Abstract
Group singing and playing of wind instruments increase COVID-19 transmission risk. After a pause during the initial period of the COVID-19 pandemic, The Tabernacle Choir at Temple Square organization (hereinafter, Choir) resumed musical events in September 2021 with prevention protocols, including required vaccination and pre-event rapid antigen testing. We investigated potential SARS-CoV-2 transmission at Choir events during September 21–November 7, 2021. We interviewed COVID-19–positive members (hereinafter, case-members) and identified members exposed when a case-member attended a Choir event during his or her infectious period. We compared whole genome sequencing results to assess the genetic relatedness of available SARS-CoV-2 specimens obtained from case-members. We identified 30 case-members through pre-event testing (n = 10), self-reported positive test results (n = 18), and a review of Utah’s disease surveillance system (n = 2). All 30 case-members reported symptoms; 21 (70%) were women and 23 (77%) received a positive test result by nucleic acid amplification test. No hospitalizations or deaths were reported. We identified 176 test-eligible exposed members from 14 instances of case-members attending events during their infectious periods. All were tested at least once 2 to 14 days after exposure: 74 (42%) by rapid antigen test only (all negative) and 102 (58%) by nucleic acid amplification test (4 positive, 97 negative, and 1 equivocal). Among viral sequences available from 15 case-members, the smallest single-nucleotide polymorphism distance between 2 sequences was 2, and the next-smallest distance was 10. The lack of disease detected in most exposed members suggests that minimal, if any, transmission occurred at Choir events. When community COVID-19 incidence is high, prevention protocols might help limit SARS-CoV-2 transmission during group musical activities.
Keywords: COVID-19, choir, prevention, contacts, whole genome sequencing
Group singing and playing of wind instruments elevate SARS-CoV-2 transmission risk because of the increased emission of respiratory particles that occurs during these activities.1-3 Multiple COVID-19 outbreaks after exposure during choral singing have been reported, including 2 outbreaks described in the literature, both in March 2020. One outbreak occurred in Washington State with an 87% attack rate; 3 Choir members were hospitalized and 2 died. 4 The other outbreak, in France, had a 70% attack rate with 7 hospitalizations and no deaths. 5
The Tabernacle Choir at Temple Square organization (hereinafter, Choir) in Salt Lake City, Utah, comprises the separate components of the Tabernacle Choir, Orchestra, and Bells at Temple Square and associated staff. After performing on March 8, 2020, the Choir paused activities because of COVID-19. On September 21, 2021, the Choir resumed group musical activities with layered COVID-19 prevention protocols, 6 with the primary goal of preventing SARS-CoV-2 transmission during Choir events.
We investigated potential SARS-CoV-2 transmission at Choir events in the context of these protocols during the initial 48-day period after the Choir resumed activities (September 21–November 7, 2021). During this time in Utah, the 7-day median (range) COVID-19 incidence rate was high (301.5 [262.0-355.6] per 100 000 population 7 ), and the Delta variant (or its sublineages) predominated. We describe the Choir’s COVID-19 prevention protocols and outcomes of our transmission investigation.
Methods
Choir Event Timeline and Investigation Period
We defined a Choir event as ≥1 rehearsal or performance on the same day. The first Choir event after the pause for COVID-19 was on September 21, 2021, with the choral ensemble and associated staff participating. The Orchestra and Bells resumed on October 14, 2021. The first performance with the entire ensemble during the investigation period was on October 24, 2021; we continued the investigation for a 2-week period after this performance (through November 7, 2021). On the basis of these dates, we defined the investigation periods as September 21–November 7, 2021, for the choral ensemble and staff and October 14–November 7, 2021, for Orchestra and Bells.
Prevention Protocol Description
The Choir’s COVID-19 prevention protocols included the following.
Vaccination: To participate in Choir events, members were required to prove receipt of a 2-dose mRNA series or a single dose of Janssen COVID-19 vaccine.
Pre-event testing: Members were required to receive a rapid antigen test ≤2 hours before the start of each event; only members who received a negative test result could participate in person. For each event, members who had received a positive test result in the 90 days before the event were exempt from testing and did not need to test to participate, although some exempt members were tested. Pre-event, on-site, rapid antigen testing was managed by the Brigham Young University Health Center and staffed by Choir members and affiliated individuals trained to conduct COVID-19 testing. Testers wore face masks, gloves, face shields or goggles, and gowns. Recorders wore face masks, gloves, and gowns. On October 28, 2021, members began self-testing, observed by personnel in face masks and gloves.
Exclusion and isolation: Members were asked to exclude themselves from events if they had symptoms of COVID-19, exposure to a COVID-19–positive household member, or a COVID-19–related health concern. Members who received a positive test result were asked to not attend events during a 10-day isolation period.
Postexposure notification and testing: In many instances, members exposed at events were notified of the exposure, and many were asked to be tested outside pre-event testing before returning to the Choir.
Leave policy: During the investigation period, the Choir granted long-term leave to members for COVID-19–related reasons. Additionally, starting on October 14, online rehearsal was available for the choral ensemble.
Reporting of symptoms and positive test results: Members were encouraged to self-report symptoms and positive test results to the Choir’s medical director.
Wearing face masks: Members were required to wear face masks indoors during events when not rehearsing or performing.
Physical distancing: During September 21–October 3, 2021, the choral loft was kept only approximately half-full to allow for increased physical distancing while singing.
Case-Member Identification and Investigation
We defined a case-member as a Choir member who received a positive result with a COVID-19 rapid antigen test or nucleic acid amplification test (NAAT) with a positive test collection or symptom onset date during the investigation period. To identify case-members, we used results of pre-event testing, self-reports of positive test results, and data in Utah’s disease surveillance system, EpiTrax. We gathered information about case-member symptoms, exposures, and attendance at events by interviewing case-members identified through pre-event testing and self-reporting, querying EpiTrax, and reviewing written and photographic attendance records. The Utah Department of Health and Human Services reviewed this activity and determined that it was not subject to institutional review board review. The Centers for Disease Control and Prevention reviewed and conducted this activity consistent with applicable federal law and Centers for Disease Control and Prevention policy (45 CFR part 46, 21 CFR part 56; 42 USC §241(d); 5 USC §552a; 44 USC §3501 et seq).
Exposed Member Identification and Investigation
We defined a case-member’s infectious period as 2 days before his or her symptom onset (or collection of the positive test result if the person did not have symptoms at the time of the test) through the end of the isolation period. When a case-member attended a Choir event during his or her infectious period, we identified members who were possibly exposed to that person (exposed members) and assessed their testing and symptom outcomes for 14 days. To identify exposed members at events, we used the following: seating assignment charts; written, photographic, and videographic attendance records; and/or testing records in EpiTrax. If a Choir member was exposed to the same case-member on multiple dates during his or her infectious period, we used the earliest exposure date as the reference date. We identified exposed members in an area around the case-member, with a larger exposure area in front of than behind the case-member. Through October 3, 2021, this area was approximately 6 feet in front and to the side of and approximately 3 feet behind the case-member (6′ × 6′ × 3′). After October 3, to improve the chance of detecting people who might have become infected because of exposure at an event, the size of this area was increased to approximately 10 feet in front and to the side of and approximately 3 feet behind the case-member (10′ × 10′ × 3′). We collected self-reported information about the symptoms of exposed members. We determined test results of the exposed member based on self-reports and data in EpiTrax.
Whole Genome Sequencing
We compared whole genome sequencing (WGS) results to assess the genetic relatedness of available SARS-CoV-2 specimens obtained from case-members’ positive NAATs. We used data from 1 person’s positive NAAT result collected immediately before the investigation period; this person also received a positive test result early in the investigation period for the same illness.
We performed RNA extractions with the TaqPath COVID-19 Combo Kit (Thermo Fisher). We prepared extracted RNA for next-generation sequencing using the COVIDSeq RUO Kit (Illumina Inc) and sequenced the resulting libraries on the NovaSeq 6000 platform (Illumina Inc). We performed Fastq generation and consensus sequence generation via DRAGEN COVID Pipeline (RUO) version 1.0.0 (Illumina Inc). RNA extraction, preparation, and sequencing were performed at the Utah Public Health Laboratory, except for viral RNA extraction for 3 case-members and viral genome sequencing for 1 case-member, which were performed at different laboratories with unknown methods. We used the Cecret workflow 8 version 2.4.20220407 for PANGO lineage determination and phylogenetic analysis. We assigned PANGO lineages with pangolin 9 version 3.1.17, aligned consensus sequences with MAFFT, 10 and created a phylogenetic tree via IQ-TREE2 11 and visualized it with ggtree 12 (open-source software). We measured relatedness by single-nucleotide polymorphism (SNP) differences between viral sequences, with a difference of 0 or 1 SNP indicating a close genetic relationship13,14 and with larger differences suggesting less genetic relatedness.
Outcomes
According to available records, there were 709 members of the Choir as of September 21, 2021, of whom 380 (54%) were women, 703 (99%) were Utah residents, and the median (range) age was 51 (21-91) years. The Choir comprised 4 components: choral ensemble (n = 409, 58%), Orchestra (n = 180, 25%), Bells (n = 32, 5%), and staff (n = 89, 13%) (1 person was a member of 2 components).
The Choir held 20 events, each lasting ≥2 hours, during the investigation period. The median (range) per-event participant number was 294 (29-488).
Case-Member Identification and Investigation
We identified 30 case-members: 10 through pre-event testing, 18 through self-reporting to the Choir’s medical director, and 2 through a review of EpiTrax. All 30 case-members reported symptoms and 21 (70%) were women. Of the 30 case-members, 23 (77%) received a positive result by NAAT, 6 (20%) by rapid antigen test only (no NAAT), and 1 (3%) received a positive result by rapid antigen test and a negative result by NAAT. Of the 28 case-members identified by the Choir through pre-event testing and self-reporting, 19 (68%) were in the choral ensemble, 8 (29%) were in the Orchestra, 1 (4%) was among staff, and none were in the Bells. No hospitalizations or deaths were reported.
Exposed Member Identification and Investigation
Fourteen case-members attended at least 1 Choir event during their infectious periods. From these events, we identified 178 exposed members and assessed their postexposure test results and symptoms. The 2 case-members found through a review of EpiTrax were not among the identified exposed members, and available records indicate that these 2 case-members did not attend an event during their infectious periods. Two exposed members had received a positive test result during the investigation period and, thus, were exempt from testing. All 176 test-eligible exposed members were tested at least once 2 to 14 days after exposure: 74 (42%) by rapid antigen test only and 102 (58%) by NAAT. Four case-members received a positive result by NAAT, and all others received a negative test result, except for 1 case-member who received an equivocal NAAT result and a negative result by rapid antigen test multiple times 2 to 14 days after exposure. Additionally, of the 176 test-eligible exposed members, 171 (97%) were tested at least once 3 to 7 days after exposure; of these, 85 (50%) were tested by rapid antigen test only (all negative) and 86 (50%) by NAAT (3 received a positive result; these are a subset of the aforementioned 4 NAAT-positive exposed members, 1 of whom initially received a negative result by NAAT 3 to 7 days after exposure and later received a positive result by NAAT).
The 4 NAAT-positive exposed members reported symptoms beginning 8, 8, 0, and 2 days after an at-event exposure (Table). Additionally, 1 NAAT-negative exposed member reported symptoms beginning 2 days after exposure. The remaining 173 exposed members did not report symptoms beginning 2 to 14 days after exposure.
Table.
Characteristics of potential COVID-19 transmission instances at The Tabernacle Choir at Temple Square organization events, Salt Lake City, Utah, September–November 2021
| Potential transmission instance no. a | Viral sequence SNP distance between COVID-19–positive members | Reported non-Choir exposures | No. of days after exposure, reported symptoms began |
|---|---|---|---|
| 1 | 18 | None | 8 |
| 2 | WGS comparison not possible (specimen discarded) | Multiple | 8 |
| 3 | WGS comparison not possible (sequencing failed) | None | 0 b |
| 4 | WGS comparison not possible (sequencing failed) | None | 2 |
| 5 | 2 | None | 4 |
Abbreviations: SNP, single-nucleotide polymorphism; WGS, whole genome sequencing.
Instances 1-4 were identified by exposure investigation. Instance 5 was identified by WGS comparison.
In instance 3, the exposed COVID-19–positive member reported symptoms beginning on the day of the at-event exposure.
WGS Comparison
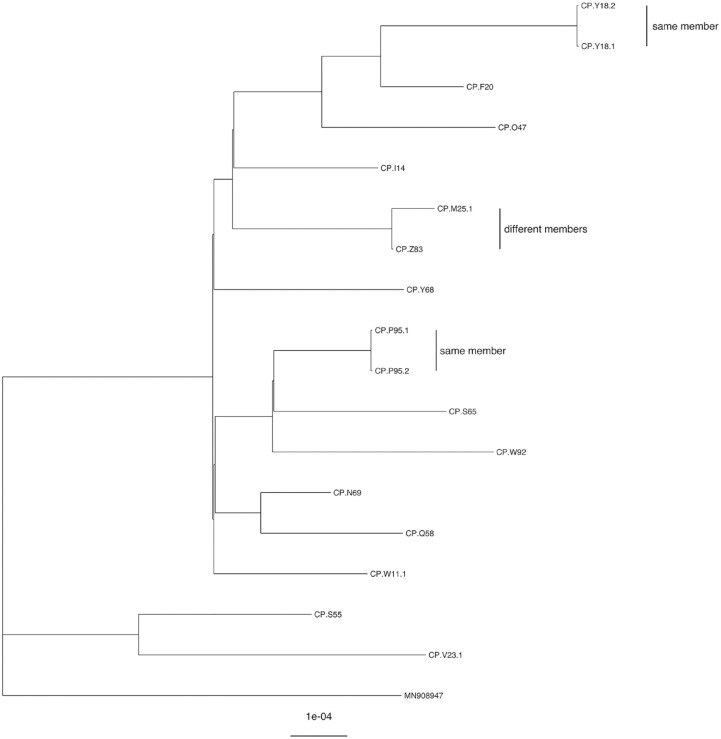
Of the 23 case-members with positive NAAT results, 15 had viral specimen genomic sequences available for comparison; all were the Delta variant or its sublineages (Figure). For 8 case-members with positive NAAT results, WGS comparison was not possible because the specimen was discarded or lost before we could perform WGS or because sequencing failed. The smallest SNP distance between viral specimens from different members was 2 (Table) and the next smallest was 10 (range, 2-45).
Figure.
Phylogenetic tree of SARS-CoV-2 genetic sequences from 15 members of The Tabernacle Choir at Temple Square organization, Salt Lake City, Utah, September–November 2021. In 2 instances, a member received 2 nucleic acid amplification tests, each yielding 2 specimens that were 0 SNPs apart from each other (sequences CP.Y18.1 and CP.Y18.2 and sequences CP.P95.1 and CP.P95.2). Sequences CP.M25 and CP.Z83 are from different members and are 2 SNPs apart. Sequence MN908947 is from the original Wuhan reference strain. Abbreviation: SNP, single-nucleotide polymorphism.
Instances of Potential Transmission
We identified 5 instances in which a case-member might have transmitted COVID-19 to another case-member at a Choir event: 4 based on exposure investigation and 1 based on WGS comparison (Table). In the first instance of potential at-event transmission, the viral sequence of the exposed case-member differed from that of the exposing case-member by 18 SNPs. WGS comparison was not possible for the other 3 potential transmission instances identified based on exposure investigation. In the second instance, the exposed case-member reported multiple non-Choir exposures. In instances 3 and 4, the respective exposed case-members reported symptoms beginning 0 and 2 days after exposure. In the fifth potential transmission instance, the viral genomic sequence between 2 case-members (case-members A and B) differed by 2 SNPs. Case-member A attended an event during this case-member’s infectious period; case-member B also attended this event, but we did not identify case-member B as having been in case-member A’s exposure area (ie, 10′ × 10′ × 3′).
Lessons Learned
We reported the results of our investigation to assess evidence of potential SARS-CoV-2 transmission at Choir events with prevention protocols in place, including vaccination, testing, exclusion for symptoms or exposure, case-member isolation, lenient leave, virtual rehearsal, and self-reporting of symptoms and positive test results. Despite hundreds of musicians rehearsing and performing in close proximity for hours at a time during a period of high community incidence, 7 our findings suggest limited or no SARS-CoV-2 transmission at events in the context of these prevention policies. It is also noteworthy that among the 30 identified case-members in this universally vaccinated population, no hospitalizations or deaths were reported, suggesting a relatively low level of illness severity.
COVID-19 was not detected in most exposed members. Of the 4 potential event-related COVID-19 transmissions identified by exposure investigation, transmission in instance 1 was ruled out by WGS. Transmission in instances 2, 3, and 4 could be neither definitively demonstrated nor ruled out because WGS comparisons were not possible. In instance 2, transmission to the exposed case-member could have occurred at Choir or via other reported non-Choir exposures. In instance 3, because the exposed case-member reported symptoms beginning on the day of exposure, we considered it very unlikely that this exposed member became infected at the event. In instance 4, the exposed case-member could have been infected at Choir; however, this case-member reported symptoms starting 2 days after exposure, which is the minimum value of the 2- to 14-day incubation period.
WGS was valuable during this investigation. In addition to ruling out 1 of the 4 potential transmissions to identified exposed members, WGS allowed us to assess possible transmission between case-members when no evident epidemiologic association existed. Indeed, we identified a fifth instance of potential at-event transmission between 2 case-members without an established exposure relationship; the viral genomic sequences of the 2 case-members differed by 2 SNPs. Given SARS-CoV-2’s low estimated mutation rate,15,16 direct transmission events would typically manifest as a 0- or 1-SNP difference between sequences.13,14 The observed 2-SNP difference could indicate direct transmission with a greater-than-expected SNP difference or independent transmission. Other available WGS data did not suggest direct transmission between case-members.
The fact that all infected members reported symptoms is noteworthy, given the known occurrence of asymptomatic COVID-19 infections during the Delta variant period17-19 and the high testing coverage among Choir members. A partial explanation for this finding could be that a person who has received a positive test result might be more likely to remember and report symptoms than a person who has not received a positive test result.
This investigation had several limitations. First, some infections might not have been detected, and some symptoms and positive home test results might not have been reported to the Choir’s medical director; however, the fact that we identified only 2 additional case-members through a search of EpiTrax suggests that nonreporting of positive test results was minimal. Second, it is possible that we did not correctly identify all exposed members; hence, there may have been additional exposed people whose postexposure testing and symptom status we did not assess. Finally, WGS comparison was possible for half the case-members, so we could not characterize the genetic relationship between the viruses from all case-members.
Our evidence suggests that, with prevention protocols in place, minimal, if any, transmission occurred at Choir events during the investigation period. This finding is in contrast to previously reported outbreaks in choral settings; these settings, in which layered preventive measures were not in place, were characterized by high attack rates and severe illness.4,5 Although we do not know the potential extent of SARS-CoV-2 transmission at Choir events if prevention protocols had not been in place, adherence to these protocols—including required vaccination, self-reporting, and exclusion of symptomatic and household-exposed members—likely helped prevent transmission at events. Likewise, vaccination likely helped limit the severity of illness. The Choir’s experience returning to musical activities with prevention protocols in place can inform other musical ensembles as they navigate COVID-19. During times of high community COVID-19 incidence, organizations hosting activities associated with increased risk of transmission, including in-person musical ensemble rehearsal and performance, should consider using prevention protocols.
Acknowledgments
The authors acknowledge The Tabernacle Choir at Temple Square organization members, Medical Committee, and Testing Team for their participation in and assistance with this investigation. We also thank the Utah Department of Health and Human Services’ epidemiology and next-generation sequencing teams for technical assistance.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
ORCID iD: William A. Lanier, DVM, MPH  https://orcid.org/0000-0002-1281-5691
https://orcid.org/0000-0002-1281-5691
References
- 1.Bahl P, de Silva C, Bhattacharjee S, et al. Droplets and aerosols generated by singing and the risk of coronavirus disease 2019 for choirs. Clin Infect Dis. 2021;72(150):e639-e641. doi: 10.1093/cid/ciaa1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mürbe D, Kriegel M, Lange J, Schumann L, Hartmann A, Fleischer M. Aerosol emission of adolescents voices during speaking, singing and shouting. PLoS One. 2021;16(2):e0246819. doi: 10.1371/journal.pone.0246819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stockman T, Zhu S, Kumar A, et al. Measurements and simulations of aerosol released while singing and playing wind instruments. ACS Environ Au. 2021;1:71-84. doi: 10.1021/acsenvironau.1c00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamner L, Dubbel P, Capron I, et al. High SARS-CoV-2 attack rate following exposure at a choir practice—Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(19):606-610. doi:10/15585/mmwr.mm6919e6 [DOI] [PubMed] [Google Scholar]
- 5.Charlotte N. High rate of SARS-CoV-2 transmission due to choir practice in France at the beginning of the COVID-19 pandemic. J Voice. Published online December23, 2020. doi: 10.1016/j.jvoice.2020.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Tabernacle Choir at Temple Square. Choir COVID plan aids in return to singing. September17, 2021. Accessed April 13, 2022. https://www.thetabernaclechoir.org/articles/choir-covid-plan-aids-return-to-singing.html
- 7.Utah Department of Health and Human Services. Overview of COVID-19 surveillance. 2022. Accessed March 30, 2022. https://coronavirus.utah.gov/case-counts
- 8.Utah Department of Health and Human Services, Utah Public Health Laboratory. Nextflow workflow for our analysis of SARS-CoV-2—Cecret. Accessed April 16, 2022. https://github.com/UPHL-BioNGS/Cecret
- 9.O’Toole Á, Scher E, Underwood A, et al. Assignment of epidemiological lineages in an emerging pandemic using the pangolin tool. Virus Evol. 2021;7(2):veab064. doi: 10.1093/ve/veab064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772-780. doi: 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minh BQ, Schmidt HA, Chernomor O, et al. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evol. 2020;37(5):1530-1534. doi: 10.1093/molbev/msaa015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu G. Using ggtree to visualize data on tree-link structures. Curr Protoc Bioinformatics. 2020;69(1):e96. doi: 10.1002/cpbi.96 [DOI] [PubMed] [Google Scholar]
- 13.Lumley SF, Constantinides B, Sanderson N, et al. Epidemiological data and genome sequencing reveals that nosocomial transmission of SARS-CoV-2 is underestimated and mostly mediated by a small number of highly infectious individuals. J Infect. 2021;83(4):473-482. doi: 10.1016/j.jinf.2021.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hare D, Meaney C, Powell J, et al. Repeated transmission of SARS-CoV-2 in an overcrowded Irish emergency department elucidated by whole-genome sequencing. J Hosp Infect. 2022;126:1-9. doi: 10.1016/j.jhin.2022.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bar-On YM, Flamholz A, Phillips R, Milo R. SARS-CoV-2 (COVID-19) by the numbers. Elife. 2020;9:e57309. doi: 10.7554/eLife.57309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amicone M, Borges V, Alves MJ, et al. Mutation rate of SARS-CoV-2 and emergence of mutators during experimental evolution. Evol Med Public Health. 2022;10(1):142-155. doi: 10.1093/emph/eoac010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novazzi F, Taborelli S, Baj A, Focosi D, Maggi F. Asymptomatic SARS-CoV-2 vaccine breakthrough infections in health care workers identified through routine universal surveillance testing. Ann Intern Med. 2021;174(12):1770-1772. doi: 10.7326/M21-3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoon SK, Hegmann KT, Thiese MS, et al. Protection with a third dose of mRNA vaccine against SARS-CoV-2 variants in frontline workers. N Engl J Med. 2022;386(9):1855-1857. doi: 10.1056/NEJMc2201821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen C, Kleynhans J, von Gottberg A, et al. SARS-CoV-2 incidence, transmission, and reinfection in a rural and an urban setting: results of the PHIRST-C cohort study, South Africa, 2020-21. Lancet Infect Dis. 2022;22(6):821-834. doi: 10.1016/S1473-3099(22)00069-X [DOI] [PMC free article] [PubMed] [Google Scholar]