Abstract
Purpose:
To identify the prevalence of drusen outside the macula and their role in progression of age-related macular degeneration (AMD).
Design:
Retrospective analysis of a prospective cohort study
Participants:
4168 eyes (2998 participants) with intermediate AMD in one or both eyes enrolled in the Age-Related Eye Disease Study 2 (AREDS2), a 5-year multicenter study of nutritional supplements were included.
Method:
Baseline 3 field 30-degree color photographs were evaluated for drusen characteristics outside the macular grid including size, area and location. The characteristics of extramacular drusen were compared to drusen within the macula.
Main Outcome Measures:
Progression rates to late AMD
Results:
Extramacular drusen were seen in 3624 (86.9% eyes) but represented a small area (< 0.5mm2) in 50.3% of eyes with only 17.5% having an area > 1 disc area (DA). Eyes with extramacular drusen had larger macular drusen size and larger macular drusen area compared to eyes without (p < 0.001). Extramacular drusen were not associated with progression to late AMD; hazard ratio adjusted for baseline age, gender, smoking, AMD severity level and reticular pseudodrusen for 4043 eyes at risk of developing late AMD over 5 years was 1.17 (95% confidence interval [CI], 0.88,1.54; P = 0.27) for geographic atrophy and 0.96 (95% CI, 0.76,1.2; P = 0.7) for neovascular AMD.
Conclusion:
Drusen outside the macula are commonly seen in eyes with AMD and are more frequent with increasing drusen burden within the macula. In eyes with intermediate AMD, extramacular drusen do not confer additional risk to previously identified risk factors in progression to late AMD.
Precis:
Eyes with intermediate AMD in the AREDS2 study have been well characterized for macular drusen. This analysis evaluated drusen outside the macula and found that they were frequently seen in AMD and were associated with increasing macular drusen area. Extramacular drusen provided no additional risk of progressing to late AMD over 5 years, after adjusting for known risk factors including AMD severity scale.
Introduction:
Age-related macular degeneration (AMD) is prevalent in 8.7% of worldwide population and is the leading cause of blindness in developed countries.1 Drusen are considered the hall mark of AMD and extracellular deposits in the sub-retinal pigment epithelial (RPE) space that are identifiable on both retinal examination and retinal imaging.2 The Wisconsin Age-Related Maculopathy Grading System (WARMGS) and the Age-Related Eye Disease Study (AREDS) standardized the classification of drusen within the macula based on color fundus photographs (CFP).3, 4 Drusen are classified based on size as small (<63 microns), medium (>63 <125 microns) and large (>125 microns). AMD is classified as early and intermediate based on drusen size; early AMD includes eyes with small or few medium drusen and intermediate AMD in eyes with extensive medium or at least one large druse. The Beckman Initiative identified small drusen as druplets and associated them with signs of aging.5 The 5-year risk of AMD progression ranges from 0.4% in normal aging up to 47.3% with bilateral, large drusen with pigmentary changes. 6
Drusen evaluation is restricted to the macula within the macular grid and most of the literature on natural history of drusen is based on studies using the macular grid.7, 8 Drusen outside the macula, aka extramacular drusen (EMD) have been documented but the significance of these drusen has not been firmly established.9 With the advent of ultrawidefield imaging, drusen have been seen in far periphery in eyes with and without AMD.10 These were cross sectional analysis and the significance of the peripheral drusen in progression of AMD remains unknown.
The Age-Related Eye Disease Study2 (AREDS2) study used three field imaging which allows evaluation of retina outside the macula.11 With carefully characterized macular features and a 5 year follow up on eyes with AMD, the AREDS2 study provides an opportunity to further our understanding on EMD and their role in progression of AMD.
Methods:
AREDS2 was a multicenter randomized clinical trial designed to study the effects of oral supplements on progression to advanced AMD.12 The study was conducted under institutional review board approval at each site and written informed consent was obtained from all study participants. The research was conducted under the Declaration of Helsinki and complied with the Health Insurance Portability and Accountability Act. Participants at high risk of developing late AMD due to either bilateral large drusen or late AMD in one eye and large drusen in the fellow eye were enrolled in the AREDS2 study. Development of central geographic atrophy(GA) or neovascular AMD was the primary AREDS2 study outcome.
Annual stereoscopic color photographs were obtained from all participants over the course of the study. This included a 30 degree stereo pair centered on the temporal edge of the optic nerve (field 1), centered on the macula (field 2) and about 1 disc diameter temporal to the macula ( field 3). Evaluation within the macula utilized previously published methods and included assessment of drusen size, area, pigment changes, geographic atrophy, neovascular AMD and the AREDS severity scale within the macular grid at all visits. 13 Presence of drusen outside the grid was documented at all study visits. For the purpose of this study, eyes with advanced AMD, both GA and neovascular AMD at baseline were excluded. Additional image evaluation was done at baseline specifically aimed at detailed drusen characteristics outside the macular grid using all three fields available (Figure 1). The images were not montaged but evaluated side by side accounting for duplication of retina across the fields. Standard disc to macula calibration was used for quantitative assessment outside the macula. 14
Figure 1:
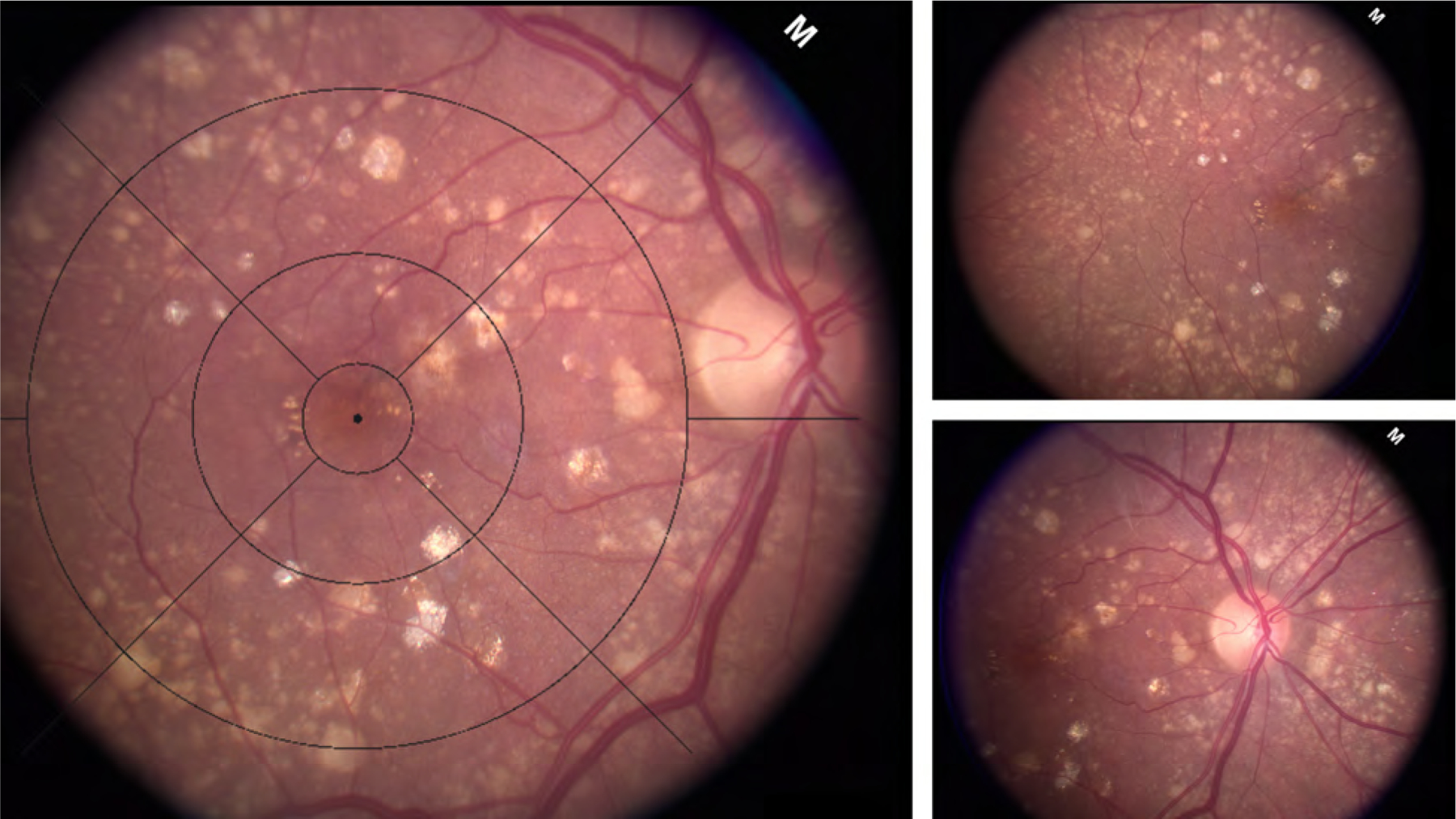
Field 2 macula centered fundus photograph with macular grid overlay. Extramacular drusen were assessed in the region outside the grid using temporal and nasal fields shown on the right.
The AREDS grading methodology guidelines were used to identify EMD.11 A grade of questionable was given if the graders were 50–90% certain that the abnormality was present and a grade of definite was given if the graders were ≥90% certain. A grade of cannot grade was given when the abnormality was not visible and <50% of the area being evaluated was not visible due to image quality issues.
Drusen like changes outside the macula were categorized as EMD continuous with macular drusen or separated from macular drusen. In addition, presence of only cuticular drusen or reticular pseudodrusen were documented and these eyes were not included in detailed drusen characterization. Eyes with reticular pseudodrusen or cuticular drusen in conjunction with drusen were included in the evaluation. In eyes with dual presentation, graders attempted their best to restrict detailed features to drusen only. EMD characterization included size, area, location, and pattern. Largest drusen size was documented as small (<63 um), medium (>63 um <125 um) or large (≥125 um). Presence of > 5 small drusen was required to be categorized as EMD. Drusen pattern (consolidated vs discrete) was documented in each quadrant – superior, nasal, inferior and temporal. Drusen area was categorized based on drusen circles as < O2(< 0.5mm2), < 1 DA (< 2.54 mm2), ≥ 1 DA and ≥ 3 DA ( > 7.62mm2).11 Presence of calcification and pigment changes was noted.
A team of 4 graders certified at Wisconsin Reading Center participated in the study and evaluated the baseline images independently. Presence of EMD was evaluated by two graders and disagreements were adjudicated by a senior grader (AD/JP). Subsequently, detailed EMD characteristics were evaluated by a single grader. Graders were masked to all patient demographics and also to follow up images. Intergrader agreement for presence of EMD was 89% ( kappa 0.73)
Statistical analysis:
Demographics at the primary AREDS2 baseline visit were tabulated for participants with and without EMD. Ocular findings at the AREDS2 baseline visit were tabulated for eyes with and without EMD. The difference between the two groups was analyzed using Chi square test. Fisher’s exact test was used for small numbers. Risk factors for progression to late AMD over 5 years were analyzed using multivariate proportional hazards model with repeated measures. These included age, gender, smoking status, AMD severity scale, presence of reticular pseudodrusen and EMD. Adjustment for correlation between eyes was made by using the robust sandwich estimate for the covariance matrix in the Wald tests.15 All analysis were performed using the SAS system.
Results:
Of the 4168 eyes (2998 participants) evaluated, EMD were seen in 3624 (86.9%) of eyes. Cuticular drusen and reticular pseudodrusen in the absence of EMD were in 38 (0.9%) and 77 (1.8%) respectively. Of the eyes with EMD, 209 (5.7%) had coexisting reticular pseudo drusen. The baseline demographics in eyes with and without EMD is shown in table 1. The mean age of participants with and without EMD was 72.3(SD 8.0) and 72.7 (8.0) respectively. There was no difference in age, gender, smoking status, diabetes, hypertension, angina, statin use and AREDS randomization arm between participants with and without EMD ( all p values > 0.05).
Table 1:
Baseline demographics of eyes with and without extramacular drusen
| Total | Absent* | Present | |
|---|---|---|---|
| N (Col%) | N (Col%) | N (Col%) | |
| Total | 4168 (100%) | 544 (100%) | 3624 (100%) |
| Age (mean SD) | 72.4 (8.0) | 72.7(8) | 72.3(8) |
| Sex | |||
| Female | 2540 (60.9%) | 298 (54.8%) | 2242 (61.9%) |
| Male | 1628 (39.1%) | 246 (45.2%) | 1382 (38.1%) |
| Smoking | |||
| Never | 1948 (46.7%) | 221 (40.6%) | 1727 (47.7%) |
| Former | 1976 (47.4%) | 291 (53.5%) | 1685 (46.4%) |
| Current | 244 (5.9%) | 32 (5.9%) | 212 (5.8%) |
| History of diabetes | |||
| No | 3694 (88.6%) | 456 (83.8%) | 3238 (89.3%) |
| Yes | 474 (11.4%) | 88 (16.2%) | 386 (10.7%) |
| History of hypertension | |||
| Unknown | 11 (0.3%) | 0 (0%) | 11 (0.3%) |
| No | 1847 (44.3%) | 220 (40.4%) | 1627 (44.9%) |
| Yes | 2310 (55.4%) | 324 (59.6%) | 1986 (54.8%) |
| History of angina | |||
| Unknown | 20 (0.5%) | 3 (0.6%) | 17 (0.5%) |
| No | 3976 (95.4%) | 510 (93.8%) | 3466 (95.6%) |
| Yes | 172 (4.1%) | 31 (5.7%) | 141 (3.9%) |
| Statin use | |||
| No | 2431 (58.3%) | 297 (54.6%) | 2134 (58.9%) |
| Yes | 1737 (41.7%) | 247 (45.4%) | 1490 (41.1%) |
| AREDS2 treatment | |||
| Control | 1010 (24.2%) | 124 (22.8%) | 886 (24.4%) |
| Lutein/Zeaxanthin | 1028 (24.7%) | 126 (23.2%) | 902 (24.9%) |
| DHA/EPA | 1065 (25.6%) | 144 (26.5%) | 921 (25.4%) |
| Combo | 1065 (25.6%) | 150 (27.6%) | 915 (25.2%) |
| Baseline Visual Acuity | |||
| <20/200 | 32 (0.8%) | 4(0.7%) | 28(0.8%) |
| <20/40–20/200 | 394 (9.5%) | 59(10.8%) | 335(9.2%) |
| <20/20–20/40 | 2104 (50.5%) | 306(56.3%) | 1798(49.6%) |
| 20/20+ | 1630 (39.1%) | 174(32.0%) | 1456(40.2%) |
| Missing | 8(0.2%) | 1(0.2%) | 7(0.2%) |
No significant different in all variables between eyes with and without EMD (p > 0.05)
Absent includes eyes with no drusen and with reticular pseudodrusen only and cuticular drusen only. AREDS2 = Age-related Eye Disease Study 2; DHA/EPA= docosahexaenoic acid /eicosapentaenoic acid
Table 2 shows the characteristics of EMD. Of the 3624 eyes with EMD, 727 (20%) were distinctly separated from macular drusen by at least 500 microns. The remaining represented a continuum of macular drusen extending beyond the grid. Detailed characterization of EMD showed large drusen in 1247 (29.9%), medium in 1448 (34.7%) and small in 913 (21.9%). The drusen were most commonly seen temporally (87.0%) and least common inferiorly (50.9%). Drusen were mostly discrete in all quadrants (60.5%) with only 1.3% consolidated (2 or more druse merged). Drusen area was small (< 0.5mm2) in 50.3% of eyes with only 14.5% having an area ≥ 1 DA. Pigment changes were seen in only 3.6% of eyes in extramacular region.
Table 2:
Characteristics of Extramacular Drusen
| Features of Extramacular Drusen | N (Col%) |
|---|---|
| Total | 4168 (100.0%) |
| Extramacular drusen | |
| Absent/Questionable | 429 (10.3%) |
| Definite, continuous with macular drusen | 2897 (69.5%) |
| Definite, separated from macular drusen | 727 (17.4%) |
| Reticular pseudodrusen only | 77 (1.8%) |
| Cuticular drusen only | 38 (0.9%) |
| Maximum extramacular drusen size | |
| Large | 1247 (34.4%) |
| Medium | 1448 (39.9%) |
| Small | 913 (25.1%) |
| Pattern of Extramacular Drusen in Superior Quadrant | |
| Absent/Questionable* | 892 (21.45) |
| Definite mixed | 36 (0.9%) |
| Definite, predominantly consolidated | 60 (1.4%) |
| Definite, predominantly discrete | 2671 (64.1%) |
| Missing | 509 (12.2%) |
| Pattern of Extramacular Drusen in Nasal Quadrant | |
| Absent/Questionable* | 1023 (24.5%) |
| Definite mixed | 22 (0.5%) |
| Definite, predominantly consolidated | 39 (0.9%) |
| Definite, predominantly discrete | 2598 (62.3%) |
| Missing | 486 (11.6%) |
| Pattern of Extramacular Drusen in Inferior Quadrant | |
| Absent/Questionable* | 1881 (40.0%) |
| Definite mixed | 11 (0.3%) |
| Definite, predominantly consolidated | 29 (0.7%) |
| Definite, predominantly discrete | 1807 (43.4%) |
| Missing | 656 (15.7%) |
| Pattern of Extramacular Drusen in Temporal Quadrant | |
| Absent/Questionable* | 544 (13.0%) |
| Definite mixed | 71(1.7%) |
| Definite, predominantly consolidated | 81 (1.9% |
| Definite, predominantly discrete | 3001 (72.0%) |
| Missing | 471 (11.3%) |
| Extramacular Drusen Area | |
| < O2 (<0.5mm2) | 2097 (50.3%) |
| < 1 DA (<2.54mm2 ) | 900 (21.6%) |
| >= 1 DA, < 3DA (2.54 – 7.62mm2) | 500 (12.0%) |
| >= 3DA (7.62mm2) | 105 (2.5%) |
| Missing | 566 (13.6%) |
| Pigmentary changes in Extramacular Region | |
| Absent/Questionable** | 3455 (82.9%) |
| Both hyper and hypo | 19 (0.5%) |
| Hyperpigmentation | 104 (2.5%) |
| Hypopigmentation | 23 (0.6%) |
| Missing | 567 (13.6%) |
| Baseline VA | |
| <20/200 | 32 (0.8%) |
| <20/40–20/200 | 394 (9.5%) |
| <20/20–20/40 | 2104 (50.5%) |
| 20/20+ | 1630 (39.1%) |
| Missing | 8 (0.2%) |
Missing includes eyes without extramacular drusen and ungradable eyes.
Absent/questionable indicates EMD present in the eye but not in the specific subfield
Absent/questionable indicates EMD present but no pigmentary changes DA = Disc Area
Table 3 shows the distribution of ocular characteristics within the macular grid in eyes with and without EMD. There was a significant difference in drusen characteristics within the macula between the two groups (p < 0.0001) with larger drusen size (> 250 microns) and larger macular drusen area (≥ 1 DA) in eyes with EMD. Baseline AREDS severity level was higher in eyes with EMD; with 59.2% of eyes with EMD having AREDS severity level 7–8 compared to 47.8% without EMD. (p < 0.0001). The mean visual acuity in eyes with and without EMD was similar at 79.5 letters (SD 10.6) and 78.4 (SD 9.9).
Table 3:
Macular characteristics of eyes with and without extramacular drusen
| Features within the macula | Extramacular drusen | P value | |
|---|---|---|---|
| Absent | Present | ||
| N (Col%) | N (Col%) | ||
| Total | 544 (100.0%) | 3624 (100.0%) | |
| Max drusen size with macular grid | 3 (0.6%) | 1 (0.0%) | |
| Absent/Questionable | P < 0.0001 | ||
| Definite, <63 um | 1 (0.2%) | 14 (0.4%) | |
| Definite, <125 um | 23 (4.2%) | 117 (3.2%) | |
| Definite, <250 um | 283 (52.0%) | 1538 (42.4%) | |
| Definite, >=250 um | 234 (43.0%) | 1954 (53.9%) | |
| Increased pigment within macular grid | |||
| Absent/Questionable | 188 (34.5%) | 1178 (32.5%) | P=0.64 |
| Definite, <C2 (0.07mm2) | 210 (38.6%) | 1445 (39.9%) | |
| Definite, >=C2 | 146 (26.8%) | 1001 (27.6%) | |
| Decreased pigment within macular grid | |||
| Absent/Questionable | 407 (74.8%) | 2632 (72.6%) | P=0.19 |
| Definite, <I2 (0.15mm2) | 48 (8.8%) | 380 (10.5%) | |
| Definite, <1/2 DA (1.27mm2) | 72 (13.2%) | 440 (12.1%) | |
| Definite, >=1/2 DA | 17 (3.1%) | 172 (4.7%) | |
| Drusen area within center subfield | |||
| Absent/Questionable | 30 (5.5%) | 198 (5.5%) | P = 0.09 |
| Definite, <C1 (0.02mm2) | 22 (4.0%) | 158 (4.4%) | |
| Definite, <C2 (0.07mm2) | 65 (11.9%) | 485 (13.4%) | |
| Definite, <I2 (0.15mm2) | 105 (19.3%) | 651 (18.0%) | |
| Definite, <O2 (0.50mm2) | 218 (40.1%) | 1263 (34.9%) | |
| Definite, <1/2 DA (1.27mm2) | 104 (19.1%) | 867 (23.9%) | |
| Cannot grade | 0 (0.0%) | 2 (0.1%) | |
| Drusen area within macular grid | |||
| Absent, Questionable, | 4 (0.7%) | 9 (0.2%) | P<0.0001 |
| Definite, <C1 (0.02mm2) | 2 (0.4%) | 5 (0.1%) | |
| Definite, <C2 (0.07mm2) | 9 (1.7%) | 47 (1.3%) | |
| Definite, <I2 (0.15mm2) | 20 (3.7%) | 85 (2.3%) | |
| Definite, <O2 (0.50mm2) | 65 (11.9%) | 281 (7.8%) | |
| Definite, <1/2 DA (1.27mm2) | 120 (22.1%) | 543 (15.0%) | |
| Definite, <1 DA (2.54mm2) | 126 (23.2%) | 900 (24.8%) | |
| Definite, >=1 DA | 198 (36.4%) | 1754 (48.4%) | |
| Baseline AREDS severity scale | |||
| 1 – 5 | 123 (22.6%) | 590 (16.3%) | P<0.0001 |
| 6 | 161 (29.6%) | 887 (24.5%) | |
| 7 | 206 (37.9%) | 1643 (45.3%) | |
| 8 | 54 (9.9%) | 504 (13.9%) | |
AREDS= Age-Related Eye Disease Study
Progression to late AMD (any GA or Neovascular AMD) over 5 years was seen in 1259 (35.8%) of eyes with EMD and 178 (34.1%) of eyes without EMD. Progression to GA, central GA and neovascular AMD in eyes with and without EMD was 19.4% vs 17.0%, 9.7% vs 8.2% and 19.1% vs 19.9 % respectively. Of the 2908 participants included in the multivariate model, age, smoking status (current vs never), AMD severity scale (level 7,8 vs < 7) and presence of reticular pseudodrusen were the only variables associated with progression to late AMD over 5 years (Figure 2). The hazard ratio adjusted for baseline age, gender, smoking, AMD severity level and reticular pseudodrusen for 4043 eyes (2908 participants) at risk of developing late AMD over 5 years was 1.06 (95% CI 0.88,1.27; p = 0.53) for late AMD; 1.17 (95% confidence interval [CI], 0.88,1.54; P = 0.27) for geographic atrophy and 0.96 (95% CI, 0.76,1.2; P = 0.7) for neovascular AMD.
Figure 2:
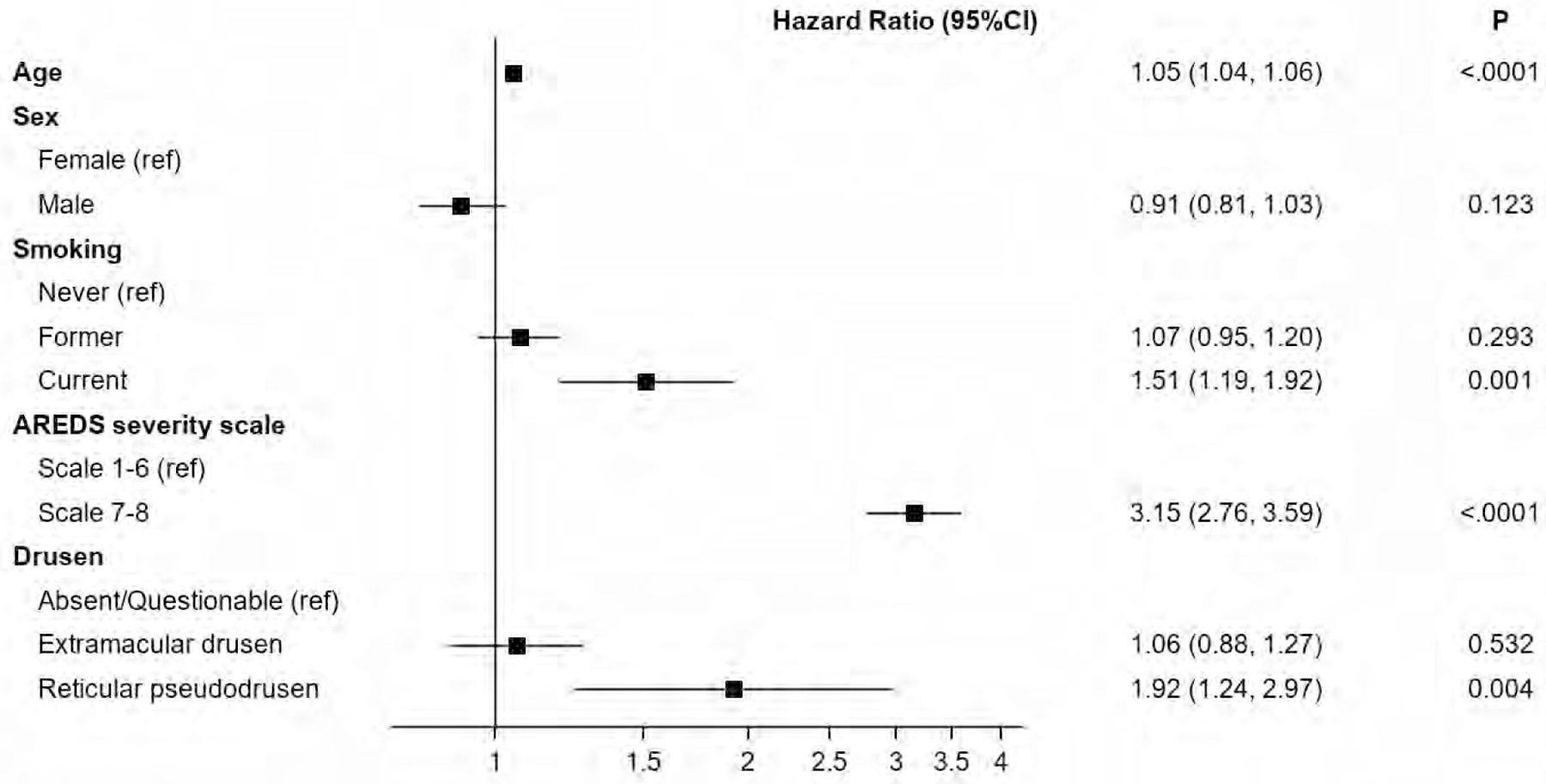
Forest plot showing the hazard ratio and 95% confidence limits of the association between known age-related macular degeneration (AMD) risk factors and progression to late AMD. Extramacular drusen were not associated with risk of progression to late AMD. Age-Related Eye Disease Study (AREDS)
Discussion:
Extramacular drusen are a common finding in AMD, seen in 87% of eyes with intermediate AMD in the AREDS2 cohort. Although frequent, they occupy a smaller area (58% < 0.2DA) relative to drusen within the macula (71.4% > 0.5DA). Prevalence of EMD increases with drusen burden within the macula. Reticular pseudodrusen are an AMD feature involving the subretinal layer, seen mostly in the extramacular space and independently contribute to risk of progression of AMD.16 Despite a shared location around vascular arcades, EMD do not share the same risk profile. After adjusting for baseline age, gender, smoking, AREDS severity level and reticular pseudodrusen for 2908 eyes at risk of developing late AMD over 5 years, EMD did not confer additional risk of progression to late AMD, both geographic atrophy and neovascular AMD.
The Wisconsin Age Related Macular Degeneration System restricts drusen evaluation to the macular grid using 30-degree retinal images. The procedure was developed on film images and provides a way to standardize the region of interest for quantification and to better understand the pattern of disease.17 Drusen size, location and area within macular grid are important variables in determining the AREDS severity scale, which is prognostic of late AMD. 4, 18 Although drusen outside the grid were documented in the AREDS study, they were not included in the AREDS severity scale. The macular grid is an artificial boundary separating retinal features within and outside the grid. It is, however, an important boundary considering the weight given to drusen area within the grid as a prognostic feature for late AMD development. This study explores moving the boundary further out and incorporating all drusen visible within the imaging field of view in risk modeling.
Evaluation of drusen patterns within the macular grid in the Beaver Dam study showed that drusen were predominantly seen in the central subfield, followed by the superior and temporal quadrants of the macular grid.17 Our study showed a similar pattern with EMD located temporally (87%) and least prevalent in the inferior retina (51%). This is expected considering EMD are an extension of the drusen within the macula. However, it is important to note that the 3 field imaging protocol includesa larger nasal and temporal area compared to superior and inferior regions and could have contributed to these results. Nearly 80% of EMD in this study were a continuation of macular drusen, indicating drusen mostly start within the macula and extend outwards. In 20% of eyes, there was a separation between clusters of drusen within the grid and outside the grid.
Histopathological studies have shown a difference in consistency between drusen within and outside the macula. 19, 20 Rudolf et al found that the periphery contained mostly hard drusen like structures that were resilient and compound drusen that had a composition between hard and soft.19 In contrast, soft drusen were exclusively seen in the macula and were fragile. The authors propose that the fragility of these central drusen contribute to the macular location of AMD. We evaluated drusen beyond 3600 microns from center of macula and found soft drusen of medium and large size. It remains to be explored at which part of the retina the drusen morphology changes from soft to a relatively harder composition, thus reducing the risk of AMD.
Ultrawide field imaging is the preferred modality to identify drusen outside the macula. A meta-analysis of 12 studies with ultrawide field imaging found peripheral lesions including drusen in 82.7% of eyes with AMD.21 Due to the varying amount of retina visible outside the macula and varying grids used in ultra-widefield imaging, definition of periphery was not comparable across studies. 22 The Age-Related Eye Disease Study 2 (AREDS2) conducted an ancillary study using ultrawide field imaging (Optos, Nikon Co. Japan) in 951 eyes (484 participants) at year 5 of the study. Using a three zone grid, the image was divided into zone 1, corresponding to the posterior pole, zone 2 in the midperiphery extending to the vortex veins and zone 3 in the far periphery. Drusen were seen in 98% of eyes in zone 1, 97% in zone 2 and 78% in zone 3. 10 Longitudinal data from ultrawide field images is limited and role of midperipheral and peripheral drusen in AMD progression is unclear.23 It remains to be seen if the current study results of lack of risk hold true for peripheral drusen also.
Peripheral drusen are known to occur in eyes without AMD; however, the prevalence is higher in eyes with AMD. The meta-analysis discussed above showed peripheral drusen in 33.3% of healthy eyes.21 The AREDS2 OPERA study enrolled controls with no drusen in macula and found 48% had drusen in midperiphery and 21% in far periphery using ultrawide field imaging.10 Seddon et al evaluated drusen at the equatorial region using 7 field color photography in 2103 family members and twins and found peripheral drusen in 7% of eyes with no AMD and 53% of eyes with AMD. 24 Ersoy et al studied EMD from 40 degree color photographs, similar to the current study, in 622 subjects and found EMD in 28% of no AMD group, 81.5% of non-neovascular AMD and 62% of neovascular AMD.25 They concluded that EMD close to the macular grid in eyes with no evidence of AMD can be considered healthy controls. The current study supports these findings with the addition of longitudinal data. More recently, the AREDS2 study reviewed the 10 year risk of advanced AMD in eyes with drusen and no pigmentary abnormalities.26 The 10 year event rate of advanced AMD was 21.7% – 37.6% in eyes with large drusen in the central and inner subfields of the macular grid vs 3.6% for in eyes with large drusen in outer subfield only. The risk of advanced AMD appears to reduce as distance of drusen from central macula increases.
Detailed categorization of drusen using stringent criteria in a large well characterized longitudinal population with a 5-year followup is the biggest strength of this study. The results clearly shows that drusen surrounding the macula do not contribute to AMD risk. The study is limited by extramacular region as defined by standard 30-degree imaging. Availability of 3 field imaging helps expand temporal to the macula but not further out. The AREDS population has intermediate AMD by inclusion criteria and is already at a high risk of progression. Macular drusen area > 0.5DA has the highest risk category in the AREDS severity scale and 70% of eyes in the study have baseline drusen area > 0.5DA. In such eyes it is unlikely that including EMD drusen into drusen area will change the risk category. It is possible that including the EMD in drusen area measurements in eyes with early AMD may have a different impact. Evaluating EMD in follow up images in future studies will help understand their natural history.
To conclude, in eyes with intermediate AMD, EMD are commonly seen and increase with drusen load within the macula. EMD are not an independent risk factor and do not provide added risk to previously identified risk factors for progression to late AMD, both geographic atrophy and neovascular AMD. The role of drusen in midperiphery and peripheral retina remains to be studied.
Financial Support:
This research was supported by the Intramural Research Program of the National Eye Institute and the National Library of Medicine, National Institutes of Health, Department of Health and Human Services, Bethesda, Maryland. It was also supported by contracts from the National Eye Institute (contract NOI-EY-0-2127 for AREDS and contracts HHS-N-260-2005-00007-C and ADB contract N01-EY-5-0007 for AREDS2). Funds were generously contributed to these contracts by the following NIH institutes: Office of Dietary Supplements; National Center for Complementary and Alternative Medicine; National Institute on Aging; National Heart, Lung, and Blood Institute; National Institute of Neurological Disorders and Stroke. The sponsor and funding organization participated in the design and conduct of the study, data collection, management, analysis, and interpretation, and preparation, review and approval of the manuscript.AD, BX and JWP were supported in part by an unrestricted grant from Research to Prevent Blindness, Inc. to the UW Madison Department of Ophthalmology and Visual Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at AAO Nov 2021
Conflicts of Interest:
AD, BX, JWP, EA, TEC, EYC: none
Fredrick L Ferris III: Bausch and Lomb, ,, , Genentech, , , Apellis, , , Novo Nordisk, Roche, Kodiak, 4D Molecular Therapeutics, Adverum, Annoxon
References
- 1.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014;2(2):e106–16. [DOI] [PubMed] [Google Scholar]
- 2.Sarks JP, Sarks SH, Killingsworth MC. Evolution of soft drusen in age-related macular degeneration. Eye (Lond) 1994;8 ( Pt 3):269–83. [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Davis MD, Magli YL, et al. The Wisconsin age-related maculopathy grading system. Ophthalmology 1991;98(7):1128–34. [DOI] [PubMed] [Google Scholar]
- 4.The Age-Related Eye Disease Study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: the Age-Related Eye Disease Study Report Number 6. Am J Ophthalmol 2001;132(5):668–81. [DOI] [PubMed] [Google Scholar]
- 5.Ferris FL 3rd Wilkinson CP, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology 2013;120(4):844–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferris FL, Davis MD, Clemons TE, et al. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch Ophthalmol 2005;123(11):1570–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarks SH. Drusen patterns predisposing to geographic atrophy of the retinal pigment epithelium. Aust J Ophthalmol 1982;10(2):91–7. [DOI] [PubMed] [Google Scholar]
- 8.Abdelsalam A, Del Priore L, Zarbin MA. Drusen in age-related macular degeneration: pathogenesis, natural course, and laser photocoagulation-induced regression. Surv Ophthalmol 1999;44(1):1–29. [DOI] [PubMed] [Google Scholar]
- 9.Seddon JM, Reynolds R, Rosner B. Peripheral retinal drusen and reticular pigment: association with CFHY402H and CFHrs1410996 genotypes in family and twin studies. Invest Ophthalmol Vis Sci 2009;50(2):586–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domalpally A, Clemons TE, Danis RP, et al. Peripheral Retinal Changes Associated with Age-Related Macular Degeneration in the Age-Related Eye Disease Study 2: Age-Related Eye Disease Study 2 Report Number 12 by the Age-Related Eye Disease Study 2 Optos PEripheral RetinA (OPERA) Study Research Group. Ophthalmology 2017;124(4):479–87. [DOI] [PubMed] [Google Scholar]
- 11.Danis RP, Domalpally A, Chew EY, et al. Methods and reproducibility of grading optimized digital color fundus photographs in the Age-Related Eye Disease Study 2 (AREDS2 Report Number 2). Invest Ophthalmol Vis Sci 2013;54(7):4548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chew EY, Clemons T, SanGiovanni JP, et al. The Age-related Eye Disease Study 2 (AREDS2) Study Design and Baseline Characteristics (AREDS2 Report Number 1). Ophthalmology 2012;119(11):2282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danis RP, Domalpally A, Chew EY, et al. Methods and Reproducibility of Grading Optimized Digital Color Fundus Photographs in the Age-Related Eye Disease Study 2 (AREDS2 Report Number 2). Investigative Ophthalmology & Visual Science 2013;54(7):4548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lujan Brandon J, Gregori G, Wang F, et al. Calibration of Fundus Images Using Spectral Domain Optical Coherence Tomography. Ophthalmic Surgery, Lasers and Imaging Retina;39(4). [DOI] [PubMed] [Google Scholar]
- 15.Wei L-J, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. Journal of the American statistical association 1989;84(408):1065–73. [Google Scholar]
- 16.Domalpally A, Agrón E, Pak JW, et al. Prevalence, Risk, and Genetic Association of Reticular Pseudodrusen in Age-related Macular Degeneration: Age-Related Eye Disease Study 2 Report 21. Ophthalmology 2019;126(12):1659–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Q, Chappell RJ, Klein R, et al. Pattern of age-related maculopathy in the macular area. The Beaver Dam Eye Study. Invest Ophthalmol Vis Sci 1996;37(11):2234–42. [PubMed] [Google Scholar]
- 18.Davis MD, Gangnon RE, Lee LY, et al. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol 2005;123(11):1484–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudolf M, Clark ME, Chimento MF, et al. Prevalence and Morphology of Druse Types in the Macula and Periphery of Eyes with Age-Related Maculopathy. Investigative Ophthalmology & Visual Science 2008;49(3):1200–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis H, Straatsma BR, Foos RY. Chorioretinal juncture. Multiple extramacular drusen. Ophthalmology 1986;93(8):1098–112. [DOI] [PubMed] [Google Scholar]
- 21.Forshaw TRJ, Minör ÅS, Subhi Y, Sørensen TL. Peripheral Retinal Lesions in Eyes with Age-Related Macular Degeneration Using Ultra-Widefield Imaging: A Systematic Review with Meta-analyses. Ophthalmol Retina 2019;3(9):734–43. [DOI] [PubMed] [Google Scholar]
- 22.Pivovar A, Oellers P. Peripheral Manifestations in Age Related Macular Degeneration: A Review of Imaging and Findings. Journal of clinical medicine 2021;10(17):3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malley CE, Agron E, Keenan TDL, et al. Progression of Age-Related Macular Degeneration measured by Ultrawidefield Imaging in the Age-Related Eye Disease Study 2 10 Year Follow-On. Investigative Ophthalmology & Visual Science 2021;62(8):215-. [Google Scholar]
- 24.Seddon JM, Reynolds R, Rosner B. Peripheral retinal drusen and reticular pigment: association with CFHY402H and CFHrs1410996 genotypes in family and twin studies. Investigative ophthalmology & visual science 2009;50(2):586–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ersoy L, Schick T, de Graft D, et al. Extramacular drusen are highly associated with age-related macular degeneration, but not with CFH and ARMS2 genotypes. Br J Ophthalmol 2016;100(8):1047–51. [DOI] [PubMed] [Google Scholar]
- 26.Chew EY, Clemons TE, Agrón E, et al. Ten-Year Follow-up of Age-Related Macular Degeneration in the Age-Related Eye Disease Study: AREDS Report No. 36. JAMA Ophthalmology 2014;132(3):272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


