Abstract
Background:
The MUC5B promoter variant (rs35705950) and telomere length are linked to pulmonary fibrosis and computed tomography (CT)-based qualitative assessments of interstitial abnormalities, but their associations with longitudinal quantitative changes of the lung interstitium among community-dwelling adults is unknown.
Methods:
We used data from participants in the Multi-Ethnic Study of Atherosclerosis with high attenuation areas (HAAs, Exams 1–6 (2000–2018)) and MUC5B genotype (n=4,552) and telomere length (n=4,488) assessments. HAA was defined as the percent of imaged lung with attenuation of −600 to −250 Hounsfield units. We used linear mixed effects models to examine associations of MUC5B risk allele (T) and telomere length with longitudinal changes in HAAs. Joint models were used to examine associations of longitudinal changes in HAAs with death and interstitial lung disease (ILD).
Results:
The MUC5B risk allele (T) was associated with an absolute change in HAAs of 2.60% (95% CI 0.36–4.86) per 10 years overall. This association was stronger among those with a telomere length below an age-adjusted percentile of 5% (p-value for interaction=0.008). A 1% increase in HAAs per year was associated with 7% increase in mortality risk (rate ratio (RR)=1.07, 95% CI 1.02–1.12) for overall death and 34% increase in ILD (RR=1.34, 95% CI 1.20–1.50). Longer baseline telomere length was cross-sectionally associated with less HAAs from baseline scans, but not with longitudinal changes in HAAs.
Conclusions:
Longitudinal increases in high attenuation areas were associated with the MUC5B risk allele and a higher risk of death and ILD.
Keywords: interstitial lung disease, imaging, Mucin-5B, telomere lengthening
INTRODUCTION
Interstitial lung disease (ILD) is a group of chronic respiratory disorders characterized by inflammation and fibrosis of the lung parenchyma.1 Non-fibrosing ILDs can become fibrotic which may lead to rapid deterioration, chronic respiratory failure, and death. By the time of diagnosis, patients often have significant physiologic impairments and decreased exercise capacity, with a poor median survival.2 3
An increasing number of studies have focused on visual, qualitative assessment of computed tomography (CT) scans for changes suggestive of ILD, termed interstitial lung abnormalities (ILA) when identified in research studies or incidentally in the clinical context.4 These studies have yielded important insights into novel risk factors for progressive fibrosing ILD. A recent Fleischner Society position paper defining the radiological criteria for ILA also highlighted the need for quantitative CT methods for evaluation of disease extent and progression as a key research priority.4
One of the strongest known risk factors for pulmonary fibrosis is the MUC5B promoter polymorphism (rs35705950); it is associated with a 4-times fold higher odds of idiopathic pulmonary fibrosis (IPF). Recent studies have shown that this gain-of-function promoter variant is linked to other types of fibrosing ILDs like chronic hypersensitivity pneumonitis and rheumatoid-arthritis related ILD.5–7 The polymorphism is associated with ILA among the general population and in cohorts of smokers, suggesting that carriers of this allele may be at higher risk of developing fibrosing ILDs.8 Shorter telomere length, a marker of accelerated cellular senescence, is another intrinsic risk factor that is associated with a higher risk of pulmonary fibrosis, more severe disease, mortality, and a higher prevalence of ILA.6 9 10 The few prior studies that have examined the MUC5B polymorphism and telomere length in IPF and chronic hypersensitivity pneumonitis did not show evidence of interaction between these genetic factors, suggesting distinct mechanisms by which they influence pathogenesis. However, study of the MUC5B promoter polymorphism and telomere length in other ILDs and in earlier stages of ILD is lacking.6 11
Whether these genetic risk factors (and their interactions) are linked to a quantitative measure of lung parenchymal changes and its progression over time among a racially/ethnic diverse sample of community-dwelling adults remains unclear. We hypothesized that the MUC5B promoter polymorphism (rs35705950) would be associated with longitudinal increases in high attenuation areas with effect modification by telomere length over time in the Multi-Ethnic Study of Atherosclerosis (MESA). We also examined whether longitudinal changes in HAAs are associated with death and ILD-related clinical events.
METHODS
Study Participants
MESA in an ongoing prospective cohort with the original intent of investigating subclinical cardiovascular disease.12 There were 6,814 U.S. adults between the ages of 45 and 84 years without clinical cardiovascular disease recruited at Exam 1 (2000–2002) and followed longitudinally, with repeat exams that included additional data collection. Participants who provided consent for genetic analyses were included in this study. All MESA participants provided written informed consent and institutional review board approval was obtained at all participating study sites.
MUC5B Genotype and Telomere Length
MUC5B (rs35705950) genotypes were obtained from whole genome sequencing (WGS) that was performed on DNA samples from whole blood in MESA as part of the National Heart Lung and Blood Institute TOPMed Program. Procedures related to the TOPMed program have been previously described in detail.13 Briefly, Illumina HiSeq X Ten instruments were used for WGS that targeted a mean depth of at least 30 × (paired-end, 150-bp reads). PCR-free library preparation kits from KAPA Biosystems were used for all sequencing. Using blood samples from MESA Exam 1, telomere length was extracted from the TOPMEd WGS using TelSeq and has been previously described. Briefly, counts of sequencing reads that contained fixed number of repeats of the telomeric nucleotide motif “TTAGGG” were used to estimate individual telomere lengths and accounted for batch corrections. TelSeq estimates of telomere length have a Pearson correlation of 0.68–0.72 with Southern blot data in TOPMed.14 There were no repeat telomere length measurements from WGS in MESA.
High Attenuation Area
MESA participants underwent cardiac CT scans at Exams 1 (2000–2002), 2 (2002–2004), 3 (2004–2005), 4 (2005–2007), and 5 (2010–2012), and full-lung scans at Exam 6 (2016–2018) using the MESA/SPIROMICS protocol.15 Sixty-six percent of the lung volume from the carina to lung bases were captured by cardiac scans. The intraclass correlation coefficient for lung density measures between cardiac and full-lung scans is 0.93.16 HAAs from Exam 6 full-lung scans were assessed in the lower two-thirds lung fields to be consistent with the previous cardiac scans; this approach has been used previously for other densitometry-based measures like percent emphysema.17 HAAs was defined as the percentage of voxels with attenuation values in the range of −600 and −250 Hounsfield units (HU), as previously described.18 The Pulmonary Analysis Software Suite (PASS) at the University of Iowa’s Advanced Pulmonary Physiomic Imaging Laboratory (APPIL) was used by trained technicians for segmentation and correction of the lung.19 Percent emphysema was defined as the percentage of lung voxels below −950 HU.20
Mortality and ILD
Vital status of participants was determined by the contact of each MESA participant or family member by interviewers every 9–12 months. The National Death Index was used to supplement this review and ensure complete follow-up. Mortality was adjudicated up until December 31, 2018. Hospitalization data were complete as of December 31, 2013. Inpatient medical records were reviewed by an adjudication panel and International Classification of Diseases, Ninth Revision (ICD-9, 495.XX, 515.X, or 516.XX) and 10th Revision (J60.X-J64.X, J67.X, or J84.X) were used to determine whether participants who were hospitalized or died had ILD as previously described.21 Further details are in the supplement.
Statistical Analysis
We used linear mixed effects models with random intercept and slope to examine associations of the MUC5B risk allele (T) with the longitudinal change in HAAs over time (Exams 1 through 6, 2000–2018). We used a genetic additive model to present our results.5 We used mixed effects model to account for within-subject correlations among repeated measurements of HAAs over time for each participant. Such modeling approach has been used in other studies.17 22 Measurements of HAAs from all six exams were used in our linear mixed effects model. Additional information is provided in the supplement.
The longitudinal analysis was adjusted for scanner parameters, principal components of genetic ancestry, baseline age, sex, race/ethnicity, smoking status, and cigarette pack-years, and time-varying covariates of height, weight, cigarettes smoked per day, and percent emphysema. We included interaction terms of covariates with the time since initial HAAs assessment to evaluate the impact of covariates on HAAs trajectories over time. We performed an analysis of the overall cohort and race/ethnic-specific analyses due to the heterogeneity of MUC5B (rs35705950) genotype frequencies.23 24 Positive and negative beta estimates from the term, “MUC5B risk allele × time since initial HAAs assessment”, were interpreted as more rapid progression and slower progression in HAAs over time, respectively. We used natural log-transformed HAAs as the outcome in all of our regression models. To improve interpretability, we exponentiated the regression coefficients of our log-transformed HAAs analysis to report our outcomes as percent change in HAAs.
We examined whether telomere length modified the association between MUC5B and HAAs based on a previous study that examined interactions between MUC5B and telomere length in pulmonary fibrosis.6 Studies have used different percentile cutoffs for telomere length as there is uncertainty of what is a relevant cutoff in general population-based cohorts.25–28 Therefore, stratified analyses were performed using age-adjusted percentile cutoffs for telomere length at 5%, 10%, and 25%. We accounted for baseline age in generating each of these cutoffs by using the percentile cutoff for each of the age groups: <50 years, 50–60, 60–70, and >80 years.
We examined associations of baseline (Exam 1) telomere length with Exam 1 HAAs using linear regression models with adjustment for baseline scanner parameters, age, sex, race/ethnicity, smoking history, cigarette pack-years, height, weight, percent emphysema, and principal components of genetic ancestry. For the longitudinal HAAs analysis, we used the same approach as our MUC5B analysis. We report results per 1-standard deviation increment in natural log-transformed telomere length.
We performed pre-specified stratified analyses by smoking status (never versus ever) based on past studies that suggest overproduction of mucin 5B may impair clearance of cigarette particles that leads to lung injury.29 Sex was also examined as a potential effect modifier due to the differential associations of sex and pulmonary fibrosis risk.1 For associations between telomere length and HAAs, we performed a stratified analysis by race/ethnicity. The log-likelihood ratio was used to test for effect modification in cross-sectional analyses. We used the F-test of the three-way interaction term, “effect modifier × primary exposure variable of interest × time since initial HAAs assessment”, to determine effect modification in longitudinal analyses.17
Joint modelling was used to examine the association of longitudinal changes of HAAs with time-to-event data (i.e., overall death and ILD-related events). This approach uses linear mixed effects models to model longitudinal changes of a time-varying covariate of interest (i.e., HAAs) and examine its association with a time-to-event outcome using a Cox regression model.30 For the ILD analysis, we used a composite binary outcome of ILD-related death or hospitalization. Due to the time when events were adjudicated, we used HAAs from Exams 1 through 5 (2010–2012) for the joint model. Results are reported as rate ratios (RR) per 1% increment in HAAs per year. A description of this method and the covariates we adjusted for in the models are in the supplement.
MUC5B and telomere length analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). The “nlme”, “survival”, and “JMbayes2” packages were used for the joint model analysis in R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
There were 4,552 MESA participants with MUC5B genotype and Exam 1 HAAs assessments (Figure S1). Table 1 shows the baseline characteristics of the study sample. Over 50 percent of the cohort were women, Asian, African-American, or Hispanic, and had a history of smoking. There was a higher proportion of self-identified non-Hispanic White participants with the GT or TT genotype compared with other races/ethnicities (Table S1). Participant characteristics among those with longer versus shorter telomere length are shown in Table S2.
Table 1.
Study Sample Characteristics
| Characteristic | Overall | MUC5B Genotype | ||
|---|---|---|---|---|
|
|
||||
| GG | GT | TT | ||
|
|
||||
| No. Participants | 4,552 | 3,959 | 569 | 24 |
| Age | 61 (10) | 61 (10) | 61 (10) | 60 (9) |
| Female sex | 51% | 51% | 53% | 50% |
| Race/ethnicity | ||||
| Non-Hispanic White | 41% | 37% | 68% | 75% |
| Asian | 13% | 15% | 2% | 0 |
| African-American | 24% | 25% | 10% | 4% |
| Hispanic | 22% | 23% | 20% | 21% |
| Smoking Status | ||||
| Never smoker | 46% | 47% | 40% | 21% |
| Former smoker | 40% | 39% | 46% | 75% |
| Current smoker | 14% | 14% | 14% | 4% |
| Cigarette pack-years | 12 (22) | 11.5 (21.2) | 13.9 (23.4) | 19.4 (30.0) |
| Height (cm) | 167 (10) | 167 (10) | 168 (10) | 169 (7) |
| Weight (kg) | 79 (17) | 78 (17) | 80 (18) | 86 (21) |
| Telomere length (kb) | 4.4 (0.9) | 4.4 (0.9) | 4.4 (0.9) | 4.1 (0.9) |
Abbreviations: HAA=high attenuation area
Continuous variables presented as mean (standard deviation)
Categorical variables presented as percentage
MUC5B and HAA
Table S3 summarizes the number of CT scans performed at each exam between 2000 and 2018 for our longitudinal analysis. There were 4,552 MESA participants with the MUC5B genotype and at least two repeat HAAs measurements between 2000 and 2018. Representative CT images of increasing HAAs between Exams 1 and 6 are shown in Figure 1.
Figure 1.
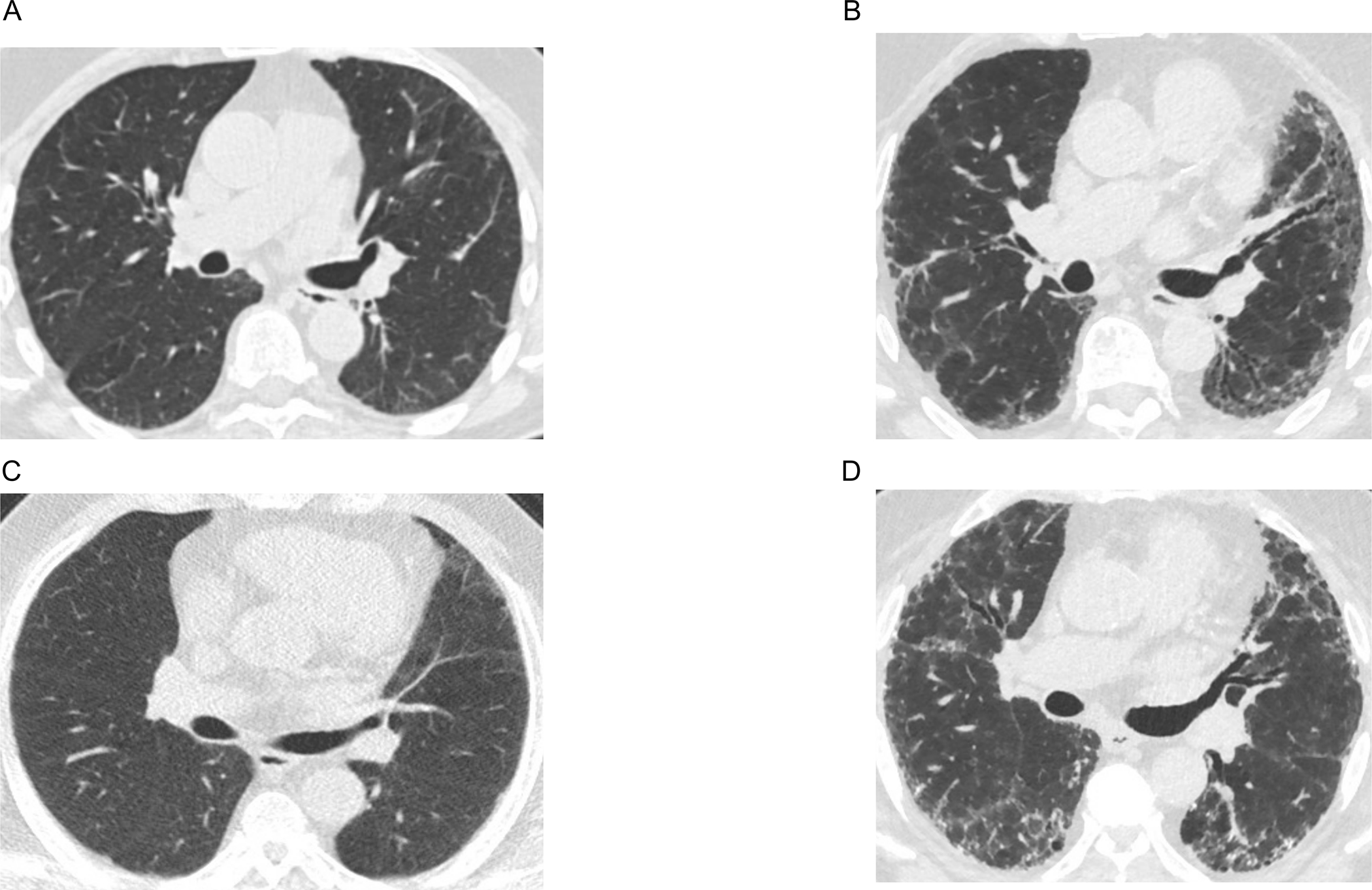
Computed tomography (CT) axial images that show an increase in high attenuation areas over time between Exams 1 (2000–2002) and 6 (2016–2018). Panels A and B are CT images of one participant with high attenuation areas percent of 4.34 at Exam 1 and 12.21 at Exam 6. Panels C and D are CT images of another participant with high attenuation areas percent of 7.38 at Exam 1 and 17.42 at Exam 6.
Each MUC5B risk allele (T) was associated with an increase in HAAs of 2.60% (95% CI 0.36 to 4.86) per 10 years (Table 2) (Figure 2). In comparison, smoking 10 cigarettes per day on average was associated with an increase in HAAs of 4.69% (95% CI 2.24 to 7.15) per 10 years (Table S4). The association between MUC5B and HAAs was stronger among those with a telomere length below an age-adjusted cutoff of 5% (p-value for interaction=0.008). There was no significant effect modification with a telomere length cutoff of 10% and 25%. There was not a significant interaction with sex or smoking (Table S5). The MUC5B and HAAs association was strongest among non-Hispanic White participants with an increase in HAAs of 3.05% (95% CI 0.64 to 5.46) per 10 years, and was significant after applying a Bonferroni corrected p-value of 0.0125 (Table S6). Associations between MUC5B and HAAs were in the same direction but weaker and not significant in the other race/ethnic subgroups (Table S6).
Table 2.
Associations of MUC5B (rs35705950) risk allele with longitudinal changes in high attenuation areas
| Model | No. Participants | % Longitudinal change in HAAs per 10 years (95% CI) per MUC5B (rs35705950) risk allele (T) | P-value* |
|---|---|---|---|
|
| |||
| Overall | 4,552 | 2.60 (0.36 to 4.86) | 0.02 |
| Stratified by telomere | |||
| length percentile cutoff | |||
| Below 5th percentile | 221 | 15.13 (5.60 to 24.75) | 0.008 |
| Above 5th percentile | 4,267 | 1.93 (−0.40 to 4.27) | |
| Below 10th percentile | 446 | 5.26 (−1.35 to 11.91) | 0.41 |
| Above 10th percentile | 4,042 | 2.30 (−0.11 to 4.71) | |
| Below 25th percentile | 1,119 | 5.03 (0.83 to 9.25) | 0.19 |
| Above 25th percentile | 3,369 | 1.72 (−0.94 to 4.38) | |
Abbreviations: CI=confidence intervals; HAAs=high attenuation areas
Overall model is adjusted for scanner parameters and principal components of genetic ancestry. Baseline age, sex, self-reported race/ethnicity, smoking status, cigarette pack-years were also adjusted for including their interaction terms with “time since initial HAAs assessment.” Time-varying covariates height, weight, percent emphysema, and cigarettes smoked per day were also adjusted for in the model.
Stratified models include their respective three-way interaction term (e.g., “telomere length percentile below 5% × MUC5B risk allele × time since initial HAAs assessment”).
P-values for stratified analysis represent p-values for interaction.
All results are reported per risk allele (T) of the MUC5B (rs35705950) promoter variant
Figure 2.

Predicted high attenuation areas (HAAs) over time by number of MUC5B (rs35705950) risk alleles (T). Linear mixed effects model with random intercept and slope adjusted for scanner parameters and principal components of genetic ancestry. Baseline sex, self-reported race/ethnicity, age, smoking status, cigarette pack-years, and their interaction term with “time since initial HAAs assessment.” Also adjusted for time-varying covariates height, weight, percent emphysema, and cigarettes smoked per day. Y-axis is log-scale.
Telomere Length and HAA
There were 4,488 MESA participants with telomere length and Exam 1 HAAs assessments (Figure S1). Shorter baseline telomere length was associated with more HAAs on Exam 1 cardiac CT. A 1-SD decrease in log-transformed telomere length was associated with a HAAs of 6.51% (95% CI 2.62 to 10.24) (Table 3). Participants with a telomere length below the 25th percentile had more baseline HAAs compared with those with longer telomeres (1.56%, 95% CI 0.04 to 3.11). Associations were not statistically significant using 5th and 10th percentile cutoffs. Baseline telomere length was not associated with a longitudinal change in HAAs overall and in stratified analyses (Table 3 and Table S7).
Table 3.
Associations of baseline telomere length with high attenuation areas
| Model | No. Participants | Mean percent change in Exam 1 HAAs (95% CI) | P-value | % Longitudinal change in HAAs per 10 years (95% CI) | P-value |
|---|---|---|---|---|---|
|
| |||||
| Absolute telomere length* | 4,488 | −6.51 (−10.24 to −2.62) | 0.001 | 0.32 (−4.68 to 5.35) | 0.90 |
| Age-adjusted telomere length percentile cutoffs | |||||
| Above 5th percentile | 4,267 | 0.0 (REF) | 0.0 (REF) | ||
| Below 5th percentile | 221 | 0.44 (−2.55 to 3.52) | 0.78 | 0.87 (−2.82 to 4.56) | 0.65 |
| Above 10th percentile | 4,042 | 0.0 (REF) | 0.0 (REF) | ||
| Below 10th percentile | 446 | 0.16 (−2.00 to 2.36) | 0.88 | 1.41 (−1.28 to 4.10) | 0.30 |
| Above 25th percentile | 3,369 | 0.0 (REF) | 0.0 (REF) | ||
| Below 25th percentile | 1,119 | 1.56 (0.04 to 3.11) | 0.04 | 0.11 (−1.74 to 1.96) | 0.91 |
Abbreviations: CI=confidence intervals; HAAs=high attenuation areas
Reported per standard deviation increment of log-transformed telomere length
Exam 1 HAAs model: Adjusted for scanner parameters, principal components of genetic ancestry, and baseline age, sex, self-reported race/ethnicity, smoking status, cigarette pack-years height, weight, and percent emphysema.
Longitudinal HAAs model: Adjusted for scanner parameters and principal components of genetic ancestry. Baseline age, sex, self-reported race/ethnicity, smoking status, cigarette pack-years were also adjusted for including their interaction terms with “time since initial HAAs assessment.” Time-varying covariates height, weight, percent emphysema, and cigarettes smoked per day were also adjusted for in the model.
HAAs, Mortality, and ILD
Among the 4,552 participants, there were 4,517 (99%) who had adjudicated death status and complete covariate data. There was a total of 827 deaths with an event rate of 111.1 (95% CI 103.8 to 118.9) per 10,000 person-years. A 1% increment in HAAs per year was associated with a 7% higher risk of overall death (RR 1.07, 95% CI 1.02 to 1.12) after adjustment for covariates (Table 4). The association between longitudinal changes in HAAs and mortality were not significantly different by MUC5B risk allele carrier status, shorter telomere length, sex, or smoking history (p-values for interaction>0.40) (Table S8). There were 4,443 participants with adjudicated ILD-related outcomes with a total of 29 events and an event rate of 5.5 (95% CI 3.7 to 7.7) per 10,000 person-years. A 1% increment in HAAs per year was associated with a 34% higher risk of ILD (i.e., ILD-related hospitalization or death) (RR 1.34, 95% CI 1.20 to 1.50).
Table 4.
Associations of longitudinal changes in high attenuation areas with mortality and interstitial lung disease
| Model | No. Participants | Total Person-years | Events | Event Rate per 10,000 Person-Years (95% CI) | Rate Ratio per 1% Increment in HAAs per year (95% CI) | P-value |
|---|---|---|---|---|---|---|
|
| ||||||
| Overall mortality | 4,517 | 74,415 | 827 | 111.1 (103.8 to 118.9) | 1.07 (1.02 to 1.12) | <0.001 |
| Interstitial lung disease | 4,443 | 53,173 | 29 | 5.5 (3.7 to 7.7) | 1.34 (1.20 to 1.50) | <0.001 |
Abbreviations: CI=confidence intervals; HAAs=high attenuation areas
Models adjusted for sex, self-reported race/ethnicity, baseline age, smoking status, cigarette pack-years, height, weight, systolic and diastolic blood pressures, total cholesterol, high-density lipoprotein cholesterol, diabetes history, cancer history, coronary artery calcium score, percent emphysema, and total intentional exercise (met-min/week).
Interstitial lung disease events defined as a hospitalization or death related to interstitial lung disease using ICD-9 ICD-10 codes (ICD-9 codes 495.XX, 515.XX, or 516.XX and ICD-10 codes J60.X-J64.X, J67.X, or J84.X)
DISCUSSION
The MUC5B (rs35705950) promoter variant was associated with more high attenuation areas over time among community-dwelling adults. This association was stronger among those with shorter telomere length using an age-adjusted 5th percentile cutoff and non-Hispanic White individuals. Shorter baseline telomere length was associated with more HAAs at baseline, but not with changes in HAAs over time. Longitudinal increases in HAAs were associated with worse survival and a higher risk of ILD-related events.
Prior studies have identified a strong association between the MUC5B (rs35705950) gain-of-function promoter variant and a higher risk of pulmonary fibrosis.5 31 MUC5B encodes the protein mucin 5B and is highly expressed in the lungs of adults with pulmonary fibrosis, particularly in the distal airways.32 Overexpression of MUC5B impairs mucosal host defenses, clearance of inhaled materials, and alveolar repair upon injury, and has been linked to a higher prevalence of ILA and its progression, further suggesting its pathogenic role in pulmonary fibrosis.8 33 Ours is the first study to report on the association of the MUC5B polymorphism with longitudinal quantitative changes in CT density (that could represent earlier stages of ILD) in a more general population cohort.
Our longitudinal analysis shows that the MUC5B (rs35705950) risk allele (T) was associated with increasing HAAs over time. This is consistent with prior studies that have identified associations between the MUC5B risk allele and other quantitative CT-based interstitial lung measures. Those previous studies have largely been cross-sectional and used cohorts comprised of individuals with a significant smoking history or first-degree relatives of adults with pulmonary fibrosis.34 35 This study extends the prior work to community-dwelling adults sampled without regard for respiratory symptoms or smoking history. Notably, the association between MUC5B and HAAs was not affected by smoking history. Previous studies did not find an association between MUC5B and HAAs assessed from a single timepoint; the focus of our study was to examine longitudinal changes in HAAs.36 37 Another difference from prior work was that we accounted for changes over time in scanner models, height, and weight. Our longitudinal analysis over an 18-year span with repeated CT measurements supports the potential unidirectional relationship between the MUC5B variant and interstitial lung changes that may represent the early stages of ILD.8
Race-ethnic specific analyses revealed that the association between MUC5B (rs35705950) and HAAs was stronger in non-Hispanic White participants compared with other groups. Consistent with prior literature, the genotypes GT and TT were rare in the Asian and African-American subgroups and less common in the Hispanic group in MESA compared with the non-Hispanic White group.23 Thus, we may have been under-powered to detect differences in the smaller racial/ethnic groups. There has been one prior study demonstrating an association between MUC5B (rs35705950) and ILA in a cohort of adults from Mexico.38 Few other studies of genetic risk factors for early ILD/ILA have focused on underrepresented racial and ethnic groups, highlighting a pressing need for research in this area. African-American patients with ILD have been shown to have a younger age at diagnosis and longer survival time, underscoring the importance of research into the determinants of racial/ethnic differences in disease risk and progression.39 The recent identification of variants related to obstructive lung disease and ILD in Hispanic/Latinx cohorts of admixed populations and African ancestry demonstrates that such studies are feasible.40–42
In addition to the MUC5B polymorphism, telomere length has been strongly implicated in pulmonary fibrosis. Adults with IPF and other fibrosing ILDs who have shorter telomere lengths have an accelerated decline in their lung function and reduced survival.9 43 In our study, shorter absolute telomere length was associated with more HAAs from Exam 1 scans, which is consistent with prior ILA studies.10 44 However, when using different telomere length thresholds, only telomere lengths below the 25th percentile showed a significant association with HAAs. Our findings highlight an important knowledge gap. More research is needed to determine what is a relevant percentile cutoff for telomere length and its relationship to chronic diseases in the general population.
In contrast to the cross-sectional analyses, baseline telomere length was not associated with longitudinal changes in HAAs. A potential reason may be the dynamic nature of telomeres, with lengths that can change over time. In addition to telomerase-related genetic variants (e.g., TERC, PARN), environmental factors influence telomere length, including socioeconomic status (i.e., neighborhood status, home ownership), nutrition, cigarette smoking, dietary supplementation, and physical activity.45–50 We did not have repeat measures of telomere length at each exam in MESA and thus were not able to examine whether longitudinal changes in telomere length and its rate of attrition may be more informative in how cellular senescence relates to longitudinal changes in lung density.
We also found that individuals with the MUC5B risk allele and a baseline telomere length below an age-adjusted 5th percentile cutoff had more HAAs over time. It has been speculated that individuals with a genetic predisposition (i.e., MUC5B risk allele carrier, telomerase mutations, etc.), in combination with other environmental risk factors, may increase one’s risk of developing pulmonary fibrosis.51 Previous studies investigating this have largely been case-control in design, with samples comprised of clinically-diagnosed patients or adults of relatives with disease.6 44 It is intriguing to consider the possibility that our finding of an interaction between MUC5B and telomere length on progression of HAA, in a prospective, population-based cohort of community-dwelling individuals, may support this hypothesis. However, the subgroup nature of this analysis is exploratory as replication in independent cohorts and investigation of the potential intersection of biological pathways of MUC5B and telomere biology are needed.
More consequential may be our finding that increased HAAs over time were associated with worse overall mortality and a higher risk of ILD-related clinical events. This finding suggests that an increase in HAAs may indicate ongoing pathological processes in the lung, which have clinical implications. Notably, the effect estimate of the association between MUC5B and HAAs was comparable to that of cigarette smoking, which is a universally accepted risk factor for ILD.52 Increases in HAAs among carriers of the MUC5B risk allele had a higher risk for overall death compared with non-carriers. However, this interaction was not statistically significant. In contrast, among patients with IPF, the MUC5B risk allele was shown to be associates with improved survival.53 However, this association has been suggested to be due to index event bias.54 Further research is needed to understand the complex relationship between MUC5B, disease progression and survival in patients with fibrosing ILD.
This study had several limitations. First, histopathological correlations of HAAs are unknown. However, HAAs are associated with ILA, biomarkers of extracellular matrix remodeling, and ILD-related outcomes, suggesting that they have construct validity as a measure of parenchymal lung injury and fibrosis.18 21 Second, one of the concerns with HAAs, and other CT densiometric-based quantitative measures, is potential confounding by body habitus, atelectasis, and artifacts generated from foreign bodies, calcifications, and lung densities unrelated to ILD.36 Although we cannot completely rule out residual confounding, we demonstrate that one of the strongest genetic risk factors for pulmonary fibrosis (i.e., MUC5B promoter variant), which does not appear to be influenced by adiposity or has a role in adipogenesis, is strongly associated with more HAAs. Notably, the effect estimate of the association between MUC5B and HAAs was comparable to that of cigarette smoking, which is a universally accepted risk factor for ILD.52 This provides further validity for HAAs as a risk factor for ILD.
Third, we did not have repeated measurements of WGS-extracted telomere length. Although telomere length from WGS correlates with laboratory-based measurements, further research is needed to improve and refine the techniques to extract telomere length from WGS, and obtain consensus on the platforms and relevant cutoffs more broad clinical use. Fourth, we may have been under-powered to detect a significant association in the underrepresented racial/ethnic subgroups.23 55 Fifth, we acknowledge the possibility of survival bias (an issue common to longitudinal population cohort studies), which may have influenced our findings. Finally, there was a very low number of ILD-related events, which is not unexpected for a population cohort without known cardiovascular disease at baseline, and adjudicated ILD diagnoses from outpatient records were not available in this study population. The increasing incidence of ILD that is likely due to growing awareness of this condition and the adjudication of ILD-related outcomes by more cohort studies will be important to determine whether radiological tools can help identify at-risk individuals.56
In conclusion, longitudinal increases in HAAs were associated with the MUC5B (rs35705950) promoter variant and a higher risk of death and ILD-related events in a population-based cohort of community-dwelling adults. This study demonstrates the feasibility and potential utility of using a quantitative assessment of lung parenchymal changes to investigate intrinsic risk factors for pulmonary fibrosis in a diverse cohort of community-dwelling adults. Quantitative CT assessments, especially more sophisticated texture-based methods that can more definitively distinguish ILD-specific characteristics from artifact, hold promise for the detection of ILD at its earlier stages and track its progression. Future studies that develop and refine quantitative methods to detect early interstitial lung changes and expand to more diverse study populations will be important in identifying novel factors that contribute to the development and progression of ILD.
Supplementary Material
What is already known on this topic
The MUC5B (rs35705950) risk allele (T) and telomere length are associated with pulmonary fibrosis and qualitative assessments of interstitial lung changes on computed tomography (CT). There are fewer studies that have examined their relationship with longitudinal interstitial lung changes among community-dwelling adults.
What this study adds
More high attenuation areas over time on computed tomography were associated with the MUC5B risk allele, particularly those with shorter baseline telomere length, and a higher risk of overall death and interstitial lung disease.
How this study might affect research, practice or policy
Our study suggests that quantitative computed tomography assessments and their longitudinal measures may be a potential tool to identify earlier stages of interstitial lung disease.
ACKNOWLEDEGMENTS
The authors thank the other investigators, staff, and participants of the MESA study for their contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. We gratefully acknowledge the studies and participants who provided biological samples and data for TOPMed. Infrastructure for the CHARGE Consortium is supported in part by the NHLBI grant R01-HL105756.
Funding:
JSK was supported by the Pulmonary Fibrosis Foundation Scholars Award and grant K23-HL-150301 from the National Heart, Lung, and Blood Institute (NHLBI). MRA was supported by grant K23-HL-150280, AJP was supported by grant K23-HL-140199, SMK was supported by grant K24-HL-103844, and AM was supported by R01-HL131565 from the NHLBI. EJB was supported by grant K23-AR-075112 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The MESA Lung Study was supported by grants R01-HL077612, R01-HL093081 and RC1-HL100543 from the NHLBI. The MESA Lung Fibrosis Study was funded by grants R01-HL-103676 from the NHLBI. The MESA project is conducted and supported by the NHLBI in collaboration with MESA investigators. Support for MESA is provided by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169, UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420. Also supported in part by the National Center for Advancing Translational Sciences, CTSI grant UL1TR001881, and the National Institute of Diabetes and Digestive and Kidney Disease Diabetes Research Center (DRC) grant DK063491 to the Southern California Diabetes Endocrinology Research Center.
Whole genome sequencing (WGS) for the Trans-Omics in Precision Medicine (TOPMed) program was supported by the National Heart, Lung and Blood Institute (NHLBI). WGS for “NHLBI TOPMed: Multi-Ethnic Study of Atherosclerosis (MESA)” (phs001416.v1.p1) was performed at the Broad Institute of MIT and Harvard (3U54HG003067-13S1). Core support including centralized genomic read mapping and genotype calling, along with variant quality metrics and filtering were provided by the TOPMed Informatics Research Center (3R01HL-117626-02S1; contract HHSN268201800002I). Core support including phenotype harmonization, data management, sample-identity QC, and general program coordination were provided by the TOPMed Data Coordinating Center (R01HL-120393; U01HL-120393; contract HHSN268201800001I). Infrastructure for the CHARGE Consortium is supported in part by the NHLBI grant R01-HL105756. We gratefully acknowledge the studies and participants who provided biological samples and data for MESA and TOPMed.
Footnotes
CONFLICTS OF INTEREST
E.A.H. is a founder of VIDA Diagnostics, Inc., a company that is commercializing lung image analysis software developed, in part, at the University of Iowa. S.M.K reports receipt of grant support from the NIH and Cardiovascular Medical Research and Education Fund and payments/honoria from Children’s Hospital of Philadelphia, University of California San Francisco, University of Minnesota, PVRI, and Stanford University, and support for attending meetings from the Pulmonary Hypertension Association, American Thoracic Society study section, Duke University, World Symposium on Pulmonary Hypertension, PVRI, Children’s Hopsital of Philadelphia, University of Miami, Wake Forest University, Aspen Lung Conference, NIH Study Section. S.M.K reports participation on a data safety monitoring board for United Therapeutics and editorial board for the European Respiratory Journal. I.N. reports receipt of personal fees and other support from Boehringer Ingelheim, HLR/Genentech, Sanofi Aventis, Global Blood Therapeutics, and Veracyte outside the scope of this work. C.K.G reports research support from the NHLBI, Department of Defense, Boehringer Ingelheim, receipt of payments/Honoria fromo the Three Lakes Foundation and Stanford University, stock options in Pliant Therapuetics, and collaboration with AstraZeneca. R.G.B. reports receipt of grants from the Alpha-1 Foundation and the COPD Foundation outside the scope of this work. A.J.P reports grant support from the NHLBI and American Lung Association and consulting fees from Regeneron and Boehringer Ingelheim outside the submitted work.
REFERENCES
- 1.Wijsenbeek M, Cottin V. Spectrum of Fibrotic Lung Diseases. N Engl J Med 2020;383(10):958–68. doi: 10.1056/NEJMra2005230 [published Online First: 2020/09/03] [DOI] [PubMed] [Google Scholar]
- 2.Raghu G, Chen SY, Yeh WS, et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001–11. Lancet Respir Med 2014;2(7):566–72. doi: 10.1016/S2213-2600(14)70101-8 [DOI] [PubMed] [Google Scholar]
- 3.du Bois RM, Weycker D, Albera C, et al. Forced vital capacity in patients with idiopathic pulmonary fibrosis: test properties and minimal clinically important difference. Am J Respir Crit Care Med 2011;184(12):1382–9. doi: 10.1164/rccm.201105-0840OC [published Online First: 2011/09/24] [DOI] [PubMed] [Google Scholar]
- 4.Hatabu H, Hunninghake GM, Richeldi L, et al. Interstitial lung abnormalities detected incidentally on CT: a Position Paper from the Fleischner Society. Lancet Respir Med 2020;8(7):726–37. doi: 10.1016/S2213-2600(20)30168-5 [published Online First: 2020/07/11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seibold MA, Wise AL, Speer MC, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med 2011;364(16):1503–12. doi: 10.1056/NEJMoa1013660 [published Online First: 2011/04/22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ley B, Newton CA, Arnould I, et al. The MUC5B promoter polymorphism and telomere length in patients with chronic hypersensitivity pneumonitis: an observational cohort-control study. Lancet Respir Med 2017;5(8):639–47. doi: 10.1016/S2213-2600(17)30216-3 [published Online First: 2017/06/27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juge PA, Lee JS, Ebstein E, et al. MUC5B Promoter Variant and Rheumatoid Arthritis with Interstitial Lung Disease. N Engl J Med 2018;379(23):2209–19. doi: 10.1056/NEJMoa1801562 [published Online First: 2018/10/23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunninghake GM, Hatabu H, Okajima Y, et al. MUC5B promoter polymorphism and interstitial lung abnormalities. N Engl J Med 2013;368(23):2192–200. doi: 10.1056/NEJMoa1216076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stuart BD, Lee JS, Kozlitina J, et al. Effect of telomere length on survival in patients with idiopathic pulmonary fibrosis: an observational cohort study with independent validation. Lancet Respir Med 2014;2(7):557–65. doi: 10.1016/S2213-2600(14)70124-9 [published Online First: 2014/06/21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Putman RK, Axelsson GT, Ash SY, et al. Interstitial lung abnormalities are associated with decreased mean telomere length. Eur Respir J 2022. doi: 10.1183/13993003.01814-2021 [published Online First: 2022/02/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dressen A, Abbas AR, Cabanski C, et al. Analysis of protein-altering variants in telomerase genes and their association with MUC5B common variant status in patients with idiopathic pulmonary fibrosis: a candidate gene sequencing study. Lancet Respir Med 2018;6(8):603–14. doi: 10.1016/S2213-2600(18)30135-8 [published Online First: 2018/06/13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002;156(9):871–81. doi: 10.1093/aje/kwf113 [published Online First: 2002/10/25] [DOI] [PubMed] [Google Scholar]
- 13.Taliun D, Harris DN, Kessler MD, et al. Sequencing of 53,831 diverse genomes from the NHLBI TOPMed Program. Nature 2021;590(7845):290–99. doi: 10.1038/s41586-021-03205-y [published Online First: 2021/02/12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taub MA, Conomos MP, Keener R, et al. Genetic determinants of telomere length from 109,122 ancestrally diverse whole-genome sequences in TOPMed. Cell Genom 2022;2(1) doi: 10.1016/j.xgen.2021.100084 [published Online First: 2022/05/10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sieren JP, Newell JD Jr., Barr RG, et al. SPIROMICS Protocol for Multicenter Quantitative Computed Tomography to Phenotype the Lungs. Am J Respir Crit Care Med 2016;194(7):794–806. doi: 10.1164/rccm.201506-1208PP [published Online First: 2016/08/03] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffman EA, Jiang R, Baumhauer H, et al. Reproducibility and validity of lung density measures from cardiac CT Scans--The Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Acad Radiol 2009;16(6):689–99. doi: 10.1016/j.acra.2008.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang M, Aaron CP, Madrigano J, et al. Association Between Long-term Exposure to Ambient Air Pollution and Change in Quantitatively Assessed Emphysema and Lung Function. JAMA 2019;322(6):546–56. doi: 10.1001/jama.2019.10255 [published Online First: 2019/08/14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Podolanczuk AJ, Oelsner EC, Barr RG, et al. High attenuation areas on chest computed tomography in community-dwelling adults: the MESA study. Eur Respir J 2016;48(5):1442–52. doi: 10.1183/13993003.00129-2016 [published Online First: 2016/11/02] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffman EA, Reinhardt JM, Sonka M, et al. Characterization of the interstitial lung diseases via density-based and texture-based analysis of computed tomography images of lung structure and function. Acad Radiol 2003;10(10):1104–18. doi: 10.1016/s1076-6332(03)00330-1 [published Online First: 2003/11/01] [DOI] [PubMed] [Google Scholar]
- 20.Barr RG, Bluemke DA, Ahmed FS, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med 2010;362(3):217–27. doi: 10.1056/NEJMoa0808836 [published Online First: 2010/01/22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Podolanczuk AJ, Oelsner EC, Barr RG, et al. High-Attenuation Areas on Chest Computed Tomography and Clinical Respiratory Outcomes in Community-Dwelling Adults. Am J Respir Crit Care Med 2017;196(11):1434–42. doi: 10.1164/rccm.201703-0555OC [published Online First: 2017/06/15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waxman A, Restrepo-Jaramillo R, Thenappan T, et al. Inhaled Treprostinil in Pulmonary Hypertension Due to Interstitial Lung Disease. N Engl J Med 2021;384(4):325–34. doi: 10.1056/NEJMoa2008470 [published Online First: 2021/01/14] [DOI] [PubMed] [Google Scholar]
- 23.Peljto AL, Selman M, Kim DS, et al. The MUC5B promoter polymorphism is associated with idiopathic pulmonary fibrosis in a Mexican cohort but is rare among Asian ancestries. Chest 2015;147(2):460–64. doi: 10.1378/chest.14-0867 [published Online First: 2014/10/03] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Putman RK, Gudmundsson G, Araki T, et al. The MUC5B promoter polymorphism is associated with specific interstitial lung abnormality subtypes. Eur Respir J 2017;50(3) doi: 10.1183/13993003.00537-2017 [published Online First: 2017/09/13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alder JK, Chen JJ, Lancaster L, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci U S A 2008;105(35):13051–6. doi: 10.1073/pnas.0804280105 [published Online First: 2008/08/30] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alter BP, Rosenberg PS, Giri N, et al. Telomere length is associated with disease severity and declines with age in dyskeratosis congenita. Haematologica 2012;97(3):353–9. doi: 10.3324/haematol.2011.055269 [published Online First: 2011/11/08] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newton CA, Zhang D, Oldham JM, et al. Telomere Length and Use of Immunosuppressive Medications in Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med 2019;200(3):336–47. doi: 10.1164/rccm.201809-1646OC [published Online First: 2018/12/20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider CV, Schneider KM, Teumer A, et al. Association of Telomere Length With Risk of Disease and Mortality. JAMA Intern Med 2022. doi: 10.1001/jamainternmed.2021.7804 [published Online First: 2022/01/19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang IV, Fingerlin TE, Evans CM, et al. MUC5B and Idiopathic Pulmonary Fibrosis. Ann Am Thorac Soc 2015;12 Suppl 2:S193–9. doi: 10.1513/AnnalsATS.201503-110AW [published Online First: 2015/11/26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y, Postmus D, Cowie MR, et al. Using joint modelling to assess the association between a time-varying biomarker and a survival outcome: an illustrative example in respiratory medicine. Eur Respir J 2021;57(2) doi: 10.1183/13993003.03206-2020 [published Online First: 2020/11/28] [DOI] [PubMed] [Google Scholar]
- 31.Allen RJ, Guillen-Guio B, Oldham JM, et al. Genome-Wide Association Study of Susceptibility to Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med 2020;201(5):564–74. doi: 10.1164/rccm.201905-1017OC [published Online First: 2019/11/12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakano Y, Yang IV, Walts AD, et al. MUC5B Promoter Variant rs35705950 Affects MUC5B Expression in the Distal Airways in Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med 2016;193(4):464–6. doi: 10.1164/rccm.201509-1872LE [published Online First: 2016/02/13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hobbs BD, Putman RK, Araki T, et al. Overlap of Genetic Risk between Interstitial Lung Abnormalities and Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med 2019;200(11):1402–13. doi: 10.1164/rccm.201903-0511OC [published Online First: 2019/07/25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathai SK, Humphries S, Kropski JA, et al. MUC5B variant is associated with visually and quantitatively detected preclinical pulmonary fibrosis. Thorax 2019;74(12):1131–39. doi: 10.1136/thoraxjnl-2018-212430 [published Online First: 2019/09/29] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ash SY, Harmouche R, Putman RK, et al. Clinical and Genetic Associations of Objectively Identified Interstitial Changes in Smokers. Chest 2017;152(4):780–91. doi: 10.1016/j.chest.2017.04.185 [published Online First: 2017/05/17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kliment CR, Araki T, Doyle TJ, et al. A comparison of visual and quantitative methods to identify interstitial lung abnormalities. BMC Pulm Med 2015;15:134. doi: 10.1186/s12890-015-0124-x [published Online First: 2015/10/31] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manichaikul A, Wang XQ, Sun L, et al. Genome-wide association study of subclinical interstitial lung disease in MESA. Respir Res 2017;18(1):97. doi: 10.1186/s12931-017-0581-2 [published Online First: 2017/05/20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buendia-Roldan I, Fernandez R, Mejia M, et al. Risk factors associated with the development of interstitial lung abnormalities. Eur Respir J 2021;58(2) doi: 10.1183/13993003.03005-2020 [published Online First: 2021/01/16] [DOI] [PubMed] [Google Scholar]
- 39.Adegunsoye A, Oldham JM, Bellam SK, et al. African-American race and mortality in interstitial lung disease: a multicentre propensity-matched analysis. Eur Respir J 2018;51(6) doi: 10.1183/13993003.00255-2018 [published Online First: 2018/05/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao X, Qiao D, Yang C, et al. Whole genome sequence analysis of pulmonary function and COPD in 19,996 multi-ethnic participants. Nat Commun 2020;11(1):5182. doi: 10.1038/s41467-020-18334-7 [published Online First: 2020/10/16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burkart KM, Sofer T, London SJ, et al. A Genome-Wide Association Study in Hispanics/Latinos Identifies Novel Signals for Lung Function. The Hispanic Community Health Study/Study of Latinos. Am J Respir Crit Care Med 2018;198(2):208–19. doi: 10.1164/rccm.201707-1493OC [published Online First: 2018/02/03] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang D, Povysil G, Newton CA, et al. Genome-wide Enrichment of TERT Rare Variants in IPF Patients of Latino Ancestry. Am J Respir Crit Care Med 2022. doi: 10.1164/rccm.202203-0622LE [published Online First: 2022/06/07] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newton CA, Oldham JM, Ley B, et al. Telomere length and genetic variant associations with interstitial lung disease progression and survival. Eur Respir J 2019;53(4) doi: 10.1183/13993003.01641-2018 [published Online First: 2019/01/13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salisbury ML, Hewlett JC, Ding G, et al. Development and Progression of Radiologic Abnormalities in Individuals at Risk for Familial Interstitial Lung Disease. Am J Respir Crit Care Med 2020;201(10):1230–39. doi: 10.1164/rccm.201909-1834OC [published Online First: 2020/02/06] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garrett-Bakelman FE, Darshi M, Green SJ, et al. The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science 2019;364(6436) doi: 10.1126/science.aau8650 [published Online First: 2019/04/13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stuart BD, Choi J, Zaidi S, et al. Exome sequencing links mutations in PARN and RTEL1 with familial pulmonary fibrosis and telomere shortening. Nat Genet 2015;47(5):512–7. doi: 10.1038/ng.3278 [published Online First: 2015/04/08] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Needham BL, Carroll JE, Diez Roux AV, et al. Neighborhood characteristics and leukocyte telomere length: the Multi-Ethnic Study of Atherosclerosis. Health Place 2014;28:167–72. doi: 10.1016/j.healthplace.2014.04.009 [published Online First: 2014/05/27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kiecolt-Glaser JK, Epel ES, Belury MA, et al. Omega-3 fatty acids, oxidative stress, and leukocyte telomere length: A randomized controlled trial. Brain Behav Immun 2013;28:16–24. doi: 10.1016/j.bbi.2012.09.004 [published Online First: 2012/09/27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cherkas LF, Hunkin JL, Kato BS, et al. The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med 2008;168(2):154–8. doi: 10.1001/archinternmed.2007.39 [published Online First: 2008/01/30] [DOI] [PubMed] [Google Scholar]
- 50.Astuti Y, Wardhana A, Watkins J, et al. Cigarette smoking and telomere length: A systematic review of 84 studies and meta-analysis. Environ Res 2017;158:480–89. doi: 10.1016/j.envres.2017.06.038 [published Online First: 2017/07/14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol 2014;9:157–79. doi: 10.1146/annurev-pathol-012513-104706 [published Online First: 2013/09/21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baumgartner KB, Samet JM, Stidley CA, et al. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1997;155(1):242–8. doi: 10.1164/ajrccm.155.1.9001319 [published Online First: 1997/01/01] [DOI] [PubMed] [Google Scholar]
- 53.Peljto AL, Zhang Y, Fingerlin TE, et al. Association between the MUC5B promoter polymorphism and survival in patients with idiopathic pulmonary fibrosis. JAMA 2013;309(21):2232–9. doi: 10.1001/jama.2013.5827 [published Online First: 2013/05/23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dudbridge F, Allen RJ, Sheehan NA, et al. Adjustment for index event bias in genome-wide association studies of subsequent events. Nat Commun 2019;10(1):1561. doi: 10.1038/s41467-019-09381-w [published Online First: 2019/04/07] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang C, Zhuang Y, Guo W, et al. Mucin 5B promoter polymorphism is associated with susceptibility to interstitial lung diseases in Chinese males. PLoS One 2014;9(8):e104919. doi: 10.1371/journal.pone.0104919 [published Online First: 2014/08/15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, et al. Trends and Patterns of Differences in Chronic Respiratory Disease Mortality Among US Counties, 1980–2014. JAMA 2017;318(12):1136–49. doi: 10.1001/jama.2017.11747 [published Online First: 2017/10/04] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


