Abstract
Mycobacterium abscessus species (MABS) is the most commonly isolated rapidly growing mycobacteria (RGM) and is one of the most antibiotic-resistant RGM with rapid progression, therefore, treatment of MABS is still challenging. We here presented a new combination treatment with sitafloxacin that targeted rough morphotypes of MABS, causing aggressive infections. Thirty-four clinical strains of MABS were isolated from various clinical samples at the Juntendo university hospital from 2011 to 2020. The susceptibility to a combination of sitafloxacin and antimicrobial agents was compared to that of the antimicrobial agents alone. Out of 34 MABS, 8 strains treated with sitafloxacin–amikacin combination, 9 of sitafloxacin–imipenem combination, 19 of sitafloxacin–arbekacin combination, and 9 of sitafloxacin–clarithromycin combination showed synergistic effects, respectively. Sitafloxacin–arbekacin combination also exhibited the synergistic effects against 10 of 22 Mycobacterium abscessus subspecies massiliense (Mma) strains and 8 of 11 Mycobacterium abscessus subspecies abscessus (Mab) strains, a highly resistant subspecies of MABS. The sitafloxacin–arbekacin combination revealed more synergistic effects in rough morphotypes of MABS (p = 0.008). We demonstrated the synergistic effect of the sitafloxacin–arbekacin combination against MABS. Further, this combination regimen might be more effective against Mab or rough morphotypes of MABS.
Subject terms: Microbiology, Antimicrobials
Introduction
Nontuberculous mycobacteria (NTM) are environmental pathogens that can cause diverse types of infectious diseases in humans. NTM are classified into RGM where colony formation requires less than seven days and slowly growing mycobacteria (SGM) forming colonies at least seven days. MABS is the most commonly isolated RGM and the third most common cause of respiratory NTM in the United States1. Pulmonary disease caused by MABS mostly occur in the setting of structural lung conditions. Most of the patients underlying disease in Japan were bronchiectasis, chronic obstructive pulmonary disease (COPD), previous pulmonary tuberculosis; whereas, that in North America and Europe was cystic fibrosis (CF)2–4. These infections are often incurable and associated with rapid lung function decline5,6. New NTM treatment guidelines were published in 20207. The guidelines introduced new treatment options, including inhaled amikacin, tigecycline, and clofazimine8–10; however, the treatment benefits were limited to negative culture conversion of sputum. Recently, the efficacy of sitafloxacin, a fluoroquinolone developed in Japan, containing regimens against MABS have been reported11,12. Sitafloxacin, with a chloro substituent at the C-8 position, is a newly developed oral quinoline, exhibiting good antimicrobial activity against extracellular and intramacrophage Mycobacterium avium complex (MAC) compared to levofloxacin in vitro and in vivo13–16. These previous papers suggest that fluoroquinolone combining regimens could have a potency for the effective treatment of MABS. Genus mycobacterium included Mycobacterium tuberculosis (M. tuberculosis), and some NTM have long been known to have both rough and smooth colony morphotypes17,18. These morphotypes are formed by the expression levels of glycopeptidolipids (GPLs). GPLs are produced by several NTMs, including RGMs (M. abscessus, M. chelonae, and M. smegmatis)19–21 and MAC members22–24. MABS can spontaneously change between a smooth form, which expresses GPLs, and a rough form, lacking GPLs. The smooth form can form biofilms and colonize surfaces; conversely, the rough morphotypes cannot form biofilms but can multiply in macrophages and cause persistent infection25. Rough morphotypes are generally more virulent than smooth variants for isolates lacking GPLs enhanced releasing TNF-α from macrophage25–27. Conversely, to form biofilms, smooth variants were related to protecting from surrounding factors28. Here, we presented the new sitafloxacin–arbekacin combination regimens, which are more effective on rough morphotypes, causing aggressive infections, and could be a potential treatment option against Mab.
Results
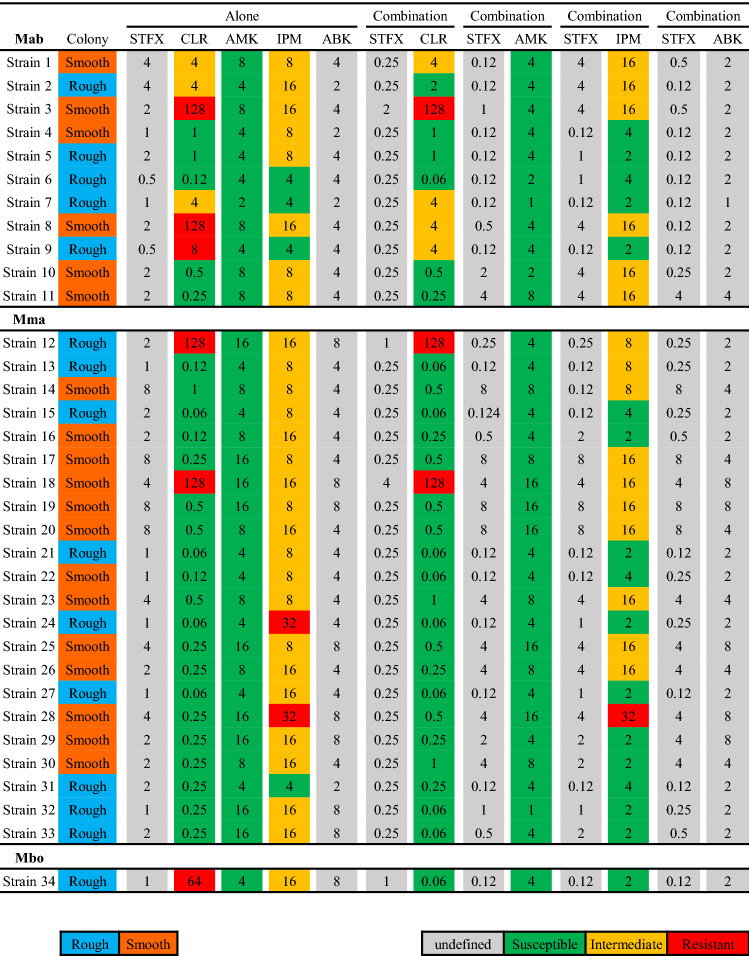
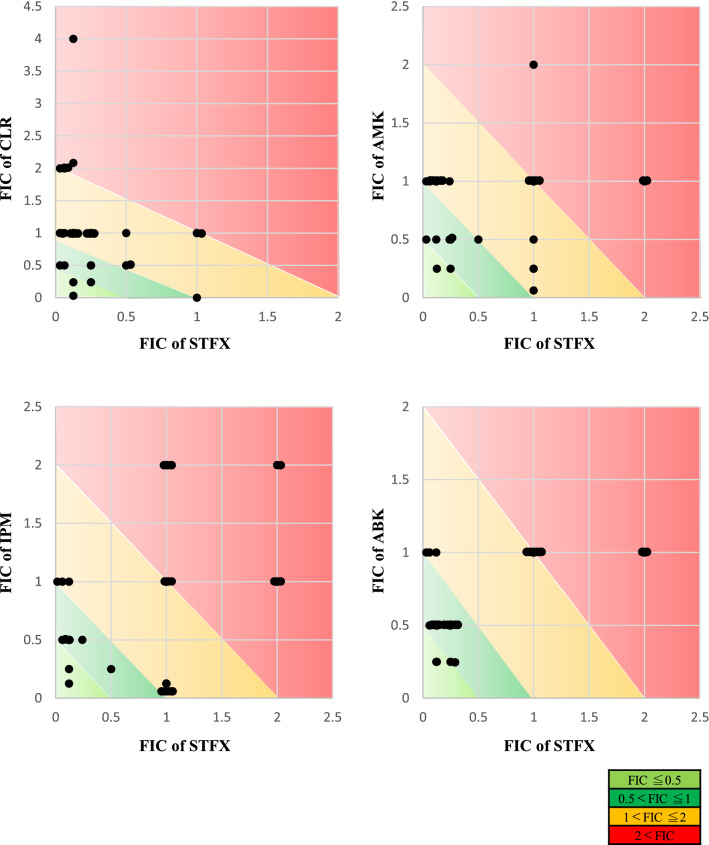
Thirty-four clinical strains of MABS were isolated from various clinical samples at the Juntendo university hospital from 2011 to 2020. The characteristics of patients isolated from MABS are shown in Table 1. Methods of incubation time and susceptibility testing were used as a reference to our previous report29. Five antimicrobials (clarithromycin, intravenous amikacin, imipenem, arbekacin, and sitafloxacin) were used for the study. The difference of MICs between both colony morphotypes was evaluated (Fig. 1), and MICs of sitafloxacin and intravenous amikacin in rough morphotypes were significantly lower than smooth morphotypes (p values of sitafloxacin and intravenous amikacin were 0.0004 and 0.002, respectively). Therefore, we investigated the best combination partners of sitafloxacin as the potential regimens for MABS especially rough morphotypes. The susceptibility to a combination of sitafloxacin and antimicrobial agents was compared to that of the antimicrobial agents alone, categorized into each subspecies of MABS (Fig. 2). The MICs of four antimicrobial agents (clarithromycin, intravenous amikacin, imipenem, and arbekacin) were measured with or without sitafloxacin. Ten of 11 Mab were susceptible to sitafloxacin in the combination administration; while, 11 of 22 Mma were susceptible. The median MICs of sitafloxacin and arbekacin in MABS were significantly lower in the combination administration (p values of sitafloxacin and intravenous amikacin were < 0.001 and 0.028, respectively, Table S2). We next evaluated the most synergistic combinations by using the fractional inhibitory concentration (FIC) index as described in previous paper30. Figure 3 showed the relation between FIC of sitafloxacin and that of the other antibiotics. The combination of sitafloxacin and amikacin tended to be obviously higher rate of synergy and additive effect. Further evaluation of FIC index of each combination was performed (Fig. 4 and Table 2). Susceptibility was divided into two classes, synergy and additive as a synergistic effect and indifference and antagonism as an antagonistic effect. Out of 34 MABS, 8 strains treated with sitafloxacin–amikacin combination, 9 of sitafloxacin–imipenem combination, 19 of sitafloxacin–arbekacin combination, and 9 of sitafloxacin–clarithromycin combination showed synergistic effects, respectively. Sitafloxacin–arbekacin combination also exhibited the synergistic effects against 10 of 22 Mma strains and 8 of 11 Mab strains, a highly resistant subspecies of MABS. We investigated whether susceptibility to the sitafloxacin–arbekacin combination might associate with clinical or isolate status. The rough colony morphotypes revealed more synergistic effects than antagonistic effects (p = 0.008) (Table 3). The other clinical parameters such as age, sex, smoking history, bronchiectasis lesion, and treatment history of antibiotics did not influence the sitafloxacin–arbekacin combination.
Table 1.
The characteristics of patients from which MABS were isolated.
| N = 34 | |
|---|---|
| Sex (male/female) | 16/18 |
| Median age (range) | 65.5 (30–83) |
| Smoking history, N (%) | 13 (38.2) |
| MABS subtype, N (%) | |
| Mycobacterium abscessus subsp. abscessus | 11 (32.4) |
| Mycobacterium abscessus subsp. masiliense | 22 (64.7) |
| Mycobacterium abscessus subsp. Bolletii | 1 (2.9) |
| Colony phenotype (rough/smooth) | 15/19 |
| MABS detected from, N (%) | |
| Sputum or bronchial lavage | 27 (79.4) |
| Others | 7 (20.6) |
| Pretreatment of antibiotics within 3 months, N (%) | |
| Macrolides | 5 (14.7) |
| Fluoroquinolones | 4 (11.8) |
| Tetracyclines | 2 (5.9) |
| Others | 12 (35.3) |
| Comorbidity, N (%) | |
| Bronchiectasis | 14 (41.2) |
| Diabetes mellitus | 4 (11.8) |
| Immunodeficiency (non HIV) | 2 (5.9) |
| Malignancy | 7 (20.6) |
| Concomitant medications, N (%) | |
| Corticosteroids | 6 (17.6) |
| Immunosupressant | 3 (8.8) |
HIV human immunodeficiency virus, MABS Mycobacterium abscessus species.
Figure 1.
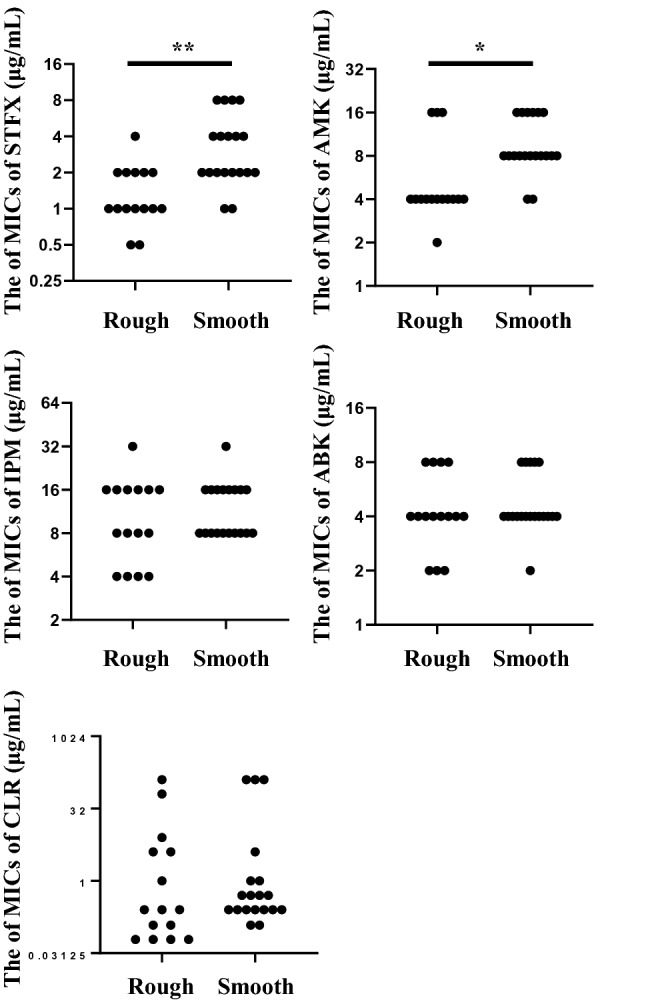
The comparison of MIC of each antimicrobial, STFX, intravenous AMK, IPM, ABK, and CLR, compared with rough and smooth colony morphotypes. *p value < 0.05, **p value < 0.01. STFX sitafloxacin, AMK amikacin, IPM imipenem, ABK arbekacin, CLR clarithromycin.
Figure 2.
MIC distributions for intravenous AMK, IPM, and ABK combined with STFX, categorized into three subspecies of MABS on day 7. Light blue color indicates rough colony morphotype, and orange color smooth colony morphotype. Green color indicates susceptibility, yellow color intermediate, and red color resistance to MABS. Gray color indicates MIC breakpoints undefined. STFX sitafloxacin, AMK amikacin, IPM imipenem, ABK arbekacin, CLR clarithromycin, Mma Mycobacterium abscessus subspecies massiliense, Mab Mycobacterium abscessus subspecies abscessus, Mbo Mycobacterium abscessus subspecies bolletii, MABS Mycobacterium abscessus species.
Figure 3.
The relation between FIC of sitafloxacin and that of the other antibiotics. Light green color indicates ≤ 0.5 of FIC value, green color indicates 0.5 < to 1, yellow color indicates 1 < to 2, and red color indicates 2< . STFX sitafloxacin, AMK amikacin, IPM imipenem, ABK arbekacin, CLR clarithromycin, FIC fractional inhibitory concentration.
Figure 4.
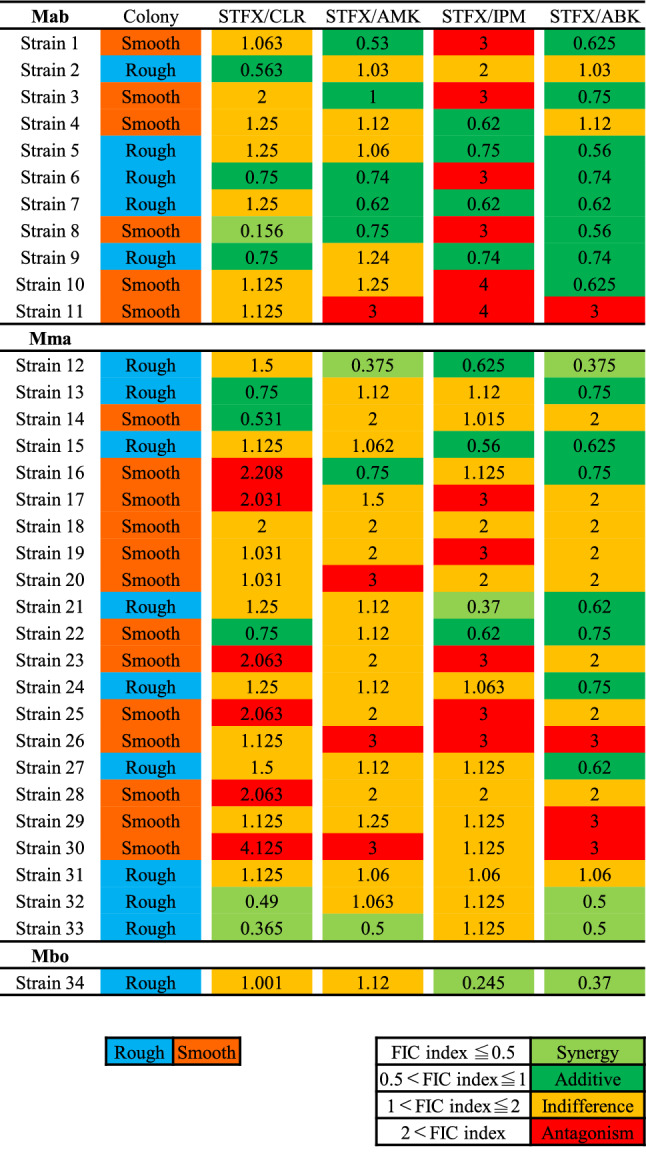
FIC index of intravenous AMK, IPM, ABK, and CLR combined with STFX categorized into three subspecies of MABS. Light blue color indicates rough colony morphotype, and orange color smooth colony morphotype. Light green color indicates synergy, green color indicates additive, yellow color indicates indifference, and red color indicates antagonism in each combination. STFX sitafloxacin, AMK amikacin, IPM imipenem, ABK arbekacin, CLR clarithromycin, FIC index fractional inhibitory concentration index, Mma Mycobacterium abscessus subspecies massiliense, Mab Mycobacterium abscessus subspecies abscessus, Mbo Mycobacterium abscessus subspecies bolletii, MABS Mycobacterium abscessus species.
Table 2.
The number of synergistic and antagonistic combination with STFX and each antimicrobial.
| Species | Categories of FIC index | STFX/CLR | STFX/AMK | STFX/IPM | STFX/ABK | p value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%, adjusted residual) | |||||||||||
| MABS | FIC index ≤1 | 9 (26.5, −0.9) | 8 (23.5, −0.9) | 9 (26.5, −1.4) | 19 (55.9, 3.3**) | 0.016* | |||||
| N = 34a | FIC index 1 < | 25 (73.5, 0.9) | 26 (76.5, 0.9) | 25 (73.5, 1.4) | 15 (44.1, −3.3**) | ||||||
| Mma | FIC index ≤1 | 5 (22.7, −0.3) | 3 (13.6, −1.4) | 4 (18.2, −0.9) | 10 (45.5, 2.6) | 0.083 | |||||
| N = 22 | FIC index 1 < | 17 (77.2, 0.3) | 19 (86.4, 1.4) | 18 (81.8, 0.9) | 12 (54.5, −2.6) | ||||||
| Mab | FIC index ≤1 | 4 (36.4, −0.9) | 5 (45.5, −0.2) | 4 (36.4, −0.9) | 8 (72.7, 1.9) | 0.26 | |||||
| N = 11 | FIC index 1 < | 7 (63.6, 0.9) | 6 (54.5, 0.2) | 7 (63.6, 0.9) | 3 (27.3, −1.9) | ||||||
FIC index fractional inhibitory concentration index, STFX sitafloxacin, CLR clarithromycin, AMK amikacin, IPM imipenem, ABK arbekacin, MABS Mycobacterium abscessus species, Mma, Mycobacterium abscessus subspecies massiliense; Mab, Mycobacterium abscessus subspecies abscessus; Mbo, Mycobacterium abscessus subsp.boletii.
*p value < 0.05, **p value < 0.01.
*Adjusted residuals >|1.96|, **adjusted residuals >|2.58|.
aIncluding Mbo(n = 1).
Table 3.
The number of synergistic and antagonistic combination with STFX and ABK in each clinical status.
| FIC index | |||
|---|---|---|---|
| Synergy + additive, N = 19 (%) | Indifference + antagonism, N = 15 (%) | p value | |
| Age | |||
| < 65 years | 7 (20.6) | 7 (20.6) | 0.56 |
| ≥ 65 years | 12 (35.3) | 8 (23.5) | |
| Sex | |||
| Male | 11 (32.4) | 5 (14.7) | 0.15 |
| Female | 8 (23.5) | 10 (29.4) | |
| Smoking history | |||
| Yes | 7 (20.6) | 6 (17.6) | 0.85 |
| No | 12 (35.3) | 9 (26.5) | |
| With bronchiectasis | |||
| Yes | 8 (23.5) | 6 (17.6) | 0.90 |
| No | 11 (32.4) | 9 (26.5) | |
| With immunosuppression | |||
| Yes | 8 (23.5) | 8 (23.5) | 0.51 |
| No | 11 (32.4) | 7 (20.6) | |
| Pretreatment of antibiotics | |||
| Yes | 8 (23.5) | 6 (17.6) | 0.90 |
| No | 11 (32.4) | 9 (26.5) | |
| Colony morphotypes | |||
| Smooth | 6 (17.6) | 13 (38.2) | 0.008** |
| Rough | 13 (38.2) | 2 (5.9) | |
Antibiotics including CLR (n = 3).
FIC index fractional inhibitory concentration index, STFX sitafloxacin, ABK arbekacin, CLR clarithromycin.
*p value < 0.05, **p value < 0.01.
Discussion
We demonstrated here the efficacy of the new sitafloxacin–arbekacin combination regimen. The combination addministration revealed MIC reduction of sitafloxacin and arbekacin, and significantly high synergistic effect against MABS. The combination regimen showed a higher rate of susceptibility and synergistic effects against Mab than all other combinations. Interestingly, the combination had relatively higher efficacy in Mma than others including clarithromycin–arbekacin combination, even though clarithromycin is the key drug of Mma treatment. These results might suggest that the combination therapy was more effective against Mab, showing a high level of antimicrobial resistance. Furthermore, the combination revealed higher efficacy for the treatment of MABS in rough morphotypes associated with aggressive infections.
Sitafloxacin is approved in Japan, and it has been clinically used against most NTM infections. Formerly, sitafloxacin has been mainly used for MAC infections among NTMs; then, in vitro studies and clinical use for MABS has increased. Bedaquiline-clofazimine-sitafloxacin combination revealed a synergistic effect against 11 isolates of 70 Mab (15.7%)11. In a Japanese retrospective study of 13 MABS pulmonary disease, all 4 patients who received sitafloxacin-containing regimens achieved negative sputum conversion after 1 year of treatment and improved radiological findings12. Japanese case series described that five cases of pulmonary MABS were successfully treated with clarithromycin and sitafloxacin combination31. Together with our data and previous reports, sitafloxacin could be an effective antimicrobial combination partner against refractory MABS. MABS exist in two distinct morphotypes, smooth and rough, that differ in their gross colony appearances when grown on solid media due to their differing amounts of cell wall GPLs. The smooth morphotype initially colonizes the airway mucosa, and had generally lower pathogenicity in this state. Subsequently switching from smooth morphotypes to rough morphotypes, aggressive pulmonary disease cause. Smooth morphotypes have an advantage in survival due to biofilm formation, leading to inhibit bacteria-induced apoptosis32. Conversely, rough morphotypes, without biofilms, induce the invasion ability mediated by apoptotic cell death33,34. Several clinical data have revealed increased pathogenicity from the rough morphotype. The rates of isolation of rough morphotype is higher in the CF patients with clinical symptoms35, and case reports describe that the CF patients with rough morphotypes lead to dramatic declines of respiratory function and/or death26,36. Thus, the development of new treatment targeted rough morphotypes has become imperative. Our study revealed that sitafloxacin–arbekacin combination had the higher synergy in rough morphotypes, the combination could be useful treatment for the patients who are isolated with rough morphotypes and/or whose disease have progressed. Interestingly, in 17 out of 19 smooth morphotypes, sitafloxacin susceptibility in the combination treatment improved as compared to alone. This data suggested that sitafloxacin–arbekacin combination could be also partially effective as the treatment for smooth morphotypes of MABS. In conclusion, our in vitro study demonstrated the synergistic effect of the sitafloxacin–arbekacin combination against MABS. Further, this combination regimen might be more effective against not only rough morphotype of MABS, causing severe disease, but also Mab, which is thought to reveal high resistance to antibiotics. The limitation of our study is that the sample size was limited to make definitive concerns and the clinical efficacy of the sitafloxacin–arbekacin combination have not been assessed. Further studies are required to clarify the validity of the combination.
Materials and methods
Determination of MABS
Three subspecies of MABS was confirmed by sequencing the 16S rRNA, rpoB, hsp65, and erm genes37,38. All strains of MABS were cultured on BD trypticase soy agar II with 5% sheep blood (Blood agar; Nippon Becton–Dickinson and Company, Japan) at 35 °C for approximately 4 to 6 days to observe colony morphology and purity and then used for species identification based on multi-locus sequence analysis. Colony morphologies were confirmed before the drug sensitivity testing, and all strains grew to maintain the same colony morphologies even after repeat-passage.
Methodological details are described in the Supplementary Materials and Methods.
PCR amplification, DNA sequencing, and MALDI–TOF MS analysis
The conditions of each analysis and primer sequences used in PCR to detect transcripts are described in the Supplementary materials and methods.
Antimicrobial susceptibility testing
Susceptibility testing was performed according to Clinical and Laboratory Standard Institute (CLSI) guideline M24-A235. The bacterial suspension was diluted at a concentration of 1–5 × 105 colony forming units (CFU)/mL in cation-adjusted Mueller–Hinton broth (CAMHB), then the final suspension was inoculated on the break-point checkerboard plate customized for the study (Eiken Chemical Co., Ltd., Japan). The ranges of antibiotic concentrations tested were as follows: clarithromycin (CLR) 0.06 to 64 μg/mL, arbekacin (ABK) 1 to 8 μg/mL, intravenous amikacin (AMK) 1 to 64 μg/mL, imipenem (IPM) 2 to 32 μg/mL, and sitafloxacin (STFX) 0.12 to 32 μg/mL. MICs of each antimicrobial agent were determined by broth microdilution methods as recommended by the CLSI. The panels were prepared with a 96-channel dispenser and stored at −80 °C until use. Sitafloxacin were dispensed alone in the first row, and arbekacin, intravenous amikacin, imipenem were dispensed in the first column. Each well was inoculated with a concentration of 1 × 105 colony-forming units (CFU)/mL. The MICs were determined after 7 days of incubation at 35 °C. The MIC breakpoints, indicating susceptible, intermediate, and resistant strains, were interpreted according to the Clinical and Laboratory Standard Institute (CLSI) criteria (Table 4)39. Sitafloxasin and arbekacin breakpoints were undefined. The effect of each agent combined with sitafloxacin was evaluated using FIC index analysis30.
Table 4.
Antimicrobial agents and MIC breakpoints for RGM.
| Antimicrobial agents | MIC (μg/mL) for category | ||
|---|---|---|---|
| Susceptible | Intermediate | Resistant | |
| Amikacin | ≤ 16 | 32 | ≥ 64 |
| Cefoxitin | ≤ 16 | 32–64 | ≥ 128 |
| Ciprofloxacin | ≤ 1 | 2 | ≥ 4 |
| Clarithromycin | ≤ 2 | 4 | ≥ 8 |
| Doxycycline | ≤ 1 | 2–4 | ≥ 8 |
| Imipenem | ≤ 4 | 8–16 | ≥ 32 |
| Linezolid | ≤ 8 | 16 | ≥ 32 |
| Moxifloxacin† | ≤ 1 | 2 | ≥ 4 |
| Trimethoprim-sulfamethoxazole | ≤ 2/38 | – | ≥ 4/76 |
| Tobramycin | ≤ 2 | 4 | ≥ 8 |
Sitafloxacinand arbekacin breakpoints were undefined.
RGM rapidly growing mycobacteria.
Statistical analysis
Categorical variables were compared using the chi-square test or Fisher's exact test. The evaluation of changes in MIC was performed using the Wilcoxon signed-rank test. Differences were considered significant at p < 0.05. When the chi-square test results were statistically significant, adjusted residuals were calculated to determine which particular associations were significant. Adjusted residuals were significant at p < 0.05 level if they were less than − 1.96 or more than 1.96 and were significant at p < 0.01 level if they were less than − 2.58 or more than 2.58. All statistical analyses were performed using the SPSS software program (version 20, IBM Japan, Japan).
Supplementary Information
Acknowledgements
This work was supported by the Grant for Cross-disciplinary Collaboration, Juntendo University (grant no. 2019-46 to T. Okabe).
Abbreviations
- MABS
Mycobacterium abscessus Species
- NTM
Nontuberculous mycobacteria
- RGM
Rapidly growing mycobacteria
- SGM
Slowly growing mycobacteria
- Mma
Mycobacterium abscessus Subspecies massiliense
- Mab
Mycobacterium abscessus Subspecies abscessus
- Mbo
Mycobacterium abscessus Subspecies bolletii
- M. tuberculosis
Mycobacterium tuberculosis
- COPD
Chronic obstructive pulmonary disease
- CF
Cystic fibrosis
- GPL
Glycopeptidolipid
- MAC
Mycobacterium avium Complex
- MALDI-TOF MS
Matrix-assisted laser-desorption/ionization time-of-flight mass spectrometry
- CLSI
Clinical and Laboratory Standard Institute
- STFX
Sitafloxacin
- AMK
Amikacin
- IPM
Imipenem
- ABK
Arbekacin
- HIV
Human immunodeficiency virus
- FIC
Fractional inhibitory concentration
- MBEC
Minimum biofilm eradication concentration
- CAMHB
Cation-adjusted Mueller–Hinton broth
- CFU
Colony forming units
Author contributions
J.W. and H.I. wrote the main manuscript text and J.W., S.T., Y.F., H.T., K.K., Y.A., K.S., I.S., and Y.O. collected the data and samples with all firures and tables. All authors reviewed the manuscript.
Data availability
The datasets used in the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-29021-0.
References
- 1.Griffith DE, Girard WM, Wallace Jr RJ. Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am. Rev. Respir. Dis. 1993;147(5):1271–1278. doi: 10.1164/ajrccm/147.5.1271. [DOI] [PubMed] [Google Scholar]
- 2.Harada T, Akiyama Y, Kurashima A, et al. Clinical and microbiological differences between Mycobacterium abscessus and Mycobacterium massiliense lung diseases. J. Clin. Microbiol. 2012;50(11):3556–3561. doi: 10.1128/JCM.01175-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagano H, Kinjo T, Nei Y, Yamashiro S, Fujita J, Kishaba T. Causative species of nontuberculous mycobacterial lung disease and comparative investigation on clinical features of Mycobacterium abscessus complex disease: A retrospective analysis for two major hospitals in a subtropical region of Japan. PLoS ONE. 2017;12(10):e0186826. doi: 10.1371/journal.pone.0186826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Floto RA, Olivier KN, Saiman L, et al. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis: Executive summary. Thorax. 2016;71(1):88–90. doi: 10.1136/thoraxjnl-2015-207983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris KA, Kenna DTD. Mycobacterium abscessus infection in cystic fibrosis: Molecular typing and clinical outcomes. J. Med. Microbiol. 2014;63(Pt 10):1241–1246. doi: 10.1099/jmm.0.077164-0. [DOI] [PubMed] [Google Scholar]
- 6.Esther CR, Jr, Esserman DA, Gilligan P, Kerr A, Noone PG. Chronic Mycobacterium abscessus infection and lung function decline in cystic fibrosis. J. Cyst. Fibros. 2010;9(2):117–123. doi: 10.1016/j.jcf.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daley CL, Iaccarino JM, Lange C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: An official ATS/ERS/ESCMID/IDSA clinical practice guideline. Clin. Infect. Dis. 2020;71(4):905–913. doi: 10.1093/cid/ciaa1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferro BE, Srivastava S, Deshpande D, et al. Tigecycline is highly efficacious against Mycobacterium abscessus pulmonary disease. Antimicrob. Agents Chemother. 2016;60(5):2895–2900. doi: 10.1128/AAC.03112-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang B, Jhun BW, Moon SM, et al. Clofazimine-containing regimen for the treatment of Mycobacterium abscessus lung disease. Antimicrob. Agents Chemother. 2017;61:6. doi: 10.1128/AAC.02052-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olivier KN, Shaw PA, Glaser TS, et al. Inhaled amikacin for treatment of refractory pulmonary nontuberculous mycobacterial disease. Ann. Am. Thorac. Soc. 2014;11(1):30–35. doi: 10.1513/AnnalsATS.201307-231OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asami T, Aono A, Chikamatsu K, et al. Efficacy estimation of a combination of triple antimicrobial agents against clinical isolates of Mycobacterium abscessus subsp. abscessus in vitro. JAC Antimicrob. Resist. 2021;3(1):dlab004. doi: 10.1093/jacamr/dlab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Namkoong H, Morimoto K, Nishimura T, et al. Clinical efficacy and safety of multidrug therapy including thrice weekly intravenous amikacin administration for Mycobacterium abscessus pulmonary disease in outpatient settings: A case series. BMC Infect. Dis. 2016;16:396. doi: 10.1186/s12879-016-1689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fung-Tomc J, Minassian B, Kolek B, Washo T, Huczko E, Bonner D. In vitro antibacterial spectrum of a new broad-spectrum 8-methoxy fluoroquinolone, gatifloxacin. J. Antimicrob. Chemother. 2000;45(4):437–446. doi: 10.1093/jac/45.4.437. [DOI] [PubMed] [Google Scholar]
- 14.Tomioka H, Sano C, Sato K, Shimizu T. Antimicrobial activities of clarithromycin, gatifloxacin and sitafloxacin, in combination with various antimycobacterial drugs against extracellular and intramacrophage Mycobacterium avium complex. Int. J. Antimicrob. Agents. 2002;19(2):139–145. doi: 10.1016/S0924-8579(01)00473-3. [DOI] [PubMed] [Google Scholar]
- 15.Tomioka H, Sato K, Akaki T, Kajitani H, Kawahara S, Sakatani M. Comparative in vitro antimicrobial activities of the newly synthesized quinolone HSR-903, sitafloxacin (DU-6859a), gatifloxacin (AM-1155), and levofloxacin against Mycobacterium tuberculosis and Mycobacterium avium complex. Antimicrob. Agents Chemother. 1999;43(12):3001–3004. doi: 10.1128/AAC.43.12.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sano C, Tatano Y, Shimizu T, Yamabe S, Sato K, Tomioka H. Comparative in vitro and in vivo antimicrobial activities of sitafloxacin, gatifloxacin and moxifloxacin against Mycobacterium avium. Int. J. Antimicrob. Agents. 2011;37(4):296–301. doi: 10.1016/j.ijantimicag.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Eckstein TM, Inamine JM, Lambert ML, Belisle JT. A genetic mechanism for deletion of the ser2 gene cluster and formation of rough morphological variants of Mycobacterium avium. J. Bacteriol. 2000;182(21):6177–6182. doi: 10.1128/JB.182.21.6177-6182.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fregnan GB, Smith DW. Description of various colony forms of mycobacteria. J. Bacteriol. 1962;83:819–827. doi: 10.1128/jb.83.4.819-827.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howard ST, Byrd TF. The rapidly growing mycobacteria: Saprophytes and parasites. Microbes Infect. 2000;2(15):1845–1853. doi: 10.1016/S1286-4579(00)01338-1. [DOI] [PubMed] [Google Scholar]
- 20.Lopez Marin LM, Laneelle MA, Prome D, Daffe M. Structures of the glycopeptidolipid antigens of two animal pathogens: Mycobacterium senegalense and Mycobacterium porcinum. Eur. J. Biochem. 1993;215(3):859–866. doi: 10.1111/j.1432-1033.1993.tb18103.x. [DOI] [PubMed] [Google Scholar]
- 21.Ripoll F, Deshayes C, Pasek S, et al. Genomics of glycopeptidolipid biosynthesis in Mycobacterium abscessus and M. chelonae. BMC Genomics. 2007;8:114. doi: 10.1186/1471-2164-8-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Field SK, Fisher D, Cowie RL. Mycobacterium avium complex pulmonary disease in patients without HIV infection. Chest. 2004;126(2):566–581. doi: 10.1378/chest.126.2.566. [DOI] [PubMed] [Google Scholar]
- 23.Wagner D, Young LS. Nontuberculous mycobacterial infections: A clinical review. Infection. 2004;32(5):257–270. doi: 10.1007/s15010-004-4001-4. [DOI] [PubMed] [Google Scholar]
- 24.Horsburgh CR., Jr The pathophysiology of disseminated Mycobacterium avium complex disease in AIDS. J. Infect. Dis. 1999;179(Suppl 3):S461–465. doi: 10.1086/314804. [DOI] [PubMed] [Google Scholar]
- 25.Howard ST, Rhoades E, Recht J, et al. Spontaneous reversion of Mycobacterium abscessus from a smooth to a rough morphotype is associated with reduced expression of glycopeptidolipid and reacquisition of an invasive phenotype. Microbiology (Reading). 2006;152(Pt 6):1581–1590. doi: 10.1099/mic.0.28625-0. [DOI] [PubMed] [Google Scholar]
- 26.Catherinot E, Roux AL, Macheras E, et al. Acute respiratory failure involving an R variant of Mycobacterium abscessus. J. Clin. Microbiol. 2009;47(1):271–274. doi: 10.1128/JCM.01478-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhoades ER, Archambault AS, Greendyke R, Hsu FF, Streeter C, Byrd TF. Mycobacterium abscessus glycopeptidolipids mask underlying cell wall phosphatidyl-myo-inositol mannosides blocking induction of human macrophage TNF-alpha by preventing interaction with TLR2. J. Immunol. 2009;183(3):1997–2007. doi: 10.4049/jimmunol.0802181. [DOI] [PubMed] [Google Scholar]
- 28.Clary G, Sasindran SJ, Nesbitt N, et al. Mycobacterium abscessus smooth and rough morphotypes form antimicrobial-tolerant biofilm phenotypes but are killed by acetic acid. Antimicrob. Agents Chemother. 2018;62(3):e02101. doi: 10.1128/AAC.01782-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takei S, Ihara H, Togo S, et al. The synergetic effect of imipenem–clarithromycin combination in the Mycobacteroides abscessus complex. BMC Microbiol. 2020;20(1):316. doi: 10.1186/s12866-020-02000-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lorian V. Antibiotics in Laboratory Medicine. 4. Williams & Wilkins; 1996. [Google Scholar]
- 31.Takano K, Shimada D, Kashiwagura S, et al. Severe pulmonary Mycobacterium abscessus cases due to co-infection with other microorganisms well treated by clarithromycin and sitafloxacin in Japan. Int. Med. Case Rep. J. 2021;14:465–470. doi: 10.2147/IMCRJ.S321969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whang J, Back YW, Lee KI, et al. Mycobacterium abscessus glycopeptidolipids inhibit macrophage apoptosis and bacterial spreading by targeting mitochondrial cyclophilin D. Cell Death Dis. 2017;8(8):e3012. doi: 10.1038/cddis.2017.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernut A, Herrmann JL, Kissa K, et al. Mycobacterium abscessus cording prevents phagocytosis and promotes abscess formation. Proc. Natl. Acad. Sci. USA. 2014;111(10):E943–952. doi: 10.1073/pnas.1321390111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Catherinot E, Clarissou J, Etienne G, et al. Hypervirulence of a rough variant of the Mycobacterium abscessus type strain. Infect Immun. 2007;75(2):1055–1058. doi: 10.1128/IAI.00835-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jonsson BE, Gilljam M, Lindblad A, Ridell M, Wold AE, Welinder-Olsson C. Molecular epidemiology of Mycobacterium abscessus, with focus on cystic fibrosis. J. Clin. Microbiol. 2007;45(5):1497–1504. doi: 10.1128/JCM.02592-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanguinetti M, Ardito F, Fiscarelli E, et al. Fatal pulmonary infection due to multidrug-resistant Mycobacterium abscessus in a patient with cystic fibrosis. J. Clin. Microbiol. 2001;39(2):816–819. doi: 10.1128/JCM.39.2.816-819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim BJ, Yi SY, Shim TS, et al. Discovery of a novel hsp65 genotype within Mycobacterium massiliense associated with the rough colony morphology. PLoS ONE. 2012;7(6):e38420. doi: 10.1371/journal.pone.0038420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshida S, Tsuyuguchi K, Suzuki K, et al. Further isolation of Mycobacterium abscessus subsp. abscessus and subsp. bolletii in different regions of Japan and susceptibility of these isolates to antimicrobial agents. Int. J. Antimicrob. Agents. 2013;42(3):226–231. doi: 10.1016/j.ijantimicag.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 39.Woods GL, Brown-Elliott BA, Conville PS, et al. Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes. Clinical and Laboratory Standards Institute; 2011. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used in the current study are available from the corresponding author on reasonable request.