Abstract
The role of previous thoracic radiation therapy as a risk factor of immune‐related pneumonitis is unclear. Furthermore, some patients develop radiation recall pneumonitis, which is characterized by a radiation pneumonitis‐like imaging pattern with consolidation progressing within a previous radiation field. In this multicenter retrospective study, we analyzed the relationship of previous thoracic radiation therapy with immune‐related pneumonitis and the characteristics of radiation recall pneumonitis. The medical records of patients with non‐small‐cell lung cancer who had received nivolumab between December 2015 and March 2017 at five institutions were retrospectively reviewed. Incidence, imaging patterns, clinical course, and risk factors of immune‐related pneumonitis and radiation recall pneumonitis were evaluated. A total of 669 patients were evaluated, and the incidences of all‐grade and grade 3 or higher immune‐related pneumonitis were 8.8% and 2.6%, respectively. The incidences of immune‐related pneumonitis were 13.2% (34/257) and 6.1% (25/412) in patients with and those without previous thoracic radiation therapy, respectively. A history of previous thoracic radiation therapy was associated with immune‐related pneumonitis (odds ratio, 2.11; 95% confidence interval, 1.21–3.69 in multivariate analysis). Among the patients with previous thoracic radiation therapy, 6.2% (16/257) showed radiation recall pattern. This study found an increased risk of nivolumab‐induced immune‐related pneumonitis associated with a history of thoracic radiation therapy. Radiation recall pattern was one of the major patterns of immune‐related pneumonitis among the patients with previous thoracic radiation therapy. Incidence, risk factors, and clinical outcome of radiation recall pneumonitis were elucidated.
Keywords: antineoplastic agent, carcinoma, immune checkpoint inhibitor, immunological, nivolumab, non‐small‐cell lung, radiation pneumonitis
The study revealed thoracic radiation therapy as a risk factor of immune‐related pneumonitis. The characteristic imaging pattern “radiation recall pneumonitis” occurred in patients with previous radiation therapy. The incidence of this characteristic pneumonitis contributed to the higher risk of immune‐related pneumonitis in patients with a history of thoracic radiation therapy.
1. INTRODUCTION
Immune‐checkpoint inhibitors (ICIs) have improved the prognosis of cancer patients, especially for patients with non‐small‐cell lung cancer (NSCLC). Nivolumab was first approved in Japan as a second‐line treatment for metastatic or recurrent NSCLC in 2015. 1 , 2 At present, pembrolizumab and atezolizumab are used in combination with a platinum agent as a first‐line treatment, and durvalumab is also used for maintenance therapy after chemoradiation treatment for locally advanced NSCLC. 3 , 4 , 5 In the next decade, most NSCLC patients will likely receive ICIs at some point in their treatment.
Immune‐related adverse events (irAEs) are sometimes difficult to control and can be fatal in some cases. Immune‐related pneumonitis (IRP) is one of the most important irAEs and is reported more frequently among lung cancer patients than among melanoma patients. 6 Five clinical trials of NSCLC reported incidences of IRP of 1%–5% among patients receiving ICI treatment. 1 , 2 , 7 , 8 , 9 Recently, retrospective studies have reported a higher incidence of 13%–19% in routine clinical practice. 10 , 11 , 12 , 13 Patients with IRP often require the discontinuation of their anticancer treatment and sometimes die from pneumonitis despite the use of corticosteroid treatment.
The risk factors for IRP have attracted interest but remain unclear. Clinical studies have suggested a relationship between a history of thoracic radiation therapy (TRT) and IRP, 14 but the details are not fully understood. Each retrospective study analyzing ICI treatment among NSCLC patients reported different risk factors, including complications from interstitial lung disease, a nonadenocarcinoma histology, combination therapy with other ICIs (such as CTL antigen‐4 inhibitors), the use of pembrolizumab, and a low serum albumin level, but none of these studies could clearly point out the relation with previous TRT. 10 , 11 , 12 Although the relation between previous TRT and IRP has not been revealed, a few patients with previous TRT have been reported to show a characteristic imaging pattern, with consolidation progressing within the previous radiation field after ICI treatment. This type of pneumonitis was first reported by Shibaki et al. in 2017 15 and was called radiation recall pneumonitis. Although the mechanism of this pneumonitis is unknown, radiation recall pneumonitis is thought to be affected by both previous TRT and ICI treatment. Thus, we decided to focus on previous TRT as a risk factor for IRP, especially radiation recall pneumonitis.
In this multicenter study, we investigated a large series of 669 patients to analyze the incidence and risk factors of IRP by performing a central radiological analysis. We especially focused on the relation between IRP and a history of TRT and revealed that patients with previous TRT had a different IRP imaging pattern compared with patients who had not received TRT; this difference might have contributed to the higher incidence of IRP among the patients with previous TRT.
2. MATERIALS AND METHODS
2.1. Patients
We designed this retrospective study to analyze the incidence and risk factors of IRP and radiation recall pneumonitis. We used electronic medical records to analyze consecutive patients treated at five institutions in Japan who received nivolumab for metastatic or recurrent NSCLC between December 2015 and March 2017. We reviewed the patients’ electronic medical records, and the following clinical data were collected: age, sex, smoking history, ECOG performance status (PS), corticosteroid use at the time of the start of nivolumab therapy, and tumor characteristics including histology, driver mutation status, and TNM staging at the time of diagnosis. Data on the treatment history including surgery, TRT, and systemic chemotherapy were also obtained. We defined TRT as radiation therapy that included a lung field and included not only curative radiation therapy for stage III cancers but also palliative radiation therapy for metastatic or recurrent cancers and stereotactic body radiotherapy for stage I/II cancers. Palliative radiation therapy for spine metastasis or liver metastasis was also included in TRT if the radiation field included lung.
We defined IRP as pneumonitis occurring during or after nivolumab treatment, excluding other causes such as infection and the use of agents other than nivolumab. To exclude non‐nivolumab drug‐induced pneumonitis, only pneumonitis that occurred after the start of nivolumab treatment and before the next chemotherapy started was considered as nivolumab‐induced pneumonitis. Each diagnosis was made clinically by the investigator at each institution based on regular follow‐up examinations that included X‐ray and computed tomography (CT) imaging throughout the course of treatment; the diagnoses were then confirmed radiologically by board‐certified diagnostic radiologists. For patients who were diagnosed as having IRP, grading according to the Common Terminology Criteria for Adverse Events version 4.0 was carried out, and information on the dose and duration of corticosteroid use, the use of other immunosuppressive agents, and the outcome of the pneumonitis were collected. The patients whose respiratory condition and CT images fully recovered, partly recovered, and were exacerbated after the recovery were defined as “cure”, “remission”, and “exacerbation”, respectively, and patients who died because of pneumonitis as “death”. We also obtained detailed clinical data on previous TRT, including a history of radiation pneumonitis, elapsed time since the last TRT, and radiation parameters such as the total dose, volumes receiving more than 20 Gy (V20) or 30 Gy (V30), and the mean lung dose.
2.2. Independent review of imaging patterns
The imaging analysis was undertaken by the consensus of two board‐certified diagnostic radiologists. The radiologists reviewed the CT images that had been obtained before the start of nivolumab treatment, those obtained at the onset of pneumonitis, and also any subsequent images, if available. Images obtained for TRT planning were used to evaluate the relation between the previous TRT and IRP. The analysis focused on whether the pneumonitis imaging pattern did or did not support a diagnosis of radiation recall pneumonitis based on a comparison of the pneumonitis field and the radiation field. The diagnostic radiologists were blinded to all other clinical information.
As no clear definition of radiation recall pneumonitis exists, including criteria regarding the total dose of TRT and the elapsed time since the last TRT, we reviewed the cases of IRP based on the imaging patterns only and classified the findings into two patterns. The “radiation recall pattern,” or so‐called radiation recall pneumonitis, was defined as a consolidation progressing inside the previous radiation field. Immune‐related pneumonitis occurring only outside the radiation field in patients with a history of previous TRT and occurring in patients without TRT were defined as a “radiation‐independent pattern,” which included all imaging patterns other than radiation recall pattern.
2.3. Statistical analysis
We analyzed the risk factors for IRP and radiation recall pattern by undertaking univariate and multivariate logistic regression analyses. The analysis for the risk factors for radiation recall pattern compared the patients who developed radiation recall pattern and those with a history of TRT who developed a radiation‐independent pattern. To compare the incidence of specific imaging patterns between the patients with and without a history of previous TRT, we used Pearson's χ2‐test. To determine the difference in onset time since the initiation of nivolumab treatment and the last TRT between the imaging patterns, comparisons of the cumulative incidence curves between the imaging patterns were carried out using a log–rank test, and the Cox proportional hazards method was used to estimate the hazard ratio. All statistical analyses were undertaken using the JMP Pro version 16.2.0 (SAS Institute, Inc.) software package.
2.4. Ethical considerations
This research was approved by the institutional review boards of the five participating institutions (National Cancer Center Hospital and National Cancer Center Hospital East, 2018‐060; Shizuoka Cancer Center, T30‐46; Kanagawa Cancer Center, 2018‐155; and Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital, 2189). The need for informed consent was waived as this study was a retrospective analysis.
3. RESULTS
3.1. Patient characteristics
In this study, 669 patients who received nivolumab for metastatic or recurrent NSCLC were analyzed. The patient characteristics are shown in Table 1. The median age was 66 years (range, 30–87 years), and 68.3% (457/669) of patients were men. Overall, 68.0% (455/669) and 22.7% (152/669) of the patients were pathologically diagnosed as having adenocarcinoma and squamous cell carcinoma, respectively; 78.2% (523/669) were current or former smokers, and 38.4% (257/669) had a history of TRT.
TABLE 1.
Characteristics of patients with non‐small‐cell lung cancer treated with nivolumab
| IRP (N = 59) | Non‐IRP (N = 610) | All (N = 669) | |
|---|---|---|---|
| Age, years | |||
| Median (range) | 68 (45–83) | 66 (30–87) | 66 (30–87) |
| Sex | |||
| Male | 45 (76.3) | 412 (67.5) | 457 (68.3) |
| Female | 14 (23.7) | 198 (32.5) | 212 (31.7) |
| Histology | |||
| Ad | 34 (57.6) | 421 (69.0) | 455 (68.0) |
| Sq | 21 (35.6) | 131 (21.5) | 152 (22.7) |
| NSCLC | 2 (3.4) | 35 (5.7) | 37 (5.5) |
| NEC/LCC | 2 (3.4) | 13 (2.1) | 15 (2.2) |
| Spindle/pleomorphic | 0 (0.0) | 4 (0.7) | 4 (0.6) |
| Other | 0 (0.0) | 6 (1.0) | 6 (0.9) |
| Stage | |||
| IV | 29 (49.2) | 358 (58.7) | 387 (57.9) |
| III | 15 (25.4) | 127 (20.8) | 142 (21.2) |
| I and II | 2 (3.4) | 5 (0.8) | 7 (1.0) |
| Postoperative recurrence | 13 (22.0) | 120 (19.7) | 133 (19.9) |
| Biomarker mutation | |||
| EGFR | 5 (8.5) | 102 (16.7) | 107 (16.0) |
| ALK | 0 (0.0) | 10 (1.6) | 10 (1.5) |
| ROS1 | 0 (0.0) | 8 (1.3) | 8 (1.2) |
| Smoking history | |||
| Nonsmoker | 6 (10.2) | 140 (23.0) | 146 (21.8) |
| Smoker | 53 (89.8) | 470 (77.0) | 523 (78.2) |
| Median pack‐years | 43 | 41 | 41 |
| Previous chemotherapy line | |||
| Median (range) | 1 (0–6) | 2 (0–12) | 2 (0–12) |
| Previous TRT | |||
| − | 26 (44.1) | 387 (63.4) | 412 (61.6) |
| + | 33 (55.9) | 223 (36.6) | 257 (38.4) |
| ECOG PS | |||
| 0 | 18 (30.5) | 119 (19.5) | 137 (20.4) |
| 1 | 34 (57.6) | 399 (65.4) | 433 (64.7) |
| 2 | 6 (10.2) | 75 (12.3) | 81 (12.1) |
| 3 | 1 (1.7) | 17 (2.8) | 18 (2.7) |
| Corticosteroid use at initiation of Nivo | |||
| + | 2 (3.4) | 30 (4.9) | 32 (4.8) |
| Treatment duration of Nivo, months | |||
| Median (range) | 3.3 (0.0–39.0) | 1.9 (0.0–42.6) | 2.3 (0.0–42.6) |
Note: Data are shown as n (%) unless otherwise indicated.
Abbreviations: Ad, adenocarcinoma; IRP, immune‐related pneumonitis; LCC, large cell carcinoma; NEC, neuroendocrine carcinoma; Nivo, nivolumab; NSCLC, non‐small‐cell lung cancer; PS, performance status; Sq, squamous cell carcinoma; TRT, thoracic radiation therapy.
3.2. Incidence of IRP
The incidences of all grade and grade 3 or higher IRP were 8.8% (59/669) and 2.6% (18/669), respectively (Table 2). The incidences of IRP in patients with and those without a history of previous TRT were 13.2% (34/257) and 6.1% (25/412), respectively; this difference was statistically significant (p = 0.0015). Three (0.4%) patients without a history of TRT died because of pneumonitis.
TABLE 2.
Grading and outcome of immune‐related pneumonitis in patients with non‐small‐cell lung cancer treated with nivolumab
| Grade | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | All (%) |
|---|---|---|---|---|---|---|
| All | 10 | 31 | 14 | 1 | 3 | 59/669 (8.8) |
| TRT+ | ||||||
| Radiation recall pattern | 4 | 8 | 4 | 0 | 0 | 16/257 (6.2) |
| Radiation‐independent pattern | 3 | 11 | 4 | 0 | 0 | 18/257 (7.0) |
| TRT− | ||||||
| Radiation‐independent pattern | 3 | 12 | 6 | 1 | 3 | 25/412 (6.1) |
| Outcome | Cure | Remission | Exacerbation | Death | All |
|---|---|---|---|---|---|
| All | 21 | 34 | 1 | 3 | 59 |
| TRT+ | |||||
| Radiation recall pattern | 9 | 7 | 0 | 0 | 16 |
| Radiation‐independent pattern | 5 | 13 | 0 | 0 | 18 |
| TRT− | |||||
| Radiation‐independent pattern | 7 | 14 | 1 | 3 | 25 |
Abbreviations: TRT, thoracic radiation therapy.
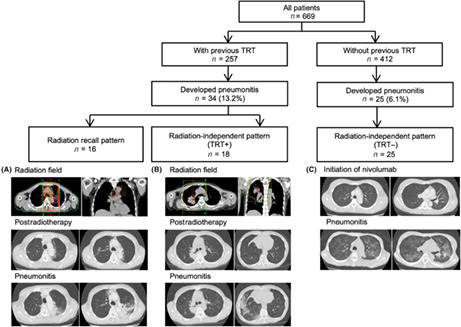
The imaging analysis undertaken by board‐certified diagnostic radiologists divided the patients who developed IRP into three groups: those with a radiation recall pattern, those with a radiation‐independent pattern and a history of previous TRT, and those without TRT. Representative images are shown in Figure 1. The incidence of a radiation recall pattern was 6.2% (16/257) among the patients with a history of previous TRT, and the incidences of radiation‐independent patterns were 7.0% (18/257) and 6.1% (25/412) among patients with and those without previous TRT, respectively (Table 2, Figure 2). Previous TRT was associated with the incidence of IRP but was not associated with the radiation‐independent pattern.
FIGURE 1.
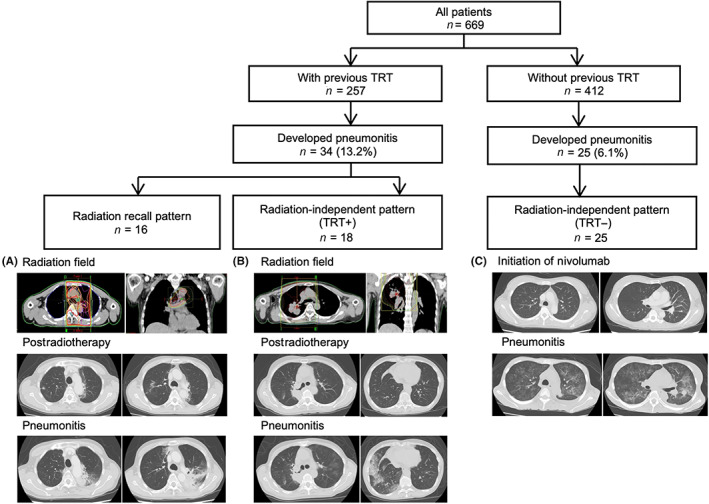
Flowchart of imaging analysis and representative images of each imaging pattern from patients with non‐small‐cell lung cancer treated with nivolumab. The flowchart shows the imaging patterns that were confirmed during an independent review by board‐certified diagnostic radiologists. Representative images of each imaging pattern are shown in the lower part of the figure. Images obtained for thoracic radiation therapy (TRT) planning and computed tomography images obtained at the onset of immune‐related pneumonitis (IRP) are shown. (A) A radiation recall pattern showing consolidation within the previous radiation field. This patient developed IRP within the radiation field. (B) A radiation‐independent pattern showing IRP outside the radiation field. This patient only developed pneumonitis in the bottom lung field, although the radiation field was in the right upper lobe. (C) IRP in a patient without a history of TRT. This patient developed a hypersensitive pneumonitis pattern in the whole lung field
FIGURE 2.
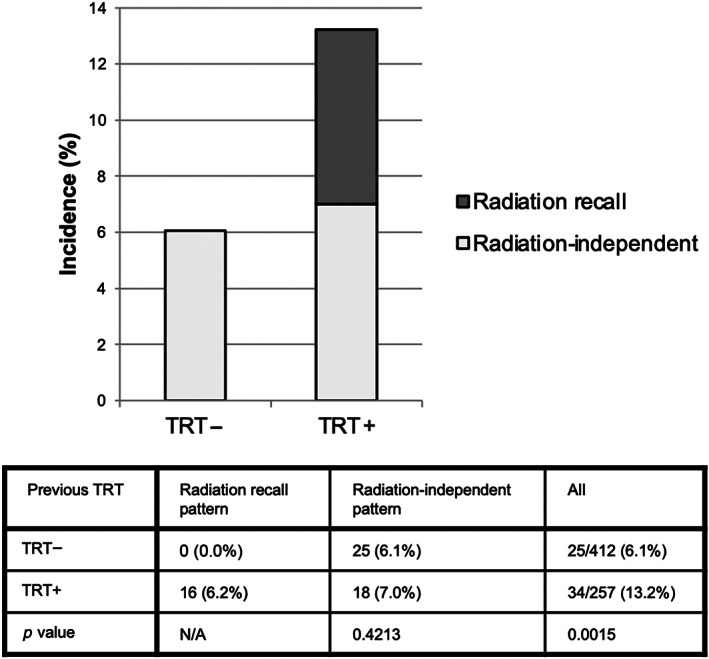
Incidence of immune‐related pneumonitis according to history of previous thoracic radiation therapy (TRT) and imaging patterns. N/A, not applicable
3.3. Risk factors of IRP
We undertook univariate and multivariate analyses to evaluate possible risk factors of IRP including age (≥65 years old or not), sex, smoking history, histology (adenocarcinoma or not), history of previous TRT, and ECOG PS (Table 3). A smoking history and a history of TRT were statistically significant risk factors in univariate analyses (smoking history: odds ratio [OR], 2.64; 95% confidence interval [CI], 1.11–6.26; previous TRT: OR, 2.36; 95% CI, 1.37–4.06), and a history of previous TRT was an independent risk factor in a multivariate analysis (OR, 2.11; 95% CI, 1.21–3.69).
TABLE 3.
Risk factors for immune‐related pneumonitis in patients with non‐small‐cell lung cancer treated with nivolumab
| Factors | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p value | Odds ratio | 95% CI | p value | |
| Age, years | ||||||
| <65 | Reference | Reference | ||||
| ≥65 | 1.66 | 0.92–2.98 | 0.0881 | 1.59 | 0.87–2.88 | 0.1266 |
| Sex | ||||||
| Female | Reference | Reference | ||||
| Male | 1.54 | 0.83–2.88 | 0.1687 | 0.99 | 0.48–2.03 | 0.9795 |
| Histology | ||||||
| Ad | Reference | Reference | ||||
| Non‐Ad | 1.64 | 0.95–2.82 | 0.0733 | 1.20 | 0.68–2.13 | 0.5293 |
| Smoking history | ||||||
| Nonsmoker | Reference | Reference | ||||
| Smoker | 2.64 | 1.11–6.26 | 0.0229 | 2.25 | 0.83–6.11 | 0.1110 |
| Previous TRT | ||||||
| − | Reference | Reference | ||||
| + | 2.36 | 1.37–4.06 | 0.0015 | 2.11 | 1.21–3.69 | 0.0089 |
| ECOG PS | ||||||
| 0–1 | Reference | Reference | ||||
| ≥2 | 0.76 | 0.33–1.72 | 0.5063 | 0.73 | 0.32–1.68 | 0.4580 |
Abbreviations: Ad, adenocarcinoma; CI, confidence interval; PS, performance status; TRT, thoracic radiation therapy.
3.4. Radiation recall pattern
Focusing on the radiation recall pattern, the incidences of all grade and grade 3 and higher pneumonitis were 6.2% (16/257) and 1.6% (4/257), respectively, and none of these patients died because of pneumonitis (Table 2). The patient characteristics are shown in Table 4. A periradiation field pattern tended to occur within 24 months after the last TRT, whereas a radiation‐independent pattern tended to appear more gradually within a period of up to 60 months after the last TRT (Figure 3C).
TABLE 4.
Characteristics of patients with non‐small‐cell lung cancer treated with nivolumab, according to radiological pattern
| Radiation recall (N = 16) | Radiation‐independent (TRT+) (N = 18) | Radiation‐independent (TRT−) (N = 25) | |
|---|---|---|---|
| Age, years | |||
| Median (range) | 69 (61–80) | 69 (59–79) | 68 (45–85) |
| Sex | |||
| Male | 13 (81.3) | 14 (77.8) | 18 (72.0) |
| Female | 3 (17.7) | 4 (22.2) | 7 (28.0) |
| Smoking history | |||
| Nonsmoker | 1 (6.2) | 2 (11.1) | 3 (12.0) |
| Smoker | 15 (93.8) | 16 (88.9) | 22 (88.0) |
| Median pack‐years | 50 | 46 | 39 |
| ECOG PS | |||
| 0 | 9 (56.3) | 2 (11.1) | 7 (28.0) |
| 1 | 5 (31.2) | 15 (83.3) | 14 (56.0) |
| 2 | 2 (12.5) | 1 (5.6) | 3 (12.0) |
| 3 | 0 (0.0) | 0 (0.0) | 1 (4.0) |
| Background lung disease | |||
| COPD | 5 (31.3) | 10 (55.6) | 8 (32.0) |
| ILD | 0 (0.0) | 0 (0.0) | 2 (8.0) |
| Purpose of radiation therapy | |||
| Curative | 11 (68.8) | 14 (77.8) | ‐ |
| Palliative | 5 (31.2) | 4 (22.2) | ‐ |
| Previous radiation pneumonitis | |||
| + | 8 (50.0) | 9 (50.0) | ‐ |
| Total dose, Gy | |||
| <40 | 2 (12.5) | 5 (27.8) | ‐ |
| 40–60 | 3 (18.8) | 1 (5.5) | ‐ |
| ≥60 | 10 (62.5) | 12 (66.7) | ‐ |
| Unknown | 1 (6.2) | 0 (0.0) | ‐ |
| V20 (%) | |||
| ≤20 | 7 (43.8) | 7 (38.9) | ‐ |
| 20–30 | 6 (37.5) | 3 (16.7) | ‐ |
| >30 | 1 (6.2) | 1 (5.5) | ‐ |
| Unknown | 2 (12.5) | 7 (38.9) | ‐ |
| V30 (%) | |||
| ≤20 | 11 (68.8) | 8 (44.4) | ‐ |
| >20 | 3 (18.7) | 3 (16.7) | ‐ |
| Unknown | 2 (12.5) | 7 (38.9) | ‐ |
| MLD, Gy | |||
| ≤10 | 8 (50.0) | 7 (38.9) | ‐ |
| >10 | 6 (37.5) | 4 (22.2) | ‐ |
| Unknown | 2 (12.5) | 7 (38.9) | ‐ |
| Last TRT to Nivo, months | |||
| <12 | 9 (56.3) | 7 (38.9) | ‐ |
| 12–24 | 5 (31.2) | 6 (33.3) | ‐ |
| 24–36 | 2 (12.5) | 1 (5.6) | ‐ |
| 36–48 | 0 (0.0) | 3 (16.6) | ‐ |
| ≥48 | 0 (0.0) | 1 (5.6) | ‐ |
Note: Data are shown as n (%) unless otherwise indicated.
Abbreviations: COPD, chronic obstructive pulmonary disease; ILD, interstitial lung disease; MLD, median lung dose; Nivo, nivolumab; PS, performance status; TRT, thoracic radiation therapy; V20, volume receiving more than 20 Gy; V30, volume receiving more than 30 Gy.
FIGURE 3.
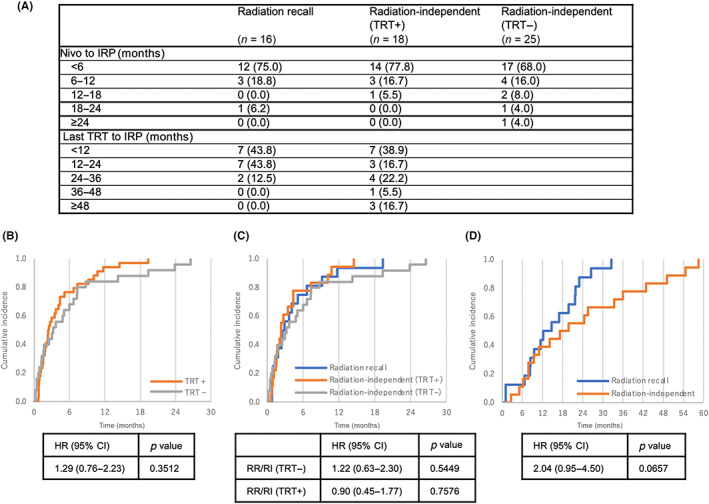
Cumulative incidences of immune‐related pneumonitis (IRP) in patients with non‐small‐cell lung cancer treated with nivolumab. (A) Duration since the initiation of nivolumab treatment or the last thoracic radiation therapy (TRT) to the occurrence of IRP. (B) Cumulative incidences of IRP showing the time since the initiation of nivolumab treatment according to the history of previous TRT. (C) Cumulative incidences of IRP showing the time since the initiation of nivolumab treatment according to imaging pattern. (D) Cumulative incidences of IRP showing the time since the last TRT according to imaging pattern. CI, confidence interval; HR, hazard ratio; RI, radiation‐independent pattern; RR, radiation recall pattern
Although the number of patients was restricted in the analysis, a shorter time (≤24 months) after the last TRT was a potential risk factor (OR, 5.60; 95% CI, 0.97–32.20; Table S1), and the same tendency was preserved when adjustments according to age, sex, PS, and V20 were performed. Focusing on the time course of nivolumab treatment after the last TRT, we also compared the cumulative incidence since the last TRT as well as since the initiation of nivolumab treatment (Figure 3). Although patients with a history of previous TRT tended to develop IRP earlier than those without a history of TRT, we could not find a statistical difference between these groups. Considering the confounding of time after the last TRT and after the initiation of nivolumab treatment, a shorter time (≤24 months) after the last TRT was a potential risk factor for a radiation recall pattern.
Patients with a radiation recall pattern had a relatively good outcome, and all 16 patients were cured or experienced remission and none of them were refractory to corticosteroid therapy (Table 2). Four of these patients were cured without requiring any drug treatment, including corticosteroid use (Table S2).
4. DISCUSSION
The current study was the first report to identify a history of previous TRT as a risk factor for IRP in a relatively large cohort of 669 patients who had been treated with nivolumab. We also undertook a central radiological analysis to distinguish the imaging patterns of IRP, and we examined the incidence and risk factors of a radiation recall pattern, which have not been previously reported.
Two clinical trials reported the incidences of IRP after nivolumab monotherapy as 3%–5%, 1 , 2 while four retrospective analyses reported incidences of 13%–19%. 10 , 11 , 12 , 13 The incidence of 8.8% in the present study was thus relatively low. This difference might be because our study only included patients who had received nivolumab, while the previous reports included all ICI treatments, including pembrolizumab, and even combination therapies, which have been reported as risk factors for IRP. 10 , 12 Only one previous study focused on the details of previous TRT, 13 and they reported in a subset analysis that IRP was more common among patients who had received curative‐intent TRT, compared with those who had received palliative‐intent TRT. However, this study only determined the different incidences of IRP between patients receiving radiotherapy with different intents, and TRT itself was not a risk factor for IRP. Our study is the first report to date to show that TRT might serve as an independent risk factor of IRP. In addition, our study was the only one to include a central imaging analysis to distinguish the imaging pattern of radiation recall pneumonitis, and this analysis revealed that the additive incidence of IRP among patients with previous TRT might be caused by this characteristic imaging pattern (Figure 2). This suggests that radiation recall pneumonitis is a subset of IRP that likely develops through a different mechanism.
The present study was the first to report the incidence and clinical characteristics of radiation recall pneumonitis. Our study showed that a radiation recall pattern occurred in 6.2% of patients with a history of previous TRT and that most of these cases occurred within 24 months after the last TRT. This result could be an indicator suggesting the need for careful observation in patients receiving ICI treatment who have a history of previous TRT. In the present study, the patients with a radiation recall pattern were responsive to corticosteroid therapy and had a relatively good outcome. Although the sample size of this study is still limited, it suggests the possibility that patients with a history of previous TRT could successfully undergo ICI treatment with careful monitoring, similar to patients without TRT.
Although the mechanism underlying IRP has not yet been fully revealed, CTLs induced by ICIs recruit lymphoid infiltration of the lung and other organs and are thought to contribute to irAEs, including IRP. 16 The synergetic effect of radiation therapy and ICIs is now attracting interest, and radiation‐induced inflammatory cytokine signaling and immune cell recruitment, as well as tumor antigens and the generation of damage‐associated molecular patterns resulting from tumor cell death, are known to stimulate the immune response in the irradiated lung field. 17 , 18 The mechanism of radiation recall pneumonitis is yet to be elucidated, but ICI‐induced radiation recall pneumonitis might involve the persistence of immune reactive products caused by previous TRT that enhance the immune response within the radiation field.
This study has some limitations. The first is that our study was evaluated only in Japanese patients. Japanese patients reportedly to have a relatively higher incidence of IRP and higher toxicity, 19 , 20 therefore evaluating the risk factors of IRP in this population is reasonable. Second, our study was retrospective, and methods of follow‐up monitoring were not standardized. However, the institutions that participated in our study were high‐volume centers, and follow‐up examinations did not differ so much by each institution. Third, despite a large cohort of 669 patients, the number of patients with previous TRT who developed IRP was relatively small to evaluate the difference of characteristics between radiation recall pattern and the other pattern. Our analysis indicated that radiation recall pneumonitis occurred within a relatively short time after the last TRT and that this pneumonitis had a relatively better outcome; however, this should be further verified in larger sample sizes. Despite these limitations, our study has significant value as this was the first report to clarify the relationship between previous TRT and risk of IRP along with imaging analysis to characterize radiation recall pneumonitis.
Our study showed that previous TRT was an independent risk factor of IRP after nivolumab treatment and that a specific IRP imaging pattern, which was previously reported as radiation recall pneumonitis, was one of the main patterns among the patients with previous TRT. Although the mechanism of radiation recall pneumonitis has not been fully investigated, our study revealed this specific type of IRP contributed to the higher risk of IRP in NSCLC patients with a history of previous TRT. The clinical characteristics of radiation recall pattern shown in this study, which tends to occur earlier after the last TRT and to have a better outcome than a radiation‐independent pattern, should be further verified in larger sample size. Further investigation to explore the mechanism of radiation recall pneumonitis in preclinical fields is warranted.
AUTHOR CONTRIBUTIONS
Hidehito Horinouchi, Yuichiro Ohe, and Shoko Noda‐Narita conceived and designed the study. Tomoyuki Naito, Hibiki Udagawa, Koichi Goto, Taichi Miyawaki, Nobuaki Mamesaya, Kazuhisa Nakashima, Hirotsugu Kenmotsu, Tadasuke Shimokawaji, Terufumi Kato, Taiki Hakozaki, Yusuke Okuma, Hidehito Horinouchi, and Shoko Noda‐Narita participated in the data collection. Masaki Nakamura and Yuko Nakayama participated in the data collection for the previous radiation therapy. Hirokazu Watanabe and Masahiko Kusumoto performed the imaging analysis. Hidehito Horinouchi and Shoko Noda‐Narita performed the overall analysis and interpreted the data. Shoko Noda‐Narita wrote the manuscript. All the authors read and approved the final article.
DISCLOSURES
Koichi Goto is an associate editor of Cancer Science. Koichi Goto received research grants from Amgen Astellas BioPharma K.K., Amgen Inc., Astellas Pharma Inc., AstraZeneca K.K., Boehringer Ingelheim Japan, Inc., Bristol‐Myers Squibb K.K., Chugai Pharmaceutical Co., Ltd, Daiichi Sankyo Co., Ltd, Eisai Co., Ltd, Eli Lilly Japan K.K., Ignyta, Inc., Janssen Pharmaceutical K.K., Kissei Pharmaceutical Co., Ltd, Kyowa Kirin Co., Ltd, Loxo Oncology, Inc., Medical & Biological Laboratories Co., Ltd, Merck Biopharma Co., Ltd, Merus N.V., MSD K.K., NEC Corporation., Novartis Pharma K.K., Ono Pharmaceutical Co., Ltd, Pfizer Japan Inc., Sumitomo Dainippon Pharma Co., Ltd, Spectrum Pharmaceuticals, Inc., Sysmex Corporation., Haihe Biopharma Co., Ltd, Taiho Pharmaceutical Co., Ltd, and Takeda Pharmaceutical Co., Ltd, and lecture fees from Amgen Inc., AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd, Eli Lilly Japan K.K., Janssen Pharmaceutical K.K., and Novartis Pharma K.K.. Nobuaki Mamesaya received a research grant from Boehringer Ingelheim. Kazuhisa Nakashima received lecture fees from AstraZeneca K.K., Pfizer Japan, Inc., Chugai Pharmaceutical Co., Ltd, and Taiho Pharmaceutical Co., Ltd Hirotsugu Kenmotsu received research grants from AstraZeneca K.K., Pfizer Japan, Inc., Chugai Pharmaceutical Co., Ltd, Novartis Pharma K.K., Pfizer K.K., and Daiichi‐Sankyo Co., Ltd Tadasuke Shimokawaji received research grants from Bristol‐Myers Squibb, Ono Pharmaceutical, AstraZeneca, and Takeda. Terufumi Kato received research grants from Bristol Myers Squibb, Ono, AbbVie, Amgen, AstraZeneca, Chugai, Eli Lilly, Merck Biopharma, MSD, Novartis, Pfizer, Taiho, and Regeneron, and lecture fees from Bristol Myers Squibb, Ono, AbbVie, Amgen, AstraZeneca, Chugai, Eli Lilly, Merck Biopharma, MSD, Novartis, Pfizer, Taiho, Boehringer Ingelheim, Daiichi‐Sankyo, Nippon Kayaku, and Takeda. Yusuke Okuma received research grants from AbbVie K.K. and Chugai Pharmaceutical Co., and lecture fees from Ono Pharmaceutical Co., Ltd, Astra Zeneca, Boehringer Ingelheim, Eli Lilly, and Chugai Pharmaceutical Co., Ltd Yuko Nakayama received lecture fees from AstraZeneca K.K.. Masahiko Kusumoto received a research grant from Canon Medical Systems Corporation, and lecture fees from AstraZeneca K.K. and Daiichi Sankyo Co., Ltd… Yuichiro Ohe is an associate editor of Cancer Science. Yuichiro Ohe received research grants from Ono Pharmaceutical, Bristol‐Myers Squibb, AstraZeneca, Chugai, Eli Lilly, Janssen, MSD, Takeda Pharmaceutical, Kissei, Ignyta, IQVIA, CMIC, and EPS, and lecture fees from AstraZeneca, Chugai, and Kyorin. Hidehito Horinouchi received research grants from BMS, Ono, MSD, AstraZeneca, Janssen, Chugai, Daiichi‐Sankyo, AbbVie, and Genomic Health, and lecture fees from BMS, Ono, MSD, AstraZeneca, Janssen, Chugai, Lilly, Merck Biopharma, Kyowa‐Kirin, and Nihonkayaku. The other authors have no conflict of interest. All authors had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
ACKNOWLEDGEMENTS
The authors would like to thank all study participants. Also, we would like to thank all the staff of each participating institution who took care of the patients.
ETHICS STATEMENT
Approval of the research protocol by and institutional review board: The study was approved by the institutional review boards of the five participating institutions (National Cancer Center Hospital and National Cancer Center Hospital East, 2018–060; Shizuoka Cancer Center, T30‐46; Kanagawa Cancer Center, 2018‐155; and Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital, 2189).
Informed consent: The need for informed consent was waived as this was a retrospective analysis.
Registry and registration no. of the study/trial: N/A.
Animal studies: N/A.
Supporting information
Table S1
Table S2
Noda‐Narita S, Naito T, Udagawa H, et al. Nivolumab‐induced radiation recall pneumonitis in non‐small‐cell lung cancer patients with thoracic radiation therapy. Cancer Sci. 2023;114:630‐639. doi: 10.1111/cas.15621
REFERENCES
- 1. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Decetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med. 2015;373(2):123‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borghaei H, Paz‐Ares L, Horn L, et al. Nivolumab versus Decetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med. 2015;373(17):1627‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gandhi L, Rodríguez D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med. 2018;378(22):2078‐2092. [DOI] [PubMed] [Google Scholar]
- 4. Paz‐Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non‐small‐cell lung cancer. N Engl J Med. 2018;379(21):2040‐2051. [DOI] [PubMed] [Google Scholar]
- 5. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first‐line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288‐2301. [DOI] [PubMed] [Google Scholar]
- 6. Nishino M, Giobbie‐Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of programmed cell death 1 Inhibotor‐related pneumonitis in patients with advanced cancer: a systematic review and meta‐analysis. JAMA Oncol. 2016;2(12):1607‐1616. [DOI] [PubMed] [Google Scholar]
- 7. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus decetaxel for preciously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): a randomised controlled trial. Lancet. 2016;387(10027):1540‐1550. [DOI] [PubMed] [Google Scholar]
- 8. Reck M, Rodríguez D, Robinson AG, et al. KEYNOTE‐024 investigators. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med. 2016;375(19):1823‐1833. [DOI] [PubMed] [Google Scholar]
- 9. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus decetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): a phase 3, open‐label, multiventre randomised controlled trial. Lancet. 2017;389(10066):255‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suresh K, Voong KR, Shankar B, et al. Pneumonitis in non‐small cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J Thorac Oncol. 2018;13(12):1930‐1939. [DOI] [PubMed] [Google Scholar]
- 11. Cho JY, Kim J, Lee JS, et al. Characteristics, incidence, and risk factors of immune checkpoint inhibitor‐related pneumonitis in patients with non‐small cell lung cancer. Lung Cancer. 2018;125:150‐156. [DOI] [PubMed] [Google Scholar]
- 12. Fukihara J, Sakamoto K, Koyama J, et al. Prognostic impact and risk factors of immune‐related pneumonitis in patients with non‐small‐cell lung cancer who received programmed death 1 inhibitors. Clin Lung Cancer. 2019;20(6):442‐450. [DOI] [PubMed] [Google Scholar]
- 13. Voong KR, Hazell SZ, Fu W, et al. Relationship between prior radiotherapy and checkpoint‐inhibitor pneumonitis in patients with advanced non‐small‐cell lung cancer. Clin Lung Cancer. 2019;20(4):e470‐e479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non‐small‐cell lung cancer: a secondary analysis of the KEYNOTE‐001 phase 1 trial. Lancet Oncol. 2017;18(7):895‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shibaki R, Akamatsu H, Fujimoto M, Koh Y, Yamamoto N. Nivolumab induced radiation recall pneumonitis after two years of radiotherapy. Ann Oncol. 2017;28(6):1404‐1405. [DOI] [PubMed] [Google Scholar]
- 16. Postow MA, Sidlow R, Hellmann MD. Immune‐related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158‐168. [DOI] [PubMed] [Google Scholar]
- 17. Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanism of synergy. Lancet Oncol. 2015;16(13):e498‐e509. [DOI] [PubMed] [Google Scholar]
- 18. Barker HE, Paget JTE, Khan AA, Harrington KJ. The tumor microenvironment after radiotherapy: mechanism of resistance and recurrence. Nat Rev Cancer. 2015;15(7):409‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kato T, Masuda N, Nakanishi Y, et al. Nivolumab‐induced interstitial lung disease analysis of two phase II studies patients with recurrent or advanced non‐small‐cell lung cancer. Lung Cancer. 2017;104:111‐118. [DOI] [PubMed] [Google Scholar]
- 20. Sata M, Sasaki S, Oikado K, et al. Treatment and relapse of interstitial lung disease in nivolumab‐treated patients with non‐small cell lung cancer. Cancer Sci. 2021;112(4):1506‐1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2


