Abstract
The synergy between drought-responsive traits across different organs is crucial in the whole-plant mechanism influencing drought resilience. These organ interactions, however, are poorly understood, limiting our understanding of drought response strategies at the whole-plant level. Therefore, we need more integrative studies, especially on herbaceous species that represent many important food crops but remain underexplored in their drought response. We investigated inflorescence stems and rosette leaves of six Arabidopsis thaliana genotypes with contrasting drought tolerance, and combined anatomical observations with hydraulic measurements and gene expression studies to assess differences in drought response. The soc1ful double mutant was the most drought-tolerant genotype based on its synergistic combination of low stomatal conductance, largest stomatal safety margin, more stable leaf water potential during non-watering, reduced transcript levels of drought stress marker genes, and reduced loss of chlorophyll content in leaves, in combination with stems showing the highest embolism resistance, most pronounced lignification, and thickest intervessel pit membranes. In contrast, the most sensitive Cvi ecotype shows the opposite extreme of the same set of traits. The remaining four genotypes show variations in this drought syndrome. Our results reveal that anatomical, ecophysiological, and molecular adaptations across organs are intertwined, and multiple (differentially combined) strategies can be applied to acquire a certain level of drought tolerance.
Keywords: Arabidopsis thaliana, chlorophyll content, drought response, embolism resistance, gene expression, intervessel pit membrane thickness, stem anatomy, stomatal control
Drought response among Arabidopsis thalianagenotypes is clearly different and determined by the interplay between stem (embolism resistance, xylem anatomy) and leaf (stomatal control, chlorophyll loss, gene regulation) traits.
Introduction
The increasing intensity and frequency of drought episodes are becoming major threats to current and future agricultural productivity around the globe. Even the countries that had not experienced drought stress during the last decades are now impacted by drought (Corso et al., 2020; Gleason et al., 2022). One of the major problems that plants experience when they are facing severe drought is that detrimental levels of drought-induced gas bubbles (embolisms) in the xylem sap generate massive obstruction of the root to shoot water transport (Sperry and Tyree, 1988; Tyree and Zimmermann, 2002; Cochard, 2006; Choat et al., 2012; Venturas et al., 2017; Johnson et al., 2022), which happens after stomata are closed (Martin-StPaul et al., 2017). Stomatal closure may result in reduced photosynthetic productivity, growth rate, and reproduction, and under conditions of intense and prolonged drought may eventually cause desiccation and dieback of tissues (Mantova et al., 2022), organs, and entire plants (Davis et al., 2002; Venturas et al., 2016; Pratt et al., 2020; Brodribb et al., 2021). Lethal levels of embolism, from which plants are unable to recover, are thought to be reached when the hydraulic conductivity is reduced to ~88% of its maximum conductance (P88) (Urli et al., 2013; Li et al., 2015; but see Hammond et al., 2019; Johnson et al., 2021), although there are probably more accurate thresholds to drought-induced mortality than P88 (Mantova et al., 2021, 2022). Due to the implications of dramatic levels of drought-induced embolism on productivity, tissue death, and long-term survival, there is increasing evidence that natural selection has shaped the hydraulic systems of plants to minimize embolism occurrence and water potential loss during periods of water shortage (Lens et al., 2022). This can be made possible when many drought-related traits from different organs act in concert (Dayer et al., 2022).
As an example, angiosperms can build more resistant xylem by modifying a whole array of xylem anatomical adaptations to prevent the spread of embolisms, such as fine-scale modifications of pits in vessel walls allowing lateral transport of water and gas between adjacent vessels (Lens et al., 2011; Li et al., 2016; Kaack et al., 2019, 2021; Levionnois et al., 2021), or increased levels of lignification (Lens et al., 2013, 2016; Thonglim et al., 2020). In addition, plants can also delay xylem sap pressures from reaching critical embolism thresholds throughout the whole-plant body by producing the stress hormone abscisic acid (ABA) that induces stomatal closure in the leaves very rapidly at the onset of drought, well before embolism events start to exponentially increase (Brodribb et al., 2017; Martin-StPaul et al., 2017; Buckley, 2019; Creek et al., 2020). Consequently, stomatal closure is one of the primary responses that helps restrict water loss, which safeguards the water potential in the leaves and buffers the negative pressure in xylem sap (Brodribb et al., 2017; Martínez-Vilalta and Garcia-Forner, 2017; Martin-StPaul et al., 2017; Knipfer et al., 2020). The regulation of water potential in leaves during drought is crucial because it influences plant metabolic processes. However, declining transpiration rates reduce not only water loss but also carbon uptake, leading to decreased photosynthetic activity, which ultimately may lead to carbon starvation when stomata remain closed for a long time (McDowell et al., 2008). In other words, the interplay between embolism resistance inside the plant’s xylem and the onset and duration of stomatal closure at the level of leaves will determine how long leaves can remain metabolically active without risk of detrimental levels of drought-induced embolism (Allen et al., 2010; Choat et al., 2012; Mitchell et al., 2013; Brodribb et al., 2017; Martínez-Vilalta and Garcia-Forner, 2017; Buckley, 2019; Creek et al., 2020; Limousin et al., 2022). Accordingly, the stomatal safety margin (SSM), which can be defined as the difference between the water potential at stomatal closure (Ψgs90) and the pressure inducing 50% loss of hydraulic conductance (P50) is physiologically more important to estimate a plant’s ability to cope with massive levels of drought-induced embolism than only P50 (Sperry and Tyree, 1988; Meinzer et al., 2009; Anderegg et al., 2016; Martin-StPaul et al., 2017; Creek et al., 2020; Dayer et al., 2020; Skelton et al., 2021). It is widely accepted that species with a narrower safety margin are operating more closely to their hydraulic threshold, while species that have a wider safety margin have a lower risk of facing a detrimental level of drought-induced embolism (Choat et al., 2012; Anderegg et al., 2016; Martin-StPaul et al., 2017; Eller et al., 2018; Creek et al., 2020; Oliveira et al., 2021; Skelton et al., 2021).
It is clear that anatomical and physiological traits need to be intertwined within and among organs, but the molecular mechanisms cross-linking different pathways remain elusive. For instance, there is increasing evidence from gene expression studies confirming the positive correlation between lignification and drought resilience in a whole range of species (Tu et al., 2020; Xu et al., 2020; Wen et al., 2021; Yan et al., 2021; Hou et al., 2022; Li et al., 2022). Regarding drought responses in plants, the ABA-mediated signalling pathway is probably the best-known pathway at the molecular level. ABA regulates the expression of stress-responsive genes via transcription factors (Bauerle et al., 2004; Cutler et al., 2010; Bauer et al., 2013; Dodd, 2013; Mehrotra et al., 2014; Chen et al., 2020). Once ABA is accumulated, it regulates ABA-responsive genes via the cis-element called ABRE (ABA-responsive element) in their promoter regions using AREB (ABRE binding) transcription factors (Choi et al., 2000; Uno et al., 2000; Yoshida et al., 2015; Chen et al., 2020). In Arabidopsis, AREB1 is mainly expressed in vegetative tissues and up-regulated during drought (Yoshida et al., 2010; Fujita et al., 2011, 2013; Singh and Laxmi, 2015; Chen et al., 2020). Other drought-responsive genes are regulated by dehydration-responsive element-binding (DREB) proteins through an ABA-independent pathway (Bartels and Sunkar, 2005; Sakuma et al., 2006; Song et al., 2018). For example, DREB2 transcription factors are induced by dehydration and are involved in gene transcription under water shortage (Agarwal et al., 2006; Song et al., 2018). Interestingly, many stress-inducible genes contain both ABREs and DREs in their promoter regions, such as Responsive to Desiccation 29 (RD29) (Shinozaki and Yamaguchi-Shinozaki, 2007). Hence, gene expression of drought-responsive genes occurs via ABA-dependent and/or ABA-independent signal transduction pathways (Umezawa et al., 2010; Rushton et al., 2012; Song et al., 2016), and allows us to evaluate the expression of drought-responsive genes during a drought experiment with a simultaneous assessment of physiological and anatomical traits involved in drought tolerance.
Most studies investigating drought-induced embolism in plants have been focusing on trees, while herbaceous plants have been largely ignored despite their importance as crops and food sources for humans and animals (Brodribb and Hill, 1999; Stiller and Sperry, 2002; Holloway-Phillips and Brodribb, 2011; Choat et al., 2012; Ahmad, 2016; Lens et al., 2016; Volaire et al., 2018). In our previous study on the herbaceous model species, Arabidopsis thaliana, including genotypes with contrasting levels of embolism resistance and lignification in the inflorescence stems (Thonglim et al., 2020), we found that the more lignified genotypes are more resistant to embolism and have thicker intervessel pit membranes. Surprisingly, in most structure–function studies published so far, the drought response is only partly observed due to methodological and time constraints. For instance, resistance to embolism in branches/twigs is often recorded in xylem physiological studies (e.g. Choat et al., 2012; Anderegg et al., 2016), and less frequently integrated with leaf P50 data (e.g. Cochard et al., 2004; Klepsch et al., 2018; Skelton et al., 2019; Levionnois et al., 2021) and/or root P50 data (e.g. Rodriguez-Dominguez et al., 2018), and sometimes linked with other leaf physiological traits such as stomatal conductance (gs) and water potential (e.g. Brodribb et al., 2003; Li et al., 2015; Cardoso et al., 2018; Charrier et al., 2018; Creek et al., 2020; Chen et al., 2021). Only occasionally are detailed hydraulic measurements in stems, leaves, and/or roots complemented with detailed anatomical traits on intervessel pits (Guan et al., 2022). Other papers only focus on the molecular pathway and gene regulation during drought (e.g. Bhargava and Sawant, 2013; Pandey et al., 2013; Janiak et al., 2016; Ebrahimian-Motlagh et al., 2017; Thirumalaikumar et al., 2018; Roca-Paixão et al., 2019; Zhang et al., 2019), while publications that integrate gene function with xylem physiology are scarce (e.g. Kitin et al., 2010; Lamarque et al., 2020). Integration of drought-related traits across organs in structure–function studies and intensive collaboration among plant anatomists, xylem physiologists, and molecular biologists will help us to make considerable progress in a holistic understanding of drought response at the whole-plant level. To contribute to that whole-plant approach, we measured hydraulic traits in stems and leaves during a drought experiment, combined with detailed stem anatomical measurements and an assessment of transcript levels of drought stress marker genes across Arabidopsis genotypes (two transgenic lines and four natural accessions).
In this study, we investigate the following two questions. (i) Is there a coupling between drought-related stem (anatomy, P50) and leaf traits (stomatal regulation, leaf water potential, expression of drought marker genes) among Arabidopsis genotypes? (ii) Can these genotypes use different combinations of drought-response traits to reach a certain level of drought tolerance? To answer these questions, we investigated six genotypes with marked differences in embolism resistance and lignification of the inflorescence stems. We examined the detailed stem anatomical traits and hydraulic traits (stem P50) of each genotype and quantified the drought response for all six genotypes using a drought experiment, during which we measured gs and leaf water potential (Ψl), allowing us to calculate the SSM (as defined by Ψgs90 minus P50). In addition, we compared the expression of four drought-responsive genes from the ABA-(in)dependent (ABI2, AREB1, RD29A, and DREB2A) pathways from the rosette leaves at the end of the drought experiment to validate the level of drought stress among the six genotypes. By integrating all traits mentioned above, we want to assess how anatomical and ecophysiological traits across organs are intertwined to acquire a certain level of drought tolerance, and how these traits relate to the drought stress level at the end of the drought experiment based on a limited number of drought stress marker genes.
Materials and methods
Plant material
In addition to the four A. thaliana genotypes with contrasting levels of stem P50 and stem lignification, we studied before the ecotypes Columbia-0 (Col-0; wild type with intermediate stem lignification), Shadarah (Sha; wild type with a higher level of stem lignification), Cape Verde Islands (Cvi; least lignified wild type), and the double loss-of-function mutant SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 and FRUITFULL (soc1ful; most lignified genotype) (see Thonglim et al., 2020); we added one more wild type [Kelsterbach-4 Kel-4)] and a p35S:AHL15 line (AHL15 overexpression) in the Col-0 background (Rahimi et al., 2022). The two additional genotypes were selected based on their inflorescence length (at least 27 cm required for the centrifuge method used to estimate embolism resistance measurements) and their increased lignification in the basal parts of the inflorescence stem, respectively (Supplementary Fig. S1A, B, G, H). Indeed, Kel-4, an early flowering ecotype from Germany, shows a relatively high proportion of lignification at the base of the inflorescence stem (Ak, 2020), and has been reported to be more drought tolerant compared with many other wild-type accessions (Bac-Molenaar et al., 2016; Kooke et al., 2016). The AT-HOOK MOTIF CONTAINING NUCLEAR LOCALIZED 15 (AHL15) gene has been found to suppress axillary meristem maturation, and its overexpression extends plant longevity (Karami et al., 2021), and promotes secondary growth in the inflorescence stem to a similar extent as the soc1ful mutant (Rahimi et al., 2022).
Growing conditions
The plants were grown at the Institute of Biology Leiden (Leiden University, The Netherlands) under the same controlled conditions as in Thonglim et al. (2020) to ensure comparable datasets. Briefly, we germinated the two additional genotypes from seeds directly into a mixture of soil and sand (4.5:1). After 10 d of germination, the healthy seedlings were transferred into pots. Plants were grown in a controlled growth chamber with the following parameters: 20 °C temperature during the day and 17 °C temperature at night, 70% relative humidity, and 16 h photoperiod condition with 100 µmol m−2 s−1 light intensity. Sampling was synchronized based on differences in flowering time and subsequent inflorescence development. To synchronize flowering, p35S:AHL15 plants were planted earlier (harvesting inflorescence stems 85 d after sowing). The Kel-4 individuals were planted slightly later (harvesting inflorescence stems 65 d after sowing) (Supplementary Fig. S1A, B).
Drought experiment
A drought experiment was performed to assess the link between the anatomical and hydraulic traits and investigate the differences in drought tolerance across the six A. thaliana genotypes studied. The six genotypes were selected based on a previous screening of drought tolerance and the differences in stem lignification (Melzer et al., 2008; Bac-Molenaar et al., 2016; Thoen et al., 2017; Thonglim et al., 2020). The seeds of each genotype were directly sown in 6 cm pots (27 g) with the same amount of soil and sand mixture (4.5:1) at different times to synchronize flowering. The weight of the pot with dry and saturated soil was controlled (807 g and 1097 g, respectively). The pots were kept in a growth-controlled chamber under the same conditions as the individuals grown for stem P50 measurements. After germination, when seedings were 10 d old, they were thinned to one healthy seeding per pot and remained well watered. We equally divided 30 individuals of each genotype into a control and a drought batch during the experiment. The control plants were well irrigated every day to keep the soil constantly hydrated (Ψl was around –0.5 MPa to –0.6 MPa). The drought batch was subjected to water deficit by completely withholding watering for 3 weeks (Ψl values ranged between –1.85 MPa to –3.4 MPa among genotypes), starting 1 week before all the genotypes began to flower. When most genotypes started developing an inflorescence stem (7 d after watering was stopped), drought measurements were initiated. Rosette leaves were harvested on the last day of the drought experiment (depending on the water potential and phenotype), immediately frozen io liquid nitrogen, and stored in a –80 °C freezer for further gene expression and chlorophyll analyses.
We initially intended to have three biological replicates per genotype. However, during sample preparation, some tubes containing ground leaf material popped open in the freezer. We assume that some liquid nitrogen used for grinding the samples was still left in the tubes, causing several closed tubes to burst open and potentially contaminate the other open tubes containing different genotypes. We opted to discard all the open tubes due to potential contamination, and use only the closed tubes. We were able to still use three biological replicates for Cvi, Sha, and soc1ful, but only two for Col-0, Kel-4, and p35S:AHL15. For the latter genotypes, we included two biological and two technical replicates.
Chlorophyll content
Chlorophyll content was determined based on three biological replications for Cvi, Sha, and soc1ful, and four replicates (two biological and two technical) for Col-0, Kel-4, and p35S:AHL15, using the 80% acetone method (Porra et al., 1989). Ground leaf samples of ~0.5 mg were transferred into 1.5 ml tubes containing 1 ml of 80% acetone. The mixtures were gently vibrated using a vortex to extract chlorophyll, and centrifuged at 1000 g for 5 min to remove debris. The supernatants (800 µl) were then transferred to UV-transparent microplates. The absorbance was measured at 647 nm (A647), 664 nm (A664), and 750 nm (A750) using the DMF-chl conc._YU program. Chl a and b contents (µg Chl ml–1) in the extract were calculated with the following formulas:
RNA isolation and qRT–PCR
Total RNA was extracted using the RNeasy Plant Mini kit (Qiagen, Hilden, Germany). Synthesis of cDNA, quantitative reverse transcription–PCR (qRT–PCR) using SYBR Green, and data analysis were performed as previously described (Balazadeh et al., 2008). Gene expression was normalized with two reference genes (ACTIN2 and GADPH). qRT–PCR primers were designed using QuantPrime (www.quantprime.de) (Arvidsson et al., 2008). Primer sequences are given in Supplementary Table S1. Experiments were conducted in three biological replications for Cvi, Sha, and soc1ful, and two biological replicates with two technical replicates for Col-0, Kel-4, and p35S:AHL15.
Leaf water potential (Ψl) and stomatal conductance (gs)
After 7 d of water deficit (i.e. the time required to dehydrate the moisturized soil in the pots of the drought batch), Ψl was measured in both control and drought batches every day during the drought period until harvesting (15–17 d). The daily measurements were carried out using three mature leaves (one from control and two from drought treatment) for each method. Before the measurements, the leaves were covered with aluminium foil for 30 min. Subsequently, leaf discs were cut from the bagged leaves and placed in the PSYPRO leaf water potential system (Wescor, Inc., Logan, UT, USA) to measure the leaf water potential. At the same time, gs (mmol H2O m−2 s−1) was measured on single mature rosette leaves that were close to the leaves used for water potential measurements, using an SC-1 leaf porometer (METER Group, Pullman, WA, USA) that was calibrated every other day. The gs was measured using Auto Mode configuration with desiccant. gs, depending on leaf water potential, was fit according to the following sigmoid function for each genotype using the NLIN procedure in SAS:
g sm is the maximal stomatal conductance for Ψl=0, S the slope of the curve, and Ψgs50 the water potential inducing 50% stomatal closure. We then estimated the water potential inducing 90% of the stomatal closure (Ψgs90).
Stomatal safety sargin (SSM)
The SSM was defined as the difference between the leaf water potential at 90% stomatal closure (Martin-StPaul et al., 2017) calculated from the fitted curve (Ψgs90) and the water potential at 50% loss of stem conductivity (P50):
Generating vulnerability curves (VCs) in stems
Sample preparation of inflorescence stems
All individuals (80 individuals per genotype) were harvested at the Institute of Biology Leiden with roots, leaves, and flowers still attached and immediately wrapped in wet tissue papers. They were then enclosed in plastic bags to avoid dehydration during the shipment to the PHENOBOIS platform (INRAE, University of Bordeaux, France), where the Cavitron centrifuge measurements were performed. Before the Cavitron measurements, the roots were cut off at the basal part of inflorescence stems and trimmed on both sides, obtaining a stem segment of 27 cm in length that matches a standard Cavitron rotor. The length of the stem segments exceeds by far the maximum vessel length of Col-0, reaching only 4 cm according to Tixier et al. (2013) to avoid potential open-vessel artefacts (Cochard et al., 2013). Next, all siliques, leaves, and flowers were removed underwater immediately before placing the inflorescence stems in the Cavitron rotor (7–9 stem segments per VC).
Xylem vulnerability to embolism was evaluated using the Cavitron method, a custom-built centrifuge that allows measuring the water flow through the inflorescence stems while spinning them to create a negative pressure in the middle part of the stem segments (Cochard, 2002; Cochard et al., 2005, 2013). The negative pressure was gradually increased in each spinning step, as described in Thonglim et al. (2020). The degree of embolism in the xylem segment was quantified as the percentage loss of conductivity (PLC), calculated as follows:
where Kmax (m2 MPa−1 s−1) is the maximum hydraulic conductivity which was calculated when stem segments were fully functioning (no embolism) at low spinning speed (near 0 MPa), and K is the decreased hydraulic conductivity due to embolisms. The extent of embolism formation at every rotation speed was measured using the Cavisoft software (Cavisoft v1.5, University of Bordeaux, France). We fitted the data points to reconstruct the VCs using a sigmoid function based on the NLIN procedure in SAS 9.4 (SAS Institute, Cary, NC, USA) (Pammenter and Van der Willigen, 1998):
where P is the xylem pressure used at each rotation step, P50 is xylem pressure inducing 50% loss of hydraulic conductivity, and S (MPa−1) is the slope of the VC at P50.
Due to the low hydraulic conductivity of Arabidopsis, we measured vulnerability to embolism of 7–9 inflorescence stems to generate one vulnerability curve. Eight VCs were constructed for each genotype.
Stem anatomy
Three stems from three representative VCs per genotype (nine stems per genotype) were randomly selected for light microscopy (LM) observations and one stem per VC from three VCs (three individuals per genotype) for TEM observations (Supplementary Fig. S1C, D). Both basal and central parts of the 27 cm inflorescence stem segments were sectioned because they differ in the amount of lignification (Supplementary Fig. S1E–H). We, however, invested more time in measuring trait data from the middle part than in the basal segment because that is the region where the negative pressures were applied during the Cavitron experiments, allowing us to accurately link the anatomical traits with embolism resistance (P50). The anatomical traits are represented in Supplementary Table S2. ImageJ (National Institutes of Health, Bethesda, MD, USA) was used, and the guidance of Scholz et al. (2013a) was followed to measure the anatomical features in digital images from both LM and TEM observations.
Light microscopy
Inflorescence stems were cut into 1 cm long pieces and submerged in 70% ethanol. The samples were then gradually infiltrated in LR-white resin (Hamann et al., 2011). After embedding in LR-white, specimens were sectioned with a rotary microtome (Leica RM 2265, Leica, Eisenmark, Wetzlar, Germany) with disposable tungsten carbon blades at 4 µm thickness. Next, the sections were heat-fixed onto the slides, stained with 1% (w/v) toluidine blue (VWR Chemicals BDH®, Radnor, PA, USA), and mounted with DPX new-100579 mounting medium (Merck Chemicals, Amsterdam, the Netherlands). Finally, various anatomical traits (Supplementary Table S2) were observed using a Leica DM2500 light microscope equipped with a Leica DFC-425 digital camera (Leica microscopes, Wetzlar, Germany).
TEM
The middle parts of inflorescence stem segments were collected immediately after Cavitron measurements and fixed in Karnovsky’s fixative (Karnovsky, 1965). Subsequently, the samples were washed in 0.1 M cacodylate buffer and post-fixed with 1% buffered osmium tetroxide. The samples were then prepared for semi-thin and ultra-thin sectioning according to the protocol described in Thonglim et al. (2020), and were observed with a JEM-1400 Plus TEM (JEOL, Tokyo, Japan) with an 11 megapixel digital camera (Quemesa, Olympus). TEM observations were conducted to measure the intervessel pit membrane thickness and the pit chamber depth (Supplementary Table S2).
Statistical analysis
R version 3.6.3 in R Studio version 1.2.5033 was used for the statistical analyses of all traits studied, of which all the differences were considered significant when the P-value was <0.05. First, general linear models with a Newman–Keuls post-hoc test were used to check the differences in embolism resistance (P50, P12, and P88), anatomical features, leaf physiological traits, chlorophyll content, and gene expression among Arabidopsis genotypes studied. Then, multiple linear regression was applied to assess the anatomical traits (predictive variables) that explain the differences in embolism resistance (responsive variables, including P50, P12, and P88). The collinearity between variables was firstly checked to select the predictors. Then, the ‘step’ function (stats package; R Core Team, 2016) was applied to achieve the most parsimonious linear regression model based on the least Akaike information criterion (AIC). Subsequently, the model’s residuals, heteroscedasticity, skewness and kurtosis, and variance inflation factor (VIF) were checked. Once we obtained the best model, the relative importance of each explanatory variable was analysed to assess the variable that explains the best P50. Pearson’s correlation was applied to plot the relationship between P50 and predictive variables and leaf physiological traits, and among the variables. Lastly, we investigated whether the different Arabidopsis genotypes presented different gs in well-watered control conditions using a generalized linear mixed model with the accession as a fixed effect, with the GLIMMIX procedure in SAS software (SAS 9.4; SAS Institute).
Gene codes
Arabidopsis gene codes are: ACTIN2, AT3G18780; GAPDH, AT1G13440; RD29A, AT5G52310; ABI2, AT5G57050; AREB1, AT1G45249; and DREB2A, AT5G05410.
Results
Drought-response phenotyping, chlorophyll content, and expression of drought-responsive genes in the basal rosette leaves
After 3 weeks of non-watering, we found differences in phenotypes of the drought-treated batch compared with the well-watered controls. The soc1ful mutant and the p35S:AHL15 overexpression line were least affected by drought based on the rosette phenotype (less wilting of leaves, less reduction of rosette size) and the small reduction of chlorophyll content when compared with the control individuals. The droughted individuals of Sha showed intermediate phenotypic drought stress-related signs compared with the control batch, such as a minor reduction in leaf rosette size, more wilting of leaves, and a slightly higher decrease of chlorophyll content (Fig. 1A, B). In contrast, the rosette leaves were more reduced in size in the droughted individuals of Col-0, Kel-4, and Cvi compared with the well-watered control plants (Fig. 1A); likewise, leaves and inflorescence stems in the droughted batch of these three genotypes were considerably more wilted compared with the control plants (Fig. 1A), along with the stronger chlorophyll reduction in the rosette leaves (Fig. 1B). With regards to Chl b reduction during the drought experiment, two significantly different genotype groups could be defined: one group comprising Col-0, Cvi, and Kel-4 (62, 67, and 46% reduction, respectively) and the other comprising Sha, soc1ful, and p35S:AHL15 (31, 13, and 27% reduction, respectively) (F=15.83, P=0.00212). For Chl a reduction, significant differences were detected among the genotypes (F=181.6, P=1.84e−06), except for soc1ful and p35S:AHL15 that presented a similar reduced value (10% and 12% reduction). This is also the case for total chlorophyll (Chl a+b) reduction (F=168.1, P=2.32e−06) (Fig. 1B).
Fig. 1.
(A) Phenotypic variation in response to drought. The phenotype of six Arabidopsis genotypes subjected to drought, by water withholding at the end of a 3 week period, and their untreated counterparts. (B) The variation in chlorophyll contents (Chl a, Chl b, and Chl a+b) among genotypes studied. The y-axis represents the percentage reduction of chlorophyll content in drought compared with the well-watered control batch. (C) qRT–PCR analysis of the expression of selected drought-responsive genes (RD29A, DREB2A, ABI2, and AREB1) across six Arabidopsis genotypes. The y-axis represents the log2 fold change of the gene expression between drought and control conditions. The genes are significantly less up-regulated by drought in Sha, p35S:AHL15, and soc1ful plants. A Newman–Keuls post-hoc test was performed, showing the differences in chlorophyll reduction and gene expression level between each genotype. Different letters indicate significant differences in means of replications among genotypes; P-value <0.05. The error bars show the SEs based on three biological replications for Cvi, Sha, and soc1ful, and two biological and two technical replications for Col-0, Kel-4, and p35S:AHL15.
In order to estimate how each Arabidopsis genotype senses drought stress at the molecular level, we measured the expression of four selected drought marker genes at the end of the 15–17 d drought treatment. In the ecotypes with an intermediate level of stem lignification (Col-0 and Kel-4) and the one with the least lignified stems (Cvi), all four drought-responsive genes were up-regulated under drought compared with well-watered conditions (Fig. 1C). In contrast, the four drought-response genes in the more lignified genotypes Sha, the overexpression line p35S:AHL15, and soc1ful were significantly less induced under drought treatment. Interestingly, p35S:AHL15 showed no difference in ABI2 and AREB1 expression level between drought and control conditions (–0.45 and –1.37 log2 fold change, respectively). Regarding the changes in the expression of each gene between drought and control conditions among genotypes studied, we found that the change of RD29A expression was similar between Col-0 and Cvi (~6.9 log2 old change). Still, these two genotypes were significantly different from the rest (2.8–4.7 log2 fold change) (F=10.2, P=0.00021). For DREB2A, two significantly different groups were defined: one comprising Col-0, Cvi, and Kel-4 (4.55, 5.6, and 5.57, respectively) and the other comprising Sha, soc1ful, and p35S:AHL15 (3.37, 2.75, and 2.87, respectively) (F=21.05, P=2.71e−06). The changes of AREB1 were significantly different among genotypes (F=13.28, P=4.63e−05), except for Col-0, Cvi, and Kel-4 (3.48, 3.22 and 3.19 log2 fold change, respectively). Likewise, for ABI2, there was a significant difference among genotypes (F =40.95, P=3.2e−08), except for Col-0 and Kel-4 (6.22 and 5.93), and Sha and soc1ful (4.57 and 3.58 log2 fold change) (Fig. 1C).
Leaf water potential (Ψl) and stomatal conductance (gs) dynamics during drought
Ψl under well-watered conditions was similar in every genotype, ranging between –0.5 MPa and –0.6 MPa (Fig. 2A). However, gs of control plants was significantly different among the genotypes studied (F=236.12, P<0.0001, Fig. 2B). Cvi (least lignified wild type) had the highest gs (384 mmol m−2 s−1), followed by Col-0, Sha, and Kel-4, while the more lignified soc1ful and p35S:AHL15 genotypes presented the lowest gs value (up to 216 mmol m−2 s−1); only gs values of Sha and Kel-4 were not statistically different from each other (Fig. 2B). In addition, we noticed that Col-0 closed its stomata at a less negative leaf water potential compared with the other genotypes. It reached 90% of stomatal closure (gs90) at –0.9 MPa, followed by Kel-4 (–1.13 MPa), and the more lignified Sha (–1.27 MPa), soc1ful (–1.43 MPa), and p35S:AHL15 (–1.6 MPa). The least lignified Cvi reached more negative Ψl, even before closing its stomata (–1.75 MPa; Fig. 2A). When following stomatal conductance and leaf water potential decline during the drought experiment, we found that the lignified soc1ful and Sha genotypes never reached critical water potential values (i.e. the P50) even after 17 d of drought, while other genotypes reached their respective P50 between 10 d and 14 d (Supplementary Fig. S2A, B).
Fig. 2.
Drought-response traits for the six A. thaliana genotypes studied. (A) The relationship between leaf water potential (Ψl) and stomatal conductance (gs). (B) gs (mmol s−1 m−2) in control well-watered plants for the different Arabidopsis genotypes (leaf water potential > –0.7 MPa). Larger symbols within boxes correspond to means, and smaller symbols outside boxes to outlier values. The error bars show the SE based on three biological replications. Colours refer to the genotype studied: Col-0, red; Cvi, turquoise; Sha, purple; soc1ful, green; p35S:AHL15, blue; Kel-4, brown.
Stem vulnerability to embolism
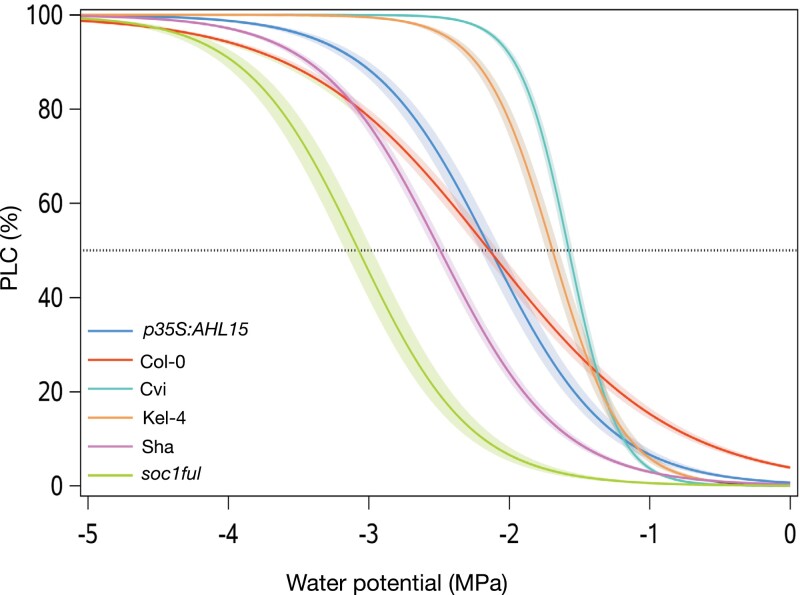
When comparing all six genotypes, the most lignified soc1ful was the most embolism resistant, with P50 of –3.07 MPa (Fig. 3; Table 1), whereas the least lignified Cvi remained the most vulnerable (P50= –1.58 MPa). For the two added genotypes, Kel-4 (wild type with intermediate lignified stems) was among the most vulnerable genotypes with P50= –1.69 MPa, whereas p35S:AHL15 (overexpression line) was intermediate, almost identical to the common wild-type Col-0 with P50= –2.13 MPa. The P12 (stem water potential at onset of embolism) values of most of the genotypes studied were different from each other (F=420.6; P<2e−16), but Cvi and Kel-4 presented similar P12 (P=0.5424). For P88, p35S:AHL15 and Kel-4 were different from other genotypes (F=75.09; P<2e−16) (Supplementary Fig. S3). The slope of the vulnerability curve was similar across the genotypes, except Col-0, which had a lower slope (see Fig. 3).
Fig. 3.
Mean vulnerability curves present the percentage loss of conductivity (PLC) as a function of xylem pressure (MPa) of each genotype studied. Shaded bands represent the SEs based on 5–10 vulnerability curves per genotype. Colours refer to the genotype studied: Col-0, red; Cvi, turquoise; Sha, purple; soc1ful, green; p35S:AHL15, blue; Kel-4, brown.
Table 1.
The hydraulic data of Arabidopsis genotypes studied measured during the drought experiment
| Genotypes |
P
50
(MPa) |
Ψgs90 (MPa) |
SSM (MPa) |
Ψlh (MPa) |
Days until 90% stomatal closure | Days until P50 | PLC after 3 weeks of non-watering |
|---|---|---|---|---|---|---|---|
| Cvi | –1.58 | –1.75 | –0.17 | –3.4 | 10 | 10 | 100% |
| Kel-4 | –1.69 | –1.13 | 0.56 | –3.4 | 11 | 11 | 100% |
| Col-0 | –2.14 | –0.9 | 1.24 | –2.97 | 10–11 | 12 | 75% |
| p35S:AHL15 | –2.13 | –1.6 | 0.53 | –3.03 | 13 | 14 | 88% |
| Sha | –2.49 | –1.27 | 1.22 | –1.85 | 12 | Does not reach P50 | 14% |
| soc1ful | –3.07 | –1.43 | 1.64 | –1.87 | 14 | Does not reach P12 | 10% |
P 50, stem water potential at 50% loss of hydraulic conductivity; Ψgs90, leaf water potential at 90% stomatal closure; SSM, stomatal safety margin; Ψlh, leaf water potential at the harvesting day; PLC, percentage loss of hydraulic conductivity.
Water potential and SSM during drought
Assuming that leaf water potential values are similar to stem water potential values in the tiny Arabidopsis herbs, we calculated the SSM as the difference between Ψgs90 and P50. The SSMs of all genotypes studied were positive (from +0.53 MPa to +1.64 MPa), except for the least lignified Cvi with a narrow and negative SSM (–0.17 MPa) (Fig. 4). Accordingly, Cvi also closed its stomata and reached a leaf water potential equivalent to P50 the soonest (10 d; Table 1). SSM was the widest in the most lignified soc1ful (+1.64 MPa), followed by Col-0 and Sha (+1.24 MPa and +1.22 MPa, respectively; Table 1; Fig. 4). Kel-4 and p35S:AHL15 had intermediate SSMs (+0.56 MPa and +0.53 MPa, respectively).
Fig. 4.
Stomatal safety margin (SSM) of each genotype studied. The graphs show the percentage loss of hydraulic conductivity (PLC) and the percentage of stomatal conductance (gs) as a function of xylem pressure (MPa). The dotted lines represent water potential at 90% loss of stomatal conductance. The dashed lines show the P50. The difference between the dashed and the dotted line refers to the SSM.
The differences in anatomical features among genotypes studied
When comparing the anatomical dataset across the six genotypes, we found that the lignified soc1ful and Sha genotypes had the thickest intervessel pit membranes (TPM), followed by an intermediate pit membrane thickness of p35S:AHL15 and Col-0 (F=3.857; P=0.0672), and thinner pit membranes in Kel-4 and the least lignified Cvi (F=4.467; P=0.0506) (Supplementary Fig. S4A). Results of vessel wall thickness (TV) showed the same pattern as that described for intervessel pit membrane thickness (F=2.546; P=0.13 and F=0.554; P=0.468, respectively) (Supplementary Fig. S4B). Vessel grouping index (VG) was markedly higher in the p35S:AHL15 overexpression line than in all the other genotypes (F=27.38; P=5.46e−13) (Supplementary Fig. S4C), which was also the case for the proportion of lignified area per total stem area (PLIG; F=28.8; P=2.25e−13) (Supplementary Fig. S4D). The lignified p35S:AHL15 overexpression line also had a higher proportion of fibre wall area per fibre cell area (PFWFA) than Kel-4, Col-0, and Cvi, but the fibres were less thick walled compared with the lignified genotypes soc1ful and Sha (F=49.05; P<2e−16) (Supplementary Fig. S4E). Surprisingly, p35S:AHL15 showed no wood formation at the stem segment investigated (Supplementary Fig. S1E) and was less lignified than soc1ful, although AHL15–SOC1–FUL belong to the same pathway. The vessel diameter (D) of Kel-4 was significantly narrower than that of the other genotypes. Among the remaining genotypes, Cvi (least lignified wild type) had the widest mean D, which was significantly different from the p35S:AHL15 overexpression line, but there was no statistical difference in D with Col-0, Cvi, Sha, and soc1ful (F= 9.46; P=2.52e−06) (Supplementary Fig. S4F). For theoretical vessel implosion resistance (TVW/DMAX)2, the lignified soc1ful and Sha showed the highest values as well, while there was no difference among p35S:AHL15, Kel-4, Col-0, and Cvi (F=3.955; P=0.0166). Finally, vessel density (VD) of p35S:AHL15, Col-0, Cvi, Sha, and soc1ful was similar (F=1.899; P=0.13) and significantly higher than that of Kel-4.
Stem anatomical traits explaining variation in embolism resistance
According to the most parsimonious model derived from multiple linear regression (AIC= –194.59), the stem anatomical predictors that explain the embolism resistance variation were TPM, TV, VG, and maximum vessel lumen diameter (DMAX) (R2=0.924; P<2.2e−16) (Supplementary Table S3). TPM was the anatomical feature explaining P50 variation best, with relative importance of 44%, followed by TV (38%), VG (9%), and DMAX (2%) (Fig. 5A). Likewise, TPM and TV together also explained most of the variation in P12, with 41% relative importance (R2=0.795; P=1.135e−14) (Supplementary Table S4; Supplementary Fig. S5A). P88 variation, on the other hand, was mostly explained by PFWFA (25% relative importance) (R2=0.516; P=1.07e−07) (Supplementary Table S5; Supplementary Fig. S5B).
Fig. 5.
(A) Relative importance of stem anatomical traits on P50 variation. The P50 variation is mainly explained by intervessel pit membrane thickness (TPM) and vessel wall thickness (TV) based on the R2 contribution averaged over orderings among regressors [based on the Lindemann, Merenda, and Gold (LMG) method]. (B) Negative correlation between TPM and P50. (C) Negative correlation between (TVW/DMAX)2 and P50. (D) Negative correlation between TV and P50. (E) Negative correlation between PFWFA and P50. The error bars show the SEs based on three biological replications for TPM and nine biological replications for other anatomical traits. Colours and styles refer to the genotype studied: Col-0, red circles; Cvi, turquoise upright triangles; Sha, inverted purple triangles soc1ful, green stars; p35S:AHL15, blue squares; Kel-4, brown diamonds.
The relationship among embolism resistance, anatomical traits, and hydraulic traits
Based on a Pearson’s correlation test, TPM was strongly positively correlated with other anatomical traits, such as TV, (TVW/DMAX)2, PFWFA, and VD (r=0.77 and P=1.108e−11; r=0.74 and P=1.956e−10; r=0.61 and P=8.96e−07, r=0.58, P=4.472e−06, respectively) (Supplementary Fig. S6). Lastly, TV and PFWFA were correlated as well (r=0.71, P=2.3e−09) (Supplementary Fig. S6). When also taking P50 into account, we saw that P50 was strongly correlated with TPM, (TVW/DMAX)2, TV, and PFWFA (r= –0.91, –0.87, –0.86, and –0.70; P<2.2e−16, respectively) (Fig. 5B–E; Supplementary Fig. S6). Similarly, P12 had strong relationships to TPM, (TVW/DMAX)2, and TV (r= –0.77 and P=6.41e−12; r= 0.84 and P=3.93e−15; r= 0.68 and P=1.38e−08, respectively) (Supplementary Fig. S6). P88 only showed a correlation with PFWFA (r= –0.54; P=2.762e−05) and TV (r= –0.44; P=0.0008146) (Supplementary Fig. S6). We also found a strong correlation between P50 and the leaf water potential at the harvesting day (Ψlh), the number of days until reaching 90% stomatal closure (Day90), and the SSM (r= –0.9, –0.85, and –0.84; P<2.2e−16, respectively), but not between P50 and Ψgs90. Subsequently, the anatomical traits that were strongly correlated to P50, such as TPM, TV, and VG, were also significantly correlated to Ψlh, Day90, and SSM (Supplementary Fig. S6).
Discussion
We performed a drought experiment including six Arabidopsis genotypes, during which we compiled a detailed xylem anatomical–hydraulic dataset of inflorescence stems (among others intervessel pit membrane thickness, proportion of lignification, and P50) and leaves (rate of stomatal conductance, leaf water potential, and chlorophyll content), and validated the drought response of the genotypes with the transcript abundance of four known drought marker genes at the end of a 15–17 d treatment without watering. Based on anatomical, hydraulic, and gene expression results, it is clear that the most lignified mutant soc1ful (Melzer et al., 2008; Lens et al., 2012, 2013) is the most drought-tolerant genotype, closely followed by the lignified ecotype Sha and the p35S:AHL15 overexpression line, while the lesser lignified Col-0, Kel-4, and especially Cvi ecotypes are much more sensitive. Interestingly, each genotype applies a unique combination of anatomical stem traits and hydraulic traits in stems and leaves to acquire a certain level of drought tolerance, as will be discussed in the following sections.
Comparing extremes in drought response: most lignified soc1ful versus least lignified Cvi
Both the most drought-tolerant soc1ful and the most drought-sensitive Cvi use a similar set of traits with contrasting trait values to reach the two extremes of the drought tolerance spectrum among the genotypes studied. The drought-tolerant strategy of soc1ful (Fig. 1A) is determined by a unique combination of traits, as exemplified by the most negative stem P50 (Fig. 3; cf. Choat et al., 2012; Lens et al., 2016; Thonglim et al., 2020), coupled with a low initial gs that gradually slowed down during drought, allowing a more stable leaf water potential (Supplementary Fig. S2A) (Li et al., 2017; Dayer et al., 2020; Lemaire et al., 2021). In addition to its low gs, soc1ful started closing its stomata rapidly at the onset of drought (at high water potential) to further reduce water loss, but at the same time it reached full stomatal closure later than in the other genotypes (Ψgs90 was reached after 14 d of non-watering, Table 1). Although we had not quantified carbon uptake during drought, we observed that stomatal closure in soc1ful occurred gradually over a longer period during drought, probably extending photosynthetic activities without risking a detrimental level of drought-induced embolism (Fig. 2A; Supplementary Fig. S2). This is further supported by a low reduction of chlorophyll content in rosette leaves of droughted soc1ful individuals compared with the well-watered control batch (Fig. 1B), Moreover, this mutant line had the widest positive SSM (Fig. 4), which is essential in estimating a plant’s drought response (Choat et al., 2012; Delzon and Cochard, 2014; Anderegg et al., 2016; Eller et al., 2018; Oliveira et al., 2021; Skelton et al., 2021). Finally, as reported in Thonglim et al. (2020), this mutant also produced the thickest intervessel pit membranes and largest wood cylinder at the base of the inflorescence stem. Both traits are thought to play an important role in preventing embolism spread (Lens et al., 2022). In contrast, the least lignified Cvi was the most vulnerable genotype as it showed the least negative stem P50 combined with a rapid drop in leaf water potential during drought, leading to rapid wilting (Fig. 1A) and a strong decrease of chlorophyll content (Fig. 1B). In addition, Cvi had the highest initial gs, and it closed its stomata at low water potential, which led to more water loss due to transpiration (Fig. 2A; Supplementary Fig. S2). Although it reached Ψgs90 earlier than the more tolerant genotypes (Table 1), it seemed like Cvi could not close its stomata in time because all the water was already consumed, giving rise to a rapid water potential drop during drought (Supplementary Fig. S2A). Due to its less negative stem P50, the Ψgs90 exceeded stem P50, leading to the only negative SSM among the six genotypes studied (Fig. 4). This implies that Cvi experiences a considerable decrease in stem hydraulic conductivity right after or even before stomatal closure. In addition to all these physiological parameters pointing to the most sensitive drought response among the genotypes studied, Cvi also had the least lignified inflorescence stems with the thinnest intervessel pit membranes (Thonglim et al., 2020).
The role of embolism resistance and stomatal regulation in drought tolerance and its impact on the stomatal safety margin
The previous section highlights the importance of embolism resistance as well as SSMs in determining drought tolerance, as has been demonstrated across many other lineages of plants (Meinzer et al., 2009; McDowell, 2011; Choat et al., 2012; Johnson et al., 2012; Cochard et al., 2013; Lens et al., 2013; Skelton et al., 2015, 2021; Martin-StPaul et al., 2017; Creek et al., 2020; Dayer et al., 2020). However, our dataset suggests that stem P50—which is probably a good proxy for whole-plant P50 based on our few leaf P50 measurements in the p35S:AHL15 overexpression line and based on other herbaceous species showing no difference in P50 across organs (e.g. Skelton et al., 2017)—outperforms SSM in explaining the responses to drought among the genotypes studied. This is because stomatal regulation in Arabidopsis genotypes that were equally drought tolerant could be substantially different, while P50 showed a more consistent pattern with whole-plant drought tolerance. However, it seems that the rate of gs in Arabidopsis under well-watered conditions is more critical than the speed of stomatal closure, as shown by Cvi, Col-0, and Kel-4 (Table 1; Supplementary Fig. S2B). Indeed, Ψgs90 is not the driving force behind drought tolerance since the more drought-tolerant genotypes closed their stomata slightly later than the sensitive ones. In other words, Cvi, Col-0, and Kel-4 lost more water because of a higher transpiration rate, but they closed their stomata sooner than the more drought-tolerant genotypes (Table 1). These results align with previous studies stating that stomatal behaviour only shows how each species respond to drought stress, but not how much they tolerate drought (Roman et al., 2015; Combe et al., 2016; Martínez-Vilalta and Garcia-Forner, 2017). Bearing this in mind, our observation shows that the two mutant genotypes studied in the Col-0 background (soc1ful and p35S:AHL15)—both belonging to the same regulatory SOC1–FUL–AHL15–cytokinin pathway that induces wood formation in stems (Rahimi et al., 2022)—also have by far the lowest initial gs values across all six genotypes studied, including the Col-0 ecotype (Fig. 2B). This makes it a promising gene regulatory pathway to discover how drought-responsive traits in stems (increased lignification or woodiness) and leaves (reduced gs) are linked to each other at the genetic level.
Our dataset aligns with earlier studies showing that safety margins across (mainly woody) angiosperms are overall positive, and considerable levels of embolisms only happen under remarkable, intense drought events (Choat et al., 2012; Delzon and Cochard, 2014; Martin-StPaul et al., 2017; Creek et al., 2020; Dayer et al., 2020; Skelton et al., 2021; Guan et al., 2022; Lens et al., 2022). The positive SSMs in five out of six genotypes indicate that stomatal closure typically occurs before embolism in order to prevent water loss and delay hydraulic dysfunction (Martin-StPaul et al., 2017; Creek et al., 2020). In contrast, Cvi—the only genotype with a negative SSM—closed its stomata at 70% loss of maximum conductance, highlighting its high sensibility to drought.
Multiple strategies to acquire drought tolerance
In addition to the drought-responsive traits discussed in soc1ful and Cvi, different combinations among these traits were observed in the remaining genotypes. This shows that even in a species with a short life cycle, multiple strategies can be applied to acquire a certain level of drought tolerance. For instance, Sha and p35S:AHL15 had a similarly high level of drought tolerance based on their phenotype after 3 weeks of water shortage (Fig. 1A), but their drought-responsive traits were different. Sha had high embolism resistance in stems combined with a relatively high initial transpiration rate in leaves that rapidly declines during drought, allowing a relatively stable leaf water potential (also confirmed by Bouchabke et al., 2008) and a large SSM. On the other hand, p35S:AHL15 had the lowest gs of all the genotypes studied (Fig. 2A), which means it can keep its leaf water potential relatively high during drought, whereas its stem P50 was intermediate and led to a smaller SSM compared with Sha (Figs 2–4). Another example is given by p35S:AHL15 (overexpression line) and Col-0 common wild type, which both had a similar stem P50 (–2.1 MPa; Fig. 3). However, Col-0 was more drought sensitive than p35S:AHL15, even though the former closed its stomata earlier during drought, resulting in a wider SSM (Fig. 4). The reason for Col-0 being more drought sensitive is that stomatal conductance is much higher, leading to more water loss and consequently a more rapid decline in leaf water potential during the drought experiment, while the leaf water potential during drought in p35S:AHL15 drops more slowly (Supplementary Fig. S2). Thus, a wider SSM does not always lead to a prolonged survival during drought since the rate of gs is not accounted for in the SSM. In other words, the width of the safety margin does not necessarily match all aspects of stomatal regulation and the resulting leaf water potential dynamics during drought (Martínez-Vilalta and Garcia-Forner, 2017; Martin-StPaul et al., 2017; Knipfer et al., 2020).
Expression levels of drought-responsive genes agree with drought-response traits
To assess the level of drought stress and compare it among the genotypes, we assessed the expression of selected drought-responsive genes on the final day of the drought treatment (15–17 d). As expected, the four drought-responsive genes RD29A, DREB2A, ABI2, and AREB1 were most up-regulated in the more sensitive genotypes Col-0, Kel-4, and Cvi, and less up-regulated in the more tolerant genotypes Sha, p35S:AHL15, and soc1ful (Fig. 1C). To study the casual relationship between physiological responses (e.g. stomatal closure) and gene activity (e.g. ABA biosynthesis genes), future work should focus on conducting a high-resolution time-course gene expression analysis, which is beyond the scope of this study.
Intervessel pit membrane thickness as an important anatomical driver of embolism resistance, and the potential effect of stem lignification on P50
Our extended database confirms our previous results that intervessel pit membrane thickness is the anatomical trait that explains best the variation in P50 across all six genotypes studied (Fig. 5A). These results are in line with several other angiosperm studies showing a strong positive correlation between embolism resistance and TPM, both at the interspecies level (Jansen et al., 2009; Lens et al., 2011, 2022; Plavcová and Hacke, 2012; Plavcová et al., 2013; Scholz et al., 2013b; Li et al., 2016; Dória et al., 2018; Trueba et al., 2019; Guan et al., 2022) and within species (Schuldt et al., 2016). The functional explanation for this relationship was intensively discussed in our previous paper (Thonglim et al., 2020). In brief, there is convincing evidence based on microCT and/or optical technique observations in stems (Brodersen et al., 2013; Knipfer et al., 2015; Choat et al., 2016; Skelton et al., 2017; Torres-Ruiz et al., 2017) and leaves (Brodribb et al., 2016; Skelton et al., 2017, 2018; Klepsch et al., 2018; Lamarque et al., 2018) that embolism spread between adjacent vessels predominantly happens via porous pit membranes located inside the bordered pits between adjacent vessels. Although this explains why the thickness of intervessel pit membrane plays an important role in embolism propagation and, by extension, also whole-plant drought tolerance, the detailed mechanisms behind this embolism spread remain poorly known due to the complex 3D structure/composition of pit membranes and the enigmatic behaviour of gas–liquid–solid–surfactant interfaces at the nano-scale (Kaack et al., 2019, 2021; Yang et al., 2020; Zhang et al., 2020; Lens et al., 2022).
It has also been shown in previous studies that intervessel pit membrane thickness is strongly linked not only with P50, but also with other anatomical traits assumed to be involved in drought-induced embolism resistance, such as vessel wall thickness (Jansen et al., 2009; Li et al., 2016), and the amount of stem lignification or woodiness (Li et al., 2016; Dória et al., 2018; Thonglim et al., 2020). How exactly lignification would impact embolism spread in stems is the subject of ongoing research. One hypothesis is that the amount of lignification in secondary cell walls may determine gas diffusion kinetics across xylem cell walls and, therefore, could reduce the speed of embolism propagation in species with increased levels of lignification or woodiness (Li et al., 2016; Dória et al., 2018; Pereira et al., 2018; Thonglim et al., 2020; Lens et al., 2022). This may imply that older stems from herbaceous species could lead to increased embolism resistance, resulting from a possible increase in stem lignification and/or the amount of wood. In our study, this may especially apply to the p35S:AHL15 overexpression line, which has the ability to develop as much wood as the soc1ful double knockout genotype (Rahimi et al., 2022). However, this study shows that wood development is delayed in p35S:AHL15 (Supplementary Fig. S1E, G) compared with soc1ful in 80-day-old plants, despite the fact that SOC1, FUL, and AHL15 belong to the same wood pathway (Rahimi et al., 2022). Older individuals of p35S:AHL15 will therefore develop more wood and probably also thicker intervessel pit membranes in their inflorescence stems, most probably resulting in both higher embolism resistance and higher SSM, which synergistically may increase total plant tolerance of the overexpression line to the level of soc1ful.
In conclusion, there is a considerable difference in drought response among the six Arabidopsis genotypes studied. The genotypes soc1ful, Sha, and p35S:AHL15 synergistically increase their drought tolerance by building lignified inflorescence stems with thick intervessel pit membranes, developing the largest SSMs, keeping the water potential in their leaves pretty stable during periods of water shortage as a result of low stomatal conductance, maintaining relatively high chlorophyll content in rosette leaves, and by showing the lowest expression levels of drought-response genes compared with the control batch. In contrast, the most sensitive genotypes to drought (Cvi, Kel-4, and Col-0) are more susceptible to drought due to the opposite extreme of the same set of drought-responsive traits. This shows that stem anatomical traits and hydraulic stem and leaf traits are intertwined to acquire a certain level of drought tolerance. To further disentangle gene regulatory networks underlying drought-responsive traits across organs and to find out how they are linked with each other and synergistically strengthen the whole-plant drought response, future studies should combine a time series of gene expression data in roots, stems, and leaves during a drought experiment followed by rewatering. During such an experiment, a range of drought-responsive (anatomical and physiological) traits in all organs should be investigated. Only with this integrative approach, will we be able to make considerable progress in securing our food production by developing breeding tools that can make crops more drought tolerant and propose solutions on how to protect our herbs and forests under the current global change scenario.
Supplementary data
The following supplementary data are available at JXB online.
Table S1. Oligonucleotide sequences.
Table S2. The anatomical characters and hydraulic values measured with acronyms, definitions, calculations, microscope techniques, and units.
Table S3. The most parsimonious multiple linear regression model (based on AIC scores) of anatomical traits, explaining stem P50 variation of the six Arabidopsis thaliana genotypes studied.
Table S4. The most parsimonious multiple linear regression model (based on AIC scores) of anatomical traits explaining stem P12 variation of the six Arabidopsis thaliana genotypes studied.
Table S5. The most parsimonious multiple linear regression model (based on AIC scores) of anatomical traits explaining stem P88 variation of the six Arabidopsis thaliana genotypes studied.
Fig. S1. Growth form and cross-sections of inflorescence stems of p35S:AHL15 and Kel-4. Fig. S2. Leaf water potential and stomatal conductance dynamics during the drought experiment for each genotype.
Fig. S3. Boxplots showing P88 and P12 variation within and between genotypes.
Fig. S4. Boxplots showing anatomical variation within and between all genotypes.
Fig. S5. The relative importance of stem anatomical traits with respect to P12 and P88.
Fig. S6. The pairwise scatter plots based on Pearson’s correlation analysis showing the correlations of P50, P12, and P88 and each stem anatomical and hydraulic trait studied, and between all the predictive variables.
Acknowledgements
We would like to thank Alex Bos (IBL) for support with the gene expression analysis, Omid Karami and Arezoo Rahimi (IBL) for providing seeds and their knowledge in taking care of Arabidopsis plants, and Gaëlle Capdeville (BIOGECO INRA) for technical support.
Glossary
Abbreviations
- D
mean diameter of vessels
- Day90
days until reaching 90% of stomatal closure
- DMAX
maximum vessel lumen diameter
- g s
stomatal conductance
- P 12
xylem pressure inducing 12% loss of hydraulic conductivity
- P 50
xylem pressure inducing 50% loss of hydraulic conductivity
- P 88
xylem pressure inducing 88% loss of hydraulic conductivity
- PFWFA
proportion of fibre wall area per fibre cell area
- PLIG
proportion of lignified area per total stem area
- SSM
stomatal safety margin
- TPM
intervessel pit membrane thickness
- TV
vessel wall thickness
- (TVW/DMAX)2
theoretical vessel implosion resistance
- VD
vessel density
- VG
vessel grouping index
- Ψgs90
leaf water potential at 90% loss of stomatal conductance
- Ψl
leaf water potential
- Ψlh
leaf water potential at the harvesting day.
Contributor Information
Ajaree Thonglim, Naturalis Biodiversity Center, Research Group Functional Traits, PO Box 9517, 2300 RA Leiden, The Netherlands.
Giovanni Bortolami, Naturalis Biodiversity Center, Research Group Functional Traits, PO Box 9517, 2300 RA Leiden, The Netherlands.
Sylvain Delzon, BIOGECO INRA, Université Bordeaux, 33615 Pessac, France.
Maximilian Larter, BIOGECO INRA, Université Bordeaux, 33615 Pessac, France.
Remko Offringa, Leiden University, Institute of Biology Leiden, Plant Developmental Genetics, Sylviusweg 72, 2333 BE Leiden, The Netherlands.
Joost J B Keurentjes, Laboratory of Genetics, Wageningen University, Droevendaalsesteeg 1, 6708 PB Wageningen, The Netherlands.
Erik Smets, Naturalis Biodiversity Center, Research Group Functional Traits, PO Box 9517, 2300 RA Leiden, The Netherlands; Leiden University, Institute of Biology Leiden, Plant Sciences, Sylviusweg 72, 2333 BE Leiden, The Netherlands.
Salma Balazadeh, Leiden University, Institute of Biology Leiden, Molecular Plant Stress Biology, Sylviusweg 72, 2333 BE Leiden, The Netherlands.
Frederic Lens, Naturalis Biodiversity Center, Research Group Functional Traits, PO Box 9517, 2300 RA Leiden, The Netherlands; Leiden University, Institute of Biology Leiden, Plant Sciences, Sylviusweg 72, 2333 BE Leiden, The Netherlands.
Jianhua Zhang, Hong Kong Baptist University.
Author contributions
AT, GB, and FL: conceptualization; AT, GB, SD, ML, and SB: formal analysis; AT and FL: funding acquisition; AT: investigation; AT, GB, SD, ML, SB, and FL: methodology; SD, JK, RO, SB, and FL: resources; ES and FL: supervision; AT: visualization and writing—original draft; AT, GB, SD, ML, RO, JK, ES, SB, and FL: writing—review and editing.
Conflict of interest
No conflict of interest declared.
Funding
This work was funded by a PhD scholarship awarded by the Institute for the Promotion of Teaching Science and Technology (IPST), Thailand, and by the Dutch Research Council NWO (grant ALWOP.488).
Data availability
All data supporting the findings of this study are available within the paper and its supplementary data published online.
References
- Agarwal PK, Agarwal P, Reddy MK, Sopory SK.. 2006. Role of DREB transcription factors in abiotic and biotic stress tolerance in plants. Plant Cell Reports 25, 1263–1274. [DOI] [PubMed] [Google Scholar]
- Ahmad P. 2016. Water stress and crop plants: a sustainable approach. Chichester, UK: John Wiley & Sons, Ltd. [Google Scholar]
- Ak D. 2020. QTL analysis of the relationship between wood formation and drought tolerance in Arabidopsis thaliana. MSc thesis, Wagenigen University, The Netherlands.
- Allen CD, Macalady AK, Chenchouni H, et al. 2010. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management 259, 660–684. [Google Scholar]
- Anderegg WRL, Klein T, Bartlett M, Sack L, Pellegrini AFA, Choat B, Jansen S.. 2016. Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proceedings of the National Academy of Sciences, USA 113, 5024–5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson S, Kwasniewski M, Riaño-Pachón DM, Mueller-Roeber B.. 2008. QuantPrime—a flexible tool for reliable high-throughput primer design for quantitative PCR. BMC Bioinformatics 9, 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bac-Molenaar JA, Granier C, Keurentjes JJB, Vreugdenhil D.. 2016. Genome-wide association mapping of time-dependent growth responses to moderate drought stress in Arabidopsis. Plant, Cell & Environment 39, 88–102. [DOI] [PubMed] [Google Scholar]
- Balazadeh S, Riaño-Pachón DM, Mueller-Roeber B.. 2008. Transcription factors regulating leaf senescence in Arabidopsis thaliana. Plant Biology 10, 63–75. [DOI] [PubMed] [Google Scholar]
- Bartels D, Sunkar R.. 2005. Drought and salt tolerance in plants. Critical Reviews in Plant Sciences 24, 23–58. [Google Scholar]
- Bauer H, Ache P, Lautner S, et al. 2013. The stomatal response to reduced relative humidity requires guard cell-autonomous ABA synthesis. Current Biology 23, 53–57. [DOI] [PubMed] [Google Scholar]
- Bauerle WL, Whitlow TH, Setter TL, Vermeylen FM.. 2004. Abscisic acid synthesis in Acer rubrum L. leaves—a vapor pressure deficit mediated response. Journal of the American Society for Horticultural Science 129, 182–187. [Google Scholar]
- Bhargava S, Sawant K.. 2013. Drought stress adaptation: metabolic adjustment and regulation of gene expression. Plant Breeding 132, 21–32. [Google Scholar]
- Bouchabke O, Chang F, Simon M, Voisin R, Pelletier G, Durand-Tardif M.. 2008. Natural variation in Arabidopsis thaliana as a tool for highlighting differential drought responses. PLoS One 3, e1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen CR, McElrone AJ, Choat B, Lee EF, Shackel KA, Matthews MA.. 2013. In vivo visualizations of drought-induced embolism spread in Vitis vinifera. Plant Physiology 161, 1820–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Bienaimé D, Marmottant P.. 2016. Revealing catastrophic failure of leaf networks under stress. Proceedings of the National Academy of Sciences, USA 113, 4865–4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb T, Brodersen CR, Carriqui M, Tonet V, Rodriguez Dominguez C, McAdam S.. 2021. Linking xylem network failure with leaf tissue death. New Phytologist 232, 68–79. [DOI] [PubMed] [Google Scholar]
- Brodribb T, Hill RS.. 1999. The importance of xylem constraints in the distribution of conifer species. New Phytologist 143, 365–372. [Google Scholar]
- Brodribb TJ, Holbrook NM, Edwards EJ, Gutiérrez MV.. 2003. Relations between stomatal closure, leaf turgor and xylem vulnerability in eight tropical dry forest trees: stomatal closure and xylem cavitation. Plant, Cell & Environment 26, 443–450. [Google Scholar]
- Brodribb TJ, McAdam SA, Carins Murphy MR.. 2017. Xylem and stomata, coordinated through time and space: functional linkages between xylem and stomata. Plant, Cell & Environment 40, 872–880. [DOI] [PubMed] [Google Scholar]
- Buckley TN. 2019. How do stomata respond to water status? New Phytologist 224, 21–36. [DOI] [PubMed] [Google Scholar]
- Cardoso AA, Brodribb TJ, Lucani CJ, DaMatta FM, McAdam SAM.. 2018. Coordinated plasticity maintains hydraulic safety in sunflower leaves. Plant, Cell & Environment 41, 2567–2576. [DOI] [PubMed] [Google Scholar]
- Charrier G, Delzon S, Domec J-C, et al. 2018. Drought will not leave your glass empty: low risk of hydraulic failure revealed by long-term drought observations in world’s top wine regions. Science Advances 4, eaao6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Li G, Bressan RA, Song C, Zhu J, Zhao Y.. 2020. Abscisic acid dynamics, signaling, and functions in plants. Journal of Integrative Plant Biology 62, 25–54. [DOI] [PubMed] [Google Scholar]
- Chen Z, Zhang Y, Yuan W, Zhu S, Pan R, Wan X, Liu S.. 2021. Coordinated variation in stem and leaf functional traits of temperate broadleaf tree species in the isohydric–anisohydric spectrum. Tree Physiology 41, 1601–1610. [DOI] [PubMed] [Google Scholar]
- Choat B, Badel E, Burlett R, Delzon S, Cochard H, Jansen S.. 2016. Noninvasive measurement of vulnerability to drought-induced embolism by x-ray microtomography. Plant Physiology 170, 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choat B, Jansen S, Brodribb TJ, et al. 2012. Global convergence in the vulnerability of forests to drought. Nature 491, 752–755. [DOI] [PubMed] [Google Scholar]
- Choi H, Hong J, Ha J, Kang J, Kim SY.. 2000. ABFs, a family of ABA-responsive element binding factors. Journal of Biological Chemistry 275, 1723–1730. [DOI] [PubMed] [Google Scholar]
- Cochard H. 2002. A technique for measuring xylem hydraulic conductance under high negative pressures. Plant, Cell & Environment 25, 815–819. [Google Scholar]
- Cochard H. 2006. Cavitation in trees. Comptes Rendus Physique 7, 1018–1026. [Google Scholar]
- Cochard H, Badel E, Herbette S, Delzon S, Choat B, Jansen S.. 2013. Methods for measuring plant vulnerability to cavitation: a critical review. Journal of Experimental Botany 64, 4779–4791. [DOI] [PubMed] [Google Scholar]
- Cochard H, Damour G, Bodet C, Tharwat I, Poirier M, Améglio T.. 2005. Evaluation of a new centrifuge technique for rapid generation of xylem vulnerability curves. Physiologia Plantarum 124, 410–418. [Google Scholar]
- Cochard H, Froux F, Mayr S, Coutand C.. 2004. Xylem wall collapse in water-stressed pine needles. Plant Physiology 134, 401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combe M, de Arellano JV-G, Ouwersloot HG, Peters W.. 2016. Plant water-stress parameterization determines the strength of land–atmosphere coupling. Agricultural and Forest Meteorology 217, 61–73. [Google Scholar]
- Corso D, Delzon S, Lamarque LJ, Cochard H, Torres-Ruiz JM, King A, Brodribb T.. 2020. Neither xylem collapse, cavitation, or changing leaf conductance drive stomatal closure in wheat. Plant, Cell & Environment 43, 854–865. [DOI] [PubMed] [Google Scholar]
- Creek D, Lamarque LJ, Torres-Ruiz JM, Parise C, Burlett R, Tissue DT, Delzon S.. 2020. Xylem embolism in leaves does not occur with open stomata: evidence from direct observations using the optical visualization technique. Journal of Experimental Botany 71, 1151–1159. [DOI] [PubMed] [Google Scholar]
- Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR.. 2010. Abscisic acid: emergence of a core signaling network. Annual Review of Plant Biology 61, 651–679. [DOI] [PubMed] [Google Scholar]
- Davis SD, Ewers FW, Sperry JS, Portwood KA, Crocker MC, Adams GC.. 2002. Shoot dieback during prolonged drought in Ceanothus (Rhamnaceae) chaparral of California: a possible case of hydraulic failure. American Journal of Botany 89, 820–828. [DOI] [PubMed] [Google Scholar]
- Dayer S, Herrera JC, Dai Z, Burlett R, Lamarque LJ, Delzon S, Bortolami G, Cochard H, Gambetta GA.. 2020. The sequence and thresholds of leaf hydraulic traits underlying grapevine varietal differences in drought tolerance. Journal of Experimental Botany 71, 4333–4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer S, Lamarque LJ, Burlett R, Bortolami G, Delzon S, Herrera JC, Cochard H, Gambetta GA.. 2022. Model-assisted ideotyping reveals trait syndromes to adapt viticulture to a drier climate. Plant Physiology 190, 1673–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delzon S, Cochard H.. 2014. Recent advances in tree hydraulics highlight the ecological significance of the hydraulic safety margin. New Phytologist 203, 355–358. [DOI] [PubMed] [Google Scholar]
- Dodd IC. 2013. Abscisic acid and stomatal closure: a hydraulic conductance conundrum? New Phytologist 197, 6–8. [DOI] [PubMed] [Google Scholar]
- Dória LC, Podadera DS, Arco M, Chauvin T, Smets E, Delzon S, Lens F.. 2018. Insular woody daisies (Argyranthemum, Asteraceae) are more resistant to drought-induced hydraulic failure than their herbaceous relatives. Functional Ecology 32, 1467–1478. [Google Scholar]
- Ebrahimian-Motlagh S, Ribone PA, Thirumalaikumar VP, Allu AD, Chan RL, Mueller-Roeber B, Balazadeh S.. 2017. JUNGBRUNNEN1 confers drought tolerance downstream of the HD-Zip I transcription factor AtHB13. Frontiers in Plant Science 8, 2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eller C, de Barros FV, Bittencourt P RL, Rowland L, Mencuccini M, Oliveira R S.. 2018. Xylem hydraulic safety and construction costs determine tropical tree growth: tree growth vs hydraulic safety trade-off. Plant, Cell & Environment 41, 548–562. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Fujita M, Shinozaki K, Yamaguchi-Shinozaki K.. 2011. ABA-mediated transcriptional regulation in response to osmotic stress in plants. Journal of Plant Research 124, 509–525. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Yoshida T, Yamaguchi-Shinozaki K.. 2013. Pivotal role of the AREB/ABF–SnRK2 pathway in ABRE-mediated transcription in response to osmotic stress in plants. Physiologia Plantarum 147, 15–27. [DOI] [PubMed] [Google Scholar]
- Gleason SM, Barnard DM, Green TR, et al. 2022. Physiological trait networks enhance understanding of crop growth and water use in contrasting environments. Plant, Cell & Environment 45, 2554–2572. [DOI] [PubMed] [Google Scholar]
- Guan X, Werner J, Cao KF, Pereira L, Kaack L, McAdam SAM, Jansen S.. 2022. Stem and leaf xylem of angiosperm trees experiences minimal embolism in temperate forests during two consecutive summers with moderate drought. Plant Biology plb.13384. [DOI] [PubMed] [Google Scholar]
- Hamann T, Smets E, Lens F.. 2011. A comparison of paraffin and resin-based techniques used in bark anatomy. Taxon 60, 841–851. [Google Scholar]
- Hammond WM, Yu K, Wilson LA, Will RE, Anderegg WRL, Adams HD.. 2019. Dead or dying? Quantifying the point of no return from hydraulic failure in drought-induced tree mortality. New Phytologist 223, 1834–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway-Phillips M-M, Brodribb TJ.. 2011. Minimum hydraulic safety leads to maximum water-use efficiency in a forage grass. Plant, Cell & Environment 34, 302–313. [DOI] [PubMed] [Google Scholar]
- Hou N, Li C, He J, Liu Y, Yu S, Malnoy M, Mobeen Tahir M, Xu L, Ma F, Guan Q.. 2022. MdMTA-mediated m6 A modification enhances drought tolerance by promoting mRNA stability and translation efficiency of genes involved in lignin deposition and oxidative stress. New Phytologist 234, 1294–1314. [DOI] [PubMed] [Google Scholar]
- Janiak A, Kwaśniewski M, Szarejko I.. 2016. Gene expression regulation in roots under drought. Journal of Experimental Botany 67, 1003–1014. [DOI] [PubMed] [Google Scholar]
- Jansen S, Choat B, Pletsers A.. 2009. Morphological variation of intervessel pit membranes and implications to xylem function in angiosperms. American Journal of Botany 96, 409–419. [DOI] [PubMed] [Google Scholar]
- Johnson DM, Katul G, Domec J.. 2022. Catastrophic hydraulic failure and tipping points in plants. Plant, Cell & Environment 45, 2231–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KM, Lucani C, Brodribb TJ.. 2021. In vivo monitoring of drought-induced embolism in Callitris rhomboidea trees reveals wide variation in branchlet vulnerability and high resistance to tissue death. New Phytologist 233, 207–218. [DOI] [PubMed] [Google Scholar]
- Johnson DM, McCulloh KA, Woodruff DR, Meinzer FC.. 2012. Hydraulic safety margins and embolism reversal in stems and leaves: why are conifers and angiosperms so different? Plant Science 195, 48–53. [DOI] [PubMed] [Google Scholar]
- Kaack L, Altaner CM, Carmesin C, et al. 2019. Function and three-dimensional structure of intervessel pit membranes in angiosperms: a review. IAWA Journal 40, 673–702. [Google Scholar]
- Kaack L, Weber M, Isasa E, et al. 2021. Pore constrictions in intervessel pit membranes provide a mechanistic explanation for xylem embolism resistance in angiosperms. New Phytologist 230, 1829–1843. [DOI] [PubMed] [Google Scholar]
- Karami O, Rahimi A, Mak P, Horstman A, Boutilier K, Compier M, van der Zaal B, Offringa R.. 2021. An Arabidopsis AT-hook motif nuclear protein mediates somatic embryogenesis and coinciding genome duplication. Nature Communications 12, 2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky MJ. 1965. A formaldehyde–glutaraldehyde fixative of high osmolality for use in electron microscopy. Journal of Cell Biology 27, 137–138A. [Google Scholar]
- Kitin P, Voelker SL, Meinzer FC, Beeckman H, Strauss SH, Lachenbruch B.. 2010. Tyloses and phenolic deposits in xylem vessels impede water transport in low-lignin transgenic poplars: a study by cryo-fluorescence microscopy. Plant Physiology 154, 887–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepsch M, Zhang Y, Kotowska MM, et al. 2018. Is xylem of angiosperm leaves less resistant to embolism than branches? Insights from microCT, hydraulics, and anatomy. Journal of Experimental Botany 69, 5611–5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipfer T, Bambach N, Hernandez MI, Bartlett MK, Sinclair G, Duong F, Kluepfel DA, McElrone AJ.. 2020. Predicting stomatal closure and turgor loss in woody plants using predawn and midday water potential. Plant Physiology 184, 881–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipfer T, Eustis A, Brodersen C, Walker AM, Mcelrone AJ.. 2015. Grapevine species from varied native habitats exhibit differences in embolism formation/repair associated with leaf gas exchange and root pressure: contrasting response of wild grapevines to drought stress. Plant, Cell & Environment 38, 1503–1513. [DOI] [PubMed] [Google Scholar]
- Kooke R, Kruijer W, Bours R, et al. 2016. Genome-wide association mapping and genomic prediction elucidate the genetic architecture of morphological traits in Arabidopsis. Plant Physiology 170, 2187–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarque LJ, Corso D, Torres-Ruiz JM, et al. 2018. An inconvenient truth about xylem resistance to embolism in the model species for refilling Laurus nobilis L. Annals of Forest Science 75, 1–5. [Google Scholar]
- Lamarque LJ, Delzon S, Toups H, et al. 2020. Over-accumulation of abscisic acid in transgenic tomato plants increases the risk of hydraulic failure. Plant, Cell & Environment 43, 548–562. [DOI] [PubMed] [Google Scholar]
- Lemaire C, Blackman CJ, Cochard H, Menezes-Silva PE, Torres-Ruiz JM, Herbette S.. 2021. Acclimation of hydraulic and morphological traits to water deficit delays hydraulic failure during simulated drought in poplar. Tree Physiology 41, 2008–2021. [DOI] [PubMed] [Google Scholar]
- Lens F, Gleason SM, Bortolami G, Brodersen C, Delzon S, Jansen S.. 2022. Functional xylem characteristics associated with drought-induced embolism in angiosperms. New Phytologist doi: 10.1111/nph.18447. [DOI] [PubMed] [Google Scholar]
- Lens F, Picon-Cochard C, Delmas CE, et al. 2016. Herbaceous angiosperms are not more vulnerable to drought-induced embolism than angiosperm trees. Plant Physiology 172, 661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lens F, Smets E, Melzer S.. 2012. Stem anatomy supports Arabidopsis thaliana as a model for insular woodiness: Letter. New Phytologist 193, 12–17. [DOI] [PubMed] [Google Scholar]
- Lens F, Sperry JS, Christman MA, Choat B, Rabaey D, Jansen S.. 2011. Testing hypotheses that link wood anatomy to cavitation resistance and hydraulic conductivity in the genus Acer. New Phytologist 190, 709–723. [DOI] [PubMed] [Google Scholar]
- Lens F, Tixier A, Cochard H, Sperry JS, Jansen S, Herbette S.. 2013. Embolism resistance as a key mechanism to understand adaptive plant strategies. Current Opinion in Plant Biology 16, 287–292. [DOI] [PubMed] [Google Scholar]
- Levionnois S, Jansen S, Wandji RT, Beauchêne J, Ziegler C, Coste S, Stahl C, Delzon S, Authier L, Heuret P.. 2021. Linking drought-induced xylem embolism resistance to wood anatomical traits in Neotropical trees. New Phytologist 229, 1453–1466. [DOI] [PubMed] [Google Scholar]
- Li D, Yang J, Pak S, Zeng M, Sun J, Yu S, He Y, Li C.. 2022. PuC3H35 confers drought tolerance by enhancing lignin and proanthocyanidin biosynthesis in the roots of Populus ussuriensis. New Phytologist 233, 390–408. [DOI] [PubMed] [Google Scholar]
- Li S, Feifel M, Karimi Z, Schuldt B, Choat B, Jansen S.. 2015. Leaf gas exchange performance and the lethal water potential of five European species during drought. Tree Physiology 36, 179–192. [DOI] [PubMed] [Google Scholar]
- Li S, Lens F, Espino S, Karimi Z, Klepsch M, Schenk HJ, Schmitt M, Schuldt B, Jansen S.. 2016. Intervessel pit membrane thickness as a key determinant of embolism resistance in angiosperm xylem. IAWA Journal 37, 152–171. [Google Scholar]
- Li Y, Li H, Li Y, Zhang S.. 2017. Improving water-use efficiency by decreasing stomatal conductance and transpiration rate to maintain higher ear photosynthetic rate in drought-resistant wheat. The Crop Journal 5, 231–239. [Google Scholar]
- Limousin J, Roussel A, Rodríguez-Calcerrada J, Torres-Ruiz JM, Moreno M, de Jalon LG, Ourcival J, Simioni G, Cochard H, Martin-StPaul N.. 2022. Drought acclimation of Quercus ilex leaves improves tolerance to moderate drought but not resistance to severe water stress. Plant, Cell & Environment 45, 1967–1984. [DOI] [PubMed] [Google Scholar]
- Mantova M, Herbette S, Cochard H, Torres-Ruiz JM.. 2022. Hydraulic failure and tree mortality: from correlation to causation. Trends in Plant Science 27, 335–345. [DOI] [PubMed] [Google Scholar]
- Mantova M, Menezes-Silva PE, Badel E, Cochard H, Torres-Ruiz JM.. 2021. The interplay of hydraulic failure and cell vitality explains tree capacity to recover from drought. Physiologia Plantarum 172, 247–257. [DOI] [PubMed] [Google Scholar]
- Martínez-Vilalta J, Garcia-Forner N.. 2017. Water potential regulation, stomatal behaviour and hydraulic transport under drought: deconstructing the iso/anisohydric concept. Plant, Cell & Environment 40, 962–976. [DOI] [PubMed] [Google Scholar]
- Martin-StPaul N, Delzon S, Cochard H.. 2017. Plant resistance to drought depends on timely stomatal closure. Ecology Letters 20, 1437–1447. [DOI] [PubMed] [Google Scholar]
- McDowell NG. 2011. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiology 155, 1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell N, Pockman WT, Allen CD, et al. 2008. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytologist 178, 719–739. [DOI] [PubMed] [Google Scholar]
- Mehrotra R, Bhalothia P, Bansal P, Basantani MK, Bharti V, Mehrotra S.. 2014. Abscisic acid and abiotic stress tolerance—different tiers of regulation. Journal of Plant Physiology 171, 486–496. [DOI] [PubMed] [Google Scholar]
- Meinzer FC, Johnson DM, Lachenbruch B, McCulloh KA, Woodruff DR.. 2009. Xylem hydraulic safety margins in woody plants: coordination of stomatal control of xylem tension with hydraulic capacitance. Functional Ecology 23, 922–930. [Google Scholar]
- Melzer S, Lens F, Gennen J, Vanneste S, Rohde A, Beeckman T.. 2008. Flowering-time genes modulate meristem determinacy and growth form in Arabidopsis thaliana. Nature Genetics 40, 1489–1492. [DOI] [PubMed] [Google Scholar]
- Mitchell PJ, O’Grady AP, Tissue DT, White DA, Ottenschlaeger ML, Pinkard EA.. 2013. Drought response strategies define the relative contributions of hydraulic dysfunction and carbohydrate depletion during tree mortality. New Phytologist 197, 862–872. [DOI] [PubMed] [Google Scholar]
- Oliveira RS, Eller CB, Barros F de V, Hirota M, Brum M, Bittencourt P.. 2021. Linking plant hydraulics and the fast–slow continuum to understand resilience to drought in tropical ecosystems. New Phytologist 230, 904–923. [DOI] [PubMed] [Google Scholar]
- Pammenter NW, Van der Willigen C.. 1998. A mathematical and statistical analysis of the curves illustrating vulnerability of xylem to cavitation. Tree Physiology 18, 589–593. [DOI] [PubMed] [Google Scholar]
- Pandey N, Ranjan A, Pant P, Tripathi RK, Ateek F, Pandey HP, Patre UV, Sawant SV.. 2013. CAMTA 1 regulates drought responses in Arabidopsis thaliana. BMC Genomics 14, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L, Domingues-Junior AP, Jansen S, Choat B, Mazzafera P.. 2018. Is embolism resistance in plant xylem associated with quantity and characteristics of lignin? Trees 32, 349–358. [Google Scholar]
- Plavcová L, Hacke UG.. 2012. Phenotypic and developmental plasticity of xylem in hybrid poplar saplings subjected to experimental drought, nitrogen fertilization, and shading. Journal of Experimental Botany 63, 6481–6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plavcová L, Jansen S, Klepsch M, Hacke UG.. 2013. Nobody’s perfect: can irregularities in pit structure influence vulnerability to cavitation? Frontiers in Plant Science 4, 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE.. 1989. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochimica et Biophysica Acta 975, 384–394. [Google Scholar]
- Pratt RB, Castro V, Fickle JC, Madsen A, Jacobsen AL.. 2020. Factors controlling drought resistance in grapevine (Vitis vinifera, Chardonnay): application of a new micro ct method to assess functional embolism resistance. American Journal of Botany 107, 618–627. [DOI] [PubMed] [Google Scholar]
- Rahimi A, Karami O, Lestari AD, de Werk T, Amakorová P, Shi D, Novák O, Greb T, Offringa R.. 2022. Control of cambium initiation and activity in Arabidopsis by the transcriptional regulator AHL15. Current Biology 32, 1764–1775. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Roca-Paixão JF, Gillet F-X, Ribeiro TP, Bournaud C, Lourenço-Tessutti IT, Noriega DD, de Melo BP, de Almeida-Engler J, Grossi-de-Sa MF.. 2019. Improved drought stress tolerance in Arabidopsis by CRISPR/dCas9 fusion with a Histone AcetylTransferase. Scientific Reports 9, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Dominguez CM, Carins Murphy MR, Lucani C, Brodribb TJ.. 2018. Mapping xylem failure in disparate organs of whole plants reveals extreme resistance in olive roots. New Phytologist 218, 1025–1035. [DOI] [PubMed] [Google Scholar]
- Roman DT, Novick KA, Brzostek ER, Dragoni D, Rahman F, Phillips RP.. 2015. The role of isohydric and anisohydric species in determining ecosystem-scale response to severe drought. Oecologia 179, 641–654. [DOI] [PubMed] [Google Scholar]
- Rushton DL, Tripathi P, Rabara RC, et al. 2012. WRKY transcription factors: key components in abscisic acid signalling. Plant Biotechnology Journal 10, 2–11. [DOI] [PubMed] [Google Scholar]
- Sakuma Y, Maruyama K, Osakabe Y, Qin F, Seki M, Shinozaki K, Yamaguchi-Shinozaki K.. 2006. Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. The Plant Cell 18, 1292–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz A, Klepsch M, Karimi Z, Jansen S.. 2013a. How to quantify conduits in wood? Frontiers in Plant Science 4, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz A, Rabaey D, Stein A, Cochard H, Smets E, Jansen S.. 2013b. The evolution and function of vessel and pit characters with respect to cavitation resistance across 10 Prunus species. Tree Physiology 33, 684–694. [DOI] [PubMed] [Google Scholar]
- Schuldt B, Knutzen F, Delzon S, Jansen S, Müller-Haubold H, Burlett R, Clough Y, Leuschner C.. 2016. How adaptable is the hydraulic system of European beech in the face of climate change-related precipitation reduction? New Phytologist 210, 443–458. [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K.. 2007. Gene networks involved in drought stress response and tolerance. Journal of Experimental Botany 58, 221–227. [DOI] [PubMed] [Google Scholar]
- Singh D, Laxmi A.. 2015. Transcriptional regulation of drought response: a tortuous network of transcriptional factors. Frontiers in Plant Science 6, 895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton RP, Anderegg LDL, Diaz J, Kling MM, Papper P, Lamarque LJ, Delzon S, Dawson TE, Ackerly DD.. 2021. Evolutionary relationships between drought-related traits and climate shape large hydraulic safety margins in western North American oaks. Proceedings of the National Academy of Sciences, USA 118, e2008987118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton RP, Anderegg LDL, Papper P, Reich E, Dawson TE, Kling M, Thompson SE, Diaz J, Ackerly DD.. 2019. No local adaptation in leaf or stem xylem vulnerability to embolism, but consistent vulnerability segmentation in a North American oak. New Phytologist 223, 1296–1306. [DOI] [PubMed] [Google Scholar]
- Skelton RP, Brodribb TJ, Choat B.. 2017. Casting light on xylem vulnerability in an herbaceous species reveals a lack of segmentation. New Phytologist 214, 561–569. [DOI] [PubMed] [Google Scholar]
- Skelton RP, Dawson TE, Thompson SE, Shen Y, Weitz AP, Ackerly D.. 2018. Low vulnerability to xylem embolism in leaves and stems of North American oaks. Plant Physiology 177, 1066–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton RP, West AG, Dawson TE.. 2015. Predicting plant vulnerability to drought in biodiverse regions using functional traits. Proceedings of the National Academy of Sciences, USA 112, 5744–5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Huang SC, Wise A, Castanon R, Nery JR, Chen H, Watanabe M, Thomas J, Bar-Joseph Z, Ecker JR.. 2016. A transcription factor hierarchy defines an environmental stress response network. Science 354, aag1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Lee J, Kim T, Hong JC, Lim CO.. 2018. VOZ1, a transcriptional repressor of DREB2C, mediates heat stress responses in Arabidopsis. Planta 247, 1439–1448. [DOI] [PubMed] [Google Scholar]
- Sperry JS, Tyree MT.. 1988. Mechanism of water stress-induced xylem embolism. Plant Physiology 88, 581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiller V, Sperry JS.. 2002. Cavitation fatigue and its reversal in sunflower (Helianthus annuus L.). Journal of Experimental Botany 53, 1155–1161. [DOI] [PubMed] [Google Scholar]
- Thirumalaikumar VP, Devkar V, Mehterov N, Ali S, Ozgur R, Turkan I, Mueller-Roeber B, Balazadeh S.. 2018. NAC transcription factor JUNGBRUNNEN1 enhances drought tolerance in tomato. Plant Biotechnology Journal 16, 354–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoen MPM, Davila Olivas NH, Kloth KJ, et al. 2017. Genetic architecture of plant stress resistance: multi-trait genome-wide association mapping. New Phytologist 213, 1346–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thonglim A, Delzon S, Larter M, Karami O, Rahimi A, Offringa R, Keurentjes JJB, Balazadeh S, Smets E, Lens F.. 2020. Intervessel pit membrane thickness best explains variation in embolism resistance amongst stems of Arabidopsis thaliana accessions. Annals of Botany 128, 171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tixier A, Cochard H, Badel E, Dusotoit-Coucaud A, Jansen S, Herbette S.. 2013. Arabidopsis thaliana as a model species for xylem hydraulics: does size matter? Journal of Experimental Botany 64, 2295–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Ruiz JM, Cochard H, Fonseca E, Badel E, Gazarini L, Vaz M.. 2017. Differences in functional and xylem anatomical features allow Cistus species to co-occur and cope differently with drought in the Mediterranean region. Tree Physiology 37, 755–766. [DOI] [PubMed] [Google Scholar]
- Trueba S, Delzon S, Isnard S, Lens F.. 2019. Similar hydraulic efficiency and safety across vesselless angiosperms and vessel-bearing species with scalariform perforation plates. Journal of Experimental Botany 70, 3227–3240. [DOI] [PubMed] [Google Scholar]
- Tu M, Wang X, Yin W, Wang Y, Li Y, Zhang G, Li Z, Song J, Wang X.. 2020. Grapevine VlbZIP30 improves drought resistance by directly activating VvNAC17 and promoting lignin biosynthesis through the regulation of three peroxidase genes. Horticulture Research 7, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Zimmermann MH.. 2002. Xylem structure and the ascent of sap. Berlin, Heidelberg: Springer Berlin Heidelberg. [Google Scholar]
- Umezawa T, Nakashima K, Miyakawa T, Kuromori T, Tanokura M, Shinozaki K, Yamaguchi-Shinozaki K.. 2010. Molecular basis of the core regulatory network in ABA responses: sensing, signaling and transport. Plant & Cell Physiology 51, 1821–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno Y, Furihata T, Abe H, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K.. 2000. Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proceedings of the National Academy of Sciences, USA 97, 11632–11637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urli M, Porte AJ, Cochard H, Guengant Y, Burlett R, Delzon S.. 2013. Xylem embolism threshold for catastrophic hydraulic failure in angiosperm trees. Tree Physiology 33, 672–683. [DOI] [PubMed] [Google Scholar]
- Venturas MD, MacKinnon ED, Dario HL, Jacobsen AL, Pratt RB, Davis SD.. 2016. Chaparral shrub hydraulic traits, size, and life history types relate to species mortality during California’s historic drought of 2014. PLoS One 11, e0159145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturas MD, Sperry JS, Hacke UG.. 2017. Plant xylem hydraulics: what we understand, current research, and future challenges. Journal of Integrative Plant Biology 59, 356–389. [DOI] [PubMed] [Google Scholar]
- Volaire F, Lens F, Cochard H, Xu H, Chacon-Doria L, Bristiel P, Balachowski J, Rowe N, Violle C, Picon-Cochard C.. 2018. Embolism and mechanical resistances play a key role in dehydration tolerance of a perennial grass Dactylis glomerata L. Annals of Botany 122, 325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W, Wang R, Su L, Lv A, Zhou P, An Y.. 2021. MsWRKY11, activated by MsWRKY22, functions in drought tolerance and modulates lignin biosynthesis in alfalfa (Medicago sativa L.). Environmental and Experimental Botany 184, 104373. [Google Scholar]
- Xu W, Tang W, Wang C, et al. 2020. SiMYB56 confers drought stress tolerance in transgenic rice by regulating lignin biosynthesis and ABA signaling pathway. Frontiers in Plant Science 11, 785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Wang P, Lu Y, Bai Y, Wei Y, Liu G, Shi H.. 2021. MeRAV5 promotes drought stress resistance in cassava by modulating hydrogen peroxide and lignin accumulation. The Plant Journal 107, 847–860. [DOI] [PubMed] [Google Scholar]
- Yang J, Michaud J M, Jansen S, Schenk HJ, Zuo YY.. 2020. Dynamic surface tension of xylem sap lipids. Tree Physiology 40, 433–444. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Fujita Y, Maruyama K, Mogami J, Todaka D, Shinozaki K, Yamaguchi-Shinozaki K.. 2015. Four Arabidopsis AREB ABF transcription factors function predominantly in gene expression downstream of SnRK2 kinases in abscisic acid signalling in response to osmotic stress. Plant, Cell & Environment 38, 35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Fujita Y, Sayama H, Kidokoro S, Maruyama K, Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K.. 2010. AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. The Plant Journal 61, 672–685. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Carmesin C, Kaack L, et al. 2020. High porosity with tiny pore constrictions and unbending pathways characterize the 3D structure of intervessel pit membranes in angiosperm xylem. Plant, Cell & Environment 43, 116–130. [DOI] [PubMed] [Google Scholar]
- Zhang P, Fan Y, Sun X, Chen L, Terzaghi W, Bucher E, Li L, Dai M.. 2019. A large-scale circular RNA profiling reveals universal molecular mechanisms responsive to drought stress in maize and Arabidopsis. The Plant Journal 98, 697–713. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available within the paper and its supplementary data published online.