Sleep disorders, such as insomnia, are associated not only with having insufficient amounts of sleep, but also with a lack of continuous undisrupted sleep. Many insomnia patients with fragmented sleep report having poorer memory and attention, higher stress responsivity, and emotional dysregulation throughout the day (Medic et al, 2017). Additionally, sleep homeostasis processes can cause excessive daytime sleepiness to compensate for nighttime sleep loss and/or fragmentation, and this can further reduce the individual's quality life.
Healthy maintenance of continuous sleep involves transitions through several sleep stages that are thought to have different roles. The two main stages of sleep are rapid eye movement (REM) and non-rapid eye movement (NREM) sleep. It has been proposed that transitions between sleep stages (i.e., sleep promotion/initiation) and sustaining individual stages (i.e., sleep maintenance) are controlled by independent mechanisms (Teng et al, 2021). These mechanisms are in constant communication with one another to maintain healthy boundaries between each vigilant state (i.e., wake, NREM and REM sleep) and to promote a healthy sleep structure.
One region that has received particular interest for its role in sleep–wake regulation is the lateral preoptic hypothalamus (LPO). The LPO contains two main group of cells: excitatory glutamatergic neurons, which are proposed to be inactive during sleep and function to promote wakefulness when active (Chung et al, 2017; Mondino et al, 2021); and inhibitory GABAergic neurons, which promote and maintain sleep by inhibiting wake-promoting monoaminergic cells (Zhang et al, 2015; Chung et al, 2017; Mondino et al, 2021). Notably, these GABAergic neurons can be divided into multiple subtypes – each of which regulates either NREM sleep, REM sleep, or both – and these are intermingled throughout the LPO (Takahashi et al, 2009; Chung et al, 2017).
GABAergic LPO neurons are suggested to be more involved in sleep maintenance than sleep initiation because electrophysiological recordings of these neurons show lower activity during the sleep–wake transition period and increased activity throughout NREM sleep (Takahashi et al, 2009; Alam et al, 2014). Further supporting this, optogenetic and chemogenetic activation of these neurons results in long-lasting NREM sleep, while inactivation of these neurons reduces NREM sleep amounts by increasing wakefulness (Zhang et al, 2015; Chung et al, 2017; Kroeger et al, 2018). In addition to sleep maintenance, some subpopulations of GABAergic LPO neurons play a role in sleep homeostasis. These neurons show elevated activity during prolonged wakefulness periods when homeostatic sleep pressure is high, and gradually decline in activity during sleep recovery periods (Alam et al, 2014). Given the importance of LPO neurons in sleep maintenance and homeostasis, any disturbance to LPO neurons could potentially lead to sleep fragmentation, sleep debt, and an overall lack of healthy sleep structure.
LPO neurons express glutamatergic NMDARs. NMDARs are expressed globally and have previously been shown to be critical for sleep regulation. For example, pharmacological inactivation of NMDARs abolishes both NREM and REM sleep (Burgdorf et al, 2019; Pálfi et al., 2021), and their activation increases the time spent in NREM sleep in rodents (Burgdorf et al, 2019). Yet the specific contribution of LPO NMDARs in sleep maintenance is unclear. In a recent issue of J Neurosci, Miracca et al. (2022) aimed to understand whether NMDARs on LPO neurons underlie the role of LPO cells in sleep maintenance and homeostasis in a mouse model.
Using multiunit recordings and in vivo fiber photometry, the authors revealed that population activity in the LPO was normally greater during REM sleep than during NREM sleep or wakefulness. In contrast, when LPO NMDARs were knocked out by genetic deletion of GluN1 subunits of NMDARs (generating ΔGluN1-LPO mice), the levels of neuronal activity were not significantly different between wake, REM, and NREM sleep. Additionally, the elimination of NMDARs reduced average time spent in NREM and REM sleep and increased wakefulness compared with control mice. Not only were total sleep amounts decreased, but ΔGluN1-LPO mice also exhibited sleep fragmentation. Specifically, the number of wake and NREM sleep episodes increased and the duration of each episode was reduced. Interestingly, the power of cortical theta oscillations during REM sleep decreased in ΔGluN1-LPO mice compared with their control, suggesting that NMDARs on LPO neurons may be responsible for regulating cortical theta oscillations during REM sleep, as well as for maintaining continuous sleep.
To assess the role of LPO neuronal NMDARs in sleep homeostasis, the authors conducted a 6 h sleep deprivation protocol. During sleep deprivation, the ΔGluN1-LPO mice fell asleep significantly more frequently and had shorter latency to fall asleep compared with control mice. These results suggest elevated sleepiness in mutant mice. In addition, the power of delta oscillations during the first hour of NREM sleep recovery after sleep deprivation was higher than baseline levels measured before sleep deprivation in both mutant and control mice. Because increased power in delta oscillations is a marker of sleep recovery, these results suggest that sleep homeostasis was intact in mutant mice. However, ΔGluN1-LPO mice still exhibited high sleep fragmentation during sleep recovery, as the number of NREM sleep and wake occurrences was significantly greater, while the duration of each episode was shorter than control mice. These findings indicate that NMDARs on LPO neurons play a role in sleep maintenance but not sleep homeostasis.
The remaining question was which NMDAR-expressing LPO neuronal populations are responsible for sleep maintenance. To address this question, short hairpin RNA (shRNA) targeting the GluN1 subunit was used to selectively reduce the expression of NMDARs in either GABAergic or glutamatergic LPO neurons. Knocking down GluN1 selectively in glutamatergic neurons resulted in a typical sleep–wake structure similar to that in control mice. In contrast, knocking down GluN1 selectively in GABAergic neurons increased the number of transitions between wake and NREM sleep while decreasing the duration of each episode, similar to the phenotype observed in ΔGluN1-LPO mice. Interestingly, unlike the ΔGluN1-LPO mice, REM sleep amounts remained similar to the control mice, and there was no significant change in cortical theta oscillations in these mice.
A sleep deprivation procedure was conducted on mice with reduced GluN1 expression in GABAergic LPO neurons. Unexpectedly, during the sleep deprivation period, mutant mice did not exhibit the sleepiness phenotype observed in ΔGluN1-LPO mice, and the phenotype was more comparable to the control mice. During sleep recovery in mice which had reduced NMDARs in GABAergic neurons, the power of delta oscillations during NREM sleep was higher than at baseline levels, demonstrating that sleep homeostasis was intact. Additionally, the mutant mice showed greater sleep fragmentation than controls, thus suggesting that NMDARs on GABAergic LPO neurons are required for sleep maintenance.
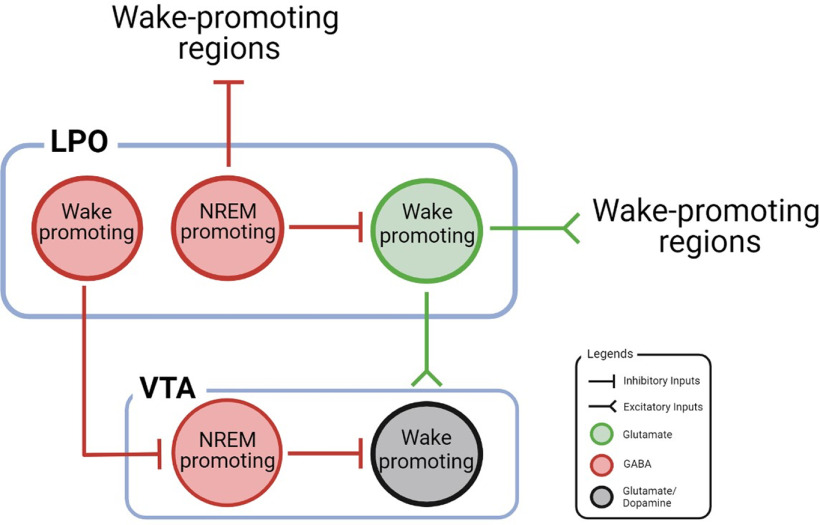
These results align with previous studies in which optogenetic activation or inactivation of GABAergic LPO neurons increased or decreased amounts of NREM sleep, respectively, without impacting the overall sleep–wake homeostasis (Chung et al, 2017; Kroeger et al, 2018). Moreover, Mondino et al. (2021) demonstrated that optogenetic activation of glutamatergic LPO neurons promotes wakefulness while destabilizing NREM sleep (Mondino et al, 2021). Based on these studies, we speculate that the GABAergic LPO neurons project to and inhibit glutamatergic LPO neurons during NREM sleep, promoting and stabilizing NREM sleep via local inhibition (Fig. 1). Furthermore, previous studies have shown that NREM-promoting nuclei, such as the perioculomotor region (Zhang et al, 2019) and subthalamic nucleus (Liu et al, 2018), send excitatory inputs to the LPO. Therefore, it is possible that, during NREM sleep, the NMDARs on GABAergic LPO neurons are activated by these NREM-promoting nuclei, which thereby inhibit the glutamatergic LPO cells. Without this local inhibition, the wake-promoting glutamatergic LPO neurons would actively fire during NREM sleep, even while other NREM-promoting nuclei are activated, which may result in the sleep–wake fragmentation phenotype observed by Miracca et al. (2022).
Figure 1.
A schematic diagram of the neural circuitry involving LPO and VTA cells. NREM sleep is induced by subpopulations of GABAergic LPO neurons through inhibition of wake-promoting regions. To maintain continuous NREM sleep, we hypothesize that GABAergic LPO neurons inhibit the activity of local wake-promoting glutamatergic LPO cells. Further, we propose that wake-promoting GABAergic LPO cells inhibit NREM-promoting GABAergic VTA cells to promote transitions from NREM to wakefulness. Wakefulness may be further facilitated by excitation of wake-promoting glutamate/dopamine VTA neurons by glutamatergic LPO cells.
Another potential pathway by which LPO neurons may regulate NREM sleep is by their projections to the VTA. It has previously been shown that GABAergic LPO neurons inhibit GABAergic VTA cells which promote NREM sleep (Yu et al., 2019; Gordon-Fennell et al, 2020). Since some GABAergic neurons in the LPO are wake-promoting (Chung et al, 2017), it is possible that these neurons inhibit NREM-promoting GABAergic VTA during wakefulness. Thus, we speculate that the removal of this inhibition by knocking down NMDARs on GABAergic LPO neurons would have increased the activity of GABAergic VTA cells, leading to an increase in NREM sleep. On the other hand, glutamatergic LPO neurons project to glutamatergic and dopaminergic VTA cells that initiate wakefulness (Eban-Rothschild et al, 2016; Yu et al, 2019; Gordon-Fennell et al, 2020). Therefore, removal of this excitation would lower the activity of wake-promoting VTA cells. Consequently, when both GABAergic and glutamatergic LPO cells are inactive due to NMDAR knock out, sleep-promoting VTA neurons would have increased activity, while wake-promoting VTA neurons would have decreased activity, resulting in the greater sleepiness behavior observed in the study by Miracca et al. (2022) (Fig. 1). Future studies should investigate the function of LPO projections to VTA neurons in sleep and wakefulness regulation.
It may be puzzling that GABAergic LPO neurons are most active during REM sleep if they play a role in NREM sleep maintenance. Consistent with the current paper, previous studies have confirmed that the majority of LPO cells display maximal activity during REM sleep, with lower discharge during NREM sleep (Alam et al, 2014; Chung et al, 2017). As previously stated, there are many GABAergic subpopulations within the LPO, including CRH- and CCK-expressing neurons, which, upon optogenetic activation, increase REM sleep amounts in addition to increasing NREM sleep (Chung et al, 2017). Given that the authors expressed calcium indicators in all GABAergic neurons as a whole and the different subpopulations of GABAergic neurons are intermingled in the LPO, it was technically challenging to determine whether different subpopulations exhibited different temporal patterns of activity. Thus, targeting specific subpopulations of LPO neurons expressing NMDARs will enhance our understanding of which cell types are involved in the regulation of REM and NREM sleep.
An interesting finding by Miracca et al. (2022) was that nonspecific knock out of NMDARs in LPO resulted in reduced duration of REM sleep, power of theta oscillations during REM sleep, as well as increased sleepiness during the sleep deprivation experiment, whereas cell-specific knockdown of NMDARs in either GABAergic or glutamatergic LPO neurons did not result in these phenotypes. These results suggest that silencing both glutamatergic and GABAergic LPO neurons resulted in a synergic effect, such that, when all glutamate inputs to LPO neurons and hence LPO outputs onto other sleep- and theta-regulating neurons were abolished, more dramatic effects were observed.
In conclusion, Miracca et al. (2022) uncovered a novel role of NMDARs in sleep maintenance and cortical oscillation regulation during REM sleep. These results are significant because they open up a new avenue of research into mechanisms that might prevent sleep fragmentation and may provide an opportunity to treat insomnia and other related sleep disorders.
Footnotes
Editor's Note: These short reviews of recent JNeurosci articles, written exclusively by students or postdoctoral fellows, summarize the important findings of the paper and provide additional insight and commentary. If the authors of the highlighted article have written a response to the Journal Club, the response can be found by viewing the Journal Club at www.jneurosci.org. For more information on the format, review process, and purpose of Journal Club articles, please see http://jneurosci.org/content/jneurosci-journal-club.
We thank Brittany Dugan, Sara Pintwala, and Hanhee Lee for support and feedback on the writing process.
The authors declare no competing financial interests.
References
- Alam MA, Kumar S, McGinty D, Alam MN, Szymusiak R (2014) Neuronal activity in the preoptic hypothalamus during sleep deprivation and recovery sleep. J Neurophysiol 111:287–299. 10.1152/jn.00504.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf JS, Vitaterna MH, Olker CJ, Song EJ, Christian EP, Sørensen L, Turek FW, Madsen TM, Khan MA, Kroes RA, Moskal JR (2019) NMDAR activation regulates the daily rhythms of sleep and mood. Sleep 42:zsz135. 10.1093/sleep/zsz135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Weber F, Zhong P, Tan CL, Nguyen TN, Beier KT, Hörmann N, Chang WC, Zhang Z, Do JP, Yao S, Krashes MJ, Tasic B, Cetin A, Zeng H, Knight ZA, Luo L, Dan Y (2017) Identification of preoptic sleep neurons using retrograde labelling and gene profiling. Nature 545:477–481. 10.1038/nature22350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eban-Rothschild A, Rothschild G, Giardino WJ, Jones JR, de Lecea L (2016) VTA dopaminergic neurons regulate ethologically relevant sleep–wake behaviors. Nat Neurosci 19:1356–1366. 10.1038/nn.4377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Fennell A, Gordon-Fennell L, Desaivre S, Marinelli M (2020) The lateral preoptic area and its projection to the VTA regulate VTA activity and drive complex reward behaviors. Front Syst Neurosci 14:581830. 10.3389/fnsys.2020.581830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeger D, Absi G, Gagliardi C, Bandaru SS, Madara JC, Ferrari LL, Arrigoni E, Münzberg H, Scammell TE, Saper CB, Vetrivelan R (2018) Galanin neurons in the ventrolateral preoptic area promote sleep and heat loss in mice. Nat Commun 9:4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Ma C, Zheng W, Yao Y, Dan Y (2018) Sleep and motor control by a basal ganglia circuit. BioRxiv. [Google Scholar]
- Medic G, Wille M, Hemels ME (2017) Short- and long-term health consequences of sleep disruption. Nat Sci Sleep 9:151–161. 10.2147/NSS.S134864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miracca G, Anuncibay-Soto B, Tossell K, Yustos R, Vyssotski AL, Franks NP, Wisden W (2022) NMDA receptors in the lateral preoptic hypothalamus are essential for sustaining NREM and REM sleep. J Neurosci 42:5389–5409. 10.1523/JNEUROSCI.0350-21.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondino A, Hambrecht-Wiedbusch VS, Li D, York AK, Pal D, González J, Torterolo P, Mashour GA, Vanini G (2021) Glutamatergic neurons in the preoptic hypothalamus promote wakefulness, destabilize NREM sleep, suppress REM sleep, and regulate cortical dynamics. J Neurosci 41:3462–3478. 10.1523/JNEUROSCI.2718-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pálfi E, Lévay G, Czurkó A, Lendvai B, Kiss T (2021) Acute blockade of NR2C/D subunit-containing N-methyl-D-aspartate receptors modifies sleep and neural oscillationsin mice. J Sleep Res 30:e13257. 10.1111/jsr.13257 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Lin JS, Sakai K (2009) Characterization and mapping of sleep–waking specific neurons in the basal forebrain and preoptic hypothalamus in mice. Neuroscience 161:269–292. 10.1016/j.neuroscience.2009.02.075 [DOI] [PubMed] [Google Scholar]
- Teng S, Zhen F, Schalchli JC, Chen X, Jin H, Wang L, Peng Y (2021) Medulla glutamatergic neurons control wake-sleep transitions. BioRxiv 434263. 10.1101/2021.03.07.434263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Li W, Ma Y, Tossell K, Harris JJ, Harding EC, Ba W, Miracca G, Wang D, Li L, Guo J, Chen M, Li Y, Yustos R, Vyssotski AL, Burdakov D, Yang Q, Dong H, Franks NP, Wisden W (2019) GABA and glutamate neurons in the VTA regulate sleep and wakefulness. Nat Neurosci 22:106–119. 10.1038/s41593-018-0288-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Ferretti V, Güntan İ, Moro A, Steinberg EA, Ye Z, Zecharia AY, Yu X, Vyssotski AL, Brickley SG, Yustos R, Pillidge ZE, Harding EC, Wisden W, Franks NP (2015) Neuronal ensembles sufficient for recovery sleep and the sedative actions of α2 adrenergic agonists. Nat Neurosci 18:553–561. 10.1038/nn.3957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhong P, Hu F, Barger Z, Ren Y, Ding X, Li S, Weber F, Chung S, Palmiter RD, Dan Y (2019) An excitatory circuit in the perioculomotor midbrain for non-REM sleep control. Cell 177:1293–1307.e16. 10.1016/j.cell.2019.03.041 [DOI] [PubMed] [Google Scholar]