Abstract
Background:
Idiopathic spinal epidural arachnoid cysts (SEACs) are rare and may cause myelopathy and cord compression. They typically arise from a congenital defect in the dura that communicates with the intrathecal subarachnoid space. Although the ideal treatment of SEACs is direct dural repair and cyst excision, there is as yet no clear standard of care for the management of these lesions.
Methods:
A 47-year-old female presented with myelopathy attributed to an magnetic resonance imaging-documented posterior epidural T12-L2 cyst (i.e., 1.1 × 6 × 3.3 cm) lesion. The patient underwent a direct dural repair of the fistulous communication between the subarachnoid space and the cyst, along with cyst drainage/ excision through a right-sided laminotomy. Postoperatively, the patient was asymptomatic. We additionally reviewed the literature regarding the management of SEACs.
Results:
Our review yielded 14 articles involving 18 patients with predominantly thoracolumbar (57%) SEACs that were either communicating (61%) or not communicating (39%) with the subarachnoid space. They averaged 35.5 years of age and exhibited a male preponderance (66%). Symptoms typically included pain (78%), followed by weakness/myelopathy (42%). Surgery frequently included bilateral laminectomies (57%) followed by unilateral laminectomies (50%) that typically resulted in symptom resolution.
Conclusion:
SEACs are rare typically thoracolumbar lesions that may cause myelopathy which resolves following direct dural closure/subarachnoid fistulous occlusion
Keywords: Arachnoid cysts, Epidural, Idiopathic, Spine
INTRODUCTION
Spinal epidural arachnoid cysts (SEACs), characterized by a dural defect, typically arise without a previous history of trauma, surgery, infection, or inflammation.[1,2] They account for 1% to 3% of all spinal tumors and are usually localized to the thoracic spine in adult males where they may contribute to myelopathy.[1,2,7,9,11] SEACs are classified using Nabor’s 3 criteria: (1) Type I: Extradural meningeal cysts that contain no neural tissue; with two subtypes, extradural arachnoid cysts (Type Ia) and sacral meningoceles (Type Ib); (2) Type II: Extradural meningeal cysts with verve root fibers (Tarlov perineurial cysts); and (3) Type III: Intradural meningeal cysts.[2,4] Here, we present a 47-year-old female with a T12-L2 SEAC and reviewed the literature findings that included 18 other cases.
MATERIALS AND METHODS
Literature search strategy
We used the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines and performed a literature review of PubMed (1976–2021) using “spinal,” “epidural,” and “arachnoid cyst.” Multiple variables were studied, along with a review of one or our own cases.
Case presentation
A 47-year-old female presented with a progressive lower extremity paraparesis of 4-month duration (i.e., pain, numbness, urge incontinence, weakness left 3/5- right 4/5 proximal/left 1/5 and right 2/5 distal), and a L1 pin level. The thoracolumbar magnetic resonance imaging (MRI) revealed a posterior epidural T12-L2 cystic lesion measuring 1.1 × 6 × 3.3 cm [Figure 1]. She underwent a T12-L2 right unilateral laminotomy for bilateral decompression/occlusion of the cyst [Figure 2]. Pathologically, the lesion was a Class 2 Nabor arachnoid cyst [Figure 3]. Immediately postoperatively, and 1 year later, the patient’s symptoms improved/resolved with follow-up MR studies confirming complete cyst resection [Figures 4 and 5].
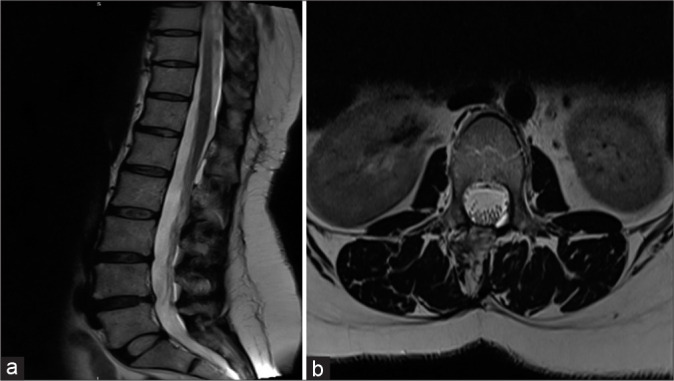
Figure 1:
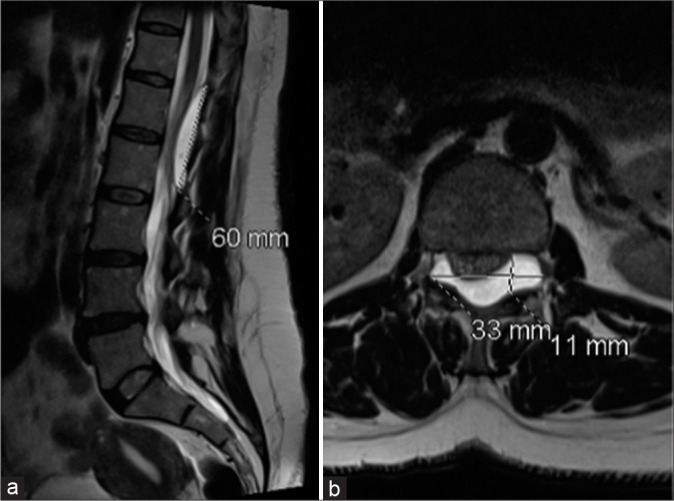
(a) T2-W magnetic resonance imaging (MRI) sagittal (a) and axial (b) images disclose a well-defined uniformly hyperintense (identical to CSF) cystic lesion extending from the lower end of the T12 vertebral body to the lower end of L2 vertebral body causing a mass effect and compression over the cord. The cystic structure is exiting through the bilateral L1-L2 exiting neural foramina and causing mass effect on the exiting nerve roots.
Figure 2:
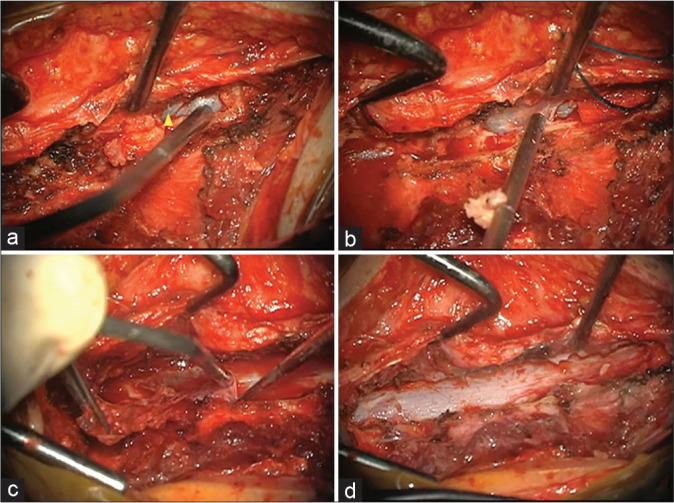
(a) Intraoperative images of surgical exposure and cyst resection. (a) Unilateral right approach and preliminary exposure of the cranial portion of the cyst (Arrowhead). (b) Progressive resection of the cyst capsule. (c) Dissection and resection of the lateral recess portion of the cyst. (d) Complete bilateral resection of the cyst through a unilateral approach.
Figure 3:
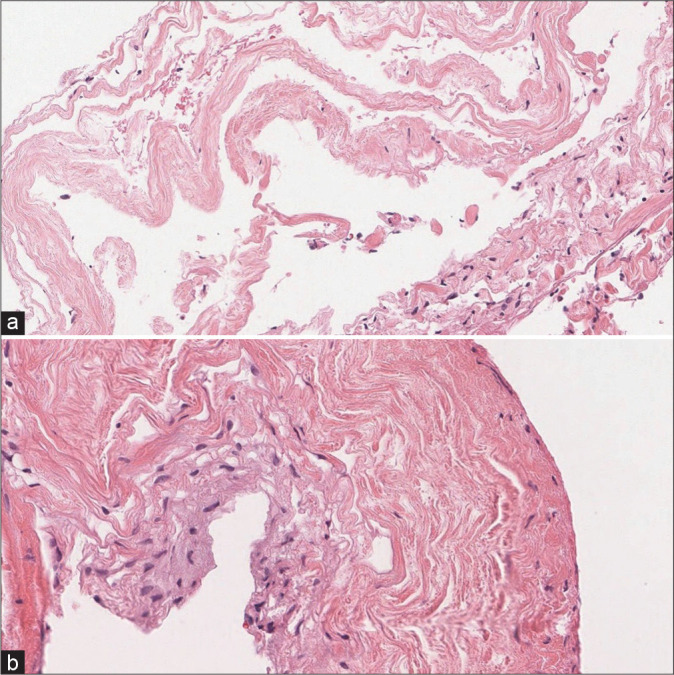
Histopathological examination revealing (a) the inner layer is the cyst lining, composed of meningothelial cells and is partially denuded. (b) The outer layer is composed of supportive fibrous tissue consisting of arachnoid cyst.
Figure 4:
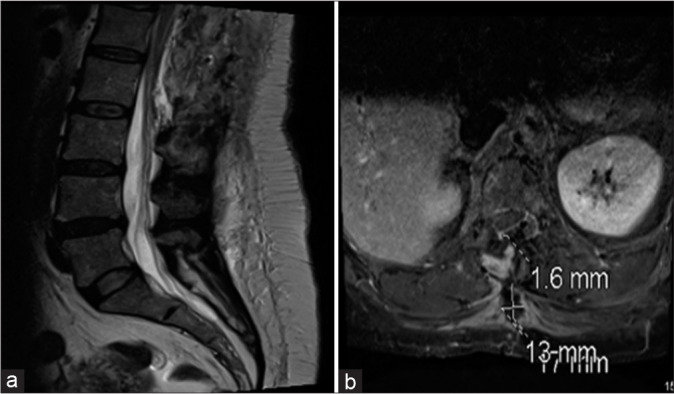
Magnetic resonance imaging lumbosacral spine sagittal (a) and axial (b) showing complete cyst resection with small fluid collection in the subcutaneous soft tissues at the site of recent surgery.
Figure 5:
T2-W magnetic resonance imaging sagittal (a) and axial (b) images show resolution of postoperative changes with decrease in focal extradural CSF collection size.
RESULTS
Our search yielded 107 results from PubMed. There were no duplicate records found. All studies were screened based on their titles and abstracts, and nonrelevant studies (n = 86) were removed. The remaining articles (n = 21) were then fully read for eligibility. Overall, 14 studies met the eligibility criteria for the final review [Figure 6].
Figure 6:
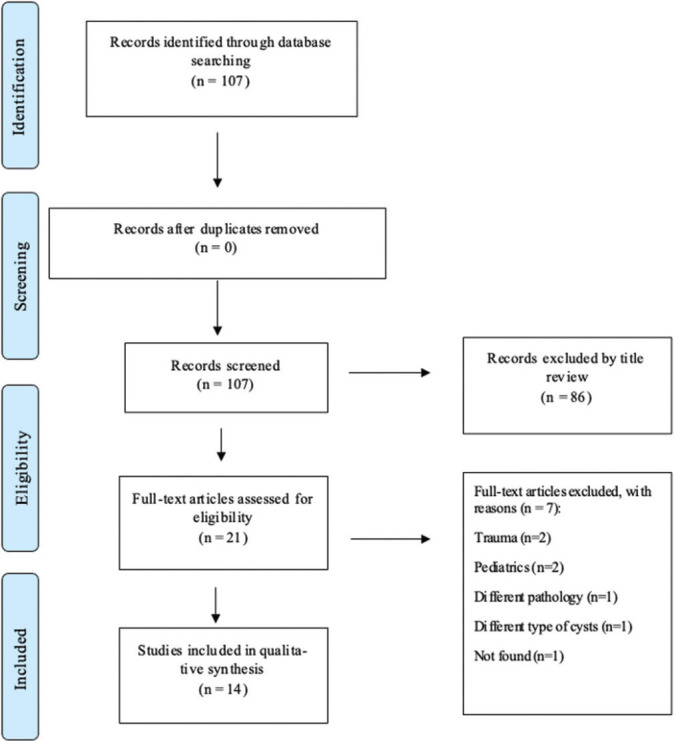
Flow diagram demonstrating the systematic analysis process (PRISMA flow diagram).
LITERATURE REVIEW
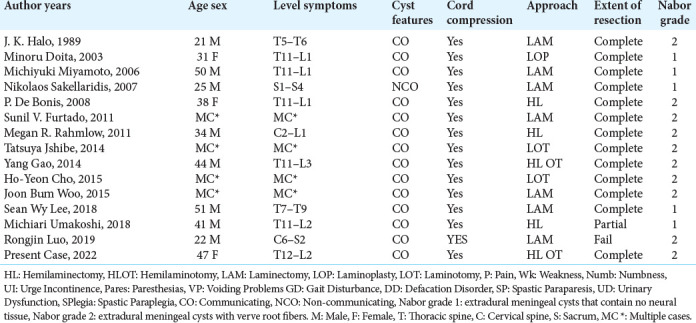
Fourteen articles involving 18 cases with idiopathic SEACs met our inclusion criteria [Table 1]. Patients averaged 35.5 years (range 20–83 years) of age at the time of diagnosis, and there was a male predominance (12:6). About 55% of SEACs were found at the thoracolumbar junction, followed by 22% located in the thoracic spine. The most common presenting symptom was pain (11/18 [61%]) followed by weakness (6/18 [33%]), numbness (5/18 [27%]), paresthesia (4/18 [22%]), urge incontinence (3/18 [16%]), and gait instability (2/18 [11%]). Most reported cases were Nabor Type 2 meningeal cysts 10/18 (55%), while the remaining 6/10 (45%) were extradural arachnoid cysts without nerve root fiber involvement (Nabor 1). The findings on MRI were divided into communicating 11/18 (61%) or non-communicating 7/18 (38%) SEAC. Treatment of SEACs included bilateral laminectomy alone (8/18 [44%]), unilateral laminectomy (6/18 [33%]), one case of unilateral laminectomy with laminoplasty, and one other case utilizing laminoplasty alone.
Table 1:
Summary of studies characteristics.
DISCUSSION
History
Idiopathic SEACs are rare cystic lesions that originate as protrusions of the arachnoid membrane through a dura defect. They may increase in size due to CSF pulsations and consequently enlarge to the point that they compress the spinal cord and/or nerve roots resulting in neurological deficits.[2] Notably, due to their slow progression, they are usually asymptomatic until late in their clinical course.[5,7] The most common complaints include pain and weakness that slowly increases over time with deficits reflecting their level or origin.[1,2] Our search found that 55% of idiopathic SEACs cases were located in the thoracolumbar area, as it is demonstrated in our case.[10]
Etiology of SEACs
The pathogenesis of idiopathic SEACs most often results from defects in the dura due to a congenital dural membrane defect.[9,11] The causative gene, FOXC2 (a protein that is expressed in the development of the mesoderm), was found to be mutated in some of the patients with familial SEACs and was associated with an early presentation (i.e., mean age of 23). HOXD4, another protein that plays a role in the determination of positional values during spine and dura matter development, was detected on Northern blots of human fetuses 5–9-weeks-old.[10]
Pathophysiology
The pathophysiology of idiopathic SEAC has been variably described.[7,8] Kim et al. reported that the pulsatile movement of CSF over time can come through the dura defect.[5] Halani et al. cited a similar mechanism for cranial arachnoid cyst enlargement.[3] Third, as the subarachnoid space is responsive to venous pressure exertion or valsalva maneuvers, and these may contribute to cyst enlargement by increasing/continued cyst filing.[1,9]
Imaging of SEACs
MRI is typically used to determine the communication site between the spinal subarachnoid space and the cyst cavity.[1,9] Notably, myelograms and myelo-CT studies have often been used to directly visualize the sites of these defects as well.
Treatment of SEAC
Treatment for asymptomatic patients consists of conservative management but symptomatic lesions require direct surgical occlusion of the fistulous defect (i.e., laminotomy, hemilaminectomy, laminectomy with complete cyst wall excision, and dural repair).[3,6] Other management techniques have included cyst aspiration, and the placement of shunts. We performed a unilateral laminotomy at T12–L2 with gross total resection and dural repair.
CONCLUSION
Idiopathic spinal epidural arachnoid cysts (SEAC) are rare. When diagnosed with MR, they should be treated with direct surgical occlusion of fistulous communication to achieve optimal postoperative resolution of myelopathy.
Footnotes
How to cite this article: Alanazi RF, Namer TS, Almalki A, AlSufiani F, Arias DP. Idiopathic thoracolumbar spinal epidural arachnoid cysts: A case report and systematic review. Surg Neurol Int 2022;13:599.
Contributor Information
Rahaf F. Alanazi, Email: alanazirfd@gmail.com.
Thana S. Namer, Email: thana.s.namer@gmail.com.
Abdulrahman Almalki, Email: Dr.abdulrahman09@hotmail.com.
Fahd AlSufiani, Email: sufianif@mngha.med.sa.
David Pinilla Arias, Email: ariasda@ngha.med.sa.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
REFERENCES
- 1.Furtado S, Thakar S, Murthy G, Dadlani R, Hegde A. Management of complex giant spinal arachnoid cysts presenting with myelopathy. J Neurosurg Spine. 2011;15:107–12. doi: 10.3171/2011.3.SPINE10672. [DOI] [PubMed] [Google Scholar]
- 2.Funao H, Nakamura M, Hosogane N, Watanabe K, Tsuji T, Ishii K, et al. Surgical treatment of spinal extradural arachnoid cysts in the thoracolumbar spine. Neurosurgery. 2012;71:278–84. doi: 10.1227/NEU.0b013e318257bf74. discussion 284. [DOI] [PubMed] [Google Scholar]
- 3.Halani S, Safain M, Heilman C. Arachnoid cyst slit valves: The mechanism for arachnoid cyst enlargement. J Neurosurg Pediatr. 2013;12:62–6. doi: 10.3171/2013.4.PEDS12609. [DOI] [PubMed] [Google Scholar]
- 4.Hatashita S, Kondo A, Shimizu T, Kurosu A, Ueno H. Spinal extradural arachnoid cyst-case report. Neurol Med Chir (Tokyo) 2001;41:318–21. doi: 10.2176/nmc.41.318. [DOI] [PubMed] [Google Scholar]
- 5.Kim I, Hong J, Son B, Lee S. Noncommunicating spinal extradural meningeal cyst in thoracolumbar spine. J Korean Neurosurg Soc. 2010;48:534–7. doi: 10.3340/jkns.2010.48.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kulkarni AG, Goel A, Thiruppathy SP, Desai K. Extradural arachnoid cysts: A study of seven cases. Br J Neurosurg. 2004;18:484–8. doi: 10.1080/02688690400012368. [DOI] [PubMed] [Google Scholar]
- 7.Lee C, Hyun S, Kim K, Jahng T, Kim H. What is a reasonable surgical procedure for spinal extradural arachnoid cysts: Is cyst removal mandatory? Eight consecutive cases and a review of the literature. Acta Neurochir (Wien) 2012;154:1219–27. doi: 10.1007/s00701-012-1356-7. [DOI] [PubMed] [Google Scholar]
- 8.Liu JK, Cole CD, Kan P, Schmidt MH. Spinal extradural arachnoid cysts: Clinical, radiological, and surgical features. Neurosurg Focus. 2007;22:E6. doi: 10.3171/foc.2007.22.2.6. [DOI] [PubMed] [Google Scholar]
- 9.Netra R, Min L, Hui M, Wang J, Bin Y, Ming Z. Spinal extradural meningeal cysts. J Spinal Disord Tech. 2011;24:132–6. doi: 10.1097/BSD.0b013e3181e47b47. [DOI] [PubMed] [Google Scholar]
- 10.Ogura Y, Miyake N, Kou I, Iida A, Nakajima M, Takeda K, et al. Identification of HOXD4 mutations in spinal extradural arachnoid cyst. PLoS One. 2015;10:e0142126. doi: 10.1371/journal.pone.0142126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh J, Lee D, Kim T, Yi S, Ha Y, Kim K, et al. Thoracolumbar extradural arachnoid cysts: A study of 14 consecutive cases. Acta Neurochir (Wien) 2011;154:341–8. doi: 10.1007/s00701-011-1110-6. [DOI] [PubMed] [Google Scholar]


