Abstract
Background
The prognosis and therapeutic responses are worse for pulmonary arterial hypertension associated with systemic sclerosis (SSc-PAH) compared with idiopathic pulmonary arterial hypertension (IPAH). This discrepancy could be driven by divergence in underlying metabolic determinants of disease.
Research Question
Are circulating bioactive metabolites differentially altered in SSc-PAH vs IPAH, and can this alteration explain clinical disparity between these PAH subgroups?
Study Design and Methods
Plasma biosamples from 400 patients with SSc-PAH and 1,082 patients with IPAH were included in the study. Another cohort of 100 patients with scleroderma with no PH and 44 patients with scleroderma with PH was included for external validation. More than 700 bioactive lipid metabolites, representing a range of vasoactive and immune-inflammatory pathways, were assayed in plasma samples from independent discovery and validation cohorts using liquid chromatography/high-resolution mass spectrometry-based approaches. Regression analyses were used to identify metabolites that exhibited differential levels between SSc-PAH and IPAH and associated with disease severity.
Results
From hundreds of circulating bioactive lipid molecules, five metabolites were found to distinguish between SSc-PAH and IPAH, as well as associate with markers of disease severity. Relative to IPAH, patients with SSc-PAH carried increased levels of fatty acid metabolites, including lignoceric acid and nervonic acid, as well as eicosanoids/oxylipins and sex hormone metabolites.
Interpretation
Patients with SSc-PAH are characterized by an unfavorable bioactive metabolic profile that may explain the poor and limited response to therapy. These data provide important metabolic insights into the molecular heterogeneity underlying differences between subgroups of PAH.
Key Words: biomarkers, metabolomics, pulmonary hypertension, scleroderma
Abbreviations: 6MWD, 6-min walk distance; FAHFA, fatty acyl esters of hydroxy fatty acid; IPAH, idiopathic pulmonary arterial hypertension; LC/MS, liquid chromatography/high-resolution mass spectrometry; LTB4, leukotriene B4; PAH, pulmonary arterial hypertension; PGF2α, prostaglandin F2α; PVR, pulmonary vascular resistance; SSc, systemic sclerosis; SSc-no PH, systemic sclerosis without associated pulmonary arterial hypertension; SSc-PAH, systemic sclerosis-associated pulmonary arterial hypertension; SVI, stroke volume index; WHO FC, World Health Organization functional class
Take-home Points.
Study Question: Are circulating bioactive metabolites differentially altered in SSc-PAH vs IPAH, and can this alteration explain clinical disparity between these PAH subtypes?
Results: Using robust design and large sample size, we identified and validated five metabolic plasma biomarkers that differentiate SSc-PAH from IPAH and associate with markers of disease severity. The selected biomarkers were increased in SSc-PAH compared vs SSC-alone, indicating these biomarkers are related to PAH condition and not simply due to the presence of scleroderma itself.
Interpretation: Patients with SSc-PAH are characterized by an unfavorable bioactive metabolic profile that may explain the poor and limited response to therapy. These data provide important metabolic insights into the pathogenesis of SSc-PAH molecular heterogeneity of subgroups of PAH and may be used for precision medicine approaches in PAH.
Pulmonary arterial hypertension (PAH) is a debilitating disease with enigmatic origins leading to elevated pulmonary arterial pressures and pulmonary vascular resistance (PVR). The most common subgroups are idiopathic PAH (IPAH) and systemic sclerosis-associated PAH (SSc-PAH).1 Systemic sclerosis is a complex, immunologic disease, characterized by autoimmunity, fibrosis of the skin and internal organs, and small vessel vasculopathy.2 Importantly, PAH in patients with SSc compared with patients with IPAH have a threefold higher mortality risk.3, 4, 5, 6 Furthermore, patients with SSc-PAH stand out from those with other types of PAH, given their impaired response to traditional therapies and worse overall clinical prognosis despite exhibiting similar end-organ pathology and often presenting with milder hemodynamic impairment.3,7 Proposed factors explaining these disparities include more pronounced inflammation,8 autoimmunity, the distinct nature of the underlying vasculopathy,9 and differing abilities of the right ventricle to adapt to the increased afterload.10 Metabolic dysregulation has been proposed as a key mechanism by which IPAH and SSc-PAH differ and could control such disparities.11,12 Interrogating such metabolic dysregulation that also reflects contributory immune-inflammatory and vasoactive pathway activation is now possible by profiling circulating levels of bioactive lipid metabolites.13, 14, 15 However, the extent to which bioactive lipid profiles may specifically differentiate between SSc-PAH and IPAH phenotypes remains unknown. The ability to clarify the molecular mechanisms underlying SSc-PAH, in particular, could accelerate the development of more effective approaches to managing and treating this especially challenging PAH subtype.
Amid the broad diversity of metabolites that may be studied in relation to disease pathogenesis, lipidomic analytes include a subset of bioactive lipids that warrant focused interrogation in relation to pulmonary vascular disorders given the known, yet still understudied role of bioactive lipids in modulating inflammation, immune regulation, vascular function, and hemostasis.13, 14, 15 To date, early studies of these bioactive metabolites in PAH have revealed changes in key energetic pathways, including abnormal lipid oxidation products, oxidative stress, and lipid metabolism. Notwithstanding these prior studies, limited data are available on how bioactive lipid activity may differentiate between the pathobiological processes underlying subgroups of PAH and specifically SSc-PAH.
In the current study, we hypothesized that patients with SSc-PAH exhibit unfavorable bioactive plasma metabolomic derangements that are associated with worse functional capacity compared with IPAH and which could explain the rapid decline and disease pathogenesis. The primary aim of this study was to determine whether there is a bioactive lipid signature of SSc-PAH. The secondary aim was to determine if this signature is associated with markers of disease severity. We applied liquid chromatography/high-resolution mass spectrometry (LC/MS) to characterize the plasma metabolic profiles from patients with IPAH, SSc-PAH, and systemic sclerosis without associated pulmonary arterial hypertension (SSc-no PH).
Study Design and Methods
Cohorts and Sample Collection
We conducted primary analyses in a prespecified discovery cohort followed by confirmatory analyses in a prespecified validation cohort, in accordance with a study design that we have used in prior human metabolomics studies and to evaluate the potential generalizability of our findings.16 Cohorts 1 and 2 included patients with IPAH and SSc-PAH and were obtained from the PAH Biobank resource. Cohort 3 included patients with SSc-no PH and patients with SSc-PAH obtained from Boston University. Details on study cohorts and sample collection are included in e-Appendix 1.
Metabolite Profiling
Bioactive metabolite analysis was performed on plasma samples by LC/MS using a Vanquish UPLC coupled to a high-resolution, Q Exactive Orbitrap mass spectrometer (Thermo Fisher Scientific), as described elsewhere14,17 (details are provided in the e-Appendix). Metabolites identified as xenobiotics or detected in < 20% of samples were excluded from the analysis, leaving 690 well-quantified biological metabolites. Following normalization, metabolite peaks were further compressed for multiple adducts and in source fragments. Metabolites missing values were imputed to the one-quarter of the lowest observed value of that molecule. Normalized, aligned, filtered data sets were subsequently used for statistical analyses, as described in the following section.
Statistical Analyses
Initial group comparisons between patients with SSc-PAH and IPAH, and between those with SSc-no PH and SSc-PAH, were performed by using the Student t test or the Mann-Whitney test for continuous variables and the χ2 test for categorical variables. Prior to all analyses, metabolite values were natural logarithmically transformed, as needed, and later standardized with mean = 0 and SD = 1 to facilitate comparisons. Logistic regression analysis was used to determine metabolites that were significantly different between SSc-PAH and IPAH (analysis I) in models adjusting for age, sex, BMI, and potential confounders, including use of prostaglandin therapy, corticosteroids, immunosuppression therapy, warfarin, or thyroid hormone, as well as renal insufficiency and cirrhosis. Renal insufficiency and cirrhosis were determined based on treating physicians’ discretion. The selection of variables included for all analyses was based on clinical expert identification of potentially confounding factors. Variables for inclusion in multivariable-adjusted analyses were also selected based on significant results observed in the unadjusted analyses. To determine if the prioritized metabolites were not driven by disease severity, 6-min walk distance (6MWD) was included in the logistic regression model.
To determine significance, a Bonferroni-corrected P value threshold of .05 divided by a conservative estimate of the total number of unique small molecules (ie, P < 10–4) was used. False discovery rate using the Benjamini-Hochberg method was also calculated, and metabolites not meeting a q value threshold of < 0.05 were excluded. Receiver-operating characteristic curve was used to assess discriminating value of metabolites against diagnosis. To determine if the significant metabolites were related to scleroderma disease or scleroderma-PAH, Student t test analysis was performed between the significant metabolites in SSc-no PH and SSc-PAH in Cohort 3. Logistic regression analysis was performed between the metabolites meeting significance threshold (P < .05) in the pairwise analysis. Regression analysis was performed between the significant metabolites from analysis I and markers of disease severity in SSc-PAH, IPAH, and SSc-PAH combined. Linear regression analysis was used between the selected metabolites and 6MWD, right atrial pressure, PVR, cardiac index, and stroke volume index (SVI), and logistic regression analysis was used between the selected metabolites and World Health Organization functional class (WHO FC; analysis II). All analyses were performed in models adjusting for age, sex, and BMI. Statistical analysis was performed with R with RStudio.18
Results
Baseline demographic, clinical, and hemodynamic characteristics and medications for patients enrolled in the study are summarized in Table 1, Table 2, and e-Table 1. At the time of enrollment, patients with SSc-PAH had significantly lower mean right atrial pressure and PVR than IPAH counterparts. In Cohort 3, patients with SSc-no PH were younger and had less disease duration compared with patients with SSc-PAH. There was no difference in immunosuppression medication usage between the groups in Cohort 3.
Table 1.
Demographic Characteristics and Clinical Features of Patients With IPAH and SSc-PAH
| Characteristic | Discovery Cohort (Cohort 1) |
Validation Cohort (Cohort 2) |
||||
|---|---|---|---|---|---|---|
| IPAH (n = 864) | SSc-PAH (n = 310) | P Value | IPAH (n = 213) | SSc-PAH (n = 91) | P Value | |
| Female | 663 (76.7) | 267 (86.1) | .001 | 166 (77.9) | 82 (90.1) | .019 |
| Age, y | 51.90 ± 18.47 | 64.10 ± 11.03 | < .001 | 53.22 ± 14.95 | 63.74 ± 10.29 | < .001 |
| BMI, kg/m2 | 30.36 ± 19.14) | 28.24 ± 11.40 | .068 | 30.78 ± 9.23 | 27.32 ± 8.17 | .002 |
| Renal insufficiency | 32 (3.7) | 28 (9.0) | < .001 | 11 (5.2) | 7 (7.7) | .555 |
| Cirrhosis | 13 (1.5) | 5 (1.6) | 1 | 2 (0.9) | 4 (4.4) | .125 |
| WHO functional class | .093 | .51 | ||||
| I | 46 (7.1) | 14 (5.7) | 5 (3.9) | 0 (0.0) | ||
| II | 182 (28.3) | 81 (33.1) | 42 (32.6) | 20 (36.4) | ||
| III | 345 (53.6) | 135 (55.1) | 72 (55.8) | 31 (56.4) | ||
| IV | 71 (11.0) | 15 (6.1) | 10 (7.8) | 4 (7.3) | ||
| 6MWD, m | 353.91 ± 138.36 | 312.44 ± 118.06 | .001 | 348.80 ± 125.44 | 312.30 ± 130.54 | .121 |
| mRAP, mm Hg | 9.16 ± 5.85 | 8.33 ± 5.06 | .028 | 8.38 ± 4.99 | 7.48 ± 4.94 | .154 |
| mPAP, mm Hg | 50.82 ± 14.25 | 43.30 ± 11.32 | < .001 | 52.73 ± 13.8 | 41.53 ± 9.47 | < .001 |
| PAWP, mm Hg | 9.58 ± 3.24 | 9.38 ± 3.35 | .36 | 9 ± 3.52 | 8.38 ± 3.39 | .15 |
| PVR, Woods units | 10.74 ± 6.84 | 8.50 ± 5.13 | < .001 | 12.18 ± 6.38 | 8.48 ± 4.06 | < .001 |
| Cardiac index, L/min/m | 2.48 ±1 | 2.60 ± 0.78 | .08 | 2.26 ± 0.83 | 2.49 ± 0.74 | .03 |
| SVI, mL/m2 | 32.65 ± 13.88 | 33.01 ± 11.70 | .77 | 29.64 ± 12.15 | 32.72 ± 10.48 | .17 |
| Prostanoid use | 415 (48.0) | 130 (41.9) | .075 | 83 (39.0) | 25 (27.5) | .074 |
Data are expressed as No. (%) or mean ± SD. 6MWD = 6-min walk distance; IPAH = idiopathic pulmonary arterial hypertension; mPAP = mean pulmonary artery pressure; mRAP = mean right atrial pressure; PAWP = pulmonary artery wedge pressure; PVR = pulmonary vascular resistance; SSc-PAH = systemic sclerosis-associated pulmonary arterial hypertension; SVI = stroke volume index.
Table 2.
Demographic Characteristics and Clinical Features of Patients With SSc-No PH and SSc-PAH (Cohort 3)
| Characteristic | SSc-no PH (n = 100) | SSc-PAH (n = 44) | PValue |
|---|---|---|---|
| Age, y | 53.35 ± 14.86 | 59.05 ± 11.31 | .025 |
| Female | 87 (87.0) | 38 (86.4) | .91 |
| Disease duration, y | 7.65 ± 6.62 | 11.69 ± 8.33 | .01 |
| BMI, kg/m2 | 27.44 ± 5.67 | 29.21 ± 7.22 | .123 |
| Immunosuppression therapy | 21 (21.0) | 8 (18.2) | .871 |
| WHO functional class | < .001 | ||
| I | 45 (71.4) | 5 (16.7) | |
| II | 17 (27.0) | 14 (46.7) | |
| III | 1 (1.6) | 10 (33.3) | |
| IV | 0 | 1 (3.3) | |
| FVC, % predicted | 87.82 ± 21.61 | 76.55 ± 19.72 | .009 |
| FEV1, % predicted | 85.60 ± 20.85 | 71.17 ± 19.47 | .001 |
| Dlco, % predicted | 66.63 ± 20.93 | 41.44 ± 20.62 | < .001 |
| RVSP, mm Hg | 27.97 ± 7.49 | 62.32 ± 22.78 | < .001 |
| mRAP, mm Hg | 3.17 ± 3.19 | 5.88 ± 4.69 | .18 |
| PAWP, mm Hg | 8.33 ± 3.61 | 10.12 ± 5.61 | .455 |
| mPAP, mm Hg | 23.29 ± 9.05 | 36.86 ± 13.94 | .017 |
| Cardiac index, L/min/m | 2.82 ± 0.48 | 2.82 ± 0.70 | .982 |
| PVR, Woods units | 3.2 ± 2.4 | 6.2 ± 5.4 | .195 |
Data are expressed as mean ± SD or No. (%). Dlco = diffusing capacity of the lung for carbon monoxide; mPAP = mean pulmonary artery pressure; mRAP = mean right atrial pressure; PAWP = pulmonary artery wedge pressure; PVR = pulmonary vascular resistance; RVSP = right ventricular systolic pressure; SSc-no PH = systemic sclerosis without associated pulmonary arterial hypertension; SSc-PAH = systemic sclerosis-associated pulmonary arterial hypertension.
Metabolites Differentiating Between SSc-PAH and IPAH
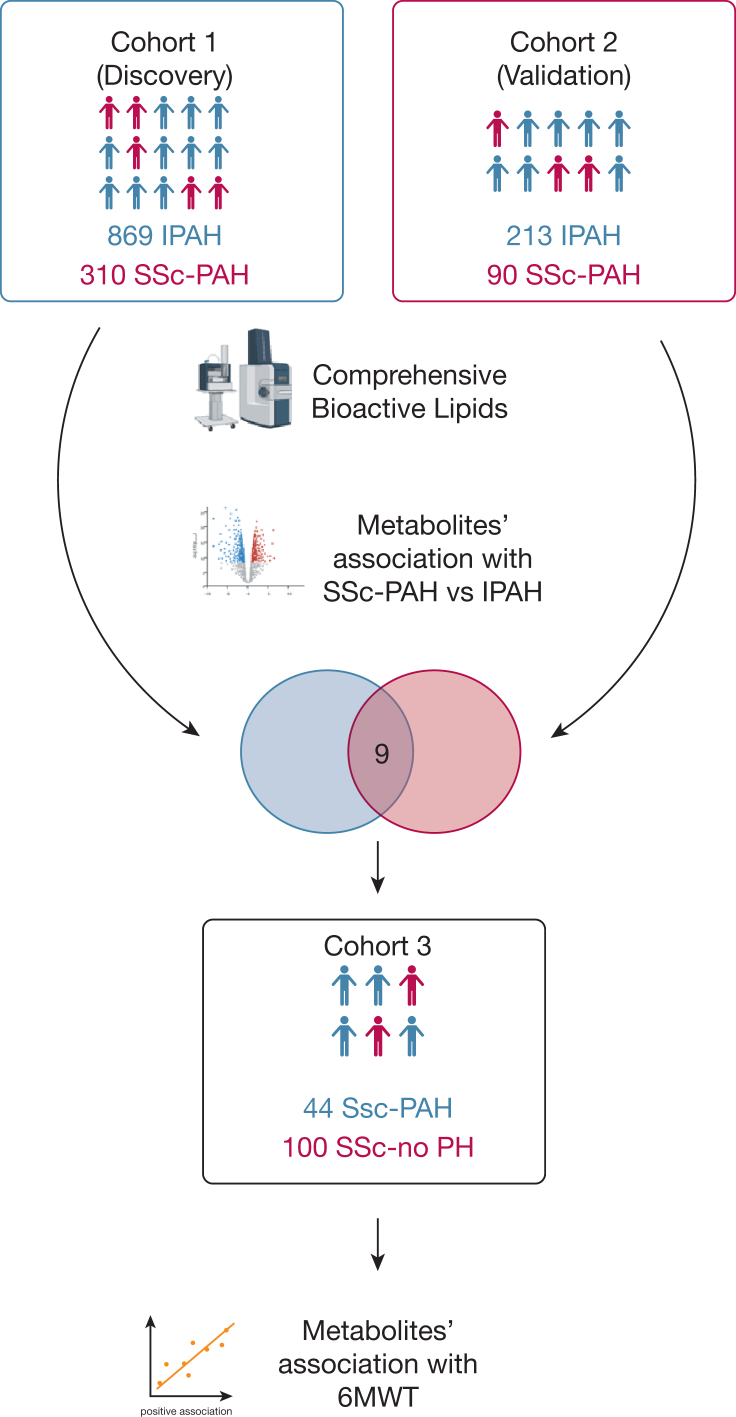
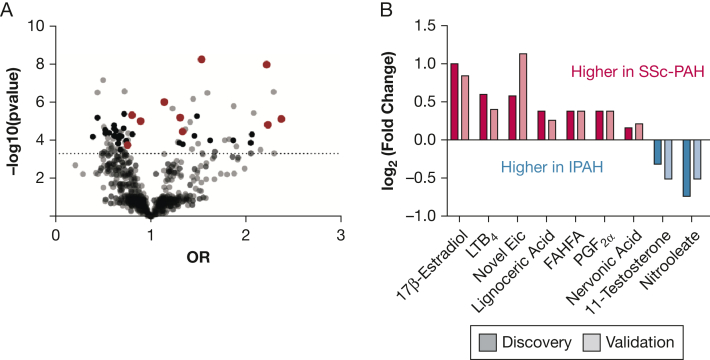
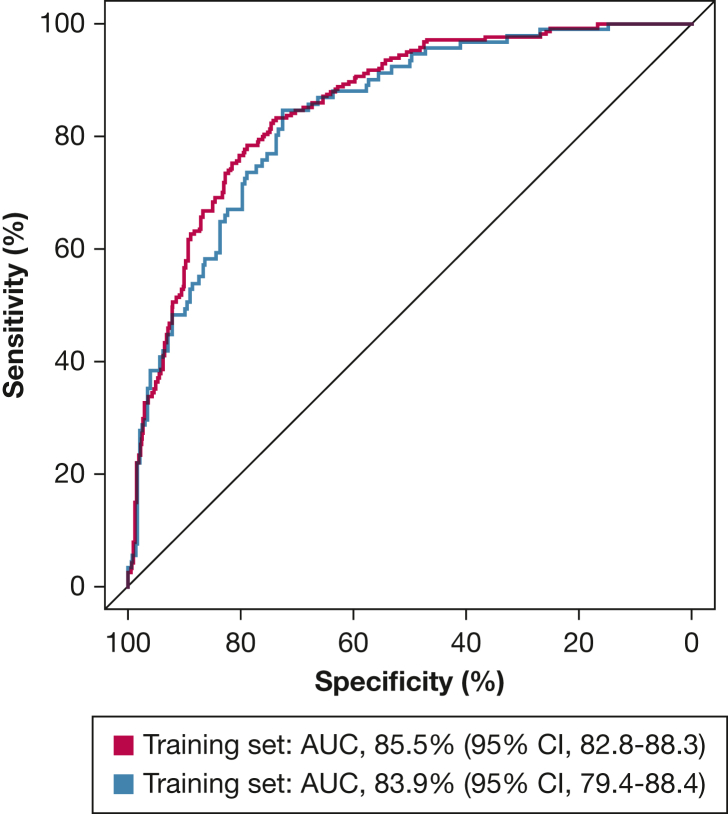
The study overview is described in Figure 1. Circulating levels of nine bioactive lipid metabolites distinguished SSc-PAH from IPAH at a “metabolome-wide” statistical threshold of P < 10–4 (e-Table 2, Table 3) after adjusting for age, sex, and BMI. Further adjustment for potential confounders, including use of prostaglandin therapy, corticosteroids, immunosuppression therapy, warfarin, or thyroid hormone, as well as renal insufficiency and cirrhosis, did not significantly affect the analyses for all of the metabolites but one. The directionality of association for a novel eicosanoid remained the same, although the significance of the association was attenuated after adjustment for warfarin use. After adjusting for 6MWD in the model, all nine metabolites remained significant. All the metabolites were able to distinguish SSc-PAH from IPAH in an independent validation cohort. These metabolites included alterations in fatty acid oxidation, eicosanoid metabolism, and sex hormones. (Fig 2). In combination, these metabolites distinguished patients with SSc-PAH and IPAH at an area under the curve of 85.5% of accuracy (95% CI, 82.8-88.3) (Fig 3).
Figure 1.
Study overview. Summary of study workflow and data analysis plan. 6MWT = 6-min walk test; IPAH = idiopathic pulmonary arterial hypertension; SSc-PAH = systemic sclerosis-associated pulmonary arterial hypertension; SSc-no PH = systemic sclerosis without associated pulmonary arterial hypertension.
Figure 2.
Bioactive metabolite analysis of SSc-PAH vs IPAH. A, Volcano plot of metabolites distinguishing SSc-PAH from IPAH in the discovery and validation cohorts. ORs and P values are derived from multivariable regression analyses. Red dots indicate metabolites significant in both discovery and validation cohorts; black dots indicate metabolites significant only in the discovery cohort; and gray dots indicate all metabolites measured in the discovery and validation cohorts. B, Waterfall plot of significant metabolites distinguishing SSc-PAH from IPAH. Values are plotted as log2 fold change of metabolite levels in SSc-PAH relative to IPAH. FAHFA = fatty acyl esters of hydroxy fatty acid; Eic = eicosanoid; IPAH = idiopathic pulmonary arterial hypertension; LTB4 = leukotriene B4; PGF2α = prostaglandin F2α; SSc-PAH = systemic sclerosis-associated pulmonary arterial hypertension.
Figure 3.
Receiver-operating characteristic curves show the performance of the model in distinguishing idiopathic pulmonary arterial hypertension from systemic sclerosis-associated pulmonary arterial hypertension using nine metabolites. The blue curve represents the training set, and the red curve represents the testing set. AUC = area under the curve.
Metabolites Distinguishing SSc-PAH From Scleroderma Disease
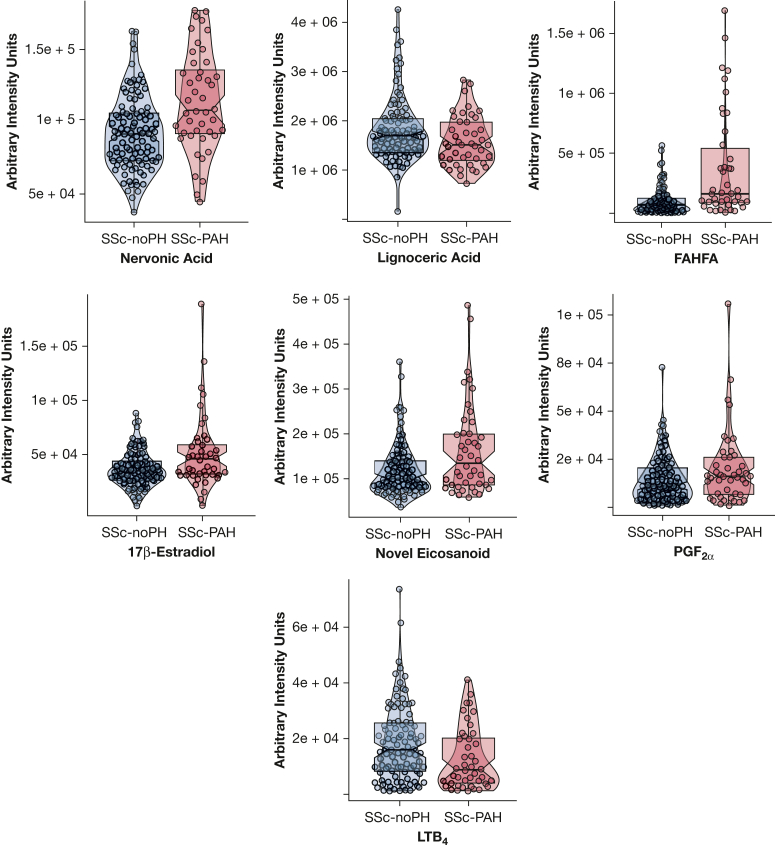
To determine if the selected metabolites were related to SSc per se, metabolomics analysis was performed comparing Cohort 3 (consisting of SSc-no PH) vs SSc-PAH. When considering the plasma metabolite signature differentiating between SSc-PAH and IPAH, levels of fatty acyl esters of hydroxy fatty acid (FAHFA), nervonic acid, 17β estradiol, prostaglandin F2α (PGF2α), and a novel eicosanoid were significantly higher in SSc-PAH vs SSc-no PH (Fig 4, Table 4). Levels of lignoceric acid and leukotriene B4 (LTB4) were significantly elevated in SSc-no PH compared with SSc-PAH. The difference in lignoceric acid and LTB4 attenuated after adjusting for disease duration (e-Fig 1, e-Table 3).
Figure 4.
Metabolite levels in SSc-no PH vs SSc-PAH. Violin plots of nervonic acid, lignoceric acid, FHAFA, 17β estradiol, novel eicosanoid, PGF2α, and LTB4 levels in SSc-no PH vs SSc-PAH. All displayed metabolites had a P value < .05. FAHFA = fatty acyl esters of hydroxy fatty acid; LTB4 = leukotriene B4; PGF2α = prostaglandin F2α; SSc-PAH = systemic sclerosis-associated pulmonary arterial hypertension; SSc-no PH = systemic sclerosis without associated pulmonary arterial hypertension.
Table 4.
Comparisons Between Metabolite Levels in SSc-PAH and SSc-no PH
| Metabolite | FC | OR | CI-L | CI-U | P Value | FDR |
|---|---|---|---|---|---|---|
| Lignoceric acid | 0.8 | 0.7 | 0.5 | 1.0 | 5E-02 | 0.05 |
| Nervonic acid | 1.2 | 2 | 1.3 | 3.0 | 2E-03 | 0.01 |
| FAHFA | 5 | 2.8 | 1.7 | 4.5 | 5E-05 | < 0.001 |
| 17β estradiol | 1.4 | 1.6 | 1.1 | 2.8 | 2E-02 | 0.03 |
| Novel eicosanoid | 1.3 | 1.9 | 1.2 | 2.9 | 4E-03 | 0.01 |
| PGF2α | 1.3 | 1.6 | 1.0 | 2.4 | 3E-02 | 0.05 |
| LTB4 | 0.6 | 0.7 | 0.5 | 1.0 | 5E-02 | 0.05 |
P values originated from multivariable logistic regression analysis. Fold change (FC) reflects the mean average of metabolite level in systemic sclerosis-associated pulmonary arterial hypertension (SSc-PAH) over systemic sclerosis without associated pulmonary arterial hypertension (SSc-no PH). ORs originated from multivariable logistic regression analysis. CI-L = lower CI; CI-U = upper CI; FAHFA = fatty acyl esters of hydroxy fatty acid; FDR = false discovery rate; LTB4 = leukotriene B4; PGF2α = prostaglandin F2α.
Associations of SSc-PAH Differentiating Metabolites With Markers of Disease Severity
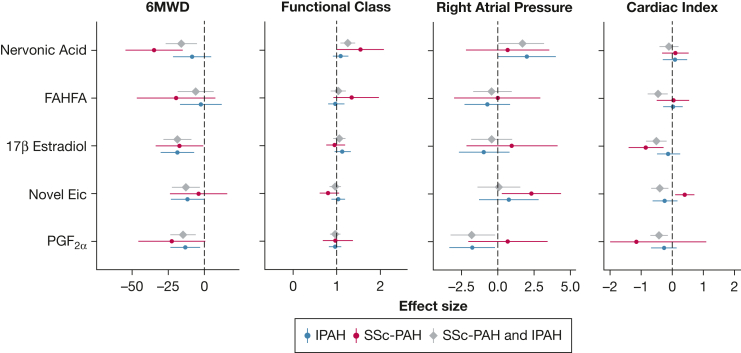
To determine if certain distinguishing metabolites associate with worse functional capacity and markers of disease severity in PAH, we next performed association of the five metabolite biomarkers with 6MWD and WHO FC. Only 58 patients (18 of them had SSc-PAH) had a right heart catheterization within 14 days of sample collection, and their hemodynamics data were included in the analysis with hemodynamic measures of disease severity: right atrial pressure, PVR, cardiac index, and SVI. As shown in e-Figure 1 and Figure 5, three of the metabolites were associated with at least one marker of disease severity in SSc-PAH, and all five metabolites were associated with at least one marker of disease severity when combining SSc-PAH and IPAH (P < .05). Two metabolites (nervonic acid and 17β estradiol) associated with decreased 6MWD in SSc-PAH, and four of the five metabolites associated with decreased 6MWD in combined SSc-PAH and IPAH (nervonic acid, 17β estradiol, novel eicosanoid, and PGF2α) and were significantly higher in SSc-PAH. Intriguingly, 17β estradiol associated with lower cardiac index and SVI in SSc-PAH but not in IPAH. Four metabolites (FAHFA, 17β estradiol, novel eicosanoid, and PGF2α) associated with lower cardiac index in combined SSc-PAH and IPAH (e-Table 4).
Figure 5.
Forest plots of metabolites association with markers of disease severity. Forest plots display the relative effect sizes and 95% CIs of significant metabolites and 6MWD, functional class, right atrial pressure, and Fick cardiac index. P values and effect sizes were derived from linear regression analysis for 6MWD, right atrial pressure, and cardiac index, and from logistic regression analysis for functional class. 6MWD = 6-minute walk distance; FAHFA = fatty acyl esters of hydroxy fatty acid; Eic = eicosanoid; IPAH = idiopathic pulmonary arterial hypertension; PGF2α = prostaglandin F2α; SSc-PAH = systemic sclerosis-associated pulmonary arterial hypertension; SSc-no PH = systemic sclerosis without associated pulmonary arterial hypertension.
Discussion
In this study, we identified significant bioactive lipid alterations that distinguish patients with SSc-PAH from those with IPAH. We assayed hundreds of circulating bioactive lipid metabolites using LC/MS approaches in 400 patients with SSc-PAH and 1,082 patients with IPAH in independent discovery and validation cohorts. We observed a set of bioactive metabolite biomarkers that independently differentiated SSc-PAH from IPAH after adjusting for multiple potential confounders. In combination, these metabolites were able to distinguish SSc-PAH from IPAH with a high degree of accuracy (area under the curve, 85.5%; 95% CI, 82.8-88.3). Importantly, levels of the differentiating metabolites were found to be altered in an independent cohort of SSc-PAH compared with SSc-no PH, and the majority of these analytes were also associated with at least one marker of disease severity. Taken together, these findings provide molecular insights into the heterogeneity that is consistently seen across PAH subgroups, and they offer viable directions for further investigation of mechanisms underlying the worse prognosis and response to therapy seen in patients with SSc-PAH.
Although there is an advancing appreciation that PAH is a heterogeneous disease with clinical differences within subgroups, still missing is a comprehensive catalogue of molecular and metabolic profiles underlying the clinical manifestations of PAH subgroup. Namely, plasma metabolomic profiles have been reported in PAH,19, 20, 21, 22 and a few reports have examined circulating metabolites that may point to potential metabolic pathways altered in SSc (with or without PAH).23,24 These studies indicated that distinct metabolic signatures exist between PAH and healthy or disease control subjects. In the largest of these studies, Rhodes et al19 performed a comprehensive metabolomics analysis in patients with IPAH and control subjects. The investigators identified that the measurements of a combination of seven circulating metabolites can be used to distinguish PAH from control subjects. Interestingly, alterations in fatty acids, steroids, and RNA-based nucleoside levels correlated with clinical outcomes, and correction of several metabolites over time was associated with better clinical outcome. Our study adds substantially to this existing compendium of metabolites by making comparisons between subgroups of PAH and focusing on a putative difference between these subgroups in metabolic dysregulation. Notably, a prior small study of eight patients with SSc without PAH and 10 patients with SSc-PAH using nuclear MRI identified an increase in glycolysis and altered fatty acid profiles in SSc-PAH.19 However, none of these studies offered a broad plasma bioactive metabolite analysis with the intent of a comparison across independent cohorts of IPAH vs SSc-PAH and SSc-PAH vs SSc-no PH. Specific molecular signatures, perhaps indicating immune-inflammatory or vasoactive targets, could be instrumental in guiding the development and tailoring of more effective management strategies. Notably, none of the top differentiating metabolites in our study was measured in prior published work, potentially related to technical differences (use of nuclear magnetic resonance vs LC/MS) and smaller sample sizes of the previous studies. Thus, our findings now set the stage for precision medicine practices in PAH clinical trial development and management based on these plasma metabolomic signatures.
Compared with IPAH, patients with SSc-PAH in the current study displayed differentially elevated metabolites of fatty acid oxidation, eicosanoid metabolism, and sex hormones (Fig 2, Table 3). Most of these elevations were persistent when comparing patients with SSc-PAH vs those with SSc-no PH (Fig 4, Table 4), indicating that these alterations are specific to the condition of SSc-PAH and not the presence of SSc alone. Future work can be envisioned to determine if such markers may define early stages of PAH in asymptomatic patients with SSc. Of particular interest, FAHFAs are a newly discovered class of complex lipid species with known antiinflammatory activities in cancer.25 Eicosanoids are small bioactive lipid species that serve as upstream mediators of inflammation and are known to modulate endothelial cell function as well as exert vasoactive properties.26 Certain eicosanoids are known to be central mechanistic triggers and drivers of PAH,27 but the full range of eicosanoid pathobiology in PAH is not defined. Until recently, sensitive methods for comprehensively detecting and quantifying eicosanoids in large sample sizes have been lacking. In this study, newly developed methods were deployed to more comprehensively measure eicosanoid metabolites14 that can now distinguish SSc-PAH compared with IPAH. Future work should be prioritized to determine how such novel metabolites may promote and potentially relate to the biology of canonical eicosanoids in both PAH subtypes.
Table 3.
Metabolites Distinguishing SSc-PAH From IPAH
| Metabolite | Pathway | Discovery |
Validation |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FC | OR | CI-L | CI-U | P Value | FDR | FC | OR | CI-L | CI-U | P Value | FDR | ||
| Lignoceric acid | Fatty acid metabolism | 1.3 | 1.4 | 1.2 | 1.6 | 7E-05 | 8E-05 | 1.2 | 1.5 | 1.1 | 2.0 | 5E-03 | 9E-03 |
| Nervonic acid | Fatty acid metabolism | 1.1 | 1.6 | 1.3 | 1.9 | 3E-07 | 9E-07 | 1.16 | 1.6 | 1.2 | 2.1 | 3E-03 | 7E-03 |
| FAHFA | Fatty acid metabolism | 1.3 | 1.6 | 1.4 | 2.0 | 9E-07 | 2E-06 | 1.3 | 1.6 | 1.1 | 2.5 | 2E-02 | 3E-02 |
| Nitrooleate | Fatty acid metabolism | 0.6 | 0.8 | 0.7 | 0.9 | 1E-04 | 1E-04 | 0.7 | 0.8 | 0.6 | 1.0 | 5E-02 | 5E-02 |
| 11-Testosterone | Steroid hormones metabolism | 0.8 | 0.7 | 0.6 | 0.8 | 3E-06 | 4E-06 | 0.7 | 0.5 | 0.4 | 0.7 | 2E-05 | 2E-04 |
| 17β estradiol | Steroid hormones metabolism | 2 | 1.5 | 1.3 | 1.7 | 1E-08 | 6E-08 | 1.8 | 1.7 | 1.3 | 2.1 | 2E-04 | 6E-04 |
| Novel eicosanoid | Arachidonic acid metabolism | 1.5 | 1.6 | 1.4 | 1.8 | 9E-09 | 6E-08 | 2.2 | 1.8 | 1.3 | 2.4 | 2E-04 | 6E-04 |
| PGF2α | Arachidonic acid metabolism | 1.3 | 1.6 | 1.3 | 1.9 | 2E-06 | 3E-06 | 1.3 | 1.4 | 1.1 | 1.9 | 9E-03 | 1E-02 |
| LTB4 | Arachidonic acid metabolism | 1.5 | 1.4 | 1.2 | 1.6 | 8E-06 | 9E-06 | 1.3 | 1.4 | 1.0 | 1.7 | 2E-02 | 3E-02 |
P values originated from multivariable regression analysis. ORs reflect multivariable regression analysis. Fold change (FC) reflects the mean average of the metabolite level in systemic sclerosis-associated pulmonary arterial hypertension (SSc-PAH) over idiopathic pulmonary arterial hypertension (IPAH). CI-L = lower confidence interval; CI-U = upper confidence interval; FAHFA = fatty acyl esters of hydroxy fatty acid; FDR = false discovery rate; LTB4 = leukotriene B4; PGF2α = prostaglandin F2α.
Intriguingly, nervonic acid was associated with worse functional capacity (both higher WHO FC and lower 6MWD) and higher right atrial pressure in SSc-PAH, despite the fact that patients with SSc-PAH displayed milder hemodynamic profiles. Previous reports have shown that patients with SSc-PAH have depressed rest and reserve right ventricular contractility.28 Correspondingly, nervonic acid is a long-chain monounsaturated omega-9 fatty acid involved in energy metabolism, antioxidant reactions, and apoptosis.29 It also modulates cardiac function and has been positively associated with greater congestive heart failure, poor performance, and increased cardiovascular mortality.30 In our cohort, nervonic acid was not associated with cardiac index or SVI; however, our hemodynamic analysis was limited by sample size. Future work should be prioritized to define any causative links of nervonic acid in cardiac impairment in SSc-PAH.
Higher levels of 17β estradiol and PGF2α were also associated with worse functional capacity in SSc-PAH. In general, 17β estradiol is considered cardioprotective, but 17β estradiol is highly pleiotropic with respect to immune function, displaying proinflammatory and antiinflammatory activity under different conditions.31,32 Intriguingly, 17β estradiol associated with lower cardiac index and SVI in SSc-PAH but associated with higher SVI in IPAH. Future studies should be geared toward defining the balance of protective vs proinflammatory effects in SSc-PAH in the absence of normal immune regulation. PGF2α is a potent pulmonary vasoconstrictor33 and marker of inflammation and oxidative stress.34 Consistent with our findings, levels of PGF2α are known to increase with both acute35 and chronic inflammation, including in connective tissue disease.36 In animal models, PGF2α promoted cardiomyocyte hypertrophy and fibrosis,37 suggesting the potential relevance of right ventricular pathobiology with PGF2α as with nervonic acid. In our study, PGF2α associated with lower cardiac index and SVI and higher PVR in SSc-PAH and IPAH combined.
It is possible that a number of the metabolic alterations associated with SSc-PAH in the current study were driven by SSc per se rather than PAH. For example, levels of lignoceric acid and LTB4 were significantly elevated in SSc-PAH compared with IPAH, but levels were more elevated in patients with SSc-no PH compared with those with SSc-PAH. Both biomarkers have been implicated in inflammation and autoimmune processes, with levels correlating with the degree of inflammation.38, 39, 40 LTB4 also induces pulmonary vascular inflammation, endothelial cell apoptosis, and vascular smooth muscle cell proliferation.40 Levels of LTB4 were significantly elevated in BAL fluid from patients with SSc-related lung disease compared with SSc patients without SSc-related lung disease and healthy control subjects.41 Interestingly, lignoceric acid was associated with a decreased 6MWD, and both lignoceric acid and LTB4 were associated with lower cardiac index and higher PVR in SSc-PAH (e-Fig 2, e-Table 3), suggesting a role of these proinflammatory biomarkers in vascular remodeling and perhaps impaired cardiac function. Studies have shown that cardiac involvement in SSc is not only linked to PAH but that SSc may have a direct impact on the right ventricular structure and function42; however, it is often difficult to differentiate between primary heart involvement and secondary impairment in the setting of PAH. More importantly, these changes may remain silent for a long time and are thus frequently underdiagnosed.
In the current cohort, patients with SSc-PAH had a longer disease duration compared with patients with SSc-no PH; in fact, the differences in lignoceric acid and LTB4 levels attenuated after adjusting for disease duration (data not shown). This molecular alteration could represent an opportunity for early detection of either PAH or cardiac involvement in patients with scleroderma and will need further prospective investigations. Another explanation is that patients with SSc-no PH had advanced SSc and a higher level of inflammation, explaining higher levels of these proinflammatory biomarkers. Unfortunately, information on the degree of inflammation was not available in this cohort. Nonetheless, our findings shed light on possible molecular mechanisms of how SSc contributes to accelerated vascular remodeling and impaired cardiac function and could potentially have therapeutic implications. Inhibition of LTB4 by bestatin (leukotriene A4 hydrolase inhibitor) prevented and reversed severe PAH in animal models,43 but a large, randomized trial of bestatin in severe PAH did not suggest drug benefit.44 Our results support a hypothesis that patients with SSc-PAH displaying high LTB4 levels may respond more robustly to bestatin.
The current study has limitations. Importantly, given the study design, adjustment for all potential confounders between IPAH and SSc-PAH was a challenge. Second, although metabolite markers were found to distinguish SSc-PAH from IPAH and independently associate with markers of disease severity, exploration of a clear causal relationship for the role of these metabolic pathways in SSc-PAH is pending. We acknowledge that 6MWD could be affected by other factors such as loss of muscle tone or arthritis, especially in patients with scleroderma. We did not have data available on loss of muscle tone or arthritis to account for. Because a significant number of right heart catheterizations were performed prior to sample collection, which limited our statistical power specifically in the SSc-PAH group, we also performed the same analysis in IPAH patients only and combined IPAH and SSc-PAH. Even though most of our selected metabolites were not significantly associated with hemodynamic markers of right ventricular dysfunction in SSc-PAH only, they became significant when combining both SSc-PAH and IPAH, and we assume that this is due to underpowering in the SSc-PAH group. Future studies with more detailed phenotyping of patients with scleroderma will be needed to validate our findings. Despite these acknowledged limitations, our findings provide more comprehensive molecular insight on the metabolic alterations present in SSc-PAH and their potential role in disease pathobiology.
Interpretation
SSc-PAH is characterized by significant metabolomic alterations that associate with markers of disease severity, which may explain accelerated disease course and contribute to poor response to therapy compared with IPAH. Our observations now offer a more comprehensive metabolic guide to much-needed diagnostic, prognostic, and therapeutic strategies of precision medicine in patients with SSc-PAH.
Acknowledgments
Author contributions: M. A. and M. J. designed the research studies, acquired data, analyzed data, and drafted the manuscript; W. C. N., M. W. P., A. A. D., A. M. B., R. L., and A. R. H. acquired data and drafted the manuscript; N. H. K., J. X.-J. Y., T. F., K. M. K., L. A., and A. M. designed the research studies and drafted the manuscript; J. D. W. conducted experiments, designed research studies, and analyzed data; T. L., S. C., and J. S. analyzed data and drafted the manuscript; and S. Y. C. designed the research studies, analyzed the data, drafted the manuscript, and is responsible for the integrity of the work as a whole.
Funding/Support: This work was supported by the National Institutes of Health grants S10OD020025 and R01ES027595 to M. J.; K01DK116917 to J. D. W.; R01 HL124021 and HL105333 to W. C. N.; R01 HL136603 to A. A. D.; P01 HL108800 and R01 HL142720 to A. R. H.; HL 122596 and HL 124021 to S. Y. C.; T32-HL134632 R01-HL154926, R01-AG063925; R01-HL148436, and R01-HL157985 to A. M.; R01-HL134168, R01-HL143227, R01-HL142983, and U54-AG065141 to S. C.; and R01-HL155955-01A1 to A. M. B. S. Y. C. was also supported by the American Heart Association Established Investigator Award 18EIA33900027. M. A. was supported by a postdoctoral fellowship award from the Chest Foundation. ResMed provided a philanthropic donation to the University of California San Diego.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: S. Y. C. has served as a consultant to United Therapeutics and Acceleron Pharma; has held research grants from Actelion, Bayer, and Pfizer; is a director, officer, and shareholder of Synhale Therapeutics; and has submitted patent applications regarding metabolism in pulmonary hypertension. N. H. K. has served as consultant for Bayer, Janssen, Merck, and United Therapeutics; has received lecture fees for Bayer and Janssen; and has received research support from Acceleron, Eiger, Gossamer Bio, Lung Biotechnology, and SoniVie. A. M. B. served as a consultant to Biogen. A. M. reports income related to medical education from LivaNova, Equillium, and Corvus. K. M. K. received university grant money from Bayer; and served as a consultant for Actelion. None declared (M. A., J. S., M. W. P., W. C. N., A. R. H., J. X.-J. Y., T. F., L. A., A. A. D., R. L., J. D. W., T. L., S. C., M. J.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: Samples and/or data from the National Biological Sample and Data Repository for PAH, which receives government support under an investigator-initiated grant [R24 HL105333] awarded by the National Heart Lung and Blood Institute were used in this study. The authors thank contributors, including the Pulmonary Hypertension Centers who collected samples used in this study, as well as patients and their families, whose help and participation made this work possible.
Additional information: The e-Appendix, e-Figures, and e-Tables are available online under ”Supplementary Data.“
Footnotes
Drs Chan and Jain contributed equally to this manuscript.
Supplementary Data
References
- 1.Badesch D.B., Raskob G.E., Elliott C.G., et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137(2):376–387. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 2.Allanore Y., Simms R., Distler O., et al. Systemic sclerosis. Nat Rev Dis Primers. 2015;1:15002. doi: 10.1038/nrdp.2015.2. [DOI] [PubMed] [Google Scholar]
- 3.Fisher M.R., Mathai S.C., Champion H.C., et al. Clinical differences between idiopathic and scleroderma-related pulmonary hypertension. Arthritis Rheum. 2006;54(9):3043–3050. doi: 10.1002/art.22069. [DOI] [PubMed] [Google Scholar]
- 4.Chung L., Liu J., Parsons L., et al. Characterization of connective tissue disease-associated pulmonary arterial hypertension from REVEAL: identifying systemic sclerosis as a unique phenotype. Chest. 2010;138(6):1383–1394. doi: 10.1378/chest.10-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed S., Palevsky H.I. Pulmonary arterial hypertension related to connective tissue disease: a review. Rheum Dis Clin North Am. 2014;40(1):103–124. doi: 10.1016/j.rdc.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Gashouta M.A., Humbert M., Hassoun P.M. Update in systemic sclerosis-associated pulmonary arterial hypertension. Presse Med. 2014;43(10 Pt 2):e293–e304. doi: 10.1016/j.lpm.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Clements P.J., Tan M., McLaughlin V.V., et al. Investigators PAHQERIP-Q The pulmonary arterial hypertension quality enhancement research initiative: comparison of patients with idiopathic PAH to patients with systemic sclerosis-associated PAH. Ann Rheum Dis. 2012;71(2):249–252. doi: 10.1136/annrheumdis-2011-200265. [DOI] [PubMed] [Google Scholar]
- 8.Chaisson N.F., Hassoun P.M. Systemic sclerosis-associated pulmonary arterial hypertension. Chest. 2013;144(4):1346–1356. doi: 10.1378/chest.12-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Overbeek M.J., Vonk M.C., Boonstra A., et al. Pulmonary arterial hypertension in limited cutaneous systemic sclerosis: a distinctive vasculopathy. Eur Respir J. 2009;34(2):371–379. doi: 10.1183/09031936.00106008. [DOI] [PubMed] [Google Scholar]
- 10.Kelemen B.W., Mathai S.C., Tedford R.J., et al. Right ventricular remodeling in idiopathic and scleroderma-associated pulmonary arterial hypertension: two distinct phenotypes. Pulm Circ. 2015;5(2):327–334. doi: 10.1086/680356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forfia P.R., Mathai S.C., Fisher M.R., et al. Hyponatremia predicts right heart failure and poor survival in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;177(12):1364–1369. doi: 10.1164/rccm.200712-1876OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams M.H., Handler C.E., Akram R., et al. Role of N-terminal brain natriuretic peptide (N-TproBNP) in scleroderma-associated pulmonary arterial hypertension. Eur Heart J. 2006;27(12):1485–1494. doi: 10.1093/eurheartj/ehi891. [DOI] [PubMed] [Google Scholar]
- 13.Hannun Y.A., Obeid L.M. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9(2):139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 14.Watrous J.D., Niiranen T.J., Lagerborg K.A., et al. Directed non-targeted mass spectrometry and chemical networking for discovery of eicosanoids and related oxylipins. Cell Chem Biol. 2019;26(3):433–442. doi: 10.1016/j.chembiol.2018.11.015. e434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lagerborg K.A., Watrous J.D., Cheng S., Jain M. High-throughput measure of bioactive lipids using non-targeted mass spectrometry. Methods Mol Biol. 2019;1862:17–35. doi: 10.1007/978-1-4939-8769-6_2. [DOI] [PubMed] [Google Scholar]
- 16.Wang T.J., Larson M.G., Vasan R.S., et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17(4):448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kantz E.D., Tiwari S., Watrous J.D., Cheng S., Jain M. Deep neural networks for classification of LC-MS spectral peaks. Anal Chem. 2019;91(19):12407–12413. doi: 10.1021/acs.analchem.9b02983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.RStudio Team. RStudio: Integrated Development for R. RStudio, PBC, Boston, MA. 2020. http://www.rstudio.com/
- 19.Rhodes C.J., Ghataorhe P., Wharton J., et al. Plasma metabolomics implicates modified transfer RNAs and altered bioenergetics in the outcomes of pulmonary arterial hypertension. Circulation. 2017;135(5):460–475. doi: 10.1161/CIRCULATIONAHA.116.024602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemnes A.R., Luther J.M., Rhodes C.J., et al. Human PAH is characterized by a pattern of lipid-related insulin resistance. JCI Insight. 2019;4(1) doi: 10.1172/jci.insight.123611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He Y.Y., Yan Y., Jiang X., et al. Spermine promotes pulmonary vascular remodelling and its synthase is a therapeutic target for pulmonary arterial hypertension. Eur Respir J. 2020;56(5) doi: 10.1183/13993003.00522-2020. [DOI] [PubMed] [Google Scholar]
- 22.Sanders J.L., Han Y., Urbina M.F., Systrom D.M., Waxman A.B. Metabolomics of exercise pulmonary hypertension are intermediate between controls and patients with pulmonary arterial hypertension. Pulm Circ. 2019;9(4) doi: 10.1177/2045894019882623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bengtsson A.A., Trygg J., Wuttge D.M., et al. Metabolic profiling of systemic lupus erythematosus and comparison with primary Sjögren’s syndrome and systemic sclerosis. PLoS One. 2016;11(7) doi: 10.1371/journal.pone.0159384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deidda M., Piras C., Cadeddu Dessalvi C., et al. Distinctive metabolomic fingerprint in scleroderma patients with pulmonary arterial hypertension. Int J Cardiol. 2017;241:401–406. doi: 10.1016/j.ijcard.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 25.Liu T., Tan Z., Yu J., et al. A conjunctive lipidomic approach reveals plasma ethanolamine plasmalogens and fatty acids as early diagnostic biomarkers for colorectal cancer patients. Expert Rev Proteomics. 2020;17(3):233–242. doi: 10.1080/14789450.2020.1757443. [DOI] [PubMed] [Google Scholar]
- 26.Auch-Schwelk W., Katusic Z.S., Vanhoutte P.M. Thromboxane A2 receptor antagonists inhibit endothelium-dependent contractions. Hypertension. 1990;15(6 Pt 2):699–703. doi: 10.1161/01.hyp.15.6.699. [DOI] [PubMed] [Google Scholar]
- 27.Bowers R., Cool C., Murphy R.C., et al. Oxidative stress in severe pulmonary hypertension. Am J Respir Crit Care Med. 2004;169(6):764–769. doi: 10.1164/rccm.200301-147OC. [DOI] [PubMed] [Google Scholar]
- 28.Hsu S., Kokkonen-Simon K.M., Kirk J.A., et al. Right ventricular myofilament functional differences in humans with systemic sclerosis-associated versus idiopathic pulmonary arterial hypertension. Circulation. 2018;137(22):2360–2370. doi: 10.1161/CIRCULATIONAHA.117.033147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwingshackl L., Strasser B., Hoffmann G. Effects of monounsaturated fatty acids on cardiovascular risk factors: a systematic review and meta-analysis. Ann Nutr Metab. 2011;59(24):176–186. doi: 10.1159/000334071. [DOI] [PubMed] [Google Scholar]
- 30.Imamura F., Lemaitre R.N., King I.B., et al. Long-chain monounsaturated fatty acids and incidence of congestive heart failure in 2 prospective cohorts. Circulation. 2013;127(14):1512–1521. doi: 10.1161/CIRCULATIONAHA.112.001197. 1521e1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lahm T., Crisostomo P.R., Markel T.A., et al. The effects of estrogen on pulmonary artery vasoreactivity and hypoxic pulmonary vasoconstriction: potential new clinical implications for an old hormone. Crit Care Med. 2008;36(7):2174–2183. doi: 10.1097/CCM.0b013e31817d1a92. [DOI] [PubMed] [Google Scholar]
- 32.Straub R.H. The complex role of estrogens in inflammation. Endocr Rev. 2007;28(5):521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 33.Vane J.R., Botting R.M. Pharmacodynamic profile of prostacyclin. Am J Cardiol. 1995;75(3):3A–10A. doi: 10.1016/s0002-9149(99)80377-4. [DOI] [PubMed] [Google Scholar]
- 34.Basu S. Oxidative injury induced cyclooxygenase activation in experimental hepatotoxicity. Biochem Biophys Res Commun. 1999;254(3):764–767. doi: 10.1006/bbrc.1998.9956. [DOI] [PubMed] [Google Scholar]
- 35.Basu S., Eriksson M. Lipid peroxidation induced by an early inflammatory response in endotoxaemia. Acta Anaesthesiol Scand. 2000;44(1):17–23. doi: 10.1034/j.1399-6576.2000.440104.x. [DOI] [PubMed] [Google Scholar]
- 36.Basu S., Whiteman M., Mattey D.L., Halliwell B. Raised levels of F(2)-isoprostanes and prostaglandin F(2alpha) in different rheumatic diseases. Ann Rheum Dis. 2001;60(6):627–631. doi: 10.1136/ard.60.6.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding W.Y., Ti Y., Wang J., et al. Prostaglandin F2α facilitates collagen synthesis in cardiac fibroblasts via an F-prostanoid receptor/protein kinase C/Rho kinase pathway independent of transforming growth factor β1. Int J Biochem Cell Biol. 2012;44(6):1031–1039. doi: 10.1016/j.biocel.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 38.Armstrong A.W., Wu J., Johnson M.A., et al. Metabolomics in psoriatic disease: pilot study reveals metabolite differences in psoriasis and psoriatic arthritis. F1000Res. 2014;3:248. doi: 10.12688/f1000research.4709.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsumori R., Miyazaki T., Shimada K., et al. High levels of very long-chain saturated fatty acid in erythrocytes correlates with atherogenic lipoprotein profiles in subjects with metabolic syndrome. Diabetes Res Clin Pract. 2013;99(1):12–18. doi: 10.1016/j.diabres.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 40.Chwieśko-Minarowska S., Kowal K., Bielecki M., Kowal-Bielecka O. The role of leukotrienes in the pathogenesis of systemic sclerosis. Folia Histochem Cytobiol. 2012;50(2):180–185. doi: 10.5603/fhc.2012.0027. [DOI] [PubMed] [Google Scholar]
- 41.Kowal-Bielecka O., Distler O., Kowal K., et al. Elevated levels of leukotriene B4 and leukotriene E4 in bronchoalveolar lavage fluid from patients with scleroderma lung disease. Arthritis Rheum. 2003;48(6):1639–1646. doi: 10.1002/art.11042. [DOI] [PubMed] [Google Scholar]
- 42.Cucuruzac R., Muntean I., Benedek I., et al. Right ventricle remodeling and function in scleroderma patients. Biomed Res Int. 2018;2018 doi: 10.1155/2018/4528148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tian W., Jiang X., Tamosiuniene R., et al. Blocking macrophage leukotriene b4 prevents endothelial injury and reverses pulmonary hypertension. Sci Transl Med. 2013;4(200):200ra117. doi: 10.1126/scitranslmed.3006674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melao A. Eiger BioPharmaceuticals Discontinues Ubenimex for PAH Clinical Trial. https://pulmonaryhypertensionnews.com/2018/01/17/eiger-biopharmaceuticals-discontinues-ubenimex-pah-clinical-trial/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.