Abstract
Background
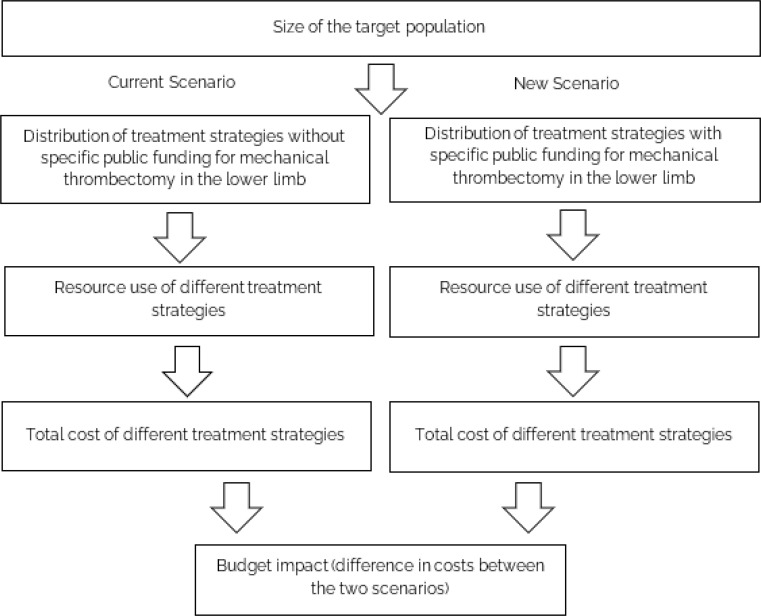
A blockage to the blood vessels in the lower extremities may cause pain and discomfort. If left unmanaged, it may lead to amputation or chronic disability, such as in the form of post-thrombotic syndrome. We conducted a health technology assessment of mechanical thrombectomy (MT) devices, which are proposed to remove a blood clot, which may form in the arteries or veins of the lower legs. This evaluation considered blockages in the veins and arteries separately, and included an evaluation of effectiveness, safety, cost-effectiveness, the budget impact of publicly funding MT for lower limb blockages, patient preferences and values, and clinical and health system stakeholders’ perspectives.
Method
We performed a systematic literature search of the clinical evidence. We assessed the risk of bias of each included study using the Cochrane tool for randomized controlled trials or the risk of bias among non-randomized studies (RoBANS) tool for nonrandomized studies, and the quality of the body of evidence according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria. We performed a systematic economic literature search. We did not conduct a primary economic evaluation since the clinical evidence is highly uncertain. We also analyzed the budget impact of publicly funding MT treatment for inpatients with arterial acute limb ischemia and acute deep vein thrombosis (DVT) in the lower limb in Ontario. To contextualize the potential value of MT, we spoke with people with acute DVT. To understand the barriers and facilitators of accessing MT, we surveyed clinical and health system stakeholders to gain their perspectives.
Results
We included 40 studies (3 randomized controlled trials and 37 observational studies) in the clinical evidence review. For patients who experience arterial acute limb ischemia, compared with catheter-directed thrombolysis (CDT) alone, MT has greater technical success and patency and reduced hospital length of stay, but the evidence for these outcomes is uncertain (GRADE: Very low). Mechanical thrombectomy may reduce the volume of thrombolytic medication required and CDT infusion time (a determinant for intensive care unit [ICU] need) in patients experiencing acute DVT, but it is uncertain if this is to a meaningful degree (GRADE: Moderate to Very low). It may also reduce the proportion of people who experience post-thrombotic syndrome and overall hospital length of stay, but it is uncertain (GRADE: Very low).
We estimated that publicly funding MT for people with arterial acute limb ischemia in Ontario would lead to an annual cost savings of $0.17 million in year 1 to $0.14 million in year 5, for a total savings of $0.83 million over 5 years. This cost savings was mainly attributed to reduced ICU stays among people who received MT, but the results had considerable uncertainty. For the population with acute DVT, publicly funding MT would lead to an additional cost of $0.77 million in year 1 to $1.44 million in year 5, for a total additional cost of $5.5 million over 5 years.
The people with acute DVT with whom we spoke reported that MT was generally seen as a positive option, and those who had undergone the procedure reported positively on its value as a treatment to quickly remove a clot. Accessing treatment for DVT could be a barrier, especially in more remote areas of Ontario.
Clinicians using the technology advised that facilitators to accessing the technology included perceived improvements in patient outcomes, resourcing requirements, addressing unmet needs, and avoidance of ICU stay. The main barrier identified was cost. Clinicians who were not using the technology advised that barriers were low case-use volume, along with costs for the equipment and for health human resources.
Conclusions
Mechanical thrombectomy may have greater technical success and patency and reduce hospital length of stay for patients experiencing an arterial acute limb ischemia and, for patients with an acute DVT, it may reduce CDT volume and infusion time, the proportion of people who experience post-thrombotic syndrome, and hospital length of stay. Mechanical thrombectomy may reduce the associated ICU costs, but it has higher equipment costs compared with usual care. Publicly funding MT in Ontario for populations with arterial acute limb ischemia may not lead to a substantial budget increase to the province. Publicly funding MT for acute DVT would lead to an additional cost of $5.5 million over 5 years. For people with acute DVT, MT was seen as a potential positive treatment option to remove the clot quickly. Overall, the majority of clinical stakeholders we engaged with (including both those with and without experience with MT) were supportive of the use of the technology.
Objective
This health technology assessment evaluates the effectiveness, safety, and cost-effectiveness of mechanical thrombectomy (MT) for people with arterial acute lower limb ischemia or acute deep vein thrombosis. It also evaluates the budget impact of publicly funding MT, the perspectives of the system stakeholders who are familiar with the technology, and the experiences, preferences, and values of people with acute deep vein thrombosis.
Background
Health Conditions
A blockage to the blood flow in the lower limbs (legs) may cause pain and discomfort and, if left unmanaged, may lead to chronic disability, amputation, or death. Blood flow may become impeded for a number of reasons, including compression on the outside of a blood vessel or, more commonly, blockage from within. Such blockage may be caused by a thrombus or an embolus, both of which are forms of blood clot. A thrombus is a clot that forms locally in the blood vessel, causing blockage at the site of the clot formation.4 An embolus is a blood clot that forms somewhere else in the body (most commonly in the heart) and breaks free to travel through the blood vessels, eventually becoming stuck in a more distant vessel, usually at a branch point, after which it impedes blood flow.4 4An occlusion is a place in a vein or artery where the flow of blood is completely impeded. This can cause ischemia, which is blood circulation that is inadequate to keep the affected tissues alive.4–7 This review focuses on blockages in the veins as well as arteries of the lower limbs. For the purposes of this review, arterial blockages are referred to as “arterial acute limb ischemia” and venous blockages as “acute deep vein thrombosis” (DVT).
Arterial Acute Limb Ischemia
Ischemia could present as chronic limb-threatening ischemia (CLTI), which describes a progressive condition where constant pain or wounds result from the development over weeks to months of insufficient blood flow to the affected limb.8 However, sometimes there is a more serious state in which there is a sudden decrease in blood flow through a limb. This is referred to as acute limb ischemia (ALI) and is a medical emergency.8
Acute ischemia presents as pain, pallor, pulselessness, paresthesia, poikilothermia (inability to regulate temperature and cold), and paralysis (together, they are known as the six Ps).9 There are many classification systems used to describe the degree of ischemia. A common one is the Rutherford classification system, which qualifies acute limb ischemia by the degree of limb ischemia, ranging from Class I, a non-threatened extremity, to Class III, where ischemia has progressed and limb salvage is not possible.10,11
Peripheral arterial disease (PAD) also known as lower extremity occlusive disease (LEOD), refers to a condition that can lead to blockages in the arteries of the limbs. It is the cause of arterial acute limb ischemia in the event of thrombosis. One key clinical measure of LEOD is the degree of peripheral arterial ischemia, commonly assessed using the ankle-brachial index (ABI);12 which is the ratio of the blood pressure measured in the arm to that measured at the ankle. Risk factors for peripheral arterial ischemia include increasing age, lifestyle (smoking/drinking), as well as obesity and conditions such as high blood pressure, high cholesterol, diabetes, atherosclerotic heart disease, arrhythmia, and other clotting disorders such as atrial fibrillation.6,7,10,13,14
A leading cause of peripheral vascular disease is atherosclerosis, which can also cause coronary artery disease and therefore affect blood supply to the heart.5,10,14 This build-up of atherosclerosis plaque is made up of fat, cholesterol, and other waste tissues from the cells and can stiffen and narrow arteries, making blockages more likely to occur.5
Acute Deep Vein Thrombosis
When a blood clot forms in a vein of the leg, it is referred to as deep vein thrombosis—an acute blockage of the veins in the leg that impairs drainage of blood and results in leg pain, redness, and swelling. If a DVT that formed in the leg dislodges, it becomes an embolus and can be driven through the vein by blood flow from the legs towards the heart and lungs. If it gets stuck in the lungs, it causes a pulmonary embolism and may result in death.5,15 Infrequently, DVT can be so extensive that it causes total occlusion of the major deep venous system, which leads to severe venous congestions, acute leg ischemia, and venous gangrene. A DVT can cause chronic venous insufficiency, resulting in post-thrombotic syndrome, a condition in which there are long-lasting leg problems, including swelling and pain.5,7,16 Risk factors for venous thromboembolism (which is composed of DVT and pulmonary embolism) include familial predispositions, medical conditions such as cancer, major surgery, hospitalization or illness, pregnancy, and lifestyle risks such as obesity and smoking.14 Cardiovascular disease risk factors of high cholesterol and high blood pressure may not be as relevant for venous thrombosis embolism (VTE) as obesity and age.17,18 In as many as 50% of VTEs, there are no clear risk factors identified; this type of VTE is referred to as an unprovoked VTE.14
Clinical Need and Target Population
Arterial Acute Limb Ischemia
Lower extremity occlusive disease is defined by an ABI of < 0.9 and is estimated to affect 4.3% of people > 40 years old,19 (increasing with age such that it affects 7.0% of people aged 60-69 years and 23.2% of people > 80 years of age).12 It is estimated that 1% to 2% of people experiencing LEOD will have a critical limb-threatening ischemia (CLTI).12 Based on an examination of Ontario administrative health databases, we estimate 2,740 unique adult patients were admitted for peripheral arterial ischemia between 2015 and 2020, with a mean age of 70 years (range 19-103 y), 41% of whom were female (see Appendix 4 for details). An Ontario study estimated that hospitalization rates of people > 40 years of age between 2006 and 2019 who had peripheral arterial ischemia was 2,665 per 100,000 people.20 Acute arterial limb ischemia is considered a medical emergency and people experiencing acute lower limb ischemia are at risk of losing their limb (20% to 50% experience amputation) and death (1-y mortality is 15% to 20%).21 Among people with ALI who do not receive treatment, the risk of amputation is 80% to 90%.12
Acute Deep Vein Thrombosis
Deep vein thrombosis affects 45,000 Canadians per year, with an estimated 1.29 acute venous thromboembolic events per 1,000 person-years (95% confidence interval [CI]: 1.06-1.53).22 Incidence increases with age in both males and females, with a higher overall incidence rate of thromboembolic events in females (1.44; 95% CI: 1.19-1.69).22 Deep vein thrombosis is a risk factor for pulmonary embolism and chronic thromboembolic hypertension. About 0.3% of people who have had a thromboembolic event go on to have chronic thromboembolic pulmonary hypertension after 2 years, and 1.3% within 10 years.14 The number of hospitalizations for DVT as the primary diagnosis in the United State remained relatively stable between 2005 and 2016, and there were an estimated 857,000 DVT events in 2016.14 Based on an examination of Ontario administrative health databases, we estimate that 4,009 unique adult patients were admitted for DVT between 2015 and 2020.
Patients had a mean age of 68 years (range: 18-105 y), and 55% were female (see Appendix 4 for details). Mortality rates in people with DVT have been relatively stable over time at 5.1% at 30 days and 19.6% at 1 year in 2010.14 Major bleeding occurs in 2.8% of patients and is the most common complication after DVT thrombus removal.7
Equity
We considered relevant equity issues across different populations defined by the PROGRESS-Plus23 categories identified during the review process, and we did not detect any potential health inequities related to the effect of MT for lower limb ischemia during scoping. However, inequities may exist in access to care as MT is not currently the standard of care in Ontario, and it is up to individual hospitals to determine how to fund the devices based on their individual global budgets. If MT for urgent blockages in lower limbs were found to be safe and effective, it may offer improved outcomes and recovery over the current standard of care.
Based on US data, the lifetime risk of experiencing a VTE at 45 years of age was higher for individuals who are Black compared to the overall cohort (11.5% vs. 8.1%). This risk is even higher among people with sickle cell traits (18.2%).14 However, it is unknown how this may compare to Black people living elsewhere in the African diaspora such as the Caribbean, and what the impacts of the social determinants of health may be and how that affects the statistics from the United States.19
Peripheral arterial disease is a risk factor of arterial ischemia and is as prevalent in women as men. 24,25 One study, based on 7-year data from Ontario, found no differences in outcomes between men and women with PAD who visited a vascular surgeon (hazard ratio: 0.99; 95% CI: 0.92-1.05). Nor were differences observed in rates of major amputation, but women were less likely to have minor amputation than men (hazard ratio 0.73; 95% CI: 0.62-.85).26 Other research has demonstrated that women have faster deterioration of functional capacity upon symptom onset and overall more complex disease progression.24 Women also tend to have more severe adverse effects after revascularization interventions, but are generally underrepresented in related research studies.24
Peripheral arterial disease is also more prevalent among individuals from certain ethnic backgrounds. One systematic review found there was lower prevalence of PAD among people with an Afro-Caribbean background compared to White people, but it was higher than that of South Asians.25 First Nations people of Ontario with diabetes receive revascularization procedures (angioplasty or bypass surgery) at a rate similar to other Ontarians, but they experience higher amputation rates (3-5 times higher) and higher mortality (adjusted hazard ratio 1.15; 95% CI: 1.05-1.26) after lower-limb procedures.27 However, one Canadian study examining three major centres found that there were no observed differences in the prevalence of PAD among people with diabetes who were also undergoing hemodialysis.28
Current Treatment Options
When a patient presents with arterial acute lower limb ischemia, it is considered an emergency. To avoid delays, Thrombosis Canada recommends immediate treatment with heparin (an anticoagulant) even before confirmatory diagnostic imaging.29 Heparin is used to prevent the progression of occlusion and secondary thrombosis.30 From there, the course of action depends on the severity of the blockage, patient clinical presentation, and comorbidities.5,6,30 Regardless of the cause of the acute blockage, the primary object for all patients is to remove the blood clot and restore blood flow as quickly as possible.
For patients with arterial acute limb ischemia blockages, patient management is typically the responsibility of the vascular surgeon and treatment could be conducted by a vascular surgeon or an interventional radiologist. Open surgery, whether thrombectomy or bypass, is required in the most severe time-sensitive cases of ischemia.5,6 Interventions to repair flow to the affected blood vessel and to prevent recurrence include using a stent or bypass graft. Patients may be treated with the less invasive endovascular approach of CDT. Catheter-directed thrombolysis refers to the administration of thrombolytic treatment (clot-busting drugs) to support revascularization (return of blood flow).5 Surgery may be required when a person cannot receive thrombolytic therapy due to a blood clotting disorder or if there is a very severe blockage, the limb is at risk, and the time required to administer catheter-directed thrombolysis (CDT; approximately 8-36 h) is considered too high a risk.5,6After the immediate concern of an acute blockage has been alleviated through endovascular interventions, it is recommended that antiplatelet therapy and, in some cases, anticoagulation be continued, with ongoing cardiovascular risk reduction therapies also critical to support patient recovery and to prevent future blockages.29
For people with acute DVT, anticoagulation therapy for at least 1-3 months is the standard of care. In some patients at higher risk of recurrence, anticoagulation can be continued if there are no contraindications. Typically, acute DVT is treated with anticoagulation as an outpatient therapy and managed by a hematologist.29 However, if there is a more extensive venous blockage, such as those caused by very large blood clots that affect proximal leg veins in the pelvis or thigh, and more severe (limb threatening) symptoms, such as acute limb ischemia, then MT with CDT can be considered on top of anticoagulation therapy. Thrombolytic treatments such as CDT with infusion of clot-busting drugs dissolve the clot causing a blockage, but they can take a significant amount of time to resolve the clot. Thrombolysis is also associated with a high risk of bleeding and other complications, such as stroke.16,31 As such, these treatments require careful monitoring throughout the duration of administration (12-72 h) and require patients to be admitted to the intensive care unit for monitoring.31
Health Technology Under Review
Mechanical thrombectomy devices are intended to break up and remove clots in a blood vessel more rapidly, and thus it is proposed that doing so will improve patient outcomes while reducing treatment time and the intensity of complementary interventions (Figure 1). There are a variety of MT devices available that employ various mechanisms of action. See Table 1 for a summary of four brands that are either currently available in Ontario or were found in the clinical literature review. Mechanisms of action, in no particular order, include (1) pharmacomechanical thrombectomy (PMT), which uses a saline jet to break up the clot and suction to remove the pieces while delivering thrombolytics,32 (2) vacuum assisted aspiration to suction out the clot,33,34 (3) physically breaking up the clot with a rotational guide wire,35 and (4) techniques that use ablation and ultrasound-assisted devices to break up the clot before removal.36–42 Given the different mechanisms of action, the devices differ in length of time required for treatment, recovery time, level of invasiveness, and impact on patient outcomes.
Figure 1: Simplified Clinical Pathway With Proposed Use of Mechanical Thrombectomy.
Abbreviation: DVT, deep vein thrombosis.
Table 1:
Overview of Select Mechanical Thrombectomy Devices
| Device | ||||
|---|---|---|---|---|
| Brand name | AngioJet32 | Indigo33 | Rotarex43 | EkoSonic (EKOS)36,44 |
| Mechanism of action | Pharmacomechanical thrombectomy | Aspiration system | Rotational mechanical thrombectomy | Ultrasound enhanced lysis |
| Uses pressurized saline to provide active aspiration and lytic delivery | Uses continuous vacuum aspiration suction to remove thrombus and monitor blood flow in real time | Uses modifying beveled tip, a rotating abrading vortex, and a continuous active aspiration with fixed inner serrated cylinder | Applies acoustic pulse to speed the dispersion of thrombolytic agent, break up the target, and accelerate the dissolution of a clot | |
| Minimum vessel diameter | 1.5 mm | 2.3 mm | 3 mm | 2 mm |
| Availability | Used by select centers in Ontario. Also available in the United States and Europe 45 | Used by select centers in Ontario. Also available in the United States, Europe, and Australia33 | Used in the United States and Europe43 | Covered in the United States by the Centers for Medicare & Medicaid Services. Also available in Europe45 |
Source: Data extracted from individual company websites, as well as from a summary by Lichtenberg, 2019.46
When MT is used, there typically is companion use of thrombolytic therapy.16 Thrombolytic therapy can be administered simultaneously with MT to shrink the size of the blood clot prior to removal, making removal easier and more complete.16 Alternatively, thrombolytic therapy may be administered after MT, if needed, to finish clearing out any remaining part of a clot. However, the required length of use of thrombolytics after MT is expected to be shorter overall, requiring less time in the intensive care unit (Dheeraj Rajan, Jeff Jaskolka, Charles de Mestral, email communication, May 2022). Currently, MT is available through the Provincial Vascular Services framework in some level 1 and level 2 hospitals (Ontario Health—CorHealth, email communication, June 29, 2021). In some cases, there is potential for MT to be used as a stand-alone treatment to avoid the need for CDT and, therefore, ICU admissions entirely.
Safety and Harm
Safety and harm outcomes are central concerns of the condition and of the interventions of interest. People experiencing lower limb ischemia are at risk of losing their limb, of mortality, of pulmonary embolism, and of chronic post-thrombotic syndrome. Furthermore, two Cochrane reviews47,48 found that systemic administration of thrombolysis had similar outcomes for patients as the initial treatment for acute limb ischemia compared to open surgery, and as acute deep vein thrombosis compared to CDT. It has been proposed that MT with or without thrombolytic medications is safer than thrombolytic medications alone due to the risk of complications from the medications, requiring hospital stay.31
Regulatory Information
At this time, there are five MT devices with Health Canada approval for lower limb ischemia (Table 2). There are other brands approved for use in the United States, Europe, and elsewhere.
Table 2:
Mechanical Thrombectomy Systems Approved by Health Canada
| Device name | Primary mechanism of action | Manufacturer | Health Canada licence number | Device class |
|---|---|---|---|---|
| AngioJet Ultra Thrombectomy System | Saline jet | Boston Scientific Corp | 79037 | 4 |
| AngioVac System | Aspiration | Angiodynamics Inc | 93477 | 2 |
| Cleaner 15 Rotational Thrombectomy System | Rotational | Argon Medical Devices Inc | 97664 | 3 |
| ClotTriever | Capture | Inari Medical, Inc. | 106967 | 2 |
| Indigo Aspiration System | Aspiration | Penumbra Inc | 98661 | 4 |
Ontario, Canadian, and International Context
Ontario Context
Mechanical thrombectomy devices are available and used for a variety of indications, including cerebral, coronary, hemodialysis fistula/graft thrombosis, and sometimes pulmonary embolism (Dheeraj Rajan, Jeff Jaskolka, and Charles de Mestral, email communications, May 2022). Currently, 14 of the 20 vascular programs in the province are using MT devices for lower limb ischemia, with costs being absorbed through hospital global budgets (Ontario Health-CorHealth, email communication, April 20, 2021). Under the current funding model, it is up to each hospital individually to determine how they spend their global budgets. As well, observed differences in access to the technology across the province may be due to differences in supported specialties, as the technology is typically used by interventional radiologists.
Most (89.5%) of the peripheral arterial ischemia cases that required inpatient management between 2018 and 2020 were treated in one of the 20 hospitals in Ontario with a vascular program; however, approximately half (54.1%) of the DVTs requiring inpatient management during the same time period were not treated at one of these centres (see Appendix 4).
Canadian Context
Mechanical thrombectomy is widely used as a procedural device to remove emboli in the brain (stroke)49, lung (pulmonary embolism), and heart (coronary ischemia). Mechanical thrombectomy is also being used for lower limb ischemia in the Calgary, Alberta area (Elisabeth Smitko, CADTH liaison officer, email communication, May 20, 2021). However, while we were able to confirm that MT is available in Manitoba, Saskatchewan, and select hospitals in British Columbia, it is unclear if the indications for use include lower-limb ischemia or are limited to other conditions such as stroke and coronary ischemia at this time. (Elisabeth Smitko, CADTH Liaison officer, email communication, November 23, 2020).
International Context
There is some adoption of MT for ischemia in the peripheral cardiovascular system, specifically the lower limbs. Mechanical thrombectomy for lower limbs is covered by several US health insurance providers and is sometimes specified when pharmacologic thrombosis is contraindicated, or was attempted but failed.50–52 In 2019, the UK National Institute for Health and Care Excellence (NICE) published guidance that acknowledged the limited available evidence for MT for acute iliofemoral DVT, but supported its use for this indication under special arrangements that included case tracking by entering details into the British Society of Interventional Radiology's Venous Registry.53
Guidelines
The recent Canadian Cardiovascular Society 2022 guideline for arterial ischemia states that treatments should be individualized to the presentation of symptoms, the likelihood of limb viability, and the patient risk profile, with an emphasis on reducing delays in treatment.54 Options for treatment include CDT or mechanical thrombus removal/aspiration and surgical thrombectomy or reconstruction.54 The 2020 clinical practice guidelines for the European Society for Vascular Surgery recommend that aspiration and MT be considered for people with acute limb ischemia. The recommendations are based on expert consensus as the evidence was found to be conflicting and based on small, retrospective, and registry studies.55,56 The 2016 American Heart Association/American College of Cardiology's guideline recommends MT as an adjunctive therapy to thrombolysis in people with salvageable limbs. This is a moderate recommendation based on moderate quality evidence of non-randomized studies.57
The Canadian CHEST guideline for VTE from 2016 recommends that oral anticoagulation alone is likely the most appropriate treatment for most people with DVT. Catheter-directed thrombolysis may be an option, in consideration of patient preference, for the prevention of post-thrombotic syndrome. Complexity of treatment weighed against the risk of bleeding from CDT.58 Similarly, the American Society of Hematology guideline recommends oral anticoagulation alone over thrombolytic therapy in additional to anticoagulation for most patients, with a caveat that it may be appropriate to use thrombolytic therapy for select patients in consideration of their condition and preferences and values.59 The American College of Radiology, 2020 guidelines, notes that it is usually appropriate to use CDT/PMT for some people, such as otherwise healthy individuals presenting with moderate to severe symptoms or who are pregnant, or if imaging is consistent with May-Thurner syndrome (external compression on left common iliac vein). It is also usually appropriate if symptoms include limb-threatening ischemia or are persistent for a prolonged period of time after an initial treatment with anticoagulation. However, it is usually not appropriate for otherwise healthy individuals presenting with iliofemoral or femoropopliteal DVT with mild to moderate symptoms.60
Patient Preferences and Values
Patient preferences, values, and experiences are explored elsewhere in this report.
Expert Consultation
We engaged with experts in the specialty areas of vascular surgery and interventional radiology; specifically, clinicians with expertise in using MT, as well as hematology, to help inform our understanding of aspects of the health technology and our methodologies and to contextualize the evidence. Experts were asked to review the clinical and economic plans, support patient engagement efforts, help ensure all relevant published literature is appropriately captured, and reviewed draft reports. Select partners in industry were also actively sought out to ensure appropriate understanding of the technology and Ontario dissemination.
PROSPERO Registration
This health technology assessment has been registered in PROSPERO, the international prospective register of systematic reviews (CRD 42021283970), available at crd.york.ac.uk/PROSPERO.
Clinical Evidence
Research Question
What are the effectiveness and safety of mechanical thrombectomy (MT) compared with usual care for the treatment of people with arterial acute limb ischemia or acute deep vein thrombosis?
Methods
Clinical Literature Search
On the advice of clinical experts, studies published before January 1, 2010, were considered to be too outdated to be relevant in the Ontario context. We performed a clinical literature search on August 20, 2021, to retrieve studies published from January 1, 2010, until the search date. We used the Ovid interface in the following databases: MEDLINE, Embase, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, the Health Technology Assessment database, and the National Health Service Economic Evaluation Database (NHS EED).
A medical librarian developed the search strategies using controlled vocabulary (e.g., Medical Subject Headings) and relevant keywords. The final search strategy was peer-reviewed using the PRESS Checklist.61
We created database auto-alerts in MEDLINE and Embase and monitored them until April 2022. We also performed a targeted grey literature search of health technology assessment agency websites as well as clinical trial and systematic review registries. See Appendix 1 for our literature search strategies, including all search terms.
Eligibility Criteria
STUDIES
Inclusion Criteria
English-language full-text publications
Studies published since January 1, 2010 (based on clinical expert advice that prior publications would be outdated and not applicable to the current Ontario context)
Systematic reviews, health technology assessments, randomized controlled trials, and comparative observational studies. s
Exclusion Criteria
Animal and in vitro studies
Nonsystematic reviews, narrative reviews, abstracts, editorials, letters, case reports, and commentaries
We made a post hoc decision to exclude single arm observational studies to focus on better quality study designs.
PARTICIPANTS
Inclusion Criteria
Adults (≥ 18 years of age) experiencing an acute or subacute (< 14 or 14-28 days from symptom onset, respectively) blockage to the blood flow in the lower limbs due to a thrombus or embolus (i.e., a clot)
Exclusion Criteria
Chronic limb-threatening ischemia
Non-obstructive cause to blood flow blockage (e.g., trauma or iatrogenic injury)
Children (< 18 years of age). Our examination of the administrative data led us to conclude that lower limb blockage among minors is not widespread in Ontario
INTERVENTIONS
Inclusion Criteria
Mechanical thrombectomy devices as the primary intervention to target the removal of a blockage in the blood flow in the lower limbs
Any adjunctive endovascular technique (e.g., balloon, stent, additional MT), with or without companion pharmaceutical intervention (pharmacomechanical, as thrombolysis either by bolus or infusion technique)
All mechanisms of action (e.g., vacuum aspiration, rotational, retriever, ultrasound, ablation) Exclusion Criteria
Devices targeting revascularization of a blood vessel that are not specified for removal of a blockage in the lower limbs (e.g., a stent to improve blood flow)
Devices used as a preventative or adjunctive therapy, such as to monitor portal vein thrombosis or to filter clots during and after a procedure
Devices that are not supported for the treatment of lower limbs or are no longer supported due to safety or efficacy
COMPARATORS
Inclusion Criteria
-
Usual care, with an emphasis on usual care in the Ontario context
Surgical (embolectomy/surgical bypass)
Pharmacological treatment alone (thrombolytic therapy as IV or CDT)
Anticoagulation therapy alone or with compression therapy
Non-mechanical aspiration techniques (e.g., manual aspiration)
Exclusion Criteria
Comparison of MT devices to each other
OUTCOME MEASURES
-
Measure of effectiveness, in order of most clinically meaningful as per our clinical expert advisors:
Limb salvage/amputation rate (including amputation-free survival)
Post thrombotic syndrome (Villalta score or any measure)
Venographic success (> 50% reduction in thrombus burden)
Arterial success (> 75% reduction in thrombus burden)
Recanalization
Pulmonary embolism
Recurrent DVT
Pain
Patency (lysis grade) from 30 days to 6 months
Re-thrombosis
Revision rates (e.g., additional treatments)
Valvular reflux
Resolution of symptoms and functional outcomes not otherwise specified
Quality of life
Activities of daily living
-
Measures of safety, for example:
Stroke
Mortality (up to 1 year)
Major bleeding (at access site or retroperitoneal or intracranial)
Intraprocedural blood loss
Hematuria
Rehospitalization rates
Other adverse effects reported (see Appendix 4)
-
Measures related to health care utilization, for example:
Hospital length of stay
ICU length of stay
Intensity and duration of thrombolytic infusion (e.g., number of hours or days), used as a determinant for ICU length of stay
Time to intervention from presentation (e.g., 8 hours to operating room vs. 4 hours to interventional radiology suite)
Literature Screening
Two reviewers followed the Cochrane rapid review methods62 to screen titles and abstracts using Covidence63 and obtained the full text of studies that appeared eligible for the review, according to the inclusion criteria. One reviewer examined the full-text articles and select studies that met the inclusion criteria. Reference lists of included studies were examined by one reviewer for any additional relevant studies not identified through the search. Citation flow and reasons for exclusion for full text articles were reported according to the PRISMA statement.64
Data Extraction
We extracted relevant data on study characteristics and risk-of-bias items using a data form to collect information on the following:
Source (e.g., citation information, study type)
Methods (e.g., study design, study duration and years, participant allocation, allocation sequence concealment, blinding, reporting of missing data, reporting of outcomes, whether the study compared two or more groups)
Outcomes (e.g., outcomes measured, number of participants for each outcome, number of participants missing for each outcome, outcome definition and source of information, unit of measurement, upper and lower limits [for scales], time points at which the outcomes were assessed)
One reviewer extracted relevant data and we considered contacting study authors to provide clarification as needed.
Statistical Analysis
Data from the original studies was used as much as possible. Where required, error rates were calculated using Review Manager65 and the Cochrane handbook approach.66 Unless otherwise stated, statistical significance was defined at P < .05 and findings were sought on an intention-to-treat basis. Where evidence synthesis was considered unfeasible or inappropriate, results are reported narratively. Where data were available and pooling was considered appropriate based on minimal methodological heterogeneity (e.g., study design, follow-up time point), statistical heterogeneity, or clinical diversity (e.g., disease severity, vein diameter), we used Review Manager models to generate pooled summary estimates.65 We calculated risk ratios for frequent events, odds ratios for infrequent events, and mean differences for continuous outcomes along with 95% CI.67 A standardized mean difference was used for continuous outcomes that were measured in different ways across the various studies. A fixed effects approach was used unless there were ongoing concerns with heterogeneity, in which case random-effects modeling was applied.68 Where multiple subgroups and reports for an outcome were provided within a study, we made efforts to decrease heterogeneity in the meta-analysis by selecting the outcome reporting that was most aligned (e.g., for Gong et al, 2021,1 a technical success metric with adjunctive therapies such as stents was selected for the meta-analysis due to that being most similar to the other available studies’ methodological approaches). To ensure transparency, findings of all outcomes are also presented narratively, with additional details along with any meta-analyses conducted.
Critical Appraisal of Evidence
We assessed risk of bias using the Cochrane tool for randomized controlled trials66 and the risk of bias among non-randomize studies (RoBANS) tool69 for nonrandomized studies (Appendix 2).
We evaluated the quality of the body of evidence for each outcome according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Handbook.70 The body of evidence was assessed based on the following considerations: risk of bias, inconsistency, indirectness, imprecision, and publication bias. The overall rating reflects our certainty in the evidence.
Results
Clinical Literature Search
The database search of the clinical literature yielded 1,293 citations published between January 1, 2010, and August 20, 2021, including grey literature sources and after duplicates were removed. We identified three additional eligible studies from other sources, such as auto alerts of the databases (monitored until April 2022). In total, we identified 40 publications (12 in the arterial population and 29 in the venous population, with one publication including both populations) that met our inclusion criteria. See Appendix 3 for a list of selected studies excluded after full-text review. Figure 2 presents the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for the clinical literature search.
Figure 2: PRISMA Flow Diagram—Clinical Search Strategy.
Abbreviations: DVT, deep vein thrombosis; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses. Source: Adapted from Page et al.72
aOne publication, Morrow et al, 201773 included both arterial and venous populations, and thus is double counted in the breakdown by population groups.
bIncludes multiple publications for the same study, such as updates and subgroup analyses.
A total of 21 systematic reviews were reviewed and assessed for eligibility. All reviews evaluated MT in acute or subacute lower limb ischemia in some form. Some reviews were limited to a specific type of MT, others were focused on a particular patient population, such as people with iliofemoral DVTs. None were determined to be of adequate relevance, comprehensiveness, recency, or of sufficient quality for us to leverage their findings for the purposes of our research question. One review was a network meta-analysis of randomized controlled trials (RCTs) for one of our populations of interest-acute DVT.71 However, this review included interventions beyond our interest, such as manual aspiration, and we could not separate these findings from our interventions of interest. As such, we conducted our own review of the primary literature. See Appendix 3 for a list of the excluded studies. Additionally, after conducting the preliminary screening of studies, we limited our analysis to comparative study designs to focus on the best quality of evidence available.
Characteristics of Included Studies
A total of 40 publications were included—12 publications evaluated MT effectiveness in arterial occlusions and 29 evaluated the venous thrombosis population (one publication73 included both populations of interest and so appears twice in our count). Most studies were conducted in the United States. Other study locations included Brazil, China, Germany, Korea, the Netherlands, Norway, Singapore, Switzerland, Taiwan, Thailand, Turkey, and the United Kingdom. The studies had some variation in inclusion and exclusion criteria, such as whether they included or excluded pregnant patients, or limited the location or type of occlusion. Studies also differed in their definitions of usual care and adjunctive therapies using differing thrombolytics, doses, and protocols, as well as compression stockings, balloons, or stents, which were typically used at the discretion of the treating physician. While our review was open to any brand of MT device for studies that met our inclusion criteria, only three brands were represented among the included studies: AngioJet, Rotarex, and EKOS.
This review was limited to studies evaluating MT as the primary intervention; however, many studies allowed for adjunctive therapies to be used at the discretion of the treating clinicians to ensure complete removal of a thrombus. Such adjunctive therapies sometimes included MT devices in the control groups. Two studies included AngioJet in both study arms as the adjunctive therapy. We included both studies as they were designed to evaluate the effectiveness of other MT devices.74,75 See Tables 3 and 14 for a summary of the included studies by population type.
Arterial Acute Limb Ischemia
We identified 12 comparative observational studies, but no randomized controlled trials that evaluated MT among people with arterial acute limb ischemia (Table 3).
Table 3:
Characteristics of Included Arterial Acute Limb Ischemia Studies
| Author, year, country | Study design (recruitment period) | Population | Methodological approach (inclusion/exclusion criteria) | Study groups MT |
Comparator | Follow-up period |
|---|---|---|---|---|---|---|
| Byrne et al, 201476 United States |
Retrospective, single centre (2005-2011) | N = 154 Acute limb ischemia
|
|
n = 71
|
n = 83
|
Mean: 15.2 mo (range: 56-56.84 mo) |
| Chait et al, 201974 United States |
Retrospective, single centre (2006-2008) | N = 91 Intra-arterial thrombolysis in acute limb ischemia
|
|
n = 22
|
n = 69
|
30 d |
| de Athayde Soares et al, 202077 Brazil | Retrospective, single centre (July 2015 to December 2018) |
N = 49 Acute limb ischemia
|
|
n = 18
|
n = 31
|
Mean: 760 d (SD ± 80 d) |
| Escobar et al, 201778 United States |
Retrospective, single centre (2007-2013) | N = 102 People treated with endovascular techniques for thrombotic syndromesa
|
|
n = 52, (34 arterial and 18 venous)
|
n = 50, (44 arterial and 6 venous)
|
3 d |
| Gandhi et al, 201879 United States |
Retrospective single centre (January 2008 to April, 2014 |
N = 83 Acute limb ischemia
|
|
n = 54
|
n = 29
|
Median: 15.8 and 24.0 mo for MT and control groups, respectively |
| Gong et al, 20211 China | Retrospective single centre (January 2015 to July 2019) |
N = 98 Acute limb ischemia (mean time from symptoms to presentation, 31-37 h)
|
|
n = 57
|
n = 41
|
12 mo |
| Hundt et al, 201380 Germany | Retrospective single centre (2007-2012) | N = 75 Acute and subacute femoropopliteal bypass occlusions
|
|
n = 35
|
n = 40
|
6 mo |
| Kronlage et al, 201781 Germany | Retrospective single centre (2006-2015) | N = 202 (Sub)acute limb ischemia
|
|
n = 146
n = 28
|
n = 28
|
1 y |
| Morrow et al, 201773 United States |
Retrospective, single centre (January 2009 to December 2014) |
N = 53 Arterial thrombosisc |
|
n = 10
n = 16
n = 14
|
n = 13
|
6 mo |
| Muli Jogi et al, 201882 Singapore |
Retrospective, single centre (2006-2015) |
N = 94 Acute limb ischemia |
|
n = 28
|
n = 89
|
30 d |
| Puangpunngam et al, 202083 Thailand | Retrospective, single centre (November 2014 to April 2017) |
N = 34 Acute and subacute lower limb ischemia (<30 d from symptom onset)
|
|
n = 12
|
n = 22
|
3 mo |
| Schernthaner et al, 201484 United States |
Retrospective, single centre (August 2005 to February 2012) |
N = 102 Acute ischemia
|
|
n = 75
|
n = 27
|
Mean 8 mo (range: 1.5-20.5 mo) |
Abbreviations: CDT, catheter-directed thrombolysis; MT, mechanical thrombectomy; PCDT, pharmacomechanical catheter-directed thrombolysis; PMT, pharmacomechanical MT; rtPA, recombinant tPA; tPA, tissue plasminogen activator; UAT, ultrasound-accelerated thrombolysis.
The population is included in this analysis because while mixed, it is largely arterial.
Study also reported on patients with subacute occlusions, defined as 14-42 days, which is beyond our inclusion criteria and therefore excluded from scope of this review.
Study included an additional 92 patients with venous thrombosis, which we excluded from our review on arterial population
RISK OF BIAS IN THE INCLUDED STUDIES
There are some concerns with risk of bias in the studies. All studies were of retrospective design, and thus the selection of intervention was at the treating physician's discretion, leading to concerns with potential confounding. There is also the possibility of a natural progression and learning effect, which may impact the effectiveness of MT device use over time, which may in turn also impact patient selection and outcomes.
The reported study traits were generally similar in baseline characteristics and there were no concerns with measurements of exposure or lack of blinding of the outcome assessments. In all studies, data was obtained through trustworthy sources such as medical records. While blinding was not present, we judged that its absence would have no effect on outcome measures such as limb loss, patency, or severe adverse effects. Finally, the quantity of missing data was similar in both study groups and, therefore, not a concern for a risk of bias. Additional details about the risk of bias are presented in Appendix 2.
OUTCOMES
Outcomes are reported in three broad categories of interest: measures of effectiveness, measures of safety, and health care utilization. Limb salvage is a key clinically important outcome; patients with arterial acute limb ischemia are not expected to experience post-thrombotic syndrome.
MEASURES OF EFFECTIVENESS
Limb Salvage
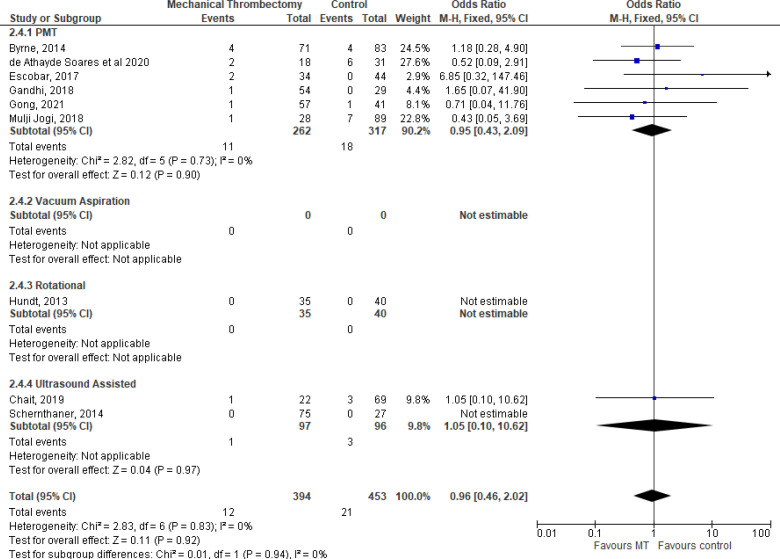
All studies reported on amputation and limb salvage rates. Rates of amputation were around 15% to 20% with no significant difference reported between those who received MT devices and control groups (Table 4, Figure 3). Our GRADE certainty for the evidence was Very low, with downgrading for risk of bias and imprecision (see Appendix 2, Table A2).
Table 4:
Limb Salvage Rates With Mechanical Thrombectomy Use in Arterial Acute Limb Ischemia
| Results | ||||
|---|---|---|---|---|
| Author, year | Outcome measurement | MT | Comparator | P value |
|
PMT
(AngioJet) | ||||
| de Athayde Soares et al, 202077 | Limb salvage rate at 720 d post-procedure (2 y) | 89.7% (n = 16) | 87.8% (n = 27) | Kaplan-Meier curve: P = .784 |
| Multivariate Cox regression analysis of factors associated with limb salvage rate | Rutherford classification, gender, kidney disease, diabetes, segment occluded, fasciotomy, type of surgery | None were significant P > .05 | ||
| Escobar et al, 201778 | Major amputation | 3% (n = 1) | 5% (n = 2) | NR |
| Gandhi et al, 201879 | Amputation | 14.8% (n = 8) | 20.7% (n = 6) | P = .55 |
| Limb salvage up to 60 mo | 85% (n = 46) | 79% (n = 23) | P = .55 | |
| Amputation free survival, median | 4.2 y (range: 3.0-5.9 y) |
4.6 y (range: 3.2-5.7 y) |
P = .91 | |
| Gong et al, 20211 Included large bore catheters, AngioJet, and Rotarex | Limb salvage at 6 mo | 93.0% (n = 53) | 90.2% (n = 37) | P = .625 |
| Limb salvage at 12 mo | 89.5% (n = 51) | 82.9% (n = 34) | P = .346 | |
| Muli Jogi et al, 201882 Included both AngioJet and Rotarex |
Major amputation at 30 d | 7.1% (n = 2) | 16.9% (n = 15) | P = .323 |
| Minor amputation at 30 d | 3.6% (n = 1) | 1.1% (n = 1) | NR | |
|
Vacuum
Aspiration (Indigo) | ||||
| No studies met our inclusion criteria for this mechanical thrombectomy device | ||||
|
Rotational
(Rotarex) | ||||
| Gong et al, 2021,1 included large bore catheters, AngioJet, and Rotarex (findings discussed elsewhere in this assessment)a | ||||
| Hundt et al, 201380 | Amputations | 0 | 0 | NR |
| Kronlage et al, 201781 | Amputation-free survival among non-critically ill, at 12 mo | NRb | NRb | Kaplan-Meier curve: P = .21 |
| Amputation-free survival among critically ill, at 12 mo | NRb | NRb | Kaplan-Meier curve: P = .14 |
|
| Muli Jogi et al, 2018,82 included both AngioJet and Rotarex (findings discussed elsewhere in this assessment)c | ||||
| Puangpunngam et al, 202083 | Limb salvage at 1 mo | 100% (n = 12) | 94.7% (n = 21) | P = .65 |
| Limb salvage at 3 mo | 80% (n = 9) | 80% (n = 17) | P = .751 | |
|
Ultrasound
Assisted (EKOS) | ||||
| Chait et al, 201974d | Major limb loss | 9% (n = 2) | 14% (n = 10) | P = .46 |
| Schernthaner et al, 201484 | Amputation | 1.3% (n = 1) | 0 | NR |
Abbreviations: ABI, ankle-brachial index; MT, mechanical thrombectomy; NR, not reported; PMT, pharmacomechanical thrombectomy.
Gong et al1 compared treatment groups of large bore catheters versus AngioJet versus Rotarex.
Data presented as Kaplan-Meier curves, specific details not obtainable.
Muli Jogi et al, 201882 compared catheter-directed thrombolysis versus AngioJet and Rotarex.
While all patients in both groups also received AngioJet, the study design isolated the effect of EKOS.
Figure 3: Limb Salvage Rates With Mechanical Thrombectomy Use in Arterial Acute Limb Ischemia, by Mechanical Thrombectomy Device.
Abbreviations: CI, confidence interval; PMT, pharmacomechanical thrombectomy.
Byrne et al, 201476 reported an overall amputation rate of 15%, but findings were not presented separately for patients who received MT and those who did not. We were thus unable to determine the effectiveness of the MT device intervention. The subgroup analysis by Gong et al1 comparing treatment groups of large bore catheter versus Rotarex versus AngioJet catheters found no difference in limb salvage at 6 months (P = .988) or 12 months (P = .915). Morrow et al73 reported limb salvage, but only at the level of detail to support a comparison of rates among patients who had kidney injury versus those who did not. They did not provide the level of detail necessary to assess the effectiveness of the interventions of interest.
Technical Success (Reduction of Thrombus Burden)
Technical success is a common term in the literature, but with no standard definition. However, all definitions include a measure of blockage reduction. They are related to patency in that they share the objective of measuring if the blockage is reduced and blood flow restored. Table 5 and Figure 4 summarize our findings. Our GRADE certainty for the evidence was Very low, with downgrading for risk of bias and imprecision (see Appendix 2, Table A2).
Table 5:
Technical Success With Mechanical Thrombectomy Use in Arterial Acute Limb Ischemia
| Results | ||||
|---|---|---|---|---|
| Author, year | Outcome measurement | MT | Comparator | P value |
| PMT (AngioJet) | ||||
| Byrne et al, 201476 | Proportion of patients who achieved restoration of blood flow to the foot, and to what was believed to be the baseline flow | 90.1% (n = 64) | 78.3% (n = 65) | P = .047 |
| de Athayde Soares et al, 202077 | Proportion of patients with no more than 30% residual stenosis and restoration of blood flow | 81.6% (n = 15) | 77.7% (n = 24) | P = .45 |
| Gandhi et al, 201879 | Technical success | 87% (n = 47) | 89% (n = 26) | P = 1.00 |
| Gong et al, 20211 Included large bore catheters, AngioJet and Rotarex | Technical success among patients who had adjuvant thrombolysis | 100% (n = 57) | 100% (n = 41) | P = 1.000 |
| Technical success among patients who had adjunctive stents or angioplasty after removal | 82.5% (n = 47) | 80.5% (n = 33) | P = .804 | |
| Muli Jogi et al, 201882 Included both AngioJet and Rotarex |
Technical success | 67.9% (n = 19) | 47.2% (n = 42) | P = .056 |
| Vacuum Aspiration (Indigo) | ||||
| No studies met our inclusion criteria for this mechanical thrombectomy device | ||||
| Rotational (Rotarex) | ||||
| Gong et al, 2021,1 included large bore catheters, AngioJet, and Rotarex | ||||
| Hundt et al, 201380 | TIMI scoresa | 1,625 | 1,121 | P < .0001 |
| Overall total scores | ||||
| Immediately after procedure | 133 | 0 | P < .0001 | |
| At 24 h | 342 | 136 | P < .0001 | |
| At 48 h | 550 | 396 | P < .0001 | |
| At 72 h | 600 | 588 | NS | |
| Muli Jogi et al, 2018,82 included both AngioJet and Rotarex (see findings discussed elsewhere in this assessment)b | ||||
| Technical success | 100% (n = 12) | 85.7% (n = 19) | P = .268 | |
| Puangpunngam et al, 202083 | Complete clot removal | 87.1% (n = 10) | 57.1% (n = 13) | P = .2 |
| Ultrasound Assisted (EKOS) | ||||
| Chait et al, 201974,c | Proportion of patients who achieved complete, or near complete, resolution of thrombus burden | 86% (n = 19) | 72% (n = 52) | P = .31 |
| Schernthaner et al, 201484 | Technical success | 100% (n = 75) | 100% (n = 27) | NS |
| Proportion with complete angiographic success | 72.0% (n = 54) | 63.0% (n = 17) | P = .542 | |
Abbreviations: NS, not significant; MT, mechanical thrombectomy; PMT, pharmacomechanical thrombectomy; TIMI, thrombosis in myocardial infarction.
A classification system borrowed from coronary perfusion assessments where 0 is complete blockage and 3 is the highest score of complete reperfusion achieved.
Muli Jogi et al, 201882 compared catheter-directed thrombolysis versus AngioJet and Rotarex.
While all patients in both groups also received AngioJet, study design was to isolate the effect of EKOS.
Figure 4: Complete Thrombus Removal With Mechanical Thrombectomy Use in Arterial Acute Limb Ischemia, by Mechanical Thrombectomy Device.
Abbreviations: CI, confidence interval; PMT, pharmacomechanical thrombectomy.
Gong et al1 conducted various subgroup analyses and found that the technical success among patients who received no adjunctive therapy was statistically significant in favour of CDT (the control group, P = .0). They also found no difference in technical or clinical success (P = .584) between patients in the intervention group who received large bore catheter, Rotarex, or AngioJet catheters.
Kronlage et al, 201781 reported that primary revascularization was achieved in over 98% of all cases, but their findings were not discernible by intervention group.
Patency
Patency is an outcome measuring the return of blood flow to a blood vessel area. It is related to technical success in that they share the objective of measuring if the blockage is reduced and blood flow restored. Sometimes this was reported as the ankle-brachial index (ABI)—a non-invasive method of comparing the blood pressure in the ankle with that in the arm. Primary patency is the measure of patency without repeat revascularization intervention. Secondary patency is used only if the initial intervention fails to maintain long-term patency and reintervention is required. For the purposes of this review, we include measures of clinical success as part of the outcome measure as, while it is defined slightly differently in the various studies in which it is reported, the outcome of clinical success is a measure related to the return of blood flow. Table 6 and Figure 5 summarize our findings. Our GRADE certainty in the evidence was Very low, with downgrading for risk of bias and imprecision (see Appendix 2, Table A2).
Table 6:
Patency With Mechanical Thrombectomy Use in Arterial Acute Limb Ischemia
| Author, year | Outcome measurement | Results | P value | |
|---|---|---|---|---|
| MT | Comparator | |||
| PMT (AngioJet) | ||||
| Byrne et al, 201476 | Primary patency at: | |||
| 12 mo | 59% (n = 42) | 54% (n = 45) | Kaplan-Meier curve P = .524 | |
| 24 mo | 50% (n = 36) | 41% (n = 34) | ||
| Multivariate analysis of primary patency, PMT use | HR 0.85; 95% CI: 0.52-1.40 | P = .525 | ||
| Primary assisted patency at: | ||||
| 12 mo | 70% (n = 50) | 62% (n = 51) | Kaplan-Meier curve P = .288 | |
| 24 mo | 60% (n = 42) | 50% (n = 42) | ||
| Secondary patency at: 12 mo 24 mo | ||||
| 12 mo | 87% (n = 62) | 74% (n = 61) | Kaplan-Meier curve P = .197 | |
| 24 mo | 80% (n = 57) | 62% (n = 51) | ||
| de Athayde Soares et al, 202077 | Ankle-brachial index postoperativea | .9 | .87 | P = .14 |
| Secondary patency | 81.9% (n = 15) | 78.8% (n = 24) | Kaplan-Meier curve P = .664 | |
| Gandhi et al, 201879 | Primary patency, 30 d | 72.2% (n = 39) | 75.9% (n = 22) | P = .92 |
| Primary patency at 1 y | 40.7% (n = 22) | 48.3% (n = 14) | P = 79 | |
| Long-term primary patency | Mean follow up 480 d: 33.3% (n = 18) | Mean follow up 728 d: 24.1% (n = 7) | P = .54 | |
| Gong et al, 20211 Included large bore catheters, AngioJet, and Rotarex | Ankle-brachial index at treatment completiona | .72 (SD ± 0.16) | .66 (SD ± 0.13) | P = .101 |
| Muli Jogi et al, 201882 Included both AngioJet and Rotarex | Clinical success defined as return to premorbid Rutherford score without amputation by 30 d | 75% (n = 21) | 73% (n = 65) | P = .837 |
| Vacuum Aspiration (Indigo) | ||||
| No studies met our inclusion criteria for this mechanical thrombectomy device | ||||
| Rotational (Rotarex) | ||||
| Gong et al, 2021,1 included large bore catheters, AngioJet, and Rotarex | ||||
| Hundt et al, 201380 | Ankle-brachial indexa | |||
| At 24 h | Acute: 0.63 (SD ± 0.14) Subacute: 0.43 (SD ± 0.08) | Acute: 0.51 (SD ± 0.11) Subacute: 0.41 (SD ± 0.04) | Acute: P = .0001 Subacute: P = .2080 | |
| At 48 h | Acute: 0.79 (SD ± 0.04) Subacute: 0.67 (SD ± 0.14) | Acute: 0.69 (SD ± 0.14) Subacute: 0.55 (SD ± 0.09) | Acute: P = .0001 Subacute: P = .0001 | |
| At 72 h | Acute: 0.81 (SD ± 0.03) Subacute: 0.79 (SD ± 0.02) | Acute: 0.79 (SD ± 0.02) Subacute: 0.79 (SD ± 0.06) | Acute: P = .0010 Subacute: P = 1.0 | |
| Kronlage et al, 201781 | Primary patency, up to 12 mo | Higher patency among patients who received MT alone compared to those who received lysis, or combined MT + lysis | Kaplan-Meier curve P < .0001 | |
| Secondary patency, up to 12 mo | Greatest in the MT alone group (85%) compared to the lysis alone or combined MT + lysis groups | Kaplan-Meier curve P < .05 | ||
| Ankle-brachial index, up to 12 moa | MT alone: 0.87 (SD ± 0.23) MT + lysis: 0.88 (SD ± 0.28) | 0.71 (SD ±0.31) | P > .05 | |
| Muli Jogi et al, 2018,82 included both AngioJet and Rotarex (findings discussed elsewhere in this assessment)b | ||||
| Ultrasound Assisted (EKOS) | ||||
| Schernthaner et al, 201484 | Proportion with patency achieved at last follow up (mean 8 mo) | 75.9% (n = 41) | 64.3% (n = 9) | P = 379 |
| Media ankle-brachial indexa | .96 (IQR: 0.72, 1.07) | .84 (IQR: 0.79, 1.000 | P = .572 | |
| Hemodynamic success by .1 ankle-brachial index | 95.9% (n = 47) | 92.3% (n = 12) | P = .590 | |
Abbreviations: CI, confidence interval; HR, hazard ratio; IQR, interquartile index; MT, mechanical thrombectomy; PMT, pharmacomechanical thrombectomy; SD, standard deviation.
Baseline values were similar between groups.
Muli Jogi et al, 201882 compared catheter-directed thrombolysis versus AngioJet and Rotarex.
Figure 5: Long-term Patency With Mechanical Thrombectomy Use in Arterial Acute Limb Ischemia by Mechanical Thrombectomy Device Compared With Control Groups.
Abbreviations: CI, confidence interval; PMT, pharmacomechanical thrombectomy.
The subgroup analysis by Gong et al1 comparing treatment groups of large bore catheter versus Rotarex versus AngioJet catheters found no difference in ABI scores post treatment completion (P = .179).
Morrow et al73 reported on patency, but only at the level of detail to support a comparison of rates among patients who had kidney injury versus those who did not. They did not provide the level of detail necessary to assess the effectiveness of the interventions of interest.
Re-Thrombosis (and Revision Rates)
The rate of re-thrombosis is an important measure indicating potential long-term success. Alternative measures include revision rates, which are an indication of re-thrombosis or incomplete thrombus removal. It is possible that the use of adjunctive therapies such as stenting may impact the outcome, but that is expected to be more relevant in the long-term revision rates, with less immediate-term effects. Table 7 and Figure 6 summarize the findings. Our GRADE certainty in the evidence was Very low, with downgrading for risk of bias and imprecision (see Appendix 2, Table A2).
Table 7:
Re-Thrombosis and Revision Rates With Mechanical Thrombectomy Use in Arterial Acute Limb Ischemia
| Author, year | Outcome measurement | Results | P value | |
|---|---|---|---|---|
| MT | Comparator | |||
| PMT (AngioJet) | ||||
| de Athayde Soares et al, 202077 | Reinterventions | 11.8% (n = 2) | 35.5% (n = 11) | P = .03 |
| Escobar et al, 201778 | Thromboembolectomy | 9.6% (n = 5) | 18% (n = 9) | P > .05 |
| Gandhi et al, 201879 | Reinterventions | 42.6% (n = 23) | 51.7% (n = 15) | P = .57 |
| Time to reintervention, mean | 76 d (range 32-355 d) | 74 d (range 28-426 d) | P = .89 | |
| Vacuum aspiration (Indigo) | ||||
| No studies met our inclusion criteria for this mechanical thrombectomy device | ||||
| Rotational (Rotarex) | ||||
| Gong et al, 2021,1 included large bore catheters, AngioJet, and Rotarex | ||||
| Hundt et al, 201380 | Reintervention at 1, 3, and 6 mo | 1 mo: 11.6% (n = 8) 3 mo: 17.4% (n = 12) 6 mo: 30.4% (n = 21) |
1 mo: 8.3% (n = 6) 3 mo: 19.4% (n = 14) 6 mo: 33.3% (n = 24) |
NR |
| Muli Jogi et al, 2018,82 included both AngioJet and Rotarex (see findings discussed elsewhere in this assessment)a | ||||
| Puangpunngam et al, 202083 | Number of operations | 8.3% (n = 1) | 13.6% (n = 3) | P = .002 |
| Ultrasound assisted (EKOS) | ||||
| Schernthaner et al, 201484 | Median reinterventions | 28.0% (n = 21) | 40.7% (n = 11) | P = .221 |
Abbreviations: MT, mechanical thrombectomy; NR, not reported; PMT, pharmacomechanical thrombectomy.
Muli Jogi et al, 201882 compared catheter-directed thrombolysis versus AngioJet and Rotarex.
Figure 6: Re-interventions With Mechanical Thrombectomy Use in Arterial Acute Limb Ischemia by Mechanical Thrombectomy Device Compared With Control Groups.
Abbreviations: CI, confidence interval; PMT, pharmacomechanical thrombectomy.
Morrow et al73 reported on re-thrombosis, but only at the level of detail to support a comparison of rates among patients who had kidney injury versus those who did not. They did not provide the level of detail necessary to assess the effectiveness of the interventions of interest.
Pain
No study reported on pain after acute arterial ischemia of the lower limb.
Quality of Life, Activities of Daily Living, Resolution of Symptoms, and Functional Outcomes Not Otherwise Specified
No study reported on outcomes of quality of life or activities of daily living after MT in acute arterial ischemia of the lower limb.
MEASURES OF SAFETY
Mortality
Mortality is a key measure of safety (summarized in Table 8). Meta-analysis of perioperative mortality, including up to 30-day mortality if that was the best available evidence, demonstrated no difference between study groups (Figure 7). Our GRADE certainty in the evidence was Very low, with downgrading for risk of bias and imprecision (see Appendix 2, Table A2).
Table 8:
Mortality With Mechanical Thrombectomy Use in Arterial Acute Limb Ischemia
| Author, year | Outcome measurement | Results | P value | |
|---|---|---|---|---|
| MT | Comparator | |||
| PMT (AngioJet) | ||||
| Byrne et al, 201476 | 1 y survival 2 y survival Overall 30-d mortality |
86% (n = 61) 81% (n = 56) 5.6% (n = 4) |
82% (n = 68) 67% (n = 56) 4.8% (n = 4) |
P = .341 P = .82 |
| de Athayde Soares et al, 202077 | Perioperative mortality | 11.1% (n = 2) | 19.3% (n = 6) | P = .03 |
| Multivariate Cox regression analysis of factors associated with survival | Rutherford classification, gender, kidney disease, diabetes, segment occluded, fasciotomy | P > .05 | ||
| Type of surgery: CDT related to increased mortality; HR 1.330 (95% CI: 0.542-1.543) | P = .26 | |||
| Overall survival at 720 d post intervention | 84.7% | 69.2% | Kaplan-Meier curve P = .822 | |
| Escobar et al, 201778 | Perioperative mortality | 3.8% (n = 2) Mesenteric ischemia and pulmonary embolus |
0 | NR |
| Gandhi et al, 201879 | Perioperative mortality | 1.9% (n = 1) | 0 | P = 1.0 |
| Gong et al, 20211 Included large bore catheters, AngioJet, and Rotarex | Mortality | 1.8% (n = 1) | 2.4% (n = 1) | P = 1.0 |
| Muli Jogi et al, 201882 Included both AngioJet and Rotarex] | Overall 30-d mortality | 3.6% (n = 1) | 8% (n = 7) | P = .425 |
| Vacuum Aspiration (Indigo) | ||||
| No studies met our inclusion criteria for this mechanical thrombectomy device | ||||
| Rotational (Rotarex) | ||||
| Gong et al, 202,11 included large bore catheters, AngioJet, and Rotarex (see findings discussed elsewhere in this assessment)a | ||||
| Hundt et al, 201380 | Mortality | 0 | 0 | NS |
| Kronlage et al, 201781 | Survival among non-critically ill, at 12 mo | Overall survival across study group: 96.02%b | Kaplan-Meier curve P = .86 | |
| Survival among critically ill, at 12 mo | Overall survival across study group: 65%b | Kaplan-Meier curve P = .12 | ||
| Muli Jogi et al, 2018,82 included both AngioJet and Rotarex (see findings discussed elsewhere in this assessment)c | ||||
| Ultrasound Assisted (EKOS) | ||||
| Chait et al, 201974,d | 30-d mortality | 4% (n = 1) | 4% (n = 3) | P = .97 |
| Schernthaner et al, 201484 | Perioperative mortality | 0 | 0 | NS |
| Event free survival | Median event-free survival: 43 mo (range: 29-64 mo) | Median event-free survival: 21 mo (range: 16-59 mo) | P = .061 | |
Abbreviations: CDT, catheter-directed thrombosis; CI, confidence interval; HR, hazard ration; MT, mechanical thrombectomy; NR, not reported; NS, not significant; PMT, pharmacomechanical thrombectomy.
Gong et al1 compared treatment groups of large bore catheters versus AngioJet versus Rotarex.
Data for each study group presented as Kaplan-Meier curves, specific details not provided.
Muli Jogi et al, 201882 compared catheter-directed thrombolysis versus AngioJet and Rotarex.
While all patients in both groups also received AngioJet, the study was designed to isolate the effects of EKOS.
Figure 7: Perioperative Mortality With Mechanical Thrombectomy Use in Arterial Acute Limb Ischemia by Mechanical Thrombectomy Device Compared With Control Groups.
Abbreviations: CI, confidence interval; PMT, pharmacomechanical thrombectomy.
Morrow et al73 reported on mortality, but only at the level of detail to support a comparison of rates among patients who had kidney injury versus those who did not. They did not provide the level of detail necessary to assess the effectiveness of the interventions of interest.
Adverse Effects and Complications
Most of the studies we examined reported adverse effects and complications as lists or counts of observed events in the study groups. The most common events reported were bleeding and kidney dysfunction (Table 9). Our GRADE certainty in the evidence was Very low, with downgrading for risk of bias and inconsistency (see Appendix 2, Table A2).
Table 9:
Adverse Effects and Complications With Mechanical Thrombectomy Use in Arterial Acute Limb Ischemia
| Author, year | Outcome measurement | Results | P value | |
|---|---|---|---|---|
| MT | Comparator | |||
| PMT (AngioJet) | ||||
| Byrne et al, 201476 | Systematic bleed | 7.0% (n = 5) | 3.6% (n = 3) | P = .34 |
| Acute renal failure | 4.2% (n = 3) | 0 | P = .06 | |
| Hematoma | 8.4% (n = 6) | 1.2% (n = 1) | P = .03 | |
| Embolization | 14.1% (n = 10) | 6.0% (n = 5) | P = .09 | |
| de Athayde Soares et al, 202077 | Specific complications leading to death | 1 acute renal failure 1 acute myocardial infarction |
2 myocardial infarction 2 pneumonia 2 hemorrhagic stroke |
NR |
| Compartment syndrome | 0 | 2 (1 resulted in infection) | NR | |
| Escobar et al, 201778 | Acute kidney injury | 29% (n = 15) | 8% (n = 4) | P = .007 |
| Fasciotomy | 5 events | 5 events | NR | |
| Bypass/endarterectomy | 3 events | 3 events | NR | |
| Mean blood loss | 12% | 5% | P = .009 | |
| Gandhi et al, 201879 | Any complication | 27.8% (n = 15) | 13.8% (n = 4) | P = .179 |
| Embolism, hematoma, bleeding, respirator distress, dissection or perforation, DVT, pseudoaneurysm, acute renal failure | No difference between groups for any specific type of complication reported | P > .5 | ||
| Gong et al, 20211 Included large bore catheters, AngioJet, and Rotarex |
Minor complications (no therapy, no consequences) | 10.5% (10) | 23.8% (11) | P = .36 |
| Major complications (requires therapy or permanent sequelae) | 5.3% (3) | 4.9% (2) | P = 1.00 | |
| Procedure related distal embolization | 24.6% (14) | 4.9% (2) | P = .009 | |
| Muli Jogi et al, 201882 Included both AngioJet and Rotarex |
Complications, reported using the CIRSE classification system | 28.6% (8) Groin hematoma, pseudoaneurysm, chest wall hematoma, fasciotomy for compartment syndrome |
30.3% (27) Groin hematoma, gastro-intestinal bleed requiring infusion, compartment syndrome requiring fasciotomy, distal embolism |
NR |
| Vacuum Aspiration (Indigo) | ||||
| No studies met our inclusion criteria for this mechanical thrombectomy device | ||||
| Rotational (Rotarex) | ||||
| Gong et al, 2021,1 included large bore catheters, AngioJet, and Rotarex (see findings discussed elsewhere in this assessment)a | ||||
| Hundt et al, 201380 | Complications | Pseudoaneuryma (3), distal embolism (9), local bleeding (2) 0 cases of systemic bleeding, arteriovenous fistula, dissection, or perforation |
Pseudoaneuryma (1), local bleeding (10), systemic bleeding (1) 0 cases of arteriovenous fistula or distal embolism |
NR |
| Kronlage et al, 201781 | Complications in non-critically ill patients (data for critically ill patients are limited): | |||
| Major bleeding | 3.6% (5) | 22.2% (4) | P < .05 | |
| Aneurysma | 2.9% (4) | 0 | NS | |
| AV-fistula | 0.7% (1) | 0 | NS | |
| Compartment syndrome | 0 | 0 | NS | |
| Muli Jogi et al, 2018,82 included both AngioJet and Rotarex (see findings discussed elsewhere in this assessment)b | ||||
| Puangpunngam et al, 202083 | Complications: | |||
| Aneurysm/pseudoaneurysm at the puncture site | 18.2% (2) | 4.8% (1) | NS | |
| Minor bleeding | 18.2% (2) | 31.8% (7) | NS | |
| Major bleeding requiring operation to stop bleeding | 0 | 2 events | NS | |
| Cardiopulmonary complications | 0 | 0 | NR | |
| Ultrasound assisted (EKOS) | ||||
| Chait et al, 201974c | Compartment syndrome | 14% (3) | 10% (7) | P = .65 |
| Significant bleeding requiring more than 4 units of blood | 0 | 4% (3) | P = 1.0 | |
| Other complications | 14% | 14% | P = .92 | |
| Schernthaner et al, 201484 | Proportion of people with bleeding complications | 6.7% (5) | 22.2% (6) | P = .025 |
| Minor complications | 2.7% (2) | 7.4% (1) | P = .276 | |
| Major complications | 4.0% (3) | 14.8% (4) | P = .57 | |
| Distal embolization | 21.7% (15) | 12.0% (3) | P = .289 | |
| Other complications | 18.7% (14) | 22.2% (6) | P = .690 | |
Abbreviations: CDT, catheter directed thrombosis; DVT, deep vein thrombosis; MT, mechanical thrombectomy; NR, not reported; NS, not significant; PMT, pharmacomechanical thrombectomy.
Gong et al1 compared treatment groups of large bore catheters versus AngioJet versus Rotarex.
Muli Jogi et al, 201882 compared catheter-directed thrombolysis versus AngioJet and Rotarex.
While all patients in both groups also received AngioJet, study design was to isolate the effect of EKOS.
The subgroup analysis by Gong et al1 comparing treatment groups of large bore catheter versus Rotarex versus AngioJet catheters found no difference in procedure-related complications, whether mild (P = .912), major (P = .841), or procedure-related distal embolization (P = .765).
Escobar et al78 reported several other metrics of kidney health and found worse outcomes among patients who received AngioJet compared to those who received CDT alone. There was a higher rise in creatinine (P = .003), which could not be accounted for by baseline levels. As well, the blood loss (noted to be higher in people who had MT) was driven mostly by those who experienced acute kidney disease.
Morrow et al73 reported greater rates of renal dysfunction among people who received MT and none in those who received the control CDT; however, their data reporting did not allow us to analyze their findings by arterial versus deep vein thrombosis (DVT) population types.
MEASURES OF HEALTH CARE UTILIZATION
Measures of health care utilization reported by the identified studies include the volume and duration of thrombolytic infusion, as well as hospital length of stay.
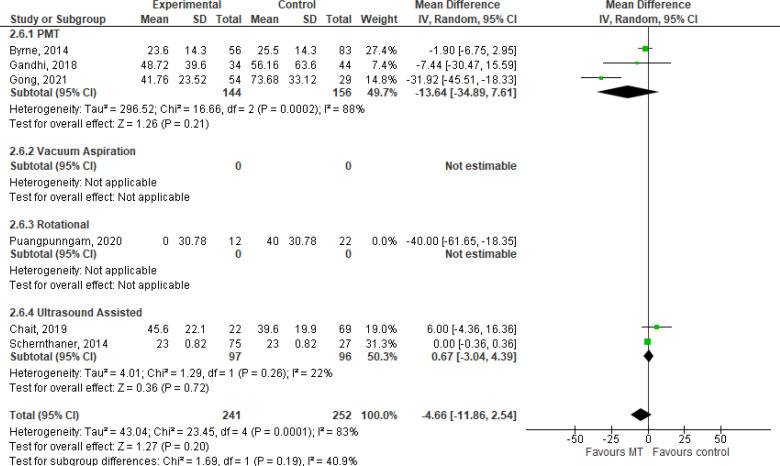
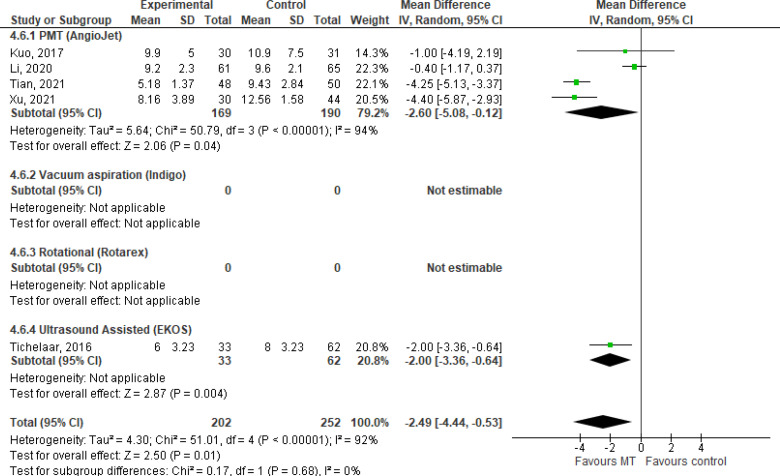
Volume of Thrombolytic Infusion
Volume of thrombolytic infusion is typically reported in milligrams (our findings are summarized in Table 10). Our GRADE certainty in the evidence was Very low, with downgrading for risk of bias and imprecision (see Appendix 2, Table A2). Due to methodological design and outcome reporting, findings are presented narratively only.
Table 10:
Volume of Thrombolytic Infusion With Mechanical Thrombectomy Use in Arterial Acute Limb Ischemia
| Author, year | Outcome measurement | Results | P value | |
|---|---|---|---|---|
| MT | Comparator | |||
| PMT (AngioJet) | ||||
| de Athayde Soares et al, 202077 | Unfractionated heparin use postoperatively | NR | NR | P = .15 |
| IV prostavasin | 11% (2) | 6% (2) | P = .40 | |
| Gong et al, 20211 Included large bore catheters, AngioJet, and Rotarex] |
Mean rtPA dose | 14.14 mg (SD ± 5.75) | 29.27 mg (SD ± 11.70) | P = .000 |
| Vacuum Aspiration (Indigo) | ||||
| No studies met our inclusion criteria for this mechanical thrombectomy device | ||||
| Rotational (Rotarex) | ||||
| Gong et al, 2021,1 included large bore catheters, AngioJet, and Rotarex (see findings discussed elsewhere in this assessment)a | ||||
| Muli Jogi et al, 2018,82 included both AngioJet and Rotarex (see findings discussed elsewhere in this assessment)a | ||||
| Puangpunngam et al, 202083 | Dose of rtPA | 0 | 30 mg | P = .001 |
| Ultrasound Assisted (EKOS) | ||||
| Chait et al, 201974,c | Mean volume of tPA administered | 48.2 mg | 44.6 mg | P = .6 |
| Schernthaner et al, 201484 | Median total rtPA lytic dose | 8.4 mg (IQR: 4.9-13.2) | 6.8 (IQR: 5.8-11.1) | P = .479 |
Abbreviations: IQR, interquartile range; PMT, pharmacomechanical thrombectomy; NR, not reported; PMT, pharmacomechanical thrombectomy; SD, standard deviation.
Gong et al1 compared treatment groups of large bore catheters versus AngioJet versus Rotarex.
Muli Jogi et al, 201882 compared catheter-directed thrombolysis versus AngioJet and Rotarex.
While all patients in both groups also received AngioJet, the study design isolated the effect of EKOS.
Morrow et al73 reported on duration of CDT, tPA dose, and aspiration volume, but only at the level of detail to support a comparison of rates among patients who had kidney injury versus those who did not. They did not provide the level of detail necessary to assess the effectiveness of the interventions of interest.
Schernthaner et al84 found no difference between intervention groups for the total dose or the infusion time for patients who received alternative medications of heparin, urokinase, or tenecteplase.
Time of Thrombolytic Infusion
Duration of thrombolytic infusion is considered to be representative of the time in the ICU, and findings are summarized in Table 11 and Figure 8. Our GRADE certainty in the evidence was Very low, with downgrading for risk of bias and imprecision (see Appendix 2, Table A2).
Table 11:
Time of Thrombolytic Infusion With Mechanical Thrombectomy Use in Arterial Acute Limb Ischemia
| Results | ||||
|---|---|---|---|---|
| Author, year | Outcome measurement | MT | Comparator | P value |
| PMT (AngioJet) | ||||
| Byrne et al, 201476 | Mean duration of lysis | Among those who received PMT and lysis (n = 56) Mean time: 23.6 h |
Mean time: 25.5 h | P =.445 |
| de Athayde Soares et al, 202077 | Mean duration of procedure | NRa | 12 h (range 1-36 h) | NR |
| Gandhi et al, 201879 | Lysis time, mean | 2.03 d (SD ± 1.65) | 2.38 d (SD ± 2.65) | P = .58 |
| Proportion of patients who required > 24 h lysis time | 56.4% (22) | 57.1% (12) | P = .96 | |
| Gong et al, 20211 Included large bore catheters, AngioJet, and Rotarex |
Mean duration of operation procedureb Mean total duration of thrombolysis |
1.89 h (SD ± 0.52) 1.74 d (SD ± 0.98) |
1.32 h (SD ± 0.44) 3.07 d (SD ± 1.38) |
P = .000 P = .00 |
| Vacuum Aspiration (Indigo) | ||||
| No studies met our inclusion criteria for this mechanical thrombectomy device | ||||
| Rotational (Rotarex) | ||||
| Gong et al, 2021,1 included large bore catheters, AngioJet, and Rotarex | ||||
| Puangpunngam et al, 202083 | Time to lysis | 0 | 40 h | P = .001 |
| Ultrasound Assisted (EKOS) | ||||
| Chait et al, 201974,c | Mean duration of thrombolysis | 45.6 h (SD ± 22.1) | 39.6 h (SD ± 19.9) | P = .22 |
| Schernthaner et al, 201484 | Median total rtPA infusion time | 23 h (IQR: 20.5-38.0) | 23 h (IQR: 20.3-32.3) | P = .787 |
Abbreviations: IQR, interquartile range; MT, mechanical thrombectomy; NR, not reported; PMT, pharmacomechanical thrombectomy; SD, standard deviation.
Reported following protocol of 20-min dwell time and 300-s activation.
Large-bore catheters had slightly shorter procedure lengths than the AngioJet or Rotarex.
While all patients in both groups also received AngioJet, study design was isolating the effect of EKOS.
Figure 8: Time of Thrombolytic Infusion With Mechanical Thrombectomy Use in Arterial Acute Limb Ischemia by Mechanical Thrombectomy Device Compared to Control Groups.
Abbreviations: CI, confidence interval; PMT, pharmacomechanical thrombectomy; SD, standard deviation.
The subgroup analysis by Gong et al1 comparing treatment groups of large bore catheter versus Rotarex versus AngioJet catheters found no difference in procedure length (P = .103), duration of thrombolysis (P = .92), or rtPA dosage (P = .76). Schernthaner et al84 found no difference between intervention groups for infusion time for patients who received alternative medications of heparin, urokinase, or tenecteplase.
Puangpunngam et al83 reported that among the subgroup of patients with Rutherford class IIb occlusions, there was statistically significantly less time in operation among those who received MT compared to those who received CDT alone (1.15 vs. 5.85 hours, respectively, P = .014).
Due to the methodological design and outcome reporting, Puangpunngam et al83 was omitted from our meta-analyses. However, sensitivity analyses we conducted that included this study found significantly in favour of MT upon its inclusion (mean difference −8.43; 95% CI: −16.59 to −.27).
Hospital Length of Stay and Other Health Care Utilization
Hospital length of stay includes all time in hospital, both in and outside the ICU, and may account for recovery from the procedure or adverse events like renal dysfunction. Table 12 and Figure 9 summarize our findings. Our GRADE certainty for the evidence was Very low, with downgrading for risk of bias (see Appendix 2, Table A2).
Table 12:
Hospital Length of Stay and Other Health Care Utilization After Mechanical Thrombectomy Use in Arterial Acute Limb Ischemia
| Results | ||||
|---|---|---|---|---|
| Author, year | Outcome measurement | MT | Comparator | P value |
| PMT (AngioJet) | ||||
| de Athayde Soares et al, 202077 | Mean hospital length of stay | 6.6 d | 8.6 d | P = .56 |
| Gong et al, 20211 Included large bore catheters, AngioJet, and Rotarex] |
Mean hospital length of stay | 4.97 d (SD ± 0.13) | 6.04 (SD ± 0.95) | P = .000 |
| Muli Jogi et al, 201882 | Mean hospital length of stay | 6.0 d | 12.6 d | P = .001 |
| Vacuum Aspiration (Indigo) | ||||
| No studies met our inclusion criteria for this mechanical thrombectomy device | ||||
| Rotational (Rotarex) | ||||
| Gong et al, 2021,1 included large bore catheters, AngioJet, and Rotarex | ||||
| Kronlage et al, 201781 | Mean hospital length of stay among non-critically ill patients | MT alone: 1.4 d (SD ± 0.9 d) MT + lysis: 4.4 d (SD ± 1.8 d) |
4.6 d (SD ± 3 d) |
MT alone: P < .001 MT + lysis: NS |
| Mean hospital length of stay among critically ill patients | MT alone: 21.7 d (SD ± 34.4 d) MT + lysis: 18.3 d (SD ± 9.36 d) |
13.3 d (SD ± 4.5 d) |
NS | |
| Muli Jogi et al, 2018,82 included both AngioJet and Rotarex (see findings discussed elsewhere in this assessment)a | ||||
| Puangpunngam et al, 202083 | Mean hospital length of stay | 16 d | 8.5 d | P = .27 |
| Ultrasound Assisted (EKOS) | ||||
| We did not identify any studies that reported this outcome of interest | ||||
Abbreviations: MT, mechanical thrombectomy; NS, not significant; PMT, pharmacomechanical thrombectomy; SD, standard deviation.
Muli Jogi et al, 201882 compared catheter-directed thrombolysis versus AngioJet and Rotarex.
Figure 9: Hospital Length of Stay After Mechanical Thrombectomy Use in Arterial Acute Limb Ischemia by Mechanical Thrombectomy Device Compared With Control Groups.
Abbreviations: CI, confidence interval; IV, inverse variance; PMT, pharmacomechanical thrombectomy; SD, standard deviation.
The subgroup analysis by Gong et al1 comparing treatment groups of large bore catheter versus Rotarex versus AngioJet catheters found no difference in hospital length of stay (P = .165).
Morrow et al73 reported hospital admission length of stay, but only at the level of detail to support a comparison of rates among patients who had kidney injury versus those who did not. They did not provide the level of detail necessary to assess the effectiveness of the interventions of interest.73
Due to the methodological design and outcome reporting, Puangpunngam et al83 was omitted from our analyses. However, sensitivity analyses demonstrated that our findings are robust when we include their findings. Our meta-analysis was conducted using the Kronlage et al81 values that we felt best represented the current Ontario context as it included patients who had both mechanical thrombectomy and lysis used in their treatment. It is possible this represents a conservative estimate of effect.
SUMMARY OF FINDINGS FOR ARTERIAL ACUTE LIMB ISCHEMIA
This summary is based on pooled estimates where conducted, and otherwise based on narrative findings as reported in data tables. We included 12 observational studies and did not identify any RCTs.
Table 13:
Summary of Findings of Effect of Mechanical Thrombosis in Arterial Acute Limb Ischemia
| Outcome | No. of participants (studies) | Effect | ||
|---|---|---|---|---|
| Relative (95% CI) | Absolute (95% CI) | GRADEa | ||
| Limb salvage | 727 (Obs 9) | OR: 1.49 (0.87-2.57) | 38 more per 1,000 (16 fewer to 74 more) | ⊕ Very low |
| Post thrombotic Syndrome | Not relevant for the arterial blockages population. We did not identify any studies that reported on this outcome of interest | |||
| Technical success: complete thrombus removal | 728 (Obs 8) | OR: 1.79 (1.21-2.64) | 108 more per 1,000(39 more to 162 more) | ⊕ Very low |
| Patency | 471 (Obs 5) | OR: 1.77 (1.13-2.77) | 120 more per 1,000 (28 more to 193 more) | ⊕ Very low |
| Re-thrombosis (and revision rates) | 421 (Obs 6) | OR: 0.64 (0.41-1.01) | 98 fewer per 1,000 (179 fewer to 2 more) | ⊕ Very low |
| Pain | We did not identify any studies that reported on this outcome of interest | — | ||
| Quality of life | We did not identify any studies that reported on this outcome of interest | — | ||
| Perioperative mortality | 847 (Obs 9) | OR: 0.96 (0.46-2.02) | 2 fewer per 1,000 (24 fewer to 43 more) | ⊕ Very low |
| Adverse events | 1.106 (Obs 12) | There are inconsistent findings in adverse events across the different MT interventions compared to control groups | ⊕ Very low | |
| Volume of thrombolytic (mg) | 359 (Obs 5) | There are inconsistent findings of volume of thrombolytics used among people who received MT compared to control groups | ⊕ Very low | |
| Time of thrombolytic infusion (h) | 493 (Obs 5) | — | MD: 4.66 lower (11.86 lower to 2.54 higher) | ⊕ Very low |
| Hospital length of stay | 466 (Obs 4) | — | MD: 1.05 lower (1.33 lower to 0.77 lower) | ⊕ Very low |
Abbreviations: CI, confidence interval; GRADE, Grading of Recommendations Assessment, Development, and Evaluation working group criteria; MD, mean difference; MT, mechanical thrombectomy; Obs, observational studies; OR, odds ratio.
Note: Summary of findings table developed using GRADEpro GDT. GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime, 2022. Available from gradepro.org.
We evaluated the quality of the body of evidence for each outcome according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Handbook.70 See Appendix 2 for further details.
Acute Deep Vein Thrombosis
We identified 29 comparative study publications evaluating MT in people with acute or subacute deep vein thrombosis. Three were RCTs, while the rest were observational study designs. While RCTs are generally considered to be a better methodological design to evaluate effectiveness with greater certainty, the observational studies are value added representations of real-world evidence. We determined them to be equally important evidence in this review. Table 14 summarizes key aspects of the included studies. Where studies included multiple publications, these were collated into one row.
Table 14:
Characteristics of Included Acute or Subacute Deep Vein Thrombosis Studies
| Author, year, country | Study design (recruitment period) | Population (inclusion/exclusion criteria) | Methodological approach | Study groups | Follow-up period | |
|---|---|---|---|---|---|---|
| MT | Comparator | |||||
| Baker et al, 201294 United States | Retrospective, single centre (June 2004 to October 2011) | N = 83 Iliofemoral DVT |
|
n = 64
|
n = 19
|
Median 35 mo (range 20-55 mo) |
| Engelberger et al, 20152 Switzerland | Randomized controlled clinical trial, single centre (November 2011 to November 2013) | N = 48 Symptomatic acute (symptoms < 2 wk) proximal DVT
|
|
n = 24
|
n = 24
|
3 mo |
| Ezelsoy et al, 201595 Turkey |
Retrospective, single centre (June 2013 to June 2014) | N = 50 Acute DVT (symptoms < 14 d)
|
|
n = 25
|
n = 25
|
Median: 14 mo (range 6-18 mo) |
| PEARL registry Garcia et al, 201585 United States and Europe |
Multisite, international registry (January 2007 to June 2013) | N = 329 Lower extremity DVT |
|
n = 115
n = 172
|
n = 13
n = 29
|
12 mo |
| Huang et al, 201596 China |
Retrospective, single centre (November 2010 to November 2013) | N = 34 Acute proximal DVT
|
|
n = 16
|
n = 18
|
12 mo |
| Huang et al, 202197 China | Retrospective, single centre (September 2013 to September 2015) | N = 131 Acute iliofemoral DVT |
|
n = 65
|
n = 66
|
5 y |
| Kuo et al, 201798 Taiwan |
Prospective, single centre (January 2009 to December 2013) | N = 61 Acute iliofemoral DVT
|
|
n = 30
|
n = 31
|
2 y |
| Lee et al, 202099 Korea | Retrospective, before/after, single centre (May 2017 to December 2018) | N = 40 Acute iliofemoral DVT
|
|
n = 20
|
n = 20
|
Mean 14.3 mo (SD ± 5.3 mo) |
| Li et al, 2020100 China | Retrospective, single centre (September 2015 to August 2019) | N = 126 (among 120 individuals) Acute iliofemoral DVT
|
|
n = 61
|
n = 65
|
Median: 6.4 mo (range: 4-12 mo) |
| Liu et al, 2018101 China | Retrospective, single centre (June 2014 to December 2016) | N = 112 Acute (< 15 d) or subacute (15-30 d) of iliofemoral or iliocaval DVT |
|
n = 52
|
n = 60
|
≤ 12 mo |
| Lu et al, 201775 United States | Retrospective, single centre (2008-2012) | N = 76 limbs (among 67 individuals) Iliofemoral or femoral popliteal DVT
|
|
n = 51
|
n = 25
|
Mean: 20 mo (SD ± 2.5) |
| Morrow et al, 201773 United States |
Retrospective, single centre (January 2009 to December 2014) | N = 92 Acute venous thrombusab |
|
n = 5
n = 26
n = 56 Percutaneous MT with CDT |
n = 5 CDT alone |
6 mo |
| CAVA trial Notton et al, 2020102; 20213 Netherlands |
Multicentre, randomized single blind trial (May 2010 to September 2017) |
N = 181 Acute iliofemoral DVT
|
|
n = 91
|
n = 93
|
Median: 39 mo (IQR range: 23.3-63.8) |
| Pouncey et al, 2020103 United Kingdom | Retrospective, single centre (November 2011 to November 2017) | N = 151 Acute or subacute iliofemoral DVT
|
|
n = 70
|
n = 81
|
12 mo |
| Shen et al, 2019104 China |
Retrospective, single centre (January 2014 to September 2017) | N = 198 Acute iliofemoral DVT
|
|
n = 79
|
n = 119
|
72 h |
| Tian et al, 2021105 China |
Retrospective, single centre (March 2016 to January 2018) | N = 98 Acute, unilateral DVT
|
|
n = 48
|
n = 50
|
Median: 28 mo (SD ± 5.2) |
| Tichelaar et al, 2016106 Norway | Retrospective, single centre (2002-2011) | N = 94 Iliofemoral or more proximal DVT |
|
n = 33
|
n = 62
|
Median: 65 mo (range: 15-141 mo) |
| ATTRACT study Vedantham et al, 201391; 201790; Weinberg et al, 201993; Kahn et al, 202087; Razavi et al, 202089 Subgroup analysis: Comerota et al, 201986; Kearon et al, 201988; Vedantham et al, 202192 United States |
Randomized controlled trial, multicentre (December 2009 to December 2014) |
N = 692 Acute (< 14 d from symptom onset) proximal DVT
Subgroup analysis: Acute Iliofemoral DVT only86 Acute femoral-popliteal deep vein thrombosis88 AngioJet MT only92 |
|
n = 336 If the test with rtPA results in good inflow to the popliteal vein:
OR
If the popliteal vein is occluded or the IVC is involved:
|
n = 355
|
2 y |
| Xu et al, 2021107 China | Retrospective, 3 institutions (January 2015 to December 2018) | N = 424 Acute lower extremity DVT
|
|
n = 186
|
n = 238
|
12 mo |
| Xu et al, 2020108 China | Retrospective, single centre (December 2015 to May 2018) | N = 74 Subacute iliofemoral DVT
|
|
n = 30
|
n = 44
|
12 mo |
| Zhu et al, 2020109 China |
Case controlled study, 2 centres (February 2015 to October 2016) |
N = 65 Acute lower extremity DVT
|
|
n = 32
|
n = 33
|
Unclear |
Abbreviations: ATTRACT, Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis; CDT, catheter directed thrombolysis; DVT, deep vein thrombosis; IQR, interquartile range; IVC, inferior vena cava; LMWH, low molecular weight heparin; MT, mechanical thrombectomy; PEARL, Peripheral Use of AngioJet Rheolytic Thrombectomy with a Variety of Catheter Lengths; PMT, pharmacomechanical thrombectomy; PTS, post-thrombotic syndrome; RT, rheolytic thrombectomy; rtPA, recombinant tissue plasminogen activator; SD, standard deviation; UAT, ultrasound-accelerated thrombolysis; UFH, unfractionated heparin.
Outcomes are reported under the arterial population group elsewhere in this report (see Table 3 for additional details).
Full study included additional 145 patients with arterial thrombosis, evaluated elsewhere in this report.
One study (Escobar et al78) included approximately 25% of its population as venous occlusions. However, they did not report outcomes for patients experiencing the venous occlusions separately from those who experienced arterial ischemia. Given that the population was largely arterial (approximately 75%), we have chosen to exclude their findings from our analysis for the venous population.
Garcia et al85 included patients with acute and subacute (≤ 14 and ≤ 30 d from symptom onset, respectively), as well as 46 people (14%) with chronic (> 30 d from symptom onset) deep vein thrombosis. Although most outcomes for this study were not discernible for the acute and subacute populations separate from the chronic population, we opted to include the study data. We felt that the group of patients with chronic symptoms comprised only a small portion of the overall study population. Additionally, the evidence where reported did not demonstrate differences in outcomes by duration of symptoms such as the rate of recurrence of thrombosis (P = .7572, log rank test).
The ATTRACT trial study design was not specifically aligned to our review's intervention and control groups of interest.86–93 It was designed to determine the effectiveness of oral anticoagulation alone compared to the additional use of one of three endovascular catheter interventions, only one of which would be our intervention of interest (PMT, AngioJet). Fifty-eight percent (n = 194) of patients in their intervention group had infusion-first rtPA, which is the equivalent of CDT, and thus serves as a control group for the research focus of our review. However, the ATTRACT trial reports several of their findings by subgroups from which we could pull the relevant data of interest; furthermore, it reports 88% (n = 297) of patients had additional endovascular treatment. However, it is unclear what portion of these patients received the MT device of interest to us (AngioJet.). We determined that the ATTRACT trial's pharmacomechanical group is too heterogenous to be included in this review in its entirety; we report only those findings that could be discernible by study groups of interest throughout this review.
RISK OF BIAS IN THE INCLUDED STUDIES
There are some concerns with risk of bias in the studies. There were three RCTs that had low-to-moderate concerns with risk of bias. The rest of the studies were observational, typically retrospective in design, and, as such, the selection of intervention was at the treating physician's discretion. Most studies reported baseline characteristics between study groups that were well balanced. There is a possibility of a natural progression and learning effect, which may impact the effectiveness of MT device use over time, as well as patient selection and outcomes.
The reported study traits were generally similar in baseline characteristics and there were no concerns with measurements of exposure or the lack of blinding of the outcome assessments. In all studies, data was obtained through trustworthy sources such as medical records and, while blinding was not present, its absence was judged to have no effect on outcome measurements such as limb loss, patency, and severe adverse effects. Finally, the quantity of missing data was similarly likely in both study groups and therefore not a concern for risk of bias. Additional details about the risk of bias are presented in Appendix 2.
OUTCOMES
Outcomes are reported in three broad categories of interest: measures of effectiveness, measures of safety, and measures of hospital utilization. Post thrombotic syndrome is considered a key clinically important outcome.
MEASURES OF EFFECTIVENESS
Post-Thrombotic Syndrome
Post-thrombotic syndrome describes long-term pain and discomfort, potentially leading to complications. There are several metrics for measuring post-thrombotic syndrome,110 the most common one seen in the included studies was the Villalta score. The Villalta score is a continuous measure ranging from 0 to 33 that accounts for physical symptoms such as pain and cramps, as well as clinical symptoms such as hyperpigmentation. A higher score indicates a more severe condition for patients. A standard interpretation of post-thrombotic syndrome is a Villalta score ≥ 5, with moderate or severe post-thrombotic syndrome defined as a Villalta score ≥ 10 or ≥ 15, respectively.3,89 Another commonly reported score is the venous clinical severity scale (VCSS), which ranges from 0 to 27; higher values indicate greater severity for patients. Post-thrombotic syndrome effects quality of life, as discussed elsewhere in this assessment. Table 15 and Figure 10 summarize the findings from the RCTs. Our GRADE certainty in the evidence was Moderate, with downgrading for imprecision (see Appendix 2, Table A5). Table 16 and Figure 11 summarize the findings of the observational studies. Our GRADE certainty in the evidence was Very low, with downgrading for risk of bias and inconsistency (see Appendix 2, Table A5).
Table 15:
Post-Thrombotic Syndrome With Mechanical Thrombectomy Use in Acute and Subacute DVT in Randomized Controlled Trials
| Results | ||||
|---|---|---|---|---|
| Author, year | Outcome measurement | MT | Comparator | P value |
| PMT (AngioJet) | ||||
| ATTRACT trial92 PMT vs. control (no procedure, anticoagulation alone) |
Proportion with post-thrombotic syndrome, 24 mo | 44.0% (n = 33) | 41.4% (n = 79) | P = .69 |
| Iliofemoral subgroupa | 28.1% (n = 9) | 30.3% (n = 20) | P = .82 | |
| Popliteal subgroupa | 37.9% (n = 11) | 42.9% (n = 39) | P = .64 | |
| Vacuum Aspiration (Indigo) | ||||
| No randomized studies met our inclusion criteria for this mechanical thrombectomy device | ||||
| Rotational (Rotarex or Cleaner) | ||||
| No randomized controlled trials met our conclusion criteria for this mechanical thrombectomy device | ||||
| Ultrasound Assisted (EKOS) | ||||
| Engelberger et al, 20152 | Mean Villalta score | 3.0 (SD ± 3.9) | 1.9 (SD ± 1.9) | P = .21 |
| CAVA trial, Notton et al, 20203,102 | Proportion of patients with post-thrombotic syndrome, at final visit, by Villalta score | 30.6% (n = 19) | 44.8% (n = 26) | P = .11 |
Abbreviations: ATTRACT, Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis; DVT, deep vein thrombosis; MT, mechanical thrombectomy; PMT, pharmacomechanical thrombectomy; SD, standard deviation.
Per protocol analysis.
Figure 10: Post-Thrombotic Syndrome With Mechanical Thrombectomy Use in Acute and Subacute DVT by Mechanical Thrombectomy Device Compared With Control Groups, in Randomized Controlled Trials.
Abbreviations: ATTRACT, Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-directed Thrombolysis; CI, confidence interval; PMT, pharmacomechanical thrombectomy.
Table 16:
Post Thrombotic Syndrome With Mechanical Thrombectomy Use in Acute and Subacute DVT in Observational Studies
| Results | ||||
|---|---|---|---|---|
| Author, year | Outcome measurement | MT | Comparator | P value |
| PMT (AngioJet) | ||||
| Huang et al 201596 | Villalta score at 12 mo | 2.06 (SD ± 2.95) | 5.06 (SD ± 4.07) | P = .030 |
| Venous reflux at 12 mo | 1.31 (SD ± 0.48) | 1.39 (SD ± 0.61) | P = .851 | |
| Huang et al, 202197 | PTS, by 5-y follow up | 45.0% (n = 27) | 57.7% (n = 34) | P = .201 |
| Severe PTS (Villalta score ≥ 15) by 5-y follow up | 11.7% (n = 7) | 27.1% (n = 16) | P = .039 | |
| Kuo et al, 201798 | Post-thrombotic syndrome | 20% (n = 6) | 19.4% (n = 6) | P = 1.0 |
| Villalta score | 1.87 (SD ± 2.7) | 3.13 (SD ± 3.0) | P = .042 | |
| Lee et al, 202099 | Villalta score at 6 mo | 1.12 (SD ± 0.92) | 1.47 (SD ± 1.24) | P = .372 |
| Pouncey et al, 2020103 | PTS at 6 mo | 21.4% (n = 15) | 24.6% (n = 20) | P = .64 |
| PTS at 1 y | 20% (n = 14) | 22.2% (n = 18) | P = .74 | |
| Villalta score at 1 ya | 1 (range: 0-3) | 3 (range: 3-4) | P = .31 | |
| Tian et al, 2021105 | Villalta score at 24 mo | 3.65 (SD ± 2.73) | 3.70 (SD ± 0.62) | P = .225 |
| Post-thrombotic syndrome at 24 mo | 0 | 4% (n = 2) | P = .493 | |
| Xu et al, 2021107 | Patients who developed PTS (Villalta score > 5) Median 19-mo follow up | 5.5% (n = 10) | 15.3% (n = 31) | P < .05 |
| Estimate of incidence of PTS at 4 y | 19.8% | 60% | Kaplan-Meier curve NR |
|
| Xu et al, 2020108 | Incidence of PTS at 12 mo | 20% (n = 6) | 29.5% (n = 13) | P = .852 |
| Vacuum Aspiration (Indigo) | ||||
| No studies met our inclusion criteria for this mechanical thrombectomy device | ||||
| Rotational (Rotarex or Cleaner) | ||||
| Ezelsoy et al, 201595 | Rate of post-thrombotic syndrome | 28% (n = 7) | 56% (n = 14) | P = .045 |
| Ultrasound Assisted (EKOS) | ||||
| Tichelaar et al, 2016106 | Proportion with severe post-thrombotic syndromeb |
5% (n = 1) | 10% (n = 5) | NS |
Abbreviations: DVT, deep vein thrombosis; MT, mechanical thrombectomy; PMT, pharmacomechanical thrombectomy; NR, not reported; NS, not significant; PTS, post-thrombotic syndrome; SD, standard deviation.
Similar finding at 6 months.
Similar findings for mild and moderate post-thrombotic syndrome.
Figure 11: Post-Thrombotic Syndrome With Mechanical Thrombectomy Use in Acute and Subacute DVT by Mechanical Thrombectomy Device Compared With Control Groups in Published Observational Studies.
Abbreviations: CI, confidence interval; DVT, deep vein thrombectomy; M-H, Mantel-Haenszel; PMT, pharmacomechanical thrombectomy.
Randomized Controlled Trials
The ATTRACT trial found that, among those who received any procedure, including rtPA alone with no MT, there was no statistically significant difference in post-thrombotic syndrome by 24 months compared to those who had no procedure and anticoagulation alone.92
The CAVA trial by Notton et al3,102 reported trends towards improved post-thrombotic syndrome over time among those who received MT compared to those who did not; however, findings did not reach statistical significance and the study authors acknowledged this may be due to the relatively small sample size. There was an observed statistically significant difference when re-evaluating post-thrombotic syndrome using the International Society on Thrombosis consensus definition (OR: 0.40; 95% CI: 0.19-84).3
Engelberger et al2 also reported no significant findings for the VCSS or clinical etiologic anatomic pathophysiological class.
Kuo et al 98 found significantly reduced thrombus scores and improved thrombolysis rates among those who did not have post-thrombotic syndrome compared to those who did, but their findings are not reported discretely for each intervention group. Huang et al97 reported similar findings using VCSS and CIVIQ score evaluations.
Technical Success (Reduction of Thrombus Burden)
Technical success is a common term in the literature, but with no standard definition. However, all definitions include a measure of blockage reduction. They are related to patency in that they share the objective of measuring if the blockage is reduced and blood flow restored. One of the more common measures was reported as the degree of thrombus removal on a graded scale of I to III, where grade III indicates little to no remaining thrombus. Table 17 and Figure 12 summarize the findings from the RCTs. Our GRADE certainty in the evidence was Very low, with downgrading for risk of bias, inconsistency, and imprecision (see Appendix 2, Table A5). Table 18 and Figure 13 summarize the findings of the observational studies. Our GRADE certainty in the evidence was Very low, with downgrading for risk of bias and imprecision (see Appendix 2, Table A5).
Table 17:
Technical Success With Mechanical Thrombectomy Use in Acute and Subacute DVT in Randomized Controlled Trials
| Results | ||||
|---|---|---|---|---|
| Author, year | Outcome measurement | MT | Comparator | P value |
| PMT (AngioJet) | ||||
| ATTRACT trial89,92 (PMT vs. infusion first rtPA) | Post-procedure thrombus score | 1.7 (SD ± 2.8) | 3.1 (SD ± 4.0) | P = .001 |
| Thrombus score change from baseline | 6.7 (SD ± 4.5) | 9.8 (SD ± 6.3) | P < .001 | |
| Proportion with clot lysis > 90% | 75% (n = 56) | 72% (n = 138) | P = .64 | |
| Vacuum Aspiration (Indigo) | ||||
| No randomized studies met our inclusion criteria for this mechanical thrombectomy device | ||||
| Rotational (Rotarex or Cleaner) | ||||
| No randomized controlled trials met our inclusion criteria for this mechanical thrombectomy device | ||||
| Ultrasound Assisted (EKOS) | ||||
| Engelberger et al, 20152 | Length-adjusted thrombus score at 15 ha | 27 (SD ± 24) | 25 (SD ± 16) | P = .68 |
| Mean percentage of thrombus reduction at 15 h | 55% (SD ± 27%) | 54% (SD ± 27%) | P = .91 | |
| Proportion of patients who had a thrombus load reduction ≥ 50% | 58% (n = 14) | 63% (n =15) | P > .99 | |
Abbreviations: ATTRACT, Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis; DVT, deep vein thrombosis; MT, mechanical thrombectomy; PMT, pharmacomechanical thrombectomy; SD, standard deviation.
A measure of thrombus size. Lower scores indicate clear vessel segments. Baseline scores were similar (P = .86) between study groups. Both groups showed statistically significant improvement (P < .01) from baseline.
Figure 12: Technical Success With Mechanical Thrombectomy Use in Acute and Subacute DVT by Mechanical Thrombectomy Device Compared to Control Groups in Published Randomized Controlled Trials.
Abbreviations: ATTRACT, Acute venous Thrombosis: Thrombus Removal with Adjunctive Catheter-directed Thrombolysis; CI, confidence interval; DVT, deep vein thrombosis; M-H, Mantel-Haenszel; PMT, pharmacomechanical thrombectomy.
Table 18:
Technical Success With Mechanical Thrombectomy Use in Acute and Subacute DVT in Observational Studies
| Results | ||||
|---|---|---|---|---|
| Author, year | Outcome measurement | MT | Comparator | P value |
| PMT (AngioJet) | ||||
| Garcia et al, 201585 | Grade III (complete lysis) | 57.7% (n = 165) | 64.2% (n = 27) | P = .42 |
| Huang et al 201596 | Grade III (complete lysis) | 0 | 0 | NS |
| Thrombus score at 12 moa | 0.56 (SD ± 0.93) | 2.22 (SD ± 3.49) | P = .224 | |
| Huang et al, 202197 | Technical success at 5 y | 38.3% (n = 23) | 25.4% (n = 15) | P = .169 |
| Grade III | 45% (n = 27) | 28.8% (n = 17) | P = .07 | |
| Kuo et al, 201798 | Functional venous obstruction | 30% (n = 9) | 28.7% (n = 12) | P = .474 |
| Mean thrombus score at 24 mo | 0.70 (SD ± 1.3) | 0.90 (SD ± 1.3) | P = .526 | |
| Lee et al, 202099 | Grade III | 95% (n = 19) | 95% (n = 19) | P = 1.0 |
| Li et al, 2020100 | Technical success rate | 100% | 100% | P = 1.0 |
| Grade III | 75.4% (n = 46) | 69.2% (n = 45) | P = .44 | |
| Liu et al, 2018101 | Grade III or higher | 53.8% (n = 28) | 63.3% (n = 38) | P = .32 |
| Pouncey et al, 2020103 | Proportion of patients who achieved lytic success | 79% (n = 55) | 83% (n = 67) | P = .30 |
| Tian et al, 2021105 | Lysis efficacy rate | 100% (n = 48) | 96% (n = 48) | P = .162 |
| Grade III | 84.5% (n = 42) | 76% (n = 36) | P = .057 | |
| Xu et al, 2021107 | Technical success | 100% | 100% | NR |
| Grade III (100%) | 23.1% (n = 43) | 17.6% (n = 32) | P = .01 | |
| Xu et al, 2020108 | Technical success | 100% | 100% | NR |
| Grade III | 36.7% (n = 11) | 15.9% (n = 7) | P = .04 | |
| Zhu et al, 2020109 | Complete removal of thrombi | 72% (n = 23) | 82% (n = 27) | P = .34 |
| Vacuum Aspiration (Indigo) | ||||
| No studies met our inclusion criteria for this mechanical thrombectomy device | ||||
| Rotational (Rotarex or Cleaner) | ||||
| We did not identify any studies that reported on this outcome of interest | ||||
| Ultrasound Assisted (EKOS) | ||||
| Baker et al, 201294 | Percentage thrombus resolution | 82% (IQR 55-92) | 89% (IQR 70-100) | P = .560 |
| Grade III (complete lysis) | 21.9% (n = 14) | 36.8% (n = 7) | P = .17 | |
| Lu et al, 201775 | Grade III (100%) | 59.5% (n = 25) | 20% (n = 5) | P = .002 |
| Proportion with clot burden reduced by > 50% | 92% (n = 218) | 56% (n = 14) | NRb | |
| Tichelaar et al, 2016106 | Grade III (> 90%) | 76% (n = 25) | 88% (n = 54) | P = .15 |
Abbreviations: DVT, deep vein thrombosis; IQR, interquartile range; MT, mechanical thrombectomy; NR, not reported; NS, not significant; PMT, pharmacomechanical thrombectomy; SD, standard deviation.
Both groups statistically significantly improved from baseline.
Both groups had statistically significant reductions in clot burden compared to baseline.
Figure 13: Technical Success With Mechanical Thrombectomy Use in Acute and Subacute DVT, by Mechanical Thrombectomy Device Compared to Control Groups in Published Observational Studies.
Abbreviations: CI, confidence interval; DVT, deep vein thrombosis; M-H, Mantel-Haenszel; MT, mechanical thrombectomy.
The ATTRACT trial reported that both intervention and control study groups showed statistically significant improvement from baseline values. They also reported a composite outcome of major treatment failure. This outcome was comprised of an unplanned endovascular procedure to treat severe venous symptoms or gangrene within 6 months, or an amputation within 24 months. They reported no significant difference (P ≤ 0.01) between study groups; however, their findings are not discernible by the intervention groups of interest in our assessment as the results for patients who received infusion first (CDT alone) are combined with the results of those who received MT.90 Patients were not randomized into these subgroups, a limitation that is reflected in the GRADE assessment.
Engelberger et al2 conducted a regression analysis and found that neither the symptom duration in days nor the rtPA dose were correlated with thrombus load reduction.
Huang et al, 2015,96 reported that most patients experienced a grade II thrombolysis event after their procedure, but findings were not significantly different between the MT and control groups.
Patency
Patency is an outcome measuring the return of blood flow to a blood vessel area. It is related to technical success in that they share the objective of measuring if the blockage is reduced and blood flow restored. Sometimes this was reported as the ankle-brachial index (ABI), a non-invasive method of comparing the blood pressure in the ankle with that in the arm. Primary patency is the measure of patency without repeat revascularization intervention. Secondary patency is used only if the initial intervention fails to maintain long term patency and reintervention is required. For the purposes of this review, we include measures of clinical success as part of the outcome measure as, while it is defined slightly differently in the various studies where it is reported, the outcome of clinical success is a measure related to the return of blood flow. Table 19 summarizes the findings from the RCTs. Our GRADE certainty in the evidence was Low, with downgrading for risk of bias and publication bias (see Appendix 2, Table A5). Table 20 and Figure 14 summarize the findings of the observational studies. Our GRADE certainty in the evidence was Very low, with downgrading for risk of bias (see Appendix 2, Table A5).
Table 19:
Patency With Mechanical Thrombectomy Use in Acute and Subacute DVT in Randomized Controlled Trials
| Author, year | Outcome measurement | Results | P value | |
|---|---|---|---|---|
| MT | Comparator | |||
| PMT (AngioJet) | ||||
| We did not identify any randomized studies that reported on this outcome of interest | ||||
| Vacuum Aspiration (Indigo) | ||||
| No randomized studies met our inclusion criteria for this mechanical thrombectomy device | ||||
| Rotational (Rotarex or Cleaner) | ||||
| No randomized controlled trials met our inclusion criteria for this mechanical thrombectomy device | ||||
| Ultrasound Assisted (EKOS) | ||||
| Engelberger et al, 20152 | Primary patency at 3 mo | 100% (n = 24) | 96% (n = 23) | P = .49 |
Abbreviations: DVT, deep vein thrombosis; MT, mechanical thrombectomy; PMT, pharmacomechanical thrombectomy.
Table 20:
Patency With Mechanical Thrombectomy Use in Acute and Subacute DVT in Observational Studies
| Results | ||||
|---|---|---|---|---|
| Author, year | Outcome measurement | MT | Comparator | P value |
| PMT (AngioJet) | ||||
| Huang et al, 201596 | Post-procedural patency, venous registry indexa | 2.44 (SD ± 1.46) | 3.56 (SD ± 2.17) | P = .126 |
| Primary patency rate at 12 mo | 93.8% (n = 15) | 88.9% (n = 16) | P = .648 | |
| Huang et al, 202197 | Primary patency rate at 5 y | 52% (n = 26) | 45% (n = 19) | P = .538 |
| Kuo et al, 201798 | Mean thrombolysis rate at 24 mo | -93.25% (SD ± 11.7) | -89.46% (SD ± 15.9) | P = .378 |
| Lee et al, 202099 | Primary patency rate | 100% (n = 20) | 95% (n = 19) | P = .317 |
| Pouncey et al, 2020103 | Primary patency rate at 2 y | 25.0% (n = 17) | 28.75% (n = 23) | Kaplan-Meier curve P = .24 |
| Tian et al, 2021105 | Clinical efficacy rate at discharge | 100% (n = 48) | 96% (n = 48) | P = .162 |
| Xu et al, 2020108 | Primary patency rate at 12 mo | 93.3% (n = 28) | 88.6% (n=39) | P = .694 |
| Vacuum Aspiration (Indigo) | ||||
| No studies met our inclusion criteria for this mechanical thrombectomy device | ||||
| Rotational (Rotarex or Cleaner) | ||||
| Ezelsoy et al, 201595 | Recanalization within 6 mo | 84% (n = 21) | 56% (n = 14) | P = .031 |
| Femoral venous insufficiency | 36% (n = 9) | 60% (n = 15) | P = .089 | |
| Ultrasound Assisted (EKOS) | ||||
| Lu et al, 201775 | Primary patency rate at 24 mo | 41.1% (n = 21) | 23.8% (n = 5) | P = .10 |
| Tichelaar et al, 2016106 | Patency, open | 76% (n = 13) | 79% (n = 30) | P = .88 |
Abbreviations: DVT, deep vein thrombosis; MT, mechanical thrombectomy; PMT, pharmacomechanical thrombectomy; SD, standard deviation.
Baseline rates were not statistically different between intervention groups, although the post-procedural rates are statistically significantly lower in both groups from baseline.
Figure 14: Patency With Mechanical Thrombectomy Use in Acute and Subacute DVT, by Mechanical Thrombectomy Device Compared to Control Groups in Published Observational Studies.
Abbreviations: CI, confidence interval; DVT, deep vein thrombosis; M-H, Mantel-Haenszel; MT, mechanical thrombectomy; PMT, pharmacomechanical thrombectomy.
Morrow et al73 reported on patency, but only at the level of detail to support a comparison of rates among patients who had kidney injury versus those who did not. They did not provide the level of detail necessary to assess the effectiveness of the interventions of interest.
Re-Thrombosis (and Revision Rates)
Rate of re-thrombosis is an important measure indicating potential long-term success. Revision rates is an alternative measure used to indicate re-thrombosis or incomplete thrombus removal. Adjunctive therapies such as stenting may impact outcomes, but they are expected to be more relevant in the long-term. They are not expected to impact immediate revision rates. Table 21 summarizes the findings from the RCTs. Our GRADE certainty in the evidence was Low, with downgrading for risk of bias and imprecision (see Appendix 2, Table A5). Table 22 summarizes the findings of the observational studies. Our GRADE certainty in the evidence was Very low, with downgrading for risk of bias and imprecision (see Appendix 2, Table A5).
Table 21:
Re-Thrombosis and Revision Rates With Mechanical Thrombectomy Use in Acute and Subacute DVT in Randomized Controlled Trials
| Results | ||||
|---|---|---|---|---|
| Author, year | Outcome measurement | MT | Comparator | P value |
| PMT (AngioJet) | ||||
| No randomized studies met our inclusion criteria for this mechanical thrombectomy device | ||||
| Vacuum Aspiration (Indigo) | ||||
| No randomized studies met our inclusion criteria for this mechanical thrombectomy device | ||||
| Rotational (Rotarex or Cleaner) | ||||
| No randomized controlled trials met our inclusion criteria for this mechanical thrombectomy device | ||||
| Ultrasound Assisted (EKOS) | ||||
| Engelberger et al, 20152 | Adjunctive thrombus removal | 29% (n = 7) | 46% (n = 11) | P = .37 |
| Re-thrombosis rate at 3 mo | None reported | 4.2% (n = 1) | NS | |
| CAVA trial Notton et al, 20203,102 |
Ulceration at any follow-up visit | 0 | 5.2% (n = 3) | OR: 0.13 (95% CI: .01-2.51); P = .18 |
| Recurrent DVT (no stent) rate at 12 mo | 6% (n = 5) | 5% (n = 4) | OR: 1.23 (95% CI: .32-4.78); P = .76 | |
| In-stent thrombosis rate at 12 mo | 13% (n = 10) | NR | NR | |
| Recurrent DVT (no stent) rate beyond 12 mo | 4.8% (n = 3) | 10.3% (n = 6) | OR: 0.44 (95% CI: .11-1.85); P = .26 | |
| In-stent thrombosis rate beyond 12 mo | 3.2% (n = 2) | 1.7% (n = 1) | OR: 1.90 (95% CI: 0.17-21.5); P = .60 | |
Abbreviations: DVT, deep vein thrombosis; MT, mechanical thrombectomy; NR, not reported; NS, not significant; OR, odds ratio; PMT, pharmacomechanical thrombectomy; SD, standard deviation.
Table 22:
Re-Thrombosis and Revision Rates With Mechanical Thrombectomy Use in Acute and Subacute DVT in Observational Studies
| Results | ||||
|---|---|---|---|---|
| Author, year | Outcome measurement | MT | Comparator | P value |
| PMT (AngioJet) | ||||
| Garcia et al, 201585 | Freedom from re-thrombosis rate at 12 mo | 83% (95% CI: 77-88) | NR | NR |
| Recurrence rate | Log rank test assessing if differences between intervention groups, specific data not reported | P = .07 | ||
| Huang et al, 201596 | Recurrent DVT, and repeat procedure within 12 mo | 1 | 2 | NR |
| Huang et al, 202197 | Success rate where reintervention required | 72% (13, of 18 reinterventions) | 54% (6 of 11 reinterventions) | P =.432 |
| Vacuum Aspiration (Indigo) | ||||
| No studies met the inclusion criteria for this mechanical thrombectomy device | ||||
| Rotational (Rotarex or Cleaner) | ||||
| We did not identify any studies that reported on this outcome of interest | ||||
| Ultrasound Assisted (EKOS) | ||||
| Baker et al, 201294 | Additional MT procedures | 11% (n = 7) | 21.1% (n = 4) | NS |
| Lu et al, 201775 | Recurrences at 30 d | 3 | 1 | P = .60 |
Abbreviations: DVT, deep vein thrombosis; MT, mechanical thrombectomy; NR, not reported; NS, not significant; PMT, pharmacomechanical thrombectomy.
The ATTRACT trial92,111 reported symptomatic recurrent VTE at 24 months as a metric of safety as it included any VTE. We interpreted it to include pulmonary embolism. See Adverse Effects and Complications for discussion.
Garcia et al 85 reported that 86% of all patients had two or fewer catheter sessions and that 9% required repeat intervention within 12 months, but their findings were not discernible by intervention used.
Morrow et al73 reported on reoperations but only at the level of detail to support a comparison of rates among patients who had kidney injury versus those who did not. They did not provide the level of detail necessary to assess the effectiveness of the interventions of interest.
Xu et al 108 reported that seven cases required additional CDT; however, their findings were not discernible by initial intervention type received.
Pain
While no study explicitly reported on pain as its own outcome, pain is captured as part of the quality-of-life metrics discussed elsewhere in this report.
Quality of Life, Activities of Daily Living, Resolution of Symptoms, and Functional Outcomes Not Otherwise Specified
The Venous Insufficiency Epidemiologic and Economic Quality of Life Survey (VEINES-QoL) is a disease-specific quality of life metric. Quality of life in this population is related to post-thrombotic syndrome (discussed elsewhere in this assessment). Table 23 and Figure 15 summarize the findings from the RCTs. Our GRADE certainty in the evidence was Low, with downgrading for risk of bias and imprecision (see Appendix 2, Table A5). Table 24 summarizes the findings of the observational studies. Our GRADE certainty in the evidence was Very low, with downgrading for risk of bias and imprecision (see Appendix 2, Table A5).
Table 23:
Quality of Life After Mechanical Thrombectomy Use in Acute and Subacute DVT in Randomized Controlled Trials
| Results | ||||
|---|---|---|---|---|
| Author, year | Outcome measurement | MT | Comparator | P value |
| PMT (AngioJet) | ||||
| ATTRACT trial92 PMT vs. control (no procedure, anticoagulation alone) |
VEINES-QOL at 24 mo | 30.53 (SE ± 3.11) | 26.17 (SE ± 2.17) | P = .21 |
| Vacuum Aspiration (Indigo) | ||||
| No randomized studies met the inclusion criteria for this MT device | ||||
| Rotational (Rotarex or Cleaner) | ||||
| No randomized controlled trials met the inclusion criteria for this MT device | ||||
| Ultrasound Assisted (EKOS) | ||||
| Engelberger et al, 20152 | Chronic VEINES-QoL | 28.0 (SD ± 11.6) | 26.2 (SD ± 7.5) | P = .55 |
| CAVA trial Notton et al, 20203,102 |
VEINES-QoL at 12 mo | 50.0 (SD ± 11.1) | 50.2 (SD ± 8.8) | P = .92 |
Abbreviations: ATTRACT, Acute venous Thrombosis: Thrombus Removal with Adjunctive Catheter-directed Thrombolysis; DVT, deep vein thrombosis; MT, mechanical thrombectomy; PMT, pharmacomechanical thrombectomy; SD, standard deviation; SE, standard error;
VEINES-QoL, Venous Insufficiency Epidemiologic and Economic Quality of Life Survey score.
Figure 15: Quality of Life With Mechanical Thrombectomy Use in Acute and Subacute DVT, by Mechanical Thrombectomy Device Compared to Control Groups in Published RCTs.
Abbreviations: ATTRACT, Acute venous Thrombosis: Thrombus Removal with Adjunctive Catheter-directed Thrombolysis; CI, confidence interval; IV, inverse variance; MT, mechanical thrombectomy; PMT, pharmacomechanical thrombectomy; SD, standard deviation.
Table 24:
Quality of Life After Mechanical Thrombectomy Use in Acute and Subacute DVT in Observational Studies
| Author, year | Outcome measurement | Results | P value | |
|---|---|---|---|---|
| MT | Comparator | |||
| PMT (AngioJet) | ||||
| We did not identify any studies that reported on this outcome of interest | ||||
| Vacuum Aspiration (Indigo) | ||||
| No studies met the inclusion criteria for this MT device | ||||
| Rotational (Rotarex or Cleaner) | ||||
| We did not identify any randomized studies that reported on this outcome of interest | ||||
| Ultrasound Assisted (EKOS) | ||||
| Tichelaar et al, 2016106 | SF-36 | 45 (SD ± 12) | 40 (SD ± 13) | NS |
| Physical subscale | ||||
| Mental subscale | 48 (SD ± 12) | 53 (SD ± 9) | NS | |
| VEINES-QoL/Sym | 51 (SD ± 6) | 50 (SD ± 7) | NS | |
| QoL subscale | ||||
| Sym subscale | 50 (SD ± 7) | 48 (SD ± 7) | NS | |
Abbreviations: DVT, deep vein thrombosis; MT, mechanical thrombectomy; NS, not significant; PMT, pharmacomechanical thrombectomy; QoL, quality of life; SD, standard deviation; VEINES-QoL/Sym, Venous Insufficiency Epidemiologic and Economic Quality of Life Survey score/symptoms.
The ATTRACT trial92 found a statistically significant difference in quality of life scores at 1 month, but the difference was not sustained at any other time point evaluated. The CAVA trial (Notton et al3,102) measured quality of life using a number of different metrics, including the SF-36 mental, physical, and general health scores, as well as the EQ-5D, PDI, and VEINES-QoL intrinsic measures. Most metrics found no statistically significant difference between groups. Where differences were statistically significant, they were found to be not clinically meaningful.3,102
Garcia et al,85 reported SF-12 quality of life scores by age of thrombus (acute, subacute, and chronic), but not by intervention.
Limb Salvage
Only one study (Pouncey et al103) reported on limb loss in the control group. Other studies did not report findings for this outcome of interest. Our GRADE certainty in the evidence was Very low, with downgrading for risk of bias and imprecision (Appendix 2, Table A5).
Morrow et al73 also reported on limb salvage, but only at the level of detail to support a comparison of rates among patients who had kidney injury versus those who did not. They did not provide the level of detail necessary to assess the effectiveness of the interventions of interest. The ATTRACT trial90 reported amputations as part of their composite outcome of major treatment failure (reported elsewhere in this assessment).
MEASURES OF SAFETY
Mortality
Randomized Controlled Trials
Mortality is a key measure of safety. Table 25 and Figure 16 summarize the findings from the RCTs. Our GRADE certainty in the evidence was Very low, with downgrading for risk of bias and imprecision (see Appendix 2, Table A5). Table 26 summarizes the findings of the observational studies. Our GRADE certainty in the evidence was Very low, with downgrading for risk of bias and imprecision (see Appendix 2, Table A5).
Table 25:
Mortality With Mechanical Thrombectomy Use in Acute and Subacute DVT in Randomized Controlled Trials
| Results | ||||
|---|---|---|---|---|
| Author, year | Outcome measurement | MT | Comparator | P value |
| PMT (AngioJet) | ||||
| ATTRACT trial92 | Deaths at 24 mo | 1.3% (n =1) | 1.6% (n = 3) | P = .99 |
| PMT vs. control (no procedure, anticoagulation alone) | ||||
| Vacuum Aspiration (Indigo) | ||||
| No randomized studies met our inclusion criteria for this MT device | ||||
| Rotational (Rotarex or Cleaner) | ||||
| No randomized controlled trials met our inclusion criteria for this MT device | ||||
| Ultrasound Assisted (EKOS) | ||||
| CAVA trial Notton et al, 20203,102 |
Deaths at 12 mo Deaths beyond 12 mo | 1% (n =1) 0 | 4% (n=3) 0 | P = 0.69 NS |
Abbreviations: ATTRACT, Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis; DVT, deep vein thrombosis; MT, mechanical thrombectomy; NS, not significant.
Figure 16: Mortality With Mechanical Thrombectomy Use in Acute and Subacute DVT, by Mechanical Thrombectomy Device Compared to Control Groups in Published RCTs.
Abbreviations: ATTRACT, Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis; CI, confidence interval; M-H, Mantel-Haenszel; MT, mechanical thrombectomy; PMT, pharmacomechanical thrombectomy.
Table 26:
Mortality With Mechanical Thrombectomy Use in Acute and Subacute DVT in Observational Studies
| Results | ||||
|---|---|---|---|---|
| Author, year | Outcome measurement | MT | Comparator | P value |
| PMT (AngioJet) | ||||
| Huang et al, 201596 | Mortality, 30 d Mortality, 6 mo |
0 | 0 3 |
NS |
| Huang et al, 202197 | Fatal hemorrhage | 0 | 0 | NS |
| Lee et al, 202099 | Mortality, mean 14 mo | 0 | 0 | NS |
| Liu et al, 2018101 | Mortality, mean 6 mo | 0 | 0 | NS |
| Xu et al, 2020108 | Mortality, 12 mo | 0 | 0 | NS |
| Vacuum Aspiration (Indigo) | ||||
| No studies met our inclusion criteria for this MT device | ||||
| Rotational (Rotarex or Cleaner) | ||||
| Ezelsoy et al, 201595 | Mortality, median 14 mo | 0 | 0 | NR |
| Ultrasound Assisted (EKOS) | ||||
| Baker et al, 201294 | Event-free survival | 33 mo (95% CI: 26-41) | 69 mo (95% CI: 55-84) | Kaplan-Meier curve P = .310 |
| Lu et al, 201775 | Mortality, 30 d | 1 | 0 | P = .44 |
| Tichelaar et al, 2016106 | Mortality, any cause, < 1 ya | 0 | 8% (n = 5) | NS |
Abbreviations: CI, confidence interval; DVT, deep vein thrombosis; NR, not reported; NS, not significant; MT, mechanical thrombectomy; PMT, pharmacomechanical thrombectomy.
Not significant when cumulative over entire follow up period.
Garcia et al85 reported one death; however, the data was incomplete and it is not possible to determine the cause of the death or the potential impact of thrombus or the intervention of choice.
Morrow et al73 reported mortality, but only at a level of detail to support a comparison of rates among patients who had kidney injury versus those who did not. They did not provide the level of detail necessary to assess the effectiveness of the interventions of interest.73
Adverse Effects and Complications
Adverse effects and complications are reported in most studies as lists or counts of observed events in the study groups. The most common events were bleeding and kidney dysfunction. Table 27 summarizes the findings from the RCTs. Our GRADE certainty in the evidence was Moderate, with downgrading for risk of bias (see Appendix 2, Table A5). Table 28 summarizes the findings of the observational studies. Our GRADE certainty in the evidence was Very low, with downgrading for risk of bias and imprecision (see Appendix 2, Table A5).
Table 27:
Adverse Effects and Complications With Mechanical Thrombectomy Use in Acute and Subacute DVT in Randomized Controlled Trials
| Results | ||||
|---|---|---|---|---|
| Author, year | Outcome measurement | MT | Comparator | P value |
| PMT (AngioJet) | ||||
| ATTRACT trial92 PMT vs. control (among the subset of sites that used AngioJet: no procedure, anticoagulation alone) |
Major bleeding at 30 d | 0 | 0 | P = 1 |
| Major bleeding at 24 mo | 5.3% (n = 4) | 3.1% (n = 6) | P = .40 | |
| Any bleeding at 24 mo | 9.3% (n = 7) | 9.9% (n = 19) | P = .88 | |
| Device-related adverse event | 13.6% (n = 25) | NA | NA | |
| Recurrent VTE at 24 mo | 13.9% (n = 24) | 6.8% (n = 13) | P = .03 | |
| Vacuum Aspiration (Indigo) | ||||
| No randomized studies met our inclusion criteria for this MT device | ||||
| Rotational (Rotarex or Cleaner) | ||||
| No randomized controlled trials met our conclusion criteria for this MT device | ||||
| Ultrasound Assisted (EKOS) | ||||
| Engelberger et al, 20152 | Treatment-related complications (major bleeding, hematoma, minor bleeding) | 12.5% (n = 3) | 8.3% (n = 2) | P > .99 |
| CAVA trial Notton et al, 20203,102 |
Major bleeding at 12 mo | 5% (n = 4) | 0 | OR: 9.25 (95% CI: 0.49-174.7) P = .14 |
| Pulmonary embolism at 12 mo | 0 | 3% (n = 2) | OR: 0.19 (95% CI: 0.01-4.02) P = .29 |
|
| Major bleeding beyond 12 mo | 0 | 0 | NS | |
| Pulmonary embolism beyond 12 mo | 4.8% (n = 3) | 3.4% (n = 2) | OR: 1.42 (95% CI: 0.23-8.84) P = .70 | |
Abbreviations: ATTRACT, Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis; CI, confidence interval; MT, mechanical thrombectomy NA, not applicable; NS, not significant; OR, odds ratio; PMT, pharmacomechanical thrombectomy; VTE, venous thrombosis embolism.
Table 28:
Adverse Effects and Complications With Mechanical Thrombectomy Use in Acute and Subacute DVT in Observational Studies
| Results | ||||
|---|---|---|---|---|
| Author, year | Outcome measurement | MT | Comparator | P value |
| PMT (AngioJet) | ||||
| Garcia et al, 201585 | Adverse eventsa | 9 (1 serious—renal failureb) | 0 | NR |
| Huang et al 201596 | Complications (major bleeding, pulmonary embolism, renal failure) | 0 | 0 | P = 1.0 |
| Minor bleeding | 0 | 1 | P = .798 | |
| Huang et al, 202197 | Acute kidney injury | 2 | 0 | P = 1.0 |
| Pulmonary embolism | 4 | 0 | P = 1.0 | |
| Minor bleeding | 9 | 16 | P = .182 | |
| Kuo et al, 201798 | Proportion with adverse effects (gross or micro hemoglobinuria, ecchymosis, or mucosal bleeding) | 46.7% (n = 14) | 22.6% (n = 7) | P = .048 |
| Proportion who received blood transfusion | 36.7% (n = 11) | 29.0% (n = 9) | P = .704 | |
| Lee et al, 202099 | Gross hemoglobinuria, recovered within 24 h | 0 | 85% (17) | NR |
| Major bleeding, renal dysfunction, symptomatic pulmonary embolism | 0 | 0 | NS | |
| Li et al, 2020100 | Minor complications Transient hemoglobinuria | 30 | 0 | P = .0 |
| Fever | 5 | 6 | P = .837 | |
| Gingival bleeding | 1 | 0 | P = .484 | |
| Severe complications Venous damage (bleeding) | 0 | 4.6% (n = 3) | P = .245 | |
| Kidney damage, fatal pulmonary embolism, intracranial hemorrhage | 0 | 0 | P = 1 | |
| Liu et al, 2018101 | Complications (minor bleeding) | 0 | 1 | P = .55 |
| Symptomatic pulmonary embolism, major bleeding | 0 | 0 | P = 1 | |
| Morrow et al, 201773 | Renal dysfunction | 11 patients developed or progressed in their renal dysfunction | no patients progressed to renal dysfunction | NR |
| Pouncey et al, 2020103 | Acute kidney injury, bacteremia, minor bleed, major bleed, cardiac | Range: 0-13 events in each group | P > .05 | |
| Haemoglobinuria | 19% (n = 12) | 4% (n = 3) | P = .006 | |
| Shen et al, 2019104 | Gross hematuria | 100% 0% | NR | |
| Incidence of post operative acute kidney injuryc | 22.8% (n = 18) | 9.2% (n = 11) | P = .013 | |
| Tian et al, 2021105 | Systemic complications | 37.5% (n = 18) | 4% (n = 2) | P = .007 |
| Major complications | 0 | 0 | NS | |
| Minor bleeding | 2 | 6 | P = .157 | |
| Xu et al, 2021107 | Complication rates | 10.2% (n = 19) | 9.6% (n = 23) | P = 0.85 |
| Serious adverse events | 0 | 0 | NS | |
| Xu et al, 2020108 | Complications | A few incidences in both study groups of minor bleeding, puncture site bleeding, hemoglobinuria (recovered after 24 h), acute kidney injury, and infection. No reported major bleeding, gingival bleeding, or symptomatic pulmonary embolism | P > .05 | |
| Zhu et al, 2020109 | Bleeding | 0 | 18% (n = 6) | P = .024 |
| Hemoglobinuria | 19% (n = 6) No other complications (e.g., no pulmonary embolisms) |
0 | P = .011 | |
| Vacuum Aspiration (Indigo) | ||||
| No studies met our inclusion criteria for this MT device | ||||
| Rotational (Rotarex or Cleaner) | ||||
| Ezelsoy et al, 201595 | Major complications | 0 | 0 | NS |
| Ultrasound Assisted (EKOS) | ||||
| Baker et al, 201294 | Major bleeding | 7.8% (n = 5) | 10.5% (n = 2) | P = .709 |
| Minor bleeding | 4.7% (n = 3) | 5.3% (n = 1) | P = .918 | |
| Lu et al, 201775 | Post operative complications (bleeding, intracranial bleeding, hypotension) | 7 | 1 | NS |
| Tichelaar et al, 2016106 | Bleeding, minor or major | 24% (n = 15) | 39% (n = 13) | NS |
Abbreviations: MT, mechanical thrombectomy; NR, not reported; NS, not significant; PMT, pharmacomechanical thrombectomy.
A diverse events were not discernible by intervention type.
Reported to occur in a patient with MT run-time beyond what was recommended manufacturer.
Other metrics of kidney health and function reported, with all findings aligned with this outcome.
Observational Studies
Morrow et al73 reported on stroke, chronic limb swelling, and ischemia but only at the level of detail to support a comparison of rates among patients who had kidney injury versus those who did not. They did not provide the level of detail necessary to assess the effectiveness of the interventions of interest.
Xu et al 107 reported that, among those who received MT, metrics of red blood cell, hemoglobin, serum creatinine, and erythrocyte (P < .05) were statistically significantly reduced. However, there were no statistically different findings for blood urea nitrogen or serum potassium 48 hours after the procedure compared to baseline values.
MEASURES OF HEALTH CARE UTILIZATION
Reported measures of health care utilization included volume and duration of thrombolytic infusion and hospital length of stay. Duration of infusion was considered by clinical experts to be a determinant for time in the ICU.
Volume of Thrombotic Infusion
Volume of thrombolytic infusion is reported in milligrams and is summarized in Table 29 for RCTs. Our GRADE certainty in the evidence was Moderate, with downgrading for risk of bias (see Appendix 2, Table A5). Table 30 and Figure 17 summarize the findings of the observational studies. Our GRADE certainty in the evidence was Very low, with downgrading for risk of bias and inconsistency (see Appendix 2, Table A5).
Table 29:
Volume of Thrombolytic Infusion With Mechanical Thrombectomy Use in Acute and Subacute DVT in Randomized Controlled Trials
| Results | ||||
|---|---|---|---|---|
| Author, year | Outcome measurement | MT | Comparator | P value |
| PMT (AngioJet) | ||||
| ATTRACT trial90 (PMT vs. infusion first rtPA group) |
Median rtPA total dose | 21 mg (IQR: 12-28) | 21 mg (IQR: 18-26) | NS |
| Vacuum Aspiration (Indigo) | ||||
| No randomized studies met our inclusion criteria for this MT device | ||||
| Rotational (Rotarex or Cleaner) | ||||
| No randomized controlled trials met our inclusion criteria for this MT device | ||||
| Ultrasound Assisted (EKOS) | ||||
| We did not identify any randomized studies that reported on this outcome of interest | ||||
Abbreviations: ATTRACT, Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis; DVT, deep vein thrombosis; IQR, interquartile range; MT, mechanical thrombectomy; NS, not significant; PMT, pharmacomechanical thrombectomy.
Table 30:
Volume of Thrombolytic Infusion With Mechanical Thrombectomy Use in Acute and Subacute DVT in Observational Studies
| Results | ||||
|---|---|---|---|---|
| Author, year | Outcome measurementa | MT | Comparator | P value |
| PMT (AngioJet) | ||||
| Huang et al, 202197 | Mean urokinase dosing postoperatively | 5.22 (SD ± 3.49) | 24.97 (SD ± 4.60) | P = .000 |
| Kuo et al, 201798 | Mean urokinase dose | 179.33 (SD ± 23.1) | 276.35 (SD ± 67.8) | P < .001 |
| Lee et al, 202099 | Mean total urokinase | 1.32 (SD ± 0.75) | 2.03 (SD ± 0.96) | P = .014 |
| Li et al, 2020100 | Mean total urokinase | 0.71 (SD ± 0.12) | 0.69 (SD ± 0.15) | P = .412 |
| Pouncey et al, 2020103 | Lysis volume used | 43.0 mg | 57.5 mg | P = .011 |
| Tian et al, 2021105 | Urokinase dose | 0.17 (SD ± 0.05) | 1.08 (SD ± 0.40) | P < .0001 |
| Xu et al, 2021107 | Urokinase dose | 95.16 (SD ± 45.89) | 293.76 (SD ±42.71) | P = .0 |
| Xu et al, 2020108 | Among those with Grade IIIb Dose of thrombolytic |
135 (SD ± 29.8) | 178.9 (SD ± 44.6) | P = .048 |
| Zhu et al, 2020109 | Urokinase amount | 0.26 (SD ± 0.14) | 1.87 (SD ± 0.53) | P = .0 |
| Vacuum Aspiration (Indigo) | ||||
| No studies met our inclusion criteria for this MT device | ||||
| Rotational (Rotarex or Cleaner) | ||||
| We did not identify any studies that reported on this outcome of interest | ||||
| Ultrasound Assisted (EKOS) | ||||
| Baker et al, 201294 | Mean total urokinase dosec | 1.7 (IQR: 1.4-2.4) | 2.1 (IQR: 1.6-2.7) | P = .10 |
| Tichelaar et al, 2016106 | Proportion of patients who had reduced thrombolytic doses compared to rtPA standard dosing | 27% (n = 9) | 21% (n = 13) | P = .49 |
Abbreviations: DVT, deep vein thrombosis; IQR, interquartile range; MT, mechanical thrombectomy; PMT, pharmacomechanical thrombectomy; SD, standard deviation.
The urokinase dose unit of measurement was inconsistent across the studies; however, this did not inhibit our analysis as the findings of interest are relative dose differences between study groups within any individual study.
Findings were similar for those with Grade II, but not significantly different among those patients with Grade I.
Findings were similar for rtPA and Tenecteplase use.
Figure 17: Volume of Thrombolytic With Mechanical Thrombectomy Use in Acute and Subacute DVT, by Mechanical Thrombectomy Device Compared to Control Groups in Published Observational Studies.
Abbreviations: CI, confidence interval; IV, inverse variance; MT, mechanical thrombectomy; PMT, pharmacomechanical thrombectomy; SD, standard deviation.
The ATTRACT trial provided details for us to evaluate patients who received AngioJet compared to those who received rtPA only for this outcome90; however, patients were not randomized into these subgroups, which led to a downgrade in our GRADE certainty.
Ezelsoy et al95 reported the mean duration of the procedure, but as a combined metric. Findings were not discernible by individual study groups.
Morrow et al73 reported total tPA dose and duration of thrombolysis, but only at the level of detail to support a comparison of rates among patients who had kidney injury versus those who did not. They did not provide the level of detail necessary to assess the effectiveness of the interventions of interest.73 Shen et al104 reported searching for this data, but it was not available or was incomplete for most patients in the administrative data sources.
Duration of Thrombotic Infusion
Duration of thrombolytic infusion is considered representative of time in the ICU and findings are summarized in Table 31 for the findings from the RCTs. Our GRADE certainty in the evidence was Moderate, with downgrading for risk of bias (see Appendix 2, Table A5). Table 32 and Figure 18 summarize the findings of the observational studies. Our GRADE certainty in the evidence was Very low, with downgrading for risk of bias and inconsistency (see Appendix 2, Table A5).
Table 31:
Duration of Thrombolytic Infusion With Mechanical Thrombectomy Use in Acute and Subacute DVT in Randomized Controlled Trials
| Author, year | Outcome measurement | Results | P value | |
|---|---|---|---|---|
| MT | Comparator | |||
| PMT (AngioJet) | ||||
| ATTRACT trial90 (PMT vs. infusion first rtPA group) |
rtPA duration | AngioJet group: 20 h (SD ± 5.3) | Infusion first group, rtPA only: 22 h (SD ± 6.5) |
P = .009 |
| Proportion with procedural length < 4h | 45% (n = 34) | 0% (n = 194) | NR | |
| Vacuum Aspiration (Indigo) | ||||
| No randomized studies met our inclusion criteria for this MT device | ||||
| Rotational (Rotarex or Cleaner) | ||||
| No randomized controlled trials met our inclusion criteria for this MT device | ||||
| Ultrasound Assisted (EKOS) | ||||
| No randomized studies met our inclusion criteria for this MT device | ||||
Abbreviations: ATTRACT, Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis; MT, mechanical thrombectomy; NR, not reported; PMT, pharmacomechanical thrombectomy; SD, standard deviation.
Table 32:
Duration of Thrombolytic Infusion With Mechanical Thrombectomy Use in Acute and Subacute DVT in Observational Studies
| Results | ||||
|---|---|---|---|---|
| Author, year | Outcome measurementa | MT | Comparator | P value |
| PMT (AngioJet) | ||||
| Garcia et al, 201585 | Mean procedure time | PMT: 2 h PMT + CDT: 22 h | RT alone: 1.4 h RT + CDT: 41 h | P < .0001 |
| Procedures completed in less than 24 h | 73% | NR | NA | |
| Average run time with AngioJet | 7.2 min (SD ± 6.3) | NA | NA | |
| Huang et al, 202197 | Mean time of thrombolytic | 1.42 d (SD ± 0.32) | 4.20 d (SD ± 1.25) | P = .000 |
| Lee et al, 202099 | Duration of thrombolysis | 27.1 h (SD ± 16.5) | 35.3 (SD ± 18.2) | P = .018 |
| Li et al, 2020100 | Operation procedure time | 101.6 min (SD ± 47.2) | 121.3 min (SD ± 17.6) | P = .002 |
| Pouncey et al, 2020103 | Lysis duration | PCDT: 41.5 h (95% CI: 25-47) AngioJet PowerPulse: 24.5 h (95% CI: 20-29) | 48.0 h (95% CI: 47-61) | P < .001 |
| Tian et al, 2021105 | Mean treatment duration | 0.97 h (SD ± 0.20) | 32.48 h (SD ± 7.46) | P < .0001 |
| Xu et al, 2020108 | Among those with Grade III,b time of treatment | 2.2 d (SD ± 5.8) | 3.1 d (SD ± 0.8) | P = .01 |
| Zhu et al, 2020109 | Time of thrombolysis | 4.2 h (SD ± 1.7) | 73.6 h (SD ± 18.3) | P = .0 |
| Vacuum Aspiration (Indigo) | ||||
| No studies met our inclusion criteria for this MT device | ||||
| Rotational (Rotarex or Cleaner) | ||||
| We did not identify any observational studies that reported on this outcome of interest | ||||
| Ultrasound Assisted (EKOS) | ||||
| Baker et al, 201294 | Mean overall infusion time | 27 h (IQR: 21 − 27) | 25 h (IQR: 22 − 39) | P = .39 |
| Lu et al, 201775 | Mean lysis time | 21 h (SD ± 1.7) | 24 (SD ± 1.8) | P = .26 |
| Tichelaar et al, 2016106 | Proportion who had a duration of intervention < 48 hc | 27% (n = 9) | 10% (n = 6) | P < .005 |
Abbreviations: CI, confidence interval; DVT, deep vein thrombosis; IQR, interquartile range; MT, mechanical thrombectomy; NA, not applicable; NR, not reported; PCDT, pharmacomechanical catheter-directed thrombolysis; PMT, pharmacomechanical thrombectomy; RT, rheolytic thrombectomy; SD, standard deviation.
Urokinase dose unit of measurement was inconsistent across the studies; however, this did not inhibit our analysis as the findings of interest are relative dose differences between study groups within any individual study.
Findings were similar for those with Grade II, but not significantly differently among those patients with Grade I.
Other timeframes up to 120 hours were not significantly different between study groups.
Figure 18: Time, In Hours, With Mechanical Thrombectomy Use in Acute and Subacute DVT by Mechanical Thrombectomy Device Compared to Control Groups in Published Observational Studies.
Abbreviations: CI, confidence interval; DVT, deep vein thrombosis; IV, inverse variance; MT, mechanical thrombectomy; PMT, pharmacomechanical thrombectomy; SD, standard deviation.
Engelberger et al2 reported that both study groups used a protocol of administering rtPA over 15 hours.
The ATTRACT trial provided sufficient detail to evaluate people who received AngioJet specifically compared to those who received rtPA only for this outcome.90 Note, however, that patients were not randomized into these subgroups, which led to a downgrade in our GRADE assessment.
Ezelsoy et al95 reported the mean duration of the procedure, but as a combined metric. Findings were not discernible by individual study groups.
Morrow et al73 reported total tPA dose and duration of thrombolysis, but only at the level of detail to support a comparison of rates among patients who had kidney injury versus those who did not. They did not provide the level of detail necessary to assess the effectiveness of the interventions of interest.
Shen et al104 reported searching for this data, but it was not available or was incomplete for most patients in the administrative data sources.
We also conducted a sensitivity analysis calculating the standardized mean difference using the original data as reported in the studies and demonstrated that the findings were consistent.
Hospital Length of Stay and Other Health Care Utilization
Hospital length of stay includes time outside the ICU and may include time for recovery from the procedure or from adverse events such as renal dysfunction. Table 33 summarizes the findings from the RCTs. Our GRADE certainty in the evidence was Moderate, with downgrading for risk of bias (see Appendix 2, Table A5). Table 34 and Figure 19 summarize the findings of the observational studies. Our GRADE certainty in the evidence was Very low, with downgrading for risk of bias, inconsistency, and imprecision (Appendix 2, Table A5).
Table 33:
Hospital Length of Stay and Other Health Care Utilization After Mechanical Thrombectomy Use in Acute and Subacute DVT in Randomized Controlled Trials
| Results | ||||
|---|---|---|---|---|
| Author, year | Outcome measurement | MT | Comparator | P value |
| PMT (AngioJet) | ||||
| We did not identify any randomized studies that reported on this outcome of interest | ||||
| Vacuum Aspiration (Indigo) | ||||
| No randomized studies met our inclusion criteria for this MT device | ||||
| Rotational (Rotarex or Cleaner) | ||||
| No randomized controlled trials met our inclusion criteria for this MT device | ||||
| Ultrasound Assisted (EKOS) | ||||
| Engelberger et al, 20152 | Mean hospital duration | 2.7 d (SD ± 1.4) | 2.8 d (SD ± 1.3) | P = .83 |
Abbreviations: DVT, deep vein thrombosis; MT, mechanical thrombectomy; PMT, pharmacomechanical thrombectomy; SD, standard deviation.
Table 34:
Hospital Length of Stay and Other Health Care Utilization After Mechanical Thrombectomy Use in Acute and Subacute DVT in Observational Studies
| Results | ||||
|---|---|---|---|---|
| Author, year | Outcome measurement | MT | Comparator | P value |
| PMT (AngioJet) | ||||
| Kuo et al, 201798 | Mean hospital length of stay | 9.9 d (SD ± 5.0) | 10.9 d (SD ± 7.5) | P = .579 |
| Li et al, 2020100 | Mean length of stay | 9.2 d (SD ± 2.3) | 9.6 d (SD ± 2.1) | P = .310 |
| Pouncey et al, 2020103 | Mean hospital length of stay | 5 d | 5 d | NS |
| Tian et al, 2021105 | Mean hospitalization length of stay | 5.18 d (SD ± 1.37) | 9.43 d (SD ± 2.84) | P < .0001 |
| Xu et al, 2021107 | Mean length of stay | 8.16 d (SD ± 3.89) | 12.56 d (SD ± 1.58) | P = .01 |
| Vacuum Aspiration (Indigo) | ||||
| No studies met our inclusion criteria for this MT device | ||||
| Rotational (Rotarex or Cleaner) | ||||
| We did not identify any studies that reported on this outcome of interest | ||||
| Ultrasound Assisted (EKOS) | ||||
| Tichelaar et al, 2016106 | Hospitalization time | 6.0 d (IQR: 5-9) | 8.0 d (IQR: 5.8-12) | P < .005 |
Abbreviations: DVT, deep vein thrombosis; IQR, interquartile range; MT, mechanical thrombectomy; NS, not significant; PMT, pharmacomechanical thrombectomy; SD, standard deviation.
Figure 19: Hospitalization Length of Stay, in Days, With Mechanical Thrombectomy Use in Acute and Subacute DVT by Mechanical Thrombectomy Device Compared to Control Groups in Published Observational Studies.
Abbreviations: CI, confidence interval; IV, inverse variance; MT, mechanical thrombectomy; PMT, pharmacomechanical thrombectomy; SD, standard deviation.
Morrow et al73 reported hospital length of stay, but only at the level of detail to support a comparison of rates among patients who had kidney injury versus those who did not. They did not provide the level of detail necessary to assess the effectiveness of the interventions of interest.
SUMMARY OF FINDINGS FOR ACUTE DVT
Our summary of findings is based on pooled estimates, where conducted, and otherwise based on narrative findings as reported in data tables. There were 3 RCTs and 26 observational studies.
Table 35:
Summary of Findings of the Effect of Mechanical Thrombectomy in Acute DVT
| Outcome | No. of participants (studies) | Effect | GRADEa | |
|---|---|---|---|---|
| Relative (95% CI) | Absolute (95% CI) | |||
| Limb salvage | 151 (1 Obs) | OR: 2.63 (0.11-65.53) | 8 fewer per 1,000 (90 fewer to 12 more) | ⊕ Very low |
| Post- thrombotic syndrome | 386 (2 RCTs) 1,113 (8 Obs) | OR: 1.14 (0.70-1.86) OR: 0.37 (0.26-0.52) | 30 more per 1,000 (75 fewer to 149 more) 129 fewer per 1,000 (156 fewer to 95 fewer) | ⊕⊕⊕ Moderate ⊕ Very low |
| Technical success, complete thrombus removal | 478 (2 RCTs) 1,898 (15 Obs) | RR: 1.03 (0.88-1.19) RR: 1.06 (0.93-1.20) | 21 more per 1,000 (85 fewer to 135 more) 36 more per 1,000 (27 fewer to 105 more) | ⊕ Very low ⊕ Very low |
| Patency | 48 (1 RCT) | RR: 1.04 (0.93-1.17) | 38 more per 1,000 (67 fewer to 163 more) | ⊕⊕ Low |
| 683 (9 Obs) | RR: 1.10 (0.99-1.21) | 61 more per 1,000 (6 fewer to 127 more) | ⊕ Very low | |
| Re-thrombosis (and revision rates) | 232 (2 RCTs) 324 (5 Obs) | Low event rates and heterogeneity of the outcomes reported precluded pooling of findings. Overall, there were no significant findings between study groups on any metric reported | ⊕⊕ Low ⊕ Very low | |
| Pain | We did not identify any studies that reported on this outcome of interest | |||
| Quality of life | 662 (3 RCTs) | — | MD: 0.74 higher (1.67 lower to 3.14 higher) | ⊕⊕ Low |
| 95 (1 Obs) | — | MD: 1.00 higher (1.69 lower to 3.69 higher) | ⊕ Very low | |
| Perioperative mortality | 614 (2 RCTs) | OR: 1.31 (0.22-7.85) | 3 more per 1,000 (7 fewer to 57 more) | ⊕ Very Low |
| 927 (8 Obs) | There were no significant differences in mortality, with most studies reporting 0 in both study arms | ⊕ Very Low | ||
| Adverse events | 589 (3 RCTs) | There were no significant differences reported in the rates of adverse events across the different MT interventions compared to control groups | ⊕⊕⊕ Moderate | |
| (17 Obs) | There were inconsistent findings for other adverse events between study groups | ⊕ Very low | ||
| 269 (1 RCT) | — | MD: 0 (same mean volume reported in both study groups) | ⊕⊕⊕ Moderate | |
| Volume of thrombolytics (mg) | 1,226 (10 Obs) | — | SMD: 2.1 lower (3.32 lower to 0.87 lower) | ⊕ Very low |
| Time of thrombolytic infusion (hours) | 269 (1 RCT) | — | MD: 2.0 lower (3.51 lower to 0.49 lower) | ⊕⊕⊕ Moderate |
| 1,395 (10 Obs) | — | MD: 22.38 lower (29.85 lower to 14.91 lower) | ⊕ Very low | |
| Hospital length of stay (days) | 48 (1 RCT) | — | MD: 0.10 lower (0.86 lower to 0.66 higher) | ⊕⊕⊕ Moderate |
| 454 (5 Obs) | — | MD: 2.49 lower (4.44 lower to 0.53 lower) | ⊕ Very low | |
Abbreviations: CI, confidence interval; DVT, deep vein thrombosis; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; MD, mean difference; MT, mechanical thrombectomy OR, odds ratio; Obs, observational study; RCT, randomized controlled trial; RR, relative risk; SMD, standardized mean difference.
Note: summary of findings table developed using GRADEpro GDT. GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime, 2022. Available from gradepro.org
We evaluated the quality of the body of evidence for each outcome according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Handbook.70 See Appendix 2 for further details.
SUMMARY OF FINDINGS, BY TYPE OF MECHANICAL THROMBECTOMY DEVICE
There are several types of MT device; however, each hospital is likely to have only one type available for use. Tables 36-38 summarize the subgroup analyses we conducted for each MT device. They include our findings for our two populations of interest—arterial acute limb ischemia and acute DVT.
Table 37:
Summary of Findings of Effect of Rotational Mechanical Thrombectomy Device in Acute Lower Limb Ischemia (Arterial or Venous)
| Outcome | No. of participants (studies) | Effect | GRADEa | |
|---|---|---|---|---|
| Relative (95% CI) | Absolute (95% CI) | |||
| Arterial Acute Limb Ischemia | ||||
| Limb salvage | 109 (2 Obs) | OR: 0.88 (0.17-4.57) | 10 fewer per 1,000 (from 260 fewer to 62 more) | ⊕ Very low |
| Complete thrombus removal | 34 (1 Obs) | OR: 3.46 (0.61-19.72) | 242 more per 1,000 (123 fewer to 375 more) | ⊕ Very low |
| Re-thrombosis (and revision rates) | 109 (2 Obs) | OR: 0.92 (0.39-2.18) | 20 fewer per 1,000 (204 fewer to 192 more) | ⊕ Very low |
| Perioperative mortality | 75 (1 Obs) | Not estimable | 0 fewer per 1,000 (0 fewer to 0 fewer) | ⊕ Very low |
| Adverse events | 311 (3 Obs) | Fewer bleeding events among those who received MT. Otherwise no significant difference reported between study groups for any other adverse outcome | ⊕ Very low | |
| Volume of thrombolytic (mg; Rotational) | 34 (1 Obs) | People who received MT had lower volumes of thrombolytic compared to control groups | ⊕ Very low | |
| Hospital length of stay (days after PMT) | 202 (1 Obs) | — | MD: 0.2 lower (1.34 lower to 0.94 higher) | ⊕ Very low |
| Acute Deep Vein Thrombosis | ||||
| Post thrombotic syndrome | 50 (1 Obs) | OR: 0.31 (0.09-0.99) | 277 fewer per 1,000 (457 fewer to 2 fewer) | ⊕ Very low |
| Perioperative mortality | 50 (1 Obs) | There were no significant differences in mortality reported, with 0 in both study arms | ⊕ Very low | |
| Adverse events | 50 (1 Obs) | There were no cases of adverse events reported in either study group | ⊕ Very low | |
Abbreviations: CI, confidence interval; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; MD, mean difference; MT, mechanical thrombectomy; OR, odds ratio; Obs, observational study; PMT, pharmacomechanical thrombectomy.
Note: summary of findings table developed using GRADEpro GDT. GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime, 2022. Available from gradepro.org
We evaluated the quality of the body of evidence for each outcome according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Handbook.70 See Appendix 2 for further details.
Table 38:
Summary of Findings of Effect of Ultrasound-assisted Mechanical Thrombectomy Device in Acute Lower Limb Ischemia (Arterial or Venous)
| Outcome | No. of participants (studies) | Effect | GRADEa | |
|---|---|---|---|---|
| Relative (95% CI) | Absolute (95% CI) | |||
| Arterial Acute Limb Ischemia | ||||
| Limb salvage | 193 (2 Obs) | OR: 1.51 (0.37-6.12) | 33 more per 1,000 (from 135 fewer to 86 more) | ⊕ Very low |
| Complete thrombus removal | 193 (2 Obs) | OR: 1.70 (0.80-3.61) | 13 fewer per 1,000 (47 fewer to 183 more) | ⊕ Very low |
| Patency | 68 (1 Obs) | OR: 1.75 (0.50-6.17) | 116 more per 1,000 (169 fewer to 275 more) | ⊕ Very low |
| Re-thrombosis (and revision rates) | 102 (1 Obs) | OR: 0.57 (0.23-1.42) | 126 fewer per 1,000 (271 fewer to 87 more) | ⊕ Very low |
| Perioperative mortality | 193 (2 Obs) | OR: 1.05 (0.10-10.62) | 2 more per 1,000 (28 fewer to 224 more) | ⊕ Very low |
| Adverse events | 193 (2 Obs) | Fewer bleeding events among those who received MT, otherwise no significant difference reported between study groups for any other adverse outcome | ⊕ Very low | |
| Volume of thrombolytic (mg) | 193 (2 Obs) | People who received MT had no difference in volume of thrombolytic compared to control groups | ⊕ Very low | |
| Time of thrombolytic infusion (hour) | 193 (2 Obs) | — | MD: 0.67 higher (3.04 lower to 4.39 higher) | ⊕ Very low |
| Acute Deep Vein Thrombosis | ||||
| Post-thrombotic syndrome | 120 (1 RCT) | OR: 1.28 (0.42-3.95) | 25 more per 1,000 (57 fewer to 210 more) | ⊕⊕⊕ Moderate |
| 70 (1 Obs) | OR: 0.47 (0.05-4.33) | 50 fewer per 1,000 (94 fewer to 225 more) | ⊕ Very low | |
| Complete thrombus removal | 48 (1 RCT) | RR: 0.93 (0.59-1.48) | 44 fewer per 1,000 (256 fewer to 300 more) | ⊕⊕⊕ Moderate |
| 254 (3 Obs) | RR: 1.03 (0.52-2.05) | 19 more per 1,000 (299 fewer to 654 more) | ⊕ Very low | |
| Patency | 48 (1 RCT) | RR: 1.04 (0.93-1.17) | 38 more per 1,000 (67 fewer to 163 more) | ⊕⊕ Low |
| 144 (2 Obs) | RR: 1.26 (0.86-1.85) | 126 more per 1,000 (68 fewer to 413 more) | ⊕ Very low | |
| Re-thrombosis (and revision rates) | 232 (2 RCTs) | There were no significant findings between study groups for recurrent DVT without stent, in-stent thrombosis, or ulceration reported | ⊕⊕ Low | |
| 159 (2 Obs) | There were no significant findings between study groups for recurrence rates or additional MT procedures | ⊕ Very low | ||
| Quality of life | 232 (2 RCTs) | — | MD: 0.23 higher (2.34 lower to 2.8 higher) | ⊕⊕ Low |
| 95 (1 Obs) | — | MD: 1.00 higher (1.69 lower to 3.69 higher) | ⊕ Very low | |
| Perioperative Mortality | 184 (1 RCT) | OR: 1.02 (0.06-16.59) | 0 fewer per 1,000 (10 fewer to 142 more) | ⊕ Very low |
| 254 (3 Obs) | There were no significant findings between study groups for mortality or time, in months, of event free survival | ⊕ Very low | ||
| Adverse events | 159 (2 RCTs) | There were no significant differences in rates of bleeding, pulmonary embolism, or hematoma between those who received MT and those who did not | ⊕⊕⊕ Moderate | |
| 412 (3 Obs) | There were no statistically significant differences in the rates of bleeding between study groups | ⊕ Very low | ||
| Volume of thrombolytic (mg) | 56 (1 Obs) | — | SMD: 0.51 lower (1.13 lower to 0.1 higher) | ⊕ Very low |
| Time of thrombolytic infusion (hour) | 159 (2 Obs) | — | MD: 1.02 lower (5.81 lower to 3.78 higher) | ⊕ Very low |
| Hospital length of stay, ultrasound assisted | 48 (1 RCT) | — | MD: 0.10 lower (0.86 lower to 0.66 higher) | ⊕⊕⊕ Moderate |
| 95 (1 Obs) | — | MD: 2 lower (3.36 lower to 0.64 lower) | ⊕ Very low | |
Abbreviations: CI, confidence interval; DVT, deep vein thrombosis; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; MD, mean difference; MT, mechanical thrombectomy; OR, odds ratio; Obs, observational study; RR, relative risk; SMD, standardized mean difference.
Note: summary of findings table developed using GRADEpro GDT. GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime, 2022. Available from gradepro.org
We evaluated the quality of the body of evidence for each outcome according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Handbook.70 See Appendix 2 for further details.
Ongoing Studies
We are aware of the following ongoing studies and protocols that have potential relevance and may impact the findings of this review:
The Prism Trial: a retrospective case review of technical success using the penumbra and Indigo systems for mechanical thrombectomy in the periphery. https://clinicaltrials.gov/ct2/show/NCT02085551
Chronic venous thrombosis: relief with adjunctive catheter-directed therapy (The C-TRACT Trial) (C-TRACT). https://clinicaltrials.gov/ct2/show/NCT03250247
ClotTriever Outcomes (CLOUT) Register. https://clinicaltrials.gov/ct2/show/NCT03575364
Ebben HP, Nederhoed JH, Lely RJ, Wisselink W, Yeung K, Must C. Microbubbles and UltraSound-accelerated Thrombolysis (MUST) for peripheral arterial occlusions: protocol for a phase II single arm trial. BMJ Open. 20177(8):e014365.
de Donato G, Pasqui E, Giannace G, Setacci F, Benevento D, Palasciano G, et al. The Indigo system in acute lower-limb malperfusion (INDIAN) registry: protocol. JMIR Research Protocols. 2019;8(3):e9972
Discussion
We included two distinct populations in this review—arterial and venous. For the arterial acute limb population, we found 0 RCTs and 12 observational studies that met our inclusion criteria. For the acute deep vein thrombosis population, we included a total of 3 RCTs and 18 observational studies from 29 publications. While RCTs are generally considered a better methodological design with greater certainty to evaluate effectiveness, our inclusion of observational studies enables a more comprehensive review of the evidence and can provide a better understanding of real-world applicability. The most common comparator group in the included studies was CDT; however, due to the retrospective observational study design of the included studies, which looked at medical charts, they do not necessarily reflect the fact that patients who went on to receive MT would have been equally eligible for CDT. Additionally, this may not reflect the current standard of care in Ontario, especially for patients experiencing an arterial acute limb ischemia, who may receive open surgery, and those with DVT, who are commonly managed with oral anticoagulation alone. The included studies were also inconsistent in their inclusion criteria for the location of blockage. Some studies included any lower limb ischemia, while others were limited to people with an iliofemoral obstruction.
Arterial Acute Limb Ischemia
Overall, the studies of the arterial population found that there was no difference between MT compared to control groups for outcomes of limb salvage, patency, or re-thrombosis. There was significantly more technical success among patients who received MT compared to the control groups, but the evidence is uncertain. There were no statistically significant findings on the impact of MT on the use of thrombolytics (volume and time). Regarding outcomes related to safety, there was no difference in mortality rates and there were inconsistent findings for adverse events. In terms of health care utilization, people who received MT compared to the control groups experienced a reduced hospital length of stay of approximately 1 day. These findings were largely driven by Gong et al,1 who reported mean stays between 5 and 6 days overall. For all MT devices, the certainty in the evidence was Very low for all outcomes.
BY MECHANICAL THROMBECTOMY TYPE
Subgroup analysis by MT type, presented in Tables 36-38, found differences by MT device, but the evidence is uncertain. The evidence suggests that, for some of the MT devices, there was more renal dysfunction and acute kidney injury, as well as hematoma, distal embolization, and mean blood loss among patients who received MT compared to control groups. There was also a reduced volume of thrombolytics used (approximately 15 mg) among patients who received MT compared to control groups.
For rotational MT devices, there were nonsignificant findings about the impact of complete thrombus removal and hospitalization, which differs from the overall findings where significant results were observed. There were no deaths reported in either study group and there was a reduced rate of bleeding events among people who received rotational MT compared to control groups. For the ultrasound-assisted group, there were no significant findings on complete thrombus removal. Otherwise, subgroup findings by type of MT device were aligned to the overall results reported.
Acute Deep Vein Thrombosis
Overall, the studies in acute deep vein thrombosis reported no difference between MT compared to control groups for limb salvage/amputation rates, technical success, patency, re-thrombosis rates, or quality of life. There were no significant findings in post-thrombotic syndrome rates among the randomized controlled trials. The observational studies reported a reduction, but the evidence is uncertain. There was also a reduced time of thrombolytics use, which is a determinant for time in the ICU (approximately 2 hours, with a greater proportion of patients having procedural times < 4 hours), but these findings may not be clinically meaningfully different. Our clinical experts advised that patients are commonly checked on every 6-24 hours (email communication, Dheeraj Rajan, May 2022), with 12 hours typically representing one nursing shift (email communication, Charles de Mestral, May 2022). The observational studies found a reduction of about 22 hours for time in the ICU; however, the evidence is uncertain according to our GRADE assessment. Clinical experts advised the observational studies aligned with their clinical experiences in Ontario (email communication, Jeff Jaskolka, May 2022). There was no observed difference in volume of thrombolytics used in the randomized studies, but there was a reduction of 2 mg in the observational studies. This evidence is uncertain. No difference in mortality rates or adverse events were observed in the RCTs; however, findings were inconsistent in the observational studies. Any potential effects did not reflect differences in overall hospitalization stays in the randomized trials. There was a reduction of approximately 2.5 days, but the evidence is uncertain.
BY MECHANICAL THROMBECTOMY TYPE
Subgroup analysis by MT type, presented in Tables 36-38, found that, for the PMT devices, there was a significant reduction in complete thrombus removal (compared to no significant difference overall). There were higher rates of renal dysfunction in people who received PMT in the observational studies, but the evidence is uncertain. For rotational MT devices, there were fewer findings reported compared to the other devices and significantly fewer people with post-thrombotic syndrome, but the evidence was very uncertain. There were no deaths or adverse events reported in either study arm. Finally, there were no significant findings for the rates of adverse events for ultrasound-assisted MT devices, nor for volume or time of thrombolytic infusion. There was also no significant difference in hospital length of stay for the randomized controlled trial evidence; it was lower in the observation study by 2 days, but the evidence is uncertain. Otherwise, subgroup findings were aligned to the overall results reported.
Strengths and Limitations
The overall body of evidence had several limitations, and the certainty was evaluated to be low. Our focus on comparable studies resulted in the exclusion of numerous single arm studies and case reports. As a result, we did not find any studies that met the inclusion criteria for several known commercially available devices, including the Indigo, which, according to our clinical experts, is increasing in use in Ontario (email communication, Dheeraj Rajan, May 2022). There are published studies, but as they are not comparative in design, we were unable to discern the isolated impact of MT. We consider them preliminary evidence that may support further studies. The strength of focusing on comparative studies is that the findings were sought from the highest quality evidence and thereby provide the greatest certainty in the observed effect estimates.
There were no randomized trials identified in the arterial population. This is typically an urgent patient population; study design and recruitment would be incredibly challenging. However, that does not preclude the inherent bias that occurs with the observational study design approach. For example, we cannot say whether the observed reduction in hospital length of stay is due to MT being more effective, or if it is an effect of bias from clinicians choosing a longer observation time for patients in the control groups.
There were randomized controlled studies in the acute venous occlusion population, but these studies have limitations. The ATTRACT trial is the most widely cited controlled trial. It was large, randomized, and the first to evaluate endovascular interventions compared to anticoagulation alone. However, the study design did not isolate for the effect of MT as their intervention group included three options to choose from, and so some patients received infusion first as catheter-directed thrombolysis. Based on how the findings are reported, it is not possible to ascertain if MT or endovascular thrombolytic infusion was the cause of the observed decrease in post-thrombotic syndrome severity by 24 months among people with DVT in the iliofemoral segment, compared to oral anticoagulation alone.86 Additionally, the ATTRACT trial has been criticized for including femoropopliteal DVT. One international survey of clinicians found 60% agreeing the ATTRACT study was generally well done, but only 20% felt that, with the inclusion of femoral popliteal, it was a well designed and informative study. Survey responses supported the idea that a new trial limited to iliofemoral DVT with a 5-year follow up should be conducted.112 The CAVA trial also has limitations. Its findings for post-thrombotic syndrome were not significant when using the Villalta scale but were found to be significantly in favour of MT using the International Society on Thrombosis consensus definition. This disagreement among standards demonstrates a need for further investigation into establishing reliable tools for diagnosing and describing the disease states in this area for patients.113
Overall, the body of evidence in this area is complex and heterogenous. There are as many confounding variables as patient populations. The subjective nature of patient selection for MT device or other endovascular treatment was based on clinician preference, availability and access, and possibly even comfort and experience. Some studies indicated that MT device use increased in more recent years compared to the earlier years of the study.74,78,103,106 The subjective nature of adjunctive therapy use (e.g., a stent), may have impacted observable longer-term outcomes. The heterogeneity of the body of evidence included in this review precluded our planned fulsome subgroup analyses evaluating the effectiveness by different occlusion locations or subpopulations such as pregnant individuals.
We surmised that the age and wellbeing of a patient may also impact outcomes as a younger population may have reduced risk of bleeding and a greater likelihood of favourable outcomes. However, the data from the RCTs did not allow for this type of subgroup analyses and we felt that observational studies were too susceptible to selection bias to produce reliable subgroup analyses. The heterogeneity in the interpretation of the body of evidence is further shown by the more than 20 other systematic reviews identified with very little overlap in their included studies lists. Our review required judgement about when it was appropriate to collate the evidence and when the heterogeneity was reasonable. Summary estimates presented in forest plots should be considered complimentary to the data reported in the tables, which are intended as a comprehensive review of the body of evidence and are considered an equally important presentation of the available data.
Our review makes judgements about heterogeneity, particularly for pooled meta-analyses, that may be subject to differences of opinion about the most appropriate manner of interpreting the evidence. Furthermore, our focus on comparative studies as a means of evaluating the evidence can offer interpretations of effectiveness. However, we recognize that there is a large body of single arm studies that may offer insights into the gaps of this review, such as the use of MT in select specific populations.
This review examined whether MT is safe and effective and also estimated its impact on health care resources. Further investigation is required to determine the population that would benefit most from its use. Some studies included an analysis to identify the factors most likely to predict long-term patency or technical success.76,84,114 However, while understanding the extent to which body mass index, diabetes, and other traits are independent factors of success and may be important for implementation and patient selection, this question was beyond the scope of our review. Arguably, none of the clinical trials in this area confirm that removing an obstruction from a vein improves patient outcomes over the longer term.113 There is still much to be learned about the effectiveness of MT, and further research will help determine the criteria for the most appropriate use of the technology.
Implementation Considerations
In Ontario, the patient population that would be eligible for MT is complex. Common comorbid conditions include pulmonary embolism, type 2 diabetes, kidney failure, atrial fibrillation, and recent surgical events (see Appendix 4 for details). Mechanical thrombectomy in the lower limbs is typically done in interventional radiology suites. The procedure may require the interventional radiologist to spend more time with patients than is usual for the alternative of catheter-directed thrombolysis (estimated to be less than 1 additional hour; email communication, Dheeraj Rajan and Jeff Jaskolka, May 2022). Any benefit from reduced ICU time due to decreased thrombolytic needs should be balanced against increased demands on interventional suite capacity and interventional radiologist time. Studies have proposed that delays in patient care in accessing MT led to longer lysis time and hospital stays.103 This possibility is supported by differences in acute compared to subacute findings in select studies101—an effect that has also been demonstrated in stroke patients.115,116 Current estimates are that specialized care unit stays in Ontario after open or endovascular revascularization procedures range from 7.7 to 40 hours.12 Proposed eligibility for MT includes situations where patients are considered to not have enough time for CDT to be effective before serious, irreversible effects of a blood clot take place (email communication, Dheeraj Rajan, May 2022). This would require an implementation plan that considers the requirement to have skilled teams available in hospital within the proposed window of benefit.
The techniques for removing clots from blood vessels is evolving and alternatives to MT may offer similar effectiveness, but they also have their own challenges for implementation. For example, manual aspiration, the creation of negative pressure suction through a catheter inserted at the site of a clot,117 may be considered a safe and effective technique,117 but it has also been called improvisation—a technique that is difficult to master and consistently apply (email communication, Dheeraj Rajan and Jeff Jaskolka, May 2022). Another alternative technique is the use of a Fogarty balloon to pull the clot out of a blood vessel. The Fogarty balloon requires an open surgical site to aid in the removal of the clot and is therefore typically applied by a vascular surgeon, not an interventional radiologist (email communication, Dheeraj Rajan, May 2022). One network meta-analysis reported that, among people with proximal and iliofemoral DVT, no treatment demonstrated superiority to another, including CDT and forms of MT.71 More research is needed to refine the population most likely to benefit, such as acute DVT in the iliofemoral regions only, and the mechanisms of delivery of MT.
With regard to MT devices, there is not an established accepted mechanism of action, and there are many different brands available that attack the problem with novel approaches. The most popular devices in Ontario are pharmacomechanical thrombectomy with saline to break up and wash away a clot. Another is vacuum aspiration to suck up and remove clot pieces. However, we identified several other mechanisms, such as rotational and ultrasound assisted devices, which are reimbursed in the United States and are becoming sufficiently widespread that their reimbursement levels were adjusted in 2021 to encourage greater use. 118 In addition to the cost considerations of these alternative technologies, Ontario clinical experts we spoke to expressed personal choice preferences among different mechanisms of action (email communication, Jeff Jaskolka and Dheeraj Rajan, May 2022). New devices are entering the Canadian market, most recently with the addition of the Inari ClotTriever, approved by Health Canada in November 2021. However, the identified published evidence is limited to preliminary findings of a registry and case reports.119,120 About a third of these patients were experiencing chronic DVT, which is beyond the scope of this review.120
Techniques employed with the existing MT devices are also evolving and some studies make efforts to demonstrate effectiveness in the absence of thrombolytics altogether.121,122 During the course of conducting this review, we found registries estimating that about half of the patients who received MT may not require CDT.85,123,124 New devices are being developed125,126 and indications of use are expanding, with growing interest in the use of MT for pulmonary embolism (email communication, Dheeraj Rajan and Jeff Jaskolka, May 2022).
As these devices become more prolific in-patient treatment of all types, the skills, comfort, and access for all patient types are expected to improve, including in the treatment of blocked arteries and veins in the lower limbs. The decision to use MT must not be done in isolation, but in consideration of the related clinical disciplines and an understanding of the overall management of a patient's experience.
Conclusions
Based on the clinical evidence identified (3 RCTs and 37 observational studies), MT for people experiencing a blockage (blood clot) in blood vessels of their lower extremities may demonstrate greater technical success and patency for patients experiencing arterial acute limb ischemia compared to alternatives such as CDT infusion, but the evidence is uncertain (GRADE: Very low). In acute DVT, MT may reduce thrombolytic medication volume used (GRADE: Very low) and the duration of thrombolytic infusion (a determinant of intensive care unit stay duration) compared to not using MT, but it is uncertain if use of MT leads to a meaningful reduction in transfusion time (GRADE: Moderate to Very low). It may also reduce the proportion of people who experience post-thrombotic syndrome (GRADE: Moderate to Very low). Additionally, MT may reduce hospital length of stay for both populations (GRADE: Very low).
Economic Evidence
Research Question
What is the cost-effectiveness of mechanical thrombectomy (MT) compared with usual care for people with arterial acute limb ischemia or acute deep vein thrombosis (DVT)?
Methods
Economic Literature Search
We performed an economic literature search on August 24, 2021, to retrieve studies published from January 1, 2010, until the search date. To retrieve relevant studies, we developed a search using the clinical search strategy with an economic and costing filter applied.
We created database auto-alerts in MEDLINE and Embase and monitored them for the duration of the assessment period. We also performed a targeted grey literature search of health technology assessment agency websites, systematic review registries, and the Tufts Cost-Effectiveness Analysis Registry. See Clinical Literature Search, above, for further details on methods used. See Appendix 1 for our literature search strategies, including all search terms.
Eligibility Criteria
STUDIES
Inclusion Criteria
English-language full-text publications
Studies published between January 2010 and August 2021
Cost-benefit analyses, cost-effectiveness analyses, cost analyses, or cost-utility analyses
Exclusion Criteria
Narrative reviews, letters/editorials, case reports, commentaries, abstracts, posters, unpublished studies
PARTICIPANTS/POPULATION
Inclusion Criteria
Adults (≥ 18 years old) experiencing an acute (≤ 14 days from symptom onset) or subacute (1428 days from symptom onset) blockage to the blood flow in the lower limbs due to a thrombus or embolus (i.e., a clot)
Exclusion Criteria
Chronic limb-threatening ischemia
Non-obstructive cause to blood flow blockage (e.g., trauma or iatrogenic injury)
Children (< 18 years old)
INTERVENTIONS
Inclusion Criteria
Mechanical thrombectomy device as the primary intervention to target the removal of a blockage in the blood flow in the lower limbs
With or without companion pharmaceutical intervention (pharmacomechanical)
All mechanisms of action (e.g., aspiration, rotational, retriever, ultrasound, ablation)
Exclusion Criteria
Devices targeting revascularization of a blood vessel, not specified to use MT
COMPARATORS
Inclusion Criteria
Surgical interventions (embolectomy/surgical bypass)
Pharmacological thrombolytic therapy as intravenous or catheter-directed treatment (CDT) alone
Anticoagulant therapy alone or with compression therapy
Non-mechanical aspiration techniques (e.g., manual aspiration)
Exclusion Criteria
Studies that compare two different MT technologies or devices
OUTCOME MEASURES
Costs
Health outcomes (e.g., quality-adjusted life-years)
Incremental costs
Incremental effectiveness
Incremental cost-effectiveness ratios (ICERs)
Literature Screening
A single reviewer conducted an initial screening of titles and abstracts and then obtained the full texts of studies that appeared eligible for review according to the inclusion criteria. The same reviewer then examined the full-text articles and selected studies eligible for inclusion.
Data Extraction
We extracted relevant data on study characteristics and outcomes to collect information about the following:
Source (e.g., citation information, study type)
Methods (e.g., study design, analytic technique, perspective, time horizon, population, intervention[s], comparator[s])
Outcomes (e.g., health outcomes, costs, incremental cost-effectiveness ratios)
Study Applicability and Limitations
We determined the usefulness of each identified study for decision-making by applying a modified quality appraisal checklist for economic evaluations originally developed by the National Institute for Health and Care Excellence (NICE) in the United Kingdom to inform the development of NICE's clinical guidelines.127 We modified the wording of the questions to remove references to guidelines and to make it specific to Ontario. Next, we separated the checklist into two sections. In the first section, we assessed the applicability of each study to the research question (directly, partially, or not applicable). In the second section, we assessed the limitations (minor, potentially serious, or very serious) of the studies that we found to be directly applicable.
Results
Economic Literature Search
The database search of the economic literature yielded 116 citations published between January 1, 2010, and August 24, 2021. We identified three additional studies from other sources, for a total of 84 after removing duplicates. See Appendix 5 for a list of selected studies excluded100,128-130 after full-text review. Figure 20 presents the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for the economic literature search.64
Figure 20: PRISMA Flow Diagram—Economic Search Strategy.
PRISMA flow diagram showing the economic search strategy. The database search of the economic literature yielded 116 citations published between January 1, 2010, and August 24, 2021. We identified 3 additional eligible studies from other sources. After removing duplicates, we screened the abstracts of 84 studies and excluded 78. We assessed the full text of 6 articles and excluded a further 3. In the end, we included 4 articles in the qualitative synthesis after an additional eligible study was identified from autoalerts.
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses. Source: Adapted from Moher et al, 2009.64
Overview of Included Economic Studies
We included two cost-utility analyses131,132 and two retrospective cohort studies133,134 that included both health and cost outcomes. We present the study design, populations, outcomes, time horizons, and study results in Table 39. We further summarized their findings below.
Table 39:
Results of Economic Literature Review—Summary
| Author, year, country | Analytic technique, study design, perspective, time horizon | Population | Intervention(s) and comparator(s) | Results | ||
|---|---|---|---|---|---|---|
| Health outcomes | Costs | Cost-effectiveness | ||||
| Magnuson et al, 2019131 United States | Type of economic analysis: cost-utility Study design: RCT for first 24 mo in combination with Markov model after 24 mo Perspective: US health care system Lifetime horizon | Inpatients with acute proximal DVT N = 692 n = 337: PMT and/or CDT n = 355 anticoagulation alone Median age: IQR: 53 (42-62) Male: 426 (62%) |
Intervention: PMT and/or CDT in conjunction with anticoagulation therapy Mechanical device: AngioJet Rheolytic Thrombectomy System: Boston Scientific, or the Trellis Peripheral Infusion System (Covidien) Comparator: Anticoagulation alone |
Total lifetime QALYs: PMT and/or CDT: 17.15 Anticoagulation alone: 17.07 Incremental QALYs: 0.08 Discount rate: 3% annually | 2017 USD Total lifetime cost PMT and/or CDT: $168,496 Anticoagulation alone: $151,756 Incremental cost: $16,740 Discount rate: 3% annually Cumulative 24 mo cost (95% CI) PMT and/or CDT: $30,591 ($27,714 to $34,043) Anticoagulation alone: $10,546 ($8,156 to $13,445) Incremental cost: $20,045 ($16,093 to $24,120) Index hospitalization cost (95% CI) PMT and/or CDT: $21,509 ($20,327 to $22,843) Anticoagulation alone: $3,877 ($3,069 to $4,803) Incremental cost: $17,632 ($16,117 to $19,243) |
Lifetime model results ICER: $222, 041 per QALY gained Probabilistic analysis: The probability of PMT and/or CDT being cost-effective was 25% at a willingness to pay of $150,000 per QALY gained |
| Kwok et al, 2018133 Australia | Type of economic analysis: cost analysis Study design: retrospective study Perspective: a hospital in Australia Time horizon: 30 d | Inpatients with noniatrogenic acute lower limb ischemia N = 42 n = 15, primary vacuum aspiration n = 27, primary CDT Mean age ± SD: | Intervention: Primary percutaneous aspiration thrombectomy (vacuum aspiration) Mechanical device: Indigo Aspiration System (Penumbra Inc) | Technical success of thrombus/embolus removal Primary vacuum aspiration: 8 (53%) by the primary intervention only 15 (100%) after the | Costs were converted to US dollars in 2017 using exchange rates The procedural costs were incurred from the initial reperfusion procedure until the return of the patient to a standard nursing ward after | NA |
| Primary vacuum aspiration: 69.0 ± 14.5 Primary CDT: 65.0 ± 99 Male: Primary vacuum aspiration: n = 10 (67%) Primary CDT: n = 22 (82%) | Comparator: Primary CDT | primary and adjunctive interventions Primary CDT: 25 (93%) by the primary intervention only 27 (100%) after the primary and adjunctive interventions Limb salvage (avoidance of amputation) at 30 d: Primary vacuum aspiration: 15 (100%) Primary CDT: 27 (100%) |
completing treatment Mean ± SD: Primary vacuum aspiration: 16,259 ± 7,452 Primary CDT: 15,175 ± 4,719 | |||
| Li et al, 2020100 China | Type of economic analysis: cost analysis Study design: retrospective study Perspective: a hospital in China Time horizon: median follow up time of 6.4 mo for clinical outcomes, but the costs were index hospitalization costs (do not include costs during the follow up) | Inpatients with acute iliofemoral DVT N: 126 PMT + CDT: n = 61; MAT + CDT: n = 65 Mean age ± SD: PMT + CDT: 53.3 ± 14.3 MAT + CDT: 55.3 ± 12.5 Male: PMT + CDT: 33 (54%) MAT + CDT: 34 (52% |
Intervention: PMT + CDT Mechanical device: AngioJet Rheolytic Thrombectomy System (Boston Scientific) Comparator: MAT + CDT |
Technical success rate: 100% in both groups Thrombus clearance rate: PMT + CDT: 60 (98.4%) MAT + CDT: 65 (100%) P = .311 |
Authors did not specify the monetary unit or year; we assume USD Costs of the index hospitalization: Mean ± SD: PMT + CDT: $8,291.7 ± 471.4 MAT + CDT: $4,632.5 ± 441.7 P < .001 |
NA |
| Li et al, 2021132 China | Type of economic analysis: cost-utility Study design: Markov model Perspective: 3rd-party payer, China Time horizon: Lifetime (20 Markov cycles) | Inpatients with lower extremity deep vein thrombosis Age: not reported |
Intervention: PMT plus anticoagulation and elastic compression stockings Mechanical device: AngioJet Thrombectomy System (Boston Scientific) Comparator: CDT plus anticoagulation and elastic compression stockings |
Total lifetime QALYsa PMT: 22.56 CDT: 23.83 Discount rate: unknown |
Costs are in 2021 USD Total lifetime costs PMT: $24,018 CDT: $49,570 Hospitalization costs of index procedure: PMT: $11,958 CDT: $10,198 Discount rate: unknown |
Authors did not calculate ICER, but concluded that PMT would be more cost-effective based on the average cost effectiveness ratio ACER PMT: $1,065/QALY CDT: $2,080/QALY |
Abbreviations: ACER, average cost effectiveness ratio; CDT, catheter-directed thrombolysis; DVT, deep vein thrombosis; ICER, incremental cost-effectiveness ratio; IQR, interquartile range; MAT, manual aspiration thrombectomy; NA, not applicable; PMT, pharmacomechanical thrombectomy; QALYs, quality-adjusted life-years; RCT, randomized controlled trial; SD, standard deviation.
The time horizon was 20 Markov cycles (the cycle length was unspecified; we assume 1 year), but it was unclear how they calculated the total effectiveness in both groups to be greater than 20 QALYs given that the maximum value of QALYs after 20 years, without discounting, should be 20. Given this issue, the results of this study may not be reliable.
Magnuson et al131 conducted a cost-utility analysis to compare the combination treatment of pharmacomechanical thrombectomy (PMT) and/or CDT in conjunction with anticoagulation therapy with anticoagulation therapy alone in patients with acute DVT from a US health care payer perspective. The authors termed the interventions “pharmacomechanical catheter-directed thrombolysis.” In this review, we refer to the interventions as PMT and/or CDT, as appropriate. Magnuson et al131 conducted their economic evaluation in tandem with the ATTRACT clinical trial135 (see our clinical review, above, for details on the ATTRACT trial). Although the duration of this trial was 24 months, the authors developed a Markov model based on in-trial results and the US life table to project the lifetime cost-effectiveness. Both direct medical care costs and indirect costs (time lost from work, informal caregiver time, etc.) were included.
We summarize the results by three stages: the index PMT procedure and hospitalization costs, cumulative in-trial costs (24 months), and lifetime costs in Table 39. In brief, the costs of PMT and/or CDT strategies were higher than anticoagulation therapy alone in all three stages. Magnuson et al131 used the Short Form-36 instrument to obtain health utilities. Compared with the baseline utilities, both groups showed a considerable increase in utility at 6 months (about 0.12), which continued increasing slightly at 12, 18, and 24 months. However, there was no significant difference in utilities between the two groups in all visits. While the quality-adjusted life years (QALYs) gained at 24 months (the trial duration) were not reported, the lifetime QALYs gained was 0.08 and the incremental cost was $16,740 USD for the PMT and/or CDT strategy, compared with anticoagulation therapy alone (lifetime cost of anticoagulation therapy alone: $151,756 USD per person). For the overall DVT population, the ICER of PMT and/or CDT was $222,041 USD (about $292,136 CAD) per QALY gained in the lifetime model, compared with anticoagulation therapy alone. For people with iliofemoral DVT, the incremental QALYs for PMT and/or CDT versus anticoagulation therapy alone increased to 0.12, and the ICER was $137,526 USD (about $180,941 CAD) per QALY gained. For people with femoral-popliteal DVT, PMT and/or CDT was dominated by anticoagulation therapy alone due to the higher costs and lower QALYs.
Kwok et al133 conducted a retrospective study to examine the effectiveness and procedural costs of percutaneous aspiration thrombectomy (termed vacuum aspiration in this review) as a first-line treatment for noniatrogenic acute lower limb ischemia, compared with conventional CDT. This study was conducted at a tertiary referral health center in Australia from January 2015 to August 2017. Kwok et al133 defined technical success as complete removal of the thrombus and a substantial improvement in the TIMI score.1 The authors defined substantial improvement as a final score ≥ 2 that is at least 1 higher than the initial scoring.
Including both the primary and the adjunctive interventions, Kwok et al133 reported that overall technical success rates were 100% for both groups. However, in the group receiving vacuum aspiration as a first-line treatment, eight patients (53%) achieved technical success by the primary intervention and seven (47%) received adjunctive CDT. In the group receiving CDT as a first-line treatment, 25 patients (93%) achieved technical success by the primary intervention and two (7%) received adjunctive vacuum aspiration. There were no significant differences in the rates of procedure-related 30-day complications or in the overall 30-day complication rate. The procedural costs covered the period from the initial reperfusion procedure until the patient's return to a standard nursing ward after completing treatment. Procedural costs included the costs of disposable equipment, overhead (the angiography suite and nursing care unit), and medical professionals (medical staff, nursing staff, radiographers, and health care assistants), but the authors did not report separate costs for each category. Costs were converted to 2017 USD. The procedure costs for people receiving vacuum aspiration as the first-line treatment were slightly higher than for those receiving CDT ($16,259 versus $15,175 per person), but this difference was not statistically significant. However, for people receiving vacuum aspiration as the first-line treatment without adjunctive CDT, the average costs were $12,757 per person—lower than the average costs in the CDT group.
Li et al100 conducted a retrospective study to compare the clinical efficacy and cost of PMT plus CDT (collectively referred to as PMT) with manual aspiration thrombectomy (MAT) plus CDT (collectively referred to as MAT) for people with acute iliofemoral DVT in China.100 The authors found that both the PMT and MAT groups had good and similar health outcomes (e.g., technical success and thrombus clearance), but PMT was associated with greater procedure costs ($8,291.7 per person, compared to $4,632.5 for MAT), mainly due to the higher catheter costs of PMT ($3,380.3 vs. $3,94.4 per person; currency unspecified).
Li et al132 conducted a model-based cost-utility analysis to compare PMT with CDT for inpatient treatment of lower extremity DVT from the perspective of a Chinese third-party payer. The study concluded that PMT is more cost-effective based on the average cost-effectiveness ratio. Note that economic evaluations generally use ICERs to evaluate the cost-effectiveness. In addition, we found some errors with the model results. For example, Table 1 of the study showed that, compared with the CDT group, the PMT group had the more favorable health outcomes, or lower chances of experiencing unfavorable outcomes (probability of experience post-thrombotic syndrome: PMT of 0.2159 vs. CDT of 0.411; probability of disabling complications: PMT of 0.0016 vs. CDT of 0.0021; and probability of death: PMT of 0.0016 vs. CDT of 0.0042). However, in Table 2, they reported that the cumulative lifetime QALYs in the PMT group were lower than that in the CDT group (22.56 vs. 23.83 QALYs, respectively). Also, the model time horizon was 20 Markov cycles (although the cycle length was unspecified, generally a Markov cycle length in medical research is less than or equal to 1 year). It was unclear how the authors calculated the total effectiveness in both groups to be greater than 20 QALYs given that the maximum value of QALYs after 20 years, without discounting, should be 20. Given these issues, the results of this study may not be reliable.
Applicability and Limitations of the Included Studies
Appendix 6 provides the results of the applicability checklists for the included studies. Two of four studies were partially applicable to the research question.131,133 Given that the costs in the United States and Australia were different from those in Canada, the results of these studies were not generalizable to Ontario.131,133 The remaining two studies, from China, were not applicable to the Ontario setting.100,132
Discussion
We did not identify any economic studies conducted from a Canadian perspective. Of the four studies included in our review, two were not applicable to the Ontario setting. The other two studies did not demonstrate the cost-effectiveness of MT for people with blocked arteries and veins in the lower limbs.131,133 Since MT is often used together with CDT and anticoagulation therapy, it is difficult to evaluate role of MT alone. Magnuson et al131 estimated the cost-effectiveness of PMT and/or CDT using three different approaches based on the ATTRACT trial (discussed in the clinical analysis, above), which did not show PMT and/or CDT to have favourable clinical outcomes. The economic analysis showed that the incremental QALYs associated with PMT and/or CDT were relatively small over a lifetime horizon, while the incremental cost was relatively large. As a result, the ICER was high and PMT and/or CDT was considered not cost-effective. In addition, although the device costs varied across three methods of thrombolytic agent delivery (mean ± SD in USD of Trellis: $3,399 ± 2,558, AngioJet: $5,239 ± 2,755, and infusion-first: $4,191 ± 3,171), the total index hospitalization costs by the three approaches were similar (mean ± SD in USD of Trellis: $21,558 ± 12,196; AngioJet: $21,291 ± 9,375; and infusion-first: $22,908 ± 11,149). Similarly, Kwok et al133 found that the costs of index hospitalization were similar for primary interventions of MT and primary interventions of CDT.
In summary, the published studies showed that the treatment group including MT did not substantially improve health outcomes but was associated with increased costs compared with anticoagulation therapy alone. Compared with CDT, the hospitalization costs of MT were only slightly higher.133 However, as the technology of MT continues to improve over time, the studies published to date may not adequately reflect the most recent experiences of providers using this technology. For instance, PMT may be conducted in the outpatient setting for selected patients; MT may also be more often conducted without CDT. Mechanical thrombectomy may potentially reduce costs in some situations.
Conclusions
Our systematic review of the economic literature identified four economic studies, two of which were partially applicable to the Ontario setting. The cost-utility study showed that PMT and/or CDT was not cost-effective compared with anticoagulation therapy alone for patients with acute DVT from the US health care system perspective. The cost analysis study showed that vacuum aspiration as the first-line treatment had slightly higher procedure costs than CDT as first-line treatment for patients with acute lower limb ischemia. We did not identify any studies conducted in a Canadian setting.
Primary Economic Evaluation
There are several challenges in assessing the cost-effectiveness of mechanical thrombectomy (MT) in Ontario.
First, there is significant heterogeneity in the clinical studies evaluating the effectiveness of MT. In the observational studies, intervention selection and the use of adjuvant treatments were often at the discretion of the treating physicians. As a result, treatment choices and sequences were different from study to study, making it difficult to determine the effectiveness of MT alone.
Second, our Clinical Evidence Review did not identify any high-quality clinical studies in the arterial acute limb ischemia population. All 12 studies were single-center retrospective studies. Also, in Ontario, the usual treatment for people hospitalized with arterial acute limb ischemia is open surgery (e.g., surgical thrombectomy or bypass). We did not identify any studies that compared MT with open surgery. Instead, the studies identified in our review used other endovascular techniques, most notably CDT, for the comparator group. In addition to the very low certainty of the evidence, the published studies showed no significant difference between MT and CDT in important clinical outcomes such as limb salvage and perioperative mortality.
Third, there is likely no statistically significant difference in important health outcomes (e.g., post-thrombotic syndrome and limb salvage) associated with using MT in the acute DVT population. The Thrombus Removal with Adjunctive Catheter-directed Thrombolysis (ATTRACT) trial was a milestone study that evaluated PMT and/or CDT for the prevention of post-thrombotic syndrome following acute DVT.135 This trial did not demonstrate that PMT and/or CDT reduces the risk of post-thrombotic syndrome. A published economic evaluation based on the ATTRACT trial showed that, compared with anticoagulation therapy alone, PMT and/or CDT in addition to anticoagulation therapy was not cost-effective (incremental cost-effectiveness ratio of PMT and/or CDT versus anticoagulant therapy alone: $222,041 USD, or approximately $292,136 CAD, per QALY gained).131 While there are more recent published studies that evaluated newer versions of devices in different subpopulations, most are either non-RCTs or they do not have as large of a study population as did the ATTRACT trial. To date, the ATTRACT trial remains the best quality evidence on MT for people with acute DVT. In addition, the results from non-randomized studies have not shown the benefits from MT treatment in important clinical outcomes, compared with other treatments (CDT or anticoagulation therapy alone).
Finally, compared with CDT alone, MT as an adjunct to CDT may impact health care resource use, such as shorter length of use of thrombolytics, shorter intensive care unit stays, and potentially shorter inpatient hospital stays. However, these benefits are captured in our budget impact analysis.
Owing to these limitations, we decided not to conduct a primary economic evaluation.
Budget Impact Analysis
Research Question
What is the potential 5-year budget impact for the Ontario Ministry of Health of publicly funding mechanical thrombectomy (MT) for people with arterial acute limb ischemia or acute deep vein thrombosis (DVT)?
Methods
Analytic Framework
We estimated the budget impact of publicly funding MT in the lower limb using the cost difference between two scenarios: (1) current clinical practice without specific public funding for MT (the Current Scenario), and (2) anticipated clinical practice with specific public funding for MT (the New Scenario). Mechanical thrombectomy for a blocked blood vessel in the lower extremities is currently being performed in 14 of the 20 hospitals in Ontario with established vascular programs. However, MT is currently funded by hospitals’ global budgets, as there is no specific public funding for this treatment. Figure 21 presents the budget impact model schematic.
Figure 21: Schematic Model of Budget Impact.
Flow chart describing the model for the budget impact analysis. Based on the size of the target population, we created two scenarios: the Current Scenario, which would explore the distribution of treatment strategies, resource use and total costs without public funding for mechanical thrombectomy in the lower limb, and the New Scenario, which would explore the distribution of treatment strategies, resource use and total costs with public funding for mechanical thrombectomy in the lower limb. The budget impact would represent the difference in costs between the two scenarios.
Please note that in this budget impact analysis, MT refers generically to any MT device. Pharmacomechanical MT (PMT) refers to a specific type of MT—PMT treatment using AngioJet Rheolytic Thrombectomy System (Boston Scientific Corp.), and vacuum aspiration refers to MT treatment using the Indigo Aspiration System (Penumbra Inc.).
Key Assumptions
MT was conducted in the inpatient setting
Under specific public funding, MT would be performed at hospitals with existing infrastructure required for this treatment (i.e., advanced imaging technologies and radiology suites). We did not include the additional capital investment for imaging equipment and/or treatment rooms
People with arterial acute limb ischemia (ALI) and acute DVT were monitored at the intensive care unit (ICU) throughout the duration of their thrombolytic therapy. When ICU durations were not reported, we assumed that the duration of thrombolytic therapy is equal to the ICU time
The size of our target population will remain stable in Ontario over the next 5 years
Because there is limited published data on vacuum aspiration treatment using the Indigo Aspiration System for the population of interest while there are several publications on PMT, we assumed that the savings in ICU time and ward time by PMT would be the same as that by vacuum aspiration
Target Population
Our target population is adults (≥ 18 years old) who are hospitalized for arterial acute limb ischemia or acute DVT in the lower limb. While MT can be performed in the outpatient setting, it is typically an inpatient procedure in Ontario, and most clinical evidence identified was obtained from inpatients. In November 2021, we used the IntelliHealth Ontario portal (IntelliHealth Ontario; intellihealth.moh.gov.on.ca) to search the Canadian Institute for Health Information (CIHI) Discharge Abstract Database (DAD) to identify inpatient cases for blood clots in Ontario. We identified these cases using the following ICD-10 codes (International Statistical Classification of Disease, 10th Revision, Canadian Version) of the most responsible diagnosis for hospital admissions:
I74.3: Embolism and thrombosis of arteries of the lower extremities
I80.2: Phlebitis and thrombophlebitis of other deep vessels of lower extremities, including DVT
We considered the conditions associated with the two ICD-10 codes as the proxy diagnoses for the indications of our target population. We excluded individuals who were younger than 18 years old at admission, as well as individuals who did not have a valid health card number in Ontario. If an individual was hospitalized two or more times in one fiscal year, we counted them once as a “unique” person in a single fiscal year. The number of unique individuals in fiscal years 2015-2020 are presented in Table 40. On average, there were 457 and 668 individuals hospitalized annually for arterial acute limb ischemia or DVT in the lower limb, respectively.
Table 40:
The Number of Adults Hospitalized for a Blocked Blood Vessel in the Lower Limb in Ontario, Fiscal Years 2015-2020
| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | Average | |
|---|---|---|---|---|---|---|---|
| Overall | 1,160 | 1,147 | 1,142 | 1,153 | 1,075 | 1,072 | 1,125 |
| Arterial acute limb ischemia (ICD-10: I74.3) | 424 | 482 | 435 | 520 | 466 | 413 | 457 |
| Acute DVT (ICD-10: I80.2) | 736 | 665 | 707 | 633 | 609 | 659 | 668 |
Abbreviations: DVT, deep vein thrombosis; ICD-10, International Statistical Classification of Disease, 10th Revision, Canadian Version.
Source: Data provided by IntelliHealth Ontario.
We may be slightly underestimating the size of our target population for two reasons. First, the volume of hospital admissions in 2020 were impacted by the COVID-19 pandemic and may not be predictive of admission rates going forward. Second, a small proportion of hospitalized adults may have a different most responsible diagnosis code (e.g., embolism and thrombosis of unspecified arteries of extremities, or veins). Some hospitalized adults may have a secondary diagnosis with I74.3 or I80.2, and they may be also part of our target population. To account for this, we increased the population size in our scenario analysis.
Not all people hospitalized for arterial acute limb ischemia or acute DVT are suitable for MT. Other treatments, such as surgical bypass or anticoagulation therapy alone may be more appropriate for some individuals. The population hospitalized with the ICD-10 diagnosis code I80.2 may include cases of superficial phlebitis, although it is rare to be hospitalized for superficial thrombophlebitis. Furthermore, MT therapy may be more appropriate for people with iliofemoral DVT (i.e., Magnuson et al131 showed that the iliofemoral DVT population had greater QALYs gained than the overall DVT population), but not for people with femoral-popliteal DVT.88,131,135,136 Many studies of MT included a mixed patient population with both iliofemoral and femoral-popliteal DVT. In the ATTRACT trial, 391 of 691 individuals (57%) had thrombosis involving the common femoral or iliac veins,86 and the remaining 300 individuals (43%) had femoral-popliteal DVT.88 The proportion of iliofemoral and femoral-popliteal DVT among the cases we identified from the CIHI database (i.e., ICD-10: I80.2) was unknown.
Given the considerations above, we estimated that up to 75% of hospitalized arterial acute limb ischemia cases and 60% of hospitalized DVT cases are suitable for MT (email communications, Dheeraj Rajan and Jeff Jaskolka, December 2021; Charles de Mestral, April 2022). Using these estimates, we estimated that each year, there will be 343 hospitalized individuals with arterial acute limb ischemia and 401 hospitalized individuals with acute DVT who are eligible for MT as either the primary or adjunctive treatment.
Current Intervention Mix
Mechanical thrombectomy for a blocked blood vessel in the lower extremities is performed in several Ontario hospitals and is funded through each hospital's global budget. There is no specific intervention code for MT in the lower extremities137 and we have not found any published data on the volume of this treatment in Ontario. Therefore, we were not able to reliably determine the current volume of MT procedures taking place. To estimate the budget impact of specific public funding for MT for the lower limbs, we assumed that the treatment share of MT therapy is zero in the current intervention mix.
We consulted with clinical experts to obtain information on current interventions for people who are eligible for MT (email communications, Dheeraj Rajan and Jeff Jaskolka, December 2021; Charles de Mestral, May 2021). Given the wide range and possible combinations of treatment options available,133,135,138 for simplicity we limited the current treatment mix to one primary treatment per person, based on the main intervention received. While additional interventions (including medical imaging) may be offered, we did not specify the additional interventions in our analysis. Current treatments considered for our target populations (see Figure 22) include:
Figure 22: Process of Estimating the Volumes of Interventions.
Volumes of interventions are estimated by comparing hospitalized adults with a blocked blood vessel in the lower limb annually, multiplying by the percentage of hospitalized adults who are eligible for mechanical thrombectomy annually. This number is compared under the Current Scenario versus the New Scenario in year 1.
Abbreviations: ALI, arterial acute limb ischemia; CDT, catheter-directed treatment; DVT, deep vein thrombosis; MT, mechanical thrombectomy.
Note: the market share of MT was estimated including any intervention strategy involving MT, including pharmacomechanical thrombectomy with or without CDT and vacuum aspiration with or without CDT.
For people with arterial acute limb ischemia, the main treatments are open surgery (75% of patients [257 of 343]; e.g., surgical thrombectomy and bypass), and catheter-directed thrombolysis (CDT; 25% of patients [86 of 343])
For people with acute DVT, the main treatments are anticoagulant therapy only (70% of patients [281 of 401]), and CDT (30% of patients [120 of 401])
Anticoagulants do not break up existing blood clots; instead they reduce formation of further clots.48 Anticoagulant therapy is typically provided to all hospitalized DVT patients regardless other treatments received. Thrombolytic therapy, on the other hand, is aimed at dissolving blood clots and can be administered through a peripheral vein, loco-regionally via a vein close to the clot, or CDT.48 Typically, thrombolytic therapy for DVT is administered by the CDT (Amol Mujoomdar, oral communication, March, 2022).
Uptake of the New Intervention and New Intervention Mix
The market shares of MT were estimated by counting any intervention strategy involving MT regardless of whether it is the main treatment or the adjunct treatment (e.g., MT with or without CDT) or the order of treatment (e.g., CDT provided before or after). Mechanical thrombectomy is often performed with the companion use of thrombolytic therapy (i.e., pre-thrombectomy to soften the thrombus and/or post-thrombectomy to complete clot clearance).135 Also, the complexity of multiple interventions will sometimes prevent a clear determination of the primary intervention. We aimed to propose the treatment mix in our New Scenario for the budget impact analysis only, and the treatment classifications proposed may not be scientifically rigorous.
We assumed that the market share of all treatments involving MT will be 25% in year 1, gradually increasing 5% annually to 45% in year 5 for both arterial acute limb ischemia and acute DVT. See Table 41. The interventions used in the Current Scenarios will decrease accordingly.
Table 41:
Market Share of Mechanical Thrombectomy in the New Scenario
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
|---|---|---|---|---|---|
| Arterial Acute Limb Ischemia | |||||
| All mechanical thrombectomy (%) | 25 | 30 | 35 | 40 | 45 |
| PMT w/wo CDT (%) | 15 | 16.5 | 17.5 | 18 | 18 |
| Vacuum aspiration w/wo CDT (%) | 10 | 13.5 | 17.5 | 22 | 27 |
| Acute DVT | |||||
| All mechanical thrombectomy (%) | 25 | 30 | 35 | 40 | 45 |
| PMT w/wo CDT (%) | 17.5 | 20.1 | 22.4 | 24.8 | 27 |
| Vacuum aspiration w/wo CDT (%) | 7.5 | 9.9 | 12.6 | 15.2 | 18 |
Abbreviations: CDT, catheter-directed treatment; DVT, deep vein thrombosis; PMT, pharmacomechanical thrombectomy; w/wo, with or without.
Note: numbers may be inexact due to rounding.
Globally, there are a range of MT technologies available. In Ontario, the AngioJet Rheolytic Thrombectomy System (Boston Scientific Corp.) and the Indigo Aspiration System (Penumbra Inc.) are the most commonly used devices. The AngioJet System includes a console (control system), catheter, and thrombolytic agent. The AngioJet delivers the thrombolytic agent and then uses mechanical devices to fragment and aspirate the thrombus. On the other hand, vacuum aspiration (Indigo) delivers a continuous vacuum to the catheters for thrombus removal. To date, there are more published studies on AngioJet and, in Ontario, it is used more often than the Indigo Aspiration System. However, the market share of the Indigo Aspiration System may increase in next a few years due to some unique features (e.g., MT treatment without CDT) and ongoing clinical trials (email communications, Jeff Jaskolka and Amol Mujoomdar, April 2022).
For people with arterial acute limb ischemia, we estimated that 60% of MT procedures will be performed using PMT (AngioJet System) in Year 1, decreasing 5% annually to 40% in Year 5. At the same time, the proportion of MT procedures using the vacuum aspiration will increase 5% annually, from 40% in Year 1 to 60% in Year 5 for people with arterial acute limb ischemia (see Table 41).
According to the PEARL (Peripheral Use of AngioJet Rheolytic Thrombectomy with a Variety of Catheter Lengths) registry data for people with arterial acute limb ischemia, 52% of PMT procedures were completed without the conjunctive CDT (i.e., PMT alone).122 A single arm case series study found that about 53% of patients receiving vacuum aspiration did not use CDT.139 See Table 42 for the volumes of interventions in the New Scenario for arterial acute limb ischemia.
Table 42:
Volumes of Interventions in the Current and New Scenarios for Hospitalized Adults With Arterial Acute Limb Ischemia in Ontario
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total | |
|---|---|---|---|---|---|---|
| Current Scenario | 343 | 343 | 343 | 343 | 343 | 1,715 |
| Open surgery, n | 257 | 257 | 257 | 257 | 257 | 1,285 |
| CDT without MT, n | 86 | 86 | 86 | 86 | 86 | 430 |
| New Scenario | 343 | 343 | 343 | 343 | 343 | 1,715 |
| Open surgery, n | 193 | 180 | 167 | 154 | 142 | 836 |
| CDT without MT, n | 64 | 60 | 56 | 52 | 47 | 279 |
| All MT,a n | 86 | 103 | 120 | 137 | 154 | 600 |
| PMT w/wo CDT,a n | 52 | 57 | 60 | 62 | 62 | 293 |
| Vacuum aspiration w/wo CDT,a n | 34 | 46 | 60 | 75 | 92 | 307 |
Abbreviations: CDT, catheter-directed treatment; MT, mechanical thrombectomy; PMT, pharmacomechanical thrombectomy; w/wo, with or without.
Note: numbers may be inexact due to rounding.
All treatments involved in mechanical thrombectomy, regardless of whether mechanical thrombectomy is the primary or secondary intervention.
For people with acute DVT, we estimated that 70% of MT procedures will be performed using PMT (AngioJet) in Year 1, which will gradually decrease to 60% in Year 5. The proportion of MT procedures using vacuum aspiration will gradually increase from 30% in Year 1 to 40% in Year 5 (see in Table 41). According to the PEARL registry data for people with DVT, 39% of PMT procedures were completed without CDT.85 Although a case series study showed that vacuum aspiration can be conducted without CDT,140 MT is typically conducted as an adjunct to thrombolytic therapy for hospitalized people with DVT. We assumed that in next 5 years, 80% of vacuum aspiration procedures will be conducted with conjunctive CDT and the other 20% will be treated with vacuum aspiration alone. See Table 43 for the volumes of interventions for acute DVT under the New Scenario.
Table 43:
Volumes of Interventions in the Current and New Scenarios for Hospitalized Adults With Acute Deep Vein Thrombosis in Ontario
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total | |
|---|---|---|---|---|---|---|
| Current Scenario | 401 | 401 | 401 | 401 | 401 | 2,005 |
| Anticoagulant therapy alone, n | 281 | 281 | 281 | 281 | 281 | 1,405 |
| CDT without MT, n | 120 | 120 | 120 | 120 | 120 | 600 |
| New Scenario | 401 | 401 | 401 | 401 | 401 | 2,005 |
| Anticoagulant therapy alone, n | 211 | 197 | 183 | 169 | 155 | 915 |
| CDT without MT, n | 90 | 84 | 78 | 72 | 66 | 390 |
| All MT,a n | 100 | 120 | 140 | 160 | 180 | 700 |
| PMT w/wo CDTa, n | 70 | 80 | 90 | 99 | 108 | 447 |
| Vacuum aspiration w/wo CDTa, n | 30 | 40 | 50 | 61 | 72 | 253 |
Abbreviations: CDT, catheter-directed treatment; MT, mechanical thrombectomy; PMT, pharmacomechanical thrombectomy; w/wo, with or without.
Note: numbers may be inexact due to rounding.
All treatments involved in mechanical thrombectomy, regardless of whether mechanical thrombectomy is the primary or secondary intervention.
Resources and Costs
It was difficult to accurately estimate the average costs and budget impact of publicly funding MT for several reasons:
There is limited published Canadian cost data on arterial acute limb ischemia and DVT inpatients
We have not found estimates for the average costs of CDT and MT treatments in Canada. Also, device and consumable costs (e.g., catheters, guidewires, and tubes) and ICU/ward stay costs related to MT were challenging to estimate and may be specific to individual patients
The wide range and possible combinations of treatment options increases the complexity of cost estimates of interventions
We made a rough estimate of treatment costs based on the Ontario Case Costing Initiative (OCCI) data in 2016 and 2017141 and the information provided by manufacturers (see below). The OCCI hospital costs included direct costs (e.g., nursing, operating room, pharmacy, and laboratory costs) and indirect costs (e.g., overhead costs such as administration, finance, human resources, etc.).141 However, OCCI data does not include physician service fees. Based on the OCCI costs of CDT, we calculated the hospital costs for MT (e.g., increased costs for the MT devices, but with offsetting decreased costs due to reductions in ICU time). Lastly, we used the Patient Cost Estimator from CIHI to estimate the physician fees for different interventions strategies and calculate the total costs (hospital plus physician) for each intervention.142
This analysis was conducted from the perspective of the Ontario Ministry of Health. All costs were reported in 2022 Canadian dollars.
HOSPITAL COSTS OF INTERVENTIONS IN THE CURRENT SCENARIO, WITHOUT MECHANICAL THROMBECTOMY
Arterial Acute Limb Ischemia
We searched the hospitalization costs of three of the most frequent surgical interventions for people with ICD-10-CA diagnosis of I74.3 (embolism and thrombosis of arteries of the lower extremities) in the OCCI database.141 See Table 44. We estimated the average cost of open surgery by weighting the three most common surgical interventions to arrive at an average hospital cost of $17,732 per procedure. There is a Canadian Classification of Health Intervention (CCI) code for general thrombolytic therapy by approach (e.g., intravenous or catheter-directed).143 Intravenous thrombolysis is not typically performed for arterial peripheral ischemia populations (Amol Mujoomdar, oral communication, March, 2022). As such, we assumed that the cost of the thrombolytic therapy could be used as a proxy for the cost of CDT therapy, $15,582 per procedure.
Table 44:
OCCI Hospital Costs (Excluding Physician Fees) of Current Treatments
| Cost (CAD)a | Weighting for cost estimate | CCI code | CCI code description | |
|---|---|---|---|---|
| Arterial Acute Limb Ischemia | ||||
| Open surgery | 17,732 | We approximated the weighted costs of three surgical procedures as a proxy for the cost of open surgery | ||
| Extraction | 17,659 | 0.5 | 1.KG.57.LA-GX (principal CCI) | Extraction, arteries of leg by the open approach. |
| Bypass | 16,023 | 0.35 | 1.KG.76.MI-XX-A (principal CCI) | Bypass terminating in lower limb artery using autograft |
| Bypass | 21,962 | 0.15 | 1.KG.76.MI-XX-N (principal CCI) | Bypass terminating in lower limb artery using synthetic material. |
| CDT without MT | 15,582 | 1 | 1.KG.35.HH-1C (principal CCI) | Pharmacotherapy (local), arteries of leg, using thrombolytic agent by percutaneous infusion approach We used the cost of thrombolytic therapy as a proxy for the cost of CDT therapy |
| Acute DVT | ||||
| Anticoagulant therapy alone | 4,781 | 1 | No principal CCI code assigned | Not assigning the intervention code suggests no invasive procedures conducted, or only receiving medical treatment We used the costs of these patients as a proxy for the main intervention with anticoagulant therapy (i.e., the standard treatment) |
| CDT without MT | 16,736 | 1 | 1.KX.35.HH-1C (principal CCI) | Pharmacotherapy (local), vein, using thrombolytic agent by percutaneous infusion approach We used the cost of thrombolytic therapy as a proxy for the cost of CDT therapy |
Abbreviations: CCI, Canadian Classification of Health Intervention; CDT, catheter-directed treatment; DVT, deep vein thrombosis; OCCI, Ontario Case Costing Initiative; MT, mechanical thrombosis.
Acute DVT
Since medical treatments are often not assigned a CCI intervention code in DAD, we have not found the intervention code of anticoagulant therapy for hospitalized DVT patients (ICD-10 code I80.2: phlebitis and thrombophlebitis of other deep vessels of lower extremities, including DVT). Individuals who were hospitalized due to DVT but not assigned an intervention code are not likely to have undergone any invasive interventions. Since anticoagulant therapy is the standard therapy for individuals with DVT,59 we assumed that those people who were not assigned the principal intervention code received anticoagulant therapy alone. We then assumed that the average hospital cost for individuals who received anticoagulant therapy alone was the same as the hospital cost for those who were not assigned the principal intervention codes, $4,781 (Table 44). The hospital cost for thrombolytic therapy was $16,736, which was assumed to be the cost of CDT treatment. Note that the hospitalization costs of CDT treatments were different for adults with arterial acute limb ischemia and acute DVT.
HOSPITAL COSTS OF INTERVENTIONS IN THE NEW SCENARIO, WITH MECHANICAL THROMBECTOMY
There was limited Canadian data available on the various costs related to MT. Mechanical thrombectomy is typically performed concurrently with CDT at a radiology suite by an interventional radiologist. As such, we used hospital costs associated with CDT as the baseline, added the cost of MT devices, and then subtracted the cost savings associated with MT (i.e., reduced ICU and ward time compared with CDT alone, and savings in CDT device use due to a proportion of MT procedures being performed without CDT). The average cost of an ICU stay was $4,005 per day (in 2022 CAD), based on 2,239 ICU encounters between April, 2012, and March, 2013, at The Ottawa Hospital.145 This unit cost included nursing, operating room, laboratory, medical imaging, pharmacy, etc., but not physician fees. Our clinical evidence review conducted a meta-analysis for the reduction in ICU stay after MT treatment for two indications (assuming that ICU stay is same as the time of thrombolytic infusion). Although the comparators in the observational studies included in the meta-analysis were not the same, the most common comparator in these studies was CDT. For simplicity, we assumed that the meta-analysis results reflected the differences in ICU and ward durations for MT (with or without CDT) versus CDT alone. The savings in ICU and ward stay parameters were based on point estimates of meta-analyses for the studies using the PMT device (see Table 36, above). The savings in ICU time in the meta-analysis was consistent with the observations in clinical practice. The meta-analysis showed that the reduced time of ICU stay was 0.57 days (13.64 hours) for adults with arterial acute limb ischemia, and the corresponding cost saving was $2,276 ($4,005 × 0.57). The reduced ICU time was about 1.18 days for the acute DVT population, and the corresponding cost saving was $4,730 ($4,005 × 1.18). Using the same approach, we estimated the cost saving for the ward stay. We present the parameters used to estimate the hospitalization costs of MT treatment in Table 45.
Table 36:
Summary of Findings of the Effect of Pharmacomechanical Thrombectomy Devices in Acute Lower Limb Ischemia (Arterial or Venous)
| Outcome | No. of participants (studies) | Effect | GRADEa | |
|---|---|---|---|---|
| Relative (95% CI) | Absolute (95% CI) | |||
| Arterial Acute Limb Ischemia | ||||
| Limb salvage | 425 (5 Obs) | OR: 1.61 (0.85-3.02) | 51 more per 1,000 (from 22 fewer to 95 more) | ⊕ Very low |
| Complete thrombus removal | 501 (5 Obs) | OR: 1.72 (1.07-2.77) | 101 more per 1,000 (14 more to 168 more) | ⊕ Very low |
| Patency | 403 (4 Obs) | OR: 1.77 (1.09-2.86) | 120 more per 1,000 (20 more to 198 more) | ⊕ Very low |
| Re-thrombosis (and revision rates) | 210 (3 Obs) | OR: 0.55 (0.29-1.06) | 118 fewer per 1,000 (208 fewer to 13 more) | ⊕ Very low |
| Perioperative mortality | 579 (6 Obs) | OR: 0.95 (0.43-2.09) | 3 fewer per 1,000 (32 fewer to 55 more) | ⊕ Very low |
| Adverse events | 602 (7 Obs) | More renal dysfunction/acute kidney injury, hematoma, distal embolization, and mean blood loss among patients who received MT compared to control groups | ⊕ Very low | |
| Volume of thrombolytic (mg) | 147 (2 Obs) | There are inconsistent findings of volume of thrombolytics used among people who received MT compared to control groups | ⊕ Very low | |
| Time of thrombolytic infusion (hours) | 300 (3 Obs) | — | MD: 13.64 lower (34.89 lower to 7.61 higher) | ⊕ Very low |
| Hospital length of stay (days after PMT | 264 (3 Obs) | — | MD: 1.10 lower (1.40 lower to 0.81 lower) | ⊕ Very low |
| Acute Deep Vein Thrombosis | ||||
| Limb Salvage | 151 (1 Obs) | OR: 2.63 (0.11-65.53) | 8 fewer per 1,000 (90 fewer to 12 more) | ⊕ Very low |
| Post thrombotic syndrome | 266 (1 RCT) | OR: 1.11 (0.65-1.91) | 26 more per 1,000 (99 fewer to 160 more) | ⊕⊕⊕ Moderate |
| 993 (6 Obs) | OR: 0.37 (0.26-0.54) | 127 fewer per 1,000 (154 fewer to 89 fewer) | ⊕ Very low | |
| Complete thrombus Removal | 430 (1 RCT) | RR: 1.04 (0.89-1.22) | 29 more per 1,000 (79 more to 158 more) | ⊕ Very low |
| 1,644 (12 Obs) | RR: 1.07 (0.94-1.21) | 43 more per 1,000 (17 fewer to 112 more) | ⊕ Very low | |
| Patency | 489 (6 Obs) | RR: 1.03 (0.94-1.14) | 19 more per 1,000 (39 fewer to 90 more) | ⊕ Very low |
| Re-thrombosis (and revision rates) | 165 (3 Obs) | There were no significant findings between study groups for freedom of re-thrombosis at 12 months, recurrence rates, or success rate where reintervention was required. | ⊕ Very low | |
| Quality of life | 430 (1 RCT) | — | MD: 4.33 higher (2.52 lower to 11.18 higher) | ⊕ Very low |
| Perioperative mortality | 430 (1 RCT) | OR: 1.59 (0.16-15.46) | 5 more per 1,000 (7 fewer to 108 more) | ⊕⊕ Low |
| 623 (4 Obs) | There were no significant differences in mortality reported, with most studies reporting 0 in both study arms | ⊕ Very Low | ||
| Adverse events | 430 (1 RCT) | There were fewer recurrent VTE and no significant difference in rates of bleeding between those who received MT and those who did not. Additionally, there were reported device-related events among 13.6% of patients who had received AngioJet | ⊕⊕⊕ Moderate | |
| 1,823 (13 Obs) | There were more cases of renal dysfunction, acute kidney injury and haemoglobinuria in some studies, while other studies had no statistically significant findings There were no statistically significant findings reported in bleedings or other complications | ⊕ Very low | ||
| Volume of thrombolytic (mg) | 269 (1 RCT) | — | MD: 0 (both study groups reported the same mean volume) | ⊕⊕⊕ Moderate |
| 1,170 (9 Obs) | — | SMD: 2.27 lower (3.6 lower to 0.95 lower) | ⊕ Very low | |
| Time of thrombolytic infusion (hours) | 269 (1 RCT) | MD: 2.0 lower (3.51 lower to 0.49 lower) | ⊕⊕⊕ Moderate | |
| 1,236 (8 Obs) | — | MD: 28.35 lower (45.64 lower to 11.05 lower) | ⊕ Very low | |
| Hospital length of stay (days after PMT) | 359 (4 Obs) | — | MD: 2.6 lower (5.08 lower to 0.12 lower) | ⊕ Very low |
Abbreviations: CI, confidence interval; DVT, deep vein thrombosis; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; MD, mean difference; MT, mechanical thrombectomy; OR, odds ratio; Obs, observational study; PMT, pharmacomechanical thrombectomy; RCT, randomized controlled trial; RR, relative risk; SMD, standardized mean difference; VTE, venous thrombosis embolism.
Note: summary of findings table developed using GRADEpro GDT. GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime, 2022. Available from gradepro.org
We evaluated the quality of the body of evidence for each outcome according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Handbook.70 See Appendix 2 for further details.
Table 45:
Description of Variables for Estimating Hospital Costs for Mechanical Thrombectomy Interventions
| Variables | Value (2022 CAD) | Description | Reference |
|---|---|---|---|
| C_CDT_I743 | $15,582 | Hospital costs of the CDT treatment for patients with arterial acute limb ischemia. We assumed it was same as the costs of the treatments of CCI code of 1.KG.35.HH-1C. | OCCI,141 Table 44 |
| C_CDT_I802 | $16,736 | Hospital costs of the CDT treatment for patients with acute DVT. We assumed it was same as the costs of the treatments of CCI code of 1.KX.35.HH-1C. | OCCI,141 Table 44 |
| C_PMT_ALI | $1,670 | The list price of AngioJet disposables (the Solent family [$1,500] and PowerPulse kit [$170]) for treating blocked arteries. We assumed that it is used for patients with arterial acute limb ischemia. | Shelley McFeetors, Boston Scientific |
| C_PMT_DVT | $3,070 | The list price of AngioJet disposables for PMT per procedure for DVT population. Zelante DVT ($2,900), and PowerPulse kit ($170). We assumed that it was used for patients with deep vein thrombosis. | Shelley McFeetors, Boston Scientific |
| C_MT_ Indigo | $5,725 | The approximate costs of disposable material using Indigo System is about $4,523 USD ($5,725 CAD) per vacuum aspiration procedure. There was no breakdown cost information. | Brian Sneek, Penumbra |
| C_CDT_device | $500 | Cost of CDT supplies for arterial or vein diseases. Note: this cost is included in hospital costs of CDT treatment (C_CDT_I743 or C_CDT_I802). For those who received the PMT or vacuum aspiration alone (without CDT), the cost of CDT supplies needs to be subtracted from CDT treatment costs. | Dheeraj Rajan, email communication, April, 2022 |
| Pct_PMTA_ALI | 52% | The proportion of PMT (AngioJet) procedures completed without concurrent CDT (i.e., PMT alone) for patients with arterial acute limb ischemia. | PEARL registry122 |
| Pct_MTA_ALI | 53% | The proportion of vacuum aspiration (Indigo) procedures completed without concurrent CDT (i.e., MT alone) patients with arterial acute limb ischemia. | Lopez et al, 2020139 |
| Pct_PMTA_DVT | 39% | The proportion of PMT (AngioJet) procedures completed without the concurrent CDT (i.e., PMT alone) for patients with acute DVT. | PEARL registry85 |
| Pct_MTA_DVT | 20% | The proportion of the vacuum aspiration (Indigo) procedures completed without the concurrent CDT (i.e., MT alone) patients with DVT. | Assumed |
| SD_ICU_ALI | 0.57 d (13.64 h) | Saving of ICU time (assuming ICU time is the duration of thrombolytic therapy), MT with or without CDT versus CDT alone for adults with arterial acute limb ischemia. | Meta-analysis (see clinical review, above) |
| SD_ICU_DVT | 1.18 d (28.35 h) | Saving of ICU time (assuming ICU time is the duration of thrombolytic therapy), MT with or without CDT versus CDT alone for adults with deep vein thrombosis. | Meta-analysis (see clinical review, above) |
| UC_ICU | $4,005 | Cost per day in ICU. Includes nursing, operating room, laboratory, medical imaging, pharmacy, etc.), but does not include physician fees. | Evans et al, 2018145 |
| SD_ward_ALI | 0.53 d | Saving of ward time, MT with or without CDT versus CDT alone (1.10-.57 d; savings in total hospital stay minus the savings in ICU time) for adults with arterial acute limb ischemia. | Meta-analysis (see clinical review, above) |
| SD_ward_DVT | 1.42 d | Saving of ward time, MT with or without CDT versus CDT alone (2.60-1.18 d, excluding the savings in ICU time) for adults with DVT. | Meta-analysis (see clinical review, above) |
| UC_ward | $1,224 | Cost per day in ward. Includes nursing, laboratory, medical imaging, pharmacy, etc.), but does not include physician fees. | Evans et al, 2018145 |
Abbreviations: ALI, acute lower limb ischemia; CCI, Canadian Classification of Health Intervention; CDT, catheter-directed treatment; CIHI, Canadian Institute for Health Information; DVT, deep vein thrombosis; ICD-10-CA, International Statistical Classification of Disease, 10th Revision, Canadian Version; ICU, Intensive care unit; MT, mechanical thrombectomy; OCCI, Ontario Case Costing Initiative; PMT, pharmacomechanical thrombectomy.
We note the following issues related to the estimated costs of MT treatment:
Since the unit cost of an ICU stay likely has included most health care costs (except physician fees), we did not consider cost of medications for thrombolytic therapy or the costs of the radiology suite stay. However, given that the global average unit costs of ICU time may inadequately reflect the true costs for MT and CDT treatment, we captured this uncertainty (e.g., greater savings from the medications) in our scenario analysis
We cannot distinguish the differences in ICU and ward stay reduction for MT with versus without CDT. However, we considered the cost savings of CDT supplies for MT alone
-
We did not include the capital costs of the equipment (the console for AngioJet PMT and the ENGINE Pump for Indigo) in the reference case for the following reasons:
Fourteen hospitals have conducted MT treatment in clinical practice. Since they have the equipment presently, the future need for replacement equipment is too speculative to quantify
Compared with the costs of disposable devices, the per-procedure cost of equipment is relatively low and hard to quantify. For example, the AngioJet console is used to set-up and monitor PMT procedures. The service life of console equipment may be more than 10 years and can be used for procedures other than PMT
We considered the capital costs of the equipment in the scenario analysis
Based on the parameters in Table 45, we developed the formulae to calculate the hospitalization costs for PMT in Table 46. For example, the average hospital costs of PMT (AngioJet) therapy for patients with arterial acute limb ischemia is C_CDT_I743 + C_PMT_ALI - (Pct_PMTA_ALI × C_CDT_device) - (SD_ICU_ALI × UC_ICU) - (SD_ward_ALI × UC_ward) = $15,582 + $1,670 - (52% × $500) - (0.57 × $4,005) - (0.53 × $1,224) = $14,065 (numbers may be inexact due to rounding).
Table 46:
Hospital Costs (Excluding Physician Fees) for Mechanical Thrombectomy Interventions
| Intervention | Cost (2022 CAD)a | Formula to calculate costa,b |
|---|---|---|
| Arterial Acute Limb Ischemia | ||
| MT (PMT w/wo CDT) | 14,065 | C_CDT_I743 + C_PMT_ALI - (Pct_PMTA_ALI × C_CDT_device) - (SD_ICU_ALI × UC_ICU) - (SD_ward_ALI × UC_ward) |
| MT (vacuum aspiration w/wo CDT) | 18,115 | C_CDT_I743 + C_MT_ Indigo - (Pct_MTA_ALI × C_CDT_device) - (SD_ICU_ALI × UC_ICU) - (SD_ward_ALI × UC_ward) |
| Acute DVT | ||
| MT (PMT w/wo CDT) | 13,143 | C_CDT_I802 + C_PMT_DVT - (Pct_PMTA_DVT × C_CDT_device) - (SD_ICU_DVT × UC_ICU) - (SD_ward_DVT × UC_ward) |
| MT (vacuum aspiration w/wo CDT) | 15,893 | C_CDT_ I802 + C_MT_ Indigo - (Pct_MTA_DVT × C_CDT_device) - (SD_ICU_DVT × UC_ICU) - (SD_ward_DVT × UC_ward) |
Abbreviations: CDT, catheter-directed treatment; DVT, deep vein thrombosis; MT, mechanical thrombectomy; PMT, pharmacomechanical thrombectomy; w/wo, with or without.
Some numbers may appear inexact due to rounding.
Descriptions of variables were presented in Table 45.
TOTAL COSTS OF INTERVENTIONS IN THE CURRENT AND NEW SCENARIOS
We determined total costs by adding hospital and physician costs. We used the CIHI Patient Cost Estimator tool to estimate the average cost of physician services. We took this approach because it is difficult to estimate the costs of physician services for major procedures (e.g., operation, diagnostic imagining, and interventional radiology procedures), as well as secondary procedures that may occur during hospitalization for people in our target population. Another contributing factor is that hospital costs may vary greatly depending on a person's individual characteristics (e.g., age) and comorbidities. There was also a lack of reliable data sources to allow us to quantify the physician fees incurred during hospitalization. Using the Patient Cost Estimator tool, we were able to obtain the average hospital costs and physician costs by Case Mix Group (CMG).142 Since we obtained the OCCI hospital costs in 2016, we calculated the ratio of physician costs to hospital costs for CMG 182 (bypass/extraction of vein/artery of limb) for that year. We used the resulting ratio (0.312) to derive the physician costs for the patients treated by open surgery, CDT, or MT treatment options. For example, the average cost of physician services associated with open surgery was calculated to be $5,532 ($17,732 × 0.312). The total overall cost of open surgery was estimated to be $23,264 ($17,732 + $5,532). Similarly, the total per-patient cost of PMT therapy was estimated to be $18,453 (hospital cost of $14,065 plus physician costs of $4,381 [$14,065 × 0.312]). For people treated with anticoagulant therapy alone, we used the ratio of physician to hospital costs for CMG 211 (deep vein thrombophlebitis). The average cost of physician services associated with anticoagulant therapy alone was estimated to be $1,042 ($4,781 × 0.218). The total overall cost of anticoagulant therapy alone was therefore estimated to be $5,823 ($4,781 + $1,042 [$4,781 × 0.218]). The average total cost of interventions of interest for both indications are presented in Table 47.
Table 47:
Estimated Total Average Costs of Treatments (Hospital and Physician Costs)
| Total cost (2022 CAD)a | Reference | |
|---|---|---|
| Arterial Acute Limb Ischemia | ||
| Open surgical treatment | 23,264 | OCCI and CIHI141,142 |
| CDT without MT | 20,444 | OCCI and CIHI141,142 |
| MT (PMT w/wo CDT) | 18,453 | Calculated |
| MT (vacuum aspiration w/wo CDT) | 23,767 | Calculated |
| Acute DVT | ||
| Anticoagulant therapy alone | 5,823 | OCCI and CIHI141,142 |
| CDT without MT | 21,957 | OCCI and CIHI141,142 |
| MT (PMT w/wo CDT) | 17,244 | Calculated |
| MT (vacuum aspiration w/wo CDT) | 20,852 | Calculated |
Abbreviations: CDT, catheter-directed treatment; CIHI, The Canadian Institute for Health Information; DVT, deep vein thrombosis; MT, mechanical thrombectomy; OCCI, Ontario Case Costing Initiative; PMT, pharmacomechanical thrombectomy; w/wo, with or without.
Some numbers may be inexact due to rounding.
In our analysis, we did not include the costs of adverse events post-treatment due to the following considerations:
The clinical evidence review showed that health outcomes (e.g., limb salvage and post-thrombotic syndrome) between MT treatment and comparators (typically CDT) were not significantly different and the certainty of the evidence was Low or Very low. We did not identify any studies comparing MT with open surgery
In practice, many adults hospitalized due to arterial acute limb ischemia or acute DVT often receive multiple interventions. It is difficult to specify which complications are caused by which intervention
Overall, it is difficult to assign cause to adverse events, whether due to MT or other treatment
Internal Validation
The secondary health economist conducted formal internal validation. This process included checking for errors and ensuring the accuracy of parameter inputs and equations in the budget impact analysis.
Analysis
We conducted a reference case analysis and sensitivity analyses. We presented the results of the budget impact for the populations with arterial acute limb ischemia and acute DVT separately. Our reference case analysis represents the analysis with the most likely set of input parameters and model assumptions. Our sensitivity analyses explored how the results are affected by varying input parameters and model assumptions. Our scenario analyses included:
The costs of AngioJet and Indigo disposable devices for MT only. We did not consider the potential savings from reduced ICU time, but focused on the direct expenditures of devices because the savings in ICU time would be allocated to other treatments and would not lead to direct financial savings for the hospital. Also, the costs of disposable devices for MT were much greater than the disposables for other interventions (see Table 45, above)
The size of target population is 86 for arterial acute limb ischemia and 120 for acute DVT (considering MT as an alternative to CDT only). The market share of MT was assumed to be the same as the reference case (Table 43), increasing from 25% in Year 1 to 45% in Year 5 for both populations
A target population 20% higher than in the reference cases (e.g., including some patients with the secondary diagnosis of a disease of interest). All other parameters were the same as in the reference case.
Lower costs of the disposable devices (25% lower than the list prices of devices) for MT treatment
Greater and lesser cost savings for ICU and ward stays (± 25% of the savings [$732 per person for arterial acute limb ischemia and $1,617 per person for acute DVT]). There was uncertainty around reduced ICU and ward time, as well as the unit costs of ICU time (e.g., the high costs of thrombolytic medications, tissue-type plasminogen activator [tPA] and the costs in the radiology suite) and ward time. Changes in ICU and ward durations or the ICU and ward unit costs would impact the costs of index hospitalization, so we used the ± 25% changes of savings to capture changes in two directions (i.e., increased and decreased costs). The other parameters were same as in the reference cases
Costs of the console of AngioJet and the Penumbra ENGINE of Indigo. We assumed that three AngioJet consoles and five Indigo Penumbra ENGINEs will be purchased for the whole province in next 5 years after public funding of MT. The list price of the console for AngioJet PMT procedures was about $47,500 (Shelley McFeetors, email communication, March 2022). The cost of Penumbra ENGINE Pump (re-useable, capital) is $15,000 (Brian Sneek, Penumbra Inc., email communication, March 2022)
Results
Reference Case
Tables 48 and 49 present the total projected costs over 5 years of the New and Current Scenarios in our reference case for the populations with arterial acute limb ischemia and acute DVT, respectively.
For the population with arterial acute limb ischemia, the total costs of the New and Current Scenarios were similar. The budget impact of publicly funding MT (PMT and vacuum-assisted MT) was associated with cost savings ranging from $0.17 million in year 1 to $0.14 million in year 5. The total 5-year budget impact was a cost saving of $0.83 million. Savings accrued in this analysis is attributed to reduced ICU and ward time and can be reallocated by hospitals to fund other priority areas. However, the magnitude of savings was small. Given the assumptions in our cost estimates, we may not interpret the results as cost saving. However, we may conclude that publicly funding MT treatment may not substantially increase the budget for the population with arterial acute limb ischemia.
Table 48:
Budget Impact Analysis Results for Adults Hospitalized Due to Arterial Acute Limb Ischemia, Reference Case
| Budget impact, in millions (2022 CAD)a,b | ||||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total | |
| Current Scenario | 7.74 | 7.74 | 7.74 | 7.74 | 7.74 | 38.68 |
| Open surgery | 5.98 | 5.98 | 5.98 | 5.98 | 5.98 | 29.89 |
| CDT without MT | 1.76 | 1.76 | 1.76 | 1.76 | 1.76 | 8.79 |
| New Scenario | 757 | 756 | 756 | 757 | 7.60 | 3786 |
| Open surgery | 4.49 | 4.19 | 3.89 | 3.58 | 3.30 | 19.45 |
| CDT without MT | 1.31 | 1.23 | 1.14 | 1.06 | 0.96 | 5.70 |
| Any MT | 1.77 | 2.15 | 2.53 | 2.93 | 3.33 | 12.70 |
| MT (PMT w/wo CDT) | 0.96 | 1.05 | 1.11 | 1.14 | 1.14 | 5.41 |
| MT (vacuum aspiration w/wo CDT) | 0.81 | 1.09 | 1.43 | 1.78 | 2.19 | 7.30 |
| Budget impact | -0.17 | -0.18 | -0.17 | -0.16 | -0.14 | -0.83 |
Abbreviations: CDT, catheter-directed treatment; MT, mechanical thrombectomy; PMT, pharmacomechanical thrombectomy; w/wo, with or without.
Negative costs indicate savings.
Some numbers may appear inexact due to rounding.
For the population with acute DVT, the New Scenario would lead to a total additional cost of $5.52 million over 5 years. The cost of anticoagulant therapy alone was much lower than MT treatments. The market share of anticoagulant therapy alone would reduce over time, and the market share of MT would increase. Thus, it led an increase in total budget.
Table 49:
Budget Impact Analysis Results for Adults Hospitalized Due to Acute Deep Vein Thrombosis, Reference Case
| Budget impact, in millions (2022 CAD) a | ||||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total | |
| Current Scenario | 4.27 | 4.27 | 4.27 | 4.27 | 4.27 | 21.36 |
| Anticoagulant therapy alone | 1.64 | 1.64 | 1.64 | 1.64 | 1.64 | 8.18 |
| CDT without MT | 2.63 | 2.63 | 2.63 | 2.63 | 2.63 | 13.17 |
| New Scenario | 5.04 | 5.21 | 5.37 | 5.54 | 5.72 | 26.87 |
| Anticoagulant therapy alone | 1.23 | 1.15 | 1.07 | 0.98 | 0.90 | 5.33 |
| CDT without MT | 1.98 | 1.84 | 1.71 | 1.58 | 1.45 | 8.56 |
| Any MT | 1.83 | 2.21 | 2.59 | 2.98 | 3.36 | 12.98 |
| MT (PMT w/wo CDT) | 1.21 | 1.38 | 1.55 | 1.71 | 1.86 | 7.71 |
| MT (vacuum aspiration w/wo CDT) | 0.63 | 0.83 | 1.04 | 1.27 | 1.50 | 5.28 |
| Budget impact | 0.77 | 0.93 | 1.10 | 1.27 | 1.44 | 5.52 |
Abbreviations: CDT, catheter-directed treatment; MT, mechanical thrombectomy; PMT, pharmacomechanical thrombectomy; w/wo, with or without.
Some numbers may appear inexact due to rounding.
Sensitivity Analysis
Tables 50 and 51 summarize the results of several scenario analyses for the populations with arterial acute limb ischemia and acute DVT, respectively. If only considering the costs of disposable devices of MT, the 5-year budget impact would be an additional $2.25 and $2.82 million for arterial acute limb ischemia and acute DVT, respectively. If considering MT as an alternative to CDT, the budget impact is very small for both populations. If purchasing the equipment for MT (e.g., console and pump) within 5 years, it will cost $0.22 million for three AngioJet consoles and five Indigo Penumbra ENGINEs.
Table 50:
Budget Impact Analysis Results for Adults Hospitalized Due to Arterial Acute Limb Ischemia, Sensitivity Analyses
| Budget impact, in millions (2022 CAD)a,b | ||||||
|---|---|---|---|---|---|---|
| Scenario | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total |
| Reference case | ||||||
| Budget impact | -0.17 | -0.18 | -0.17 | -0.16 | -0.14 | -0.83 |
| 1. Including only the costs of disposable devices for MT | ||||||
| Budget impact | 0.28 | 0.36 | 0.44 | 0.53 | 0.63 | 2.25 |
| 2. Considering MT only as the alternative to CDT | ||||||
| Budget impact | 0.00 | 0.01 | 0.02 | 0.03 | 0.04 | 0.11 |
| 3. Target population 20% larger than the reference case | ||||||
| Budget impact | -0.21 | -0.21 | -0.21 | -0.20 | -0.17 | -0.99 |
| 4. Costs of disposable devices 25% lower than the reference case | ||||||
| Budget impact | -0.24 | -0.27 | -0.28 | -0.30 | -0.30 | -1.39 |
| 5-1. Cost savings of ICU and ward stay of MT treatment 25% higher than the reference case | ||||||
| Budget impact | -0.23 | -0.25 | -0.26 | -0.26 | -0.25 | -1.27 |
| 5-2. Cost savings of ICU and ward stay of MT treatment 25% lower than the reference case | ||||||
| Budget impact | -0.11 | -0.10 | -0.09 | -0.06 | -0.03 | -0.39 |
| 6. Additional purchases of equipment for MT in next 5 years | ||||||
| Budget impact | — | — | — | — | — | 0.22 |
Abbreviations: CDT, catheter-directed treatment; ICU, Intensive care unit; MT, mechanical thrombectomy.
Negative costs indicate savings.
Some numbers may appear inexact due to rounding.
Table 51:
Budget Impact Analysis Results for Adults Hospitalized Due to Acute Deep Vein Thrombosis, Sensitivity Analyses
| Scenario | Budget impact, in millions (2022 CAD)a,b,c | |||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total | |
| Reference case | ||||||
| Budget impact | 0.77 | 0.93 | 1.10 | 1.27 | 1.44 | 5.52 |
| 1. Including only the costs of disposable devices for MT | ||||||
| Budget impact | 0.39 | 0.47 | 0.56 | 0.65 | 0.74 | 2.82 |
| 2. Considering MT only as the alternative of CDT | ||||||
| Budget impact | -0.11 | -0.13 | -0.14 | -0.16 | -0.18 | -0.72 |
| 3. Target population 20% larger than the reference case | ||||||
| Budget impact | 0.92 | 1.12 | 1.32 | 1.53 | 1.73 | 6.62 |
| 4. Costs of disposable devices 25% lower than the reference case | ||||||
| Budget impact | 0.67 | 0.82 | 0.96 | 1.11 | 1.26 | 4.81 |
| 5-1. Cost savings of ICU and ward stay of MT treatment 25% higher than the reference case | ||||||
| Budget impact | 0.60 | 0.74 | 0.88 | 1.01 | 1.15 | 4.39 |
| 5-2. Cost savings of ICU and ward stay of MT treatment 25% lower than the reference case | ||||||
| Budget impact | 0.93 | 1.13 | 1.33 | 1.53 | 1.74 | 6.65 |
Abbreviations: CDT, catheter-directed treatment; DVT, deep vein thrombosis; ICU, Intensive care unit; MT, mechanical thrombectomy.
Negative costs indicate savings.
Some numbers may appear inexact due to rounding.
If a hospital purchases equipment for MT, the equipment can be used for both populations—arterial acute limb ischemia and acute DVT. Since we included the scenario of additional purchases of equipment for MT treatment in next 5 years in the sensitivity analysis of arterial acute limb ischemia (Table 50), we did not include it in this table.
Discussion
We made a rough estimate of the budget impact of publicly funding MT for arterial acute limb ischemia and acute DVT in Ontario. Isolating costs and outcomes for MT treatment was complicated by the fact that MT is often used together with other treatments, such as CDT, balloon, and stent.135 The order and combinations of treatments can impact health care resource use. Also, clinical practices have some variation. There is insufficient data to quantify the health care resource use under these differing conditions. Mechanical thrombectomy has been shown to reduce the duration of thrombolytic infusion (or ICU time). Our analyses showed that, compared with CDT, the savings from ICU and ward time by MT treatment may compensate for the additional cost of the disposable devices and not lead to a substantial cost increase. However, if MT replaces anticoagulation therapy alone, it is likely to increase the budget since the cost of anticoagulation therapy alone is low.
The ICU and ward time reductions achieved by MT are likely to be reallocated to the other hospitalized patients, and so may not translate into actual monetary benefit. Therefore, eventually, publicly funding MT will likely increase costs due to purchasing the disposable device, but it will also create opportunities to improve the efficiency of hospitals and/or the health care system.
Mechanical thrombectomy may be used in hospitals that have established vascular programs. In addition to vascular surgeons, interventional radiologists, venous thrombosis specialists, and other professionals, the following facilities are often required for vascular care and management: medical imaging, vascular testing facilities, interventional radiology suites, operating rooms, anesthesia, critical care, and catheterization laboratories.146 Presently, there are 20 hospitals in Ontario that have established vascular programs.12 The assessment, diagnosis, and treatment (including endovascular interventions such as MT) of people with arterial acute limb ischemia and acute DVT can all be performed at these hospitals. The individuals who are inpatients at hospitals that do not have established vascular programs can be transferred to a hospital with an established vascular program for the MT treatment.
Strengths and Limitations
Our study had the following strengths:
Key parameters and main assumptions were verified by clinical experts in Ontario
Extensive sensitivity analyses covering many possible scenarios
Estimates of economic implications with consideration of the savings from ICU and ward time
The following limitations should be noted when interpreting the findings of this analysis:
There is a lack of published data on adults receiving MT treatment in Ontario. Thus, information on the volume, health care resource use, and cost of MT treatment in Ontario are all estimates
We used ICD-10-CA codes to identify the most appropriate cases for our analysis. However, due to the complexity and variability of clinical practice, it is difficult to accurately estimate the size of the eligible population for MT
Conclusions
Publicly funding MT in Ontario for populations with arterial acute limb ischemia may not lead to a substantial cost increase for the province. Publicly funding MT for acute DVT would be associated with an additional cost of $5.5 million over 5 years.
Preferences and Values Evidence
Objective
The objective of this analysis was to explore the underlying values, needs, and priorities of those who have lived experience of acute deep vein thrombosis (DVT) or arterial acute limb ischemia, as well as the preferences and perceptions of patients and family members of people who have mechanical thrombectomy (MT) as a treatment option.
Background
Exploring patient preferences and values provides a unique source of information about people's experiences of a health condition and the health technologies or interventions used to manage or treat that health condition. It includes the impact of the condition and its treatment on the person with the health condition, their family and other caregivers, and the person's personal environment. Engagement also provides insights into how a health condition is managed by the province's health system.
Information shared from lived experience can also identify gaps or limitations in published research (e.g., outcomes important to those with lived experience that are not reflected in the literature).147–149 Additionally, lived experience can provide information and perspectives on the ethical and social values implications of health technologies or interventions.
Because the needs, preferences, priorities, and values of those with lived experience in Ontario are important to consider to understand the impact of the technology in people's lives, we may speak directly with people who live with a given health condition, including those with experience of the technology or intervention we are exploring.
For this analysis, we examined the preferences and values of people with DVT or arterial acute limb ischemia who may or may not receive MT as a treatment option. We explored these preferences and values through direct engagement by Ontario Health with people with these conditions through interviews.
Direct Patient Engagement
Methods
PARTNERSHIP PLAN
The partnership plan for this health technology assessment focused on consultation to examine the experiences of people with arterial acute limb ischemia or DVT and those of their families. We engaged people via phone interviews.
We used a qualitative interview, as this method of engagement allowed us to explore the meaning of central themes in the experiences of people with arterial acute limb ischemia or DVT as well as those of their families.150 The sensitive nature of exploring people's experiences of a health condition and their quality of life are other factors that support our choice of an interview methodology.
PARTICIPANT OUTREACH
We used an approach called purposive sampling,151–154 which involves actively reaching out to people with direct experience of the health condition and the health technology or intervention being reviewed. We approached a variety of partner organizations to spread the word about this engagement activity and to contact people with experience with arterial acute limb ischemia or DVT and family members, including those with experience of MT.
Inclusion Criteria
We sought to speak with adults with lived experience of arterial acute limb ischemia or DVT and treatments such as MT. Participants did not need to have direct experience with MT to participate.
Exclusion Criteria
We did not set exclusion criteria.
Participants
For this project, we spoke with nine people with DVT living in Ontario (we were not able to speak with anyone who had lived experience with arterial acute limb ischemia), as well as one family member. We spoke with people who had experience with both MT and anticoagulation medications and people who had experience with anticoagulation medications only.
APPROACH
At the beginning of the interview, we explained the role of our organization, the purpose of this health technology assessment, the risks of participation, and how participants’ personal health information would be protected. We gave this information to participants both verbally and in a letter of information (Appendix 8), if requested. We then obtained participants’ verbal consent before starting the interview. With participants’ consent, we audio-recorded and then transcribed the interviews.
Interviews lasted approximately 20 to 40 minutes. The interview was loosely structured and consisted of a series of open-ended questions. Questions were based on a list developed by the Health Technology Assessment International Interest Group on Patient and Citizen Involvement in Health Technology Assessment.155 Questions focused on the impact of DVT on the quality of life of people, their experiences with treatments to manage or treat DVT, their experiences with MT, and their perceptions of the benefits or limitations of MT. For family members, questions focused on their perceptions of the impact of DVT and treatments on the quality of life of the person with the condition, as well as the impact of the person's health condition and treatments on the family members and caregivers themselves. See Appendix 9 for our interview guide.
DATA EXTRACTION AND ANALYSIS
We used a modified version of a grounded-theory methodology to analyze interview transcripts. The grounded-theory approach allowed us to organize and compare information on experiences across participants. This method consists of a repetitive process of obtaining, documenting, and analyzing responses while simultaneously collecting, analyzing, and comparing information.156,157 We used the qualitative data analysis software program NVivo158 to identify and interpret patterns in the data. The patterns we identified allowed us to highlight the impact of DVT and treatments on the patients and family members we interviewed.
Results
OCCURRENCE AND PROGRESSION OF ACUTE DEEP VEIN THROMBOSIS
Participants generally reported a mild onset of their DVT, often believing that it was of little concern and attributing symptoms to muscle aches or soreness due to physical activities. Participants were generally fairly young and described themselves as active and healthy individuals for the most part. Several participants commented on the physical activities and sports that they participate in and that occurrences of tightness or pain in muscles or joints were not unexpected.
I remember the feeling in my left calf…. It just felt kind of like a sore muscle and I thought I had strained it. I had done a long walk in the winter, the day before, and I thought, “oh, maybe it was too cold out” and “I did too much of a walk” and I just kind of wrote it off.
[I] played squash the night before, on a Tuesday. On Wednesday, I had a cramp, what appeared to be a cramp, in my left calf. And it didn't go away. It wasn't hard like a normal muscular cramp, but it felt just like a cramp. Had it most of the day.
And I was having almost like really bad tightness. Almost like when you work out super, super hard and do a bunch of squats or whatnot and your leg just kind of gets super tight and swells up. Except it was only in my left leg.
Due to the active nature of many of those who were interviewed, some participants reported feeling surprised when the initial mild pain or soreness did not dissipate and new, more worrying, symptoms began to emerge. Several people spoke about symptoms such as localized swelling, increased pain, changes in colour, or decreased sensation in the limb.
The DVT was found in my leg, and I remember seeing even my foot starting to turn colours. Like the bottom of my foot would be purple and blue.
The muscle wasn't firm, it felt like a normal uncramped muscle. But it wouldn't go away [with] hand massages. So when my [wife] got home, she took a look and said, “your calf and foot are the colour of white paper, unusually. “ It felt frozen and cold.
And I remember one day in class, standing and having to run to the washroom to take the compression stockings off because it was a really searing pain. I didn't see any anything…and] forgot about it. I did notice a hard lumpy area [later] and then it got swollen and then it got red.
More serious symptoms could also impact an individual's quality of life. Increasing pain, numbness, and swelling were attributed to a decrease in physical activity as well as challenges sleeping and participating in certain daily activities such as walking outdoors.
Many times, I couldn't sleep. It felt like someone squeezing [my leg] and squeezing it, or they're twisting and squeezing at the same time. And it'd be really painful.
Yes, I could limp around. So my leg wasn't collapsing. At the time, I was very fit, played squash, golf, whatever…so it wasn't unusual to have a cramp. The fact that I could limp around on it was normal. Except for the colour and the lack of feeling.
It just kept getting increasingly painful—like at this point I was using crutches because I can't put any weight on it.
Generally, participants did not report a great deal of anxiety surrounding their symptoms of DVT. While some people reported mild concerns and a desire to relieve their symptoms, especially as they became more severe, most reported few negative emotions until they were urged by others to seek medical attention. Some expressed surprise that their health condition would require a visit to an emergency department.
I got concerned and I went to my primary doctor, my family physician, and he immediately sent me to [the emergency department], which I was shocked at!
I was sitting in my doctor's office—I had just had an appointment—and he noticed…that my one lower leg was red and not the other one. And it was hot to the touch as compared to my other lower leg. And so, he thought I was having a DVT, and he told me to go to the emergency [department] right away—which I was surprised at…He called the emergency [department], so they were waiting for me.
I tried walking around and on the treadmill and the knee was getting stiffer and stiffer. I didn't really notice a size difference until one morning, early morning, I noticed that the leg was all puffed up…The leg was really ballooned up, really obvious… [and] they rushed me to the hospital.
DIAGNOSIS AND CARE JOURNEY WITH ACUTE DEEP VEIN THROMBOSIS
Most participants reported that their DVT was diagnosed quickly once examined in hospital. Participants described being assessed by medical staff and several reported receiving an ultrasound at the area of pain and discoloration and the clot was detected. For those people who received a quick and successful diagnosis, there could be feelings of relief.
I went to [the emergency department, and] got looked after right away, because they said, “oh that looks like a blood clot. “ It had stopped blood flow to my lower left leg, so I was admitted that night.
Thank goodness I went to him and right away he sent me that day for an ultrasound and they found a centimeter and a half blood clot.
My mother's nurse friend said, “go to the hospital immediately. “ She was pretty sure it was blood clots. So I went to the ER, ended up getting a shot of blood thinners that night.
Not everyone experienced a quick diagnosis, however. A few participants spoke of miscommunication, multiple consults, and delays, resulting in the diagnosis taking several weeks or months and contributing to longer periods of pain and diminished quality of life.
They ended up booking an ultrasound and, after getting the ultrasound, that's where we kind of ran into problems…We were told they're sending it to a specialist, it's going to take a little while to get an appointment with the hematologist. So we waited. I'll probably say we waited for like 4 months.
Finally, I was trying to get it addressed and they were just giving me different diagnoses. And that wasn't it. And the medication they tried to give me for those diagnoses, that's not it. And so finally, when I came to my family doctor, that's when they did more tests again…That's when it was diagnosed as DVT.
I went to my family doctor and told him,… “I think I went for a walk and might've hurt my calf and it's really hurting. “ So, he right away just prescribed me a Tylenol. I took [the] Tylenol that week and it's still… It just kept getting worse, and then [I] went back to him, he gave me [Tylenol 3], and then I went back a third time, and he gave me Percocet.
Receiving a diagnosis of a DVT could be an emotional experience. Some participants talked about their feelings of anxiety and fear after learning about their clot and its potential consequences, as well as surprise at the escalating seriousness of the condition. Others lamented their own previous disregard for their symptoms and were grateful that they received diagnosis before more serious consequences occurred. Still others reported that they were relatively calm and were focused on their treatment.
When it came to the blood clot, it was like “finally, we found out what was wrong, okay, let's treat it. “ It's probably different from other people's reactions. Some people would be really upset and nervous and scared. I wasn't. I was just annoyed with the other [clinic that failed to diagnose the clot].
I'd never even heard of a DVT before! And once I learned about what it was, it was kind of freaky. I didn't like to have that happen [and] I was worried that it would happen again…So, yeah, that's an emotional concern I had.
I didn't appreciate the seriousness of my situation. I just thought it was part and parcel of varicose vein pain. I did not [understand] a blood clot that could kill me.
Some participants expressed a desire to understand where the blood clot had come from, why it had formed, and what they had potentially done wrong to allow it to occur. While this type of clarity may have been helpful for participants, most reported that no cause was determined, which could lead to feelings of frustration and concern that the clot may return in the future.
The other thing is frustrating in a way, that the vascular team could suggest was maybe [a cause]—[they pointed] a finger at my smoking history. So that's a little frustrating…not being able to be definitive [about the cause], but they [can] read the tea leaves like other people do and say, “here are the predispositions, of which you had one and possibly that was sufficient to block it off. “ Anyway, [I] haven't experienced it since, [and] hopefully won't.
I think for me what I would have liked to have known is how do blood clots [form]? Why are they coming? I would like to know the source, of where it's coming from.
Additionally, some participants and family members commented on the impact a DVT diagnosis could have on mental health. The impact the condition has on quality of life and the requirements for treatment led some participants to report feelings of depression and overall frustration.
Knowing you're probably going to be on blood thinners for the rest of your life. It could have been dealt with sooner, [but it] wasn't based on communication errors, I guess I'll say. That hit me pretty hard because I did feel like I wasn't going to be able to actually go, you know, achieve my dreams.
I was very depressed about what I was going through…I know my family was upset. The whole COVID situation made [it] worse, everybody depressed and worried and stressed.
I was concerned for his well being for a while. Mainly because his life, he's been active since he was, like, two, playing sports. And a very active kid goes from that to a hard stop. [He] couldn't even climb a set of stairs. So that was very difficult for him.
TREATMENTS AND DECISION-MAKING
After diagnosis, most participants reported that their DVTs were managed and treated with anticoagulation medication. Some reported that they received these medications intravenously in hospital for a time, while others received regular injections in the stomach or took the medications orally at home. In addition to the anticoagulants, some participants also reported having regular bloodwork done to check their status and make sure the medications were effective. Others reported having to return for regular appointments for additional testing or imaging, such as ultrasounds.
Other than the shots in my belly, I don ‘t remember being on anything else. I think I did have some kind of drip on me in the hospital, [but] I don't know what was in there…. But yeah, definitely nothing physical to try to break it up.
I don't recall having anything other than blood work done to check the INR [International Normalized Ratio]. The first day I remember it being 2.2 and there was some concern. And then it was 1 point something on the subsequent INR and they were okay with that. That was never explained to me. Those were the three tracking tests that they did for me.
I was taking a needle in my stomach for it and then, after about three weeks, four weeks…I had to do it once or twice a day…I stayed on the needles for about a month, then they put me on warfarin and from there…they transitioned [me] to baby aspirin.
Participants reported that the required injections could be painful and left bruising on their stomachs.
Oh yeah, my stomach was dark purple, like grey-purple. And I can't figure out why you could give yourself a needle in other areas of your body and it doesn't turn dark purple but in your stomach it turns dark purple. The bruising didn't hurt; it just wasn't the best.
No Iconcernsl. Just the “ouch “ of the needle [laughs]. I didn't like being poked in the belly all the time [laughs]. So, other than fear and anxiety, no, I didn't have any [concerns].
Some participants reported that they were instructed to monitor their DVT and the progression of symptoms on a regular basis while taking anticoagulants at home. They were generally aware that their clot had the potential to move from their lower limbs to elsewhere in the body and that it could have serious negative consequences. While participants received instructions on what to do in case of symptom progression, some reported feeling scared and anxious at the lack of definitive treatment or close monitoring.
I was sent home scared because of that 20-minute window. [I was] told it was life threatening; that the clot could “break and go up to the lungs and you've got 20 minutes to do something about it.” So, I felt very unprotected and uncared for. So, yeah, it caused a lot of anxiety and very [poor] sleep!
It was in my calf, so they were really reassuring that, you know, “it's not in your lungs. “ [Laughs] But they definitely told me that [it was a] “we don't want it to go there” kind-of-thing.
Overall, when it came to treatment options for their DVT, participants generally reported that there was little decision-making on their part that went into choosing one treatment over another. Participants accepted the treatment option provided by their care provider and didn't report exploring other options or having discussions with physicians about other treatment options. For some, the emergency nature of the situation made such discussion unlikely. A few participants commented on their concerns about the chemical effects of the medications they were required to take, but did not believe they had any alternative.
No [discussion] at all. It was just, “this is what we're doing. The name of the drug is warfarin and we're going to give it to you by injection, “ and that was about it. I don't remember., there were no choices available or any lengthy explanation. It was just “this is what we're doing. “
Because supposedly that is what they use warfarin for—killing rats or something. But anyways, I just remember thinking “oh my gosh, and I'm putting that in my in my body!” I definitely did not like the idea of it, but at the same time it was., I just remembered them saying, “that's what we have” right? Like “this is what you do when you have a blood clot; you try to thin your blood, and this is a blood thinner. “
Yeah, I think they just immediately assumed it was a DVT and took that action. Everything was done before I even talked., you know, had a chance to discuss what they were doing. They just did it.
MECHANICAL THROMBECTOMY
With only a few exceptions, participants reported that the relative lack of choice in treatment decision-making included a lack of information around MT as a treatment option. Given the relatively small number of interview participants, the narrow clinical appropriateness of this treatment option, and its unavailability at most medical centres, it is not surprising that most people we spoke with reported that MT was not presented as a treatment option. During interviews, participants were prompted with information about MT and asked if this treatment option would have been of interest, had they been appropriate clinical candidates. Most told us they had little to no knowledge of the technique and where that the technology was available in Ontario.
There's some kind of way to do it by physically breaking [it] up? There was definitely no talk of that in 2006, so it's good to know!
No, I wasn ‘t consulted at all or told there were any other options like [MT]. They move very quickly.
No, I wasn't aware of what the options were or [that] there was even an option to have [the clot] removed. I wasn't aware till they did it on my lungs. Even then, I wasn't aware they could do it on my leg.
When presented with the hypothetical option of receiving MT treatment, participants were generally positive towards the concept and the potential impact of the treatment. Some of the positive components of the treatment that participants identified were the perceived potential for a quicker resolution to their symptoms as well as the belief that perhaps MT would decrease the amount of blood thinner medication required or reduce the duration that this medication would be necessary. Some participants lamented the fact that their care journey did not allow them to access this technique.
I think it's faster to get rid of it rather than taking injections or eventually taking pills. I think it would be faster to just have it removed.
But I think I was in the hospital for about 2 weeks, and, at the end, the clots were gone, but they put me on warfarin pill form, and I was on that for like 6 months. And I had to go and have my blood tested so they knew how much warfarin to give me. So, I had my blood tested like every 2 days or so. It was very inconvenient [laughs]. I would have much rather have the blood clot removed and not have to worry about all that crap.
This could have been dealt with much [more easily] if we knew about this 6 months ago. By the time we had actually gotten in, the clots had like dried up and were stuck to the inside of my veins. They were no longer like active clots. I don't know if that's the best way to describe it, but I think that's pretty accurate.
A few participants expressed concern at the prospect of the MT procedure. There was general concern at the potential pain of the procedure and a desire to be conservative when exploring treatment options. Participants also acknowledged their inability to comment on the clinical benefits of MT given their current understanding of the procedure. They expressed trust that their physician would choose the best options in an emergency situation.
I wouldn't want to be the first to try it out for a couple of reasons; threading that wire through a vein, it's got to be painful and you're already in exquisite pain. It was severe pain! So, that's one thing that would worry me. The other is, you say that it's going to suck up all the debris, or if it's going to exacerbate the chance of a piece of that clot going up to the heart and lungs. So, you haven't told me yet, but if 1,000 people had no trouble then I would have no trouble…. But I don't want to be [the first].
I don't want to be a “Debbie Downer” about mechanical [thrombectomy]. As I said, you have to show me the evidence, you have to convince me if I'm given a choice. And I'm honestly… based on my experience in that situation, I don't think you're given the choice. It's going to be whatever the emergency doctor is more comfortable with and more experienced with. That's what you're going to get as your treatment. First, do no harm.
Participants who received MT to treat their DVT reported learning about this alternative treatment in hospital early in the diagnosis stage. One individual reported that he advocated for the procedure, wanting an alternative anticoagulation medication. There was a perception that the treatment would result in quicker resolution of the clot than would be achieved with medication alone. Several participants commented on their desire to be rid of the clot as quickly as possible.
For me, even though they said it was risky., I wanted something else because I was so uncomfortable. I didn't know how long I would be like this. And it might take a longer process for me to use those blood thinners., but I definitely wanted to walk again, so that was the main idea. I said, “Please, something else. “
Anyway, after they decided and after I signed the papers, I was very happy that at least something was going to be different, be improved. I was lucky to get that second chance. I was lucky I pushed for it.
With all the issues I was having with my blood, my body was basically black and blue. [I] was like, “just do something to get it done”.because I just wanted it dealt with. So whenever they told me that they were going to take it out, it was the best thing for me.
People we spoke with who had MT felt that they were well-informed about the procedure and that it met their expectations. They described it as a relatively short procedure, but it required a hospital stay of a few days for recovery and monitoring. Some reported that the procedure caused some pain and discomfort, along with bruising afterwards. One patient reported that the procedure had to be done twice, as the first time did not break the clots completely. But, overall, participants reported being satisfied with the procedure. They were fascinated by the ability to observe the catheter in their blood vessels as it traveled to break up the clots.
I think it was probably what you'd call “mechanical thrombectomy” because it was inserting a tool into my main vein in my leg at my groin. And I gather in addition to the thinners that were being pumped into me, I think it was also they were driving some kind of…a tool, a device, that goes into the blood vessel and breaks up the clot [and] suctions it out. The only reason I know that [is] that I was semi-conscious.
It was just painful for that moment during the operation, but it was also fascinating, and I was able to see the big screen while they were doing it. But I figured it was coming to an end there, s o I didn't complain.
I look to my side and I'm looking at Google Maps. And Google Maps happened to be the veins in my leg. That's what they used, I guess, to drive the tool they use to break it up.
Once the clots were removed or broken up by the procedure, participants reported an almost immediate sensation of returning blood flow to the affected area, which could sometimes be quite painful. Some people also noted that bruising occurred during the procedure. These participants reported staying in hospital for several days after the procedure, depending on the circumstances and the degree of recovery needed.
I know from my mid-thigh to my waist I was totally black and blue. You know from the subcutaneous hematoma. In recovery, I was in pain. They gave me a self-injection—if you feel pain, push the button. I was leaning on the pain relief because…as the blood flow started to get through my blood vessels, it was like pins and needles, except in extremis. It was very very painful.
The one thing that [concerned them] was that I [didn't have] a proper pulse in my foot at the time. They were really worried about the blood flow going to my foot. And so they were constantly checking the pulse in my foot. Once they had surgically removed [the clot], the blood flow to my foot started to be a lot better.
The circulation started right away once they blew that dam of clots. And I started getting a lot more energy, my pulse went down a bit. I guess with the clot, the heart was working harder, right?
IMPACT OF ACUTE DEEP VEIN THROMBOSIS AND ITS TREATMENTS
Whether they received medical management of their DVT with blood thinners or the MT procedure, participants generally reported an eventual resolution of their clots and an improvement in their quality of life. The swelling in their lower limbs was diminished and the pain and soreness due to the DVT also resolved. However, improvement could take several weeks or months and most participants reported weakness in the limb that persisted for some time. Some participants who had been admitted to hospital reported receiving physiotherapy to help improve their return to function.
By the fall of that year—May was when I got discharged—I remember in my head thinking., “oh I can actually sit down and get up without feeling any kind of,” I wouldn ‘t say pain, but [there] was definitely like a tightness in my leg for months after.
Six years later [I'm probably] still not back to the same physical condition that I was in. Because it put me at rest for a couple of months at least—2, maybe 3 months—I didn't do much. It wasn't a total restriction; probably I took it more seriously than necessary. And then, that being the case, you actually end up becoming a couch potato instead of a little more active lifestyle. So that's longer term and you know it's., I blame having had the clots.
Several participants reported longer-term consequences of having a DVT. They were informed by physicians that the occurrence of a DVT increased the risk of it happening again in the future. Several participants mentioned being told that activities such as flying would be an increased risk, and others reduced or restricted their participation in physical activities due to fear of reoccurrence. Additionally, some women reported being advised that they would need to take extra precautions in the event of pregnancy.
Before I even got pregnant, I remember my doctor saying, “you know, if you ever want to start a family, you just need to consider…he literally told me “try not to be on bed rest. “ [Laughs] Not that you can help that.
I ended up…I quit the rugby team. I figured it was probably not a good idea to [play] with blood or potential blood clots. But outside of that, it was just a lot of aches and deep…I don ‘t know how to explain it. Like, deep in my leg. The pain wasn ‘t constant, at least.
While some interview participants reported that they felt they had recovered fully and were not distracted by a fear of recurrence, others spoke about the mental challenges of longer-term recovery and the frustration that can occur when quality of life hasn't returned to levels enjoyed previously.
Once I was cleared with the final ultrasound and blood work. I just had a follow up with my family physician for extra blood work, probably about 2 to 3 months later. And everything had settled down and I never gave it another thought. I was told that “if you've made a clot once, you tend to be more vulnerable to making one again” and I kept that in the back of my mind. And then that's it—it was one and done.
Does it affect your emotions? I think it's a whole package. That you are originally like “oh, I'm going to be better in less than a year and things are OK. “ You don't worry too much. And then [a] year turns into 2 years or 2 years turns into 3 years. And then you get frustrated over different things.
One participant who received MT later had reoccurrence of DVT. This person's clots were managed with anticoagulation medication, but he expressed a desire to have the MT again, believing it could resolve the issue more quickly.
Because my blood issues had been corrected and everything else like the platelets, they did it [by medication]. They said if it came to it, they would do [mechanical thrombectomy again]. I believe that if they had done [mechanical thrombectomy], I probably wouldn't have been in hospital as long. I was there for a week before they could release me because…the blood tests that they were taking, and everything were showing that the blood clot hadn't broken up enough…. I think if they had done the mechanical thrombectomy at the time, it would have been a lot quicker for me in the hospital, so I would probably have been the released a lot earlier.
BARRIERS
Participants were asked about barriers they experienced in accessing care for their DVTs or the potential barriers that may exist for seeking the MT procedure. Several reported on the difficulty treating their DVTs with medication. The requirement for regular bloodwork and injections could be somewhat burdensome if it required travel or time away from employment or other activities. A few people from northern Ontario commented on access to services such as ultrasounds, which are necessary for the diagnosis and treatment of DVT.
I had to go 21/2 to 3 hours to the next city. Or the next city would have been like 6 hours away. I was in northern Ontario, so the next city would have been Sault Ste Marie or Thunder Bay, and Thunder Bay was a lot farther. It's winter, so it's hit and miss when you can drive, because you have to go by the weather too. Yeah, barriers I think were just…I wasn't in a city, I was just out in this small town. And they had a clinic, but it was your family doctors. They didn't have a specialist.
I had to measure out the medication into the needle myself. I wouldn't have wanted to go back and forth to the hospital every day just to get injections. It takes like 30 seconds…. I did it myself, and then when they were going to transition me to warfarin, then I would go [in] for routine blood tests.
They put me on warfarin pill form, and I was on that for like 6 months. And I had to go and have my blood tested so they knew how much warfarin to give me. So, I had my blood tested like every 2 days or so. It was very inconvenient
One participant reported that it was difficult to access MT in smaller communities, where the expertise required to do the procedure might not be available.
I'd like to know the human resource costs, because you've got to have an expert that can thread [the wire] through a vein—that's not easy. Those things are smaller in the legs, so that takes a vascular surgeon or whatever. And in Thunder Bay, I'm not sure we even have a vascular surgeon…. We're a city of 120,000. It's a regional hospital. How many have the expertise for mechanical [thrombectomy]?
Discussion
Engaging participants through direct interviews allowed for an examination of the experiences, preferences, and values of patients regarding DVT and treatment options such as MT. All participants had experienced DVT or were family members of someone who had. Participants also had experience with anticoagulation medication as a treatment for DVT and some had direct experience with MT. Participants were able to comment on the impact and consequences of DVT and changes to their quality of life due to this health issue, both for themselves and for family members.
A limitation of this work was that we could not speak with anyone who had lived experience with arterial acute limb ischemia, and it is unknown whether the preferences and values for this population would be comparable to those with DVT.
Those participants who were family members of someone who had experience with MT and could speak to their values and preferences about treatment options and whether MT fulfils those preferences. In this way, the interviews allowed for a thematic analysis of a wide variety of perspectives and for a full consideration of the perceived value of MT.
Mechanical thrombectomy requires specialized skills and is provided only in certain medical centres. The low number of participants with direct experience of this treatment option was a limitation of this engagement. Further interviews may have provided additional context around decision-making and values when it comes to treatment options for DVT.
Conclusions
Acute deep vein thrombosis may initially present with mild symptoms, but can progress and have a significant impact on the health and quality of life of patients. Participants reported having anxiety about the potential health consequences of DVT and they trusted their physicians to choose the most appropriate treatment option. While most participants were not aware of and had not received MT, it was generally seen as a positive option and those who had received it reported positively on its value as a treatment to quickly remove a clot. Accessing treatments for DVT could be a barrier, especially for people living in more remote areas of Ontario. We did not speak with anyone who had lived experience with arterial acute limb ischemia.
Health System Stakeholders’ Perspectives Summary
We conducted a stakeholders’ perspectives analysis for this health technology assessment (HTA) to provide contextual information from clinical and health system stakeholders’ perspectives on the use and non-use in Ontario of mechanical thrombectomy (MT) for blocked blood vessels in the lower limbs.
Research Question
What are the clinical and health system stakeholders’ perspectives on the barriers and facilitators of accessing MT for blocked blood vessels in the lower limbs?
Methods
PERSPECTIVES PLAN
Stakeholders were directly engaged to gain perspectives on the use and non-use of MT through a survey (Appendix 7). For this project, hospital administrators were considered health system stakeholders.
From stakeholders who are using the technology, we sought to learn what the drivers and facilitators of use are for MT for blocked blood vessels in the lower limbs, along with the barriers they encountered, including resourcing needs, equity implications, and the expectations for the future state of the technology.
From stakeholders who are not using the technology, we sought to learn why it was not being used, if there was interest in using it, and what they saw as the barriers to adoption. Other contextual considerations that may also be discussed include where the technology is used in the pathway of care, who the receptor (the stakeholder responsible for implementing the technology in the health system) of the HTA might be, how the technology might be funded by the health system, what resources are or are not in place to adopt the technology, and the potential disinvestment opportunities.
The Context and Implementation of Complex Interventions (CICI) Framework was leveraged to inform the development of this analysis and the engagement survey questions.159 The CICI framework was developed to assess and document the context and implementation of complex interventions and address limitations around evaluating a complex technology. The framework includes eight domains of context (setting, geographical, epidemiological, socio-cultural, socio-economic, ethical, legal, and political) and four domains of implementation (provider, organization and structure, funding, and policy).
The survey questions were revised based on feedback from health technology assessment receptors in Ontario, who are responsible for implementing the technology. The survey methodology was chosen as the method of engagement to reach variety of audiences and provide flexibility for respondents to participate.
PARTICIPANT OUTREACH
We used an approach called purposeful sampling, which involves engaging stakeholders who are especially knowledgeable or experienced with using the health technology.160 Purposeful sampling is also commonly used in implementation research. We also used snowball sampling to identify additional contacts through the survey. Ontario Health-CorHealth assisted with identifying stakeholders from vascular centres. The engagement questionnaire was sent through Alchemer.161
Inclusion Criteria
We approached a variety of clinical and health system stakeholders located at hospital sites across Ontario who may be familiar with the use and non-use of MT for blocked vessels in the lower limbs. There are three levels of vascular centres in Ontario (Figure 23). Sites were selected to achieve a cross-section of centre levels, geography, and experience with the technology. We also reached out to sites that are not vascular centres, but may be using MT technology to treat lower limb ischemia.
Figure 23: Questionnaire Reply Map.
There are three vascular centre levels:
-
Level 1 (9 hospitals)
Level 2 and 3 criteria plus:
Have infrastructure, equipment, and clinical composition to perform advanced open aortic aneurysm repair and advanced endovascular aneurysm repair
Cardiac surgery program
-
Level 2 (10 hospitals)
Level 3 criteria plus:
Have infrastructure, equipment, and clinical composition to perform endovascular aneurysm repair
Maintain annual aortic aneurysm repair volume greater than or equal to 30 standard and/or moderate cases, where greater than or equal to 15 are endovascular aneurysm repair
Service availability 24/7, stand alone or with other vascular centres
-
Level 3 (1 hospital)
Baseline of services: perform abdominal aortic aneurysm surgical repair, carotid endarterectomy, lower extremity revascularization; composite volume greater than or equal to 50 cases per year
Exclusion Criteria
We did not set specific exclusion criteria.
Participants
The engagement questionnaire was sent to 14 administrators of hospitals and other medical sites in Ontario. They were asked to complete the survey themselves or have it completed by someone in their centre.
APPROACH
The questionnaire introduced the HTA and stakeholder perspectives summary and advised recipients that the objective was to gain further contextual information from a clinician and health system stakeholder perspective on the use and non-use of MT for blocked vessels in the lower limbs. The questionnaire was sent out in November 2021, and recipients were asked to return the completed questionnaire within 6 weeks. An additional 6 weeks was provided on follow up. The questionnaire was designed to take approximately 10 minutes to complete. There were 13 questions for facilities using the MT device, and 9 for facilities not using the device (Appendix 7).
Of the 14 sites chosen to receive the questionnaire, 12 were vascular centres (there are 20 vascular centres in Ontario). Nine sites were identified as using MT for acute and subacute blocked arteries and veins in the lower limbs, three were not using the technology, and two were of unknown status. Sites were located in all regions of Ontario except the North East region.
DATA EXTRACTION AND ANALYSIS
A descriptive thematic analysis was used to analyze questionnaire results. The qualitative data analysis software Nvivo was used to identify and interpret themes in the data.
Results
Of the 14 sites that were sent the questionnaire, eight clinicians responded from eight different sites. Four were Level 1 vascular centres and four were Level 2 vascular centres. Five of the centres were using the technology and three were not. Figure 23 provides further details of the questionnaire respondents.
OUTCOMES
STAKEHOLDERS USING MECHANICAL THROMBECTOMY
Five sites advised on the use of MT. Level 1 sites were using MT for acute limb ischemia and DVT, in addition to pulmonary embolism. Level 2 sites were using MT for these indications as well as mesenteric arteries.
Facilitators and Barriers
There were several drivers and facilitators to using this technology at the five sites. According to questionnaire respondents, cost is the main barrier to use of the device. Only one other barrier was mentioned: training for nurses to manage the arterial and venous sheath.
Facilitators for using MT:
Provides an alternative to surgery/OR
Perceived improvement in outcomes, including mortality, morbidity, and limb salvage
Addresses an unmet need
Avoidance or decrease in the amount of time a patient may spend in ICU
A rebate program from industry that addressed cost challenges to using the technology
Funding available for the use of the technology
Resources to use the technology were available at the hospital site, including equipment and training for nurses, clinicians, and interventional radiologists to perform the surgery
Inability to transfer patients to other sites for emergent treatment
Meets a need to deliver more acute therapy
Perceived fewer complications
Thought to provide more timely treatment
Barriers
The costs associated with using the technology
Training for nurses to manage the arterial and venous sheath
Resourcing Requirements
Numerous resourcing requirements were identified by centres using the device, including:
Infrastructure resources: the intervention radiology suite, ICU step down unit, ICU, OR
Equipment resources: thrombectomy device, catheters, pumps, tubing
Human resources: interventional radiology (IR) nurse, IR technicians, medical radiation therapist
Training and education: IR nurses and physicians trained on the device, nurses trained to manage arterial and venous sheaths
Expanded funding
Equity Considerations
Access to the MT device is not consistent across the province, creating perceived equity concerns. Three questionnaire respondents, two of whom are from a level one vascular centre, and one from a level two centre (all of whom have access to the technology) identified equity as an issue. These respondents advised that different places have different access to the device, and access to different supporting vendors. Larger sites may have the budget to access the technology, while smaller sites may not. Access may also be variable due to different facility priorities. Questionnaire respondents felt this may lead to poorer outcomes for patients in areas or in facilities where the device is not available.
Two questionnaire respondents, a level one and a level two vascular centre, who also have access to the technology, indicated that there were no perceived equity issues. The level one site advised that adoption was not an issue for them. The level two site advised that they have the equipment and trained interventional radiologists, along with the funding and trained nurses to use MT.
Future State
When asked about the future state of MT, some respondents indicated that they expect massive and submassive pulmonary embolisms (PEs) to be an area of growth for MT treatment. As the device improves, it may also be used for outpatient management of leg DVT, PE, and more challenging cases/clot load versus catheter-directed thrombolysis. Funding was also mentioned as a support need for future use of MT, either through direct program funding, volume funding, or quality-based procedure funding. One stakeholder advised that they did not foresee any expanded use of the device beyond the current indication and patient population. This respondent's facility already has access to the technology and is using it for acute limb ischemia and deep vein thrombosis, and did not indicate why they did not expect to see any expanded use.
One questionnaire respondent advised that MT needs to be supported and funded as an essential tool when dealing with acute limb ischemia, typically avoiding an OR or catheter-directed thrombolysis (freeing an ICU bed for other patients). It was also suggested that further study is required to show actual cost-savings and outcomes compared to conventional therapy (e.g., surgical thrombectomy or catheter-directed drip thrombolysis).
STAKEHOLDERS NOT USING MECHANICAL THROMBECTOMY
All three sites who advised they are not using the technology indicated that they were interested in its use, although one respondent indicated that their site handled too few procedures to justify the cost of acquiring the device. They reported feeling that larger centres are better suited for MT. They noted that they felt that the evidence shows benefits for patients with the right indications.
The respondents indicated that MT is not available due to human resourcing and cost implications, particularly a lack of sufficient funds and the personnel required for after-hours coverage. One respondent indicated that their site is in discussion with vendors to implement MT with daytime/planned procedures. Another opined that this technology should be mandatory at all level one vascular centres and thought it to be cost-effective and reduces ICU time, particularly for venous procedures and acute occlusions. This site, a level one vascular centre, indicated that funding to maintain equipment is the primary barrier to adoption.
Barriers to Adoption
When asked about barriers to adopting the technology, respondents provided reasons similar to those explaining why it's not available. Low case-use volume and cost of the equipment and device were common barriers, along with the cost of staff for urgent procedures.
One respondent indicated they had difficulty identifying which version of the technology was the best fit for their site. The respondent noted the expected value of Ontario Health's review of the technology to assist them in their decision-making.
Discussion
Outreach for this stakeholder perspectives summary yielded engagement with eight clinicians who have experience with the use of MT or are familiar with the technology.
Most stakeholders from level one and level two vascular centres, whether using the technology in their facility or not, are supportive of its use. Sites that are supportive of the technology perceived that MT offers an alternative to surgery, improves outcomes, meets an unmet need, decreases ICU stays, can offer timely treatment, and reduces complications from treatment. However, volume and cost, along with human resources, including training, are barriers to adoption.
For those who are using the technology, all the required resources were available to support adoption. However, cost and training seem to still be barriers for expanded use. For those centres not using the technology, two were supportive of its use at their centre, and a third indicated that the cost was not justifiable due to the low volume of procedures.
To support further adoption of MT in Ontario, respondents felt that infrastructure, funding, equipment, training and education, and health human resources (including after-hours coverage) require consideration. Perceived inequities of access may be addressed if MT is adopted more widely.
LIMITATIONS
The small number of respondents who had awareness of the technology (either through use or experience with the technology) may limit the generalizability of their perspectives. The questionnaire was initially distributed to administrators, who forwarded it onto clinicians. This may have contributed to bias due to the clinicians having an interest in using the technology, Additionally, the level of expertise of the clinicians who completed the questionnaire was unknown. All questionnaire responses were from vascular centres. We did not receive responses from other sites we reached out to.
Conclusions
The majority of clinical stakeholders with whom we engaged were supportive of the use of mechanical thrombectomy. For those currently using the technology, facilitators to accessing the technology included perceived improvements in patient outcomes, existing resources, addressing unmet needs, and reduction/avoidance of ICU stay. The main barrier was cost.
For those not using the technology, the barriers were low case-use volume, costs for the equipment, and health human resources.
Conclusions of the Health Technology Assessment
Based on the clinical evidence identified (3 RCTs and 37 observational studies), MT for people experiencing a blockage (blood clot) in blood vessels of their lower extremities may demonstrate greater technical success and patency for patients experiencing arterial acute limb ischemia compared to alternatives such as CDT infusion, but the evidence is uncertain (GRADE: Very low). In acute DVT, MT may reduce thrombolytic medication volume used (GRADE: Very low) and the duration of thrombolytic infusion (a determinant of intensive care unit stay duration) compared to not using MT, but it is uncertain if use of MT leads to a meaningful reduction in transfusion time (GRADE: Moderate to Very low). It may also reduce the proportion of people who experience post-thrombotic syndrome (GRADE: Moderate to Very low). Additionally, MT may reduce hospital length of stay for both populations (GRADE: Very low).
We identified one published cost-utility analysis that was partially applicable to the Ontario context. This study showed that the combination treatment of PMT and/or CDT was not cost-effective compared with anticoagulation therapy alone for patients with DVT in the United States.
Publicly funding MT in Ontario for populations with arterial acute limb ischemia may not lead to a substantial cost increase for the province. Publicly funding MT for acute DVT would be associated with an additional cost of $5.5 million over 5 years.
We also used a questionnaire tool to engage with clinical stakeholders in Ontario. While there was a limited population involved in the clinician and health system stakeholder summary, questionnaire results allowed for the exploration of the barriers and facilitators surrounding the use and non-use of MT for blocked blood vessels in the lower limbs. Most questionnaire participants are supportive of the technology; however, case-use volume needs to be considered if implementing the procedure. Funding mechanisms, resourcing needs, equity of access, and the model for care delivery would also need to be considered if Ontario were to adopt this technology more broadly.
People with acute DVT with whom we spoke reported that they generally saw MT as a positive option. Those who had undergone the procedure reported positively on its value as a treatment to quickly remove a clot. Accessing treatment for DVT could be a barrier, especially in more remote areas of Ontario. These results may not be applicable to people with arterial acute limb ischemia as we were not able to speak with anyone who had lived experience with this condition.
Acknowledgments
This report was developed by a multidisciplinary team from Ontario Health. The primary clinical epidemiologist was Stacey Vandersluis, the primary medical librarian was Corinne Holubowich, the secondary medical librarian was Caroline Higgins, the primary health economist was Xuanqian Xie, the secondary health economist was Jennifer Guo, the primary patient engagement analyst was David Wells, and the stakeholder perspectives liaison officer was Elisabeth Smitko.
The medical editor was Tim Maguire. Others involved in the development and production of this report were Claude Soulodre, Susan Harrison, Sarah McDowell, Chunmei Li, Andrée Mitchell, Charles de Mestral, and Nancy Sikich.
We would like to thank the following people and organizations for lending their expertise to the development of this report:
Noel Chan, Division of Hematology and Thromboembolism, Department of Medicine, McMaster University
Charles de Mestral, Division of Vascular Surgery, Unity Health Toronto
Jeff Jaskolka, Brampton Civic Hospital, Clinical Adjunct Professor, University of Toronto
Shelley McFeetors, Boston Scientific
Brian Norton, Penumbra Inc
Dheeraj Rajan, Professor and Division Head of Vascular and Interventional Radiology, University Health Network, University of Toronto
Brian Sneek, Penumbra Inc.
Ontario Health-CorHealth
We also thank our lived experience participants who generously gave their time to share their stories with us for this report.
The statements, conclusions, and views expressed in this report do not necessarily represent the views of those we consulted.
Parts of this health technology assessment are based on data and information compiled and provided by the Canadian Institute for Health Information. However, the analyses, conclusions, opinions, and statements expressed in this assessment are those of the authors and not necessarily those of the Institute.
Citation
Ontario Health. Mechanical thrombectomy for acute and subacute blocked arteries and veins in the lower limbs: a health technology assessment. Ont Health Technol Assess Ser [Internet]. 2023 Jan;23(1):1-244. Available from: https://www.hqontario.ca/Evidence-to-Improve-Care/Health-Technology-Assessment/Reviews-And-Recommendations/Mechanical-Thrombectomy-for-Acute-and-Subacute-Blocked-Arteries-and-Veins-in-the-Lower-Limbs
Abbreviations
- ABI:
ankle-brachial index
- ALI:
acute limb ischemia
- CCI:
Canadian Classification of Health Intervention
- CDT:
catheter-directed thrombolysis
- CI:
confidence interval
- CIHI:
Canadian Institute for Health Information
- DAD:
Discharge Abstract Database
- DVT:
deep vein thrombosis
- GRADE:
Grading of Recommendations Assessment, Development, and Evaluation
- ICER:
incremental cost-effectiveness ratio
- ICU:
intensive care unit
- MT:
mechanical thrombectomy
- OCCI:
Ontario Case Costing Initiative
- OR:
odds ratio
- PAD:
Peripheral arterial disease
- PMT:
pharmacomechanical thrombectomy
- QALY:
quality-adjusted life-year
- RCT:
randomized controlled trial
- rtPA:
recombinant tissue plasminogen activator
- VCSS:
venous clinical severity scale
- VEINES-QoL:
The Venous Insufficiency Epidemiologic and Economic Quality of Life Survey
- VTE:
venous thrombosis embolism
Glossary
- Acute deep vein thrombosis:
A sudden loss of blood flow in a lower limb that occurs when a blood clot forms in a deep vein (distinguished from a superficial vein, usually small and near the surface of the body, a deep vein is larger and runs through the muscle). Sometimes there are no noticeable symptoms, but it can cause leg pain or swelling.
- Acute limb ischemia:
A sudden decrease in blood flow through an artery in a lower limb. If untreated, it may lead to loss of limb. The ischemia is usually caused by a blockage, such as a blood clot.
- Adverse event:
An adverse event is an unexpected medical problem that happens during treatment for a health condition. Adverse events may be caused by something other than the treatment.
- Base case:
In economic evaluations, the base case is the “best guess” scenario, including any assumptions, considered most likely to be accurate. In health technology assessments conducted by Ontario Health, the reference case is used as the base case.
- Budget impact analysis:
A budget impact analysis estimates the financial impact of adopting a new health care intervention on the current budget (i.e., the affordability of the new intervention). It is based on predictions of how changes in the intervention mix will impact the level of health care spending for a specific population. Budget impact analyses are typically conducted for a short-term period (e.g., 5 years). The budget impact, sometimes referred to as the net budget impact, is the estimated cost difference between the current scenario (i.e., the anticipated amount of spending for a specific population without using the new intervention) and the new scenario (i.e., the anticipated amount of spending for a specific population following the introduction of the new intervention).
- Cohort model:
In economic evaluations, a cohort model is used to simulate what happens to a homogeneous cohort (group) of patients after receiving a specific health care intervention. The proportion of the cohort who experiences certain health outcomes or events is estimated, along with the relevant costs and benefits. In contrast, a microsimulation model follows the course of individual patients.
- Cost-benefit analysis:
A cost-benefit analysis is a type of economic evaluation that expresses the effects of a health care intervention in terms of a monetary value so that these effects can be compared with costs. Results can be reported either as a ratio of costs to benefits or as a simple sum that represents the net benefit (or net loss) of one intervention over another. The monetary valuation of the different intervention effects is based on either prices that are revealed by markets or an individual or societal willingness-to-pay value.
- Cost-consequence analysis:
A cost-consequence analysis is a type of economic evaluation that estimates the costs and consequences (i.e., the health outcomes) of two or more health care interventions. In this type of analysis, the costs are presented separately from the consequences.
- Cost-effective:
A health care intervention is considered cost-effective when it provides additional benefits, compared with relevant alternatives, at an additional cost that is acceptable to a decisionmaker based on the maximum willingness-to-pay value.
- Cost-effectiveness acceptability curve:
In economic evaluations, a cost-effectiveness acceptability curve is a graphical representation of the results of a probabilistic analysis. It illustrates the probability of health care interventions being cost-effective over a range of willingness-to-pay values. Willingness-to-pay values are plotted on the horizontal axis of the graph, and the probability of the intervention of interest and its comparator(s) being cost-effective at corresponding willingness-to-pay values is plotted on the vertical axis.
- Cost-effectiveness acceptability frontier:
In economic evaluations, a cost-effectiveness acceptability frontier is a graph summarizing the probability of a number of health care interventions being cost-effective over a range of willingness-to-pay values. Like cost-effectiveness acceptability curves, cost-effectiveness acceptability frontiers plot willingness-to-pay values on the horizontal axis and the probability of the interventions being cost-effective at particular willingness-to-pay values on the vertical axis.
- Cost-effectiveness analysis:
Used broadly, “cost-effectiveness analysis” may refer to an economic evaluation used to compare the benefits of two or more health care interventions with their costs. It may encompass several types of analysis (e.g., cost-effectiveness analysis, cost-utility analysis). Used more specifically, “cost-effectiveness analysis” may refer to a type of economic evaluation in which the main outcome measure is the incremental cost per natural unit of health (e.g., life-year, symptom-free day) gained.
- Cost-effectiveness plane:
In economic evaluations, a cost-effectiveness plane is a graph used to show the differences in cost and effectiveness between a health care intervention and its comparator(s). Differences in effects are plotted on the horizontal axis, and differences in costs are plotted on the vertical axis.
- Cost-minimization analysis:
In economic evaluations, a cost-minimization analysis compares the costs of two or more health care interventions. It is used when the intervention of interest and its relevant alternative(s) are determined to be equally effective.
- Cost-utility analysis:
A cost-utility analysis is a type of economic evaluation used to compare the benefits of two or more health care interventions with their costs. The benefits are measured using quality-adjusted life-years, which capture both the quality and quantity of life. In a cost-utility analysis, the main outcome measure is the incremental cost per quality-adjusted life-year gained.
- Decision tree:
A decision tree is a type of economic model used to assess the costs and benefits of two or more alternative health care interventions. Each intervention may be associated with different outcomes, which are represented by distinct branches in the tree. Each outcome may have a different probability of occurring and may lead to different costs and benefits.
- Deterministic sensitivity analysis:
Deterministic sensitivity analysis is an approach used to explore uncertainty in the results of an economic evaluation by varying parameter values to observe the potential impact on the cost-effectiveness of the health care intervention of interest. One-way sensitivity analysis accounts for uncertainty in parameter values one at a time, whereas multiway sensitivity analysis accounts for uncertainty in a combination of parameter values simultaneously.
- Disability-adjusted life-year (DALY):
The disability-adjusted life-year (DALY) is a health-related quality-of-life measure used to quantify the burden of disease from ill health, disability, or premature death. One disability-adjusted life-year represents the loss of one year of full health. Disability-adjusted life-years enable comparisons across different diseases, such that a disease that may cause premature death (e.g., measles) can be compared with a disease that may cause disability (e.g., cataracts).
- Discounting:
Discounting is a method used in economic evaluations to adjust for the differential timing of the costs incurred and the benefits generated by a health care intervention over time. Discounting reflects the concept of positive time preference, whereby future costs and benefits are reduced to reflect their present value. The health technology assessments conducted by Ontario Health use an annual discount rate of 1.5% for both future costs and future benefits.
- Disease-specific preference-based measures:
Disease-specific preference-based measures are instruments used to obtain the quality-adjusted weight (i.e., the utility value) of being in a particular health state or having a specific health condition. Disease-specific preference-based measures are often thought to be more sensitive than generic preference-based measures in capturing condition-specific health effects. Like generic preference-based measures, disease-specific preference-based measures typically consist of a self-completed questionnaire, a health-state classification system, and a scoring formula that calculates the utility value. The key difference is that health states in disease-specific preference-based measures are important for the health condition of interest but may not apply to all patient populations. Examples of disease-specific preference-based measures include the Diabetes Utility Index (DUI) and the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30).
- Disutility:
A disutility is a decrease in utility (i.e., a decrease in preference for a particular health outcome) typically resulting from a particular health condition (e.g., experiencing a symptom or complication).
- Dominant:
A health care intervention is considered dominant when it is more effective and less costly than its comparator(s).
- Embolus:
A blood clot that forms somewhere else in the body (most commonly in the heart) and breaks free to travel through the blood vessels.
- EQ-5D:
The EQ-5D is a generic health-related quality-of-life classification system widely used in clinical studies. In economic evaluations, it is used as an indirect method of obtaining health state preferences (i.e., utility values). The EQ-5D questionnaire consists of five questions relating to different domains of quality of life: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. For each domain, there are three response options: no problems, some problems, or severe problems. A newer instrument, the EQ-5D-5L, includes five response options for each domain. A scoring table is used to convert EQ-5D scores to utility values.
- Extended dominance:
A health care intervention is considered to be extendedly dominated when it has an incremental cost-effectiveness ratio higher than that of the next most costly or effective comparator. Interventions that are extendedly dominated are ruled out.
- Generic preference-based measures:
Generic preference-based measures are generic (i.e., not disease specific) instruments used to obtain the quality-adjusted weight (i.e., the utility value) of being in a given health state. Generic preference-based measures typically consist of a self-completed questionnaire, a health-state classification system, and a scoring formula that calculates the utility value. Examples include the Health Utilities Index Mark 3 (HUI3), the EuroQol-Five Dimensions (EQ-5D), and the Short Form-Six Dimensions (SF-6D). The quality-adjusted weights are obtained from the public or from patients, who are provided with a descriptive profile of each predefined health state and asked to fill out a questionnaire. The benefit of using a generic instrument is the ability to obtain utility values that are comparable across different health care interventions and diseases.
- Health-related quality of life:
Health-related quality of life is a measure of the impact of a health care intervention on a person's health. It includes the dimensions of physiology, function, social life, cognition, emotions, sleep and rest, energy and vitality, health perception, and general life satisfaction.
- Health state:
A health state is a particular status of health (e.g., sick, well, dead). A health state is associated with some amount of benefit and may be associated with specific costs. Benefit is captured through individual or societal preferences for the time spent in each health state and is expressed in quality-adjusted weights called utility values. In a Markov model, a finite number of mutually exclusive health states are used to represent discrete states of health.
- Health Utilities Index Mark 3 (HUI3):
The HUI3 is a generic health-related quality-of-life classification system widely used in clinical studies. In economic evaluations, it is used as an indirect method of obtaining health state preferences (i.e., utility values). The HUI3 was developed in Canada and is used in major Canadian population health surveys. The HUI3 comprises eight attributes: vision, hearing, speech, ambulation, dexterity, emotion, cognition, and pain and discomfort. Each attribute is associated with five or six defined functional levels, thus producing a total of 972,000 unique health states. A predefined scoring formula is used to convert HUI3 scores to utility values.
- Human capital approach:
In economic evaluations, the human capital approach is used to estimate a monetary value that represents a person's loss of productivity due to disability, illness, or premature death.
- Incremental cost:
The incremental cost is the additional cost, typically per person, of a health care intervention versus a comparator.
- Incremental cost-effectiveness ratio (ICER):
The incremental cost-effectiveness ratio (ICER) is a summary measure that indicates, for a given health care intervention, how much more a health care consumer must pay to get an additional unit of benefit relative to an alternative intervention. It is obtained by dividing the incremental cost by the incremental effectiveness. Incremental cost-effectiveness ratios are typically presented as the cost per life-year gained or the cost per quality-adjusted life-year gained.
- Incremental net benefit:
Incremental net benefit is a summary measure of cost-effectiveness. It incorporates the differences in cost and effect between two health care interventions and the willingness-to-pay value. Net health benefit is calculated as the difference in effect minus the difference in cost divided by the willingness-to-pay value. Net monetary benefit is calculated as the willingness-to-pay value multiplied by the difference in effect minus the difference in cost. An intervention can be considered cost-effective if either the net health or net monetary benefit is greater than zero.
- Market distribution:
When evaluating more than two technologies, the market distribution is the proportion of the population that uses each technology.
- Mechanical Thrombectomy:
A medical procedure to remove a blockage (a blood clot) from a vein or artery. First, a thin, flexible tube (known as a catheter) is inserted into the affected artery or vein, and then a clot-disrupting device is fed through the tube to the location of the clot. The device then breaks up and/or removes the blockage.
- Markov model:
A Markov model is a type of decision-analytic model used in economic evaluations to estimate the costs and health outcomes (e.g., quality-adjusted life-years gained) associated with using a particular health care intervention. Markov models are useful for clinical problems that involve events of interest that may recur over time (e.g., stroke). A Markov model consists of mutually exclusive, exhaustive health states. Patients remain in a given health state for a certain period of time before moving to another health state based on transition probabilities. The health states and events modelled may be associated with specific costs and health outcomes.
- Microsimulation model:
In economic evaluations, a microsimulation model (e.g., an individual-level or patient-level model) is used to simulate the health outcomes for a heterogeneous group of patients (e.g., patients of different ages or with different sets of risk factors) after receiving a particular health care intervention. The health outcomes and health events of each patient are modelled, and the outcomes of several patients are combined to estimate the average costs and benefits accrued by a group of patients. In contrast, a cohort model follows a homogeneous cohort of patients (e.g., patients of the same age or with the same set of risk factors) through the model and estimates the proportion of the cohort who will experience specific health events.
- Ministry of Health perspective:
The perspective adopted in economic evaluations determines the types of costs and health benefits to include. Ontario Health develops health technology assessment reports from the perspective of the Ontario Ministry of Health. This perspective includes all costs and health benefits attributable to the Ministry of Health, such as treatment costs (e.g., drugs, administration, monitoring, hospital stays) and costs associated with managing adverse events caused by treatments. This perspective does not include out-of-pocket costs incurred by patients related to obtaining care (e.g., transportation) or loss of productivity (e.g., absenteeism).
- Monte Carlo simulation:
Monte Carlo simulation is an economic modelling method that derives parameter values from distributions rather than fixed values. The model is run several times, and in each iteration, parameter values are drawn from specified distributions. This method is used in microsimulation models and probabilistic analysis.
- Multiway sensitivity analysis:
A multiway sensitivity analysis is used to explore uncertainty in the results of an economic evaluation. It is done by varying a combination of model input (i.e., parameter) values simultaneously between plausible extremes to observe the potential impact on the cost-effectiveness of the health care intervention of interest.
- Natural history of a disease:
The natural history of a disease is the progression of a disease over time in the absence of any health care intervention.
- One-way sensitivity analysis:
A one-way sensitivity analysis is used to explore uncertainty in the results of an economic evaluation. It is done by varying one model input (i.e., a parameter) at a time between its minimum and maximum values to observe the potential impact on the cost-effectiveness of the health care intervention of interest.
- Patency:
A measurement of the return of blood flow to a blood vessel area. The ankle-brachial index, which compares the blood pressure in the ankle with that in the arm, is a common tool to determine patency.
- Probabilistic analysis:
A probabilistic analysis (also known as a probabilistic sensitivity analysis) is used in economic models to explore uncertainty in several parameters simultaneously and is done using Monte Carlo simulation. Model inputs are defined as a distribution of possible values. In each iteration, model inputs are obtained by randomly sampling from each distribution, and a single estimate of cost and effectiveness is generated. This process is repeated many times (e.g., 10,000 times) to estimate the number of times (i.e., the probability) that the health care intervention of interest is cost-effective.
- Quality-adjusted life-year (QALY):
The quality-adjusted life-year (QALY) is a generic health outcome measure commonly used in cost-utility analyses to reflect the quantity and quality of life-years lived. The life-years lived are adjusted for quality of life using individual or societal preferences (i.e., utility values) for being in a particular health state. One year of perfect health is represented by one quality-adjusted life-year.
- Reference case:
The reference case is a preferred set of methods and principles that provide the guidelines for economic evaluations. Its purpose is to standardize the approach of conducting and reporting economic evaluations, so that results can be compared across studies.
- Return on investment:
Return on investment is a type of economic evaluation that values the financial return, or benefits, of a health care intervention against the total costs of its delivery. Return on investment is the benefit minus the cost, expressed as a proportion of the cost.
- Risk difference:
Risk difference is the difference in the risk of an outcome occurring between one health care intervention and an alternative intervention.
- Scenario analysis:
A scenario analysis is used to explore uncertainty in the results of an economic evaluation. It is done by observing the potential impact of different scenarios on the cost-effectiveness of a health care intervention. Scenario analyses include varying structural assumptions from the reference case.
- Sensitivity analysis:
Every economic evaluation contains some degree of uncertainty, and results can vary depending on the values taken by key parameters and the assumptions made. Sensitivity analysis allows these factors to be varied and shows the impact of these variations on the results of the evaluation. There are various types of sensitivity analysis, including deterministic, probabilistic, and scenario.
- Short-Form-Six Dimensions (SF-6D):
The SF-6D is a generic health-related quality-of-life classification system widely used in clinical studies. In economic evaluations, it is used as an indirect method of obtaining health state preferences (i.e., utility values). The classification system consists of six attributes (physical functioning, role limitations, social functioning, pain, mental health, and vitality), each associated with four to six levels, thus producing a total of 18,000 possible unique health states. A scoring table is used to convert SF-6D scores to health state values.
- Societal perspective:
The perspective adopted in an economic evaluation determines the types of costs and health benefits to include. The societal perspective reflects the broader economy and is the aggregation of all perspectives (e.g., health care payer and patient perspectives). It considers the full effect of a health condition on society, including all costs (regardless of who pays) and all benefits (regardless of who benefits).
- Standard gamble:
In economic evaluations, standard gamble is a direct method of measuring people's preferences for various health states. In a standard gamble, respondents are asked about their preference for either (a) remaining in a certain health state for the rest of their life, or (b) a gamble scenario in which there is a chance of having optimal health for the rest of one's life but also a chance of dying immediately. Respondents are surveyed repeatedly, with the risk of immediate death varying each time (e.g., 75% chance of optimal health, 25% chance of immediate death) until they are indifferent about their choice. The standard gamble is considered the gold standard for eliciting preferences as it incorporates individual risk attitudes, unlike other methods of eliciting preferences.
- Time horizon:
In economic evaluations, the time horizon is the time frame over which costs and benefits are examined and calculated. The relevant time horizon is chosen based on the nature of the disease and health care intervention being assessed, as well as the purpose of the analysis. For instance, a lifetime horizon would be chosen to capture the long-term health and cost consequences over a patient's lifetime.
- Time trade-off:
In economic evaluations, time trade-off is a direct method of measuring people's preferences for various health states. In a time-trade off, respondents are asked about their preference for either (a) living with a chronic health condition for a certain amount of time, followed by death, or (b) living in optimal health but for less time than in scenario (a). That is, respondents decide how much time in good health they would be willing to “trade off” for more time spent in poorer health. Respondents are surveyed repeatedly, with the amount of time spent in optimal health varying each time until they are indifferent about their choice.
- Tornado diagram:
In economic evaluations, a tornado diagram is used to determine which model parameters have the greatest influence on results. Tornado diagrams present the results of multiple one-way sensitivity analyses in a single graph.
- Uptake rate:
In instances where two technologies are being compared, the uptake rate is the rate at which a new technology is adopted. When a new technology is adopted, it may be used in addition to an existing technology, or it may replace an existing technology.
- Utility:
A utility is a value that represents a person's preference for various health states. Typically, utility values are anchored at 0 (death) and 1 (perfect health). In some scoring systems, a negative utility value indicates a state of health valued as being worse than death. Utility values can be aggregated over time to derive quality-adjusted life-years, a common outcome measure in economic evaluations.
- Value-of-information analysis:
In economic evaluations, value-of-information analysis is used to estimate the value of investing in future research to minimize uncertainty in input parameters.
- Visual analogue scale (VAS):
The visual analogue scale (VAS) is a direct method of measuring people's preferences for various health states. Respondents are first asked to rank a series of health states from least to most preferable. Then, they are asked to place the health states on a scale with intervals reflecting the differences in preference among the given health states. The scale ranges from 0 (worst imaginable health) to 100 (best imaginable health). The value of a respondent's preference for each health state is given by their placement of each health state on the scale.
- Willingness-to-pay value:
A willingness-to-pay value is the monetary value a health care consumer is willing to pay for added health benefits. When conducting a cost-utility analysis, the willingness-to-pay value represents the cost a consumer is willing to pay for an additional quality-adjusted life-year. If the incremental cost-effectiveness ratio is less than the willingness-to-pay value, the health care intervention of interest is considered cost-effective. If the incremental cost-effectiveness ratio is more than the willingness-to-pay value, the intervention is considered not to be cost-effective.
Appendices
Appendix 1: Literature Search Strategies
Clinical Evidence Search
Clinical Literature Search
Search date: August 20, 2021
Databases searched: Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Centre for Reviews and Dissemination (CRD) Health Technology Assessment Database, and National Health Service (NHS) Economic Evaluation Database
Database segments: EBM Reviews - Cochrane Central Register of Controlled Trials <July 2021>, EBM Reviews - Cochrane Database of Systematic Reviews <2005 to August 18, 2021>, EBM Reviews - Health Technology Assessment <4th Quarter 2016>, EBM Reviews - NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2021 Week 32>, Ovid MEDLINE(R) ALL <1946 to August 19, 2021> Search Strategy:
-
1
Peripheral Arterial Disease/ (32625)
-
2
((peripheral adj2 arter* adj3 (thrombos?s or thrombi or thrombus or emboli* or embolus or disease* or obstruct* or occlus* or isch?emia* or stenos* or aneurysm* or insufficien*) or peripheral vascular disease* or vascular occlus* disease* or lower extremit* arter* disease* or lower extremit* occlus* disease*).ti,ab,kf. (81928)
-
3
Venous Thrombosis/ (41818)
-
4
((deep adj3 ((vein or venous) adj3 (thrombos?s or thrombus or thrombi or occlus* or clot*))) or phlebothrombos* or phlebo thrombos* or deep thrombophlebitis or deep thrombo phlebitis or DVT).ti,ab,kf. (87644)
-
5
Thrombosis/ (204733)
-
6
(blood clot* or thrombos?s or thrombus or thrombi).ti,ab,kf. (483375)
-
7
Embolism/ (36517)
-
8
Thromboembolism/ (93009)
-
9
Venous Thromboembolism/ (55209)
-
10
(embolism* or embolus or thromboembol* or thrombo embol* or VTE).ti,ab,kf. (317583)
-
11
exp Ischemia/ and Acute Disease/ (20406)
-
12
Arterial Occlusive Diseases/ (44884)
-
13
(((acute* or subacute* or urgent* or emergen*) adj3 isch?emia*) or ((arterial or large vessel) adj3 (thrombos?s or thrombus or thrombi or emboli* or occlus* or isch?emia*)) or vasular occlus*).ti,ab,kf. (116858)
-
14
or/5-13 (963170)
-
15
exp Lower Extremity/ (586170)
-
16
((low* adj2 (extremit* or limb*)) or leg or legs or feet or foot or thigh* or calf or calves or iliofemoral or femoral or femoropopliteal or popliteal or iliac or proximal).ti,ab,kf. (1799324)
-
17
or/15-16 (2062820)
-
18
14 and 17 (122634)
-
19
((acute limb* or subacute limb* or acute lower limb* or subacute lower limb* or critical limb* or urgent limb* or emergen* limb*) adj3 isch?emia*).ti,ab,kf. (15937)
-
20
or/1-4,18-19 (294523)
-
21
Mechanical Thrombolysis/ (9411)
-
22
(((mechanic* or pharmacomechanic*) adj4 (thromboly* or thrombectom* or thromboembolect*)) or ((mechanic* or pharmacomechanic*) adj4 (thromb* adj3 (aspirat* or rotat* or retriev* or remov* or clear* or clot* or disrupt*))) or ((thrombectom* or thromboly*) adj3 (aspiration* or rotation* or retriever* or percutaneous or endovascular or catheter based)) or thromboaspirat* or PMT).ti,ab,kf. (27432)
-
23
(angiojet* or angiovac* or ((indigo* or penumbra*) adj4 (thromb* or aspiration* or rotation* or retriever* or catheter* or device* or mechanic* or separator*)) or (cleaner* adj3 rotation*) or cleaner xt* or aspirex* or rotarex* or flowtriever* or flow triever* or clottriever* or clot triever* or triever20* or jeti8* or (jeti* adj3 thromb*) or peripheral thrombect* or quickclear* or quick clear* or ekos*).ti,ab,kf. (3109)
-
24
or/21-23 (31678)
-
25
20 and 24 (4876)
-
26
exp Animals/ not Humans/ (15664220)
-
27
25 not 26 (3880)
-
28
Case Reports/ or Comment.pt. or Editorial.pt. or (Letter not (Letter and Randomized Controlled Trial)).pt. or Congress.pt. (5875894)
-
29
27 not 28 (3350)
-
30
limit 29 to english language [Limit not valid in CDSR; records were retained] (3044)
-
31
limit 30 to yr=“2010 -Current” (2492)
-
32
31 use medall,cctr,coch,clhta,cleed (937)
-
33
peripheral occlusive artery disease/ (37368)
-
34
((peripheral adj2 arter* adj3 (thrombos?s or thrombi or thrombus or emboli* or embolus or disease* or obstruct* or occlus* or isch?emia* or stenos* or aneurysm* or insufficient) or peripheral vascular disease* or vascular occlus* disease* or lower extremit* arter* disease* or lower extremit* occlus* disease*).tw,kw. (84949)
-
35
deep vein thrombosis/ (94241)
-
36
lower extremity deep vein thrombosis/ (1766)
-
37
leg thrombosis/ (2150)
-
38
((deep adj3 ((vein or venous) adj3 (thrombos?s or thrombus or thrombi or occlus* or clot*))) or phlebothrombos* or phlebo thrombos* or deep thrombophlebitis or deep thrombo phlebitis or DVT).tw,kw. (90026)
-
39
vein thrombosis/ (36286)
-
40
thrombosis/ (204733)
-
41
(blood clot* or thrombos?s or thrombus or thrombi).tw,kw. (496866)
-
42
embolism/ (36517)
-
43
thromboembolism/ (93009)
-
44
venous thromboembolism/ (55209)
-
45
(embolism* or embolus or thromboembol* or thrombo embol* or VTE).tw,kw. (322703)
-
46
ischemia/ and acute disease/ (3258)
-
47
artery occlusion/ (25322)
-
48
(((acute* or subacute* or urgent* or emergen*) adj3 isch?emia*) or ((arterial or large vessel) adj3 (thrombos?s or thrombus or thrombi or emboli* or occlus* or isch?emia*)) or vasular occlus*).tw,kw. (119138)
-
49
or/39-48 (955800)
-
50
exp lower limb/ (586170)
-
51
((low* adj2 (extremit* or limb*)) or leg or legs or feet or foot or thigh* or calf or calves or iliofemoral or femoral or femoropopliteal or popliteal or iliac or proximal).tw,kw. (1802895)
-
52
or/50-51 (2067105)
-
53
49 and 52 (116963)
-
54
leg ischemia/ and acute disease/ (100)
-
55
critical limb ischemia/ (5567)
-
56
((acute limb* or subacute limb* or acute lower limb* or subacute lower limb* or critical limb* or urgent limb* or emergen* limb*) adj3 isch?emia*).tw,kw. (16231)
-
57
or/33-38,53-56 (320663)
-
58
mechanical thrombectomy/ (8680)
-
59
(((mechanic* or pharmacomechanic*) adj4 (thromboly* or thrombectom* or thromboembolect*)) or ((mechanic* or pharmacomechanic*) adj4 (thromb* adj3 (aspirat* or rotat* or retriev* or remov* or clear* or clot* or disrupt*))) or ((thrombectom* or thromboly*) adj3 (aspiration* or rotation* or retriever* or percutaneous or endovascular or catheter based)) or thromboaspirat* or PMT).tw,kw,dv. (27835)
-
60
(angiojet* or angiovac* or ((indigo* or penumbra*) adj4 (thromb* or aspiration* or rotation* or retriever* or catheter* or device* or mechanic* or separator*)) or (cleaner* adj3 rotation*) or cleaner xt* or aspirex* or rotarex* or flowtriever* or flow triever* or clottriever* or clot triever* or triever20* or jeti8* or (jeti* adj3 thromb*) or peripheral thrombect* or quickclear* or quick clear* or ekos*).tw,kw,dv. (3960)
-
61
or/58-60 (32013)
-
62
57 and 61 (5201)
-
63
(exp animal/ or nonhuman/) not exp human/ (11123885)
-
64
62 not 63 (5094)
-
65
Case Report/ or Comment/ or Editorial/ or (letter.pt. not (letter.pt. and randomized controlled trial/)) or conference abstract.pt. or conference review.pt. (12043310)
-
66
64 not 65 (2733)
-
67
limit 66 to english language [Limit not valid in CDSR; records were retained] (2381)
-
68
limit 67 to yr=“2010 -Current” (1878)
-
69
68 use emez (1028)
-
70
32 or 69 (1965)
-
71
70 use medall (799)
-
72
70 use coch (2)
-
73
70 use cctr (133)
-
74
70 use clhta (3)
-
75
70 use cleed (0)
-
76
70 use emez (1028)
-
77
remove duplicates from 70 (1331)
Economic Evidence Search
Economic Evaluation and Cost Effectiveness Search
Search date: August 24, 2021
Databases searched: Ovid MEDLINE, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Centre for Reviews and Dissemination (CRD) Health Technology Assessment Database, and National Health Service (NHS) Economic Evaluation Database
Database segments: EBM Reviews - Cochrane Central Register of Controlled Trials <July 2021>, EBM Reviews - Cochrane Database of Systematic Reviews <2005 to August 18, 2021>, EBM Reviews - Health Technology Assessment <4th Quarter 2016>, EBM Reviews - NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2021 Week 33>, Ovid MEDLINE(R) ALL <1946 to August 23, 2021> Search Strategy:
-
1
Peripheral Arterial Disease/ (32342)
-
2
((peripheral adj2 arter* adj3 (thrombos?s or thrombi or thrombus or emboli* or embolus or disease* or obstruct* or occlus* or isch?emia* or stenos* or aneurysm* or insufficient) or peripheral vascular disease* or vascular occlus* disease* or lower extremit* arter* disease* or lower extremit* occlus* disease*).ti,ab,kf. (78932)
-
3
Venous Thrombosis/ (40377)
-
4
((deep adj3 ((vein or venous) adj3 (thrombos?s or thrombus or thrombi or occlus* or clot*))) or phlebothrombos* or phlebo thrombos* or deep thrombophlebitis or deep thrombo phlebitis or DVT).ti,ab,kf. (84870)
-
5
Thrombosis/ (207582)
-
6
(blood clot* or thrombos?s or thrombus or thrombi).ti,ab,kf. (469295)
-
7
Embolism/ (38295)
-
8
Thromboembolism/ (94800)
-
9
Venous Thromboembolism/ (52249)
-
10
(embolism* or embolus or thromboembol* or thrombo embol* or VTE).ti,ab,kf. (307424)
-
11
exp Ischemia/ and Acute Disease/ (21328)
-
12
Arterial Occlusive Diseases/ (42315)
-
13
(((acute* or subacute* or urgent* or emergen*) adj3 isch?emia*) or ((arterial or large vessel) adj3 (thrombos?s or thrombus or thrombi or emboli* or occlus* or isch?emia*)) or vasular occlus*).ti,ab,kf. (113500)
-
14
or/5-13 (948526)
-
15
exp Lower Extremity/ (588743)
-
16
((low* adj2 (extremit* or limb*)) or leg or legs or feet or foot or thigh* or calf or calves or iliofemoral or femoral or femoropopliteal or popliteal or iliac or proximal).ti,ab,kf. (1754258)
-
17
or/15-16 (2035439)
-
18
14 and 17 (118970)
-
19
((acute limb* or subacute limb* or acute lower limb* or subacute lower limb* or critical limb* or urgent limb* or emergen* limb*) adj3 isch?emia*).ti,ab,kf. (15105)
-
20
or/1-4,18-19 (286903)
-
21
Mechanical Thrombolysis/ (8023)
-
22
(((mechanic* or pharmacomechanic*) adj4 (thromboly* or thrombectom* or thromboembolect*)) or ((mechanic* or pharmacomechanic*) adj4 (thromb* adj3 (aspirat* or rotat* or retriev* or remov* or clear* or clot* or disrupt*))) or ((thrombectom* or thromboly*) adj3 (aspiration* or rotation* or retriever* or percutaneous or endovascular or catheter based)) or thromboaspirat* or PMT).ti,ab,kf. (25439)
-
23
(angiojet* or angiovac* or ((indigo* or penumbra*) adj4 (thromb* or aspiration* or rotation* or retriever* or catheter* or device* or mechanic* or separator*)) or (cleaner* adj3 rotation*) or cleaner xt* or aspirex* or rotarex* or flowtriever* or flow triever* or clottriever* or clot triever* or triever20* or jeti8* or (jeti* adj3 thromb*) or peripheral thrombect* or quickclear* or quick clear* or ekos*).ti,ab,kf. (2912)
-
24
or/21-23 (29295)
-
25
20 and 24 (4515)
-
26
exp Animals/ not Humans/ (15973556)
-
27
25 not 26 (3521)
-
28
Case Reports/ or Comment.pt. or Editorial.pt. or (Letter not (Letter and Randomized Controlled Trial)).pt. or Congress.pt. (5807614)
-
29
27 not 28 (2996)
-
30
limit 29 to english language [Limit not valid in CDSR; records were retained] (2705)
-
31
limit 30 to yr=“2010 -Current” (2153)
-
32
31 use coch,clhta,cleed (5)
-
33
economics/ (261621)
-
34
economics, medical/ or economics, pharmaceutical/ or exp economics, hospital/ or economics, nursing/ or economics, dental/ (897102)
-
35
economics.fs. (450054)
-
36
(econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmaco-economic*).ti,ab,kf. (1038421)
-
37
exp “costs and cost analysis”/ (622016)
-
38
(cost or costs or costing or costly).ti. (291377)
-
39
cost effective*.ti,ab,kf. (375472)
-
40
(cost* adj2 (util* or efficacy* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog*)).ab,kf. (244300)
-
41
models, economic/ (14415)
-
42
markov chains/ or monte carlo method/ (91778)
-
43
(decision adj1 (tree* or analy* or model*)).ti,ab,kf. (50682)
-
44
(markov or markow or monte carlo).ti,ab,kf. (148500)
-
45
quality-adjusted life years/ (45288)
-
46
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).ti,ab,kf. (88362)
-
47
((adjusted adj1 (quality or life)) or (willing* adj2 pay) or sensitivity analys*s).ti,ab,kf. (147125)
-
48
or/33-47 (2873402)
-
49
31 and 48 (101)
-
50
49 use medall,cctr (54)
-
51
32 or 50 (59)
-
52
peripheral occlusive artery disease/ (37070)
-
53
((peripheral adj2 arter* adj3 (thrombos?s or thrombi or thrombus or emboli* or embolus or disease* or obstruct* or occlus* or isch?emia* or stenos* or aneurysm* or insufficient) or peripheral vascular disease* or vascular occlus* disease* or lower extremit* arter* disease* or lower extremit* occlus* disease*).tw,kw. (81751)
-
54
deep vein thrombosis/ (90274)
-
55
lower extremity deep vein thrombosis/ (1551)
-
56
leg thrombosis/ (2090)
-
57
((deep adj3 ((vein or venous) adj3 (thrombos?s or thrombus or thrombi or occlus* or clot*))) or phlebothrombos* or phlebo thrombos* or deep thrombophlebitis or deep thrombo phlebitis or DVT).tw,kw. (87121)
-
58
vein thrombosis/ (35190)
-
59
thrombosis/ (207582)
-
60
(blood clot* or thrombos?s or thrombus or thrombi).tw,kw. (482254)
-
61
embolism/ (38295)
-
62
thromboembolism/ (94800)
-
63
venous thromboembolism/ (52249)
-
64
(embolism* or embolus or thromboembol* or thrombo embol* or VTE).tw,kw. (312230)
-
65
ischemia/ and acute disease/ (3354)
-
66
artery occlusion/ (24264)
-
67
(((acute* or subacute* or urgent* or emergen*) adj3 isch?emia*) or ((arterial or large vessel) adj3 (thrombos?s or thrombus or thrombi or emboli* or occlus* or isch?emia*)) or vasular occlus*).tw,kw. (115685)
-
68
or/58-67 (941058)
-
69
exp lower limb/ (588743)
-
70
((low* adj2 (extremit* or limb*)) or leg or legs or feet or foot or thigh* or calf or calves or iliofemoral or femoral or femoropopliteal or popliteal or iliac or proximal).tw,kw. (1757376)
-
71
or/69-70 (2039353)
-
72
68 and 71 (113789)
-
73
leg ischemia/ and acute disease/ (93)
-
74
critical limb ischemia/ (4827)
-
75
((acute limb* or subacute limb* or acute lower limb* or subacute lower limb* or critical limb* or urgent limb* or emergen* limb*) adj3 isch?emia*).tw,kw. (15354)
-
76
or/52-57,72-75 (311854)
-
77
mechanical thrombectomy/ (7291)
-
78
(((mechanic* or pharmacomechanic*) adj4 (thromboly* or thrombectom* or thromboembolect*)) or ((mechanic* or pharmacomechanic*) adj4 (thromb* adj3 (aspirat* or rotat* or retriev* or remov* or clear* or clot* or disrupt*))) or ((thrombectom* or thromboly*) adj3 (aspiration* or rotation* or retriever* or percutaneous or endovascular or catheter based)) or thromboaspirat* or PMT).tw,kw,dv. (25774)
-
79
(angiojet* or angiovac* or ((indigo* or penumbra*) adj4 (thromb* or aspiration* or rotation* or retriever* or catheter* or device* or mechanic* or separator*)) or (cleaner* adj3 rotation*) or cleaner xt* or aspirex* or rotarex* or flowtriever* or flow triever* or clottriever* or clot triever* or triever20* or jeti8* or (jeti* adj3 thromb*) or peripheral thrombect* or quickclear* or quick clear* or ekos*).tw,kw,dv. (3693)
-
80
or/77-79 (29550)
-
81
76 and 80 (4805)
-
82
(exp animal/ or nonhuman/) not exp human/ (11358842)
-
83
81 not 82 (4703)
-
84
Case Report/ or Comment/ or Editorial/ or (letter.pt. not (letter.pt. and randomized controlled trial/)) or conference abstract.pt. or conference review.pt. (11582665)
-
85
83 not 84 (2600)
-
86
limit 85 to english language [Limit not valid in CDSR; records were retained] (2262)
-
87
limit 86 to yr=“2010 -Current” (1759)
-
88
Economics/ (261621)
-
89
Health Economics/ or Pharmacoeconomics/ or Drug Cost/ or Drug Formulary/ (139561)
-
90
Economic Aspect/ or exp Economic Evaluation/ (485323)
-
91
(econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmaco-economic*).tw,kw. (1064885)
-
92
exp “Cost”/ (622016)
-
93
(cost or costs or costing or costly).ti. (291377)
-
94
cost effective*.tw,kw. (388307)
-
95
(cost* adj2 (util* or efficac* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog*)).ab,kw. (256752)
-
96
Monte Carlo Method/ (71860)
-
97
(decision adj1 (tree* or analy* or model*)).tw,kw. (54494)
-
98
(markov or markow or monte carlo).tw,kw. (153414)
-
99
Quality-Adjusted Life Years/ (45288)
-
100
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw,kw. (92270)
-
101
((adjusted adj1 (quality or life)) or (willing* adj2 pay) or sensitivity analys*s).tw,kw. (168511)
-
102
or/88-101 (2474797)
-
103
87 and 102 (126)
-
104
103 use emez (57)
-
105
51 or 104 (116)
-
106
105 use medall (39)
-
107
105 use emez (57)
-
108
105 use coch (2)
-
109
105 use cctr (15)
-
110
105 use cleed (0)
-
111
105 use clhta (3)
-
112
remove duplicates from 105 (88)
Grey Literature Search
Performed on: August 26 - September 2, 2021
Websites searched:
Alberta Health Evidence Reviews, Alberta Health Services, BC Health Technology Assessments, Canadian Agency for Drugs and Technologies in Health (CADTH), Institut national d'excellence en santé et en services sociaux (INESSS), Institute of Health Economics (IHE), McGill University Health Centre Health Technology Assessment Unit, Centre Hospitalier de l'Universite de Quebec-Universite Laval, Health Technology Assessment Database, Agency for Healthcare Research and Quality (AHRQ) Evidence-based Practice Centers, Centers for Medicare & Medicaid Services Technology Assessments, Veterans Affairs Health Services Research and Development, Institute for Clinical and Economic Review, Oregon Health Authority Health Evidence Review Commission, Washington State Health Care Authority Health Technology Reviews, National Institute for Health and Care Excellence (NICE), Healthcare Improvement Scotland, Health Technology Wales, Ireland Health Information and Quality Authority Health Technology Assessments, Australian Government Medical Services Advisory Committee, Council of Australian Governments Health Technologies, Australian Safety and Efficacy Register of New Interventional Procedures -Surgical (ASERNIP-S), Italian National Agency for Regional Health Services (AGENAS), Belgian Health Care Knowledge Centre, Ludwig Boltzmann Institute for Health Technology Assessment, Swedish Agency for Health Technology Assessment and Assessment of Social Services, Ministry of Health Malaysia Health Technology Assessment Section, Tuft's Cost-Effectiveness Analysis Registry, PROSPERO, EUnetHTA, clinicaltrials.gov
Keywords used:
mechanical thrombectomy, pharmacomechanical thrombectomy, thrombectomy, peripheral arterial disease, deep vein thrombosis, venous thrombosis, occlusion, thrombosis, embolism, blood clot, acute limb ischemia, critical limb ischemia, lower extremity, lower limb, angiojet, angiovac, indigo, penumbra, aspirex, rotarex, flowtriever, clottriever, jeti, ekos, thrombectomie mécanique, pharmacomécanique, maladie artérielle périphérique, thrombose veineuse profonde, caillot de sanguin, ischémie aiguës, extrémité inférieure
Clinical results (included in PRISMA): 4 Economic results (included in PRISMA): 3 Ongoing HTAs (PROSPERO/EUnetHTA/): 2 Ongoing RCTs (clinicaltrials.gov): 30
Appendix 2: Critical Appraisal of Clinical Evidence
Arterial Acute Limb Ischemia Population
Table A1:
Risk of Biasa Among Non-randomized Studies (RoBANS Tool)—in the Arterial Acute Limb Ischemia Population
| Author, year | Selection of participants | Confounding variables | Measurement of exposure | Blinding of outcome assessments | Incomplete outcome data |
|---|---|---|---|---|---|
| Arterial Acute Limb Ischemia Population | |||||
| Byrne et al, 201476 | Lowb | Highc | Lowd | Lowe | Lowf |
| Chait et al, 201974 | Lowb | Highc | Lowd | Lowe | Lowf |
| de Athayde Soares et al, 202077 | Lowb | Highg | Lowd | Lowe | Lowf |
| Escobar et al, 201778 | Lowb | Highc | Lowd | Lowe | Lowf |
| Gandhi et al, 201879 | Lowb | Highc | Lowd | Lowe | Lowf |
| Gong et al, 20211 | Lowb | Highh | Lowd | Lowe | Lowf |
| Hundt et al, 201380 | Lowb | Highc | Lowd | Lowe | Lowf |
| Kronlage et al, 201781 | Lowb | Highc | Lowd | Lowe | Lowf |
| Morrow et al, 201773 | Lowb | Highi | Lowd | Lowe | Lowf |
| Muli Jogi et al, 201882 | Lowb | Highc | Lowd | Lowe | Lowf |
| Puangpunngam et al, 202083 | Lowb | Highj | Lowd | Lowe | Lowf |
| Schernthaner et al, 201484 | Lowb | Highc | Lowd | Lowe | Lowf |
Abbreviations: CDT, catheter-directed thrombolysis; MT, mechanical thrombectomy; RoBANS, Risk of Bias Assessment Tool for Non-randomized Studies.
Possible risk-of-bias levels: low, high, unclear.
Reported study traits demonstrated baseline characteristics that patients in both study groups do not substantially differ, unless otherwise stated.
Major confounding variables were not adequately confirmed and considered during study design because intervention selection was at the treating physician's discretion and patients selected for experimental intervention may have been different than those selected for control intervention.
In all studies, data was obtained through trustworthy sources such as medical records.
While blinding was not present, its absence was judged to have no effect on outcome measurements such as limb loss, patency, and severe adverse effects.
The quantity of missing data was considered to be similarly likely in both study groups and therefore not a concern for risk of bias.
Patients were allocated to treatment groups based on their risk factors, with MT selected for patients with recent major surgery, major trauma, or > 70 years of age.
There were more patients in the MT group who had a history of pulmonary embolism and May-Thurner, but otherwise the groups were similar.
People in both groups were mostly similar, with more people who received CDT having a history of coronary artery disease. No one had renal dysfunction.
Study title is case-controlled; however, the text states that patients were randomly allocated. No other details provided. Given the inconsistency in reporting the methodological approach, we have interpreted this paper to be an observational study design, but one that accounted for confounding variables through allocation to treatment.
Table A2:
GRADE Evidence Profile for Mechanical Thrombectomy in Arterial Acute Limb Ischemia
| Outcome and subgroups (number of studies, design) | Risk of biasa | Inconsistency | Indirectness | Imprecision | Publication bias | Upgrade considerations | Quality |
|---|---|---|---|---|---|---|---|
| Limb Salvage | |||||||
| Overall (9 observational) | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| PMT (5 observational) | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Rotational (2 observational) | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Ultrasound Assisted (2 observational) | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Technical Success (Reduction of Thrombus Burden) | |||||||
| Overall (8 observational) | Serious limitations (-1) | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕ Very low |
| PMT (5 observational) | Serious limitations (-1) | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕ Very low |
| Rotational (1 observational) | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Ultrasound Assisted (2 observational) | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Patency | |||||||
| Overall (5 observational) | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| PMT (4 observational) | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Ultrasound Assisted (1 observational) | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Re-Thrombosis (and Revision rates) | |||||||
| Overall (6 observational) | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| PMT (3 observational) | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Rotational (2 observational) | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Ultrasound Assisted (1 observational) | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Perioperative Mortality | |||||||
| Overall (9 observational) | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| PMT (6 observational) | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Rotational (1 observational) | Serious limitations (-1) | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕ Very low |
| Ultrasound Assisted (2 observational) | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Adverse Effects and Complications | |||||||
| Overall (9 observational) | Serious limitations (-1) | Serious limitations (-1)c | No serious limitations | No serious limitations | Undetected | None | ⊕ Very low |
| PMT (5 observational) | Serious limitations (-1) | Serious limitations (-1)c | No serious limitations | No serious limitations | Undetected | None | ⊕ Very low |
| Rotational (2 observational) | Serious limitations (-1) | Serious limitations (-1)c | No serious limitations | No serious limitations | Undetected | None | ⊕ Very low |
| Ultrasound Assisted (2 observational) | Serious limitations (-1) | Serious limitations (-1)c | No serious limitations | No serious limitations | Undetected | None | ⊕ Very low |
| Volume of Thrombotic Infusion | |||||||
| Overall (5 observational) | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| PMT (2 observational) | Serious limitations (-1) | No serious limitations | No serious limitations | No serious limitations | Strongly suspected (-1)d | None | ⊕ Very low |
| Rotational (1 observational) | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Ultrasound Assisted (2 observational) | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Time of Thrombotic Infusion | |||||||
| Overall (5 observational) | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| PMT (3 observational) | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Ultrasound Assisted (2 observational) | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Hospital Length of Stay | |||||||
| Overall (3 observational) | Serious limitations (-1) | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕ Very low |
| PMT (2 observational) | Serious limitations (-1) | No serious limitations | No serious limitations | No serious limitations | Strongly suspected (-1)d | None | ⊕ Very low |
| Rotational (2 observational) | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; PMT, pharmacomechanical thrombectomy.
Risk of bias assessment details in Tables A3 and A4.
The confidence interval crosses the clinical decision threshold between recommending and not recommending treatment.
There were inconsistent findings reported in the literature.
Though showing benefit, published evidence is limited to a small number of small trials.
Deep Vein Thrombosis Population
Table A3:
Risk of Biasa Among Randomized Controlled Trials (Cochrane Risk-of-Bias Tool) in the Deep Vein Thrombosis Population
| Author, year | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|
| ATTRACT trial86–93 | Lowb | Low | Lowc | Low | Low | None |
| Engelberger et al, 20152 | Low | Low | Lowc | Low | Lowd | None |
| CAVA trial, Notton et al, 2020,102 20213 | Low | Low | Highe | Lowf | Lowd | None |
Possible risk-of-bias levels: low, high, and unclear.
Trial was randomized; however, the patients who were randomized to the intervention group may have received 1 of 3 interventions selected in a non-perfectly random way. For the purposes of this review, subgroups from within the intervention group were selected to be compared for select analyses, thereby selecting for groups that were not necessarily randomized to allocation. This was accounted for in the GRADE analysis.
Assessors blinded to group assignment where possible. Some outcomes, such as mortality, are not subject to bias, while others, such as hospitalization length of stay, could possibly be subjected to bias of the treating clinician.
The authors did not identify the protocol; however, all clinically meaningful outcomes expected were reported in a transparent manner, including both significant and nonsignificant findings. The authors included additional outcomes in the appendices.
Single blind. Patients were aware of their allocation and were asked to not disclose their allocation to monitoring and long-term treating local assessors and physicians.
Some loss to follow up, but balanced between study groups.
Table A4:
Risk of Biasa Among Non-randomized Studies (RoBANS Tool) in the Deep Vein Thrombosis Population
| Author, year | Selection of participants | Confounding variables | Measurement of exposure | Blinding of outcome assessments | Incomplete outcome data |
|---|---|---|---|---|---|
| Baker et al, 201294 | Lowb | Highc | Lowd | Lowe | Lowf |
| Escobar et al, 201778 | Lowb | Highc | Lowd | Lowe | Lowf |
| Ezelsoy et al, 201595 | Lowb | Highc | Lowd | Lowe | Lowf |
| Garcia et al, 201585 | Lowb | Highc | Lowd | Lowe | Lowf |
| Huang et al 201596 | Lowb | Highc | Lowd | Lowe | Lowf |
| Huang et al, 202197 | Lowb | Highc | Lowd | Lowe | Lowf |
| Kuo et al, 201798 | Lowb | Highc | Lowd | Lowe | Lowf |
| Lee et al, 202099 | Lowb | Highc | Lowd | Lowe | Lowf |
| Li et al, 2020100 | Lowb | Highc | Lowd | Lowe | Lowf |
| Liu et al, 2018101 | Highg | Highc | Lowd | Lowe | Lowf |
| Lu et al, 201775 | Lowh | Highc | Lowd | Lowe | Lowf |
| Morrow et al, 201773 | Lowi | Highc | Lowd | Lowe | Lowf |
| Pouncey et al, 2020103 | Lowb | Highc | Lowd | Lowe | Lowf |
| Shen et al, 2019104 | Lowb | Highc | Lowd | Lowe | Lowf |
| Tian et al, 2021105 | Lowb | Highc | Lowd | Lowe | Lowf |
| Tichelaar et al, 2016106 | Lowb | Highc | Lowd | Lowe | Lowf |
| Xu et al, 2021107 | Lowb | Highc | Lowd | Lowe | Lowf |
| Xu et al, 2020108 | Lowb | Highc | Lowd | Lowe | Lowf |
| Zhu et al, 2020109 | Lowb | Lowj | Lowd | Lowe | Lowf |
Abbreviations: CDT, catheter-directed thrombolysis; PMT, pharmacomechanical thrombectomy; RoBANS, Risk of Bias Assessment Tool for Non-randomized Studies.
Possible risk-of-bias levels: low, high, unclear.
Reported study traits demonstrated baseline characteristics that patients in both study groups do not substantially differ, unless otherwise stated.
Major confounding variables were not adequately confirmed and considered during study design because intervention selection was at the treating physician's discretion and patients selected for experimental intervention may have been different than those selected for control intervention.
In all studies, data was obtained through trustworthy sources, such as medical records.
While blinding was not present, its absence was judged to have no effect on outcome measurements such as limb loss, patency, and severe adverse effects.
The quantity of missing data was considered to be similarly likely in both study groups and therefore not a concern for risk of bias.
There was a higher rate of thrombophilia in the intervention group than in the comparator group, but otherwise, patients were similar in both groups.
The intervention group had significantly more patients with a Rutherford IIb classification lesion, while the control group had more with a diagnosis of Rutherford IIa.
Those who received the intervention had higher rates of arrhythmia, thrombophilia, history of cancer, and renal dysfunction; however, those who received CDT alone were more likely to have coronary artery disease.
People who received PMT were more likely to have a history of peripheral vascular disease than the comparator group.
Table A5:
GRADE Evidence Profile for Mechanical Thrombectomy in Acute Deep Vein Thrombosis— Overall
| Number of studies (design) | Risk of biasa | Inconsistency | Indirectness | Imprecision | Publication bias | Upgrade considerations | Quality |
|---|---|---|---|---|---|---|---|
| Limb Salvage | |||||||
| 1 Observational | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Post Thrombotic Syndrome | |||||||
| 2 RCTs | No serious limitations | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕⊕⊕ Moderate |
| 8 Observational | Serious limitations (-1) | Serious limitations (-1)c | No serious limitations | No serious limitations | Undetected | None | ⊕ Very low |
| Technical Success (Reduction of Thrombus Burden) | |||||||
| 2 RCT | Serious limitations (-1) | Serious limitations (-1)c | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| 15 Observational | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Patency | |||||||
| 1 RCTs | Serious limitations (-1) | No serious limitations | No serious limitations | No serious limitations | Strongly suspected (-1)d | None | ⊕⊕ Low |
| 9 Observational | Serious limitations (-1) | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕ Very low |
| Re-Thrombosis (and Revision Rates) | |||||||
| 2 RCTs | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕⊕ Low |
| 5 Observational | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Quality of Life, Activities of Daily Living and Resolution of Symptoms and Function Not Otherwise Specified | |||||||
| 3 RCTs | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕⊕ Low |
| 1 Observational | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Mortality | |||||||
| 2 RCTs | Serious limitations (-1) | Serious limitations (-1)c | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very Low |
| 7 Observational | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Adverse Effects and Complications | |||||||
| 3 RCTs | Serious limitations (-1) | No serious limitations | No serious limitations | No serious limitationse | Undetected | None | ⊕⊕⊕ Moderate |
| 13 Observational | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Volume of Thrombotic Infusion | |||||||
| 1 RCT | Serious limitations (-1) | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕⊕ Moderate |
| 10 Observational | Serious limitations (-1) | Serious limitations (-1)c | No serious limitations | No serious limitations | Undetected | None | ⊕ Very low |
| Time of Thrombotic Infusion | |||||||
| 1 RCT | Serious limitations (-1) | No serious limitations | No serious limitations | No serious limitations | Undetectedf | None | ⊕⊕⊕ Moderate |
| 10 Observational | Serious limitations (-1) | Serious limitations (-1)c | No serious limitations | No serious limitations | Undetected | None | ⊕ Very low |
| Hospital Length of Stay | |||||||
| 1 RCTs | Serious limitations (-1) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | None | ⊕⊕⊕ Moderate |
| 5 Observational | Serious limitations (-1) | Serious limitations (-1)c | No serious limitations | Serious limitations (-1)b | No serious limitations | None | ⊕ Very low |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; RCT, randomized controlled trial.
Risk of bias assessment details in Tables A3 and A4.
The confidence interval crosses the clinical decision threshold between recommending and not recommending treatment.
There is minimal or no overlap of the confidence intervals, indicating that there may be heterogeneity that is also seen with the I2 statistical test.
Though showing benefit, published evidence is limited to a small number of small trials.
Very low event rates in both study arms leads to the judgement that there are no concerns with imprecision in spite of confidence intervals that cross the clinical decision threshold.
While there was only one RCT published, the outcome of interest was not a primary outcome of the study. As a result, we determined that the reported benefit was unlikely to be a sign of publication bias.
Table A6:
GRADE Evidence Profile for Pharmacomechanical Thrombectomy Devices in Acute Deep Vein Thrombosis
| Number of studies (design) | Risk of biasa | Inconsistency | Indirectness | Imprecision | Publication bias | Upgrade considerations | Quality |
|---|---|---|---|---|---|---|---|
| Limb Salvage | |||||||
| 1 Observational | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Post Thrombotic Syndrome | |||||||
| 1 RCT | No serious limitations | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕⊕⊕ Moderate |
| 6 Observational | Serious limitations (-1) | Serious limitations (-1)c | No serious limitations | No serious limitations | Undetected | None | ⊕ Very low |
| Technical Success (Reduction of Thrombus Burden) | |||||||
| 1 RCT | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Strongly suspected (-1)d | None | ⊕ Very low |
| 11 Observational | Serious limitations (-1) | Serious limitations (-1)c | No serious limitations | No serious limitations | Undetected | None | ⊕ Very low |
| Patency | |||||||
| 6 Observational | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Re-Thrombosis (and Revision Rates) | |||||||
| 3 Observational | Serious limitations (-1) | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕ Very low |
| Quality of Life, Activities of Daily Living and Resolution of Symptoms and Function Not Otherwise Specified | |||||||
| 1 RCT | Serious limitations (-1) | Serious limitations (-1)c | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Mortality | |||||||
| 1 RCT | No serious limitations | No serious limitations | No serious limitations | Serious limitations (-1)b | Strongly suspected (-1)d | None | ⊕⊕ Low |
| 4 Observational | Serious limitations (-1) | No serious limitations | No serious limitations | No serious limitationse | Undetected | None | ⊕ Very Low |
| Adverse Effects and Complications | |||||||
| 1 RCT | No serious limitations | No serious limitations | No serious limitations | No serious limitationse | Strongly suspected (-1)d | None | ⊕⊕⊕ Moderate |
| 13 Observational | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Volume of Thrombotic Infusion | |||||||
| 1 RCT | No serious limitations | No serious limitations | No serious limitations | No serious limitations | No serious limitations | None | ⊕⊕⊕ Moderate |
| 9 Observational | Serious limitations (-1) | Serious limitations (-1)c | No serious limitations | No serious limitations | Undetected | None | ⊕ Very low |
| Time of Thrombotic Infusion | |||||||
| 1 RCT | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetectedf | None | ⊕⊕⊕ Moderate |
| 8 Observational | Serious limitations (-1) | Serious limitations (-1)c | No serious limitations | No serious limitations | Undetected | None | ⊕ Very low |
| Hospital Length of Stay | |||||||
| 4 Observational | Serious limitations (-1) | Serious limitations (-1)c | No serious limitations | Serious limitations (-1)b | No serious limitations | None | ⊕ Very low |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; PMT, pharmacomechanical thrombectomy; RCT, randomized controlled trial.
Risk of bias assessment details in Tables A3 and A4.
The confidence interval crosses the clinical decision threshold between recommending and not recommending treatment.
There is minimal or no overlap of the confidence intervals, indicating that there may be heterogeneity that is also seen with the I2 statistical test.
Though showing benefit, published evidence is limited to a small number of small trials.
Very low event rates in both study arms leads to the judgement that there are no concerns with imprecision in spite of confidence intervals that cross the clinical decision threshold.
While there was only one RCT published, the outcome of interest was not a primary outcome of the study. As a result, we determined that the reported benefit was unlikely to be a sign of publication bias.
Table A7:
GRADE Evidence Profile for Rotational Mechanical Thrombectomy Devices in Acute Deep Vein Thrombosis
| Number of studies (design) | Risk of biasa | Inconsistency | Indirectness | Imprecision | Publication bias | Upgrade considerations | Quality |
|---|---|---|---|---|---|---|---|
| Post Thrombotic Syndrome | |||||||
| 1 Observational | Serious limitations (-1) | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕ Very low |
| Patency | |||||||
| 1 Observational | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Mortality | |||||||
| 1 Observational | Serious limitations (-1) | No serious limitations | No serious limitations | No serious limitationsc | Undetected | None | ⊕ Very low |
| Adverse Effects and Complications | |||||||
| 1 Observational | Serious limitations (-1) | No serious limitations | No serious limitations | No serious limitationsc | Undetected | None | ⊕ Very low |
Abbreviation: GRADE, Grading of Recommendations Assessment, Development, and Evaluation.
Risk of bias assessment details in Tables A3 and A4.
The confidence interval crosses the clinical decision threshold between recommending and not recommending treatment.
Very low event rates in both study arms led to our judgement that there are no concerns with imprecision in spite of confidence intervals that cross the clinical decision threshold.
Table A8:
GRADE Evidence Profile for Ultrasound-assisted Mechanical Thrombectomy Devices in Acute Deep Vein Thrombosis
| Number of studies (design) | Risk of biasa | Inconsistency | Indirectness | Imprecision | Publication bias | Upgrade considerations | Quality |
|---|---|---|---|---|---|---|---|
| Post Thrombotic Syndrome | |||||||
| 1 RCT | No serious limitations | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕⊕⊕ Moderate |
| 1 Observational | Serious limitations (-1) | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕ Very low |
| Technical Success (Reduction of Thrombus Burden) | |||||||
| 1 RCT | No serious limitations | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕⊕⊕ Moderate |
| 3 Observational | Serious limitations (-1) | Serious limitations (-1)c | No serious limitations | No serious limitations | Undetected | None | ⊕ Very low |
| Patency | |||||||
| 1 RCT | Serious limitations (-1) | No serious limitations | No serious limitations | No serious limitations | Strongly suspected (-1)d | None | ⊕⊕ Low |
| 2 Observational | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Re-Thrombosis (and Revision Rates) | |||||||
| 2 RCTs | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕⊕ Low |
| 2 Observational | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Quality of Life, Activities of Daily Living and Resolution of Symptoms and Function Not Otherwise Specified | |||||||
| 2 RCTs | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕⊕ Low |
| 1 Observational | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Mortality | |||||||
| 1 RCT | Serious limitations (-1) | Serious limitations (-1)c | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| 3 Observational | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Adverse Effects and Complications | |||||||
| 2 RCTs | Serious limitations (-1) | No serious limitations | No serious limitations | No serious limitationse | Undetected | None | ⊕⊕⊕ Moderate |
| 3 Observational | Serious limitations (-1) | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕ Very low |
| Volume of Thrombotic Infusion | |||||||
| 1 Observational | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Time of Thrombotic Infusion | |||||||
| 2 Observational | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | None | ⊕ Very low |
| Hospital Length of Stay | |||||||
| 1 RCTs | Serious limitations (-1) | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕⊕ Moderate |
| 1 Observational | Serious limitations (-1) | No serious limitations | No serious limitations | Serious limitations (-1)b | Strongly suspected (-1)d | None | ⊕ Very low |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; RCT, randomized controlled trial.
Risk of bias assessment details in Tables A3 and A4.
The confidence interval crosses the clinical decision threshold between recommending and not recommending treatment.
There is minimal or no overlap of the confidence intervals, indicating that there may be heterogeneity that is also seen with the I2 statistical test.
Though showing benefit, published evidence is limited to a small number of small trials.
Very low event rates in both study arms leads to the judgement that there are no concerns with imprecision in spite of confidence intervals that cross the clinical decision threshold.
Appendix 3: Selected Excluded Studies—Clinical Evidence
For transparency, we provide a list of select excluded studies. Table A9 is a list of systematic reviews that were identified but not used in preference for conducting our own evaluation of primary studies. Table A10 is a list of primary studies that we surmise readers might have expected to see but did not meet the inclusion criteria, along with the primary reason for exclusion.
Table A9:
Excluded Systematic Reviews
| Citation |
| Ashrafi M, Ahmad SB, Antoniou SA, Khan T, Antoniou GA. Treatment strategies for proximal deep vein thrombosis: a network meta-analysis of randomised controlled trials. Eur J Vasc Endovasc Surg. 2022:63(2)323-34. |
| Dasari TW, Pappy R, Hennebry TA. Pharmacomechanical thrombolysis of acute and chronic symptomatic deep vein thrombosis: a systematic review of literature. Angiology. 2012;63(2):138-45. |
| Diniz J, Coelho A, Mansilha A. Endovascular treatment of iliofemoral deep venous thrombosis: is there enough evidence to support it? A systematic review with meta-analysis. Int Angiol. 2020;39(2):93-104. |
| Doomernik DE, Schrijver AM, Zeebregts CJ, De Vries JPPM, Reijnen MMPJ. Advancements in catheter-directed ultrasound-accelerated thrombolysis. J Endovasc Ther. 2011;18(3)418-34. |
| lerardi AM, Xhepa G, Piffaretti G, Bacuzzi A, Tozzi M, Carbone M, et al. Clinical experience with AngioJet: a comprehensive review. Int Angiol. 2015;34(6 Suppl 1):1-14. |
| Karthikesalingam A, Young EL, Hinchliffe RJ, Loftus IM, Thompson MM, Holt PJ. A systematic review of percutaneous mechanical thrombectomy in the treatment of deep venous thrombosis. Eur J Vasc Endovasc Surg. 2011;41(4):554-65. |
| Li GQ, Wang L, Zhang XC. AngioJet thrombectomy versus catheter-directed thrombolysis for lower extremity deep vein thrombosis: a meta-analysis of clinical trials. Clin Appl Thromb Hemost. 2021;27:10760296211005548. |
| Lichtenberg M, Stahlhoff FW, Boese D. Endovascular treatment of acute limb ischemia and proximal deep vein thrombosis using rotational thrombectomy: a review of published literature. Cardiovasc Revasc Med. 2013;14(6):343-8. |
| Lichtenberg MKW, Stahlhoff S, Mlynczak K, Golicki D, Gagne P, Razavi MK, et al. Endovascular mechanical thrombectomy versus thrombolysis in patients with iliofemoral deep vein thrombosis—a systematic review and meta-analysis. Vasa. 2021;50(1):59-67. |
| Loffroy R, Falvo N, Galland C, Frechier L, Ledan F, Midulla M, et al. Percutaneous rotational mechanical atherectomy plus thrombectomy using Rotarex S device in patients with acute and subacute lower limb ischemia: a review of safety, efficacy, and outcomes. Front Cardiovasc Med. 2020;7:557420. |
| Malgor RD, Gasparis AP. Pharmaco-mechanical thrombectomy for early thrombus removal. Phlebology. 2012;27 Suppl 1:155-62. |
| Marietta M, Romagnoli E, Cosmi B, Coluccio V, Luppi M. Is there a role for intervention radiology for the treatment of lower limb deep vein thrombosis in the era of direct oral anticoagulants? A comprehensive review. Eur J Int Med. 2018;52:13-21. |
| Ng TT, Sigman M, Weaver FA. Basic data related to thrombolytic therapy for acute venous thrombosis. Ann Vasc Surg. 2014;28(4):1039-44. |
| Robertson L, McBride O, Burdess A. Pharmacomechanical thrombectomy for iliofemoral deep vein thrombosis. Cochrane Database Syst Rev. 2016;11:CD011536. |
| Shi Y, Shi W, Chen L, Gu J. A systematic review of ultrasound-accelerated catheter-directed thrombolysis in the treatment of deep vein thrombosis. J Thromb Thrombolysis. 2018;45(3):440-51. |
| Tang T, Chen L, Chen J, Mei T, Lu Y. Pharmacomechanical thrombectomy versus catheter-directed thrombolysis for iliofemoral deep vein thrombosis: a meta-analysis of clinical trials. Clin Appl Thromb Hemost. 2019:25:1076029618821190. |
| Thomas M, Hollingsworth A, Mofidi R. Endovascular management of acute lower limb deep vein thrombosis: a systematic review and meta-analysis. Ann Vasc Surg. 2019;58:363-70. |
| Veenstra EB, van der Laan MJ, Zeebregts CJ, de Heide EJ, Kater M, Bokkers RPH. A systematic review and meta-analysis of endovascular and surgical revascularization techniques in acute limb ischemia. J Vasc Surg. 2020;71(2):654-68 e3. |
| Wang CN, Deng HR. Percutaneous endovenous intervention plus anticoagulation versus anticoagulation alone for treating patients with proximal deep vein thrombosis: a meta-analysis and systematic review. Ann Vasc Surg. 2018;49:39-48. |
| Wang W, Sun R, Chen Y, Liu C. Meta-analysis and systematic review of percutaneous mechanical thrombectomy for lower extremity deep vein thrombosis. J Vasc Surg Venous Lymphat Disord. 2018;6(6):788-800. |
| Wong PC, Chan YC, Law Y, Cheng SWK. Percutaneous mechanical thrombectomy in the treatment of acute iliofemoral deep vein thrombosis: a systematic review. Hong Kong Med J. 2019;25(1):48-57. |
Table A10:
Select Excluded Primary Studies
| Citation | Primary reason for exclusion |
|---|---|
| Selected Excluded Studies in Arterial Occlusions | |
| Leung DA, Blitz LR, Nelson T, Amin A, Soukas PA, Nanjundappa A, et al. Rheolytic pharmacomechanical thrombectomy for the management of acute limb ischemia: results from the PEARL registry. J Endovasc Ther. 2015;22(4):546-57. | Wrong intervention, both groups had mechanical thrombectomy; evaluating the effect of additional CDT or not |
| Liu J, Li T, Huang W, Zhao N, Liu H, Zhao H, et al. Percutaneous mechanical thrombectomy using Rotarex catheter in peripheral artery occlusion diseases—experience from a single center. Vascular. 2019;27(2):199-203. | Wrong population, defined subacute patients as up to 60 days after symptom onset |
| Schrijver AM, van Leersum M, Fioole B, Reijnen MM, Hoksbergen AW, Vahl AC, et al. Dutch randomized trial comparing standard catheter-directed thrombolysis and ultrasound-accelerated thrombolysis for arterial thromboembolic infrainguinal disease (DUET). J Endovasc Ther. 2015;22(1):87-95. | Wrong population, defined subacute patients as up to 49 days after symptom onset |
| Zamboni M, Scrivere P, Silvestri A, Vit A, Pellegrin A, Sponza M, et al. Hybrid approach to popliteal artery aneurysm with thromboembolic symptoms. a pilot study. Ann Vasc Surg. 2021;72:270-5. | Wrong population, included > 50% critical limb ischemia, a chronic condition |
| Selected Excluded Studies in Acute Deep Vein Thrombosis | |
| Benarroch-Gampel J, Pujari A, Aizpuru M, Rajani RR, Jordan WD, Crawford R. Technical success and short-term outcomes after treatment of lower extremity deep vein thrombosis with the ClotTriever system: a preliminary experience. J Vasc Surg Venous Lymphat Disord. 2020;8(2):174-81. | Wrong study design, non-comparative study design |
| de Donato G, Pasqui E, Giannace G, Setacci F, Benevento D, Palasciano G, et al. The Indigo system in acute lower-limb malperfusion (INDIAN) registry: protocol. JMIR Res Protoc. 2019;8(3):e9972. | Wrong study design, protocol |
| Haig Y, Enden T, Grotta O, Klow NE, Slagsvold CE, Ghanima W, et al. Post-thrombotic syndrome after catheter-directed thrombolysis for deep vein thrombosis (CaVenT): 5-year follow-up results of an open-label, randomised controlled trial. Lancet Haematol. 2016;3(2):e64-71. | Wrong intervention groups, no mechanical thrombectomy |
| Kumar R, Rodriguez V, Matsumoto JMS, Khan SP, Weaver AL, McBane RD, et al. Prevalence and risk factors for post thrombotic syndrome after deep vein thrombosis in children: a cohort study. Thromb Res. 2015;135(2):347-51. | Wrong population, not adults |
| Leung DA, Blitz LR, Nelson T, Amin A, Soukas PA, Nanjundappa A, et al. Rheolytic pharmacomechanical thrombectomy for the management of acute limb ischemia: results from the PEARL registry. J Endovasc Ther. 2015;22(4):546-57. | Wrong intervention groups, evaluated the effectiveness of CDT with MT |
| Lopez R, DeMartino R, Fleming M, Bjarnason H, Neisen M. Aspiration thrombectomy for acute iliofemoral or central deep venous thrombosis. J Vasc Surg. 2019;7(2):162-8. | Wrong study design, noncomparative study design |
| Paz T, Bloom A, Roth B, Kalish Y, Rottenstreich A, Elchalal U, et al. Pharmacomechanical catheter thrombolysis for pregnancy-related proximal deep venous thrombosis: prevention of post-thrombotic syndrome. J Matern Fetal Neonatal Med. 2021;34(9):1441-7. | Wrong population, chronic DVT (mean 50 mo) |
Appendix 4: Additional Analyses and Summary Tables—Clinical Evidence Administrative Health Data
Some data presented in the background was gathered through the IntelliHealth Ontario portal (IntelliHealth Ontario; intellihealth.moh.gov.on.ca), in November, 2021. We searched the Canadian Institute for Health Information (CIHI) Discharge Abstract Database (DAD) to identify inpatient cases of people hospitalized for blood clots in Ontario. We identified these cases using the following ICD-10 codes (International Statistical Classification of Disease, 10th Revision, Canadian Version) of the most responsible diagnosis for hospital admissions:
I74.3: Embolism and thrombosis of arteries of the lower extremities
I80.2: Phlebitis and thrombophlebitis of other deep vessels of lower extremities, including DVT
We excluded individuals who were < 18 years old at admission, as well as individuals who did not have a valid health number in Ontario (i.e., OHIP). If an individual was hospitalized two or more times in one fiscal year, we counted them once as a “unique” person in a single fiscal year. The number of unique individuals in fiscal years 2015-2020 are presented in Table 40. On average, there were 668 and 457 individuals hospitalized annually for DVT and peripheral arterial ischemia in the lower limb, respectively. The size of our target population may be slightly underestimated for two reasons. First, due to the impact of COVID-19, the volume of hospitalizations in 2019 and 2020 were lower than those before the start of the pandemic. Second, a small proportion of hospitalized adults with a different most responsible diagnosis code (e.g., embolism and thrombosis of unspecified arteries of extremities, or veins) or hospitalized adults who have a secondary diagnosis with I74.3 or I80.2 may be part of our target population. To account for this, we will conduct a scenario analysis with a larger target population size.
All Findings From Clinical Evidence Review
Table A11:
Summary of Findings of Effect of Mechanical Thrombectomy in Arterial Acute Limb Ischemia—All Results
| Outcome | No. of participants (studies) | Effect | GRADEa | |
|---|---|---|---|---|
| Relative (95% CI) | Absolute (95% CI) | |||
| Limb Salvage | 727 (9 Obs) |
OR: 1.49 (.87-2.57) |
38 more per 1,000 (16 fewer to 74 more) |
⊕ Very low |
| PMT | 425 (5 Obs) |
OR: 1.61 (.85-3.02) |
51 more per 1,000 (from 22 fewer to 95 more) | ⊕ Very low |
| Vacuum aspiration | (0 studies) | — | — | — |
| Rotational | 109 (2 Obs) |
OR: 0.88 (.17-4.57) |
10 fewer per 1,000 (from 260 fewer to 62 more) |
⊕ Very low |
| Ultrasound assisted | 193 (2 Obs) |
OR: 1.51 (.37-6.12) |
33 more per 1,000 (from 135 fewer to 86 more) |
⊕ Very low |
| Post thrombotic syndrome | We did not identify any studies that reported on this outcome of interest | |||
| Technical Success, Complete Thrombus Removal | 728 (8 Obs) |
OR: 1.79 (1.21-2.64) |
108 more per 1,000 (39 more to 162 more) |
⊕ Very low |
| PMT | 501 (5 Obs) |
OR: 1.72 (1.07-2.77) |
101 more per 1,000 (14 more to 168 more) |
⊕ Very low |
| Vacuum aspiration | (0 studies) | — | — | — |
| Rotational | 34 (1 Obs) |
OR: 3.46 (.61-19.72) |
242 more per 1,000 (123 fewer to 375 more) |
⊕ Very low |
| Ultrasound assisted | 193 (2 Obs) |
OR: 1.70 (.80-3.61) |
13 fewer per 1,000 (47 fewer to 183 more) |
⊕ Very low |
| Patency | 471 (5 Obs) |
OR: 1.77 (1.13-2.77) |
120 more per 1,000 (28 more to 193 more) |
⊕ Very low |
| PMT | 403 (4 Obs) |
OR: 1.77 (1.09-2.86) |
120 more per 1,000 (20 more to 198 more) |
⊕ Very low |
| Vacuum aspiration | (0 studies) | — | — | — |
| Rotationalb | (0 studies) | — | — | — |
| Ultrasound assisted | 68 (1 Obs) |
OR: 1.75 (-0.50-6.17) |
116 more per 1,000 (169 fewer to 275 more) |
⊕ Very low |
| Re-Thrombosis (and Revision Rates) | 421 (6 Obs) |
OR: 0.64 (.41-1.01) |
98 fewer per 1,000 (179 fewer to 2 more) |
⊕ Very low |
| PMT | 210 (3 Obs) |
OR: 0.55 (.29-1.06) |
118 fewer per 1,000 (208 fewer to 13 more) |
⊕ Very low |
| Vacuum aspiration | (0 studies) | — | — | — |
| Rotational | 109 (2 Obs) |
OR: 0.92 (.39-2.18) |
20 fewer per 1,000 (204 fewer to 192 more) |
⊕ Very low |
| Ultrasound assisted | 102 (1 Obs) |
OR: 0.57 (.23-1.42) |
126 fewer per 1,000 (271 fewer to 87 more) |
⊕ Very low |
| Pain | We did not identify any studies that reported on this outcome of interest | |||
| Quality of Life | We did not identify any studies that reported on this outcome of interest | |||
| Perioperative Mortality | 847 (9 Obs) |
OR: 0.96 (.46-2.02) |
2 fewer per 1,000 (24 fewer to 43 more) |
⊕ Very low |
| PMT | 579 (6 Obs) |
OR: 0.95 (.43 2.09) |
3 fewer per 1,000 (32 fewer to 55 more) |
⊕ Very low |
| Vacuum aspiration | (0 studies) | — | — | — |
| Rotational | 75 (1 Obs) |
Not estimable | 0 fewer per 1,000 (0 fewer to 0 fewer) |
⊕ Very low |
| Ultrasound assisted | 193 (2 Obs) |
OR: 1.05 (.10-10.62) |
2 more per 1,000 (28 fewer to 224 more) |
⊕ Very low |
| Adverse Events | 1.106 (12 Obs) |
There are inconsistent findings in adverse events across the different MT interventions compared to control groups | ⊕ Very low | |
| PMT | 602 (7 Obs) |
MT had more renal dysfunction/acute kidney injury, hematoma, distal embolization, and mean blood loss compared to control groups | ⊕ Very low | |
| Vacuum aspiration | (0 studies) | — | — | — |
| Rotational | 311 (3 Obs) |
There were fewer bleeding events among those who received MT; otherwise, no significant difference between study groups for any other adverse outcome reported | ⊕ Very low | |
| Ultrasound assisted | 193 (2 Obs) |
There were fewer bleeding events among those who received MT; otherwise, no significant difference between study groups for any other adverse outcome reported | ⊕ Very low | |
| Volume of Thrombolytic (mg) | 359 (5 Obs) |
There are inconsistent findings of volume of thrombolytics used among people who received MT compared to control groups | ⊕ Very low | |
| PMT | 147 (2 Obs) |
There are inconsistent findings of volume of thrombolytics used among people who received MT compared to control groups | ⊕ Very low | |
| Vacuum Aspiration | (0 studies) | — | — | — |
| Rotational | 34 (1 study) |
People who received MT had lower volume of thrombolytic compared to control groups | ⊕ Very low | |
| Ultrasound Assisted | 193 (2 Obs) |
People who received MT had no difference in volume of thrombolytic compared to control groups | ⊕ Very low | |
| Time of Thrombolytic Infusion (h) | 493 (5 Obs) |
— | MD: 4.66 lower (11.86 lower to 2.54 higher) |
⊕ Very low |
| PMT | 300 (3 Obs) |
— | MD: 13.64 lower (34.89 lower to 7.61 higher) |
⊕ Very low |
| Vacuum aspiration | (0 studies) | — | — | — |
| Rotational | (0 studies) | — | — | — |
| Ultrasound assisted | 193 (2 Obs) |
— | MD: 0.67 higher (3.04 lower to 4.39 higher) |
⊕ Very low |
| Hospital Length of Stay | 466 (4 Obs) |
— | MD: 1.05 lower (1.33 lower to 0.77 lower) |
⊕ Very low |
| PMT | 264 (3 Obs) |
— | MD: 1.10 lower (1.40 lower to 0.81 lower) |
⊕ Very low |
| Vacuum Aspiration | (0 studies) | — | — | — |
| Rotational | 202 (1 Obs) |
— | MD: 0.2 lower (1.34 lower to 0.94 higher) |
⊕ Very low |
| Ultrasound assisted | (0 studies) | — | — | — |
Abbreviations: CI, confidence interval; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; MD, mean difference; MT, mechanical thrombectomy; OR, odds ration; Obs, observational study; PMT, pharmacomechanical thrombectomy.
Note: Summary of findings table developed using GRADEpro GDT. GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime, 2022. Available from gradepro.org
See Appendix 2 for details about GRADE determination.
Reported metric is brachial index.
Table A12:
Summary of Findings of Effect of Mechanical Thrombectomy in Acute Deep Vein Thrombolysis—All Results
| Outcome | No. of Participants | Effect | GRADEa | |
|---|---|---|---|---|
| Relative (95% CI) | Absolute (95% CI) | |||
| Limb Salvage | (1 Obs) | OR: 2.63 (.11-65.53) |
8 fewer per 1,000 (90 fewer to 12 more) |
⊕ Very low |
| PMT | (0 RCTs) | — | — | — |
| 151 (1 Obs) |
OR: 2.63 (.11-65.53) |
8 fewer per 1,000 (90 fewer to 12 more) |
⊕ Very low | |
| Vacuum aspiration | (0 RCTs) | — | — | — |
| (0 Obs) | — | — | — | |
| Rotational | (0 RCTs) | — | — | — |
| (0 Obs) | — | — | — | |
| Ultrasound assisted | (0 RCTs) | — | — | — |
| (0 Obs) | — | — | — | |
| Post-Thrombotic Syndrome | 386 (2 RCTs) |
OR: 1.14 (.70-1.86) |
30 more per 1,000 (75 fewer to 149 more) |
⊕⊕⊕ Moderate |
| 1,113 (8 Obs) |
OR: 0.37 (.26-52) |
129 fewer per 1,000 (156 fewer to 95 fewer) |
⊕ Very low | |
| PMT | 266 (1 RCT) |
OR: 1.11 (0.65 to 1.91) |
26 more per 1,000 (99 fewer to 160 more) |
⊕⊕⊕ Moderate |
| 993 (6 Obs) |
OR: 0.37 (.26-.54) |
127 fewer per 1,000 (154 fewer to 89 fewer) |
⊕ Very low | |
| Vacuum Aspiration | (0 RCTs) | — | — | — |
| (0 Obs) | — | — | — | |
| Rotational | (0 RCTs) | — | — | — |
| 50 (1 Obs) |
OR: 0.31 (.09-.99) |
277 fewer per 1,000 (457 fewer to 2 fewer) |
⊕ Very low | |
| Ultrasound Assisted | 120 (1 RCT) |
OR: 1.28 (.42-3.95) |
25 more per 1,000 (57 fewer to 210 more) |
⊕⊕⊕ Moderate |
| 70 (1 Obs) |
OR: 0.47 (.05-4.33) |
50 fewer per 1,000 (94 fewer to 225 more) |
⊕ Very low | |
| Technical Success, Complete Thrombus Removal | 478 (2 RCTs) |
RR: 1.03 (.88-1.19) |
21 more per 1,000 (85 fewer to 135 more) |
⊕⊕⊕ Moderate |
| 1,898 (15 Obs) |
RR: 1.06 (.93-1.20) |
36 more per 1,000 (27 fewer to 105 more) |
⊕ Very low | |
| PMT | 430 (1 RCT) |
RR: 1.04 (.89-1.22) |
29 more per 1,000 (79 more to 158 more) |
⊕ Very low |
| 1,644 (12 Obs) |
RR: 1.07 (.94-1.21) |
43 more per 1,000 (17 fewer to 112 more) |
⊕ Very low | |
| Vacuum aspiration | (0 RCTs) | — | — | — |
| (0 Obs | — | — | — | |
| Rotational | (0 RCTs) | — | — | — |
| (0 Obs | — | — | — | |
| Ultrasound assisted | 48 (1 RCT) |
RR: 0.93 (.59-1.48) |
44 fewer per 1,000 (256 fewer to 300 more) |
⊕⊕ Low |
| 254 (3 Obs) |
RR: 1.03 (.52-2.05) |
19 more per 1,000 (299 fewer to 654 more) |
⊕ Very low | |
| Patency | 48 (1 RCT) |
RR: 1.04 (.93-1.17) |
38 more per 1,000 (67 fewer to 163 more) |
⊕⊕ Low |
| 683 (9 studies) |
RR: 1.10 (.99-1.21) |
61 more per 1,000 (6 fewer to 127 more) |
⊕ Very low | |
| PMT | (0 RCTs) | — | — | — |
| 489 (6 Obs) |
RR: 1.03 (.94-1.14) |
19 more per 1,000 (39 fewer to 90 more) |
⊕ Very low | |
| Vacuum aspiration | (0 RCTs) | — | — | — |
| (0 Obs) | — | — | — | |
| Rotational | (0 RCTs) | — | — | — |
| 50 (1 Obs) |
RR: 1.50 (1.02-2.21) |
280 more per 1,000 (11 more to 678 more) |
⊕ Very low | |
| Ultrasound assisted | 48 (1 RCT) |
RR: 1.04 (.93-1.17) |
38 more per 1,000 (67 fewer to 163 more) |
⊕⊕ Low |
| 144 (2 Obs) |
RR: 1.26 (.86-1.85) |
126 more per 1,000 (68 fewer to 413 more) |
⊕ Very low | |
| Re-Thrombosis (and Revision Rates) | 232 (2 RCTs) |
Low event rates and heterogeneity of reported outcomes precluded pooling of findings. Overall, there were no significant findings between study groups on any metric | ⊕⊕ Low | |
| 324 (5 Obs |
⊕ Very low | |||
| PMT | (0 RCTs) | — | — | — |
| 165 (3 Obs) |
There were no significant findings between study groups for freedom from re-thrombosis at 12 months, recurrence rates, or the success rate where reintervention was required | ⊕ Very low | ||
| Vacuum aspiration | (0 RCTs) | — | — | — |
| (0 Obs) | — | — | — | |
| Rotational | (0 RCTs) | — | — | — |
| (0 Obs) | — | — | — | |
| Ultrasound assisted | 232 (2 RCTs) |
There were no significant findings reported between study groups for recurrent DVT without stent, in-stent thrombosis, or ulceration | ⊕⊕ Low | |
| 159 (2 Obs) |
There were no significant findings reported between study groups for need of additional mechanical thrombectomy procedures or for recurrence rates | ⊕ Very low | ||
| Pain | We did not identify any studies that reported on this outcome of interest | |||
| Quality of Life | 662 (3 RCTs) |
— | MD: 0.74 higher (1.67 lower to 3.14 higher) |
⊕⊕ Low |
| 95 (1 Obs) |
— | MD: 1.00 higher (1.69 lower to 3.69 higher) |
⊕ Very low | |
| PMT | 430 (1 RCT) |
— | MD: 4.33 higher (2.52 lower to 11.18 higher) |
⊕ Very low |
| (0 Obs) | — | — | — | |
| Vacuum aspiration | (0 RCTs) | — | — | — |
| (0 Obs) | — | — | — | |
| Rotational | (0 RCTs) | — | — | — |
| (0 Obs) | — | — | — | |
| Ultrasound assisted | 232 (2 RCTs) |
— | MD: 0.23 higher (2.34 lower to 2.8 higher) |
⊕⊕ Low |
| 95 (1 Obs |
— | MD: 1.00 higher (1.69 lower to 3.69 higher) |
⊕ Very low | |
| Perioperative Mortality | 614 (2 RCTs) |
OR: 1.31 (.22-7.85) |
3 more per 1,000 (7 fewer to 57 more) |
⊕Very Low |
| 927 (8 Obs) |
There were no significant differences in mortality, with most studies reporting 0 in both study arms | ⊕Very Low | ||
| PMT | 430 (1 RCT) |
OR: 1.59 (.16-15.46) |
5 more per 1,000 (7 fewer to 108 more) |
⊕⊕ Low |
| 623 (4 Obs) |
There were no significant differences in mortality, with most studies reporting 0 in both study arms | ⊕Very Low | ||
| Vacuum aspiration | (0 RCTs) | — | — | — |
| (0 Obs) | — | — | — | |
| Rotational | (0 RCTs) | — | — | — |
| 50 (1 Obs) |
There were no significant differences in mortality, with 0 reported in both study arms | ⊕ Very low | ||
| Ultrasound assisted | 184 (1 RCT) |
OR: 1.02 (.06-16.59) |
0 fewer per 1,000 (10 fewer to 142 more) |
⊕ Very low |
| 254 (3 Obs) |
There were no significant findings between study groups for mortality or time, in months, of event-free survival | ⊕ Very low | ||
| Adverse Events | 589 (3 RCTs) |
There were no significant differences reported in the rates of adverse events across the different MT interventions compared to control groups | ⊕⊕⊕ Moderate | |
| (17 Obs) | There were inconsistent findings for other adverse events between study groups | ⊕ Very low | ||
| PMT | 430 (1 RCT) |
There were fewer recurrent VTE and no significant differences in rates of bleeding between those who received MT and those who did not. Additionally, there were reported device-related events among 13.6% of patients who had received AngioJet | ⊕⊕⊕ Moderate | |
| 1,823 (13 Obs) |
|
⊕ Very low | ||
| Vacuum aspiration | (0 RCTs) | — | — | — |
| (0 Obs) | — | — | — | |
| Rotational | (0 RCTs) | — | — | — |
| 50 (1 Obs) |
There were no cases of adverse events reported in either study group | ⊕ Very low | ||
| Ultrasound assisted | 159 (2 RCTs) |
There were no significant differences in rates of bleeding, pulmonary embolism, or hematoma between those who received MT and those who did not | ⊕⊕⊕ Moderate | |
| 412 (3 Obs) |
There were no statistically significant differences in the rates of bleeding between study groups | ⊕ Very low | ||
| Volume of Thrombolytic (mg) | 269 (1 RCT) |
MD 0 (same mean volume reported in both study groups) | ⊕⊕⊕ Moderate | |
| 1,226 (10 Obs) |
— | SMD: 2.1 lower (3.32 lower to 0.87 lower) |
⊕ Very low | |
| PMT | 269 (1 RCT) |
— | MD: 0 (same mean volume reported in both study groups) | ⊕⊕⊕ Moderate |
| 1,170 (9 Obs |
— | SMD: 2.27 lower (3.6 lower to 0.95 lower) |
⊕ Very low | |
| Vacuum aspiration | (0 RCTs) | — | — | — |
| (0 Obs) | — | — | — | |
| Rotational | (0 RCTs) | — | — | — |
| (0 Obs) | — | — | — | |
| Ultrasound assisted | (0 RCTs) | — | — | — |
| 56 (1 Obs) |
— | SMD: 0.51 lower (1.13 lower to 0.1 higher) |
⊕ Very low | |
| Time of Thrombolytic Infusion (h) | 269 (1 RCT) |
MD: 2.0 lower (3.51 lower to 0.49 lower) |
⊕⊕⊕ Moderate | |
| 1,395 (10 Obs) |
— | MD: 22.38 lower (29.85 lower to 14.91 lower) |
⊕ Very low | |
| PMT | 269 (1 RCT) |
MD: 2.0 lower (3.51 lower to 0.49 lower) |
⊕⊕⊕ Moderate | |
| 1,236 (8 Obs) |
— | MD 28.35 lower (45.64 lower to 11.05 lower) |
⊕ Very low | |
| Vacuum aspiration | (0 RCTs) | — | — | — |
| (0 Obs) | — | — | — | |
| Rotational | (0 RCTs) | — | — | — |
| (0 Obs) | — | — | — | |
| Ultrasound assisted | (0 RCTs) | — | — | — |
| 159 (2 Obs) |
— | MD: 1.02 lower (5.81 lower to 3.78 higher) |
⊕ Very low | |
| Hospital Length of Stay (d) | 48 (1 RCT) |
— | MD: 0.10 lower (0.86 lower to 0.66 higher) |
⊕⊕⊕ Moderate |
| 454 (5 Obs) |
— | MD: 2.49 lower (4.44 lower to 0.53 lower) |
⊕ Very low | |
| PMT | (0 RCTs) | — | — | — |
| 359 (4 Obs) |
— | MD: 2.6 lower (5.08 lower to 0.12 lower) |
⊕ Very low | |
| Vacuum aspiration | (0 RCTs) | — | — | — |
| (0 Obs) | — | — | — | |
| Rotational | (0 RCTs) | — | — | — |
| (0 Obs) | — | — | — | |
| Ultrasound assisted | 48 (1 RCT) |
— | MD: 0.10 lower (0.86 lower to 0.66 higher) |
⊕⊕⊕ Moderate |
| 95 (1 Obs) |
— | MD: 2 lower (3.36 lower to 0.64 lower) |
⊕ Very low | |
Abbreviations: CI, confidence interval; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; MD, mean difference; OR, odds ratio; Obs, observational study; PMT, pharmacomechanical thrombectomy; RCT, randomized control trial; RR, relative risk; SMD, standard mean difference; VTE, venous thrombosis embolism.
Note: Summary of findings table developed using GRADEpro GDT. GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime, 2022. Available from gradepro.org
See Appendix 2 for details about GRADE determination.
Appendix 5: Selected Excluded Studies—Economic Evidence
For transparency, we provide a list of studies that readers might have expected to see but that did not meet the inclusion criteria, along with the primary reason for exclusion.
Table A13:
Select Excluded Economic Studies
| Citation | Primary Reason for Exclusion |
|---|---|
| Migliara B, Cappellari TF, Mirandola M, Griso A, Kolasa K, Zah V, et al. Treatment of bypass failure in patients with chronic limb threatening ischemia - open surgery vs. percutaneous mechanical thrombectomy. Vasa Eur J Vasc Med. 2020;49(5):395-402. | Target population is focused on chronic limb ischemia |
| Peters CML, de Vries J, Redeker S, Timman R, Eijck GV, Steunenberg SL, et al. Cost-effectiveness of the treatments for critical limb ischemia in the elderly population. J Vasc Surg. 2019;70(2):530-538 e531. | Does not specify the specific endovascular procedures |
| Vaidya V, Gangan N, Comerota A, Lurie F. Cost-effectiveness analysis of initial treatment strategies for nonembolic acute limb ischemia using real-world data. Ann Vasc Surg. 2017;39:276-283. | Does not specify the specific endovascular procedures |
Appendix 6: Results of Applicability Checklists for Studies Included in the Economic Literature Review
Table A14:
Assessment of the Applicability of Studies Evaluating the Cost-Effectiveness of Mechanical Thrombectomy for Arterial Acute Limb Ischemia and Acute DVT in the Lower Limbs
| Author, year, country | Is the study population similar to the question? | Are the interventions similar to the question? | Is the health care system studied sufficiently similar to Ontario? | Were the perspectives clearly stated? If yes, what were they? | Are all direct effects included? Are all other effects included where they are material? | Are all future costs and outcomes discounted? If yes, at what rate? | Is the value of health effects expressed in terms of quality-adjusted life-years? | Are costs and outcomes from other sectors fully and appropriately measured and valued? | Overall Judgmenta |
|---|---|---|---|---|---|---|---|---|---|
| Magnuson et al, 2019131 United States |
Yes | Yes | No | Yes. The US health care system | Yes | Yes. 3% annually | Yes | Yes | Partially applicable |
| Kwok et al, 2018133 Australia |
Yes | Yes | No | Yes. A hospital in Australia | Partially | No. Included procedure costs only | No | No | Partially applicable |
| Li et al, 2020100 China | Yes | Yes | No | Yes. A hospital in China | Partially | No. Included index hospitalization costs only | No | No | Not applicable |
| Li et al, 2021132 China | Yes | Yes | No | Yes. Third-party payer in China | Yes | Unknown | Yes | No | Not applicable |
Note: Response options for all items were “yes,” “partially,” “no,” “unclear,” and “NA” (not applicable).
Overall judgment may be “directly applicable,” “partially applicable,” or “not applicable.”
Appendix 7: Stakeholder Engagement Questionnaire
Appendix 8: Letter of Information
Appendix 9: Interview Guide
Author contributions
This report was developed by a multidisciplinary team from Ontario Health. The primary clinical epidemiologist was Stacey Vandersluis, the primary medical librarian was Corinne Holubowich, the secondary medical librarian was Caroline Higgins, the primary health economist was Xuanqian Xie, the secondary health economist was Jennifer Guo, the primary patient engagement analyst was David Wells, and the stakeholder perspectives liaison officer was Elisabeth Smitko.
Key Messages
What Is This Health Technology Assessment About?
Blood flow to the legs may become blocked, either because a blood clot forms in a blood vessel (arteries or veins) in the leg, or a clot that formed somewhere else in the body comes free and travels to the leg arteries, where it gets stuck and interferes with blood flow. If left untreated, it may lead to disability or loss of the limb.
Some blockages require immediate treatment, others can be managed with blood thinners or other medication. Where surgery is considered necessary, common procedures include inserting a balloon into the blood vessel to pull out the blockage and open the passage for blood flow or replacing or bypassing the affected portion of the vessel. Minimally invasive mechanical thrombectomy delivered through skin puncture into the vessel involves the use of a device to break up and remove a blood clot, which may alleviate the need for more invasive surgery or prolonged administration of clot-busting medication.
This health technology assessment looked at how safe, effective, and cost-effective mechanical thrombectomy is for adults with blocked arteries and veins in the legs. It also looked at the budget impact of publicly funding mechanical thrombectomy, at the perspectives of system stakeholders, and at the experiences, preferences, and values of people with blocked blood vessels in the lower limbs.
What Did This Health Technology Assessment Find?
Mechanical thrombectomy for people experiencing a blockage (blood clot) in the arterial blood vessels of their legs may improve the effectiveness of clot removal and return to normal blood flow, as well as reduce time in hospital compared to alternatives such as catheter administration of clot-busting medications. For severe blockages in veins, it may reduce the proportion of patients experiencing post-thrombotic syndrome and reduce volume for thrombolytic medications, which are administered in the intensive care unit. It has been shown to reduce time in the ICU as well as overall hospital stay.
We estimate that publicly funding mechanical thrombectomy for people with a blockage in an artery may not lead to a substantial cost increase as additional device costs are offset by reduced time in intensive care. For people with a blockage in a vein, we estimate that publicly funding mechanical thrombectomy would cost an additional $5.5 million over the next 5 years. People with whom we spoke reported that they generally saw mechanical thrombectomy as a positive option, especially as a treatment to quickly remove a blood clot. Most respondents to the system stakeholder survey were supportive of the technology; however, volume of cases, funding mechanisms, resourcing needs, access, and model for delivery need to be considered if Ontario is to adopt this technology across the province.
The authors developed the Thrombolysis in Myocardial Infarction (TIMI) score from the cardiac literature to rate perfusion according to a scale from 0 to 3, in which a score of 0 indicates no perfusion distal to the lesion, 1 indicates a faint flow beyond the occlusion without capillary bed filling, 2 indicates delayed distal perfusion with capillary bed filling; and 3 indicates normal perfusion.
Contributor Information
Ontario Health:
Stacey Vandersluis, Corinne Holubowich, Caroline Higgins, Xuanqian Xie, Jennifer Guo, David Wells, and Elisabeth Smitko
About Us
Ontario Health is an agency of the Government of Ontario. Our mandate is to connect and coordinate our province's health care system in ways that have not been done before to help ensure that Ontarians receive the best possible care. We work to support better health outcomes, patient experiences, provider experiences and value for money spent.
For more information about Ontario Health, visit ontariohealth.ca.
References
- (1).Gong M, He X, Zhao B, Kong J, Gu J, Chen G. Endovascular revascularization strategies using catheter-based thrombectomy versus conventional catheter-directed thrombolysis for acute limb ischemia. Thromb J. 2021;19(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Engelberger RP, Spirk D, Willenberg T, Alatri A, Do DD, Baumgartner I, et al. Ultrasound-assisted versus conventional catheter-directed thrombolysis for acute iliofemoral deep vein thrombosis. Circ Cardiovasc Interv. 2015;8(1). [DOI] [PubMed] [Google Scholar]
- (3).Notten P, de Smet A, Tick LW, van de Poel MHW, Wikkeling ORM, Vleming LJ, et al. CAVA (ultrasound-accelerated catheter-directed thrombolysis on preventing post-thrombotic syndrome) Trial: long-term follow-up results. J Am Heart Assoc. 2021;10(11):e018973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Healthwise Staff. Venous thrombus and embolus [Internet]. Alberta: MyHealth.Alberta.ca; 2019. [June 22, 2021]. Available from: https://myhealth.alberta.ca/health/Pages/conditions.aspx?hwid=tp12576&lang=en-ca [Google Scholar]
- (5).Patel NH, Krishnamurthy VN, Kim S, Saad WE, Ganguli S, Walker TG, et al. Quality improvement guidelines for percutaneous management of acute lower-extremity ischemia. J Vasc Interv Radiol. 2013;24(1):3–15. [DOI] [PubMed] [Google Scholar]
- (6).Thrombosis Canada. Periperhal arterial disease 2020 June 18, 2021]. Available from: https://thrombosiscanada.ca/wp-content/uploads/2020/02/Peripheral-Arterial-Disease_18Feb2020.pdf
- (7).Vedantham S, Sista AK, Klein SJ, Nayak L, Razavi MK, Kalva SP, et al. Quality improvement guidelines for the treatment of lower-extremity deep vein thrombosis with use of endovascular thrombus removal. J Vasc Interv Radiol. 2014;25(9):1317–25. [DOI] [PubMed] [Google Scholar]
- (8).Olinic DM, Stanek A, Tataru DA, Homorodean C, Olinic M. Acute limb ischemia: an update on diagnosis and management. J Clin Med. 2019;8(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Smith DA, Lilie CJ. Acute arterial occlusion. StatPearls. Treasure Island (FL): StatPearls Publishing; 2022 Jan. [PubMed] [Google Scholar]
- (10).Smith DA, Lilie CJ. Acute Arterial Occlusion. StatPearls. Treasure Island (FL) 2021. [PubMed]
- (11).Hardman RL, Jazaeri O, Yi J, Smith M, Gupta R. Overview of classification systems in peripheral artery disease. Semin Intervent Radiol. 2014;31(4):378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).CorHealth Ontario and The Ministry of Health. Quality-based procedures clinical handbook: non-cardiac vascular (lower extremities occlusive disease) [Internet]. Toronto (ON): CorHealth; 2021. Available from: https://www.corhealthontario.ca/NCV-LEOD-QBP-Clinical-Handbook-Feb-2021.pdf [Google Scholar]
- (13).CorHealth Ontario and Ministry of Health. Quality-Based Procedures Clinical Handbook: Non-Cardiac Vascular (lower extremities occlusive disease). Ontario: 2021.
- (14).Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–e596. [DOI] [PubMed] [Google Scholar]
- (15).Thrombosis Canada. Deep Vein Thrombosis (DVT): Treatment [Internet]. 2016. [June 18, 2021]. Available from: https://thrombosiscanada.ca/wp-content/uploads/2016/05/3_Deep-Vein-Thrombosis-Treatment-2016May19-FINAL.pdf
- (16).Liu D, Peterson E, Dooner J, Baerlocher M, Zypchen L, Gagnon J, et al. Diagnosis and management of iliofemoral deep vein thrombosis: clinical practice guideline. CMAJ. 2015;187(17):1288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Mahmoodi BK, Cushman M, Naess IA, Allison MA, Bos WJ, Braekkan SK, et al. Association of traditional cardiovascular risk factors with venous thromboembolism: an individual participant data meta-analysis of prospective studies. Circulation. 2017;135(1):7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Gregson J, Kaptoge S, Bolton T, Pennells L, Willeit P, Burgess S, et al. Cardiovascular risk factors associated with venous thromboembolism. JAMA Cardiol. 2019;4(2):163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Hackler E, Hamburg N, White Solaru K. Racial and ethnic disparities in peripheral artery disease. Circ Res. 2021;128(12):1913–26. [DOI] [PubMed] [Google Scholar]
- (20).Jacob-Brassard J, Al-Omran M, Hussain MA, Mamdani M, Stukel TA, Lee DS, et al. Temporal trends in hospitalization for lower extremity peripheral artery disease in Ontario: the importance of diabetes. Can J Cardiol. 2021;37(10):1507–12. [DOI] [PubMed] [Google Scholar]
- (21).Lind B, Morcos O, Ferral H, Chen A, Aquisto T, Lee S, et al. Endovascular strategies in the management of acute limb ischemia. Vasc Specialist Int. 2019;35(1):4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Payne JG, Tagalakis V, Wu C, Lazo-Langner A, Can VN. Current estimates of the incidence of acute venous thromboembolic disease in Canada: a meta-analysis. Thromb Res. 2021;197:8–12. [DOI] [PubMed] [Google Scholar]
- (23).O'Neill J, Tabish H, Welch V, Petticrew M, Pottie K, Clarke M, et al. Applying an equity lens to interventions: using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. J Clin Epidemiol. 2014;67(1):56–64. [DOI] [PubMed] [Google Scholar]
- (24).Paudel S, Zaitoun A, Al-Najafi S, Musa T, Szpunar S, Light D, et al. The association of gender with quality of health in peripheral arterial disease following peripheral vascular intervention. Vasc Dis Manag. 2017;14(10):E218–E24. [DOI] [PubMed] [Google Scholar]
- (25).Francis DK, Bennett NR, Ferguson TS, Hennis AJ, Wilks RJ, Harris EN, et al. Disparities in cardiovascular disease among Caribbean populations: a systematic literature review. BMC Public Health. 2015;15):828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Hussain MA, Lindsay TF, Mamdani M, Wang X, Verma S, Al-Omran M. Sex differences in the outcomes of peripheral arterial disease: a population-based cohort study. CMAJ Open. 2016;4(1):E124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Shah BR, Frymire E, Jacklin K, Jones CR, Khan S, Slater M, et al. Peripheral arterial disease in Ontario First Nations people with diabetes: a longitudinal population-based cohort study. CMAJ Open. 2019;7(4):E700–E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Sebastianski M, Tonelli M, Tsuyuki RT. Ethnic differences in prevalence of peripheral artery disease in patients undergoing hemodialysis. J Racial Ethn Health Disparities. 2015;2(3):275–9. [DOI] [PubMed] [Google Scholar]
- (29).Periperhal arterial disease [Internet]. Whitby (ON): Thrombosis Canada; 2020. [cited 2021 June 18]. Available from: https://thrombosiscanada.ca/clinicalguides/?search=arterial%20disease# [Google Scholar]
- (30).Obara H, Matsubara K, Kitagawa Y. Acute limb ischemia. Ann Vasc Dis. 2018;11(4):443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Kohi MP, Kohlbrenner R, Kolli KP, Lehrman E, Taylor AG, Fidelman N. Catheter directed interventions for acute deep vein thrombosis. Cardiovasc Diagn Ther. 2016;6(6):599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).AngioJet Periperhal Thrombectomy System [Internet]. Boston (MA): Boston Scientitific Corp.; (c)2022. [cited 2021 June 17]. Available from: https://www.bostonscientific.com/en-US/products/thrombectomy-systems/angiojet-thrombectomy-system.html [Google Scholar]
- (33).Indigo System [Internet]. Alameda (CA): Penumbra, Inc.; (c)2022. [June 17, 2021]. Available from: https://www.penumbrainc.com/peripheral-device/indigo-system [Google Scholar]
- (34).AngioVac Cannula & Circuit [Internet]. Latham (NY): AngioDynamics, Inc.; (c)2022. [cited 2021 June 17]. Available from: https://angiovac.com/product-features-gen-3 [Google Scholar]
- (35).Cleaner rotation thrombectomy system [Internet]. Argon Medical Devices; 2020. [cited 2021 June 17]. Available from: https://www.argonmedical.com/products/cleaner-rotational-thrombectomy-system [Google Scholar]
- (36).EkoSonic endovascular system brief summary [Internet]. Boston (MA): Boston Scientific; [cited 2021 June 17]. Available from: https://www.bostonscientific.com/en-US/products/thrombectomy-systems/ekosonic-endovascular-system/ekosonic-endovascular-system-brief-summary.html [Google Scholar]
- (37).Straub Endovascular System & Straub Endovascular Tools [Internet]. Vilters-Wangs (SW): Straub Medical; 2019. [cited 2021 July 16]. Available from: http://www.straubmedical.com/userdata/Produkte/bro-en-komplett.pdf [Google Scholar]
- (38).FlowTriever - Inari Medical INC - In Depth guide [Internet]. Miami (FL): Dexur; (c)2022. [cited 2021 July 16]. Available from: https://dexur.com/md/5070327/#physician-metrics [Google Scholar]
- (39).ClotTriever Bold [Internet]. Irvine (CA): Inari Medical; (c)2022. [July 16, 2021]. Available from: https://www.inarimedical.com/clottriever/ [Google Scholar]
- (40).Amplatz Goose Neck Snares and Microsnares [Internet]. [July 16, 2021]. Available from: https://europe.medtronic.com/xd-en/healthcare-professionals/products/cardiovascular/snares/amplatz.html
- (41).Jeti peripheral thrombectomy system [Internet]. Chicago: Abbott; (2)2022. [cited 2021 July 16]. Available from: https://www.jeti.tv [Google Scholar]
- (42).QuickClear mechanical thrombectomy system [Internet]. Amsterdam (NL): Koninklijke Philips N.V.; (c)2004-2022. [cited 2021 July 16]. Available from: https://www.usa.philips.com/healthcare/product/HSIGTDQKCLRTHRM/quickclear-thrombectomy-system [Google Scholar]
- (43).Rotarex rotational excisional atherectomy system [Internet]. Franklin Lakes (NJ): Becton, Dickenson and Co; (c)2022. [cited 2022 March 9]. Available from: https://www.bd.com/en-us/products-and-solutions/products/product-families/rotarex-rotational-excisional-atherectomy-system [Google Scholar]
- (44).EKOS Endovascular System [Internet]. Boston Scientific; 2022. [March 9, 2022]. Available from: https://www.bostonscientific.com/en-US/products/thrombectomy-systems/ekosonic-endovascular-system.html [Google Scholar]
- (45).Thrombectomy Systems [Internet]. Marlborough (MA): Boston Scientific Corp; 2022. [March 9, 2022]. Available from: https://www.bostonscientific.com/en-EU/products/thrombectomy-systems.html [Google Scholar]
- (46).Lichtenberg MKW. Evolving evidence for acute and subacute limb ischemia treatment with a purely mechanical thrombectomy approach. J Endovasc Ther. 2019;26(3):302–4. [DOI] [PubMed] [Google Scholar]
- (47).Darwood R, Berridge DC, Kessel DO, Robertson I, Forster R. Surgery versus thrombolysis for initial management of acute limb ischaemia. Cochrane Database Syst Rev. 2018;8):CD002784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Broderick C, Watson L, Armon MP. Thrombolytic strategies versus standard anticoagulation for acute deep vein thrombosis of the lower limb. Cochrane Database Syst Rev. 2021;1):CD002783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Lambrinos A, Schaink AK, Dhalla I, Krings T, Casaubon LK, Sikich N, et al. Mechanical thrombectomy in acute ischemic stroke: a systematic review. Can J Neurol Sci. 2016;43(4):455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Toyoda K, Yamagami H, Koga M. Consensus guides on stroke thrombolysis for anticoagulated patients from Japan: application to other populations. J Stroke. 2018;20(3):321–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Cordis. 2017 Endovascular Reimbursement Coding Fact sheet [Internet]. Vaughan (ON): Cardinal Health; 2016. [July 23, 2021]. Available from: https://pdf4pro.com/cdn/2017-endovascular-reimbursement-coding-fact-sheet-56534d.pdf [Google Scholar]
- (52).GuidePoint Reimbursement Resources [Internet]. Boston (MA): Boston Scientific; (c)2016. [cited 2021 July 23]. Available from: https://www.bostonscientific.com/content/dam/ThrombusU/Resources/DVT%20Reimbursement%20Guide.pdf [Google Scholar]
- (53).The National Institute for Health and Care Excellence. Interventional procedures programme: interventional procedure overview of percutaneous mechnical thrombectomy for acute deep vein thrombosis of the leg [Internet]. London: NICE; 2019. Available from: https://www.nice.org.uk/guidance/ipg651/evidence/overview-final-pdf-6833014813 [Google Scholar]
- (54).Primary Panel, Abramson BL, Al-Omran M, Anand SS, Albalawi Z, Coutinho T, et al. Canadian Cardiovascular Society 2022 guidelines for peripheral arterial disease. Can J Cardiol. 2022;38(5):560–87. [DOI] [PubMed] [Google Scholar]
- (55).Bjorck M, Earnshaw JJ, Acosta S, Bastos Goncalves F, Cochennec F, Debus ES, et al. Editor's Choice - European Society for Vascular Surgery (ESVS) 2020 clinical practice guidelines on the management of acute limb ischaemia. Eur J Vasc Endovasc Surg. 2020;59(2):173–218. [DOI] [PubMed] [Google Scholar]
- (56).Wanhainen A, Verzini F, Van Herzeele I, Allaire E, Bown M, Cohnert T, et al. Corrigendum to ‘European Society for Vascular Surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms’ [European Journal of Vascular & Endovascular Surgery 57/1 (2019) 8-93]. Eur J Vasc Endovasc Surg. 2020;59(3):494. [DOI] [PubMed] [Google Scholar]
- (57).Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2017;69(11):e71–e126. [DOI] [PubMed] [Google Scholar]
- (58).Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149(2):315–52. [DOI] [PubMed] [Google Scholar]
- (59).Ortel TL, Neumann I, Ageno W, Beyth R, Clark NP, Cuker A, et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020;4(19):4693–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Expert Panel on Interventional Radiology, Farsad K, Kapoor BS, Fidelman N, Cain TR, Caplin DM, et al. ACR Appropriateness criteria radiologic management of iliofemoral venous thrombosis. J Am Coll Radiol. 2020;17(5S):S255–S64. [DOI] [PubMed] [Google Scholar]
- (61).McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6. [DOI] [PubMed] [Google Scholar]
- (62).Garritty C, Gartlehner G, Nussbaumer-Streit B, King VJ, Hamel C, Kamel C, et al. Cochrane Rapid Reviews Methods Group offers evidence-informed guidance to conduct rapid reviews. J Clin Epidemiol. 2021;130:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Covidence (systematic review software) [Computer program]. Veritas Health Innovation. Melbourne (Australia). Available at: https://www.covidence.org/home. [Google Scholar]
- (64).Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(6):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Review Manager meta-analysis software [Internet]. London: The Cochrane Collaboration; (c)2022. [cited 2021 Jun 17]. Available from: https://training.cochrane.org/online-learning/core-software/revman [Google Scholar]
- (66).Cochrane handbook for systematic reviews of interventions version 6.3. Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor. Ottawa (ON): The Cochrane Collaboration; (c)2022. [Google Scholar]
- (67).Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. [DOI] [PubMed] [Google Scholar]
- (68).Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009 Jan;172(1):137–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Kim SY, Park JE, Lee YJ, Seo HJ, Sheen SS, Hahn S, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013;66(4):408–14. [DOI] [PubMed] [Google Scholar]
- (70).GRADE handbook [Internet]. Hamilton (ON): Grade Working Group; 2013. [cited 2017 Dec]. Available from: http://gdt.guidelinedevelopment.org/app/handbook/handbook.html [Google Scholar]
- (71).Ashrafi M, Ahmad SB, Antoniou SA, Khan T, Antoniou GA. Treatment strategies for proximal deep vein thrombosis: a network meta-analysis of randomised controlled trials. Eur J Vasc Endovasc Surg. 2022;63(2):323–34. [DOI] [PubMed] [Google Scholar]
- (72).Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol [Internet]. 2021. [cited 2021 May 27]; 134: 178–89. Available from: https://www.sciencedirect.com/science/article/pii/S0895435621000731 [DOI] [PubMed] [Google Scholar]
- (73).Morrow KL, Kim AH, Plato SA, 2nd, Shevitz AJ, Goldstone J, Baele H, et al. Increased risk of renal dysfunction with percutaneous mechanical thrombectomy compared with catheter-directed thrombolysis. J Vasc Surg. 2017;65(5):1460–6. [DOI] [PubMed] [Google Scholar]
- (74).Chait J, Aurshina A, Marks N, Hingorani A, Ascher E. Comparison of ultrasound-accelerated versus multi-hole infusion catheter-directed thrombolysis for the treatment of acute limb ischemia. Vasc Endovasc Surg. 2019;53(7):558–62. [DOI] [PubMed] [Google Scholar]
- (75).Lu T, Loh TM, El-Sayed HF, Davies MG. Single-center retrospective review of ultrasound-accelerated versus traditional catheter-directed thrombolysis for acute lower extremity deep venous thrombosis. Vascular. 2017;25(5):525–32. [DOI] [PubMed] [Google Scholar]
- (76).Byrne RM, Taha AG, Avgerinos E, Marone LK, Makaroun MS, Chaer RA. Contemporary outcomes of endovascular interventions for acute limb ischemia. J Vasc Surg. 2014;59(4):988–95. [DOI] [PubMed] [Google Scholar]
- (77).de Athayde Soares R, Matielo MF, Brochado Neto FC, Pereira de Carvalho BV, Sacilotto R. Analysis of the safety and efficacy of the endovascular treatment for acute limb ischemia with percutaneous pharmacomechanical thrombectomy compared with catheter-directed thrombolysis. Ann Vasc Surg. 2020;66:470–8. [DOI] [PubMed] [Google Scholar]
- (78).Escobar GA, Burks D, Abate MR, Faramawi MF, Ali AT, Lyons LC, et al. Risk of acute kidney injury after percutaneous pharmacomechanical thrombectomy using angiojet in venous and arterial thrombosis. Ann Vasc Surg. 2017;42:238–45. [DOI] [PubMed] [Google Scholar]
- (79).Gandhi SS, Ewing JA, Cooper E, Chaves JM, Gray BH. Comparison of low-dose catheter-directed thrombolysis with and without pharmacomechanical thrombectomy for acute lower extremity ischemia. Ann Vasc Surg. 2018;46:178–86. [DOI] [PubMed] [Google Scholar]
- (80).Hundt W, Kalinowski M, Stamm AC, Portig I, Swaid Z, Dietz C, et al. Combined treatment of subacute and acute synthetic and venous bypass-graft occlusions with percutaneous mechanical thrombectomy and thrombolysis. Eur J Radiol. 2013;82(12):e807–15. [DOI] [PubMed] [Google Scholar]
- (81).Kronlage M, Printz I, Vogel B, Blessing E, Muller OJ, Katus HA, et al. A comparative study on endovascular treatment of (sub)acute critical limb ischemia: mechanical thrombectomy vs thrombolysis. Drug Des Devel Ther. 2017;11:1233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Muli Jogi RK, Damodharan K, Leong HL, Tan ACS, Chandramohan S, Venkatanarasimha NKK, et al. Catheter-directed thrombolysis versus percutaneous mechanical thrombectomy in the management of acute limb ischemia: a single center review. CVIR Endovascular. 2018;1(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Puangpunngam N, Pleehachinda P, Ruangsetakit C, Wongwanit C, Sermsathanasawadi N, Chinsakchai K, et al. Outcome of percutaneous mechanical thrombectomy compare with catheter directed thrombolysis in acute and subacute lower limb ischemia patients. J Med Assoc Thai. 2020;103(5):55–60. [Google Scholar]
- (84).Schernthaner MB, Samuels S, Biegler P, Benenati JF, Uthoff H. Ultrasound-accelerated versus standard catheter-directed thrombolysis in 102 patients with acute and subacute limb ischemia. J Vasc Interv Radiol. 2014;25(8):1149–56. [DOI] [PubMed] [Google Scholar]
- (85).Garcia MJ, Lookstein R, Malhotra R, Amin A, Blitz LR, Leung DA, et al. Endovascular management of deep vein thrombosis with rheolytic thrombectomy: final report of the prospective multicenter PEARL (peripheral use of angiojet rheolytic thrombectomy with a variety of catheter lengths) registry. J Vasc Intervent Radiol. 2015;26(6):777–85. [DOI] [PubMed] [Google Scholar]
- (86).Comerota AJ, Kearon C, Gu CS, Julian JA, Goldhaber SZ, Kahn SR, et al. Endovascular thrombus removal for acute iliofemoral deep vein thrombosis. Circulation. 2019;139(9):1162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Kahn SR, Julian JA, Kearon C, Gu CS, Cohen DJ, Magnuson EA, et al. Quality of life after pharmacomechanical catheter-directed thrombolysis for proximal deep venous thrombosis. J Vasc Surg. 2020;8(1):8–23.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Kearon C, Gu CS, Julian JA, Goldhaber SZ, Comerota AJ, Gornik HL, et al. Pharmacomechanical catheter-directed thrombolysis in acute femoral-popliteal deep vein thrombosis: analysis from a stratified randomized trial. Thromb Haemost. 2019;119(4):633–44. [DOI] [PubMed] [Google Scholar]
- (89).Razavi MK, Salter A, Goldhaber SZ, Lancia S, Kahn SR, Weinberg I, et al. Correlation between post-procedure residual thrombus and clinical outcome in deep vein thrombosis patients receiving pharmacomechanical thrombolysis in a multicenter randomized trial. J Vasc Interv Radiol. 2020;31(10):1517–28.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Vedantham S, Goldhaber SZ, Julian JA, Kahn SR, Jaff MR, Cohen DJ, et al. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med. 2017;377(23):2240–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Vedantham S, Goldhaber SZ, Kahn SR, Julian J, Magnuson E, Jaff MR, et al. Rationale and design of the ATTRACT study: a multicenter randomized trial to evaluate pharmacomechanical catheter-directed thrombolysis for the prevention of postthrombotic syndrome in patients with proximal deep vein thrombosis. Am Heart J. 2013;165(4):523–30.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Vedantham S, Salter A, Lancia S, Lewis L, Thukral S, Kahn SR. Clinical outcomes of a pharmacomechanical catheter-directed venous thrombolysis strategy that included rheolytic thrombectomy in a multicenter randomized trial. J Vasc Interv Radiol. 2021;11):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Weinberg I, Vedantham S, Salter A, Hadley G, Al-Hammadi N, Kearon C, et al. Relationships between the use of pharmacomechanical catheter-directed thrombolysis, sonographic findings, and clinical outcomes in patients with acute proximal DVT: results from the ATTRACT multicenter randomized trial. Vasc Med. 2019;24(5):442–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Baker R, Samuels S, Benenati JF, Powell A, Uthoff H. Ultrasound-accelerated vs standard catheter-directed thrombolysis - a comparative study in patients with iliofemoral deep vein thrombosis. J Vasc Interv Radiol. 2012;23(11):1460–6. [DOI] [PubMed] [Google Scholar]
- (95).Ezelsoy M, Turunc G, Bayram M. Early outcomes of pharmacomechanical thrombectomy in acute deep vein thrombosis patients. Heart Surg Forum. 2015;18(6):E222–5. [DOI] [PubMed] [Google Scholar]
- (96).Huang CY, Hsu HL, Kuo TT, Lee CY, Hsu CP. Percutaneous pharmacomechanical thrombectomy offers lower risk of post-thrombotic syndrome than catheter-directed thrombolysis in patients with acute deep vein thrombosis of the lower limb. Ann Vasc Surg. 2015;29(5):995–1002. [DOI] [PubMed] [Google Scholar]
- (97).Huang T, Ding W, Chen Z, Yin Y, Yu J, Jin Y, et al. Comparison of pharmacomechanical catheter-directed thrombolysis versus catheter-directed thrombolysis for the treatment of acute iliofemoral deep vein thrombosis: measures of long-term clinical outcome and quality of life. Ann Vasc Surg. 2021;25):25. [DOI] [PubMed] [Google Scholar]
- (98).Kuo TT, Huang CY, Hsu CP, Lee CY. Catheter-directed thrombolysis and pharmacomechanical thrombectomy improve midterm outcome in acute iliofemoral deep vein thrombosis. J Chin Med Assoc. 2017;80(2):72–9. [DOI] [PubMed] [Google Scholar]
- (99).Lee JK, Kim KY, Byun SJ. Safety and efficacy of aspiration thrombectomy or pharmacomechanical thrombectomy after catheter-directed thrombolysis for the treatment of acute iliofemoral deep vein thrombosis. Vasc Specialist Int. 2020;36(3):144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Li X, Xie H, Zhang Y, Li H. Individual choice for the aspiration thrombectomy treatment of acute iliofemoral deep venous thrombosis. Ann Vasc Surg. 2020;69:237–45. [DOI] [PubMed] [Google Scholar]
- (101).Liu X, Cao P, Li Y, Zhao J, Li L, Li H, et al. Safety and efficacy of pharmacomechanical thrombolysis for acute and subacute deep vein thrombosis patients with relative contraindications. Medicine. 2018;97(43):e13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Notten P, Ten Cate-Hoek AJ, Arnoldussen C, Strijkers RHW, de Smet A, Tick LW, et al. Ultrasound-accelerated catheter-directed thrombolysis versus anticoagulation for the prevention of post-thrombotic syndrome (CAVA): a single-blind, multicentre, randomised trial. Lancet Haematol. 2020;7(1):e40–e9. [DOI] [PubMed] [Google Scholar]
- (103).Pouncey AL, Gwozdz AM, Johnson OW, Silickas J, Saha P, Thulasidasan N, et al. AngioJet pharmacomechanical thrombectomy and catheter directed thrombolysis vs. catheter directed thrombolysis alone for the treatment of iliofemoral deep vein thrombosis: a single centre retrospective cohort study. Eur J Vasc Endovasc Surg. 2020;60(4):578–85. [DOI] [PubMed] [Google Scholar]
- (104).Shen Y, Wang X, Jin SS, Zhang RL, Zhao WJ, Chen G. Increased risk of acute kidney injury with percutaneous mechanical thrombectomy using AngioJet compared with catheter-directed thrombolysis. J Vasc Surg. 2019;7(1):29–37. [DOI] [PubMed] [Google Scholar]
- (105).Tian Y, Huang Z, Luo K, Zhang N, Yuan B. A retrospective comparison of catheter-directed thrombolysis versus pharmacomechanical thrombolysis for treatment of acute lower extremity deep venous thrombosis. Ann Vasc Surg. 2021;74:306–14. [DOI] [PubMed] [Google Scholar]
- (106).Tichelaar VYIG, Brodin EE, Vik A, Isaksen T, Skjeldestad FE, Kumar S, et al. A retrospective comparison of ultrasound-assisted catheter-directed thrombolysis and catheter-directed thrombolysis alone for treatment of proximal deep vein thrombosis. Cardiovasc Intervent Radiol. 2016;39(8):1115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (107).Xu Y, Wang X, Shang D, Liu J, Chen W, Han X. Outcome of AngioJet mechanical thrombus aspiration in the treatment of acute lower extremities deep venous thrombosis. Vascular. 2021;29(3):415–23. [DOI] [PubMed] [Google Scholar]
- (108).Xu YD, Zhong BY, Yang C, Cai XS, Hu B, Wang XY, et al. Comparison of catheter-directed thrombolysis with and without percutaneous mechanical thrombectomy for subacute iliofemoral deep vein thrombosis. Phlebology. 2020;35(8):589–96. [DOI] [PubMed] [Google Scholar]
- (109).Zhu J, Ni CF, Dai ZY, Yao LZ, Li WH. A case-controlled study on AngioJet rheolytic thrombectomy and catheter-directed thrombolysis in the treatment of acute lower extremity deep venous thrombosis. Vascular. 2020;28(2):177–82. [DOI] [PubMed] [Google Scholar]
- (110).Soosainathan A, Moore HM, Gohel MS, Davies AH. Scoring systems for the post-thrombotic syndrome. J Vasc Surg. 2013;57(1):254–61. [DOI] [PubMed] [Google Scholar]
- (111).Vedantham S, Salter A, Lancia S, Lewis L, Thukral S, Kahn SR. Clinical Outcomes of a Pharmacomechanical Catheter-Directed Venous Thrombolysis Strategy that Included Rheolytic Thrombectomy in a Multicenter Randomized Trial. Journal of Vascular and Interventional Radiology. 2021;11):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Staniszewska A, Onida S, Lane T, Davies AH. The good, bad and the ugly of the acute venous thrombosis: thrombus removal with adjunctive catheter-directed thrombolysis trial from the viewpoint of clinicians. J Vasc Surg. 2020;8(6):912–8. [DOI] [PubMed] [Google Scholar]
- (113).Obi A, Barnes GD. Continuing to advance the venous agenda: long-term insights from the CAVA trial. J Am Heart Assoc. 2021;10(11):e021659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Hemingway J, Emanuels D, Aarabi S, Quiroga E, Tran N, Starnes B, et al. Safety of transfer, type of procedure, and factors predictive of limb salvage in a modern series of acute limb ischemia. J Vasc Surg. 2019;69(4):1174–9. [DOI] [PubMed] [Google Scholar]
- (115).Weddell J, Muddegowda G, Natarajan I, Nayak S, Jadun C, Hashim Z, et al. Mechanical thrombectomy and the ‘weekend effect’: does admission time influence outcomes? Future Healthc J. 2020;7(Suppl 1):s3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Weddell J, Parr E, Knight S, Muddegowda G, Natarajan I, Chembala J, et al. Mechanical thrombectomy: can it be safely delivered out of hours in the UK? BMC Neurol. 2020;20(1):326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (117).Cakir V, Gulcu A, Akay E, Capar AE, Gencpinar T, Kucuk B, et al. Use of percutaneous aspiration thrombectomy vs. anticoagulation therapy to treat acute iliofemoral venous thrombosis: 1-year follow-up results of a randomised, clinical trial. Cardiovasc Intervent Radiol. 2014;37(4):969–76. [DOI] [PubMed] [Google Scholar]
- (118).EKOS™ endovascular system - EKOS reimbursement hospital inpatient final rule update [Internet]. MA, USA: Boston Scientific; 2020. [March 24, 2022]. Available from: https://www.bostonscientific.com/content/dam/bostonscientific/Reimbursement/peripheral-intervention/pdf/Ekos_Reimbursement_Updates_PI-830305-AB.pdf [Google Scholar]
- (119).Jones S. New mechanical thrombectomy devices designed to treat venous thromboembolism [Internet]. Ottawa (ON): CADTH; 2020. [updated Oct 22 2020; cited 2022 March 24]. Available from: https://www.cadth.ca/new-mechanical-thrombectomy-devices-designed-treat-venous-thromboembolism [Google Scholar]
- (120).Dexter DJ, Kado H, Schor J, Annambhotla S, Olivieri B, Mojibian H, et al. Interim outcomes of mechanical thrombectomy for deep vein thrombosis from the All-Comer CLOUT registry. J Vasc Surg Venous Lymphat Disord. 2022. [DOI] [PubMed]
- (121).Rodriguez LE, Aboukheir-Aboukheir A, Figueroa-Vicente R, Soler-Bernardini H, Bolanos-Avila G, Torruella-Bartolomei LJ, et al. Hybrid operative thrombectomy is noninferior to percutaneous techniques for the treatment of acute iliofemoral deep venous thrombosis. J Vasc Surg Venous Lymphat Disord. 2017;5(2):177–84. [DOI] [PubMed] [Google Scholar]
- (122).Leung DA, Blitz LR, Nelson T, Amin A, Soukas PA, Nanjundappa A, et al. Rheolytic pharmacomechanical thrombectomy for the management of acute limb ischemia: results from the PEARL registry. J Endovasc Ther. 2015;22(4):546–57. [DOI] [PubMed] [Google Scholar]
- (123).Lopez R, DeMartino R, Fleming M, Bjarnason H, Neisen M. Aspiration thrombectomy for acute iliofemoral or central deep venous thrombosis. Journal of Vascular Surgery. 2019;7(2):162–8. [DOI] [PubMed] [Google Scholar]
- (124).Lopez R, Yamashita TS, Neisen M, Fleming M, Colglazier J, Oderich G, et al. Single-center experience with Indigo aspiration thrombectomy for acute lower limb ischemia. Journal of Vascular Surgery. 2020;72(1):226–32. [DOI] [PubMed] [Google Scholar]
- (125).Cao W, Liu Y, Ran P, He J, Xie S, Weng J, et al. Ultrasound-propelled Janus Rod-shaped micromotors for site-specific sonodynamic thrombolysis. ACS Appl Mater Interfaces. 2021;13(49):58411–21. [DOI] [PubMed] [Google Scholar]
- (126).Sista AK, Bhatheja R, Rali P, Natarajan K, Green P, Piazza G, et al. First-in-human study to assess the safety and feasibility of the bashir endovascular catheter for the treatment of acute intermediate-risk pulmonary embolism. Circ Cardiovasc Interv. 2021;14(1):e009611. [DOI] [PubMed] [Google Scholar]
- (127).National Institute for Health and Care Excellence. Process and methods guides. Appendix I: quality appraisal checklist-economic evaluations [Internet]. London: The Institute; 2012. [cited 2016 Jan]. Available from: https://www.nice.org.uk/process/pmg4/chapter/appendix-i-quality-appraisal-checklist-economic-evaluations [Google Scholar]
- (128).Peters CML, de Vries J, Redeker S, Timman R, Eijck GV, Steunenberg SL, et al. Cost-effectiveness of the treatments for critical limb ischemia in the elderly population. J Vasc Surg. 2019;70(2):530–8 e1. [DOI] [PubMed] [Google Scholar]
- (129).Migliara B, Cappellari TF, Mirandola M, Griso A, Kolasa K, Zah V, et al. Treatment of bypass failure in patients with chronic limb threatening ischemia - open surgery vs. percutaneous mechanical thrombectomy. Eur J Vasc Med. 2020;49(5):395–402. [DOI] [PubMed] [Google Scholar]
- (130).Vaidya V, Gangan N, Comerota A, Lurie F. Cost-effectiveness analysis of initial treatment strategies for nonembolic acute limb ischemia using real-word data. Ann Vasc Surg. 2017;39:276–83. [DOI] [PubMed] [Google Scholar]
- (131).Magnuson EA, Chinnakondepalli K, Vilain K, Kearon C, Julian JA, Kahn SR, et al. Cost-Effectiveness of Pharmacomechanical Catheter-Directed Thrombolysis Versus Standard Anticoagulation in Patients With Proximal Deep Vein Thrombosis: Results From the ATTRACT Trial. Circ Cardiovasc Qual Outcomes. 2019;12(10):e005659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Li G, Xu M, Xu Z, Sun Y, Zhang J, Zhang X. Cost-effectiveness analysis of AngioJet and CDT for lower extremity deep vein thrombosis among Chinese population. Clin Appl Thromb Hemost. 2021;27):10760296211061147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).Kwok CHR, Fleming S, Chan KKC, Tibballs J, Samuelson S, Ferguson J, et al. Aspiration Thrombectomy versus Conventional Catheter-Directed Thrombolysis as First-Line Treatment for Noniatrogenic Acute Lower Limb Ischemia. J Vasc Interv Radiol. 2018;29(5):607–13. [DOI] [PubMed] [Google Scholar]
- (134).Li X, Xie H, Zhang Y, Li H. Individual Choice for the Aspiration Thrombectomy Treatment of Acute Iliofemoral Deep Venous Thrombosis. Ann Vasc Surg. 2020;69:237–45. [DOI] [PubMed] [Google Scholar]
- (135).Vedantham S, Goldhaber SZ, Julian JA, Kahn SR, Jaff MR, Cohen DJ, et al. Pharmacomechanical Catheter-Directed Thrombolysis for Deep-Vein Thrombosis. N Engl J Med. 2017;377(23):2240–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (136).Comerota AJ, Kearon C, Gu CS, Julian JA, Goldhaber SZ, Kahn SR, et al. Endovascular Thrombus Removal for Acute Iliofemoral Deep Vein Thrombosis. Circulation. 2019;139(9):1162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (137).Canadian Institute for Health Information. Canadian classification of health interventions. Volume four-alphabetical index [Internet]. Ottawa (ON): The Institute; (c)1996-2022. Available from: https://www.cihi.ca/en/submit-data-and-view-standards/codes-and-classifications [Google Scholar]
- (138).de Donato G, Setacci F, Sirignano P, Galzerano G, Massaroni R, Setacci C. The combination of surgical embolectomy and endovascular techniques may improve outcomes of patients with acute lower limb ischemia. J Vasc Surg. 2014;59(3):729–36. [DOI] [PubMed] [Google Scholar]
- (139).Lopez R, Yamashita TS, Neisen M, Fleming M, Colglazier J, Oderich G, et al. Single-center experience with Indigo aspiration thrombectomy for acute lower limb ischemia. J Vasc Surg. 2020;72(1):226–32. [DOI] [PubMed] [Google Scholar]
- (140).Lopez R, DeMartino R, Fleming M, Bjarnason H, Neisen M. Aspiration thrombectomy for acute iliofemoral or central deep venous thrombosis. J Vasc Surg. 2019;7(2):162–8. [DOI] [PubMed] [Google Scholar]
- (141).Ministry of Health and Long-Term Care. Health data branch web portal: Ontario case costing [Internet]. Toronto (ON): Queen's Printer for Ontario. 2022. Available from: https://hsim.health.gov.on.ca/HDBPortal [Google Scholar]
- (142).Canadian Institute for Health Information (CIHI). Patient cost estimator [Internet]. 2022. [cited 2022 Feb 25]. Available from: https://www.cihi.ca/en/patient-cost-estimator
- (143).Canadian Institute for Health Information. Care in Canadian ICUs [Internet]. Ottawa (ON): The Institute; 2016. Available from: https://secure.cihi.ca/freeproducts/ICUReportEN.pdf [Google Scholar]
- (144).Statistics Canada. Consumer price index: table 326-0020 [Internet]. Ottawa (ON): Statistics Canada; 2022. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1810000401 [Google Scholar]
- (145).Evans J, Kobewka D, Thavorn K, D'Egidio G, Rosenberg E, Kyeremanteng K. The impact of reducing intensive care unit length of stay on hospital costs: evidence from a tertiary care hospital in Canada. Can J Anaesth. 2018;65(6):627–35. [DOI] [PubMed] [Google Scholar]
- (146).Ontario current state assessment and proposed program framework: acute care vascular services [Internet]. Toronto (ON): Cardiac Care Network; 2015. Available from: https://www.corhealthontario.ca/resources-for-healthcare-planners-&-providers/vascular-health-general/CCN-Vascular-Services-Curent-State-Assessment-&-Proposed-Program-Framework-2015.pdf [Google Scholar]
- (147).Barham L. Public and patient involvement at the UK National Institute for Health and Clinical Excellence. Patient. 2011;4(1):1–10. [DOI] [PubMed] [Google Scholar]
- (148).Messina J, Grainger DL. A pilot study to identify areas for further improvements in patient and public involvement in health technology assessments for medicines. Patient. 2012;5(3):199–211. [DOI] [PubMed] [Google Scholar]
- (149).Ontario Health Technology Advisory Committee Public Engagement Subcommittee. Public engagement for health technology assessment at Health Quality Ontario—final report from the Ontario Health Technology Advisory Committee Public Engagement Subcommittee [Internet]. Toronto (ON): Queen's Printer for Ontario; 2015 Apr. [cited 2018 Apr 30]. Available from: http://www.hqontario.ca/Portals/0/documents/evidence/special-reports/report-subcommittee-20150407-en.pdf [Google Scholar]
- (150).Kvale S. Interviews: an introduction to qualitative research interviewing. Thousand Oaks (CA): Sage; 1996. [Google Scholar]
- (151).Kuzel AJ. Sampling in qualitative inquiry. In: Miller WL, Crabtree BF, editors. Doing qualitative research. Thousand Oaks (CA): Sage; 1999. p. 33–45. [Google Scholar]
- (152).Morse J. Emerging from the data: cognitive processes of analysis in qualitative research. In: Morse J, editor. Critical Issues in Qualitative Research Methods. Thousand Oaks (CA): Sage; 1994. p. 23–41. [Google Scholar]
- (153).Patton MQ. Qualitative research and evaluation methods. 3rd ed. Thousand Oaks (CA): Sage; 2002. [Google Scholar]
- (154).Strauss AL, Corbin JM. Basics of qualitative research: techniques and procedures of developing a grounded theory. 2nd ed. Thousand Oaks (CA): Sage; 1998. [Google Scholar]
- (155).Health Technology Assessment International. Introduction to health technology assessment [Internet]. Edmonton (AB): Health Technology Assessment International; 2015. [cited 2018 Apr 30]. Available from: http://www.htai.org/fileadmin/HTAi_Files/ISG/PatientInvolvement/v2_files/Resource/PCISG-Resource-Intro_to_HTA_KFacey_Jun13.pdf [Google Scholar]
- (156).Strauss AL, Corbin JM. Grounded theory research: procedures, canons, and evaluative criteria. Qual Sociol. 1990;13(1):3–21. [Google Scholar]
- (157).Strauss AL, Corbin JM. Grounded theory methodology: an overview. In: Denzin NK, Lincoln YS, editors. Handbook of qualitative research. Thousand Oaks (CA): Sage; 1994. p. 273–85. [Google Scholar]
- (158).NVivo qualitative data analysis software. QSR International. Doncaster, Victoria (Australia). Available at: https://www.qsrinternational.com/nvivo/home. [Google Scholar]
- (159).Pfadenhaur L, Rohwer A, Burns J, Booth A, Lysdahl KB, Hofmann B, et al. Guidance for the assessment of context and implementation in health technology assessments (HTA) and Systematic reviews of complex interventions: the context and implementation of complex interventions (CICI) framework [Internet] 2016. Available from: https://www.researchgate.net/publication/298340571
- (160).Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health. 2015;42(5):533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (161).Alchemer Survey Software [Internet]. Louisville (CO): Alchemer LLC; (c)2022. [cited 2022 Aug 18]. Available from: https://www.alchemer.com [Google Scholar]