Abstract
Although effective, immune checkpoint blockade induces response in only a subset of cancer patients. There is an urgent need to discover new immune checkpoint targets. Recently, it was found that a class of sialic acid–binding immunoglobulin‐like lectins (Siglecs) expressed on the surface of T cells in cancer patients inhibit T cell activation through their intracellular immunosuppressive motifs by recognizing sialic acid–carrying glycans, sialoglycans. However, ligands of Siglecs remain elusive. Here, we report sialylated IgG (SIA‐IgG), a ligand to Siglec‐7, that is highly expressed in epithelial cancer cells. SIA‐IgG binds Siglec‐7 directly and inhibits TCR signals. Blocking of either SIA‐IgG or Siglec‐7 elicited potent antitumor immunity in T cells. Our study suggests that blocking of Siglec‐7/SIA‐IgG offers an opportunity to enhance immune function while simultaneously sensitizing cancer cells to immune attack.
Keywords: immune checkpoint, sialylated IgG (SIA‐IgG), Siglec, tumor immunology, tumor microenvironment
Here, we reported sialylated‐IgG (SIA‐IgG), a ligand to Siglec‐7, binds Siglec‐7 directly and inhibits TCR signals, and the inhibitory signal is dependent on its sialylation level. Blocking of either SIA‐IgG or Siglec‐7 elicited potent antitumor immunity in tumor‐killing T cells. Our study suggests that blocking of Siglec‐7/SIA‐IgG offers an opportunity to enhance immune function while simultaneously sensitizing tumor cells to immune attack.
Abbreviations
- Asn
asparagine
- CTLA‐4
cytotoxic T lymphocyte–associated protein‐4
- His
histidine
- ITIM
immunoreceptor tyrosine–based inhibition motif
- IVIG
intravenous immunoglobulin
- LAG‐3
lymphocyte activation gene 3
- PD‐1
programmed death‐1
- SHP‐1
Src homology region 2 domain–containing phosphatase‐1
- SIA‐IgG
sialylated IgG
- Siglecs
sialic acid–binding immunoglobulin‐like lectins
- TME
tumor microenvironment
1. INTRODUCTION
Breakthroughs in cancer immunotherapy using checkpoint blockade have been demonstrated experimentally and translated into treatment of many cancers in clinic. 1 A number of inhibitory immune receptors have been identified and studied in cancer in the past decades, including but not limited to PD‐1, CTLA‐4, and LAG‐3, which are named “immune checkpoints” referring to molecules that act as gatekeepers of immune responses. 2 , 3 However, as clinical data accumulate worldwide, the low response rate in most cancers showed up as a drawback in immune checkpoint blockade therapy, with a range of 10%‐30%. 1 Thus, intensive research aimed at exploring novel immune checkpoint targets has been ongoing.
Siglecs are found on most immune cells and have an N‐terminal Ig domain that recognizes sialic acid–containing glycans commonly found on glycoproteins and glycolipids. Siglecs regulate immune cell functions by binding to sialoglycans, which are considered markers of “self.” 4 In humans, the family of Siglecs comprises 14 members. Based on sequence similarity, they can be divided into conserved Siglecs (Siglec‐1, ‐2, ‐4, and ‐15) and rapidly evolving CD33‐related Siglecs (CD33 or Siglec‐3, Siglec‐5, ‐6, ‐7, ‐8, ‐9, ‐10, ‐11, ‐14, and ‐16), while Siglec‐12 and Siglec‐13 are missing in humans. 5 Unlike the conserved Siglecs, CD33‐related Siglecs do not have clear mammalian homologs and exhibit a broader expression across immune cells. 6 CD33‐related Siglecs are mostly inhibitory receptors, which contain at least one immunoreceptor tyrosine–based inhibition motif (ITIM). 5 , 7 By recognizing sialoglycans, the inhibitory Siglecs have been considered to mainly downregulate antitumor responses of innate immune cells, including NK cells, 8 macrophages, 9 or neutrophils, 10 but rarely in T cells. 11 In recent years, the inhibitory Siglecs, such as Siglec‐9 12 , 13 and Siglec‐10, 14 , 15 have also been reported to be expressed on human T cells and mediate T cell inhibition and tumor immune escape in lung cancer and melanoma, suggesting that inhibitory Siglecs act as new immune checkpoints. 12 , 13 , 14 Although human Siglecs primarily bind to sialic acids on diverse types of glycans, many of the natural ligands of Siglecs have not been fully identified.
Increased level of immunoglobulin (Ig) in patients with cancer has been observed for decades. 16 These Igs are generally considered to be the result of increased expression of B cell–derived antitumor antibodies. However, there is increasing evidence that IgG in the tumor microenvironment (TME) usually indicates a poor prognosis. Our group and others have been investigating IgG overexpressed in many cancer cells since 1996. 17 , 18 , 19 , 20 We have already found that cancer‐derived IgG displays growth factor–like activity and promotes the progression of cancer cells. 21 , 22 , 23 In our recent studies, we used a monoclonal antibody, RP215, which was developed in 1987, and found that RP215 can distinguish cancer‐derived IgG from B‐IgG. 24 , 25 RP215‐recognized cancer‐derived IgG was further revealed to be specifically N‐glycosylated at Asn‐162, which located on the CH1 domain of IgG heavy chain and carried a sialic acid modification. 26 , 27 Cancer‐derived SIA‐IgG can be secreted in an autocrine manner by cancer cells, and can specifically interact with the integrin α6β4 complex and subsequently activate the FAK‐Src pathway. 26 , 28 Moreover, we found that SIA‐IgG can directly inhibit T cell proliferation and significantly promote tumor growth by reducing T cell infiltration. These effects depend on sialylation and binding to Siglecs on T cells, indicating Siglecs/SIA‐IgG act as new checkpoints to inhibit T cell function. 27
In this study, we first found that CD4+T cells, but not CD8+T cells from peripheral blood, expressed inhibitory Siglec‐3, ‐6, ‐7, ‐9, and ‐10. By analyzing expression frequency of inhibitory Siglecs on tumor‐infiltrating T cells in colon cancer tissues and ovarian cancer ascites, we found that only Siglec‐7, but not other Siglecs, was prominently upregulated on tumor‐infiltrating CD8+T cells. Moreover, Siglec‐7 was also coexpressed with PD‐1 and LAG‐3, which indicates the exhaustion of tumor‐infiltrating CD8+T cells. We then proved that SIA‐IgG served as a ligand of Siglec‐7, activating downstream signals and suppressing TCR pathways. Blockade of Siglec‐7 or SIA‐IgG enhanced the killing effects of T cells for autologous cancer cells in vitro and in vivo. These findings revealed that SIA‐IgG expressed by epithelial cancer cells inhibits T cell function directly through binding Siglec‐7 on T cells and Siglec‐7/SIA‐IgG is a potential target of immune checkpoint therapy.
2. MATERIALS AND METHODS
2.1. Tissue and samples
Colon tumors and margin tissue samples were obtained from Tumor Hospital of the Chinese Academy of Medical Sciences. Peripheral blood and ovarian cancer ascites samples for SIA‐IgG purification and coculture were obtained from Peking University Third Hospital.
2.2. Cell proliferation and coculture
Sorted CD3+T cells were suspended in 1640 containing 10% FBS and stimulated by precoated 3 μg/ml anti‐CD3 antibody (317,303, BioLegend) and 1 μg/ml anti‐CD28 antibody (302,913, BioLegend). EpCAM+ cancer cells from the same individuals were added. Cells were cocultured for 24 hours, and 10 μg/ml anti‐Siglec‐7 blocking antibodies (347,702, Biolegend) or RP215 was added. Tumor apoptosis was analyzed by staining for annexin V/7AAD.
2.3. Purification of cancer‐derived SIA‐IgG
The SIA‐IgG–specific antibody RP215 was coupled to CNBr‐activated Sepharose™ 4B (71‐7086‐00 AF, GE Healthcare) according to the manufacturer's recommendations. IgG from the TME was incubated with the RP215‐coupled CNBr‐activated sepharose column for RP215 recognition–based SIA‐IgG purification.
2.4. Neuraminidase digestion
IgG from the TME was combined with H2O in a total reaction volume of 9 μl, and then 2 μl 10 × GlycoBuffer and 2 μl neuraminidase (P0720, New England Biolabs) were added and incubated at 37°C for 1 hour.
2.5. Animal experiments
Female nude mice and female NOD‐SCID mice were purchased from Beijing Vital River Laboratory Animal Technology Company and used at 6‐8 weeks of age.
EpCAM+ cancer cells were sorted from ovarian cancer ascites, amplified in vitro, and then injected into nude mice (5 × 106 cells each tumor) or NOD‐SCID mice (3 × 106 cells each tumor). After 6 days, the nude mice were randomly allocated into three groups. Each group was injected subcutaneously around the tumors with PBS or 5 × 105 T cells from ovarian cancer ascites, with 10 μg/ml anti‐Siglec‐7 antibodies or control IgG every 3 days. Likewise, NOD‐SCID mice were randomly allocated into five groups, and each group was injected subcutaneously around the tumors with PBS or 20 μg/ml RP215 or 3 × 105 T cells from ovarian cancer ascites, with 20 μg/ml anti‐Siglec‐7 or RP215 or control IgG every 4 days. The mice were then sacrificed and analyzed for tumor volume and weight and the proportions of T cells in the tumor tissue.
2.6. TIMER database analysis
TIMER (http://timer.cistrome.org/) is a comprehensive resource for systematic analysis of immune infiltrates across diverse cancer types from TCGA. We analyzed the correlation of SIGLEC7 expression and T cell infiltration across 38 cancer types.
2.7. Statistics
All data were analyzed with GraphPad Prism software and are presented as the mean ± s.d. or s.e.m. Statistical significance was determined by a two‐tailed paired or unpaired Student's t test or one‐way analysis of variance (ANOVA) followed by Tukey's multiple comparisons test, with significance levels of *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 and ns, not significant (P ≥ 0.05).
Detailed materials and methods, including cell culture and reagents, antibodies for multicolor flow cytometry, immunohistochemistry, immunofluorescence, purification of IgG from the TME, glycopeptide analysis, SDS‐PAGE and Western blotting, coimmunoprecipitation (Co‐IP) analysis, microscale thermophoresis (MST), can be found in the supplementary document.
3. RESULTS
3.1. Siglec‐7 was highly expressed on tumor‐infiltrating CD8 +T cells
To analyze the expression frequency of inhibitory Siglecs on T cells of patients with cancer, we first analyzed inhibitory Siglec‐3, ‐6, ‐7, ‐9, and ‐10, on T cells in peripheral blood of 10 kinds of tumor patients (Table S1). The results revealed that all these five inhibitory Siglecs were expressed on CD4+T cells of patients with cancer; however, the expression frequency of each Siglec showed significant differences: The expression of Siglec‐3, ‐6, ‐7, ‐9, and ‐10 was markedly increased in patients with lung cancer; increased Siglec‐3, ‐9, and ‐10 were found in cervical cancer; and Siglec‐3 and Siglec‐9 were found in rectal cancer (Figure 1A). In contrast, none of the five inhibitory Siglecs displayed difference on CD8+T cells between healthy donors and patients (Figure S1A). To further investigate the expression of inhibitory Siglecs on T cells in TME, we collected nine ovarian cancer ascites and analyzed the expression of Siglec‐6, ‐7, ‐9, and ‐10 on T cells in ascites. Surprisingly, we found that Siglec‐6, ‐7, ‐9, and ‐10 were highly expressed on both CD4+ and CD8+T cells in ovarian cancer ascites compared with those in peripheral blood of healthy donors (Figure 1B). Obviously, the finding is different from that in peripheral blood of patients with cancers.
FIGURE 1.
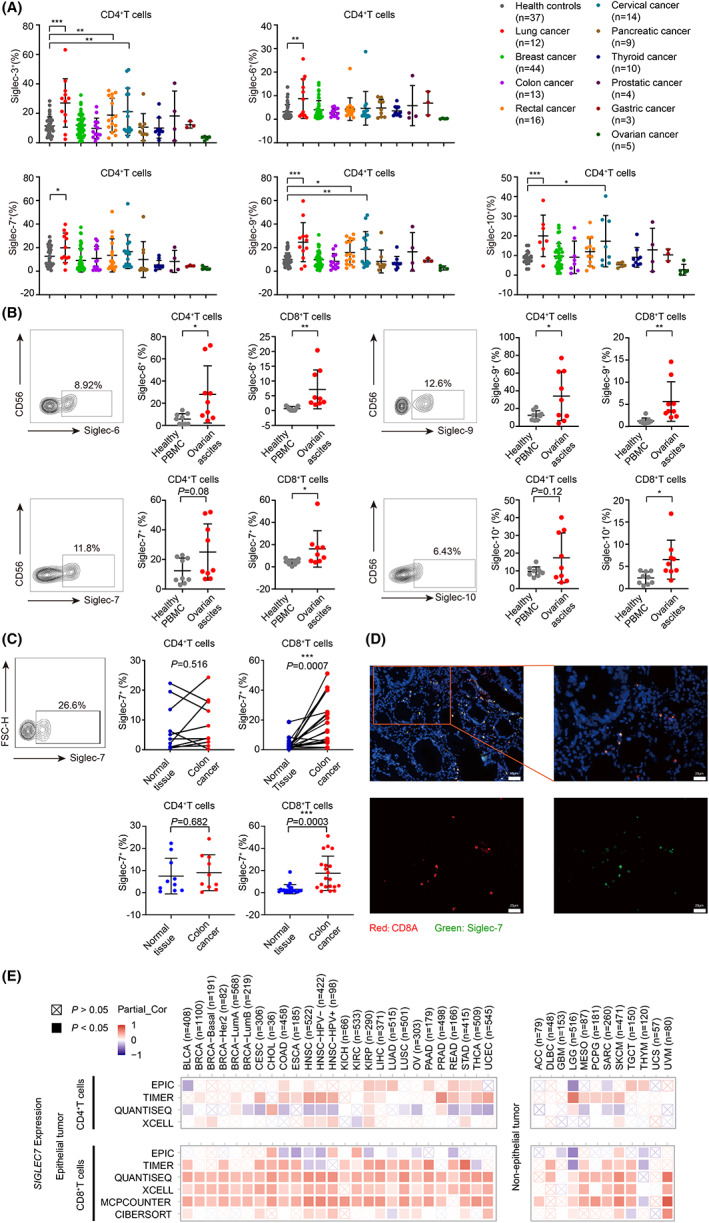
Expression of Siglecs on T cells. (A) Analysis of Siglecs on CD4+T cells from healthy people or patients with different tumors. (B) Quantification of Siglec‐6, ‐7, ‐9, and ‐10 expression on CD4+ T or CD8+T cells from ovarian cancer ascites (n = 9). (C) Analysis of Siglec‐7 on CD4+T or CD8+T cells from colon cancer or normal tissues. (D) Representative immunofluorescence analysis of CD8 and Siglec‐7 in colon cancer. (E) Functional heatmap of the association between Siglec‐7 expression and immune infiltration level of CD4+ or CD8+T cells across 38 cancer types. Red: significant positive association. Blue: significant negative association. Crossed square: non‐significant.
To further address if inhibitory Siglecs were expressed differently on T cells in tumor tissues and normal tissues, we collected 20 colon cancer tissues, using the surgery margin colon tissues from the same individual as normal tissue control, and then analyzed T cells isolated from both tissues. Surprisingly, our results showed that only Siglec‐7 was dominantly expressed on CD8+T cells in TILs (tumor infiltrating T cells), but not in normal tissues in 17 of 20 cases (Figure 1C, Figure S1B). We further analyzed Siglec‐7 location in colon cancer tissues by immunohistochemistry and found that Siglec‐7 staining was shown on lymphocytes (Figure S1C). Moreover, the double immunofluorescence staining showed that Siglec‐7 were colocalized with CD8+T cells (Figure 1D).
Subsequently, we analyzed the correlation of SIGLEC7 expression level with CD4+ and CD8+T cells infiltration in 38 cancer types, including 26 epithelial cancers and 12 nonepithelial cancers, using the Tumor Immune Estimation Resource (TIMER2.0) website (http://timer.cistrome.org/). 29 We first analyzed the correlation between CD4+T cell infiltration and the expression of SIGLEC7 by four different tools (XCELL, TIMER, QUANTISEQ, and EPIC). As revealed in the heat map, the positive correlation of SIGLEC7 expression and CD4+T cell infiltration was shown in several epithelial cancers, such as head and neck cancer and pancreatic cancer, and nonepithelial cancers, such as low‐grade glioma, only by TIMER, but not by the other three tools (Figure 1E). Subsequently, we analyzed the correlation between CD8+T cell infiltration level and expression of SIGLEC7 using six different tools (MCPCOUNTER, CIBERSORT, QUANTISEQ, XCELL, TIMER, and EPIC). We found that the expression of SIGLEC7 displayed significantly positive correlation with CD8+T cells in most epithelial cancers and 6 of 12 nonepithelial cancers by MCPCOUNTER, QUANTISEQ, and XCELL (Figure 1E).
3.2. Siglec‐7 expression was increased on activated T cells, especially on memory CD8 +T cells
Next, we want to answer whether Siglec‐7 can be upregulated on activated T cells. Immune cells were isolated from colon cancer tissues, and T cells were activated with anti‐CD3/CD28; then, Siglec‐7 expression on CD4+ and CD8+T cells was determined. The results showed that Siglec‐7 expression level was significantly increased on both CD4+ and CD8+T cells (Figure 2A). Similarly, in T cells isolated from ovarian cancer ascites, Siglec‐7 was also upregulated upon stimulation on both CD4+ and CD8+T cells (Figure 2B). Aiming to further characterize the coexpression of Siglec‐7 and the well‐known immune checkpoint PD‐1, multicolor flow cytometry was carried out in colon cancer tissues. The results showed that Siglec‐7 was coexpressed with PD‐1 in both CD4+ and CD8+T cells (Figure 2C,D). However, most PD‐1+CD8+ TILs were found to highly express Siglec‐7 (Figure 2D), but PD‐1+CD4+ TILs expressed lower Siglec‐7 (Figure 2C). We also sorted Siglec‐7+ and Siglec‐7− T cells from ovarian cancer ascites for stimulation. Siglec‐7+CD8+ TILs expanded poorly upon anti‐CD3/CD28 stimulation and expressed higher PD‐1 and LAG‐3 (Figure 2E).
FIGURE 2.
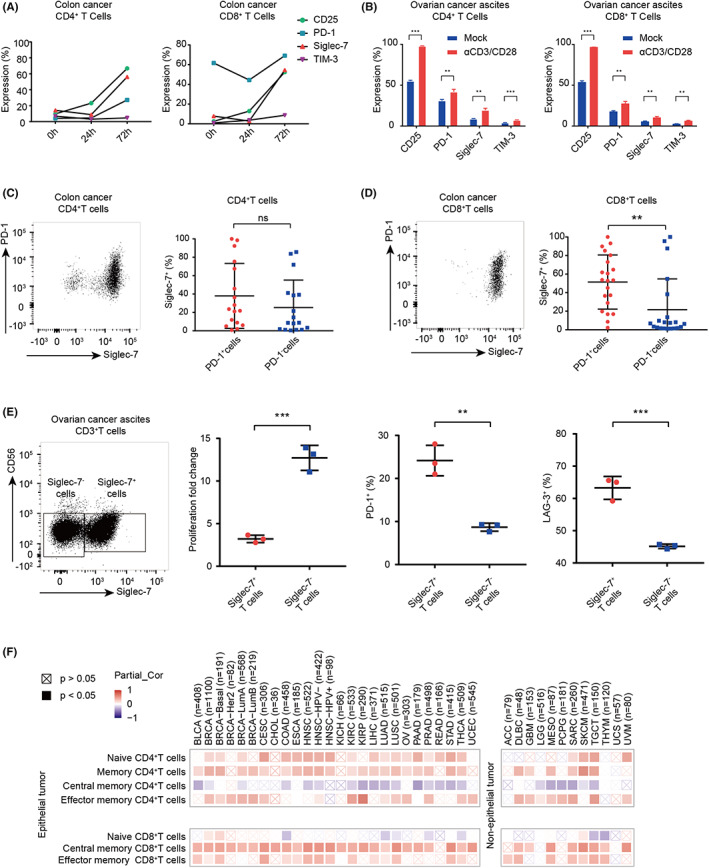
Functional analysis of Siglec‐7+ CD8+ T cells. (A) Time course of Siglec‐7 and checkpoint molecule expression on CD4+ and CD8+T cells from colon cancer tumor tissues. (B) Siglec‐7 and checkpoint molecule expression on CD4+T and CD8+T cells form ovarian cancer ascites. (C and D) Expression of Siglec‐7 on PD‐1+T cells or PD‐1−T cells on CD4+ (C) and CD8+T cells (D) in colon cancer. (E) Proliferation fold change and expression of PD‐1 and LAG‐3 on Siglec‐7+ or Siglec‐7− T cells from ovarian cancer ascites after 72 h activating. (F) Functional heatmap of the association between SIGLEC7 expression and immune infiltration level of CD4+ or CD8+T cells across different cancer types. Red: significant positive association. Blue: significant negative association. Crossed square: non‐significant.
We then used XCELL to divide T cells into naïve or memory subsets, including central memory T cells and effector memory T cells, to analyze if SIGLEC7 expression was correlated with distinct T cell subsets. We found that, in CD4+T cells, SIGLEC7 expression was positively correlated with both naïve and memory T cells (Figure 2F). Interestingly, in CD8+T cells, the expression of SIGLEC7 was found to be negatively correlated with naïve CD8+T cells but positively correlated with memory CD8+T cells, especially with central memory CD8+T cells (Figure 2F). To further investigate the subsets of Siglec‐7+T cells in TME, we carried out CD45RA and CCR7 staining on T cells in colon cancer or ovarian cancer ascites. Results showed that the CD4+T cells in both TMEs were mainly CD45RA−CCR7− effector memory T cells (TEM), while the CD8+T cells were mainly TEM and CD45RA+CCR7− effector memory (TEMRA) in colon cancer and TEMRA in ovarian cancer ascites (Figure S1D).
3.3. SIA‐IgG acts as ligand of Siglec‐7 in TME
Ligands of Siglec receptors are vaguely described as sialoglycans. However, which protein is sialylated and bound to Siglecs in TME remains unclear. Recently, our group found that epithelial cancer cells, but not B cells, can specifically produce IgG with high sialic acid modification (SIA‐IgG). As known, IgG is a glycoprotein, and has N‐glycosylation on its CH2 Asn‐297 site, but with little sialylation. However, SIA‐IgG has a nonclassical N‐glycosylation on its CH1 Asn‐162 site, with extremely high sialylation, which was firstly reported by us. 27 Glycopeptide analysis is the only alternative for profiling site–specific glycosylation; therefore, we further carried out glycopeptide analysis for SIA‐IgG. SIA‐IgG purified from ovarian cancer ascites was digested with papain to obtain Fab and Fc fragments and then analyzed by liquid chromatography–mass spectrometry (LC‐MS). The results showed that the Fab fragments of SIA‐IgG displayed a large number of three‐branched structure and significantly high sialylation on the terminal of glycans (Figure 3A, Table S3). In contrast, the Fc fragments of SIA‐IgG rarely displayed three‐branched structure; moreover, there was no or low sialylation on the terminal of glycans (Figure 3A bottom, Table S4). Compared with Fc fragments of SIA‐IgG, up to 98% glycans on Fab fragments of SIA‐IgG was sialylated (Figure 3B). To further determine the glycosylation sites of SIA‐IgG Fab fragments, we then analyzed characteristic fragments of glycopeptides, which are known to be the most important basis to determine glycopeptides. 30 Obviously, results also confirmed that the exact Asn‐162 site of SIA‐IgG was rich in three‐branched N‐glycosylation with high sialylation on the glycan terminal, including single or double sialylation (Figure 3C).
FIGURE 3.
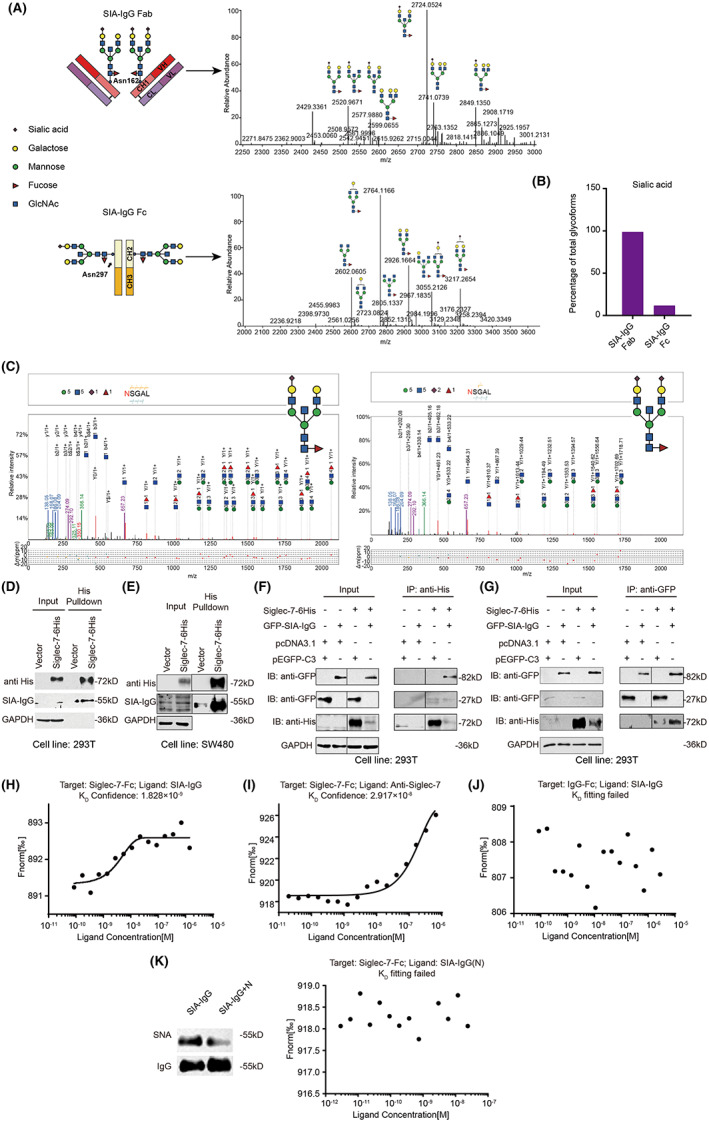
SIA‐IgG is a ligand of Siglec‐7. (A) Mass spectrometry (MS) spectrum of SIA‐IgG Fab (top) and Fc fragments (bottom). GlcNAc, N‐acetylglucosamine. (B) Relative composition of sialic acids of Fab and Fc in SIA‐IgG. (C) MS/MS of characteristic glycopeptide fragments of SIA‐IgG Fab. (D) Interaction between purified SIA‐IgG and Siglec‐7. (E) Interaction between endogenous SIA‐IgG and Siglec‐7. (F and G) Interaction between Siglec‐7‐6His and GFP‐SIA‐IgG. (H‐K) Binding kinetics of Siglec‐7‐Fc and purified SIA‐IgG (H) or anti‐Siglec‐7 (I), IgG‐Fc and purified SIA‐IgG (J), and Siglec‐7‐Fc and neuraminidase treated SIA‐IgG (K).
The significantly high sialylation of SIA‐IgG on Asn‐162 suggested that SIA‐IgG can act as a ligand of Siglecs. In this study, to further verify the interaction between SIA‐IgG and Siglec‐7, we first expressed Siglec‐7 in 293 T cells (Figure S2A) and found that Siglec‐7 interacted significantly with exogenous purified SIA‐IgG by immunoprecipitation (IP) (Figure 3D). Similarly, when Siglec‐7 was overexpressed in colon cancer cell line SW480, we also found that endogenous SIA‐IgG interacted with Siglec‐7 (Figure 3E). Correspondingly, we coexpressed Siglec‐7 and SIA‐IgG as described previously 26 and proved that GFP‐SIA‐IgG interacted with Siglec‐7‐His by Co‐IP (Figure 3F,G).
We then determined if SIA‐IgG can bind to Siglec‐7 by MST. Siglec‐7‐Fc was labeled with NHS kit 31 and was incubated with SIA‐IgG or anti‐Siglec‐7 antibodies, which were used as a positive control. Results showed that SIA‐IgG could bind to Siglec‐7‐Fc with high affinity (K D = 1.8 × 10−9; Figure 3H,I). In contrast, SIA‐IgG did not bind to IgG‐Fc alone (Figure 3J). SIA‐IgG treated with neuraminidase no longer bound to Siglec‐7‐Fc either (Figure 3K). These results demonstrated that SIA‐IgG directly binds Siglec‐7 in a sialylation‐dependent manner, proving that SIA‐IgG is a ligand of Siglec‐7.
3.4. SIA‐IgG triggers downstream inhibitory pathways of Siglec‐7
We next explored if SIA‐IgG serves as a ligand of Siglec‐7 to activate the downstream inhibitory signals. Firstly, Siglec‐7 was expressed on the surface of Jurkat cells (Sig7+ Jurkat; Figure S3A). Purified SIA‐IgG or IVIG (which is reported to have a relatively low level of sialylation 32 ) was used to bind Sig7+ or mock Jurkat. Results showed that SIA‐IgG, but not IVIG, specifically binds to Sig7+ Jurkat (Figure 4A). Given that Siglec‐7 contains an ITIM known to recruit tyrosine phosphatases, 33 the phosphorylation of the downstream SHP‐1 was determined after adding SIA‐IgG. Our results showed that SIA‐IgG caused a rapid phosphorylation of SHP‐1 only in Sig7+ Jurkat, but not mock Jurkat (Figure 4B). Notably, IVIG did not cause SHP‐1 phosphorylation in either cells (Figure 4C). Next, to further prove the inhibitory effect of SIA‐IgG in T cells, we isolated T cells from ovarian ascites and stimulated them with SIA‐IgG. Results showed that the phosphorylation of SHP‐1 increased immediately, while blockade of Siglec‐7 significantly inhibited the phosphorylation (Figure 4D).
FIGURE 4.
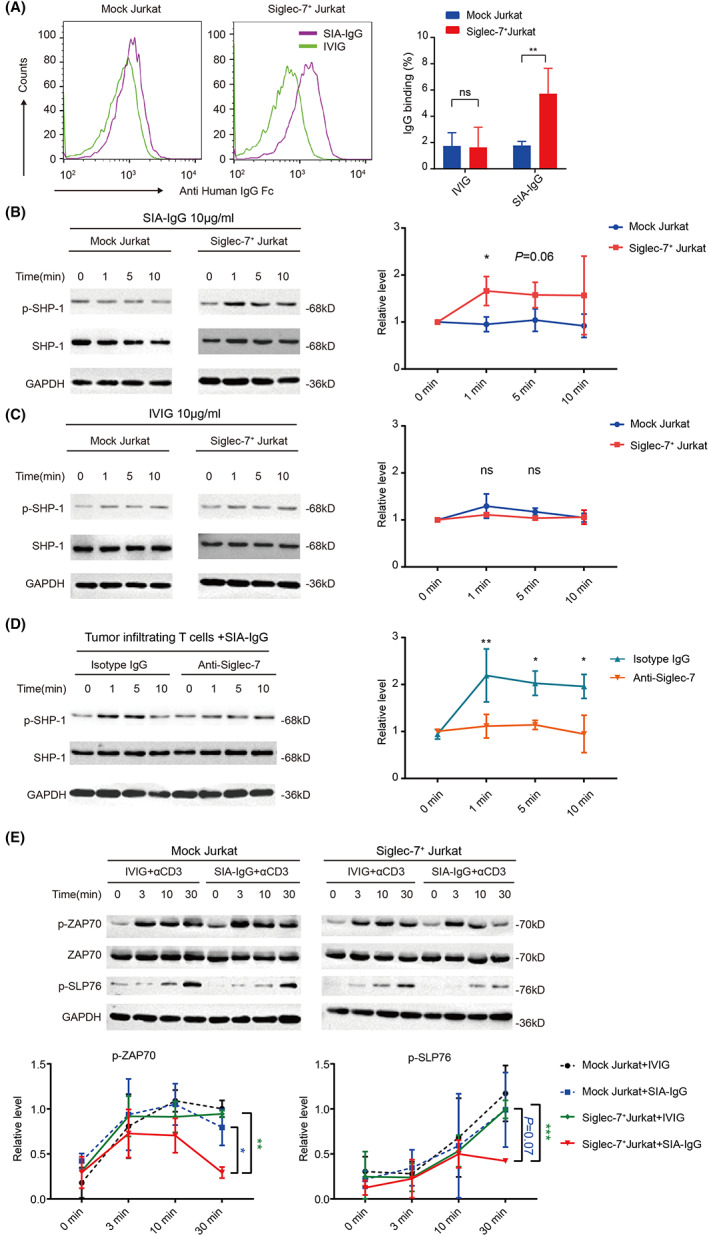
SIA‐IgG regulates Siglec‐7 downstream signals. (A) The binding of SIA‐IgG to Sig‐7+ Jurkat. (B and C) Mock or Sig‐7+ Jurkat cells were treated with SIA‐IgG (B) or IVIG (C). Cells were harvested for SHP1 phosphorylation. (D) SHP1 phosphorylation results in T cells sorted from ovarian cancer ascites. (E) Western blot of ZAP‐70 and SLP‐76 in mock or Sig‐7+ Jurkat stimulated by anti‐CD3 (2 μg/ml) with SIA‐IgG or IVIG. Data are representative of at least three experiments.
Based on these findings, we also evaluated the effect of SIA‐IgG on activation of downstream molecules ZAP‐70 and SLP‐76 following the engagement of TCR. Result showed that the phosphorylation of ZAP‐70 and SLP‐76 reduced at all time points following TCR engagement with SIA‐IgG in Sig7+ Jurkat compared with mock Jurkat, but IVIG showed no interruption (Figure 4E). These findings suggested a role of Siglec‐7–dependent, SHP‐1–associated inhibitory function of SIA‐IgG engagement in T cells.
3.5. Siglec‐7/SIA‐IgG is a potential immune checkpoint in T cells
Next, we wanted to explore the function of Siglec‐7/SIA‐IgG as checkpoint inhibitor in the tumor‐killing function of T cells. In consideration of that Siglec‐7 does not have a known murine homolog, we tried to evaluate the tumor‐killing function of T cells in human cancer models. Ovarian cancer ascites is a TME which contains a large number of both cancer cells and T cells, allowing us to investigate the tumor‐killing ability of T cells for cancer cells from the same individual in vitro. As shown above, Siglec‐7 was expressed wildly on T cells in ovarian cancer ascites (Figure 1B). The expression of SIA‐IgG on cancer cell membrane was also confirmed by both flow cytometry and immunofluorescence staining (Figure 5A,B, Table S2). Next, T cells were sorted from ovarian cancer ascites and stimulated; then, EpCAM+ cancer cells were sorted and cocultured with T cells, and the apoptosis of cancer cells was measured by annexin V/7AAD staining (Figure S3). Our results revealed that activated T cells showed a strong effect of inducing tumor apoptosis (Figure 5C). Next, the blocking antibodies of Siglec‐7 or SIA‐IgG were added to the coculture medium of T cells and cancer cells. Obviously, blockade of Siglec‐7 displayed stronger apoptosis of cancer cells in all six ovarian ascites cases. Similarly, blocking of SIA‐IgG significantly increased the apoptosis ratio of cancer cells in four of six cases (Figure 5D).
FIGURE 5.
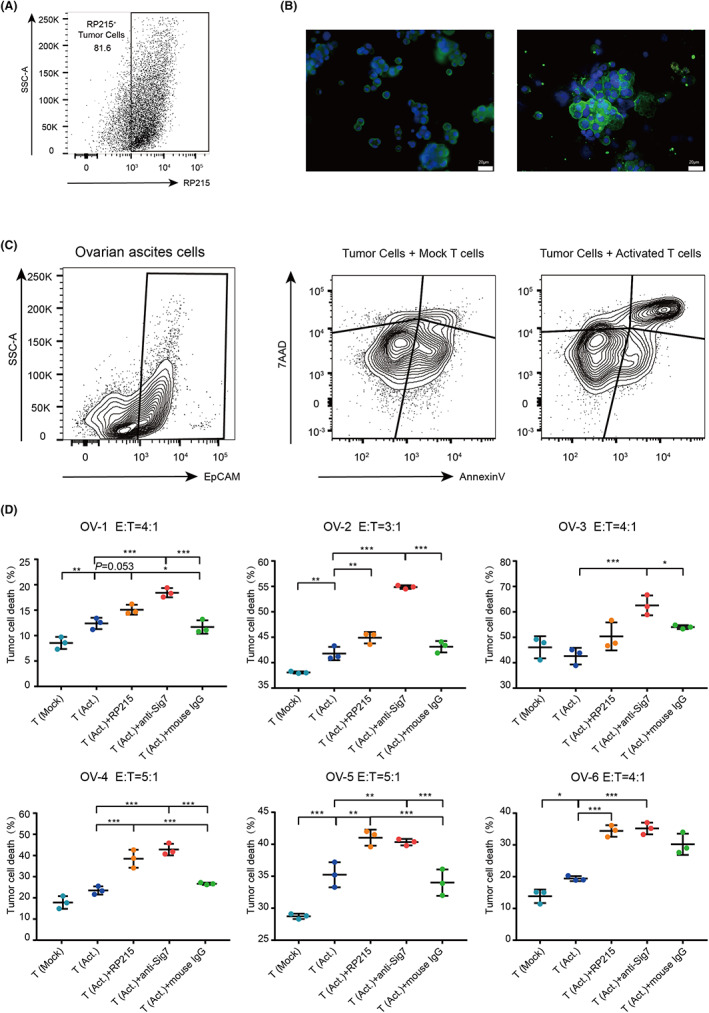
Targeting Siglec‐7/SIA‐IgG improved T cell–mediated tumor cell killing in vitro. (A) Flow cytometry analysis of SIA‐IgG expression on tumor cell in ovarian cancer ascites using RP215. (B) Immunofluorescence study in ovarian cancer ascites cells. (C) Flow cytometry analysis of cancer cells cocultured with mock T cells or activated T cells. (D) Percentage of annexin V+7AAD+ cancer cells after coculturing with untreated T cells (T [Mock]) or activated T cells (T [Act.]), with addition of anti‐Siglec‐7, RP215, or control IgG.
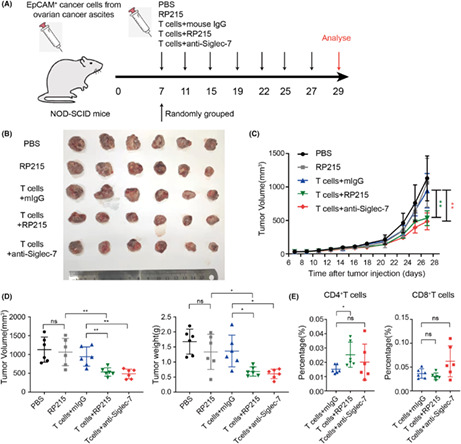
Next, we wanted to know if anti‐Siglec‐7 or anti‐SIA‐IgG can enhance the tumor‐killing effect of T cells in vivo. EpCAM+ cancer cells were sorted from ovarian cancer ascites, cultured for amplification in vitro, and then injected into nude mice subcutaneously to construct xenograft tumor models. After the tumors had grown to 40 mm3, the T cells sorted from ovarian cancer ascites were activated by anti‐CD3/CD28 alone or in combination with anti‐Siglec‐7 in vitro for 48 hours and then injected peritumorally (5 × 105 every 3 days; Figure S4A). Clearly, activated T cells could significantly inhibit xenograft tumors. The treatment with anti‐Siglec‐7 partially enhanced the antitumor function of T cells, although without statistical difference (Figure S4B–D). However, the ratio of infiltrated CD8+T cells was significantly increased in tumors treated with anti‐Siglec‐7 (Figure S4D). To further prove if the anti‐SIA‐IgG or anti‐Siglec‐7 can enhance the antitumor effect of T cells, subsequently, we established the same xenografts in NOD‐SCID mice. T cells were activated, and RP215 or anti‐Siglec‐7 was added in vitro. Then, activated T cells (3 × 105 every 4 days) were injected peritumorally (Figure 6A). The results showed that RP215 or T cells alone could not inhibit the growth of tumor; however, T cells treated with RP215 could significantly inhibit tumor growth (Figure 6B–D). Similarly, anti‐Siglec‐7 also enhanced the antitumor function of T cells. Interestingly, the number of tumor‐infiltrating CD4+T cells was increased by RP215 treatment, while that of tumor‐infiltrating CD8+T cells was increased by anti‐Siglec‐7 (Figure 6E).
FIGURE 6.
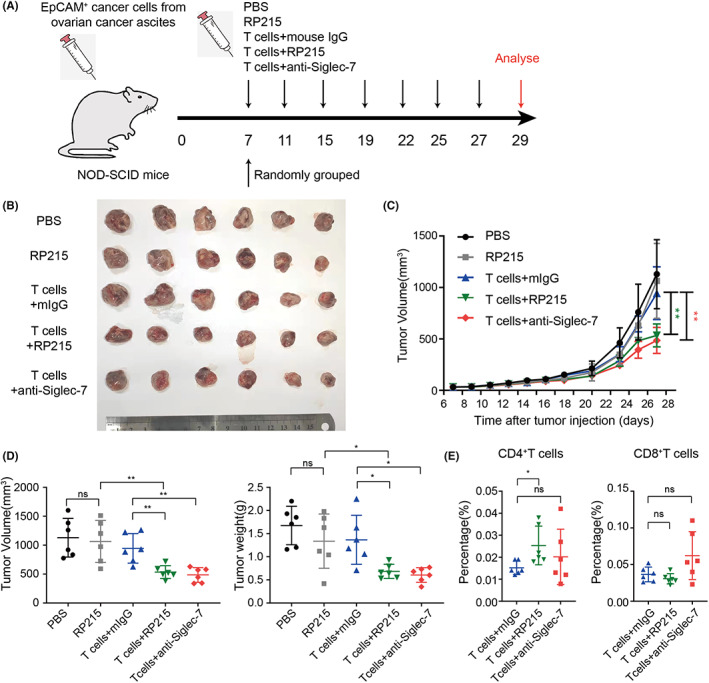
Blockade of Siglec‐7/SIA‐IgG promotes the tumor‐killing function of T cells in vivo. (A) Establishment of mouse models used in (B‐E). Isolated tumors are presented (B) together with growth curve (C) and tumor volume and weight (D). E, Proportions of human CD4+ and CD8+T cells in the tumors treated with T cells.
4. DISCUSSION
In this study, we demonstrated that Siglec‐7/SIA‐IgG is a new checkpoint and SIA‐IgG acts as a newly discovered sialylated ligand of Siglec‐7 and inhibits the tumor‐killing function of T cells by inhibiting the TCR downstream signals, leading to tumor immune escape. Blocking of both SIA‐IgG and Siglec‐7 can rescue T cell function and enhance antitumor immunity.
Siglec‐7 belongs to CD33‐related Siglecs, carrying an intracellular ITIM motif to mediate immunosuppression. CD33‐related Siglecs have been reported to be mainly expressed on innate immune cells, such as macrophages, 9 , 34 neutrophils, 10 and NK cells, 35 , 36 and rarely expressed on T cells. Recently, Siglec‐9 was reported to be expressed on tumor‐infiltrating T cells in lung cancer and melanoma, 12 , 13 and Siglec‐10 was reported to be expressed on activated T cells 14 , 15 and to inhibit T cell activation, indicating the possible roles of inhibitory Siglecs as new immune checkpoints. 37 However, to date, ligands of inhibitory Siglecs have not been fully explored. 38
In this study, we first identified Siglec‐3, ‐6, ‐7, ‐9, and ‐10 on CD4+ and CD8+T cells in the blood of tumor patients. Our results revealed that inhibitory Siglecs expression was significantly increased on CD4+T cells in lung cancer, cervical cancer, and rectal cancer patients. In contrast, none of the five inhibitory Siglecs’ expression on CD8+T cells increased in any patients. To further address if the inhibitory Siglecs were expressed on T cells in TME, we analyzed Siglec‐6, ‐7, ‐9, and ‐10 on T cells in ovarian cancer ascites. These Siglecs were all highly expressed on both CD4+ and CD8+T cells in ovarian cancer ascites. This finding was obviously different from that in blood of tumor patients, suggesting that inhibitory Siglecs might be upregulated on T cells in TME. To further address this hypothesis, we analyzed Siglec‐3, ‐6, ‐7, ‐9, and ‐10 on T cells in colon cancer tissues. Interestingly, only Siglec‐7 was dominantly expressed on CD8+T cells, but not on CD4+T cells, in cancer tissues or in normal tissues from the same individual. Next, using the TIMER2.0 database, we found that the expression of SIGLEC7 displayed significantly positive correlation with infiltration of CD8+T cells, but not CD4+T cells.
Siglec‐7 expression was significantly increased on T cells, especially on tumor‐infiltrating CD8+T cells, which suggested that Siglec‐7 might be related to antigen‐specific T cell activation. Thus, we explored whether Siglec‐7 can be upregulated by activation of TCR signals. Siglec‐7 expression was markedly upregulated upon TCR stimulation on both CD4+ and CD8+T cells from colon cancer tissues or ovarian cancer ascites. We also noticed that Siglec‐7 was significantly coexpressed with PD‐1 on T cells in colon cancer tissues, especially on CD8+T cells, which was related to T cell exhaustion. Moreover, compared with Siglec‐7−T cells, Siglec‐7+T cells expanded poorly upon TCR stimulation and expressed higher PD‐1 and LAG‐3, which suggested that Siglec‐7 was related to T cell exhaustion. Bioinformatics analysis showed that Siglec‐7 was negatively correlated with the naïve CD8+T cells but positively correlated with central memory CD8+T cells in TME.
SIA‐IgG was reported to be highly secreted by epithelial cancer cells instead of B cells. Depending on its high sialic acid modification on its CH1 domain, SIA‐IgG can directly inhibit T cell proliferation by binding to Siglec‐10. 27 In this study, to confirm the sialylation modification on Fab fragments of SIA‐IgG, we further analyzed the glycopeptides of SIA‐IgG. As expected, the results showed that the exact Asn‐162 site of Fab fragments of SIA‐IgG displayed a large amount of three‐branched glycans, with highly sialylated terminals. Next, we further confirmed that SIA‐IgG interacts with Siglec‐7. MST allows quantitative analysis of protein interaction in free solution, 31 and this method has been used to quantify weak affinities of Siglecs and synthetic sialic acid ligands. 39 We proved that SIA‐IgG can bind Siglec‐7 directly by MST. Moreover, SIA‐IgG promoted the phosphorylation of downstream SHP‐1 to inhibit TCR signals. These findings suggested that Siglec‐7/SIA‐IgG is a so far unidentified immune checkpoint in epithelial cancers.
We then explored whether blocking Siglec‐7/SIA‐IgG can enhance the antitumor effect of T cells. Because Siglec‐7 has no homologs in mice, we had to establish tumor immunotherapy models with human T cells. We used the cancer cells as target cells and activated T cells as effector cells to explore antitumor effect in ovarian cancer ascites in vivo and in vitro. Blocking Siglec‐7 can significantly enhance the antitumor effect in all six cases. Similarly, RP215 also enhanced T cell function in four of six cases. Why did the two cases show poor treatment effect? We found that the expression level of SIA‐IgG on cancer cells in these two cases was extremely high, and the concentration of RP215 might have been insufficient. Next, in in vivo experiments, fortunately, we obtained a case of ovarian cancer cells from ascites that can form tumors in immunodeficient mice. The activated T cells from ovarian cancer ascites had strong antitumor effect in vivo, and both anti‐Siglec‐7 and RP215 showed strong promotion in the tumor‐killing function of T cells. It is worth noting that our previous study showed RP215 itself can directly inhibit tumor. 26 So, in order to highlight the significance of immunotherapy, a low dose of RP215 was used as a control. Clearly, our results showed that RP215 alone cannot inhibit tumor growth, but the addition of RP215 can significantly increase the antitumor effect of T cells. Both anti‐Siglec‐7 and RP215 increased CD4+T cell infiltration, with RP215 being more significant, while anti‐Siglec‐7 increased the infiltration of CD8+T cells. We attribute this difference to that Siglec‐7 is highly expressed in memory CD8+T cells in TME, according to the bioinformatics analysis. Thus, blocking Siglec‐7 has a more direct effect on CD8+T cells, while blockade of SIA‐IgG increased the infiltration of both CD4+ and CD8+T cells. We speculate that the expression of other Siglecs, such as Siglec‐6, 9, or 10, on CD4+T cells in this model may be higher than that on CD8+T cells because SIA‐IgG can also bind to other Siglecs. 27 However, the detailed mechanism needs to be further explored. Our results suggested that Siglec‐7/SIA‐IgG acts as a novel immune checkpoint. In particular, Siglec‐7/SIA‐IgG can be used as a common immune checkpoint of most epithelial tumors in immunotherapy.
FUNDING INFORMATION
This work was supported by research grants to Xiaoyan Qiu from the Major Research Plan of the National Natural Science Foundation of China (No. 82030044).
DISCLOSURE
The authors have no conflict of interest.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Reviewer Board: The study was conducted according to an institutional review board–approved protocol and was approved by the Peking University Third Hospital Medical Science Research Ethics Committee.
Informed Consent: Informed consent was obtained from all the participants prior to the publication of this study.
Registry and the Registration No. of the study/trial: Peking University Third Hospital Medical Science Research Ethics Committee, IRB00006761‐M2019291.
Animal Studies: All mice were housed in a pathogen‐free facility at the Peking University Health Science Center (reference number for the ethic offices: LA2019091).
Supporting information
Appendix S1.
ACKNOWLEDGMENTS
We would like to thank G. Lee (Andrology Lab, University of British Columbia Centre for Reproductive Health, Vancouver, BC V5Z 4H4, Canada) for developing RP215. We also thank Xiaoyu Li (National Center for Protein Sciences, Beijing) for the glycopeptide analysis of SIA‐IgG Fab and Fc fragments.
Fan T, Liao Q, Zhao Y, et al. Sialylated IgG in epithelial cancers inhibits antitumor function of T cells via Siglec‐7. Cancer Sci. 2023;114:370‐383. doi: 10.1111/cas.15631
Tianrui Fan, Qinyuan Liao, and Yang Zhao contributed equally to this work.
Contributor Information
Zexian Zeng, Email: zexianzeng@pku.edu.cn.
Hongyan Guo, Email: bysyghy@163.com.
Haizeng Zhang, Email: haizengzhang@cicams.ac.cn.
Xiaoyan Qiu, Email: qiuxy@bjmu.edu.cn.
REFERENCES
- 1. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;23(359):1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. He X, Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020;30(8):660‐669. doi: 10.1038/s41422-020-0343-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Egen JG, Ouyang W, Wu LC. Human anti‐tumor immunity: insights from immunotherapy clinical trials. Immunity. 2020;52(1):36‐54. doi: 10.1016/j.immuni.2019.12.010 [DOI] [PubMed] [Google Scholar]
- 4. Duan S, Paulson JC. Siglecs as immune cell checkpoints in disease. Annu Rev Immunol. 2020;38:365‐395. doi: 10.1146/annurev-immunol-102419-035900 [DOI] [PubMed] [Google Scholar]
- 5. Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7(4):255‐266. doi: 10.1038/nri2056 [DOI] [PubMed] [Google Scholar]
- 6. Cao H, Bono B, Belov K, et al. Comparative genomics indicates the mammalian CD33rSiglec locus evolved by an ancient large‐scale inverse duplication and suggests all Siglecs share a common ancestral region. Immunogenetics. 2009;61(5):401‐417. doi: 10.1007/s00251-009-0372-0 [DOI] [PubMed] [Google Scholar]
- 7. Jandus C, Simon HU, von Gunten S. Targeting siglecs‐a novel pharmacological strategy for immuno‐ and glycotherapy. Biochem Pharmacol. 2011;82(4):323‐332. doi: 10.1016/j.bcp.2011.05.018 [DOI] [PubMed] [Google Scholar]
- 8. Hudak JE, Canham SM, Bertozzi CR. Glycocalyx engineering reveals a Siglec‐based mechanism for NK cell immunoevasion. Nat Chem Biol. 2014;10(1):69‐75. doi: 10.1038/nchembio.1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barkal AA, Brewer RE, Markovic M, et al. CD24 signalling through macrophage Siglec‐10 is a target for cancer immunotherapy. Nature. 2019;572(7769):392‐396. doi: 10.1038/s41586-019-1456-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Laubli H, Pearce OM, Schwarz F, et al. Engagement of myelomonocytic Siglecs by tumor‐associated ligands modulates the innate immune response to cancer. Proc Natl Acad Sci U S A. 2014;111(39):14211‐14216. doi: 10.1073/pnas.1409580111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nguyen DH, Hurtado‐Ziola N, Gagneux P, Varki A. Loss of Siglec expression on T lymphocytes during human evolution. Proc Natl Acad Sci USA. 2006;103(20):7765‐7770. doi: 10.1073/pnas.0510484103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stanczak MA, Siddiqui SS, Trefny MP, et al. Self‐associated molecular patterns mediate cancer immune evasion by engaging Siglecs on T cells. J Clin Invest. 2018;128(11):4912‐4923. doi: 10.1172/JCI120612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haas Q, Boligan KF, Jandus C, et al. Siglec‐9 regulates an effector memory CD8(+) T‐cell subset that congregates in the melanoma tumor microenvironment. Cancer Immunol Res. 2019;7(5):707‐718. doi: 10.1158/2326-6066.CIR-18-0505 [DOI] [PubMed] [Google Scholar]
- 14. Bandala‐Sanchez E, Bediaga NG, Naselli G, Neale AM, Harrison LC. Siglec‐10 expression is up‐regulated in activated human CD4(+) T cells. Hum Immunol. 2020;81(2‐3):101‐104. doi: 10.1016/j.humimm.2020.01.009 [DOI] [PubMed] [Google Scholar]
- 15. Bandala‐Sanchez E, G Bediaga N, Goddard‐Borger ED, et al. CD52 glycan binds the proinflammatory B box of HMGB1 to engage the Siglec‐10 receptor and suppress human T cell function. Proc Natl Acad Sci USA. 2018;115(30):7783‐7788. doi: 10.1073/pnas.1722056115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gercel‐Taylor C, Bazzett LB, Taylor DD. Presence of aberrant tumor‐reactive immunoglobulins in the circulation of patients with ovarian cancer. Gynecol Oncol. 2001;81(1):71‐76. doi: 10.1006/gyno.2000.6102 [DOI] [PubMed] [Google Scholar]
- 17. Qiu X, Zhu X, Zhang L, et al. Human epithelial cancers secrete immunoglobulin G with unidentified specificity.Pdf. Cancer Res. 2003;63(19):6488‐6495. [PubMed] [Google Scholar]
- 18. Babbage G, Ottensmeier CH, Blaydes J, Stevenson FK, Sahota SS. Immunoglobulin heavy chain locus events and expression of activation‐induced cytidine deaminase in epithelial breast cancer cell lines. Cancer Res. 2006;66(8):3996‐4000. doi: 10.1158/0008-5472.CAN-05-3704 [DOI] [PubMed] [Google Scholar]
- 19. Cassetta L, Fragkogianni S, Sims AH, et al. Human tumor‐associated macrophage and monocyte transcriptional landscapes reveal cancer‐specific reprogramming, biomarkers, and therapeutic targets. Cancer Cell. 2019;35(4):588‐602.e510. doi: 10.1016/j.ccell.2019.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhu X, Wu L, Zhang L, et al. Distinct regulatory mechanism of immunoglobulin gene transcription in epithelial cancer cells. Cell Mol Immunol. 2010;7(4):279‐286. doi: 10.1038/cmi.2010.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li M, Zheng H, Duan Z, et al. Promotion of cell proliferation and inhibition of ADCC by cancerous immunoglobulin expressed in cancer cell lines. Cell Mol Immunol. 2011;9(1):54‐61. doi: 10.1038/cmi.2011.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Crespo HJ, Lau JT, Videira PA. Dendritic cells: a spot on sialic acid. Front Immunol. 2013;4:491. doi: 10.3389/fimmu.2013.00491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ding Y, Guo Z, Liu Y, et al. The lectin Siglec‐G inhibits dendritic cell cross‐presentation by impairing MHC class I‐peptide complex formation. Nat Immunol. 2016;17(10):1167‐1175. doi: 10.1038/ni.3535 [DOI] [PubMed] [Google Scholar]
- 24. Lee G, Laflamme E, Chien C‐H, Ting HH. Molecular identity of a pan cancer marker, CA215. Cancer Biol Ther. 2014;7(12):2007‐2014. doi: 10.4161/cbt.7.12.6984 [DOI] [PubMed] [Google Scholar]
- 25. Lee G. Cancer cell‐expressed immunoglobulins: CA215 as a pan cancer marker and its diagnostic applications. Cancer Biomark. 2009;5(3):137‐142. doi: 10.3233/CBM-2009-0610 [DOI] [PubMed] [Google Scholar]
- 26. Tang J, Zhang J, Liu Y, et al. Lung squamous cell carcinoma cells express non‐canonically glycosylated IgG that activates integrin‐FAK signaling. Cancer Lett. 2018;430:148‐159. doi: 10.1016/j.canlet.2018.05.024 [DOI] [PubMed] [Google Scholar]
- 27. Wang Z, Geng Z, Shao W, et al. Cancer‐derived sialylated IgG promotes tumor immune escape by binding to Siglecs on effector T cells. Cell Mol Immunol. 2020;17(11):1148‐1162. doi: 10.1038/s41423-019-0327-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mitra SK, Schlaepfer DD. Integrin‐regulated FAK‐Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18(5):516‐523. doi: 10.1016/j.ceb.2006.08.011 [DOI] [PubMed] [Google Scholar]
- 29. Li T, Fu J, Zeng Z, et al. TIMER2.0 for analysis of tumor‐infiltrating immune cells. Nucleic Acids Res. 2020;48(W1):W509‐W514. doi: 10.1093/nar/gkaa407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang Q, Li Z, Wang Y, Zheng Q, Li J. Mass spectrometry for protein sialoglycosylation. Mass Spectrom Rev. 2018;37(5):652‐680. doi: 10.1002/mas.21555 [DOI] [PubMed] [Google Scholar]
- 31. Seidel SA, Dijkman PM, Lea WA, et al. Microscale thermophoresis quantifies biomolecular interactions under previously challenging conditions. Methods. 2013;59(3):301‐315. doi: 10.1016/j.ymeth.2012.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Griciuc A, Serrano‐Pozo A, Parrado AR, et al. Alzheimer's disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron. 2013;78(4):631‐643. doi: 10.1016/j.neuron.2013.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ikehara Y, Ikehara SK, Paulson JC. Negative regulation of T cell receptor signaling by Siglec‐7 (p70/AIRM) and Siglec‐9. J Biol Chem. 2004;279(41):43117‐43125. doi: 10.1074/jbc.M403538200 [DOI] [PubMed] [Google Scholar]
- 34. Beatson R, Tajadura‐Ortega V, Achkova D, et al. The mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec‐9. Nat Immunol. 2016;17(11):1273‐1281. doi: 10.1038/ni.3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jandus C, Boligan KF, Chijioke O, et al. Interactions between Siglec‐7/9 receptors and ligands influence NK cell‐dependent tumor immunosurveillance. J Clin Invest. 2014;124(4):1810‐1820. doi: 10.1172/JCI65899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Belisle JA, Horibata S, Jennifer GA, et al. Identification of Siglec‐9 as the receptor for MUC16 on human NK cells, B cells, and monocytes. Mol Cancer. 2010;9:118. doi: 10.1186/1476-4598-9-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van de Wall S, Santegoets KCM, van Houtum EJH, Bull C, Adema GJ. Sialoglycans and Siglecs can shape the tumor immune microenvironment. Trends Immunol. 2020;41(4):274‐285. doi: 10.1016/j.it.2020.02.001 [DOI] [PubMed] [Google Scholar]
- 38. Jiang HS, Zhuang SC, Lam CH, Chang LY, Angata T. Recent Progress in the methodologies to identify physiological ligands of Siglecs. Front Immunol. 2021;12:813082. doi: 10.3389/fimmu.2021.813082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu GJ, Zhang Y, Zhou L, et al. A water‐soluble AIE‐active polyvalent glycocluster: design, synthesis and studies on carbohydrate‐lectin interactions for visualization of Siglec distributions in living cell membranes. Chem Commun (Camb). 2019;55(66):9869‐9872. doi: 10.1039/c9cc05008f [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.


