Abstract
Purpose
Accurate knowledge of greater palatine foramen (GPF) and greater palatine canal (GPC) anatomy is necessary to avoid injury to the greater palatine artery (GPA) when performing a variety of anesthesiologic, dental or surgical procedures. The aim of this paper was to perform a systematic review and meta-analysis of literature on the anatomy and localization of bony structures associated with the GPA, namely the GPF and GPC.
Methods
A systematic literature search was performed using PubMed, Embase, ScienceDirect, and Web of Science databases. Seventy-five studies were included in the meta-analysis (n = 22,202 subjects).
Results
The meta-analysis showed that the GPF is positioned 17.21 mm (95% CI = 16.34–18.09 mm) from the posterior nasal spine, 2.56 mm (95% CI = 1.90–3.22 mm) from the posterior border of the hard palate, 46.24 mm (95% CI = 44.30–48.18 mm) from the anterior nasal spine, 15.22 mm (95% CI = 15.00–15.43 mm) from the midline maxillary suture, 37.32 mm (95% CI = 36.19–38.45 mm) from the incisive foramen, and opposite the third maxillary molar (M3) in 64.9% (58.7–70.7%) of the total population.
Conclusion
An up-to-date, comprehensive analysis of GPF and GPC clinical anatomy is presented. The results from this evidence-based anatomical study provides a unified set of data to aid clinicians in their practice.
Keywords: Greater palatine artery, Greater palatine foramen, Greater palatine canal, Hard palate, Meta-analysis, Systematic review
Introduction
The hard palate is formed by the fusion of the palatine processes of the maxilla and the horizontal plates of the palatine bone at the so-called transverse palatine suture [20].
The mucosa of the hard palate is predominantly supplied by the greater palatine artery (GPA), which originates from the descending palatine artery in the pterygopalatine fossa, descends through the greater palatine canal (GPC), and emerges from the greater palatine foramen (GPF) near the posterior border of the hard palate [46, 51, 52]. The location of the GPF varies, but it can generally be identified by palpation of the palate opposite the third maxillary molar teeth [35, 60, 74]. Viveka et al. [83] concluded that the utilization of multiple anatomical reference points, such as the incisive foramen, the midline maxillary suture, and the second and third maxillary molars, simplifies identification of the GPF. Adequate identification of the GPF allows for visualization of arterial pulsations, and confirms the location of the GPA.
At the hard palate, the GPA courses anteriorly in close proximity to the alveolar ridge. The greater palatine nerve traverses a groove medial to the artery, from which it is separated by a palpable crest, which can be used by clinicians to localize both structures [13, 60]. The main trunk of the GPA—the lateral branch—enters the nasal cavity through the incisive foramen [50, 60], where it anastomoses with the posterior septal branch of the sphenopalatine artery to supply the anteroinferior portion of the nasal septum. The diameter of the GPA is greatest at the site of its emergence from the GPF, and then decreases gradually as it courses toward the incisive foramen. The GPA gives off most of its branches in the premolar area, and more commonly toward the alveolar side, rather than to the hard palate [28].
An accurate appreciation of the GPA’s location and size is essential to avoid its injury and the resulting surgical and post-surgical complications [69]. Bleeding from the GPA can be difficult to control, with the potential to cause significant blood loss and palatal tissue necrosis [16]. The injury itself, or damage caused by attempts to arrest hemorrhage, may lead to postoperative pseudoaneurysms, or injury to the greater palatine nerve, resulting in paresthesia or insufficient anesthesia of the ipsilateral hard palate [17], and in rare cases, transient ophthalmoplegia [21, 22].
Injury to the GPA occurs most commonly during subepithelial connective tissue graft harvesting and can result in prolonged intraoperative bleeding and postoperative wound healing complications related to impaired blood flow [14, 72]. In fact, the position of the GPA, along with the thickness of the palatal mucosa, are the two main factors that dictate the size of subepithelial connective tissue grafts that can be safely harvested from the hard palate [16].
GPA injury may also be implicated during down-fracture of the maxilla [12], or in other surgical procedures such as osteotomy of the medial and lateral maxillary sinus walls, pterygomaxillary disjunction, endoscopic medial maxillectomy [13], and pterygopalatine fossa infiltration [12]. The last procedure involves injecting either a vasoconstricting agent into the greater palatine canal––to prophylactically induce hemostasis and limit posterior epistaxis during endoscopic sinus surgery and septorhinoplasty––or an anesthetic solution through the greater palatine canal into the pterygopalatine fossa, to achieve anesthesia of the hemi-maxilla during dental procedures by maxillary nerve block [11]. Clinicians can increase the efficiency and safety of these procedures by referring to the anatomical structures in the oral cavity when determining the adequate position, angle, and length of the needle used for pterygopalatine fossa infiltration [16].
Lastly, the morphological parameters discussed are of clinical significance in the mobilization of GPA for closure of oroantral fistula using mucoperiosteal pedicled palatal flaps [16]; radical release of the GPA during cleft palate repair and reconstruction [26]; and endoscopic cauterization of the GPA at the incisive foramen for the purpose of controlling recurrent or uncontrolled anterior epistaxis [15].
We aimed to update and extend the methodology outlined by Tomaszewska to conduct the meta-analysis on the location of the GPF relative to the maxillary molars, by applying it to other anatomical data extracted from the studies. The objective of our review was to update and extend that of Tomaszewska et al. [75] in 2014. The protocol was methodologically planned and followed, although it was not registered. An updated search strategy was utilized to broaden the scope of the research question to include all available anatomical data to synthesize as evidence by introducing more keyword phrases that describe other related anatomical structures than the GPF.
The main objective was to synthesize evidence from all available studies reporting anatomical data, including cadaveric (i.e., dry skulls) and CT-imaging studies of adult patients (i.e., ≥ 21 years old), combining the results into a comprehensive set of readily available data. The primary outcomes to be measured were the pooled mean estimates of the distances between the center of the GPF and five major anatomical reference points, GPF and GPC diameters, and length and angle of the GPC; and the pooled prevalence estimates of the location of the GPF relative to the maxillary molar teeth, morphology of the GPF, and direction of GPF opening into the oral cavity. Secondary outcome measures included subgroup analysis based on the geographical region of the studies included in the analysis, to probe for sources of heterogeneity.
Materials and methods
Search strategy
The authors strictly followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [57] guidelines throughout the literature search [Online Supplementary File 1].
The major electronic databases (PubMed, Embase, ScienceDirect, and Web of Science) were searched extensively to identify articles eligible for inclusion in our meta-analysis up to July 2022. No lower date limit was applied. The following search terms: “greater palatine artery”, “greater palatine canal”, “greater palatine foramen”, “pterygopalatine fossa anatomy”, “pterygopalatine canal”, and “descending palatine canal” were used in different combinations, as shown in Table 1. The references in all included articles were searched manually to identify any further relevant publications. We included only published studies, relying on the journal review process as one step of quality control.
Table 1.
Full database search strategies
| Database | Full search strategy |
|---|---|
| PubMed | (greater palatine artery) OR (greater palatine canal) OR (greater palatine foramen) OR (pterygopalatine fossa anatomy) OR (pterygopalatine canal) OR (descending palatine canal) |
| EMBASE | (greater AND palatine AND artery) OR (greater AND palatine AND canal) OR (greater AND palatine AND foramen) OR (pterygopalatine AND fossa AND anatomy) OR (pterygopalatine AND canal) OR (descending AND palatine AND canal) |
| ScienceDirect | (“greater palatine artery”) OR (“greater palatine canal”) OR (“greater palatine foramen”) OR (“pterygopalatine fossa anatomy”) OR (“pterygopalatine canal”) OR (“descending palatine canal”) |
| Web of Science Core Collection/SciELO/BIOSIS/Current Content Connect/Korean Journal Database/Russian Citation Index | (((((ALL = (greater palatine artery)) OR ALL = (greater palatine canal)) OR ALL = (greater palatine foramen)) OR ALL = (pterygopalatine fossa anatomy)) OR ALL = (pterygopalatine canal)) OR ALL = (descending palatine canal) |
Full search strategies used to search major electronic databases. Databases were accessed on July 2022
Eligibility
Study eligibility for inclusion in our meta-analysis was assessed independently by two reviewers (J.R. and W.R.). Studies were considered eligible for inclusion if they (1) were cadaveric or imaging studies, and (2) reported relevant and extractable data on the clinical anatomy of the greater palatine artery, foramen, or canal. The reviewers did not consider (1) case reports, systematic reviews, animal studies, letters to editors, or meta-analyses, (2) studies that provided missing, unclear, or incomplete results, and (3) studies that did not clearly define (by text or figures) the descriptive anatomy used in the study [33]. Review of full-text articles was limited to the ones published in English language. All differences of opinion among the reviewers concerning the eligibility of the studies were resolved by consensus through consultation with a third reviewer (D.K.).
Data extraction
The studies were analyzed looking for all numerical parameters that could be directly compared between studies. This meant that the same parameter was used in at least two different studies and measured with a comparable degree of precision. The following parameters were included:
Distance between the GPF and the posterior nasal spine (GPF–PNS)
Distance between the GPF and the posterior border of hard palate (GPF–PBHP)
Distance between the GPF and the anterior nasal spine (GPF–ANS)
Distance between the GPF and the midline maxillary suture (GPF–MMS)
Distance between the GPF and the incisive foramen (GPF–IF)
Location of the GPF in relation to the second (M2) and third (M3) maxillary molars
Diameter of the GPF in anteroposterior (AP) and lateromedial (LM) dimensions
Shape of the GPF
Direction of GPF opening into the oral cavity
Angle of the GPC relative to the vertical plane and to the transverse plane
Length of the GPC
Diameter of the GPC upper opening in the anteroposterior (AP) dimension
Quality assessment
The authors used the AQUA tool to evaluate both the quality and accuracy of the anatomical studies incorporated into this meta-analysis, as well as to properly classify their quality and risk of biases [32]. The assessment covers five domains: (1) objective(s) and study characteristics, (2) study design, (3) methodology characterization, (4) descriptive anatomy, and (5) reporting of results. The potential risk for bias in each domain is appraised by judging it as “low,” “high,” or “unclear” using the signaling questions with answers “yes,” “no,” or “unclear,” respectively. In other words, all queries answered with “yes” place the corresponding domain in the “low” risk of bias category, whereas all queries answered with “no” place the corresponding domain in the “high” risk of bias category. Inadequate data that did not allow for clear scrutiny were placed in the “unclear” risk of bias category.
Statistical analysis
The extracted data were pooled into a meta-analysis using R software, with the ‘meta’ package (R Foundation for Statistical Computing). The inverse-variance, random-effects model was used to calculate the pooled effect size estimate across the studies, and the DerSimonian–Laird method was used to estimate the between-study variance, τ2. Statistical heterogeneity was assessed using the I2 statistic and interpreted according to the guidelines in Chapter 9.5.2 of the Cochrane Handbook (Higgins 2011). This statistic expresses the percentage of variation across studies. Heterogeneity of I2 < 25% was considered low, between 25 and 75% was considered moderate, and > 75% was considered high. Subgroup analyses based on the geographic regions in which the studies were performed were conducted to detect sources of heterogeneity. To assess statistically significant differences between two or more subgroups, confidence intervals were compared. If the confidence intervals overlapped, then the differences were considered statistically insignificant [33].
Results
Study identification
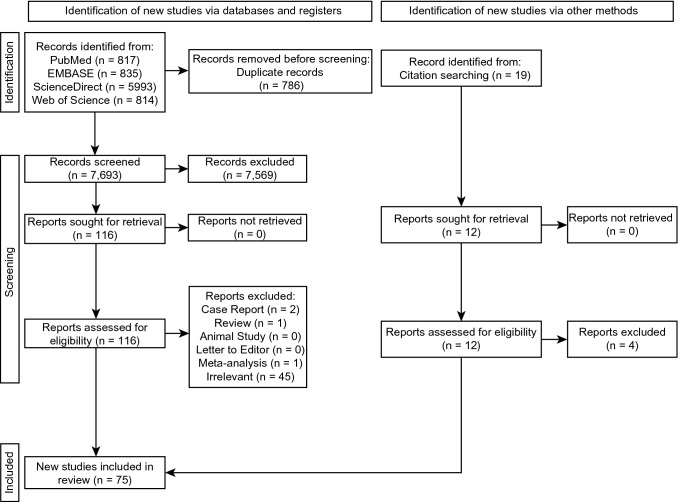
The study identification process is presented in Fig. 1. After extensive searching through the major databases (PubMed, Embase, ScienceDirect, Web of Science), 7,693 studies were initially identified. A further 19 were identified through citation searching; 124 studies were assessed by full text for potential eligibility, of which 49 were deemed ineligible. Thus, 75 studies were included in the meta-analysis.
Fig. 1.
A flowchart depicting the study selection process according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) 2020 Guidelines
Characteristics of included studies
The characteristics of included studies are presented in Table 2. A total of 75 studies (n = 22,202 subjects) were considered eligible and were included in the meta-analysis. In total, there were 29 imaging studies and 46 cadaveric studies. The studies spanned the years 1982 to 2022 and originated from Africa, Asia, Europe, North America, and South America.
Table 2.
Characteristics of studies included in the meta-analysis
| Study | Country of origin | Type of investigation | Sample size by subjects (n) | Sample size by sides |
|---|---|---|---|---|
| Ajmani et al. [1] Indian | India | Cadaveric | 86 | 172 |
| Ajmani et al. [1] Nigerian | Nigeria | Cadaveric | 34 | 68 |
| Anjankar et al. [2] | India | Cadaveric | 65 | 130 |
| Aoun et al. [5] | Lebanon | Imaging | 58 | 116 |
| Aoun and Nasseh [3] | Lebanon | Imaging | 79 | 158 |
| Aoun et al. [4] | Lebanon | Imaging | 74 | 148 |
| Apinhasmit et al. [6] | Thailand | Cadaveric | 55 | 110 |
| Ashwini and Jaishree [7] | India | Cadaveric | 100 | 200 |
| Awad et al. [8] | Egypt | Imaging | 200 | 400 |
| Ayoub et al. [9] | United States | Imaging | 50 | 100 |
| Badshah et al. [10] | Pakistan | Cadaveric | 85 | 170 |
| Bahşi et al. [11] | Turkey | Imaging | 150 | 300 |
| Beetge et al. [12] | South Africa | Imaging | 77 | 154 |
| Cagimni et al. [16] | Turkey | Cadaveric | 120 | 240 |
| Campbell et al. [17] | United States | Imaging | 50 | 100 |
| Cheung et al. [18] | China | Cadaveric | 30 | 60 |
| Chopra et al. [19] | India | Cadaveric | 100 | 200 |
| Chrcanovic and Custódio [20] | Brazil | Cadaveric | 80 | 160 |
| Das et al. [22] | United States | Imaging | 100 | 200 |
| Dave et al. [23] | India | Cadaveric | 100 | 200 |
| D’Souza et al. [21] | India | Cadaveric | 40 | 80 |
| Douglas and Wormald [24] | Australia | Cadaveric | 21 | 42 |
| Duruel et al. [25] | United States | Imaging | 131 | 262 |
| Fonseka et al. [27] | Sri Lanka | Imaging | 50 | 100 |
| Fu et al. [28] | United States | Cadaveric | 11 | 22 |
| Gibelli et al. [29] | Italy | Cadaveric | 100 | 200 |
| Hassanali and Mwaniki [31] | Africa | Cadaveric | 125 | 250 |
| Howard-Swirzinski et al. [34] | United States | Imaging | 500 | 1000 |
| Hwang et al. [35] | South Korea | Imaging | 50 | 100 |
| Ikuta et al. [36] | Brazil | Imaging | 50 | 100 |
| Ilayperuma et al. [37] | Sri Lanka | Cadaveric | 136 | 272 |
| Jaffar and Hamadah [38] | Iraq | Cadaveric | 50 | 100 |
| Kaffe et al. [39] | Israel | Imaging | 58 | 116 |
| Kang et al. [40] | South Korea | Imaging | 107 | 214 |
| Kaur et al. [41] | India | Cadaveric | 100 | 200 |
| Klosek and Rungruang [42] | Thailand | Cadaveric | 41 | 82 |
| Kumar et al. [43] | India | Cadaveric | 100 | 200 |
| Lacerda-Santos et al. [44] | Brazil | Imaging | 60 | 120 |
| Langenegger et al. [45] | South Africa | Cadaveric | 100 | 200 |
| Lim et al. [47] | South Korea | Imaging | 147 | 294 |
| Lopes et al. [48] | Brazil | Cadaveric | 94 | 188 |
| Malamed and Trieger [49] | Mixed origin | Cadaveric | 204 | 408 |
| McKinney et al. [50] | United States | Imaging | 10 | 20 |
| Methathrathip et al. [52] | Thailand | Cadaveric | 105 | 210 |
| Narayan et al. [53] | India | Cadaveric | 35 | 70 |
| Nascimento et al. [54] | Brazil | Cadaveric | 100 | 200 |
| Nimigean et al. [55] | Romania | Cadaveric | 100 | 200 |
| Ortug and Uzel [56] | Turkey | Cadaveric | 97 | 194 |
| Piagkou et al. [57] | Greece | Cadaveric | 71 | 142 |
| Priya et al. [58] | India | Cadaveric | 132 | 264 |
| Rapado-González et al. [59] | Spain | Imaging | 150 | 300 |
| Rapado-González et al. [60] | Spain | Imaging | 110 | 220 |
| Renu [61] | India | Cadaveric | 100 | 200 |
| Reshmi [62] | India | Cadaveric | 50 | 100 |
| Safavi et al. [63] | Iran | Imaging | 128 | 256 |
| Salcedo et al. [64] | Chile | Cadaveric | 31 | 62 |
| Saralaya and Nayak [65] | India | Cadaveric | 132 | 264 |
| Sharma and Garud [66] | India | Cadaveric | 100 | 200 |
| Sheikhi et al. [67] | Iran | Imaging | 138 | 276 |
| Siddiqui et al. [68] | India | Cadaveric | 98 | 196 |
| Soto et al. [70] | Colombia | Cadaveric | 50 | 100 |
| Suzuki et al. [71] | Japan | Cadaveric | 20 | 40 |
| Teixeira et al. [73] | Brazil | Cadaveric | 141 | 282 |
| Thunyacharoen et al. [74] | Thailand | Cadaveric | 200 | 400 |
| Tomaszewska et al. [75] | Poland | Imaging | 1200 | 2400 |
| Tomaszewska et al. [77] | Poland | Imaging | 1350 | 2700 |
| Tomaszewska et al. [76] | Poland | Imaging | 1500 | 3000 |
| Urbano et al. [78] | Brazil | Cadaveric | 43 | 86 |
| Valizadeh et al. [79] | Iran | Imaging | 148 | 296 |
| Vidulasri and Thenmozhi [80] | India | Cadaveric | 50 | 100 |
| Vikraman et al. [81] | India | Cadaveric | 30 | 60 |
| Vinay et al. [82] | India | Cadaveric | 150 | 300 |
| Viveka and Kumar [83] | India | Imaging | 44 | 88 |
| Wang et al. [84] | China | Cadaveric | 100 | 200 |
| Westmoreland and Blanton [85] | India | Cadaveric | 300 | 600 |
| Wu et al. [86] | China | Imaging | 120 | 240 |
Table displaying characteristics of the 75 studies that were included in the meta-analysis, sorted in alphabetical order (A–Z) by last names of the first authors of the studies. The study characteristics included country of study origin, whether the study subjects were either dry skulls (i.e., cadaveric studies) or CBCT (cone-beam computed tomography) scans (i.e., imaging studies), number of subjects in the studies, and the number of sides included in each study
Quality assessment
Application of the AQUA tool criteria revealed that 41 studies (54.7%) in this meta-analysis had a “low” risk of bias while 34 studies (45.3%) had a “high” risk of bias in domain one (objective(s) and characteristics of the subject). In domain two (study design), 70 studies (93.3%) presented a “low” risk of bias and 5 studies (6.7%) a “high” risk. In contrast, 60 studies (80%) were assessed as having “low” risk of bias in domain three (methodology characterization) and 15 studies (20%) were assessed as having “high” risk of bias. In domain four (descriptive anatomy), 61 studies (81.3%) had a “low” risk of bias while the remaining 14 studies (18.7%) had a “high” risk of bias. Lastly, in domain five (reporting of results), 58 studies (77.3%) had a “low” risk of bias and 17 studies (22.7%) had a “high” risk of bias. Details of the risk of bias assessment using the AQUA tool criteria are shown in Table 3.
Table 3.
Summary of results of the AQUA tool used to evaluate the risk of bias assessment
| Study | Risk of bias | ||||
|---|---|---|---|---|---|
| Objective(s) and study characteristics | Study design | Methodology characterization | Descriptive anatomy | Reporting of results | |
| Ajmani et al. [1] | High | Low | Low | High | High |
| Anjankar et al. [2] | High | Low | Low | Low | Low |
| Aoun et al. [5] | Low | Low | Low | Low | Low |
| Aoun and Nasseh [3] | Low | Low | Low | Low | Low |
| Aoun et al. [4] | Low | Low | Low | Low | Low |
| Apinhasmit et al. [6] | Low | Low | Low | Low | High |
| Ashwini and Jaishree [7] | High | Low | Low | Low | Low |
| Awad et al. [8] | Low | Low | Low | Low | Low |
| Ayoub et al. [9] | Low | Low | Low | Low | Low |
| Badshah et al. [10] | High | Low | High | Low | Low |
| Bahşi et al. [11] | Low | Low | Low | Low | Low |
| Beetge et al. [12] | High | Low | Low | Low | Low |
| Cagimni et al. [16] | High | Low | Low | Low | High |
| Campbell et al. [17] | High | Low | High | Low | Low |
| Cheung et al. [18] | High | High | High | High | Low |
| Chopra et al. [19] | High | Low | Low | Low | High |
| Chrcanovic and Custódio [20] | High | Low | Low | Low | Low |
| Das et al. [22] | High | Low | High | Low | Low |
| Dave et al. [23] | High | Low | High | High | Low |
| D’Souza et al. [21] | Low | Low | Low | Low | Low |
| Douglas and Wormald [24] | High | Low | Low | Low | Low |
| Duruel et al. [25] | Low | Low | Low | Low | Low |
| Fonseka et al. [27] | Low | Low | Low | Low | Low |
| Fu et al. [28] | High | Low | Low | Low | High |
| Gibelli et al. [29] | Low | Low | Low | Low | Low |
| Hassanali and Mwaniki [31] | Low | Low | Low | Low | Low |
| Howard-Swirzinski et al. [34] | Low | Low | Low | Low | Low |
| Hwang et al. [35] | Low | Low | Low | Low | Low |
| Ikuta et al. [36] | Low | Low | Low | Low | Low |
| Ilayperuma et al. [37] | High | Low | Low | Low | Low |
| Jaffar and Hamadah [38] | High | Low | High | Low | Low |
| Kaffe et al. [39] | High | High | Low | Low | Low |
| Kang et al. [40] | High | Low | Low | Low | High |
| Kaur et al. [41] | Low | Low | Low | Low | Low |
| Klosek and Rungruang [42] | Low | Low | Low | Low | Low |
| Kumar et al. [43] | High | Low | Low | Low | Low |
| Lacerda-Santos, et al. [44] | Low | Low | Low | Low | Low |
| Langenegger et al. [45] | Low | Low | Low | Low | High |
| Lim et al. [47] | Low | Low | Low | Low | Low |
| Lopes et al. [48] | Low | Low | High | Low | Low |
| Malamed and Trieger [49] | High | High | High | High | High |
| McKinney et al. [50] | High | Low | Low | Low | High |
| Methathrathip et al. [52] | Low | Low | Low | High | High |
| Narayan et al. [53] | Low | Low | Low | Low | Low |
| Nascimento et al. [54] | Low | Low | Low | Low | Low |
| Nimigean et al. [55] | High | Low | Low | Low | Low |
| Ortug and Uzel [56] | Low | Low | Low | Low | Low |
| Piagkou et al. [57] | High | Low | Low | Low | High |
| Priya et al. [58] | High | Low | Low | Low | Low |
| Rapado-González et al. [59] | Low | Low | Low | Low | Low |
| Rapado-González et al. [60] | Low | Low | High | High | Low |
| Renu [61] | High | Low | Low | Low | Low |
| Reshmi [62] | High | High | High | High | High |
| Safavi et al. [63] | Low | Low | Low | Low | Low |
| Salcedo et al. [64] | Low | Low | Low | Low | High |
| Saralaya and Nayak [65] | High | Low | Low | Low | Low |
| Sharma and Garud [66] | High | Low | Low | Low | Low |
| Sheikhi et al. [67] | Low | Low | Low | High | Low |
| Siddiqui et al. [68] | High | Low | Low | High | High |
| Soto et al. [70] | High | High | High | High | Low |
| Suzuki et al. [71] | Low | Low | Low | Low | Low |
| Teixeira et al. [73] | Low | Low | Low | High | Low |
| Thunyacharoen et al. [74] | Low | Low | Low | Low | Low |
| Tomaszewska et al. [75] | Low | Low | Low | Low | Low |
| Tomaszewska et al. [77] | Low | Low | Low | Low | High |
| Tomaszewska et al. [76] | Low | Low | Low | Low | Low |
| Urbano et al. [78] | High | Low | High | High | High |
| Valizadeh et al. [79] | Low | Low | Low | Low | Low |
| Vidulasri and Thenmozhi [80] | High | Low | Low | Low | Low |
| Vikraman et al. [81] | High | Low | High | High | High |
| Vinay et al. [82] | High | Low | Low | Low | Low |
| Viveka and Kumar [83] | Low | Low | Low | Low | Low |
| Wang et al. [84] | Low | Low | High | Low | Low |
| Westmoreland and Blanton [85] | Low | Low | High | High | Low |
| Wu et al. [86] | Low | Low | Low | Low | Low |
Distance between the greater palatine foramen and selected anatomical landmarks
The results of the meta-analysis regarding the distance between the greater palatine foramen and surrounding anatomical landmarks are presented in Table 4. A total of 8 studies [8, 11, 29, 35, 56, 63, 77, 83] (n = 2358 subjects) reported data on the distance from the greater palatine foramen to the posterior nasal spine (GPF–PNS). The pooled mean, across the eight studies, was calculated to be 17.21 mm (95% CI = 16.34–18.09 mm). The Q test showed high heterogeneity (Q = 345.96; p < 0.0001), which was confirmed by the I2 test (98.0%; 95% CI = 97.2–98.6%). To explore the source of heterogeneity, the studies were subdivided into groups based on geographical location. For both subgroups, heterogeneity was still high: for Asian studies Q = 198.31 (p < 0.0001), I2 = 98.5% (95% CI = 97.6–99.0%) and for European studies Q = 105.84 (p < 0.0001), I2 = 99.1% (95% CI = 98.2–99.5%).
Table 4.
Distance between the greater palatine foramen and surrounding anatomical landmarks
| Total number of studies | Total number of subjects | Pooled mean (95% CI) [mm] |
Cochrane’s Q | I2 (95% CI) [%] | p value | |
|---|---|---|---|---|---|---|
| GPF–PNSa | 8 | 2358 | 17.21 (16.34–18.09) | 345.96 | 98.0 (97.2–98.6) | p < 0.0001 |
| Asia | 4 | 380 | 17.06 (15.57–18.56) | 198.31 | 98.5 (97.6–99.0) | p < 0.0001 |
| Europe | 2 | 1450 | 18.04 (15.94–20.15) | 105.84 | 99.1 (98.2–99.5) | p < 0.0001 |
| GPF–PBHP | 24 | 4349 | 2.56 (1.90–3.22) | 276,374.89 | 100.0 | p = 0 |
| Africa | 2 | 465 | 3.71 (0.83–6.59) | 43.02 | 97.7 (94.3–99.0) | p < 0.0001 |
| Asia | 17 | 2030 | 4.16 (3.17–5.15) | 262,382.79 | 100.0 | p = 0 |
| Europe | 3 | 1600 | 4.18 (1.83–6.54) | 79.92 | 97.5 (95.1–98.7) | p < 0.0001 |
| South America | 2 | 254 | 3.41 (0.53–6.30) | 0.11 | 0.0 | p = 0.74 |
| GPF–ANSb | 4 | 365 | 46.24 (44.30–48.18) | 90.68 | 96.7 (94.1–98.2) | p < 0.0001 |
| GPF–MMS | 38 | 5379 | 15.22 (15.00–15.43) | 10,090.72 | 99.6 (99.6–99.7) | p = 0 |
| Africa | 3 | 565 | 15.13 (14.36–15.89) | 42.13 | 95.3 (89.4–97.9) | p = 0.075 |
| Asia | 26 | 2506 | 15.14 (14.88–15.40) | 9776.26 | 99.7 (99.7–99.8) | p = 0 |
| Europe | 4 | 1773 | 15.76 (15.10–16.42) | 68.67 | 95.6 (91.7–97.7) | p < 0.0001 |
| South America | 5 | 535 | 15.21 (14.61–15.81) | 56.23 | 92.9 (86.4–96.3) | p < 0.0001 |
| GPF–IF | 23 | 4404 | 37.32 (36.19–38.45) | 3837.15 | 99.4 (99.4–99.5) | p = 0 |
| Africa | 2 | 520 | 38.23 (37.70–38.75) | 2.68 | 62.6 (62.5–62.6) | p < 0.0001 |
| Asia | 16 | 2113 | 36.87 (35.51–38.22) | 2078.70 | 99.3 (99.0–99.5) | p < 0.0001 |
| Europe | 2 | 1450 | 36.79 (31.69–41.89) | 353.06 | 99.7 (99.6–99.8) | p < 0.0001 |
| South America | 3 | 321 | 39.47 (35.81–43.12) | 173.63 | 98.8 (98.1–99.3) | p < 0.0001 |
CI confidence interval, GPF greater palatine foramen, PNS posterior nasal spine, PBHP posterior border of hard palate, ANS anterior nasal spine, MMS midline maxillary suture, IF incisive foramen
aTwo studies [8, 63] were excluded from the subgroup analysis due to being the only studies in their own respective subgroups
bSubgroup analysis for GPF–ANS was not performed due to the low number of studies
A total of 24 studies [1, 7, 8, 10, 16, 19–21, 29, 37, 38, 41, 43, 48, 58, 59, 65, 66, 77, 80, 82, 84, 85] (n = 4349 subjects) reported data on the distance from the greater palatine foramen to the posterior border of the hard palate (GPF–PBHP). The pooled mean, across the 24 studies, was calculated to be 2.56 mm (95% CI = 1.90–3.22 mm). The Q test showed high heterogeneity (Q = 274,522.83; p < 0.0001), which was confirmed by the I2 test (100.0%). Subgroup analysis, based on geographical region, was performed to investigate heterogeneity. For South American studies, the Q test showed almost no heterogeneity (Q = 0.11; p = 0.74), confirmed by the I2 test (0.0%). For the other geographical regions, heterogeneity was still high: for Asian studies Q = 261,423.78 (p < 0.0001), I2 = 100.0%, and for European studies Q = 79.92 (p < 0.0001), I2 = 97.5% (95% CI = 95.1–98.7%).
A total of 4 studies [5, 27, 40, 59] (n = 365 subjects) reported data on the distance from the greater palatine foramen to the anterior nasal spine (GPF–ANS). The pooled mean, across the four studies, was calculated to be 46.24 mm (95% CI = 44.30–48.18 mm). The Q test showed high heterogeneity (Q = 90.68; p < 0.0001), which was confirmed by the I2 test (96.7%). Subgroup analysis based on geographical region was not performed due to the low number of studies; there were only two possible subgroups and one of these contained only one study, precluding the possibility of pooling the mean using meta-analysis.
A total of 38 studies [1, 5, 7, 8, 10, 11, 16, 19–22, 27, 29, 36–38, 40–45, 48, 56–59, 63, 65, 66, 73, 77, 80–85] (n = 5479 subjects) reported data on the distance from the greater palatine foramen to the median maxillary suture (GPF–MMS). The pooled mean, across the 38 studies, was calculated to be 15.22 mm (95% CI = 15.00–15.43 mm). The Q test showed high heterogeneity (Q = 10,090.72; p = 0), which was confirmed by the I2 test (99.6%; 95% CI = 99.6–99.7%). Subgroup analysis, based on geographical location of the studies, was performed to explore the source of this heterogeneity. For African studies, the Q test showed high heterogeneity (Q = 42.13; p = 0.075), confirmed by the I2 test (95.3%; 95% CI = 89.4–97.9%). For the other geographical regions, heterogeneity was significantly higher: for Asian studies Q = 9776.26 (p < 0.0001), I2 = 99.7% (95% CI = 99.7–99.8%), for European studies Q = 68.67 (p < 0.0001), I2 = 95.6% (95% CI = 91.7–97.7%), and for South American studies Q = 56.23 (p < 0.0001), I2 = 92.9% (95% CI = 86.4–96.3%).
A total of 23 studies [7, 8, 10, 11, 20, 29, 40, 41, 43, 44, 47, 54, 56, 58, 65, 66, 73, 74, 77, 80, 82, 83, 86] (n = 3164 subjects) reported data on the distance from the greater palatine foramen to the incisive fossa (GPF–IF). The pooled mean, across the 23 studies, was calculated to be 37.32 mm (95% CI = 36.19–38.45 mm). The Q test showed high heterogeneity (Q = 3837.15; p = 0), which was confirmed by the I2 test (99.4%; 95% CI = 99.4–99.5%). Subgroup analysis, based on geographic location of the studies, was performed to explore sources of heterogeneity. For African studies, the Q test showed high heterogeneity (Q = 2.68; p < 0.0001), confirmed by the I2 test (62.6%; 95% CI = 62.5–62.6%), for Asian studies Q = 2078.70 (p < 0.0001), I2 = 98.7% (95% CI = 99.0–99.5%), for European studies Q = 353.06 (p < 0.0001), I2 = 99.7% (95% CI = 99.6–99.8), and for South American studies Q = 173.63 (p < 0.0001), I2 = 98.8% (95% CI = 98.1–99.3%).
Location of the greater palatine foramen in relation to maxillary molars
The results of the meta-analysis regarding the location of the GPF in relation to the maxillary molar teeth are presented in Table 5. Only two studies [19, 27] (n = 284 subjects) reported data on the prevalence of the greater palatine foramen being located “anterior to the 2nd maxillary molar teeth”. The pooled prevalence, across the two studies, was calculated to be 3.27% (95% CI = 0.45–20.29%). The statistical significance of the Q test (Q = 6.67, df = 1, p = 0.0098) allowed the null hypothesis of homogeneity to be rejected. The I2 test showed moderate to high heterogeneity (I2 = 85.0%; 95% CI = 58.5–94.6%).
Table 5.
The location of the greater palatine foramen in relation to maxillary molars
| Total number of studies | Total number of subjects | Pooled prevalence (95% CI) [%] |
Cochrane’s Q | I2 (95% CI) [%] | p value | |
|---|---|---|---|---|---|---|
| Anterior to M2a | 2 | 284 | 3.3 (0.5–20.3) | 6.67 | 85.0 (58.5–94.6) | p = 0.0098 |
| Opposite M2b | 33 | 8852 | 5.0 (3.2–3.9) | 371.78 | 91.4 (89.0–93.3) | p < 0.0001 |
| Africa | 4 | 980 | 6.2 (1.0–30.0) | 10.86 | 72.4 (48.5–93.3) | p = 0.0045 |
| Asia | 23 | 4731 | 3.8 (2.2–6.3) | 160.52 | 86.3 (80.9–90.7) | p < 0.0001 |
| Europe | 2 | 2800 | 16.0 (9.9–26.9) | 3.68 | 72.8 (30.3–89.4) | p = 0.055 |
| South America | 2 | 162 | 4.3 (2.2–9.6) | 1.05 | 5.0 (0.0–11.4) | p = 0.30 |
| Between M2 and M3 | 37 | 9496 | 19.3 (15.3–24.0) | 547.71 | 94.5 (93.2–95.6) | p < 0.0001 |
| Africa | 4 | 980 | 11.4 (2.9–35.6) | 54.62 | 96.3 (92.5–98.2) | p < 0.0001 |
| Asia | 27 | 5366 | 20.6 (17.3–24.4) | 155.95 | 86.5 (81.0–90.5) | p < 0.0001 |
| Europe | 3 | 2907 | 11.8 (5.9–22.1) | 21.87 | 90.9 (77.5–96.3) | p < 0.0001 |
| South America | 2 | 222 | 18.1 (2.1–69.6) | 32.83 | 97.0 (92.9–98.7) | p < 0.0001 |
| Opposite M3 | 38 | 9754 | 64.9 (58.7–70.7) | 610.61 | 94.8 (93.5–95.8) | p < 0.0001 |
| Africa | 4 | 980 | 49.5 (46.4–52.6) | 76.94 | 97.4 (95.0–98.6) | p < 0.0001 |
| Asia | 27 | 5366 | 66.7 (65.4–67.9) | 363.88 | 94.0 (92.4–95.6) | p < 0.0001 |
| Europe | 3 | 2907 | 74.7 (73.1–76.2) | 0.21 | 0.0 | p = 0.90 |
| South America | 3 | 322 | 65.8 (60.5–70.8) | 33.96 | 94.1 (86.7–97.4) | p < 0.0001 |
| Distal to M3 | 31 | 8608 | 6.0 (3.7–9.6) | 1183.69 | 96.7 (96.1–97.3) | p < 0.0001 |
| Asia | 24 | 4841 | 7.7 (7.0– 8.6) | 263.34 | 89.0 (84.5–92.2) | p < 0.0001 |
| Europe | 3 | 2907 | 2.4 (1.9–3.0) | 10.93 | 81.7 (49.2–93.4) | p = 0.0042 |
| South America | 2 | 260 | 25.8 (20.8–31.4) | 26.11 | 96.2 (90.7–98.4) | p < 0.0001 |
A total of 33 studies [1, 2, 5, 7, 8, 10, 11, 19, 21, 22, 27, 28, 31, 36–39, 41, 43, 45, 49, 52, 55, 58, 61, 63–65, 77, 79, 81, 82, 85] (n = 8,852 subjects) reported data on the prevalence of the greater palatine foramen being located “opposite the 2nd maxillary molar teeth”. The pooled prevalence, across the 33 studies, was calculated to be 5.0% (95% CI = 3.2–3.9%). The statistical significance of the Q test (Q = 371.78, p < 0.0001) allowed the null hypothesis of homogeneity to be rejected. The I2 test showed high heterogeneity (I2 = 91.4%; 95% CI = 89.0–93.3%).
A total of 37 studies [1, 2, 5, 7, 8, 10, 11, 19–22, 27, 28, 31, 37–39, 41, 43, 45, 52, 53, 55, 57, 58, 61, 63–65, 68, 74, 77, 79, 81, 82, 85] (n = 9,496 subjects) reported data on the greater palatine foramen being located “between the 2nd and 3rd maxillary molar teeth”. The pooled prevalence, across the 37 studies, was calculated to be 19.3% (95% CI = 15.3–24.0%). The statistical significance of the Q test (Q = 547.61, p < 0.0001) allowed the null hypothesis of homogeneity to be rejected. The I2 test showed high heterogeneity (I2 = 94.5%; 95% CI = 93.2–95.6%).
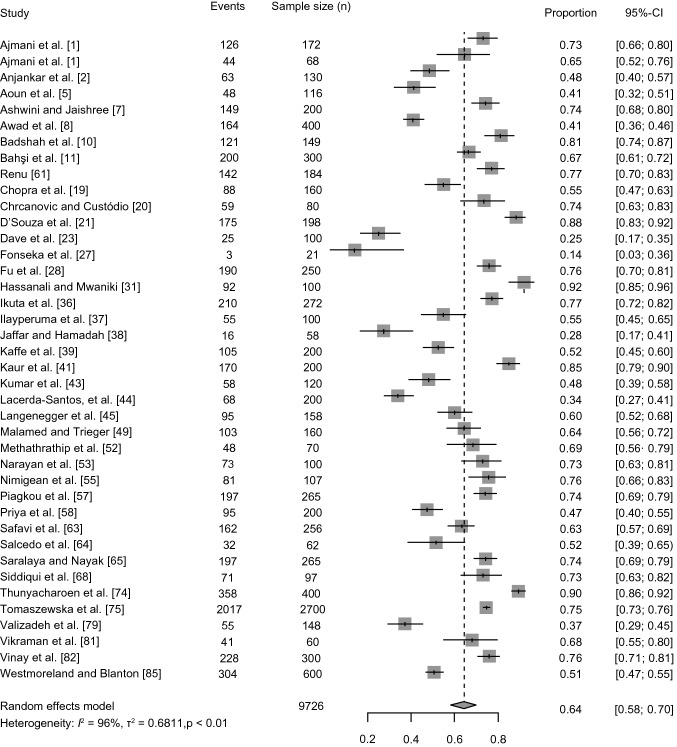
A total of 38 studies [1, 2, 5, 7, 8, 10, 11, 19–22, 27, 28, 31, 36–39, 41, 43, 45, 49, 52, 53, 55, 57, 58, 61, 63–65, 68, 74, 77, 79, 81, 82, 85] (n = 9754 subjects) reported data on the greater palatine foramen being located “opposite the 3rd maxillary molar teeth”. The pooled prevalence, across the 38 studies, was calculated to be 64.9% (95% CI = 58.7–70.7%). The statistical significance of the Q test (Q = 610.61, p < 0.0001) allowed the null hypothesis of homogeneity to be rejected. The I2 test showed high heterogeneity (I2 = 94.8%; 95% CI = 93.5–95.8%). The results of the meta-analysis are shown as a forest plot in Fig. 2.
Fig. 2.
Forest plot depicting the prevalence of the greater palatine foramen positioned opposite the third maxillary molar teeth. Studies were sorted in order of the reported proportion, defined as the ratio of the number of events (GPF being located opposite M3) to the total number of subjects in the study
A total of 31 studies [1, 2, 5, 7, 8, 11, 19–21, 27, 36–39, 41, 43, 45, 52, 53, 55, 57, 58, 61, 63, 65, 68, 74, 77, 81, 82, 85] (n = 7,282 subjects) reported data on the greater palatine foramen (GPF) being located “distal to the 3rd maxillary molar”. The pooled prevalence, across the 31 studies, was calculated to be 6.0% (95% CI = 3.7–9.6%). The statistical significance of the Q test (Q = 1183.6, p < 0.0001) allowed the null hypothesis of homogeneity to be rejected. The I2 test showed high heterogeneity (I2 = 96.7%; 95% CI = 96.1–97.3%).
Morphometric parameters of the greater palatine foramen
The results of the meta-analysis regarding the morphometric parameters of the GPF are presented in Table 6. A total of 13 studies [4, 5, 8, 11, 12, 25, 27, 35, 41, 57, 59, 66, 77] (n = 3,066 subjects) reported data on the anteroposterior (AP) diameter of the greater palatine foramen. The pooled mean, across the 11 studies, was calculated to be 5.34 mm (95% CI = 4.99–5.68 mm). The statistical significance of the Q test (Q = 491.85, df = 10, p < 0.0001) allowed the null hypothesis of homogeneity to be rejected. The I2 test showed high heterogeneity (I2 = 98.0%; 95% CI = 97.3–98.5%).
Table 6.
Size and shape of the greater palatine foramen
| Total number of studies | Total number of subjects | Pooled estimate (95% CI) |
Cochrane’s Q | I2 (95% CI) [%] | p value | |
|---|---|---|---|---|---|---|
| AP diametera (mm) | 13 | 3066 | 5.34 (4.99–5.68) | 491.85 | 98.0 (97.3–98.5) | p < 0.0001 |
| Africa | 2 | 477 | 4.64 (3.88–5.40) | 76.48 | 98.7 (97.2–99.4) | p < 0.0001 |
| Asia | 6 | 537 | 5.29 (4.84–5.74) | 390.81 | 98.7 (98.2–99.1) | p < 0.0001 |
| Europe | 3 | 1721 | 5.50 (4.88–6.13) | 91.48 | 97.8 (95.9–98.8) | p < 0.0001 |
| LM diameter (mm) | 14 | 4803 | 2.77 (2.58–2.96) | 967.40 | 98.2 (98.3–98.9) | p < 0.0001 |
| Africa | 2 | 677 | 2.51 (2.12–2.90) | 62.59 | 96.8 (93.5–98.4) | p = 0.0002 |
| Asia | 5 | 855 | 2.65 (2.34–2.96) | 341.01 | 98.5 (97.9–99.0) | p < 0.0001 |
| Europe | 5 | 3271 | 3.04 (2.74–3.35) | 105.66 | 96.2 (93.5–97.8) | p < 0.0001 |
| Oval/ovoid shapeb (%) | 6 | 986 | 77.8 (57.6–90.0) | 150.22 | 96.7 (94.7–97.9) | p < 0.0001 |
| Asia | 3 | 516 | 93.2 (74.1–98.5) | 35.28 | 94.3 (87.3–97.5) | p < 0.0001 |
| South America | 2 | 250 | 52.2 (40.9–63.2) | 2.57 | 61.1 (12.0–82.8) | p = 0.11 |
| Round shapec (%) | 4 | 670 | 9.4 (3.3–23.8) | 44.43 | 91.0 (82.3–95.4) | p < 0.0001 |
| Slit/lancet shapec (%) | 4 | 676 | 8.4 (2.4–25.8) | 56.20 | 92.9 (86.6–96.2) | p < 0.0001 |
| Other shapec (%) | 2 | 330 | 35.3 (14.3–64.0) | 20.78 | 95.2 (87.9–98.1) | p < 0.0001 |
CI confidence interval, AP anteroposterior, LM lateromedial
aTwo studies [12, 25] were excluded from the subgroup analysis because they were the only studies in their own respective subgroups
bOne study [60] was excluded from the subgroup analysis
cMeta-analysis for the greater palatine foramen shapes “Round”, “Slit/lancet”, and “Other” were not followed up by subgroup analysis due to the low number of studies
A total of 14 studies [6, 8, 10–12, 35, 41, 45, 52, 55, 57, 59, 76, 77] (n = 4,803 subjects) reported data on the lateromedial (LM) diameter of the greater palatine foramen. The pooled mean, across the 12 studies, was calculated to be 2.77 mm (95% CI = 2.58–2.96 mm). The statistical significance of the Q test (Q = 967.40, p < 0.0001) allowed the null hypothesis of homogeneity to be rejected. The I2 test showed high heterogeneity (I2 = 98.2%; 95% CI = 98.3–98.9%).
Morphology of the greater palatine foramen
The results of the meta-analysis regarding the morphology of the GPF are presented in Table 6. A total of 6 studies [6–8, 19, 41, 48, 60, 64] (n = 986 subjects) reported data on the prevalence of the greater palatine foramen being “oval/ovoid” in shape. The pooled prevalence, across the six studies, was calculated to be 77.8% (95%CI = 57.6–90.0%). The statistical significance of the Q test (Q = 150.22, p < 0.0001) allowed the null hypothesis of homogeneity to be rejected. The I2 test showed high heterogeneity (I2 = 96.7%; 95% CI = 94.7–97.9%).
A total of 4 studies [7, 8, 41, 48, 60, 64] (n = 670 subjects) reported data on the prevalence of the greater palatine foramen being “round” in shape. The pooled prevalence, across the four studies, was calculated to be 9.4% (95% CI = 3.3–23.8%). The statistical significance of the Q test (Q = 44.43, p < 0.0001) allowed the null hypothesis of homogeneity to be rejected. The I2 test showed moderate to high heterogeneity (I2 = 91.0%; 95% CI = 82.3–95.4%).
A total of 4 studies [19, 41, 48, 60, 64] (n = 676 subjects) reported data on the prevalence of the greater palatine foramen being “slit/lancet” in shape. The pooled prevalence, across the four studies, was calculated to be 8.4% (95% CI = 2.4–25.8%). The statistical significance of the Q test (Q = 56.20, p < 0.0001) allowed the null hypothesis of homogeneity to be rejected. The I2 test showed moderate to high heterogeneity (I2 = 92.9%; 95% CI = 86.6–96.2%).
Only 2 studies [6, 41, 60] (n = 330 subjects) reported data on the prevalence of the greater palatine foramen being “other” in shape. The pooled prevalence, across the two studies, was calculated to be 35.3% (95% CI = 14.3–64.0%). The statistical significance of the Q test (Q = 20.78, p < 0.0001) allowed the null hypothesis of homogeneity to be rejected. The I2 test showed high heterogeneity (I2 = 95.2%; 95% CI = 87.9–98.1%).
Direction of opening of the greater palatine foramen
The results of the meta-analysis regarding the direction of opening of the GPF into the oral cavity are presented in Table 7. A total of 10 studies [1, 2, 7, 37, 43, 65, 66, 68, 77, 82] (n = 4,534 subjects) reported data on the prevalence of the greater palatine foramen opening into the oral cavity in the inferior–anterior–lateral direction. The pooled prevalence, across the ten studies, was calculated to be 14.41% (95% CI = 4.91–35.43%). The statistical significance of the Q test (Q = 873.78, p < 0.0001) allowed the null hypothesis of homogeneity to be rejected. The I2 test showed high heterogeneity (I2 = 99.0%; 95% CI = 98.7–99.2%).
Table 7.
Direction of the opening of the greater palatine foramen into the oral cavity
| Total number of studies | Total number of subjects | Pooled prevalence (95% CI) [%] |
Cochrane’s Q | I2 (95% CI) [%] | p value | |
|---|---|---|---|---|---|---|
| I–A–La | 10 | 4534 | 14.41 (4.91–35.43) | 873.78 | 99.0 (98.7–99.2) | p < 0.0001 |
| Anterior | 15 | 5864 | 30.11 (17.67–46.37) | 1063.43 | 98.7 (98.4–98.9) | p < 0.0001 |
| Asia | 12 | 2804 | 32.74 (20.40–48.05) | 453.05 | 97.6 (96.8–98.2) | p < 0.0001 |
| Europe | 2 | 2900 | 9.58 (5.45–16.31) | 7.87 | 87.3 (64.8–95.4) | p = 0.0050 |
| I–A–M | 14 | 5312 | 54.54 (40.53–67.87) | 886.74 | 98.4 (98.0–98.7) | p < 0.0001 |
| Asia | 10 | 1872 | 48.88 (36.66–61.24) | 216.68 | 95.8 (94.0–97.1) | p < 0.0001 |
| Europe | 2 | 2900 | 82.55 (81.13–83.89) | 0.05 | 0.0 | p = 0.83 |
| Vertical | 13 | 5490 | 15.95 (5.78–37.00) | 1406.94 | 99.1 (99.0–99.3) | p < 0.0001 |
| Asia | 9 | 2180 | 19.62 (5.25–51.84) | 741.85 | 98.9 (98.6–99.2) | p < 0.0001 |
| Europe | 2 | 2900 | 5.17 (4.42–6.04) | 0.01 | 0.0 | p = 0.91 |
CI confidence interval, I–A–L inferior–anterior–lateral, I–A–M, inferior–anterior–medial
aSubgroup analysis for I–A–L was not performed due to the low number of studies; there were only two possible subgroups and one of these contained only one study, precluding the possibility of pooling the prevalence using meta-analysis
A total of 15 studies [1, 7, 20, 21, 37, 38, 41, 43, 55, 65, 66, 68, 77, 82, 84, 85] (n = 5,864 subjects) reported data on the prevalence of the greater palatine foramen opening anteriorly into the oral cavity. The pooled prevalence, across the 15 studies, was calculated to be 30.11% (95% CI = 17.67–46.37%). The statistical significance of the Q test (Q = 1063.43, p < 0.0001) allowed the null hypothesis of homogeneity to be rejected. The I2 test showed high heterogeneity (I2 = 98.7%; 95% CI = 98.4–98.9%).
A total of 15 studies [1, 2, 7, 20, 31, 37, 38, 41, 43, 55, 65, 66, 68, 77, 82] (n = 5,312 subjects) reported data on the prevalence of the greater palatine foramen opening in the inferior–anterior–medial direction into the oral cavity. The pooled prevalence, across the 15 studies, was calculated to be 54.54% (95% CI = 40.53–67.87%). The statistical significance of the Q test (Q = 886.74, p < 0.0001) allowed the null hypothesis of homogeneity to be rejected. The I2 test showed high heterogeneity (I2 = 98.4%; 95% CI = 98.0–98.7%).
A total of 13 studies [1, 20, 21, 31, 38, 43, 52, 55, 66, 77, 82, 84, 85] (n = 5,490 subjects) reported data on the prevalence of the greater palatine foramen opening in the vertical direction into the oral cavity. The pooled prevalence, across the 13 studies, was calculated to be 15.94% (95% CI = 5.78–37.00%). The statistical significance of the Q test (Q = 1406.94, p < 0.0001) allowed the null hypothesis of homogeneity to be rejected. The I2 test showed high heterogeneity (I2 = 99.2%; 95% CI = 99.0–99.3%).
Other characteristics of the greater palatine canal
The results of the meta-analysis regarding other characteristics of the GPC are presented in Table 8. A total of 13 studies [3, 4, 23, 25, 34, 35, 50, 52, 59, 67, 70, 75, 76] (n = 4,798 subjects) reported data on the length of the greater palatine canal. The pooled mean, across the 13 studies, was calculated to be 26.97 mm (95% CI = 23.65–30.29 mm). The statistical significance of the Q test (Q = 17,900.35, df = 12, p < 0.0001) allowed the null hypothesis of homogeneity to be rejected. The I2 test showed high heterogeneity (I2 = 99.93%; 95% CI = 99.93–99.94%).
Table 8.
Characteristics of the greater palatine canal
| Total number of studies | Total number of subjects | Pooled estimate (95% CI) |
Cochrane’s Q | I2 (95% CI) [%] | p value | |
|---|---|---|---|---|---|---|
| Length (mm) | 13 | 4798 | 26.97 (23.65–30.29) | 17,900.35 | 100.0 | p = 0 |
| Asia | 5 | 597 | 28.19 (19.58–36.80) | 6434.65 | 100.0 | p = 0 |
| Europe | 3 | 2850 | 24.86 (16.01–33.71) | 10,945.60 | 100.0 | p = 0 |
| North America | 4 | 1251 | 25.78 (23.44–28.13) | 280.19 | 98.9 (98.4–99.3) | p < 0.0001 |
| AP diametera (mm) | 3 | 360 | 4.61 (2.74–6.47) | 486.64 | 99.6 (99.4–99.7) | p < 0.0001 |
| Angle between the vertical plane and the axis of the GPC (°) | 4 | 710 | 19.09 (9.20–28.99) | 1297.44 | 99.7 (99.6–99.8) | p < 0.0001 |
| Asia | 2 | 510 | 10.76 (2.80–18.72) | 225.37 | 99.6 (99.3–99.7) | p < 0.0001 |
| North America | 2 | 200 | 27.45 (18.74–36.16) | 91.84 | 98.9 (97.9–99.4) | p < 0.0001 |
| Angle between the transverse plane and the axis of the GPC* (°) | 2 | 310 | 62.63 (53.32–71.94) | 141.84 | 99.3 (98.7–99.6) | p < 0.0001 |
CI confidence interval, AP anteroposterior, GPC greater palatine canal
aSubgroup analyses were not performed due to the low number of studies; for each parameter, there were only two possible subgroups and one of these contained only one study, precluding the possibility of pooling the prevalence using meta-analysis
A total of 3 studies [3, 25, 59] (n = 360) reported data on the anteroposterior diameter of the upper opening of the greater palatine canal. The pooled mean, across the three studies, was calculated to be 3.88 mm (95% CI = 3.77–3.99 mm). The statistical significance of the Q test (Q = 486.64, df = 2, p < 0.0001) allowed the null hypothesis of homogeneity to be rejected. The I2 test showed high heterogeneity (I2 = 99.59%; 95% CI = 99.41–99.71%).
A total of 5 studies [9, 11, 17, 44, 52] (n = 710 subjects) reported data on the angle between the vertical plane and the axis of the greater palatine canal. The pooled mean, across the four studies, was calculated to be 19.09° (95% CI = 9.20–28.99°). The statistical significance of the Q test (Q = 1297.44, df = 4, p < 0.0001) allowed the null hypothesis of homogeneity to be rejected. The I2 test showed high heterogeneity (I2 = 99.69%; 95% CI = 99.61–99.75%).
A total of 2 studies [35, 52] (n = 310 subjects) reported data on the measured angle between the transverse plane and the axis of the greater palatine canal. The pooled mean, across the two studies, was calculated to be 62.63° (95% CI = 53.32–71.94°). The statistical significance of the Q test (Q = 141.84, df = 1, p < 0.0001) allowed the null hypothesis of homogeneity to be rejected. The I2 test showed high heterogeneity (I2 = 99.29%; 95% CI = 98.75–99.60%).
Discussion
To date, the leading anesthesiology and surgery textbooks have offered only general descriptions regarding clinical localization of the greater palatine foramen (GPF) and greater palatine canal (GPC), often leading to inconsistencies in physician training [82]. Though a large number of studies have been conducted concerning the location and morphometric characteristics of the GPF and GPC, many of these publications report an ongoing difficulty in localizing these structures, and therefore identifying the GPA in clinical settings [30].
Locating the GPF in relation to maxillary molar teeth remains a fast and effective way for clinicians to estimate the location of the GPF. Our findings were consistent with those of a similar review by Tomaszewska et al. [75], which also revealed that the GPF is most commonly located opposite the third maxillary molar (M3). Our results add substantial value to the findings of Tomaszewska et al. [75]. The analysis of the prior review contained only 23 studies (n = 6927 subjects) and the pooled prevalence was estimated to be 63.9% with a 95% confidence interval ranging from 56.5 to 70.9%. Our review, which contained a total of 38 studies (n = 9,754 subjects) and a pooled prevalence of 64.9%, with a 95% confidence interval from 58.7 to 70.7% strengthens the validity of the findings of Tomaszewska et al. [75–77] with the addition of 15 studies, adding significantly to the overall sample size, and narrowing the 95% confidence interval.
An additional aspect to consider when referencing the GPF to the maxillary molars is the size and shape of the GPF. Our meta-analysis revealed that the GPF has an anteroposterior (AP) diameter of 5.34 mm and lateromedial (LM) diameter of 2.82 mm, representing the major and minor axes, respectively. This is consistent with our other findings that the GPF was described as “oval or ovoid” in shape in 77.78% of the population. A possible explanation for such AP elongation of the GPF, is that the AP dimension of the palate increases with the eruption of the posterior teeth.
In edentulous patients, the location of the GPF can be accurately triangulated using measured distances to easily identifiable landmarks, the most reliable of which are the median maxillary suture (MMS), the posterior border of the hard palate (PBHP), and the incisive foramen (IF), rather than the posterior nasal septum (PNS) and the anterior nasal septum (ANS). The topography of the hard palate with reference to the anatomical landmarks is of clinical importance also when obtaining free gingival and connective tissue grafts [42], where the distance from the GPF to the incisive foramen (GPF–IF) is used to estimate the possible length of the graft [20, 42, 64].
Furthermore, using GPF–IF and GPF–MMS, it is possible to derive the angle between the MMS and the line from the IF to the GPF, which Tomaszewska et al. [75] called the MMS–IF–GPF angle. Utilizing our findings for GPF–IF and GPF–MMS, we found the MMS–IF–GPF angle to be 24.07 degrees, which is consistent with the angle calculated by Saralaya and Nayak (21.1 and 21.2 degrees) [64] and Chrcanovic and Custódio (22.12 and 23.30 degrees) [20]. Knowing the MMS–IF–GPF angle may also be useful in determining the angle to be made by the needle for anesthetic infiltration into the GPF [20, 64].
In the setting of maxillary nerve block and hemostasis using the GPC approach, the length of the GPC is particularly relevant. For anesthesia, the needle must advance 30 mm, while for hemostasis, specifically during sinus surgery, it is recommended to infiltrate the needle as deep as 25 mm [75].
Our meta-analysis results suggest that anatomical variation of the direction of opening of the GPF may occur more frequently than previously thought. An inferior–anterior–medial (I–A–M) opening relative to the sagittal plane was found 54.54% of the time, considerably less than that was previously estimated at 82.1% [75]. The second most common direction of opening was in the anterior direction, occurring 30.11% of the time in our study, in stark contrast to 7.6% in the 2015 study by Tomaszewska et al. [77] The most common method of administering anesthesia via the GPF was to bend the needle to an angle of 30–45 degrees. In light of our findings, it may be advisable to administer anesthesia to the maxillary nerve by bending the needle to an angle closer to 30 degrees, as the smaller angle would mitigate the risk of puncturing the hard palate soft tissue in the case that the GPF opens in the anterior direction.
One notable variation in GPF anatomy, as shown in ultrasonographic imaging studies [22], is a bony ledge that partially covers the opening of the foramen; in the presence of this variation, the data collected and pooled on the direction of opening becomes a clinically difficult statistic, and represents another challenge the clinician must be aware of when inserting a needle into the GPF.
We met with several limitations during our systematic review which was the lack of studies which directly described the anatomy of the GPA; this prevents us from making conclusions about the course of the artery itself, at least distal to the GPF. Another issue was the heterogeneity of the included studies, both in terms of the parameters measured and the modalities used to measure them (e.g., imaging versus cadaveric studies). For instance, as mentioned above, different studies used different categories to report the location of the GPF in relation to the maxillary molars, as well as its shape (see also Tables 3, 4). On the other hand, some parameters—such as the distance between the GPF and the nasal spines—were only reported in a small number of studies (Table 2). The main limitation of the meta-analysis was the substantial heterogeneity among the included studies, which persisted even after subgroup analysis based on geographical region. The included studies featured little information on individual patient characteristics, such as gender, precluding a more detailed subgroup analysis. The majority of the studies were performed on dry adult skulls, and consequently, the majority of these studies also did not report gender or age, which posed a limitation when probing for possible sources of heterogeneity.
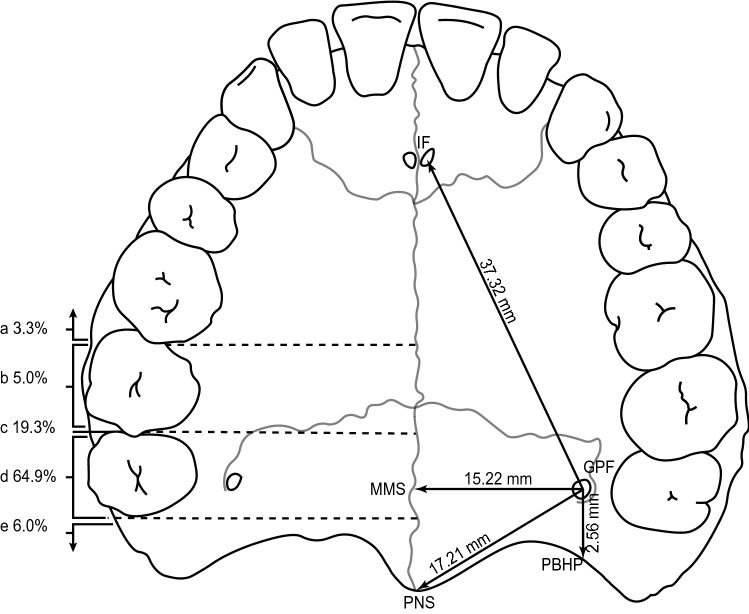
We propose that the maxillary molar teeth, midline maxillary suture, posterior border of the hard palate, and the incisive foramen are the most reliable anatomical landmarks to accurately locate the GPF. Clinicians may expect to locate the foramen 15.00–15.44 mm from the midline maxillary suture, 1.90–3.22 mm from the posterior border of the hard palate, and 36.19–38.45 mm from the incisive foramen. The main findings are summarized in Fig. 3.
Fig. 3.
Illustration of the hard palate, displaying the greater palatine foramen in relation to anatomical landmarks and the maxillary molar teeth. The pooled mean distances from the greater palatine foramen (GPF) to four major anatomical landmarks (IF, MMS, PBHP, PNS) are shown on the right side of the diagram, while the pooled prevalence of the greater palatine foramen location in relation to the maxillary molar teeth are shown on the left side, (a–e). GPF greater palatine foramen, IF incisive foramen, MMS midline maxillary suture, PBHP posterior border of hard palate, PNS posterior nasal spine; a anterior to the mesial surface of the second maxillary molar; b opposite to the second maxillary molar; c between the second and the third maxillary molar; d opposite to the third maxillary molar; e distal to the third maxillary molar
Acknowledgements
The illustration (Fig. 3) is an original work done by the corresponding author of this study, Dong Woon Kim.
Author contributions
DWK: data collection or management, data analysis, manuscript writing/editing. JT: manuscript writing/editing. JS: manuscript writing/editing. JR: data collection or management. WR: data collection or management. IŚ: data collection or management. JRP: data collection or management. IMT: protocol/project development.
Funding
The authors declare that they did not receive any funding.
Data availability
All of the data that were extracted from the included studies during the data collection process and used to perform our analyses are stored in a repository and publicly available on Open Science Framework (https://osf.io/64thm/?view_only=d472458ec3084d9da9efefdfd396b605).
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ajmani ML. Anatomical variation in position of the greater palatine foramen in the adult human skull. J Anat. 1994;184(Pt 3):635–637. [PMC free article] [PubMed] [Google Scholar]
- 2.Anjankar V, Gupta D, Nair S, Thaduri N, Trivedi G, Budhiraja V. Analysis of position of greater palatine foramen in central Indian adult skulls: a consideration for maxillary nerve block. Indian J Pharm Biol Res (IJPBR) 2014;2:51–54. doi: 10.30750/ijpbr.2.1.8. [DOI] [Google Scholar]
- 3.Aoun G, Nasseh I. The length of the greater palatine canal in a Lebanese population: a radio-anatomical study. Acta Inform Med. 2016;24:397–400. doi: 10.5455/aim.2016.24.397-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoun G, Nasseh I, Sokhn S. Radio-anatomical study of the greater palatine canal and the pterygopalatine fossa in a Lebanese population: a consideration for maxillary nerve block. J Clin Imaging Sci. 2016;6:35. doi: 10.4103/2156-7514.190862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aoun G, Nasseh I, Sokhn S, Saadeh M. Analysis of the greater palatine foramen in a Lebanese population using cone-beam computed tomography technology. J Int Soc Prev Community Dent. 2015;5:S82–S88. doi: 10.4103/2231-0762.171594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apinhasmit W, Chompoopong S, Methathrathip D, Sangvichien S, Karuwanarint S. Clinical anatomy of the posterior maxilla pertaining to Le Fort I osteotomy in Thais. Clin Anat. 2005;18:323–329. doi: 10.1002/ca.20131. [DOI] [PubMed] [Google Scholar]
- 7.Ashwini H, Jaishree H. The morphometric study of greater palatine foramen in dry adult skulls. Indian J Med Case Rep. 2014;3:73–77. [Google Scholar]
- 8.Awad AS, Tohamy HMA, Gadallah HN, Ibrahim MEE-D, Raafat TA. Role of multi-detector CT in analysis of the greater and lesser palatine foramina. Egypt J Radiol Nucl Med. 2020;51:150. doi: 10.1186/s43055-020-00272-5. [DOI] [Google Scholar]
- 9.Ayoub N, Thamboo A, Hwang PH, Walgama ES. Radioanatomic study of the greater palatine canal relevant to endoscopic endonasal surgical landmarks. Otolaryngol Head Neck Surg. 2017;157:731–736. doi: 10.1177/0194599817711883. [DOI] [PubMed] [Google Scholar]
- 10.Badshah M, Soames R, Khan MJ, Hasnain J. Morphology of the human hard palate: a study on dry skulls. Ital J Anat Embryol. 2018;123:55–63. [Google Scholar]
- 11.Bahşi İ, Orhan M, Kervancıoğlu P, Yalçın ED. Morphometric evaluation and clinical implications of the greater palatine foramen, greater palatine canal and pterygopalatine fossa on CBCT images and review of literature. Surg Radiol Anat. 2019;41:551–567. doi: 10.1007/s00276-019-02179-x. [DOI] [PubMed] [Google Scholar]
- 12.Beetge MM, Todorovic VS, Oettlé A, Hoffman J, van Zyl AW. A micro-CT study of the greater palatine foramen in human skulls. J Oral Sci. 2018;60:51–56. doi: 10.2334/josnusd.16-0783. [DOI] [PubMed] [Google Scholar]
- 13.Benninger B, Andrews K, Carter W. Clinical measurements of hard palate and implications for subepithelial connective tissue grafts with suggestions for palatal nomenclature. J Oral Maxillofac Surg. 2012;70:149–153. doi: 10.1016/j.joms.2011.03.066. [DOI] [PubMed] [Google Scholar]
- 14.Brasher WJ, Rees TD, Boyce WA. Complications of free grafts of masticatory mucosa. J Periodontol. 1975;46:133–138. doi: 10.1902/jop.1975.46.3.133. [DOI] [PubMed] [Google Scholar]
- 15.Butrymowicz A, Weisstuch A, Zhao A, Agarwal J, Pinheiro-Neto CD. Endoscopic endonasal greater palatine artery cauterization at the incisive foramen for control of anterior epistaxis. Laryngoscope. 2016;126:1033–1038. doi: 10.1002/lary.25677. [DOI] [PubMed] [Google Scholar]
- 16.Cagimni P, Govsa F, Ozer MA, Kazak Z. Computerized analysis of the greater palatine foramen to gain the palatine neurovascular bundle during palatal surgery. Surg Radiol Anat. 2017;39:177–184. doi: 10.1007/s00276-016-1691-0. [DOI] [PubMed] [Google Scholar]
- 17.Campbell RG, Solares CA, Mason EC, Prevedello DM, Carrau RL. Endoscopic endonasal landmarks to the greater palatine canal: a radiographic study. J Neurol Surg B Skull Base. 2018;79:325–329. doi: 10.1055/s-0037-1607966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheung LK, Fung SC, Li T, Samman N. Posterior maxillary anatomy: implications for Le Fort I osteotomy. Int J Oral Maxillofac Surg. 1998;27:346–351. doi: 10.1016/s0901-5027(98)80062-3. [DOI] [PubMed] [Google Scholar]
- 19.Chopra V, Singh AP, Chopra R, Joshi H. Location of greater palatine foramen in the Indian population. SMU Med J. 2016;3:205–214. [Google Scholar]
- 20.Chrcanovic BR, Custódio AL. Anatomical variation in the position of the greater palatine foramen. J Oral Sci. 2010;52:109–113. doi: 10.2334/josnusd.52.109. [DOI] [PubMed] [Google Scholar]
- 21.D’Souza AS, Mamatha H, Jyothi N. Morphometric analysis of hard palate in south Indian skulls. Biomed Res. 2012;23:173–175. [Google Scholar]
- 22.Das S, Kim D, Cannon TY, Ebert CS, Jr, Senior BA. High-resolution computed tomography analysis of the greater palatine canal. Am J Rhinol. 2006;20:603–608. doi: 10.2500/ajr.2006.20.2949. [DOI] [PubMed] [Google Scholar]
- 23.Dave MR, Yagain VK, Anadkat S. A study of the anatomical variations in the position of the greater palatine foramen in adult human skulls and its clinical significance. Int J Morphol. 2013;31:578–583. doi: 10.4067/S0717-95022013000200036. [DOI] [Google Scholar]
- 24.Douglas R, Wormald PJ. Pterygopalatine fossa infiltration through the greater palatine foramen: where to bend the needle. Laryngoscope. 2006;116:1255–1257. doi: 10.1097/01.mlg.0000226005.43817.a2. [DOI] [PubMed] [Google Scholar]
- 25.Duruel O, Kulkarni V, Ataman-Duruel ET, Tözüm MD, Tözüm TF. Radio-morphometric evaluation of greater palatine canal and pterygopalatine fossa component: maxillary anesthetic implications. J Craniofac Surg. 2019;30:863–867. doi: 10.1097/scs.0000000000005260. [DOI] [PubMed] [Google Scholar]
- 26.Fayyaz GQ, Gill NA, Chaudry A, Ishaq I, Aslam M, Shamim R, Kafeel MM, Aazam M, Sailer H, Ganatra MA. Radical dissection of greater palatine artery and dynamic reconstruction of cleft palate. Plast Reconstr Surg Glob Open. 2017;5:e1235. doi: 10.1097/gox.0000000000001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fonseka MCN, Hettiarachchi P, Jayasinghe RM, Jayasinghe RD, Nanayakkara CD. A cone beam computed tomographic analysis of the greater palatine foramen in a cohort of Sri Lankans. J Oral Biol Craniofac Res. 2019;9:306–310. doi: 10.1016/j.jobcr.2019.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu JH, Hasso DG, Yeh CY, Leong DJ, Chan HL, Wang HL. The accuracy of identifying the greater palatine neurovascular bundle: a cadaver study. J Periodontol. 2011;82:1000–1006. doi: 10.1902/jop.2011.100619. [DOI] [PubMed] [Google Scholar]
- 29.Gibelli D, Borlando A, Dolci C, Pucciarelli V, Cattaneo C, Sforza C. Anatomical characteristics of greater palatine foramen: a novel point of view. Surg Radiol Anat. 2017;39:1359–1368. doi: 10.1007/s00276-017-1899-7. [DOI] [PubMed] [Google Scholar]
- 30.Hafeez NS, Sondekoppam RV, Ganapathy S, Armstrong JE, Shimizu M, Johnson M, Merrifield P, Galil KA. Ultrasound-guided greater palatine nerve block: a case series of anatomical descriptions and clinical evaluations. Anesth Analg. 2014;119:726–730. doi: 10.1213/ane.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 31.Hassanali J, Mwaniki D. Palatal analysis and osteology of the hard palate of the Kenyan African skulls. Anat Rec. 1984;209:273–280. doi: 10.1002/ar.1092090213. [DOI] [PubMed] [Google Scholar]
- 32.Henry BM, Tomaszewski KA, Ramakrishnan PK, Roy J, Vikse J, Loukas M, Tubbs RS, Walocha JA. Development of the anatomical quality assessment (AQUA) tool for the quality assessment of anatomical studies included in meta-analyses and systematic reviews. Clin Anat. 2017;30:6–13. doi: 10.1002/ca.22799. [DOI] [PubMed] [Google Scholar]
- 33.Henry BM, Tomaszewski KA, Walocha JA. Methods of evidence-based anatomy: a guide to conducting systematic reviews and meta-analysis of anatomical studies. Ann Anat. 2016;205:16–21. doi: 10.1016/j.aanat.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Howard-Swirzinski K, Edwards PC, Saini TS, Norton NS. Length and geometric patterns of the greater palatine canal observed in cone beam computed tomography. Int J Dent. 2010 doi: 10.1155/2010/292753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hwang SH, Seo JH, Joo YH, Kim BG, Cho JH, Kang JM. An anatomic study using three-dimensional reconstruction for pterygopalatine fossa infiltration via the greater palatine canal. Clin Anat. 2011;24:576–582. doi: 10.1002/ca.21134. [DOI] [PubMed] [Google Scholar]
- 36.Ikuta CR, Cardoso CL, Ferreira-Júnior O, Lauris JR, Souza PH, Rubira-Bullen IR. Position of the greater palatine foramen: an anatomical study through cone beam computed tomography images. Surg Radiol Anat. 2013;35:837–842. doi: 10.1007/s00276-013-1151-z. [DOI] [PubMed] [Google Scholar]
- 37.Ilayperuma I, Nanayakkara G, Palahepitiya N. Evaluación morfométrica del foramen palatino mayor en craneos adultos de Sri Lanka. Int J Morphol. 2014;32:1418–1422. doi: 10.4067/S0717-95022014000400046. [DOI] [Google Scholar]
- 38.Jaffar AA, Hamadah HJ. An analysis of the position of the greater palatine foramen. J Basic Med Sci. 2003;3:24–32. [Google Scholar]
- 39.Kaffe I, Littner MM, Tamse A, Yechezkeli N, Arensburg B. The greater palatine foramen in periapical radiographs imaged with the bisecting angle technique. Dentomaxillofac Radiol. 1984;13:117–124. doi: 10.1259/dmfr.1984.0013. [DOI] [PubMed] [Google Scholar]
- 40.Kang SH, Byun IY, Kim JH, Park HK, Kim MK. Three-dimensional analysis of maxillary anatomic landmarks for greater palatine nerve block anesthesia. J Craniofac Surg. 2012;23:e199–e202. doi: 10.1097/SCS.0b013e31824de71b. [DOI] [PubMed] [Google Scholar]
- 41.Kaur A, Singla RK, Sharma R. An integrative anatomical evaluation of greater palatine foramen in human skull base in north Indian population with clinical implications. J Cardiovasc Dis Res. 2022;13:488–495. [Google Scholar]
- 42.Klosek SK, Rungruang T. Anatomical study of the greater palatine artery and related structures of the palatal vault: considerations for palate as the subepithelial connective tissue graft donor site. Surg Radiol Anat. 2009;31:245–250. doi: 10.1007/s00276-008-0432-4. [DOI] [PubMed] [Google Scholar]
- 43.Kumar A, Sharma A, Singh P. Assessment of the relative location of greater palatine foramen in adult Indian skulls: consideration for maxillary nerve block. Eur J Anat. 2011;15:150–154. [Google Scholar]
- 44.Lacerda-Santos JT, Granja GL, de Freitas GB, Manhães LRC, Jr, de Melo DP, Dos Santos JA. The influence of facial types on the morphology and location of the greater palatine foramen: a CBCT study. Oral Radiol. 2022;38:337–343. doi: 10.1007/s11282-021-00563-1. [DOI] [PubMed] [Google Scholar]
- 45.Langenegger JJ, Lownie JF, Cleaton-Jones PE. The relationship of the greater palatine foramen to the molar teeth and pterygoid hamulus in human skulls. J Dent. 1983;11:249–256. doi: 10.1016/0300-5712(83)90197-5. [DOI] [PubMed] [Google Scholar]
- 46.Li KK, Meara JG, Alexander A., Jr Location of the descending palatine artery in relation to the Le Fort I osteotomy. J Oral Maxillofac Surg. 1996;54:822–825. doi: 10.1016/s0278-2391(96)90528-5. [DOI] [PubMed] [Google Scholar]
- 47.Lim BD, Choi DS, Jang I, Cha BK. Application of the foramina of the trigeminal nerve as landmarks for analysis of craniofacial morphology. Korean J Orthod. 2019;49:326–337. doi: 10.4041/kjod.2019.49.5.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lopes PT, Santos AMP, Pereira GA, Oliveira VCBD. Análisis morfométrico del foramen palatino mayor en cráneos de individuos adultos del sur de Brasil. Int J Morphol. 2011;29:420–423. doi: 10.4067/S0717-95022011000200019. [DOI] [Google Scholar]
- 49.Malamed SF, Trieger N. Intraoral maxillary nerve block: an anatomical and clinical study. Anesth Prog. 1983;30:44–48. [PMC free article] [PubMed] [Google Scholar]
- 50.McKinney KA, Stadler ME, Wong YT, Shah RN, Rose AS, Zdanski CJ, Ebert CS, Jr, Wheless SA, Senior BA, Drake AF, Zanation AM. Transpalatal greater palatine canal injection: radioanatomic analysis of where to bend the needle for pediatric sinus surgery. Am J Rhinol Allergy. 2010;24:385–388. doi: 10.2500/ajra.2010.24.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mercer NS, MacCarthy P. The arterial supply of the palate: implications for closure of cleft palates. Plast Reconstr Surg. 1995;96:1038–1044. doi: 10.1097/00006534-199510000-00005. [DOI] [PubMed] [Google Scholar]
- 52.Methathrathip D, Apinhasmit W, Chompoopong S, Lertsirithong A, Ariyawatkul T, Sangvichien S. Anatomy of greater palatine foramen and canal and pterygopalatine fossa in Thais: considerations for maxillary nerve block. Surg Radiol Anat. 2005;27:511–516. doi: 10.1007/s00276-005-0016-5. [DOI] [PubMed] [Google Scholar]
- 53.Narayan RK, Ghosh SK. Can the morphological attributes of greater palatine foramen have implications in maxillary nerve block? An analytical study using anatomical planes. Transl Res Anat. 2021;22:100093. doi: 10.1016/j.tria.2020.100093. [DOI] [Google Scholar]
- 54.Nascimento Correia Lima N, Fortes de Oliveira O, Sassi C, Picapedra A, Francesquini L, Jr, Daruge E., Jr Sex determination by linear measurements of palatal bones and skull base. J Forensic Odontostomatol. 2012;30:37–43. [PMC free article] [PubMed] [Google Scholar]
- 55.Nimigean V, Nimigean VR, Buţincu L, Sălăvăstru DI, Podoleanu L. Anatomical and clinical considerations regarding the greater palatine foramen. Rom J Morphol Embryol. 2013;54:779–783. [PubMed] [Google Scholar]
- 56.Ortug A, Uzel M. Greater palatine foramen: assessment with palatal index, shape, number and gender. Folia Morphol (Warsz) 2019;78:371–377. doi: 10.5603/FM.a2018.0088. [DOI] [PubMed] [Google Scholar]
- 57.Piagkou M, Xanthos T, Anagnostopoulou S, Demesticha T, Kotsiomitis E, Piagkos G, Protogerou V, Lappas D, Skandalakis P, Johnson EO. Anatomical variation and morphology in the position of the palatine foramina in adult human skulls from Greece. J Craniomaxillofac Surg. 2012;40:e206–e210. doi: 10.1016/j.jcms.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 58.Priya SB, Johnson W, Lakshmanan P, Merlin J. The relative position of greater palatine foramen in dried human adult unsexed skull bones. Res J Pharm Biol Chem Sci. 2015;6:869–876. [Google Scholar]
- 59.Rapado-González O, Suárez-Quintanilla JA, Otero-Cepeda XL, Fernández-Alonso A, Suárez-Cunqueiro MM. Morphometric study of the greater palatine canal: cone-beam computed tomography. Surg Radiol Anat. 2015;37:1217–1224. doi: 10.1007/s00276-015-1511-y. [DOI] [PubMed] [Google Scholar]
- 60.Rapado-González O, Suárez-Quintanilla JA, Suárez-Cunqueiro MM. Anatomical variations of the greater palatine canal in cone-beam computed tomography. Surg Radiol Anat. 2017;39:717–723. doi: 10.1007/s00276-016-1791-x. [DOI] [PubMed] [Google Scholar]
- 61.Renu C. The position of greater palatine foramen in the adult human skulls of North Indian origin. J Surg Acad. 2013;3:54–57. [Google Scholar]
- 62.Reshmi B. Morphometric analysis of greater palatine foramen. Res J Pharm Technol. 2015;8:1171–1172. doi: 10.5958/0974-360X.2015.00212.7. [DOI] [Google Scholar]
- 63.Safavi M, Tehranchi M, Shabab S, Ganji SM, Taleghani F. CBCT evaluation of the position of palatal neurovascular bundle and the greater palatine foramen in an Iranian population. J Iran Dent Assoc. 2021;33:44–50. [Google Scholar]
- 64.Salcedo A, Araya C, Silva J, Barraza N, Latín A. Contribución al estudio descriptivo del foramen y canal palatino mayor. Int J Odontostomatol. 2019;13:40–45. doi: 10.4067/S0718-381X2019000100040. [DOI] [Google Scholar]
- 65.Saralaya V, Nayak SR. The relative position of the greater palatine foramen in dry Indian skulls. Singapore Med J. 2007;48:1143–1146. [PubMed] [Google Scholar]
- 66.Sharma NA, Garud RS. Greater palatine foramen–key to successful hemimaxillary anaesthesia: a morphometric study and report of a rare aberration. Singapore Med J. 2013;54:152–159. doi: 10.11622/smedj.2013052. [DOI] [PubMed] [Google Scholar]
- 67.Sheikhi M, Zamaninaser A, Jalalian F. Length and anatomic routes of the greater palatine canal as observed by cone beam computed tomography. Dent Res J (Isfahan) 2013;10:155–161. doi: 10.4103/1735-3327.113324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Siddiqui AU, Gandhi KR, Daimi SRH, Saxena S, Trivedi S, Sinha MB, Rathore M. Morphometric assessment of the greater palatine foramen with the adjacent anatomical landmarks. Indian J Anat. 2013;2:61–65. doi: 10.5402/2013/803853. [DOI] [Google Scholar]
- 69.Smith BG, Pratt AM, Anderson JA, Ray JJ. Targeted endodontic microsurgery: implications of the greater palatine artery. J Endod. 2021;47:19–27. doi: 10.1016/j.joen.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 70.Soto RA, Cáceres F, Vera C. Morphometry of the greater palatal canal in adult skulls. J Craniofac Surg. 2015;26:1697–1699. doi: 10.1097/scs.0000000000001600. [DOI] [PubMed] [Google Scholar]
- 71.Suzuki M, Omine Y, Shimoo Y, Yamamoto M, Kaketa A, Kasahara M, Serikawa M, Rhee S, Matsubayashi T, Matsunaga S, Abe S. Regional anatomical observation of morphology of greater palatine canal and surrounding structures. Bull Tokyo Dent Coll. 2016;57:223–231. doi: 10.2209/tdcpublication.2016-1100. [DOI] [PubMed] [Google Scholar]
- 72.Tavelli L, Barootchi S, Ravidà A, Oh TJ, Wang HL. What is the safety zone for palatal soft tissue graft harvesting based on the locations of the greater palatine artery and foramen? A systematic review. J Oral Maxillofac Surg. 2019;77:271.e1–271.e9. doi: 10.1016/j.joms.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 73.Teixeira CS, Souza VR, Marques CP, Junior WS, Pereira KF. Topography of the greater palatine foramen in macerated skulls. J Morphol Sci. 2017;27:88–92. [Google Scholar]
- 74.Thunyacharoen S, Iamaroon A, Mahakkanukrauh P. Morphometric study of incisive, greater and lesser palatine foramina: a novel point of maxillary nerve block in a Thai population. Int J Morphol. 2021;39:994–1000. doi: 10.4067/S0717-95022021000400994. [DOI] [Google Scholar]
- 75.Tomaszewska IM, Frączek P, Gomulska M, Pliczko M, Sliwińska A, Sałapa K, Chrzan R, Kowalski P, Nowakowski M, Walocha JA. Sex determination based on the analysis of a contemporary Polish population's palatine bones: a computed tomography study of 1,200 patients. Folia Morphol (Warsz) 2014;73:462–468. doi: 10.5603/fm.2014.0069. [DOI] [PubMed] [Google Scholar]
- 76.Tomaszewska IM, Kmiotek EK, Pena IZ, Średniawa M, Czyżowska K, Chrzan R, Nowakowski M, Walocha JA. Computed tomography morphometric analysis of the greater palatine canal: a study of 1,500 head CT scans and a systematic review of literature. Anat Sci Int. 2015;90:287–297. doi: 10.1007/s12565-014-0263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tomaszewska IM, Tomaszewski KA, Kmiotek EK, Pena IZ, Urbanik A, Nowakowski M, Walocha JA. Anatomical landmarks for the localization of the greater palatine foramen–a study of 1200 head CTs, 150 dry skulls, systematic review of literature and meta-analysis. J Anat. 2014;225:419–435. doi: 10.1111/joa.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Urbano ES, Melo KA, Costa ST. Morphologic study of the greater palatine canal. J Morphol Sci. 2017;27:102–104. [Google Scholar]
- 79.Valizadeh S, Ahmadi SM, Ahsaie MG, Vasegh Z, Jamalzadeh N. The anatomical position and size of greater palatine foramen and canal in an Iranian sample using cone beam computed tomography. J Long Term Eff Med Implants. 2022;32:73–80. doi: 10.1615/JLongTermEffMedImplants.2022040974. [DOI] [PubMed] [Google Scholar]
- 80.Vidulasri N, Thenmozhi MS. Morphometric analysis of greater palatine foramen in dry skulls. Int J Pharm Sci Res. 2015;6:4779–4782. [Google Scholar]
- 81.Vikraman KS, Thenmozhi MS, Lakshmanan G. Greater palatine foramen: assessment with palatal index, shape, number, and gender in South Indian population. Drug Invent Today. 2019;12:1714–1716. [Google Scholar]
- 82.Vinay K, Beena D, Vishal K. Morphometric analysis of the greater palatine foramen in south Indian adult skulls. Int J Basic Appl Med Sci. 2012;2:5–8. [Google Scholar]
- 83.Viveka S, Kumar M. Radiological localization of greater palatine foramen using multiple anatomical landmarks. Anat Physiol. 2016;2:187–189. doi: 10.15406/mojap.2016.02.00073. [DOI] [Google Scholar]
- 84.Wang TM, Kuo KJ, Shih C, Ho LL, Liu JC. Assessment of the relative locations of the greater palatine foramen in adult Chinese skulls. Acta Anat (Basel) 1988;132:182–186. doi: 10.1159/000146572. [DOI] [PubMed] [Google Scholar]
- 85.Westmoreland EE, Blanton PL. An analysis of the variations in position of the greater palatine foramen in the adult human skull. Anat Rec. 1982;204:383–388. doi: 10.1002/ar.1092040412. [DOI] [PubMed] [Google Scholar]
- 86.Wu B, Li H, Fan Y, Wang X, Li W, Zhong S, Ren J, Chen Y, Zhang L, Zhao G. Clinical and anatomical study of foramen locations in jaw bones and adjacent structures. Medicine (Baltimore) 2020;99:e18069. doi: 10.1097/md.0000000000018069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All of the data that were extracted from the included studies during the data collection process and used to perform our analyses are stored in a repository and publicly available on Open Science Framework (https://osf.io/64thm/?view_only=d472458ec3084d9da9efefdfd396b605).