Abstract
To assess and describe the aetiology and management of febrile illness in children with primary or acquired immunodeficiency at high risk of serious bacterial infection, as seen in emergency departments in tertiary hospitals. Prospective data on demographics, presenting features, investigations, microbiology, management, and outcome of patients within the ‘Biomarker Validation in HR patients’ database in PERFORM, were analysed. Immunocompromised children (< 18 years old) presented to fifteen European hospitals in nine countries, and one Gambian hospital, with fever or suspected infection and clinical indication for blood investigations. Febrile episodes were assigned clinical phenotypes using the validated PERFORM algorithm. Logistic regression was used to assess the effect size of predictive features of proven/presumed bacterial or viral infection. A total of 599 episodes in 482 children were analysed. Seventy-eight episodes (13.0%) were definite bacterial, 67 episodes probable bacterial (11.2%), and 29 bacterial syndrome (4.8%). Fifty-five were definite viral (9.2%), 49 probable viral (8.2%), and 23 viral syndrome (3.8%). One hundred ninety were unknown bacterial or viral infections (31.7%), and 108 had inflammatory or other non-infectious causes of fever (18.1%). Predictive features of proven/presumed bacterial infection were ill appearance (OR 3.1 (95% CI 2.1–4.6)) and HIV (OR 10.4 (95% CI 2.0–54.4)). Ill appearance reduced the odds of having a proven/presumed viral infection (OR 0.5 (95% CI 0.3–0.9)). A total of 82.1% had new empirical antibiotics started on admission (N = 492); 94.3% proven/presumed bacterial (N = 164), 66.1% proven/presumed viral (N = 84), and 93.2% unknown bacterial or viral infections (N = 177). Mortality was 1.9% (N = 11) and 87.1% made full recovery (N = 522).
Conclusion: The aetiology of febrile illness in immunocompromised children is diverse. In one-third of cases, no cause for the fever will be identified. Justification for standard intravenous antibiotic treatment for every febrile immunocompromised child is debatable, yet effective. Better clinical decision-making tools and new biomarkers are needed for this population.
|
What is Known: • Immunosuppressed children are at high risk for morbidity and mortality of serious bacterial and viral infection, but often present with fever as only clinical symptom. • Current diagnostic measures in this group are not specific to rule out bacterial infection, and positivity rates of microbiological cultures are low. | |
|
What is New: • Febrile illness and infectious complications remain a significant cause of mortality and morbidity in HR children, yet management is effective. • The aetiology of febrile illness in immunocompromised children is diverse, and development of pathways for early discharge or cessation of intravenous antibiotics is debatable, and requires better clinical decision-making tools and biomarkers. |
Supplementary Information
The online version contains supplementary material available at 10.1007/s00431-022-04642-1.
Keywords: Immunocompromised, Paediatric, Fever, Infection, Antibiotics
Introduction
Complex comorbidities render a growing number of children who attend the emergency department (ED) at increased risk of infection [1]. This includes children with primary (PID) or acquired immunodeficiencies, but also those who are dependent on total parenteral nutrition (TPN) with central venous lines. They are at high risk (HR) for serious bacterial infection (SBI) and life-threatening infectious complications [1, 2]. Some of these children are neutropenic. SBI during febrile neutropenia (FN) is a medical emergency associated with significant morbidity and mortality if left untreated [3, 4]. One-third of neutropenic episodes in paediatric patients on cancer treatment or during haematopoietic stem cell transplant (HSCT) are associated with fever [5].
Differentiating viral, bacterial, and inflammatory illnesses on admission is challenging in HR patients: clinical syndromes are often non-specific, and at least 36–48 h is needed to culture microorganisms [4, 6]. Because the risk of having SBI is significant [4, 7], immunocompromised patients with febrile illness are virtually always admitted for intravenous antibiotic treatment, awaiting microbiological results, yet only around 20% will have a microbiologically documented infection [3, 8–10]. 41.3–62.3% will have no cause identified [11–14].
This approach has led to a significant reduction in mortality and morbidity [15], but consequently antibiotic overuse, increased risk of antimicrobial resistance, and prolonged hospitalisation. Fever accounts for 60.2% of emergency department (ED) attendance in paediatric cancer [16] and is a significant burden for caregivers [17] and healthcare systems. Commonly used biomarkers such as C-reactive protein (CRP) and procalcitonin aid the diagnostic process, but are often not sensitive enough to rule out bacterial infection [18, 19]. It is suspected that a large proportion of HR fever has a viral aetiology, is drug-induced, or is caused by an underlying disease [20–25].
We describe the aetiology and management of fever in immunocompromised children, and assess the risk of bacterial and viral infections in a mixture of immunocompromised patients as seen by paediatricians in tertiary healthcare centre EDs, where they present primarily with fever.
Material and methods
This prospective, international, multicentre, observational study assessed children recruited to the ‘Biomarker Validation in HR patients’ cohort within Personalised Risk assessment in Febrile illness to Optimise Real-life Management across the European Union (PERFORM) between 2 June 2016 and 31 December 2019.
Participants
Children, < 18 years of age, immunocompromised due to primary or secondary immunodeficiency, were eligible upon presentation to ED, ward, or intensive care unit (PICU) admission from their usual place of residence for community-acquired infections, or from wards or PICU for suspected hospital-acquired infections with (history of) fever (< 72 h prior to admission, T ≥ 38.0 °C) or suspected infection, and clinical indication for blood investigations as per treating clinician’s decision. Participants could have multiple episodes, with a 2-week minimum between the end and start of consecutive episodes. They were recruited between 2 June 2016 and 31 December 2019.
Participants were recruited from sixteen tertiary centres in ten countries: four each from the United Kingdom (UK) and the Netherlands, and one each from Austria, the Gambia, Germany, Greece, Latvia, Slovenia, Spain, and Switzerland.
Data collection
We collected in-depth clinical data on standardised forms, including clinical symptoms, laboratory results, management, clinical syndromes, 28-day follow-up by phone call or in outpatient clinic, severity, and mortality. Regular data quality control was performed on the data in the online database. Missing values, outliers, abnormal laboratory results, and dates were double checked to ensure they were correct for the respective episode.
Study outcomes
All episodes were assigned final phenotypes using the validated algorithm in the PERFORM protocol [26] (Supplementary Information Fig. S1), previously described by Nijman et al. [27], and assigned one of eleven phenotypes: definite bacterial, probable bacterial, bacterial syndrome, unknown bacterial/viral, viral syndrome, probable viral, definite viral, trivial, other infection, uncertain infection/inflammation, or inflammatory syndrome. Episodes assigned definite bacterial, probable bacterial, or unknown bacterial/viral phenotypes could also have viral or fungal co-infection identified. Phenotypes for all episodes were reviewed by two experienced paediatricians before definite assignment. In case of disagreement or uncertainty by the assigning paediatricians, episodes were anonymously discussed with paediatricians from the other sites within the consortium before assigning the final phenotype.
To evaluate determinants of bacterial and viral infection, we combined definite bacterial, probable bacterial, and bacterial syndromes to a proven/presumed bacterial infection group, and definite viral, probable viral, and viral syndromes to a proven/presumed viral infection group. The grouping was performed as these children would receive the same initial treatment and management, and it would allow us to assess the wider spectrum of bacterial and viral diseases. The definite bacterial phenotype could only be assigned if the bacterium was isolated from a sterile site.
First, we described our cohort and compared clinical features of proven/presumed bacterial, and proven/presumed viral groups versus the other phenotypes. Neutropenia was defined as absolute neutrophil count (ANC) < 0.5 × 109/L or < 1.0 × 109/L but expected < 0.5 × 109/L within 48 h, or, if no ANC available, white cell count < 1.0 × 109/L [28] and lymphopenia defined as lymphocyte count < 1.0 × 109/L. Second, we described microbiology results and empirical antimicrobial management, utilising the AWaRe classification [29], categorising antibiotics in ‘access’, ‘watch’, and ‘reserve’ groups. Last, we described clinical syndromes, severity, and outcome.
Statistical analysis
Data was analysed using IBM SPSS Statistics, version 27 (Armonk, USA 2020). For descriptive data, absolute frequencies and percentages were used. Data was not normally distributed; non-parametric tests, medians, and interquartile ranges (IQR) were used. The Mann–Whitney U tests were used for continuous variables and Pearson’s χ2 or Fisher’s exact tests for categorical data. To assess the size effect of significantly associated clinical features for proven/presumed bacterial or viral infections, odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using univariate binary logistic regression. Subsequently, multivariate binary logistic regression was performed on variables with significant ORs after univariate binary logistic regression. P-values < 0.05 were considered significant.
Results
A total of 599 episodes in 482 children were analysed. Eighty-four children had multiple febrile episodes, with a maximum of six episodes in one child. A total of 343 episodes were in males (57.3%), and median age at admission was 7.7 years (IQR 4.1–12.8 years). Eight patients were from the Gambia (1.3%).
Final phenotype diagnoses
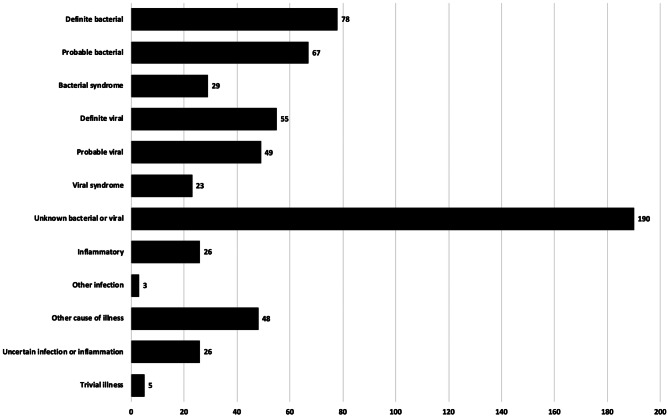
A total of 174 episodes (29.0%) were proven/presumed bacterial, of which 78 were definite bacterial (13.0%). A total of 127 episodes were proven/presumed viral (21.2%), of which 55 were definite viral (9.2%). A total of 190 episodes (31.7%) were unknown bacterial or viral infections (Fig. 1).
Fig. 1.
Final phenotypes assigned by episode as per PERFORM protocol (N = 599 episodes)
Demographics
Table 1 gives a demographic overview, with detailed data on demographics per phenotype in Supplementary Information Table S1 and underlying conditions in Supplementary Information Table S2. Most common underlying conditions were malignancies in 354 episodes (59.2%), followed by non-malignant haematological disease in 79 (13.2%), and inflammatory disease and PID with 47 episodes (7.8%) each. Of the Gambian patients, 7 had sickle cell disease, and 1 HIV, as an underlying condition.
Table 1.
Cohort demographics at admission
| All (N = 599) | Proven/presumed bacterial (N = 174) |
Proven/presumed viral (N = 127) |
Unknown bacterial or viral infection (N = 190) | Other phenoypes (N = 108) |
Proven/presumed bacterial vs all other phenotypes (p-value) |
Proven/presumed viral vs all other phenotypes (p-value) | Missing values (N = 599) | |
|---|---|---|---|---|---|---|---|---|
| Male | 343 (57.3%) | 101 (58.0%) | 70 (55.1%) | 107 (56.3%) | 65 (60.2%) | 0.80 | 0.58 | - |
| Age (years) | 7.7 (4.1–12.8) | 7.9 (3.5–12.9) | 7.2 (4.4–12.2) | 6.5 (4.1–11.7) | 10.0 (4.6–14.9) | 0.91 | 0.61 | - |
| HSCT patient | 69 (11.5%) | 15 (8.6%) | 19 (15.0%) | 15 (7.9%) | 20 (18.5%) | 0.16 | 0.17 | - |
| Underlying condition | ||||||||
| Malignancy | 354 (59.2%) | 98 (56.4%) | 69 (54.3%) | 150 (78.9%) | 37 (34.2%) | 0.38 | 0.22 | - |
| Haematological disease | 79 (13.2%) | 21 (12.1%) | 19 (15.0%) | 19 (10.0%) | 20 (18.5%) | 0.60 | 0.51 | - |
| Inflammatory syndromes | 47 (7.8%) | 7 (4.0%) | 9 (7.1%) | 5 (2.6%) | 26 (24.1%) | 0.03 | 0.72 | - |
| Primary immunodeficiency | 47 (7.8%) | 10 (5.7%) | 14 (11.0%) | 8 (4.2%) | 15 (13.9%) | 0.22 | 0.13 | - |
| Solid organ transplant | 30 (5.0%) | 19 (10.9%) | 4 (3.1%) | 3 (1.6%) | 4 (3.7%) | < 0.001 | 0.28 | - |
| HIV | 8 (1.3%) | 6 (3.4%) | 0 (0.0%) | 0 (0.0%) | 2 (1.9%) | 0.004 | 0.21 | - |
| Nephrotic syndrome | 6 (1.0%) | 3 (1.7%) | 1 (0.8%) | 1 (0.5%) | 1 (0.9%) | 0.36 | 1.00 | - |
| Cystic fibrosis | 5 (0.8%) | 2 (1.2%) | 1 (0.8%) | 2 (1.1%) | 0 (0.0%) | 0.63 | 1.00 | - |
| Short bowel syndrome | 4 (0.7%) | 2 (1.2%) | 1 (0.8%) | 1 (0.5%) | 0 (0.0%) | 0.58 | 1.00 | - |
| Other conditions | 19 (3.2%) | 6 (3.4%) | 9 (7.1%) | 1 (0.5%) | 3 (2.8%) | 0.81 | 0.01 | - |
| Clinical features | ||||||||
| Ill appearance | 176 (29.4%) | 83 (47.7%) | 26 (20.5%) | 39 (20.5%) | 28 (25.9%) | < 0.001 | 0.01 | - |
| Lifesaving intervention required | 54 (9.0%) | 26 (14.9%) | 8 (6.3%) | 9 (4.7%) | 11 (10.2%) | 0.001 | 0.23 | - |
| Diarrhoea | 45 (7.5%) | 14 (8.0%) | 10 (7.9%) | 9 (4.7%) | 12 (11.1%) | 0.75 | 0.86 | - |
| Increased work of breathing | 36 (6.0%) | 11 (6.3%) | 10 (7.9%) | 6 (3.2%) | 9 (8.3%) | 0.84 | 0.32 | - |
| Vomiting | 28 (4.7%) | 12 (6.9%) | 6 (4.7%) | 5 (2.6%) | 5 (4.6%) | 0.10 | 0.98 | - |
| Non-blanching rash | 15 (2.5%) | 6 (3.4%) | 2 (1.6%) | 2 (1.1%) | 5 (4.6%) | 0.39 | 0.75 | - |
| Clinical dehydration | 15 (2.5%) | 6 (3.4%) | 4 (3.1%) | 3 (1.6%) | 2 (1.9%) | 0.39 | 0.54 | - |
| Seizures | 8 (1.3%) | 4 (2.3%) | 0 (0.0%) | 2 (1.1%) | 2 (1.9%) | 0.24 | 0.21 | - |
| Meningism | 3 (0.5%) | 1 (0.6%) | 1 (0.8%) | 0 (0.0%) | 1 (0.9%) | 0.87 | 0.51 | - |
| Vital parameters, age adjusted* | ||||||||
| Tachypnoea | 79 (13.2%) | 32 (18.4%) | 15 (11.8%) | 16 (8.4%) | 16 (14.8%) | 0.02 | 0.61 | 151 |
| Bradypnoea | 9 (1.5%) | 5 (2.9%) | 1 (0.8%) | 2 (1.1%) | 1 (0.9%) | 0.13 | 0.69 | 151 |
| Low saturation (< 94% in air) | 39 (6.5%) | 11 (6.3%) | 8 (6.3%) | 9 (4.7%) | 11 (10.2%) | 0.91 | 0.91 | 123 |
| Tachycardia | 189 (31.6%) | 69 (39.7%) | 35 (37.6%) | 63 (33.2%) | 22 (20.4%) | 0.01 | 0.28 | 63 |
| Bradycardia | 3 (0.5%) | 1 (0.6%) | 0 (0.0%) | 1 (0.5%) | 1 (0.9%) | 1.00 | 1.00 | 63 |
| Hypotension | 48 (8.0%) | 14 (8.0%) | 11 (8.7%) | 15 (7.9%) | 8 (7.4%) | 0.99 | 0.76 | 190 |
| Hypertension | 179 (29.9%) | 56 (32.2%) | 36 (28.3%) | 43 (22.6%) | 44 (40.7%) | 0.43 | 0.67 | 190 |
| Prolonged capillary refill time (> 2 s) | 16 (2.7%) | 8 (5.5%) | 2 (1.6%) | 5 (2.6%) | 1 (0.9%) | 0.11 | 0.54 | 142 |
| Decreased consciousness (AVPU < A, GCS < = 13) | 5 (0.8%) | 2 (1.1%) | 0 (0.0%) | 2 (1.1%) | 1 (0.9%) | 0.63 | 0.59 | - |
| Fever (documented/history = > 38.0 °C) | 528 (88.1%) | 155 (89.1%) | 115 (90.6%) | 178 (93.7%) | 80 (74.1%) | 0.65 | 0.35 | - |
| Blood investigations | ||||||||
| Neutropenia | 212 (35.4%) | 61 (35.1%) | 33 (26.0%) | 101 (53.2%) | 17 (15.7%) | 0.87 | 0.01 | 3 |
| Lymphopenia | 265 (44.2%) | 75 (51.7%) | 63 (49.6%) | 89 (46.8%) | 38 (35.2%) | 0.63 | 0.65 | 103 |
| Immunomodulating drug use | ||||||||
| Biologicals | 34 (5.7%) | 6 (3.4%) | 5 (3.9%) | 6 (3.2%) | 17 (15.7%) | 0.13 | 0.34 | - |
| Ciclosporin | 35 (5.8%) | 10 (5.7%) | 9 (7.1%) | 5 (2.6%) | 11 (10.2%) | 0.95 | 0.50 | - |
| Colchicine | 1 (0.2%) | 0 (0.0%) | 1 (0.8%) | 0 (0.0%) | 0 (0.0%) | 1.00 | 0.21 | - |
| Immunoglobulin | 39 (6.5%) | 9 (5.2%) | 10 (7.9%) | 8 (4.2%) | 12 (11.1%) | 0.4 | 0.48 | - |
| Methotrexate | 118 (19.7%) | 31 (17.8%) | 32 (25.2%) | 45 (23.7%) | 10 (9.3%) | 0.46 | 0.08 | - |
| Steroids | 112 (20.4%) | 41 (23.6%) | 21 (16.5%) | 35 (18.4%) | 25 (23.1%) | 0.21 | 0.23 | - |
| Tacrolimus | 32 (5.3%) | 18 (10.3%) | 5 (3.9%) | 3 (1.6%) | 6 (5.6%) | < 0.001 | 0.43 | - |
| Other immunomodulating drug | 262 (43.7%) | 78 (44.8%) | 65 (51.2%) | 76 (40.0%) | 43 (39.8%) | 0.73 | 0.06 | - |
GCS, Glasgow Coma Scale, HIV human Immunodeficiency Virus, HSCT Haematopoietic Stem Cell Transplant
*Age-adjusted vital parameters as per APLS 2017 (> 95th centile or < 5th centile). Data is presented as N = episodes (%) or median (IQR). p-values were calculated using χ2, Fisher’s exact, or Mann–Whitney U tests as appropriate
In univariate binary logistic regression, the following clinical features at admission were associated with proven/presumed bacterial infections versus all other phenotypes: ill appearance (OR 3.3 (95% CI 2.2–4.7)), tachypnoea (OR 1.8 (95% CI 1.1–2.9)), tachycardia (OR 1.6 (95% CI 1.1–2.4)), requiring a lifesaving intervention (OR 2.5 (95% CI 1.4–4.4)), solid organ transplant recipients (OR 4.7 (95% CI 2.1–9.9)), HIV (OR 7.6 (95% CI 1.5–37.8)), inflammatory disease (OR 0.4 (95% CI 0.2–0.9)), and tacrolimus use (OR 3.4 (95% CI 1.6–6.9)) (Table 2). After the multivariate binary logistic regression, HIV (OR 10.4 (95% CI 2.0–54.4)) and ill appearance (OR 3.1 (95% CI 2.1–4.6)) were the two covariates remaining significant. The model achieved an area under ROC of 0.69 (95% CI 0.65–0.74).
Table 2.
Univariate and multivariate regression for clinically associated features and proven/presumed bacterial infection and proven/presumed viral infection. Data reported as odds ratio (95% confidence interval)
| Clinical features | Univariate logistic regression | Multivariate logistic regression |
|---|---|---|
| Proven/presumed bacterial infection vs other phenotypes | ||
| Underlying inflammatory condition | 0.4 (0.2–0.9) | 0.5 (0.2–1.2) |
| Solid organ transplant | 4.7 (2.1–9.9) | 3.0 (0.9–10.7) |
| HIV | 7.6 (1.5–37.8) | 10.4 (2.0–54.4) |
| Ill appearance | 3.3 (2.2–4.7) | 3.1 (2.1–4.6) |
| Lifesaving intervention required | 2.5 (1.4–4.4) | 1.4 (0.7–2.6) |
| Tachypnoea | 1.8 (1.1–2.9) | 1.3 (0.7–2.2) |
| Tachycardia | 1.6 (1.1–2.4) | 1.4 (1.0–2.2) |
| Tacrolimus use | 3.4 (1.6–6.9) | 1.5 (0.4–5.1) |
| Proven/presumed viral infection vs other phenotypes | ||
| Other underlying conditions | 3.5 (1.4–8.9) | 3.9 (1.5–10.0) |
| Ill appearance | 0.6 (0.3–0.9) | 0.5 (0.3–0.9) |
| Neutropenia at admission | 1.0 (0.7–1.4) | - |
Ill appearance reduced the odds of having a proven/presumed viral infection (OR 0.6 (95% CI 0.3–0.9)), and other underlying conditions increased the odds (OR 3.5 (95% CI 1.4–8.9)) in univariate binary logistic regression (Table 2). Both covariates remained significant after multivariate binary logistic regression, with an achieved area under ROC of 0.58 (95% CI 0.52–0.63).
Microbiology
Blood cultures were obtained in 563 episodes (94.0%), with a positive yield of 15.1% (N = 85). Urine cultures were performed in 165 episodes (27.5%), with a yield of 13.9% (N = 23). Polymerase chain reactions, primarily utilised for the detection of viral pathogens, were performed in 258 episodes (43.1%), with a yield of 46.9% (N = 121) for any tested pathogen. Rapid antigen testing (N = 86, 14.4%), serology (N = 61, 10.2%), and tuberculosis testing (N = 14, 2.3%) were less frequently performed, with yields of 15.1%, 39.3%, and 7.1%, respectively.
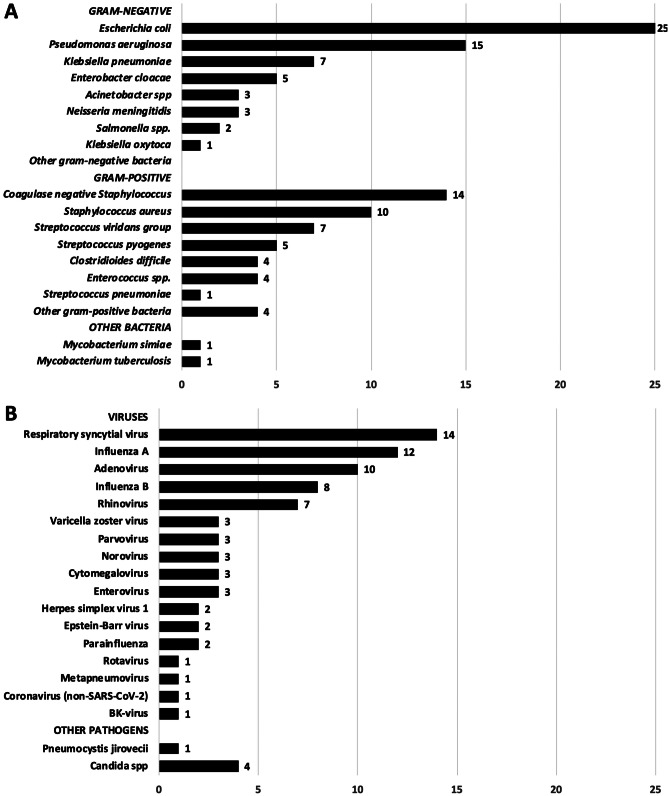
An overview of identified causative pathogens from blood and sterile site cultures is given in Fig. 2. In the definite bacterial group, with positive cultures from sterile sites, 118 bacterial isolates were cultured, of which 67 were gram-negative (56.7%) and 49 were gram-positive (41.5%). Two patients (1.8%) had mycobacterial pathogens identified. Common pathogens in our cohort were Escherichia coli (N = 25), Pseudomonas aeruginosa (N = 15), and Staphylococcus aureus (N = 10). Coagulase-negative staphylococci were the most common gram-positive pathogen, in 14 episodes (Fig. 2A).
Fig. 2.
Causative pathogens isolated or detected by episode. In 5 episodes, ≥ 1 causative bacteria were isolated, and in 10 episodes, ≥ 1 virus was detected. A Bacteria from blood or other sterile site cultures: other gram-negative: Burkholderia cepacia complex, Citrobacter freundii, Delftia acidovorans, Fusobacterium nucleatum, Haemophilus influenzae (unspecified), Serratia marcescens, all once isolated. Other gram-positive: Corynebacterium spp., Kytococcusschroeteri, Lactobacillus rhamnosus
For viral pathogens, respiratory syncytial virus (N = 14), influenza A (N = 12), and adenovirus (N = 10) were detected most frequently. In 5 patients, a fungal pathogen was deemed causative (Fig. 2B). Co-infection was documented in 31 episodes, of which 29 were bacterial-viral and 2 bacterial-fungal.
Empirical antimicrobial treatment
In 492 episodes (82.1%), new empirical antibiotics were started on admission (group by antibiotic class in Table 3, more detailed in Supplementary Information Table S3). A total of 164 proven/presumed bacterial, 84 proven/presumed viral, and 177 unknown bacterial or viral episodes had new antibiotics started on admission. A total of 270 episodes had been treated with non-prophylactic antibiotics within 7 days prior to admission (45.1%). Most given empirical antibiotics were piperacillin-tazobactam (N = 197, 40.0%) and teicoplanin (N = 115, 23.4%). A total of 440 episodes were started on ‘watch’ antibiotics (73.5%) empirically, and one was started on linezolid, a ‘reserve’ antibiotic, to use only as a last resort drug, according to the World Health Organization AWaRe classification [29].
Table 3.
Empirical antimicrobials started on admission by episodes (N = 599), antibiotics are grouped by class. The total number of antimicrobials exceeds the number of episodes as ≥ 1 antimicrobial could be started for a single episode
| Antimicrobial group | N = 599 | % |
|---|---|---|
| Penicillins | 257 | 42.9 |
| Glycopeptides | 138 | 23.0 |
| Aminoglycosides | 109 | 18.2 |
| 3rd-generation cephalosporins | 106 | 17.7 |
| 4th-generation cephalosporins | 71 | 11.9 |
| Carbapenems | 41 | 6.8 |
| Macrolides | 29 | 4.8 |
| Imidazoles | 19 | 3.2 |
| 2nd-generation cephalosporins | 15 | 2.5 |
| Fluroquinolones | 15 | 2.5 |
| Other antibiotics | 12 | 2.0 |
| Lincosamides | 10 | 1.7 |
| DHFR inhibitors | 9 | 1.5 |
| 1st-generation cephalosporins | 3 | 0.5 |
| Amphenicols | 2 | 0.3 |
| Oxazolidinones | 1 | 0.2 |
| Antivirals | 37 | 6.2 |
| Antifungals | 23 | 3.8 |
| No antimicrobials | 77 | 12.9 |
Median duration of antibiotic treatment was 7 days (IQR 4–10 days). The proven/presumed bacterial group was treated significantly longer (median 10 days (IQR 7–14 days), p < 0.001) and the proven/presumed viral group significantly shorter (median 5 days (IQR 3–8 days), p = 0.001). The unknown bacterial or viral group was treated for a median of 5 days (IQR 3–8 days).
Clinical syndromes
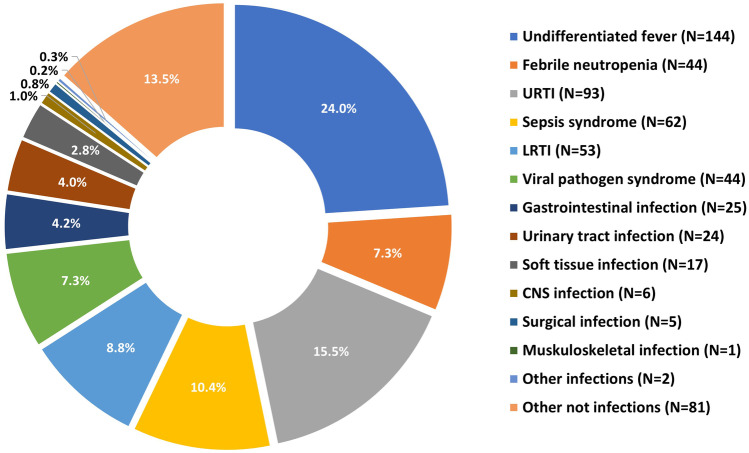
Common foci for febrile illness were upper respiratory tract infections (N = 93, 15.5%), and sepsis syndromes (10.4%), who had no specific localised focus for fever, but did have a positive blood culture. A total of 144 episodes were classed as undifferentiated fever, and 42 episodes had febrile neutropenia only, meaning 31.1% of febrile episodes in children at high risk for SBI had no source for the fever identified (Fig. 3, detailed in Supplementary Information Table S4). Eighty-one episodes (13.5%) had non-infectious causes of fever.
Fig. 3.
Clinical syndromes by group and by episodes (N = 599)
Severity and outcome
Mortality within the febrile illness episode was 1.9% (11 children). Four had a malignancy, three PID, two solid organ transplant, and one sickle cell disease, and one was on prolonged steroids following ischaemic brain injury. Three children died due to viral infection: one had disseminated adenoviraemia, one congenital cytomegalovirus reactivation, and one influenza A whilst developing multi-organ failure due to chemotoxicity from HSCT medication. Two died of sepsis: one Streptococcus pneumoniae, one Candida albicans. Two children had clinical lower respiratory tract infections but no pathogen isolated. One child died of drug reaction with eosinophilia and systemic symptom (DRESS) syndrome and one of gastrointestinal infection already in palliative care. Two children died of non-infectious cancer-related complications.
In 522 episodes (87.1%), patients made full recovery on the 28-day follow-up, with no significant difference between the proven/presumed bacterial and proven/presumed viral groups. Median length of in-patient stay (LOS) was 5 days (IQR 2–13 days), with longer admissions in the proven/presumed bacterial group (median 7 days (IQR 4–25 days, p < 0.001)), and shorter admissions in the proven/presumed viral group (median 2 days (IQR 1–6 days), p < 0.001) compared to other phenotypes. PICU admissions were required for 54 episodes (9.0%), which was associated with a proven/presumed bacterial infection (p = 0.005). Median admission duration to PICU was 6 days (IQR 2–15). A proven/presumed viral infection was associated with a shorter PICU admission duration (p = 0.007) versus other phenotypes. In 17 episodes (2.8%), inotropic support was required, of which 12 had proven/presumed bacterial infections. Sixty-nine episodes required supplemental oxygen (11.5%), 24 (4.0%) non-invasive ventilation, and 27 (4.5%) invasive ventilation. Inotropic support, non-invasive ventilation, and invasive ventilation were associated with proven/presumed bacterial infection when compared to other phenotypes (p < 0.001, p = 0.001, and p = 0.008, respectively).
Discussion
Our study provides insights into current aetiology and management of febrile illness in immunocompromised children at HR for SBI. In one-third of febrile episodes, no focus for the fever was identified, regardless of advances in laboratory and microbiological investigations. This is lower than previously reported in children with FN [5]; however, our cohort includes non-neutropenic febrile illness, which could partly explain this difference. Our 13.0% rate of definite bacterial infection was comparable to 11.4–37.0% reported in recent literature [8, 30–32].
Objective clinical features and laboratory investigations at admission did not discriminate well between bacterial and viral infections in our cohort, and neutropenia at admission in our cohort did not significantly change the risk of having a proven/presumed bacterial or viral infection.
Looking at any immunosuppressant use, we did not observe significant associations. Ill appearance was associated with proven/presumed bacterial infection and is known to be a risk factor for bacterial infection [33, 34]. Ill appearance also reduced the risk of having a proven/presumed viral infection. HIV increased the odds of having a proven/presumed bacterial infection; however, we acknowledge that the number of patients with HIV was low and that the results may be skewed by inclusion bias.
We observed considerable variability in empirical antibiotic use across sites, with 29 empirical antibiotics used. This can be partially explained by protocol differences, some centres preferring different glycopeptides, and some children requiring specific antibiotic cover, e.g. for Burkholderia in chronic granulomatous disease. A significant proportion of patients, mainly oncology or HSCT patients, were empirically treated with piperacillin-tazobactam with or without teicoplanin, in accordance with guidance on treatment of suspected FN sepsis [35, 36].
There are grounds to assume that a significant proportion of HR children are overtreated with intravenous antibiotics and, similar to the general paediatric population, have self-limiting febrile illness [20, 21, 37]. However, infections remain the main cause of morbidity and mortality in the HR population [7, 38]. Withholding or early discontinuation of antibiotics remains controversial. We do not have sufficient evidence to effectively alter current practice [35, 39, 40]. Immunocompromised children remain frequently hospitalised for intravenous antibiotic treatment, which has a negative impact on patient and family quality of life [17].
We acknowledge that the small proportion of Gambian patients represent different epidemiology and aetiology, and that these patients have less access to biologicals, and specialised diagnostic tests compared to the other sites in this cohort, a known issue in LMIC.
Currently, there is no validated risk stratification tool for this population [41]. In adults, there is a well-used risk stratification for high-risk patients, allowing for short-course, oral, and outpatient parenteral antibiotics that reduced both hospital admission and broad-spectrum antibiotic use [42]. It is not yet proven helpful in children [43].
For patients with T cell deficiencies, seen in certain PID and HSCT patients, viral infections are just as significant as bacterial infections requiring antiviral or immunoglobulin treatment [44, 45]. Both SBI and ‘serious viral infection’ cause significant morbidity and mortality, as demonstrated by the fatal cases in our cohort.
In our cohort, mortality was low at 1.9%, but 9% PICU admission rate demonstrates significant morbidity.
The high percentage of children with no definitive diagnosis demonstrates the need for better diagnostic tests to optimise early, effective, and targeted treatment.
Strengths and limitations
The study strengths lie in the international and multicentre approach, allowing us to evaluate management across Europe and the Gambia. We collected in-depth patient data, with 28-day follow-up, and included a wide range of immunocompromised children reflecting the clinical spectrum at university hospital EDs. Study limitations lie in the nature of recruitment: episodes in this cohort are biased by referrals and inclusion rates of participating centres across different countries. Therefore, it cannot be judged as a general epidemiological perspective or estimate for proportional incidence rates, nor is management generalisable to other LMIC as the availability of LMIC data in our cohort was low.
The use of experienced paediatricians as reviewers of the assigned final phenotypes potentially induced observer and outcome bias due to intra- and inter-rater differences. This is a known problem and leads to a low inter-rater reliability, as demonstrated in the process of peer reviewing by scientific journals [46] or by assessing performance scores in oncology patients [47]. We mitigated the potentially induced bias to the best of our ability by having two reviewers and an independent consortium episode review in case of disagreement, and using a validated algorithm [27]. Validated scores or algorithms increase inter-rater reliability and have been reported in evaluation of paediatric early warning scores [48].
Conclusions
Febrile illness and infectious complications remain a significant cause of morbidity and mortality in immunocompromised children. Current management is effective and mortality low, but a significant proportion of children require PICU care. Swift and accurate diagnosis of febrile illness in this population remains challenging. Justifying broad-spectrum intravenous antibiotic treatment of fever for every high-risk patient is costly in terms of drugs, burden of antibiotic resistance, hospitalisation, and costs to families and overburdened healthcare systems. Identifying low-risk febrile patients could reduce hospital admission in this patient population. Future research should focus on development of new rapid clinical decision-making tools and biomarkers targeting immunocompromised paediatric population.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- ANC
Absolute neutrophil count
- CI
Confidence interval
- CRP
C-reactive protein
- ED
Emergency department
- FN
Febrile neutropenia
- HIV
Human immunodeficiency virus
- HR
High risk
- HSCT
Haematopoietic stem cell transplant
- IQR
Interquartile range
- LMIC
Low- and middle-income country
- LOS
Length of in-patient stay
- PERFORM
Personalised Risk assessment in Febrile illness to Optimise Real-life Management across the European Union
- OR
Odds ratio
- PICU
Paediatric intensive care unit
- PID
Primary immunodeficiency
- SBI
Serious bacterial infection
- UK
United Kingdom
Authors’ contributions
FvdV was the main author of this manuscript and performed the analyses on the data supervised by EL and ME. GdV and AM were involved in the preparation of the final database and involved in patient recruitment. EL, UvB, LK, EC, AK, JH, TW, FMT, IRC, CV, NH, MP, AP, LS, MT, IE, SY, DZ, CF, MV, WZ, BK, PA, EU, FS, RdG, ML, and MvdF were all responsible for the conduct of the PERFORM study and patient recruitment for their respective sites. TD was responsible for the digital database system and its maintenance. RG and JH were responsible for the ethics surrounding this study and the wider PERFORM study. All authors have read and provided valuable input during the writing of this manuscript. All authors agree to submission of this manuscript.
Funding
This project received funding under the European Union’s Horizon2020 research and innovation programme, grant agreement number 668303. UK enrolment was supported by NIHR Biomedical Research Centres at Imperial College London, and Newcastle.
Declarations
Ethics approval
Ethical approval was obtained for all countries via respective national ethics committees, for the UK: IRAS 209035, REC 16/LO/1684. Informed consent was obtained from all participants or their legal guardians, assent where appropriate.
Competing interests
The authors declare no competing interests.
Footnotes
Affiliation for Marko Pokorn has been corrected.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
1/23/2023
A Correction to this paper has been published: 10.1007/s00431-022-04788-y
Contributor Information
Marieke Emonts, Email: Marieke.emonts@ncl.ac.uk.
PERFORM consortium:
Michael Levin, Aubrey Cunnington, Tisham De, Jethro Herberg, Myrsini Kaforou, Victoria Wright, Lucas Baumard, Evangelos Bellos, Giselle D’Souza, Rachel Galassini, Dominic Habgood-Coote, Shea Hamilton, Clive Hoggart, Sara Hourmat, Heather Jackson, Ian Maconochie, Stephanie Menikou, Naomi Lin, Samuel Nichols, Ruud Nijman, Oliver Powell, Ivonne Pena Paz, Priyen Shah, Ching-Fen Shen, Ortensia Vito, Clare Wilson, Amina Abdulla, Ladan Ali, Sarah Darnell, Rikke Jorgensen, Sobia Mustafa, Salina Persand, Molly M. Stevens, Nayoung Kim, Eunjung Kim, Katy Fidler, Julia Dudley, Vivien Richmond, Emma Tavliavini, Ching-Fen Shen, Ching-Chuan Liu, Shih-Min Wang, Federico Martinón-Torres, Antonio Salas, Fernando Álvez González, Cristina Balo Farto, Ruth Barral-Arca, María Barreiro Castro, Xabier Bello, Mirian Ben García, Sandra Carnota, Miriam Cebey-López, María José Curras-Tuala, Carlos Durán Suárez, Luisa García Vicente, Alberto Gómez-Carballa, Jose Gómez Rial, Pilar Leboráns Iglesias, Federico Martinón-Torres, Nazareth Martinón-Torres, José María Martinón Sánchez, Belén Mosquera Pérez, Jacobo Pardo-Seco, Lidia Piñeiro Rodríguez, Sara Pischedda, Sara Rey Vázquez, Irene Rivero Calle, Carmen Rodríguez-Tenreiro, Lorenzo Redondo-Collazo, Miguel Sadiki Ora, Antonio Salas, Sonia Serén Fernández, Cristina Serén Trasorras, Marisol Vilas Iglesias, Dace Zavadska, Anda Balode, Arta Bārzdiņa, Dārta Deksne, Dace Gardovska, Dagne Grāvele, Ilze Grope, Anija Meiere, Ieva Nokalna, Jana Pavāre, Zanda Pučuka, Katrīna Selecka, Aleksandra Rudzāte, Dace Svile, Urzula Nora Urbāne, Effua Usuf, Kalifa Bojang, Syed M. A. Zaman, Fatou Secka, Suzanne Anderson, Anna RocaIsatou Sarr, Momodou Saidykhan, Saffiatou Darboe, Samba Ceesay, Umberto D’alessandro, Henriëtte A. Moll, Clementien L Vermont, Dorine M. Borensztajn, Nienke N. Hagedoorn, Chantal Tan, Joany Zachariasse, W Dik, Philipp KA Agyeman, Christoph Berger, Eric Giannoni, Martin Stocker, Klara M Posfay-Barbe, Ulrich Heininger, Sara Bernhard-Stirnemann, Anita Niederer-Loher, Christian R. Kahlert, Giancarlo Natalucci, Christa Relly, Thomas Riedel, Christoph Aebi, Luregn J Schlapbach, Enitan D Carrol, Elizabeth Cocklin, Rebecca Jennings, Joanne Johnston, Aakash Khanijau, Simon Leigh, Nadia Lewis-Burke, Karen Newall, Sam Romaine, Maria Tsolia, Irini Eleftheriou, Maria Tambouratzi, Antonis Marmarinos, Marietta Xagorari, Kelly Syggelou, Colin Fink, Marie Voice, Leo Calvo-Bado, Werner Zenz, Benno Kohlmaier, Nina A. Schweintzger, Manfred G. Sagmeister, Daniela S. Kohlfürst, Christoph Zurl, Alexander Binder, Susanne Hösele, Manuel Leitner, Lena Pölz, Glorija Rajic, Sebastian Bauchinger, Hinrich Baumgart, Martin Benesch, Astrid Ceolotto, Ernst Eber, Siegfried Gallistl, Gunther Gores, Harald Haidl, Almuthe Hauer, Christa Hude, Markus Keldorfer, Larissa Krenn, Heidemarie Pilch, Andreas Pfleger, Klaus Pfurtscheller, Gudrun Nordberg, Tobias Niedrist, Siegfried Rödl, Andrea Skrabl-Baumgartner, Matthias Sperl, Laura Stampfer, Volker Strenger, Holger Till, Andreas Trobisch, Sabine Löffler, Shunmay Yeung, Juan Emmanuel Dewez, Martin Hibberd, David Bath, Alec Miners, Ruud Nijman, Elizabeth Fitchett, Ronald de Groot, Michiel van der Flier, Marien I. de Jonge, Koen van Aerde, Wynand Alkema, Bryan van den Broek, Jolein Gloerich, Alain J. van Gool, Stefanie Henriet, Martijn Huijnen, Ria Philipsen, Esther Willems, G.P.J.M. Gerrits, M. van Leur, J. Heidema, L. de Haan, C.J. Miedema, C. Neeleman, C.C. Obihara, G.A. Tramper-Stranders, Andrew J. Pollard, Rama Kandasamy, Stéphane Paulus, Michael J. Carter, Daniel O’Connor, Sagida Bibi, Dominic F. Kelly, Meeru Gurung, Stephen Thorson, Imran Ansari, David R. Murdoch, Shrijana Shrestha, Zoe Oliver, Marieke Emonts, Emma Lim, Lucille Valentine, Karen Allen, Kathryn Bell, Adora Chan, Stephen Crulley, Kirsty Devine, Daniel Fabian, Sharon King, Paul McAlinden, Sam McDonald, Anne McDonnell, Ailsa Pickering, Evelyn Thomson, Amanda Wood, Diane Wallia, Phil Woodsford, Frances Baxter, Ashley Bell, Mathew Rhodes, Rachel Agbeko, Christine Mackerness, Bryan Baas, Lieke Kloosterhuis, Wilma Oosthoek, Tasnim Arif, Joshua Bennet, Kalvin Collings, Ilona van der Giessen, Alex Martin, Aqeela Rashid, Emily Rowlands, Gabriella de Vries, Fabian van der Velden, Joshua Soon, Lucille Valentine, Mike Martin, Ravi Mistry, Ulrich von Both, Laura Kolberg, Manuela Zwerenz, Judith Buschbeck, Christoph Bidlingmaier, Vera Binder, Katharina Danhauser, Nikolaus Haas, Matthias Griese, Tobias Feuchtinger, Julia Keil, Matthias Kappler, Eberhard Lurz, Georg Muench, Karl Reiter, Carola Schoen, François Mallet, Karen Brengel-Pesce, Alexandre Pachot, Marine Mommert, Marko Pokorn, Mojca Kolnik, Katarina Vincek, Tina Plankar Srovin, Natalija Bahovec, Petra Prunk, Veronika Osterman, Tanja Avramoska, Taco Kuijpers, Ilse Jongerius, J. M. van den Berg, D. Schonenberg, A. M. Barendregt, D. Pajkrt, M. van der Kuip, A. M. van Furth, Evelien Sprenkeler, Judith Zandstra, G. van Mierlo, and J. Geissler
References
- 1.Borensztajn DM, Hagedoorn NN, Carrol ED, von Both U, Emonts M, van der Flier M, et al. Febrile children with comorbidities at the emergency department - a multicentre observational study. Eur J Pediatr. 2022;181(9):3491–3500. doi: 10.1007/s00431-022-04552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viscoli C, Varnier O, Machetti M. Infections in patients with febrile neutropenia: epidemiology, microbiology, and risk stratification. Clin Infect Dis. 2005;40(Suppl 4):S240–S245. doi: 10.1086/427329. [DOI] [PubMed] [Google Scholar]
- 3.Aviles-Robles M, Schnur JJ, Dorantes-Acosta E, Marquez-Gonzalez H, Ocampo-Ramirez LA, Chawla NV (2022) Predictors of septic shock or bacteremia in children experiencing febrile neutropenia post-chemotherapy. J Pediatric Infect Dis Soc [DOI] [PMC free article] [PubMed]
- 4.Mora-Capin A, Lorente-Romero J, Hernanz-Lobo A, Rivas-Garcia A, Vazquez-Lopez P, Carrascosa-Garcia P, et al. Risk factors of serious bacterial infection in previously healthy children older than 90 days old with fever and neutropenia. Pediatr Emerg Care. 2022;38(7):e1378–e1383. doi: 10.1097/PEC.0000000000002758. [DOI] [PubMed] [Google Scholar]
- 5.Castagnola E, Fontana V, Caviglia I, Caruso S, Faraci M, Fioredda F, et al. A prospective study on the epidemiology of febrile episodes during chemotherapy-induced neutropenia in children with cancer or after hemopoietic stem cell transplantation. Clin Infect Dis. 2007;45(10):1296–1304. doi: 10.1086/522533. [DOI] [PubMed] [Google Scholar]
- 6.Craig JC, Williams GJ, Jones M, Codarini M, Macaskill P, Hayen A, et al. The accuracy of clinical symptoms and signs for the diagnosis of serious bacterial infection in young febrile children: prospective cohort study of 15 781 febrile illnesses. BMJ. 2010;340:c1594. doi: 10.1136/bmj.c1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinon-Torres F, Salas A, Rivero-Calle I, Cebey-Lopez M, Pardo-Seco J, Herberg JA, et al. Life-threatening infections in children in Europe (the EUCLIDS Project): a prospective cohort study. Lancet Child Adolesc Health. 2018;2(6):404–414. doi: 10.1016/S2352-4642(18)30113-5. [DOI] [PubMed] [Google Scholar]
- 8.van der Galiën HT, Loeffen EAH, Miedema KGE, Tissing WJE. Predictive value of PCT and IL-6 for bacterial infection in children with cancer and febrile neutropenia. Support Care Cancer. 2018;26(11):3819–3826. doi: 10.1007/s00520-018-4249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Şahbudak Bal Z, Karadaş Özdemir N, Şen S, Yılmaz Karapınar D, Azarsız E, Aydemir Ş, et al. Diagnostic accuracy of interleukin-6, interleukin-8, and interleukin-10 for predicting bacteremia in children with febrile neutropenia. Turk J Haematol. 2017;34(3):254–257. doi: 10.4274/tjh.2016.0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alali M, David MZ, Danziger-Isakov LA, Elmuti L, Bhagat PH, Bartlett AH. Pediatric febrile neutropenia: change in etiology of bacteremia, empiric choice of therapy and clinical outcomes. J Pediatr Hematol Oncol. 2020;42(6):e445–e451. doi: 10.1097/MPH.0000000000001814. [DOI] [PubMed] [Google Scholar]
- 11.Kitanovski L, Jazbec J, Hojker S, Derganc M. Diagnostic accuracy of lipopolysaccharide-binding protein for predicting bacteremia/clinical sepsis in children with febrile neutropenia: comparison with interleukin-6, procalcitonin, and C-reactive protein. Support Care Cancer. 2014;22(1):269–277. doi: 10.1007/s00520-013-1978-1. [DOI] [PubMed] [Google Scholar]
- 12.Aimoto M, Koh H, Katayama T, Okamura H, Yoshimura T, Koh S, et al. Diagnostic performance of serum high-sensitivity procalcitonin and serum C-reactive protein tests for detecting bacterial infection in febrile neutropenia. Infection. 2014;42(6):971–979. doi: 10.1007/s15010-014-0657-6. [DOI] [PubMed] [Google Scholar]
- 13.Baraka A, Zakaria M. Presepsin as a diagnostic marker of bacterial infections in febrile neutropenic pediatric patients with hematological malignancies. Int J Hematol. 2018;108(2):184–191. doi: 10.1007/s12185-018-2447-x. [DOI] [PubMed] [Google Scholar]
- 14.Urbonas V, Eidukaitė A, Tamulienė I. The diagnostic value of interleukin-6 and interleukin-8 for early prediction of bacteremia and sepsis in children with febrile neutropenia and cancer. J Pediatr Hematol Oncol. 2012;34(2):122–127. doi: 10.1097/MPH.0b013e3182446a60. [DOI] [PubMed] [Google Scholar]
- 15.Cennamo F, Masetti R, Largo P, Argentiero A, Pession A, Esposito S (2021) Update on febrile neutropenia in pediatric oncological patients undergoing chemotherapy. Children (Basel) 8(12) [DOI] [PMC free article] [PubMed]
- 16.Burcham MD, Cochrane AR, Jacob SA, Carroll AE, Mueller EL. Emergency department chief complaints among children with cancer. J Pediatr Hematol Oncol. 2018;40(6):445–449. doi: 10.1097/MPH.0000000000001223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crothers A, Haeusler GM, Slavin MA, Babl FE, Mechinaud F, Phillips R, et al. Examining health-related quality of life in pediatric cancer patients with febrile neutropenia: factors predicting poor recovery in children and their parents. EClinicalMedicine. 2021;40:101095. doi: 10.1016/j.eclinm.2021.101095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herberg JA, Kaforou M, Wright VJ, Shailes H, Eleftherohorinou H, Hoggart CJ, et al. Diagnostic test accuracy of a 2-transcript host RNA signature for discriminating bacterial vs viral Infection in febrile children. JAMA. 2016;316(8):835–845. doi: 10.1001/jama.2016.11236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Velden FJS, Gennery AR, Emonts M. Biomarkers for diagnosing febrile illness in immunocompromised children: a systematic review of the literature. Front Pediatr. 2022;10:828569. doi: 10.3389/fped.2022.828569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Licciardello M, Pegoraro A, Cesaro S. Prophylaxis and therapy of viral infections in pediatric patients treated for malignancy. Pediatr Rep. 2011;3(1):e5. doi: 10.4081/pr.2011.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindblom A, Bhadri V, Soderhall S, Ohrmalm L, Wong M, Norbeck O, et al. Respiratory viruses, a common microbiological finding in neutropenic children with fever. J Clin Virol. 2010;47(3):234–237. doi: 10.1016/j.jcv.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen SN, Vu LT, Vu QV, Tran TT, Dinh VTT. Clinical epidemiology characteristics and etiology of febrile neutropenia in children: analysis of 421 cases. Hematol Rep. 2022;14(3):245–252. doi: 10.3390/hematolrep14030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cordonnier C, Ljungman P, Cesaro S, Hirsch HH, Maschmeyer G, von Lilienfeld-Toal M, et al. The EHA research roadmap: infections in hematology. Hemasphere. 2021;5(12):e662. doi: 10.1097/HS9.0000000000000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith JM, Dharnidharka VR. Viral surveillance and subclinical viral infection in pediatric kidney transplantation. Pediatr Nephrol. 2015;30(5):741–748. doi: 10.1007/s00467-014-2866-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Englund J, Feuchtinger T, Ljungman P. Viral infections in immunocompromised patients. Biol Blood Marrow Transplant. 2011;17(1 Suppl):S2–5. doi: 10.1016/j.bbmt.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.PERFORM Consortium (2016) PERFORM Clinical Protocol, Version 1.0
- 27.Nijman RG, Oostenbrink R, Moll HA, Casals-Pascual C, von Both U, Cunnington A, et al. A novel framework for phenotyping children with suspected or confirmed infection for future biomarker studies. Front Pediatr. 2021;9:688272. doi: 10.3389/fped.2021.688272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):e56–93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization (2020) WHO electronic essential medicines list (eEML) 2020 [beta version 1.0:[Available from: https://list.essentialmeds.org
- 30.Xia T, Xu X, Zhao N, Luo Z, Tang Y. Comparison of the diagnostic power of cytokine patterns and procalcitonin for predicting infection among paediatric haematology/oncology patients. Clin Microbiol Infect. 2016;22(12):996–1001. doi: 10.1016/j.cmi.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 31.Cabanillas Stanchi KM, Queudeville M, Malaval C, Feucht J, Schlegel P, Dobratz M, et al. Comparison of procalcitonin and C-reactive protein as early diagnostic marker for the identification of transplant-related adverse events after allogeneic hematopoietic stem cell transplantation in pediatric patients. J Cancer Res Clin Oncol. 2019;145(11):2779–2791. doi: 10.1007/s00432-019-03008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobs L, Berrens Z, Stenson EK, Zackoff M, Danziger-Isakov L, Lahni P, et al. Interleukin-27 as a candidate diagnostic biomarker for bacterial infection in immunocompromised pediatric patients. PLoS ONE. 2018;13(11):e0207620. doi: 10.1371/journal.pone.0207620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kara SS, Tezer H, Polat M, Cura Yayla BC, Bedir Demirdag T, Okur A, et al. Risk factors for bacteremia in children with febrile neutropenia. Turk J Med Sci. 2019;49(4):1198–1205. doi: 10.3906/sag-1901-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hakim H, Flynn PM, Srivastava DK, Knapp KM, Li C, Okuma J, et al. Risk prediction in pediatric cancer patients with fever and neutropenia. Pediatr Infect Dis J. 2010;29(1):53–59. doi: 10.1097/INF.0b013e3181c3f6f0. [DOI] [PubMed] [Google Scholar]
- 35.Lehrnbecher T, Robinson P, Fisher B, Alexander S, Ammann RA, Beauchemin M, et al. Guideline for the management of fever and neutropenia in children with cancer and hematopoietic stem-cell transplantation recipients: 2017 Update. J Clin Oncol. 2017;35(18):2082–2094. doi: 10.1200/JCO.2016.71.7017. [DOI] [PubMed] [Google Scholar]
- 36.CG151 Ng (2012) Neutropenic sepsis: prevention and management in people with cancer. Available from: https://www.nice.org.uk/guidance/cg151 [PubMed]
- 37.Hakim H, Flynn PM, Knapp KM, Srivastava DK, Gaur AH. Etiology and clinical course of febrile neutropenia in children with cancer. J Pediatr Hematol Oncol. 2009;31(9):623–629. doi: 10.1097/MPH.0b013e3181b1edc6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Randolph AG, McCulloh RJ. Pediatric sepsis: important considerations for diagnosing and managing severe infections in infants, children, and adolescents. Virulence. 2014;5(1):179–189. doi: 10.4161/viru.27045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oude Nijhuis C, Kamps WA, Daenen SM, Gietema JA, van der Graaf WT, Groen HJ, et al. Feasibility of withholding antibiotics in selected febrile neutropenic cancer patients. J Clin Oncol. 2005;23(30):7437–7444. doi: 10.1200/JCO.2004.00.5264. [DOI] [PubMed] [Google Scholar]
- 40.Stern A, Carrara E, Bitterman R, Yahav D, Leibovici L, Paul M (2019) Early discontinuation of antibiotics for febrile neutropenia versus continuation until neutropenia resolution in people with cancer. Cochrane Database Syst Rev 1:CD012184 [DOI] [PMC free article] [PubMed]
- 41.Stabell N, Nordal E, Stensvold E, Gammelsrud KW, Lund B, Taxt A, et al. Febrile neutropenia in children with cancer: a retrospective Norwegian multicentre study of clinical and microbiological outcome. Scand J Infect Dis. 2008;40(4):301–307. doi: 10.1080/00365540701670436. [DOI] [PubMed] [Google Scholar]
- 42.Phillips RS, Bhuller K, Sung L, Ammann RA, Tissing WJ, Lehrnbecher T, et al. Risk stratification in febrile neutropenic episodes in adolescent/young adult patients with cancer. Eur J Cancer. 2016;64:101–106. doi: 10.1016/j.ejca.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 43.Phillips B, Morgan JE, Haeusler GM, Riley RD, Collaborative P. Individual participant data validation of the PICNICC prediction model for febrile neutropenia. Arch Dis Child. 2020;105(5):439–445. doi: 10.1136/archdischild-2019-317308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al-Herz W, Essa S. Spectrum of viral infections among primary immunodeficient children: report from a national registry. Front Immunol. 2019;10:1231. doi: 10.3389/fimmu.2019.01231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ljungman P. Molecular monitoring of viral infections after hematopoietic stem cell transplantation. Int J Hematol. 2010;91(4):596–601. doi: 10.1007/s12185-010-0570-4. [DOI] [PubMed] [Google Scholar]
- 46.Bornmann L, Mutz R, Daniel HD. A reliability-generalization study of journal peer reviews: a multilevel meta-analysis of inter-rater reliability and its determinants. PLoS ONE. 2010;5(12):e14331. doi: 10.1371/journal.pone.0014331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chow R, Chiu N, Bruera E, Krishnan M, Chiu L, Lam H, et al. Inter-rater reliability in performance status assessment among health care professionals: a systematic review. Ann Palliat Med. 2016;5(2):83–92. doi: 10.21037/apm.2016.03.02. [DOI] [PubMed] [Google Scholar]
- 48.Jensen CS, Aagaard H, Olesen HV, Kirkegaard H. A multicentre, randomised intervention study of the paediatric early warning score: study protocol for a randomised controlled trial. Trials. 2017;18(1):267. doi: 10.1186/s13063-017-2011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.