Abstract
To identify autonomic neuropathy (AN) phenotypes, we used principal component analysis on data from participants (N=209) who underwent standardized autonomic testing including quantitative sudomotor axon reflex testing, and heart rate and blood pressure at rest and during tilt, Valsalva, and standardized deep breathing. The analysis identified seven clusters: 1) normal, 2) hyperadrenergic features without AN, 3) mild AN with hyperadrenergic features, 4) moderate AN, 5) mild AN with hypoadrenergic features, 6) borderline AN with hypoadrenergic features, 7) mild balanced deficits across parasympathetic, sympathetic and sudomotor domains. These findings demonstrate a complex relationship between adrenergic and other aspects of autonomic function.
Keywords: principal component analysis (PCA), autonomic neuropathy, autonomic function tests, physiology, phenotyping
Introduction
The peripheral autonomic nervous system can be divided functionally into afferent (i.e. sensory), parasympathetic and sympathetic branches, with each branch being comprised of distinct groups of nerve fibers. Autonomic neuropathy (AN) is a common neurologic disorder that typically occurs in the context of systemic diseases which impact peripheral nerve dysfunction more generally, e.g. diabetes or HIV.1,2 Peripheral neuropathy affecting somatic sensory and motor function has a well-described typical presentation, i.e. a distal distribution of sensory predominant symptoms including neuropathic pain and paresthesias. Owing to the length dependency of the neuropathy, with time signs and symptoms may progress to involve more proximal parts of the lower limbs as well as the hands.
In contrast, the anatomic distribution of AN and its progression over time cannot be established based on symptoms and neurologic examination findings because these are less clearly localizable than their somatic counterparts. Thus, alternative methods must be used to establish phenotypes of AN that might correspond to different stages of disease progression. The most widely used methods for doing so involve scoring systems based on the results of batteries of autonomic function tests, for example the Composite Autonomic Scoring Scale (CASS) which produces a score from 0–10 where zero is normal and ten is most severe, and includes subscores for cardiovagal, adrenergic and sudomotor hypofunction.3 Although very valuable, such scoring systems have not been used previously to develop descriptive phenotypes. They also fail to capture the hyperadrenergic features which may be observed during autonomic testing such as tachycardia and increased blood pressure (BP) with upright tilt. These hyperadrenergic features have largely been described in the context of the postural orthostatic tachycardia syndrome (POTS).4,5 However, they have also been proposed as a feature of early AN,6 given that sympathetic activity is normally reflexively modulated by the autonomic afferent system, and so if these autonomic afferents and sympathetic fibers are both partially impaired, a hyper- or hypoadrenergic state might result depending on the balance between the two. Hyperadrenergic states may be particularly important because of their association with cardiovascular diseases.7
Principal component analysis (PCA) is a technique to reduce dimensionality in complex datasets while preserving the information they contain. It has been used in multiple disease states as an unbiased technique to uncover phenotypes from multidimensional biomarker data, with the ultimate goal of using increasingly specific phenotypes as a springboard for personalized medicine.8 In this study we used PCA to analyze autonomic function testing data, seeking phenotypes that could suggest underlying differences in autonomic pathophysiology between groups of patients.
Methods
Participants and autonomic testing procedures.
This is a secondary analysis of autonomic testing data collected during two contemporaneous studies which have been previously described.1,9 The parent studies had identical procedures, but differed in recruitment location and in purpose. One study recruited patients from a primary care HIV clinic to document the prevalence of AN in people living with HIV.1 The second study recruited patients from a general neurology clinic with the purpose of examining the correlation of autonomic symptoms with objective abnormalities on autonomic testing.9 In both studies, participants were recruited from the clinic waiting rooms, thus the sample was likely enriched for AN, which is fairly common in its milder forms, but not for rarer and more severe forms of autonomic dysfunction which would have required a more purposive sampling strategy. All participants were seen for a single visit during which they underwent a standardized battery of autonomic function tests including quantitative sudomotor axon reflex testing (QSART), heart rate response to deep breathing (HRDB), Valsalva maneuver (VM), and head-up tilt table testing.3 All procedures were performed according to protocols approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai. All participants provided written informed consent.
Calculation of autonomic indices.
Autonomic indices were based on the data elements used to calculate the CASS.3 These included elements reflecting sympathetic (adrenergic and sudomotor) and parasympathetic (cardiovagal) function. To quantify adrenergic function, the CASS uses changes in blood pressure during VM and tilt which are summarized as an adrenergic index (scored 0–4). Sudomotor function is quantified by QSART, and cardiovagal function is quantified by HRDB and the Valsalva ratio (VR). HRDB is the difference between the maximum and minimum heart rate averaged over six consecutive deep breaths; VR is the ratio of the highest heart rate during VM to the lowest heart rate immediately following release of VM. To these we added three additional heart rate variability (HRV) parameters. The first was change in HR during tilt, which is the cardinal feature used to diagnose POTS.10 The other two were measures of resting HRV which were calculated based on the five minute time period immediately prior to the tilt table testing during which time the participant was resting supine. These were: the heart rate adjusted root mean square of successive differences between normal heartbeats (cvRMSSD),11 and the percentage of NN intervals that are different by more than 50ms (pNN50).12
Statistical methods.
The following variables were included in the PCA: HRDB, VR, cvRMSSD, pNN50, baseline systolic (SBP) and diastolic blood pressure (DBP) prior to tilt, minimum and maximum SBP and DBP during tilt, maximum change in SBP and DBP during tilt, baseline HR prior to tilt, maximum HR during tilt, maximum change in HR during tilt, minimum mean arterial pressure (MAP) during VM, minimum pulse pressure during VM, maximum change in MAP during VM, QSART volume from the forearm, proximal leg, distal leg and foot, CASS adrenergic subscore. CASS cardiovagal and sudomotor subscores were not included because they are derived directly from other included variables (HRDB, VR and QSART).
The principal components were then used to define clusters of participants. Using each point as a possible central point and k-means as a distance metric, the within sum of squares determined the max amount of variance explained at varying number of clusters. Similarly, the silhouette method was used to confirm the number of clusters in the data that explained the most variation. Lastly, the gap statistic was used to establish how closely related the clusters are.
Results
Participant characteristics.
Demographics of the sample have been described previously.13 Briefly, the sample had an average age of 48 years, even representation of men and women (50% each), and was representative of our patient population with regard to race and ethnicity (50% Black/African-American, 39% Hispanic/Latinx, 9% non-Hispanic white). Overall, 39% had evidence of peripheral neuropathy on neurologic examination, mostly attributable to HIV (54%) or diabetes (19%). No participants were known to have a neurodegenerative disorder associated with autonomic dysfunction (e.g. Parkinson’s disease, multiple system atrophy).
Results of PCA.
The PCA identified two principal components (see figure) which together explained 36% of the variance. Principal component #1 (PC1) explained 22% of the variance and was most strongly influenced by the absolute BP parameters (as opposed to changes in BP) recorded during tilt and to a slightly lesser extent by the change in MAP and pulse pressure during VM. The directionality of this influence was negative, such that participants with higher values of PC1 tended to have lower BPs.
Figure.
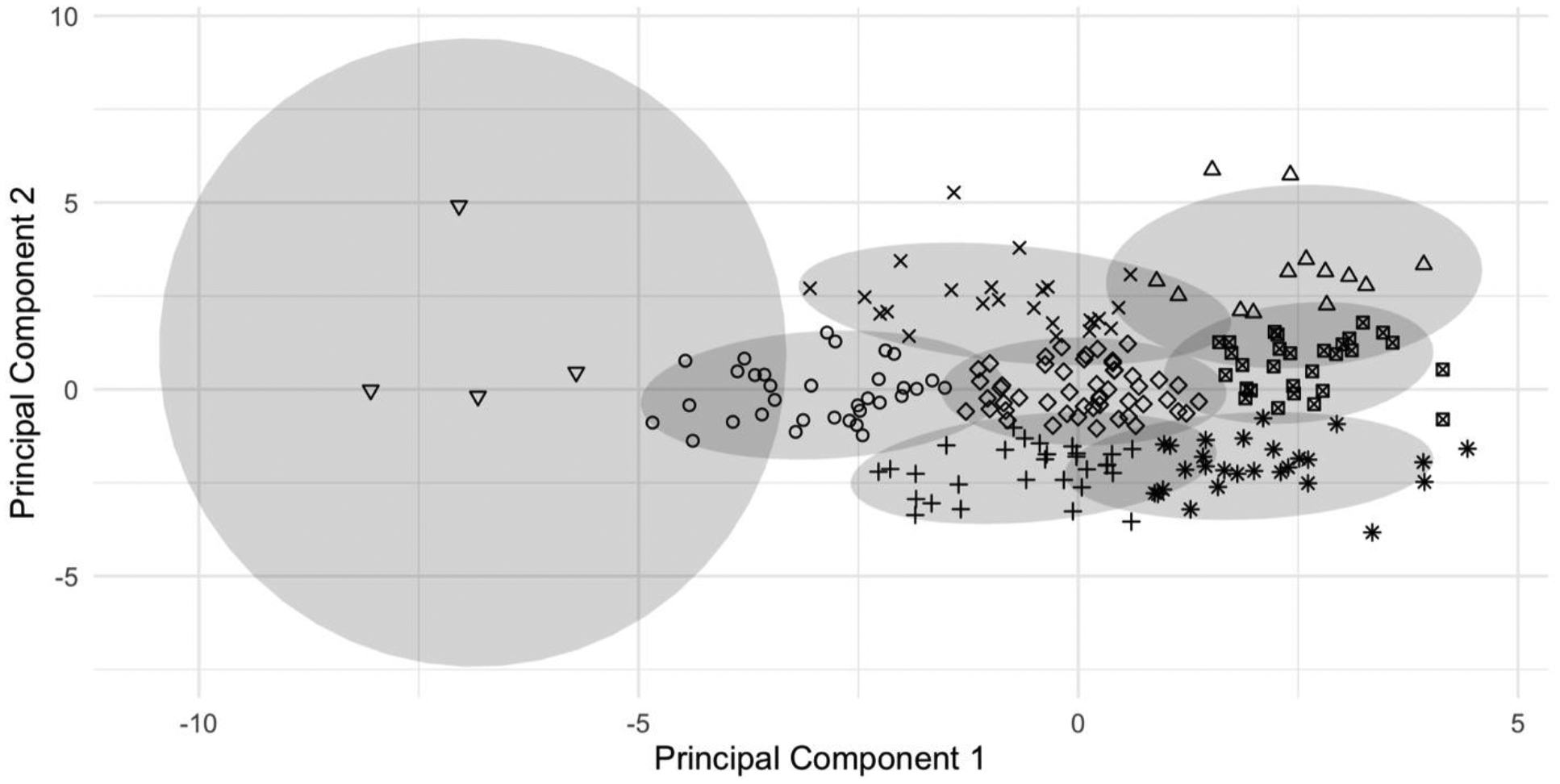
Clusters identified by the principal components analysis. Each individual participant is indicated by a symbol determined by their cluster.
Principal component #2 (PC2) explained 14% of the variance and was most strongly positively influenced by all four measures of HRV (resting and reflexive), and all four QSART measures. PC2 was also strongly negatively influenced by HR parameters during tilt (baseline and max HR) as well as the CASS adrenergic subscore and the drop in SBP during VM. Thus, participants with higher values of PC2 tended to have higher (i.e., more normal) HRV and QSART values, lower HRs during tilt, and were less likely to exhibit hypoadrenergic features such as an exaggerated BP drop during VM.
Characteristics of the clusters.
Eight clusters were initially identified; cluster features are detailed in the table. The clusters were numbered beginning with the upper right quadrant of the graph and proceeding counterclockwise finishing with the centermost cluster. The outlier cluster (inverted triangles at the far left of the figure) contained four participants for whom beat-to-beat BP readings were so high that they were suspected of being spurious. Thus, this cluster is not discussed further.
Table.
Summary of autonomic indices by cluster
| Clusters | 1 (N=13) | 2 (N=24) | 3 (N=33) | 4 (N=29) | 5 (N=27) | 6 (N=29) | 7 (N = 50) | Total (N=209) |
|---|---|---|---|---|---|---|---|---|
| Cluster symbol | Upright triangle | x | Circle | + | * | Box with x | Diamond | Inverted triangle |
| Age | 41.4 (12.8) | 43.8 (11.1) | 51.8 (8.6) | 51.1 (7.2) | 51.7 (9.8) | 42.1 (11.8) | 50.0 (12.1) | 48.4 (11.2) |
| Total CASS | 1.39 (1.39) | 1.46 (0.83) | 3.00 (1.16) | 4.35 (1.14) | 3.59 (1.15) | 2.21 (1.15) | 2.60 (1.31) | 2.77 (1.47) |
| Vagal parameters | ||||||||
| HRDB | 23.0 (10.0) | 24.7 (8.73) | 14.9 (10.1) | 11.6 (5.54) | 13.0 (6.44) | 21.9 (9.37) | 17.7 (8.18) | 17.35 (9.39) |
| Valsalva ratio | 1.904 (0.330) | 1.774 (0.262) | 1.565 (0.344) | 1.474 (0.251) | 1.551 (0.344) | 1.928 (0.424) | 1.689 (0.352) | 1.673 (0.368) |
| cv-RMSSD | 7.957 (9.538) | 5.989 (3.997) | 3.687 (4.052) | 2.322 (1.112) | 3.306 (3.682) | 5.465 (2.834) | 3.708 (2.024) | 4.245 (3.979) |
| pNN50 | 27.065 (18.939) | 21.628 (19.320) | 8.788 (13.982) | 2.153 (3.531) | 3.242 (5.395) | 24.535 (19.564) | 9.980 (11.275) | 12.424 (16.182) |
| Blood pressure values during tilt | ||||||||
| Clusters | 1 (N=13) | 2 (N=24) | 3 (N=33) | 4 (N=29) | 5 (N=27) | 6 (N=29) | 7 (N = 50) | Total (N=209) |
| Baseline SBP before tilt | 113.204 (8.574) | 135.931 (15.469) | 156.385 (13.569) | 133.282 (12.811) | 109.809 (11.804) | 109.243 (8.615) | 127.885 (11.196) | 129.303 (21.355) |
| Maximum SBP during tilt | 126.903 (9.634) | 155.491 (17.770) | 174.232 (13.926) | 150.707 (11.854) | 127.160 (17.408) | 123.503 (10.611) | 145.808 (14.882) | 146.629 (23.713) |
| Minimum SBP during tilt | 105.145 (7.080) | 122.628 (16.082) | 134.579 (17.788) | 115.157 (17.718) | 92.440 (14.394) | 94.403 (11.016) | 113.341 (13.695) | 112.790 (20.750) |
| Baseline DBP before tilt | 67.527 (7.162) | 82.509 (7.105) | 94.907 (9.993) | 80.151 (7.237) | 66.891 (7.937 | 66.497 (7.239) | 77.431 (8.162) | 78.460 (13.757) |
| Maximum DBP during tilt | 75.739 (6.776) | 99.292 (9.192) | 108.636 (12.094) | 96.070 (12.928) | 80.507 (11.655) | 80.656 (9.619) | 91.353 (9.460) | 92.864 (16.603) |
| Minimum DBP during tilt | 59.881 (5.844) | 73.360 (5.663) | 83.183 (8.780) | 70.228 (8.614) | 57.754 (7.938) | 57.350 (7.134) | 69.433 (9.225) | 68.967 (12.628) |
| Heart rate values during tilt | ||||||||
| Baseline HR | 60.238 (9.401) | 66.313 (8.400) | 71.448 (11.497) | 75.274 (10.303) | 77.127 (11.010) | 61.992 (8.852) | 65.929 (10.004) | 68.723 (11.264) |
| Maximum HR | 69.932 (10.859) | 74.058 (9.036) | 78.889 (11.568) | 81.507 (11.212) | 83.162 (11.928) | 72.105 (11.037) | 74.559 (10.025) | 76.613 (11.392) |
| Change in HR | 9.694 (5.883) | 7.744 (5.357) | 7.441 (4.801) | 6.233 (5.047) | 6.035 (4.566) | 10.114 (5.418) | 8.630 (4.166) | 7.890 (5.018) |
| Adrenergic parameters | ||||||||
| Clusters | 1 (N=13) | 2 (N=24) | 3 (N=33) | 4 (N=29) | 5 (N=27) | 6 (N=29) | 7 (N = 50) | Total (N=209) |
| Minimum MAP during VM | 86.154 (8.934) | 102.958 (11.816) | 106.758 (11.219) | 87.241 (10.422) | 75.185 (10.122) | 80.276 (7.387) | 93.920 (8.388) | 91.914 (15.180) |
| Minimum pulse pressure during VM | 30.923 (12.586) | 31.958 (8.595) | 25.364 (12.617) | 21.448 (6.179) | 16.593 (7.266) | 21.241 (12.483) | 25.220 (12.077) | 24.388 (11.653) |
| Change in MAP during VM | −2.385 (7.489) | 0.542 (10.253) | 7.030 (12.378) | 13.690 (8.848) | 10.222 (9.870) | 3.724 (8.263) | 3.800 (7.754) | 5.722 (10.362) |
| 2 | 0 (0%) | 0 (0%) | 2 (6%) | 3 (10%) | 2 (7%) | 0 (0%) | 0 (0%) | 7 (3%) |
| QSART parameters | ||||||||
| Forearm | 1.612 (0.894) | 1.219 (0.749) | 0.661 (0.644) | 0.297 (0.357) | 0.597 (0.672) | 0.798 (0.491) | 0.599 (0.598) | 0.727 (0.690) |
| Proximal leg | 1.085 (0.533) | 0.819 (0.526) | 0.360 (0.386) | 0.109 (0.139) | 0.207 (0.247) | 0.401 (0.331) | 0.316 (0.263) | 0.403 (0.437) |
| Distal leg | 1.876 (0.711) | 1.587 (0.740) | 0.758 (0.516) | 0.212 (0.257) | 0.385 (0.391) | 0.965 (0.572) | 0.698 (0.518) | 0.818 (0.713) |
| Foot | 1.095 (0.751) | 0.812 (0.609) | 0.373 (0.371) | 0.124 (0.182) | 0.196 (0.219) | 0.428 (0.328) | 0.367 (0.339) | 0.415 (0.462) |
Members of cluster 1 (upright triangles in the upper right of the figure) had the highest values for both PC1 and PC2 and had generally normal autonomic function with a mean CASS of 1.7 (SD=1.1). This was the youngest group with a mean age of 41 (SD=12.8) years as compared to a mean of 48 (SD=11.2) years for the overall sample. They generally had the highest (i.e. most normal) values for the cardiovagal and QSART parameters, and had normal HR and BP at rest and during the testing. Similarly, members of cluster 2 (indicated by “x” in the figure) also displayed normal autonomic testing results with a mean CASS of 1.4 (SD=1.4). The main feature distinguishing cluster 2 from cluster 1 was higher BP parameters, indicating a possible hyperadrenergic state. Also, although measures of reflexive HRV (i.e., VR and HRDB) were similar between clusters 1 and 2, measures of resting HRV (i.e., cv-RMSSD and pNN50) were lower in cluster 2. Clusters 1 and 2 also had the lowest percentage of participants meeting any hypoadrenergic criteria (based on the CASS adrenergic subscore).
Cluster 3 (indicated by circles) had a mean CASS consistent with mild AN (3.0, SD=1.6), with vagal and sudomotor parameters which were lower than clusters 1 and 2. Cluster 3 also had the highest BP parameters of all the groups. Cluster 4 (indicated by the “+” sign) displayed typical features of AN with relatively balanced hypofunction across the domains measured. The mean total CASS was the highest in this cluster (4.3, SD=1.1), and they also displayed the greatest deficits in sudomotor function and cardiovagal parameters. Moreover, all of the participants in this group had an abnormal CASS adrenergic subscore, indicating hypoadrenergic function. Cluster 5 (asterisks) also displayed a mean CASS which met criteria for AN (3.6, SD=1.2), albeit somewhat milder than in cluster 4. The main distinguishing feature of cluster 5 was that its members on average displayed the lowest BPs, and 89% had an abnormal CASS adrenergic subscore indicating hypoadrenergic function.
Clusters 6 (boxes with an “x” inside) and cluster 7 (diamonds) both displayed borderline autonomic dysfunction with mean CASS of 2.2 (SD=1.2) and 2.6 (SD=1.3) respectively. The main differences between these groups were that cluster 6 was younger and overall had slightly better autonomic function in terms of cardiovagal and QSART parameters. However, the proportion of participants in cluster 6 with an abnormal CASS adrenergic index was essentially the same as cluster 7, and cluster 6 also had significantly lower BPs, indicating a relative hypoadrenergic state for the overall degree of autonomic impairment.
Thus, the phenotypic features of the seven clusters can be summarized as follows: 1) normal autonomic function, 2) hyperadrenergic features without AN, 3) mild AN with hyperadrenergic features, 4) moderate AN, 5) mild AN with hypoadrenergic features, 6) borderline AN with relative hypoadrenergic features, 7) borderline AN with balanced deficits across parasympathetic, sympathetic/adrenergic and sudomotor domains.
Discussion
In this study a principal components analysis (PCA) identified seven main phenotypes of autonomic function and dysfunction in a sample of 209 patients attending general neurology or HIV-focused primary care clinics. Although this methodology is unsupervised, i.e. generates clusters without any initial hypotheses, the resulting phenotypes were clinically interpretable and provide evidence for significant diversity in the neurophysiology of AN. Specifically, the data suggest that the sympathetic adrenergic system may become over- or underactive as the function of other branches of the autonomic nervous system decline.
Over-activity of the sympathetic nervous system (SNS) is not a new idea. It has been described as a feature of aging,14 and has been linked to various disease states including hypertension, obesity, and heart disease.7 However, this literature does not typically address whether SNS over-activity is occurring as part of an early AN, focusing instead on other potential contributing factors such as changes in angiotensin 2, blood volume and osmolarity.7 The possibility that SNS over-activity could be part of early AN has been posited,6 although not extensively explored. The most directly relevant work pertains to diabetes, for which early literature described a hyperadrenergic state with or without accompanying othostatic hypotension,15,16 and subsequent work documented increased muscle sympathetic nerve activity (MSNA) using microneurography in diabetics compared to controls.17–19 Our findings suggest that hyperadrenergic features may be present in a significant proportion of patients meeting typical diagnostic criteria for AN. The present study design does not permit inference regarding the mechanism underlying this pattern, although a logical explanation would be lack of appropriate inhibition of the tonically active SNS due to dysfunction in the afferent arms of regulatory reflexes such as the baroreflex.7,10 Interestingly, cluster 2, which we characterized as “hyperadrenergic features without AN,” also had lower measures of resting HRV (i.e., cv-RMSSD and pNN50) with preserved reflexive HRV (i.e., VR and HRDB). Such a pattern can be seen in neurologically normal persons with stress-related psychiatric/psychological conditions; peripheral vagal pathways are intact (as reflected by normal markers of reflexive HRV) but resting HRV is decreased due to dysfunction in cortical circuits subserving self-regulatory capacity.13
Use of multidimensional datasets to create phenotypes, as we did here, has sometimes been described as a person-centered approach. In contrast to variable-centered approaches which may address questions such as the relative importance of predictor variables in explaining the variance of outcome variables, a person-centered approach focuses on differences between individuals in how variables are related to each other.20 Although originating in the field of developmental psychology, such approaches have been applied to the analysis of heart rate variability data to identify patterns associated with predetermined patient characteristics such as age and frailty.21,22 In addition, one study used PCA to select BP and HR variables (obtained during a period of supine rest) to predict of the presence or absence of AN.23 However, to our knowledge this is the first study to use data from a comprehensive standardized autonomic function testing battery to identify AN phenotypes. Previous work has employed a more traditional descriptive approach to characterize the natural history of the most common form of AN, diabetic AN, including the rate of change of autonomic test scores over time, and the value of these scores for predicting important outcomes such as morbidity and mortality.24,25
This study has limitations. It is a secondary analysis of data collected for other purposes, derived from two separate studies one of which recruited only people living with HIV. However, the proportion of people living with HIV did not differ among the clusters, suggesting that the observed phenotypes are not affected by HIV status. We have also inferred a possible hyperadrenergic state based on BP parameters. Adrenergic function is often assessed in this manner, e.g. in the adrenergic subscore of the CASS, however, we did not have measures such as MSNA or catecholamine measurements which would have provided additional information regarding potential etiology of this phenotype. Thus, it is entirely possible that elevations in BP reflected essential hypertension and not necessarily a hyperadrenergic state. Likely due to our recruitment strategy, our sample did not include any patients with more severe forms of autonomic dysfunction and so it is uncertain how the presence of such patients might have influenced the clusters. Finally, the PCA/cluster technique applied in this study creates distinct groups, however it is recognized that group membership can be “fuzzy” and is not a full characterization of any one individual.26
Despite these limitations, this work raises interesting questions regarding the spectrum of SNS function and dysfunction in AN. Under normal physiologic conditions control of SNS activity to different organs is highly differentially regulated, with output to some organs under a high degree of baroreflex control, and others not.7 It is currently unknown whether such differentiation is generally maintained in pathologic states or if, for example, increased cardiovascular SNS activity arising from an impaired baroreflex might also affect organ systems (such as the immune system) in which SNS activity is not normally under classically reflexive control due to a lack of significant afferent innervation.27 In addition, given that the current analysis is cross-sectional, it is unclear whether an individual’s autonomic phenotype is static or representative of a step on a pathway of disease progression, and whether there might be multiple different such paths passing through these phenotypes, for example, hyperadrenergic (clusters 1→2→3→4), balanced (clusters 1→7→4), and hypoadrenergic (clusters 1→6→4) pathways. Future research, including advanced modelling techniques applied to longitudinal data is needed to explore the pathophysiologic underpinnings and potential clinical implications of these autonomic phenotypes and to determine whether they are reproducible in samples containing participants with more severe autonomic dysfunction and more diverse underlying etiologies including α-synucleinopathies, amyloidosis, postural tachycardia syndrome, autonomic ganglionopathies.
Funding sources:
This work was supported by the following grants (PI: Robinson-Papp): R01DK122853, K23NS066789.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Robinson-Papp J, Sharma S, Simpson DM, Morgello S. Autonomic dysfunction is common in HIV and associated with distal symmetric polyneuropathy. JNeurovirol. 2013/04// 2013;19(2):172–180. doi: 10.1007/s13365-013-0160-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziegler D, Dannehl K, Mühlen H, Spüler M, Gries Fa. Prevalence of Cardiovascular Autonomic Dysfunction Assessed by Spectral Analysis, Vector Analysis, and Standard Tests of Heart Rate Variation and Blood Pressure Responses at Various Stages of Diabetic Neuropathy. Diabetic Medicine. 1992/11/01/ 1992;9(9):806–814. doi: 10.1111/j.1464-5491.1992.tb01898.x [DOI] [PubMed] [Google Scholar]
- 3.Low PA. Composite autonomic scoring scale for laboratory quantification of generalized autonomic failure. Mayo ClinProc. 1993/08// 1993;68(8):748–752. [DOI] [PubMed] [Google Scholar]
- 4.Crnošija L, Krbot Skorid M, Adamec I, et al. Hemodynamic profile and heart rate variability in hyperadrenergic versus non-hyperadrenergic postural orthostatic tachycardia syndrome. Clin Neurophysiol. Feb 2016;127(2):1639–1644. doi: 10.1016/j.clinph.2015.08.015 [DOI] [PubMed] [Google Scholar]
- 5.Taub PR, Zadourian A, Lo HC, Ormiston CK, Golshan S, Hsu JC. Randomized Trial of Ivabradine in Patients With Hyperadrenergic Postural Orthostatic Tachycardia Syndrome. J Am Coll Cardiol. Feb 23 2021;77(7):861–871. doi: 10.1016/j.jacc.2020.12.029 [DOI] [PubMed] [Google Scholar]
- 6.Freeman R. Chapter 6 - Diabetic autonomic neuropathy. In: Zochodne DW, Malik RA, eds. HandbClinNeurol. Elsevier; 2014:63–79. [DOI] [PubMed] [Google Scholar]
- 7.Malpas SC. Sympathetic Nervous System Overactivity and Its Role in the Development of Cardiovascular Disease. Physiological Reviews. 2010/04/01 2010;90(2):513–557. doi: 10.1152/physrev.00007.2009 [DOI] [PubMed] [Google Scholar]
- 8.Kherif F, Latypova A. Chapter 12 - Principal component analysis. In: Mechelli A, Vieira S, eds. Machine Learning. Academic Press; 2020:209–225. [Google Scholar]
- 9.Robinson-Papp J, Sharma SK, George MC, Simpson DM. Assessment of autonomic symptoms in a medically complex, urban patient population. Clin Auton Res. Feb 2017;27(1):25–29. doi: 10.1007/s10286-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Autonomic Neuroscience: Basic and Clinical. 2011;161(1):46–48. doi: 10.1016/j.autneu.2011.02.004 [DOI] [PubMed] [Google Scholar]
- 11.de Geus EJC, Gianaros PJ, Brindle RC, Jennings JR, Berntson GG. Should heart rate variability be “corrected” for heart rate? Biological, quantitative, and interpretive considerations. Psychophysiology. Feb 2019;56(2):e13287. doi: 10.1111/psyp.13287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaffer F, Ginsberg JP. An Overview of Heart Rate Variability Metrics and Norms. Review. Frontiers in Public Health. 2017-September-28 2017;5 doi: 10.3389/fpubh.2017.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon PM, Lawrence S, Mueller BR, Thayer JF, Benn EKT, Robinson-Papp J. Interpreting resting heart rate variability in complex populations: the role of autonomic reflexes and comorbidities. Clin Auton Res. May 14 2022; doi: 10.1007/s10286-022-00865-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shoemaker JK, Klassen SA, Badrov MB, Fadel PJ. Fifty years of microneurography: learning the language of the peripheral sympathetic nervous system in humans. Journal of Neurophysiology. 2018;119(5):1731–1744. doi: 10.1152/jn.00841.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cryer PE, Silverberg AB, Santiago JV, Shah SD. Plasma catecholamines in diabetes: The syndromes of hypoadrenergic and hyperadrenergic postural hypotension. AmJMed. 1978;64(3):407–416. doi: 10.1016/0002-9343(78)90220-6 [DOI] [PubMed] [Google Scholar]
- 16.Tohmeh JF, Shah SD, Cryer PE. The pathogenesis of hyperadrenergic postural hypotension in diabetic patients. AmJMed. 1979;67(5):772–778. doi: 10.1016/0002-9343(79)90733-2 [DOI] [PubMed] [Google Scholar]
- 17.Huggett RJ, Scott EM, Gilbey SG, Bannister J, Mackintosh AF, Mary DA. Disparity of autonomic control in type 2 diabetes mellitus. Diabetologia. Jan 2005;48(1):172–9. doi: 10.1007/s00125-004-1601-6 [DOI] [PubMed] [Google Scholar]
- 18.Straznicky NE, Grima MT, Sari CI, et al. Neuroadrenergic dysfunction along the diabetes continuum: a comparative study in obese metabolic syndrome subjects. Diabetes. Oct 2012;61(10):2506–16. doi: 10.2337/db12-0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holwerda SW, Restaino RM, Manrique C, Lastra G, Fisher JP, Fadel PJ. Augmented pressor and sympathetic responses to skeletal muscle metaboreflex activation in type 2 diabetes patients. American journal of physiology Heart and circulatory physiology. 2016;310(2):H300–H309. doi: 10.1152/ajpheart.00636.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laursen B, Hoff E. Person-Centered and Variable-Centered Approaches to Longitudinal Data. Merrill-Palmer Quarterly. 2006;52(3):377–389. doi: 10.1353/mpq.2006.0029 [DOI] [Google Scholar]
- 21.Trevizani GA, Nasario-Junior O, Benchimol-Barbosa PR, Silva LP, Nadal J. Cardiac autonomic changes in middle-aged women: identification based on principal component analysis. Clin Physiol Funct Imaging. Jul 2016;36(4):269–73. doi: 10.1111/cpf.12222 [DOI] [PubMed] [Google Scholar]
- 22.Varadhan R, Chaves PHM, Lipsitz LA, et al. Frailty and Impaired Cardiac Autonomic Control: New Insights From Principal Components Aggregation of Traditional Heart Rate Variability Indices. The Journals of Gerontology: Series A. 2009;64A(6):682–687. doi: 10.1093/gerona/glp013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petry D, Mirian de Godoy Marques C, Brum Marques JL. Baroreflex sensitivity with different lags and random forests for staging cardiovascular autonomic neuropathy in subjects with diabetes. Comput Biol Med. Dec 2020;127:104098. doi: 10.1016/j.compbiomed.2020.104098 [DOI] [PubMed] [Google Scholar]
- 24.Steger A, Müller A, Barthel P, et al. Polyscore of Non-invasive Cardiac Risk Factors. Original Research. Frontiers in Physiology. 2019-February-04 2019;10 doi: 10.3389/fphys.2019.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ewing DJ, Campbell IW, Clarke BF. The natural history of diabetic autonomic neuropathy. Q J Med. Winter 1980;49(193):95–108. [PubMed] [Google Scholar]
- 26.Ferraro MB. Fuzzy k-Means: history and applications. Econometrics and Statistics. 2021/11/26/ 2021; doi: 10.1016/j.ecosta.2021.11.008 [DOI] [Google Scholar]
- 27.Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007). Brain Behav Immun. Aug 2007;21(6):736–45. doi: 10.1016/j.bbi.2007.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]


