Abstract
This prospective phase I trial aimed to determine the recommended dose of 3‐day total marrow and lymphoid irradiation (TMLI) for a myeloablative conditioning regimen by increasing the dose per fraction. The primary end‐point of this single‐institution dose escalation study was the recommended TMLI dose based on the frequency of dose‐limiting toxicity (DLT) ≤100 days posthematopoietic stem cell transplantation (HSCT); a 3 + 3 design was used to evaluate the safety of TMLI. Three dose levels of TMLI (14/16/18 Gy in six fractions over 3 days) were set. The treatment protocol began at 14 Gy. Dose‐limiting toxicities were defined as grade 3 or 4 nonhematological toxicities. Nine patients, with a median age of 42 years (range, 35–48), eight with acute lymphoblastic leukemia and one with chronic myeloblastic leukemia, received TMLI followed by unrelated bone marrow transplant. The median follow‐up period after HSCT was 575 days (range, 253–1037). Three patients were enrolled for each dose level. No patient showed DLT within 100 days of HSCT. The recommended dose of 3‐day TMLI was 18 Gy in six fractions. All patients achieved neutrophil engraftment at a median of 19 days (range, 14–25). One‐year overall and disease‐free survival rates were 83.3% and 57.1%, respectively. Three patients experienced relapse, and no nonrelapse mortality was documented during the observation period. One patient died due to disease relapse 306 days post‐HSCT. The recommended dose of 3‐day TMLI was 18 Gy in six fractions. The efficacy evaluation of this regimen is currently being planned in a phase II study.
Keywords: allogeneic hematopoietic stem cell transplantation, intensity‐modulated radiation therapy, myeloablative conditioning regimen, total body irradiation, total marrow and lymphoid irradiation
This phase I dose escalation study evaluated the safety of 3‐day total marrow and lymphoid irradiation (TMLI, 14/16/18 Gy in 6 fractions) as myeloablative conditioning for hematopoietic stem cell transplantation. Among enrolled 9 patients (8 with acute lymphoblastic leukemia and 1 with chronic myeloid leukemia), no dose‐limiting toxicity 100‐day after transplant was observed; the one‐year overall survival and non‐relapse mortality were 83.3% and 0%, respectively. The recommended dose of 3‐day TMLI was 18 Gy in 6 fractions, which is currently being planned in a phase II study.
Abbreviations
- 1MMUD
HLA 1‐locus‐mismatched unrelated donor
- ATG
antithymocyte globulin
- BMT
bone marrow transplantation
- CR
complete remission
- CTCAE
Common Terminology Criteria for Adverse Events
- CT
computed tomography
- CTV
clinical target volume
- CY
cyclophosphamide
- DFS
disease‐free survival
- DLT
dose‐limiting toxicity
- GVHD
graft‐versus‐host disease
- HSCT
hematopoietic stem cell transplantation
- IMRT
intensity‐modulated radiation therapy
- MUD
HLA allele‐matched unrelated donor
- NRM
nonrelapse mortality
- MRD
measurable residual disease
- OS
overall survival
- PTV
planning target volume
- TBI
total body irradiation
- TMLI
total marrow and lymphoid irradiation
1. INTRODUCTION
In patients undergoing HSCT, TBI plays an important role in the conditioning regimens. Total body irradiation exerts an antitumor effect by eradicating malignant cells from the bone marrow, and inducing immunosuppression to prevent the rejection of donor cells. 1 Total body irradiation can reach sanctuary sites such as the central nervous system or testes. Additionally, unlike chemotherapy, efficacy of radiation does not depend on blood supply, metabolism, or clearance kinetics of the tumor. 2 Compared to the busulfan/CY without TBI regimen, the TBI‐containing regimen demonstrated significantly fewer transplant‐related deaths 3 and better survival. 4 Higher‐dose irradiation also has the potential to decrease the relapse rate. 5 , 6 , 7 Nevertheless, higher doses of TBI increase toxicity and long‐term morbidities. 7 , 8 , 9 , 10 Consequently, higher‐dose TBI did not improve OS in certain studies, despite a lower relapse rate. 7 , 8 , 9
Total marrow and lymphoid irradiation is an emerging treatment, using a more selective targeted irradiation technique. It can deliver a high dose to the target volume, while sparing healthy tissues such as lungs, kidneys, heart, and intestines. Intensity‐modulated radiation therapy is a high‐precision radiotherapy technique that allows TMLI to be delivered while avoiding risk to such organs. A phase I trial of TMLI achieved a reduction in the median organ dose (D50), with doses of 6.8, 6.1, 6.8, and 7.5 Gy to the lungs, kidneys, heart, and intestines, 11 respectively. Total marrow and lymphoid irradiation might therefore reduce toxicities 12 and improve disease control, and it is a promising treatment in terms of its potential to prolong survival.
Some clinical trials have evaluated dose‐escalated TMLI of ≤20 Gy at 2 Gy per fraction. 11 , 13 , 14 , 15 , 16 , 17 , 18 These trials escalated the doses by increasing fraction numbers rather than the dose per fraction. The treatment time for TMLI is 1 h or longer. The myeloablative regimen is usually delivered twice per day for 3 more consecutive days (over six fractions). This duration is considerably long for pretransplant patients and increasing the number of fractions poses an undesirable burden. Maintaining the number of fractions by escalating the fraction size could reduce the undesirable burden to some extent. Only one trial has reported the safety of larger fraction sizes, delivering up to 8 Gy at 4 Gy per fraction (administering fractions two times per day). 16 Ideally, the treatment intensity should be increased without increasing the number of fractions. Conversely, the α/β value for progenitor and terminally dividing leukemic cells was assumed to be 1.49 and 3.12, respectively. 19 For these values, the biological equivalent dose in 2 Gy fractions was 23.2 Gy and 21.5 Gy for 18 Gy in six fractions, respectively. The efficacy of 18 Gy in six fractions was assumed to be greater than or equal to 20 Gy in 10 fractions. Therefore, the present clinical trial aimed to determine the recommended radiation dose of TMLI for leukemia, by escalating the dose per fraction over 3 days. Our target TMLI dose was 18 Gy or less in six fractions over 3 days.
2. MATERIALS AND METHODS
2.1. Patients
The eligibility criteria were as follows: (i) presence of a hematologic malignancy, (ii) planned HSCT with a myeloablative regimen, (iii) age between 20 and 60 years, (iv) ECOG performance status of <3, and (v) adequacy of clinical parameters for HSCT (a cardiac ejection fraction of ≥50% vital capacity and forced expiratory volume in 1 s of ≥70%, serum bilirubin of ≤2 mg/dl, alanine aminotransferase and aspartate aminotransferase of at least five‐fold higher than the upper limits of normal, and a calculated creatinine clearance of ≥30 ml/min/m2). Patients fulfilling the following criteria were considered ineligible for TMLI: (i) non‐CR status at pretransplantation, or presence of extramedullary disease at the time of HSCT, (ii) a history of any HSCT, (iii) presence of another malignancy, and (iv) difficulty in holding still in the supine position for 1 h (during radiation therapy).
2.2. Study design
This single‐center phase I study evaluated different TMLI dose levels among patients with hematological malignancies in CR pre‐HSCT. The primary end‐point was the recommended dose, based on the frequency of DLT ≤100 days of HSCT. Dose‐limiting toxicities were defined as grade 3 or 4 nonhematological toxicities including those of cardiac, bladder, renal, pulmonary, hepatic, and central nervous system tissues, oral mucositis, gastrointestinal toxicities according to Bearman's criteria, 20 and other grade 3 or higher nonhematological toxicities according to CTCAE version 4.0. Grade 4 neutropenia (as per CTCAE version 4.0) associated with fever or infection lasting >3 weeks, or grade 4 neutropenia persisting for ≥28 days were also considered DLTs. The secondary end‐points were the engraftment rate, OS, DFS, NRM, and incidence of acute or chronic GVHD.
This trial used a 3 + 3 design. 21 There were three dose levels of TMLI (14/16/18 Gy in six fractions), and treatment started at level 1 (14 Gy). Three patients were treated at the same level; the next level of treatment was administered if DLT was not observed ≤100 days post‐HSCT. Three additional patients were enrolled at the same level, and six were evaluated at the same level if one of the first three patients developed DLT. If a DLT was documented in only one of six patients at the same level, the dose level was increased. However, the dose was reduced if two or more DLTs were recorded at the same level. Three additional patients were needed to evaluate the reduced dose level if it was used in only three patients. If fewer than two patients experienced DLT at the reduced dose level, or the reduced dose level had already been evaluated in six patients, the recommended dose was reduced. If a maximum of one DLT was observed at the highest dose level, the highest dose was considered the recommended dose. However, trial treatment was rejected if two or more DLTs occurred at the lowest dose level.
The study protocol was approved by the institutional ethical review board of the institute (number 2332). This study was registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN 000037581). All the participants provided written informed consent in accordance with the Declaration of Helsinki.
2.3. Procedures
All TMLIs were delivered using Radixact (Accuray, Inc.). Patients were immobilized using a full‐body evacuated cushion (CIVCO Medical Solutions) and a thermoplastic mask (CIVCO Medical Solutions) over the head and neck, in a stable supine position. Treatment planning CT images were obtained at a slice thickness of 5 mm. The CTV was defined as the volume encompassing the bones, major lymph node chains, brain, spleen, liver, and testes. Waldeyer ring lymph nodes and the mandible were excluded from the CTV to minimize the dose to the oral cavity. Additionally, mesenteric lymph nodes were excluded from the CTV to protect intestines. The lenses, oral cavity, parotid glands, lungs, heart, esophagus, stomach, kidneys, intestines, and breasts were delineated as organs at risk, and dose constraints were set (Table 1). The ribs, sternum, liver, spleen, and kidneys were contoured considering the respiratory motion; a 5–10 mm margin was added to the CTV bone excluding important risk organs to create the PTV for bone. Subsequently, the combined volume of the lymph node chains and the PTV for bones was defined as the primary PTV. The brain and liver were prescribed doses of ≤12 Gy at each dose level. For the PTV excluding the brain and liver, the minimum doses received by 80% (D80%) and maximum doses (Dmax) received were set 98%–105% and 115% of the prescription doses, respectively. Considering the maximum movement range of the Radixact bed, the radiation field was divided into two parts: the cranial and caudal. The gap between the two fields was adjusted with reference to the dose distributions, to minimize the volume exceeding 110% of the prescription dose. Total marrow and lymphoid irradiation was administered in six fractions twice per day for 3 consecutive days, with an interval of at least 6 h between fractions at each dose level. Whole‐body megavoltage CT was used for localization.
TABLE 1.
Dose constraints and dose parameter for organs at risk in a trial of myeloablative conditioning with 3‐day total marrow and lymphoid irradiation for leukemia
| Organ | Constraints | Median Dmean (range) | Median Dmax/D10%/D2% (range) | |
|---|---|---|---|---|
| Lenses | Dmean ≤ 6 Gy | Dmax ≤ 10 Gy | 4.98 Gy (3.46–5.73 Gy) | Dmax 6.61 Gy (4.66–7.86 Gy) |
| Oral cavity | Dmean ≤ 5.5 Gy | D2% ≤ 10 Gy | 4.17 Gy (3.96–5.13 Gy) | D2% 9.61 Gy (9.29–9.92 Gy) |
| Parotid grands | Dmean ≤ 7.5 Gy | — | 6.52 Gy (6.08–7.47 Gy) | — |
| Lungs | Dmean ≤ 8 Gy | D10% ≤ 12 Gy | 7.68 Gy (7.10–7.84 Gy) | D10% 11.71 Gy (11.19–11.97 Gy) |
| Heart | Dmean ≤ 8 Gy | D10% ≤ 12 Gy | 7.77 Gy (7.34–7.99 Gy) | D10% 11.71 Gy (10.82–11.91 Gy) |
| Esophagus | Dmean ≤ 7 Gy | D2% ≤ 12 Gy | 6.76 Gy (6.33–6.83 Gy) | D2% 11.45 Gy (10.64–11.97 Gy) |
| Stomach | D10% ≤ 12 Gy | — | — | D10% 11.26 Gy (9.02–11.88 Gy) |
| Kidneys | Dmean ≤ 10 Gy | D2% ≤ 12 Gy | 6.78 Gy (6.33–8.79 Gy) | D2% 11.52 Gy (10.45–11.93 Gy) |
| Intestine | Dmean ≤ 10 Gy | — | 8.58 Gy (7.86–9.83 Gy) | — |
| Breasts (n = 1) | Dmean ≤ 15 Gy | — | 14.71 Gy | — |
The standard conditioning regimen consisted of CY (60 mg/kg/day) for 2 days and TMLI (total of 14/16/18 Gy) for 3 days. Both the TMLI and CY lead regimens were used, and relied on the HSCT day of the week. The GVHD prophylaxis comprised a calcineurin inhibitor (tacrolimus), short‐term methotrexate, and in some cases added rabbit ATG (2.5 mg/kg). These are our institutional standard regimens, as previously described in a TBI pilot study. 22
2.4. Evaluation
As mentioned earlier, the DLTs were evaluated based on the Bearman scale and CTCAE criteria. Neutrophil engraftment was defined as the first of 3 consecutive days when the absolute neutrophil count was ≥0.5 × 109/L, and platelet engraftment was defined as the first of 3 consecutive days when the platelet count was ≥50 × 109/L over ≥7 days without transfusion. Graft‐versus‐host disease was scored according to previously published criteria. 23 , 24 Dose‐limiting toxicities were evaluated from the start day of TMLI until post‐HSCT day 100. The OS was measured from the time of HSCT to that of death from any cause. The DFS was defined as the time from HSCT to that of recurrence or death. The OS and DFS rates were estimated using the Kaplan–Meier method. The cumulative incidence of grade II–IV acute GVHDs was calculated by accounting for death and relapse as competing risks. The Gray analysis was used to evaluate the cumulative incidence of acute GVHD. Patients were followed‐up for a minimum of 100 days, and the radiation‐induced toxicities of TMLI and the late effects of HSCT were recorded. All statistical analyses were undertaken using EZR (Saitama Medical Center, Jichi Medical University). 25
3. RESULTS
3.1. Patients
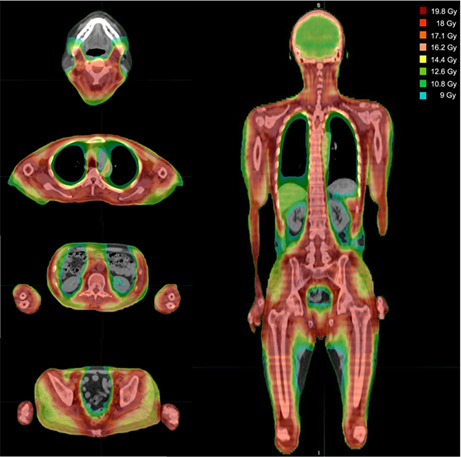
Between July 2019 and October 2021, nine patients were enrolled in this study at three levels, with a median age of 42 years (range, 35–48); among these, eight had acute lymphoblastic leukemia. All patients achieved hematologic CR at the time of transplantation, meanwhile, seven patients had MRD. No patients had extramedullary disease at HSCT. All patients received BMT from MUD (n = 4) or 1MMUD (n = 5). The patient characteristics are shown in Table 2. Figure 1 shows the dose distribution in a typical case of 18 Gy.
TABLE 2.
Characteristics of patients with leukemia treated with myeloablative conditioning with 3‐day total marrow and lymphoid irradiation
| Case | Dose | Age (years)/sex | PS | Disease | Disease status | MRD status | Donor source | Donor Age/sex | HLA disparity | HCT‐CI | GVHD prophylaxis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 14 | 35/M | 0 | CML | CP2 | (+) | UBM | 34/M | 8/8 | 0 | FK + sMTX |
| 2 | 14 | 47/M | 0 | Ph+ ALL | CR1 | (+) | UBM | 47/M | 7/8 | 0 | FK + sMTX |
| 3 | 14 | 44/M | 0 | Ph+ ALL | CR1 | (+) | UBM | 26/M | 8/8 | 0 | FK + sMTX |
| 4 | 16 | 42/M | 0 | Ph+ ALL | CR2 | (−) | UBM | 40/M | 7/8 | 0 | FK + sMTX |
| 5 | 16 | 38/M | 0 | B‐ALL | CR1 | (+) | UBM | 24/M | 7/8 | 0 | FK + sMTX |
| 6 | 16 | 39/M | 0 | Ph+ ALL | CR1 | (+) | UBM | 36/M | 8/8 | 1 | FK + sMTX |
| 7 | 18 | 46/M | 1 | B‐ALL | CR1 | (+) | UBM | 31/F | 8/8 | 1 | FK + sMTX |
| 8 | 18 | 36/M | 0 | Ph+ ALL | CR1 | (−) | UBM | 24/M | 7/8 | 1 | FK + sMTX + ATG |
| 9 | 18 | 48/F | 0 | B‐ALL | CR2 | (+) | UBM | 32/F | 7/8 | 5 | FK + sMTX + ATG |
Abbreviations: ALL, acute lymphoblastic leukemia; ATG, antithymocyte globulin; B‐ALL, B‐cell acute lymphoblastic leukemia; CML, chronic myeloid leukemia; CP, chronic phase; CR, complete remission; F, female; FK, tacrolimus; GVHD, graft‐versus‐host disease; HCT‐CI, hematopoietic cell transplantation‐comorbidity index; HLA, human leukocyte antigen; M, male; MRD, measurable residual disease; Ph, Philadelphia chromosome; PS, performance status; sMTX, short‐term methotrexate; UBM, unrelated bone marrow.
FIGURE 1.
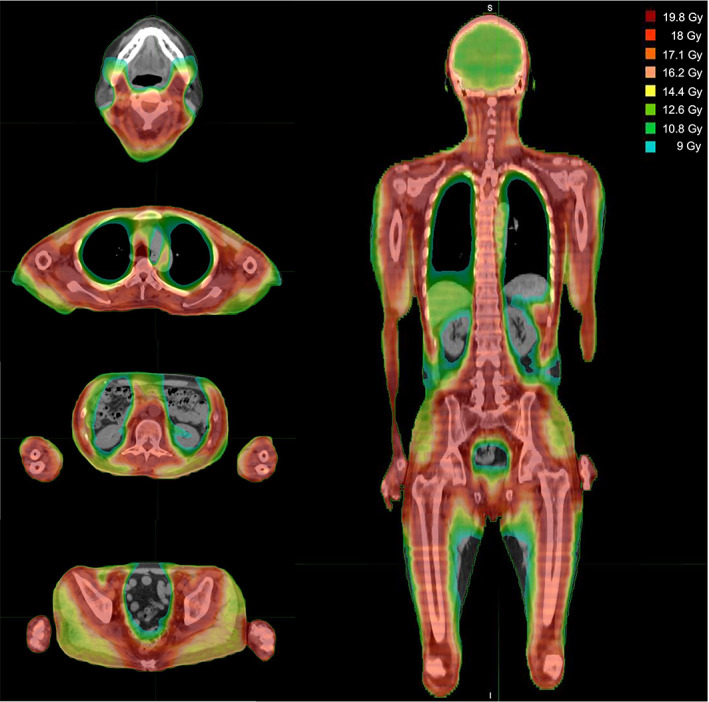
Dose distribution of 18 Gy total marrow and lymphoid irradiation
3.2. Clinical outcomes
The median follow‐up period after HSCT was 575 days (range, 253–1037). In each of the three levels, no patient had documented DLT ≤100 days post‐HSCT. All nine patients showed hematologic or molecular CR in the bone marrow test 30 days post‐HSCT. The toxicities of TMLI and the clinical outcomes are summarized in Table 3 and the toxicities by dose levels are presented in Table 4.
TABLE 3.
Toxicities and clinical outcomes in patients with leukemia treated with myeloablative conditioning with 3‐day total marrow and lymphoid irradiation
| Case | Dose | Neutrophil engraftment (days) | Toxicities | aGVHD max grade (organ, stage) | cGVHD | Relapse (days after HSCT) | Outcome | Follow‐up (days) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Bearman grade ≤2 (grade) | CTCAE Grade ≤2 (grade) | Grade ≥3 | ||||||||
| 1 | 14 | 21 | Renal (2) Oral mucositis (2) | Nausea (2) | — | II (GI 1) | Mouth (mild) Skin (mild) | — | Alive | 1037 |
| 2 | 14 | 19 |
Renal (2) Oral mucositis (2) |
Nausea (2) Sinusitis (2) Otitis media (2) |
— | — | — | — | Alive | 932 |
| 3 | 14 | 18 | Renal (2) |
Nausea (2) Skin infection (2) Arthritis (2) |
— | — | — | Yes (day 176) | Dead | 306 |
| 4 | 16 | 19 | Oral mucositis (2) | Abdominal pain (1) | — | IV (skin 4) |
Mouth (mild) Eye (mild) |
— | Alive | 617 |
| 5 | 16 | 16 | GI (1) | Nausea (2) | — | IV (skin 4) | — | Yes (day 298) | Alive | 601 |
| 6 | 16 | 19 | GI (1) | Nausea (1) | — | — | — | Yes (day 90) | Alive | 575 |
| 7 | 18 | 25 | Oral mucositis (2) | Otitis media (2) | — | I (skin 1) | — | — | Alive | 318 |
| 8 | 18 | 14 | GI (1) | Nausea (2) | — | II (skin 1, GI 1) | — | — | Alive | 304 |
| 9 | 18 | 23 |
Oral mucositis (2) GI (1) |
Anal pain (2) | — | I (skin 1) | — | — | Alive | 253 |
Abbreviations: aGVHD, acute graft‐versus‐host disease; cGVHD, chronic graft‐versus‐host disease; CTCAE, Common Terminology Criteria for Adverse Events; GI, gastrointestinal; HSCT, hematopoietic stem cell transplantation; max, maximum.
TABLE 4.
Toxicities by dose level
| Toxicity | 14 Gy (n = 3) | 16 Gy (n = 3) | 18 Gy (n = 3) | |||
|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 1 | Grade 2 | Grade 1 | Grade2 | |
| Pulmonary | 0 | 0 | 0 | 0 | 0 | 0 |
| Renal | 0 | 3 | 0 | 0 | 0 | 0 |
| Gastrointestinal | 0 | 0 | 2 | 0 | 2 | 0 |
| Oral mucositis | 0 | 2 | 0 | 1 | 0 | 2 |
| Nausea | 0 | 3 | 1 | 1 | 0 | 1 |
| Other | 0 | 4 a | 1 b | 0 | 0 | 2 c |
Sinusitis, otitis media, skin infection, and arthritis.
Abdominal pain.
Otitis media and anal pain.
All patients achieved neutrophil engraftment, and the median time to neutrophil engraftment was 19 days (range, 14–25); platelet engraftment was recorded in all patients at a median of 30 days (range, 20–118) post‐HSCT. The 1‐year OS and DFS rates were 83.3% and 58.3%, respectively. Figure 2 shows swimmer plots of survival durations. Three patients relapsed on days 84, 90, and 298, respectively, and only one patient died 306 days post‐HSCT due to relapse. All relapse patients had MRD in the time of HSCT. Except for extramedullary relapse in the submental lymph node in one case, all relapses were hematologic in nature; no NRM was documented during the observation period. Grade II or higher acute GVHD was observed in five patients and the cumulative incidence of grade II–IV and grade III–IV acute GVHD were 44.4% and 22.2% at 100 days post‐HSCT, respectively. Two patients developed mild chronic GVHD. All acute GVHDs requiring treatment responded well to steroids. We experienced a human herpesvirus 6 encephalitis and no other infections, including pneumonitis. No early death was recorded ≤30 days post‐HSCT in any of the patients.
FIGURE 2.
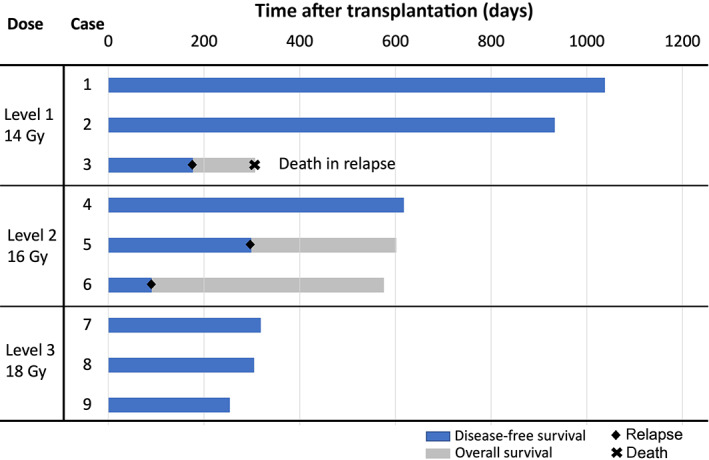
Swimmer plots of overall and disease‐free survival duration in patients with leukemia treated with myeloablative conditioning with 3‐day total marrow and lymphoid irradiation
The progress of relapse in the patients was as follows. One patient (case 3), who received 14 Gy TMLI, experienced hematological relapse 176 days post‐HSCT; he received a second course of HSCT 257 days after the first HSCT, but died 306 days after the first HSCT. The second patient (case 5) received 16 Gy TMLI, and experienced hematological relapse 298 days post‐HSCT; the second HSCT course was administered 447 days after the first HSCT. The third patient (case 6) was diagnosed with hematological relapse 90 days post‐HSCT; submental lymph node relapse was detected 109 days post‐HSCT. He subsequently received a second course of HSCT 240 days after the first, but a second relapse occurred 384 days after the first HSCT. We are currently preparing for a third course of HSCT.
4. DISCUSSION
This paper describes the results of a phase I dose escalation trial of TMLI with increased fraction sizes. The TMLI doses could be safely escalated to a total of 18 Gy, with 3 Gy per fraction delivered twice per day; DLTs were not observed at any dose level. All patients in this cohort achieved neutrophil engraftment post‐HSCT. Furthermore, no early deaths were observed ≤100 days. Moreover, no NRMs occurred during any observation period.
Numerous clinical trials on TMLI 11 , 13 , 14 , 15 , 16 , 17 , 18 , 26 (Table 5) used dose escalation, by increasing the number of fractions and using additional chemotherapy other than CY. Hui et al. approached dose escalation with 3 Gy per fractions up to 18 Gy in 6 days for high‐risk patients. 15 Their study differed from the current study in the numbers of daily fractions. Their study concluded that a feasible dose of TMLI was 15 Gy in 5 days, because they experienced treatment‐related mortality at 18 Gy. The current study presents a unique approach for increasing the dose of TMLI without schedule extension. Escalating a single fraction dose of TMLI could achieve high‐intensity conditioning without increasing the treatment burden, which is an issue of concern with long‐time fixed radiotherapy or numerous radiotherapy sessions. Moreover, unlike 5‐day TMLI, 3‐day TMLI could provide hematological oncologists with a flexible HSCT schedule, by permitting the selection of a suitable treatment start day in the week, especially in institutions that provide radiotherapy only on weekdays. Three‐day TMLI allows us to deliver the same schedule as the most popular myeloablative 3‐day TBI regimen.
TABLE 5.
Trials of total marrow irradiation (TMI) and total marrow and lymphoid irradiation (TMLI) for acute leukemia
| Trial | Number of patients | Treatment | Patients | Median follow‐up | Dose fractionation | Dose per fraction (Gy) | Chemotherapy | NRM/OS | |
|---|---|---|---|---|---|---|---|---|---|
| Rosenthal et al, 2011 18 | Phase I/II | 61 | TMLI | RIC | 13.1 months |
12 Gy in 8 (twice daily) |
1.5 | Flu + Mel |
1‐year NRM 8.1% 1‐year OS 75% |
| Jensen et al, 2018 26 | Prospective | 61 | TMLI | RIC | 7.4 years |
12 Gy in 8 (twice daily) |
1.5 | Flu + Mel |
5‐year NRM 33% 5‐year OS 42% |
| Wong et al, 2013 13 |
Phase I/ phase I |
12/20 | TMLI |
MAC (non‐CR) |
14.75 months/ 7.3 months |
12–15Gy in 8–10 (twice daily) |
1.5 |
CY + VP16/ BU + VP16 |
100‐day NRM 8%/20% Death 6/15 |
| Patel et al, 2014 14 | Phase I | 14 | TMI |
MAC (high‐risk) |
37.0 months |
3–12 Gy in 2–8 (twice daily) |
1.5 | Flu + BU |
TRM 29% OS 50% |
| Hui et al, 2017 15 | Phase I | 12 | TMI |
MAC (refractory/MRD) |
3.3 months |
15–18 Gy in 5–6 (once daily) |
3 | CY + Flu |
1‐year NRM 42% 1‐year OS 42% |
| Stein et al, 2017 11 | Phase I | 51 | TMLI | MAC (relapse/refractory) | 24.6 months |
12–20 Gy in 10 (twice daily) |
1.5–2 | CY + VP16 |
100‐day NRM 3.9% 1‐year OS 55.5% |
| Shi et al, 2021 16 | Retrospective | 61 | TMLI | MAC | N/A |
8 Gy in 2 (twice daily) |
4 | N/A |
2‐year NRM 5% 2‐year OS 74.7% |
| Current study | Phase I | 9 | TMLI |
MAC (CR/CP/MRD) |
18.9 months |
14–18 Gy in 6 (twice daily) |
2.33–3 | CY |
NRM 0% 1‐year OS 83.3% |
Abbreviations: BU, busulfan; CP, chronic phase; CR, complete remission; CY, cyclophosphamide; Flu, fludarabine; MAC, myeloablative conditioning; Mel, melphalan; MRD, measurable residual disease; N/A, not available; NRM, nonrelapse mortality; OS, overall survival; RIC, reduced‐intensity conditioning; TRM, treatment‐related mortality; VP16, etoposide.
We previously reported on the delivery of 12 Gy of TBI with IMRT. 22 Overall, 6/10 patients receiving TBI experienced Bearman grade 2 oral mucositis and 7/10 patients experienced gastrointestinal toxicities of any grade. In the present study, five and four cases showed oral mucositis and gastrointestinal toxicities, respectively. Despite escalation of prescription dose, the reduced doses to the oral cavity and intestines avoided an increase in these toxicities. Our dose constraints for each organ were set based on the report of a phase I trial on TMLI, 13 which were modified for this study. We set higher‐dose constraints for some organs, especially the oral cavity and intestines, to deliver a dose to the bone marrow. As no increase in severe oral mucositis or gastrointestinal toxicities occurred in this study, dose constraints for the oral cavity and intestines were effective in preventing severe toxicities. For pulmonary toxicities, we strictly maintained the dose constraint of a mean lung dose of ≤8 Gy. 12 No pulmonary toxicities, including pulmonary infection or pneumonitis, were observed; maintaining a mean lung dose of 8 Gy is therefore important in dose‐escalated TMLI. In this study, dose escalation was safe using our dose constraints, and the results showed that maintaining the dose constraints leads to safe treatment with TMLI, despite increasing the dose to the target. However, long‐term follow‐up will be needed to evaluate long‐term radiation‐related toxicities.
The α/β value for progenitor and terminally dividing leukemic cells was assumed to be 1.49 and 3.12, respectively. 19 For these values, 18 Gy in six fractions was converted to 23.1 Gy and 21.5 Gy, respectively, in 2‐Gy‐per‐fraction equivalent doses. As these doses were higher than those in previously reported trials, 11 , 13 , 15 , 16 18 Gy in six fractions was expected to have a high antitumor effect. In this context, the antitumor effect of TMLI with 18 Gy in six fractions will be evaluated in our phase II trial.
Higher dose rate irradiation of TMLI was one of the concerning issues. Helical tomotherapy can deliver a maximum of 1000 cGy/min. Many studies of TMLI 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 used the same treatment delivery system, and no study reported critical toxicities or lower rate of engraftment after TMLI. In this study, we observed no critical toxicities and all patients achieved neutrophil engraftment. Fractionated treatment and dose constraints for risk organs might be reduce the effect of the dose rate.
We did not set dose constraints for the ovaries in this study as the aim was to evaluate the safety of TMLI. Reducing the dose delivered to the ovaries is essential for fertility preservation. 27 Such a reduction is technically feasible, and ovarian‐sparing irradiation is potentially adoptable for patients in hematological remission at transplantation. However, this approach carries the potential risk of relapse given the reduced dose delivered to the area surrounding the ovaries. Therefore, ovarian‐sparing irradiation must only be adopted after careful consideration in high‐risk patients. Nevertheless, fertility‐sparing TMLI will become an increasingly adopted treatment technique in the near future.
One patient in this cohort experienced extramedullary relapse in the submental lymph nodes. These nodes are kept outside the target volume of TMLI, to reduce the risk of oral mucositis; this could be a potential risk factor for out‐of‐field relapse. In the context of risk of out‐of‐field relapses, the risk of extramedullary relapse has been discussed in a report. 28 In this report, 13/101 patients treated with total marrow irradiation developed extramedullary relapse at 19 sites; nine sites were within the target volume and received ≥12 Gy. The extramedullary relapse incidence was as frequent in regions receiving ≥10 Gy as in those receiving <10 Gy, and the only significant predictor of extramedullary relapse was pretransplantation extramedullary disease. The risk of extramedullary relapse did not appear to be greater after TMLI than that after TBI. Although the patients with pretransplantation extramedullary disease were excluded in this cohort, pretransplantation disease status needs to be carefully evaluated in pre‐HSCT patients.
Notably, we encountered two cases of skin stage 4, grade IV acute GVHD (Table 2). Both received BMT from 1MMUD without using ATG as a GVHD prophylaxis (Table 3); thus, they were at risk for severe acute GVHD. Recent Japanese registry‐based studies have revealed that the incidence of grade II–IV and grade III–IV acute GVHD of this cohort were 40–50% and over 10%, respectively, which resulted in their higher NRM rates than those of the BMT recipients from MUD 29 , 30 ; adding low‐dose ATG for this high‐risk cohort was associated with reduced incidence of severe acute GVHD and NRM. 31 In accordance with the above reports, our two cases in the 18 Gy TMLI cohort receiving BMT from 1MMUD with low‐dose ATG did not develop severe GVHD. In terms of the influence on skin GVHD occurrence, TMLI can spare the skin from the radiation field as opposed to TBI; however, the radiation dose is higher where it involves the radiation field. Total body irradiation is known as a risk factor for GVHD 32 ; however, whether a wide radiation field or high‐dose irradiation is the greater contributor remains unclear. Currently, there are no reports supporting that increased dose of TMLI induces a high rate of GVHD. 1 , 11 , 13 , 14 , 15 , 16 , 18 , 26 Further research is needed to reveal the effect of TMLI for GVHD. In any case, sufficient consideration for appropriate GVHD prophylaxis is pivotal.
The limitations of the present study included the small sample size and short observation period. Therefore, the efficacy of TMLI could not be reliably assessed. However, this study confirmed the acceptable safety of TMLI at a dose of 18 Gy in six fractions over 3 days, delivered within 100 days post‐HSCT. Another limitation is that all included patients were in CR at HSCT. To evaluate safety of TMLI itself, the target was set only in CR patients. As a result, we experienced no NRM and no DLT. However, seven patients had MRD at the time of HSCT. Although MRD is an important risk factor for disease relapse, 33 , 34 no relapse was observed in the two patients treated with 18 Gy TMLI who had MRD. A phase II study is needed to evaluate the efficacy of 3‐day TMLI.
This phase I study serves as an important cornerstone to establishing the treatment of TMLI with IMRT. The outcomes from this phase I study on TMLI indicate the recommended dose to be 18 Gy in six fractions. A phase II study is currently being planned to assess the efficacy of TMLI with 18 Gy in six fractions at our institution.
AUTHOR CONTRIBUTIONS
H.O., T. Konishi, Y.N., and K.O. designed the study. H.O., S.K., S.H., and K.N.M performed TMLI. H.O., T. Konishi, Y.N., N.D., and K.N.M wrote the manuscript. T. Konishi, Y.N., C.K., S.S., Y.K., Y.A., R.K., A.W., D.M., S.N., Y.U., D.O., A.H., A.N., N.S., T.T., H.S., T. Kobayashi, and N.D. took care of the patients. All authors reviewed and approved the final version of the manuscript.
FUNDING INFORMATION
The authors have no financial interests to disclose.
CONFLICT OF INTEREST
The authors have no conflict of interest.
ETHICAL APPROVAL
All the participants provided written informed consent in accordance with the Declaration of Helsinki. The study protocol was approved by the institutional ethical review board of the institute (number 2332). This study was registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN 000037581).
ACKNOWLEDGMENTS
Dr. Katsuyuki Karasawa, the former director of the Division of Radiation Oncology, Department of Radiology, Komagome Hospital, sadly passed away on April 27, 2021. We would like to express our deepest sympathy and heartfelt condolences to his family. The authors would like to thank Masao Hagiwara and Daisuke Kudo, as part of the Efficacy and Safety Assessment Committee, for their support. The authors also thank the nursing staff at Tokyo Metropolitan Cancer and Infectious Diseases Center, Komagome Hospital for their excellent patient care. We would like to thank Editage for English language editing.
Ogawa H, Konishi T, Najima Y, et al. Phase I trial of myeloablative conditioning with 3‐day total marrow and lymphoid irradiation for leukemia. Cancer Sci. 2023;114:596‐605. doi: 10.1111/cas.15611
Hiroaki Ogawa and Tatsuya Konishi contributed equally to this work.
REFERENCES
- 1. Wong JYC, Filippi AR, Scorsetti M, Hui S, Muren LP, Mancosu P. Total marrow and total lymphoid irradiation in bone marrow transplantation for acute leukaemia. Lancet Oncol. 2020;21(10):e477‐e487. doi: 10.1016/S1470-2045(20)30342-9 [DOI] [PubMed] [Google Scholar]
- 2. Brochstein JA, Kernan NA, Groshen S, et al. Allogeneic bone marrow transplantation after Hyperfractionated Total‐body irradiation and cyclophosphamide in children with acute leukemia. New Engl J med. 1987;317(26):1618‐1624. doi: 10.1056/NEJM198712243172602 [DOI] [PubMed] [Google Scholar]
- 3. Gupta T, Kannan S, Dantkale V, Laskar S. Cyclophosphamide plus total body irradiation compared with busulfan plus cyclophosphamide as a conditioning regimen prior to hematopoietic stem cell transplantation in patients with leukemia: a systematic review and meta‐analysis. Hematol Oncol Stem Cell Ther. 2011;4(1):17‐29. doi: 10.5144/1658-3876.2011.17 [DOI] [PubMed] [Google Scholar]
- 4. Hartman AR, Williams SF, Dillon JJ. Survival, disease‐free survival and adverse effects of conditioning for allogeneic bone marrow transplantation with busulfan/cyclophosphamide vs total body irradiation: a meta‐analysis. Bone Marrow Transplant. 1998;22(5):439‐443. doi: 10.1038/sj.bmt.1701334 [DOI] [PubMed] [Google Scholar]
- 5. Kal HB, Loes van Kempen‐Harteveld M, Heijenbrok‐Kal MH, Struikmans H. Biologically effective dose in total‐body irradiation and hematopoietic stem cell transplantation. Strahlenther Onkol. 2006;182(11):672‐679. doi: 10.1007/s00066-006-1528-6 [DOI] [PubMed] [Google Scholar]
- 6. Marks DI, Forman SJ, Blume KG, et al. A comparison of cyclophosphamide and total body irradiation with etoposide and total body irradiation as conditioning regimens for patients undergoing sibling allografting for acute lymphoblastic leukemia in first or second complete remission. Biol Blood Marrow Transplant. 2006;12(4):438‐453. doi: 10.1016/j.bbmt.2005.12.029 [DOI] [PubMed] [Google Scholar]
- 7. Clift RA, Buckner CD, Appelbaum FR, Sullivan KM, Storb R, Thomas ED. Long‐term follow‐up of a randomized trial of two irradiation regimens for patients receiving allogeneic marrow transplants during first remission of acute myeloid leukemia. Blood. 1998;92(4):1455‐1456. [PubMed] [Google Scholar]
- 8. Clift RA, Buckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation in patients with acute myeloid leukemia in first remission: a randomized trial of two irradiation regimens. Blood. 1990;76(9):1867‐1871. [PubMed] [Google Scholar]
- 9. Clift RA, Buckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation in patients with chronic myeloid leukemia in the chronic phase: a randomized trial of two irradiation regimens. Blood. 1991;77(8):1660‐1665. [PubMed] [Google Scholar]
- 10. Bradley J, Reft C, Goldman S, et al. High‐energy total body irradiation as preparation for bone marrow transplantation in leukemia patients: treatment technique and related complications. Int J Radiat Oncol Biol Phys. 1998;40(2):391‐396. doi: 10.1016/s0360-3016(97)00578-6 [DOI] [PubMed] [Google Scholar]
- 11. Stein A, Palmer J, Tsai NC, et al. Phase I trial of Total marrow and lymphoid irradiation transplantation conditioning in patients with relapsed/refractory acute leukemia. Biol Blood Marrow Transplant. 2017;23(4):618‐624. doi: 10.1016/j.bbmt.2017.01.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shinde A, Yang D, Frankel P, et al. Radiation‐related toxicities using organ sparing Total marrow irradiation transplant conditioning regimens. Int J Radiat Oncol Biol Phys. 2019;105(5):1025‐1033. doi: 10.1016/j.ijrobp.2019.08.010 [DOI] [PubMed] [Google Scholar]
- 13. Wong JY, Forman S, Somlo G, et al. Dose escalation of total marrow irradiation with concurrent chemotherapy in patients with advanced acute leukemia undergoing allogeneic hematopoietic cell transplantation. Int J Radiat Oncol Biol Phys. 2013;85(1):148‐156. doi: 10.1016/j.ijrobp.2012.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Patel P, Aydogan B, Koshy M, et al. Combination of linear accelerator‐based intensity‐modulated total marrow irradiation and myeloablative fludarabine/busulfan: a phase I study. Biol Blood Marrow Transplant. 2014;20(12):2034‐2041. doi: 10.1016/j.bbmt.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 15. Hui S, Brunstein C, Takahashi Y, et al. Dose escalation of Total marrow irradiation in high‐risk patients undergoing allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2017;23(7):1110‐1116. doi: 10.1016/j.bbmt.2017.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shi L, Lu X, Deng D, et al. The safety and efficacy of a novel hypo‐fractionated total marrow and lymphoid irradiation before allogeneic stem cell transplantation for lymphoma and acute leukemia. Clin Transl Radiat Oncol. 2021;26:42‐46. doi: 10.1016/j.ctro.2020.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bao Z, Zhao H, Wang D, et al. Feasibility of a novel dose fractionation strategy in TMI/TMLI. Radiat Oncol. 2018;13(1):248. doi: 10.1186/s13014-018-1201-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rosenthal J, Wong J, Stein A, et al. Phase ½ trial of total marrow and lymph node irradiation to augment reduced‐intensity transplantation for advanced hematologic malignancies. Blood. 2011;117(1):309‐315. doi: 10.1182/blood-2010-06-288357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cowen D, Richaud P, Landriau S, et al. Radiobiological features of acute myeloblastic leukemia: comparison of self‐renewal versus terminally differentiated populations. Int J Radiat Oncol Biol Phys. 1994;30(5):1133‐1140. doi: 10.1016/0360-3016(94)90320-4 [DOI] [PubMed] [Google Scholar]
- 20. Bearman SI, Appelbaum FR, Back A, et al. Regimen‐related toxicity and early posttransplant survival in patients undergoing marrow transplantation for lymphoma. J Clin Oncol. 1989;7(9):1288‐1294. doi: 10.1200/JCO.1989.7.9.1288 [DOI] [PubMed] [Google Scholar]
- 21. Le Tourneau C, Lee JJ, Siu LL. Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst. 2009;101(10):708‐720. doi: 10.1093/jnci/djp079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Konishi T, Ogawa H, Najima Y, et al. Safety of total body irradiation using intensity‐modulated radiation therapy by helical tomotherapy in allogeneic hematopoietic stem cell transplantation: a prospective pilot study. J Radiat Res. 2020;61(6):969‐976. doi: 10.1093/jrr/rraa078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825‐828. [PubMed] [Google Scholar]
- 24. Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft‐versus‐host disease: I. the 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant. 2015;21(3):389‐401. doi: 10.1016/j.bbmt.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452‐458. doi: 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jensen LG, Stiller T, Wong JYC, Palmer J, Stein A, Rosenthal J. Total marrow lymphoid irradiation/fludarabine/melphalan conditioning for allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2018;24(2):301‐307. doi: 10.1016/j.bbmt.2017.09.019 [DOI] [PubMed] [Google Scholar]
- 27. Kanda Y, Wada H, Yamasaki R, et al. Protection of ovarian function by two distinct methods of ovarian shielding for young female patients who receive total body irradiation. Ann Hematol. 2014;93(2):287‐292. doi: 10.1007/s00277-013-1852-8 [DOI] [PubMed] [Google Scholar]
- 28. Kim JH, Stein A, Tsai N, et al. Extramedullary relapse following total marrow and lymphoid irradiation in patients undergoing allogeneic hematopoietic cell transplantation. Int J Radiat Oncol Biol Phys. 2014;89(1):75‐81. doi: 10.1016/j.ijrobp.2014.01.036 [DOI] [PubMed] [Google Scholar]
- 29. Terakura S, Atsuta Y, Tsukada N, et al. Comparison of outcomes of 8/8 and 7/8 Allelee matched unrelated bone marrow transplantation and single‐unit cord blood transplantation in adults with acute leukemia. Biol Blood Marrow Transplant. 2016;22(2):330‐338. doi: 10.1016/j.bbmt.2015.10.006 [DOI] [PubMed] [Google Scholar]
- 30. Kawamura K, Kanda J, Murata M, et al. Impact of the presence of HLA 1‐locus mismatch and the use of low‐dose antithymocyte globulin in unrelated bone marrow transplantation. Bone Marrow Transplant. 2017;52(10):1390‐1398. doi: 10.1038/bmt.2017.153 [DOI] [PubMed] [Google Scholar]
- 31. Miyao K, Terakura S, Kimura F, et al. Updated comparison of 7/8 HLA allele‐matched unrelated bone marrow transplantation and single‐unit umbilical cord blood transplantation as alternative donors in adults with acute leukemia. Biol Blood Marrow Transplant. 2020;26(11):2105‐2114. doi: 10.1016/j.bbmt.2020.08.001 [DOI] [PubMed] [Google Scholar]
- 32. Nakasone H, Fukuda T, Kanda J, et al. Impact of conditioning intensity and TBI on acute GVHD after hematopoietic cell transplantation. Bone Marrow Transplant. 2015;50(4):559‐565. doi: 10.1038/bmt.2014.293 [DOI] [PubMed] [Google Scholar]
- 33. Bassan R, Bruggemann M, Radcliffe HS, et al. A systematic literature review and meta‐analysis of minimal residual disease as a prognostic indicator in adult B‐cell acute lymphoblastic leukemia. Haematologica. 2019;104(10):2028‐2039. doi: 10.3324/haematol.2018.201053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Berry DA, Zhou S, Higley H, et al. Association of Minimal Residual Disease with Clinical Outcome in pediatric and adult acute lymphoblastic leukemia: a meta‐analysis. JAMA Oncol. 2017;3(7):e170580. doi: 10.1001/jamaoncol.2017.0580 [DOI] [PMC free article] [PubMed] [Google Scholar]


