] | ||
| Oxygen | Neisseria meningitidis | Oxygenation increased release of OMVs | [83,84] | ||
| Temperature Stress | Heat shock | Yersinia pestis; | Increased production of OMVs | [50] | |
| Escherichia coli | Increased production of OMVs | [49] | |||
| Escherichia coli | Increased membrane blebs | [68] | |||
| Cold shock | Yersinia pestis | Increased production of OMVs in low temperature | [50] | ||
| Growth Conditions | Bacterial density | Shigella sonnei | Bacterial growth density mediated production of OMVs | [94] | |
| Increased volumes | Neisseria meningitidis | Continuous culture achieved 4.0 × 1014 OMVs per liter per day | [88] | ||
| IMVs | Physical | Extrusion | Escherichia coli | Production of IMVs | [51] |
| DMVs | Physical | Cavitation | Pseudomonas aeruginosa | High yield of BMVs | [19,37] |
Note: Bacterial membrane vesicles (BMVs); Outer membrane vesicles (OMVs); Inner membrane vesicles (IMVs); Double membrane vesicles (DMVs); Isopropyl ß-D-1-thiogalactopyranoside (IPTG).
2.1.2. Inner membrane vesicles (IMVs)
The inner membrane of Gram-negative bacteria is covered by the outer membrane and the peptidoglycan layer, so it is not easy to form membrane vesicles naturally. Because OMVs contain LPS, they may cause the immune toxicity in their medical applications. Kim et al. [51,52] proposed to generate inner membrane vesicles from Gram-negative bacteria, so called bacterial protoplast-derived nanovesicles (PDNVs). Protoplast is a state of bacteria without a toxic outer membrane and a peptidoglycan layer (cell wall) that were removed by lysozyme. After the outer membrane was removed, the protoplast generated IMVs. The IMVs were successfully produced by a serial extrusion approach and were used to develop a universal adjuvant-free vaccine. The IMVs showed the higher efficacy and a safety profile than the vaccine developed using OMVs. In addition, IMVs induced the strong antigen-specific humoral and cellular immune responses.
2.1.3. DMVs
OMVs or IMVs are made from a single lipid membrane, thus their stability and cargo loading may be problematic as drug delivery systems. Gram-negative bacteria have a distinct structure with a double-layer lipid membrane linked by peptidoglycans, and integrated membrane contains the functions of parent bacteria. Developing vesicles made from integrated bacterial membrane may be critical for medical applications of BMVs [53,54]. Wang et al. [37] proposed an innovative approach using nitrogen cavitation to generate DMVs. Using cryogenic transmission electron microscopy (cryo-TEM), biochemistry and proteomics, the authors have shown that DMVs possessed the whole bacterial membrane including the inner and outer lipid membranes and two membrane linkers of peptidoglycans. The unique feature is that DMVs contained many antigens required for development of vaccines. In a mouse model of sepsis induced by Pseudomonas aeruginosa, it was found that DMVs dramatically increased mouse survival compared to OMVs made from Pseudomonas aeruginosa. The molecular mechanism of improved survival is associated with the enhanced adaptive immunity and distinct biodistribution of DMVs compared to OMVs, possibly due to more pathogen-associated molecular patterns (PAMPs) on DMVs. This study shows that the nitrogen cavitation approach would be a promising technology to make DMVs from a wide range of bacteria.
The same research group extended DMVs to target multiple cells (immune cells and endothelium) in tumor microenvironments [20]. In the study, the authors designed bacterial (Escherichia coli) DMVs expressing arginine-glycine-aspartate (RGD) peptides and with endogenous targeting ligands from bacteria. The DMVs can spontaneously bind to neutrophils, monocytes, and endothelial cells in tumor microenvironments. Importantly, the authors can load doxorubicin (Dox) inside DMVs via a pH gradient, and the loading efficiency of Dox was very high at 12% (w/w). In a mouse model of melanoma, DMVs loaded with Dox dramatically shrank the tumor sizes compared to DMVs without expression of targeting ligands. This study demonstrates that DMVs are novel targeted drug delivery carriers to efficiently deliver therapeutics for cancer therapies.
2.2. BMVs made from Gram-positive bacteria
Compared to Gram-negative bacteria, Gram-positive bacterial membrane vesicles are recently attractive and become an emerging topic [55]. There are more than 30 species of Gram-positive bacteria have shown that they are capable of secreting BMVs [56].
Gram-positive BMVs contain a wide range of cargo molecules, such as nucleic acids, proteins, lipids, enzymes, and toxins [55,57] (Fig. 2). Compared to OMVs, Gram-positive BMVs lack of LPS and periplasmic components (Fig. 3). Studies have shown that the formation of BMVs is regulated by an active metabolic process.
The mechanism of biogenesis of Gram-positive BMVs remains unclear. Unlikely the biogenesis of OMVs, OMVs are pinched off the outer membrane of Gram-negative bacteria because Gram-positive bacteria possess a thick cell wall made of peptidoglycans that may inhibit the budding of lipid membrane from Gram-positive bacteria. One hypothesis is that enzymes may destroy the peptidoglycan layer, and then exposed lipid membrane may be easy to bud to form BMVs [55,58].
The functions of Gram-positive BMVs include transport of molecules and increased bacterial survival [59] (Fig. 2). For example, it was observed that bacterial chromosomal DNA was transported in Ruminococcin spp [60] and BMVs transported surface receptors [61]. Interestingly, Gram-positive bacteria also secrete BMVs containing nutrient uptake enhancer (Iron-binding factor) [62], antibiotic degrading enzymes (β-lactamase) [63] and even toxic factor to enhance bacterial survival [64,65]. The sizes of Gram-positive BMVs range in 10–400 nm [56].
3. Engineering BMVs
Advances in bioengineering and nanotechnology enable to engineer BMVs to solve the limitations on BMVs, for example, low production yields, toxicity and tissue targeting. Here we discuss several approaches and methods used in engineering BMVs for solving these barriers.
3.1. Enhanced production of BMVs
BMVs are secreted during bacterial growth, but this type of production of BMVs results in the challenges for biomedical applications. Various strategies have been developed to solve these problems. Several approaches and methods were used by selecting bacterial strains and changing culture environments.
OMVs constitutively release during the bacterial growth [66], and interestingly, bacterial strains strongly affect the production of OMVs. For example, Escherichia coli [67,68] Salmonella typhimurium [69,70], Borrelia burgdorferi [71], and Gallibacterium anatis [72] were observed to produce more OMVs. In addition, it was reported that the late culture phase in Neisseria meningitides [73] and Francisella novicida [74] produced more OMVs. Another bacterium, Legionella pneumophila formed OMVs and their growth was related to all phases of the cell growth cycle [75], while for Helicobacter pylori, the release of OMVs was inversely dependent on the cell growth. Interestingly, the production of OMVs was also correlated with bacterial shape transitions [76].
The generation of OMVs is very sensitive to culture environments, such as temperature [49,50,68], lacking of amino acids [77], antibiotics [78], or phage infection [79]. It was shown that the cysteine depletion caused the oxidative stress that mediated the secretion of OMVs in Neisseria meningitidis [77]. Interestingly, adding chemicals in culture media can also induce more production of OMVs, such as supplement of hexadecane in Acinetobacter calcoaceticus [80], glucose in Yersinia pestis [81] and sodium carbonate in Vibrio cholera [82]. Oxidative stress can also induce the release of OMVs in Neisseria meningitidis [83].
To increase the yield of OMVs, detergents or ethylenedi-aminetetraacetic acid (EDTA) [40,84] was used to extract more OMVs from bacterial membrane, but the production is still limited by the culture process. Thus, the fermentation with bioreactors [85–87], were employed. A continuous generation of Neisseria meningitidis OMVs is a way to increase culture volumes. This process can be performed for more than 600 h and the production yield was at 4.0 × 1014 OMVs per liter a day. The results showed that features of OMVs were similar to those produced by traditional culture procedures, suggesting that the bioreactors may be feasible for the scalable production of OMVs for medical applications [88].
The generation of OMVs is dependent on peptidoglycans between inner and outer lipid membrane, and some proteins regulate the peptidoglycans. The Tol-Pal system regulated the membrane peptidoglycans [89]. The studies show that the Tol-pal operon regulated seven downstream genes, and five genes encoded proteins for a Tol-Pal system, called tolQ, tolR, tolA, tolB, and pal. These 5 proteins regulated the assembly a complex of peptidoglycan, and controlled the outer membrane tightness [90]. Mutation of these genes may cause a malfunction of the protein complex, resulting in increased vesiculation of bacteria [91,92]. For example, deletion of tolB increased vesiculation of Helicobacter pylori [93]. Similarly, simultaneous mutation of the tolR gene and the galU gene was also found to increase the production of OMVs in Shigella sonnei [94]. Local protein aggregations may also impair the Tof-Pal system, leading to increased OMV production. It was also observed that a high level of TolR induced vesiculation in Escherichia coli [95].
Recently, the protoplast membrane vesicles [51] and double membrane vesicles derived from a whole bacterial membrane [20,37] were also created. These new types of BMVs demonstrated the high production and scalability for potential medical applications.
We summarized the approaches and methods used in increasing the production of BMVs in Table 1. Current technologies including chemical and biological methods show a promise on translating BMVs in clinic.
3.2. Reduction of virulence in BMVs
BMVs can cause immune responses when they are used as drug carriers because they contain antigens expressing on bacterial membrane. The research is focused on how to reduce this virulence when BMVs are produced. There are two major resources of toxins: LPS (lipopolysaccharide) and toxic proteins in BMVs, either on the membrane or inside BMVs. It is needed to develop novel approaches and methods to attenuate immunogenetic agents in BMVs when they are used as drug delivery carriers.
LPS is a major component of the outer membrane on Gram-negative bacteria, comprising lipid A, core oligosaccharides, and O-antigen [96,97]. When LPS binds to a receptor complex consisting of TLR4, CD14 and MD2, myeloid differentiation factor (MyD88) activates nuclear factor-κB (NF-κB) and mitogen-activated protein kinases, leading to inflammatory responses. The inflammatory responses may cause a severe disease, such as sepsis [98,99]. Thus, removing LPS from BMVs is critical to develop BMVs as drug delivery vehicles.
To reduce LPS levels on BMVs, controlling the expression of LPS on bacteria may be a strategy. Genetic modification of acylation of the lipid A moiety showed the reduced inflammatory responses in BMVs [100]. Lipid A needs enzymes for its biosynthesis [96], therefore, downregulating these enzymes may be a novel approach to control the synthesis of lipid A. Knockout of lpxL and lpxM have demonstrated the low toxicity [101–103]. The modified phosphorylation of a lipid A component may be also a novel approach to lower the toxicity of LPS. The lipid A was phosphorylated, and it was observed that monophosphorylated lipid A is less immunogenic [104]. Monophosphorylated lipid A (MPL) has been approved by FDA for the use as an adjuvant [105].
Endotoxin removal by detergent-extraction was used in the first vaccine made from OMVs, and this vaccine has successfully prevented the outbreak of Neisseria meningitidis serogroup B [106]. Based on the structural features of LPS, non-ionic detergents and chelating agents were used for sequential reduction of LPS during the production of OMVs.
There are other virulence factors including collagenase and hyaluronate lyase that disrupt extracellular matrix (ECM) [107], and serine proteases [108]. The exfoliative toxins [109] can disrupt blood barriers. It is shown that BMVs made from Bacillus anthracis, Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus pyogenes, and Streptococcus agalactiae, carried hemolysins and/or pore forming toxins [64,109–111]. Other membrane proteins, VacA, OstA, FilC, pilA, PagL, EstA and aaaA were also reported to be toxic [38]. Genetic engineering, as well as natural strain selectivity, may be a good strategy for the reduction of toxins.
3.3. Engineering BMVs for antigen presentation
Peptides and proteins can be engineered on the bacterial surface by fusing them with an anchoring motif. For example, the Mycobacterium tuberculosis antigens ESAT6, Ag85B, and Rv2660c were linked to the surface of Escherichia coli OMVs via the fusion to hemoglobin protease (Hbp). The OMVs decorated with multiple heterologous antigens caused antigen-specific immune responses, thus they may be novel vaccines to prevent the infection from Mycobacterium tuberculosis [112].
It is noticed that configuration of antigens on the surface of OMVs is important in immune responses. For example, OspA (outer surface protein A) was fused to different regions of factor H binding protein (fHbp) (a meningococcal surface-exposed lipoprotein) and was tested whether OspA was displayed on the surface of OMVs. When mice were immunized with the OMVs, the OspA-specific antibodies were observed. This study indicated that the correct expression of antigen on the surface of OMVs was critical to develop a vaccine system [113].
To extend the Hbp platform, heterologous proteins were coupled to internal sites of the Hbp passenger. For example, SpyTags were coupled to the Salmonella OMV surface and efficiently interacted with the SpyCatcher fusion proteins. These studies show that a modular Hbp system was established for developing recombinant OMVs-based vaccines and therapies [114,115].
3.4. Engineering BMVs for targeting
Living bacteria naturally exhibit tumor tropism because bacteria favor the hypoxia where tumor cells exist [116], thus bacterium-based anticancer therapies demonstrate the benefits overcoming the limitations of current cancer therapies. Clinical trials showed that it was safe to administrate attenuated strain of bacteria, but with the limited antitumor effect [117]. These studies suggest that it is needed to increase bacterial targeting. Genetical modifications using bioengineering allows to develop new and novel bacterium-derived therapeutic systems.
Proteins or peptides can be genetically engineered on the surface of BMVs. Bacterial OMVs fused with heterologous proteins were studied using a system of vesicle-associated toxin ClyA of Escherichia coli [118]. In the study, it is found that chimeric ClyA fusion proteins were localized on the surface of bacterial OMVs and retained its activity, demonstrating that ClyA is a useful tool to modify the surface of bacteria and their OMVs. Using this tool, they fused a fragment of anti-digoxin single-chain Fv to the C terminus of ClyA to make a designer, so called “immuno-MVs”.
Based on this concept, Kim et al. [51] fused epithelial growth factor (EGF) with PrsA, an inner membrane protein, and prepared EGF-decorated IMVs. The A549 lung cancer expresses an EGF receptor (EGFR), therefore EGF-expressed IMVs would target the tumors. The in vitro and in vivo studies confirmed this hypothesis. Similarly, Gao et al. [20] fused a RGD peptide to the ClyA protein in bacteria, and subsequently they prepared RGD-expressed DMVs using the nitrogen cavitation method. The in vitro and in vivo studies indicated that RGD-expressed DMVs dramatically increased their tumor accumulation via multiple targeting of immune cells, tumor vasculature and tumor cells.
The motif of RGD peptide is a novel targeting molecule because they are small and specific to certain tissues [119,120]. For example, Chen et al. [121] conjugated RGD to the surface of OMVs for increased targeting of OMVs. The OMVs also carried polymeric micelles and they were tested in the synergistic therapeutic effect of OMVs. The nanocomplexes were able to accumulate in tumor tissues in the mouse breast xenograft model.
3.5. Drug loading in BMVs
The current approaches for loading drugs in BMVs include incubation, extrusion and sonication [36,38], but the loading efficiency is very low because the methods are the passive loading processes. Bacteria have a function to eliminate antibiotics via secretion of OMVs as a mechanism for antimicrobial resistance, so it is possible that antibiotics may be loaded in OMVs when bacteria secrete OMVs in the presence of antibiotics. To verify this hypothesis, the authors added gentamicin during culturing Pseudomonas aeruginosa strain PAO1, and then they harvested OMVs. They found that gentamicin was incorporated in OMVs at 4 ng of drug per μg of proteins [122,123]. Tashiro et al. [124] changed the timing of adding antibiotics during the culture of bacteria. Buttiauxella agrestis - was grown until the late phase, and then gentamicin was added at a concentration of 4 folds higher than the minimum inhibition concentration (MIC). After the OMVs were collected, they observed the loading of gentamicin in OMVs, but they did not quantitatively analyze the actual loading yield of gentamicin in OMVs. Similarly, Huang et al. [125] used Acinetobacter baumanii and added levofloxacin in the culture media at sub-MIC concentrations. They found that OMVs incorporated levofloxacin. These studies suggest that the strains, drugs, and culture conditions may affect the drug loading in OMVs.
BMVs are composed of phospholipid layers, thus hydrophobic drugs may be incorporated in the lipophilic leaflets. Using this approach, Kuerban et al. [53] loaded Dox in OMVs after Dox was incubated with OMVs in PBS at 37 °C for 4 h. The loading efficiency of Dox was estimated to be 2% after Dox was determined by LC-MS. Similarly, after the prodrug of taxol, DSEP-PEG-CA-PTX was dissolved in a small volume of OMVs, the drug was successfully loaded in OMVs [126].
BMVs are made of lipid bilayer vesicles. The lipid layer is a barrier to load therapeutics inside BMVs, so the passive loading of drugs in BMVs is not an efficient method to load the drugs. It is critical to establish novel concepts and methods to load therapeutics. Inspired by the remote loading of Dox in liposomes (Doxil® and Doxebo®) [127,128], Wang lab proposed to load Dox in BMVs using pH gradient [20]. To achieve the pH gradient between inner and outer BMVs, they incorporated cholesterol in lipid layers of BMVs. They found that establishing pH gradient required the incorporation of cholesterol. Using this approach, they found that Dox loading in DMVs was 12% (w/w). This drug loading level in BMVs offers the potential translation of BMVs in clinic. In addition, genetical methods were used to express the interest of proteins or genes in BMVs. We summarize the current approaches and methods used in loading drugs in BMVs as shown in Table 2.
Table 2.
Drug loading strategies of BMVs and the results.
| Types | Methods | Cargo | Key results | Ref. |
|---|---|---|---|---|
| OMVs | Incubation | Gentamicin | 4 ng/μg OMVs (protein weight) | [122] |
| Incubation | Gentamicin | 146 μg/ml (unknown in OMVs) | [124] | |
| Incubation | Levofloxacin | 23.2 mg per 1.4 × 1014 OMVs | [125] | |
| Incubation | Dox | 2% (w/w) | [53] | |
| Incubation/genetical | TRAIL; ICG | 632 pg/μg (TRAIL) | [129] | |
| Incubation/electroporation | siRNA; PTX | Unknown | [126] | |
| Cloaking | Tegafur/micelles | 7.1% (w/w) | [121] | |
| Hybrid/cloaking | ICG/NPs | Unknown | [130] | |
| IMVs | Genetically engineering | GFP/OmpA SAcoagulase | Unknown | [51] |
| DMVs | Remote loading | Dox | 12% (w/w) | [20] |
Note: BMVs, bacterial membrane vesicles; OMVs, outer membrane vesicles; IMVs, inner membrane vesicles; DMVs, double membrane vesicles; Dox, doxorubicin; GFP, Green Fluorescence Protein; SAcoagulase, Staphylococcus aureus specific coagulase; ICG, indocyanine green; TRAIL, tumor necrosis factor related apoptosis-inducing ligand; PTX, paclitaxel.
4. Infectious diseases
4.1. BMVs as antigens for vaccination
Bacterial membrane vesicles, especially OMVs are derived from various pathogens, so they have been developed for immunogenic vaccines against the respective or closely related organisms [29]. Upon administration of BMVs, they induce the innate and adaptive immune responses. Antigen presentation cells (such as dendritic cells (DCs)) encounter BMVs and subsequently DCs activate T cells and B cells to produce cytokines and antibodies, thus equipping the immune system to combat pathogen infections [37].
There are several advantages of OMVs as vaccines: 1) Easily decorating OMVs with heterologous proteins for enhanced vaccine features. 2) Incorporating heterologous glycans to OMVs for development of glycoconjugate vaccines. 3) Engineering OMVs for desired immune responses. 4) Incorporating multiple antigens in BMVs to make effective vaccines and 5) Possible scalability and translation of OMVs for clinic [40]. There are several reviews [33,40,131,132] have summarized recent progress on engineering OMVs in vaccine development. Although the virulence derived from bacteria membrane is necessary to initiate immune responses, management of the toxicity levels is critical in the development of OMVs-based vaccines. Removal of other antigens is a method before OMVs are produced, for example, detergents are used to remove lipoxins of Gram-negative bacteria for the vaccines of OMVs [133].
IMVs and DMVs were designed to increase vaccine performance. Kim et al. [51] generated an adjuvant-free vaccine delivery platform by depleting the toxic outer membrane of bacteria, called PDNVs. The PDNVs exhibited the higher production and better safety profile than OMVs and induced strong immune responses. After bacterial antigens were loaded in PDNVs and PDNVs were administered to mice, immunized mice showed the effective protection to sepsis. Wang et al [37] fabricated a new novel vaccine platform, so-called DMVs (double layer membrane vesicles) derived from Pseudomonas aeruginosa, and mice were treated with DMVs against the infection by Pseudomonas aeruginosa. Compared with OMVs, the platform of DMVs increased the mouse survival and the immune response to the infection.
4.2. BMVs as adjuvants in vaccination
Protein-based vaccines often require adjuvants for increased immune responses [134,135]. The vaccine adjuvants include cholera toxin, alum, and diphtheria toxin, but they exhibit several adverse side effects such as, inflammation, toxicity, and less effective mucosal immunity [136,137]. BMVs may be the potential adjuvants due to their ability to stimulate the innate immune system. LPS and peptidoglycan present in BMVs can activate sensors of the innate immune system [138–140]. BMVs were capable of effectively inducing immune responses [141]. BMVs can also act as antigen carriers [142].
Antigens, AnAPN1 and Pfs48/45, or ovalbumin were used to evaluate OMVs derived from Escherichia coli as an adjuvant in intranasal vaccination. In the study, they compared with the adjuvant cholera toxin (CT) and parenteral adjuvant MF59C.1. The vaccine was administered intranasally or subcutaneously. When OMVs were used as an adjuvant in intranasal immunization, the antibody production and cellular responses were observed. This study provides the rationale for the future development of OMVs-based adjuvants in other infectious diseases [143].
Influenza is a major of cause for acute respiratory diseases, resulting in global health issues. The immunization is considered a promising route for vaccination [141]. OMVs can be manipulated to produce low endotoxic vaccines. This can be done through modifying the structure of LPS. In this study, the modified OMVs showed the decreased toxicity compared to native OMVs. In the immunization studies, intranasal administration of OMVs dramatically enhanced antibody production and T cell functions. Interestingly, vaccinated mice increased the survival when they were challenged at a lethal dose of viruses. These studies indicate that OMVs may be used in intranasal vaccines.
4.3. Anti-microbial effects
During the OMV formation in many gram-negative bacteria, OMVs entrap several periplasmic components, such as alkaline phosphatase, phospholipase C and peptidoglycan hydrolase [78,122,144]. For example, a study demonstrated that BMVs made from Pseudomonas aeruginosa PAO1 were able to lyse Staphylococcus aureus, Escherichia coli, and another Pseudomonas strain [122]. Pseudomonas aeruginosa OMVs possessed the most lytic activity [145].
Goes et al. [146] generated OMVs from Cystobacter velatus Cbv34 and Cystobacter ferrugineus Cbfe23 and analyzed their effects in intracellular killing of Staphylococcus aureus. The results showed that both Cbv34 and Cbfe23 OMVs killed Staphylococcus aureus via the intracellular mechanism after OMVs were taken up by macrophages and epithelial cells. Interestingly, the uptake of OMVs did not kill macrophages. The molecular mechanism of the antimicrobial feature of myxobacterial OMVs may be related to cystobactamid [147].
4.4. Probiotic-related BMVs for host defense
Lactobacilli are probiotics, and they can prevent cellular apoptosis from cytokines, thus decreasing the pathogenicity, such as Escherichia coli and vancomycin-resistant enterococcus (VRE) [148,149]. For example, Lactobacillus acidophilus NCFM prolonged the survival of nematodes when exposed to VRE. Lactiplantibacillus plantarum-derived BMVs prevented the worms from VRE invasion. The molecular mechanism for this protection may be related to upregulation of host defense genes by BMVs made from Lactiplantibacillus plantarum. The development of probiotic derived BMVs may be a new approach to treat antimicrobial resistant pathogens [150].
4.5. BMVs as antibiotic carriers
When Pseudomonas aeruginosa PAO1 bacteria were incubated with gentamicin, the bacteria secreted membrane vesicles containing gentamicin (g-MVs) and peptidoglycan hydrolase. The studies showed that g-MVs delivered gentamicin to IIIa Burkholderia cepacian. The further studies showed that strain CEP0248 was killed by g-MVs, but the strain C5424 was not because the second strain was resistant to gentamicin [123]. This passive loading of antibiotics to BMVs has the low drug loading, thus the therapeutic outcome was low [89]. To resolve this issue, Wu et al.[151] developed strategy in which they first loaded rifampicin in mesoporous silica nanoparticles and then coated the nanoparticles with OMVs. In this approach, mesoporous silica nanoparticles increased the drug loading. The authors coated the rifampicin nanoparticles using - Escherichia coli OMVs and the nanoparticle complexes had a longer drug release. The OMV-coated MSNs were taken up by Escherichia coli cells and this uptake was more efficient than uncoated nanoparticles or free drugs. However, it is hard to eliminate bioactive molecules during the production of BMVs, therefore these molecules may degrade antibiotics resulting in low efficacy in treating infectious diseases [63,152].
4.6. Phage therapy reinforcement
Phages can interact with bacterial cells. To limit Vibrio cholerae infection and the spread of cholera, new preventative methods are still required. Due to the quick production of phages, a bacteriophage (phage)-based therapy is an alternative to treat bacterial infections. However, a recent study showed that bacteria secreted OMVs to neutralize phage binding for prevention of phage predation to Vibrio cholerae. This finding supported that OMVs secreted by bacteria are decoys to prevent phage therapy [153].
We summarized the current technologies and methods used in treating infectious diseases using BMVs (as shown in Fig. 4 and Table 3). The studies are mainly focused on developing effective vaccines and delivering antibiotics using BMVs.
Fig. 4.

Molecular mechanisms in which BMVs treat infectious diseases through vaccination and direct killing bacteria. For vaccination, BMVs are recognized by DCs and subsequently DCs activate T cells and B cells to recognize and neutralize bacteria, and finally macrophages phagocytose bacteria to resolve infections. In addition, engineering BMVs may directly kill bacteria. BMVs, bacteria membrane vesicles. DCs, dendritic cells.
Table 3.
The applications of BMVs in infectious diseases.
| Types | Sources | Regimens | Antigens/Drugs | Targets | Ref. |
|---|---|---|---|---|---|
| Gram-negative | |||||
| OMVs | Vibrio cholerae | Vaccination | Natural PAMPs | Vibrio cholerae | [154] |
| Escherichia coli | Vaccination | Genetically engineering of exogeneous FhuD2/ Csa1A/LukE/ SpAkkaa/ Hla | Staphylococcus aureus | [155] | |
| Campylobacter jejuni | Vaccination | Genetically overexpression of cjaA | Campylobacter jejuni | [156] | |
| Escherichia coli | Vaccination | Genetically engineering of SpyCEP/StrepO/ Spy0269/SAM1372/R-TEM b-lac | Streptoccus pyogenes | [157] | |
| Yersinia pestis | Vaccination | Genetically overexpression of LcrV | Yersinia pestis | [158] | |
| Neisseria meningitidis | Vaccination | Natural PAMPs with LPS detoxification | Neisseria meningitidis | [159] | |
| Burkholderia pseuomallei | Vaccination | Natural PAMPs | Burkholderia pseuomallei | [160] | |
| Edwardsiella tarda | Vaccination | Natural PAMPs | Edwardsiella tarda | [161] | |
| Salmonella typhimurium | Vaccination | Natural PAMPs | Falmonella enterritidis; Escheerichia coli | [162] | |
| Burkholderia pseudomallei | Vaccination | Natural PAMPs | Burkholderia pseudomallei | [163] | |
| Escherichia coli | Vaccination | Natural PAMPs | Escherichia coli | [164] | |
| Burkholderia pseuomallei | Vaccination | Condition tuned OMVs; Intracellular-stage proteins | Burkholderia pseuomallei | [165] | |
| Acinetobacter baumanni | Vaccination | Natural PAMPs with LPS detoxification | Acinetobacter baumanni | [166] | |
| Gallibacterium anatis | Vaccination | Natural PAMPs Hydrostatic filtration | Gallibacterium anatis | [167] | |
| Helicobater pylori | Vaccination | Natural PAMPs | Helicobater pylori | [168] | |
| Escherichia coli | Vaccination | ESTA6, Ag85BN, Ag85BC Rv2660c | Escherichia coli | [112] | |
| Cystobacter ferrugineus | Therapy | Anti-microbial potency | Cystobacter ferrugineus | [146] | |
| Cystobacter velatus | Therapy | Anti-microbial potency | Escherichia coli | [147] | |
| Pseudomonas aeruginosa | Therapy | In vivo gentamicin loading | Burkholderia cepacia | [123] | |
| Pseudomonas aeruginosa | Therapy | Loading ceftriaxone, amikacin, azithromycin, ampicillin, levofloxacin, ciprofloxacin and norfloxacin by incubation | Lactobacillus acidophilus | [125] | |
| Klebsiella pneumoniae | Therapy | BSA nanoparticle cloaking | Klebsiella pneumoniae | [169] | |
| Aggregatibacter actinomycetemcomitans | Therapy | Nano decoy | Aggregatibacter actinomycetemcomitans | [170] | |
| Pseudomonas aeruginosa | Therapy | Loading gentamicin via incubation | Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa | [122] | |
| Pseudomonas aeruginosa | Therapy | Loading gentamicin via incubation | Bacillus subtilis; Staphylococcus aureus | [171] | |
| Buttiauxella. agrestis | Therapy | Loading gentamicin via incubation | Buttiauxella agrestis, Escherichia coli | [124] | |
| Helicobater pylori | As adjuvant | Not appliable | Helicobater pylori | [172] | |
| Escherichia coli | Therapy | Rifampicin-loaded nanoparticle coating | Escherichia coli | [151] | |
| IMVs | Escherichia coli | Vaccination | Genetical engineering of OmpA | Escherichia coli | [51] |
| DMVs | Pseudomonas aeruginosa | Vaccination | Natural PAMPs; Prepared by nitrogen cavitation | Pseudomonas aeruginosa | [37] |
| Gram-positive | |||||
| BMVs | Lactobacillus acidophilus | Vaccination | Natural PAMPs | Enterococcus faecium | [150] |
| Staphylococcus aureus | Therapy | Vancomycin-loaded nanoparticle cloaking | Staphylococcus aureus | [173] | |
Note: BMVs, bacterial membrane vesicles; OMVs, outer membrane vesicles; IMVs, inner membrane vesicles; DMVs, double membrane vesicles; LPS, lipopolysaccharide; PAMPs, pathogen-associated molecular patterns; BSA, bovine serum albumin; Hbp, hemoglobin protease.
5. Cancer therapies and diagnosis
5.1. Cancer immunotherapy using BMVs
The tremendous progress on cancer immunotherapy over the past few decades has shown that cancer can evade the immune system and metastasize. This understanding offers new ways to treat cancer by targeting tumor microenvironments rather than targeting cancer cells. The present cancer immunotherapeutic strategies include cancer vaccines, immune checkpoint blockade therapies, targeting immunosuppressive myeloid cells and chimeric antigen receptor (CAR)-T cell adoptive immunotherapy [174–176].
During cancer immunotherapy, BMVs are able to induce both innate and adaptive immune responses, leading to direct and immunoglobulin-mediated killing of cancer cells. However, a significant barrier in cancer immunotherapy is how to specifically target and deliver immune modulators to tumor microenvironments.
Bacteria and BMVs possess the natural tumor tropism because they can interact with tumor tissues. To verify this idea in which OMVs can reprogram tumor microenvironments (TME), Kim et al. [177]designed a regimen with multiple doses of OMVs derived from Escherichia coli in a mouse CT26 tumor model and found that OMVs effectively induced antitumor immune responses to completely eliminate established tumors. In addition, the authors addressed the molecular mechanism of this antitumor effect. They found that administered OMVs accumulated in tumor locations, and subsequently induced the production of antitumor cytokines, CXCL10 and interferon-γ. The further study in interferon-γ-deficient mice showed that this antitumor effect was interferon-γ dependent. The results showed that OMVs may be anticancer agents to regulate tumor immunity.
Aiming to enhance the tumor checkpoint therapy efficiency and tumor targeting of OMVs, Li et al. [178] reported the genetic engineering of OMVs in which the surface of OMVs was modified by adding the ectodomain of programmed death 1 (PD1). The engineered OMV-PD1 can bind the programmed death ligand 1 (PD-L1) on the tumor cell surface and facilitate its reduction of PD-L1 on the surface, thereby inhibiting the interactions of PD1-PD-L1 between T cells and cancer cells. This study suggests that bioengineered BMVs would be effective immunotherapeutic agents to improve cancer immunotherapies.
BMVs may be as antigen carriers to develop cancer vaccines. A eukaryotic-prokaryotic vesicle (EPV) nanoplatform was constructed by fusing melanoma cytomembrane vesicles (CMVs) to attenuated Salmonella OMVs. This design allowed EPV to integrate melanoma antigens with natural adjuvants to form a single therapeutic agent. In vivo prophylactic studies revealed that the EPV nano formulation stimulated the immune system and triggered the antitumor immune response, combating tumorigenesis. The eukaryotic-prokaryotic fusion strategy provides new methods to design hybrid vaccine platforms [130].
5.2. BMVs as drug carriers
The challenges to translate BMVs are the low drug loading because BMVs have the membrane barriers to prevent drug loading inside BMVs. Nanoparticles (NPs) possess the high drug loading efficiency and nanoparticle coated with eukaryotic cell membrane vesicles show the improved drug loading for cancer therapies [179–181], thus the similar concepts have been applied to BMVs for combination therapies.
Chen et al. [121] prepared OMVs made from attenuated Salmonella and fused DSPE-PEG-RGD to OMVs through the coextrusion of OMVs and DSPE-PEG-RGD, named OMV-DSPE-PEG-RGD (OR). Tegafur-loaded micelles (FT) were encapsulated with OR for the formation of OR-coated F127 micelles (ORFT). It was hypothesized that ORFT nanoparticles could possess the antitumor effect in a mouse melanoma model because OMVs stimulated the host immune response and the drugs sensitized tumor cells to CTLs for tumor killing. The experiments were designed to test this hypothesis and the results supported it. The studies show that bioinspired immunomodulatory nanomedicine may be a novel strategy to the improve current cancer immunotherapies.
Another study is to harness the benefit of immunotherapy from OMVs through the design of hollow polydopamine (HPDA)-mediated photothermal therapy (PTT) in the mouse model of melanoma. An OMV-CC hybrid membrane comprised of a bacterial OMV and B16-F10 cancer cell (CC) membrane was coated onto HPDA nanoparticles. When injected intravenously, nanoparticles of HPDA@[OMV-CC] targeted melanoma tissues and mediated the immune response via activation of dendritic cells (DC) in lymph nodes. The results showed that combinatory therapies of immunotherapy and PTT are needed to eliminate tumor in this mouse model of melanoma [182].
BMVs are liposome-like structures, therefore their enclosed compartments provide various space for loading of hydrophilic and hydrophobic chemotherapeutics. Guo et al. [126] designed a pH-sensitive drug delivery system made of OMVs derived from Gram-negative bacteria loaded with paclitaxel (PTX) and DNA damage response 1 (Redd1)-siRNA to simultaneously regulate tumor metabolism microenvironment and suppressing tumor growth. In a series of in vitro and in vivo experiments, the co-loaded OMVs repolarized tumor-associated macrophages (TAM), suppressed tumor growth, and remodeled TME in the triple-negative breast cancer model. This study shows that co-delivery of multiple drugs using OMVs may be a novel platform to enhance cancer treatment. Furthermore, Gao et al. [20] expressed RGD peptides on bacterial surface, and generated a drug delivery system made of bacterial (Escherichia coli) DMVs to tumor vasculature. Furthermore, the authors loaded DOX in DMVs using a pH gradient driven drug loading method. In a melanoma xenograft mouse model, the authors found that DMVs can target neutrophils and monocytes in vivo and they mediated the transport of DMVs across blood vessel barriers. Interestingly, DMVs can also bind to the tumor vasculature and tumor cells, leading to enhanced deposition of DMVs in tumor microenvironments (TMEs). Notably, DMVs were genetically conferred a RGD targeting group by fusing the RGD-4C peptide to ClyA, a common membrane anchoring protein on bacterial surface, and this strategy significantly enhanced the delivery efficiency of Dox to TME. The study reveals that DMVs are a powerful tool to develop drug carriers for enhanced tumor targeting.
5.3. Probiotic BMVs for tumor inhibition
Probiotic BMVs derived from Gram-positive Lactobacillus rhamnosus were isolated from conditioned medium and HepG2 cells were treated with BMVs. The studies showed that BMVs regulated the expression of Bcl-2 and Bax genes in HepG2. Interestingly, at only 100 μg/ml BMVs showed a significant killing to cancer cells. They studied the apoptotic factors (Bax/Bcl-2 expression ratio) after treating cancer cells with BMVs at 50 and 100 μg/ml, and the increased ratio of Bax/Bcl-2 was related to cancer cell killing, suggesting that the cellular death may be mediated by apoptotic signaling [183]. However, the findings are limited by the in vitro studies, but it is not clear whether this observation can be applied to in vivo cancer treatment.
5.4. Tumor diagnosis
BMVs can be used for cancer diagnosis like EVs [184]. Gujrati et al. [185] investigated bioengineered OMVs derived from Escherichia coli for enhanced optoacoustic (photoacoustic) imaging. The authors prepared OMVs encapsulating biopolymer-melanin (OMVMel) comprised of a tyrosinase transgene. The in vivo results in a 4 T1 breast cancer harboring model showed that OMVMel generated strong optoacoustic signals for tumor imaging.
Chen et al. [186] reported a one-pot synthetic strategy to engineer OMVs-based sensors for both antigen binding and signal generation. INP-Scaf3 surface scaffold was fused to the Z-domain for antibody recruiting. To assess the capability of the OMV sensor for cancer cell detection, the cancer-specific surface marker, MUC1, was selected as a target. Meanwhile, a GFP was assembled onto the displayed INP-Scaf3-Z scaffold. The results showed that HeLa cells were detected only when the anti-MUC1 antibody was added, with low background interaction between OMVs and HeLa cells.
We have summarized the current technologies and approaches using BMVs to improve the cancer treatment in Fig. 5 and Table 4. These approaches demonstrate the impact of BMVs in biomedical applications via harnessing tumor immunity.
Fig. 5.

Several platforms of BMVs used in cancer therapies. (A) BMVs as cancer antigen carriers for cancer immunotherapy. (B) BMVs as drug carriers to target cancer cells. (C) BMVs are naturally tumoricidal agents to induce cancer cell apoptosis. (D) BMVs as imaging carriers for cancer imaging and diagnosis. (E) BMVs combine with drug nanoparticles to enhance cancer therapy. BMVs, bacteria membrane vesicles. DCs, dendritic cells.
Table 4.
The applications of BMVs in cancer therapy.
| Types | Bacterial strain | Engineering and regimens | Therapeutical cargo | Diseases | Ref. |
|---|---|---|---|---|---|
| OMVs | Escherichia coli; Vibro cholera; Shigella flexneri | CaP decorating/Immunotherapy | NA | 4 T1 breast cancer; CT26 colon cancer | [187] |
| Escherichia coli | Co-loading of OMVs | PTX; siRNA | 4 T1 breast cancer | [126] | |
| Escherichia coli | Modified OMVs | PD1 | B16 melanoma | [178] | |
| Escherichia coli | Drug carrier | 5-FU | CT26 colon cancer | [188] | |
| Escherichia coli | Decoration/conjugation drug loading | ICG; TRAIL | B16 melanoma | [189] | |
| Escherichia coli | Hybrid OMVs | ICG | B16 melanoma | [130] | |
| Escherichia coli | Transdermal delivery | ICG; PEP | B16 melanoma | [129] | |
| Escherichia coli | Photothermal therapy | Melanin | 4 T1 breast cancer | [185] | |
| Escherichia coli | OMVs/B16 melanoma cell membrane vesicles. | HPDA | B16 melanoma | [182] | |
| Klebsiella pneumonia | Attenuated strain/drug loading | Dox | A549 non-small-cell lung cancer | [53] | |
| Salmonella Typhimurium | Therapy in combination with PTX; Immunotherapy | NA | HTC116 colorectal carcinoma; MCF-7 breast cancer; HepG2 hepatocellular carcinoma | [190] | |
| Salmonella typhimurium; VNP20009; Staphylococcus aureus | Photothermal/Immunotherapy | NA | CT26 colon cancer | [191] | |
| IMVs | Escherichia coli | Targeting ligand decoration Drug loading | Dox | A549 lung carcinoma | [52] |
| DMVs | Escherichia coli | RGD ligand decoration Drug loading | Dox | B16 melanoma | [20] |
| BMVs | Lactobacillus rhamnosus | Immunotherapy | NA | HepG2 liver cancer | [183] |
Note: BMVs, bacterial membrane vesicles; OMVs, outer membrane vesicles; IMVs, inner membrane vesicles; DMVs, double membrane vesicles; Dox, doxorubicin; TRAIL, tumor necrosis factor related apoptosis-inducing ligand; ICG, indocyanine green; PD1, programmed cell death protein 1; 5-FU, fluorouracil; RGD, arginine-glycine-aspartate peptide; NA, not appliable.
6. Conclusion and perspective
In this review, we have discussed the unique features of BMVs and their functions. There are two types of bacteria, Gram-negative and Gram-positive. They have the different membrane structures, so the formation of BMVs is different. For example, Gram-negative bacteria can form OMVs. Advances in nanotechnology enable to design and generate many types of BMVs. We can make OMVs, IMVs and DMVs from Gram-negative bacteria. To generate the translational products, a novel approach of nitrogen cavitation was developed to fabricate DMVs, and this approach may be applied to any bacteria. We also discussed the current approaches and methods to generate BMVs using chemical, physical, and biological tools. Biological engineering is a novel and appealing tool to design BMVs for improved their tissue targeting and therapeutic outcomes. Furthermore, we discussed the status on loading of therapeutics in BMVs. There is a challenge to effectively load therapeutics inside BMVs because BMVs form a lipid bilayer barrier. The loading of drug inside BMVs using passive methods was less than 2% (w/w). This is far away to translate them in clinic. To resolve this problem, Gao et al. [20] proposed the remote loading of drugs via pH-gradient between outer and insider membrane of BMVs. The approach improved the drug loading to 12% (w/w). Finally, we discussed the biomedical applications of BMVs in infectious diseases and cancers. The current data demonstrate that the technologies of BMVs could be applied to treat a wide range of diseases even though we only focused on discussing infectious diseases and cancers. Bacteria and BMVs have the natural tumor tropism, therefore, they would be novel drug delivery systems to deliver a wide range of cargo including chemo drugs and genetic materials to tumor microenvironments.
Although BMVs-based therapeutics seem promising, many questions remain studied. 1) Drug loading is too low to make the impact of BMVs in clinic. Current research is mainly focused on developing new structures of BMVs. There is lacking effort to develop novel approaches and methods to increase the drug loading to BMVs. Remote loading of therapeutics in BMVs may be a new direction for translational research. 2) Reproducibility of BMVs. There are so many methods to generate BMVs, and there are no standard protocols. Most importantly, the current methods generate the heterogeneous BMVs (such as sizes and compositions), thus it is difficult to warrant the batch-to-batch consistency of products required in clinic [192,193]. It is needed to address what factors impact on generation of BMVs. Can we develop physical methods to produce BMVs? Can we develop proteomics and lipidomics to analyze the compositions of BMVs? We need to develop rigorous methods to address those questions. 3) Immunotoxicity of BMVs. BMVs are derived from pathogens, so BMVs may contain toxins that may cause the side effects. We need to develop methods to control immunotoxicity of BMVs when they are used in clinic. 4) Spontaneous tumor targeting of BMVs. The studies show that BMVs have the tumor tropism and preclinical studies show that BMVs may be a novel drug carrier to improve cancer treatments. We need to understand the molecular mechanism of tumor targeting of BMVs.
In summary, we have discussed the current studies on BMVs including their generation, varied types and therapies for infectious diseases and cancers. The unique structures of bacteria (Gram-negative and Gram-positive bacteria) offer us to design and produce a wide range of types of BMVs compared to mammal cells. This feature may allow us to develop the translational BMVs-based products. There is the lacking information of how BMVs interact with the host. This knowledge would provide the opportunities to develop tissue targeting drug delivery systems. Finally, we expect that the research of BMVs will lead to the translational therapeutics for treating a wide range of diseases, such as inflammatory disorders, autoimmune diseases, and cardiovascular diseases.
Acknowledgments
This work was financially supported by NIH grants R01EB027078 to Z. W.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Domingues S, Nielsen KM, Membrane vesicles and horizontal gene transfer in prokaryotes, Curr. Opin. Microbiol 38 (2017) 16–21. [DOI] [PubMed] [Google Scholar]
- [2].Yurkin ST, Wang Z, Cell membrane-derived nanoparticles: emerging clinical opportunities for targeted drug delivery, Nanomedicine (Lond) 12 (2017) 2007–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhang X, Zhang H, Gu J, Zhang J, Shi H, Qian H, Wang D, Xu W, Pan J, Santos HA, Engineered Extracellular Vesicles for Cancer Therapy, Adv. Mater 33 (2021) e2005709. [DOI] [PubMed] [Google Scholar]
- [4].Wang S, Dong X, Gao J, Wang Z, Targeting Inflammatory Vasculature by Extracellular Vesicles, AAPS J 20 (2018) 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hong CW, Extracellular Vesicles of Neutrophils, Immune network 18 (2018) e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yang M, Walker SA, Aguilar Diaz De Leon JS, Davidovich I, Broad K, Talmon Y, Borges CR, Wolfram J, Extracellular vesicle glucose transporter-1 and glycan features in monocyte-endothelial inflammatory interactions, Nanomedicine, (2022) 102515. [DOI] [PubMed] [Google Scholar]
- [7].Vakili S, Ahooyi TM, Yarandi SS, Donadoni M, Rappaport J, Sariyer IK, Molecular and Cellular Impact of Inflammatory Extracellular Vesicles (EVs) Derived from M1 and M2 Macrophages on Neural Action Potentials, Brain Sci 10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kuo WP, Tigges JC, Toxavidis V, Ghiran I, Red Blood Cells: A Source of Extracellular Vesicles, Methods Mol. Biol 1660 (2017) 15–22. [DOI] [PubMed] [Google Scholar]
- [9].Tripisciano C, Weiss R, Karuthedom George S, Fischer MB, Weber V, Extracellular Vesicles Derived From Platelets, Red Blood Cells, and Monocyte-Like Cells Differ Regarding Their Ability to Induce Factor XII-Dependent Thrombin Generation, Front. Cell Developm. Biol 8 (2020) 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wu Q, Wang J, Tan WLW, Jiang Y, Wang S, Li Q, Yu X, Tan J, Liu S, Zhang P, Tiang Z, Chen Z, Foo RS, Yang HT, Extracellular vesicles from human embryonic stem cell-derived cardiovascular progenitor cells promote cardiac infarct healing through reducing cardiomyocyte death and promoting angiogenesis, Cell Death Dis 11 (2020) 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V, Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases, Cells 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang X, Pham A, Kang L, Walker SA, Davidovich I, Iannotta D, TerKonda SP, Shapiro S, Talmon Y, Pham S, Wolfram J, Effects of Adipose-Derived Biogenic Nanoparticle-Associated microRNA-451a on Toll-like Receptor 4-Induced Cytokines, Pharmaceutics 14 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Iannotta D, Yang M, Celia C, Di Marzio L, Wolfram J, Extracellular vesicle therapeutics from plasma and adipose tissue, Nano Today 39 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gao J, Chu D, Wang Z, Cell membrane-formed nanovesicles for disease-targeted delivery, Journal of controlled release : official journal of the Controlled Release Society 224 (2016) 208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gao J, Dong X, Su Y, Wang Z, Human neutrophil membrane-derived nanovesicles as a drug delivery platform for improved therapy of infectious diseases, Acta Biomater 123 (2021) 354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gao J, Wang S, Dong X, Leanse LG, Dai T, Wang Z, Co-delivery of resolvin D1 and antibiotics with nanovesicles to lungs resolves inflammation and clears bacteria in mice, Commun. Biol 3 (2020) 680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dong XY, Zhang CY, Gao J, Wang ZJ, Targeting of Nanotherapeutics to Infection Sites for Antimicrobial Therapy, Adv Ther-Germany 2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dong X, Gao J, Zhang CY, Hayworth C, Frank M, Wang Z, Neutrophil Membrane-Derived Nanovesicles Alleviate Inflammation To Protect Mouse Brain Injury from Ischemic Stroke, ACS Nano (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gao J, Wang S, Wang Z, High yield, scalable and remotely drug-loaded neutrophil-derived extracellular vesicles (EVs) for anti-inflammation therapy, Biomaterials 135 (2017) 62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gao J, Wang S, Dong X, Wang Z, RGD-expressed bacterial membrane-derived nanovesicles enhance cancer therapy via multiple tumorous targeting, Theranostics 11 (2021) 3301–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gao J, Dong X, Wang Z, Generation, purification and engineering of extracellular vesicles and their biomedical applications, Methods (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Vatter FAP, Cioffi M, Hanna SJ, Castarede I, Caielli S, Pascual V, Matei I, Lyden D, Extracellular vesicle- and particle-mediated communication shapes innate and adaptive immune responses, J. Exp. Med 218 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang S, Gao J, Wang Z, Outer membrane vesicles for vaccination and targeted drug delivery, Wiley Interdiscip Rev Nanomed. Nanobiotechnol (2018) e1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bladen HA, Waters JF, Electron Microscopic Study of Some Strains of Bacteroides, J. Bacteriol 86 (1963) 1339–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Konings WN, Bisschop A, Veenhuis M, Vermeulen CA, New procedure for the isolation of membrane vesicles of Bacillus subtilis and an electron microscopy study of their ultrastructure, J. Bacteriol 116 (1973) 1456–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dorward DW, Garon CF, DNA Is Packaged within Membrane-Derived Vesicles of Gram-Negative but Not Gram-Positive Bacteria, Appl. Environ. Microbiol 56 (1990) 1960–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gao L, Veen SVD, Role of Outer Membrane Vesicles in Bacterial Physiology and Host Cell Interactions, Infect. Microbes Diseases 2 (2020) 7. [Google Scholar]
- [28].Beveridge TJ, Structures of gram-negative cell walls and their derived membrane vesicles, J. Bacteriol 181 (1999) 4725–4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Acevedo R, Fernandez S, Zayas C, Acosta A, Sarmiento ME, Ferro VA, Rosenqyise E, Campal C, Cardoso D, Garcia L, Perez JL, Bacterial outer membrane vesicles and vaccine applications, Front. Immunol 5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Klimentova J, Pavkova I, Horcickova L, Bavlovic J, Kofronova O, Benada O, Stulik J, Francisella tularensis subsp. holarctica Releases Differentially Loaded Outer Membrane Vesicles Under Various Stress Conditions, Front Microbiol 10 (2019) 2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kulp A, Kuehn MJ, Biological functions and biogenesis of secreted bacterial outer membrane vesicles, Annu. Rev. Microbiol 64 (2010) 163–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Baker JL, Chen L, Rosenthal JA, Putnam D, DeLisa MP, Microbial biosynthesis of designer outer membrane vesicles, Curr. Opin. Biotechnol 29 (2014) 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Li M, Zhou H, Yang C, Wu Y, Zhou X, Liu H, Wang Y, Bacterial outer membrane vesicles as a platform for biomedical applications: An update, Journal of controlled release : official journal of the Controlled Release Society 323 (2020) 253–268. [DOI] [PubMed] [Google Scholar]
- [34].Huang Y, Nieh MP, Chen W, Lei Y, Outer membrane vesicles (OMVs) enabled bio-applications: A critical review, Biotechnol. Bioeng (2021). [DOI] [PubMed] [Google Scholar]
- [35].Naskar A, Cho H, Lee S, Kim KS, Biomimetic Nanoparticles Coated with Bacterial Outer Membrane Vesicles as a New-Generation Platform for Biomedical Applications, Pharmaceutics 13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gao J, Dong X, Wang Z, Generation, purification and engineering of extracellular vesicles and their biomedical applications, Methods 177 (2020) 114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang S, Gao J, Li M, Wang L, Wang Z, A facile approach for development of a vaccine made of bacterial double-layered membrane vesicles DMVs, Biomaterials 187 (2018) 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wang S, Gao J, Wang Z, Outer membrane vesicles for vaccination and targeted drug delivery, Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 11 (2019) e1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Toyofuku M, Nomura N, Eberl L, Types and origins of bacterial membrane vesicles, Nat. Rev. Microbiol 17 (2019) 13–24. [DOI] [PubMed] [Google Scholar]
- [40].Gerritzen MJH, Martens DE, Wijffels RH, van der Pol L, Stork M, Bioengineering bacterial outer membrane vesicles as vaccine platform, Biotechnol. Adv 35 (2017) 565–574. [DOI] [PubMed] [Google Scholar]
- [41].Hu R, Liu H, Wang M, Li J, Lin H, Liang M, Gao Y, Yang M, An OMV-Based Nanovaccine Confers Safety and Protection against Pathogenic Escherichia coli via Both Humoral and Predominantly Th1 Immune Responses in Poultry, Nanomaterials 10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lee EY, Choi DS, Kim KP, Gho YS, Proteomics in Gram-Negative Bacterial Outer Membrane Vesicles, Mass Spectrom Rev 27 (2008) 535–555. [DOI] [PubMed] [Google Scholar]
- [43].Biller SJ, Schubotz F, Roggensack SE, Thompson AW, Summons RE, Chisholm SW, Bacterial vesicles in marine ecosystems, Science 343 (2014) 183–186. [DOI] [PubMed] [Google Scholar]
- [44].Schertzer JW, Whiteley M, A bilayer-couple model of bacterial outer membrane vesicle biogenesis, mBio, 3 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kohl P, Zingl FG, Eichmann TO, Schild S, Isolation of Outer Membrane Vesicles Including Their Quantitative and Qualitative Analyses, Methods Mol Biol 2018 (1839) 117–134. [DOI] [PubMed] [Google Scholar]
- [46].Alzahrani H, Winter J, Boocock D, De Girolamo L, Forsythe SJ, Characterization of outer membrane vesicles from a neonatal meningitic strain of Cronobacter sakazakii, Fems Microbiol Lett 362 (2015). [DOI] [PubMed] [Google Scholar]
- [47].Badamchi A, Bahrami F, Tasbiti AH, Yari S, Shafiei M, Shahcheraghi F, Siadat SD, Immuno-proteomics analysis between OMV of vaccine and dominant wild type strains of Bordetella pertussis in Iran, Iran, J. Microbiol 12 (2020) 77–88. [PMC free article] [PubMed] [Google Scholar]
- [48].Schmitt S, Prokisch H, Schlunck T, Camp DG, Ahting U, Waizenegger T, Scharfe C, Meitinger T, Imhof A, Neupert W, Oefner PJ, Rapaport D, Proteome analysis of mitochondrial outer membrane from Neurospora crassa, Proteomics 6 (2006) 72–80. [DOI] [PubMed] [Google Scholar]
- [49].McBroom AJ, Kuehn MJ, Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response, Mol. Microbiol 63 (2007) 545–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Eddy JL, Gielda LM, Caulfield AJ, Rangel SM, Lathem WW, Production of outer membrane vesicles by the plague pathogen Yersinia pestis, PLoS ONE 9 (2014) e107002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kim OY, Choi SJ, Jang SC, Park KS, Kim SR, Choi JP, Lim JH, Lee SW, Park J, Di Vizio D, Lotvall J, Kim YK, Gho YS, Bacterial Protoplast-Derived Nanovesicles as Vaccine Delivery System against Bacterial Infection, Nano Lett 15 (2015) 266–274. [DOI] [PubMed] [Google Scholar]
- [52].Kim OY, Dinh NTH, Park HT, Choi SJ, Hong K, Gho YS, Bacterial protoplast-derived nanovesicles for tumor targeted delivery of chemotherapeutics, Biomaterials 113 (2017) 68–79. [DOI] [PubMed] [Google Scholar]
- [53].Kuerban K, Gao X, Zhang H, Liu J, Dong M, Wu L, Ye R, Feng M, Ye L, Doxorubicin-loaded bacterial outer-membrane vesicles exert enhanced antitumor efficacy in non-small-cell lung cancer, Acta Pharm. Sinica. B 10 (2020) 1534–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zhang D, Qin X, Wu T, Qiao Q, Song Q, Zhang Z, Extracellular vesicles based self-grown gold nanopopcorn for combinatorial chemo-photothermal therapy, Biomaterials 197 (2019) 220–228. [DOI] [PubMed] [Google Scholar]
- [55].Brown L, Wolf JM, Prados-Rosales R, Casadevall A, Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi, Nat. Rev. Microbiol 13 (2015) 620–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Liu Y, Defourny KAY, Smid EJ, Abee T, Gram-Positive Bacterial Extracellular Vesicles and Their Impact on Health and Disease, Front. Microbiol 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kim JH, Lee J, Park J, Gho YS, Gram-negative and Gram-positive bacterial extracellular vesicles, Semin. Cell Dev. Biol 40 (2015) 97–104. [DOI] [PubMed] [Google Scholar]
- [58].Toyofuku M, Carcamo-Oyarce G, Yamamoto T, Eisenstein F, Hsiao CC, Kurosawa M, Gademann K, Pilhofer M, Nomura N, Eberl L, Prophage-triggered membrane vesicle formation through peptidoglycan damage in Bacillus subtilis, Nat. Commun 8 (2017) 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Marsollier L, Brodin P, Jackson M, Kordulakova J, Tafelmeyer P, Carbonnelle E, Aubry J, Milon G, Legras P, Andre JP, Leroy C, Cottin J, Guillou ML, Reysset G, Cole ST, Impact of Mycobacterium ulcerans biofilm on transmissibility to ecological niches and Buruli ulcer pathogenesis, PLoS Pathog 3 (2007) e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Klieve AV, Yokoyama MT, Forster RJ, Ouwerkerk D, Bain PA, Mawhinney EL, Naturally occurring DNA transfer system associated with membrane vesicles in cellulolytic Ruminococcus spp. of ruminal origin, Appl. Environ. Microbiol 71 (2005) 4248–4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Tzipilevich E, Habusha M, Ben-Yehuda S, Acquisition of Phage Sensitivity by Bacteria through Exchange of Phage Receptors, Cell, 168 (2017) 186-+. [DOI] [PubMed] [Google Scholar]
- [62].Rodriguez GM, Prados-Rosales R, Functions and importance of mycobacterial extracellular vesicles, Appl. Microbiol. Biotechnol 100 (2016) 3887–3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lee J, Lee EY, Kim SH, Kim DK, Park KS, Kim KP, Kim YK, Roh TY, Gho YS, Staphylococcus aureus Extracellular Vesicles Carry Biologically Active beta-Lactamase, Antimicrob Agents Ch 57 (2013) 2589–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Olaya-Abril A, Prados-Rosales R, McConnell MJ, Martin-Pena R, Gonzalez-Reyes JA, Jimenez-Munguia I, Gomez-Gascon L, Fernandez J, Luque-Garcia JL, Garcia-Lidon C, Estevez H, Pachon J, Obando I, Casadevall A, Pirofski LA, Rodriguez-Ortega MJ, Characterization of protective extracellular membrane-derived vesicles produced by Streptococcus pneumoniae, J. Proteomics 106 (2014) 46–60. [DOI] [PubMed] [Google Scholar]
- [65].Lee WH, Choi HI, Hong SW, Kim KS, Gho YS, Jeon SG, Vaccination with Klebsiella pneumoniae-derived extracellular vesicles protects against bacteria-induced lethality via both humoral and cellular immunity, Exp. Mol. Med 47 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Manning AJ, Kuehn MJ, Functional advantages conferred by extracellular prokaryotic membrane vesicles, J. Mol. Microbiol. Biotechnol 23 (2013) 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Mug-Opstelten D, Witholt B, Preferential release of new outer membrane fragments by exponentially growing Escherichia coli, BBA 508 (1978) 287–295. [DOI] [PubMed] [Google Scholar]
- [68].Katsui N, Tsuchido T, Hiramatsu R, Fujikawa S, Takano M, Shibasaki I, Heat-induced blebbing and vesiculation of the outer membrane of Escherichia coli, J Bacteriol 151 (1982) 1523–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Deatherage BL, Lara JC, Bergsbaken T, Rassoulian Barrett SL, Lara S, Cookson BT, Biogenesis of bacterial membrane vesicles, Mol. Microbiol 72 (2009) 1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Alaniz RC, Deatherage BL, Lara JC, Cookson BT, Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo, J. Immunol 179 (2007) 7692–7701. [DOI] [PubMed] [Google Scholar]
- [71].Charon NW, Goldstein SF, Marko M, Hsieh C, Gebhardt LL, Motaleb MA, Wolgemuth CW, Limberger RJ, Rowe N, The flat-ribbon configuration of the periplasmic flagella of Borrelia burgdorferi and its relationship to motility and morphology, J. Bacteriol 191 (2009) 600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Bager RJ, Persson G, Nesta B, Soriani M, Serino L, Jeppsson M, Nielsen TK, Bojesen AM, Outer membrane vesicles reflect environmental cues in Gallibacterium anatis, Vet. Microbiol 167 (2013) 565–572. [DOI] [PubMed] [Google Scholar]
- [73].Santos S, Arauz LJ, Baruque-Ramos J, Lebrun I, Carneiro SM, Barreto SA, Schenkman RP, Outer membrane vesicles (OMV) production of Neisseria meningitidis serogroup B in batch process, Vaccine 30 (2012) 6064–6069. [DOI] [PubMed] [Google Scholar]
- [74].McCaig WD, Koller A, Thanassi DG, Production of outer membrane vesicles and outer membrane tubes by Francisella novicida, J. Bacteriol 195 (2013) 1120–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Galka F, Wai SN, Kusch H, Engelmann S, Hecker M, Schmeck B, Hippenstiel S, Uhlin BE, Steinert M, Proteomic characterization of the whole secretome of Legionella pneumophila and functional analysis of outer membrane vesicles, Infect. Immun 76 (2008) 1825–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Olofsson A, Vallstrom A, Petzold K, Tegtmeyer N, Schleucher J, Carlsson S, Haas R, Backert S, Wai SN, Grobner G, Arnqvist A, Biochemical and functional characterization of Helicobacter pylori vesicles, Mol. Microbiol 77 (2010) 1539–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].van de Waterbeemd B, Zomer G, van den IJssel J, van Keulen L, Eppink MH, van der Ley P, van der Pol LA, Cysteine Depletion Causes Oxidative Stress and Triggers Outer Membrane Vesicle Release by Neisseria meningitidis; Implications for Vaccine Development, Plos One, 8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kadurugamuwa JL, Beveridge TJ, Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion, J Bacteriol 177 (1995) 3998–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Loeb MR, Kilner J, Release of a special fraction of the outer membrane from both growing and phage T4-infected Escherichia coli B, BBA 514 (1978) 117–127. [DOI] [PubMed] [Google Scholar]
- [80].Borneleit P, Hermsdorf T, Claus R, Walther P, Kleber HP, Effect of hexadecane-induced vesiculation on the outer membrane of Acinetobacter calcoaceticus, J. Gen. Microbiol 134 (1988) 1983–1992. [DOI] [PubMed] [Google Scholar]
- [81].Kolodziejek AM, Caplan AB, Bohach GA, Paszczynski AJ, Minnich SA, Hovde CJ, Physiological levels of glucose induce membrane vesicle secretion and affect the lipid and protein composition of Yersinia pestis cell surfaces, Appl. Environ. Microbiol 79 (2013) 4509–4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Altindis E, Fu Y, Mekalanos JJ, Proteomic analysis of Vibrio cholerae outer membrane vesicles, Proceedings of the National Academy of Sciences of the United States of America, 111 (2014) E1548–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Gerritzen MJH, Maas RHW, van den Ijssel J, van Keulen L, Martens DE, Wijffels RH, Stork M, High dissolved oxygen tension triggers outer membrane vesicle formation by Neisseria meningitidis, Microb. Cell Fact 17 (2018) 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Gerritzen MJH, Salverda MLM, Martens DE, Wijffels RH, Stork M, Spontaneously released Neisseria meningitidis outer membrane vesicles as vaccine platform: production and purification, Vaccine 37 (2019) 6978–6986. [DOI] [PubMed] [Google Scholar]
- [85].Watson DC, Bayik D, Srivatsan A, Bergamaschi C, Valentin A, Niu G, Bear J, Monninger M, Sun M, Morales-Kastresana A, Jones JC, Felber BK, Chen XY, Gursel I, Pavlakis GN, Efficient production and enhanced tumor delivery of engineered extracellular vesicles, Biomaterials 105 (2016) 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Xia P, Gao J, Guan W, Li J, Yu X, Wang F, He H, Deng Q, Zhou L, Yuan Y, Han W, Yu Y, Production of bioactive recombinant rat soluble receptor for advanced glycation end products (rrsRAGE) in Pichia pastoris, Protein Expr. Purif 138 (2017) 81–87. [DOI] [PubMed] [Google Scholar]
- [87].Xia P, He H, Kristine MS, Guan W, Gao J, Wang Z, Hu J, Han L, Li J, Han W, Yu Y, Therapeutic effects of recombinant human S100A6 and soluble receptor for advanced glycation end products(sRAGE) on CCl4-induced liver fibrosis in mice, Eur. J. Pharmacol 833 (2018) 86–93. [DOI] [PubMed] [Google Scholar]
- [88].Gerritzen MJH, Stangowez L, van de Waterbeemd B, Martens DE, Wijffels RH, Stork M, Continuous production of Neisseria meningitidis outer membrane vesicles, Appl. Microbiol. Biotechnol 103 (2019) 9401–9410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Collins SM, Brown AC, Bacterial Outer Membrane Vesicles as Antibiotic Delivery Vehicles, Front. Immunol 12 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Szczepaniak J, Press C, Kleanthous C, The multifarious roles of Tol-Pal in Gram-negative bacteria, Fems Microbiol. Rev 44 (2020) 490–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Bernadac A, Gavioli M, Lazzaroni JC, Raina S, Lloubes R, Escherichia coli tol-pal mutants form outer membrane vesicles, J. Bacteriol 180 (1998) 4872–4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].McBroom AJ, Johnson AP, Vemulapalli S, Kuehn MJ, Outer membrane vesicle production by Escherichia coli is independent of membrane instability, J. Bacteriol 188 (2006) 5385–5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Turner L, Praszkier J, Hutton ML, Steer D, Ramm G, Kaparakis-Liaskos M, Ferrero RL, Increased Outer Membrane Vesicle Formation in a Helicobacter pylori tolB Mutant, Helicobacter 20 (2015) 269–283. [DOI] [PubMed] [Google Scholar]
- [94].Scorza FB, Colucci AM, Maggiore L, Sanzone S, Rossi O, Ferlenghi I, Pesce I, Caboni M, Norais N, Di Cioccio V, Saul A, Gerke C, High Yield Production Process for Shigella Outer Membrane Particles, PLoS ONE 7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Henry T, Pommier S, Journet L, Bernadac A, Gorvel JP, Lloubes R, Improved methods for producing outer membrane vesicles in Gram-negative bacteria, Res Microbiol 155 (2004) 437–446. [DOI] [PubMed] [Google Scholar]
- [96].Wang X, Quinn PJ, Lipopolysaccharide: Biosynthetic pathway and structure modification, Prog. Lipid Res 49 (2010) 97–107. [DOI] [PubMed] [Google Scholar]
- [97].Manissorn J, Sitthiyotha T, Montalban JRE, Chunsrivirot S, Thongnuek P, Wangkanont K, Biochemical and Structural Investigation of GnnA in the Lipopolysaccharide Biosynthesis Pathway of Acidithiobacillus ferrooxidans, Acs Chem. Biol 15 (2020) 3235–3243. [DOI] [PubMed] [Google Scholar]
- [98].Dauphinee SM, Karsan A, Lipopolysaccharide signaling in endothelial cells, Lab. Investig. J. Tech. Methods Pathol 86 (2006) 9–22. [DOI] [PubMed] [Google Scholar]
- [99].Matsuura M, Ito Y, Kiso M, Hasegawa A, Homma JY, Tolerance-inducing mechanisms to lethal toxicity of LPS by synthetic lipid A analogs, Jpn. J. Med. Sci. Biol 43 (1990) 274–275. [PubMed] [Google Scholar]
- [100].Needham BD, Trent MS, Fortifying the barrier: the impact of lipid A remodelling on bacterial pathogenesis, Nat. Rev. Microbiol 11 (2013) 467–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].van der Ley P, Steeghs L, Hamstra HJ, ten Hove J, Zomer B, van Alphen L, Modification of lipid A biosynthesis in Neisseria meningitidis lpxL mutants: influence on lipopolysaccharide structure, toxicity, and adjuvant activity, Infect. Immun 69 (2001) 5981–5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Lee DH, Kim SH, Kang W, Choi YS, Lee SH, Lee SR, You S, Lee HK, Chang KT, Shin EC, Adjuvant effect of bacterial outer membrane vesicles with penta-acylated lipopolysaccharide on antigen-specific T cell priming, Vaccine 29 (2011) 8293–8301. [DOI] [PubMed] [Google Scholar]
- [103].Anisimov AP, Shaikhutdinova RZ, Pan’kina LN, Feodorova VA, Savostina EP, Bystrova OV, Lindner B, Mokrievich AN, Bakhteeva IV, Titareva GM, Dentovskaya SV, Kocharova NA, Senchenkova SN, Holst O, Devdariani ZL, Popov YA, Pier GB, Knirel YA, Effect of deletion of the lpxM gene on virulence and vaccine potential of Yersinia pestis in mice, J. Med. Microbiol 56 (2007) 443–453. [DOI] [PubMed] [Google Scholar]
- [104].Berezow AB, Ernst RK, Coats SR, Braham PH, Karimi-Naser LM, Darveau RP, The structurally similar, penta-acylated lipopolysaccharides of Porphyromonas gingivalis and Bacteroides elicit strikingly different innate immune responses, Microb. Pathog 47 (2009) 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Wang YQ, Bazin-Lee H, Evans JT, Casella CR, Mitchell TC, MPL Adjuvant Contains Competitive Antagonists of Human TLR4, Front. Immunol 11 (2020) 577823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Matthias KA, Reveille A, Connolly KL, Jerse AE, Gao YS, Bash MC, Deletion of major porins from meningococcal outer membrane vesicle vaccines enhances reactivity against heterologous serogroup B Neisseria meningitidis strains, Vaccine 38 (2020) 2396–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Surve MV, Anil A, Kamath KG, Bhutda S, Sthanam LK, Pradhan A, Srivastava R, Basu B, Dutta S, Sen S, Modi D, Banerjee A, Membrane Vesicles of Group B Streptococcus Disrupt Feto-Maternal Barrier Leading to Preterm Birth, PLoS Pathog 12 (2016) e1005816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Jeon J, Mok HJ, Choi Y, Park SC, Jo H, Her J, Han JK, Kim YK, Kim KP, Ban C, Proteomic analysis of extracellular vesicles derived from Propionibacterium acnes, Proteom. Clin. Appl 11 (2017). [DOI] [PubMed] [Google Scholar]
- [109].Jeon H, Oh MH, Jun SH, Kim SI, Choi CW, Kwon HI, Na SH, Kim YJ, Nicholas A, Selasi GN, Lee JC, Variation among Staphylococcus aureus membrane vesicle proteomes affects cytotoxicity of host cells, Microb. Pathog 93 (2016) 185–193. [DOI] [PubMed] [Google Scholar]
- [110].Rivera J, Cordero RJ, Nakouzi AS, Frases S, Nicola A, Casadevall A, Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins, PNAS 107 (2010) 19002–19007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Thay B, Wai SN, Oscarsson J, Staphylococcus aureus alpha-toxin-dependent induction of host cell death by membrane-derived vesicles, PLoS ONE 8 (2013) e54661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Daleke-Schermerhorn MH, Felix T, Soprova Z, Ten Hagen-Jongman CM, Vikstrom D, Majlessi L, Beskers J, Follmann F, de Punder K, van der Wel NN, Baumgarten T, Pham TV, Piersma SR, Jimenez CR, van Ulsen P, de Gier JW, Leclerc C, Jong WS, Luirink J, Decoration of outer membrane vesicles with multiple antigens by using an autotransporter approach, Appl. Environ. Microbiol 80 (2014) 5854–5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Salverda MLM, Meinderts SM, Hamstra HJ, Wagemakers A, Hovius JWR, van der Ark A, Stork M, van der Ley P, Surface display of a borrelial lipoprotein on meningococcal outer membrane vesicles, Vaccine 34 (2016) 1025–1033. [DOI] [PubMed] [Google Scholar]
- [114].van den Berg HB, van Saparoea D, Houben C, Kuijl J, Luirink WSPJ, Combining Protein Ligation Systems to Expand the Functionality of Semi-Synthetic Outer Membrane Vesicle Nanoparticles, Front Microbiol 11 (2020) 890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].van den Berg HB, van Saparoea D, de Houben MI, Jonge WSP, Jong JL, Display of Recombinant Proteins on Bacterial Outer Membrane Vesicles by Using Protein Ligation, Appl. Environ. Microbiol 84 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Park SH, Zheng JH, Nguyen VH, Jiang SN, Kim DY, Szardenings M, Min JH, Hong Y, Choy HE, Min JJ, RGD Peptide Cell-Surface Display Enhances the Targeting and Therapeutic Efficacy of Attenuated Salmonella-mediated Cancer Therapy, Theranostics 6 (2016) 1672–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Toso JF, Gill VJ, Hwu P, Marincola FM, Restifo NP, Schwartzentruber DJ, Sherry RM, Topalian SL, Yang JC, Stock F, Freezer LJ, Morton KE, Seipp C, Haworth L, Mavroukakis S, White D, MacDonald S, Mao J, Sznol M, Rosenberg SA, Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma, Journal of clinical oncology : official journal of the American Society of, Clin. Oncol 20 (2002) 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Kim JY, Doody AM, Chen DJ, Cremona GH, Shuler ML, Putnam D, DeLisa MP, Engineered bacterial outer membrane vesicles with enhanced functionality, J. Mol. Biol 380 (2008) 51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Danhier F, Vroman B, Lecouturier N, Crokart N, Pourcelle V, Freichels H, Jerome C, Marchand-Brynaert J, Feron O, Preat V, Targeting of tumor endothelium by RGD-grafted PLGA-nanoparticles loaded with Paclitaxel, J. Control. Release 140 (2009) 166–173. [DOI] [PubMed] [Google Scholar]
- [120].Temming K, Schiffelers RM, Molema G, Kok RJ, RGD-based strategies for selective delivery of therapeutics and imaging agents to the tumour vasculature, Drug Resist Update 8 (2005) 381–402. [DOI] [PubMed] [Google Scholar]
- [121].Chen Q, Bai H, Wu W, Huang G, Li Y, Wu M, Tang G, Ping Y, Bioengineering Bacterial Vesicle-Coated Polymeric Nanomedicine for Enhanced Cancer Immunotherapy and Metastasis Prevention, Nano Lett 20 (2020) 11–21. [DOI] [PubMed] [Google Scholar]
- [122].Kadurugamuwa JL, Beveridge TJ, Bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria including pathogens: conceptually new antibiotics, J. Bacteriol 178 (1996) 2767–2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Allan ND, Beveridge TJ, Gentamicin delivery to Burkholderia cepacia group IIIa strains via membrane vesicles from Pseudomonas aeruginosa PAO1, Antimicrob. Agents Ch 47 (2003) 2962–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Tashiro Y, Hasegawa Y, Shintani M, Takaki K, Ohkuma M, Kimbara K, Futamata H, Interaction of Bacterial Membrane Vesicles with Specific Species and Their Potential for Delivery to Target Cells, Front. Microbiol 8 (2017) 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Huang WW, Zhang QS, Li WR, Yuan MC, Zhou JX, Hua LQ, Chen YJ, Ye C, Ma YB, Development of novel nanoantibiotics using an outer membrane vesicle-based drug efflux mechanism, J. Control. Release 317 (2020) 1–22. [DOI] [PubMed] [Google Scholar]
- [126].Guo Q, Li X, Zhou W, Chu Y, Chen Q, Zhang Y, Li C, Chen H, Liu P, Zhao Z, Wang Y, Zhou Z, Luo Y, Li C, You H, Song H, Su B, Zhang T, Sun T, Jiang C, Sequentially Triggered Bacterial Outer Membrane Vesicles for Macrophage Metabolism Modulation and Tumor Metastasis Suppression, ACS Nano (2021). [DOI] [PubMed] [Google Scholar]
- [127].Sekiya N, Imamura A, [Doxil–pegylated liposomal doxorubicin], Gan to kagaku ryoho, Cancer Chemotherapy 35 (2008) 1439–1443. [PubMed] [Google Scholar]
- [128].Bavli Y, Winkler I, Chen BM, Roffler S, Cohen R, Szebeni J, Barenholz Y, Doxebo (doxorubicin-free Doxil-like liposomes) is safe to use as a pre-treatment to prevent infusion reactions to PEGylated nanodrugs, Journal of controlled release : official journal of the Controlled Release Society 306 (2019) 138–148. [DOI] [PubMed] [Google Scholar]
- [129].Gu TW, Wang MZ, Niu J, Chu Y, Guo KR, Peng LH, Outer membrane vesicles derived from E. coli as novel vehicles for transdermal and tumor targeting delivery, Nanoscale 12 (2020) 18965–18977. [DOI] [PubMed] [Google Scholar]
- [130].Chen Q, Huang G, Wu W, Wang J, Hu J, Mao J, Chu PK, Bai H, Tang G, A Hybrid Eukaryotic-Prokaryotic Nanoplatform with Photothermal Modality for Enhanced Antitumor Vaccination, Adv. Mater 32 (2020) e1908185. [DOI] [PubMed] [Google Scholar]
- [131].Cheng K, Zhao R, Li Y, Qi Y, Wang Y, Zhang Y, Qin H, Qin Y, Chen L, Li C, Liang J, Li Y, Xu J, Han X, Anderson GJ, Shi J, Ren L, Zhao X, Nie G, Bioengineered bacteria-derived outer membrane vesicles as a versatile antigen display platform for tumor vaccination via Plug-and-Display technology, Nat. Commun 12 (2021) 2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].van der Pol L, Stork M, van der Ley P, Outer membrane vesicles as platform vaccine technology, Biotechnol. J 10 (2015) 1689–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Holst J, Martin D, Arnold R, Huergo CC, Oster P, O’Hallahan J, Rosenqvist E, Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis, Vaccine 27 (2009) B3–B12. [DOI] [PubMed] [Google Scholar]
- [134].Li L, Petrovsky N, Molecular Adjuvants for DNA Vaccines, Current Issues Mol. Biol 22 (2017) 17–40. [DOI] [PubMed] [Google Scholar]
- [135].Golos A, Lutynska A, Aluminium-adjuvanted vaccines–a review of the current state of knowledge, Przegl. Epidemiol 69 (731–734) (2015) 871–1734. [PubMed] [Google Scholar]
- [136].Pulendran B, P SA, O’Hagan DT, Emerging concepts in the science of vaccine adjuvants, Nature reviews. Drug discovery, 20 (2021) 454–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Guerrero Manriquez GG, Tuero I, Adjuvants: friends in vaccine formulations against infectious diseases, Hum. Vaccines Immunotherap 17 (2021) 3539–3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Zheng Y, Ye PP, Zhou Y, Wu SY, Liu XT, Du B, Tang BH, Kan M, Nie AQ, Yin R, Wang M, Hao GX, Song LL, Yang XM, Huang X, Su LQ, Wang WQ, van den Anker J, Zhao W, LPS-Induced Inflammation Affects Midazolam Clearance in Juvenile Mice in an Age-Dependent Manner, J. Inflam. Res 14 (2021) 3697–3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Wang Y, Goossens E, Eeckhaut V, Perez Calvo E, Lopez-Ulibarri R, Eising I, Klausen M, Debunne N, De Spiegeleer B, Ducatelle R, Van Immerseel F, Dietary muramidase degrades bacterial peptidoglycan to NOD-activating muramyl dipeptides and reduces duodenal inflammation in broiler chickens, British J. Nutr 126 (2021) 641–651. [DOI] [PubMed] [Google Scholar]
- [140].Needham BD, Carroll SM, Giles DK, Georgiou G, Whiteley M, Trent MS, Modulating the innate immune response by combinatorial engineering of endotoxin, PNAS 110 (2013) 1464–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Lee TY, Kim CU, Bae EH, Seo SH, Jeong DG, Yoon SW, Chang KT, Kim YS, Kim SH, Kim DJ, Outer membrane vesicles harboring modified lipid A moiety augment the efficacy of an influenza vaccine exhibiting reduced endotoxicity in a mouse model, Vaccine 35 (2017) 586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Sanders H, Feavers IM, Adjuvant properties of meningococcal outer membrane vesicles and the use of adjuvants in Neisseria meningitidis protein vaccines, Expert Rev. Vaccines 10 (2011) 323–334. [DOI] [PubMed] [Google Scholar]
- [143].Pritsch M, Ben-Khaled N, Chaloupka M, Kobold S, Berens-Riha N, Peter A, Liegl G, Schubert S, Hoelscher M, Loscher T, Wieser A, Comparison of Intranasal Outer Membrane Vesicles with Cholera Toxin and Injected MF59C.1 as Adjuvants for Malaria Transmission Blocking Antigens AnAPN1 and Pfs48/45, J. Immunol. Res 2016 (2016) 3576028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Li Z, Clarke AJ, Beveridge TJ, A major autolysin of Pseudomonas aeruginosa: subcellular distribution, potential role in cell growth and division and secretion in surface membrane vesicles, J. Bacteriol 178 (1996) 2479–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Li ZS, Clarke AJ, Beveridge TJ, Gram-negative bacteria produce membrane vesicles which are capable of killing other bacteria, J. Bacteriol 180 (1998) 5478–5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Goes A, Lapuhs P, Kuhn T, Schulz E, Richter R, Panter F, Dahlem C, Koch M, Garcia R, Kiemer AK, Muller R, Fuhrmann G, Myxobacteria-Derived Outer Membrane Vesicles: Potential Applicability Against Intracellular Infections, Cells 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Schulz E, Goes A, Garcia R, Panter F, Koch M, Muller R, Fuhrmann K, Fuhrmann G, Biocompatible bacteria-derived vesicles show inherent antimicrobial activity, Journal of controlled release : official journal of the Controlled Release Society 290 (2018) 46–55. [DOI] [PubMed] [Google Scholar]
- [148].Medellin-Pena MJ, Griffiths MW, Effect of molecules secreted by Lactobacillus acidophilus strain La-5 on Escherichia coli O157:H7 colonization, Appl. Environ. Microbiol 75 (2009) 1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Manley KJ, Fraenkel MB, Mayall BC, Power DA, Probiotic treatment of vancomycin-resistant enterococci: a randomised controlled trial, Med. J. Australia 186 (2007) 454–457. [DOI] [PubMed] [Google Scholar]
- [150].Li M, Lee K, Hsu M, Nau G, Mylonakis E, Ramratnam B, Lactobacillus-derived extracellular vesicles enhance host immune responses against vancomycin-resistant enterococci, BMC Microbiol 17 (2017) 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Wu S, Huang Y, Yan JC, Li YZ, Wang JF, Yang YY, Yuan PY, Ding X, Bacterial Outer Membrane-Coated Mesoporous Silica Nanoparticles for Targeted Delivery of Antibiotic Rifampicin against Gram-Negative Bacterial Infection In Vivo, Adv. Funct. Mater 31 (2021). [Google Scholar]
- [152].Kulkarni HM, Nagaraj R, Jagannadham MV, Protective role of E coli outer membrane vesicles against antibiotics, Microbiol. Res 181 (2015) 1–7. [DOI] [PubMed] [Google Scholar]
- [153].Reyes-Robles T, Dillard RS, Cairns LS, Silva-Valenzuela CA, Housman M, Ali A, Wright ER, Camilli A, Vibrio cholerae Outer Membrane Vesicles Inhibit Bacteriophage Infection, J. Bacteriol 200 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Sedaghat M, Siadat SD, Mirabzadeh E, Keramati M, Vaziri F, Shafiei M, Shahcheraghi F, Evaluation of antibody responses to outer membrane vesicles (OMVs) and killed whole cell of Vibrio cholerae O1 El Tor in immunized mice, Iran, J. Microbiol 11 (2019) 212–219. [PMC free article] [PubMed] [Google Scholar]
- [155].Irene C, Fantappie L, Caproni E, Zerbini F, Anesi A, Tomasi M, Zanella I, Stupia S, Prete S, Valensin S, Konig E, Frattini L, Gagliardi A, Isaac SJ, Grandi A, Guella G, Grandi G, Bacterial outer membrane vesicles engineered with lipidated antigens as a platform for Staphylococcus aureus vaccine, Proceedings of the National Academy of Sciences of the United States of America, 116 (2019) 21780–21788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Godlewska R, Kuczkowski M, Wyszynska A, Klim J, Derlatka K, Wozniak-Biel A, Jagusztyn-Krynicka EK, Evaluation of a protective effect of in ovo delivered Campylobacter jejuni OMVs, Appl. Microbiol. Biotechnol 100 (2016) 8855–8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Fantappie L, de Santis M, Chiarot E, Carboni F, Bensi G, Jousson O, Margarit I, Grandi G, Antibody-mediated immunity induced by engineered Escherichia coli OMVs carrying heterologous antigens in their lumen, J. Extracell. Vesicles 3 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Wang X, Singh AK, Zhang X, Sun W, Induction of Protective Antiplague Immune Responses by Self-Adjuvanting Bionanoparticles Derived from Engineered Yersinia pestis, Infect. Immun 88 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Jessouroun E, da Silveira IF, Larangeira AP, Pereira S, Fernandes SA, Rabinovitch L, Frasch CE, Castro-Faria-Neto HC, Bozza PT, Outer membrane vesicles (OMVs) and detoxified lipooligosaccharide (dLOS) obtained from Brazilian prevalent N. meningitidis serogroup B strains protect mice against homologous and heterologous meningococcal infection and septic shock, Vaccine 22 (2004) 2617–2625. [DOI] [PubMed] [Google Scholar]
- [160].Nieves W, Petersen H, Judy BM, Blumentritt CA, Russell-Lodrigue K, Roy CJ, Torres AG, Morici LA, A Burkholderia pseudomallei Outer Membrane Vesicle Vaccine Provides Protection against Lethal Sepsis, Clin. Vaccine Immunol 21 (2014) 747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Park SB, Jang HB, Nho SW, Cha IS, Hikima J, Ohtani M, Aoki T, Jung TS, Outer membrane vesicles as a candidate vaccine against edwardsiellosis, PLoS ONE 6 (2011) e17629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Chen Y, Jie K, Li B, Yu H, Ruan H, Wu J, Huang X, Liu Q, Immunization With Outer Membrane Vesicles Derived From Major Outer Membrane Protein-Deficient Salmonella Typhimurium Mutants for Cross Protection Against Salmonella Enteritidis and Avian Pathogenic Escherichia coli O78 Infection in Chickens, Front. Microbiol 11 (2020) 588952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Nieves W, Asakrah S, Qazi O, Brown KA, Kurtz J, Aucoin DP, McLachlan JB, Roy CJ, Morici LA, A naturally derived outer-membrane vesicle vaccine protects against lethal pulmonary Burkholderia pseudomallei infection, Vaccine 29 (2011) 8381–8389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Hu RJ, Li J, Zhao YZ, Lin H, Liang L, Wang MM, Liu HJ, Min YN, Gao YP, Yang MM, Exploiting bacterial outer membrane vesicles as a cross-protective vaccine candidate against avian pathogenic Escherichia coli (APEC), Microb. Cell Fact 19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Baker SM, Settles EW, Davitt C, Gellings P, Kikendall N, Hoffmann J, Wang YH, Bitoun J, Lodrigue KR, Sahl JW, Keim P, Roy C, McLachlan J, Morici LA, Burkholderia pseudomallei OMVs derived from infection mimicking conditions elicit similar protection to a live-attenuated vaccine, npj Vaccines 6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Pulido MR, Garcia-Quintanilla M, Pachon J, McConnell MJ, A lipopolysaccharide-free outer membrane vesicle vaccine protects against Acinetobacter baumannii infection, Vaccine 38 (2020) 719–724. [DOI] [PubMed] [Google Scholar]
- [167].Antenucci F, Arak H, Gao J, Allahgadry T, Thofner I, Bojesen AM, Hydrostatic Filtration Enables Large-Scale Production of Outer Membrane Vesicles That Effectively Protect Chickens against Gallibacterium anatis, Vaccines 8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Liu Q, Li X, Zhang Y, Song Z, Li R, Ruan H, Huang X, Orally-administered outer-membrane vesicles from Helicobacter pylori reduce H. pylori infection via Th2-biased immune responses in mice, Pathogens and disease 77 (2019). [DOI] [PubMed] [Google Scholar]
- [169].Wu G, Ji H, Guo X, Li Y, Ren T, Dong H, Liu J, Liu Y, Shi X, He B, Nanoparticle reinforced bacterial outer-membrane vesicles effectively prevent fatal infection of carbapenem-resistant Klebsiella pneumoniae, Nanomedicine 24 (2020) 102148. [DOI] [PubMed] [Google Scholar]
- [170].Lindholm M, Metsaniitty M, Granstrom E, Oscarsson J, Outer membrane vesicle-mediated serum protection in Aggregatibacter actinomycetemcomitans, Journal of oral microbiology 12 (2020) 1747857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].MacDonald KL, Beveridge TJ, Bactericidal effect of gentamicin-induced membrane vesicles derived from Pseudomonas aeruginosa PAO1 on gram-positive bacteria, Can. J. Microbiol 48 (2002) 810–820. [DOI] [PubMed] [Google Scholar]
- [172].Song Z, Li B, Zhang Y, Li R, Ruan H, Wu J, Liu Q, Outer Membrane Vesicles of Helicobacter pylori 7.13 as Adjuvants Promote Protective Efficacy Against Helicobacter pylori Infection, Front. Microbiol 11 (2020) 1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Gao F, Xu LL, Yang BQ, Fan F, Yang L, Kill the Real with the Fake: Eliminate Intracellular Staphylococcus aureus Using Nanoparticle Coated with Its Extracellular Vesicle Membrane as Active-Targeting Drug Carrier, Acs Infect. Dis 5 (2019) 218–227. [DOI] [PubMed] [Google Scholar]
- [174].Yang Y, Cancer immunotherapy: harnessing the immune system to battle cancer, J. Clin. Investig 125 (2015) 3335–3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Riley RS, June CH, Langer R, Mitchell MJ, Delivery technologies for cancer immunotherapy, Nat. Rev. Drug Discovery 18 (2019) 175–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Atsavapranee ES, Billingsley MM, Mitchell MJ, Delivery technologies for T cell gene editing: Applications in cancer immunotherapy, Ebiomedicine 67 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Kim OY, Park HT, Dinh NTH, Choi SJ, Lee J, Kim JH, Lee SW, Gho YS, Bacterial outer membrane vesicles suppress tumor by interferon-gamma-mediated antitumor response, Nat. Commun 8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Li Y, Zhao R, Cheng K, Zhang K, Wang Y, Zhang Y, Li Y, Liu G, Xu J, Xu J, Anderson GJ, Shi J, Ren L, Zhao X, Nie G, Bacterial Outer Membrane Vesicles Presenting Programmed Death 1 for Improved Cancer Immunotherapy via Immune Activation and Checkpoint Inhibition, ACS Nano (2020). [DOI] [PubMed] [Google Scholar]
- [179].Liu CM, Chen GB, Chen HH, Zhang JB, Li HZ, Sheng MX, Weng WB, Guo SM, Cancer cell membrane-cloaked mesoporous silica nanoparticles with a pH-sensitive gatekeeper for cancer treatment, Colloid Surface B 175 (2019) 477–486. [DOI] [PubMed] [Google Scholar]
- [180].Wang P, Kankala RK, Chen BQ, Zhang Y, Zhu MZ, Li XM, Long RM, Yang DY, Krastev R, Wang SB, Xiong X, Liu YG, Cancer Cytomembrane-Cloaked Prussian Blue Nanoparticles Enhance the Efficacy of Mild-Temperature Photothermal Therapy by Disrupting Mitochondrial Functions of Cancer Cells, Acs Appl. Mater. Inter 13 (2021) 37563–37577. [DOI] [PubMed] [Google Scholar]
- [181].Chang ZM, Zhang R, Yang C, Shao D, Tang YG, Dong WF, Wang Z, Cancer-leukocyte hybrid membrane-cloaked magnetic beads for the ultrasensitive isolation, purification, and non-destructive release of circulating tumor cells, Nanoscale 12 (2020) 19121–19128. [DOI] [PubMed] [Google Scholar]
- [182].Wang DD, Liu CH, You SQ, Zhang K, Li M, Cao Y, Wang CT, Dong HF, Zhang XJ, Bacterial Vesicle-Cancer Cell Hybrid Membrane-Coated Nanoparticles for Tumor Specific Immune Activation and Photothermal Therapy, Acs Appl. Mater. Inter 12 (2020) 41138–41147. [DOI] [PubMed] [Google Scholar]
- [183].Behzadi E, Mahmoodzadeh Hosseini H, Imani Fooladi AA, The inhibitory impacts of Lactobacillus rhamnosus GG-derived extracellular vesicles on the growth of hepatic cancer cells, Microb. Pathog 110 (2017) 1–6. [DOI] [PubMed] [Google Scholar]
- [184].Hu T, Wolfram J, Srivastava S, Extracellular Vesicles in Cancer Detection: Hopes and Hypes, Trends in cancer 7 (2021) 122–133. [DOI] [PubMed] [Google Scholar]
- [185].Gujrati V, Prakash J, Malekzadeh-Najafabadi J, Stiel A, Klemm U, Mettenleiter G, Aichler M, Walch A, Ntziachristos V, Bioengineered bacterial vesicles as biological nano-heaters for optoacoustic imaging, Nat. Commun 10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Chen Q, Rozovsky S, Chen W, Engineering multi-functional bacterial outer membrane vesicles as modular nanodevices for biosensing and bioimaging, Chem. Commun 53 (2017) 7569–7572. [DOI] [PubMed] [Google Scholar]
- [187].Qing S, Lyu C, Zhu L, Pan C, Wang S, Li F, Wang J, Yue H, Gao X, Jia R, Wei W, Ma G, Biomineralized Bacterial Outer Membrane Vesicles Potentiate Safe and Efficient Tumor Microenvironment Reprogramming for Anticancer Therapy, Adv. Mater 32 (2020) e2002085. [DOI] [PubMed] [Google Scholar]
- [188].Shi J, Ma Z, Pan H, Liu Y, Chu Y, Wang J, Chen L, Biofilm-encapsulated nano drug delivery system for the treatment of colon cancer, J. Microencapsul 37 (2020) 481–491. [DOI] [PubMed] [Google Scholar]
- [189].Peng LH, Wang MZ, Chu Y, Zhang L, Niu J, Shao HT, Yuan TJ, Jiang ZH, Gao JQ, Ning XH, Engineering bacterial outer membrane vesicles as transdermal nanoplatforms for photo-TRAIL-programmed therapy against melanoma, Sci. Adv 6 (2020) eaba2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Aly RG, El-Enbaawy MI, Abd El-Rahman SS, Ata NS, Antineoplastic activity of Salmonella Typhimurium outer membrane nanovesicles, Exp. Cell Res 399 (2021) 112423. [DOI] [PubMed] [Google Scholar]
- [191].Zhuang Q, Xu J, Deng D, Chao T, Li J, Zhang R, Peng R, Liu Z, Bacteria-derived membrane vesicles to advance targeted photothermal tumor ablation, Biomaterials 268 (2021) 120550. [DOI] [PubMed] [Google Scholar]
- [192].Alves NJ, Turner KB, Medintz IL, Walper SA, Emerging therapeutic delivery capabilities and challenges utilizing enzyme/protein packaged bacterial vesicles, Therap. Delivery 6 (2015) 873–887. [DOI] [PubMed] [Google Scholar]
- [193].Gujrati VB, Jon S, Bioengineered bacterial outer membrane vesicles: what is their potential in cancer therapy?, Nanomedicine 9 (2014) 933–935 [DOI] [PubMed] [Google Scholar]


